Abstract
Extracellular matrix (ECM) derived components are emerging sources for the engineering of biomaterials that are capable of inducing desirable cell-specific responses. This review explores the use of biomaterials derived from naturally occurring ECM proteins and their derivatives in approaches that aim to regulate cell function. Biomaterials addressed are grouped into six categories: purified single ECM proteins, combinations of purified ECM proteins, cell-derived ECM, tissue-derived ECM, diseased and modified ECM, and ECM-polymer coupled biomaterials. Purified ECM proteins serve as a material coating for enhanced cell adhesion and biocompatibility. Cell-derived and tissue-derived ECM, generated by cell isolation and decellularization technologies, can capture the native state of the ECM environment and guide cell migration and alignment patterns as well as stem cell differentiation. We focus primarily on recent advances in the fields of soft tissue, cardiac, and dermal repair, and explore the utilization of ECM proteins as biomaterials to engineer cell responses.
Keywords: Extracellular Matrix, Acellular matrix, Decellularization, Scaffolds, Biomaterials, Cell therapy, Tissue engineering, Biopolymers, Repair
1. Introduction
1.1. Brief history and trends
In tissue, the ECM provides functionality, structural integrity, and suitable conditions for cell growth. The mechanical and biochemical cues within ECM can guide many cellular functions, such as cell migration and lineage commitment (Engler et al., 2006; Rho et al., 2006). More recent discoveries have strongly implicated the ECM in altering cellular behavior during disease progression (Walraven and Hinz, 2018), stem cell proliferation and differentiation (Reilly and Engler, 2010), and growth factor retention (Klingberg et al., 2018). Therefore, native ECM proteins are considered as candidates for the development of biomaterials capable of inducing specific cellular behavior.
Beginning with purified proteins as biomaterial surface coatings, native ECM has evolved into more complex systems, such as cell-derived and whole-tissue-derived constructs. For example, the early commercial wound healing product Biobrane®, was a synthetic mesh conjugated with porcine collagen. It provided a temporary barrier between the wound bed and the air to protect the underlying cellular environment (Smith, 1995; Tavis et al., 1980). Somewhat more complex, the bilayer artificial skin product Integra® was introduced in the 1980s. It consisted of purified ECM components from cow and shark. The porous material allowed cells to migrate towards the open wound and begin regenerative processes (Burke et al., 1982). Harvested from cadaver skin, AlloDerm® became the first acellular skin substitute for the treatment of burn wounds and breast reconstruction (Wainwright, 1995). AlloDerm® largely retained the native dermal structure while possessing an enhanced regenerative potential. ECM proteins have also been explored for the creation of wound dressings and as carriers for delivering cells. Approved in 2001, Graftskin was one of the first “living” materials to employ bovine type I collagen to encapsulate viable fibroblasts to promote healing. In addition to providing material support, it preconditioned the wound bed with fibroblasts (Veves et al., 2001). Recently, special acellular materials, enriched in growth factors and derived from urinary bladder and placenta matrix, were used to improve wound healing (Choi et al., 2013; Meng et al., 2015; Rameshbabu et al., 2018). Of note, multiple clinical trials are currently investigating the efficacy of amniotic-membrane-derived material in wound healing (Moore et al., 2017)
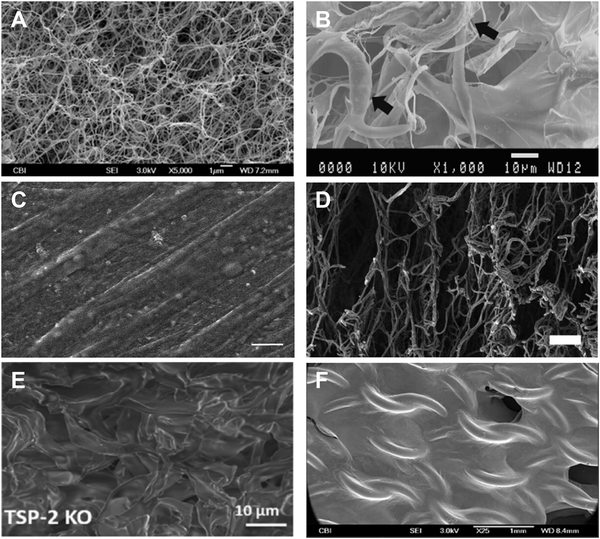
Recent technological advances in decellularizing tissue and isolating cells allow the creation of functional complex biomaterials that take advantage of ECM proteins in their natural form. Bioabsorbable ECM polymers from decellularized tissue provide architecture and retain growth factors and protein modifications that are difficult to reproduce in non-native biomaterials. Moreover, in vitro cell-derived matrix (CDM) provides flexibility in manipulating matrix production and assembly via genetic or pharmacological modification without compromising its overall integrity. Finally, ECM constructs, when delivered in situ, provide a dynamic microenvironment capable of engineering specific cell responses. Figure 1 depicts representative scanning electron microscopy (SEM) images for the six categories of materials covered in this review.
Figure 1.
Representative SEM images of each of the six classes of biomaterials covered in this review.
1.2. Sources for isolating ECM
Various organs and tissues are regarded as suitable reservoirs for several ECM proteins. Table 1 lists the commonly used tissue sources of ECM proteins. Typically, tissues are isolated and processed through mechanical disruption, enzymatic digestion, chromatography, and precipitation. Though these traditional purification methods are effective, they lack the flexibility afforded by adding specific modifications to proteins. Recombinant technology has enabled the rapid production of ECM proteins in vitro with the ability to tune, modify, and manipulate the expressed protein (Even-Ram and Artym, 2011; Trabbic-carlson et al., 2004). Bacterial and eukaryotic cell lines have been used as carriers for producing ECM proteins such as recombinant fibronectin fragments (Amaral et al., 2013; Neubauer et al., 2016; Yun et al., 2015), various types of collagen (Bulleid et al., 2000; Que et al., 2018; Stoichevska et al., 2016), human tropoelastin (Martin et al., 1995; Wang et al., 2016), and laminins (Kortesmaa et al., 2000; Rodin et al., 2010). Such in vitro expression systems are highly reproducible and inexpensive. However, species-specific post-translational modifications have been shown to induce undesirable host responses in vivo, including allergic reactions to gelatin-stabilized vaccines (Olsen et al., 2003).
Table 1.
Source for isolating common ECM proteins
| ECM Protein | Source | Reference |
|---|---|---|
| Collagen | Skin | (McPherson et al., 1986) |
| Tail tendon | (Light and Bailey, 1979; Rajan et al., 2007) | |
| Fish Scale | (Nagai et al., 2004) | |
| Fibronectin | Plasma | (Vuento and Vaheri, 1979)(Ruoslahti et al., 1982) |
| Laminin | Placenta | (Wondimu et al., 2006) |
| Heart | (Paulssons and Saladin, 1989) | |
| Elastin | Aorta | (Rasmussen et al., 1975) |
To create biomaterials for tissue specific regeneration, ECM can be extracted from organs and tissues using decellularization and isolation techniques. However, allogenic and xenogeneic sources exhibit limited long-term efficacy due to the innate and adaptive immune response. Skin substitute products, like TransCyte® and OrCel®, delivered allogeneic fibroblasts to the wound site and exhibited regenerative capacity in short-term studies. Nevertheless, an unfavorable host response and the products’ inability to vascularize the implant resulted in massive cell death and limit the long-term efficacy of these constructs (Varkey et al., 2015).
1.3. Advantages and disadvantages compared to synthetic materials
Native ECM-derived biomaterials, unlike synthetic materials, constitute a dynamic environment that can be processed, remodeled, and replaced during cell therapy (Gattazzo et al., 2014). Synthetic materials such as polyethylene meshes and silicone devices can trigger a foreign body response when implanted, leading to the generation of a fibrotic capsule that isolates the material from the surrounding tissue (Morais et al., 2010). ECM-derived biopolymers can mitigate the foreign body response by presenting ECM molecules at the interface between material and tissue. ECM-derived materials can evoke an innate immune response to replace the implanted matrices with new ECM for host integration. However, materials using allogenic and xenogeneic sources can evoke an adaptive immune response and cytokine release that might shorten the lifespan of the materials. Badylak and Gilbert (2008), Lopresti and Brown (2015), and Morris et al. (2017) have extensively discussed immune response to biomaterials.
As alluded to in section 1.1., native ECM constructs preserve the overall architecture of the matrix. The use of proper enzymatic and non-enzymatic agents, as well as a gentle detergent such as Triton X-100, can retain ECM proteins and matrix-bound components, such as growth factors, to a great extent. Reviews from the Badylak group (Crapo et al., 2011; Gilbert et al., 2006) has comprehensively addressed the effects of the decellularization process on the mechanical and biochemical integrity of ECM. Decellularized constructs have been shown to provide structural support, adhesion sites for cell attachment, and growth factors for cell proliferation (Kim et al., 2017; Wilson et al., 2016). In general, these materials are considered to be more desirable than ECM-modified synthetic constructs fabricated from electrospinning and electrospraying. This is due to the inability of these two techniques to create the multiplex assembly needed to resemble the native ECM. In terms of engineering specific cellular responses, polymers can be modified via functional group chemistry to decorate them with ECM proteins that can increase biorecognition (Benoit et al., 2007; Chu et al., 2018b; Ghosh et al., 2006). Nevertheless, native ECM constructs are considered superior to polymers in supporting tissue regeneration.
Tissues exhibit different physiological stiffnesses in order to provide the optimal environment for cell growth and function (Discher et al., 2009). Such variation cannot be precisely reproduced in native ECM materials due to limitations in processability. Partial modification can be achieved by chemical crosslinking reactions via glutaraldehyde and EDC/NHS chemistry to increase ECM stiffness. However, such changes were shown to be associated with decreased porosity and degradation rate, making the material suboptimal for cell infiltration and host integration (Ye et al., 2010). On the other hand, synthetic polymers, such as polyethylene glycol (PEG), can be easily made into controlled thickness and porosity. As a result, hybrid materials that augment ECM proteins with stronger synthetic polymers were invented to control porosity and the rate of degradation by modifying the polymer backbone while still retaining biocompatibility through surface modification or a coating (Chen et al., 2018; Goyal et al., 2017; Noh et al., 2016).
2. Naturally derived biomaterials and their applications
2.1. Purified single ECM protein biomaterials
Type I collagen is the most abundant structural protein in soft tissue and one of the earliest ECM components to be identified and isolated. It provides cells with a three-dimensional environment that supports cell growth and influences morphology and function. During tissue repair, collagen was shown to promote cell adhesion and migration by engaging cell surface receptors such as integrin β1 subunit (Hynes, 1992), glycoprotein VI (Smethurst et al., 2007), non-specific receptors, and integrin-like receptors (Gullberg et al., 1989). The triple helix structure of collagen offered several favorable characteristics for engineering cellular functions, including thermal stability and mechanical strength (Shoulders and Raines, 2010).
Based on the properties mentioned above, type I collagen is the most popular native material for delivering cells or inducing specific cellular behavior for soft tissue regeneration. Collagen can self-assemble under physiological conditions, absorb onto surfaces through nonspecific interactions (Wallace and Rosenblatt, 2003), and be electrospun into biomaterials (Joshi et al., 2018; Rho et al., 2006). Type I collagen has been used in cell culture as a substrate to study and promote angiogenesis (Cross et al., 2010; Luwang et al., 2018; Ryan et al., 2019), as a wound dressing to treat burn wounds (Oryan et al., 2018), as a vehicle to encapsulate stem cells to induce dermal regeneration (Altman et al., 2008), and as a scaffold to generate a skin substitute for grafting (Trottier et al., 2008). Through integrin signaling and growth factor sensing at its interface with cells, a collagen matrix has been shown to maintain mesenchymal stem cells (MSCs) stemness (Engler et al., 2006; Mauney et al., 2005) or direct stem cells towards specific lineages for tissue regeneration (Farrell et al., 2006; Grier et al., 2018; Laiva et al., 2018). The compatibility between collagen matrices and MSCs enabled a collagen scaffold to serve as a cell delivery vehicle for diabetic wounds to promote repair via improved angiogenesis (Holst et al., 2013). Chattopadhyay & Raines (2014) have generated a comprehensive list of collagen-based biomaterial and commercial products.
Fibronectin promotes cell adhesion and controls cellular functions via peptide domains, including various binding sites for cell surface molecules and other biological molecules such as heparin, fibrin, collagen, and growth factors (Klingberg et al., 2018; Pankov, 2002). The soluble form of fibronectin mainly circulates in plasma. The application of plasma fibronectin is limited to direct application of an aqueous solution, passive absorption, and covalent linkage to the surface of a biomaterial to promote bio-recognition and biocompatibility (MacDonald et al., 2002; Macdonald et al., 1998; Okada et al., 1985; Qiu et al., 2007). These methods lack proper spatial control over deposition. Electrospraying was developed to deposit soluble fibronectin onto the surface of a material with controlled density, thickness, and pore size for optimal cell migration and adhesion (Martyn et al., 2011). Another difficulty in developing fibronectin-based biomaterials is that soluble fibronectin in an aqueous solution cannot be utilized directly to constitute a biomaterial. Thus, recent work has focused on engineering biomaterials for cell therapy from lyophilized powder and precipitated plasma fibronectin. By using a rotary jet spinning technique, human fibronectin was made into nanofibers and assembled into meshes that could be used to improve dermal wound healing (Chantre et al., 2018). By using concentrated fibronectin solution, a large fibronectin mesh was produced after mixing with 0.25 M hydrochloric acid and 2% calcium chloride solution, or from passing through an ultrafiltration cell under constant stirring (Ahmed et al., 2003). Such fabrication methods retain the ability of fibronectin to promote cell adhesion. More importantly, these approaches allow for the production of engineered fibronectin-based three-dimensional constructs.
Other ECM proteins such as (tropo)elastin and laminin present in basement membranes are used in strategies to elicit desired phenotypes for cell therapy. Specifically, different peptide sequences in elastin are employed to generate scaffolding for cell regeneration therapy. These elastin-like polypeptides self-assemble into a gelatinous material above their transition temperature due to the unique thermo-responsive properties. Alternatively, they can be crosslinked by enzymatic reactions. Previously, such aggregates were applied in cartilage regeneration (Betre et al., 2002; McHale et al., 2005) and were also used to encapsulate adipose-derived stem cells (ASCs) for full thickness dermal wound healing (Choi et al., 2016). The effect of an elastin construct on maintaining stemness was also interrogated; the author found that the elasticity and tensegrity of the construct were necessary to provide key mechanical cues for maintaining and expanding hematopoietic stem cells (Holst et al., 2011). A novel manufacturing process called HeaTro, which utilizes tropoelastin as the sole ingredient to manufacture scaffold in different shapes and forms, was invented in the lab of Dr. Anthony Weiss. Mouse and pig full-thickness wound models were used to evaluate the in vivo performance of the HeaTro product. When compared to the Integra Dermal Regeneration Template, increased vascularization was observed in the proximity of an implanted HeaTro-generated scaffold, pointing to the great therapeutic potential of elastin-based products in wound healing (Mithieux et al., 2018).
