Abstract
Repetitive transcranial magnetic stimulation (rTMS), a non-invasive brain stimulation technique, has emerged as a promising treatment for mild cognitive impairment (MCI) and Alzheimer’s disease (AD). Currently, however, the effectiveness of this therapy is unclear due to the low statistical power and heterogeneity of previous trials. The purpose of the meta-analysis was to systematically characterize the effectiveness of various combinations of rTMS parameters on different cognitive domains in patients with MCI and AD. Thirteen studies comprising 293 patients with MCI or AD were included in this analysis. Random effects analysis revealed an overall medium-to-large effect size (0.77) favoring active rTMS over sham rTMS in the improvement of cognitive functions. Subgroup analyses revealed that 1) high-frequency rTMS over the left dorsolateral prefrontal cortex (DLPFC) and low-frequency rTMS at the right DLPFC significantly improved memory functions; 2) high-frequency rTMS targeting the right inferior frontal gyrus significantly enhanced executive performance; and 3) the effects of 5–30 consecutive rTMS sessions could last for 4–12 weeks. Potential mechanisms of rTMS effects on cognitive functions are discussed.
Keywords: mild cognitive impairment, Alzheimer’s disease, transcranial magnetic stimulation, plasticity, dorsolateral prefrontal cortex, intervention, memory, treatment, therapy, cognition
INTRODUCTION
Transcranial magnetic stimulation (TMS) is a non-invasive brain stimulation technique that is increasingly utilized for a growing number of research and clinical applications. To perform TMS, a magnetic field that reaches a strength of up to 2 Tesla is rapidly generated in <1 ms. Typically, this transient magnetic field is focally applied with a Figure-of-Eight coil that is carefully placed on the surface of the scalp over a targeted stimulation site. Leveraging Faraday’s principle of electromagnetic induction, this magnetic field penetrates the skull and generates an electric current in the more conductive brain tissue in a direction that is perpendicular to the applied magnetic field (Barker, et al., 1985, Hallett, 2007). If this orthogonal electrical current is strong enough to surpass a physiological threshold, it can depolarize neurons in the targeted cortical tissue. Due to the nature of the exponentially decaying electromagnetic field, the penetration depth is limited to 2–3 centimeters. Thus, only superficial brain tissue can be directly stimulated by TMS, but this excitation can be propagated to distal targets through circuits that are structurally and/or functionally connected to the stimulation site (Chou, et al., 2015b, Liston, et al., 2014, Wang, et al., 2014). In the corticospinal system, for example, when the motor cortex is stimulated with a suprathreshold TMS pulse, this direct excitation evokes a series of descending corticospinal volleys in the form of Direct (D-) and Indirect (I-) waves, which ultimately elicits a motor response in the corresponding limb (Bestmann and Krakauer, 2015).
Contrary to single-pulse TMS, patterned repetitive TMS (rTMS) can produce long-lasting effects on neural activity and behavior beyond the stimulation period (Chou, et al., 2015a, Fitzgerald, et al., 2006). Careful manipulation of the parameters comprising these patterned rTMS pulse trains can induce neuroplastic changes that resemble either Long-Term Potentiation (LTP) or Depression (LTD) (Chen, et al., 1997, Pascual-Leone, et al., 1994). Early studies targeting the motor cortex helped elucidate which rTMS parameters promote particular responses and their neurophysiological underpinnings (Klomjai, et al., 2015). More recently, this ability to evoke distinct long-lasting changes in neural activity has been leveraged for therapeutic applications in neuropsychiatric disease. In 2008, for example, TMS devices were cleared for market by the Food and Drug Administration (FDA) for the clinical treatment of medication-resistant Major Depression Disorder. The specific FDA approved protocol is comprised of a high-frequency stimulation protocol, which is known to produce an excitatory LTP-like effect, over the Left Dorsolateral Prefrontal Cortex (DLFPC) (O’Reardon, et al., 2007). However, as an extension of the prefrontal asymmetry phenomenon, it has also been reported that inhibitory LTD-like low-frequency rTMS over the Right DLPFC is equally efficacious in producing anti-depressive outcomes in this patient population (Fitzgerald, et al., 2009, Sutton and Davidson, 1997). In other words, prefrontal activity in patients with MDD is abnormally imbalanced with right-sided hyperactivity and left-sided hypoactivity. Thus, distinct rTMS protocols that are known to produce opposite neurophysiological effects must be specifically applied depending on the targeted site of stimulation. This interaction between rTMS protocol and stimulation site has also been documented in the treatment of neurodegenerative disease (Chou, et al., 2015a). Accordingly, it is necessary to carefully discern the rTMS parameters when evaluating its potential efficacy in all neurologic and psychiatric conditions.
In recent years, rTMS has been closely investigated to evaluate its potential to modulate cognitive functions in AD and MCI. Here, we extend previous important reviews (Birba, et al., 2017, Cheng, et al., 2017, Dong, et al., 2018, Hsu, et al., 2015, Liao, et al., 2015, Nardone, et al., 2014) by including rTMS studies among both patients with AD or MCI to evaluate and update the efficacy of rTMS intervention compared to sham controls. Additionally, we sought to give greater attention to the methodological components of these existing studies. Considerable heterogeneity exists among the various rTMS treatment protocols that have been reported for cognitive enhancement for AD and MCI in the literature (e.g., different combinations of stimulation site, pulse frequency, stimulation intensity, number of stimuli delivered, and number of treatment sessions). A systematic and quantitative review that delineates rTMS effects based on various rTMS treatment protocols is not available. It is important to integrate these findings in a manner that accounts for this methodological heterogeneity to more accurately estimate the effects of rTMS in AD and MCI. Furthermore, we systematically characterize the effectiveness of various combinations of rTMS parameters on different cognitive domains in patients with AD and MCI. The distilled findings presented herein can be used to improve the experimental design of future rTMS clinical trials.
METHODS
Search Strategy
Our meta-analysis was conducted in accordance with the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement (Moher, et al., 2009) (Figure 1). To identify studies for inclusion in this meta-analysis we searched PubMed, Web of Science, Current Contents Connect, and SciELO Citation Index through March 2019. Databases were searched using combinations of the following terms: Alzheimer’s disease or mild cognitive impairment and repetitive transcranial magnetic stimulation (or rTMS or repetitive TMS). Additionally, we searched reference lists of previous reviews on rTMS for AD/MCI (Birba, et al., 2017, Cheng, et al., 2017, Dong, et al., 2018, Hsu, et al., 2015, Liao, et al., 2015, Nardone, et al., 2014) to identify additional relevant articles.
Figure 1.
Flow diagram showing the search and selection procedure that was used for this meta-analysis. Diagram adapted from Moher et al.(Moher, et al., 2009).
Inclusion Criteria for the Selection of Studies
We included studies that met all of the following criteria: 1) clinical population of patients previously diagnosed with AD or MCI; 2) rTMS was the only intervention being investigated; 3) cognitive functions were assayed as a behavioral outcome measure; 4) parallel or cross-over design that utilized a sham-controlled group or condition; and 5) articles written in English. Studies identified through database searches were initially screened on the basis of their title and abstract. They were subsequently excluded if it was clear from the title or abstract that the study was not relevant or did not meet the inclusion criteria (see Supplementary Table 1). If it remained unclear, the paper was assessed in its entirety. Additionally, studies were excluded if they were conference abstracts/papers.
Data Extraction
Two authors (YHC and VTT) independently performed the data extraction and any disagreements were resolved by joint discussion. Extracted data included sample size, sample characteristics, study design, rTMS protocol, statistical data of the score of cognitive performance for effect size estimation, and timing of outcome measurements (short-term: within 1 hour of the final rTMS session or long-term: ≥ 4 weeks post-rTMS). When published data were insufficient for data analysis, the original study author(s) were contacted with requests for access to additional data.
Statistical Analysis
Effect size calculation.
We used standardized mean difference (SMD, also known as Cohen’s d) to express the effect size of rTMS on cognitive functions. A random-effects model was used to calculate pooled effect sizes and examine whether the averaged effect size was significantly different from zero (p < 0.05, two-tailed). The mean effect was expressed as SMD with 95% confidence intervals. Generally, one effect size was derived from each study. If a study had multiple effect sizes from the same patient group (e.g., short-term and long-term rTMS effects; or different cognitive measures), we obtained one averaged effect size across multiple effect sizes within that particular study. If the effect sizes were reported from mild AD and severe AD separately within a single study or if the effect sizes were estimated from 2 independent subgroups of patients who received rTMS with different protocols, then the data were included as 2 independent units in the meta-analysis.
