Abstract
Background:
The functional luminal imaging probe (FLIP) can evaluate esophagogastric junction (EGJ) distensibility and esophageal peristalsis in real time. FLIP measurements performed during diagnostic endoscopy can accurately discriminate between healthy controls and patients with achalasia, based on EGJ distensibility and distinct motility patterns termed repetitive antegrade contractions (RACs) and repetitive retrograde contractions (RRCs). We sought to evaluate real time motility changes in patients undergoing surgical myotomy for achalasia.
Methods:
FLIP measurements using a stepwise volumetric distention protocol were performed at three time points during assessment and performance of laparoscopic Heller myotomy and POEM: 1) During preoperative outpatient endoscopy, 2) Intraoperatively following induction of anesthesia, and 3) Intraoperatively after myotomy completion. EGJ-Distensibility, contractility, RACs, and RRCs were measured.
Results:
FLIP measurements were performed in 32 patients. The EGJ-distensibility index was similar between the preoperative and initial operative measurements (1.1 vs 1.4 mm2/mmHg, p = NS). There was a significant increase in distensibility following surgical myotomy (1.1 to 4.7 mm2/mmHg, p < 0.01). Intraoperative contractile patterns varied between achalasia subtypes. Contractility was seen in < 20% of assessments in patients with types I and II achalasia. Type III patients demonstrated contractility in 100% of assessments, with 70% exhibiting RRCs and 60% RACs. There was a reduction in the frequency of RRC presence (70% to 20%), and contractile vigor (80% to 0% of patients with lumen occluding contractions) in type III patients following surgical myotomy.
Conclusions:
This first report of real time intraoperative measurement of esophageal motility using FLIP demonstrates the feasibility of such assessments during surgical myotomy for achalasia. Patients with type I and II achalasia exhibited rare intraoperative contractility, while the presence of motility was the norm in those with type III. Patients with type III achalasia demonstrated an immediate reduction in repetitive contraction motility patterns and contractile vigor following myotomy.
Keywords: Achalasia, Esophagus, FLIP, Functional Luminal Imaging Probe, Esophageal Motility, POEM, Per-oral Esophageal Myotomy
Introduction
Achalasia is a rare esophageal motility disorder that is characterized by impaired lower esophageal sphincter (LES) relaxation and absent or greatly altered esophageal body peristalsis [1]. Treatment modalities include medications (e.g. calcium channel blockers), endoscopic botulinum toxin injection, and palliative disruption of the LES via pneumatic dilation or surgical myotomy. Laparoscopic Heller myotomy with partial fundoplication (LHM) and per-oral esophageal myotomy (POEM) are now both standard options for surgical myotomy.
The functional luminal imaging probe (FLIP) is a catheter-based system that has recently been introduced and used to evaluate esophageal compliance in patients with achalasia. FLIP utilizes impedance planimetry to calculate the EGJ-distensibility index (DI), which has been shown to correlate with symptom burden and barium retention in treated achalasia patients [2]. We have shown that DI increases sequentially during surgical myotomy and intraoperative values are predictive of symptomatic outcomes [3–5]. FLIP data can also be displayed as a function of time (FLIP topography) in order to visualize distention-mediated esophageal peristalsis and categorize motility (FLIP Panometry) [6]. This approach can accurately identify patients with achalasia and reveal contractile patterns that are not seen on HRM [7, 8].
There are several aspects of FLIP utilization that remain uncertain. It is unclear how varied anesthetic regimens affect distensibility metrics and contractile patterns. This complicates the comparison of FLIP data obtained with moderate sedation to measurements procured under general anesthesia. Moreover, FLIP Panometry contractile patterns have yet to be described in the operative setting. In this study, we obtained FLIP measurements during outpatient endoscopy and surgical myotomy for achalasia. Our aims were twofold – (1) to compare FLIP measurements obtained in these two anesthetic settings, and (2) describe intraoperative contractile patterns observed before and after myotomy.
Materials and Methods
Patient Selection
Patients ≥ 18 years old who were diagnosed with achalasia or EGJ outflow obstruction with partially preserved peristalsis via high-resolution manometry and scheduled to undergo either POEM or LHM were eligible for study inclusion. The FLIP measurements were conducted according to a protocol approved by the Northwestern Institutional Review Board.
