Abstract
The Women’s Health Initiative (WHI), a longitudinal study of more than 161,000 post-menopausal women across the United States, provides an opportunity to investigate the link between sleep health and healthy aging. The purpose of this paper was to systematically review all published WHI papers investigating sleep as a predictor of health outcomes and health behaviors/quality of life outcomes. A strength of the WHI, is that for most participants, sleep measures were completed before a major health diagnosis, with a significant portion of participants also providing sleep measures after diagnosis. Twenty-three WHI articles were identified and examined for this review. The combination of sleep duration and insomnia symptoms was the most commonly investigated sleep measure. Results indicated that both short (≤6 hours) and long sleep (≥9 hours) duration were associated with a higher risk of cardiovascular disease, colorectal cancer, mortality, cognitive decline, and poor diet. Insomnia symptoms, frequent snoring, and risk of sleep-disordered breathing (SDB) were also associated with increased risk for ischemic stroke and cardiovascular disease. However, many significant results were attenuated after multivariable adjustment. Limitations of these WHI investigations include the use of different categories for sleep measures across studies and a lack of examination by race/ethnicity. Due to the longitudinal study design, large sample size, and long-term follow-up for health outcomes, the WHI serves as a rich resource for examining associations between sleep characteristics, demographics, and health in post-menopausal women.
Keywords: Women’s Health Initiative, post-menopausal women, sleep, insomnia, cancer, cardiovascular disease, sleep-disordered breathing, sleep duration
INTRODUCTION
Sufficient, restorative sleep is critical for the optimal functioning of all body systems (1). Poor sleep quality and sleep duration extremes (short or long) are associated with worse cardiovascular and cardiometabolic outcomes (e.g., hypertension, heart attack, stroke, obesity, metabolic dysfunction) and increased incidence of type 2 diabetes (2–6) and cancer (7–9). Poor sleep habits are also associated with increased mortality (10–13), as well as increased inflammation and inflammatory disorders (14). In addition, mental health is tied to sleep quality and duration, and sleep disturbances, whether preceding or coexisting, are very common in psychiatric disorders (15–18).
In general, sleep problems increase as people age. Sleep duration typically decreases over time, and sleep may become more fragmented. Early morning awakenings or trouble falling back asleep are common (19,20). For women, many sleep problems begin in menopause (20,21) and can be associated with vasomotor symptoms (e.g., hot flashes and night sweats) (22,23). Peri- and post-menopausal women have high rates of sleep disturbance and insomnia complaints, ranging from 35–60% (24), in addition to diagnosable sleep disorders like insomnia, obstructive sleep apnea (OSA), and restless leg syndrome, which are often underdiagnosed (20,25).
Studies on midlife and post-menopausal women are important to understand health during transition stages of life. Seminal work from the Nurse’s Health Study (NHS) (26), the Women’s Health Initiative (WHI) (27) and the Study of Women’s Health Across the Nation (SWAN) (28) are strong examples of the importance of studying older adults in the growing aging U.S. population. Across all of these study cohorts, the importance of sleep to health has been documented (21,29–32). For this review, we will focus on the examination of sleep and health outcomes within the WHI cohort.
The WHI offers rich data regarding the impact of sleep quality and sleep duration on major health outcomes, such as cardiovascular and cancer, in post-menopausal women. The purpose of this review is to examine the results of published WHI papers to date that use sleep as a predictor of health outcomes and health behaviors/quality of life outcomes (i.e., mortality, cardiovascular-related, cancer-related, mental health/cognition-related, and diet). In addition, we identify gaps in the literature and recognize opportunities for additional analyses within the WHI. More broadly, given the large national sample and extensive follow-up, this review of WHI studies on sleep can serve as a reference for further research on sleep among aging women.
METHODS
The WHI is a longitudinal study of 161,809 post-menopausal women recruited between the ages of 50–79 from 40 clinical centers across the U.S. from 1993–1998. The recruitment and follow-up procedures for the WHI have been described elsewhere (27,33,34). Briefly, women were enrolled in one or more of three clinical trials (i.e., hormone therapy, dietary modification, calcium and vitamin D supplementation) or the WHI observational study (OS). All clinical trial (CT) components ended by 2005, with three extension studies for additional outcomes assessments and self-report questionnaires conducted from 2005–2010, 2010–2015, and 2015–2020. The Institutional Review Boards at all participating WHI institutions approved the study protocols.
The WHI uses a highly organized article tracking and review system for the proposal and development of manuscripts and ancillary studies. All researchers desiring to use WHI data for analyses, manuscripts, presentations, or grant applications must submit a structured proposal to the WHI Publications and Presentations (P&P) Committee for review and comment. Prior to the development of the proposal, the first author is responsible for reviewing the online listings of completed and proposed papers, and proposals from WHI data, by searching an author and topic database designed for WHI researchers. If overlap is identified, the author may discontinue the proposal, contact the other author to determine if overlap can be avoided by differing data analysis, or consider joining the writing group of the other proposal if the lead author agrees. New WHI members are required to work with a sponsoring WHI Principal Investigator or established WHI member. At least two members of the WHI P&P Committee review each proposal and provide feedback/questions to the authors. Authors must submit revised proposals if P&P reviewers require any changes or clarifications. Once the paper has been approved, the lead author of the paper is responsible for forming the writing or research group, working with the paper statisticians (generally provided by WHI), and updating the WHI P&P Committee on the manuscript’s status. Before submitting a paper to a journal, the lead author must re-submit the manuscript to the P&P for review and approval to submit for publication. The WHI P&P takes great effort to ensure all analyses are unique, methodologically and scientifically sound, and recorded in an accessible online location to move the science of the topic forward.
Authors with approved proposals are assigned a statistician, if necessary, and given access to a dataset after signing a WHI Data Use Agreement form. Datasets are created by the WHI Clinical Coordinating Center or Regional Centers. The datasets compiled consist of the WHI participants of interest and only relevant variables necessary for the proposed data analysis as outlined in the approved proposal. Given the longitudinal nature and the concurrent clinical trials within WHI, no standard or single dataset is compiled to examine WHI research questions of interest. Each dataset is unique and designed to efficiently answer research questions.
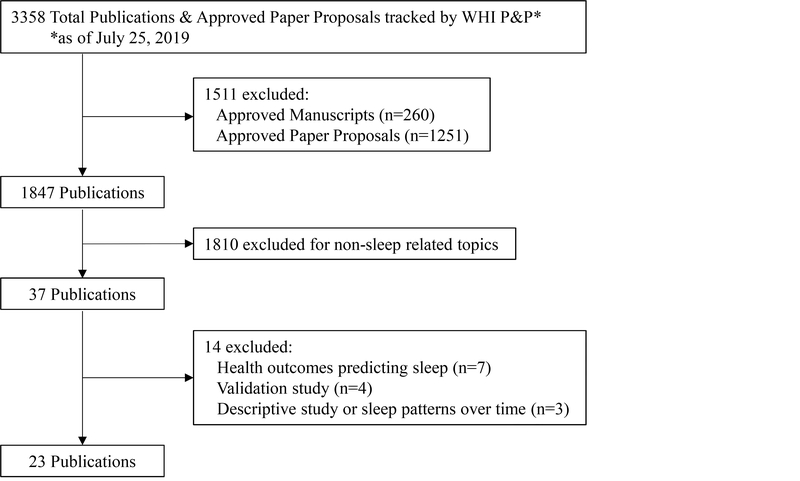
For this review, which was approved by the P&P process described above, only peer-reviewed, English-language, original articles published from 2000 through July 25, 2019 were examined. We used the WHI publications’ database, as well as PubMed to identify all relevant articles. The following search keywords were used: sleep, duration, insomnia, fatigue, tired, snoring, breathing, apnea, and obstructive sleep apnea, (in addition to variations on all the words above, for example, “snore”). There were a total of 3,358 approved proposals, manuscripts, and publications within the WHI database (Figure 1). Approved proposals and manuscripts were excluded (n=1,511), then an additional 1,810 publications were excluded for non-sleep related topics. This resulted in 37 published papers related to sleep that were identified from the database searches. For the purposes of this review, we further refined our criteria to identify WHI articles that investigated sleep as a predictor of health outcomes or health behaviors/quality of life outcomes (n=23). The excluded papers (n=14) assessed health outcomes that predicted sleep (n=7), were validation sleep papers (n=4), or papers describing sleep and sleep patterns over time (n=3). The health outcomes or health behavior/quality of life outcomes included in the 23 selected papers were: cardiovascular disease (n=6), cancer (n=6), cognition (n=2), mortality (n=2), diet (n=2), telomeres/epigenetic age (n=2), nocturnal enuresis (n=1), sexual satisfaction (n=1), and falls/fractures (n=1). The three most recent of these articles were published while prior versions of this manuscript were under peer-review.
Figure 1.
Flow diagram for identifying eligible studies.
WHI Sleep Measures
Individual Sleep Measures
Sleep duration was assessed with a single item: “About how many hours of sleep did you get on a typical night during the past 4 weeks?” Response categories included: ≤5 hours, 6 hours, 7 hours, 8 hours, 9 hours, and ≥10 hours. Snoring was also assessed with a single item: “Over the past 4 weeks, did you snore?” Response categories included “No, not in the past 4 weeks; “Yes, less than once a week”; “Yes, 1 or 2 times a week”; “Yes, 3 or 4 times a week”; “Yes, 5 or more times a week”; or “Do not know.”