Due to the heterogeneity of cognitive measures included in each study, we first derived an effect size from the main outcome measures reported in each study. We then performed a sensitivity analysis that included additional secondary or exploratory cognitive measures (Please refer to the Sensitivity Analysis Section below). The cognitive tasks used for the estimation of effect size (i.e., the main outcome measures reported in each study) included the mini-mental status evaluation (MMSE), AD assessment scale cognitive subscale (ADAS-cog), Rey auditory verbal learning test (RAVLT), Rivermead behavioral memory test (RBMT), Battery for analysis of aphasic deficits, trail making task, Stroop color-word task, word-image association task, recognition memory task, and action naming and object naming task.
Additionally, although our overall effect was estimated across diagnosis (AD and MCI), number of rTMS sessions (i.e., short and long rTMS manipulation), rTMS site, rTMS frequency (i.e., low frequency: ≤ 1 Hz and high frequency: ≥ 5 Hz), cognitive measures, and timing of outcome measurements (i.e., short-term: within 1 hour of the final rTMS session and long-term: ≥ 4 weeks post-rTMS), these potential rTMS moderating effects were investigated in the subgroup analyses (Please refer to the Subgroup Analyses Section below).
Heterogeneity analysis.
We used the Q statistics and the I2 index to assess heterogeneity. A probability value less than 0.05 and I2 greater than 40% is indicative of heterogeneity between included studies as it exceeds what is expected by chance (Higgins, et al., 2003).
Publication or selection bias.
Publication/selection bias was evaluated with Egger’s Test of Asymmetry (Egger, et al., 1997) and Orwin’s fail-safe N approach (Borenstein, et al., 2009). In the absence of publication/selection bias, effect sizes are symmetrically distributed around the overall average effect size, since the sampling error is random. Egger’s test evaluated whether the amount of asymmetry is significant. In addition, studies that demonstrated lack of benefit might not have been submitted and/or accepted for publication. Therefore, we used the Orwin’s fail-safe N to estimate the number of missing studies (with a mean effect size of 0) that would need to be incorporated in our meta-analysis to make the summary effect become trivial.
Subgroup analyses.
Our pre-specified categories for subgroup analyses included diagnosis (AD and MCI), rTMS site, rTMS frequency (low: ≤ 1 Hz and high: ≥ 5 Hz), cognitive measures, timing of outcome measurements (short-term: within 1 hour of the final rTMS session vs. long-term: ≥ 4 weeks post-rTMS), number of rTMS sessions (i.e., short vs. long rTMS manipulation), and the interaction between rTMS site, rTMS frequency and outcome measures.
Sensitivity analysis.
The process of undertaking a meta-analysis involves making decisions about which outcome measures to include in the analysis. We performed a sensitivity analysis to examine whether our results would have differed if we had included other secondary or exploratory cognitive measures in our estimation of effect sizes.
Risk of Bias Assessment in Individual Studies
We used the Physiotherapy Evidence Database (PEDro) scale (de Morton, 2009, Maher, et al., 2003) to quantify the quality of included studies. The PEDro scale scored 11 items (Supplementary Table 1) as either present or absent. The final score is the number of positive answers on all questions. We considered a PEDro score of 11 to represent an excellent-quality study, a score between 8 and 10 a good-quality study, a score of 6 and 7 a fair-quality study, and a score of ≤ 5 a low-quality study (Franssen, et al., 2014).
Risk of Bias Assessment across Studies
We utilized the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach to assess the quality of evidence (Atkins, et al., 2004). Four levels of quality of evidence are specified: high quality, moderate quality, low quality, and very low quality. A high level of evidence is initially assumed, and subsequently downgraded for meeting any of the following criteria (O’Connell, et al., 2014):
Risk of bias: downgrade once if less than 75% of included studies are excellent or good quality.
Heterogeneity: downgrade once if heterogeneity between the included studies is significant and the I2 value is greater than 40%.
Indirectness: downgrade once if more than 50% of the participants were outside the target group.
Imprecision: downgrade once if fewer than 400 participants (Guyatt, et al., 2011).
Publication/selection bias: downgrade once if the publication/selection bias is significant.
RESULTS
Search Results
Our initial search of all databases retrieved 124 studies and 1 additional article was identified from previous reviews (Figure 1). After rejecting articles based off the contents of the title and abstract, the full texts of 28 articles were obtained for further examination. Of these, 15 studies were excluded (see Supplementary Table 1). The remaining 13 studies (Ahmed, et al., 2012, Anderkova, et al., 2015, Cotelli, et al., 2011, Cotelli, et al., 2008, Drumond Marra, et al., 2015, Eliasova, et al., 2014, Koch, et al., 2018, Padala, et al., 2018, Rutherford, et al., 2015, Sole-Padulles, et al., 2006, Turriziani, et al., 2012, Wu, et al., 2015, Zhao, et al., 2017) that met the inclusion criteria were included for this meta-analysis. Among the 13 included studies, Cotelli et al. (2008) separately reported statistics from mild AD and moderate-to-severe AD, and Ahmed et al. (2012) distinctly described statistics from independent subgroups of AD patients who received rTMS with different protocols (i.e., inhibitory or excitatory). Therefore, those data (Ahmed, et al., 2012, Cotelli, et al., 2008) were included as multiple independent units in the meta-analysis. We only needed to request additional data from one group of authors to calculate effect sizes for the sensitivity analysis. The authors responded to our request within 10 days. Thus, no study was excluded due to the lack of data required for the effect size estimation
Study Characteristics
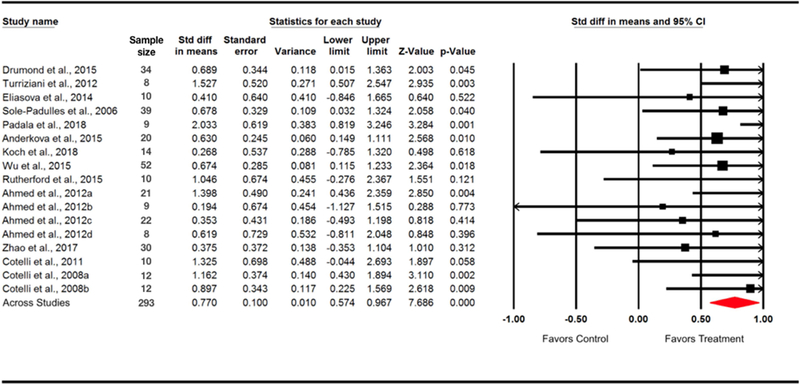
The 13 eligible studies included 293 participants (age range = 50–87 years, 45% males). The total effect size (pooled across all parameters) of rTMS on cognitive performance was 0.77 (95% CI = [0.57, 0.97]), indicating a medium-to-large effect size favoring active rTMS over sham rTMS (z = 7.69, p < 0.0001). The main characteristics of the included studies are described in Tables 1, 2, 3, and the distribution of effect sizes is illustrated in Figure 2.
Table 1.
Characteristics of included studies – participants and design
| Source | Sample size | Diagnosis | Age (years) | Gender (M/F) | DD (years) | Study design |
|---|---|---|---|---|---|---|
| Padala et al., 2018 | 9a | MCI | 66 ± 9 | 8/1 | NA | RAN_CO_DB_SC |
| Koch et al., 2018 | 14 | aMCI | 70 ± 5 | 7/7 | 1 | RAN_CO_DB_SC |
| Zhao et al., 2017 | 30 | AD | 71 ± 6 | 15/15 | NA | RAN_PA_DB_SC |
| Anderkova et al., 2015 | 20 | 12 AD, 8 MCI | 73 ± 7 | 9/11 | 3 ± 2 | RAN_CO_SB_SC |
| Wu et al., 2015 | 52 | AD | 72 ± 5 | 21/31 | 5 ± 2 | RAN_PA_DB_SC |
| Rutherford et al., 2015 | 10 | AD | 57 – 87 | 3/7 | NA | RAN_CO_DB_SC |
| Drumond Marra et al., 2015 | 34 | MCI | 60 – 74 | 12/22 | NA | RAN_PA_DB_SC |
| Eliasova et al., 2014 | 10 | 7 Mild AD, 3 aMCI | 75 ± 8 | 6/4 | ~4 | RAN_CO_SC |
| Ahmed et al., 2012 | 45 | Probable AD | 68 ± 6 | 16/29 | NA | RAN_PA_SB_SC |
| Turriziani et al., 2012 | 8 | aMCI | 66 ± 6 | 8/0 | 1–2 | RAN_CO_SC |
| Cotelli et al., 2011 | 10 | AD | 76 ± 5 | NA | NA | RAN_PA_SB_SC |
| Cotelli et al., 2008, mAD | 12 | mild AD | 75 ± 6 | NA | NA | RAN_CO_SC |
| Cotelli et al., 2008, msAD | 12 | moderate to severe AD | 78 ± 6 | NA | NA | RAN_CO_SC |
| Sole-Padulles et al., 2006 | 39 | aMCI | > 50 | 1½8 | >1 year | RAN_PA_DB_SC |
Note. AD = Alzheimer’s disease; aMCI = amnestic mild cognitive impairment; CO = cross-over; DB = double-blind; DD = Disease duration; mAD = mild AD; ms AD = moderate to severe AD; MCI = mild cognitive impairment; NA = not available; PA = parallel; RAN = randomized; SB = single-blind; SC = sham-controlled.
