Abstract
Background:
Post-transplant diabetes mellitus (PTDM) is common after liver transplantation (LT). Yet, how PTDM relates to graft outcomes and survival needs elucidation as more individuals are transplanted for non-alcoholic fatty liver disease (NAFLD).
Methods:
This single center, retrospective study of adult LT recipients (2003-2016) identified PTDM incidence and associations with graft steatosis, rejection, and post-LT patient survival. Multivariable analysis investigated predictors of PTDM. Kaplan-Meier curves depicted patient survival 5 years post-LT.
Results:
Among 415 adult LT recipients, 23% had pre-LT DM and 13% were transplanted for NAFLD. PTDM incidence was 34.7%, 46.9%, and 56.2% and overall survival was 90%, 80.9%, and 71.7% at 1, 3, and 5 years, respectively. Over a third of non-NAFLD patients developed PTDM. Half of PTDM cases developed by 6 months and 75% by 12 months. The PTDM group had more rejection episodes compared to no PTDM (31.9% vs. 21.8%, p=0.055), with trends towards worse patient survival 5 years post-LT (log-rank test p=0.254). Age was the only significant predictor of PTDM.
Conclusions:
PTDM occurs rapidly in the post-LT period and is a significant problem for both NAFLD and non-NAFLD LT recipients. Age is a significant risk factor for PTDM. Outcomes trended towards increased rejection and worse survival among PTDM individuals, suggesting the benefit of early strategies targeting glucose control.
Keywords: liver transplantation, post-transplant diabetes mellitus, hyperglycemia, transplant outcomes
Background
Post-transplant diabetes mellitus (PTDM) occurs in 12-45% of liver transplant (LT) recipients,1,2 and is reportedly associated with worse patient and graft survival potentially due to infections, rejection episodes, and late onset hepatic artery thrombosis.1,3,4 Long-term achievement of lower glucose levels post-LT has been associated with lower rates of rejection, infection, and hospital readmission rates,1,3,5–7 suggesting that tight glucose control is essential to minimizing poor post-LT outcomes.
With the global epidemic of obesity and metabolic syndrome, the prevalence of non-alcoholic fatty liver disease (NAFLD) is on the rise and becoming one of the most common causes of chronic liver disease and LT.8 While LT may address the underlying intrinsic liver disease, concomitant metabolic syndrome and extrahepatic comorbidities may contribute to persistent diabetes mellitus (DM) or de novo PTDM.8 Patients with NAFLD and insulin resistance pre-LT may continue to have difficulty managing blood glucose after LT due to the addition of immunosuppressant medications, thus leading to poor glucose control that predisposes individuals to PTDM and NAFLD recurrence.5 In fact, approximately 30-60% of NAFLD LT recipients have recurrence of fatty liver disease. How PTDM mitigates this risk remains unclear.
Non-NAFLD LT recipients are also at risk for poor glucose control given the side effects of immunosuppressive medications and rapid weight gain during the post-LT period. The presence of PTDM may promote or worsen already existing metabolic abnormalities, leading to post-LT metabolic syndrome and de novo NAFLD.5,8 In fact, de novo NAFLD occurs in 20-30% of patients undergoing LT for non-NAFLD etiologies including alcohol and viral hepatitis.8–10 LT recipients are still at risk for PTDM, HTN and chronic kidney disease a decade post-LT, controlling for the potential confounder of obesity, suggesting that all LT recipients (not just NAFLD patients) are at risk for metabolic syndrome, PTDM and associated poor outcomes.11
What remains unclear is: 1) whether PTDM differentially affects NAFLD patients as compared to non-NAFLD patients, 2) the degree to which PTDM influences other specific transplant outcomes including de novo or recurrent steatosis and the implications of this relationship on developing NAFLD post-LT, and 3) at what timepoint post-LT individuals are most vulnerable for PTDM—acutely peri-operatively in the setting of surgical stress or years after LT after chronic immunosuppression.
In this study, we sought to investigate the relationship between PTDM and post-LT outcomes, especially among non-NAFLD individuals. The aims of this study were: 1) to determine the incidence of PTDM in a large cohort of LT recipients at our transplant center, 2) to assess the relationship between PTDM and post-LT outcomes including recurrent or de novo steatosis, as well as graft and patient survival, and 3) to investigate predictors of PTDM including patient-specific characteristics, primary indication for LT, and steroid duration.
Methods
Patient Populatio
This single center, retrospective cohort study included adult LT recipients at University of North Carolina (UNC) Medical Center between January 1, 2003 and March 31, 2016. Patients were identified via electronic medical record. Repeat LT, recipient age < 18 years old, multi-organ transplants, and LT due to fulminant liver failure were excluded. Transplant-specific data (i.e. donor and operative characteristics) were obtained via the United Network of Organ Sharing (UNOS) database, and all other data (i.e. sociodemographic and laboratory data) were obtained via UNC electronic medical record chart review. Data collection was performed after approval from the UNC Institutional Review Board.