Demographic information including age, sex, body mass index, prior achalasia treatment, duration of symptoms, and Eckardt symptom score [9] was collected preoperatively. Patients underwent preoperative evaluation with high-resolution manometry (HRM) for the diagnosis and categorization of achalasia according to the Chicago classification of esophageal motility disorders v3.0 [10].
FLIP Measurements
Preoperative Endoscopy Measurements
Preoperative FLIP was performed during upper endoscopy using moderate sedation (midazolam, fentanyl) after initial diagnostic evaluation per the distention protocol listed below.
Intraoperative Measurements
Intraoperative FLIP measurements were performed using an identical distension protocol. Measurements were performed at two intraoperative time points: 1) after induction of general anesthesia and endotracheal intubation (succinylcholine/rocuronium, propofol) and 2) after completion of the operation, including partial fundoplication in the case of LHM. During LHM all measurements were performed with the peritoneal cavity completely deinsufflated.
Our techniques for POEM and LHM were previously described in detail [11, 12]. POEM was performed under general endotracheal anesthesia in the supine position. An anterior selective circular myotomy was utilized in all but one case (posterior myotomy). The standard myotomy length was 8–10 cm in total, including a 2–3 cm extension onto the stomach. The proximal aspect of the myotomy was extended in cases of Type III achalasia, to ablate the contractile segment as defined by preoperative HRM. The LHM included both circular and longitudinal muscle fibers. The total myotomy length was 8–10 cm, with a 2–3 extension onto the stomach. A partial fundoplication was fashioned in every case. The type of partial fundoplication was dictated by patient factors and surgeon preference.
FLIP System and Distension Protocol
Impedance planimetry data were obtained with a commercially available FLIP system (EndoFLIP 2.0, Medtronic, Minneapolis, MN). The probe consisted of a 240 cm catheter with a distally mounted bag that assumed a 16 cm cylindrical shape (EF-322 N, Medtronic). The bag housed 17 ring electrodes spaced at 1 cm intervals and a solid-state pressure transducer. The probe was placed across the esophagogastric junction (EGJ) and filled incrementally with saline. The system used electrode-based impedance data to generate cross-sectional areas (CSA) at the level of each electrode pair. An intrabag pressure was derived from the solid-state pressure transducer.
Prior to probe insertion, the catheter was purged of any air and zeroed to atmospheric pressure. The catheter was inserted trans-orally and placed under direct endoscopic vision, such that 1–2 sensors were positioned distal to the EGJ. Proper placement was confirmed through observation of an hourglass shape on the FLIP display, with the area of narrowing representing the EGJ. The bag was progressively filled to 30-, 40-, 50-, 60, and 70 mL distension volumes, with a 30–60 second pause at each volume.
Data Analysis and FLIP Panometry Definitions
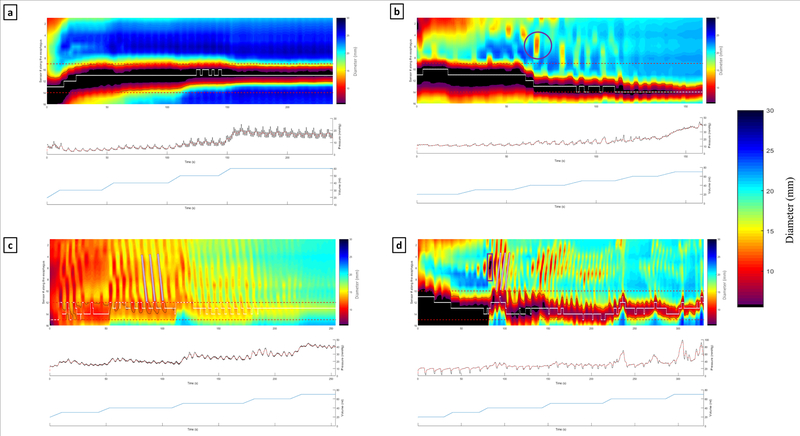
Data from each study were exported to MATLAB and analyzed using a custom program (MathWorks, Natick, MA). After filtering for vascular and respiratory artifacts, the program plotted each channel’s luminal diameter as a function of time. Diameters were expressed as a color-coded topography plot. The midline EGJ was identified as the minimal CSA of the distal impedance channels. Figure 1 shows examples of the FLIP topograms used in the motility analysis.