Data from enrolled participants
Participants who completed the rTMS sessions and the 1-hour after-rTMS evaluation
Median disease duration
Data analyzed
Table 2.
Characteristics of included studies – rTMS parameters
| Source | rTMS site | rTMS frequency | Intensity | No. of pulsesa | Treatment duration | Sham rTMS |
|---|---|---|---|---|---|---|
| Padala et al., 2018 | L-DLPFC | 10 Hz | 120% RMT | 30000 (3000 × 10) | 2 weeks | Tilted coil |
| Koch et al., 2018 | L parietal region (precuneus) | 20 Hz | 100% RMT | 16000 (1600 × 10) | 1 weeks | Sham coil |
| Zhao et al., 2017 | Bilateral parietal region and posterior temporal areas | 20 Hz | NA | 120000 (4000 × 30) | 6 weeks | Inactive coil with sound |
| Anderkova et al., 2015 | R IFG and R STG | 10 Hz | 90% RMT | 2250 (2250 × 1) | 1 session for each target | Stimulating vertex |
| Wu et al., 2015 | L DLPFC | 20 Hz | 80% RMT | 24000 (1200 × 20) | 4 weeks | Tilted coil |
| Rutherford et al., 2015 | Bilateral DLPFC | 20 Hz | 90%–100% RMT | 26000 (2000 × 13) | 4 weeks | Wooden block |
| Drumond Marra et al., 2015 | L DLPFC | 10 Hz | 110% RMT | 20000 (2000 × 10) | 2 weeks | Sham coil |
| Eliasova et al., 2014 | R IFG | 10 Hz | 90% RMT | 2250 (2250 × 1) | 1 day | Stimulating vertex |
|
Ahmed et al., 2012, 20Hz Ahmed et al., 2012, 1Hz |
Bilateral DLPFC | 20 Hz | 90% RMT | 10000 (2000 × 5) | 5 days | Elevated coil |
| Bilateral DLPFC | 1 Hz | 100% RMT | 10000 (2000 × 5) | 5 days | Elevated coil | |
| Turriziani et al., 2012 | L DLPFC | 1 Hz | 90% RMT | 600 (600 × 1) | 1 day | Tilted coil |
| R DLPFC | 1 Hz | 90% RMT | 600 (600 × 1) | 1 day | Tilted coil | |
|
Cotelli et al., 2008, mAD Cotelli et al., 2008, msAD |
L and R DLPFC | 20 Hz | 90% RMT | NA | 1 session for each target | Stimulating vertex |
| L and R DLPFC | 20 Hz | 90% RMT | NA | 1 session for each target | Stimulating vertex | |
| Cotelli et al., 2011 | L DLPFC | 20 Hz | 100% RMT | 20000 (2000 × 10) | 2 weeks | Sham coil |
| Sole-Padulles et al., 2006 | L DLPFC | 5 Hz | 80% RMT | 500 (500 × 1) | 1 day | Tilted coil |
Note. AMT = active motor threshold; M1 = primary motor cortex, mAD = mild Alzheimer’s disease; msAD = mild to severe Alzheimer’s disease; SMA = supplementary motor area; DLPFC = dorsolateral prefrontal cortex; PMd = dorsal premotor cortex; RMT = resting motor threshold
Total number of pulses (number of pulses per session x number of sessions).
Table 3.
Characteristics of included studies – outcome measurements
| Source | Cognitive measures used for the estimation of effect size (Type of cognitive domain) | Post-rTMS evaluation | Seizure due to rTMS |
|---|---|---|---|
| Padala et al., 2018 | MMSE (General cognitive function) and TMT (Executive function) | 1 hour after | No |
| Koch et al., 2018 | Delayed recall of the RAVLT (Memory) and MMSE (General cognitive function) | Immediately after | No |
| Zhao et al., 2017 | ADAS-cog (General cognitive function) and MMSE (General cognitive function) | Immediately after and 6 weeks after | No |
| Anderkova et al., 2015 | Stroop color-word task (Executive function) and TMT | 1 hour after | No |
| Wu et al., 2015 | ADAS-cog (General cognitive function) | Immediately after | No |
| Rutherford et al., 2015 | Word-image association (Memory), ADAS-cog (General cognitive function), and associative memory (Memory) | Immediately after | No |
| Drumond Marra et al., 2015 | RBMT (Memory), letter-number sequencing test (Executive function), and TMT-B (Executive function) | Immediately after and 1 month after | No |
| Eliasova et al., 2014 | TMT-A, TMT-B (Executive function) and Stroop task (Executive function) | Immediately after | No |
| Ahmed et al., 2012, 20Hz | MMSE (General cognitive function) | Immediately after, 1 month after, and 3 months after | No |
| Ahmed et al., 2012, 1Hz | MMSE (General cognitive function) | Immediately after, 1 month after, and 3 months after | No |
| Turriziani et al., 2012, LDLPFC | Recognition memory test (Memory) | Immediately after | No |
| Turriziani et al., 2012, RDLPFC | Recognition memory test (Memory) | Immediately after | No |
| Cotelli et al., 2008, mAD | Action naming and object naming (Language) | Immediately after | No |
| Cotelli et al., 2008, msAD | Action naming and object naming (Language) | Immediately after | No |
| Cotelli et al., 2011 | SC-BADA (Language) and MMSE (General cognitive function) | Immediately after | No |
| Sole-Padulles et al., 2006 | Associative memory (Memory) | Immediately after | No |
Abbreviations. ADAS-cog = AD assessment scale cognitive subscale; MMSE = mini-mental status evaluation; RAVLT = Rey auditory verbal learning test; RBMT = Rivermead behavioral memory test; SC-BADA = Battery for analysis of aphasic deficits; TMT = trail making task. Note. Cognitive measures listed in this table were included in the sensitivity analysis. Cognitive measures in bold were the primary outcome measures used to derive the overall effect size. The italicized tasks were included in the sensitivity analysis.
Figure 2.
Forest plot. Individual and pooled rTMS effect sizes (SMDs) for cognitive function in patients with MCI or AD. The size of the squares increases with increasing sample size. Cotelli et al. (2008) reported statistics from mild AD (Cotelli et al., 2008a) and moderate-to-severe AD (Cotelli et al., 2008b) separately, and Ahmed et al. (2012) described statistics from 4 groups (Ahmed et al., 2012a = high-frequency rTMS in mild AD; Ahmed et al., 2012b = high-frequency rTMS in severe AD; Ahmed et al., 2012c = low-frequency rTMS in mild AD; Ahmed et al., 2012d = low-frequency rTMS in severe AD;), therefore, those data were included as multiple independent units in the meta-analysis.
Heterogeneity between the included studies did not exceed that expected by chance, Q = 14.55, df(Q) = 16, p = 0.56, I2 = 0.00, which indicate that the results across the included studies were statistically homogeneous. Publication bias was evaluated using Egger’s Test of Asymmetry and Orwin’s fail-safe N approach. Egger’s Test did not reveal significant asymmetry across included studies (intercept = 0.76, df = 15, t = 1.08, two-tailed p = 0.30, Supplementary Figure 1). Orwin’s fail-safe N analysis showed that 239 studies with a mean effect size of 0 would be needed to offset the conclusion that we are able to draw from the 13 studies included in this analysis (i.e., to bring p-value greater than 0.05). This suggests that our findings are robust, and it reduces concerns of potential publication bias.
Subgroup Analyses
Patient population.