Laminin 111, a major epithelial ECM component, has been used as a component of Matrigel® to provide a basement membrane-like scaffold. In addition, it has been used in electrospinning to construct fibrous meshes on a charged surface with tunable pore size and fiber diameter. The engineered meshes could maintain ASCs attachment and viability in vitro, and could induce them to develop neurite-like structure (Neal et al., 2009). Laminin 111 has also been shown to participate in other biological processes, including angiogenesis and neural differentiation (Li and Chau, 2010; Neal et al., 2009; Nicosia et al., 1994). However, in recent years, more effort has been dedicated towards investigating the remainder of the 16 known laminin isoforms and their subunits. Laminin 511 was found to maintain the pluripotency of mouse embryonic stem cells through β1 integrins, while laminin 411, 332, and 111 caused either rapid differentiation or cell death (Domogatskaya et al., 2008). By ablating laminin α1 chains in a murine skeletal muscle injury model, it was found that it plays a critical role in maintaining and activating satellite cells (Rayagiri et al., 2018). For further discussion on this topic, please refer to the book chapter on laminin isoforms written by Dr. Anna Domogtskaya and Dr. Sergey Rodin (Domogatskaya and Rodin, 2018).
ECM proteins like collagen, fibronectin, elastin, and laminin are readily accessible through isolation or transgenic expression. The availability of pure ECM proteins allows researchers to investigate how they are essential for cell survival and growth. Moreover, the experimental approaches mentioned above utilize these proteins to generate biorecognizable surfaces to promote integration of the material as well as to direct stem cell differentiation by tuning material properties. Therefore, these simple protein constructs still hold importance in engineering biomaterials.
2.2. Biomaterials made from combinations of purified ECM proteins
ECM proteins like fibronectin, elastin, entactin, and laminin lack the material properties needed for generating macroscale biomaterials. Thus, purified ECM proteins are commonly mixed with other native proteins, such as collagen and fibrinogen, to produce more structurally sound biomaterials. These composite biomaterials provide structural integrity and biomolecular cues that can be easily manipulated both in vivo and in vitro. In an early attempt to mimic the basement membrane, laminin-entactin complexes were added to a collagen scaffold. This resulting material induced greater microvessel formation in vitro (Nicosia et al., 1994). In recent years, a basement membrane mimic containing laminin-111, collagen IV, entactin, and heparin sulfate proteoglycans was shown to support endothelial progenitor cells to form tubular structures and bone-marrow derived stem cells to form capillary-like structures (Arnaoutova et al., 2012). A biomaterial composed of collagen and laminin augmented with chitosan was shown to support the viability of circulating angiogenic cells (McEwan et al., 2016). Different mechanical and biochemical properties of these proteins provided multiplex signals and enhanced the biomaterial’s capability to engineer cell functions through novel special arrangements, such as layer-by-layer assembly (Mauquoy and Dupont-Gillain, 2016) and homogenous gel formation (McEwan et al., 2016). These laminin-containing composite biomaterials could also support multiple cell behaviors including angiogenic function both in vitro and in vivo. Similarly, Floren and Tan (2015) explored the effect of multiprotein materials made of collagen, fibronectin, laminin, and elastin on MSC differentiation. By modifying the material properties, they found a correlation between the elasticity of the material and the extent of MSC vascular commitments.
Fibrin and fibrinogen matrices, harvested from the body’s clotting cascade, have been used as substrates for cell expansion in situ (Stevens et al., 2017) and as delivery vehicles for cells and growth factors (Whelan et al., 2014). When fibronectin was mixed with fibrinogen, the resulting material possessed a greater elasticity (Okada et al., 1985) due to the inter-linked spatial arrangement of the fibronectin and fibrinogen network. This elasticity was found to be more relevant for directing cell functions (Engler et al., 2006). Gelatin, a biopolymer derived from denatured collagen, can be formed into a biomaterial via various methods including phase separation, electrospinning, and solvent casting (Hoque et al., 2014). An injectable synthetic ECM composed of gelatin, hyaluronan, and chondroitin sulfate supported the proliferation of encapsulated NIH3T3 cells and induced matrix deposition by these cells in vivo (Shu et al., 2006). Bovine gelatin displayed lower tensile stiffness compared to collagen, but interacted with soluble elastin with higher affinity, favoring fibrosarcoma (HT-1080) cell line adhesion and growth (Grover et al., 2012). Recently, a multi-compartment system composed of keratin, fibrin, and gelatin was constructed as drug delivery vehicle to release encapsulated substances, such as Mupirocin and Curcumin, in a controlled manner. The fabricated material was capable of accelerating dermal wound healing in a silicone splint animal model by releasing therapeutic agents, facilitating gas exchange, and absorbing exudate (Singaravelu et al., 2017). Other materials that focus on utilizing biopolymers for material backbone, such as polysaccharides and glycosaminoglycans (GAGs), have been reviewed extensively by others (Andersen et al., 2015; Custódio et al., 2014; Lutolf and Hubbell, 2005; Van Vlierberghe et al., 2011).
The building blocks for these multi-protein systems are purified ECM proteins. By combining several of these naturally derived proteins, researchers can adjust the material properties to expose epitopes for biorecognition and induce desirable cellular behaviors. By employing various fabrication methods, different proteins can be layered to create multiplex architectures in engineered biomaterials.
2.3. Cell-derived ECM biomaterials
Even with the most advanced engineering tools, extracted and purified proteins cannot capture the three-dimensional complexity and spatial presentation of growth factors found in native tissue. To create native-like structures, cells like fibroblasts and ASCs were shown to deposit collagen-enriched ECM in vitro when treated with L-ascorbate 2-phosphate (A2-P) or growth factors (Franceschi et al., 2009; Reichenberger et al., 1999). The resulting ECM could be decellularized to provide a cell-free construct capable of modulating cell functions (Pérez-Castrillo et al., 2018; Yu et al., 2018). This method of generating ECM was first adopted to produce native matrix for investigating cellular functions in vitro. Specifically, primary lung fibroblasts were stimulated by TGFβ−1 and TGFβ−3 to produce ECM that captured some features of native tissue and was used to model aspects of lung fibrosis (Reichenberger et al., 1999). Similarly, by spatially arranging fibroblasts, Wilks et al. (2018) employed normal human dermal fibroblasts to produce highly aligned matrix in a ring shape by using a toroid apparatus. Once decellularized, this matrix instructed fibroblasts to realign with the acellular scaffold. Multiple studies showed that ECM derived from fibroblasts reduced MSC proliferation and levels of stem cell markers via α2 and β1 integrins, while promoting chondrogenic differentiation of MSCs in vitro (Dzobo et al., 2016; Zhou et al., 2016). The approach of producing physiologically relevant ECM components has opened other avenues to interrogate the tissue microenvironment. ECM produced by papillary fibroblasts, reticular fibroblasts, and dermal papilla fibroblasts isolated from patients produced matrices that differentially affected the functions of seeded keratinocytes (Ghetti et al., 2018).
Decellularized matrix produced by stem cells shows great potential for maintaining stem cell multipotency and inducing a particular lineage (Antebi et al., 2015; Lin et al., 2012; Ng et al., 2014; Rakian et al., 2015). The following examples highlight this feature of in vitro-derived ECM produced by stem cells. Acellular matrix from fetal mesenchymal stem cells (fMSCs) supported MSCs growth and expansion ex vivo. Specifically, MSCs seeded on the fMSC-derived matrix exhibited osteogenic, adipogenic, and chondrogenic markers (Ng et al., 2014). ECM derived from human umbilical cord MSCs was able to increase MSC proliferation compared to tissue culture plastic. More importantly, MSC-derived ECM improved MSC survival under oxidative stress by inducing the expression of intracellular antioxidative enzymes (X. Liu et al., 2016; Zhou et al., 2018). Decellularized ECM derived from placenta MSCs supported the proliferation and growth of MSCs and induced the cells into osteogenic differentiation (Kusuma et al., 2017). Finally, ECM derived from ASCs and bone marrow stem cells (BMSCs) directed these two types of stem cells to either a chondrogenic or osteogenic phenotype, respectively (Pérez-Castrillo et al., 2018).
Aside from in vitro applications, CDM offers a biocompatible material that can transiently support cell adhesion and viability, thus extending the effective duration of a cell therapy. ASCs have been explored for the repair of damaged tissue (Traktuev et al., 2006), including cardiac tissue (Przybyt et al., 2015), dermal tissue (Yu et al., 2018), and cartilage tissue (Pizzute et al., 2016). Similar to fibroblasts, ASCs were reported to produce abundant ECM following A2-P treatment (Arslan et al., 2018; Yu et al., 2018). ASCs treated with 250 μM A2-P not only deposited dense ECM, but also displayed enhanced stem cell markers critical for proliferation and downstream differentiation at the wound site. The stem cell sheets upregulated CTRP3 expression in ASCs, and the conditioned media suppressed TGF-β1 and TNF-α expression from macrophages. When transplanted as a “living” material onto full thickness dermal wounds, stem cell sheets provided a longer therapeutic effect than cells alone (Yu et al., 2018). Trottier et al. combined ASC cell sheets with keratinocyte and dermal sheets to produce a three-layered skin substitute composed of simulated epidermis, dermis, and hypodermis. When applied in vivo, the skin substitute developed a well-differentiated epidermis and induced cell proliferation as detected by Ki-67 (Trottier et al., 2008). A2-P preconditioning of stem cells was also shown to help regenerate tissues such as cartilage (Pizzute et al., 2016) and tendon (Lui et al., 2016). It is believed that the underlying ECM produced from these treatments provides the essential mechanical and molecular signals that enhance stem cell function in cell therapy.
2.4. Tissue-derived ECM biomaterials
The process of engineering biomaterials using tissue-derived ECM has been facilitated by advances in decellularization. When these techniques were used, tissue-derived ECM was able to retain most of the major protein components and the complex three-dimensional architecture that can serve as a prototyping scaffold for regeneration (Song and Ott, 2011). However, the usage of SDS and harsh mechanical processing in many decellularization protocols can also wash away ECM and matrix-bound proteins and damage the microscopic structure. The overall effect of the decellularization process on protein preservation and structure retention is detailed in reviews mentioned in section 1. 3.. As a result of excessive depletion of structural proteins, the decellularized organs have different mechanical properties than the native tissue. Structurally impaired decellularized products have been shown to fail in vivo or elicit undesirable cellular responses in vitro (Petersen et al., 2012; Tsuchiya et al., 2014). More importantly, the ultimate goal of organ decellularization is to provide an alternative source for transplantation. The immune response of the host is the key determinant of the functional outcome of the decellularized construct. A decellularized organ should encourage host cell infiltration and ECM remodeling so that it can be best suited for seamless integration. Dziki et al. (2017) have compiled recent literature on the immunomodulatory effect of decellularized materials. They observed that the initial anti-inflammatory macrophage response and the Th-2 cell response are critical in promoting the viability of the biomaterial post implantation. Evidently, the choice of decellularization process is important not only in retaining the structural and biochemical integrity of the scaffold, but also in ensuring host integration. When proper approaches are taken to decellularize a tissue, the resulting ECM can add layers of both microscopic and macroscopic complexity to biomaterials. Aside from its three-dimensional structure, decellularized ECM can also capture the biomechanical and biochemical modifications present in the native tissue under physiological conditions (Reing et al., 2010). Much of the discussion below concerns recent research on using decellularized native tissue either intact or as hydrogels, powders, surface coatings, and bio-inks that can elicit desirable cellular responses in vivo for the purpose of tissue repair.
The dermis is the most abundant source of ECM in the body. Dermal matrix is ideal for skin reconstruction because it stimulates the native dermis microenvironment and contains growth factors that support the repopulation of a variety of cell types (Reing et al., 2010). Additionally, the degradation products from the construct can be readily turned over to produce new dermal tissue (Chu et al., 2018a). Commercial products such as AlloDerm® take advantage of the accessibility of this material. Skin matrices have been made into acellular sheets (Gamboa-Bobadilla, 2006), hydrogels (Wolf et al., 2012), and bio-inks (Kim et al., 2018) for different applications. Specifically, acellular dermal matrix (ADM) has been used for skin reconstruction, especially in breast reconstruction (Krishnan et al., 2014). Acellular dermal sheets mimicked native tissue in elastic properties to provide integration with the surrounding tissue and increased patients’ quality of life (Gamboa-Bobadilla, 2006). Use of ADM in sheep showed fibroblast infiltration and vascularization near the implant (Nafisi et al., 2017), implicating ADM in potentially inducing fibroblast and endothelial cell functions for better material integration. ADM was also shown to facilitate dermal wound healing through maintaining ASC survival and directing ASC differentiation into endothelial, epithelial, and fibroblastic lineages that are necessary for closure (Altman et al., 2008; Chu et al., 2018a; Mohamed et al., 2018). As mentioned above, decellularized dermal matrix was also fabricated into a gel form and used as a wound dressing to accelerate wound healing (Wolf et al., 2012), or used as bio-ink for 3D printing in an organogenesis approach (Kim et al., 2018).
Adipose tissue is another plentiful source of ECM. Human decellularized lipoaspirate and its derivatives were reported to have strong mechanical properties (Choi et al., 2011; Ki et al., 2014) and were shown to support autologous ASC survival and expansion, making it a suitable substrate for in situ autologous cell expansion (Young et al., 2011). Decellularized adipose tissue was also shown to support adipogenic differentiation of ASCs (Tan et al., 2017; Turner et al., 2012) and infiltration of these stem cells along with adipose tissue macrophages, which are necessary for adipose tissue regeneration (Kim et al., 2017). Decellularized adipose tissue was enzymatically digested into a thermally responsive hydrogel, which was applied in vivo to improve full thickness dermal wound healing (Wu et al., 2018), promoting adipose tissue formation (Adam Young et al., 2014; Kim et al., 2017), and neovascularization (Adam Young et al., 2014). However, it is unclear whether residual growth factors are present in the decellularized adipose construct to encourage cell proliferation and migration.
In the dynamic and biomechanically complex environment of the heart, cells have limited regenerative capacity, as seen following myocardial infarction and other cardiac diseases (Seif-Naraghi et al., 2013). However, decellularized cardiac tissue possesses desirable properties such as presence of the basement membrane (Roderjan et al., 2019), strong mechanical properties, and inclusion of matrisome proteins to support cardiac repair (Perea-gil et al., 2018). Whole cardiac ECM (Jeffords et al., 2015; Seo et al., 2017; Taylor et al., 2018), dissected ventricle ECM (Arslan et al., 2018; Baghalishahi et al., 2018; Becker et al., 2018; Duan et al., 2011; Grover et al., 2014; Johnson et al., 2011; Kappler et al., 2016; Seif-Naraghi et al., 2013; Singelyn et al., 2012; Ungerleider et al., 2015; Williams et al., 2015), and dissected pericardial ECM (Bielli et al., 2018; Seif-Naraghi et al., 2012; Sonnenberg et al., 2015) have all been studied for cellular repair post myocardial infarction or for coating other materials. Heart tissue possesses tremendous potential in angiogenesis and in directing the differentiation of human embryonic stem cells (hESCs), MSCs, and cardiac progenitor cells into cardiomyocytes (Arslan et al., 2018; Baghalishahi and Piryaei, 2018; Grover et al., 2014; Seif-Naraghi et al., 2013; Williams et al., 2015). Acellular cardiac tissue was also shown to instruct the endothelial, adipogenic, and chondrogenic differentiation of MSCs (Jeffords et al., 2015; Z. Z. Liu et al., 2016). In particular, a hybrid material made from a 3:1 mixture of myocardial ECM and collagen induced hESC contractility on the matrix and expression of Troponin C, a marker for cardiomyocytes (Duan et al., 2011). However, cardiac tissue incorporated materials have only been tested in animal models for short durations ranging from hours to days (Seif-Naraghi et al., 2012; Singelyn et al., 2012). Long term efficacy experiments are still needed to evaluate the material’s ability for cardiac tissue regeneration.