Figure 1:
Esophageal diameter (FLIP) Panometry contraction types and contractile patterns. Examples of contraction types include: [a] absent contractility, [b] non-occluding contractions (purple circle), and [d] occluding contractions (purple rectangle). Examples of contractile patterns include: [c] repetitive antegrade contractions and [d] repetitive retrograde contractions (checkered boxes highlight propagation direction).
Our method for FLIP Panometry interpretation was previously described in detail [6, 8]. All studies were examined by 2 independent raters (RC and DC), and the final classifications represented a consensus opinion. Esophageal contractions were defined as a transient luminal diameter decrease of ≥ 5 mm in 2 or more adjacent impedance channels. Contractions were further delineated as either occluding or non-occluding if their nadir diameter was ≤ 6 mm or > 6 mm, respectively. Contractions were considered repetitive when 3 or more occurred consecutively. The propagation direction of repeating contractions was noted as antegrade (towards the EGJ) or retrograde (towards the upper esophageal sphincter). The presence or absence of contractions, RACs, and RRCs were noted as a dichotomous variable.
Statistical Analysis
Analysis was performed using SPSS Statistics v25 (IBM, Armonk, NY). Comparisons of continuous data at different time points were performed with a paired t test. Dichotomous variables were compared with a McNemar’s or Fischer Exact test. A two-tailed p value < 0.05 was considered statistically significant. Results are presented as mean ± standard deviation unless otherwise noted.
Results
Intraoperative FLIP measurements were performed in 32 consecutive patients from May 2018 - January 2019 (8 type I achalasia, 7 type II achalasia, 10 type III achalasia, 7 EGJ outflow obstruction with partially preserved peristalsis). Nineteen participants were male (59%) and the mean age was 53 years (range 20 – 83). The median pre-operative Eckardt score was 7 ± 2. Ten patients (31%) had previously received endoscopic achalasia therapy (botox injection or pneumatic dilation). All patients underwent intraoperative FLIP measurement before and after completion of the operation and 28 patients (88%) additionally underwent FLIP evaluation during preoperative outpatient endoscopy. 24 patients (75%) were treated with POEM, and 8 with LHM.
EGJ-Distensibility
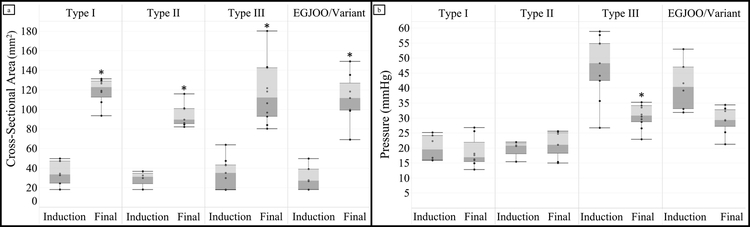
Results for EGJ-CSA, intrabag pressure, and EGJ-DI with 60 mL distention volume at each time point are listed in Table 1. CSA and DI values did not differ significantly between the preoperative outpatient examination (preop) and the baseline intraoperative measure (induction), while intrabag pressure was lower at induction than preop evaluation; p < 0.05. Figure 2 shows that CSA increased for all achalasia subtypes between induction and the conclusion of the operation (final). A difference in pressure at the conclusion of the procedure was observed among patients with type III achalasia (41.7 ± 8 mmHg vs 30.7 ± 4 mmHg, p < 0.001), but not in patients with type I, II, or EGJOO. Mean EGJ-DI increased between induction and final measurements for each subtype and for the group as a whole, when compared to induction values (overall 1.5 ± 0.9 mm2/mmHg vs 4.7 ± 1.6 mm2/mmHg, p < 0.001). Patients with type I achalasia had the largest increase in DI (1.6 ± 0.5 vs 6.8 ± 1.6 mm2/mmHg, p < 0.01). There was no significant difference in mean pressure, mean CSA, or mean EGJ-DI between the POEM and LHM groups.
Table 1:
FLIP distensibility measurements taken before and during surgical myotomy
| Measurement | Time Point | ||
|---|---|---|---|
| Pre-Op | Induction | Final | |
| Cross-Sectional Area (mm2) | 32.70 ± 12.7 | 35.55 ± 17.0 | 111.28 ±24.0* |
| Pressure (mmHg) | 34.74 ± 14.7 | 30.76 ± 11.9* | 25.29 ± 6.8* |
| Distensibility Index (mm2/mmHg) | 1.10 ± 0.6 | 1.39 ± 0.9 | 4.74 ± 1.62* |
Significant (p<0.05) change from previous time point
Figure 2:
Changes in EGJ cross-sectional area [a] and pressure [b] after surgical myotomy. The mean cross-sectional increased after myotomy in each achalasia subtype, as compared to baseline intraoperative values. The mean pressure decreased in patients with type III achalasia and EGJ outflow obstruction, but not in type I and II patients.