The WHIIRS is a measure of perceived insomnia symptoms (35–37) and consists of five questions that assess insomnia and sleep quality during the past four weeks: “Did you have trouble falling asleep?”; “Did you wake up several times at night?”; “Did you wake up earlier than you planned to?”; “Did you have trouble getting back to sleep after you woke up too early?”; and “Overall, was your typical night’s sleep during the past 4 weeks: Very sound or restful, sound or restful, average quality, restless, or very restless?” Response categories ranged from 0 (“No, not in the past 4 weeks”) to 4 (“Yes, 5 or more times a week”), with a summed sleep quality score of the 5 items ranging from 0 to 20. A higher WHIIRS score indicates poorer sleep quality. A WHIIRS score of ≥ 9 is used as a cut off for having a high risk of insomnia (36).
Timing
Participants enrolled in the WHI CT completed the sleep duration and the WHIIRS measures at baseline, year 1, and study closeout (on average at year 9). A subsample of CT participants also completed sleep measures at year 3, year 6, and year 9 of follow-up. The WHI OS participants completed sleep measures at baseline and year 3. Participants who enrolled in the WHI extension studies, which began in 2005 and consisted of participants from both the CT and OS, completed an additional sleep measure in 2011–2012.
Other sleep measures in WHI
A SDB risk score was created by researchers (38,39) based on questions from WHI data and adapted from the Berlin Questionnaire (40,41). One point was awarded for each of the following items: (1) self-reported snoring ≥3 times a week; (2) self-reported falling asleep during quiet activities ≥3 times a week; and (3) either hypertension (self-reported physician diagnosis or measured systolic blood pressure [BP] ≥140 mm Hg or diastolic BP ≥90 mm Hg) or measured obesity (body mass index [BMI] >30 kg/m2). Scores range from 0 to 3 points, with scores ≥ 2 defined as high risk for SDB and < 2 as low risk. Participants’ baseline values were used in both studies calculating a SDB risk score. Another related score was created for Obstructive Sleep Apnea (OSA) risk (42). It incorporated BMI ≥30 kg/m2, snoring ≥ 3 times per week, restless or worse sleep quality, wake up at night ≥ 3 times per week, diagnosis of hypertension, and presence of daytime sleepiness ≥ 3 times per week. One point was given for each risk factor, for a score ranging from 0–6. Baseline values were also used for the OSA risk.
Actigraphy was also collected in a subset of women (n=459) from the WHI Observational Study. This ancillary study recorded additional sleep details (objective total sleep time, nap time, sleep efficiency, clock time of sleep acrophase) by having participants wear a wrist actigraph for seven days and also completed subjective sleep diaries. Actigraphy recordings generally started a few months after the baseline assessment.
RESULTS
Overall
There were 23 WHI publications included in this review. The majority of the papers (21 out of 23) were published after 2010, with the earliest paper published in 2008. Most authors used the WHIIRS questionnaire and the single sleep duration question as their sleep measures. Five articles used sleep duration alone as a primary exposure (43–47), 2 used the WHIIRS alone (48,49) and 10 articles used both sleep duration and WHIIRS (50–59). The remaining 6 papers used the snoring item (60), calculated risk score for OSA (42), SDB scale and sleep duration (38), SDB scale and WHIIRS (39), or actigraphy (61,62). All papers used baseline sleep measures for analysis, except for two (48,57). One paper used baseline and year 1 or year 3 sleep measures, depending on study arm follow up schedule (48), and the other paper used the repeated sleep measures in a time-dependent analysis (57).
Cardiovascular health and diabetes outcomes
Six published articles investigated the association between sleep and cardiovascular health outcomes, including ischemic stroke (43,50,60), coronary heart disease (CHD), (44,49,50,60), and cardiovascular disease (CVD) more broadly defined (38,50). All of the CVD studies used sleep-related measures assessed at baseline. We present these results in the following clusters of sleep-related predictor variables: short sleep duration, long sleep duration, insomnia symptoms, snoring, and SDB risk score.
One paper included in this review was completed as part of a journal special issue on Veteran health. Veterans often have greater co-morbidities and higher rates of heart disease, diabetes, and depressive symptoms (63–65). Additionally, occupational exposures are different between Veterans and non-Veterans (e.g., muscular, joints, exposure to chemicals, psychosocial risks) (66). Participants who responded affirmatively to the following baseline question were self-identified as Veterans, “Have you served in the U.S. armed forces on active duty for a period of 180 days or more.” The authors examined the impact of sleep disturbance on cardiometabolic health in Veterans and non-Veterans.
Short sleep duration results
Three of the WHI studies demonstrated a positive association between short sleep duration and increased risk of cardiovascular-related health outcomes, although most did not reach statistical significance after adjusting for covariates (43,44,60). Chen et al. observed a modest positive association in the relative risk for ischemic stroke for those with ≤ 6 hours of sleep and no previous history of CVD. However, in the fully-adjusted model there was not a significant association between risk of stroke and ≤6 hours of sleep (43). Hale et al. also found no statistically significant associations between short sleep (≤5 hours) and CHD in partially adjusted and fully adjusted models (44). In a study by Sands-Lincoln and colleagues, those with short sleep (≤5 hours) were at a higher risk of incident CHD and CVD compared to those with 7–8 hours, but consistent with the Chen and Hale results, these associations were attenuated in fully-adjusted models (60). However, research by Rissling et al. comparing sleep among Veterans and non-Veterans with cardiometabolic outcomes observed significant, but modest, higher risk of CVD among non-Veterans who slept ≤5 hours or 6 hours, but no significant association among Veterans. They also found very short sleep (≤5 hours) to be significantly associated with higher risk for diabetes among non-Veterans (38).
Long sleep duration results
Several studies found more evidence of a higher risk of CVD-related outcomes in post-menopausal women with long sleep compared to short sleep (38,43,44,50). Risk of ischemic stroke was 24% higher for those who slept 8 hours and 70% higher for those who slept ≥9 hours compared to those who slept 7 hours in fully adjusted models (43). Hale et al. found ≥9 hours of sleep to be associated with an higher odds of CHD, however when levels of fibrinogen (an inflammatory protein which promotes blood clotting) were added to the model, the odds ratio (OR) diminished from 2.05 to 1.97 and was no longer statistically significant (44). Similarly, Sands-Lincoln and colleagues observed a higher risk of incidence CHD and CVD for those individuals with long sleep (≥10 hours) compared to 7–8 hours of sleep, however this was also attenuated in fully-adjusted models (50). Rissling et al. found that long sleep duration (≥9 hours) was only significantly associated with diabetes in non-Veterans, but not among Veterans (38).
Insomnia Symptoms
Three studies investigated the association between sleep quality and cardiovascular health, and found that higher insomnia rating scores (WHIIRS) were associated with increased risk of CVD (38,49,50). Sands-Lincoln et al. demonstrated that women with WHIIRS scores ≥9 had the highest risk of CHD and CVD, and were still significant after models were adjusted for confounders and mediators. In a stratified analysis, high levels of insomnia symptoms (≥9 WHIIRS score) and long sleep duration (≥10 hours) also revealed a significant increased risk in CHD, but a non-statistically significant increased risk of CVD. Rissling et al. observed a small, yet significant, increased risk of CVD among non-Veterans who had WHIIRS scores ≥9. Huang et al. investigated poor sleep quality (using only four questions from the WHIIRS) and plasma metabolites to help explain the pathway from poor sleep quality to CHD (49). There were 69 metabolites associated with sleep quality, most (n=59) being lipid-related species and a smaller subset of the metabolites were considered sleep-related (n=9). Women with poor sleep quality had 1.36 times the risk of CHD and about 20% of the association was mediated by the sleep-related metabolites.
Snoring
Two studies assessed the association between snoring status and CVD and CHD (43,60). Both studies revealed a higher risk of CVD and CHD in WHI participants who snored. In women without prevalent CVD, Chen et al. saw an association between frequent snoring and frequent sleepiness (both defined as occurring more than once per week), with a higher risk of ischemic stroke (43). Sands and associates defined frequent snorers as those snoring ≥5 times per week and observed that frequent snorers had an increased risk of CHD, CVD, and stroke compared to those who were not snorers, after adjusting for age and race (60). In fully adjusted models, these associations were attenuated and more modest in magnitude, but still statistically significant. A stratified analysis of the same population by body mass index (BMI) categories revealed that frequent snoring in overweight and obese women was a significant predictor of CHD, but not statistically significant in fully adjusted models.
SDB risk score
Rissling and colleagues also assessed how SDB was associated with CVD (38). Veterans and non-Veterans at high risk for SDB had almost a 30% increased risk of CVD. There was also a 37% increased risk of diabetes in non-Veterans at high risk for SDB. When high risk of SDB was paired with a high risk of insomnia, non-Veterans had a significantly increased risk of CVD and of diabetes, whereas Veterans were only at increased risk of diabetes.
Cancer health outcomes
Six published papers assessed sleep and cancer outcomes, including breast, thyroid, colorectal, and liver cancer (45,46,51–54).