Six studies reported rTMS effects in AD (Ahmed, et al., 2012, Cotelli, et al., 2011, Cotelli, et al., 2008, Rutherford, et al., 2015, Wu, et al., 2015, Zhao, et al., 2017), 5 studies presented rTMS efficacy in MCI (Drumond Marra, et al., 2015, Koch, et al., 2018, Padala, et al., 2018, Sole-Padulles, et al., 2006, Turriziani, et al., 2012), and 2 studies included data on both AD and MCI (Anderkova, et al., 2015, Eliasova, et al., 2014) (Table 1). Our subgroup analysis revealed that the effect sizes of rTMS estimated from both MCI (SMD = 0.91, p < 0.0001) and AD (SMD = 0.75, p < 0.0001) were significant.
Stimulation site x frequency x outcome measures.
For the stimulation site, 9 studies stimulated left, right, or bilateral DLPFC, 2 studies targeted the right inferior frontal gyrus (IFG), and the remaining 2 studies stimulated the temporo-parietal regions (Table 2). Pertaining to the stimulation frequency, the majority of included studies (12/13) used high-frequency (≥ 5 Hz) rTMS protocols, while only 2 studies employed low-frequency (≤ 1 Hz) rTMS protocols (Table 2). Regarding the outcome measures, memory, executive, language, and general cognitive functions have been included for the assessments of the rTMS effects. Please see Table 3 for a complete list of neuropsychological tests that were administered in each study, and the respective time points at which each was assessed.
To parse the effectiveness of various combinations of rTMS parameters on different cognitive domains, we ran Stimulation site x frequency x outcome measures subgroup analyses across the included studies. The estimated effect sizes and p values are illustrated in Figure 3. First, the effects of rTMS at the DLPFC were lateralized. High-frequency rTMS over the left DLPFC (SMD = 0.68, p < 0.005) and low-frequency rTMS at the right DLPFC (SMD = 1.53, p < 0.005) significantly improved memory functions. Second, high-frequency rTMS targeting the right IFG significantly improved executive functions. Additionally, the effects of high-frequency rTMS over the left DLPFC on language (SMD = 1.33, p = 0.06) and general cognitive function (SMD = 1.24, p = 0.06) were marginally significant. Although the effects of high-frequency rTMS at bilateral DLPFC on memory and general cognitive function were not significant, it should be noted that all of these effect sizes were greater than 0.8, which may be indicative of a large effect of active rTMS over the sham-condition. Future studies are warranted to further investigate these rTMS protocols in patients with AD or MCI.
Figure 3.
rTMS effect sizes for various combinations of stimulation site, frequency, and outcome measures. DLPFC = dorsolateral prefrontal cortex; ES = effect size; High-F = high-frequency rTMS; IFG = inferior frontal gyrus; Low-F = low-frequency rTMS.
Number of rTMS sessions (i.e., short vs. long rTMS manipulation).
Most of the included studies administered rTMS for multiple sessions on consecutive days, but 5 studies only consisted of a single rTMS session (Anderkova, et al., 2015, Cotelli, et al., 2008, Eliasova, et al., 2014, Sole-Padulles, et al., 2006, Turriziani, et al., 2012) (Table 2). Our subgroup analysis showed that both single-session rTMS (SMD = 0.83, p < 0.001) and multi-session rTMS (SMD = 0.72, p < 0.001) improved cognitive functions.
Timing of outcome measurements.
All 13 studies assessed cognitive functions acutely (≤ 1 hour after the final rTMS session), and 3 studies additionally reported follow-up behavioral assessments as a long-term outcome measure (≥ 4 weeks) (Ahmed, et al., 2012, Drumond Marra, et al., 2015, Zhao, et al., 2017). The acute assessment was administered within 1 hour of the final rTMS session, while the chronic assessment occurred either at 4 weeks, 6 weeks, or 12 weeks after the final rTMS session (Table 3). Results of our subgroup analysis revealed that both short-term (SMD = 0.71, p < 0.0001) and long-term rTMS effects on cognitive functions (SMD = 0.71, p < 0.0001) were significant, suggesting that with 5–30 consecutive rTMS sessions, the effects could last 4–12 weeks.
Sensitivity Analysis
We included secondary or exploratory cognitive measures from 8 studies (Anderkova, et al., 2015, Cotelli, et al., 2011, Drumond Marra, et al., 2015, Eliasova, et al., 2014, Koch, et al., 2018, Padala, et al., 2018, Rutherford, et al., 2015, Zhao, et al., 2017) into our meta-analysis to examine whether the results would change (Table 3). The pooled rTMS effect remained medium-to-large and significant (SMD = 0.65, 95% CI = [0.46, 0.84], z = 6.61, p < .0001), as did the pooled effect from the primary cognitive measures (SMD = 0.77, 95% CI = [0.57, 0.97], z = 7.69, p < .0001).
Adverse Events
No incidents of seizure were reported in this patient population across the 13 studies. Eight studies further assessed the incidence of other minor adverse responses related to the application of rTMS. Among them, patients in the Ahmed et al. (2012) and Cotelli et al. (2011) studies did not report any adverse events during or after rTMS sessions. The minor adverse events reported due to rTMS are summarized in Supplementary Table 2.
Risk of Bias Assessment in Individual Studies
The risk of bias assessment for all included studies is summarized in Supplementary Table 1. Overall, our analysis indicates that 2 studies were of excellent methodological quality (PEDro scale 11/11), 10 studies were of good methodological quality (8– 10/11), and one study was of fair methodological quality (7/11).
Risk of Bias Assessment across Studies
Using the GRADE criteria, we characterized the quality of evidence presented in this meta-analysis as moderate quality. The initial presumption for a high level of evidence was downgraded once because fewer than 400 participants were included in this meta-analysis. Despite this imperfection, our meta-analysis exhibited low risk of bias, adequate homogeneity, directedness (i.e., all participants were patients with AD or MCI), and it is free from publication/selection bias across included studies.
DISCUSSION
Overall, our meta-analysis provides evidence supporting a beneficial effect of rTMS on cognitive functions in both patients with MCI and AD. Subgroup analyses indicate that 1) high-frequency rTMS over the left DLPFC and low-frequency rTMS at the right DLPFC significantly improved memory functions; 2) high-frequency rTMS targeting the right IFG significantly enhanced executive performance; and 3) the effects of 5–30 consecutive rTMS sessions could last for 4–12 weeks.
What are the potential mechanisms underlying the rTMS effects on cognitive functions?
Previous animal models of dementia indicate that both high-frequency (Ma, et al., 2017, Wang, et al., 2010, Zhang, et al., 2015) and low-frequency (Huang, et al., 2017, Wang, et al., 2010, Yang, et al., 2015, Zhang, et al., 2018) rTMS (daily sessions for 2–4 weeks) can significantly improve hippocampal dependent functions of learning and memory. These findings are congruent with those presented in this meta-analysis, and a closer examination of such experimental models may yield additional insights as to the underlying mechanisms of rTMS. Here, we will explore the multifaceted neurobiological effects of rTMS that may be interacting in some capacity to influence cognitive functions.
Numerous in vitro and in vivo experimental models provide evidence that rTMS can increase long-term potentiation (LTP) (Thickbroom, 2007). Of particular relevance, this has been documented in various murine models of dementia where rTMS rescues LTP deficits (Tan, et al., 2013, Wang, et al., 2015, Zhang, et al., 2015, Zhen, et al., 2017). These improvements in neural plasticity are observed in conjunction with improved performance on hippocampal dependent measures of spatial cognition (Huang, et al., 2017, Ma, et al., 2014, Tan, et al., 2013, Wang, et al., 2015). Further, in murine models of AD, the increase in LTP and improvement in spatial cognition following rTMS are observed concomitantly with reduced amyloid-β load (Huang, et al., 2017, Tan, et al., 2013, Wang, et al., 2015). Reported mechanisms to explain this reduction in pathology by rTMS include 1) the reduction of amyloid precursor protein (APP) (Huang, et al., 2017), and 2) facilitation of the large-conductance calcium-activated potassium channel via increased expression of the Homer1a scaffold protein (Wang, et al., 2015).
On more granular level, there are numerous rTMS induced effects that likely mediate this enhanced synaptic function. Numerous studies, for example, report that hippocampal expression of brain derived neurotrophic factor (BDNF) and vascular endothelial growth factor (VEGF) are increased following rTMS (Gersner, et al., 2011, Ma, et al., 2014, Muller, et al., 2000, Shang, et al., 2016, Zhang, et al., 2015). In addition to these neurotrophic factors, rTMS reportedly increases expression of N-methyl-D-aspartate (NMDA) receptors and other proteins that facilitate synaptic plasticity (e.g., synaptophysin, post-synaptic density protein-95, cyclin dependent kinase 5, GAP43) (Etievant, et al., 2015, Kole, et al., 1999, Ma, et al., 2017, Ma, et al., 2014, Shang, et al., 2016, Wang, et al., 2010, Yang, et al., 2015, Zhang, et al., 2015).
