Abstract
Workers involved in battery manufacturing or recycling factories are occupationally exposed to high concentrations of lead. In humans, lead can cause a wide range of biological effects depending upon the level and duration of exposure. The purpose of this study was to find out the blood lead levels (BLL) in occupationally exposed workers involved in battery industry in Delhi NCR region and to study whether lead affected the vitamin D (vit D) and calcium metabolism. For this study 100 occupationally lead-exposed battery workers (LEBW) and 100 non-lead exposed controls (NLEC) were recruited. BLL were measured using inductively coupled plasma optical emission spectrometer (ICP-OES) technique while ELISA was performed to quantify the serum vit D levels in the study subjects. Routine biochemical parameters were measured by chemistry autoanalyzers. Statistical analysis was done using appropriate statistical tools. Results showed that BLL were significantly higher in LEBW as compared to NLEC (p < 0.0001). Serum vitamin D, calcium and phosphorus levels were significantly decreased in battery workers as compared to controls (p < 0.005). Spearman’s rank correlation analysis showed significant negative correlation of BLL with serum Vitamin D and calcium levels. Significant positive correlation was observed between BLL and duration of lead exposure. Weak negative correlation was also observed between BLL and vit D even after adjusting for smoking status. In conclusion, this study demonstrated that higher BLL significantly alters the vit D and calcium metabolism.
Keywords: Occupational hazard, Battery workers, Blood lead levels, Vitamin D, Serum calcium
Introduction
Lead (Pb) is a ubiquitous and versatile metal, which is being used by humans for over 9000 years. However, due to its non-biodegradable nature and widespread use, it has grown to become an omnipresent pollutant. Despite stringent regulation and policies, it is one of the major environmental and occupational pollutants present in the developed and developing countries including India. Pb demonstrates properties such as corrosion resistance, pliability, high density, low elasticity, high thermal expansion, low melting point, easy workability, and others which lead to its use in painting industries, battery-manufacturing industries, silver jewelry making, soldering cans, folk remedies, petrol additives, soldering water distribution pipes, ceramic glazes, paper industries etc. [1, 2].
Pb exposure may occur from several sources including drinking water, traditional medicines, cosmetics, paints, ceramic dinnerware, ornaments, metallic products, automobile batteries and many more. Smoking or usage of tobacco in any other form is also known to be associated with high blood lead levels [3]. Environmental and occupational exposure, both constitute major modes of Pb toxicity caused mainly through dermal contact, ingestion and inhalation [4]. Upon exposure it is readily absorbed by the gastrointestinal tract and is rapidly taken into blood and soft tissues (half-life 28–36 days) and then to bones (half-life 27 years). Pb mainly accumulates in red blood cells, soft tissue such as brain, kidney and bone marrow and mineralized tissues like bone and teeth. Excretion of Pb from body is mainly through urine (> 90%) while partly it can be through sweat, hair, feces and nails [1, 2].
It has also been associated with several adverse health effects and hence currently has become a global health issue [1]. As per the reports of Centers for Disease Control and Prevention (CDC, 2011), effects of lead exposure range from acute to chronic, which in some cases can also be life-threatening [5]. As per the reports given by International Agency for Research on Cancer (IARC) [6],inorganic form of Pb acts as a potential human carcinogen. Major Pb-induced toxicities include central nervous system disorders [7] such as depression, panic attacks, anxiety, cognitive deficits [8], intelligence, memory, and attention disorders [9]; anemia, oxidative stress [10]; effects on fetus and child growth [11], cardiovascular effects [12], skeletal system disorders, and have adverse effects on hematological, respiratory, gastrointestinal, reproductive, and endocrine systems.
Over the past few decades, there has been significant advancement in the regulation and policies framed to determine the critical limit of Pb in the blood. As per the Center for Disease Control (CDC, 2011), reference blood lead levels of < 10 μg/dL in adults is generally acceptable [[4, 5] ]. However in 2015, The National Institute of Occupational Safety and Health (NIOSH)/CDC designated 5 µg/dL of whole blood, in a venous blood sample, as the reference blood lead level for adults [13].
Blood lead concentrations are currently regarded as the most reliable index of exposure to lead. Over 95% of blood lead is bound to the erythrocytes and seems to be in dynamic equilibrium with plasma lead [14].
