Abstract
Microphthalmia/TFE (MiT) transcription factors such as TFEB and TFE3, are emerging as key regulators of innate immunity and inflammation. Rapid progress in the field requires a focused update on the latest advances. Recent studies show that TFEB and TFE3 function in innate immune cells to regulate antibacterial and antiviral responses downstream of phagocytosis, IFN-γ, LPS, and adenosine receptors. Moreover, overexpression of TFEB or TFE3 can drive inflammation in vivo, such as in atherosclerosis, but in other scenarios, can perform anti-inflammatory functions. MiT factors may constitute potential therapeutic targets for a broad range of diseases; however, to harness their therapeutic potential, sophisticated ways to manipulate MiT factor activity safely and effectively must be developed.
Keywords: TFEB, TFE3, transcription, inflammation, innate immunity, infection
MiT Factors Regulate Innate Immunity
Transcription of pro- and anti-inflammatory genes in the innate immune system is tightly regulated. Appropriate induction of gene expression occurs during infection or injury and is essential for organismal homeostasis and innate immunity. Inappropriate gene expression can drive inflammation, autoimmune disease, and oncogenesis [1,2]. Thus, an assessment of the transcription factors (TFs) that regulate innate immunity and inflammation is essential for understanding homeostasis and pathogenesis.
The MiT family of TFs includes microphthalmia transcription factor (MITF), which binds to enhancer 3 (TFE3), transcription factor EC (TFEC), and transcription factor EB (TFEB)[3]. MiT factors are known to play important roles in melanoma (e.g. MITF [4]) and in autophagy, lysosomal biogenesis, and lipid catabolism (e.g. TFEB and TFE3 [5–10]). Several recent and excellent review articles have highlighted the basic molecular biology of MiT factors including their functions during cellular stress, nutrient limitation [3,11–13], and innate immunity [14,15]. However, the rapid pace of progress in this area requires an update on the most recent advances, placing TFEB and TFE3 downstream of important signaling pathways in innate immune cells, such as the toll-like receptor (TLR) and γ interferon (IFN-γ) pathways (Figure 1). These advances also provide a better understanding of the TFEB- and TFE3-mediated regulation of innate immune cell function, including macrophage polarization and cytokine production (Key Figure, Figure 2), and may provide a link between TFEB and TFE3 and the gut-brain axis (Glossary and Box 2). In addition to these advances, this review highlights the recently established relevance of TFEB and/or TFE3 in several mammalian inflammatory diseases (Table 1). Indeed, MiT factors are rapidly emerging as key contributors to the regulation of innate immunity and inflammation through several mechanisms.
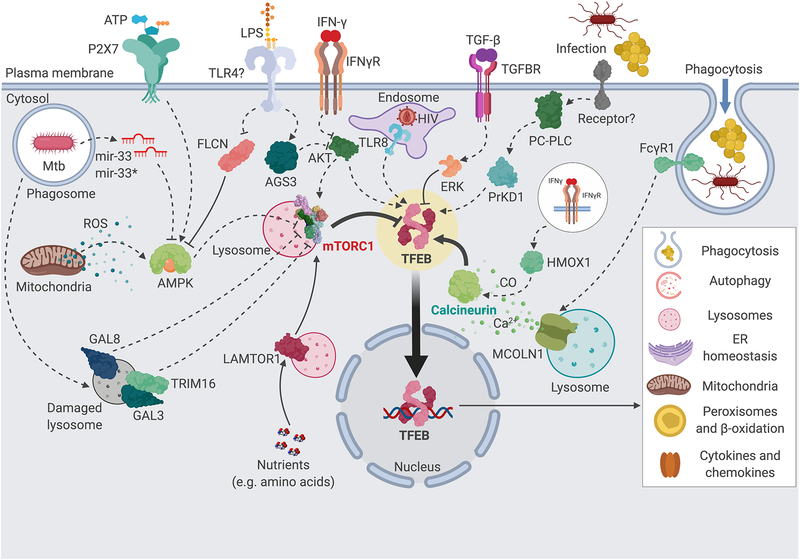
Figure 1. Upstream regulation of TFEB in murine and human macrophages.
TFEB (highlighted in yellow) is known to be regulated by a large number of stimuli and pathways in mammalian macrophages. At the center, mTORC1 (red) and calcineurin (green) are the two major post-translational regulators of TFEB. Several upstream pathways feed into the regulation of mTORC1 and calcineurin, as indicated. Dashed arrows indicate unknown mechanisms or several steps; full arrows indicate direct interactions. Activated TFEB induces many downstream cellular processes important for phagocyte function, including phagocytosis, lysosomes and autophagy, restoration of ER and mitochondrial homeostasis, lipid and glucose metabolism, and cytokine and chemokine production. Transcriptional mechanisms of TFEB regulation are not represented. GAL3, 8, galectins 3 and 8; TRIM16, tripartite motif containing 16; AMPK, AMP-activated protein kinase; FLCN, folliculin; TLR4, Toll-like receptor 4; TLR8, Toll-like receptor 8; LPS, lipopolysaccharide; AGS3, activator of G-protein signaling 3; AKT, v-Akt murine thymoma viral oncogene homolog; mTORC1, mechanistic target of rapamycin, complex 1; LAMTOR1, late endosomal/lysosomal adaptor, MAPK and mTOR activator 1; IFN-γ, interferon γ; IFNγR, INF-γ receptor; HIV, human immunodeficiency virus; TFEB, transcription factor EB; TGF-β, transforming growth factor β; TGFBR, TGF-β receptor; ERK, extracellular signal regulated protein kinase; PC-PLC, phosphatidyl choline-directed phospholipase C; PrKD1, protein kinase D1; FcγR1, antibody Fcγ fragment receptor 1; HMOX1, heme oxidase 1; MCOLN1, mucolipin 1; ROS, reactive oxygen species; CO, carbon monoxide; Mtb, M. tuberculosis. This figure was created using BioRender (https://biorender.com/).
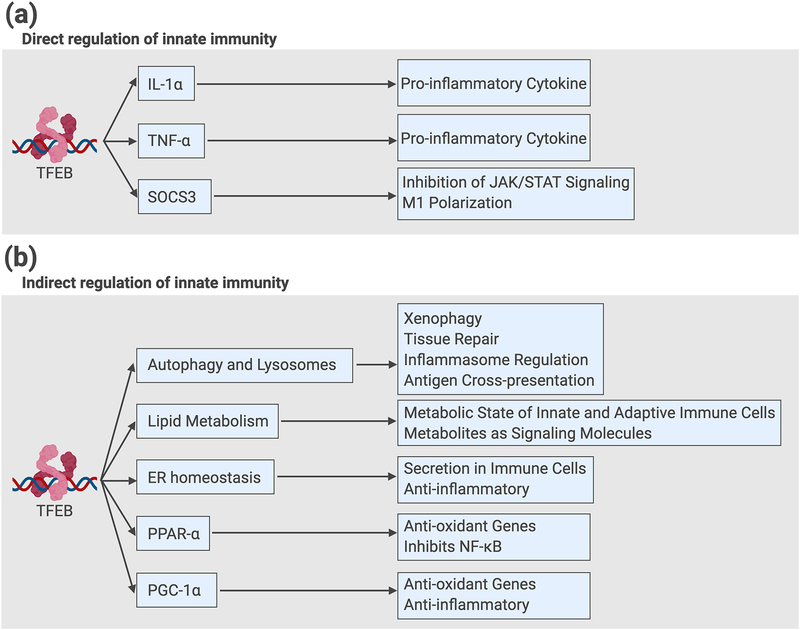
Key Figure, Figure 2. Direct and Indirect Regulation of Innate Immunity and Inflammation by TFEB in mammalian cells.
(a) Examples of direct mechanisms: TFEB and TFE3 bind directly to target gene promoters encoding cytokines IL-1β and TNF-α, and SOCS3, a negative regulator of JAK/STAT signaling and pro-classical activation factor. (b) Examples of cellular processes and gene targets that mediate indirect regulation of innate immune cell function: TFEB and TFE3 promote autophagy and lysosomal expansion, lipid catabolism, and ER homeostasis. Collectively, these processes function to reduce inflammation in a cell-intrinsic manner. In addition, TFEB directly induces expression of PPAR-α and PGC-1α, which promote mitochondrial biogenesis, inhibit NF-κB, and reduce reactive oxygen species (ROS) production and inflammation. TFEB, transcription factor EB; IL-1α, interleukin 1α; TNF-α, tumor necrosis factor α; SOCS3, suppressor of cytokine signaling 3; JAK, Janus kinase; STAT, signal transducer and activator of transcription; PPAR-α, peroxisome proliferator activator receptor α; PGC-1α, PPAR-γ coactivator 1α; NF-κB, nuclear factor κB. This figure was created using BioRender (https://biorender.com/).
Box 2: TFEB and host defense in Caenorhabditis elegans.