Regarding the breast cancer studies, on the whole in multivariable models, there were no statistically significant associations between sleep duration, insomnia scores, and breast cancer risk in WHI studies. A positive trend was shown for estrogen receptor (ER) positive breast cancer risk and sleep duration (p-trend=0.02), but it was not statistically significant for individual sleep duration categories (51). Hormone receptor status for breast cancer is important for treatment implications. Most breast cancer therapies attach to estrogen receptor sites and therefore women without estrogen receptor breast cancer (ER negative) have fewer treatment options. Soucise et al. found that non-Hispanic white women who slept 6 hours/night had 1.25 times the odds of regional/distant breast cancer compared to those who slept 7–8 hours/night in adjusted models. Regional breast cancer has moved outside the breast to nearby lymph nodes whereas distant breast cancer has moved to other parts of the body. Both regional and distant are more advanced and higher stages of breast cancer. No other sleep measures were significant in this population though (54). African-American women had no statistically significant associations between any sleep measures and tumor stage at diagnosis (54). Yet African-American women who reported restless or very restless sleep quality had 3.74 times the odds of triple negative breast cancer compared to those with sound or restful sleep quality. Triple negative breast cancer cells do not have estrogen receptors, progesterone receptors, and very little hormone epidermal growth factor receptor 2 (HER2), making common hormone treatments ineffective. These cancers also tend to be more aggressive than other types of breast cancer.
Colorectal cancer risk was found to be significantly higher among women who slept ≤5 hours or ≥9 hours. Women who reported having trouble falling asleep ≥3 times/week had 1.25 times the risk of colorectal cancer compared to those reporting <3 times/week. There was also a 26% increased risk of colorectal cancer in women who reported trouble getting back to sleep at night ≥3 times per week (45).
One team examined the relationship between sleep disturbance and thyroid cancer risk. Obesity is a risk factor for thyroid cancer (67) and sleep disturbances may interrupt appetite regulation (68), leading authors to also investigate the sleep-obesity interaction within thyroid cancer risk. However, thyroid cancer risk was found to be higher in non-obese WHI women who had more sleep disturbance (52). When divided into quartiles of insomnia scores, higher WHIIRS scores (≥11) were significantly associated with a higher risk of thyroid cancer compared to women with the lowest insomnia scores (<4). Stratified analyses showed significant results only for non-obese women with higher insomnia scores compared to obese women with higher insomnia scores. Sleep duration was not associated with thyroid cancer risk (52).
Liver cancer risk was higher in obese women with long sleep (46). Among obese WHI participants, those with long sleep duration (≥9 hours) had an increased risk of liver cancer compared to those who slept between 6–8 hours/night. The association was not significant among non-obese women. There was also no association between short sleep duration (≤5 hours) and liver cancer risk. Insomnia rating scores, snoring, and daytime napping were also not associated with liver cancer risk (46).
In examining survival rates across all cancer sites, no sleep characteristics at WHI baseline (pre-diagnosis) were significantly associated with overall cancer survival. However, in combination, those who slept ≤6 hours and reported frequent snoring (≥5 times/week) had significantly poorer overall cancer survival compared to those who slept 7–8 hours/night and did not snore. This association was stronger in women with breast cancer. Women with breast cancer who had short sleep duration (≤5 hours) had significantly poorer breast cancer-specific survival (53).
Cognitive/Mental health outcomes
Two papers addressed the relationships between sleep and cognitive health, emotional well-being, and physical functioning (47,48). Chen et al. examined sleep among WHI participants in the Women’s Health Initiative Memory Study (WHIMS). WHIMS was an ancillary study to the WHI to test the effects of hormone therapy on all-cause dementia. Cognitive decline in this cohort was measured by the mini-mental state (3MS) examination (69), and mild cognitive impairment (MCI) or probable dementia was determined by the validated 4-phase WHIMS protocols (70,71). Cognitive exams were conducted annually. Short sleep duration (≤6 hours) was significantly associated with an increase in risk for cognitive decline, but long sleep duration (≥8 hours) was not associated with cognitive decline in fully adjusted models. A similar pattern was seen for MCI/dementia. Those with short sleep had 1.36 times the risk of MCI/dementia and those with long sleep duration did not have a significantly higher risk. Results did not change significantly even after adjusting for cardiovascular disease and other related risk factors (47).
Zaslavsky et al. addressed changes in insomnia status with physical functioning and emotional well-being, by using sleep measurements from two time points: baseline and follow-up (year 1 for CT participants or year 3 for OS participants). Physical impairments were determined by the SF-36 physical functioning (scores of ≤60) and the emotional well-being (scores of ≤60) subscales cut-offs based on other comparable-age groups. Emotional well-being was also defined using a Center for Epidemiological Studies Depression (CESD) short form (72) to categorize women as having a higher number of depressive symptoms (score of >0.06). Women who developed insomnia at study follow up (incident insomnia, WHIRS ≥9) had significantly higher odds of physical impairment, higher depressive symptoms, and mixed impairments (both physical impairments and depressive symptoms) compared to those with no insomnia at follow up. Women with insomnia at baseline and follow up (persistent insomnia) had significantly higher odds of physical impairments, higher depressive symptoms, and mixed impairments (48).
Health behaviors/quality of life and other outcomes
Several additional published WHI papers examine sleep and other health behavior/quality of life outcomes that did not fall within the previous categories (12,39,42,49,55–57,59,62).
One ancillary WHI study utilized actigraphy data as an objective measure of sleep. These data were collected in a subset of WHI participants over one week (61,62). A U-shaped relationship was observed between survival and actigraphy measures of sleep duration. Those with <300 minutes (<5 hours) of sleep had only a 61% survival rate compared to a 90% survival rate in those with 300–390 minutes of sleep. Women sleeping longer than 390 minutes (>6.5 hours) also had a reduced survival of 78% compared to those with 300–390 minutes (61). The other paper using actigraphy data by Grandner et al. examined how actigraphy and subjective sleep reports were associated with dietary nutrients (62). Sleep acrophase (the peak of a fitted 24-hour cosine), total sleep time, naps, and sleep efficiency were calculated. Objective estimates of total sleep time were negatively associated with fat intake. However, these results were not found in subjective reports of total sleep, but subjective naps were positively correlated with fat intake and nutrients associated with meat intake (62).
Stern et al. used self-reported sleep to assess dietary intake and diet quality. The authors found that women with self-reported short sleep (≤6 hours) had lower circulating leptin levels compared to women reporting ≥8 hours. Short sleep was also associated with higher dietary intake and both short sleep and long sleep (≥8 hours) were associated with lower diet quality compared to women sleeping 7 hours (55).
Similar to the mortality article using actigraphy (12), when using self-reported baseline sleep measures, Kabat et al. (57) found short and long sleep duration to be associated with a higher risk of total mortality, cardiovascular disease mortality, and “other” mortality, but not with cancer mortality. In an additional time-dependent analysis, Kabat et al. utilized all available sleep measures (up to eight measures) and found results to be unchanged, but long sleep was now associated with cancer mortality (57).
Two papers investigated biological aging with differing markers (56,58). Carroll et al. (56) examined epigenetic age (a biomarker of aging based on DNA methylation) and immune senescence with insomnia symptoms. More insomnia symptoms were related to advanced epigenetic age and late differentiated T cells, but short and long sleep durations were not related to epigenetic age. Grieshober et al. (58) examined leukocyte telomere length (repetitive, non-coding DNA structures at the end of chromosomes) with sleep duration and sleep disturbance. Each additional hour of sleep after 5 hours was associated with an increase in base pairs of the leukocyte telomere length. The association between sleep duration and telomere length was strongest among African Americans and there was no significant association among European Americans for sleep duration. Sleep disturbance was not associated with telomere length in either group.
Nocturnal enuresis, sexual satisfaction, and recurrent falls were each studied once within the WHI. Nocturnal enuresis, the uncontrollable loss of urine at nighttime, was examined with OSA risk. For every additional OSA risk factor, such as BMI ≥30 kg/m2, or snoring ≥3 times per week, the odds of nocturnal enuresis was significantly higher (42). Higher insomnia scores were associated with lower odds of sexual satisfaction, measured with the question, “How satisfied are you with your current sexual activities, either with a partner or alone?” Short sleep duration was also associated with lower odds of partnered sexual activity and less sexual satisfaction (39). Recurrent falls (≥2 in the past year) and risk of fractures were also examined with sleep (59). Short and long sleep were associated with increased odds of recurrent falls. Additionally, those with very restless sleep, more sleep disturbances, and insomnia were associated with increased odds of recurrent falls. Short sleep was associated with increased risk of fractures (at various facture sites), but other sleep measures were not.
CONCLUSION
The WHI has provided a valuable resource in which to better understand the relationship between sleep measures and certain health outcomes or health behaviors/quality of life outcomes in post-menopausal women. When we explored articles in which sleep measures predicted a health outcome or health behavior, we generally observed that findings among WHI participants supported previous research on cardiovascular, cancer, mental health/cognition outcomes, and other health behavior outcomes. However, many of these WHI studies found that significant associations were attenuated after adjusting for various covariates.