Interactions of Pb with nutrients like calcium and iron affects lead bio-availability [15]. Chronic or repetitive episodes of abdominal pain, nausea, vomiting, constipation, bloating, and anorexia are common GI symptoms seen with BLL ranging from 30 to 80 mg/dL [16]. Several studies reported that in kidney, Pb inhibits 1-α-hydroxylase enzyme required for the synthesis of calcitriol which in turn affects mineral metabolism mainly calcium and phosphorus by minimizing its absorption from the intestine and renal tubules which ultimately causes hypocalcaemia and hypophosphatemia [1, 17, 18]. Vitamin D plays an important role in bone metabolism and there are considerable evidences that BLL affects the synthesis of 1, 25-di-hydroxyvitamin D in the kidney [19]. Together these effects decrease the bone mineralization thereby decreasing bone mineral density and increase the risk of osteoporosis in occupational lead exposed population. Lead also affects the cardiovascular system and increases systolic and diastolic blood pressure [18]. Mazumdar et al. [20] observed in a Bangladeshi population of jewelry factory workers that toxic lead levels were associated with low levels of di-hydroxy vitamin D (Calcitriol). Similar findings have been observed in chronically lead exposed children as well. Kemp et al. [21] observed a seasonal variation in the BLL along with vitamin D levels.
Majority of studies in Indian subcontinent mentioned the BLL in relation to the ethnicity, age groups or occupation. Few studies have tried to correlate the BLL with serum vitamin D levels in occupationally exposed subjects. Therefore this study was designed to look into blood lead level in occupationally lead exposed battery workers and their effects on Vitamin D and calcium metabolism.
Materials and Methods
Study Subjects
In this cross-sectional study, 100 male workers in battery factories in Delhi, NCR region who were occupationally exposed to lead (LEBW, lead-exposed battery workers) and 100 age-matched male controls not exposed to lead (NLEC, non-lead exposed controls) were recruited with their informed consent after institutional ethical clearance from the SGT University, Haryana, India. Demographic details including age, smoking status, alcohol ingestion and duration of occupational exposure and clinical details, etc. were collected through a questionnaire. Alcoholics were defined as subjects who took alcohol regularly and smokers were defined as those who smoked at least 1 packet/day of cigarettes/bidis. The subjects, who were suffering from diabetes mellitus, hypertension and some surgery and had past histories of major illness, were excluded from this study.
Sample Collection
From each subject, 5 mL venous blood sample was collected. 3 mL blood was taken in plain vial for serum isolation for biochemical parameters. 2 mL blood was taken in EDTA vials for estimation of blood lead level (BLL) and stored at − 20 °C till future use. Routine biochemistry parameters including serum calcium and phosphorous were measured using Modular p Biochemistry auto-analyzer (Roche Diagnostics, Basel, Switzerland).
Estimation Blood Lead Level (BLL)
Blood lead levels were estimated by Inductively coupled plasma optical emission spectrometer (ICP-OES) (Optima 8000, Perkin Elmer, Waltham, MA) along with Microwave Digestive System 3000 (Anton Paar). 2 mL of whole blood was digested with 2 mL of nitric acid (HNO3) and 0.2 mL of hydrogen peroxide (H2O2) under proper power, temperature and time. Digested samples were made up to 5 ml using triple-distilled water and the concentration of lead was measured. A known concentration of lead standard solution was digested and analyzed for internal quality control.
Enzyme Linked Immunosorbent Assay (ELISA)
The measurement of serum 25-hydroxy vitamin D (25OH vitD) was performed using Vitamin D ELISA kit (Cal Biotech, El Cajon, CA) based on competitive binding detection method. Manufacturer’s recommended protocol was followed. The final absorbance was measured at 450 nm in an automated ELISA reader (Biotek, Winooski, VT).
Statistical Analyses
Statistical analyses was done with Graph-Pad Prism 6 and SPSS version 21.0. Data were expressed as mean ± SD or median and range as appropriate. Independent t test (for parametric data) and Mann–Whitney U test (for nonparametric data) was used to compare the available data. Spearman rank correlation test was used to determine the correlation between BLL and other parameters. p value < 0.05 was taken to be statistically significant.