In engineering orthopedic soft tissue, specifically skeletal muscle and tendon, synthetic materials, collagen matrix, and acellular dermal matrix have been extensively investigated (Docheva et al., 2015; Wolf et al., 2015). The use of native muscle tissue has recently been explored for muscle regeneration. Decellularized muscle was found to preserve the anisotropy and ECM components (laminin α2 and fibronectin) necessary to encourage the proliferation of satellite cells, which are critical in restoring muscle function (Mcclure et al., 2018; Zhao et al., 2018). Decellularized skeletal muscle tissues from different parts of the body were shown to retain the distinct mechanical properties unique to the extracted muscle groups (Patel et al., 2019; Wilson et al., 2016), providing a spectrum of material stiffnesses. In a xenotransplantation model, human decellularized skeletal muscle ECM implanted in a rabbit abdominal hernia model prevented visceral herniation but with limited muscle cell coverage at a 3-week time point (Porzionato et al., 2015). Moreover, studies have found that decellularized skeletal matrix encompasses favorable environmental signals for stem cell myogenic differentiation (Perniconi et al., 2011; Stern et al., 2009). In a rat limb volumetric muscle loss model, decellularized skeletal muscle scaffold embedded with minced autologous muscle tissue showed significant restoration of contractile forces compared to using scaffold alone. This change in cellular behavior was confirmed by the increased expression of MyoD marker (Kasukonis et al., 2016).
Advances in tendon repair and regeneration are lagging in comparison to skeletal muscle therapies due to the avascularity of the native tissue and the strict requirement for mechanical matching (Docheva et al., 2015). Despite multiple high-profile cases where tendon repair with commercial products failed (Lin et al., 2018), there is still effort in the field to engineer ECM-derived materials for this application. For example, decellularized tendon ECM was fabricated into a gel-based scaffold to provide a friendly environment for ASC and MSC growth (Farnebo et al., 2017, 2014; Rothrauff et al., 2017). However, hydrogels do not mechanically match with native tendon tissue. In small tendon defects, tendon-derived stem cells stimulated with connective tissue growth factor and AP-2 were able to deposit ECM and provided a short-term solution, showing more organized alignment compared to unstimulated stem cells or fibrin gel in vivo (Lui et al., 2016). For large defects, decellularized tendon slices used in large rotator cuff tear model in rabbits demonstrated short-term improvement of mechanical properties of regenerated tissue, as well as bone matrix formation at the tendon-bone interface (Liu et al., 2018). For additional information the reader is directed to Cheng et al. (2014) and Woo et al. (2019) who have extensively reviewed the use of decellularized tendon and muscle ECM for tissue engineering applications.
The placenta has a unique structure and contains a variety of growth factors and matricellular proteins that support embryonic growth. The innermost lining of the placenta, the amniotic membrane, possesses enriched vasculature and shares similarities with the basement membrane (Becker et al., 2018; Lobo et al., 2016). Because of these properties and its availability, this source of tissue has become a popular choice for tissue engineering. For example, the potential use of placental vasculature for acellular vascular grafts was explored (Schneider et al., 2018). Another exciting study that took advantage of the vasculature in decellularized human placenta to culture hepatic tissue showed promising results in a partial hepatectomy-induced liver failure model in sheep. The existing vasculature and growth factors in decellularized placenta tissue promoted the long-term survival of implanted hepatic tissue with functional results (Kakabadze et al., 2018). Decellularized placenta has also been explored extensively to treat full thickness wounds. The regenerative ability of this ECM-derived biomaterial has been demonstrated in the growth of hair follicles, decreased scarring, and a well-organized dermal and epidermal layer (Choi et al., 2013; Francis et al., 2017; Rameshbabu et al., 2018). A variety of reviews on biomaterials using placenta as a tissue source are available. Niknejad et al. summarized potential applications in both animal models and humans (2008). A full list of ongoing clinical trials involving commercial amniotic products, including NEOX®, Biovance®, and AmnioExcel®, was recently compiled by Moore et al. (2017).
Because of its unique structure, mechanical properties, and pool of growth factors, urinary bladder matrix (UBM) is often used for repair of the urinary bladder, the urinary tract, and hernias (Davis et al., 2017; Pokrywczynska et al., 2015; Rosario et al., 2008; Zografakis et al., 2018) Aside from these common uses, in recent years UBM has been studied to replace ADM for vaginal and abdominal wall reconstruction (Liang et al., 2017; Young et al., 2018). Both authors found that the mechanical similarity between UBM and ADM made it a good candidate to replace the latter as a surgical substitute. UBM-derived hydrogel was shown to attract perivascular stem cells and modulate the host response by downregulating macrophage-derived TNFα, uPA, and IL-1β (Slivka et al., 2014) and promoting the induction of the anti-inflammatory phenotype in macrophages (Meng et al., 2015). In addition, UBM maintained the multipotency of BMSC in culture. Upon induction, BMSCs differentiated into bone, adipose, and smooth muscle cells (Antoon et al., 2012). In summary, UBM is considered a promising surgical graft for various applications. Its abilities to induce an anti-inflammatory response and maintain the multipotency of stem cells present itself as a good carrier for stem cell transfer and implantation.
Decellularized small intestine submucosa (SIS) ECM was developed by Badylak and colleagues and has been significantly investigated for grafting applications in tissues, including blood vessel (Badylak et al., 1989), urethra (Kropp et al., 1998), and bladder (Chen and Badylak, 2001; Lin et al., 2014). Some of the advantages of SIS include its ability to retain the directionality of diffusion and the low porosity found in the native tissue (Andrée et al., 2013). Additionally, decellularized SIS ECM was found to retain higher GAG and laminin content compared to ECM derived from tendon and pericardium (Claudio-Rizo et al., 2017). The construct was found to have abundant fibroblast growth factor (FGF) and transforming growth factor beta 1 (TGFβ−1), which was shown to enhance fibroblast migration and matrix integration in models of both ligament (Fisher et al., 2012; Liang et al., 2015) and cardiac repair (Mewhort et al., 2017). SIS ECM has also shown therapeutic potential in mucosa regeneration. For example, it induced reepithelization in patients who received esophageal circumferential resection (Badylak et al., 2011). When encapsulated with gingiva-derived mesenchymal stem cells, SIS ECM induced regeneration of tongue myomucosa (Xu et al., 2017). SIS matrix also restored the mucosal barrier in an ulcerative colitis model by encouraging the pro-inflammatory phenotype of macrophages (Keane et al., 2017).
Other tissue-derived materials for engineering cell functions are also on the horizon, including omentum-derived ECM for cardiac cell delivery (Shevach et al., 2015), brain-derived ECM for inducing a pro-inflammatory macrophage phenotype (Meng et al., 2015), and liver-derived ECM for bile duct formation (Lewis et al., 2018).
2.5. Diseased and modified ECM biomaterials
Disease and genetic mutations can result in changes in molecular ECM structure and the functions and secretome of the cells residing in the space (Mor-Yossef Moldovan et al., 2018; Robert and Johnson, 2001; Walraven and Hinz, 2018). In addition, matrix remodeling, often accompanying disease progression, is associated with changes in the level of soluble factors such as TGFβ (Doyle et al., 2012), matrix metalloproteinases (MMPs), and cross-linking enzymes (Argyropoulos et al., 2016). Preparation of ECM constructs from diseased tissues provides an additional investigational tool to determine the relative contribution of abnormal ECM to cellular reprogramming and pathological features. In some cases, decellularized constructs displayed pronounced biophysical changes during the progression of the disease. For example, decellularized lung fibrotic tissue revealed the heterogeneity of stiffness in the diseased tissue, showing a pattern of focal spreading of fibrotic tissue deposition (Melo et al., 2014). In another case, decellularized lungs from patients with chronic obstructive pulmonary disease (COPD) did not support the long-term survival of bronchial epithelial cells, endothelial progenitor cells, MSCs, and lung fibroblasts. However, when the cells were plated onto tissue culture plates coated with homogenized ECM, there was no difference in survival between normal lung ECM and disease ECM, suggesting that the three dimensional ECM structure was abnormal in the COPD lung (Wagner et al., 2014). Decellularized cardiac tissue post myocardial infarction showed significant remodeling events and inhibited MSC differentiation into the myogenic lineage due to increased stiffness (Sullivan et al., 2014). Decellularized tissue taken from organisms in normal and diseased states enable the isolation of the ECM component and can reveal how three-dimensional structure is altered and in turn influences cellular function during disease progression.
Similar to diseased tissues, cells isolated from disease models produce altered ECM that is useful in analyzing the microenvironment of a variety of diseases such as fibrosis, cancer, and diabetes, and the disease CDM can offer new knowledge on how the tissue microenvironment influences cellular function and disease progression. Matrix derived from subpopulations of patients’ dermal fibroblasts (papillary fibroblasts, reticular fibroblasts, and dermal papilla fibroblasts) contained distinct fibers that were also observed in the tissue. These fibroblasts maintained “memories” that instructed the intracellular machinery to produce highly or randomly organized ECM fibers (Ghetti et al., 2018). When these matrices were repopulated with cells, scientists were able to reenact the cellular response ex vivo by analyzing cell functions, the production of secreted factors, and gene expression (Hedström et al., 2018). Moreover, analysis of ECM derived from tumor associated fibroblasts (Amatangelo et al., 2005; Castelló-Cros and Cukierman, 2009) and lymphatic endothelial cells (Ungaro et al., 2009) allowed the identification of ECM properties and growth factors that were essential for metastasis. Additional research on matrix in aging and diabetic conditions have elucidated how dysregulated ECM can facilitate disease progression. Specifically, when compared to young dermal fibroblasts, aged fibroblasts secreted less HAPLN1, a hyaluronic and proteoglycan link protein, leading to aligned ECM fibers that promoted tumor metastasis (Kaur et al., 2018). By using cell-derived ECM, scientists were also able to study the effect of obesity, a key risk factor for breast cancer, on tumor development and progression. Adipose stromal cells (ASCs) isolated from obese animals displayed an enhanced profibrotic phenotype with elevated levels of a-SMA and fibronectin. As a result, the ECM produced by diabetic ASCs showed a linearized pattern with partial unfolding and higher stiffness. Additional experiments performed on human breast cancer cell line MDA-MB231 showed that diabetic ECM promoted breast tumor cell growth via the YAP/TAZ pathway (Seo et al., 2015). These studies highlight the potential offered by studying CDM in the context of various diseases.
Tissue-derived biomaterials are normally extracted from healthy donors. However, genetic engineering offers an alternative for modulating cell functions through changing matrix composition. Galactosyl-α(1,3) galactose (alpha-gal) is a key player in IgE binding and the immune response (Steinke et al., 2015). In principle, biomaterials from alpha-gal knock-out (KO) animals should reduce the extent of immune rejection. An ECM sheet and an ECM hydrogel derived from alpha-gal deficient porcine tissue were used to treat a transected ACL goat model. The ECM sheet and the hydrogel showed increased neo-tissue generation around the injury site compared to a suture-only control group (Fisher et al., 2012). However, the immunological benefit of this material could not be fully interpreted because a wild type construct as the control was not included. Thrombospondin-2 (TSP2) is a matricellular protein that influences multiple processes including collagen assembly and angiogenesis (Calabro et al., 2015). Matrix derived from TSP2-KO animals displayed impaired von Willebrand factor adhesion, which was associated with resistance to thrombosis (Kristofik et al., 2016, 2017). Acellular dermal matrix developed from TSP2-KO mouse exhibited a greater pro-migratory effect and an enhanced healing ability in a diabetic animal model with greater vessel maturation compared to matrix derived from wild type animals (Morris et al., 2018). Similarly TSP2KO skin-derived hydrogel displayed altered mechanical properties and improved healing of skin wounds in diabetic mice (Morris et al., 2018).
ECM decellularized from diseased tissue can draw out parts of the underlying mechanisms of cellular dysfunction by recreating the ECM environment ex vivo. Additionally, genetically modified matrix holds tremendous potential in eliciting desired cellular responses for soft tissue regeneration.
2.6. ECM-polymer coupled biomaterials
Tissue- and cell-derived ECMs display enhanced host response and integration in comparison to synthetic materials. However, in most cases, their fast reabsorption prevents them from providing long-term structural support. To overcome this limitation, synthetic polymers capable of being made into organized meshes and fibers have been combined with native ECM (Fu and Wang, 2012). These polymer-ECM coupled materials, by combining the chemical stability of synthetic polymers and the biocompatibility of ECM proteins, have been shown to induce long-term cellular programming (Shu et al., 2013).
Purified ECM protein or peptide sequences conjugated to, or coated on, a polymer matrix can exhibit stronger cellular effects compared to synthetic polymers alone. For example, fibronectin domains, when coupled to a PEG scaffold, had a greater pro-migratory effect on fibroblasts in a dermal wound compared to RGD functionalized polymer or unmodified polymer scaffolds (Ghosh et al., 2006). In another example, the basement membrane component laminin-111 conjugated to PEG hydrogel induced satellite cell proliferation and myogenic differentiation in a muscle injury model (Ziemkiewicz et al., 2018). This composite material was also shown to provide greater structural integrity than laminin alone and elicited cellular responses that were similar to more complex basement membrane-based materials (Arnaoutova et al., 2012). In addition, fibronectin decorated polyvinyl alcohol (PVA) gel shielded the polymer surface for better fibroblast recognition (Nuttelman et al., 2001). Collagen has also been used in combination with synthetic polymers such as polycaprolactone (PCL) and bioactive glass nanoparticles, and the hybrid material was used as a delivery vehicle for endothelial progenitor cells in dermal wound healing (Wang et al., 2018). In another example, a collagen-alginate composite material was formulated as a cell carrier. It was found that higher collagen content of this material increased its overall elasticity and enhanced the osteogenic differentiation of MSCs (Lee et al., 2018).
Tissue-derived ECM has also been engineered with polymer backbones. PEG material covalently incorporated into myocardial matrix generated materials with a wide spectrum of stiffnesses and degradation rates for directing cellular function (Grover et al., 2014). Through electrospinning, a dermal ECM and poly(ester urethane)urea (PEUU) composite material was formed that induced repair in a full-thickness abdominal defect model (Hong et al., 2011). In a similar manner, decellularized muscle tissue ECM was electrospun with PCL to provide more structural integrity (Patel et al., 2019). When BARD™, a clinical polypropylene mesh, was coated with porcine dermal ECM, it showed a reduced host foreign body response (Wolf et al., 2014). Finally, a PEGylated graphene oxide scaffold containing dermal matrix and quercetin, a dietary bioflavonoid, was able to induce MSC adipogenic and osteogenic differentiation as well as improved diabetic wound healing (Chu et al., 2018b)
The above studies show that synthetic polymers and biopolymers can complement each other to form stable constructs with tunability in material properties, such as porosity, stiffness, and degradation rate, and can additionally be tuned through chemical crosslinking. Based on the properties of the native ECM, composite materials are expected to display increased biorecognition and a reduction in the foreign body response.
3. Conclusion and perspectives
The adoption of ECM-based biomaterials for soft tissue repair has shown great success in animal models and in some clinical settings. Through various fabrication techniques, ECM-derived materials have evolved into a variety of shapes: surface coatings, meshes, hydrogels, cell sheets, and decellularized tissues and organs. Obviously, the increase in available forms of ECM-derived biomaterials widens the pool of potential clinical applications.