Predictive role of sleep on health outcomes
Short sleep duration was generally associated with more cardiovascular disease health outcomes in the reviewed papers. However, almost all studies did not reach statistical significance after full adjustment for covariates. Short sleep has often been reported as a risk factor for CVD in the general population (2,3,73), yet it is possible post-menopausal women are less affected by short sleep and more affected by long sleep duration as it relates to CVD (74). Long sleep and sleep disturbance issues have been associated with a higher risk for CVD-related outcomes in a study of Chinese post-menopausal women (74). Another study observed that increased daytime napping was positively associated with increased risk of CHD, but did not see an association between night sleeping hours and 10-year CHD risk, even though shorter night time sleep may induce daytime sleepiness and increase the duration of daytime naps (75). Our review found more evidence in WHI for long sleep duration and sleep quality/insomnia being associated with a higher risk of CVD, although more WHI studies assessed the relationship with short sleep. Two studies (44,49) used biological markers from WHI participants to examine if there was a mediating role in the relationship between sleep and cardiovascular outcomes through biomarkers. Both found significant results and called for further research on these topics. In our review, frequent snoring and being at high risk for SDB showed some evidence of a positive association with CVD, however the definition of “frequent” snoring was inconsistent and SDB was only assessed in one CVD study. SDB and SDB comorbid with insomnia were more common in women Veterans than non-Veterans, but the risk for cardiometabolic health outcomes did not differ by Veteran status. The paper by Rissling et al. (38) on women Veterans was the only analysis in our current review to assess sleep in a special occupation group. Certain occupational exposures and restrictions can affect sleep and from this analysis Veterans were identified as a having a higher prevalence of risk for comorbid insomnia and SDB.
In terms of cancer outcomes, no sleep measures were associated with a higher risk of breast cancer in the WHI, yet some subset analyses showed associations between sleep measures and breast cancer among African-American women (54). African-American women with restless or very restless sleep had over three times the odds of triple negative breast cancer compared to women with sound or restful sleep quality. Other non-WHI studies have found an association between sleep duration and an increased risk of breast cancer in older women (76), while others have found long sleep to be associated with a decreased risk of breast cancer (77,78). In a recent publication, short and long sleep duration were not associated with an increased risk of breast cancer in post-menopausal women, however post-menopausal women with >4 nights/week of difficulty sleeping had 1.51 times the risk of breast cancer compared to women with difficulty sleeping <1 night/week (79). The null findings from the WHI are consistent with findings from several large cohort studies. The Nurses’ Health Study (80) and the Finnish Twin Cohort (78) found no evidence of an association between sleep duration and breast cancer risk or incidence. The Finnish Twin Cohort study, in addition to a Western Australian study (81), also saw no association with between sleep quality and the risk of breast cancer. The mixed results suggest a few possible avenues for exploration. Certain breast cancer risk factors may outweigh the negative side effects of poor sleep and be the primary driver for cancer development, rather than the pathway from disrupted sleep to carcinogenesis in the post-menopausal population. The negative effects of sleep disturbances on the body may take time to accumulate, therefore in post-menopausal women it is possible that newly developed sleep problems stemming from menopause or other midlife changes do not influence the more recent development of breast cancer. Finally, poor sleep may be associated with higher risk of breast cancer, but the methods to capture the exposure are inadequate in this population.
Conversely, there was an association between sleep and colorectal cancer risk among WHI participants. In general, the association between sleep health and colorectal cancer is a less studied topic area, especially in older women. A 2011 study found that short sleep duration was associated with an increased risk for colorectal adenomas in men and women (82). Another study found that colorectal cancer risk in overweight women or women who were snorers was higher for those who reported ≥9 hours of sleep (8).
Findings on overall cancer survival in WHI were not significantly associated with any individual sleep measure in our review, but when considered in combination, snoring with short sleep was associated with increased risk of mortality. Prior research demonstrating an association between sleep and poor cancer prognosis have primarily used actigraphy and post-diagnosis measures, which differs from the methods in the WHI studies reviewed (83,84).
Regarding cognitive functioning, short sleep duration and higher insomnia symptoms were found to be associated with a higher risk for cognitive decline or cognitive impairments (47,48). In a non-WHI meta-analysis (85), authors found more support for insufficient sleep affecting cognition in younger or middle age than in older adults. Others have noted less consistent findings between insomnia and cognitive impairment among older adults, but have observed that sleep duration may increase the risk for cognitive decline (86).
Lastly, in this group of WHI post-menopausal women, short and long sleep measured objectively and subjectively, were associated with a higher risk of mortality. This result has been found in other studies, including risks associated with long sleep in older adults (12,87,88). Actigraphy-measured sleep duration was also associated with fat intake and those with short sleep tended to have lower leptin levels, a hormone responsible for regulating energy expenditure. Subjectively-measured insomnia symptoms were associated with more advanced epigenetic age, lower odds of sexual satisfaction, and higher odds of recurrent falls. Additionally, an increasing OSA score was associated with increased odds for experiencing nocturnal enuresis.
Attenuated findings after adjustment
There are many potential explanations for non-significant associations. First, fully adjusted models may have accounted for many of the key demographic and health behavior differences among those with and without the outcome of interest. In many cases, we can assume that the full attenuation indicates that there is no true causal association between the sleep parameter and the health outcome. However, in other cases, adjustment for health behaviors and comorbid conditions may serve as an over-adjustment, if the adjusted comorbid conditions were related to the presence of a sleep parameter (50). A second concern is measurement error, which biases associations towards the null. Subjective measures of sleep duration, for example, may introduce differential misclassification (43). Self-reported sleep was also commonly used from baseline only and a single measure of sleep may not truly reveal long-term sleep habits, since sleep patterns change differentially with age and disease (89). There is a complex and bidirectional relationship between sleep and health, with many factors (social, behavioral, psychological) impacting both sleep patterns and morbidity.
Limitations and Strengths
This review has some limitations that should be noted. We restricted our review to include only published papers that used sleep as a predictor of health outcomes or health behaviors. First, this introduces the challenge of publication bias, where there may have been other analyses conducted that did not make it to full publication, most likely due to null findings. Second, there were more WHI published papers that examined the associations between sleep and health in other ways (n=14). However, we believe that using sleep as a predictor variable may provide more information that can be used for interventions to improve health outcomes and behaviors in older adult women. Third, researchers often used differing definitions/measures of short sleep (≤5 hours vs ≤6 hours vs <7 hours), described ‘frequent snoring’ by varying times per week, and used different cutoff points for the WHIIRS (≥9 vs ≥11). Lastly, we must also consider the timing of sleep assessments completed by study participants. For many of the WHI participants, sleep assessments occurred mainly at study baseline and the observed health outcome may have happened up to 9 years after the assessment. Despite these concerns, a strength of the longitudinal study design is that all sleep measures were assessed before the outcome was measured and therefore reverse causality can be minimized.
Conclusions
In summary, this review reveals that many health outcomes in post-menopausal women are associated with shorter sleep duration, excess sleep, insomnia, OSA and SDB risk, although some of these relationships were attenuated after adjustment in multivariate analyses. Certain outcomes were studied more frequently, like cardiovascular disease, compared to diabetes or less common cancers. Sleep habits and patterns are a modifiable risk factor to a certain extent (e.g., work schedules, sleep hygiene), and individuals, caregivers, health care providers, and institutions can make efforts to help improve sleep habits in an aging population. Taken as a whole, these results demonstrate that there are consistent associations between sleep health and stroke, colorectal cancer, cognition, dietary intake and quality, and mortality, but inconsistent associations with CHD/CVD, breast cancer, and cancer survival among the WHI Study participants. More research could be done in separate populations to replicate these studies and better understand any inconsistencies. Replication of epidemiologic findings is beneficial to accumulate evidence and assess generalizability.
Future research on sleep and health outcomes in postmenopausal women using the WHI may benefit by considering mediation models, longitudinal methods, and incorporating actigraphy or other objective sleep data into the analysis. Thus far, only two of the 23 articles included in this review focused on a mediation analysis (44,49). Additionally, changes in sleep over time may better capture the effects of sleep duration and sleep quality. Due to the long follow up of the WHI, many participants have completed multiple sleep questionnaires, but the timing of these assessments in relation to specific health outcomes needs to be taken into consideration if using these suggested longitudinal methods. Most papers in this review did not focus on the results by race/ethnicity. Sleep may differ by race/ethnicity for many reasons and is a complex relationship that must also include the role of sociodemographic differences (90), but this could be recognized more in future WHI papers. There has been a consistent underrepresentation of racial/ethnic minorities in sleep research (91), and though the WHI is composed of a majority of non-Hispanic white women (82.5%), the large number of racially/ethnically diverse participants (n=28,267) is a currently under used source. We believe these suggestions could strengthen WHI sleep papers going forward. By summarizing and critiquing the state of WHI sleep papers, this review highlights the importance of sleep in post-menopausal women and next milestones to accomplish for a more comprehensive understanding of sleep in older populations.
Table 1.
