Abstract
Reduced cerebral blood flow (CBF), an indicator of neurovascular processes and metabolic demands, is a common finding in Alzheimer’s disease (AD). However, little is known about what contributes to CBF deficits in individuals with mild cognitive impairment (MCI). We examine regional CBF differences in 17 MCI compared to 21 age-matched cognitively healthy older adults. Next, we examined associations between CBF, white matter lesions (WML) volume, amplitude of low frequency fluctuations (ALFF), and cortical thickness to better understand whether altered CBF was detectable prior to other markers as well as the potential mechanistic underpinnings of CBF deficits in MCI. MCI had significantly reduced CBF, while cortical thickness and ALFF were not affected. Reduced CBF was associated with the WML volume but not associated with other measures. Given the presumed vascular etiology of WML and relatively worsening of vascular health in MCI, it may suggest CBF deficits result from early vascular as opposed to metabolic deficits in MCI. These findings may support vascular mechanisms as an underlying component of cognitive impairment.
Keywords: Alzheimer’s disease, Mild cognitive impairments, Cerebral blood flow, White matter lesions, ALFF, Vascular
1. Introduction
There is a clear link between conditions that confer risk for vascular disease and the risk for the development of Alzheimer’s disease (AD; (Jellinger, 2010; Jellinger and Attems, 2005; Kivipelto et al., 2001)) as well as the clinical severity and progression of the clinical syndrome of AD (Mielke et al., 2007; Snowdon, D. A. et al., 1997; Snowdon, David A et al., 1997; Snyder et al., 2015). Vascular health in part influences AD through cerebrovascular pathology which is commonly found in patients (Gorelick et al., 2011; Grammas, 2011; Iadecola, 2004) and recent theories posit that vascular mechanisms may even play a major role in the development or initiation of AD pathology (Gorelick et al., 2011; Rius-Perez et al., 2018; Zlokovic, 2011).
Reduced brain perfusion has been reported in AD through quantification of cerebral blood flow (CBF) across various imaging modalities including singe photon emission computed tomography (SPECT) (Bradley et al., 2002; Hirao et al., 2005; Huang et al., 2018; Kogure et al., 2000; Matsuda, 2007), positron emission tomography (PET) (Matsuda, 2001), and magnetic resonance imaging (MRI) (Alsop, David C et al., 2000; Asllani et al., 2008; Binnewijzend et al., 2016; Ding et al., 2014; Huang et al., 2018; Johnson, Nathan A et al., 2005; Okonkwo et al., 2012; Roher et al., 2012). Arterial spin labeling (ASL) magnetic resonance imaging (MRI) (Williams et al., 1992) is a commonly used procedure for quantification of CBF typically in the resting state (Detre et al., 1992; Kety and Schmidt, 1945; Roy and Sherrington, 1890) by detecting magnetically-labeled protons in blood without injection of a contrast agent (Alsop, David C et al., 2010; Binnewijzend, Maja AA et al., 2013; Dai, Weiying et al., 2009). Reduced CBF measured by ASL is a common finding in AD (Alsop, D. C. et al., 2010; Alsop, D. C. et al., 2000; Asllani et al., 2008; Binnewijzend et al., 2016; Binnewijzend, M. A. et al., 2013; Dai, W. et al., 2009; Ding et al., 2014; Hays et al., 2016; Johnson, Nathan A et al., 2005; Johnson, N. A. et al., 2005; Leeuwis et al., 2017; Mak et al., 2012; Schuff et al., 2009; Yoshiura et al., 2009) and this reduction in CBF is associated with hypometabolism (Chen, Y. et al., 2011; Musiek et al., 2012; Riederer et al., 2018; Verfaillie et al., 2015), loss of normalized brain volume (Benedictus et al., 2014), and worse cognitive functions in AD (Benedictus et al., 2017; Binnewijzend et al., 2016; Binnewijzend, Maja AA et al., 2013; Leeuwis et al., 2017; Leijenaar et al., 2017). Thus, better understanding of altered CBF in AD may provide critical information about mechanisms of the disease that have an important clinical and pathologic impact.
Individuals with mild cognitive impairment (MCI) are at a greater risk for the subsequent development of dementia due to AD and MCI therefore representing a preclinical stage of AD in certain individuals (Petersen et al., 1999; Winblad et al., 2004). Increasing evidence supports a role for cerebral hypoperfusion in the conversion from MCI to AD (Chao et al., 2010; Chao et al., 2009; Hirao et al., 2005; Lacalle-Aurioles et al., 2014; Matsuda, 2007). However, how CBF is associated with structural and functional measures of neural health in MCI is largely unknown.
White matter lesions (WML) of presumed vascular origin measured by neuroimaging are prominent in individuals with AD and influence the course of clinical disease (Carmichael et al., 2010; Gouw et al., 2008; Lindemer et al., 2017; Prasad et al., 2011; Provenzano et al., 2013; Yoshita et al., 2006). Recent studies shows that reduced CBF is associated with increased white matter hyperintensity (WMH) lesion burden in older adults (Bastos-Leite et al., 2008; Crane et al., 2015; Van Dalen et al., 2016). These abnormalities linked to aging are thought to be complex, but to some degree caused by chronic ischemia due to reduced cerebral perfusion (Brickman, Adam M et al., 2009; Brickman, A. M. et al., 2009; Fazekas et al., 1993; Marstrand et al., 2002; Naka et al., 2006). White matter lesions therefore provide an index of vascular health and may therefore be useful in understanding the potential vascular etiology of altered CBF in AD. Although WML and reduced CBF have commonly been observed in AD, to our knowledge, no studies have investigated differential associations among CBF, WML, cortical atrophy, and functional alterations in MCI. Specific associations between WML and CBF could suggest a vascular etiology to the reduction in CBF which may precede functional deficits.
Amplitude of low frequency fluctuations (ALFF) is a measure based on regional intensity of spontaneous fluctuations in the blood-oxygen-level dependent (BOLD) signal (Yu-Feng et al., 2007; Zou et al., 2008). ALFF varies with the performance of functional tasks, and is altered in brain disorders (Guo et al., 2012; Guo et al., 2015; He et al., 2007; Hou et al., 2012; Liang et al., 2014; Liu et al., 2014), and has been suggested to reflect to some degree coherence in neural population activity (Biswal et al., 1995; Liu et al., 2011; Qiu et al., 2017; Yu-Feng et al., 2007). Here we utilized ALFF as a marker of regional functional brain activity (Zou et al., 2008), while also recognizing that this measure is influenced by multiple neurovascular properties.
Our goal was to examine differences in individuals with MCI compared to cognitively healthy older adults in imaging markers reflecting different aspects of brain health, as well as any group interactions in the associations among those imaging markers. Examination of such associations could provide information about early mechanisms of cognitive decline. Specifically, we compared MCI to cognitively health older adults on 1) pseudo-continuous arterial spin labeling (pCASL) (Liu and Brown, 2007) measured CBF previously reported to be altered in MCI (Benedictus et al., 2017; Binnewijzend, Maja AA et al., 2013; Chao et al., 2009; Dai, Weiying et al., 2009; Ding et al., 2014; Johnson, N. A. et al., 2005; Leeuwis et al., 2017), 2) WML as a marker of presumed vascular-associated brain tissue damage (Benedictus et al., 2014) and a marker of cerebrovascular disease (Wardlaw et al., 2013), 3) cortical thickness which is altered with aging as well as neurodegenerative conditions, and 4) ALFF fluctuations in the BOLD signal that has been described previously as reflecting spontaneous neuronal activity (Biswal et al., 1995; Liu et al., 2011; Qiu et al., 2017; Yu-Feng et al., 2007).
2. Materials and methods
2.1. Participants
Twenty one typically healthy older adults, and nineteen older adults with neuropsychologically defined MCI (Bondi, Mark W et al., 2014; Jak et al., 2016) were enrolled through the Brain Aging and Dementia (BAnD) Laboratory at Massachusetts General Hospital (MGH). Participants were referred for the study through the MGH Alzheimer’s Disease Research Center (MGH ADRC), enrolled from a local longitudinal cohort, or through community outreach. Clinical scales included Clinical Dementia Rating scale (CDR), Montreal Cognitive Assessment (MoCA), and Mini-Mental State Examination (MMSE). Neuropsychological testing was used to determine clinical status using operational criteria for MCI as defined previously (Bondi, M. W. et al., 2014; Bondi et al., 2008; Jak et al., 2009; Stricker et al., 2013)(Bondi et al., 2014; Bondi et al., 2008; Jak et al., 2009; Stricker et al., 2013). MCI designation was based on objective criteria of performance on at least two cognitive domains falling one standard deviation (SD) or more below published normative values. Participants were excluded for significant health concerns outside of the domains of study including major neurological or psychiatric disorders, such as vascular dementia and clinical stroke, as well as any substantial systemic illness that would prevent participation or would be likely to confound study procedures and results. Two participants were excluded due to motion artifacts in resting state functional MRI (rsfMRI) and pCASL MRI. Consequently, the total of 21 healthy older adults (mean age 68.25; SD 5.84; 66.67% female), and 17 older adults with neuropsychologically defined MCI (mean age 68.40; SD 6.28; 35.29% female) were used for this study. The Partners Healthcare institutional review board (IRB) approved this work and informed consent was obtained from each participant.
2.2. MRI acquisition
All MRIs, including T1-weighted MRI, rsfMRI, and pCASL MRI, were acquired using a Siemens Biograph mMR 3 Tesla system with 32-channel head coil.
T1-weighted MRI was acquired using multi-echo MPRAGE (van der Kouwe et al., 2008) with the following parameters: slice thickness = 1.0 mm, repetition time = 2530.0 ms, inversion time = 1100 ms, multiple echo times = 1.69 ms, 3.55 ms, 5.41 ms, and 7.27 ms, flip angle = 7°, matrix size = 256 × 256 pixels, and field of view = 256 mm × 256 mm, with no inter-slice gap. The individual echos from the multi-echo MPRAGE were combined for each participant by using the root mean square to create a single image volume with high contrast-to-noise for further imaging analysis.
The rsfMRI was acquired with whole-brain echo planar imaging (EPI) time-series scans with the following parameters: slice thickness = 3.0 mm, repetition time = 3000 ms, echo time = 30 ms, flip angle = 90°, matrix size = 72 × 72 pixels, field of view = 216 mm × 216 mm, and number of dynamics = 120.
The ASL MRI was acquired using a pCASL pulse sequence with a 2D echo planar imaging (EPI) readout and the following parameters: slice thickness = 5 mm, repetition time = 4000 ms, echo time = 12 ms, rate2 GRAPPA, matrix = 64×64, slices = 22, field of view = 220 mm × 220 mm, total labeling duration = 1500 ms, post-labeling delay (PLD) = 1200 ms, and 40 pairs of tag and control acquired in 5.5 min. An M0 scan where the imaging readout was matched to the main pCASL sequence, but with spin labeling removed, was acquired with a TR = 8000 ms for equilibrium blood water magnetization calculation and CBF calibration.
2.3. Demographic and neuropsychological testing
Clinical characteristics and neuropsychological test results were compared between the individuals with MCI and age-matched cognitively healthy older adults using Student t-test or Mann-Whitney U test according to distribution pattern of the variables.