Purified ECM proteins provide the basic biocompatibility. Their abundance and rather simple fabrication process allow for their easy adoption in engineering biomaterials. Subsets of ECM proteins, like fibronectin type III domain and laminin-111, are identified as more potent and essential ECM components that can induce a greater cellular response. By combining different ECM proteins, researchers can construct materials that closely resemble the native tissue micro-environment. Matrices derived from cells provide material that is strong enough to use as a delivery vehicle for cell transplantation. These matrices also provide opportunities to investigate the dynamic processes of ECM deposition and modification under diseased conditions ex vivo. In addition, cell lines have been genetically modified to produce ECM proteins in large quantity. Thus, CDM provides an alternative for the future of manufacturing biomaterials independent of tissue sources. Tissue-derived ECM provides the three-dimensional structure and growth factors needed for engineering functional tissues and organs. However, great care is still required when choosing the proper decellularization process to achieve the balance between protein retention and host immune response. Many studies mentioned above have shown that mechanosensing through integrins is critical in maintaining cell survival and directing stem cell lineage. Anisotropic and isotropic properties of ECM have been proven to influence cell proliferation and differentiation as seen in the case of satellite cells cultured on decellularized muscle tissue. On the other hand, decellularized organs, if matching in mechanical properties, can be used to repair defects in tissues different from their native tissue of origin as seen with acellular dermal matrix and urinary bladder matrix. This unique property of tissue-derived constructs allows for repurposing material to combat the shortage of certain organs. Hydrogels derived from this type of ECM retain growth factors and some material properties present in the original source. They have been shown to provide the necessary stiffness and microenvironment for resident cells to infiltrate and populate. More excitingly, using ECM derived from genetically modified animals or conditioned tissue in disease states can reflect the physiological changes that may benefit in vivo repair. These methods open unique avenues for studying the role of ECM in disease progression and repair, and potentially provide new therapeutic targets and ways to engineer cell functions. As shown in the previous sections, these ECM-derived materials are capable of instructing cellular behavior, especially MSCs. Table 2 summarizes the effect of recently developed materials on MSC survival, proliferation, and differentiation.
Table 2.
The effect of native ECM-derived materials on MSC function
| Function | Material | Material Category | Reference |
|---|---|---|---|
| Collagen + polydopamine | Composite (polymer) | (Razavi et al., 2018) | |
| ECM derived from BM - MSC | Cell-derived | (Rakian et al., 2015) | |
| ECM derived from BM - MSC | Cell-derived | (Lin et al., 2012b) | |
| Proliferation and maintanence of stemness | ECM derived from MSC + collagen + hydroxyapatite | Cell-derived | (Antebi et al., 2015) |
| Collagen | Purified protein | (Mauney et al., 2005) | |
| Pericardial matrix | Tissue-derived | (Z. Z. Liu et al., 2016) | |
| ECM derived from fetal MSC | Cell-derived | (Ng et al., 2014) | |
| Urinary bladder matrix | Tissue-derived | (Antoon et al., 2012) | |
| Antioxidation | ECM derived from umbillical cord MSC | Cell-derived | (X. Liu et al., 2016) (Zhou et al., 2018) |
| Neural differentiation | Dermal matrix + reduced graphene oxide | Composite (nonpolymer) | (Guo et al., 2016) |
| ECM derived from ASC | Cell-derived | (Perez-Castrillo et al., 2018) | |
| Chondrogenic differentiation | Tendon matrix and cartilage matrix | Tissue-derived | (Rothrauff et al., 2017) |
| ECM derived from fibroblast | Cell-derived | (Dzobo et al., 2016; Zhou et al., 2016) | |
| Osteogenic differentiation | ECM derived from placenta MSC | Cell-derived | (Kusuma et al., 2017) |
| Collagen + alginate | Composite (polymer) | (Lee et al., 2018) | |
| Adipogenic and osteogenic differentiation Inhibition of myogenic differentiation | Dermal matrix + graphene oxide + PEG + Quercetin | Composite (polymer and nonpolymer) | (Chu et al., 2018) |
| Infarcted myocardium matrix | Modified ECM | (Sullivan et al., 2014) | |
| Myogenic differentiation | Myocardial matrix | Tissue-derived | (Arslan et al., 2018) |
| Angiogenic differentiation | Collagen, laminin, fibronectin, and elastin | Combination of purified proteins | (Floren and Tan, 2015) |
| Angiogenic under hypoxia | Collagen + chitosan | Composite (nonpolymer) | (Tong et al., 2016) |
| Endothelial differentiation | Myocardial matrix | Tissue-derived | (Jeffords et al., 2015) |
Sources of ECM proteins have shifted from xenogeneic sources to allogeneic, even autologous sources. This adoption lowers the risk of undesirable immune responses. In particular, adipose and dermal tissues -- the most easily accessible sources for ECM proteins -- can be developed into personalized biomaterials for wound dressing or reconstructive repair. A new trend of using genetically modified animals as the source of ECM allows the investigation of the intracellular mechanisms that regulate matrix production and assembly. Animals with gene deletions or mutations can display drastically different matrix morphology, and in the case of the TSP2-KO genetic modification, the abnormal matrices can further induce the regenerative potential of the wound bed, leading to faster wound closure. Other genetic models may also possess interesting matrix phenotypes that can be exploited to engineer cell functions.
4. Acknowledgements
This work was supported by NIH Grants (GM-072194; HL083895).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- Adam Young D, Bajaj V, Christman KL, 2014. Decellularized adipose matrix hydrogels stimulate in vivo neovascularization and adipose formation. J. Biomed. Mater. Res. A 102, 1641–51. 10.1002/jbm.a.35109 [DOI] [PubMed] [Google Scholar]
- Ahmed Z, Underwood S, Brown RA, 2003. Nerve Guide Material Made from Fibronectin: Assessment of in Vitro Properties. Tissue Eng. 9, 219–231. 10.1089/107632703764664693 [DOI] [PubMed] [Google Scholar]
- Altman AM, Matthias N, Yan Y, Song YH, Bai X, Chiu ES, Slakey DP, Alt EU, 2008. Dermal matrix as a carrier for in vivo delivery of human adipose-derived stem cells. Biomaterials 29, 1431–1442. 10.1016/j.biomaterials.2007.11.026 [DOI] [PubMed] [Google Scholar]
- Amaral IF, Neiva I, Ferreira F, Sousa SR, Piloto AM, Lopes CDF, Barbosa MA, Kirkpatrick CJ, Pêgo AP, 2013. Endothelialization of chitosan porous conduits via immobilization of a recombinant fibronectin fragment ( rhFNIII 7 – 10 ). Acta Biomater. 9, 5643–5652. 10.1016/j.actbio.2012.10.029 [DOI] [PubMed] [Google Scholar]
- Amatangelo MD, Bassi DE, Klein-szanto JP, Cukierman E, 2005. Stroma-Derived Three-Dimensional Matrices Are Necessary and Sufficient to Promote Desmoplastic Differentiation of Normal Fibroblasts. Matrix Pathol. 167, 475–488. 10.1016/S0002-9440(10)62991-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen T, Auk-Emblem P, Dornish M, 2015. 3D Cell Culture in Alginate Hydrogels. Microarrays 4, 133–161. 10.3390/microarrays4020133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrée B, Bär A, Haverich A, Hilfiker A, 2013. Small Intestinal Submucosa Segments as Matrix for Tissue Engineering: Review. Tissue Eng. Part B Rev. 19, 279–291. 10.1089/ten.teb.2012.0583 [DOI] [PubMed] [Google Scholar]
- Antebi B, Zhang Z, Wang Y, Lu Z, Chen X-D, Ling J, 2015. Stromal-cell-derived extracellular matrix promotes the proliferation and retains the osteogenic differentiation capacity of mesenchymal stem cells on three-dimensional scaffolds. Tissue Eng. Part C. Methods 21, 171–181. 10.1089/ten.TEC.2014.0092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoon R, Yeger H, Loai Y, Islam S, Farhat WA, 2012. Impact of bladder-derived acellular matrix, growth factors, and extracellular matrix constituents on the survival and multipotency of marrow-derived mesenchymal stem cells. J. Biomed. Mater. Res. - Part A 100 A, 72–83. 10.1002/jbm.a.33230 [DOI] [PubMed] [Google Scholar]
- Argyropoulos AJ, Robichaud P, Balimunkwe RM, Fisher GJ, Hammerberg C, Yan Y, Quan T, 2016. Alterations of dermal connective tissue collagen in diabetes: Molecular basis of aged-appearing skin. PLoS One 11, 1–17. 10.1371/journal.pone.0153806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaoutova I, George J, Kleinman HK, Benton G, 2012. Basement Membrane Matrix (BME) has Multiple Uses with Stem Cells. Stem Cell Rev. Reports 8, 163–169. 10.1007/s12015-011-9278-y [DOI] [PubMed] [Google Scholar]
- Arslan YE, Galata YF, Sezgin Arslan T, Derkus B, 2018. Trans-differentiation of human adipose-derived mesenchymal stem cells into cardiomyocyte-like cells on decellularized bovine myocardial extracellular matrix-based films. J. Mater. Sci. Mater. Med 29, 127 10.1007/s10856-018-6135-4 [DOI] [PubMed] [Google Scholar]
- Badylak SF, Gilbert TW, 2008. Immune response to biologic scaffold materials. Semin. Immunol 20, 109–116. 10.1016/j.smim.2007.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badylak SF, Hoppo T, Nieponice A, Gilbert TW, Davison JM, Jobe BA, 2011. Esophageal Preservation in Five Male Patients After Endoscopic Inner-Layer Circumferential Resection in the Setting of Superficial Cancer: A Regenerative Medicine Approach with a Biologic Scaffold. Tissue Eng. Part A 17, 1643–1650. 10.1089/ten.tea.2010.0739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badylak SF, Lantz GC, Coffey A, Geddes L. a, 1989. Small intestinal submucosa as a large diameter vascular graft in the dog. J. Surg. Res 47, 74–80. 10.1016/0022-4804(89)90050-4 [DOI] [PubMed] [Google Scholar]
- Baghalishahi M, Efthekhar-vaghefi S. hasan, Piryaei A, Nematolahi-mahani SN, Mollaei HR, Sadeghi Y, 2018. Cardiac extracellular matrix hydrogel together with or without inducer cocktail improves human adipose tissue-derived stem cells differentiation into cardiomyocyte–like cells. Biochem. Biophys. Res. Commun 502, 215–225. 10.1016/j.bbrc.2018.05.147 [DOI] [PubMed] [Google Scholar]
- Baghalishahi M, Piryaei A, 2018. Cardiac extracellular matrix hydrogel together with or without inducer cocktail improves human adipose tissue-derived stem cells differentiation into cardiomyocyte-like cells. Biochem. Biophys. Res. Commun 502, 215–225. 10.1016/j.bbrc.2018.05.147 [DOI] [PubMed] [Google Scholar]
- Becker M, Maring JA, Schneider M, Martin AXH, Seifert M, Klein O, Braun T, Falk V, Stamm C, 2018. Towards a novel patch material for cardiac applications: Tissue-specific extracellular matrix introduces essential key features to decellularized amniotic membrane. Int. J. Mol. Sci 19, 1–20. 10.3390/ijms19041032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit DSW, Durney AR, Anseth KS, 2007. The effect of heparin-functionalized PEG hydrogels on three-dimensional human mesenchymal stem cell osteogenic differentiation. Biomaterials 28, 66–77. 10.1016/j.biomaterials.2006.08.033 [DOI] [PubMed] [Google Scholar]
- Betre H, Setton LA, Meyer DE, Chilkoti A, 2002. Characterization of a genetically engineered elastin-like polypeptide for cartilaginous tissue repair. Biomacromolecules 3, 910–916. 10.1021/bm0255037 [DOI] [PubMed] [Google Scholar]
- Bielli A, Bernardini R, Varvaras D, Rossi P, Di Blasi G, Petrella G, Buonomo OC, Mattei M, Orlandi A, 2018. Characterization of a new decellularized bovine pericardial biological mesh: Structural and mechanical properties. J. Mech. Behav. Biomed. Mater 78, 420–426. 10.1016/j.jmbbm.2017.12.003 [DOI] [PubMed] [Google Scholar]
- Bulleid NJ, John DCA, Kadler KE, 2000. Recombinant expression systems for the production of collagen. Biochem. Soc. Trans 28, 350–353. [PubMed] [Google Scholar]
- Burke JF, Yannas IV, Quinby WC, Bondoc CC, Jung W., 1982. Successful use of a physiologically acceptable artificial skin in the treatment of extensive burn injury. Plast. Reconstr. Surg 70, 784 10.1097/00006534-198212000-00092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabro NE, Kristofik NJ, Kyriakides TR, 2015. Thrombospondin-2 and extracellular matrix assembly Nicole. Biochim Biophys Acta 1840, 2396–2402. 10.1021/nl061786n.Core-Shell [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelló-Cros R, Cukierman E, 2009. Stromagenesis During Tumorigenesis: Characterization of Tumor-associated Fibroblasts and Stroma-derived 3D Matrices, in: Even-Ram S, Artym V (Eds.), Extracellular Matrix Protocols: Second Edition. Humana Press, Totowa, NJ, pp. 275–305. 10.1007/978-1-59745-413-1_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantre CO, Campbell PH, Golecki HM, Buganza AT, Capulli AK, Deravi LF, Dauth S, Sheehy SP, Paten JA, Gledhill K, Doucet YS, Abaci HE, Ahn S, Pope BD, Ruberti JW, Hoerstrup SP, Christiano AM, Parker KK, 2018. Production-scale fibronectin nanofibers promote wound closure and tissue repair in a dermal mouse model. Biomaterials 166, 96–108. 10.1016/j.biomaterials.2018.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S, Raines RT, 2014. Review collagen-based biomaterials for wound healing. Biopolymers 101, 821–833. 10.1002/bip.22486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MK, Badylak SF, 2001. Small bowel tissue engineering using small intestinal submucosa as a scaffold. J. Surg. Res 99, 352–358. 10.1006/jsre.2001.6199 [DOI] [PubMed] [Google Scholar]