WHI published sleep-related research articles, by health outcome.
| First Author, Year | Sample Size for Analysis* | WHI Study Arm | Sleep Measures | Health Outcome | |
|---|---|---|---|---|---|
| Cardiovascular outcomes | |||||
| Chen, 2008 | 93,175 | OS | Duration | Stroke | |
| Sands, 2013 | 42,244 | OS | Snoring | Stroke, CHD, CVD | |
| Hale, 2013 | 3,942 | OS & CT | Duration | CHD | |
| Sands-Lincoln, 2013 | 86,329 | OS | Duration & WHIIRS | CHD, CVD | |
| Rissling, 2016 | 145,061 | OS & CT | Duration, WHIIRS, SDB | CVD, T2DM | |
| Huang, 2018 | 1,956 | OS & CT | WHIIRS | CHD | |
| Cancer outcomes | |||||
| Vogtmann, 2013 | 110,011 | OS & CT | Duration & WHIIRS | Breast cancer | |
| Jiao, 2013 | 75,828 | OS | Duration | Colorectal cancer | |
| Luo, 2013 | 142,933 | OS & CT | Duration & WHIIRS | Thyroid cancer | |
| Phipps, 2016 | 21,230 | OS & CT | Duration & WHIIRS | Cancer survival | |
| Soucise, 2017 | 4,406 | OS | Duration & WHIIRS | Breast cancer | |
| Royse, 2017 | 139,368 | OS & CT | Duration | Liver cancer | |
| Cognition/Mental Health outcomes | |||||
| Zaslavsky, 2015 | 93,532 | OS & CT | WHIIRS | Physical Impairments, Emotional Well-being | |
| Chen, 2016 | 7,444 | CT | Duration | Dementia | |
| Health Behavior/Quality of Life and Other outcomes | |||||
| Grandner, 2010 | 459 | OS | Actigraphy | Dietary nutrients | |
| Kripke, 2011 | 459 | OS | Actigraphy | Mortality | |
| Stern, 2014 | 769 | OS | Duration & WHIIRS | Leptin, Dietary intake | |
| Koo, 2016 | 161,808 | OS & CT | OSA | Nocturnal enuresis | |
| Carroll, 2017 | 2,078 | OS & CT | Duration & WHIIRS | Epigenetic Age | |
| Kling, 2017 | 93,668 | OS | WHIIRS, OSA | Sexual satisfaction | |
| Kabat, 2018 | 158,203 | OS & CT | Duration & WHIIRS | Mortality | |
| Cauley, 2019 | 157,306 | OS & CT | Duration & WHIIRS | Falls/Fractures | |
| Grieshober, 2019 | 3,145 | OS & CT | Duration & WHIIRS | Telomere Length |
OS: observational study; CT: clinical trial; SDB: Sleep disordered breathing, WHIIRS: Women’s Health Initiative Insomnia Rating Scale; CVD: Cardiovascular disease; CHD: Coronary Heart Disease; T2DM: Type 2 Diabetes Mellitus; OSA: Obstructive Sleep Apnea
Sample sizes for each analysis vary depending on the specified WHI participants and variables of interest pre-determined in approved paper proposals
Table 2.
Summary of 23 WHI published research articles on the association between sleep and health outcomes.
| First Author, Year | Health Outcome | Results | Measure of Association | Independent Variables in Final Model |
|---|---|---|---|---|
| Cardiovascular Outcomes | ||||
| Chen, 2008 | Stroke | Long sleep duration was positively associated with increased risk of stroke. | RR: 1.70 (95% CI: l.32, 2.21) | Age, race, education, family income, employment status, depression, smoking, exercise, use of hormone therapy, relevant CVD risk factors (previous CVD, diabetes, hypertension, high cholesterol requiring pills, BMI) |
| Sands, 2013 | Stroke, CHD, CVD | Frequent snoring was modestly associated with increased risk of incident CHD, CVD, and stroke. |
CHD: HR: 1.14 (95% CI: 1.01, 1.28) CVD: HR: 1.12 (95% CI: 1.01, 1.24) Stroke: HR: 1.19 (95% CI: 1.02, 1.40) |
Age, race, education, income, smoking, physical activity, alcohol intake, depression, diabetes, high blood pressure, BMI, waist-to-hip ratio, hyperlipidemia |
| Hale, 2013 | CHD | Long sleep duration is associated with increased CHD, but not after adjustment for fibrinogen. Fibrinogen may be a mechanism through which long sleep duration is associated with CHD and mortality | Unadjusted OR: 2.05 (95% CI: 1.02–4.11); Adjusted OR: 1.88 (95% CI: .92–3.83) |
Age, race/ethnicity, education, income, fibrinogen, BMI, exercise, smoking history, alcohol intake, comorbidities (elevated blood pressure, diabetes, depression), general health, life satisfaction |
| Sands-Lincoln, 2013 | CHD, CVD | High insomnia scores were positively associated with the greatest risk of CHD and CVD |
CHD: HR: 1.19 (95% CI: 1.08, 1.30) CVD: HR: 1.11 (95% CI: 1.03, 2.00) |
Age, race, education, income, smoking, BMI, physical activity, alcohol intake, depression, diabetes, high blood pressure, hyperlipidemia, comorbid conditions |
| Rissling, 2016 | CVD, T2DM | Veterans at high risk for insomnia + SDB were positively associated with increased risk for diabetes. High risk for SDB was positively associated with increased risk for CVD in both Veterans and non-Veterans. Sleep duration was associated with greater risk of CVD and diabetes in non-Veterans. |
Veterans: Diabetes, Insomnia + SDB: HR 1.49 (95% CI: 1.11, 2.00); CVD, SDB: HR 1.28 (95% CI: 1.05, 1.56) Non-Veterans: CVD, SDB: HR 1.29 (95% CI: 1.24, 1.34) CVD, Short Sleep: HR: 1.15 (95% CI: 1.08, 1.22) Diabetes, Short Sleep: HR: 1.11 (95% CI: 1.05, 1.19) Diabetes, Long Sleep: HR: 1.12 (95% CI: 1.03, 1.22) |
Baseline age, race, education, BMI, living with partner, physical activity, vasomotor symptoms, napping, current smoker, study arm |
| Huang, 2018 | CHD | Lipid metabolites may be a mechanism through which poor sleep quality is associated with development of CHD. | CHD, Poor sleep quality: OR: 1.36 (95% CI: 1.02, 1.81); 20.2% mediated by a sleep-related metabolic score | Age, race/ethnicity, hysterectomy status, enrollment window, BMI, smoking, dietary quality, physical activity, prevalent hypertension, current hormone therapy, aspirin use, statin use and other lipid-lowering medications |
| Cancer Outcomes | ||||
| Vogtmann, 2013 | Breast cancer | No statistically significant association between duration or insomnia and breast cancer risk after adjustment. | N/A | Age, clinical trial arm assignment, number of live births, age at menarche, age at menopause, body mass index, energy expenditure, education, income, race/ethnicity, marital status, age at first birth, previous use of hormone replacement therapy, history of benign breast disease, family history of breast cancer, alcohol consumption, smoking status |
| Jiao, 2013 | Colorectal cancer | Short and long sleep were positively associated with greater risk of CRC |
Short sleep: HR: 1.36 (95% CI: 1.06, 1.74) Long sleep: HR: 1.47 (95% CI: 1.10, 1.96) |
Age, ethnicity, fatigue, hormone replacement therapy, waist to hip ratio, physical activity |
| Luo, 2013 | Thyroid cancer | Higher insomnia scores were positively associated with increased risk of thyroid cancer, especially for non-obese women. |
Higher insomnia scores: HR: 1.44 (95% CI: 1.01, 2.05) Non-obese: HR: 1.71 (95% CI: 1.12, 2.62) Obese: HR: 0.94 (95% CI: 0.48, 1.84) |
Age at enrollment, ethnicity, educational level, smoking, BMI, recreational physical activity, alcohol intake, family history of cancer, previous thyroid disease, history of hormone therapy use, depression score, and different treatment assignments for WHI clinical trials |
| Phipps, 2016 | Cancer survival | No pre-diagnosis sleep measures were significantly associated with cancer survival for all sites combined. However, in combination, short sleep with frequent snoring was positively associated with poorer cancer-specific survival. |
Short sleep + snoring: HR: 1.32 (95% CI: 1.14, 1.54) |
Age at enrollment, study arm, cancer site, marital status, household income, smoking history, recreational physical activity, and lag time between baseline data collection and cancer diagnosis |
| Soucise, 2017 | Breast cancer | Short sleep was positively associated with increased odds of regional/distant stage tumors in Non-Hispanic White women; Restless or very restless sleep quality in African American women was positively associated with increased odds for triple-negative tumors |
Short Sleep: OR: 1.25 (95% CI: 1.05, 1.48) Restless: OR: 3.74 (95% CI: 1.10, 12.77) |
Age, BMI, and HT use, income, smoking status, pack years, alcohol intake, and physical activity |
| Royse, 2017 | Liver cancer | Long sleep was positively associated with higher risk of liver cancer; Long sleep in obese women was positively associated with increased risk of liver cancer. There was no positive association in non-obese women. |
Long sleep, obese: HR: 3.18 (95% CI: 1.84, 8.60) Long sleep, non-obese: HR: 0.93 (95% CI: 0.34, 2.53) |
Age at screening, race/ethnicity, employment, pain, fatigue, coffee, alcohol servings per week, hormone replacement therapy, BMI, type 2 diabetes |
| Cognitive/Mental Health Outcomes | ||||
| Zaslavsky, 2015 | Physical Impairments, Emotional Well-being | Insomnia developed by follow up (Normal-Abnormal) or insomnia present at baseline and follow up (Abnormal-Abnormal) were positively associated with higher odds for physical impairment (PI), depressive symptoms, and mixed impairments (MI). |
Normal-Abnormal (CT): PI: OR: 1.86 (95% CI: 1.57, 2.20); Depressive symptoms: OR: 4.11 (95% CI: 3.59, 4.72); MI: OR: 6.37 (95% CI: 4.65, 8.74). Normal-Abnormal (OS): PI: OR: 1.70 (95% CI: 1.51, 1.89); Depressive symptoms: OR: 3.80 (95% CI: 3.39, 4.25); MI: OR: 4.41 (95% CI: 3.56, 5.46). |