Sociodemographic and transplant characteristics were determined a priori and included age, sex, race, primary indication for LT, Model for End Stage Liver Disease (MELD), diabetes history, presence of hepatocellular carcinoma (HCC), serum sodium, creatinine, and BMI at the time of LT. NAFLD was deemed to be the primary diagnosis for LT based on biopsy evidence of steatosis/steatohepatitis or in cases of cryptogenic cirrhosis with at least two components of the metabolic syndrome prior to LT, as previously described in the literature.12 Non-NAFLD indications for LT included hepatitis C virus (HCV), alcohol, combination HCV/alcohol, and other viral hepatitis indications. Other indications for LT included PBC, PSC, autoimmune hepatitis, neoplasm, drug-induced liver injury, among others. Pre-LT DM was defined as more than two random blood glucoses >200 mg/dL or hemoglobin A1C > 6.5% prior to LT. Donor characteristics included age, sex, donor type, cause of death, donor risk index (DRI), and presence of steatosis (defined as histologic evidence of >30% steatosis). Operative characteristics that were investigated included warm ischemia time and cold ischemia time. Other post-LT clinical characteristics included immunosuppression regimen at one year and duration of steroid use.
The primary outcome of interest was PTDM incidence at 1, 3, and 5 years post-LT. PTDM was defined as having ≥ 2 random blood glucose > 200 mg/dL, HbA1C > 6.5%, or use of oral anti-diabetic agents, non-insulin injectables or insulin at least 30 days after LT.12 For the purposes of our analysis, only patients without pre-LT DM could be eligible to develop PTDM. Thus, PTDM refers to only new onset or de novo cases of DM post-LT. Risk factors or predictors of PTDM were investigated including recipient age, sex, BMI, indication for LT, MELD and duration of steroid therapy.
Secondary outcomes included de novo or recurrent steatosis defined as radiographic evidence of steatosis in a non-NAFLD LT recipient without prior steatosis (de novo) or NAFLD patient with prior steatosis (recurrent). Additionally, persistent DM was defined as more than two random blood glucoses >200 mg/dL or hemoglobin A1C > 6.5% at the time of transplant among LT recipients with a pre-LT diagnosis of DM). Other secondary outcomes included graft rejection, and patient survival at 1, 3, and 5 years post-LT. The relationship between PTDM and these outcomes was investigated. Steatosis outcomes were identified by radiographic imaging alone, given annual protocol biopsies are not performed at our institution. Therefore, no histologic data was used to determine whether a LT recipient had recurrent or de novo graft steatosis. Graft rejection was identified by biopsy performed in the clinical setting. No biopsies were performed for research purposes in this study.
Immunosuppression Regimen
All patients received methylprednisolone or basiliximab for induction therapy based on our institution protocol. Basiliximab 20 mg intravenously on post-operative day zero and four was given if the patient had a serum creatinine ≥1.5 at the time of transplant or was on renal replacement therapy. Maintenance immunosuppression consisted primarily of tacrolimus, mycophenolate mofetil and a prednisone taper over 30 days unless the patient had a history of HCV, autoimmune hepatitis, or PSC in which case prednisone was continued for 6 months or indefinitely at a dose of 5 mg per day, respectively. Per institutional protocol, steroids are decreased to 5mg/day by post-operative day (POD) 9, 2.5 mg by POD 15 and then off by POD 30. For HCV patients, the taper stops at 5 mg daily on POD 9 and continues until POD 180 at 5 mg daily. For AIH patients it is continued indefinitely. We do not routinely modify the taper if early PTDM is detected. Target trough levels for tacrolimus were 8 to 10 ng/mL from 0 to 3 months, 6 to 8 ng/mL from 4 to 12 months, and 4 to 6 ng/mL after 12 months. Mycophenolate mofetil was administered orally at a total dose of 1000 mg per day or the equivalent mycophenolate sodium dose at the time of transplantation.
Statistical Analysis
For univariate analysis we examined means and medians of continuous variables (age, donor risk index, serum sodium, creatinine, MELD, BMI, warm/cold ischemia time, and duration of steroid use). Frequencies were reported for categorical variables (sex, ethnicity, indication for LT, DM history, HCC history, immunosuppressant type, donor characteristics including type, cause of death, and presence of steatosis). Donor risk index (DRI) was determined as described by Feng et al.13 Bivariate analysis was used to compare outcomes among patients with and without PTDM. Fisher’s exact test was used for categorical variables, Student’s t-tests were used for continuous variables, and z-tests were used to compare proportions, as appropriate. The primary outcome of interest was incidence of PTDM at 1, 3, and 5 years post-LT. To calculate incidence, the relevant denominators represented the total population at risk at the 1, 3, or 5-year mark. For example, the total population at risk (denominator) for PTDM incidence referred to the number of patients who did not have DM prior to transplant, who were alive, or who developed PTDM by the 1, 3, or 5 year mark. Secondary outcomes included de novo steatosis, persistent DM, graft rejection, and patient survival at 1, 3, and 5 years post-LT.