2.4. MR image processing
T1-weighted anatomical images were automatically processed to reconstruct cortical surfaces and to segment volume region-of-interests (ROI) volumes using the Freesurfer recon-all procedure (http://surfer.nmr.mgh.harvard.edu/) (Dale, Anders M et al., 1999; Fischl et al., 1999). The technical details of these procedures are described in prior publications (Dale, A. M. et al., 1999; Fischl et al., 2001; Fischl, B. et al., 2002; Fischl, B. et al., 2004a; Fischl, B. et al., 2004b). The shortest distance between a vertex on the inner surface and a vertex on the outer surface was calculated to measure cortical thickness (Fischl and Dale, 2000). All T1-weighted MRI were segmented as brain tissue images using watershed/surface deformation procedure (Ségonne et al., 2004), and automatically registered into a common surface template using a surface-based averaging technique by considering cortical folding patterns. Then, ROIs in a standard space were inversely mapped to each native MRI space using a high-dimensional spherical morphing procedure to define the ROIs in each individual (Fischl, Bruce et al., 2002; Fischl, Bruce et al., 2004). We used the FreeSurfer based procedure for measuring white matter hypointensities as described in prior publications (Coutu et al., 2016; Coutu et al., 2017). This procedure provides an estimate of WML that is highly correlated with traditional FLAIR WML (Coutu et al., 2016; Dadar et al., 2018). Lobar-based cortical ROIs included frontal, parietal, temporal, and occipital lobules, using the Population-Average, Landmark- and Surface-based (PALS) atlas for linear regression analysis (Van Essen, 2005). Partial volume estimation (PVE) maps for GM and WM, which consisted of the percent of each tissue type in each voxel, were calculated by using ‘mri_compute_volume_fractions’ procedure within FreeSurfer for each individual for further analysis.
The pCASL timeseries was preprocessed by motion correction (Jenkinson et al., 2002), brain extraction, and registration to the M0 calibration image within the FMRIB Software Library (FSL-http://fsl.fmrib.ox.ac.uk). A pairwise mean difference perfusion map was calculated by performing subtraction between the control and label images via Baysian Inference for Arterial Spin Labeling MRI (BASIL) procedure within FSL (Chappell et al., 2009). We coregistered the mean difference perfusion map to the corresponding T1-weighted MRI by using Boundary-Based Registration (BBR) with GM mask for the surface-based analysis in each individual (Greve and Fischl, 2009). The cerebrospinal fluid (CSF) mask defined on the native T1-weighted MRI was inversely mapped to each mean perfusion image by using the registration matrix from the coregistration and then it was used to segment a reference region from M0. Then the M0 calibration image was converted to the equilibrium magnetization of arterial blood by scaling with the blood water density as 0.87 in each individual. The difference map was normalized by the equilibrium magnetization of arterial blood estimated via the magnetization of CSF for the quantification of CBF by using the FSL command line tool ‘oxford_asl’ from the BASIL toolbox (Chappell et al., 2009). The GM and WM PVE maps defined on the T1-weighted MRIs were registered to native perfusion images. Then, those PVE maps were used for partial volume correction (PVC) by considering the global ratio between WM to GM as 0.4 (Chen, Y. et al., 2011; Du et al., 2006). We note that effects measured were substantially more conservative when applying PVC. We therefore additionally present non-PVC results as supplementary data as these data may provide insight into effects that may be detected in a more statistically powered sample.
Resting state fMRI EPI volumes were processed using Analysis of Functional NeuroImages (AFNI; http://afni.nimh.nih.gov/afni/; Cox, 1996). EPI data were screened for head-motion artifacts (Power et al., 2012) and visually examined for head-coil artifacts through examination of local WM signal time series. The first three volumes from each functional image were discarded to allow for the stabilization of the magnetic field. Slice timing correction, motion correction, and coregistration were applied by using ‘afni_proc.py’ within AFNI process stream. Cerebrospinal fluid (CSF), WM, and ventricle masks defined on the T1-weighted MRIs were inversely registered into the functional images by using the coregistration matrix to regress out the potential nuisance signals from each time series via general linear model (GLM) within AFNI process stream (Jo et al., 2010). After that, each time series was projected into the frequency domain by fast Fourier transform (FFT) to acquire the power spectrum. We calculated voxel-wise amplitude of low frequency fluctuation (ALFF), which represents the amplitude of spontaneous neural activity in the resting states (Yang et al., 2007), a signal presumed to be weighted towards cognitive function-related intrinsic neural activity (Fryer et al., 2015), by calculating the average square root of the low-frequency (0.009–0.08 Hz) power spectrum in each individual (Yu-Feng et al., 2007). Preprocessed ALFF data were co-registered to the corresponding T1-weighted anatomical images by BBR (Greve and Fischl, 2009) using the transformation obtained from the AFNI process stream to further surface-based analysis. The GM PVE maps defined on the T1-weighted MRIs were inversely registered to native ALFF images using BBR (Greve and Fischl, 2009). Given that the differential tissue contribution substantially increases the false-positive rate for rsfMRI (Dukart and Bertolino, 2014), we applied PVC in ALFF using GM PVE map in each ALFF by considering the percent of GM tissue in each voxel via one compartment model.
The preprocessed CBF and ALFF images were sampled onto the cortical surface by mapping a mean value between inner and outer surfaces in each vertex point. Sampling included data from voxels in the interior 70% of the cortex (excluding data from the outer 15% of each surface border) to reduce partial volume contamination of sampled data. For surface based statistical analyses, all surface-mapped variables, including cortical thickness, CBF and ALFF, were smoothed with a surface-based 20mm FWHM Gaussian kernel as described in our previous work (Chen et al., 2013). We also measured the regional mean values of CBF, ALFF, and cortical thickness within lobar-based cortical ROIs from PALS atlas in each individual for further linear regression analysis.
2.5. Vertex-wise group differences in CBF, ALFF, and cortical thickness
Cortical thickness, surface-mapped CBF and ALFF values across whole cortical vertices were compared between individuals with MCI and age-matched cognitively healthy older adults after controlling for sex using a GLM within the FreeSurfer software. We performed a Monte Carlo simulation-based cluster-wise procedure to correct for multiple comparisons in all surface-based group analysis by determining the distribution of the maximum cluster size under the null hypothesis as described previously (Hagler et al., 2006). All surface-mapped CBF and ALFF were corrected by PVE maps to avoid the effects of local atrophy in the perfusion measures.
2.6. Vertex-wise local correlation analysis
The vertex-wise correlations between surface-mapped CBF, ALFF, the volume of WML, and cortical thickness were performed across whole cortical vertices in each group while controlling for sex by using Pearson’s partial correlation analysis in Statistics and Machine Learning Toolbox within MATLAB.
2.6.1. Vertex-wise local correlation between cerebral perfusion measures and the volume of WML
We measured a local correlation between surface measures of CBF, ALFF, and the volume of WML in each group to examine a potential relationship between cerebrovascular dysfunction and CBF.
2.6.2. Vertex-wise local correlation between cerebral perfusion measures and cortical thickness
We measured a correlation between CBF, ALFF, and cortical thickness to identify a potential relationship between structural and functional measures.
2.6.3. Vertex-wise local correlation between CBF and ALFF
We performed vertex-wise correlation analyses between CBF and ALFF to examine whether those cerebrovascular-related measures share the common findings in the brain.
We additionally performed the same local correlation analysis by considering all participants to examine a comprehensive effect of older adults across these measures. The surface-mapped CBF and ALFF were corrected by PVE maps to avoid the effects of local atrophy in the perfusion measures, and all vertex-wise correlation analysis were adjusted for sex and corrected the false discovery rate (FDR) for multiple comparisons at a q value of 0.05 (Benjamini and Hochberg, 1995).
2.7. Lobar-ROI-based linear regression analysis
We performed linear regression analysis between measures in each lobar-based cortical ROI in each group with sex as a covariate to examine regional relationship between CBF, ALFF, the volume of WML, and cortical thickness. All linear regression analyses were performed with the SurfStat MATLAB toolbox (http://www.math.mcgill.ca/keith/surfstat). We measured the mean lobar CBF, ALFF, and cortical thickness within PALS lobar-based ROIs for regression analysis after partial volume correction.
2.7.1. Lobar-ROI-based linear associations between mean lobar CBF, ALFF, and the volume of WML
The associations between the mean lobar CBF, ALFF, and the volume of WML were performed by GLM in each lobar-ROI for each group to determine the regional linear association between the mean lobar perfusion measures and the WML volume.
2.7.2. Lobar-ROI-based linear associations between mean lobar CBF, ALFF, and cortical thickness
We examined the association between the mean lobar CBF, ALFF, and mean cortical thickness by using GLM in each lobar-ROI for each group to examine the lobar-based linear association between mean lobar perfusion measures and cortical thickness.
2.7.3. Lobar-ROI-based linear association between mean lobar CBF and ALFF
Similarly, we examined the association between the mean lobar CBF and ALFF.
Bonferroni correction was performed at a q value of 0.05 for multiple comparisons in all lobar-ROI-based linear association analyses (Bonferroni, 1936).
3. Results
3.1. Group characteristics
Detailed demographic characteristics of all groups and the results of neuropsychological tests are described in Table 1. There was a significant difference in sex distribution, pulse pressure, MoCA performance and CDR scores between groups with MCI having significantly lower MoCA, higher CDR scores as expected, and higher pulse pressure compared with cognitively healthy older adults (Table 1). In addition, increased volume of WML were observed in individuals with MCI than cognitively healthy older adults (Table 1).
Table 1.
Demographics and clinical characteristics
| Cognitively healthy older adults | MCI | |
|---|---|---|
| (n = 21) | (n = 17) | |
| Gender | F = 14/M = 7 | F = 6/M = 11* |
| Age (y) | 68.25 ± 5.84 | 68.40 ± 6.28 |
| Education (y) | 16.52 ± 2.62 | 15.24 ± 2.59 |
| MMSE | 28.80 ± 1.44 | 27.65 ± 2.12 |
| CDR | 0.0 (0.0–0.0) | 0.5 (0.0–0.5)* |
| MoCA | 27.20 ± 1.99 | 22.82 ± 2.77* |
| Total WML volume (mm3) | 2686.60 ± 1909.556 | 5008.73 ± 3739.24* |
| Diastolic Blood Pressure (mmHg) | 75.25 ± 10.54 | 74.47 ± 6.00 |
| Systolic Blood Pressure (mmHg) | 123.73 ± 14.05 | 130.53 ± 13.10 |
| Pulse Pressure (mmHg) | 47.25 ± 8.05 | 55.03 ± 8.95* |
statistically significant difference from individuals with cognitively healthy older adults at p < 0.05
Abbreviations: MCI, mild cognitive impairment; MMSE, mini-mental state examination; CDR, clinical dementia rating scale; MoCA, Montreal Cognitive Assessment; WML, white matter lesions.
3.2. Vertex-wise group differences in CBF, ALFF, and cortical thickness
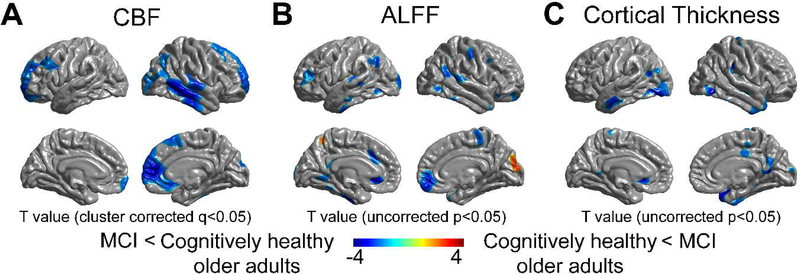
MCI had significantly lower CBF than cognitively healthy older adults in portions of both the superior frontal cortices, right medial frontal cortex, right temporal cortex, right lateral occipital cortex, right anterior cingulate cortex, left meddle frontal cortex, and a partial area of the right inferior parietal cortex, after performing PVC adjusted for sex (cluster corrected q < 0.05, Fig. 1A). In contrast, no significant group difference was noted in both ALFF and cortical thickness after performing cluster correction (uncorrected p < 0.05, Fig. 1B–C).