Age, race, income, education, hormone replacement therapy, selected chronic conditions, alcohol use, smoking, coffee consumption, BMI, sleep duration, and whether a participant lives alone |
| Chen, 2016 | Dementia | Short sleep was positively associated with higher MCI/dementia risk and cognitive decline. Long sleep was positively associated with MCI/dementia only in women without CVD. |
Short sleep, MCI/dementia: HR: 1.36 (95% CI: 1.09, 1.71) Short sleep, cognitive decline: HR: 1.36 (95% CI: 1.14, 1.62) |
Age, race, SES, lifestyle factors (smoking, alcohol consumption, and physical activities), depression, and other relevant clinical characteristics (previous HT use, BMI, prior CVD history, hypertension, diabetes, hypercholesterolemia) |
| Health Behavior/Quality of Life and Other outcomes | ||||
| Grandner, 2010 | Dietary nutrients | Total sleep time measured by actigraphy was negatively associated with intakes of fat; This was not observed in subjective total sleep time. Subjective naps were significantly correlated with fat intake. | Partial correlations for differing dietary variables listed within Table 2 of original paper | Age, income, education, total dietary gram amount, BMI, minutes of moderate-strenuous physical activity |
| Kripke, 2011 | Mortality | Actigraphic sleep of <300 minutes and >390 minutes were both negatively associated with survival compared to 300–390 min. | <300 min: 61% survival, (95% CI: 54%, 69%) >390 min: 78% survival (95% CI: 73%, 85%). |
Age squared, systolic blood pressure, major depression, diabetes, history of myocardial infarction, history of cancer, aMT6s-sleep phase angle |
| Stern, 2014 | Leptin, Dietary Intake | Short sleep was positively associated with lower leptin levels. Those with short sleep reported higher dietary intake and those with short and long sleep reported lower diet quality. |
Leptin: ≤6 hours vs ≥8 hours, p=0.04. Dietary Intake: β: 0.009; SE: 0.004, p=0.014 Diet Quality, short sleep: β: −1.634, SE: 0.779, p=0.036 Diet Quality, long sleep: β: −2.077, SE: 0.872, p=0.017 |
Age, race, total body fat mass, square root of physical activity, income, education, smoking status, alcohol intake, antidepressant use, and comorbidity status (diabetes/ hypertension/ rheumatoid arthritis), natural log of circulating leptin levels |
| Koo, 2016 | Nocturnal enuresis | Each additional OSA risk factor was positively associated with increasing odds for nocturnal enuresis | OR: 1.38, 2.0, 2.8, 3.87, 5.10, 7.02 for OSA scores of 1 to 6, respectively | Age, race, education, income, marital status, waist-to-hip ratio, diabetes, hyperlipidemia, coronary heart disease, heart failure, atrial fibrillation, stroke, hormone therapy, diuretic use, sedatives/hypnotics use, modified Charlson comorbidity index, smoking, alcohol consumption, physical activity, parity |
| Carroll, 2017 | Epigenetic age | Insomnia symptoms had modest associations with measures of advanced epigenetic age, but was positively associated with increased proportions of late differentiated T cells. Short and long sleep were not related to epigenetic age. | Epigenetic Age: β: 1.02, SE: 0.37, p=0.005. Late differentiated T cells: β: 0.59, SE: 0.21, p=0.006 |
Race (black vs. nonblack Hispanic vs. non-Hispanic), education (category), body mass index (category), snore (yes=1), comorbid chronic conditions: diabetes, hypertension, and cardiovascular disease |
| Kling, 2017 | Sexual satisfaction | Higher insomnia scores were associated with reduced odds of sexual satisfaction. Short sleep duration was associated with reduced odds of partnered sexual activity and less sexual satisfaction. |
High insomnia scores: OR:0.92 (95% CI: 0.87, 0.96) Short sleep, sexual activity: OR: 0.88 (95% CI: 0.80, 0.96) Short sleep, sexual satisfaction: OR: 0.88 (95% CI: 0.81, 0.95) |
Age, marital status, family income, race/ethnicity, education level, physical activity, self-rated health, antidepressant use including SSRI use, sedative hypnotic use, sleep medication use, CES-D/DIS, hysterectomy, smoking status, weight, alcohol use, hot flashes, night sweats, osteoporosis, physical and verbal abuse, life events scale, social strain construct, social support construct, vaginal dryness, hypertension, cardiovascular disease, arthritis, stroke, cancer (breast, ovarian, and cervical), diabetes, peripheral arterial disease, obesity (BMI>30 kg/m2), and HT |
| Kabat, 2018 | Mortality | Short and long sleep were positively associated with increased risk of total mortality, CVD mortality, and other mortality. It was not associated with cancer mortality. |
Total mortality: HRs: ≤5 h: 1.06 (95% CI: 1.01, 1.11); 9 h: 1.10 (95% CI: 1.04, 1.17); ≥10 h: 1.18, (95% CI: 1.00, 1.39). CVD mortality: HR for ≤5 h: 1.10 (95% CI: 1.01, 1.19); 9 h: 1.10 (95% CI: 0.98, 1.23); ≥10 h: 1.38, (95% CI: 1.05, 1.81). Other mortality: HR for ≤5 h: 1.10 (95% CI: 1.01, 1.19); 9 h: 1.19 (95% CI: 1.08, 1.31); ≥10 h: 1.08 (95% CI: 0.97, 1.19). |
Age, smoking status, pack-years of smoking, alcohol intake, hormone therapy, body mass index, red meat intake, physical activity, history of diabetes, history of cancer, history of cardiovascular disease, systolic blood pressure, health status, educational level, ethnicity, study participation (observational study; intervention vs. placebo arm of clinical trials) |
| Cauley, 2019 | Falls/Fractures | Short and long sleep were associated with increased odds of recurrent falls. Those with very restless sleep, more sleep disturbances, and insomnia were also associated with increased odds of recurrent falls. Short sleep was associated with increased risk of fractures, but other sleep measures were not. |
Recurrent Falls: Short sleep: OR: 1.27 (95% CI: 1.22, 1.33); Long sleep: OR: 1.24 (95% CI: 1.08, 1.42); Very restless: OR: 1.21 (95% CI: 1.13, 1.30); Sleep disturbance ≥11: OR: 1.34 (95% CI: 1.30, 1.39) Fractures: Short sleep: HRs range from 1.10 to 1.13 (see original paper, Table 3 for exact HRs and 95% CIs). |
Age, weight, height, treated diabetes, ethnicity/race, region, smoking status, general health status, current HT use, total vitamin D intake, physical activity, alcohol intake, depressive symptom score, caffeine intake, HT trial arm, diet modification trial arm, hypnotics medication use, anti-anxiety medication use, antidepressant medication use, analgesic narcotic medication use, physical function score and number of chronic conditions (stroke, MI, CHF, diabetes, Parkinson’s disease, COPD, asthma, any cancer |
| Grieshober, 2019 | Telomere Length | Each additional hour of sleep after 5 hours was associated with an increase in base pairs of the leukocyte telomere length. Sleep disturbance was not associated with telomere length. | 27 base pair (95% CI: 6, 48) longer telomere lengths. |
Sleep duration model: Age, race, LTL batch, body mass index, income, sleep aid use, cigarette pack years, and physical function Sleep disturbance model: Age, race, LTL batch, body mass index, cardiovascular disease status, comorbidity presence, depression status, education, income, marital/partner status, neighborhood socioeconomic status, cigarette pack years, physical function score, sleep aid use, social support score, and U.S. enrollment region |
ACKNOWLEDGEMENTS
The authors want to thank and acknowledge all the WHI participants and investigators. The following is a short list of WHI investigators:
Program Office: (National Heart, Lung, and Blood Institute, Bethesda, Maryland) Jacques Rossouw, Shari Ludlam, Joan McGowan, Leslie Ford, and Nancy Geller
Clinical Coordinating Center: (Fred Hutchinson Cancer Research Center, Seattle, WA) Garnet Anderson, Ross Prentice, Andrea LaCroix, and Charles Kooperberg
Investigators and Academic Centers: (Brigham and Women’s Hospital, Harvard Medical School, Boston, MA) JoAnn E. Manson; (MedStar Health Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Arizona, Tucson/Phoenix, AZ) Cynthia A. Thomson; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Iowa, Iowa City/Davenport, IA) Jennifer Robinson; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller; (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker; (University of Nevada, Reno, NV) Robert Brunner; (University of Minnesota, Minneapolis, MN) Karen L. Margolis
Women’s Health Initiative Memory Study: (Wake Forest University School of Medicine, Winston-Salem, NC) Mark Espeland
This research was supported by the WHI, which is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts, HHSN268201600018C, HHSN268201600001C, HHSN268201600002C, HHSN268201600003C, and HHSN268201600004C.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Chloe M. Beverly Hery, Division of Epidemiology, College of Public Health, The Ohio State University, Columbus, OH 43210.
Lauren Hale, Program in Public Health, Department of Family, Population, and Preventive Medicine, Stony Brook University, NY 11794-8338.
Michelle J. Naughton, Division of Cancer Prevention and Control, College of Medicine, The Ohio State University, Columbus, OH 43210.
REFERENCES
References with an asterisk (*) identify the articles included in this review.