In order to identify potential predictors of PTDM, multivariable analysis using a Cox proportional hazards model was performed and included calculated hazard ratios (HR). Clinically relevant predictors were determined a priori based on the literature and clinical expertise and included age, sex, race, primary indication for LT, duration of steroid use post-LT, BMI, and MELD at time of LT to account for patient disease severity. Kaplan-Meier curves for overall patient survival stratified by presence of PTDM were constructed for the first 5 years post-LT. For patients who developed PTDM, Kaplan-Meier curves were created to depict time-to-PTDM development. Patients with missing data or who did not have documentation of receiving steroids post-LT were excluded from the analysis. All statistical analyses were performed in R version 3.4.1, using the survival package.14
Results
Sociodemographic and Clinical Characteristics of Study Population
A total of 415 adult liver transplants took place from 2003 to 2016, 320 of whom were included in our final analysis. Donor and recipient transplant characteristics are summarized in Table 1. Of the total 415 patients, 95 patients were excluded from further analysis due to a diagnosis of pre-LT DM. Transplant characteristics for the remaining 320 patients were stratified by diabetes status (PTDM or no PTDM) and summarized in Table 1. The mean recipient age at LT was 54 years, and the majority of patients were male (68%) and Caucasian (76.9%). Mean MELD score at transplant was 20.4. Fifty-three (12.8%) LTs were performed for NAFLD, and 362 (87.2%) were performed for non-NAFLD indications including HCV (32.3%), alcohol (10.4%), combined HCV/alcohol (13.0%), non-HCV viral hepatitis (2.9%), and other (28.7%). Ninety-five patients (22.9%) had DM at the time of transplant, and overall mean BMI was 28.9 kg/m2. Fifty-five percent of NAFLD patients had DM at the time of transplant (29/53). Among non-NAFLD individuals, 18.2% had DM at LT (66/362). Approximately 4.8% of donors had evidence of steatosis at the time of transplant. Regarding immunosuppression, the majority of patients received tacrolimus (93.5%), and mycophenolate (97.6%) at 1-year post-LT. The median duration of steroid use was 6 months with an average duration of 13.65 months (range 1-150 months) post-LT. Among all LT recipients, 25% experienced a treated rejection episode with either corticosteroids or T-cell depleting agents.
Table 1:
Sociodemographic and Clinical Characteristics of Adult LT Recipients and Donors Stratified by PTDM Diagnosis at the University of North Carolina (UNC) Transplant Center (2003–2016)
| Variable | TOTAL (N = 415) |
PTDM* (N = 141) |
No PTDM (N = 179) |
p-value^ |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| LT Recipient Characteristic | ||||
| Mean age at LT, years | 54.38 | 55.05 | 52.40 | 0.008 |
| Male | 282 (68.0) | 98 (69.5) | 125 (69.8) | >0.999 |
| Caucasian | 319 (76.9) | 109 (77.3) | 143 (79.9) | 0.672 |
| Indications for LT | ||||
| NAFLD | 53 (12.8) | 10 (7.1) | 14 (7.8) | 0.939 |
| Non-NAFLD | 362 (87.2) | 131 (92.9) | 165 (92.2) | |
| HCV | 134 (32.3) | 50 (35.5) | 54 (30.2) | |
| Alcohol | 43 (10.4) | 16 (11.3) | 20 (11.2) | |
| Combined Alcohol/HCV | 54 (13.0) | 16 (11.3) | 23 (12.8) | |
| Other Viral§ | 12 (2.9) | 4 (2.8) | 7 (3.9) | |
| Other+ | 119 (28.7) | 45(31.9) | 61 (34.1) | |
| Mean MELD at LT | 20.38 | 19.39 | 21.48 | 0.051 |
| Diabetes pre-LT | 95 (22.9) | - | - | - |
| Hepatocellular carcinoma | 112 (27) | 36 (25.5) | 48 (26.8) | 0.896 |
| Mean serum sodium (mEq/L) | 136.4 | 136.5 | 136.3 | 0.716 |
| Mean creatinine (mg/dL) | 1.419 | 1.309 | 1.427 | 0.312 |
| Mean body mass index (kg/m2) | 28.87 | 28.71 | 28.57 | 0.828 |
| Donor Characteristic | ||||
| Male | 263 (63.4) | 92 (65.2) | 104 (58.1) | 0.235 |
| Mean age, years | 35.76 | 34.51 | 35.43 | 0.582 |
| Donor type | ||||
| Deceased Donor | 410 (98.8) | 138 (97.9) | 177 (98.9) | 0.788 |
| DCD | 5 (1.2) | 3 (2.1) | 2 (1.1) | |
| Donor cause of death | ||||
| Trauma | 196 (47.2) | 64 (45.4) | 87 (48.6) | 0.868 |
| Cerebrovascular accident | 151 (36.4) | 50 (35.5) | 62 (34.6) | |
| Anoxia | 53 (12.8) | 22 (15.6) | 23 (12.8) | |
| Other | 10 (2.4) | 2 (1.4) | 5 (2.8) | |
| Mean donor risk index (DRI) | 1.282 | 1.281 | 1.280 | 0.972 |
| Donors with steatosis | 37 (8.9) | 14 (9.9) | 15 (8.4) | 0.789 |
| Operative Characteristic | ||||
| Mean warm ischemia time (minutes) | 43.12 | 40.60 | 44.17 | 0.116 |
| Mean cold ischemia time (minutes) | 406.1 | 426.3 | 398.7 | 0.267 |
| Post-Operative Course | ||||
| Immunosuppression | ||||
| Tacrolimus | 388 (93.5) | 135 (95.7) | 165 (92.2) | 0.282 |
| Mycophenolate | 405 (97.6) | 139 (98.6) | 173 (96.6) | 0.460 |
| Sirolimus | 24 (5.8) | 10 (7.1) | 10 (5.6) | 0.749 |
| Mean duration of steroid use (months) | 13.65 | 14.38 | 14.27 | 0.960 |
| Median duration of steroid use (months) | 6 | 6 | 6 | - |
Among the total cohort (N=415), 95 patients were excluded given a history of pre-LT DM, and therefore were not candidates for developing PTDM (defined as de novo or new onset DM post-LT).