Results
Study Subjects Characteristics
Of the total 200 subjects recruited in this study, 100 were LEBW and 100 were age and matched male NLEC. The general characteristics are shown in Table 1. The mean duration of exposure for LEBW was 14.8 ± 9.5 year. The other general parameters obtained were smoking and alcohol consumption status. Table 2 represents results of routine biochemistry parameters. Although, statistically significant differences are being observed between the groups however, they were not found to be clinically relevant as they were within the reference ranges for our population.
Table 1.
General characteristics of study subjects
| Characteristics | NLEC | LEBW | ||
|---|---|---|---|---|
| N (%) | Mean ± SD | N (%) | Mean ± SD | |
| Age (year) | 100 | 34.7 ± 7.9 | 100 | 32.6 ± 10.3 |
| 20–30 year | 24 (24) | 24.9 ± 2.4 | 47 (47) | 23.7 ± 3.1 |
| 30–40 year | 46 (46) | 33.6 ± 3.1 | 27 (27) | 34.2 ± 2.9 |
| 40–60 year | 30 (30) | 44.1 ± 4.3 | 26 (26) | 46.8 ± 5.4 |
| Duration of lead exposure(year) | – | – | 100 | 14.8 ± 9.5 |
| > 20 year | – | – | 33 (33) | 27.4 ± 6.8 |
| >10–20 year | – | – | 39 (39) | 13.6 ± 2.4 |
| < 10 year | – | – | 28 (28) | 5.7 ± 2.5 |
| Smoking status | ||||
| Smoker (%) | 34 (34) | – | 64 (64) | – |
| Non smoker (%) | 66 (66) | – | 36 (36) | – |
| Alcohol consumption | ||||
| Drinker (%) | 52 (52) | – | 57 (57) | – |
| Non drinker (%) | 48 (48) | – | 43 (43) | – |
NLEC non lead exposed controls, LEBW lead exposed battery workers, N total number of subjects
Table 2.
Routine biochemistry parameters
| Characteristics | NLEC | LEBW | p value |
|---|---|---|---|
| AST (IU/L) | 27.3 ± 9.8 | 34.5 ± 19.6 | 0.0012* |
| ALT (IU/L) | 30.4 ± 19.1 | 40.3 ± 30.5 | 0.0066* |
| Total Protein (g/dL) | 7.3 ± 0.8 | 7.4 ± 0.4 | 0.2156 |
| Albumin (g/dL) | 3.9 ± 0.6 | 4.5 ± 0.5 | < 0.0001* |
| ALP (IU/L) | 152.5 ± 117.3 | 179.4 ± 83.6 | 0.0631 |
| Serum Urea (mg/dL) | 24.9 ± 7.6 | 27.4 ± 8.9 | 0.030* |
| Serum Creatinine (mg/dL) | 0.8 ± 0.2 | 0.9 ± 0.2 | 0.0029* |
*p ≤ 0.05 is considered as significant
NLEC non lead exposed controls, LEBW lead exposed battery workers
Blood Lead Levels, Serum Vitamin D, Calcium and Phosphorous Levels
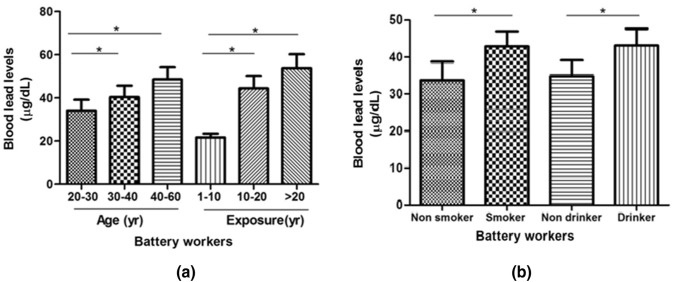
In all study subjects BLL was measured in whole blood using ICP-OES. Results demonstrate that LEBW have significantly higher (p < 0.0001) BLL compared to NLEC subjects as shown in Table 3. On dividing the LEBW on the basis of their age as depicted in Fig. 1a, we observed that subjects within 30–40 years and 40–60 years age groups had significantly higher (p < 0.005) BLL compared to 20–30 year age group. Similarly, when LEBW were stratified on the basis of duration of exposure to Pb, subjects exposed for > 20 year and those having exposure between 10 and 20 year had significantly high BLL as compare to subjects having 1–10 year exposure (Fig. 1a). Additionally, analysis of BLL in smokers and alcoholics showed very high BLL as compared to non-smokers and non-alcoholics respectively (Fig. 1b).