Figure 1.
Vertex-wise group differences between individuals with MCI and cognitively healthy older adults after performing PVC and adjusting for sex. Comparisons of (A) CBF, (B) ALFF, and (C) cortical thickness between groups. Significantly reduced CBF was found regionally in the right temporal/parietal cortex as well as lateral and medial frontal cortex (cluster corrected q <0.05), while no significant difference was found in either ALFF or cortical thickness after cluster-wise correction.
Abbreviations: MCI, mild cognitive impairment; CBF, cerebral blood flow; ALFF, amplitude of low frequency fluctuations; PVC, partial volume correction; FDR, False discovery rate.
3.3. Vertex-wise local correlation
3.3.1. Vertex-wise local correlation between cerebral perfusion measures and the volume of WML
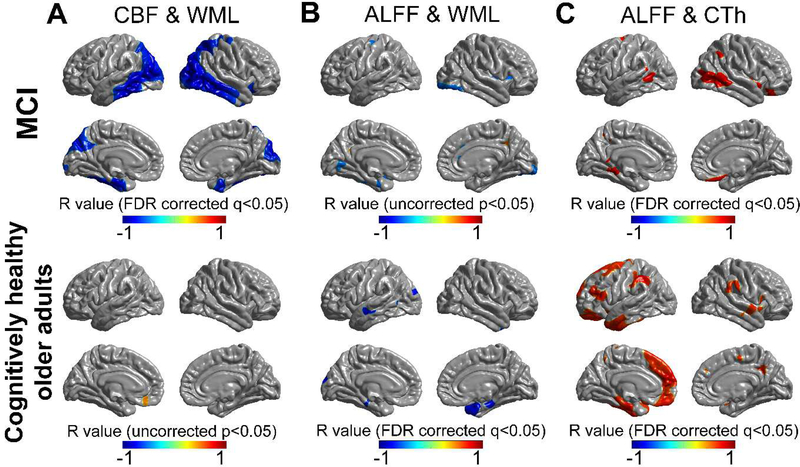
There were significant negative correlations between CBF and the volume of WML in individuals with MCI, but not in the cognitively healthy older adults (FDR corrected q < 0.05, Fig. 2A). Significant negative correlations between CBF and the volume of WML was noted in both the middle and inferior temporal cortices, both lateral occipital cortices, both entorhinal cortices, left precuneus, and right cuneus, in individuals with MCI after performing PVC adjusted for sex (FDR corrected q < 0.05, Fig. 2A). ALFF was negatively correlated with the volume of WML, mainly in the right temporal pole, in cognitively healthy older adults (FDR corrected q < 0.05, Fig. 2B), while, no correlation was observed in individuals with MCI after performing PVC and adjusting for sex (uncorrected p < 0.05, Fig. 2B).
Figure 2.
Vertex-wise local correlations between CBF, ALFF, the volume of WML, and cortical thickness in individuals with MCI and cognitively healthy older adults after performing PVC and adjusting for sex. (A) A significant negative correlation between CBF and the volume of WML was noted in MCI (FDR corrected q <0.05), while not in cognitively healthy older adults. (B) A significant negative correlation between ALFF and the volume of WML was noted in cognitively healthy older adults (FDR corrected q < 0.05), but not in MCI. (C) A significant positive correlation between ALFF and cortical thickness was noted in both groups (FDR corrected q < 0.05). Color bar indicates scale of Pearson’s correlation coefficient.
Abbreviations: MCI, mild cognitive impairment; CBF, cerebral blood flow; WML, white matter signal lesions; CTh, cortical thickness; PVC, partial volume correction.
3.3.2. Vertex-wise local correlation between cerebral perfusion measures and cortical thickness
There was no significant correlation between CBF and cortical thickness in both groups. In contrast, ALFF was significantly correlated with cortical thickness in both groups. The ALFF was positively correlated with cortical thickness in left medial frontal cortex, left entorhinal cortex, and left parahippocampal gyrus, both supramarginal gyri, and portions of the lateral temporal cortex in cognitively healthy older adults after performing PVC adjusted for sex (FDR corrected q < 0.05, Fig. 2C). There were relatively less areas that showed significant correlation between ALFF and cortical thickness, mainly in portions of the middle temporal cortices in individuals with MCI compared with healthy older adults after performing PVC adjusted for sex (FDR corrected q < 0.05, Fig. 2C).
3.3.3. Vertex-wise local correlation between CBF and ALFF
No significant correlation was found between CBF and ALFF in either group.
3.4. Lobar-ROI-based linear association
3.4.1. Lobar-ROI-based linear associations between mean lobar CBF, ALFF, and the volume of WML
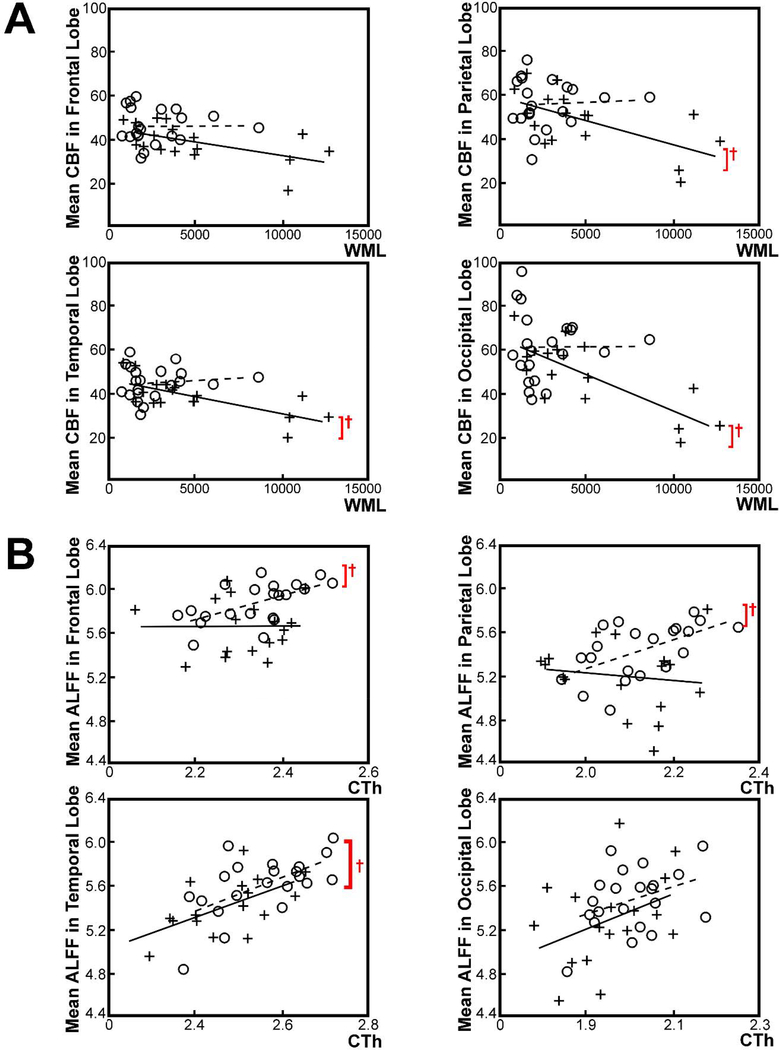
Mean CBF was significantly associated with the volume of WML in all lobar regions, including the parietal lobe (t(15) = −3.00, p = 0.009), temporal lobe (t(15) = −3.89, p =0.001), and occipital lobe (t(15) =−4.71, p < 0.001), in individuals with MCI but not frontal lobe (t(15) = −2.58, p = 0.021). No significant association was found in the cognitively healthy older adults after performing PVC adjusted for sex (Bonferroni corrected q < 0.05, Fig. 3A). Mean lobar ALFF was negatively associated with the volume of WML in the temporal lobe (t(19) = −5.16, p < 0.001) and occipital lobe (t(19) = −4.40, p < 0.001) in cognitively healthy older adults, while no significant association was noted in individuals with MCI after performing PVC and adjusting for sex (Bonferroni corrected q < 0.05).
Figure 3.
Lobar-ROI-based linear associations between mean lobar CBF, ALFF, volume of WML, and cortical thickness, in individuals with MCI and cognitively healthy older adults after performing PVC adjusted for sex. (A) A significant negative association between the mean lobar CBF (mL/100g/min) and the volume of WML (mm3) was noted across all lobar except for the frontal lobe in individuals with MCI (Bonferroni corrected q < 0.05), while not in cognitively healthy older adults. (B) A significant positive association between the mean lobar ALFF and cortical thickness (mm) was noted across all lobar except for the occipital lobe in cognitively healthy older adults (Bonferroni corrected q < 0.05), while only noted in the temporal lobe in MCI. A red cross marker indicates statistically significant association at Bonferroni corrected q < 0.05.
Abbreviations: MCI, mild cognitive impairment; ALFF, amplitude of low frequency fluctuations; WML, white matter lesions; PVC, partial volume correction.
3.4.2. Lobar-ROI-based linear associations between mean lobar CBF, ALFF, and cortical thickness
No significant association between the mean lobar CBF and the mean lobar cortical thickness was found in either group. Mean ALFF was positively associated with the mean value cortical thickness across all lobar, including the frontal lobe (t(19) = 3.27, p = 0.004), parietal lobe (t(19) = 2.99, p = 0.008), and temporal lobe (t(19) = 3.32, p = 0.004), except for the occipital lobe (t(19)= 1.75, p = 0.096), in cognitively healthy older adults (Bonferroni corrected q < 0.05, Fig. 3B). A positive association between the mean lobar ALFF and the mean lobar cortical thickness was only noted in the temporal lobe (t(15) = 3.13, p = 0.007) in individuals with MCI after performing PVC adjusted for sex (Bonferroni corrected q < 0.05, Fig. 3B).
3.4.3. Lobar-ROI-based linear association between mean lobar CBF and ALFF
No significant association was found between the mean lobar CBF and the mean lobar ALFF in either group after performing lobar-ROI-based regression analysis.