FLIP Panometry
Contractility:
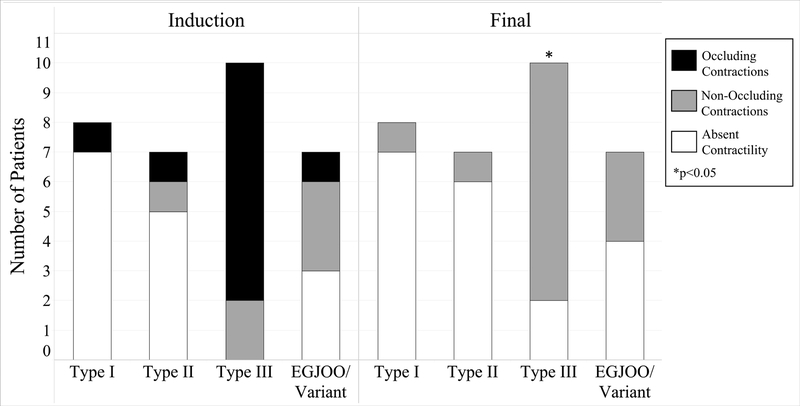
Table 2 shows the contractions and contractile patterns observed at each time point. Contractions were observed in 57% (16/28) of patients during preoperative evaluation and 53% (17/32) at induction. Absent contractility was observed in the majority of type I and II achalasia patients at induction, with only 20% (3/15) demonstrating any contractions (Figure 3). All patients with type III achalasia (10/10) and 57% (4/7) of patients with EGJOO demonstrated contractions at induction. Occluding contractions were seen in 65% (11/17) of patients with contractility at induction, including 8 of 10 patients with type III achalasia.
Table 2:
Esophageal body motility observed prior to and during surgical myotomy
| FLIP Panometry Variable | Time Pointa | ||
|---|---|---|---|
| Pre-Op | Induction | Final | |
| Contractility | 16 (57%) | 17 (53%) | 13 (41%) |
| Occluding Contractions | 11 | 11 | 0* |
| Non-Occluding Contractions | 5 | 6 | 13* |
| Contractile Patternb | 11 (39%) | 13 (41%) | 8 (25%) |
| Repetitive Antegrade Contractions | 8 | 8 | 4* |
| Repetitive Retrograde Contractions | 9 | 10 | 5* |
Significant (p<0.05) change from previous time point
Pre-Op n=28, Induction and Final n=32
Note that some patients exhibited both motility patterns
Figure 3:
FLIP Panometry contractility before and after surgical myotomy. The majority of patients with contractility demonstrated occluding contractions prior to myotomy. All patients with occluding contractions had non-occluding contractions (gray) or absent contractility (white) post-myotomy.
Following myotomy, there was a reduction in the number of patients with occluding contractions (11 to 0, p<0.01). Of the 11 patients with occluding contractions at baseline, 8 had non-occlusive contractions at final assessment, while 3 showed absent contractility. Of the 6 patients with non-occluding contractions at induction, 5 (83%) retained this designation on final assessment, and the remaining patient demonstrated absent contractility.