Table 3.
Comparison of blood lead levels (BLL), serum vitamin D, calcium and phosphorus levels between lead exposed battery workers (LEBW) and non-lead exposed controls (NLEC)
| Characteristics | NLEC (Mean ± SD) (n = 100) |
LEBW (Mean ± SD) (n = 100) |
p-value |
|---|---|---|---|
| Blood lead level (μg/dL) | 10.5 ± 12.2 | 39.5 ± 31.9 | < 0.0001 |
| Serum vitamin D (ng/mL) | 53.5 ± 11.7 | 18.9 ± 8.9 | < 0.0001 |
| Serum calcium (mg/dL) | 9.1 ± 0.8 | 8.8 ± 0.5 | 0.0005 |
| Serum phosphorus (mg/dL) | 4.2 ± 1.3 | 3.8 ± 0.9 | 0.008 |
p ≤ 0.05 is considered as significant
Fig. 1.
Graphical representation of the blood lead levels on the basis of: a age and duration of exposure in LEBW; b smokers and drinkers in LEBW; data is expressed as Mean ± SD. LEBW lead exposed battery workers;*p ≤ 0.05 is considered as significant
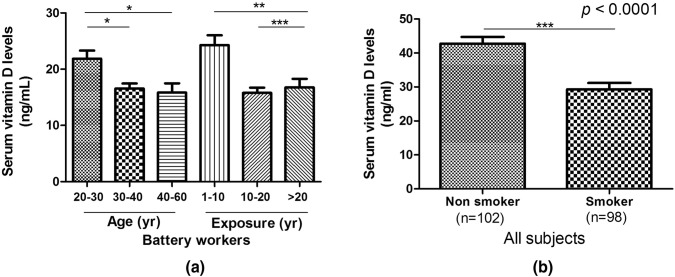
Upon analysis of serum vitamin D levels in all participants, it was observed that LEBW showed significantly low (p < 0.0001) serum vit D levels compared to NLEC subjects (Table 3). Subjects aged between 30–40 and 40–60 year demonstrated significantly low serum vitamin D levels than subjects in the 10–20 year age group. In addition, duration of Pb exposure also affected the levels of serum vitamin D, thus patients exposed for longer duration (> 20 year and 10–20 year) had significantly low levels of serum vitamin D compared to those exposed for < 10 year only (Fig. 2a).
Fig. 2.
Graphical representation of serum vitamin D levels on the basis of a age and duration of exposure in LEBW b smokers and non-smokers in all subjects. LEBW lead exposed battery workers;*p ≤ 0.05 is considered as significant
Since our study included subjects who were smokers, and smoking is known to affect vitamin D levels, we compared the vitamin D levels between smokers and non smokers (as shown in Fig. 2b). Vitamin D levels were found to be significantly lower in smokers.
Lastly, the levels of serum calcium were significantly decreased in the LEBW subjects compared to NLEC (Table 3). Significantly decreased (p = 0.008) levels of phosphorous was observed in the LEBW subjects in comparison to NLEC (Table 3).
Correlation Analysis
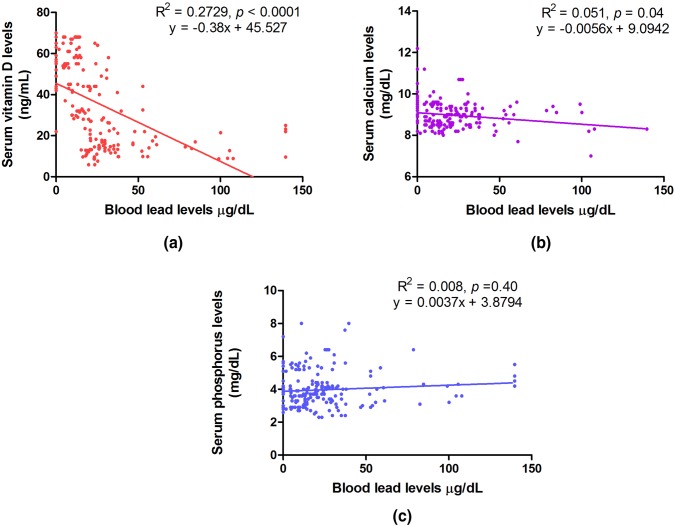
Spearman rank correlation analysis was performed between BLL and serum levels of vitamin D, calcium and phosphorus in all subjects together (N = 200) (Fig. 3a, b, c). Significant negative correlation of BLL was observed with serum vit D (R2 = 0.2729; p ≤ 0.0001). Similarly, there was an inverse correlation between BLL and serum calcium levels (R2 = 0.051; p = 0.04). Significant correlation was not observed between serum phosphorus and BLL (R2 = 0.008; p = 0.40). Spearman rank correlation analysis to study the effect of BLL on vitamin D with adjustment for smoking revealed weak negative correlation between the two (Adjusted R2 = 0.2976; p < 0.001).