Other viral includes non-Hepatitis C viral hepatitis including hepatitis A, B, and E.
Other indications for LT include PBC, PSC, autoimmune hepatitis, malignant neoplasm, drug-induced, cryptogenic/idiopathic among others.
p-value was calculated comparing PTDM patients to Non-PTDM patients; t-tests were used for continuous variables and chi-square tests were used for categorical variables
DCD = donation after cardiac death; LT = liver transplantation; MELD = model for end stage liver disease; NAFLD= non-alcoholic fatty liver disease; PTDM = post-transplant diabetes mellitus
Incidence of PTDM, Graft Steatosis and Patient Survival
Overall, the incidence of PTDM was 34.7% at 1-year, 46.8% at 3-years, and 55.9% at 5-years (Table 2). The incidence of de novo graft steatosis was 5.2%, 14.1% and 25.7% at 1, 3, and 5 years, respectively. Recurrent graft steatosis occurred in 6.8%, 31.4%, and 51.9% of LT recipients at 1, 3, and 5 years respectively. Overall survival was 90% at 1-year, 80.9% at 3-years, and 71.7% at 5-years.
Table 2.
Overall Incidence of Post-LT Outcomes Including PTDM, Persistent Diabetes, Graft Steatosis, and Survival among Adult LT Recipients at 1, 3 and 5 Years Post Transplant (N=415)
| Outcome | 1 Year n (%) | 3 Years n (%) | 5 Years n (%) |
|---|---|---|---|
|
PTDM* Total Population at Risk § |
105 (34.7) 303 |
123 (46.8) 263 |
132 (55.9) 236 |
|
Persistent Diabetes+ Total Population at Risk |
75 (82.4) 91 |
81 (92.0) 88 |
81 (93.1) 87 |
|
De novo graft steatosis^ Total Population at Risk |
17 (5.2) 327 |
37 (14.1) 262 |
55 (25.7) 214 |
|
Recurrent graft steatosis Total Population at Risk |
3 (6.8) 44 |
11 (31.4) 35 |
14 (51.9) 27 |
|
Graft rejection Total Population at Risk |
67 (18.1) 370 |
90 (30.8) 292 |
95 (41.7) 228 |
|
Patient survival Total Population at Risk |
370 (90) 411 |
292 (80.9) 361 |
228 (71.7) 318 |
PTDM = post-transplant diabetes mellitus; PTDM refers to new onset diabetes in those without DM prior to transplant.
Total population at risk refers to the relevant denominators at the 1, 3, or 5-year mark out of which percentages were calculated. For example, the total population at risk for PTDM refers to the number of patients who did not have DM prior to transplant, who were alive, or who developed PTDM by the 1, 3, or 5 year mark.
Persistent diabetes refers to ongoing or uncontrolled diabetes post-LT (glucose >200, HgB A1c >6.5) among those with diabetes at the time of transplant.
De novo steatosis refers to new onset steatosis in those without steatosis prior to transplant. Recurrent steatosis refers to steatosis in the liver graft among those transplanted for NAFLD.
PTDM = post-transplant diabetes mellitus; LT = liver transplantation
Relationship Between PTDM and Post-LT Outcomes
In order to assess the association between PTDM and graft steatosis and survival, these outcomes were stratified based on diabetes status (i.e. PTDM vs. no PTDM) in a population excluding individuals with pre-LT DM (n=320). The results of the analysis are shown in Tables 1 and 3. Patients with PTDM were older than non-PTDM patients (mean age 55 years vs. 52 years, respectively) and had lower mean MELD (19 vs. 21). Among all non-NAFLD individuals, 36.2% developed PTDM. There were no meaningful differences in donor or operative characteristics, as well as mean DRI between the PTDM and non-PTDM groups.
Table 3.