C. elegans can recognize infections by bacterial pathogens and induce pathogen-specific transcriptional defense programs independently of Toll-like receptor (TLR) and nuclear factor κB (NF-κB) pathways [56]. The first bioinformatic study implicating TFEB in the induction of host defense genes revealed an over-representation of M-box in promoters of C. elegans genes induced by Staphylococcus aureus infection; this suggested potential transcriptional regulation by an MiT factor. C. elegans harbors one MiT factor gene, hlh-30 (ceTFEB) and 80% of the host innate immune response was abrogated in ceTFEB mutants compared to WT; affected genes included autophagy and lysosomal, as well as antimicrobial genes, contributing to infection survival as revealed via RNAi and mutant analyses [36]. GFP-tagged ceTFEB translocated into somatic cell nuclei shortly after infection, suggesting that ceTFEB activation was an early event in the host response [36]. Murine RAW264.7 cells and primary BMDMs showed TFEB nuclear translocation promptly after S. aureus infection [36]. Moreover, S. aureus-infected RAW264.7 cells with silenced Tfeb were impaired in the induction of cytokine and chemokine genes including Il1b, Il6, and Tnf compared to siRNA controls (qRT-PCR) [36]. TFEB was thus deemed an evolutionarily conserved host defense transcription factor. ceTFEB was also implicated in host defense against bacterial pore-forming toxins [57]. Nematodes lacking autophagy by mutation (e.g. BECLIN1 homolog bec-1 or ATG4 double mutant atg-4.1; atg-4.2) and intestine-specific knockdown (e.g. MAP1LC3B homologs lgg-1, lgg-2, and lgg-3) exhibited defective survival due to intoxication with purified Cry toxins made by Bacillus thuringiensis, compared with vector-treated controls [57]. Consistent with ceTFEB’s known roles in autophagy and host defense [36,49], ceTFEB mutants were more susceptible to Cry-mediated killing [57]. Thus, ceTFEB is essential for defense against pathogenic bacteria and isolated pore-forming toxins.
Other studies showed that ceTFEB is essential for behavioral host defense via production of INS-11 in the intestinal epithelium: INS-11 (insulin-like neuropeptide involved in gut-to-nervous system communication) elicits protective behavioral responses in C elegans [58,59]. Enteric infection with P. aeruginosa induced an ins-11 transcriptional GFP reporter in the intestinal epithelium compared with noninfected controls [58]. In vivo, mutated ins-11 eliminated aversive behavior towards P. aeruginosa, including derepression of tph-1 (encoding tryptophan hydroxylase) in ASI neurons, and of ins-6 (encoding an insulin-like neuropeptide linked to aversive learning) in ADF neurons, compared to WT (via fluorescent transcriptional reporters) [58]. A ceTFEB mutation abrogated intestinal induction of ins-11 by P. aeruginosa, increasing tph-1 and ins-6 expression and defective aversive learning compared to WT [58]. Using the same ceTFEB mutant and ins-11 fluorescent reporter, an independent study showed that ceTFEB was required for ins-11 intestinal expression during infection by P. aeruginosa or Serratia marcescens [59]. During intestinal infection, ceTFEB induces neuropeptide production by the intestine and modulates behavior, and ceTFEB is an essential component of the gut-nervous system axis in nematodes. It will be important to determine whether TFEB and TFE3 contribute to the mammalian brain/gut/microbiota axis [60].
In an RNAi screen [61], RNAi of dkf-1/DKF-1 (homolog of mammalian protein kinase D (PrKD) activated by diacylglycerol (DAG) via phospholipase C (PLC) [62]) prevented GFP-ceTFEB nuclear import and ceTFEB target gene induction (e.g. ilys-2 and lys-5, by RT-qPCR) during S. aureus infection of C elegans [61]. Accordingly, knockdown of plc-1 (PLCε) or egl-30 (Gαq) abrogated ceTFEB nuclear translocation and target gene induction compared with control animals [61]. Chemical inhibition and knockdown experiments also showed that the PLC-PrKD pathway was required for nuclear translocation of GFP- or FLAG- tagged TFEB in stably transfected RAW264.7 cells infected with S. aureus or S. enterica Typhimurium [61]. Thus, the PLC-PrKD-TFEB pathway is evolutionarily conserved (Figure 1). A second pathway of AMP-activated kinase (AMPK) was recently described: simultaneous deletion of aak-1 and aak-2 (encoding AMPK α1 and α2 catalytic subunits), decreased nuclear translocation of ceTFEB-GFP during S. aureus infection in C elegans relative to WT controls [31]. RNAseq revealed that roughly a sixth of ceTFEB-dependent S. aureus-induced host defense genes were dependent on aak-1 and aak-2 compared to WT, causing reduced survival from infection for aak-1;aak-2 mutants relative to WT [31]. AMPK was thus required for full activation of ceTFEB during infection, via an unknown mechanism.
Table 1:
MiT Factors and Inflammatory Diseases
| Disease | Function/observation | Mechanism | References |
|---|---|---|---|
| Atherosclerosis | Anti-inflammatory | ||
| Murine peritoneal macrophage TFEB activated by cholesterol crystals. | Cholesterol crystals damage lysosomes thus activating TFEB. | [34] | |
| Genetic and chemical induction of TFEB in macrophages protects mice from atherosclerosis. | Induction of autophagy and degradation of atherosclerotic plaques. | [34] | |
| Endothelial TFEB is necessary for VEGF signaling and proliferation of murine and human endothelial cells (EC). | TFEB was required for VEGF signaling in mice. TFEB functioned cell-autonomously in human and murine EC to promote EC proliferation in vitro and endothelial repair in vivo. | [68] | |
| Endothelial TFEB induces antioxidant genes and protects mice from vascular disease. | TFEB induces expression of HMOX1 and SOD1 in endothelium, reducing ROS and preventing NF-κB induction and vascular disease in db/db mice. | [69] | |
| Colitis | Anti-inflammatory | ||
| Deletion of Tfeb in the intestinal epithelium enhanced susceptibility of mice to chemically-induced colitis. | Mechanism unknown; suspected to be mediated by decreased TFEB-dependent expression of apolipoprotein A1. | [70] | |
| Cystitis | Pro-inflammatory? | ||
| Activated by pro-inflammatory protease-activated receptors (PARs) in bladder epithelium of mice. | Unknown mechanism connecting PARs to TFEB | [55] | |
| Hepatitis | Anti-inflammatory | ||
| Knockdown of Tfeb exacerbated ethanol-induced liver steatosis and injury in mice, while Tfeb overexpression ameliorated them. | Ethanol activated mTORC1, inhibiting TFEB and autophagy. Induction of autophagy increased β-oxidation of fatty acids and decreased triglycerides. | [71] | |
| Pancreatitis | Anti-inflammatory | ||
| Deletion of Tfeb in pancreatic acinar cells enhanced cerulein-induced pancreatitis in mice; Tfeb Tfe3 double knockouts developed spontaneous pancreatitis. Human pancreatitis biopsies showed decreased expression of TFEB. | Cerulein decreased autophagic flux and TFEB expression in mice; precise connection with inflammation is not well understood. | [72] | |
Novel Regulatory Mechanisms Upstream of MiT Factors in Innate Immune Cells
Overall, two major inputs are known to regulate TFEB and TFE3 in a broad range of cell types and organisms (Figure 1). First, mechanistic target of rapamycin, complex 1 (mTORC1) is a negative regulator that phosphorylates conserved Ser/Thr residues on both factors [10,16–18]. Such phosphorylation enables 14-3-3 protein binding, which restricts TFEB and TFE3 to the cytosol [16]. Second, protein phosphatase 3 (calcineurin) is the most important enzyme that removes mTORC1-mediated phosphorylation, thus allowing TFEB and TFE3 import into the nucleus [19]. Inhibition of mTORC1 or activation of calcineurin are sufficient to trigger TFEB and TFE3 nuclear import [16–19]. Thus, mTORC1 and calcineurin act in opposition: as inhibitor and activator of TFEB function, respectively. Many signaling pathways are known to impact mTORC1 activity under various conditions (e.g. cellular energy status). Regulation of calcineurin, activated by calmodulin and cytosolic Ca2+, is similarly complex [20]. Dual regulation by mTORC1 and calcineurin enables TFEB (and TFE3) to integrate multiple inputs (Figure 1) and feedback loops (Box 1) to affect cellular and organismal physiology in ways that are not trivially predictable. The following sections include a description of recent advances in the upstream mechanisms controlling TFEB and TFE3, with a focus on mammalian phagocytes.
Box 1: Early clues of immunological roles for TFEB and TFE3.
TFEB and TFE3 were linked to the immune system upon their discovery, in a phage display screen using cDNA from human B cells to identify proteins that bound to the μE3 enhancer of the human immunoglobulin heavy chain locus [52,53]. Subsequently, T-cell specific expression of a dominant negative construct that inhibited TFEB and TFE3 in transgenic mice caused defective expression of cluster of differentiation 40 ligand (CD40L) compared with WT, leading to a defect in plasma B cell differentiation [12]. Moreover, in vitro gel shift and promoter-luciferase assays using primary murine CD4+ splenic T cells showed that TFEB and TFE3 bound to E-boxes in the Cd40lg promoter to induce its expression [12]. Thus, TFEB and/or TFE3 were directly linked to CD40L-mediated T cell help to drive B cell differentiation [54].