4. Discussion
We found a significant reduction in CBF in individuals with neuropsychologically defined MCI compared to cognitively healthy older adults in both superior frontal cortices, right medial frontal cortex, right temporal cortex, right lateral occipital cortex, and a partial area of the right inferior parietal cortex, while no significant group differences were found in ALFF and cortical thickness. CBF was strongly associated with WML volume, mainly in temporal cortex, which is overlapped with the regions with reduced CBF in MCI. These early reduced CBF may be an indicator to predict clinical status that cannot be detected by cortical thickness or ALFF in individuals with MCI. Previous CBF studies also suggested that reduced CBF may be an important indicator of AD pathophysiology in MCI (Borroni et al., 2006; Encinas et al., 2003; Hirao et al., 2005). Findings of regionally reduced CBF in MCI are partially in accord with prior studies reporting reduced CBF mainly in frontal, temporal, parietal, and medial temporal regions, in individuals with MCI and AD by using spin-labeled MRI (Alsop, David C et al., 2000; Binnewijzend, M. A. et al., 2013; Johnson, Nathan A et al., 2005; Luckhaus, C. et al., 2008; Schuff et al., 2009) as well as Single Photon Emission Computed Tomography (SPECT) procedures (Bartenstein et al., 1997; Borroni et al., 2006; Dai, Weiying et al., 2009; Encinas et al., 2003; Hirao et al., 2005; O’Brien et al., 1992; Reed et al., 1989). These results also overlap with regions known to show consistent atrophy in later stages of AD (e.g. cortical signature paper (Dickerson et al., 2009). However, unexpectedly, results of reduced CBF in the PCC or/and precuneus, which have been described as vulnerable regions with hypoperfusion in MCI and AD patients (Binnewijzend, Maja AA et al., 2013; Huang et al., 2002; Johnson et al., 2006; Riederer et al., 2018), were less apparent in our sample. It is possible that such differences could be related to the MCI in this study having relatively subtle impairment as well as potential heterogeneity in any pathophysiology of the MCI group. For example, Luckhaus et al. demonstrated hypoperfusion of medial temporal regions and anterior cingulate in amnestic MCI, and the hypoperfusion of PCC was confined to mild AD (Luckhaus, Christian et al., 2008). Mattsson et al. reported no significant regional CBF difference between controls and early or late MCI, but significantly reduced CBF of PCC-precuneus in AD (Mattsson et al., 2014). Although the literature generally describes trends towards reduced CBF in MCI, there has been some discrepancy in the timing and regional basis of these reductions.
There was a significant association between CBF and the volume of WML in individuals with MCI with greater volume of WML related to lower regional CBF. Significant local correlations as well as lobar-based associations between CBF and the volume of WML were noted mainly in the temporal and lateral occipital cortex in individuals with MCI, but not observed in cognitively health older adults. Our findings are similar to those reported in AD demonstrating decreased CBF associated with increased volume of WML (Benedictus et al., 2014; Kimura et al., 2012), although we did not find a significant relationship between CBF and the volume of WML in cognitively intact older adults. In addition, MCI had a significantly increased pulse pressure as well as the high WML volume compared to cognitively healthy older adults. Thus, reduced CBF in individuals with MCI may reflect a more systemic etiology (Shokouhi et al., 2018). These findings may suggest that cerebral perfusion compromise manifests in the early stages of cognitive impairment and is linked to the volume of WML in individuals with MCI. We additionally performed a statistical test to directly compare an interaction between groups and WML in CBF. We observed a significant group difference between the group WML slopes in CBF and this regional pattern was largely overlapping with the results of the surface-based correlation between CBF and WML (Fig 2A) yet was less statistically powered given the specifics of the test (Supplementary Fig 4A). Thus, another possibility of the lack of correlation between WML and CBF in cognitively healthy older adults might be explained by the group difference in the interaction between WML and CBF. We also observed significant associations between CBF and the volume of WML in all participants combined with the regional patterns similar to the MCI results but more wide-spread compared to the MCI (Supplementary Fig. 2A). Thus, it is possible that the lack of effect in the healthy older adults is due to lack of power and range in that group alone.
CBF was not associated with cortical thickness in either group. Ultimately, it could be expected that reduced CBF may be detrimental to neuronal health and contribute to cortical variation. However, other recent reports suggest that regional CBF is not strongly associated with regional cerebral atrophy (Benedictus et al., 2014; Lacalle-Aurioles et al., 2014; Luckhaus et al., 2010) and is in accord with our work on non-impaired aging (Chen, J.J. et al., 2011). Wirth al. demonstrated the partly divergent patterns of temporo-parietal hypoperfusion and medial-temporal atrophy in MCI (Wirth et al., 2017) which may reflect a temporal dissociation between reduced CBF and subsequent cortical thinning which will be of interest to explore in future work.
Significant positive local correlations between ALFF and cortical thickness were found including some portions of the AD ‘cortical signature’ (Dickerson et al., 2008) in cognitively healthy older adults, but was limited to the middle temporal cortex in individuals with MCI. It is possible that the measures are generally correlated in healthy individuals but that disease procession constrains these associations in MCI. Interestingly, mean lobar ALFF was associated with mean lobar cortical thickness across all ROIs except for the occipital lobe in cognitively healthy older adults. In contrast, mean ALFF and cortical thickness were only associated in the temporal lobe in MCI. These findings may suggest a general coupling of these properties that is lost with disease.
Regional CBF was not significantly associated with regional ALFF in either group, which is inconsistent with the previous BOLD imaging studies (Gottler et al., 2018; Jann et al., 2015; Li et al., 2012; Liang et al., 2013). It is not clear why there were no associations found in the current work, however the difference of the method to measure the functional activity could be a possible reason. Previous studies that showed a significant association between CBF and BOLD signal used a measure of functional connectivity, which reflects synchronous BOLD fluctuations between voxels or regions (Gottler et al., 2018; Jann et al., 2015; Li et al., 2012; Liang et al., 2013). This measure provides information about the integration of neural systems in brain functions, in contrast, ALFF, which measures the amplitude of spontaneous fluctuations in the frequency domain, reflects the level of spontaneous brain activity related to physiological states of the brain. Although several studies reported a spatial association between ALFF and CBF, compared to the results from the functional connectivity studies, these regions were limited and inconsistent (Bray, 2017; Hu et al., 2019; Li et al., 2012; Song et al., 2018). Therefore, it is possible that ALFF may provide information about different aspects of brain activity related to vascular physiology that could not be measured by CBF (Zou et al., 2015), however, such speculation relies on indirect information which must be considered with caution and further studies are needed to elucidate.
The current study has limitations that will be addressed in future work. The work employed a convenient sample and the size was limited reducing generalizability and statistical power. However, given this sample we were able to describe differential sensitivity of the measures to the effects of MCI (e.g. an effect of CBF but not ALFF) as well as specific associations among the divergent measures of neural health. The pCASL MRI was acquired with post labeling delay of 1.2s which is shorter than the recommended parameters for adults and clinical patients with compromised vascular physiology (1.8 ~ 2.0s) (Alsop et al., 2015). Thus, there is a potential possibility that the CBF results may reflect different aspects of vascular dysfunction, including arterial transit delay differences between the groups. Similarly, an association between CBF and the volume of WML may be linked to other vascular mechanisms, such as altered transit times and incomplete delivery of labeled blood to tissue. Thus, the reduced CBF in this study may suggest regionally specific macrovascular effects in individuals with MCI that may also influence the microvasculature. We include volume-based CBF maps from 6 individuals who have low and high WML volume in both groups to clarify the potential visual artifacts in CBF images caused by transit time issue (Supplementary Figure 5). All CBF maps seem to show no major visual artifacts, however, there is a subtle trend that some individuals have inhomogeneous hypo- or/and hyper- CBF in several regions in both groups, not only in MCI (Supplementary Figure 5). Therefore, these finding may suggest the potential effects of arterial transit delay differences in CBF data in both groups in the current study. However, there is another possibility that the potential hypoperfusion caused by transit time artifacts may affect the correlation between CBF and the WML volume more in MCI than the cognitively healthy older adults since MCI has a high WML volume than the cognitively healthy older adults. Even though we cannot find severe visual artifacts due to transit time differences between groups in our CBF maps, it cannot fully cover the potential problem caused by the short PLD and therefore a multi-delay sequence allowing the explicit mapping of transit times would be optimal for future work. Our ongoing work acquires novel ASL data with a multi-PLD procedure as described through the Human Connectome Project Lifespan study (Bookheimer et al., 2019; Harms et al., 2018). Future studies will more explicitly examine transit time differences in cognitively impaired individuals. Third, the CBF and ALFF measures are limited in that these images are almost two orders of magnitude lower resolution than the structural scans. We attempted to minimize signal contamination through examination of native space data limited to the data within 70% interior of the cortex as well as through PVC. However, ultimately, additional research with higher resolution imaging is warranted and this research is currently underway. Lastly, we suggested that the CBF reduction in MCI may indicate the cerebral perfusion dysfunction caused by vascular processes, because the reduced CBF was strongly associated with the volume of WML, which is presumed as an indicator of vascular health but not related to the ALFF as well as cortical thickness. However, to clarify the effects of pure vascular physiology on the CBF in the brain, additional examination of vascular measures, such as arterial transit time (ATT) (Alsop and Detre, 1996) in CBF by ASL MRI, are needed. Our ongoing work, the Human Connectome Project Lifespan study (Bookheimer et al., 2019; Harms et al., 2018), acquires novel ASL data and we will be able to calculate the transit times to characterize the hemodynamics of the brain system in future studies (Bibic et al., 2015).
5. Conclusion
Reduced CBF was observed in individuals with MCI, while cortical thickness and ALFF were minimally affected. Reduced CBF was associated with the high volume of WML in individuals with MCI but not associated with cortical thickness or ALFF. Because MCI had a relatively higher pulse pressure as well as the WML volume compared to cognitively healthy older adult, reduced CBF in MCI may provide an information of the brain perfusion related to vascular systemics that could not be measured by ALFF. Although speculative, given the presumed vascular etiology of WML, these results may suggest that deterioration in vascular health is a causal mechanism of reduced CBF in MCI which may also be an important early component of the pathological etiology of this condition. Taken together, CBF combined with WML could be a useful marker reflecting an increased risk of cognitive deterioration which often represents the early stages of AD.
Supplementary Material
Supplementary Figure 1. Vertex-wise group difference between individuals with MCI and cognitively healthy older adults without performing PVC adjusted for sex. Comparisons of (A) CBF, and (B) ALFF between groups. Significantly reduced CBF and ALFF were noted in individuals with MCI compared with cognitively healthy older adults without performing PVC adjusted for sex (cluster corrected q < 0.05).
Abbreviations: MCI, mild cognitive impairment; CBF, cerebral blood flow; ALFF, amplitude of low frequency fluctuations; PVC, partial volume correction; FDR, False discovery rate.
Supplementary Figure 2. Vertex-wise local correlations between CBF, ALFF, volume of WML, and cortical thickness in all participants after performing PVC adjusted for sex. (A) A significant negative correlation between CBF and the volume of WML was noted in all participants (FDR corrected q <0.05), while (B) no correlation between ALFF and volume of WML was noted. (C) A significant positive correlation between ALFF and cortical thickness was noted in all participants (FDR corrected q < 0.05). Color bar indicates scale of Pearson’s correlation coefficient.
Abbreviations: CBF, cerebral blood flow; WML, white matter signal lesions; CTh, cortical thickness; PVC, partial volume correction; AD, Alzheimer’s disease.
Supplementary Figure 3. Vertex-wise local correlations between CBF, ALFF, volume of WML, and cortical thickness in individuals with MCI and cognitively healthy older adults without performing PVC adjusted for sex. (A) A significant negative correlation between CBF and the volume of WML was noted in individuals with MCI (FDR corrected q <0.05), while not in cognitively healthy older adults. (B) A significant negative correlation between ALFF and the volume of WML was noted in cognitively healthy older adults (FDR corrected q < 0.05), but not in MCI. (C) A widespread pattern of positive correlation between ALFF and cortical thickness was noted in both groups (FDR corrected q < 0.05). Color bar indicates scale of Pearson’s correlation coefficient.
Abbreviations: MCI, mild cognitive impairment; CBF, cerebral blood flow; WML, white matter signal lesions; CTh, cortical thickness; PVC, partial volume correction.