- Chen Y, Ye S-H, Sato H, Zhu Y, Shanov V, Tiasha T, Amore AD, Luketich S, Wan G, Wagner WR, 2018. Hybrid scaffolds of Mg alloy mesh reinforced polymer / extracellular matrix composite for critical - sized calvarial defect reconstruction 1374–1388. 10.1002/term.2668 [DOI] [PubMed] [Google Scholar]
- Cheng CW, Solorio LD, Alsberg E, 2014. Decellularized tissue and cell-derived extracellular matrices as scaffolds for orthopaedic tissue engineering. Biotechnol. Adv 32, 462–484. 10.1016/j.biotechadv.2013.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JS, Kim BS, Kim JY, Kim JD, Choi YC, Yang H, Park K, Lee HY, Cho YW, 2011. Decellularized extracellular matrix derived from human adipose tissue as a potential scaffold for allograft tissue engineering 292–299. 10.1002/jbm.a.33056 [DOI] [PubMed] [Google Scholar]
- Choi JS, Kim JD, Yoon HS, Cho YW, 2013. Full-Thickness Skin Wound Healing Using Human Placenta-Derived Extracellular Matrix Containing Bioactive Molecules. Tissue Eng. Part A 19, 329–339. 10.1089/ten.tea.2011.0738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SK, Park JK, Kim JH, Lee KM, Kim E, Jeong KS, Jeon WB, 2016. Integrin-binding elastin-like polypeptide as an in situ gelling delivery matrix enhances the therapeutic efficacy of adipose stem cells in healing full-thickness cutaneous wounds. J. Control. Release 237, 89–100. 10.1016/j.jconrel.2016.07.006 [DOI] [PubMed] [Google Scholar]
- Chu J, Shi P, Deng X, Jin Y, Liu H, Chen M, Han X, 2018a. Dynamic multiphoton imaging of acellular dermal matrix scaffolds seeded with mesenchymal stem cells in diabetic wound healing. J. Biophotonics 11 10.1002/jbio.201700336 [DOI] [PubMed] [Google Scholar]
- Chu J, Shi P, Yan W, Fu J, Yang Z, He C, Deng X, Liu H, 2018b. PEGylated graphene oxide-mediated quercetin-modified collagen hybrid scaffold for enhancement of MSCs differentiation potential and diabetic wound healing. Nanoscale 10, 9547–9560. 10.1039/c8nr02538j [DOI] [PubMed] [Google Scholar]
- Claudio-Rizo JA, Rangel-Argote M, Castellano LE, Delgado J, Mata-Mata JL, Mendoza-Novelo B, 2017. Influence of residual composition on the structure and properties of extracellular matrix derived hydrogels. Mater. Sci. Eng C 79, 793–801. 10.1016/j.msec.2017.05.118 [DOI] [PubMed] [Google Scholar]
- Crapo PM, Gilbert TW, Badylak DVM, 2011. An overview of tissue and whole organ decellularization processes. Biomaterials 32, 3233–3243. 10.1016/j.biomaterials.2011.01.057.An [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross VL, Zheng Y, Won Choi N, Verbridge SS, Sutermaster BA, Bonassar LJ, Fischbach C, Stroock AD, 2010. Dense type I collagen matrices that support cellular remodeling and microfabrication for studies of tumor angiogenesis and vasculogenesis in vitro. Biomaterials 31, 8596–8607. 10.1016/j.biomaterials.2010.07.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Custódio CA, Reis RL, Mano JF, 2014. Engineering Biomolecular Microenvironments for Cell Instructive Biomaterials. Adv. Healthc. Mater 3, 797–810. 10.1002/adhm.201300603 [DOI] [PubMed] [Google Scholar]
- Davis NF, Cunnane EM, O’Brien FJ, Mulvihill JJ, Walsh MT, 2017. Tissue engineered extracellular matrices (ECMs) in urology: Evolution and future directions. Surgeon 16, 55–65. 10.1016/j.surge.2017.07.002 [DOI] [PubMed] [Google Scholar]
- Discher DE, Mooney DJ, Zandstra PW, 2009. Growth Factors, Matrices, and Forces Combine and Control Stem Cells Dennis E. Discher,. Science (80-. ). 324, 1673–1678. 10.1126/science.1171643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docheva D, Müller SA, Majewski M, Evans CH, 2015. Biologics for tendon repair. Adv. Drug Deliv. Rev 84, 222–239. 10.1016/j.addr.2014.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domogatskaya A, Rodin S, 2018. Biologically Relevant Laminins in Regenerative Medicine, in: Extracellular Matrix for Tissue Engineering and Biomaterials. Stem Cell Biology and Regenerative Medicine. Humana Press, Cham, pp. 59–82. [Google Scholar]
- Domogatskaya A, Rodin S, Boutaud A, Tryggvason K, 2008. Laminin-511 but Not −332, −111, or −411 Enables Mouse EmbryonicStem Cell Self-Renewal In Vitro. Stem Cells 26, 2800–2809. 10.1634/stemcells.2007-0389 [DOI] [PubMed] [Google Scholar]
- Doyle JJ, Gerber EE, Dietz HC, 2012. Matrix-dependent perturbation of TGFβ signaling and disease. FEBS Lett. 586, 2003–2015. 10.1016/j.febslet.2012.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Y, Liu Z, O’Neill J, Wan LQ, Freytes DO, Vunjak-Novakovic G, 2011. Hybrid gel composed of native heart matrix and collagen induces cardiac differentiation of human embryonic stem cells without supplemental growth factors. J. Cardiovasc. Transl. Res 4, 605–615. 10.1007/s12265-011-9304-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziki JL, Huleihel L, Scarritt ME, Badylak SF, 2017. Extracellular Matrix Bioscaffolds as Immunomodulatory Biomaterials. Tissue Eng. Part A 23, 1152–1159. 10.1089/ten.tea.2016.0538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzobo K, Turnley T, Wishart A, Rowe A, Kallmeyer K, van Vollenstee FA, Thomford NE, Dandara C, Chopera D, Pepper MS, Parker MI, 2016. Fibroblast-Derived Extracellular Matrix Induces Chondrogenic Differentiation in Human Adipose-Derived Mesenchymal Stromal/Stem Cells in Vitro. Int. J. Mol. Sci 17 10.3390/ijms17081259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, Discher DE, 2006. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 126, 677–689. 10.1016/j.cell.2006.06.044 [DOI] [PubMed] [Google Scholar]
- Even-Ram S, Artym VV, 2011. Extracellular Matrix Protocols, Methods in Molecular Biology. 10.1007/978-1-61779-166-6_2 [DOI] [Google Scholar]
- Farnebo S, Farnebo L, Kim M, Woon C, Pham H, Chang J, 2017. Optimized Repopulation of Tendon Hydrogel: Synergistic Effects of Growth Factor Combinations and Adipose-Derived Stem Cells. Hand 12, 68–77. 10.1177/1558944715628005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnebo S, Woon CYL, Schmitt T, Joubert L-M, Kim M, Pham H, Chang J, 2014. Design and Characterization of an Injectable Tendon Hydrogel: A Novel Scaffold for Guided Tissue Regeneration in the Musculoskeletal System. Tissue Eng. Part A 20, 1550–1561. 10.1089/ten.tea.2013.0207 [DOI] [PubMed] [Google Scholar]
- Farrell E, O’Brien FJ, Doyle P, Fischer J, Yannas I, Harley BA, O’Connell B, Prendergast PJ, Campbell VA, 2006. A Collagen-glycosaminoglycan Scaffold Supports Adult Rat Mesenchymal Stem Cell Differentiation Along Osteogenic and Chondrogenic Routes. Tissue Eng. 12, 459–468. 10.1089/ten.2006.12.459 [DOI] [PubMed] [Google Scholar]
- Fisher MB, Liang R, Jung HJ, Kim KE, Zamarra G, Almarza AJ, McMahon PJ, Woo SLY, 2012. Potential of healing a transected anterior cruciate ligament with genetically modified extracellular matrix bioscaffolds in a goat model. Knee Surgery, Sport. Traumatol. Arthrosc 20, 1357–1365. 10.1007/s00167-011-1800-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floren M, Tan W, 2015. Three-dimensional, soft neotissue arrays as high throughput platforms for the interrogation of engineered tissue environments. Biomaterials 59, 39–52. 10.1016/j.biomaterials.2015.04.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi RT, Iyer BS, Cui Y, 2009. Effects of ascorbic acid on collagen matrix formation and osteoblast differentiation in murine MC3T3-E1 cells. J. Bone Miner. Res 9, 843–854. 10.1002/jbmr.5650090610 [DOI] [PubMed] [Google Scholar]
- Francis MP, Breathwaite E, Bulysheva AA, Varghese F, Rodriguez RU, Dutta S, Semenov I, Ogle R, Huber A, Tichy AM, Chen S, Zemlin C, 2017. Human placenta hydrogel reduces scarring in a rat model of cardiac ischemia and enhances cardiomyocyte and stem cell cultures. Acta Biomater. 52, 92–104. 10.1016/j.actbio.2016.12.027 [DOI] [PubMed] [Google Scholar]
- Fu X, Wang H, 2012. Spatial Arrangement of Polycaprolactone/Collagen Nanofiber Scaffolds Regulates the Wound Healing Related Behaviors of Human Adipose Stromal Cells. Tissue Eng. Part A 18, 631–642. 10.1089/ten.tea.2011.0069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamboa-Bobadilla GM, 2006. Implant breast reconstruction using acellular dermal matrix. Ann. Plast. Surg 56, 22–25. 10.1097/01.sap.0000185460.31188.c1 [DOI] [PubMed] [Google Scholar]
- Gattazzo F, Urciuolo A, Bonaldo P, 2014. Extracellular matrix: A dynamic microenvironment for stem cell niche. Biochim. Biophys. Acta - Gen. Subj 1840, 2506–2519. 10.1016/j.bbagen.2014.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghetti M, Topouzi H, Theocharidis G, Papa V, Williams G, Bondioli E, Cenacchi G, Connelly JT, Higgins CA, 2018. Subpopulations of dermal skin fibroblasts secrete distinct extracellular matrix: Implications for using skin substitutes in the clinic. Br. J. Dermatol 381–393. 10.1111/bjd.16255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh K, Ren X-D, Shu XZ, Prestwich GD, Clark RAF, 2006. Fibronectin Functional Domains Coupled to Hyaluronan Stimulate Adult Human Dermal Fibroblast Responses Critical for Wound Healing. Tissue Eng. 12, 601–613. 10.1089/ten.2006.12.601 [DOI] [PubMed] [Google Scholar]
- Gilbert TW, Sellaro TL, Badylak SF, 2006. Decellularization of tissues and organs. Biomaterials 27, 3675–3683. 10.1016/j.biomaterials.2006.02.014 [DOI] [PubMed] [Google Scholar]
- Goyal R, Vega ME, Pastino AK, Singh S, Guvendiren M, Kohn J, Murthy NS, Schwarzbauer JE, 2017. Development of hybrid scaffolds with natural extracellular matrix deposited within synthetic polymeric fibers. J Biomed Mater Res Part A 105A, 2162–2170. 10.1002/jbm.a.36078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grier WK, Tiffany AS, Ramsey MD, Harley BAC, 2018. Incorporating beta-cyclodextrin into collagen scaffolds to sequester growth factors and modulate mesenchymal stem cell activity. Acta Biomater. 76, 116–125. 10.1016/j.actbio.2018.06.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover CN, Cameron RE, Best SM, 2012. Investigating the morphological, mechanical and degradation properties of scaffolds comprising collagen, gelatin and elastin for use in soft tissue engineering. J. Mech. Behav. Biomed. Mater 10, 62–74. 10.1016/j.jmbbm.2012.02.028 [DOI] [PubMed] [Google Scholar]
- Grover GN, Rao N, Christman KL, 2014. Myocardial matrix–polyethylene glycol hybrid hydrogels for tissue engineering. Nanotechnology 25, 014011 10.1088/0957-4484/25/1/014011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gullberg D, Terracioy L, Borgii TK, Rubin K, 1989. Identification of Integrin-like Matrix Receptors with Affinity for Interstitial Collagens *. J. Biol. Chem 264, 12686–12694. [PubMed] [Google Scholar]
- Hedström U, Hallgren O, Öberg L, Demicco A, Vaarala O, Westergren-Thorsson G, Zhou X, 2018. Bronchial extracellular matrix from COPD patients induces altered gene expression in repopulated primary human bronchial epithelial cells. Sci. Rep 8, 1–13. 10.1038/s41598-018-21727-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holst J, Watson S, Lord MS, Eamegdool SS, Bax DV, Nivison-smith LB, Kondyurin A, Ma L, Oberhauser AF, Weiss AS, Rasko JEJ, 2011. Substrate elasticity provides mechanical signals for the expansion of hemopoietic stem and progenitor cells. Nat. Biotechnol 28 10.1038/nbt.1687 [DOI] [PubMed] [Google Scholar]
- Holst JJ, Giaccari A, Kulkarni RN, 2013. Topical Administration of Allogeneic Mesenchymal Stem Cells Seeded in a Collagen Scaffold Augments Wound Healing and Increases Angiogenesis in the Diabetic Rabbit Ulcer. Diabetes 62, 2588–2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Y, Huber A, Takanari K, Amoroso NJ, Hashizume R, Badylak SF, Wagner WR, 2011. Mechanical properties and in vivo behavior of a biodegradable synthetic polymer microfiber-extracellular matrix hydrogel biohybrid scaffold. Biomaterials 32, 3387–3394. 10.1016/j.biomaterials.2011.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoque ME, Nuge T, Yeow TK, Nordin N, Prasad RGSV, 2014. Gelatin Based Scaffolds for Tissue Engineering – a Review. Polym. Res. J 9, 15–32. [Google Scholar]
- Hynes RO, 1992. Integrins: Versatility, modulation, and signaling in cell adhesion. Cell 69, 11–25. 10.1016/0092-8674(92)90115-S [DOI] [PubMed] [Google Scholar]
- Jeffords ME, Wu J, Shah M, Hong Y, Zhang G, 2015. Tailoring Material Properties of Cardiac Matrix Hydrogels to Induce Endothelial Differentiation of Human Mesenchymal Stem Cells. ACS Appl. Mater. Interfaces 7, 11053–11061. 10.1021/acsami.5b03195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TD, Lin SY, Christman KL, 2011. Tailoring material properties of a nanofibrous extracellular matrix derived hydrogel. Nanotechnology 22 10.1088/0957-4484/22/49/494015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi J, Brennan D, Beachley V, Kothapalli CR, 2018. Cardiomyogenic differentiation of human bone marrow-derived mesenchymal stem cell spheroids within electrospun collagen nano fiber mats. J Biomed Mater Res Part A 106A, 3303–3312. 10.1002/jbm.a.36530 [DOI] [PubMed] [Google Scholar]
- Kakabadze Z, Kakabadze A, Chakhunashvili D, Karalashvili L, Berishvili E, Sharma Y, Gupta S, 2018. Decellularized human placenta supports hepatic tissue and allows rescue in acute liver failure. Hepatology 67, 1956–1969. 10.1002/hep.29713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappler B, Anic P, Becker M, Bader A, Klose K, Klein O, Oberwallner B, Choi YH, Falk V, Stamm C, 2016. The cytoprotective capacity of processed human cardiac extracellular matrix. J. Mater. Sci. Mater. Med 27, 1–13. 10.1007/s10856-016-5730-5 [DOI] [PubMed] [Google Scholar]