- 1.Czeisler CA. Duration, timing and quality of sleep are each vital for health, performance and safety. Sleep Health. 2015; 1(1):5–8. [DOI] [PubMed] [Google Scholar]
- 2.Altman NG, Izci-Balserak B, Schopfer E, et al. Sleep duration versus sleep insufficiency as predictors of cardiometabolic health outcomes. Sleep medicine. 2012; 13(10): 1261–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoevenaar-Blom MP, Spijkerman AM, Kromhout D, van den Berg JF, Verschuren WM. Sleep duration and sleep quality in relation to 12-year cardiovascular disease incidence: the MORGEN study. Sleep. 2011;34(11):1487–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buxton OM, Marcelli E. Short and long sleep are positively associated with obesity, diabetes, hypertension, and cardiovascular disease among adults in the United States. Soc Sci Med. 2010;71(5):1027–1036. [DOI] [PubMed] [Google Scholar]
- 5.Leineweber C, Kecklund G, Akerstedt T, Janszky I, Orth-Gomer K. Snoring and the metabolic syndrome in women. Sleep medicine. 2003;4(6):531–536. [DOI] [PubMed] [Google Scholar]
- 6.Knutson KL. Sleep duration and cardiometabolic risk: a review of the epidemiologic evidence. BestPractRes Clin EndocrinolMetab. 2010;24(5):731–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hurley S, Goldberg D, Bernstein L, Reynolds P. Sleep duration and cancer risk in women. Cancer Causes Control. 2015;26(7):1037–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang X, Giovannucci EL, Wu K, et al. Associations of self-reported sleep duration and snoring with colorectal cancer risk in men and women. Sleep. 2013;36(5):681–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang P, Ren FM, Lin Y, et al. Night-shift work, sleep duration, daytime napping, and breast cancer risk. Sleep medicine. 2015;16(4):462–468. [DOI] [PubMed] [Google Scholar]
- 10.Gallicchio L, Kalesan B. Sleep duration and mortality: a systematic review and meta-analysis. J Sleep Res. 2009;18(2): 148–158. [DOI] [PubMed] [Google Scholar]
- 11.Grandner MA, Hale L, Moore M, Patel NP. Mortality associated with short sleep duration: The evidence, the possible mechanisms, and the future. Sleep medicine reviews. 2010;14(3): 191–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002;59(2): 131–136. [DOI] [PubMed] [Google Scholar]
- 13.Vgontzas AN, Fernandez-Mendoza J, Liao D, Bixler EO . Insomnia with objective short sleep duration: the most biologically severe phenotype of the disorder. Sleep medicine reviews. 2013;17(4):241–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Irwin MR. Why sleep is important for health: a psychoneuroimmunology perspective. Annu Rev Psychol. 2015;66:143–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riemann D, Berger M, Voderholzer U. Sleep and depression--results from psychobiological studies: an overview. Biol Psychol. 2001;57(1–3):67–103. [DOI] [PubMed] [Google Scholar]
- 16.Baglioni C, Battagliese G, Feige B, et al. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord. 2011;135(1–3):10–19. [DOI] [PubMed] [Google Scholar]
- 17.Fernandez-Mendoza J, Calhoun SL, Bixler EO, et al. Sleep misperception and chronic insomnia in the general population: role of objective sleep duration and psychological profiles. Psychosom Med. 2011;73(1) :88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palagini L, Baglioni C, Ciapparelli A, Gemignani A, Riemann D. REM sleep dysregulation in depression: state of the art. Sleep medicine reviews. 2013;17(5):377–390. [DOI] [PubMed] [Google Scholar]
- 19.Vitiello MV. Sleep in Normal Aging. Sleep medicine clinics. 2006;1(2): 171–176. [Google Scholar]
- 20.Jehan S, Masters-Isarilov A, Salifu I, et al. Sleep Disorders in Postmenopausal Women. J Sleep Disord Ther. 2015;4(5). [PMC free article] [PubMed] [Google Scholar]
- 21.Kripke DF, Brunner R, Freeman R, et al. Sleep Complaints of Postmenopausal Women. Clinical journal of women’s health. 2001;1(5):244–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carpenter JS, Elam JL, Ridner SH, Carney PH, Cherry GJ, Cucullu HL. Sleep, fatigue, and depressive symptoms in breast cancer survivors and matched healthy women experiencing hot flashes. Oncology nursing forum. 2004;31(3):591–5598. [DOI] [PubMed] [Google Scholar]
- 23.Kravitz HM, Avery E, Sowers M, et al. Relationships between menopausal and mood symptoms and EEG sleep measures in a multi-ethnic sample of middle-aged women: the SWAN sleep study. Sleep. 2011;34(9): 1221–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kravitz HM, Zhao X, Bromberger JT, et al. Sleep disturbance during the menopausal transition in a multi-ethnic community sample of women. Sleep. 2008;31(7):979–990. [PMC free article] [PubMed] [Google Scholar]
- 25.Joffe H, Massler A, Sharkey KM. Evaluation and management of sleep disturbance during the menopause transition. Semin ReprodMed. 2010;28(5):404–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Colditz GA, Manson JE, Hankinson SE. The Nurses’ Health Study: 20-year contribution to the understanding of health among women. J Womens Health. 1997;6(1):49–62. [DOI] [PubMed] [Google Scholar]
- 27.Design of the Women’s Health Initiative clinical trial and observational study. The Women’s Health Initiative Study Group. Controlled clinical trials. 1998;19(1):61–109. [DOI] [PubMed] [Google Scholar]
- 28.Sowers M, Crawford S, Sternfeld B, et al. SWAN: a multi-center, multi-ethnic, community-based cohort study of women and the menopausal transition In: Menopause: biology andpathobiology. Academic Press, New York, NY; 2000:175–188. [Google Scholar]
- 29.Kravitz HM, Joffe H. Sleep during the perimenopause: a SWAN story. Obstet Gynecol Clin North Am. 2011;38(3):567–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hall MH, Casement MD, Troxel WM, et al. Chronic Stress is Prospectively Associated with Sleep in Midlife Women: The SWAN Sleep Study. Sleep. 2015;38(10): 1645–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colditz GA, Philpott SE, Hankinson SE. The Impact of the Nurses’ Health Study on Population Health: Prevention, Translation, and Control. Am J Public Health. 2016;106(9):1540–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gangwisch JE, Feskanich D, Malaspina D, Shen S, Forman JP. Sleep duration and risk for hypertension in women: results from the nurses’ health study. Am J Hypertens. 2013;26(7):903–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anderson GL, Manson J, Wallace R, et al. Implementation of the Women’s Health Initiative study design. Annals of epidemiology. 2003;13(9 Suppl):S5–17. [DOI] [PubMed] [Google Scholar]
- 34.Hays J, Hunt JR, Hubbell FA, et al. The Women’s Health Initiative recruitment methods and results. Annals of epidemiology. 2003;13(9 Suppl):S18–77. [DOI] [PubMed] [Google Scholar]
- 35.Levine DW, Kaplan RM, Kripke DF, Bowen DJ, Naughton MJ, Shumaker SA. Factor structure and measurement invariance of the Women’s Health Initiative Insomnia Rating Scale. Psychological assessment. 2003;15(2): 123–136. [DOI] [PubMed] [Google Scholar]
- 36.Levine DW, Kripke DF, Kaplan RM, et al. Reliability and validity of the Women’s Health Initiative Insomnia Rating Scale. Psychological assessment. 2003;15(2): 137–148. [DOI] [PubMed] [Google Scholar]
- 37.Levine DW, Dailey ME, Rockhill B, Tipping D, Naughton MJ, Shumaker SA. Validation of the Women’s Health Initiative Insomnia Rating Scale in a multicenter controlled clinical trial. PsychosomMed. 2005;67(1):98–104. [DOI] [PubMed] [Google Scholar]
- 38.*.Rissling MB, Gray KE, Ulmer CS, et al. Sleep Disturbance, Diabetes, and Cardiovascular Disease in Postmenopausal Veteran Women. Gerontologist. 2016;56 Suppl 1:S54–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.*.Kling JM, Manson JE, Naughton MJ, et al. Association of sleep disturbance and sexual function in postmenopausal women. Menopause. 2017;24(6):604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999; 131 (7) :485–491. [DOI] [PubMed] [Google Scholar]
- 41.Mustafa M, Erokwu N, Ebose I, Strohl K. Sleep problems and the risk for sleep disorders in an outpatient veteran population. Sleep Breath. 2005;9(2):57–63. [DOI] [PubMed] [Google Scholar]
- 42.*.Koo P, McCool FD, Hale L, Stone K, Eaton CB. Association of obstructive sleep apnea risk factors with nocturnal enuresis in postmenopausal women. Menopause. 2016;23(2):175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.*.Chen JC, Brunner RL, Ren H, et al. Sleep duration and risk of ischemic stroke in postmenopausal women. Stroke. 2008;39(12):3185–3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.*.Hale L, Parente V, Dowd JB, et al. Fibrinogen may mediate the association between long sleep duration and coronary heart disease. J Sleep Res. 2013;22(3):305–314. [DOI] [PubMed] [Google Scholar]
- 45.*.Jiao L, Duan Z, Sangi-Haghpeykar H, Hale L, White DL, El-Serag HB. Sleep duration and incidence of colorectal cancer in postmenopausal women. Br J Cancer. 2013; 108( 1):213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.*.Royse KE, El-Serag HB, Chen L, et al. Sleep Duration and Risk of Liver Cancer in Postmenopausal Women: The Women’s Health Initiative Study. J Womens Health (Larchmt). 2017;26(12): 1270–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.*.Chen JC, Espeland MA, Brunner RL, et al. Sleep duration, cognitive decline, and dementia risk in older women. Alzheimers Dement. 2016;12(1):21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.*.Zaslavsky O, LaCroix AZ, Hale L, Tindle H, Shochat T. Longitudinal changes in insomnia status and incidence of physical, emotional, or mixed impairment in postmenopausal women participating in the Women’s Health Initiative (WHI) study. Sleep medicine. 2015; 16(3):364–371. [DOI] [PubMed] [Google Scholar]