Fig. 3.
Spearman rank correlation analysis between a blood lead level (BLL) and serum vitamin D, b BLL and serum calcium and c BLL and serum phosphorus in all subjects (N = 200)
Discussion
Occupational hazards are one of the greatest challenges to mankind in the modern era. Lead is one of the most important occupational pollutants in today’s environment. Due to its physico-chemical properties and non-biodegradable nature, it is accumulating in the environment at a very alarming rate. Though large number of developed countries have discouraged its use, still multiple industries such as lead based painting, lead containing pipes, battery manufacturing and recycling, refining, smelting, etc. are highly dependent on the use of lead. Due to its significant toxicity, Centers for Disease Control [CDC, 2011] has defined the standard blood lead level to be less than 10 µg/dL in adults and also instructed for routine estimation of Pb in occupationally exposed workers [1]. In this study BLL, serum vit D, serum calcium and phosphorus was estimated and compared between LEBW and NLEC. Significantly high BLL were observed in LEBW as compared to NLEC. Further, upon dividing NLEB subjects on the basis of age and duration of exposure, high BLL were observed in study subjects between 30–40 year and 40–60 year age compared to 20–30 year age group LEBW subjects. Similarly, higher duration of exposure was also associated with higher BLL in the LEBW. Subjects exposed for > 20 year and 10–20 year had significantly high BLL as compare to subjects exposed for < 10 year. Further, we have demonstrated the effects of BLL on the serum vit D, calcium and phosphorous levels in LEBW and NLEC. Lastly, Spearman rank correlation analysis was performed between BLL, vit D, calcium levels in the studied subjects.
Elevated BLLs have previously been observed in different populations as shown in Table 4. From those reports it can be observed that the mean BLLs reported from different parts of our country varied from 25.26 to 65.5 μg/dL in occupationally exposed people. In non-exposed groups like children and in pregnant women the levels varied from 6.89 to 17.87 μg/dL.
Table 4.
Reports on Blood lead levels from different parts of India
| S.No. | Lead author | Place of study | Study population | BLL Mean ± SD (μg/dL) |
References |
|---|---|---|---|---|---|
| Occupationally lead exposed workers | |||||
| 1. | Bhaskar Singamsetty, et al. | Andhra Pradesh | Battery Workers | 25.26 ± 2.121 | Int J Community Med and Public Health, 2017 |
| 2. | Mandakini K Shirsagar, et al. | Maharashtra | Battery Workers | 33.50 ± 12.90 | J Pharma, Chemical and Biological Sci, 2015 |
| 3. | Ipsita Mazumdar, K. Goswami, et al. | Kolkata | Plastic Industry Workers | 59.6 ± 6.5 | Ind J Clin Biochem (Jan-Mar 2014) |
| 4. | Nilima N. Dongre, et al. | Karnataka | Battery Workers | 65.5 ± 18.5 | Ind J Clin Biochem (Jan-Mar 2013) |
| 5. | Imran Khan Mohammad, Abbas Ali Mahdi, et al. | Lucknow | Painters | 219.2 ± 61.9 | Arh Hig Rada Toksikol, 2008 |
| Non-occupationally lead exposed subjects | |||||
| 6. | Sakshi Chaudhary, Abbas Ali Mahdi, et al. | Aligarh | Children | 44.2% Had High BLL | Indian Pediatrics, Vol-55,2017 |
| 7. | Varun Mehta, et al. | North India | Patients Uses Ayurvedic Medication | 9.5% Had High BLL | Clin Toxicology, 2016 |
| 8. | Shailja Chambial, Praveen Sharma, et al. | Jodhpur | Adult Population | 6.89 ± 9.5 | Ind J Clin Biochem (July-Sept 2015) |
| 9. | Shilpa A. Pratinidhi, et al. | Maharashtra | Children | 12.810 ± 8.951 | J Basic Clin Physiol Pharmacol, 2014 |
| 10. | Veena Kalra, et al. | Delhi | Children | 12% Had High BLL(≥ 10 μg/dl) | Ind J Pedia (August 2013) |