Relatedly, there are reports of rTMS increases hippocampal neurogenesis in the dentate gyrus (Ueyama, et al., 2011), which plays a critical role in pattern separation (Clelland, et al., 2009). In addition to the neurotrophic mediators listed above, rTMS may promote neurogenesis via the increased expression of cholecystokinin (CCK) (Muller, et al., 2000). In addition to its neuroprotective effects in rodent models of AD (Sugaya, et al., 1992), CCK has been shown to increase cell proliferation and neurogenesis in the dentate gyrus (Reisi, et al., 2015). CCK is the most abundant peptide in the mammalian brain, and there is a dipropionate density of CCK receptors found in the hippocampus (Zarbin, et al., 1983). In numerous rodent models, increased expression of CCK corresponds with increased performance on hippocampal dependent tasks of learning and memory (Croll, et al., 1999, Reisi, et al., 2015, Sebret, et al., 1999, Taghzouti, et al., 1999). In a recent human study from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) dataset, CCK concentration in the cerebrospinal fluid was identified as a novel biomarker that was associated with both cognitive status and gray matter volume in key anatomical regions associated with AD pathology (Plagman, et al., 2019).
These microscopic alterations from rTMS may also interact to produce network level changes in brain function that are more readily detected in human trials. As an example, consider the findings reported by Solé-Padullés and colleagues in their clinical trial of AD patients that utilized task-based fMRI before and after rTMS. They report that improvements in associative memory following rTMS corresponded with compensatory recruitment of additional neural networks (Sole-Padulles, et al., 2006). Similar compensatory brain activity has been previously documented in older adults who retain high cognitive functions as they age (Bangen, et al., 2012, Cabeza, et al., 2002).
Why does the DLPFC rTMS enhance memory functions in AD and MCI?
The DLPFC is a core region involved in executive functions, such as working memory and cognitive flexibility (Blumenfeld, et al., 2011, Blumenfeld and Ranganath, 2007). Previous research reported that patients with MCI and AD have executive function deficits (Baudic, et al., 2006, Chen, et al., 1998, Guarino, et al., 2018, Guarino, et al., 2019) and the impairments in executive functions are considered to exacerbate memory deficits in this population (Buckner, 2004, Zheng, et al., 2012). Our subgroup analyses provide evidence showing that the DLPFC rTMS significantly improved memory functions in patients with MCI or AD. One possible explanation for this effect is that the DLPFC may contribute to long-term memory formation through its interaction with regions within the medial temporal network (e.g., hippocampus) during memory encoding (Blumenfeld and Ranganath, 2006, Murray and Ranganath, 2007, Ranganath, et al., 2005, Simons and Spiers, 2003, Yuan, et al., 2016) and retrieval (Achim and Lepage, 2005, Balconi and Ferrari, 2012, Balconi and Ferrari, 2013, Manenti, et al., 2010, Simons and Spiers, 2003). Consistent with this idea, studies of patients with focal prefrontal lesion have reported that damage to the DLPFC can disrupt recognition memory (Duarte, et al., 2005, Gershberg and Shimamura, 1995); activity in the DLPFC during the early stage of working memory maintenance was predictive of subsequent long-term memory (Ranganath, et al., 2005); and disruption of working memory processing resulted in impaired long-term memory formation (Ranganath, et al., 2005). Collectively, previous behavioral and imaging studies suggest that successful long-term memory may depend on effective control of information in working memory (Blumenfeld and Ranganath, 2007), and this relationship might be mediated by the DLPFC (Ranganath, et al., 2005, Yuan, et al., 2016).
Additionally, our subgroup analyses revealed that both high-frequency rTMS over the left DLPFC and low-frequency rTMS at the right DLPFC significantly improved memory functions in patients with MCI or AD. These two rTMS protocols have also been examined in major depressive disorder (MDD), and previous meta-analyses in MDD show that these two rTMS protocols exhibited similarly lateralized clinical efficacy on response rate and remission rate (Cao, et al., 2018, Chen, et al., 2013). Previous brain imaging studies in MDD found reduced cerebral blood flow and metabolism in the left DLPFC and hypermetabolism in the right DLPFC in acute MDD (Mayberg, 2003, Phillips, et al., 2003). Thus, it is postulated that patients with MDD benefit from high-frequency rTMS over the left DLPFC due to increasing cortical activity and benefit from low-frequency rTMS over the right DLPFC because the cortical activity is suppressed (Avery, et al., 2006, Cao, et al., 2018, Chen, et al., 2013, George, et al., 2010). Currently, it is not clear whether patients with MCI or AD exhibit similar prefrontal asymmetry as patients with MDD do. Furthermore, functional brain imaging data examining how each rTMS protocol modulates brain activity of the DLPFC and other connected regions in MCI and AD is very limited. Future studies that integrate rTMS and brain imaging techniques will be needed to understand the prefrontal functions in MCI and AD, and better explain the DLPFC rTMS effects.
Notably, the same rTMS protocols over the respective DLPFC targets elicit positive clinical outcomes in both patients with depression and those with MCI/AD. As an additional consideration, this might also be suggestive that the reported rTMS influence on cognition may be an indirect consequence of improved affect. Scholars have long discussed the appreciable interaction and marginal phenomenological distinction between affect and cognition (Duncan and Barrett, 2007). The two behavioral phenotypes are increasingly intertwined with age (Crocco, et al., 2010), which may speak to enmeshed neurobiological mechanisms (e.g., inflammation) or it may be driven by a bidirectional interaction of the phenomenology of the conditions. Only a single study included in our analysis reported outcome measures pertaining to both affect and cognition (Ahmed, et al., 2012). Interestingly, these authors report that the cohort with appreciable improvement in cognition also exhibited a concomitant decrease in depressive symptoms (Ahmed, et al., 2012). This possibility is met with conflicting evidence in the literature. Generally, cognitive improvements are not observed in clinical trials applying rTMS for depression (Martin, et al., 2017). However, this potential effect is likely influenced by the baseline cognitive status of patients receiving DLPFC rTMS. As such, the association between neurocognitive and mood improvement following rTMS may be more likely to emerge in populations where baseline cognition is more vulnerable (Ilieva, et al., 2018).
Limitations and future directions
Regarding the limitations of the present meta-analysis, our results could be constrained by insufficient number of patients (i.e., less than 400 patients with MCI or AD) included in this meta-analysis. In addition, very few studies have stimulated brain regions other than the DLPFC, therefore, the non-significant rTMS effects of other brain regions should be interpreted with caution. Future studies should focus on establishing a more precise relationship between stimulation site, rTMS frequency, and function of cognitive domains enhanced by rTMS; as well as 2) establishing a procedure to tailor the rTMS parameters for each individual patient.
Supplementary Material
Supplementary Figure 1. Funnel plot of standard error by effect size (SMD). The funnel plot was plotted with SMD on the X axis and the standard error on the Y axis.
Highlights.