Subsequent reports uncovered links between TFEB and inflammation. The first study used mice whose bladders were infused with peptide agonists specific for three distinct protease-activated receptors (PARs), as a model for cystitis [55]. This study used protein-DNA microarrays representing 345 consensus binding sequences to identify activated TFs in nuclear extracts from the inflamed bladder mucosae. The only transcription factor whose binding was activated by all three peptides was TFEB. Immunohistochemical detection of TFEB in inflamed tissue and additional functional assays supported the idea that TFEB was activated downstream of PARs [55]. This was the first time that TFEB was proposed as a potential therapeutic target for inflammatory disorders.
Recently, another study showed that loss of cytosolic DNAse three-prime repair exonuclease 1 (TREX1) activated TFEB and lysosomal biogenesis in mouse and human fibroblasts [44]. Trex−/− mouse cells exhibited mTORC1 inhibition, measured by immunoblot of phosphorylated S6 kinase (RPS6KB) and EIF4EBP1, constitutive TFEB nuclear localization (by immunofluorescence), and lysosomal expansion (Lysotracker and LAMP1 staining). This was proposed as a trigger for interferon-independent activation of antiviral genes, such as Ifit1, Ifit2, and Ifit3, in Trex1−/− mouse fibroblasts via a pathway involving stimulator of interferon genes (STING)-TANK binding kinase 1 (TBK1)- interferon regulatory factor 3/7 (IRF3/7) [44]. However, the molecular mechanisms linking TREX1 to TFEB, and lysosomal biogenesis to the STING pathway, remain unknown [44].
Phagocytosis
A key study showed that phagocytosis is sufficient to activate TFEB. Specifically, phagocytosis of IgG-opsonized latex beads activated TFEB in murine RAW264.7 cells and primary bone-marrow derived macrophages (BMDMs), as measured by TFEB nuclear translocation in live cells using fluorescence microscopy and by the induction of TFEB target genes (e.g. Atp6v1h, Ctsb, Map1lc3b) by qRT-PCR [21]. This study showed that protein kinase Syk is activated downstream of the Fcγ receptor (FcγR), even if phagocytosis is not complete (a phenomenon called frustrated phagocytosis [21]). TFEB activation occurred only in cells that completed phagocytosis, indicating that Syk activation was not sufficient to activate TFEB [21]. By contrast, the same study found that siRNA-mediated silencing of mucolipin 1 (MCOLN1, an important ligand-gated Ca2+ channel in lysosomes [22]) prevented phagocytosis-mediated TFEB activation by phagocytosis of opsonized beads; this suggested that lysosomal Ca2+ release was a required step in this process [21]. The functional consequence of TFEB activation resulted in increased killing of subsequently phagocytosed bacteria, as evidenced from bacterial survival in untreated and in Tfeb-silenced RAW264.7 cells in vitro [21]. Therefore, MCOLN1-dependent activation of TFEB by phagocytosis (Figure 1) primed macrophages to more effectively kill ingested bacteria. However, the precise mechanism linking phagocytosis to MCOLN1 remains unknown.
Lysosomal damage
Lysosome-permeabilizing agent L-leucyl-L-leucine methyl ester (LLOMe) has been reported to induce TFEB nuclear translocation (visualized via immunofluorescence) in HeLa cells [23]. The mechanism of TFEB activation during lysosomal damage was shown to be mediated by E3 ubiquitin ligase tripartite motif containing 16 (TRIM16). For instance, co-localization analysis and affinity precipitation revealed interactions between TRIM16 and galectin GAL3, as well as their recruitment to damaged endomembranes in HeLa, HEK293T, and THP-1 cells exposed to LLOMe [23]. Moreover, similar biochemical and cell biology experiments showed that GAL3-mediated TRIM16 recruitment led to the localization of autophagic machinery (unc-52-like kinase 1, ULK1, Beclin1, and autophagy-related gene 16-like 1, ATG16L1) to damaged lysosomes; and furthermore, TRIM16 could interact with mTORC1 subunits and with calcineurin and TFEB [23]. TRIM16 deletion promoted TFEB nuclear translocation in HeLa cells, in LLOMe-treated and -untreated cells alike, presumably because of defective lysosome quality control in TRIM16−/− cells [23]. Thus, GAL3 and TRIM16 emerged as key markers of defective lysosome integrity, inhibiting mTORC1, and triggering both TFEB activation and autophagy of defective organelles [23]. Subsequent work showed similar mechanisms of TFEB activation involving galectins 8 (GAL8) and 9 (GAL9) during lysosomal damage caused by glycyl-L-phenylalanine 2-naphthylamide (GPN) in primary human peripheral blood monocyte-derived macrophages (MDMs, Figure 1) [24]. Moreover, TFEB nuclear translocation in GPN-treated murine BMDMs was partially impaired in Lgals8−/− cells relative to wild type (WT) cells [24]. Thus, these studies revealed mechanisms linking lysosomal damage to TFEB activation via galectins and mTORC1 (Figure 1).
Interferon γ
Several studies support a role for TFEB downstream of IFN-γ. For example, during infection of mice with influenza virus, ELISA and qRT-PCR of IFN-γ in sorted alveolar F4/80+ CD11c+ cells (alveolar macrophages, AMs) showed initial IFN-γ production and decreased expression of macrophage phagocytic receptor with collagenous structure (MARCO) relative to uninfected animals [25]. At later times, IFN-γ secretion was reduced and MARCO expression was increased in the infected animals [25]. RNA-seq of AMs comparing early and late times identified TFEB as a potentially late-activated TF [25]. Moreover, network analysis of the differentially expressed genes suggested that genes encoding IFN-γ, AKT, and TFEB were functionally linked [25]. Consistent with this, IFN-γ-induced decreases in MARCO expression 24h post-infection in primary human alveolar-like monocyte-derived macrophages (AM-MDMs) was prevented by chemical activation of AKT (as evidenced from immunoblot and cell staining) [25]. Moreover, TFEB knockdown reversed this effect of AKT activation, suggesting that AKT required TFEB to induce MARCO during IFN-γ treatment [25]. ChIP-qPCR of the MARCO promoter using anti-TFEB antibodies showed that TFEB directly induced MARCO transcription in AM-MDMs [25]. Moreover, relative to controls, TFEB knockdown versus overexpression in THP-1 cells, caused lower and higher MARCO qRT-PCR expression, respectively. Together, these results suggested that influenza virus might downregulate MARCO expression through IFN-γ by inactivating AKT and TFEB (Figure 1).
However, a study using murine RAW264.7 and primary peritoneal macrophages (PMs) showed that IFN-γ could activate TFEB [26]. Immunofluorescence and cell fractionation showed that 3h of IFN-γ treatment induced TFEB nuclear translocation [26]. In contrast, PMs from Hmox1−/− mice, which lack the gene encoding IFN-γ-induced heme oxidase 1 (HMOX1), showed defective IFN-γ-triggered TFEB nuclear localization, as well as TFEB target expression (immunoblotting of SQSTM1, BECN1, LAMP1, and ATG5); this indicated that HMOX1 was required for IFN-γ to activate TFEB [26]. Moreover, chemical inhibition of HMOX1 prevented IFN-γ-triggered TFEB nuclear translocation in RAW264.7 cells, while chemical elicitation of carbon monoxide (CO), a product generated by HMOX1, reversed the effect of HMOX1 inhibition on TFEB activation by IFN-γ. These results suggested that CO was necessary and sufficient to induce TFEB activation via IFN-γ in vitro [26]. Furthermore, Ca2+ imaging and chelation experiments, in addition to chemical and genetic inhibition of calcineurin, showed that Ca2+ release and calcineurin were required for CO-elicited TFEB nuclear localization in this model [26]. Thus, this study suggested that TFEB might be activated via IFN-γ through HMOX1-generated CO, Ca2+ efflux, and calcineurin in PMs (Figure 1).
The reasons for these conflicting results are not clear. The studies in [25] involved human AM-MDMs and 24h stimulation with 20 IU/ml IFN-γ. Those in [26] involved murine PMs cells and 3h stimulation with 200 IU/ml IFN-γ. It is possible that human and murine macrophages diverge in their regulation of TFEB by IFN-γ, or that IFN-γ dose and timing are important. Nonetheless, a third study examined TFEB activation in murine RAW264.7 cells using 4h treatment with approximately 170 IU/ml IFN-γ, but did not observe TFEB activation [21]. Therefore, more work is required to fully understand the regulation of TFEB via IFN-γ, as well as the functional outcomes of this specific pathway.