| 11. | Amit Kumar Mani Tiwari, AA Mahdi, et al. | Lucknow | Pregnant Anemic Women | High BLL | Ind J Clin Biochem. 2012 |
| 12. | Ananya Roy, et al. | Chennai | Children | 11.4 ± 5.3 | Env Health Perspect, 2009 |
| 13. | M. Ahamed, et al. | Lucknow | Children | 17.87 ± 9.36 | Clinica Chimica Acta 377 (2007) |
In 2018, Ahmad et al. reported significantly high levels of lead in blood, hairs and nails in automobile technicians and battery workers in certain districts of Pakistan [22]. Similarly, Basit et al. (2015) demonstrated significant association of high BLL and pathological changes in blood parameters in battery workers of Pakistan [23]. Ahmad Akhtar et al. (2014) found high Mean BLL among the workers of Bangladesh [24]. Only three studies have been reported on battery workers from India till date, all from southern India. They reported BLL from 25.26 to 65.5 μg/dL. Our’s is the first study which reports BLL in battery workers from Delhi NCR region. We found 39.5 μg/dL which is more or less corresponding with previous reports from India. Till date, there is only a single study reported from Karnataka, India which found high blood Pb levels to be associated with hypertension, hypocalcemia and low vitamin D levels in battery workers [18]. In our study, we report that BLL is significantly high in LEBW in comparison to NLEC. Further, the levels were more in higher age group workers compared to younger subjects. Additionally, duration of exposure positively impacted the BLL. Thus, LEBW exposed for longer duration had significantly higher BLL compared to the group exposed for shorter duration. This corroborates with a previous reported study claiming that duration of exposure has got significant effects on neuronal-cognitive function, bone mineral metabolism, hypertension and many more [25].
In our study we further observe that, serum total calcium was significantly decreased in the LEBW in comparison to NLEC. Serum phosphorous levels were significantly lower in the battery manufacturing worker compared to controls. Similarly, there were significantly decreased levels of serum vit D in the LEBW than NLEC. The findings corroborate the claims of Nilima et al., 2013 from Karnataka [18]. Pb ingestion has been shown to decrease serum concentration of 1,25(OH)2D and block vitamin D-dependent intestinal calcium transport in rats [26]. High BLL has also been suggested to cause disruption of the renal hydroxylation of 25-hydroxyvitamin D [25(OH)D] by 1-α-hydroxylase thereby decreasing the production of active form of vitamin D, 1,25(OH)2D [27]. This leads to reduced calcium levels and increased serum parathyearoid hormone (PTH) levels. Thus, Pb interferes with various vitamin D functions involved in calcium balance and metabolism in various tissues and organs [28]. Normally, calcitriol maintains homeostasis of calcium and phosphorous by enhanced absorption of calcium across the small intestine by stimulating the production of calcium binding protein in the intestine. Further, absorption of calcium and phosphorous across the renal tubules is modulated by calcitriol. Thus, in this study, high BLL might have lead to decreased vit D levels which in turn lead to decrease in calcium and phosphorous levels in blood.
In our study we have included both smokers and non smokers. Smoking is not only known to affect BLL but also reports are there in literature showing lower vitamin D levels in smokers [29]. Hence, we analyzed our results with adjustment for smokers and found that weak negative correlation exists between BLL and vitamin D in our subjects.