Supplementary Figure 4. Group differences in WML effects on CBF and ALFF. (A) Significant high group WML slopes in CBF were noted, mainly in the right temporal cortex, medial prefrontal cortex, and temporal poles, in cognitively healthy older adults compared with individuals with MCI (p<0.05). (B) Significant low group WML slopes in ALFF were noted, mainly in middle temporal cortex, PCC-precuneus, and left inferior parietal cortex in individuals with MCI compared with cognitively normal healthy older adults (p<0.05). Red color indicates high slope in interaction of WML on CBF in cognitively healthy older adults than the individuals with MCI and blue color indicates an opposite direction.
Abbreviations: MCI, mild cognitive impairment; CBF, cerebral blood flow; ALFF, amplitude of low frequency fluctuations; WML, white matter signal lesions; Rt, right; Lt, left; Inf Parietal, inferior parietal cortex; Lat Occi, lateral occipital cortex; mPFC, medial prefrontal cortex; Temp, temporal cortex; Temp pole, temporal pole; EC, entorhinal cortex; Inf Parietal, inferior parietal cortex; Middle Temp, middle temporal cortex; PCC-precuneus, posterior cingulate cortex-precuneus; Ling, lingual gyrus.
Supplementary Figure 5. CBF maps are shown for the 6 participants with low (WML volume<2000cm3) and high amount of WML volume (WML volume>5000cm3) in both groups, respectively. A) CBF maps from 3 cognitively healthy older adults with high WML volume. (B) CBF maps from 3 MCI with high WML volume. (C) CBF maps from 3 cognitively healthy older adults with low WML volume. (D) CBF maps from 3 MCI with low WML volume. Each CBF map was spatially normalized to standard MNI152 space by using linear registration. Red color indicates the regions with low CBF in each individual.
Abbreviations: CBF, cerebral blood flow; WML, white matter lesions; CH, cognitively healthy older adults; MCI, mild cognitive impairment.
Acknowledgements
This work supported by National Institutes of Health/National Institute of Nursing Research R01NR010827.
Footnotes
Disclosure statement
The authors have no actual or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alsop DC, Dai W, Grossman M, Detre JA, 2010. Arterial spin labeling blood flow MRI: its role in the early characterization of Alzheimer’s disease. Journal of Alzheimer’s Disease 20(3), 871–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsop DC, Dai W, Grossman M, Detre JA, 2010. Arterial spin labeling blood flow MRI: its role in the early characterization of Alzheimer’s disease. Journal of Alzheimer’s disease : JAD 20(3), 871–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsop DC, Detre JA, 1996. Reduced transit-time sensitivity in noninvasive magnetic resonance imaging of human cerebral blood flow. Journal of Cerebral Blood Flow & Metabolism 16(6), 1236–1249. [DOI] [PubMed] [Google Scholar]
- Alsop DC, Detre JA, Golay X, Günther M, Hendrikse J, Hernandez- Garcia L, Lu H, MacIntosh BJ, Parkes LM, Smits M, 2015. Recommended implementation of arterial spin- labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magnetic resonance in medicine 73(1), 102–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsop DC, Detre JA, Grossman M, 2000. Assessment of cerebral blood flow in Alzheimer’s disease by spin-labeled magnetic resonance imaging. Ann Neurol 47(1), 93–100. [PubMed] [Google Scholar]
- Alsop DC, Detre JA, Grossman M, 2000. Assessment of cerebral blood flow in Alzheimer’s disease by spin- labeled magnetic resonance imaging. Annals of neurology 47(1), 93–100. [PubMed] [Google Scholar]
- Asllani I, Habeck C, Scarmeas N, Borogovac A, Brown TR, Stern Y, 2008. Multivariate and univariate analysis of continuous arterial spin labeling perfusion MRI in Alzheimer’s disease. Journal of Cerebral Blood Flow & Metabolism 28(4), 725–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartenstein P, Minoshima S, Hirsch C, Buch K, 1997. Quantitive assessment of cerebral blood flow in patients with Alzheimer’s disease by SPECT. The Journal of Nuclear Medicine 38(7), 1095. [PubMed] [Google Scholar]
- Bastos-Leite A, Kuijer J, Rombouts S, Sanz-Arigita E, Van Straaten E, Gouw A, van der Flier W, Scheltens P, Barkhof F, 2008. Cerebral blood flow by using pulsed arterial spin-labeling in elderly subjects with white matter hyperintensities. American Journal of Neuroradiology 29(7), 1296–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedictus MR, Binnewijzend MA, Kuijer JP, Steenwijk MD, Versteeg A, Vrenken H, Scheltens P, Barkhof F, van der Flier WM, Prins ND, 2014. Brain volume and white matter hyperintensities as determinants of cerebral blood flow in Alzheimer’s disease. Neurobiology of aging 35(12), 2665–2670. [DOI] [PubMed] [Google Scholar]
- Benedictus MR, Leeuwis AE, Binnewijzend MA, Kuijer JP, Scheltens P, Barkhof F, van der Flier WM, Prins ND, 2017. Lower cerebral blood flow is associated with faster cognitive decline in Alzheimer’s disease. European radiology 27(3), 1169–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y, 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the royal statistical society. Series B (Methodological), 289–300. [Google Scholar]
- Bibic A, Knutsson L, Schmidt A, Henningsson E, Månsson S, Abul- Kasim K, Åkeson J, Gunther M, Ståhlberg F, Wirestam R, 2015. Measurement of vascular water transport in human subjects using time- resolved pulsed arterial spin labelling. NMR in Biomedicine 28(8), 1059–1068. [DOI] [PubMed] [Google Scholar]
- Binnewijzend MA, Benedictus MR, Kuijer JP, van der Flier WM, Teunissen CE, Prins ND, Wattjes MP, van Berckel BN, Scheltens P, Barkhof F, 2016. Cerebral perfusion in the predementia stages of Alzheimer’s disease. Eur Radiol 26(2), 506–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binnewijzend MA, Kuijer JP, Benedictus MR, van der Flier WM, Wink AM, Wattjes MP, van Berckel BN, Scheltens P, Barkhof F, 2013. Cerebral blood flow measured with 3D pseudocontinuous arterial spin-labeling MR imaging in Alzheimer disease and mild cognitive impairment: a marker for disease severity. Radiology 267(1), 221–230. [DOI] [PubMed] [Google Scholar]
- Binnewijzend MA, Kuijer JP, Benedictus MR, van der Flier WM, Wink AM, Wattjes MP, van Berckel BN, Scheltens P, Barkhof F, 2013. Cerebral blood flow measured with 3D pseudocontinuous arterial spin-labeling MR imaging in Alzheimer disease and mild cognitive impairment: a marker for disease severity. Radiology 267(1), 221–230. [DOI] [PubMed] [Google Scholar]
- Biswal B, Zerrin Yetkin F, Haughton VM, Hyde JS, 1995. Functional connectivity in the motor cortex of resting human brain using echo- planar MRI. Magnetic resonance in medicine 34(4), 537–541. [DOI] [PubMed] [Google Scholar]
- Bondi MW, Edmonds EC, Jak AJ, Clark LR, Delano-Wood L, McDonald CR, Nation DA, Libon DJ, Au R, Galasko D, 2014. Neuropsychological criteria for mild cognitive impairment improves diagnostic precision, biomarker associations, and progression rates. Journal of Alzheimer’s Disease 42(1), 275–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi MW, Edmonds EC, Jak AJ, Clark LR, Delano-Wood L, McDonald CR, Nation DA, Libon DJ, Au R, Galasko D, Salmon DP, 2014. Neuropsychological criteria for mild cognitive impairment improves diagnostic precision, biomarker associations, and progression rates. J Alzheimers Dis 42(1), 275–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi MW, Jak AJ, Delano-Wood L, Jacobson MW, Delis DC, Salmon DP, 2008. Neuropsychological contributions to the early identification of Alzheimer’s disease. Neuropsychol Rev 18(1), 73–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonferroni CE, 1936. Teoria statistica delle classi e calcolo delle probabilita. Libreria internazionale Seeber. [Google Scholar]
- Bookheimer SY, Salat DH, Terpstra M, Ances BM, Barch DM, Buckner RL, Burgess GC, Curtiss SW, Diaz-Santos M, Elam JS, Fischl B, Greve DN, Hagy HA, Harms MP, Hatch OM, Hedden T, Hodge C, Japardi KC, Kuhn TP, Ly TK, Smith SM, Somerville LH, Ugurbil K, van der Kouwe A, Van Essen D, Woods RP, Yacoub E, 2019. The Lifespan Human Connectome Project in Aging: An overview. Neuroimage 185, 335–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borroni B, Anchisi D, Paghera B, Vicini B, Kerrouche N, Garibotto V, Terzi A, Vignolo L, Di Luca M, Giubbini R, 2006. Combined 99m Tc-ECD SPECT and neuropsychological studies in MCI for the assessment of conversion to AD. Neurobiology of aging 27(1), 24–31. [DOI] [PubMed] [Google Scholar]
- Bradley K, O’sullivan V, Soper N, Nagy Z, King EF, Smith A, Shepstone B, 2002. Cerebral perfusion SPET correlated with Braak pathological stage in Alzheimer’s disease. Brain 125(8), 1772–1781. [DOI] [PubMed] [Google Scholar]
- Bray S, 2017. Age-associated patterns in gray matter volume, cerebral perfusion and BOLD oscillations in children and adolescents. Hum Brain Mapp 38(5), 2398–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman AM, Zahra A, Muraskin J, Steffener J, Holland CM, Habeck C, Borogovac A, Ramos MA, Brown TR, Asllani I, 2009. Reduction in cerebral blood flow in areas appearing as white matter hyperintensities on magnetic resonance imaging. Psychiatry Research: Neuroimaging 172(2), 117–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman AM, Zahra A, Muraskin J, Steffener J, Holland CM, Habeck C, Borogovac A, Ramos MA, Brown TR, Asllani I, Stern Y, 2009. Reduction in cerebral blood flow in areas appearing as white matter hyperintensities on magnetic resonance imaging. Psychiatry research 172(2), 117–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael O, Schwarz C, Drucker D, Fletcher E, Harvey D, Beckett L, Jack CR Jr., Weiner M, DeCarli C, Alzheimer’s Disease Neuroimaging I, 2010. Longitudinal changes in white matter disease and cognition in the first year of the Alzheimer disease neuroimaging initiative. Arch Neurol 67(11), 1370–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao LL, Buckley ST, Kornak J, Schuff N, Madison C, Yaffe K, Miller BL, Kramer JH, Weiner MW, 2010. ASL perfusion MRI predicts cognitive decline and conversion from MCI to dementia. Alzheimer disease and associated disorders 24(1), 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao LL, Pa J, Duarte A, Schuff N, Weiner MW, Kramer JH, Miller BL, Freeman KM, Johnson JK, 2009. Patterns of cerebral hypoperfusion in amnestic and dysexecutive MCI. Alzheimer disease and associated disorders 23(3), 245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell MA, Groves AR, Whitcher B, Woolrich MW, 2009. Variational Bayesian inference for a nonlinear forward model. IEEE Transactions on Signal Processing 57(1), 223–236. [Google Scholar]