Post-LT Outcomes Including de novo and Recurrent Graft Steatosis and Survival among LT recipients with PTDM versus No PTDM (N=320)
| Outcome | PTDM n (%) | No PTDM n (%) | p value* |
|---|---|---|---|
| De novo graft steatosis | |||
| 1 year | 3 (2.1) | 7 (6.0) | 0.115 |
| 3 years | 10 (8.9) | 12 (11.5) | 0.654 |
| 5 years | 18 (20.0) | 22 (27.2) | 0.284 |
| Recurrence of graft steatosis | |||
| 1 year | 2 (13.3) | 0 (0) | 0.484 |
| 3 years | 2 (18.2) | 3 (27.3) | 1 |
| 5 years | 2 (22.2) | 5 (50.0) | 0.350 |
| Patient survival | |||
| 1 year | 129 (91.4) | 162 (91.5) | 1 |
| 3 years | 114 (85.7) | 122 (86.5) | 0.863 |
| 5 years | 87 (72.5) | 95 (77.2) | 0.460 |
| Rejection Episodes | 45 (31.9) | 39 (21.8) | 0.055 |
p-values calculated using Fisher’s exact test.
PTDM = post-transplant diabetes mellitus; LT = liver transplantation
More rejection episodes occurred in the PTDM group versus no PTDM group (31.9% vs. 21.8%, p =0.055) (Table 3). There was no statistically significant difference in incidence of de novo graft steatosis between the PTDM and no PTDM groups at 1, 3, and 5 years post-LT (2.1% vs. 6.0%; p=0.115, 8.9% vs. 11.5%; p=0.654, and 20.0% vs. 27.2%; p=0.284, respectively) (Table 3). There was no significant difference in patient survival between PTDM vs. no PTDM, though there was a trend towards worse survival over the first five years (log-rank test p = 0.254). The Kaplan-Meier curve demonstrating time to PTDM among those who ultimately developed PTDM over the first five years is shown in Figure 1.
Figure 1:
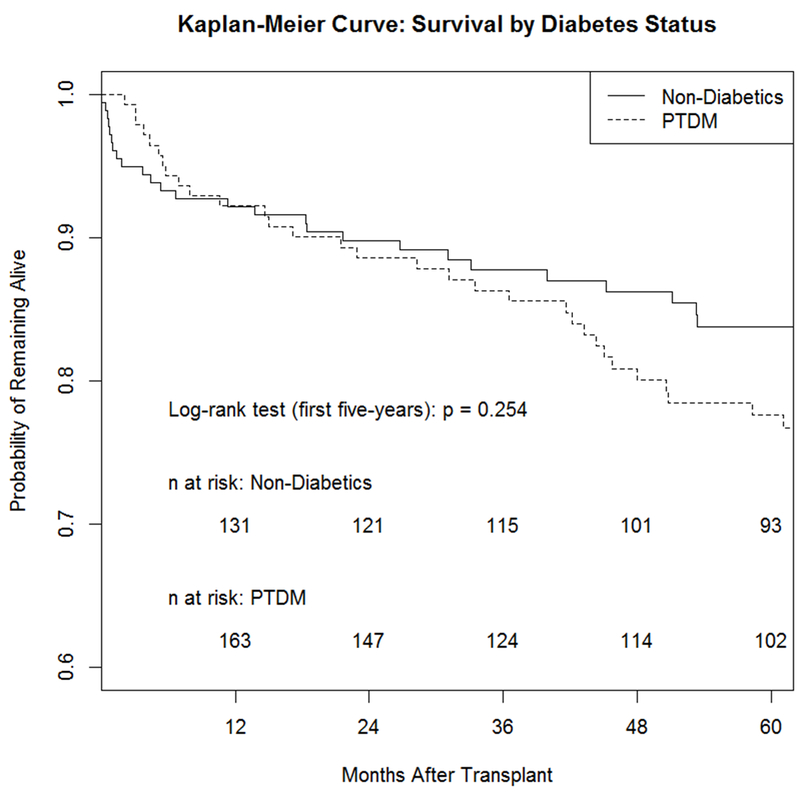
Kaplan-Meier survival curve among LT recipients with PTDM vs. Non-Diabetics
Predictors of PTDM
Of the patients who developed PTDM, onset was rapid. Approximately 50% of these patients developed PTDM by 6 months and 75% developed PTDM by 12 months, as shown in Figure 2. Age was the only significant variable associated with PTDM on bivariate analyses. A multivariable Cox proportional hazards model was used to determine potential predictors of PTDM (Table 4). Age was the only statistically significant variable associated with PTDM adjusting for covariates including gender, race, BMI, MELD score, duration of steroid use, and transplant indication. Further analysis was performed to determine associations between age and the development of PTDM, as the incidence of PTDM increased significantly with rising age. To identify a cut-off value of age as a predictor of PTDM, Youden’s index identified a threshold age of 49.0 (sensitivity 0.31; specificity 0.86).
Figure 2: Time to PTDM Onset Among LT recipients Who Developed PTDM.