To conclude, this is the first study from Delhi NCR region which reports blood lead levels in occupationally lead exposed workers from battery industry which was observed to be significantly higher BLL as compared to non-exposed controls. Decreased levels of total calcium, phosphorus and vit D were simultaneously observed in these workers. Significant negative correlation was also observed between BLL and levels of vit D and calcium probably due to the interference of lead in vit D and calcium metabolism. Thus, this study indicates that occupationally exposed workers have higher blood lead levels and are at risk for impaired calcium and vit D metabolism. However, in this study the duration of exposure to sunlight of the workers and controls were not matched and could be a potential source of error. Most workers worked indoors all through the day thereby getting limited exposure to sunlight. Hence, future studies should be conceived to address this issue before we could comment on the association of lead with low vit D levels.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Research Involving Human Participants and/or Animals
This article involves participations of human subjects with the approval from Ethical Committee, SGT University, Gurgaon, Haryana, India (No. SGTU/FMHS/D/2015/Dated 17.09.2015)
Informed Consent
Prior written informed consent was obtained from all subjects recruited in this study.
Contributor Information
Himani, Email: rathimanu29@gmail.com.
Raman Kumar, Email: raman_baliyan1987@yahoo.co.in.
Jamal Akhtar Ansari, Email: jamalakhtarindia@gmail.com.
Abbas Ali Mahdi, Email: mahdiaa@rediffmail.com.
Dilutpal Sharma, Email: dilutpal@yahoo.co.in.
Busi Karunanand, Email: karunanandbusi@gmail.com.
Sudip Kumar Datta, Email: dr.sudipdatta@gmail.com.
References
- 1.Agency for Toxic Substances and Disease Registry (ATSDR). Toxicology profilr for lead,USDepartment of health and human servies. Atlanta, Georgia, USA:US Government Printing,2005; pp 102–225.
- 2.International Programme on Chemical Safety (IPCS). Inorganic lead. Environmental health criteria 165. Geneva: WHO; 1995
- 3.Ooi PL, Goh KT, Heng BH, Sam CT, Kong KH, Rajan U. Biological monitoring of human exposure to environmental lead in Singapore. Rev Environ Health. 1991;9(4):207–213. doi: 10.1515/REVEH.1991.9.4.207. [DOI] [PubMed] [Google Scholar]
- 4.Wu S, Peng S, Zhang X, Wu D, Luo W, Zhang T, Zhou S, Yang G, Wan H, Wu L. Levels and health risk assessments of heavy metals in urban soils in Dongguan. China J Geochem Explor. 2015;148:71–78. doi: 10.1016/j.gexplo.2014.08.009. [DOI] [Google Scholar]
- 5.Centers for Disease Control and Prevention (CDC) Adult blood lead epidemiology and surveilance. MMWR Morb Mortal Wkly Rep. 2011;60:841. [PubMed] [Google Scholar]
- 6.International Agency for Research on Cancer (IARC) Inorganic and organic lead compounds. IARC Monogr Eval Carcinog Riska Hum. 2006;87:1. [PMC free article] [PubMed] [Google Scholar]
- 7.Luo W, Ruan D, Yin C, Chen J. Effects of chronic lead exposure on functions of nervous system in Chinese children and developmental rats. Neurotoxicolocy. 2012;33:862–871. doi: 10.1016/j.neuro.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 8.Braun JM, Hoffman E, Schwartz J, Sanchez B, Schnaas L, Mercado-Garcia A, et al. Assessing windows of susceptibility to lead-induced cognitive deficits in Mexican children. NeuroToxicology. 2012;33(5):1040–1047. doi: 10.1016/j.neuro.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arbuckle TE, Davis K, Boylan K, Fisher M, Fu J, Bisphenol A. Phthalates and lead and learning and behavioral problems in Canadian children 6–11 years of age: CHMS 2007–2009. NeuroToxicology. 2016;54:89–98. doi: 10.1016/j.neuro.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 10.Roy A, Queirolo E, Peregalli F, Mañay N, Martínez G, Kordas K. Association of blood lead levels with urinary F2-8α isoprostane and 8-hydroxy-2-deoxy-guanosine concentrations in first-grade Uruguayan children. Environ Res. 2015;140:127–135. doi: 10.1016/j.envres.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dallaire R, Dewailly É, Ayotte P, Forget-Dubois N, Jacobson SW, Jacobson JL, et al. Growth in Inuit children exposed to polychlorinated biphenyls and lead during fetal development and childhood. Environ Res. 2014;134:17–23. doi: 10.1016/j.envres.2014.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skröder H, Hawkesworth S, Moore SE, Wagatsuma Y, Kippler M, Vahter M. Prenatal lead exposure and childhood blood pressure and kidney function. Environ Res. 2016;151:628–634. doi: 10.1016/j.envres.2016.08.028. [DOI] [PubMed] [Google Scholar]
- 13.https://www.cdc.gov/niosh/topics/ables/description.html.