Lipopolysaccharide
Lipopolysaccharide (LPS) is a bacterial ligand for several mammalian pattern-recognition receptors, such as TLR4, murine Caspase-11, and human Caspase-4 [27]. Treatment of murine BMDM, RAW264.7, or primary microglial cells caused TFE3 nuclear import, as well as the expression of TFE3 target LAMP1 (as evidenced from microscopy, cell fractionation and immunoblotting) [28]. ChIP-seq of endogenous TFE3 in LPS-treated RAW264.7 cells compared with controls revealed multiple LPS-specific TFE3 genomic binding sites, and microarray analysis of LPS-stimulated mouse Tfe3−/− TfebΔLysM double mutant BMDMs revealed a large group of innate immunity genes whose expression was decreased compared with Tfebflox/flox controls [28]. LPS-stimulated Tfe3 or Tfeb single knockout RAW264.7 and BMDM cells produced markedly less TNF-α, IL-6, colony stimulating factor 1 (CSF1, or M-CSF), and 2 (CSF2, or GM-CSF) relative to WT cells, suggesting non-redundant functions for TFEB and TFE3 [28]. Of note, phosphorylated AKT, mTOR, and mTORC1 targets TFE3, RPS6KB, and EIF4EBP1 failed to show mTORC1 inhibition by LPS via immunoblotting, thus ruling out the possibility that LPS activated TFE3 and TFEB by inhibiting mTORC1. However, the precise mechanism of their activation by LPS remained undefined (Figure 1) [28].
A contemporaneous study showed that LPS treatment of human THP-1 and murine BMDM increased expression of activator of G-protein signaling 3 (AGS3), compared to untreated cells (immunoblotting) [29]. In transfected THP-1 cells sorted by AGS3-GFP expression, AGS3 expression directly correlated with TFEB nuclear localization, as well as target gene expression (LAMP1, CTSD, ATP6V1H) and higher lysosomal proteolysis of DQ-BSA (as evidenced from microscopy, cell fractionation, immunoblotting, and qRT-PCR) [29]. Conversely, in AGS3 knockout Gpsm−/− murine BMDM, defective LAMP1 protein expression was reported relative to WT at baseline [29]. Together, these results implicated AGS3 as a positive regulator of TFEB (Figure 1). LPS-triggered AGS3 protein expression was abrogated in Myd88−/− and Ticam−/− (TRIF) immortalized BMDMs, suggesting that TLR4-mediated LPS detection might be required for AGS3 induction [29]. Furthermore, AGS3 protein expression inversely correlated with phosphorylation of AKT, TSC2, and RPS6KB, suggesting that AGS3 might repress the AKT-mTORC1 signaling pathway [29]. Thus, a model was proposed whereby LPS activated TLR4, inducing AGS3, inhibiting AKT and mTORC1, and thus activating TFEB (Figure 1) [29]. However, TLR4 involvement, as well as the mechanisms for AGS3 induction and for a link between AGS3 and AKT were not directly shown [29].
Recently, studies uncovered a role for folliculin (FLCN) in LPS-triggered TFEB and TFE3 activation in murine macrophages. In one key study, LPS stimulation of BMDMs caused a decrease in FLCN protein expression (compared to control cells), concomitant with increased TFE3 nuclear translocation [30]. Conversely, Flcn transduction in BMDM prevented TFE3 nuclear translocation following LPS stimulation. Moreover, myeloid-specific deletion of Flcn (FlcnΔLysM) resulted in constitutive TFE3 nuclear localization in BMDMs, lysosomal gene induction (Lamp1, Ctsz, Ctsd, Mcoln1, Tpp1, Clcn1, qRT-PCR), and lysosomal expansion (LAMP2 immunofluorescence) relative to Flcnflox/flox controls [30]. However, FlcnΔLysM BMDMs did not constitutively express higher amounts of TNF-α, IL-6, IL-12, or CCL2 than controls. Nonetheless, exaggerated secretion of these cytokines was reported following LPS or CpG stimulation, suggesting that although not constitutively activated, FlcnΔLysM BMDMs were hyperresponsive to TLR ligands [30]. In vivo, FlcnΔLysM mice exhibited spontaneous alopecia, anemia, splenomegaly, and hepatomegaly by 8 months of age; moreover, whole blood analysis and cell sorting showed expansion of the monocyte precursor and neutrophil populations. Histology revealed severe macrophage infiltration in spleen, bone marrow, skin, liver, lymph nodes, and white adipose tissue [30]. Of relevance, these phenotypes were reversed by co-deletion of Tfe3 in FlcnΔLysM Tfe3−/− mice, indicating that activation of TFE3 upon loss of Flcn in myeloid cells had dramatic systemic inflammatory effects in vitro and in vivo [30]. Therefore, it FLCN has been proposed as an LPS-repressed negative regulator of TFE3 that can dampen inflammation in macrophages, although the molecular mechanisms of TFE3 control by FLCN has not been demonstrated. Additionally, overexpression of Rragd in FlcnΔLysM BMDM compared to WT was noted. Indeed, enhanced Rragd expression in FlcnΔLysM BMDMs correlated with activated mTORC1 (as evidenced from the expression of phospho-RPS6KB via immunoblotting, as well as from increased cellular proliferation, relative to controls) [30]. Such effect was diminished by Tfe3 silencing in vitro; this suggested the existence of a negative feedback loop where TFE3 induced the activation of its inhibitor mTORC1 via Rragd in mouse BMDMs (Box 1) [30].
The proposed anti-inflammatory role of FLCN through TFE3 inhibition was supported by a contemporaneous study showing that Flcn deletion in mouse embryonic fibroblasts (MEFs) caused constitutive TFEB and TFE3 nuclear translocation [31]. Mechanistically, the authors posited that TFEB/TFE3 activation in Flcn−/− cells occurred via activation of AMPK (Figure 1) [31]. Chemical activation of AMPK was sufficient to drive TFEB/TFE3 nuclear translocation in MEFs and high expression of G-CSF, IL-6, and VEGF in RAW264.7 cells relative to untreated controls [31]. However, whether the induction of these cytokines in Flcn−/− cells was dependent on TFEB/TFE3 was not tested. Overall, although the activation of TFEB and TFE3 by LPS in human and murine macrophages is well established, the precise mechanisms involved remain poorly understood (Figure 1).
Extracellular ATP
Extracellular ATP signals tissue damage and activates the purinergic receptor P2X7, which plays important roles in neuroinflammation [32]. A P2X7 chemical agonist was used to activate AMPK in BV-2 transformed murine microglial cells compared with vehicle controls [33]. Biochemical assays showed that P2X7 activation also induced rapid and transient TFEB nuclear translocation and lysosomal expansion (Lysotracker staining), compared with controls [33]. Moreover, chemical inhibition or silencing of AMPK prevented P2X7-triggered TFEB nuclear import, indicating that P2X7 activated TFEB through AMPK (Figure 1) [33]. Thus, TFEB could be activated downstream of P2X7, known to be broadly expressed in myeloid cells. It will be relevant to determine if P2X7 can also regulate TFEB and TFE3 in other innate immune cells, and what the physiological impacts of such regulatory nodes are in health vs disease.
Phagocyte Effector Functions Regulated by MiT Factors
Lysosome/autophagy enhancement
The activation of autophagy and lysosomal genes by TFEB in macrophages was first demonstrated in primary PMs from TfebLysM-OX transgenic mice overexpressing TFEB in the myeloid compartment [34]. Compared with WT controls, TfebLysM-OX PMs exhibited 8-fold induction of Tfeb and 1.5- to 3-fold induction of TFEB target genes Sqstm1, Lamp1, and Map1lc3b via qRT-PCR [34]. In addition, Tfeb overexpression ameliorated the loss of lysosomes caused by cholesterol crystals, compared to WT BMDMs (Lysotracker) [34]. These observations confirmed the observation that TFEB overexpression could be sufficient for increased lysosome biogenesis in murine macrophages, similarly to other cell types, and suggested that TFEB might be relevant to the pathogenesis of atherosclerosis [34]. This area certainly merits further investigation.
Bacterial killing
Nuclear receptor peroxisome proliferator activator receptor α (PPAR-α) is important for the control of Mycobacterium tuberculosis growth within macrophages [35]. TFEB was recently identified as a critical mediator of PPAR-α-induced autophagy in M. tuberculosis-infected murine BMDMs [35]. In this study, PPAR-α agonists drove TFEB gene expression and nuclear localization, as well as the expression of TFEB target autophagy genes (qRT-PCR of Uvrag, Map1lc3b, Vps11, and Vps34), and the induction of autophagy (as evidenced from MAP1LC3 lipidation immunoblotting and puncta formation via fluorescence microscopy) [35]. Tfeb siRNA knockdown reduced these effects of PPAR-α activation, thus hampering the control of M. tuberculosis survival within BMDM [35]. In contrast to prior findings where TFEB and TFE3 induced IL-6 and TNF-α in LPS-treated or phagocytosing mouse macrophages [28,36], M. tuberculosis-infected BMDM with silenced Tfeb produced more IL-6 and TNF-α than shRNA controls [35]. Whether this was due to a heightened response to infection or to increased bacterial burden in Tfeb shRNA-treated cells was unclear. Nonetheless, TFEB was established as a mediator of PPAR-α-enhanced control of M. tuberculosis intracellular growth [35].