- Kasukonis B, Kim J, Brown L, Jones J, Ahmadi S, Washington T, Wolchok J, 2016. Codelivery of Infusion Decellularized Skeletal Muscle with Minced Muscle Autografts Improved Recovery from Volumetric Muscle Loss Injury in a Rat Model. Tissue Eng. Part A 22, 1151–1163. 10.1089/ten.tea.2016.0134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur A, Ecker BL, Douglass SM Iii, C. HK, Webster MR, Almeida FV, Somasundaram R, Hayden J, Ban E, Ahmadzadeh H, Franco-barraza J, Shah N, Mellis IA, Keeney F, Kossenkov A, Tang H, Yin X, Liu Q, Xu X, Fane M, Brafford P, Herlyn M, Speicher DW, Wargo JA, Tetzlaff MT, Haydu LE, Raj A, Shenoy V, Cukierman E, Weeraratna AT, 2018. Remodeling of the Collagen Matrix in Aging Skin Promotes Melanoma Metastasis and Affects Immune Cell Motility. Cancer Discov. 9, 64–81. 10.1158/2159-8290.CD-18-0193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane TJ, Dziki J, Sobieski E, Smoulder A, Castleton A, Turner N, White LJ, Badylak SF, 2017. Restoring Mucosal Barrier Function and Modifying Macrophage Phenotype with an Extracellular Matrix Hydrogel: Potential Therapy for Ulcerative Colitis. J. Crohns. Colitis 11, 360–368. 10.1093/ecco-jcc/jjw149 [DOI] [PubMed] [Google Scholar]
- Ki H, Tian T, Han Y, Marecak DM, Watkins JF, Amsden BG, Flynn LE, 2014. Biomaterials Composite hydrogel scaffolds incorporating decellularized adipose tissue for soft tissue engineering with adipose-derived stem cells. Biomaterials 35, 1914–1923. 10.1016/j.biomaterials.2013.11.067 [DOI] [PubMed] [Google Scholar]
- Kim BS, Kwon YW, Kong JS, Park GT, Gao G, Han W, Kim MB, Lee H, Kim JH, Cho DW, 2018. 3D cell printing of in vitro stabilized skin model and in vivo pre-vascularized skin patch using tissue-specific extracellular matrix bioink: A step towards advanced skin tissue engineering. Biomaterials 168, 38–53. 10.1016/j.biomaterials.2018.03.040 [DOI] [PubMed] [Google Scholar]
- Kim JS, Choi JS, Cho YW, 2017. Cell-Free Hydrogel System Based on a Tissue-Specific Extracellular Matrix for In Situ Adipose Tissue Regeneration. ACS Appl. Mater. Interfaces 9, 8581–8588. 10.1021/acsami.6b16783 [DOI] [PubMed] [Google Scholar]
- Klingberg F, Chau G, Walraven M, Boo S, Koehler A, Chow ML, Olsen AL, Im M, Lodyga M, Wells RG, White ES, Hinz B, 2018. The fibronectin ED-A domain enhances recruitment of latent TGF-β-binding protein-1 to the fibroblast matrix. J. Cell Sci. 131, jcs201293 10.1242/jcs.201293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortesmaa J, Yurchenco P, Tryggvason K, 2000. Recombinant Laminin-8 (α4β1γ1). J. Biol. Chem 275, 14853–14859. [DOI] [PubMed] [Google Scholar]
- Krishnan NM, Chatterjee A, Rosenkranz KM, Powell SG, Nigriny JF, Vidal DC, 2014. The cost effectiveness of acellular dermal matrix in expander-implant immediate breast reconstruction. J. Plast. Reconstr. Aesthetic Surg. 67, 468–476. 10.1016/j.bjps.2013.12.035 [DOI] [PubMed] [Google Scholar]
- Kristofik N, Calabro NE, Tian W, Meng A, MacLauchlan S, Wang Y, Breuer CK, Tellides G, Niklason LE, Kyriakides TR, 2016. Impaired von Willebrand factor adhesion and platelet response in thrombospondin-2 knockout mice. Blood 128, 1642–1650. 10.1182/blood-2016-03-702845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristofik NJ, Qin L, Calabro NE, Dimitrievska S, Li G, Tellides G, Niklason LE, Kyriakides TR, 2017. Improving in vivo outcomes of decellularized vascular grafts via incorporation of a novel extracellular matrix. Biomaterials 141, 63–73. 10.1016/j.biomaterials.2017.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropp BP, Ludlow JK, Spicer D, Rippy MK, Badylak SF, Adams MC, Keating MA, Rink RC, Birhle R, Thor KB, 1998. Rabbit urethral regeneration using small intestinal submucosa onlay grafts. Urology 52, 138–142. 10.1016/S0090-4295(98)00114-9 [DOI] [PubMed] [Google Scholar]
- Kusuma GD, Brennecke SP, O’Connor AJ, Kalionis B, Heath DE, 2017. Decellularized extracellular matrices produced from immortal cell lines derived from different parts of the placenta support primary mesenchymal stem cell expansion. PLoS One 12, e0171488 10.1371/journal.pone.0171488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laiva AL, Raftery RM, Keogh MB, O’Brien FJ, 2018. Pro-angiogenic impact of SDF-1 α gene-activated collagen-based sca ff olds in stem cell driven angiogenesis. Int. J. Pharm 544, 372–379. 10.1016/j.ijpharm.2018.03.032 [DOI] [PubMed] [Google Scholar]
- Lee H, Woo H-M, Kang B-J, 2018. Impact of collagen-alginate composition from microbead morphological properties to microencapsulated canine adipose tissue-derived mesenchymal stem cell activities. J. Biomater. Sci. Polym. Ed 29, 1042–1052. 10.1080/09205063.2017.1399002 [DOI] [PubMed] [Google Scholar]
- Lewis PL, Su J, Yan M, Meng F, Glaser SS, Alpini GD, Green RM, Sosa-Pineda B, Shah RN, 2018. Complex bile duct network formation within liver decellularized extracellular matrix hydrogels. Sci. Rep 8, 1–14. 10.1038/s41598-018-30433-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Chau Y, 2010. Neural differentiation directed by self-assembling peptide scaffolds presenting laminin-derived epitopes. J. Biomed. Mater. Res. - Part A 94, 688–699. 10.1002/jbm.a.32707 [DOI] [PubMed] [Google Scholar]
- Liang R, Knight K, Easley D, Palcsey S, Abramowitch S, Moalli PA, 2017. Towards rebuilding vaginal support utilizing an extracellular matrix bioscaffold. Acta Biomater. 57, 324–333. 10.1016/j.actbio.2017.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang R, Yang G, Kim KE, D’Amore A, Pickering AN, Zhang C, Woo SLY, 2015. Positive effects of an extracellular matrix hydrogel on rat anterior cruciate ligament fibroblast proliferation and collagen mRNA expression. J. Orthop. Transl 3, 114–122. 10.1016/j.jot.2015.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H-K, Godiwalla SY, Palmer B, Frimberger D, Yang Q, Madihally SV, Fung K-M, Kropp BP, 2014. Understanding Roles of Porcine Small Intestinal Submucosa in Urinary Bladder Regeneration: Identification of Variable Regenerative Characteristics of Small Intestinal Submucosa. Tissue Eng. Part B Rev. 20, 73–83. 10.1089/ten.teb.2013.0126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Yang G, Tan J, Tuan RS, 2012. Influence of decellularized matrix derived from human mesenchymal stem cells on their proliferation, migration and multi-lineage differentiation potential. Biomaterials 33, 4480–4489. 10.1016/j.biomaterials.2012.03.012 [DOI] [PubMed] [Google Scholar]
- Lin J, Zhou W, Han S, Bunpetch V, Zhao K, Liu C, Yin Z, Ouyang H, 2018. Cell-material interactions in tendon tissue engineering. Acta Biomater. 70, 1–11. 10.1016/j.actbio.2018.01.012 [DOI] [PubMed] [Google Scholar]
- Liu GM, Pan J, Zhang Y, Ning LJ, Luo JC, Huang FG, Qin TW, 2018. Bridging Repair of Large Rotator Cuff Tears Using a Multilayer Decellularized Tendon Slices Graft in a Rabbit Model. Arthrosc. - J. Arthrosc. Relat. Surg 34, 2569–2578. 10.1016/j.arthro.2018.04.019 [DOI] [PubMed] [Google Scholar]
- Liu X, Zhou L, Chen X, Liu T, Pan G, Cui W, Li M, Luo Z-P, Pei M, Yang H, Gong Y, He F, 2016. Culturing on decellularized extracellular matrix enhances antioxidant properties of human umbilical cord-derived mesenchymal stem cells. Mater. Sci. Eng. C. Mater. Biol. Appl 61, 437–448. 10.1016/j.msec.2015.12.090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZZ, Wong ML, Griffiths LG, 2016. Effect of bovine pericardial extracellular matrix scaffold niche on seeded human mesenchymal stem cell function. Sci. Rep 6, 37089 10.1038/srep37089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo SE, Leonel LCPC, Miranda CMFC, Coelho TM, Ferreira GAS, Mess A, Abrão MS, Miglino MA, 2016. The placenta as an organ and a source of stem cells and extracellular matrix: A review. Cells Tissues Organs 201, 239–252. 10.1159/000443636 [DOI] [PubMed] [Google Scholar]
- Lopresti ST, Brown BN, 2015. Host Response to Naturally Derived Biomaterials, in: Host Response to Biomaterials. pp. 53–79. 10.1016/B978-0-12-800196-7.00004-9 [DOI] [Google Scholar]
- Lui PPY, Wong OT, Lee YW, 2016. Transplantation of tendon-derived stem cells pre-treated with connective tissue growth factor and ascorbic acid in vitro promoted better tendon repair in a patellar tendon window injury rat model. Cytotherapy 18, 99–112. 10.1016/j.jcyt.2015.10.005 [DOI] [PubMed] [Google Scholar]
- Lutolf MP, Hubbell JA, 2005. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat. Biotechnol 23, 47–55. 10.1038/nbt1055 [DOI] [PubMed] [Google Scholar]
- Luwang A, Raftery RM, Keogh MB, Brien FJO, 2018. Pro-angiogenic impact of SDF-1 α gene-activated collagen-based sca ff olds in stem cell driven angiogenesis. Int. J. Pharm 544, 372–379. 10.1016/j.ijpharm.2018.03.032 [DOI] [PubMed] [Google Scholar]
- MacDonald DE., Deo N, Markovic B, Stranick M, Somasundaran P, 2002. Adsorption and dissolution behavior of human plasma fibronectin on thermally and chemically modified titanium dioxide particles. Biomaterials 23, 1269–79. 10.1016/S0142-9612(01)00317-9 [DOI] [PubMed] [Google Scholar]
- Macdonald DE, Markovic B, Allen M, Somasundaran P, Boskey AL, 1998. Surface analysis of human plasma fibronectin adsorbed to commercially pure titanium materials. J Biomed Mater Res 41, 120–30. [DOI] [PubMed] [Google Scholar]
- Martin SL, Vrhovski B, Weiss AS, 1995. Total synthesis and expression in Escherichia coli of a gene encoding human tropoelastin. Gene 154, 159–166. 10.1016/0378-1119(94)00848-M [DOI] [PubMed] [Google Scholar]
- Martyn SV, Heywood HK, Rockett P, Paine MD, Wang MJ, Dobson PJ, Sheard SJ, Lee DA, Stark JPW, 2011. Electrospray deposited fibronectin retains the ability to promote cell adhesion. J. Biomed. Mater. Res. - Part B Appl. Biomater 96 B, 110–118. 10.1002/jbm.b.31745 [DOI] [PubMed] [Google Scholar]
- Mauney JR, Volloch V, Kaplan DL, 2005. Matrix-mediated retention of adipogenic differentiation potential by human adult bone marrow-derived mesenchymal stem cells during ex vivo expansion. Biomaterials 26, 6167–6175. 10.1016/j.biomaterials.2005.03.024 [DOI] [PubMed] [Google Scholar]
- Mauquoy S, Dupont-Gillain C, 2016. Combination of collagen and fibronectin to design biomimetic interfaces: Do these proteins form layer-by-layer assemblies? Colloids Surfaces B Biointerfaces 147, 54–64. 10.1016/j.colsurfb.2016.07.038 [DOI] [PubMed] [Google Scholar]
- Mcclure MJ, Cohen DJ, Ramey AN, Bivens CB, 2018. Decellularized Muscle Supports New Muscle Fibers and Improves Function Following Volumetric Injury. Tissue Eng. Part A 24, 1228–1241. 10.1089/ten.tea.2017.0386 [DOI] [PubMed] [Google Scholar]
- McEwan K, Padavan DT, Ellis C, McBane JE, Vulesevic B, Korbutt GS, Suuronen EJ, 2016. Collagen–chitosan–laminin hydrogels for the delivery of insulin-producing tissue. J. Tissue Eng. Regen. Med 10, E397–E408. 10.1002/term [DOI] [PubMed] [Google Scholar]
- McHale MK, M S, Setton LA, Chilkoti A, 2005. Synthesis and in Vitro Evaluation of Enzymatically Cross-Linked Elastin-Like Polypeptide Gels for Cartilaginous Tissue Repair. Tissue Eng. 11, 1768–1779. [DOI] [PubMed] [Google Scholar]
- Melo E, Cárdenes N, Garreta E, Luque T, Rojas M, Navajas D, Farré R, 2014. Inhomogeneity of local stiffness in the extracellular matrix scaffold of fibrotic mouse lungs. J. Mech. Behav. Biomed. Mater 37, 186–195. 10.1016/j.jmbbm.2014.05.019 [DOI] [PubMed] [Google Scholar]
- Meng FW, Slivka PF, Dearth CL, Badylak SF, 2015. Solubilized extracellular matrix from brain and urinary bladder elicits distinct functional and phenotypic responses in macrophages. Biomaterials 46, 131–140. 10.1016/j.biomaterials.2014.12.044 [DOI] [PubMed] [Google Scholar]
- Mewhort HEM, Svystonyuk DA, Turnbull JD, Teng G, Belke DD, Guzzardi DG, Park DS, Kang S, Hollenberg MD, Fedak PWM, 2017. Bioactive Extracellular Matrix Scaffold Promotes Adaptive Cardiac Remodeling and Repair. JACC Basic to Transl. Sci 2, 450–464. 10.1016/j.jacbts.2017.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mithieux SM, Aghaei-ghareh-bolagh B, Yan L, Kuppan KV, Wang Y, Garces-suarez F, Li Z, Maitz PK, Carter EA, Limantoro C, Chrzanowski W, Cookson D, Riboldi-tunnicliffe A, Baldock C, Ohgo K, Kumashiro KK, Edwards G, Weiss AS, 2018. Tropoelastin Implants That Accelerate Wound Repair 1701206, 1–12. 10.1002/adhm.201701206 [DOI] [PubMed] [Google Scholar]
- Mohamed GF, Manal MH, Omar S, Zeid AAA, Walaa BM, Assem M, Ahmed O, Sobhy M, Sabry A, Salem M, Adel O, 2018. The therapeutic role of acellular dermal matrix seeded with mesenchymal stem cells versus autologous skin graft in healing of skin defect. QJM An Int. J. Med 111 [Google Scholar]
- Moore MC, Van De Walle A, Chang J, Juran C, McFetridge PS, 2017. Human Perinatal-derived Biomaterials. Adv Heal. Mater 6, 130–135. 10.1016/j.pep.2015.11.007.Simple [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor-Yossef Moldovan L, Lustig M, Naftaly A, Mardamshina M, Geiger T, Gefen A, Benayahu D, 2018. Cell shape alteration during adipogenesis is associated with coordinated matrix cues. J. Cell. Physiol 10.1002/jcp.27157 [DOI] [PubMed] [Google Scholar]
- Morais JM, Papadimitrakopoulos F, Burgess DJ, 2010. Biomaterials/Tissue Interactions: Possible Solutions to Overcome Foreign Body Response. AAPS J. 12, 188–196. 10.1208/s12248-010-9175-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris AH, Lee H, Xing H, Stamer DK, Tan M, Kyriakides TR, 2018a. Tunable Hydrogels Derived from Genetically Engineered Extracellular Matrix Accelerate Diabetic Wound Healing. ACS Appl. Mater. Interfaces 10, 41892–41901. 10.1021/acsami.8b08920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris AH, Stamer DK, Kunkemoeller B, Chang J, Xing H, Kyriakides TR, 2018b. Decellularized materials derived from TSP2-KO mice promote enhanced neovascularization and integration in diabetic wounds. Biomaterials 169, 61–71. 10.1016/j.biomaterials.2018.03.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris AH, Stamer DK, Kyriakides TR, 2017. The host response to naturally-derived extracellular matrix biomaterials. Semin. Immunol 29, 72–91. 10.1016/j.smim.2017.01.002 [DOI] [PubMed] [Google Scholar]