- 14.De Silva PE. Determination of lead in plasma and studies on its relationship to lead in erythrocytes. Occup Environ Med. 1981;38(3):209–217. doi: 10.1136/oem.38.3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mudipalli A. Lead hepatotoxicity & potential effects. Indian J Med Res. 2007;126:518–527. [PubMed] [Google Scholar]
- 16.Janin Y, Couinaud C, Stone A, Wise L. The, “lead-induced colic” syndrome in lead intoxication. Surg Annu. 1985;17:287–307. [PubMed] [Google Scholar]
- 17.Gidlow DA. Lead toxicity. Occup Med. 2004;54(2):76–81. doi: 10.1093/occmed/kqh019. [DOI] [PubMed] [Google Scholar]
- 18.Dongre NN, Suryakar AN, Patil AJ, Hundekari IA, Devarnavadagi BB. Biochemical effects of lead exposure on battery manufacture workers with reference to blood pressure, calcium metabolism and bone mineral density. Indian J Clin Biochem. 2013;28(1):65–70. doi: 10.1007/s12291-012-0241-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hass GM, Brown DV, Eisenstein R, Hemmens A. Relations between lead poisoning in rabbit and man. Am J Pathol. 1964;45:691–727. [PMC free article] [PubMed] [Google Scholar]
- 20.Mazumdar I, Goswami K, Ali MS. Status of serum calcium, vitamin D and parathyearoid hormone and hematological indices among lead exposed jewelry workers in Dhaka, Bangladesh. Indian J Clin Biochem. 2017;32(1):110–116. doi: 10.1007/s12291-016-0582-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kemp FW, Neti PVSV, Howell RW, Wenger P, Louria DB, Bogden JD. Elevated blood lead concentrations and vitamin D deficiency in winter and summer in young urban children. Environ Health Perspect. 2006;115(4):630–635. doi: 10.1289/ehp.9389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmad I, Khan B, Khan S, Khan MT, Schwab AP. Assessment of lead exposure among automobile technicians in Khyber Pakhtunkhwa, Pakistan. Sci Total Environ. 2018;633:293–299. doi: 10.1016/j.scitotenv.2018.03.160. [DOI] [PubMed] [Google Scholar]
- 23.Basit S, Karim N, Ali SS, Solangi SUH, Khan FA, Munshi AB. Occupational lead toxicity in battery workers of Karachi. Pak J Med Sci. 2015;31(4):775. doi: 10.12669/pjms.314.7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahmad SA, Khan MH, Khandker S, Sarwar AFM, Yasmin N, Faruquee MH, et al. Blood lead levels and health problems of lead acid battery workers in Bangladesh. Sci World J. 2014;2014:1–7. doi: 10.1155/2014/974104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Juberg DR, Kleiman CF, Kwon SC. Position paper of the American council on science and health: lead and human health. Ecotoxicol Environ Saf. 1997;38(3):162–180. doi: 10.1006/eesa.1997.1591. [DOI] [PubMed] [Google Scholar]
- 26.Smith CM, DeLuca HF, Tanaka Y, Mahaffey KR. Effect of lead ingestion on functions of vitamin D and its metabolites. J Nutr. 1981;111(8):1321–1329. doi: 10.1093/jn/111.8.1321. [DOI] [PubMed] [Google Scholar]
- 27.Schwalfenberg GK, Genuis SJ. Vitamin D, essential minerals, and toxic elements: exploring interactions between nutrients and toxicants in clinical medicine. Sci World J. 2015;2015:1–8. doi: 10.1155/2015/318595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anticona C, San Sebastian M. Anemia and malnutrition in indigenous children and adolescents of the Peruvian Amazon in a context of lead exposure: a cross-sectional study. Glob Health Action. 2014;7(1):22888. doi: 10.3402/gha.v7.22888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ren W, Gu Y, Zhu L, Wang L, Chang Y, Yan M, et al. The effect of cigarette smoking on vitamin D level and depression in male patients with acute ischemic stroke. Compr Psychiatry. 2016;65:9–14. doi: 10.1016/j.comppsych.2015.09.006. [DOI] [PubMed] [Google Scholar]