Repetitive TMS is a promising tool to enhance cognitive functions in AD and MCI
High-frequency L-DLPFC and low-frequency R-DLPFC rTMS may improve memory function
High-frequency R-IFG rTMS may enhance executive performance
rTMS effects on cognition are documented at both acute and chronic time points
Chronic effects of consecutive rTMS sessions reportedly persist at 12 weeks
ACKNOWLEDGEMENTS
This work was supported by the NIH P30 AG019610 Arizona Alzheimer’s Consortium Pilot Study Program (Pilot Project PI: Y.-H. C.) and the BIO5 Team Scholars Award (PI: Y.-H. C.). The authors thank William Mennie for assistance in literature review.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Achim AM, Lepage M 2005. Dorsolateral prefrontal cortex involvement in memory post-retrieval monitoring revealed in both item and associative recognition tests. Neuroimage 24, 1113–21. [DOI] [PubMed] [Google Scholar]
- Ahmed MA, Darwish ES, Khedr EM, El Serogy YM, Ali AM 2012. Effects of low versus high frequencies of repetitive transcranial magnetic stimulation on cognitive function and cortical excitability in Alzheimer’s dementia. J Neurol 259, 83–92. [DOI] [PubMed] [Google Scholar]
- Anderkova L, Eliasova I, Marecek R, Janousova E, Rektorova I 2015. Distinct Pattern of Gray Matter Atrophy in Mild Alzheimer’s Disease Impacts on Cognitive Outcomes of Noninvasive Brain Stimulation. Journal of Alzheimer’s disease : JAD 48, 251–60. [DOI] [PubMed] [Google Scholar]
- Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, Guyatt GH, Harbour RT, Haugh MC, Henry D, Hill S, Jaeschke R, Leng G, Liberati A, Magrini N, Mason J, Middleton P, Mrukowicz J, O’Connell D, Oxman AD, Phillips B, Schunemann HJ, Edejer T, Varonen H, Vist GE, Williams JW Jr., Zaza S, Group, G.W. 2004. Grading quality of evidence and strength of recommendations. BMJ 328, 1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery DH, Holtzheimer PE 3rd, Fawaz W, Russo J, Neumaier J, Dunner DL, Haynor DR, Claypoole KH, Wajdik C, Roy-Byrne P 2006. A controlled study of repetitive transcranial magnetic stimulation in medication-resistant major depression. Biol Psychiatry 59, 187–94. [DOI] [PubMed] [Google Scholar]
- Balconi M, Ferrari C 2012. Emotional memory retrieval. rTMS stimulation on left DLPFC increases the positive memories. Brain imaging and behavior 6, 454–61. [DOI] [PubMed] [Google Scholar]
- Balconi M, Ferrari C 2013. Repeated transcranial magnetic stimulation on dorsolateral prefrontal cortex improves performance in emotional memory retrieval as a function of level of anxiety and stimulus valence. Psychiatry Clin Neurosci 67, 210–8. [DOI] [PubMed] [Google Scholar]
- Bangen KJ, Kaup AR, Mirzakhanian H, Wierenga CE, Jeste DV, Eyler LT 2012. Compensatory brain activity during encoding among older adults with better recognition memory for face-name pairs: an integrative functional, structural, and perfusion imaging study. J Int Neuropsychol Soc 18, 402–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker AT, Jalinous R, Freeston IL 1985. Non-invasive magnetic stimulation of human motor cortex. Lancet 1, 1106–7. [DOI] [PubMed] [Google Scholar]
- Baudic S, Barba GD, Thibaudet MC, Smagghe A, Remy P, Traykov L 2006. Executive function deficits in early Alzheimer’s disease and their relations with episodic memory. Arch Clin Neuropsychol 21, 15–21. [DOI] [PubMed] [Google Scholar]
- Bestmann S, Krakauer JW 2015. The uses and interpretations of the motor-evoked potential for understanding behaviour. Exp Brain Res 233, 679–89. [DOI] [PubMed] [Google Scholar]
- Birba A, Ibanez A, Sedeno L, Ferrari J, Garcia AM, Zimerman M 2017. Non-Invasive Brain Stimulation: A New Strategy in Mild Cognitive Impairment? Front Aging Neurosci 9, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld RS, Parks CM, Yonelinas AP, Ranganath C 2011. Putting the pieces together: the role of dorsolateral prefrontal cortex in relational memory encoding. Journal of cognitive neuroscience 23, 257–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld RS, Ranganath C 2006. Dorsolateral prefrontal cortex promotes long-term memory formation through its role in working memory organization. J Neurosci 26, 916–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld RS, Ranganath C 2007. Prefrontal cortex and long-term memory encoding: an integrative review of findings from neuropsychology and neuroimaging. Neuroscientist 13, 280–91. [DOI] [PubMed] [Google Scholar]
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR 2009. Publication bias. Introduction to meta-analysis John Wiley and Sons, Chichester, UK. [Google Scholar]
- Buckner RL 2004. Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron 44, 195–208. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR 2002. Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage 17, 1394–402. [DOI] [PubMed] [Google Scholar]
- Cao X, Deng C, Su X, Guo Y 2018. Response and Remission Rates Following High-Frequency vs. Low-Frequency Repetitive Transcranial Magnetic Stimulation (rTMS) Over Right DLPFC for Treating Major Depressive Disorder (MDD): A Meta-Analysis of Randomized, Double-Blind Trials. Front Psychiatry 9, 413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Zhou C, Wu B, Wang Y, Li Q, Wei Y, Yang D, Mu J, Zhu D, Zou D, Xie P 2013. Left versus right repetitive transcranial magnetic stimulation in treating major depression: a meta-analysis of randomised controlled trials. Psychiatry Res 210, 1260–4. [DOI] [PubMed] [Google Scholar]
- Chen R, Classen J, Gerloff C, Celnik P, Wassermann EM, Hallett M, Cohen LG 1997. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology 48, 1398–403. [DOI] [PubMed] [Google Scholar]
- Chen ST, Sultzer DL, Hinkin CH, Mahler ME, Cummings JL 1998. Executive dysfunction in Alzheimer’s disease: association with neuropsychiatric symptoms and functional impairment. J Neuropsychiatry Clin Neurosci 10, 426–32. [DOI] [PubMed] [Google Scholar]
- Cheng CPW, Wong CSM, Lee KK, Chan APK, Yeung JWF, Chan WC 2017. Effects of repetitive transcranial magnetic stimulation on improvement of cognition in elderly patients with cognitive impairment: a systematic review and meta-analysis. Int J Geriatr Psychiatry [DOI] [PubMed]
- Chou YH, Hickey PT, Sundman M, Song AW, Chen NK 2015a. Effects of Repetitive Transcranial Magnetic Stimulation on Motor Symptoms in Parkinson Disease: A Systematic Review and Meta-analysis. JAMA neurology 72, 432–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou YH, You H, Wang H, Zhao YP, Hou B, Chen NK, Feng F 2015b. Effect of repetitive transcranial magnetic stimulation on fMRI resting-state connectivity in multiple system atrophy. Brain Connect 5, 451–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD Jr., Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, Bussey TJ 2009. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science 325, 210–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotelli M, Calabria M, Manenti R, Rosini S, Zanetti O, Cappa SF, Miniussi C 2011. Improved language performance in Alzheimer disease following brain stimulation. J Neurol Neurosurg Psychiatry 82, 794–7. [DOI] [PubMed] [Google Scholar]
- Cotelli M, Manenti R, Cappa SF, Zanetti O, Miniussi C 2008. Transcranial magnetic stimulation improves naming in Alzheimer disease patients at different stages of cognitive decline. Eur J Neurol 15, 1286–92. [DOI] [PubMed] [Google Scholar]
- Crocco EA, Castro K, Loewenstein DA 2010. How late-life depression affects cognition: neural mechanisms. Curr Psychiatry Rep 12, 34–8. [DOI] [PubMed] [Google Scholar]
- Croll SD, Chesnutt CR, Greene NA, Lindsay RM, Wiegand SJ 1999. Peptide immunoreactivity in aged rat cortex and hippocampus as a function of memory and BDNF infusion. Pharmacol Biochem Behav 64, 625–35. [DOI] [PubMed] [Google Scholar]
- de Morton NA 2009. The PEDro scale is a valid measure of the methodological quality of clinical trials: a demographic study. Aust J Physiother 55, 129–33. [DOI] [PubMed] [Google Scholar]