Contractile Patterns:
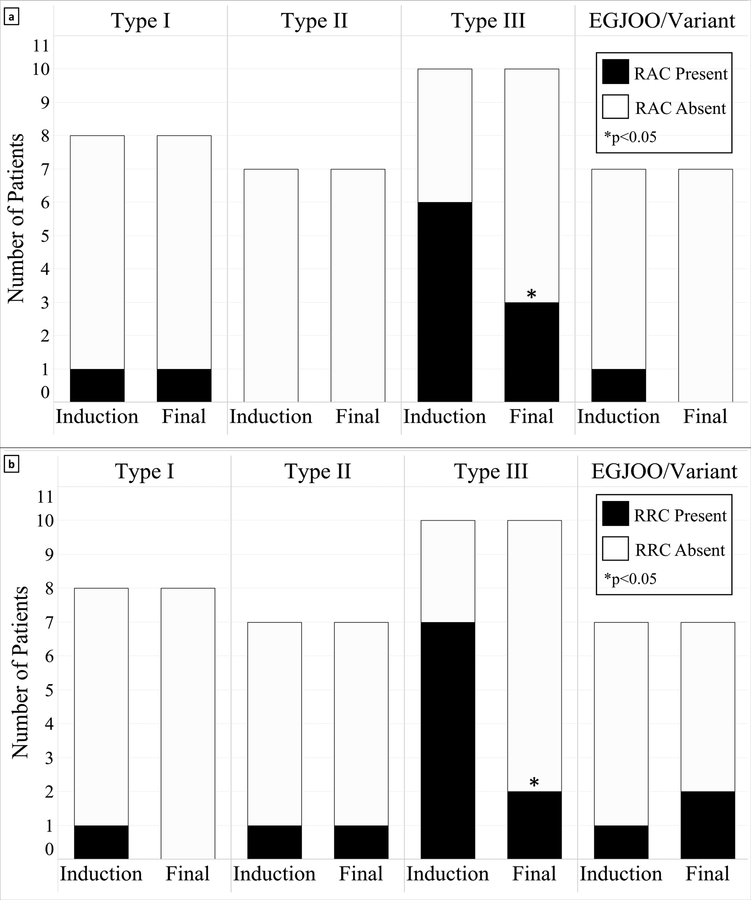
Either a RAC or RRC pattern was seen in 39% (11/28) and 41% (13/32) of patients at preop and induction, respectively. Figure 4 illustrates the distribution of contractile patterns, which varied between achalasia subtypes. The majority of patients with type III achalasia demonstrated a repetitive contractile pattern on initial evaluation: 60% exhibited a RAC pattern and 70% exhibited a RRC pattern. Following myotomy, there was a reduction of patients overall who had a RAC pattern (8 to 4, p < 0.01) or RRC pattern (10 to 5, p < 0.01). The reduction was most pronounced in patients with type III achalasia, where the frequency of patients with RAC and RRC patterns was reduced from 60% to 30% and 70% to 20%, respectively.
Figure 4:
FLIP Panometry contractile patterns before and after surgical myotomy. The number of type III achalasia patients with repetitive antegrade contractions [a] or repetitive retrograde contractions [b] contractions was reduced after surgical myotomy
Discussion
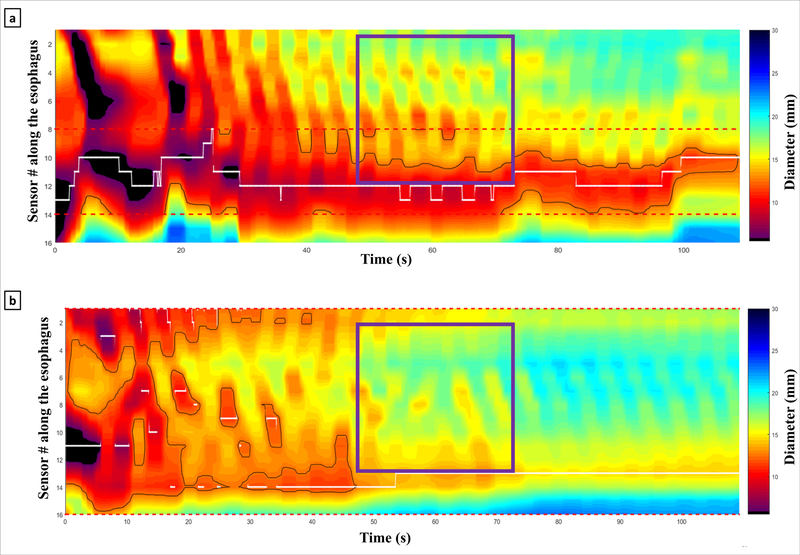
The main finding of this study is that FLIP measurements of esophageal motility were significantly altered by surgical myotomy. FLIP was able to measure esophageal motility intraoperatively, revealing a dramatic reduction in patients with occluding contractions, a RAC pattern, or a RRC pattern after myotomy. These findings are mostly attributable to the type III achalasia patients, the majority of whom demonstrated occluding contractions and a contractile pattern during the baseline evaluation. Figure 5 depicts a typical case in which the contractile vigor was diminished substantially after myotomy. Additionally, the mean DI and CSA increased with surgical myotomy, as previously reported [3, 4]. The mean intrabag pressure decreased after myotomy, but a subgroup analysis revealed this was mainly attributable to patients with type III achalasia and EGJOO.