An independent study showed that M. tuberculosis could inhibit TFEB and autophagy to enhance its intracellular survival [37]. In this study, M. tuberculosis induced expression of NF-κB-dependent micro-RNAs miR-33 and miR-33* in THP-1 cells and murine PMs [37]. qRT-PCR array profiling of PMs revealed that several autophagy genes were differentially regulated upon treatment with miR-33 and miR-33* agonists and antagonists, including Prkaa1, which encodes AMPKα and carries a miR-33 target site in its 3’ UTR [37]. At baseline, chemical or genetic inhibition of miR-33 enhanced nuclear translocation of TFEB and TFEB target gene expression (Tfeb, Ctsb, Lipa, Uvrag by qRT-PCR) in BMDM, compared with controls [37]. Conversely, transfection with miR-33 or miR-33* repressed Tfeb expression (qRT-PCR and immunoblot) in WT BMDMs compared with transfection controls, and such repression was abrogated in mouse Prkab−/− cells [37]. Therefore, AMPK was required for miR-33/33* repression of TFEB. Furthermore, sorted GFP+ AMs from animals infected with GFP-tagged M. tuberculosis showed reduced expression of Tfeb and its autophagy target genes (Map1lc3b, Lipa, and Uvrag) compared with GFP− cells [37]. Together, these observations suggested that induction of miR-33/33* by M. tuberculosis repressed AMPKα. Because AMPK represses mTORC1, it was proposed that AMPK repression might activate mTORC1, thus diminishing TFEB activity and autophagic clearance of M. tuberculosis (Figure 1) [37]. Together, these studies support the hypothesis that TFEB may be relevant to M. tuberculosis infection in mammalian macrophages, but further study is required to clearly delineate its roles and regulation in this type of infection.
Cytokine production
As mentioned, expression analysis and ChIP showed that TFEB and TFE3 directly controlled the expression of several cytokines and chemokines (e.g. TNF-α, IL-1α and β, IL-6, M-CSF, and GM-CSF) in murine macrophages infected with pathogenic bacteria or stimulated with LPS [28,36,38]. Additionally, human THP-1 and U937 cells have been documented to secrete IL-1β in response to high extracellular glucose concentrations [39]. TFEB knockdown in these cells impaired IL-1β secretion compared to siRNA controls [39]. Moreover, high glucose concentrations caused TFEB nuclear translocation, which Ca2+ imaging, chemical inhibitors, and genetic knockdowns showed was due to lysosomal Ca2+ release and calcineurin activation [39]. However, whether these effects were mediated through alterations in glucose-induced pyroptosis or if they represented a novel mechanism for IL-1β processing and secretion remains unknown. Nonetheless, these findings may have important implications for a putative roles of macrophage TFEB during hyperglycemia.
Macrophage polarization
Several studies indicate that TFEB and TFE3 are important for driving macrophages to a pro-inflammatory “polarized state” (also called “classical activation” and “M1”)[40]. As mentioned, Flcn deletion can induce TFE3 activation and have a polarizing effect on macrophages [30]. Likewise, myeloid cell-restricted deletion of late endosomal/lysosomal adaptor, MAPK and mTOR activator 1 (LAMTOR1, Figure 1) in Lamtor1ΔLysM mice can cause constitutive TFEB activation in macrophages, as evidenced from TFEB immunofluorescence and target gene and protein Lamp1, Ctsd, and Map1lc3b expression in Lamtor1ΔLysM BMDM compared to Lamtor1flox/flox cells [38]. Moreover, LPS-stimulated Lamtor1ΔLysM BMDMs have shown enhanced secretion of IL-6 and TNF-α compared with WT, an outcome that was partially suppressed by knockdown of Tfeb [38]. Of relevance, ChIP-PCR from RAW264.7 cells showed TFEB recruitment to the Il6 and Tnf promoters after treatment with LPS and mTORC1 chemical inhibitor Torin 1 [38]. Moreover, Lamtor1ΔLysM AMs secreted more IL-6 and TNF-α than WT after LPS stimulation in vivo, and Lamtor1ΔLysM animals were more susceptible than WT to weight loss and death caused by bleomycin, in an acute respiratory distress syndrome mouse model [38]. A such, Lamtor1ΔLysM macrophages displayed stronger responses to pro-inflammatory stimuli than WT, and this effect was at least partially mediated by TFEB.
A separate study found that treatment of murine PMs with murine breast or lung tumor-conditioned medium repressed Tfeb gene and protein expression compared with controls [41]. TGF-β neutralizing antibody and recombinant murine TGF-β experiments using murine PMs showed the TFEB-repressing activity to be TGF-β. Moreover, chemical inhibitor experiments showed that TGF-β required ERK signaling to repress TFEB in PMs [41]. To test the role of TFEB in the macrophage anti-inflammatory polarized state (i.e. “alternative activation” or “M2”), Tfeb was silenced in PMs treated with IL-4 or tumor-conditioned media. Tfeb knockdown cells showed increased expression of Arg1 and Chil3 (used as markers of the M2-like state), concomitant with decreased expression of suppressor of cytokine signaling 3 (Socs3) [41]. Moreover, Tfeb knockdown cells showed decreased phosphorylation of signal transducer and activator of transcription 3 (STAT3) [41]. These results suggested that TFEB might repress the M2-like state via induction of Socs3 and inactivation of STAT3, and that repression of TFEB might license M2 polarization in PMs. Moreover, TFEB activation by chemical or genetic means in IL-4- or tumor-conditioned media-treated PMs decreased Arg1 and Chil3 expression compared to untreated cells. And, TfebLysM-OX-driven macrophage-specific TFEB overexpression correlated with diminished breast cancer tumor growth in in vivo bone marrow transplant experiments, compared with WT macrophages [41]. Thus, in tumor-associated macrophages, TGF-β was reported to promote M2 polarization via ERK-mediated repression of TFEB, thus inhibiting M1 polarization and promoting an M2 state. This may have relevant implications for cancer immunotherapy, as M2-like tumor-associated macrophages are thought to limit treatment efficacy for a variety of cancers [41].
Additional support for the M1-polarizing role of TFEB was provided by a study using the anti-malarial drug chloroquine (CQ) to increase lysosomal pH [42]. CQ has been ascribed anti-tumor properties in mice and humans [43]. In one study, CQ treatment of murine M2-polarized BMDM drove their M1 polarization, as evidenced from flow cytometry markers IFN-γ, IL-12p40, TNF-α, CD80, CD86, MHC-II, in addition to NO production [42]. Immunoblot and fluorescence microscopy revealed activation of p38 MAPK, NF-κB, and TFEB by CQ in murine RAW264.7 and M2-BMDM cells [42]. In addition, chemical and genetic inhibition of NF-κB or p38 MAPK (but not of TFEB) prevented the induction of iNOS and the repression of arginase 1 (ARG1) by CQ (assessed via flow cytometry), suggesting that the NF-κB and p38 MAPK pathways drove M1 polarization. In contrast, Seahorse™ experiments showed that the switch from oxidative phosphorylation to lactic fermentation characteristic of M1 polarization was not affected by NF-κB or p38 inhibition, but rather, was prevented by Tfeb silencing [42]. In vivo, mice bearing hepatocarcinoma ascites were injected intraperitoneally with CQ-treated RAW264.7 cells, which resulted in reduced numbers of myeloid-derived suppressor cells and Tregs at the lesion compared to mice that received PBS-treated cells (assessed by flow cytometry) [42]. Moreover, Tfeb siRNA abrogated this effect, demonstrating that TFEB was required for CQ to induce macrophage-dependent antitumor activity in vivo [42]. This study concluded that TFEB could promote an M1-polarized state by promoting a metabolic switch to lactic fermentation, rather than through cell fate determination [42].
Antiviral responses
Type I IFNs are major signals in the antiviral response. TFEB can drive the constitutive induction of type I IFN response gene Ifit1 in Trex1−/− murine MEFs, which present high concentrations of cytosolic DNA (Box 1) [44]. Thus, TFEB has been proposed as an anti-viral factor. Consistent with this view, a separate study using primary human macrophages found that HIV-1 infection could activate TFEB via TLR8 (Figure 1, [45]). In this study, HIV-1 infection at early time points in human MDMs, induced autophagy (SQSTM1 degradation and MAP1LC3 lipidation by immunoblot) and inhibited autophagy at later time points [45]. At early times, TFEB translocated into the nucleus, and induced expression of its target genes UVRAG, ATG9B, and MCOLN1 (qRT-PCR) compared to uninfected cells [45]. Moreover, TFEB shRNA prevented SQSTM1 degradation, MAP1LC3 lipidation, and target gene induction by HIV-1 infection, while viral transduction of TFEB caused the opposite effects [45]. Of relevance, shRNA of TLR8 prevented TFEB nuclear translocation during HIV-1 infection and abrogated downstream gene transcription and autophagy induction; this suggested that viral detection via TLR8 was required for TFEB activation [45]. Furthermore, infection with ΔNef HIV-1 -- lacking the negative factor gene Nef -- produced the same extent of TFEB activation and autophagy induction as infection with WT HIV-1; however, while at later time points the WT virus shut down autophagy, activation of TFEB and of autophagy persisted in the ΔNef HIV-infected cells [45]. Thus, the study proposed that TLR8 could mediate initial HIV-1 recognition in human macrophages to activate TFEB and downstream autophagy; at later times, Nef appeared to impair autophagy, presumably to allow virion assembly and productive replication [45]. Future studies are warranted to further assess these observations.