- Dong X, Yan L, Huang L, Guan X, Dong C, Tao H, Wang T, Qin X, Wan Q 2018. Repetitive transcranial magnetic stimulation for the treatment of Alzheimer’s disease: A systematic review and meta-analysis of randomized controlled trials. PLoS One 13, e0205704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drumond Marra HL, Myczkowski ML, Maia Memoria C, Arnaut D, Leite Ribeiro P, Sardinha Mansur CG, Lancelote Alberto R, Boura Bellini B, Alves Fernandes da Silva A, Tortella G, Ciampi de Andrade D, Teixeira MJ, Forlenza OV, Marcolin MA 2015. Transcranial Magnetic Stimulation to Address Mild Cognitive Impairment in the Elderly: A Randomized Controlled Study. Behav Neurol 2015, 287843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte A, Ranganath C, Knight RT 2005. Effects of unilateral prefrontal lesions on familiarity, recollection, and source memory. J Neurosci 25, 8333–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan S, Barrett LF 2007. Affect is a form of cognition: A neurobiological analysis. Cogn Emot 21, 1184–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger M, Smith GD, Schneider M, Minder C 1997. Bias in meta-analysis detected by a simple, graphical test. British Medical Journal 315, 629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliasova I, Anderkova L, Marecek R, Rektorova I 2014. Non-invasive brain stimulation of the right inferior frontal gyrus may improve attention in early Alzheimer’s disease: a pilot study. J Neurol Sci 346, 318–22. [DOI] [PubMed] [Google Scholar]
- Etievant A, Manta S, Latapy C, Magno LA, Fecteau S, Beaulieu JM 2015. Repetitive transcranial magnetic stimulation induces long-lasting changes in protein expression and histone acetylation. Sci Rep 5, 16873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald PB, Fountain S, Daskalakis ZJ 2006. A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clin Neurophysiol 117, 2584–96. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB, Hoy K, Daskalakis ZJ, Kulkarni J 2009. A randomized trial of the anti-depressant effects of low- and high-frequency transcranial magnetic stimulation in treatment-resistant depression. Depress Anxiety 26, 229–34. [DOI] [PubMed] [Google Scholar]
- Franssen M, Winward C, Collett J, Wade D, Dawes H 2014. Interventions for fatigue in Parkinson’s disease: A systematic review and meta-analysis. Mov Disord 29, 1675–8. [DOI] [PubMed] [Google Scholar]
- George MS, Lisanby SH, Avery D, McDonald WM, Durkalski V, Pavlicova M, Anderson B, Nahas Z, Bulow P, Zarkowski P, Holtzheimer PE 3rd, Schwartz T, Sackeim HA 2010. Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: a sham-controlled randomized trial. Arch Gen Psychiatry 67, 507–16. [DOI] [PubMed] [Google Scholar]
- Gershberg FB, Shimamura AP 1995. Impaired use of organizational strategies in free recall following frontal lobe damage. Neuropsychologia 33, 1305–33. [DOI] [PubMed] [Google Scholar]
- Gersner R, Kravetz E, Feil J, Pell G, Zangen A 2011. Long-term effects of repetitive transcranial magnetic stimulation on markers for neuroplasticity: differential outcomes in anesthetized and awake animals. J Neurosci 31, 7521–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarino A, Favieri F, Boncompagni I, Agostini F, Cantone M, Casagrande M 2018. Executive Functions in Alzheimer Disease: A Systematic Review. Front Aging Neurosci 10, 437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarino A, Forte G, Giovannoli J, Casagrande M 2019. Executive functions in the elderly with mild cognitive impairment: a systematic review on motor and cognitive inhibition, conflict control and cognitive flexibility. Aging Ment Health, 1–18. [DOI] [PubMed]
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso-Coello P, Rind D, Devereaux PJ, Montori VM, Freyschuss B, Vist G, Jaeschke R, Williams JW Jr., Murad MH, Sinclair D, Falck-Ytter Y, Meerpohl J, Whittington C, Thorlund K, Andrews J, Schunemann HJ 2011. GRADE guidelines 6. Rating the quality of evidence--imprecision. J Clin Epidemiol 64, 1283–93. [DOI] [PubMed] [Google Scholar]
- Hallett M 2007. Transcranial magnetic stimulation: a primer. Neuron 55, 187–99. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG 2003. Measuring inconsistency in meta-analyses. BMJ 327, 557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu WY, Ku Y, Zanto TP, Gazzaley A 2015. Effects of noninvasive brain stimulation on cognitive function in healthy aging and Alzheimer’s disease: a systematic review and meta-analysis. Neurobiology of aging 36, 2348–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Tan T, Du Y, Chen L, Fu M, Yu Y, Zhang L, Song W, Dong Z 2017. Low-Frequency Repetitive Transcranial Magnetic Stimulation Ameliorates Cognitive Function and Synaptic Plasticity in APP23/PS45 Mouse Model of Alzheimer’s Disease. Front Aging Neurosci 9, 292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilieva IP, Alexopoulos GS, Dubin MJ, Morimoto SS, Victoria LW, Gunning FM 2018. Age-Related Repetitive Transcranial Magnetic Stimulation Effects on Executive Function in Depression: A Systematic Review. Am J Geriatr Psychiatry 26, 334–46. [DOI] [PubMed] [Google Scholar]
- Klomjai W, Katz R, Lackmy-Vallee A 2015. Basic principles of transcranial magnetic stimulation (TMS) and repetitive TMS (rTMS). Ann Phys Rehabil Med 58, 208–13. [DOI] [PubMed] [Google Scholar]
- Koch G, Bonni S, Pellicciari MC, Casula EP, Mancini M, Esposito R, Ponzo V, Picazio S, Di Lorenzo F, Serra L, Motta C, Maiella M, Marra C, Cercignani M, Martorana A, Caltagirone C, Bozzali M 2018. Transcranial magnetic stimulation of the precuneus enhances memory and neural activity in prodromal Alzheimer’s disease. Neuroimage 169, 302–11. [DOI] [PubMed] [Google Scholar]
- Kole MH, Fuchs E, Ziemann U, Paulus W, Ebert U 1999. Changes in 5-HT1A and NMDA binding sites by a single rapid transcranial magnetic stimulation procedure in rats. Brain research 826, 309–12. [DOI] [PubMed] [Google Scholar]
- Liao X, Li G, Wang A, Liu T, Feng S, Guo Z, Tang Q, Jin Y, Xing G, McClure MA, Chen H, He B, Liu H, Mu Q 2015. Repetitive Transcranial Magnetic Stimulation as an Alternative Therapy for Cognitive Impairment in Alzheimer’s Disease: A Meta-Analysis. Journal of Alzheimer’s disease : JAD 48, 463–72. [DOI] [PubMed] [Google Scholar]
- Liston C, Chen AC, Zebley BD, Drysdale AT, Gordon R, Leuchter B, Voss HU, Casey BJ, Etkin A, Dubin MJ 2014. Default mode network mechanisms of transcranial magnetic stimulation in depression. Biol Psychiatry 76, 517–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Wang J, Lv C, Pang J, Han B, Wang M, Gen Y 2017. The Role of Hippocampal Structural Synaptic Plasticity in Repetitive Transcranial Magnetic Stimulation to Improve Cognitive Function in Male SAMP8 Mice. Cell Physiol Biochem 41, 137–44. [DOI] [PubMed] [Google Scholar]
- Ma J, Zhang Z, Kang L, Geng D, Wang Y, Wang M, Cui H 2014. Repetitive transcranial magnetic stimulation (rTMS) influences spatial cognition and modulates hippocampal structural synaptic plasticity in aging mice. Exp Gerontol 58, 256–68. [DOI] [PubMed] [Google Scholar]
- Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M 2003. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther 83, 713–21. [PubMed] [Google Scholar]
- Manenti R, Cotelli M, Calabria M, Maioli C, Miniussi C 2010. The role of the dorsolateral prefrontal cortex in retrieval from long-term memory depends on strategies: a repetitive transcranial magnetic stimulation study. Neuroscience 166, 501–7. [DOI] [PubMed] [Google Scholar]
- Martin DM, McClintock SM, Forster JJ, Lo TY, Loo CK 2017. Cognitive enhancing effects of rTMS administered to the prefrontal cortex in patients with depression: A systematic review and meta-analysis of individual task effects. Depress Anxiety 34, 1029–39. [DOI] [PubMed] [Google Scholar]
- Mayberg HS 2003. Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. Br Med Bull 65, 193–207. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, Group, P. 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6, e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller MB, Toschi N, Kresse AE, Post A, Keck ME 2000. Long-term repetitive transcranial magnetic stimulation increases the expression of brain-derived neurotrophic factor and cholecystokinin mRNA, but not neuropeptide tyrosine mRNA in specific areas of rat brain. Neuropsychopharmacology 23, 205–15. [DOI] [PubMed] [Google Scholar]
- Murray LJ, Ranganath C 2007. The dorsolateral prefrontal cortex contributes to successful relational memory encoding. J Neurosci 27, 5515–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardone R, Tezzon F, Holler Y, Golaszewski S, Trinka E, Brigo F 2014. Transcranial magnetic stimulation (TMS)/repetitive TMS in mild cognitive impairment and Alzheimer’s disease. Acta Neurol Scand 129, 351–66. [DOI] [PubMed] [Google Scholar]