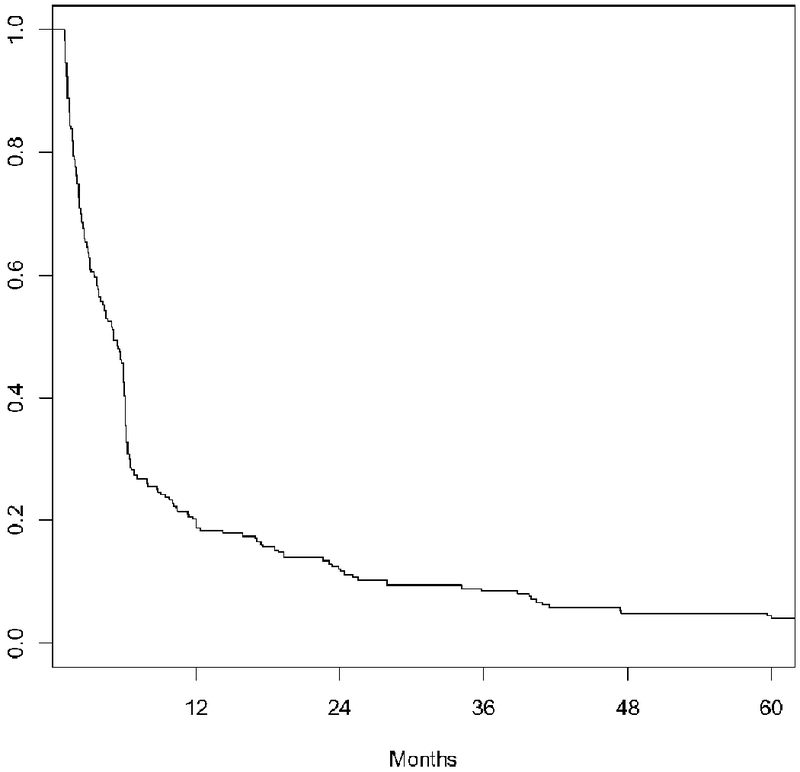
Graphical depiction of time to post-transplant diabetes mellitus (PTDM) onset among those liver transplant recipients who developed PTDM during the 5-year post-LT period. Onset of PTDM was rapid—about 50% of cases occurred by 6 months and 75% by 12 months.
Table 4.
Predictors of de novo PTDM—Multivariable Cox Proportional Hazards Model
| Variable | Adjusted HR* (95% CI) | p-value |
|---|---|---|
| Age | 1.03 (1.01, 1,05) | 0.005 |
| Gender: Male | 1.03 (0.70, 1.51) | 0.879 |
| Race: White | 0.78 (0.52, 1.18) | 0.238 |
| Indication: Alcohol | 1.20 (0.54, 2.68) | 0.654 |
| Indication: HCV | 1.20 (0.60, 2.42) | 0.606 |
| Indication: Combo Alcohol/HCV | 1.04 (0.46, 2.34) | 0.930 |
| Indication: Other viral | 1.01 (0.31, 3.29) | 0.985 |
| Indication: Other non-NAFLD | 1.16 (0.57, 2.38) | 0.686 |
| MELD Score | 0.99 (0.97, 1.01) | 0.191 |
| Steroid duration (months) | 1.00 (1.00, 1.01) | 0.755 |
| BMI | 1.00 (0.97, 1.03) | 0.884 |
Hazard ratios calculated using cox proportional hazards model adjusted for age, gender, race, indication for LT, MELD, steroid duration and BMI. Referent category for gender is female, referent category for race is non-white, referent category for indication is NAFLD
CI = confidence interval; HR = hazards ratio
Discussion
In a large cohort of 415 adult LT recipients, PTDM was common and developed very early in the acute post-LT period, even among non-NAFLD patients. Over half of all LT recipients had PTDM by 5 years post-LT, and among those who developed PTDM, the majority developed it by 6 months post-LT. More than a third of non-NAFLD individuals developed PTDM over the 5 year study period. Age was the only significant independent predictor of PTDM with older adults found to have significantly higher incidence of PTDM. The age of 49 years was identified as a potential risk cutoff for PTDM. Individuals with PTDM had higher rates of graft rejection. Our results also suggested worse survival among patients with PTDM vs. no PTDM, although these findings were non-significant.
This study highlights the salient problem of PTDM even among non-NAFLD patients, and uniquely investigates PTDM onset at different time intervals post-LT in order to capture when LT recipients are most vulnerable to develop PTDM. Moreover, this is one of the largest studies to determine PTDM incidence up to 5 years post-LT. Our finding that PTDM onset occurs early in the first year post-LT suggests the need to implement strategies to target PTDM immediately post-LT. Moreover, given increasing rates of NAFLD on the transplant waitlist,15 metabolic syndrome risk factors among donors,16,17 and more frequent LT for older individuals, special attention needs to be paid to the risk of developing PTDM, in particular among older LT recipients.
Our estimates of PTDM incidence (34.7% at 1-year, 46.9% at 3-years, and 56.2% at 5-years) were congruent with those reported in the literature,1,18,19 and may in fact underestimate rates of PTDM in the LT population given the conservative definition of PTDM used in this study. Prior studies defined PTDM more strictly (blood glucose ≥126 mg/dl or 2-h plasma glucose ≥ 200 mg/dl after an oral glucose test).2 Due to the retrospective nature of this study, fasting blood glucose and oral glucose tests were not consistently available for patients, limiting our definition to ≥ 2 random blood glucose > 200 mg/dL, HbA1C > 6.5%, or using oral anti-diabetic agents, non-insulin injectables or insulin at least 30 days after LT. Thus, even with our conservative definition, PTDM was very common in this population.