TFEB has also been implicated in the restriction of adeno-associated viruses (AAV). AAV are attractive vectors for gene therapy due to their safety profile [46]. However, to deliver cargo DNA to the nucleus, AAV must overcome several cellular barriers to gene delivery, including cellular degradation and clearance pathways. In HeLa cells, AAV2 infection was reported to activate TFEB compared with uninfected cells (as evidenced from immunofluorescence and qRT-PCR of MAP1LC3B, LAMP1, HEXA, and SQSTM1) [46]. Moreover, compared with control cells, chemical activation of TFEB reduced AAV2-mediated transduction while TFEB siRNA enhanced it, suggesting that TFEB could pose as a cell-intrinsic barrier to AAV infection [46]. Thus, TFEB inhibition might constitute a promising approach to potentially increase the efficiency of AAV-mediated gene therapies. Collectively, these studies suggest that TFEB can also perform anti-viral functions in macrophages, including the induction of autophagy and the expression of antiviral genes, and it will be interesting to further follow this area of investigation.
Dendritic cell migration
Dendritic cells (DCs) are antigen-presenting cells that when activated (or ‘matured’) migrate to draining lymph nodes to present antigens to T cells. LPS activates DCs, causing them to migrate faster and to follow chemokine gradients [47]. Compared with WT cells, tamoxifen-induced Tfeb deletion in primary bone-marrow derived DCs (BMDCs) from CAG-Cre-ERT+/− Tfebflox/flox mice resulted in defective LPS-induced speed of migration in microfabricated channels visualized by light microscopy [48]. However, Tfeb deletion did not affect LPS-induced DC maturation per se (measured by flow cytometry of CD86 and CD40). Conversely, TFEB activation using mTORC1 chemical inhibitor Torin 2 increased migration but did not increase expression of CD86 and CD40 relative to controls [48]. Thus, TFEB appeared to be important for the induction of the migratory phenotype but dispensable for DC maturation. As DC maturation entails macropinocytosis downregulation [47], it was proposed that decreased macropinocytosis could lead to mTORC1 inhibition due to decreased cellular nutrient uptake [48]. Inactive mTORC1 would cause TFEB to translocate to the nucleus, thus driving the expression of Mcoln1, and further TFEB activation in a Ca2+-mediated feedback loop (Box 1). Accordingly, chemical inhibition of macropinocytosis increased TFEB nuclear translocation and Mcoln1 expression in immature DCs, compared with treatment controls [48]. The authors concluded that LPS could induce DC maturation, reducing macropinocytosis and nutrient intake, and thus, inactivating mTORC1. mTORC1 inactivation resulted in TFEB activation, which was necessary for the induction of increased migratory speed. However, the mechanism linking TFEB to increased speed remained undefined [48].
Concluding Remarks
The emerging picture is that TFEB and TFE3 can modulate innate immunity and inflammation by direct mechanisms, controlling the transcription of inflammatory mediators (Key Figure, Figure 2, panel a); and by indirect mechanisms, controlling cellular processes (e.g. metabolism, autophagy, and secretion) that impact microbial infection, organismal metabolism, and inflammatory signaling both locally and systemically (Key Figure, Figure 2, panel b). The large number of cellular processes under TFEB control, as well as the many feedback and feedforward mechanisms involved in TFEB regulation, makes it difficult to predict the organismal effects of perturbations to this regulatory network. In vivo experiments illustrate how TFEB can perform pro-inflammatory or anti-inflammatory functions, depending on tissue and context (e.g. Table 1).
Several key mechanistic questions remain (see Outstanding Questions). A major focus of future research will undoubtedly be to address the roles of MiT factors in immunometabolism. TFEB and TFE3 may influence inflammatory status and host defense through the interlinked mechanisms of innate immune signaling and metabolic regulation of inflammatory cellular phenotypes, as has been demonstrated in macrophage polarization [42]. Given the roles that TFEB and TFE3 play in lipid catabolism and in mitochondrial homeostasis [7], it is highly likely that they might play important roles in innate immunity through cellular metabolism.
Outstanding Questions.
What are the tissue- and organ-specific functions of TFEB (and other MiT factors) in the context of acute and chronic inflammation and host defense against infection? This information is critical to understanding the tissues that might be affected by therapeutic targeting of individual MiT factors, and to manage off-target effects.
What are the specific upstream regulatory pathways in each cell type? This question is important, because although many pathways have been identified in different cell types and species, the relevance of each pathway to specific disease conditions and specific cell types is not fully understood.
Can we develop therapeutically viable strategies to manipulate MiT factors? Investigating this merits attention as MiT factors perform several essential cellular functions in homeostasis and their overactivation can be detrimental.
How do MiT metabolic functions fit into the bigger picture of immunometabolism? Indeed, MiT factors might be important regulators of innate immunity and inflammation via presumed metabolic functions.
Results in humans and nematodes (box 2) suggest that TFEB activation can reach a threshold above which TFEB activity becomes detrimental. C. elegans ceTFEB overexpression has been shown to cause autophagy-dependent lifespan extension of these nematodes [49]. In addition, C. elegans maintained on a high-glucose diet has exhibited constitutively activated ceTFEB and high induction of autophagy [50]. However, in this model, glucose-enhanced ceTFEB expression and autophagy was shown to lead to a shorter lifespan autophagy [50]; and furthermore, high glucose has induced the secretion of IL-1β in U937 human monocytes in a TFEB-dependent manner [39]. Thus, it is reasonable to speculate that inflammatory pathologies such as diabetes might be associated with evolutionarily-conserved TFEB-dependent perturbations and pro-inflammatory mechanisms, although this will require robust testing. Of note, TFEB overexpression alone can cause oncogenic transformation in mice, representing an interesting area of future investigation [51]. Indeed, work to assess MiT factors as putative therapeutic targets for a variety of maladies is currently under way, but extensive effort must be devoted to identifying viable means to modulating these factors in biologically- and clinically-relevant contexts.
Supplementary Material
Box 3: Regulation of TFEB by multiple feedback loops.
Feedback loops are pervasive mechanisms by which a regulatory step can influence the activity of an upstream step [63]. Recent work has uncovered several feedback loops that modulate TFEB activity in a variety of mammalian cell types (Figure I).
TFEB-TFEB positive loop
The TFEB promoter includes several CLEAR motifs, mediating TFEB auto-regulation in a positive feedback loop in many cell types (e.g. HeLa, human and mouse hepatocytes; Fig. Ia) [7].
TFEB-MCOLN1 positive loop
Activation of MCOLN1 is necessary and sufficient to activate TFEB in many cell types (e.g. HeLa, human hepatocytes, macrophages) mediated by Ca2+ and calcineurin [19]. In addition, MCOLN1 is directly induced by TFEB in these cells [5]. This positive feedback loop (Fig. Ib) may amplify the Ca2+ wave initiated from the lysosome, boosting TFEB nuclear import and downstream target gene activation.
TFEB-PGC-1α /PPAR-α positive loop
TFEB directly activates PGC1A and PPARA expression in HeLa and hepatocyte cells during nutrient starvation [7]. Conversely, PGC-1α can bind to the TFEB promoter, inducing TFEB expression through PPAR-α [7]. This positive feedback loop (Fig. Ic) induces β-oxidation genes and mitochondrial biogenesis in hepatocytes [7] and brain cells [64,65].
TFEB-mTORC1 negative loop
TFEB induces endocytosis in HeLa and HEK293 cells, driving the formation of signaling endosomes, modulating cell signaling, and enhancing the intake of nutrients, resulting in recovery of mTORC1 activity after starvation [66]. In addition, TFEB directly induces the expression of RRAGD in HeLa cells, thus enhancing mTORC1 activity [67]. Via these two mechanisms, TFEB participates in a negative feedback loop that tends to restore homeostatic activity (Fig. Id).
TFEB-autophagy/lysosome negative loop
Disruption of autophagy or autophagosome-lysosome fusion leads to TFEB activation in HeLa and hepatocyte cells [6]. TFEB drives the transcription of a large number of autophagy-related genes and induces autophagy, restoring autophagic flux [6]. When autophagic flux is restored by this negative feedback loop, the TFEB-activating signal diminishes and TFEB activity is reduced, returning to homeostatic equilibrium (Fig. Ie).