- 49.*.Huang T, Zeleznik OA, Poole EM, et al. Habitual sleep quality, plasma metabolites and risk of coronary heart disease in post-menopausal women. Int J Epidemiol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.*.Sands-Lincoln M, Loucks EB, Lu B, et al. Sleep duration, insomnia, and coronary heart disease among postmenopausal women in the Women’s Health Initiative. J Womens Health (Larchmt). 2013;22(6):477–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.*.Vogtmann E, Levitan EB, Hale L, et al. Association between sleep and breast cancer incidence among postmenopausal women in the Women’s Health Initiative. Sleep. 2013;36(10): 1437–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.*.Luo J, Sands M, Wactawski-Wende J, Song Y, Margolis KL. Sleep disturbance and incidence of thyroid cancer in postmenopausal women the Women’s Health Initiative. American journal of epidemiology. 2013;177(1):42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.*.Phipps AI, Bhatti P, Neuhouser ML, et al. Pre-diagnostic Sleep Duration and Sleep Quality in Relation to Subsequent Cancer Survival. J Clin Sleep Med. 2016;12(4):495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.*.Soucise A, Vaughn C, Thompson CL, et al. Sleep quality, duration, and breast cancer aggressiveness. Breast cancer research and treatment. 2017; 164(1): 169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.*.Stern JH, Grant AS, Thomson CA, et al. Short sleep duration is associated with decreased serum leptin, increased energy intake and decreased diet quality in postmenopausal women. Obesity (Silver Spring). 2014;22(5):E55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.*.Carroll JE, Irwin MR, Levine M, et al. Epigenetic Aging and Immune Senescence in Women With Insomnia Symptoms: Findings From the Women’s Health Initiative Study. Biol Psychiatry. 2017;81(2):136–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.*.Kabat GC, Xue X, Kamensky V, et al. The association of sleep duration and quality with all-cause and cause-specific mortality in the Women’s Health Initiative. Sleep medicine. 2018;50:48–54. [DOI] [PubMed] [Google Scholar]
- 58.*.Grieshober L, Wactawski-Wende J, Blair RH, et al. A Cross-Sectional Analysis of Telomere Length and Sleep in the Women’s Health Initiative. American journal of epidemiology. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.*.Cauley JA, Hovey KM, Stone KL, et al. Characteristics of Self-Reported Sleep and the Risk of Falls and Fractures: The Women’s Health Initiative (WHI). J Bone Miner Res. 2019;34(3):464–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.*.Sands M, Loucks EB, Lu B, et al. Self-reported snoring and risk of cardiovascular disease among postmenopausal women (from the Women’s Health Initiative). Am J Cardiol. 2013;111(4):540–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.*.Kripke DF, Langer RD, Elliott JA, Klauber MR, Rex KM. Mortality related to actigraphic long and short sleep. Sleep medicine. 2011;12(1):28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.*.Grandner MA, Kripke DF, Naidoo N, Langer RD. Relationships among dietary nutrients and subjective sleep, objective sleep, and napping in women. Sleep medicine. 2010;11 (2): 180–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hoerster KD, Lehavot K, Simpson T, McFall M, Reiber G, Nelson KM. Health and health behavior differences: U.S. Military, veteran, and civilian men. Am JPrevMed. 2012;43(5):483–89. [DOI] [PubMed] [Google Scholar]
- 64.Weitlauf JC, LaCroix AZ, Bird CE, et al. Prospective Analysis of Health and Mortality Risk in Veteran and Non-Veteran Participants in the Women’s Health Initiative. Womens Health Issues. 2015;25(6):649–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vimalananda VG, Miller DR, Christiansen CL, Wang W, Tremblay P, Fincke BG. Cardiovascular disease risk factors among women veterans at VA medical facilities. J Gen Intern Med. 2013;28 Suppl 2:S517–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Heppner PS, Crawford EF, Haji UA, et al. The association of posttraumatic stress disorder and metabolic syndrome: a study of increased health risk in veterans. BMC Med. 2009;7:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kitahara CM, Platz EA, Freeman LE, et al. Obesity and thyroid cancer risk among U.S. men and women: a pooled analysis of five prospective studies. Cancer Epidemiol Biomarkers Prev. 2011;20(3):464–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Knutson KL. Does inadequate sleep play a role in vulnerability to obesity? Am J Hum Biol. 2012;24(3):361–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48(8):314–318. [PubMed] [Google Scholar]
- 70.Shumaker SA, Reboussin BA, Espeland MA, et al. The Women’s Health Initiative Memory Study (WHIMS): a trial of the effect of estrogen therapy in preventing and slowing the progression of dementia. Controlled clinical trials. 1998;19(6):604–621. [DOI] [PubMed] [Google Scholar]
- 71.Shumaker SA, Legault C, Kuller L, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291(24):2947–2958. [DOI] [PubMed] [Google Scholar]
- 72.Burnam MA, Wells KB, Leake B, Landsverk J. Development of a brief screening instrument for detecting depressive disorders. Med Care. 1988;26(8):775–789. [DOI] [PubMed] [Google Scholar]
- 73.Gottlieb DJ, Redline S, Nieto FJ, et al. Association of usual sleep duration with hypertension: the Sleep Heart Health Study. Sleep. 2006;29(8): 1009–1014. [DOI] [PubMed] [Google Scholar]
- 74.Chair SY, Wang Q, Cheng HY, et al. Relationship between sleep quality and cardiovascular disease risk in Chinese post-menopausal women. BMC Womens Health. 2017;17(1):79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li F, Sun K, Lin D, et al. Longtime napping is associated with cardiovascular risk estimation according to Framingham risk score in postmenopausal women. Menopause. 2016;23(9):950–956. [DOI] [PubMed] [Google Scholar]
- 76.Kakizaki M, Kuriyama S, Sone T, et al. Sleep duration and the risk of breast cancer: the Ohsaki Cohort Study. Br J Cancer. 2008;99(9):1502–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wu AH, Wang R, Koh WP, Stanczyk FZ, Lee HP, Yu MC. Sleep duration, melatonin and breast cancer among Chinese women in Singapore. Carcinogenesis. 2008;29(6):1244–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Verkasalo PK, Lillberg K, Stevens RG, et al. Sleep duration and breast cancer: a prospective cohort study. Cancer Res. 2005;65(20):9595–9600. [DOI] [PubMed] [Google Scholar]
- 79.White AJ, Weinberg CR, Park YM, et al. Sleep characteristics, light at night and breast cancer risk in a prospective cohort. Int J Cancer. 2017;141(11):2204–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pinheiro SP, Schernhammer ES, Tworoger SS, Michels KB. A prospective study on habitual duration of sleep and incidence of breast cancer in a large cohort of women. Cancer Res. 2006;66(10):5521–5525. [DOI] [PubMed] [Google Scholar]
- 81.Girschik J, Heyworth J, Fritschi L. Self-reported sleep duration, sleep quality, and breast cancer risk in a population-based case-control study. American journal of epidemiology. 2013;177(4):316–327. [DOI] [PubMed] [Google Scholar]
- 82.Thompson CL, Larkin EK, Patel S, Berger NA, Redline S, Li L. Short duration of sleep increases risk of colorectal adenoma. Cancer. 2011;117(4): 841–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Levi F, Dugue PA, Innominato P, et al. Wrist actimetry circadian rhythm as a robust predictor of colorectal cancer patients survival. ChronobiolInt. 2014;31(8):891–900. [DOI] [PubMed] [Google Scholar]
- 84.Palesh O, Aldridge-Gerry A, Zeitzer JM, et al. Actigraphy-measured sleep disruption as a predictor of survival among women with advanced breast cancer. Sleep. 2014;37(5):837–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Scullin MK, Bliwise DL. Sleep, cognition, and normal aging: integrating a half century of multidisciplinary research. PerspectPsychol Sci. 2015;10(1):97–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yaffe K, Falvey CM, Hoang T. Connections between sleep and cognition in older adults. Lancet Neurol. 2014;13(10): 1017–1028. [DOI] [PubMed] [Google Scholar]
- 87.Youngstedt SD, Kripke DF. Long sleep and mortality: rationale for sleep restriction. Sleep medicine reviews. 2004;8(3):159–174. [DOI] [PubMed] [Google Scholar]
- 88.Suzuki E, Yorifuji T, Ueshima K, et al. Sleep duration, sleep quality and cardiovascular disease mortality among the elderly: a population-based cohort study. PrevMed. 2009;49(2–3): 135–141. [DOI] [PubMed] [Google Scholar]
- 89.Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep. 2010;33(5):585–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jehan S, Myers AK, Zizi F, et al. Sleep health disparity: the putative role of race, ethnicity and socioeconomic status. Sleep Med Disord. 2018;2(5): 127–133. [PMC free article] [PubMed] [Google Scholar]
- 91.Jackson CL, Redline S, Emmons KM. Sleep as a potential fundamental contributor to disparities in cardiovascular health. Annu Rev Public Health. 2015;36:417–440. [DOI] [PMC free article] [PubMed] [Google Scholar]