A previous study by Yadav et al. aimed to compare predictive factors of PTDM in living and deceased donor LT and also found that age > 50 years old was a significant predictor of diabetes post-LT.19 The American Diabetes Association (ADA) guidelines recommend that adults > 45 years of age undergo testing for diabetes; however, there is no consensus on diabetic testing among transplant recipients.20 There are inconsistent practices across transplant selection committees regarding diabetic screening prior to transplant listing. More rigorous screening may be warranted for older patients, especially those at risk for metabolic syndrome, and could potentially serve as an opportunity for multidisciplinary team interventions in order to prevent early PTDM during the first year post-LT. Based on our results, more robust diabetic screening protocols prior to transplant listing and stricter perioperative glucose control may be necessary given the high incidence of PTDM acutely post-LT period. Moreover, these procedures should not be limited to NAFLD LT recipients, given non-NAFLD individuals are also at risk for PTDM onset. The early post-LT period offers an important opportunity to intervene and target high-risk individuals for PTDM including elderly individuals.2
Previous studies have reported worse survival outcomes for diabetic patients post-LT presumably due to higher rates of infections, chronic rejections, and late onset hepatic artery thrombosis.1,3,4 A qualitative systematic review of perioperative glucose control also confirmed worse transplant outcomes associated with hyperglycemia.21 Our results demonstrated higher rates of graft rejection among PTDM individuals with trends toward worse overall survival also suggesting a unique relationship between glucose dysregulation, graft viability and patient survival. It is unclear based on our results whether PTDM itself leads to worse graft outcomes due to the impact of glucose dysregulation, or if rejection episodes associated with increased steroid and immunosuppressant use leads to PTDM development. Interestingly, duration of steroid use was not an independent risk factor for PTDM onset in multivariate analysis. Additionally, rejection episodes were not associated with time to PTDM onset in a Cox regression model. Moreover, trends towards recurrent and de novo graft steatosis were found in this study, suggesting the important role PTDM may play in triggering fatty changes to the graft liver, even among non-NAFLD LT recipients. Given that radiographic imaging was used as a surrogate marker for steatosis due to lack of protocol biopsies, our results may have underestimated cases of recurrent and de novo graft steatosis among LT recipients.
Regarding potential predictors of PTDM, other studies found that male gender, recipient age, body mass index (BMI), hepatitis C virus (HCV) infection, and treated rejection episodes, as well as donor factors including age and diabetes to be risk factors for PTDM.19 This study only found age to be a significant predictor of PTDM among all LT recipients regardless of their indication for LT.
The strengths of this study included investigation of a large, heterogeneous cohort of both NAFLD and non-NAFLD LT recipients many of whom were overweight or obese. Comparisons were made based on PTDM status, and our analyses investigated a variety of important outcomes including overall survival and graft steatosis for a long duration of follow-up (i.e. 5 years post-LT). While limited to a single center, our chart review allowed for investigation of much more granular patient information than what is available via large registry database studies. Moreover, we were able to quantify better at what time point post-LT recipients were at higher risk for PTDM development. Limitations included the retrospective design of this study. Our evaluation of PTDM was limited given the lack of standardized glycemic monitoring in LT recipients without a diagnosis of DM. PTDM was identified by two consecutive laboratory values any time during the post-LT period. Despite this less rigorous monitoring of PTDM, we were able to identify high rates of PTDM even among individuals not transplanted for NAFLD. We were unable to examine the relationship between pre-LT pre-diabetes and post-LT outcomes including PTDM development given lack of standardized glycemic assessments and a small population of individuals identified with pre-diabetes in this study. Lastly, graft steatosis was identified by radiographic techniques without quantification of steatosis in an objective manner using radiologic data such as with controlled attenuation parameter or magnetic resonance proton density fat fraction.
Ultimately, our results highlight that PTDM is a common complication among LT recipients, even among non-NAFLD individuals. We provided a more nuanced evaluation of PTDM incidence among LT recipients using a large, heterogeneous population of predominantly overweight or obese individuals, many of whom were transplanted for non-NAFLD indications. Moreover, the association between PTDM, graft steatosis, rejection and patient survival were investigated in this large cohort. This study was novel for describing the rapid onset of PTDM in the early post-LT period and for capturing risk of PTDM development at different time intervals post-LT. Age may be useful to identify patients at highest risk for developing early onset PTDM, among other known risk factors for metabolic syndrome. Our findings support a systematic approach to screening individuals for diabetes in the immediate post-transplant phase, especially among older LT candidates (>49 years) to minimize early onset of PTDM. Future studies need to investigate the association between different thresholds of glycemic control and outcomes in the early and late post-LT periods including more rigorous glycemic monitoring in individuals without an existing diagnosis of DM. Moreover, empiric use of anti-diabetic agents in conjunction with immunosuppressants among high risk individuals (i.e. elderly patients with metabolic syndrome or insulin resistance) could be investigated. Lastly, it needs to be further elucidated how a diagnosis of pre-diabetes before and after LT affects PTDM and graft/patient survival. A multi-disciplinary approach to monitor and control perioperative hyperglycemia and long-term glucose levels may reduce the incidence of PTDM and its associated negative outcomes including worse graft rejection and patient survival.