Figure 5:
Initial [a] and post-myotomy [b] FLIP topograms from a patient with type III achalasia. Notice the diminished contractile vigor in the antegrade contractions (purple boxes) post-myotomy.
This is the first description of intraoperative FLIP esophageal motility changes following surgical myotomy. Given the relatively recent development of FLIP topography, the clinical significance of these findings is yet to be seen [13]. In the early experience of FLIP Panometry, all of the aforementioned motility parameters - the presence of contractions, the contractile vigor, and the presence of contractile patterns - varied considerably among achalasia subtypes. Healthy patients, on the other hand, consistently demonstrated occluding contractions and a RAC pattern [8]. None of healthy controls exhibited a RRC pattern, which was observed in type I, II, and III patients. As such, the RRC pattern may be specific to achalasia or other diseases that affect esophageal innervation and/or motility [14]. Given its association with underlying pathophysiology, the drastic reduction of patients who demonstrated a RRC pattern after myotomy may be a marker of myotomy effectiveness.
The current study also demonstrates that distensibility metrics and motility patterns are largely consistent whether obtained with moderate sedation or under general anesthesia. Despite a small but significant change in the mean intrabag pressure, the mean DI was not statistically different in the preoperative and baseline intraoperative evaluations. The mean CSA was also consistent when comparing these time points. Motility parameters, including the presence of contractions, occluding contractions, and contractile patterns were strikingly similar in the preoperative and baseline intraoperative evaluations, each differing at most by one patient.
Secondary peristalsis is a firmly grounded topic within the literature [15–18], and previous studies have used impedance planimetry technology to study this phenomenon [6–8, 19]. However, this is the first report that directly compares FLIP motility data gathered from the same patient cohort in two anesthetic settings. There are a number of potential mechanisms by which differing anesthetics may alter FLIP measurements. Propofol may suppress nitric oxide-based relaxation in smooth muscle cells and activate several prominent parasympathetic pathways [20, 21]. Midazolam enhances the sympathetic nervous system, and opiates have a well-documented association with myriad esophageal motility abnormalities [22–25]. Other common anesthetic agents, such as amino amides and GABA agonists, are known to specifically affect distension-induced secondary peristalsis of the esophagus [26, 27]. However, it would seem that the pathophysiologic mechanisms of our study population were either unaffected or similarly altered with moderate sedation and general anesthesia. Continued validation of this finding is critical for the development of FLIP as a meaningful intraoperative tool.
This study has some limitations. Although the effects of pneumoperitoneum on distensibility have been described [28–30], data regarding the effect of other intraoperative variables, such as time under anesthesia and patient position, are lacking. Further careful investigation is needed to fully delineate the effects of these variables, as there is a growing call for the standardization of intraoperative FLIP protocols. The study is also limited by the specialized software used in the topography analysis, which is not widely available and thus limits the generalizability of the findings. This problem will be likely addressed in future FLIP software that performs system-integrated topography analysis [13]. A final consideration is the novelty of FLIP Panometry itself. Although the technique has validated diagnostic capabilities [7], it is yet unproven in guiding therapy or post-treatment prognostication.
In conclusion, FLIP distensibility metrics and contractile patterns were consistent in achalasia patients studied with moderate sedation or general anesthesia. Contractile vigor, as indicated by the presence of occluding contractions, was dramatically reduced after surgical myotomy. Achalasia patients under general anesthesia exhibited RAC and RRC patterns on FLIP topography. The frequency of both patterns was diminished following surgical myotomy. These findings merit further investigation of their relation to symptomatic and physiologic outcomes.
Acknowledgements
This work was supported by R01 DK079902 (JEP) and P01 DK117824 (JEP) from the Public Health service.