Figure I. Feedback regulation of TFEB in human cells. (a) TFEB drives expression of the TFEB gene. (b) MCOLN1/Ca2+/calcineurin signaling activates TFEB, driving the expression of MCOLN1. (c) PPAR-α and PGC-1α drive TFEB expression, while TFEB drives the PPARA and PGC1A expression. (d) TFEB drives expression of RRAGD and endocytosis genes, enhancing mTORC1 lysosomal recruitment and activation. Activated mTORC1 represses TFEB. (e) Lysosomal damage and disruption of autophagy activate TFEB. Activated TFEB drives lysosomal biogenesis and autophagy, diminishing the activating signal. TFEB, transcription factor EB; MCOLN1, mucolipin 1; PPAR-α, peroxisome proliferator activator receptor α; PGC-1α, PPAR-γ coactivator 1α; mTORC1, mechanistic target or rapamycin, complex 1; RRAGD, Ras-Related GTP-Binding Protein D; ATG, autophagy. Figure created via BioRender (https://biorender.com/)
Highlights.
TFEB, TFE3 and related MiT transcription factors are broadly expressed in mammals.
Under resting or homeostatic conditions, TFEB and TFE3 reside in the cytosol; their activation entails nuclear translocation, which is triggered by many distinct stimuli.
Recently identified upstream regulation of TFEB and/or TFE3 in innate immune cells includes phagocytosis, lysosome damage, IFN-γ, LPS, and extracellular ATP.
Recently identified downstream functions of TFEB and/or TFE3 in innate immune cells include autophagy and lysosomal biogenesis, bacterial killing, pro-inflammatory cytokine production, macrophage classical activation, antiviral responses, and dendritic cell migration.
Multiple feedback loops control TFEB and TFE3, mandating sophisticated approaches to safely control their activities for therapeutic purposes.
Acknowledgements
The author apologizes to researchers whose work was not cited or cited through reviews owing to space limitations. Three anonymous reviewers provided generous feedback and intellectual input. Funding for the Irazoqui laboratory was provided by NSF IOS 1457055, NIH GM101056, and NIH DK043351.
Glossary
- Acute respiratory distress syndrome
Rapidly progressive pulmonary inflammation
- ADF neurons
Sensory C. elegans neurons
- Anti-inflammatory polarized state
Macrophage activation causing secretion of pro-resolution, anti-inflammatory molecules
- ASI neurons
Sensory C. elegans neurons
- Autophagy
Commonly refers to macroautophagy, in which cells form double-membrane vesicles around portions of the cytosol, which may contain organelles, protein aggregates, or pathogens, for lysosomal degradation
- Bleomycin
Fungal antibiotic that causes lung inflammation
- Brain/gut/microbiota axis
Term used to describe bidirectional communication between the central nervous system, the gastrointestinal system and the intestinal microbiota
- Chloroquine
Synthetic anti-malarial drug
- Cry toxin
Pore-forming toxin
- Cystitis
Inflammation of the bladder
- Dominant negative
allele or construct that inhibits the function of the wild type in a dominant fashion
- E-box
The DNA sequence 5’-CACGTG-3’
- E3 ubiquitin ligase
E3 ubiquitin ligases recruit ubiquitylation substrates to the E2 ubiquitin transferase
- Folliculin
Multi-domain protein that functions as a GTPase activating protein for RRAGC and RRAGD
- Frustrated phagocytosis
Scenario where the phagocytic cup is not allowed to close, such as by coating the bottom of the well with scavenger receptor ligand
- Gag
Group-specific antigen
- Galectins
Proteins that bind to β-galactoside sugar moieties, such as those exposed on membranes of damaged lysosomes
- gut-brain axis
Term to describe bidirectional communication between the central nervous system and the gastrointestinal tract
- Lactic fermentation
The breakdown of glucose to pyruvate, followed by its reduction to lactate
- Lipopolysaccharide
Major lipid component of the outer leaflet of the outer membrane of Gram- bacteria
- Lysosome
Membrane-bound organelle in the cytoplasm that contains degradative enzymes
- M-box
The cognate sequence for MITF, 5’-TCATGTG-3’
- Macropinocytosis
Form of endocytosis; engulfment of large quantities of solutes in an actin-dependent process
- Microglia
Glial cells of the central nervous system that function as phagocytes
- Myeloid-derived suppressor cells
Type of myeloid cell generated during chronic infection or inflammation that can inhibit T cells and NK cells
- Nef
Negative factor, enhances viral titers
- Opsonization
Promotion of phagocytic uptake by modification of the surface of the target particle
- Oxidative phosphorylation
The consumption of reducing equivalents to generate a proton gradient and the dissipation of such gradient by ATP synthase to generate ATP
- Pattern-recognition receptors
Membrane or cytosolic proteins important for sensing molecules derived from microbes
- Protease-activated receptors
Transmembrane proteins that include their own ligands, which are activated by proteolysis
- Phage display
Technique to express recombinant proteins attached to the tail of a bacteriophage for interaction studies
- Phagocytosis
Process by which cells engulf large extracellular particles
- Pro-inflammatory “polarized state”
Macrophage activation that leads to the secretion of pro-inflammatory cytokines and chemokines
- Purinergic receptor
Plasma membrane ATP receptor
- Pyroptosis
Form of pro-inflammatory programmed cell death
- Seahorse™
Commercial live-cell metabolic assay platform
- T cell help
Mechanism by which T cells stimulate the differentiation of B cells into plasma cells
- Tregs
Regulatory T cells, normally identified by the expression of FOXP3
- Tryptophan hydroxylase
Enzyme catalyzing the conversion of Trp to serotonin
- Vif
Viral infectivity factor, from retrovirus, disrupts function of APOBEC proteins
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Smale ST and Natoli G (2014) Transcriptional Control of Inflammatory Responses. Csh Perspect Biol 6, a016261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Medzhitov R and Horng T (2009) Transcriptional control of the inflammatory response. Nat Rev Immunol 9, 692–703 [DOI] [PubMed] [Google Scholar]
- 3.Perera RM et al. (2018) MiT/TFE Family of TFs, Lysosomes, and Cancer. Annu Rev Cancer Biology 3, 1–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.King R et al. (1999) Microphthalmia Transcription Factor A Sensitive and Specific Melanocyte Marker for Melanoma Diagnosis. Am J Pathology 155, 731–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sardiello M et al. (2009) A Gene Network Regulating Lysosomal Biogenesis and Function. Science 325, 473–477 [DOI] [PubMed] [Google Scholar]
- 6.Settembre C et al. (2011) TFEB Links Autophagy to Lysosomal Biogenesis. Science 332, 1429–1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Settembre C et al. (2013) TFEB controls cellular lipid metabolism through a starvation-induced autoregulatory loop. Nat Cell Biol 15, 647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Medina DL et al. (2011) Transcriptional Activation of Lysosomal Exocytosis Promotes Cellular Clearance. Dev Cell 21, 421–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martina JA et al. (2014) Novel roles for the MiTF/TFE family of TFs in organelle biogenesis, nutrient sensing, and energy homeostasis. Cell Mol Life Sci 71, 2483–2497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martina JA et al. (2014) The Nutrient-Responsive Transcription Factor TFE3 Promotes Autophagy, Lysosomal Biogenesis, and Clearance of Cellular Debris. Sci Signal 7, ra9–ra9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Puertollano R et al. (2018) The complex relationship between TFEB transcription factor phosphorylation and subcellular localization. Embo J 37, e98804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raben N and Puertollano R (2015) TFEB and TFE3: Linking Lysosomes to Cellular Adaptation to Stress. Annu Rev Cell Dev Bi 32, 1–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Napolitano G and Ballabio A (2016) TFEB at a glance. J Cell Sci 129, 2475–2481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nabar N and Kehrl J (2017) The Transcription Factor EB Links Cellular Stress to the Immune Response. Yale J Biol Med 90, 301–315 [PMC free article] [PubMed] [Google Scholar]
- 15.Brady OA et al. (2017) Emerging roles for TFEB in the immune response and inflammation. Autophagy 14, 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roczniak-Ferguson A et al. (2012) The Transcription Factor TFEB Links mTORC1 Signaling to Transcriptional Control of Lysosome Homeostasis. Sci Signal 5, ra42–ra42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Settembre C et al. (2012) A lysosome-to-nucleus signalling mechanism senses and regulates the lysosome via mTOR and TFEB. Embo J 31, 1095–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martina JA et al. (2012) MTORC1 functions as a transcriptional regulator of autophagy by preventing nuclear transport of TFEB. Autophagy 8, 877–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Medina DL et al. (2015) Lysosomal calcium signalling regulates autophagy through calcineurin and TFEB. Nat Cell Biol 17, 288–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li H et al. (2011) Interaction of calcineurin with substrates and targeting proteins. Trends Cell Biol 21, 91–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gray MA et al. (2016) Phagocytosis Enhances Lysosomal and Bactericidal Properties by Activating the Transcription Factor TFEB. Curr Biol 26, 1955–1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paola S et al. (2018) TRPML1: The Ca(2+)retaker of the lysosome. Cell Calcium 69, [DOI] [PubMed] [Google Scholar]