- Nafisi N, Akbari ME, Mahjoub F, Mohseni MJ, Sabetkish S, Khorramirouz R, Tehrani M, Kajbafzadeh AM, 2017. Application of Human Acellular Breast Dermal Matrix (ABDM) in Implant-Based Breast Reconstruction: An Experimental Study. Aesthetic Plast. Surg 41, 1435–1444. 10.1007/s00266-017-0931-y [DOI] [PubMed] [Google Scholar]
- Neal RA, McClugage SG, Link MC, Sefcik LS, Ogle RC, Botchwey EA, 2009. Laminin Nanofiber Meshes That Mimic Morphological Properties and Bioactivity of Basement Membranes. Tissue Eng. Part C Methods 15, 11–21. 10.1089/ten.tec.2007.0366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubauer S, Kessler H, Gil FJ, Pegueroles M, Manero JM, 2016. Tuning Mesenchymal Stem Cell Response onto Titanium − Niobium − Hafnium Alloy by Recombinant Fibronectin Fragments. ACS Appl. Mater. Interfaces 8, 2517–2525. 10.1021/acsami.5b09576 [DOI] [PubMed] [Google Scholar]
- Ng CP, Sharif ARM, Heath DE, Chow JW, Zhang CBY, Chan-Park MB, Hammond PT, Chan JKY, Griffith LG, 2014. Enhanced ex vivo expansion of adult mesenchymal stem cells by fetal mesenchymal stem cell ECM. Biomaterials 35, 4046–4057. 10.1016/j.biomaterials.2014.01.081 [DOI] [PubMed] [Google Scholar]
- Nicosia RF, Bonanno E, Smith M, Yurchenco P, 1994. Modulation of angiogenesis in vitro by laminin-entactin complex. Dev. Biol 10.1006/dbio.1994.1191 [DOI] [PubMed] [Google Scholar]
- Niknejad H, Peirovi H, Jorjani M, Ahmadiani A, Ghanavi J, Seifalian AM, 2008. Properties of the amniotic membrane for potential use in tissue engineering. Eur. Cells Mater. 15, 88–99. 10.22203/eCM.v015a07 [DOI] [PubMed] [Google Scholar]
- Noh YK, Du P, Kim IG, Ko J, Kim SW, Park K, 2016. Polymer mesh scaffold combined with cell-derived ECM for osteogenesis of human mesenchymal stem cells. Biomater. Res 1–7. 10.1186/s40824-016-0055-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuttelman CR, Mortisen DJ, Henry SM, Anseth KS, 2001. Attachment of fibronectin to poly(vinyl alcohol) hydrogels promotes NIH3T3 cell adhesion, proliferation, and migration. J. Biomed. Mater. Res 57, 217–223. [DOI] [PubMed] [Google Scholar]
- Okada M, Blomback B, Chang MD, Horowitz B, 1985. Fibronectin and fibrin gel structure. J. Biol. Chem 260, 1811–1820. https://doi.org/0049-3848(83)90039-7[pii] [PubMed] [Google Scholar]
- Olsen D, Yang C, Bodo M, Chang R, Leigh S, Baez J, Carmichael D, Perälä M, Hämäläinen ER, Jarvinen M, Polarek J, 2003. Recombinant collagen and gelatin for drug delivery. Adv. Drug Deliv. Rev 55, 1547–1567. 10.1016/j.addr.2003.08.008 [DOI] [PubMed] [Google Scholar]
- Oryan A, Jalili M, Kamali A, Nikahval B, 2018. The concurrent use of probiotic microorganism and collagen hydrogel/scaffold enhances burn wound healing: An in vivo evaluation. Burns 1–12. 10.1016/j.burns.2018.05.016 [DOI] [PubMed] [Google Scholar]
- Pankov R, 2002. Fibronectin at a glance. J. Cell Sci. 115, 3861–3863. 10.1242/jcs.00059 [DOI] [PubMed] [Google Scholar]
- Patel KH, Dunn AJ, Talovic M, Haas GJ, Marcinczyk M, Elmashhady H, Kalaf EG, Sell SA, Garg K, 2019. Aligned nanofibers of decellularized muscle ECM support myogenic activity in primary satellite cells in vitro Aligned nano fi bers of decellularized muscle ECM support myogenic activity in primary satellite cells in vitro. [DOI] [PubMed]
- Perea-gil I, Gálvez-montón C, Prat-vidal C, Jorba I, Segú-vergés C, Roura S, Soler-botija C, Iborra-egea O, 2018. Head-to-head comparison of two engineered cardiac grafts for myocardial repair : From scaffold characterization to pre-clinical testing. Sci. Rep 1–13. 10.1038/s41598-018-25115-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Castrillo S, González-Fernández ML, López-González ME, Villar-Suárez V, 2018. Effect of ascorbic and chondrogenic derived decellularized extracellular matrix from mesenchymal stem cells on their proliferation, viability and differentiation. Ann. Anat 220, 60–69. 10.1016/j.aanat.2018.07.006 [DOI] [PubMed] [Google Scholar]
- Perniconi B, Costa A, Aulino P, Teodori L, Adamo S, Coletti D, 2011. The pro-myogenic environment provided by whole organ scale acellular scaffolds from skeletal muscle. Biomaterials 32, 7870–7882. 10.1016/j.biomaterials.2011.07.016 [DOI] [PubMed] [Google Scholar]
- Petersen TH, Calle EA, Colehour MB, Niklason LE, 2012. Matrix Composition and Mechanics of Decellularized Lung Scaffolds. Cells Tissues Organs 195, 222–231. 10.1159/000324896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzute T, Zhang Y, He F, Pei M, 2016. Ascorbate-dependent impact on cell-derived matrix in modulation of stiffness and rejuvenation of infrapatellar fat derived stem cells toward chondrogenesis. Biomed. Mater 11 10.1088/1748-6041/11/4/045009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokrywczynska M, Gubanska I, Drewa G, Drewa T, 2015. Application of bladder acellular matrix in urinary bladder regeneration: The state of the art and future directions. Biomed Res. Int 2015 10.1155/2015/613439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porzionato A, Sfriso MM, Pontini A, Macchi V, Petrelli L, Pavan PG, Natali AN, Bassetto F, Vindigni V, De Caro R, 2015. Decellularized human skeletal muscle as biologic scaffold for reconstructive surgery. Int. J. Mol. Sci 16, 14808–14831. 10.3390/ijms160714808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przybyt E, Van Luyn MJA, Harmsen MC, 2015. Extracellular matrix components of adipose derived stromal cells promote alignment, organization, and maturation of cardiomyocytes in vitro. J. Biomed. Mater. Res. - Part A 103, 1840–1848. 10.1002/jbm.a.35311 [DOI] [PubMed] [Google Scholar]
- Qiu Z, Kwon AH, Kamiyama Y, 2007. Effects of Plasma Fibronectin on the Healing of Full-Thickness Skin Wounds in Streptozotocin-Induced Diabetic Rats. J. Surg. Res 138, 64–70. 10.1016/j.jss.2006.06.034 [DOI] [PubMed] [Google Scholar]
- Que RA, Arulmoli J, Silva NA Da, Wang S, 2018. Recombinant collagen scaffolds as substrates for human neural stem / progenitor cells. J Biomed Mater Res Part A 106A, 1363–1372. 10.1002/jbm.a.36343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakian R, Block TJ, Johnson SM, Marinkovic M, Wu J, Dai Q, Dean DD, Chen X-D, 2015. Native extracellular matrix preserves mesenchymal stem cell “stemness” and differentiation potential under serum-free culture conditions. Stem Cell Res. Ther 6, 235 10.1186/s13287-015-0235-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rameshbabu AP, Bankoti K, Datta S, Subramani E, Apoorva A, Ghosh P, Maity PP, Manchikanti P, Chaudhury K, Dhara S, 2018. Silk Sponges Ornamented with a Placenta-Derived Extracellular Matrix Augment Full-Thickness Cutaneous Wound Healing by Stimulating Neovascularization and Cellular Migration. ACS Appl. Mater. Interfaces 10, 16977–16991. 10.1021/acsami.7b19007 [DOI] [PubMed] [Google Scholar]
- Rayagiri SS, Ranaldi D, Raven A, Izzah N, Mohamad F, Lefebvre O, Zammit PS, Borycki A, 2018. Basal lamina remodeling at the skeletal muscle stem cell niche mediates stem cell self-renewal. Nat. Commun 9, 1075 10.1038/s41467-018-03425-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenberger F, Eickelberg O, Ko E, Bertschin S, Woodtli T, Erne P, Perruchoud P, Roth M, Ko E, Reichen- F, Bertschin S, Woodtli T, Erne P, Perruchoud P, Extracellular MR, 1999. Extracellular matrix deposition by primary human lung fibroblasts in response to TGF-beta 1 and TGF-beta 3. Am J Physiol 276, L814–24. 10.1152/ajplung.1999.276.5.L814. [DOI] [PubMed] [Google Scholar]
- Reilly GC, Engler AJ, 2010. Intrinsic extracellular matrix properties regulate stem cell differentiation. J. Biomech 43, 55–62. 10.1016/j.jbiomech.2009.09.009 [DOI] [PubMed] [Google Scholar]
- Reing JE, Brown BN, Daly KA, Freund JM, Gilbert TW, Hsiong SX, Huber A, Kullas KE, Tottey S, Wolf MT, Badylak SF, 2010. The effects of processing methods upon mechanical and biologic properties of porcine dermal extracellular matrix scaffolds. Biomaterials 31, 8626–8633. 10.1016/j.biomaterials.2010.07.083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rho KS, Jeong L, Lee G, Seo BM, Park YJ, Hong SD, Roh S, Cho JJ, Park WH, Min BM, 2006. Electrospinning of collagen nanofibers: Effects on the behavior of normal human keratinocytes and early-stage wound healing. Biomaterials 27, 1452–1461. 10.1016/j.biomaterials.2005.08.004 [DOI] [PubMed] [Google Scholar]
- Robert P, Johnson A, 2001. ROLE OF HUMAN AIRWAY SMOOTH MUSCLE IN ALTERED EXTRACELLULAR MATRIX PRODUCTION IN ASTHMA. Clin. Exp. Pharmacol. Physiol 28, 233–236. [DOI] [PubMed] [Google Scholar]
- Roderjan JG, Noronha L De, Augusto M, Correa A, Leitolis A, Rocha R, Bueno L, Diniz F, 2019. Structural assessments in decellularized extracellular matrix of porcine semilunar heart valves : Evaluation of cell niches 1–15. 10.1111/xen.12503 [DOI] [PubMed] [Google Scholar]
- Rodin S, Domogatskaya A, Ström S, Hansson EM, Chien KR, Inzunza J, Hovatta O, Tryggvason K, 2010. Long-term self-renewal of human pluripotent stem cells on human recombinant laminin-511. Nat. Biotechnol 28, 611–615. 10.1038/nbt.1620 [DOI] [PubMed] [Google Scholar]
- Rosario DJ, Reilly GC, Salah EA, Glover M, Bullock AJ, MacNeil S, 2008. Decellularization and sterilization of porcine urinary bladder matrix for tissue engineering in the lower urinary tract. Regen. Med 3, 145–156. 10.2217/17460751.3.2.145 [DOI] [PubMed] [Google Scholar]
- Rothrauff BB, Yang G, Tuan RS, 2017. Tissue-specific bioactivity of soluble tendon-derived and cartilage-derived extracellular matrices on adult mesenchymal stem cells. Stem Cell Res. Ther 8, 133 10.1186/s13287-017-0580-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan EJ, Ryan AJ, González-vázquez A, Philippart A, Ciraldo FE, Hobbs C, Nicolosi V, Boccaccini AR, Kearney CJ, Brien FJO, Engineering T, College R, 2019. Biomaterials Collagen scaffolds functionalised with copper-eluting bioactive glass reduce infection and enhance osteogenesis and angiogenesis both in vitro and in vivo. Biomaterials 197, 405–416. 10.1016/j.biomaterials.2019.01.031 [DOI] [PubMed] [Google Scholar]
- Schneider KH, Enayati M, Grasl C, Walter I, Budinsky L, Zebic G, Kaun C, Wagner A, Kratochwill K, Redl H, Teuschl AH, Podesser BK, Bergmeister H, 2018. Acellular vascular matrix grafts from human placenta chorion: Impact of ECM preservation on graft characteristics, protein composition and in vivo performance. Biomaterials 177, 14–26. 10.1016/j.biomaterials.2018.05.045 [DOI] [PubMed] [Google Scholar]
- Seif-Naraghi SB, Horn D, Schup-Magoffin PJ, Christman KL, 2012. Injectable extracellular matrix derived hydrogel provides a platform for enhanced retention and delivery of a heparin-binding growth factor. Acta Biomater. 8, 3695–3703. 10.1016/j.actbio.2012.06.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif-Naraghi SB, Singelyn JM, Salvatore MA, Osborn KG, Wang JJ, Sampat U, Kwan OL, Strachan GM, Wong J, Schup-Magoffin PJ, Braden RL, Bartels K, DeQuach JA, Preul M, Kinsey AM, DeMaria AN, Dib N, Christman KL, 2013. Safety and efficacy of an injectable extracellular matrix hydrogel for treating myocardial infarction. Sci. Transl. Med 5 10.1126/scitranslmed.3005503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo BR, Bhardwaj P, Choi S, Gonzalez J, Eguiluz RCA, Wang K, Mohanan S, Morris PG, Du B, Zhou XK, Vahdat LT, Verma A, Elemento O, Hudis CA, Williams RM, Gourdon D, Dannenberg AJ, Fischbach C, 2015. Obesity-dependent changes in interstitial ECM mechanics promote breast tumorigenesis. Sci. Transl. Med 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo Y, Jung Y, Kim SH, 2017. Decellularized heart ECM hydrogel using supercritical carbon dioxide for improved angiogenesis. Acta Biomater. 67, 270–281. 10.1016/j.actbio.2017.11.046 [DOI] [PubMed] [Google Scholar]
- Shevach M, Zax R, Abrahamov A, Fleischer S, Shapira A, Dvir T, 2015. Omentum ECM-based hydrogel as a platform for cardiac cell delivery. Biomed. Mater 10 10.1088/1748-6041/10/3/034106 [DOI] [PubMed] [Google Scholar]
- Shoulders MD, Raines RT, 2010. Collagen Structure and Stability. Annu. Rev. Biochem 78, 929–958. 10.1146/annurev.biochem.77.032207.120833.COLLAGEN [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu JY, Panganiban B, Xu T, 2013. Peptide-Polymer Conjugates: From Fundamental Science to Application. Annu. Rev. Phys. Chem 64, 631–657. 10.1146/annurev-physchem-040412-110108 [DOI] [PubMed] [Google Scholar]
- Shu XZ, Ahmad S, Liu Y, Prestwich GD, 2006. Synthesis and evaluation of injectable, in situ crosslinkable synthetic extracellular matrices for tissue engineering. J. Biomed. Mater. Res. Part A 79, 902–912. 10.1002/jbm.a [DOI] [PubMed] [Google Scholar]
- Singaravelu S, Ramanathan G, Sivagnanam UT, 2017. Dual-layered 3D nanofibrous matrix incorporated with dual drugs and their synergetic effect on accelerating wound healing through growth factor regulation. Mater. Sci. Eng. C 76, 37–49. 10.1016/j.msec.2017.02.148 [DOI] [PubMed] [Google Scholar]