Ryan A. J. Campagna, MD is supported by T32DK101363 from the National Institutes of Health
Disclosures
Dr. Carlson: Medtronic, Inc. – speaking, consulting, shared intellectual property rights and ownership surrounding functional luminal imaging probe panometry systems, methods, and apparatus. Dr. Hungness: Cook Medical – consulting; Boston Scientific – consulting. Dr. Pandolfino: Medtronic, Inc. – consulting, grant funding, speaking, speaking, consulting, shared intellectual property rights and ownership surrounding functional luminal imaging probe panometry systems, methods, and apparatus; Sandhill Scientific - consulting, speaking; Crospon - stock options; Takeda – speaking; AstraZeneca - speaking. Dr. Soper: Miret Surgical, Inc. – scientific advisory board, stock options; FlexDex, Inc. - scientific advisory board, stock options; MeshSuture, Inc. - scientific advisory board, stock options. Dr. Teitelbaum: Cook Medical – consulting; Boston Scientific - consulting. Drs. Campagna and Holmstrom: None
References:
- 1.Boeckxstaens GE, Zaninotto G, Richter JE (2014) Achalasia. The Lancet 383:83–93 [DOI] [PubMed] [Google Scholar]
- 2.Rohof WO, Hirsch DP, Kessing BF, Boeckxstaens GE (2012) Efficacy of treatment for patients with achalasia depends on the distensibility of the esophagogastric junction. Gastroenterology 143:328–335 [DOI] [PubMed] [Google Scholar]
- 3.Teitelbaum EN, Boris L, Arafat FO, Nicodeme F, Lin Z, Kahrilas PJ, Pandolfino JE, Soper NJ, Hungness ES (2013) Comparison of esophagogastric junction distensibility changes during POEM and Heller myotomy using intraoperative FLIP. Surgical endoscopy 27:4547–4555 [DOI] [PubMed] [Google Scholar]
- 4.Ngamruengphong S, von Rahden BH, Filser J, Tyberg A, Desai A, Sharaiha RZ, Lambroza A, Kumbhari V, El Zein M, Abdelgelil A, Besharati S, Clarke JO, Stein EM, Kalloo AN, Kahaleh M, Khashab MA (2016) Intraoperative measurement of esophagogastric junction cross-sectional area by impedance planimetry correlates with clinical outcomes of peroral endoscopic myotomy for achalasia: a multicenter study. Surgical endoscopy 30:2886–2894 [DOI] [PubMed] [Google Scholar]
- 5.Teitelbaum EN, Soper NJ, Pandolfino JE, Kahrilas PJ, Hirano I, Boris L, Nicodème F, Lin Z, Hungness ES (2015) Esophagogastric junction distensibility measurements during Heller myotomy and POEM for achalasia predict postoperative symptomatic outcomes. Surgical endoscopy 29:522–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carlson DA, Lin Z, Rogers MC, Lin CY, Kahrilas PJ, Pandolfino JE (2015) Utilizing functional lumen imaging probe topography to evaluate esophageal contractility during volumetric distention: a pilot study. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society 27:981–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carlson DA, Kahrilas PJ, Lin Z, Hirano I, Gonsalves N, Listernick Z, Ritter K, Tye M, Ponds FA, Wong I, Pandolfino JE (2016) Evaluation of Esophageal Motility Utilizing the Functional Lumen Imaging Probe. The American journal of gastroenterology 111:1726–1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carlson DA, Lin Z, Kahrilas PJ, Sternbach J, Donnan EN, Friesen L, Listernick Z, Mogni B, Pandolfino JE (2015) The Functional Lumen Imaging Probe Detects Esophageal Contractility Not Observed With Manometry in Patients With Achalasia. Gastroenterology 149:1742–1751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eckardt VF (2001) Clinical presentation and complications of achalasia. Gastrointestinal Endoscopy Clinics of North America 11:281–292 [PubMed] [Google Scholar]
- 10.Kahrilas PJ, Bredenoord AJ, Fox M, Gyawali CP, Roman S, Smout AJPM, Pandolfino JE (2015) The Chicago Classification of esophageal motility disorders, v3.0. Neurogastroenterology & Motility 27:160–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campagna RAJ, Hungness ES (2018) Treatment of Idiopathic Achalasia with Per-Oral Esophageal Myotomy. Tech Gastrointest Endosc 20:114–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vaziri K, Soper NJ (2008) Laparoscopic Heller myotomy: technical aspects and operative pitfalls. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract 12:1586–1591 [DOI] [PubMed] [Google Scholar]