- 23.Chauhan S et al. (2016) TRIMs and Galectins Globally Cooperate and TRIM16 and Galectin-3 Co-direct Autophagy in Endomembrane Damage Homeostasis. Dev Cell 39, 13–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jia J et al. (2018) Galectins Control mTOR in Response to Endomembrane Damage. Mol Cell 70, 120–135 e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu M et al. (2017) Immunomodulators targeting MARCO expression improve resistance to postinfluenza bacterial pneumonia. Am J Physiol-lung C 313, L138–L153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh N et al. (2018) Antimycobacterial effect of IFNG (interferon gamma)-induced autophagy depends on HMOX1 (heme oxygenase 1)-mediated increase in intracellular calcium levels and modulation of PPP3/calcineurin-TFEB (transcription factor EB) axis. Autophagy 14, 1–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brubaker S et al. (2015) Innate immune pattern recognition: a cell biological perspective. Annu Rev Immunol 33, 257–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pastore N et al. (2016) TFEB and TFE3 cooperate in the regulation of the innate immune response in activated macrophages. Autophagy 12, 1–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vural A et al. (2016) Activator of G-Protein Signaling 3–Induced Lysosomal Biogenesis Limits Macrophage Intracellular Bacterial Infection. J Immunol 196, 846–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li J et al. (2019) Myeloid Folliculin balances mTOR activation to maintain innate immunity homeostasis. Jci Insight DOI: 10.1172/jci.insight.126939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.El-Houjeiri L et al. (2019) The TFs TFEB and TFE3 Link the FLCN-AMPK Signaling Axis to Innate Immune Response and Pathogen Resistance. Cell Reports 26, 3613–3628.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adinolfi E et al. (2017) The P2X7 receptor: a main player in inflammation. Biochemical Pharmacology 151, 234–244 [DOI] [PubMed] [Google Scholar]
- 33.Sekar P et al. (2018) AMPK-dependent and independent actions of P2X7 in regulation of mitochondrial and lysosomal functions in microglia. Cell Commun Signal 16, 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Emanuel R et al. (2014) Induction of Lysosomal Biogenesis in Atherosclerotic Macrophages Can Rescue Lipid-Induced Lysosomal Dysfunction and Downstream Sequelae. Arteriosclerosis Thrombosis Vasc Biology 34, 1942–1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim Y et al. (2017) PPAR-α Activation Mediates Innate Host Defense through Induction of TFEB and Lipid Catabolism. J Immunol 198, 3283–3295 [DOI] [PubMed] [Google Scholar]
- 36.Visvikis O et al. (2014) Innate Host Defense Requires TFEB-Mediated Transcription of Cytoprotective and Antimicrobial Genes. Immunity 40, 896–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ouimet M et al. (2016) Mycobacterium tuberculosis induces the miR-33 locus to reprogram autophagy and host lipid metabolism. Nat Immunol 17, 677–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hayama Y et al. (2018) Lysosomal Protein Lamtor1 Controls Innate Immune Responses via Nuclear Translocation of Transcription Factor EB. J Immunol 200, 3790–3800 [DOI] [PubMed] [Google Scholar]
- 39.Tseng H et al. (2017) Lysosomal Ca2+ Signaling Regulates High Glucose-Mediated Interleukin-1β Secretion via Transcription Factor EB in Human Monocytic Cells. Front Immunol 8, 1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murray PJ et al. (2014) Macrophage Activation and Polarization: Nomenclature and Experimental Guidelines. Immunity 41, 14–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fang L et al. (2017) Transcriptional factor EB regulates macrophage polarization in the tumor microenvironment. Oncoimmunology 6, 00–00 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen D et al. (2018) Chloroquine modulates antitumor immune response by resetting tumor-associated macrophages toward M1 phenotype. Nat Commun 9, 873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maes H et al. (2013) Autophagy: shaping the tumor microenvironment and therapeutic response. Trends Mol Med 19, 428–446 [DOI] [PubMed] [Google Scholar]
- 44.Hasan M et al. (2013) Trex1 regulates lysosomal biogenesis and interferon-independent activation of antiviral genes. Nat Immunol 14, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Campbell GR et al. (2015) Human Immunodeficiency Virus Type 1 Nef Inhibits Autophagy through Transcription Factor EB Sequestration. Plos Pathog 11, e1005018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Popp L et al. (2017) TFEB-mediated activation of the lysosome-autophagy system affects the transduction efficiency of adeno-associated virus 2. Virology 510, 1–8 [DOI] [PubMed] [Google Scholar]
- 47.Vargas P et al. (2015) Innate control of actin nucleation determines two distinct migration behaviours in dendritic cells. Nat Cell Biol 18, ncb3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bretou M et al. (2017) Lysosome signaling controls the migration of dendritic cells. Sci Immunol 2, eaak9573. [DOI] [PubMed] [Google Scholar]
- 49.Lapierre LR et al. (2013) The TFEB orthologue HLH-30 regulates autophagy and modulates longevity in Caenorhabditis elegans. Nat Commun 4, 2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Franco-Juárez B et al. (2018) A high glucose diet induces autophagy in a HLH-30/TFEB-dependent manner and impairs the normal lifespan of C. elegans. Aging Albany Ny 10, 2657–2667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Calcagnì A et al. (2016) Modelling TFE renal cell carcinoma in mice reveals a critical role of WNT signaling. Elife 5, e17047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beckmann H et al. (1990) TFE3: a helix-loop-helix protein that activates transcription through the immunoglobulin enhancer muE3 motif. Genes and Development 4, 167–179 [DOI] [PubMed] [Google Scholar]
- 53.Carr C and Sharp P (1990) A helix-loop-helix protein related to the immunoglobulin E box-binding proteins. Mol Cell Biol 10, 4384–4388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huan C et al. (2006) TFs TFE3 and TFEB are critical for CD40 ligand expression and thymus-dependent humoral immunity. Nat Immunol 7, ni1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saban R et al. (2007) Transcription factor network downstream of protease activated receptors (PARs) modulating mouse bladder inflammation. Bmc Immunol 8, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pujol N et al. (2001) A reverse genetic analysis of components of the Toll signaling pathway in Caenorhabditis elegans. Curr Biol 11, 809–821 [DOI] [PubMed] [Google Scholar]
- 57.Chen H-D et al. (2016) HLH-30/TFEB-mediated autophagy functions in a cell-autonomous manner for epithelium intrinsic cellular defense against bacterial pore-forming toxin in C. elegans. Autophagy 13, 00–00 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee K and Mylonakis E (2017) An Intestine-Derived Neuropeptide Controls Avoidance Behavior in Caenorhabditis elegans. Cell Reports 20, 2501–2512 [DOI] [PubMed] [Google Scholar]
- 59.Lee S-H et al. (2018) Modulatory upregulation of an insulin peptide gene by different pathogens in C. elegans. Virulence 9, 00–00 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fung T et al. (2017) Interactions between the microbiota, immune and nervous systems in health and disease. Nat Neurosci 20, 145–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Najibi M et al. (2016) An Evolutionarily Conserved PLC-PKD-TFEB Pathway for Host Defense. Cell Reports 15, 1728–1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Feng H et al. (2006) Conserved Domains Subserve Novel Mechanisms and Functions in DKF-1, a Caenorhabditis elegans Protein Kinase D. J Biol Chem 281, 17815–17826 [DOI] [PubMed] [Google Scholar]
- 63.Brandman O and Meyer T (2008) Feedback loops shape cellular signals in space and time. Science 322, 390–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tsunemi T et al. (2012) PGC-1α Rescues Huntington’s Disease Proteotoxicity by Preventing Oxidative Stress and Promoting TFEB Function. Sci Transl Med 4, 142ra97–142ra97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ghosh A et al. (2015) Activation of Peroxisome Proliferator-activated Receptor α Induces Lysosomal Biogenesis in Brain Cells IMPLICATIONS FOR LYSOSOMAL STORAGE DISORDERS. J Biol Chem 290, 10309–10324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nnah IC et al. (2018) TFEB-driven endocytosis coordinates MTORC1 signaling and autophagy. Autophagy 15, 151–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Malta C et al. (2017) Transcriptional activation of RagD GTPase controls mTORC1 and promotes cancer growth. Science 356, 1188–1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Doronzo G et al. (2019) TFEB controls vascular development by regulating the proliferation of endothelial cells. Embo J 38, e98250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Song W et al. (2019) Endothelial TFEB (Transcription Factor EB) Restrains IKK (IκB Kinase)-p65 Pathway to Attenuate Vascular Inflammation in Diabetic db/db Mice. Arteriosclerosis Thrombosis Vasc Biology DOI: 10.1161/atvbaha.119.312316 [DOI] [PubMed] [Google Scholar]
- 70.Gkouskou K et al. (2016) Apolipoprotein A-I inhibits experimental colitis and colitis-propelled carcinogenesis. Oncogene 35, 2496–2505 [DOI] [PubMed] [Google Scholar]
- 71.Chao X et al. (2018) Impaired TFEB-mediated Lysosome Biogenesis and Autophagy Promote Chronic Ethanol-induced Liver Injury and Steatosis in Mice. Gastroenterology 155, 865–879 e12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang S et al. (2019) Impaired TFEB-mediated lysosomal biogenesis promotes the development of pancreatitis in mice and is associated with human pancreatitis. Autophagy DOI: 10.1080/15548627.2019.1596486 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.