- O’Connell NE, Wand BM, Marston L, Spencer S, Desouza LH 2014. Non-invasive brain stimulation techniques for chronic pain. Cochrane Database Syst Rev 4, CD008208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reardon JP, Solvason HB, Janicak PG, Sampson S, Isenberg KE, Nahas Z, McDonald WM, Avery D, Fitzgerald PB, Loo C, Demitrack MA, George MS, Sackeim HA 2007. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry 62, 1208–16. [DOI] [PubMed] [Google Scholar]
- Padala PR, Padala KP, Lensing SY, Jackson AN, Hunter CR, Parkes CM, Dennis RA, Bopp MM, Caceda R, Mennemeier MS, Roberson PK, Sullivan DH 2018. Repetitive transcranial magnetic stimulation for apathy in mild cognitive impairment: A double-blind, randomized, sham-controlled, cross-over pilot study. Psychiatry Res 261, 312–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Leone A, Valls-Sole J, Wassermann EM, Hallett M 1994. Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain 117 ( Pt 4), 847–58. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R 2003. Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biol Psychiatry 54, 515–28. [DOI] [PubMed] [Google Scholar]
- Plagman A, Hoscheidt S, McLimans KE, Klinedinst B, Pappas C, Anantharam V, Kanthasamy A, Willette AA, Alzheimer’s Disease Neuroimaging I 2019. Cholecystokinin and Alzheimer’s disease: a biomarker of metabolic function, neural integrity, and cognitive performance. Neurobiology of aging 76, 201–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C, Cohen MX, Brozinsky CJ 2005. Working memory maintenance contributes to long-term memory formation: neural and behavioral evidence. Journal of cognitive neuroscience 17, 994–1010. [DOI] [PubMed] [Google Scholar]
- Reisi P, Ghaedamini AR, Golbidi M, Shabrang M, Arabpoor Z, Rashidi B 2015. Effect of cholecystokinin on learning and memory, neuronal proliferation and apoptosis in the rat hippocampus. Adv Biomed Res 4, 227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford G, Lithgow B, Moussavi Z 2015. Short and Long-term Effects of rTMS Treatment on Alzheimer’s Disease at Different Stages: A Pilot Study. J Exp Neurosci 9, 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebret A, Lena I, Crete D, Matsui T, Roques BP, Dauge V 1999. Rat hippocampal neurons are critically involved in physiological improvement of memory processes induced by cholecystokinin-B receptor stimulation. J Neurosci 19, 7230–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang Y, Wang X, Shang X, Zhang H, Liu Z, Yin T, Zhang T 2016. Repetitive transcranial magnetic stimulation effectively facilitates spatial cognition and synaptic plasticity associated with increasing the levels of BDNF and synaptic proteins in Wistar rats. Neurobiol Learn Mem 134 Pt B, 369–78. [DOI] [PubMed] [Google Scholar]
- Simons JS, Spiers HJ 2003. Prefrontal and medial temporal lobe interactions in long-term memory. Nat Rev Neurosci 4, 637–48. [DOI] [PubMed] [Google Scholar]
- Sole-Padulles C, Bartres-Faz D, Junque C, Clemente IC, Molinuevo JL, Bargallo N, Sanchez-Aldeguer J, Bosch B, Falcon C, Valls-Sole J 2006. Repetitive transcranial magnetic stimulation effects on brain function and cognition among elders with memory dysfunction. A randomized sham-controlled study. Cereb Cortex 16, 1487–93. [DOI] [PubMed] [Google Scholar]
- Sugaya K, Takahashi M, Kubota K 1992. Cholecystokinin protects cholinergic neurons against basal forebrain lesion. Jpn J Pharmacol 59, 125–8. [DOI] [PubMed] [Google Scholar]
- Sutton SK, Davidson RJ 1997. Prefrontal brain asymmetry: A biological substrate of the behavioral approach and inhibition systems. Psychological Science 8, 204–10. [Google Scholar]
- Taghzouti K, Lena I, Dellu F, Roques BP, Dauge V, Simon H 1999. Cognitive enhancing effects in young and old rats of pBC264, a selective CCK(B) receptor agonist. Psychopharmacology (Berl) 143, 141–9. [DOI] [PubMed] [Google Scholar]
- Tan T, Xie J, Liu T, Chen X, Zheng X, Tong Z, Tian X 2013. Low-frequency (1 Hz) repetitive transcranial magnetic stimulation (rTMS) reverses Abeta(1–42)-mediated memory deficits in rats. Exp Gerontol 48, 786–94. [DOI] [PubMed] [Google Scholar]
- Thickbroom GW 2007. Transcranial magnetic stimulation and synaptic plasticity: experimental framework and human models. Exp Brain Res 180, 583–93. [DOI] [PubMed] [Google Scholar]
- Turriziani P, Smirni D, Zappala G, Mangano GR, Oliveri M, Cipolotti L 2012. Enhancing memory performance with rTMS in healthy subjects and individuals with Mild Cognitive Impairment: the role of the right dorsolateral prefrontal cortex. Front Hum Neurosci 6, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueyama E, Ukai S, Ogawa A, Yamamoto M, Kawaguchi S, Ishii R, Shinosaki K 2011. Chronic repetitive transcranial magnetic stimulation increases hippocampal neurogenesis in rats. Psychiatry Clin Neurosci 65, 77–81. [DOI] [PubMed] [Google Scholar]
- Wang F, Geng X, Tao HY, Cheng Y 2010. The restoration after repetitive transcranial magnetic stimulation treatment on cognitive ability of vascular dementia rats and its impacts on synaptic plasticity in hippocampal CA1 area. J Mol Neurosci 41, 145–55. [DOI] [PubMed] [Google Scholar]
- Wang F, Zhang Y, Wang L, Sun P, Luo X, Ishigaki Y, Sugai T, Yamamoto R, Kato N 2015. Improvement of spatial learning by facilitating large-conductance calcium-activated potassium channel with transcranial magnetic stimulation in Alzheimer’s disease model mice. Neuropharmacology 97, 210–9. [DOI] [PubMed] [Google Scholar]
- Wang JX, Rogers LM, Gross EZ, Ryals AJ, Dokucu ME, Brandstatt KL, Hermiller MS, Voss JL 2014. Targeted enhancement of cortical-hippocampal brain networks and associative memory. Science 345, 1054–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Xu W, Liu X, Xu Q, Tang L, Wu S 2015. Adjunctive treatment with high frequency repetitive transcranial magnetic stimulation for the behavioral and psychological symptoms of patients with Alzheimer’s disease: a randomized, double-blind, sham-controlled study. Shanghai Arch Psychiatry 27, 280–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HY, Liu Y, Xie JC, Liu NN, Tian X 2015. Effects of repetitive transcranial magnetic stimulation on synaptic plasticity and apoptosis in vascular dementia rats. Behav Brain Res 281, 149–55. [DOI] [PubMed] [Google Scholar]
- Yuan B, Chen J, Gong L, Shu H, Liao W, Wang Z, Liu D, Xie C, Zhang Z 2016. Mediation of episodic memory performance by the executive function network in patients with amnestic mild cognitive impairment: a resting-state functional MRI study. Oncotarget 7, 64711–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarbin MA, Innis RB, Wamsley JK, Snyder SH, Kuhar MJ 1983. Autoradiographic localization of cholecystokinin receptors in rodent brain. J Neurosci 3, 877–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Xing M, Wang Y, Tao H, Cheng Y 2015. Repetitive transcranial magnetic stimulation enhances spatial learning and synaptic plasticity via the VEGF and BDNF-NMDAR pathways in a rat model of vascular dementia. Neuroscience 311, 284–91. [DOI] [PubMed] [Google Scholar]
- Zhang XQ, Li L, Huo JT, Cheng M, Li LH 2018. Effects of repetitive transcranial magnetic stimulation on cognitive function and cholinergic activity in the rat hippocampus after vascular dementia. Neural Regen Res 13, 1384–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Li Z, Cong Y, Zhang J, Tan M, Zhang H, Geng N, Li M, Yu W, Shan P 2017. Repetitive transcranial magnetic stimulation improves cognitive function of Alzheimer’s disease patients. Oncotarget 8, 33864–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen J, Qian Y, Weng X, Su W, Zhang J, Cai L, Dong L, An H, Su R, Wang J, Zheng Y, Wang X 2017. Gamma rhythm low field magnetic stimulation alleviates neuropathologic changes and rescues memory and cognitive impairments in a mouse model of Alzheimer’s disease. Alzheimers Dement (N Y) 3, 487–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng D, Dong X, Sun H, Xu Y, Ma Y, Wang X 2012. The overall impairment of core executive function components in patients with amnestic mild cognitive impairment: a cross-sectional study. BMC Neurol 12, 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Funnel plot of standard error by effect size (SMD). The funnel plot was plotted with SMD on the X axis and the standard error on the Y axis.