- 13.Hirano I, Pandolfino JE, Boeckxstaens GE (2017) Functional Lumen Imaging Probe for the Management of Esophageal Disorders: Expert Review From the Clinical Practice Updates Committee of the AGA Institute. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association 15:325–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Herwaarden MA, Samsom M, Smout AJ (2001) Prolonged manometric recordings of oesophagus and lower oesophageal sphincter in achalasia patients. Gut 49:813–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bardan E, Xie P, Aslam M, Kern M, Shaker R (2000) Disruption of primary and secondary esophageal peristalsis by afferent stimulation. American journal of physiology Gastrointestinal and liver physiology 279:G255–261 [DOI] [PubMed] [Google Scholar]
- 16.Pandolfino JE, Shi G, Zhang Q, Kahrilas PJ (2005) Absence of a deglutitive inhibition equivalent with secondary peristalsis. American journal of physiology Gastrointestinal and liver physiology 288:G671–676 [DOI] [PubMed] [Google Scholar]
- 17.Sifrim D, Janssens J (1996) Secondary peristaltic contractions, like primary peristalsis, are preceded by inhibition in the human esophageal body. Digestion 57:73–78 [DOI] [PubMed] [Google Scholar]
- 18.Takeda T, Nabae T, Kassab G, Liu J, Mittal RK (2004) Oesophageal wall stretch: the stimulus for distension induced oesophageal sensation. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society 16:721–728 [DOI] [PubMed] [Google Scholar]
- 19.Rao SS, Hayek B, Summers RW (1995) Impedance planimetry: an integrated approach for assessing sensory, active, and passive biomechanical properties of the human esophagus. The American journal of gastroenterology 90:431–438 [PubMed] [Google Scholar]
- 20.Miyawaki I, Nakamura K, Terasako K, Toda H, Kakuyama M, Mori K (1995) Modification of endothelium-dependent relaxation by propofol, ketamine, and midazolam. Anesthesia and analgesia 81:474–479 [DOI] [PubMed] [Google Scholar]
- 21.Deutschman CS, Harris AP, Fleisher LA (1994) Changes in heart rate variability under propofol anesthesia: a possible explanation for propofol-induced bradycardia. Anesthesia and analgesia 79:373–377 [DOI] [PubMed] [Google Scholar]
- 22.Win NN, Fukayama H, Kohase H, Umino M (2005) The different effects of intravenous propofol and midazolam sedation on hemodynamic and heart rate variability. Anesthesia and analgesia 101:97–102, table of contents [DOI] [PubMed] [Google Scholar]
- 23.Aadam AA, Abe S (2018) Endoscopic submucosal dissection for superficial esophageal cancer. Diseases of the esophagus : official journal of the International Society for Diseases of the Esophagus [DOI] [PubMed] [Google Scholar]
- 24.Kraichely RE, Arora AS, Murray JA (2010) Opiate-induced oesophageal dysmotility. Alimentary pharmacology & therapeutics 31:601–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ravi K, Murray JA, Geno DM, Katzka DA (2016) Achalasia and chronic opiate use: innocent bystanders or associated conditions? Diseases of the esophagus : official journal of the International Society for Diseases of the Esophagus 29:15–21 [DOI] [PubMed] [Google Scholar]
- 26.Chen CL, Liu TT, Yi CH (2011) Control of esophageal distension-induced secondary peristalsis by the GABA(B) agonist baclofen in humans. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society 23:612–e250 [DOI] [PubMed] [Google Scholar]
- 27.Chen CL, Liu TT, Yi CH (2010) Effects of lidocaine on esophageal secondary peristalsis in humans. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society 22:606–610 [DOI] [PubMed] [Google Scholar]
- 28.Pitt KA, Mayhew PD, Barter L, Pollard R, Kass PH, Marks SL (2017) Consistency and effect of body position change on measurement of upper and lower esophageal sphincter geometry using impedance planimetry in a canine model. Diseases of the esophagus : official journal of the International Society for Diseases of the Esophagus 30:1–7 [DOI] [PubMed] [Google Scholar]
- 29.Teitelbaum EN, Soper NJ, Pandolfino JE, Kahrilas PJ, Boris L, Nicodeme F, Lin Z, Hungness ES (2014) An extended proximal esophageal myotomy is necessary to normalize EGJ distensibility during Heller myotomy for achalasia, but not POEM. Surgical endoscopy 28:2840–2847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nathanson LK, Brunott N, Cavallucci D (2012) Adult esophagogastric junction distensibility during general anesthesia assessed with an endoscopic functional luminal imaging probe (EndoFLIP(R)). Surgical endoscopy 26:1051–1055 [DOI] [PubMed] [Google Scholar]