- Chen JJ, Rosas HD, Salat DH, 2011. Age-associated reductions in cerebral blood flow are independent from regional atrophy. Neuroimage 55(2), 468–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JJ, Rosas HD, Salat DH, 2013. The relationship between cortical blood flow and sub-cortical white-matter health across the adult age span. PloS one 8(2), e56733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Wolk D, Reddin J, Korczykowski M, Martinez P, Musiek E, Newberg A, Julin P, Arnold S, Greenberg J, 2011. Voxel-level comparison of arterial spin-labeled perfusion MRI and FDG-PET in Alzheimer disease. Neurology, WNL. 0b013e31823a31820ef31827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutu JP, Goldblatt A, Rosas HD, Salat DH, Alzheimer’s Disease Neuroimaging I, 2016. White Matter Changes are Associated with Ventricular Expansion in Aging, Mild Cognitive Impairment, and Alzheimer’s Disease. Journal of Alzheimer’s disease : JAD 49(2), 329–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutu JP, Lindemer ER, Konukoglu E, Salat DH, Alzheimer’s Disease Neuroimaging I, 2017. Two distinct classes of degenerative change are independently linked to clinical progression in mild cognitive impairment. Neurobiol Aging 54, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane DE, Black SE, Ganda A, Mikulis DJ, Nestor SM, Donahue MJ, MacIntosh BJ, 2015. Gray matter blood flow and volume are reduced in association with white matter hyperintensity lesion burden: a cross-sectional MRI study. Frontiers in aging neuroscience 7, 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadar M, Maranzano J, Ducharme S, Carmichael OT, Decarli C, Collins DL, Alzheimer’s Disease Neuroimaging I, 2018. Validation of T1w-based segmentations of white matter hyperintensity volumes in large-scale datasets of aging. Hum Brain Mapp 39(3), 1093–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai W, Lopez OL, Carmichael OT, Becker JT, Kuller LH, Gach HM, 2009. Mild cognitive impairment and alzheimer disease: patterns of altered cerebral blood flow at MR imaging. Radiology 250(3), 856–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai W, Lopez OL, Carmichael OT, Becker JT, Kuller LH, Gach HM, 2009. Mild cognitive impairment and alzheimer disease: patterns of altered cerebral blood flow at MR imaging. Radiology 250(3), 856–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI, 1999. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 9(2), 179–194. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI, 1999. Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage 9(2), 179–194. [DOI] [PubMed] [Google Scholar]
- Detre JA, Leigh JS, Williams DS, Koretsky AP, 1992. Perfusion imaging. Magnetic resonance in medicine 23(1), 37–45. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Bakkour A, Salat DH, Feczko E, Pacheco J, Greve DN, Grodstein F, Wright CI, Blacker D, Rosas HD, 2008. The cortical signature of Alzheimer’s disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cerebral cortex 19(3), 497–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Bakkour A, Salat DH, Feczko E, Pacheco J, Greve DN, Grodstein F, Wright CI, Blacker D, Rosas HD, Sperling RA, Atri A, Growdon JH, Hyman BT, Morris JC, Fischl B, Buckner RL, 2009. The cortical signature of Alzheimer’s disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb Cortex 19(3), 497–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding B, Ling H. w., Zhang Y, Huang J, Zhang H, Wang T, Yan FH, 2014. Pattern of cerebral hyperperfusion in Alzheimer’s disease and amnestic mild cognitive impairment using voxel-based analysis of 3D arterial spin-labeling imaging: initial experience. Clinical interventions in aging 9, 493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du A, Jahng G, Hayasaka S, Kramer J, Rosen H, Gorno-Tempini M, Rankin K, Miller B, Weiner M, Schuff N, 2006. Hypoperfusion in frontotemporal dementia and Alzheimer disease by arterial spin labeling MRI. Neurology 67(7), 1215–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukart J, Bertolino A, 2014. When structure affects function–the need for partial volume effect correction in functional and resting state magnetic resonance imaging studies. PloS one 9(12), e114227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Encinas M, de Juan R, Marcos A, Gil P, Barabash A, Fernández C, de Ugarte C, Cabranes JA, 2003. Regional cerebral blood flow assessed with 99m Tc-ECD SPET as a marker of progression of mild cognitive impairment to Alzheimer’s disease. European journal of nuclear medicine and molecular imaging 30(11), 1473–1480. [DOI] [PubMed] [Google Scholar]
- Fazekas F, Kleinert R, Offenbacher H, Schmidt R, Kleinert G, Payer F, Radner H, Lechner H, 1993. Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology 43(9), 1683–1689. [DOI] [PubMed] [Google Scholar]
- Fischl B, Dale AM, 2000. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences 97(20), 11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Liu A, Dale AM, 2001. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE transactions on medical imaging 20(1), 70–80. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, Van Der Kouwe A, Killiany R, Kennedy D, Klaveness S, 2002. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33(3), 341–355. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM, 2002. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33(3), 341–355. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Van Der Kouwe AJ, Makris N, Ségonne F, Quinn BT, Dale AM, 2004. Sequence-independent segmentation of magnetic resonance images. Neuroimage 23, S69–S84. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, van der Kouwe AJ, Makris N, Segonne F, Quinn BT, Dale AM, 2004a. Sequence-independent segmentation of magnetic resonance images. Neuroimage 23 Suppl 1, S69–84. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale AM, 1999. High-resolution intersubject averaging and a coordinate system for the cortical surface. Human brain mapping 8(4), 272–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM, 2004b. Automatically parcellating the human cerebral cortex. Cereb Cortex 14(1), 11–22. [DOI] [PubMed] [Google Scholar]
- Fryer SL, Roach BJ, Ford JM, Turner JA, van Erp TG, Voyvodic J, Preda A, Belger A, Bustillo J, O’Leary D, Mueller BA, Lim KO, McEwen SC, Calhoun VD, Diaz M, Glover G, Greve D, Wible CG, Vaidya J, Potkin SG, Mathalon DH, 2015. Relating Intrinsic LowFrequency BOLD Cortical Oscillations to Cognition in Schizophrenia. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 40(12), 2705–2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelick PB, Scuteri A, Black SE, DeCarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, 2011. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 42(9), 2672–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottler J, Preibisch C, Riederer I, Pasquini L, Alexopoulos P, Bohn KP, Yakushev I, Beller E, Kaczmarz S, Zimmer C, Grimmer T, Drzezga A, Sorg C, 2018. Reduced blood oxygenation level dependent connectivity is related to hypoperfusion in Alzheimer’s disease. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism, 271678X18759182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouw A, Seewann A, Vrenken H, Van Der Flier W, Rozemuller J, Barkhof F, Scheltens P, Geurts J, 2008. Heterogeneity of white matter hyperintensities in Alzheimer’s disease: post-mortem quantitative MRI and neuropathology. Brain 131(12), 3286–3298. [DOI] [PubMed] [Google Scholar]
- Grammas P, 2011. Neurovascular dysfunction, inflammation and endothelial activation: implications for the pathogenesis of Alzheimer’s disease. Journal of neuroinflammation 8(1), 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve DN, Fischl B, 2009. Accurate and robust brain image alignment using boundary-based registration. Neuroimage 48(1), 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W. b., Liu F, Xue Z. m., Xu X. j., Wu R. r., Ma C. q., Wooderson SC, Tan C. l., Sun X. l., Chen J. d., 2012. Alterations of the amplitude of low-frequency fluctuations in treatment-resistant and treatment-response depression: a resting-state fMRI study. Progress in Neuro-Psychopharmacology and Biological Psychiatry 37(1), 153–160. [DOI] [PubMed] [Google Scholar]
- Guo W, Song Y, Liu F, Zhang Z, Zhang J, Yu M, Liu J, Xiao C, Liu G, Zhao J, 2015. Dissociation of functional and anatomical brain abnormalities in unaffected siblings of schizophrenia patients. Clinical Neurophysiology 126(5), 927–932. [DOI] [PubMed] [Google Scholar]
- Hagler DJ Jr., Saygin AP, Sereno MI, 2006. Smoothing and cluster thresholding for cortical surface-based group analysis of fMRI data. Neuroimage 33(4), 1093–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms MP, Somerville LH, Ances BM, Andersson J, Barch DM, Bastiani M, Bookheimer SY, Brown TB, Buckner RL, Burgess GC, Coalson TS, Chappell MA, Dapretto M, Douaud G, Fischl B, Glasser MF, Greve DN, Hodge C, Jamison KW, Jbabdi S, Kandala S, Li X, Mair RW, Mangia S, Marcus D, Mascali D, Moeller S, Nichols TE, Robinson EC, Salat DH, Smith SM, Sotiropoulos SN, Terpstra M, Thomas KM, Tisdall MD, Ugurbil K, van der Kouwe A, Woods RP, Zollei L, Van Essen DC, Yacoub E, 2018. Extending the Human Connectome Project across ages: Imaging protocols for the Lifespan Development and Aging projects. Neuroimage 183, 972–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays CC, Zlatar ZZ, Wierenga CE, 2016. The utility of cerebral blood flow as a biomarker of preclinical Alzheimer’s disease. Cellular and molecular neurobiology 36(2), 167–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Wang L, Zang Y, Tian L, Zhang X, Li K, Jiang T, 2007. Regional coherence changes in the early stages of Alzheimer’s disease: a combined structural and resting-state functional MRI study. Neuroimage 35(2), 488–500. [DOI] [PubMed] [Google Scholar]
- Hirao K, Ohnishi T, Hirata Y, Yamashita F, Mori T, Moriguchi Y, Matsuda H, Nemoto K, Imabayashi E, Yamada M, 2005. The prediction of rapid conversion to Alzheimer’s disease in mild cognitive impairment using regional cerebral blood flow SPECT. Neuroimage 28(4), 1014–1021. [DOI] [PubMed] [Google Scholar]
- Hou J, Wu W, Lin Y, Wang J, Zhou D, Guo J, Gu S, He M, Ahmed S, Hu J, 2012. Localization of cerebral functional deficits in patients with obsessive–compulsive disorder: a resting-state fMRI study. Journal of affective disorders 138(3), 313–321. [DOI] [PubMed] [Google Scholar]
- Hu B, Yan LF, Sun Q, Yu Y, Zhang J, Dai YJ, Yang Y, Hu YC, Nan HY, Zhang X, Heng CN, Hou JF, Liu QQ, Shao CH, Li F, Zhou KX, Guo H, Cui GB, Wang W, 2019. Disturbed neurovascular coupling in type 2 diabetes mellitus patients: Evidence from a comprehensive fMRI analysis. NeuroImage. Clinical 22, 101802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Wahlund L-O, Svensson L, Winblad B, Julin P, 2002. Cingulate cortex hypoperfusion predicts Alzheimer’s disease in mild cognitive impairment. BMC neurology 2(1), 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CW, Hsu SW, Chang YT, Huang SH, Huang YC, Lee CC, Chang WN, Lui CC, Chen NC, Chang CC, 2018. Cerebral Perfusion Insufficiency and Relationships with Cognitive Deficits in Alzheimer’s Disease: A Multiparametric Neuroimaging Study. Scientific reports 8(1), 1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, 2004. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nature Reviews Neuroscience 5(5), 347. [DOI] [PubMed] [Google Scholar]