- Singelyn JM, Sundaramurthy P, Johnson TD, Schup-Magoffin PJ, Hu DP, Faulk DM, Wang J, Mayle KM, Bartels K, Salvatore M, Kinsey AM, Demaria AN, Dib N, Christman KL, 2012. Catheter-deliverable hydrogel derived from decellularized ventricular extracellular matrix increases endogenous cardiomyocytes and preserves cardiac function post-myocardial infarction. J. Am. Coll. Cardiol 59, 751–763. 10.1016/j.jacc.2011.10.888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slivka PF, Dearth CL, Keane TJ, Meng FW, Medberry CJ, Riggio RT, Reing JE, Badylak SF, 2014. Fractionation of an ECM hydrogel into structural and soluble components reveals distinctive roles in regulating macrophage behavior. Biomater. Sci 2, 1521–1534. 10.1039/c4bm00189c [DOI] [PubMed] [Google Scholar]
- Smethurst PA, Onley DJ, Jarvis GE, O’Connor MN, Graham Knight C, Herr AB, Ouwehand WH, Farndale RW, 2007. Structural basis for the platelet-collagen interaction: The smallest motif within collagen that recognizes and activates platelet Glycoprotein VI contains two glycine-proline-hydroxyproline triplets. J. Biol. Chem 282, 1296–1304. 10.1074/jbc.M606479200 [DOI] [PubMed] [Google Scholar]
- Smith DJ, 1995. Indications for Use of biobrane in wound management. J. Burn Care Rehabil. 16, 317–320. 10.1097/00004630-199505000-00018 [DOI] [PubMed] [Google Scholar]
- Song JJ, Ott HC, 2011. Organ engineering based on decellularized matrix scaffolds. Trends Mol. Med 17, 424–432. 10.1016/j.molmed.2011.03.005 [DOI] [PubMed] [Google Scholar]
- Sonnenberg SB, Rane AA, Liu CJ, Rao N, Agmon G, Suarez S, Wang R, Munoz A, Bajaj V, Zhang S, Braden R, Schup-Magoffin PJ, Kwan OL, DeMaria AN, Cochran JR, Christman KL, 2015. Delivery of an engineered HGF fragment in an extracellular matrix-derived hydrogel prevents negative LV remodeling post-myocardial infarction. Biomaterials 45, 56–63. 10.1016/j.biomaterials.2014.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinke JW, Platts-Mills TAE, Commins SP, 2015. The alpha-gal story: Lessons learned from connecting the dots. J. Allergy Clin. Immunol 135, 589–596. 10.1016/j.jaci.2014.12.1947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern MM, Myers RL, Hammam N, Stern KA, Eberli D, Kritchevsky SB, Soker S, Van Dyke M, 2009. The influence of extracellular matrix derived from skeletal muscle tissue on the proliferation and differentiation of myogenic progenitor cells ex vivo. Biomaterials 30, 2393–2399. 10.1016/j.biomaterials.2008.12.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens KR, Scull MA, Ramanan V, Fortin CL, Chaturvedi RR, Knouse KA, Xiao JW, Fung C, Mirabella T, Chen AX, Mccue MG, Yang MT, Fleming HE, Chung K, De Jong YP, Chen CS, Rice CM, Bhatia SN, 2017. In situ expansion of engineered human liver tissue in a mouse model of chronic liver disease. Sci. Transl. Med 9 10.1126/scitranslmed.aah5505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoichevska V, Peng YY, Vashi AV, Werkmeister JA, Dumsday GJ, Ramshaw JAM, 2016. Engineering specific chemical modification sites into a collagen-like protein from Streptococcus pyogenes. J Biomed Mater Res Part A 105A, 806–813. 10.1002/jbm.a.35957 [DOI] [PubMed] [Google Scholar]
- Sullivan KE, Quinn KP, Tang KM, Georgakoudi I, Black LD 3rd, 2014. Extracellular matrix remodeling following myocardial infarction influences the therapeutic potential of mesenchymal stem cells. Stem Cell Res. Ther 5, 14 10.1186/scrt403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan QW, Zhang Y, Luo JC, Zhang D, Xiong BJ, Yang JQ, Xie HQ, Lv Q, 2017. Hydrogel derived from decellularized porcine adipose tissue as a promising biomaterial for soft tissue augmentation. J. Biomed. Mater. Res. - Part A 105, 1756–1764. 10.1002/jbm.a.36025 [DOI] [PubMed] [Google Scholar]
- Tavis MJ, Thornton JW, Bartlett RH, Roth JC, Woodroof EA, 1980. A new composite skin prosthesis. Burns 7, 123–130. 10.1016/0305-4179(80)90038-8 [DOI] [Google Scholar]
- Taylor DA, Frazier OH, Elgalad A, Hochman-Mendez C, Sampaio LC, 2018. Building a Total Bioartificial Heart: Harnessing Nature to Overcome the Current Hurdles. Artif. Organs 0–3. 10.1111/aor.13336 [DOI] [PubMed] [Google Scholar]
- Trabbic-carlson K, Liu LI, Kim B, Chilkoti A, 2004. Expression and purification of recombinant proteins from Escherichia coli: Comparison of an elastin-like polypeptide fusion with an oligohistidine fusion. Protein Sci. 13, 3274–3284. 10.1110/ps.04931604.rapid [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traktuev D, Parfenova E, Tkachuk V, March K, 2006. Adipose stromal cells--plastic type of cells with high therapeutic potential. Tsitologiia 48, 83–94. [PubMed] [Google Scholar]
- Trottier V, Marceau-Fortier G, Germain L, Vincent C, Fradette J, 2008. IFATS Collection: Using Human Adipose-Derived Stem/Stromal Cells for the Production of New Skin Substitutes. Stem Cells 26, 2713–2723. 10.1634/stemcells.2008-0031 [DOI] [PubMed] [Google Scholar]
- Tsuchiya T, Balestrini JL, Mendez J, Calle EA, Zhao L, Niklason LE, 2014. Influence of pH on Extracellular Matrix Preservation During Lung Decellularization. Tissue Eng. Part C 20, 1028–1036. 10.1089/ten.tec.2013.0492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner AEB, Yu C, Bianco J, Watkins JF, Flynn LE, 2012. The performance of decellularized adipose tissue microcarriers as an inductive substrate for human adipose-derived stem cells. Biomaterials 33, 4490–4499. 10.1016/j.biomaterials.2012.03.026 [DOI] [PubMed] [Google Scholar]
- Ungaro F, Colombo P, Massimino L, Ugolini GS, Correale C, Rasponi M, Garlatti V, Rubbino F, Tacconi C, Spaggiari P, Spinelli A, Carvello M, Sacchi M, Spanò S, Vetrano S, Malesci A, Peyrin-biroulet L, Danese S, Alessio SD, 2009. Lymphatic endothelium contributes to colorectal cancer growth via the soluble matrisome component GDF11. Int. J. Cancer 10.1002/ijc.32286 [DOI] [PubMed] [Google Scholar]
- Ungerleider JL, Johnson TD, Rao N, Christman KL, 2015. Fabrication and characterization of injectable hydrogels derived from decellularized skeletal and cardiac muscle. Methods 84, 53–59. 10.1016/j.ymeth.2015.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Vlierberghe S, Dubruel P, Schacht E, 2011. Biopolymer-based hydrogels as scaffolds for tissue engineering applications: A review. Biomacromolecules 12, 1387–1408. 10.1021/bm200083n [DOI] [PubMed] [Google Scholar]
- Varkey M, Ding J, Tredget E, 2015. Advances in Skin Substitutes—Potential of Tissue Engineered Skin for Facilitating Anti-Fibrotic Healing. J. Funct. Biomater 6, 547–563. 10.3390/jfb6030547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veves A, Falanga V, Armstrong DG, Sabolinski ML, 2001. Graftskin, a human skin equivalent, is effective in the management of noninfected neuropathic diabetic foot ulcers: A prospective randomized multicenter clinical trial. Diabetes Care 24, 290–295. 10.2337/diacare.24.2.290 [DOI] [PubMed] [Google Scholar]
- Wagner DE, Bonenfant NR, Parsons CS, Sokocevic D, Brooks EM, Borg ZD, Lathrop MJ, Wallis JD, Daly AB, Wai Y, Deng B, Desarno MJ, Ashikaga T, Loi R, Weiss DJ, 2014. Comparative decellularization and recellularization of normal versus emphysematous human lungs. Biomaterials 35, 3281–3297. 10.1016/j.biomaterials.2013.12.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainwright DJ, 1995. Use of an acellular allograft dermal matrix (AlloDerm) in the management of full-thickness burns. Burns 21, 243–248. 10.1016/0305-4179(95)93866-I [DOI] [PubMed] [Google Scholar]
- Wallace DG, Rosenblatt J, 2003. Collagen gel systems for sustained delivery and tissue engineering. Adv. Drug Deliv. Rev 55, 1631–1649. 10.1016/j.addr.2003.08.004 [DOI] [PubMed] [Google Scholar]
- Walraven M, Hinz B, 2018. Therapeutic approaches to control tissue repair and fibrosis: Extracellular matrix as a game changer. Matrix Biol. 71–72, 205–224. 10.1016/j.matbio.2018.02.020 [DOI] [PubMed] [Google Scholar]
- Wang C, Wang Q, Gao W, Zhang Z, Lou Y, Jin H, Chen X, Lei B, Xu H, Mao C, 2018. Highly Efficient Local Delivery of Endothelial Progenitor Cells Significantly Potentiates Angiogenesis and Full-thickness Wound Healing. Acta Biomater. 69, 156–169. 10.1016/j.actbio.2018.01.019 [DOI] [PubMed] [Google Scholar]
- Wang R, Mithieux SM, Ozsvar J, Weiss AS, 2016. Synthetic Elastin Systems, in: Ramamurthi A, Kothapalli C (Eds.), Elastic Fiber Matrices: Biomimetic Approaches to Regeneration and Repair. Taylor & Francis. [Google Scholar]
- Whelan D, Caplice NM, Clover AJP, 2014. Fibrin as a delivery system in wound healing tissue engineering applications. J. Control. Release 196, 1–8. 10.1016/j.jconrel.2014.09.023 [DOI] [PubMed] [Google Scholar]
- Wilks BT, Evans EB, Nakhla MN, Morgan JR, 2018. Directing fibroblast self-assembly to fabricate highly-aligned, collagen-rich matrices. Acta Biomater. 10.1016/J.ACTBIO.2018.09.030 [DOI] [PubMed] [Google Scholar]
- Williams C, Budina E, Stoppel WL, Sullivan KE, Emani S, Emani SM, Black LD, 2015. Cardiac extracellular matrix-fibrin hybrid scaffolds with tunable properties for cardiovascular tissue engineering. Acta Biomater. 14, 84–95. 10.1016/j.actbio.2014.11.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson K, Terlouw A, Roberts K, Wolchok JC, 2016. The characterization of decellularized human skeletal muscle as a blueprint for mimetic scaffolds. J. Mater. Sci. Mater. Med 27, 1–15. 10.1007/s10856-016-5735-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf MT, Carruthers CA, Dearth CL, Crapo PM, Huber A, Burnsed OA, Londono R, Johnson SA, Daly KA, Stahl EC, Freund JM, Medberry CJ, Carey LE, Nieponice A, Amoroso NJ, Badylak SF, 2014. Polypropylene surgical mesh coated with extracellular matrix mitigates the host foreign body response. J. Biomed. Mater. Res. - Part A 102, 234–246. 10.1002/jbm.a.34671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf MT, Daly KA, Brennan-Pierce EP, Johnson SA, Carruthers CA, D’Amore A, Nagarkar SP, Velankar SS, Badylak SF, 2012. A hydrogel derived from decellularized dermal extracellular matrix. Biomaterials 33, 7028–7038. 10.1016/j.biomaterials.2012.06.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf MT, Dearth CL, Sonnenberg SB, Loboa EG, Badylak SF, 2015. Naturally derived and synthetic scaffolds for skeletal muscle reconstruction. Adv. Drug Deliv. Rev 84, 208–221. 10.1016/j.addr.2014.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo SL-Y, Mau JR, Kang H, Liang R, Almarza AJ, Fisher MB, 2019. Functional Tissue Engineering of Ligament and Tendon Injuries. Princ. Regen. Med 1179–1198. 10.1016/B978-0-12-809880-6.00067-9 [DOI] [Google Scholar]
- Wu S-H, Shirado T, Mashiko T, Feng J, Asahi R, Kanayama K, Mori M, Chi D, Sunaga A, Sarukawa S, Yoshimura K, 2018. Therapeutic Effects of Human Adipose-Derived Products on Impaired Wound Healing in Irradiated Tissue. Plast. Reconstr. Surg 142, 383–391. 10.1097/PRS.0000000000004609 [DOI] [PubMed] [Google Scholar]
- Xu Q, Shanti RM, Zhang Q, Cannady SB, O’Malley BW, Le AD, 2017. A Gingiva-Derived Mesenchymal Stem Cell-Laden Porcine Small Intestinal Submucosa Extracellular Matrix Construct Promotes Myomucosal Regeneration of the Tongue. Tissue Eng. Part A 23, 301–312. 10.1089/ten.tea.2016.0342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q, Harmsen MC, van Luyn MJA, Bank RA, 2010. The relationship between collagen scaffold cross-linking agents and neutrophils in the foreign body reaction. Biomaterials 31, 9192–9201. 10.1016/j.biomaterials.2010.08.049 [DOI] [PubMed] [Google Scholar]
- Young DA, Ibrahim DO, Hu D, Christman KL, 2011. Injectable hydrogel scaffold from decellularized human lipoaspirate. Acta Biomater. 7, 1040–1049. 10.1016/j.actbio.2010.09.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young DA, McGilvray KC, Ehrhart N, Gilbert TW, 2018. Comparison of in vivo remodeling of urinary bladder matrix and acellular dermal matrix in an ovine model. Regen. Med 13, 759–773. 10.2217/rme-2018-0091 [DOI] [PubMed] [Google Scholar]
- Yu J, Wang M-Y, Tai H-C, Cheng N-C, 2018. Cell sheet composed of adipose-derived stem cells demonstrates enhanced skin wound healing with reduced scar formation. Acta Biomater. 77, 191–200. 10.1016/j.actbio.2018.07.022 [DOI] [PubMed] [Google Scholar]
- Yun Y, Pham LBH, Yoo Y, Lee S, Kim H, 2015. Engineering of Self-Assembled Fibronectin Matrix Protein and Its Effects on Mesenchymal Stem Cells. Int. J. Mol. Sci 16, 19645–19656. 10.3390/ijms160819645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Wang S, Wang G, Su M, Song L, Chen J, Fan S, Lin X, 2018. Preparation of decellularized biphasic hierarchical myotendinous junction extracellular matrix for muscle regeneration. Acta Biomater. 68, 15–28. 10.1016/j.actbio.2017.12.035 [DOI] [PubMed] [Google Scholar]
- Zhou L, Chen X, Liu T, Zhu C, Si M, Jargstorf J, Li M, Pan G, Gong Y, Luo Z-P, Yang H, Pei M, He F, 2018. SIRT1-dependent anti-senescence effects of cell-deposited matrix on human umbilical cord mesenchymal stem cells. J. Tissue Eng. Regen. Med 12, e1008–e1021. 10.1002/term.2422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Zimber M, Yuan H, Naughton GK, Fernan R, Li W-J, 2016. Effects of Human Fibroblast-Derived Extracellular Matrix on Mesenchymal Stem Cells. Stem Cell Rev. 12, 560–572. 10.1007/s12015-016-9671-7 [DOI] [PubMed] [Google Scholar]
- Ziemkiewicz N, Talovic M, Madsen J, Hill L, Scheidt R, Patel A, 2018. Laminin-111 functionalized polyethylene glycol hydrogels support myogenic activity in vitro. Biomed. Mater 10.1088/1748-605X/aad915 [DOI] [PubMed] [Google Scholar]
- Zografakis J, Johnston G, Haas J, Berbiglia L, Bedford T, Spear J, Dan A, Pozsgay M, 2018. Urinary Bladder Matrix Reinforcement for Laparoscopic Hiatal Hernia Repair. JSLS J. Soc. Laparoendosc. Surg 22, e2017.00060 10.4293/JSLS.2017.00060 [DOI] [PMC free article] [PubMed] [Google Scholar]