- Jak AJ, Bondi MW, Delano-Wood L, Wierenga C, Corey-Bloom J, Salmon DP, Delis DC, 2009. Quantification of five neuropsychological approaches to defining mild cognitive impairment. Am J Geriatr Psychiatry 17(5), 368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jak AJ, Preis SR, Beiser AS, Seshadri S, Wolf PA, Bondi MW, Au R, 2016. Neuropsychological criteria for mild cognitive impairment and dementia risk in the Framingham Heart Study. Journal of the International Neuropsychological Society 22(9), 937–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jann K, Gee DG, Kilroy E, Schwab S, Smith RX, Cannon TD, Wang DJ, 2015. Functional connectivity in BOLD and CBF data: similarity and reliability of resting brain networks. Neuroimage 106, 111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellinger KA, 2010. Prevalence and impact of cerebrovascular lesions in Alzheimer and lewy body diseases. Neurodegenerative Diseases 7(1–3), 112–115. [DOI] [PubMed] [Google Scholar]
- Jellinger KA, Attems J, 2005. Prevalence and pathogenic role of cerebrovascular lesions in Alzheimer disease. Journal of the neurological sciences 229, 37–41. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S, 2002. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17(2), 825–841. [DOI] [PubMed] [Google Scholar]
- Jo HJ, Saad ZS, Simmons WK, Milbury LA, Cox RW, 2010. Mapping sources of correlation in resting state FMRI, with artifact detection and removal. Neuroimage 52(2), 571–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson NA, Jahng G-H, Weiner MW, Miller BL, Chui HC, Jagust WJ, Gorno-Tempini ML, Schuff N, 2005. Pattern of cerebral hypoperfusion in Alzheimer disease and mild cognitive impairment measured with arterial spin-labeling MR imaging: initial experience. Radiology 234(3), 851–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson NA, Jahng G-H, Weiner MW, Miller BL, Chui HC, Jagust WJ, Gorno-Tempini ML, Schuff N, 2006. Pattern of cerebral hypoperfusion in Alzheimer’s disease and mild cognitive impairment measured with arterial spin-labeling MR imaging: Initial experience, International Congress Series. Elsevier, pp. 108–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson NA, Jahng GH, Weiner MW, Miller BL, Chui HC, Jagust WJ, Gorno-Tempini ML, Schuff N, 2005. Pattern of cerebral hypoperfusion in Alzheimer disease and mild cognitive impairment measured with arterial spin-labeling MR imaging: initial experience. Radiology 234(3), 851–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kety SS, Schmidt CF, 1945. The determination of cerebral blood flow in man by the use of nitrous oxide in low concentrations. American Journal of Physiology-Legacy Content 143(1), 53–66. [Google Scholar]
- Kimura N, Nakama H, Nakamura K, Aso Y, Kumamoto T, 2012. Effect of white matter lesions on brain perfusion in Alzheimer’s disease. Dementia and geriatric cognitive disorders 34(3–4), 256–261. [DOI] [PubMed] [Google Scholar]
- Kivipelto M, Helkala EL, Laakso MP, Hanninen T, Hallikainen M, Alhainen K, Soininen H, Tuomilehto J, Nissinen A, 2001. Midlife vascular risk factors and Alzheimer’s disease in later life: longitudinal, population based study. Bmj 322(7300), 1447–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogure D, Matsuda H, Ohnishi T, Asada T, Uno M, Kunihiro T, Nakano S, Takasaki M, 2000. Longitudinal evaluation of early Alzheimer’s disease using brain perfusion SPECT. Journal of nuclear medicine : official publication, Society of Nuclear Medicine 41(7), 1155–1162. [PubMed] [Google Scholar]
- Lacalle-Aurioles M, Mateos-Pérez JM, Guzmán-De-Villoria JA, Olazarán J, Cruz-Orduña I, Alemán-Gómez Y, Martino M-E, Desco M, 2014. Cerebral blood flow is an earlier indicator of perfusion abnormalities than cerebral blood volume in Alzheimer’s disease. Journal of Cerebral Blood Flow & Metabolism 34(4), 654–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeuwis AE, Benedictus MR, Kuijer JP, Binnewijzend MA, Hooghiemstra AM, Verfaillie SC, Koene T, Scheltens P, Barkhof F, Prins ND, 2017. Lower cerebral blood flow is associated with impairment in multiple cognitive domains in Alzheimer’s disease. Alzheimer’s & Dementia 13(5), 531–540. [DOI] [PubMed] [Google Scholar]
- Leijenaar JF, van Maurik IS, Kuijer JP, van der Flier WM, Scheltens P, Barkhof F, Prins ND, 2017. Lower cerebral blood flow in subjects with Alzheimer’s dementia, mild cognitive impairment, and subjective cognitive decline using two-dimensional phase-contrast magnetic resonance imaging. Alzheimer’s & Dementia: Diagnosis, Assessment & Disease Monitoring 9, 76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Zhu Y, Childress AR, Detre JA, Wang Z, 2012. Relations between BOLD fMRI-derived resting brain activity and cerebral blood flow. PLoS One 7(9), e44556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang P, Xiang J, Liang H, Qi Z, Li K, Initiative, A.s.D.N., 2014. Altered amplitude of low-frequency fluctuations in early and late mild cognitive impairment and Alzheimer’s disease. Current Alzheimer Research 11(4), 389–398. [DOI] [PubMed] [Google Scholar]
- Liang X, Zou Q, He Y, Yang Y, 2013. Coupling of functional connectivity and regional cerebral blood flow reveals a physiological basis for network hubs of the human brain. Proceedings of the National Academy of Sciences of the United States of America 110(5), 1929–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemer ER, Greve DN, Fischl B, Augustinack JC, Salat DH, 2017. Differential regional distribution of juxtacortical white matter signal abnormalities in aging and Alzheimer’s disease. Journal of Alzheimer’s Disease 57(1), 293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu TT, Brown GG, 2007. Measurement of cerebral perfusion with arterial spin labeling: Part 1. Methods. Journal of the International Neuropsychological Society 13(03), 517–525. [DOI] [PubMed] [Google Scholar]
- Liu X, Wang S, Zhang X, Wang Z, Tian X, He Y, 2014. Abnormal amplitude of low-frequency fluctuations of intrinsic brain activity in Alzheimer’s disease. Journal of Alzheimer’s Disease 40(2), 387–397. [DOI] [PubMed] [Google Scholar]
- Liu Y, Liang P, Duan Y, Jia X, Wang F, Yu C, Qin W, Dong H, Ye J, Li K, 2011. Abnormal baseline brain activity in patients with neuromyelitis optica: a resting-state fMRI study. European journal of radiology 80(2), 407–411. [DOI] [PubMed] [Google Scholar]
- Luckhaus C, Cohnen M, Flüβ MO, Jänner M, Grass-Kapanke B, Teipel SJ, Grothe M, Hampel H, Peters O, Kornhuber J, 2010. The relation of regional cerebral perfusion and atrophy in mild cognitive impairment (MCI) and early Alzheimer’s dementia. Psychiatry Research: Neuroimaging 183(1), 44–51. [DOI] [PubMed] [Google Scholar]
- Luckhaus C, Flüß MO, Wittsack H-J, Grass-Kapanke B, Jänner M, Khalili-Amiri R, Friedrich W, Supprian T, Gaebel W, Mödder U, 2008. Detection of changed regional cerebral blood flow in mild cognitive impairment and early Alzheimer’s dementia by perfusion-weighted magnetic resonance imaging. Neuroimage 40(2), 495–503. [DOI] [PubMed] [Google Scholar]
- Luckhaus C, Fluss MO, Wittsack HJ, Grass-Kapanke B, Janner M, Khalili-Amiri R, Friedrich W, Supprian T, Gaebel W, Modder U, Cohnen M, 2008. Detection of changed regional cerebral blood flow in mild cognitive impairment and early Alzheimer’s dementia by perfusion-weighted magnetic resonance imaging. Neuroimage 40(2), 495–503. [DOI] [PubMed] [Google Scholar]
- Mak HK, Chan Q, Zhang Z, Petersen ET, Qiu D, Zhang L, Yau KK, Chu L-W, Golay X, 2012. Quantitative assessment of cerebral hemodynamic parameters by QUASAR arterial spin labeling in Alzheimer’s disease and cognitively normal Elderly adults at 3-tesla. Journal of Alzheimer’s Disease 31(1), 33–44. [DOI] [PubMed] [Google Scholar]
- Marstrand J, Garde E, Rostrup E, Ring P, Rosenbaum S, Mortensen EL, Larsson H, 2002. Cerebral perfusion and cerebrovascular reactivity are reduced in white matter hyperintensities. Stroke 33(4), 972–976. [DOI] [PubMed] [Google Scholar]
- Matsuda H, 2001. Cerebral blood flow and metabolic abnormalities in Alzheimer’s disease. Annals of nuclear medicine 15(2), 85–92. [DOI] [PubMed] [Google Scholar]
- Matsuda H, 2007. Role of neuroimaging in Alzheimer’s disease, with emphasis on brain perfusion SPECT. Journal of Nuclear Medicine 48(8), 1289–1300. [DOI] [PubMed] [Google Scholar]
- Mattsson N, Tosun D, Insel PS, Simonson A, Jack CR Jr, Beckett LA, Donohue M, Jagust W, Schuff N, Weiner MW, 2014. Association of brain amyloid-β with cerebral perfusion and structure in Alzheimer’s disease and mild cognitive impairment. Brain 137(5), 1550–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke M, Rosenberg P, Tschanz J, Cook L, Corcoran C, Hayden K, Norton M, Rabins P, Green R, Welsh-Bohmer K, 2007. Vascular factors predict rate of progression in Alzheimer disease. Neurology 69(19), 1850–1858. [DOI] [PubMed] [Google Scholar]
- Musiek ES, Chen Y, Korczykowski M, Saboury B, Martinez PM, Reddin JS, Alavi A, Kimberg DY, Wolk DA, Julin P, 2012. Direct comparison of fluorodeoxyglucose positron emission tomography and arterial spin labeling magnetic resonance imaging in Alzheimer’s disease. Alzheimer’s & Dementia 8(1), 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naka H, Nomura E, Takahashi T, Wakabayashi S, Mimori Y, Kajikawa H, Kohriyama T, Matsumoto M, 2006. Combinations of the presence or absence of cerebral microbleeds and advanced white matter hyperintensity as predictors of subsequent stroke types. AJNR. American journal of neuroradiology 27(4), 830–835. [PMC free article] [PubMed] [Google Scholar]
- O’Brien JT, Eagger S, Syed GM, Sahakian BJ, Levy R, 1992. A study of regional cerebral blood flow and cognitive performance in Alzheimer’s disease. Journal of Neurology, Neurosurgery & Psychiatry 55(12), 1182–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okonkwo OC, Xu G, Oh JM, Dowling NM, Carlsson CM, Gallagher CL, Birdsill AC, Palotti M, Wharton W, Hermann BP, 2012. Cerebral blood flow is diminished in asymptomatic middle-aged adults with maternal history of Alzheimer’s disease. Cerebral cortex 24(4), 978–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E, 1999. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 56(3), 303–308. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE, 2012. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59(3), 2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad K, Wiryasaputra L, Ng A, Kandiah N, 2011. White matter disease independently predicts progression from mild cognitive impairment to Alzheimer’s disease in a clinic cohort. Dement Geriatr Cogn Disord 31(6), 431–434. [DOI] [PubMed] [Google Scholar]
- Provenzano FA, Muraskin J, Tosto G, Narkhede A, Wasserman BT, Griffith EY, Guzman VA, Meier IB, Zimmerman ME, Brickman AM, Alzheimer’s Disease Neuroimaging I, 2013. White matter hyperintensities and cerebral amyloidosis: necessary and sufficient for clinical expression of Alzheimer disease? JAMA neurology 70(4), 455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu L, Fu X, Wang S, Tang Q, Chen X, Cheng L, Zhang F, Zhou Z, Tian L, 2017. Abnormal regional spontaneous neuronal activity associated with symptom severity in treatment-naive patients with obsessive-compulsive disorder revealed by resting-state functional MRI. Neuroscience letters 640, 99–104. [DOI] [PubMed] [Google Scholar]
- Reed BR, Jagust WJ, Seab JP, Ober BA, 1989. Memory and regional cerebral blood flow in mildly symptomatic Alzheimer’s disease. Neurology 39(11), 1537–1539. [DOI] [PubMed] [Google Scholar]
- Riederer I, Bohn KP, Preibisch C, Wiedemann E, Zimmer C, Alexopoulos P, Förster S, 2018. Alzheimer disease and mild cognitive impairment: integrated pulsed arterial spin-labeling MRI and 18F-FDG PET. Radiology, 170575. [DOI] [PubMed] [Google Scholar]
- Rius-Perez S, Tormos AM, Perez S, Talens-Visconti R, 2018. Vascular pathology: Cause or effect in Alzheimer disease? Neurologia 33(2), 112–120. [DOI] [PubMed] [Google Scholar]
- Roher AE, Debbins JP, Malek-Ahmadi M, Chen K, Pipe JG, Maze S, Belden C, Maarouf CL, Thiyyagura P, Mo H, 2012. Cerebral blood flow in Alzheimer’s disease. Vascular health and risk management 8, 599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy CS, Sherrington CS, 1890. On the regulation of the blood- supply of the brain. The Journal of physiology 11(1–2), 85–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ségonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, Fischl B, 2004. A hybrid approach to the skull stripping problem in MRI. Neuroimage 22(3), 1060–1075. [DOI] [PubMed] [Google Scholar]
- Schuff N, Matsumoto S, Kmiecik J, Studholme C, Du A, Ezekiel F, Miller BL, Kramer JH, Jagust WJ, Chui HC, Weiner MW, 2009. Cerebral blood flow in ischemic vascular dementia and Alzheimer’s disease, measured by arterial spin-labeling magnetic resonance imaging. Alzheimer’s & dementia : the journal of the Alzheimer’s Association 5(6), 454–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shokouhi M, Qiu D, Samman Tahhan A, Quyyumi AA, Hajjar I, 2018. Differential Associations of Diastolic and Systolic Pressures with Cerebral Measures in Older Individuals with Mild Cognitive Impairment. American journal of hypertension. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR, 1997. Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. JAMA 277(10), 813–817. [PubMed] [Google Scholar]
- Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR, 1997. Brain infarction and the clinical expression of Alzheimer disease: the Nun Study. Jama 277(10), 813–817. [PubMed] [Google Scholar]
- Snyder HM, Corriveau RA, Craft S, Faber JE, Greenberg SM, Knopman D, Lamb BT, Montine TJ, Nedergaard M, Schaffer CB, 2015. Vascular contributions to cognitive impairment and dementia including Alzheimer’s disease. Alzheimer’s & Dementia 11(6), 710–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song D, Chang D, Zhang J, Ge Q, Zang YF, Wang Z, 2018. Associations of brain entropy (BEN) to cerebral blood flow and fractional amplitude of low-frequency fluctuations in the resting brain. Brain imaging and behavior. [DOI] [PubMed] [Google Scholar]
- Stricker NH, Salat DH, Foley JM, Zink TA, Kellison IL, McFarland CP, Grande LJ, McGlinchey RE, Milberg WP, Leritz EC, 2013. Decreased white matter integrity in neuropsychologically defined mild cognitive impairment is independent of cortical thinning. J Int Neuropsychol Soc 19(8), 925–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dalen J, Mutsaerts H, Nederveen A, Vrenken H, Steenwijk M, Caan M, Majoie C, van Gool W, Richard E, 2016. White matter hyperintensity volume and cerebral perfusion in older individuals with hypertension using arterial spin-labeling. American Journal of Neuroradiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kouwe AJ, Benner T, Salat DH, Fischl B, 2008. Brain morphometry with multiecho MPRAGE. Neuroimage 40(2), 559–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC, 2005. A population-average, landmark-and surface-based (PALS) atlas of human cerebral cortex. Neuroimage 28(3), 635–662. [DOI] [PubMed] [Google Scholar]
- Verfaillie SC, Adriaanse SM, Binnewijzend MA, Benedictus MR, Ossenkoppele R, Wattjes MP, Pijnenburg YA, van der Flier WM, Lammertsma AA, Kuijer JP, 2015. Cerebral perfusion and glucose metabolism in Alzheimer’s disease and frontotemporal dementia: two sides of the same coin? European radiology 25(10), 3050–3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, Lindley RI, T O’Brien J, Barkhof F, Benavente OR, 2013. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. The Lancet Neurology 12(8), 822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DS, Detre JA, Leigh JS, Koretsky AP, 1992. Magnetic resonance imaging of perfusion using spin inversion of arterial water. Proceedings of the National Academy of Sciences 89(1), 212–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, Nordberg A, Backman L, Albert M, Almkvist O, Arai H, Basun H, Blennow K, de Leon M, DeCarli C, Erkinjuntti T, Giacobini E, Graff C, Hardy J, Jack C, Jorm A, Ritchie K, van Duijn C, Visser P, Petersen RC, 2004. Mild cognitive impairment--beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. Journal of internal medicine 256(3), 240–246. [DOI] [PubMed] [Google Scholar]
- Wirth M, Pichet Binette A, Brunecker P, Köbe T, Witte AV, Flöel A, 2017. Divergent regional patterns of cerebral hypoperfusion and gray matter atrophy in mild cognitive impairment patients. Journal of Cerebral Blood Flow & Metabolism 37(3), 814–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Long X-Y, Yang Y, Yan H, Zhu C-Z, Zhou X-P, Zang Y-F, Gong Q-Y, 2007. Amplitude of low frequency fluctuation within visual areas revealed by resting-state functional MRI. Neuroimage 36(1), 144–152. [DOI] [PubMed] [Google Scholar]
- Yoshita M, Fletcher E, Harvey D, Ortega M, Martinez O, Mungas D, Reed B, DeCarli C, 2006. Extent and distribution of white matter hyperintensities in normal aging, MCI, and AD. Neurology 67(12), 2192–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiura T, Hiwatashi A, Noguchi T, Yamashita K, Ohyagi Y, Monji A, Nagao E, Kamano H, Togao O, Honda H, 2009. Arterial spin labelling at 3-T MR imaging for detection of individuals with Alzheimer’s disease. Eur Radiol 19(12), 2819–2825. [DOI] [PubMed] [Google Scholar]
- Yu-Feng Z, Yong H, Chao-Zhe Z, Qing-Jiu C, Man-Qiu S, Meng L, Li-Xia T, Tian-Zi J, Yu-Feng W, 2007. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain and Development 29(2), 83–91. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV, 2011. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nature Reviews Neuroscience 12(12), 723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Q-H, Zhu C-Z, Yang Y, Zuo X-N, Long X-Y, Cao Q-J, Wang Y-F, Zang Y-F, 2008. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. Journal of neuroscience methods 172(1), 137–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Q, Yuan BK, Gu H, Liu D, Wang DJ, Gao JH, Yang Y, Zang YF, 2015. Detecting static and dynamic differences between eyes-closed and eyes-open resting states using ASL and BOLD fMRI. PLoS One 10(3), e0121757. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Vertex-wise group difference between individuals with MCI and cognitively healthy older adults without performing PVC adjusted for sex. Comparisons of (A) CBF, and (B) ALFF between groups. Significantly reduced CBF and ALFF were noted in individuals with MCI compared with cognitively healthy older adults without performing PVC adjusted for sex (cluster corrected q < 0.05).
Abbreviations: MCI, mild cognitive impairment; CBF, cerebral blood flow; ALFF, amplitude of low frequency fluctuations; PVC, partial volume correction; FDR, False discovery rate.
Supplementary Figure 2. Vertex-wise local correlations between CBF, ALFF, volume of WML, and cortical thickness in all participants after performing PVC adjusted for sex. (A) A significant negative correlation between CBF and the volume of WML was noted in all participants (FDR corrected q <0.05), while (B) no correlation between ALFF and volume of WML was noted. (C) A significant positive correlation between ALFF and cortical thickness was noted in all participants (FDR corrected q < 0.05). Color bar indicates scale of Pearson’s correlation coefficient.
Abbreviations: CBF, cerebral blood flow; WML, white matter signal lesions; CTh, cortical thickness; PVC, partial volume correction; AD, Alzheimer’s disease.
Supplementary Figure 3. Vertex-wise local correlations between CBF, ALFF, volume of WML, and cortical thickness in individuals with MCI and cognitively healthy older adults without performing PVC adjusted for sex. (A) A significant negative correlation between CBF and the volume of WML was noted in individuals with MCI (FDR corrected q <0.05), while not in cognitively healthy older adults. (B) A significant negative correlation between ALFF and the volume of WML was noted in cognitively healthy older adults (FDR corrected q < 0.05), but not in MCI. (C) A widespread pattern of positive correlation between ALFF and cortical thickness was noted in both groups (FDR corrected q < 0.05). Color bar indicates scale of Pearson’s correlation coefficient.
Abbreviations: MCI, mild cognitive impairment; CBF, cerebral blood flow; WML, white matter signal lesions; CTh, cortical thickness; PVC, partial volume correction.
Supplementary Figure 4. Group differences in WML effects on CBF and ALFF. (A) Significant high group WML slopes in CBF were noted, mainly in the right temporal cortex, medial prefrontal cortex, and temporal poles, in cognitively healthy older adults compared with individuals with MCI (p<0.05). (B) Significant low group WML slopes in ALFF were noted, mainly in middle temporal cortex, PCC-precuneus, and left inferior parietal cortex in individuals with MCI compared with cognitively normal healthy older adults (p<0.05). Red color indicates high slope in interaction of WML on CBF in cognitively healthy older adults than the individuals with MCI and blue color indicates an opposite direction.
Abbreviations: MCI, mild cognitive impairment; CBF, cerebral blood flow; ALFF, amplitude of low frequency fluctuations; WML, white matter signal lesions; Rt, right; Lt, left; Inf Parietal, inferior parietal cortex; Lat Occi, lateral occipital cortex; mPFC, medial prefrontal cortex; Temp, temporal cortex; Temp pole, temporal pole; EC, entorhinal cortex; Inf Parietal, inferior parietal cortex; Middle Temp, middle temporal cortex; PCC-precuneus, posterior cingulate cortex-precuneus; Ling, lingual gyrus.
Supplementary Figure 5. CBF maps are shown for the 6 participants with low (WML volume<2000cm3) and high amount of WML volume (WML volume>5000cm3) in both groups, respectively. A) CBF maps from 3 cognitively healthy older adults with high WML volume. (B) CBF maps from 3 MCI with high WML volume. (C) CBF maps from 3 cognitively healthy older adults with low WML volume. (D) CBF maps from 3 MCI with low WML volume. Each CBF map was spatially normalized to standard MNI152 space by using linear registration. Red color indicates the regions with low CBF in each individual.
Abbreviations: CBF, cerebral blood flow; WML, white matter lesions; CH, cognitively healthy older adults; MCI, mild cognitive impairment.