Acknowledgement:
Dr. Lieber is supported by a grant from the National Institutes of Health (T32DK007634).
Abbreviations:
- BMI
body mass index
- DRI
donor risk index
- HCV
hepatitis C virus
- LT
liver transplantation
- MELD
Model for End Stage Liver Disease
- NAFLD
non-alcoholic fatty liver disease
- PTDM
post-transplant diabetes mellitus
Footnotes
Disclosures: The authors declare no conflicts of interest.
References:
- 1.Galindo RJ, Wallia A. Hyperglycemia and Diabetes Mellitus Following Organ Transplantation. Curr Diab Rep. 2016;16(2):14. doi: 10.1007/s11892-015-0707-1. [DOI] [PubMed] [Google Scholar]
- 2.Sharif A, Hecking M, de Vries APJ, et al. Proceedings from an international consensus meeting on posttransplantation diabetes mellitus: recommendations and future directions. Am J Transplant. 2014;14(9):1992–2000. doi: 10.1111/ajt.12850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wallia A, Parikh ND, Molitch ME, et al. Posttransplant hyperglycemia is associated with increased risk of liver allograft rejection. Transplantation. 2010;89(2):222–226. doi: 10.1097/TP.0b013e3181c3c2ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moon JI, Barbeito R, Faradji RN, Gaynor JJ, Tzakis AG. Negative impact of new-onset diabetes mellitus on patient and graft survival after liver transplantation: Long-term follow up. Transplantation. 2006;82(12):1625–1628. doi: 10.1097/01.tp.0000250361.60415.96. [DOI] [PubMed] [Google Scholar]
- 5.Cauble MS, Gilroy R, Sorrell MF, et al. Lipoatrophic diabetes and end-stage liver disease secondary to nonalcoholic steatohepatitis with recurrence after liver transplantation. Transplantation. 2001;71(7):892–895. [DOI] [PubMed] [Google Scholar]
- 6.Wallia A, Parikh ND, O’Shea-Mahler E, et al. Glycemic control by a glucose management service and infection rates after liver transplantation. Endocr Pract. 17(4):546–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keegan MT, Vrchota JM, Haala PM, Timm J V. Safety and effectiveness of intensive insulin protocol use in post-operative liver transplant recipients. Transplant Proc. 2010;42(7):2617–2624. [DOI] [PubMed] [Google Scholar]
- 8.Pais R, Barritt AS, Calmus Y, et al. NAFLD and liver transplantation: Current burden and expected challenges. J Hepatol. 2016;65(6):1245–1257. doi: 10.1016/j.jhep.2016.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dureja P, Mellinger J, Agni R, et al. NAFLD recurrence in liver transplant recipients. Transplantation. 2011;91(6):684–689. doi: 10.1097/TP.0b013e31820b6b84. [DOI] [PubMed] [Google Scholar]
- 10.Seo S, Maganti K, Khehra M, et al. De novo nonalcoholic fatty liver disease after liver transplantation. Liver Transpl. 2007;13(6):844–847. doi: 10.1002/lt.20932. [DOI] [PubMed] [Google Scholar]
- 11.Simo KA, Sereika S, Bitner N, Newton KN, Gerber DA. Medical epidemiology of patients surviving ten years after liver transplantation. Clin Transplant. 2011;25(3):360–367. doi: 10.1111/j.1399-0012.2010.01305.x. [DOI] [PubMed] [Google Scholar]
- 12.Barritt AS, Dellon ES, Kozlowski T, Gerber DA, Hayashi PH. The influence of nonalcoholic fatty liver disease and its associated comorbidities on liver transplant outcomes. J Clin Gastroenterol. 2011;45(4):372–378. doi: 10.1097/MCG.0b013e3181eeaff0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng S, Goodrich NP, Bragg-Gresham JL, et al. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006;6(4):783–790. doi: 10.1111/j.1600-6143.2006.01242.x. [DOI] [PubMed] [Google Scholar]
- 14.Therneau T Survival Analysis. 2017. https://cran.r-project.org/web/packages/survival/survival.pdf. Accessed March 17, 2018.
- 15.Yi Z, Mayorga ME, Orman ES, Wheeler SB, Hayashi PH, Barritt AS. Trends in Characteristics of Patients Listed for Liver Transplantation Will Lead to Higher Rates of Waitlist Removal Due to Clinical Deterioration. Transplantation. 2017;101(10):2368–2374. doi: 10.1097/TP.0000000000001851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orman ES, Barritt AS, Wheeler SB, Hayashi PH. Declining liver utilization for transplantation in the United States and the impact of donation after cardiac death. Liver Transplant. 2013;19(1):59–68. doi: 10.1002/lt.23547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orman ES, Mayorga ME, Wheeler SB, et al. Declining liver graft quality threatens the future of liver transplantation in the United States. Liver Transplant. 2015;21(8):1040–1050. doi: 10.1002/lt.24160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wallia A, Illuri V, Molitch ME. Diabetes Care After Transplant: Definitions, Risk Factors, and Clinical Management. Med Clin North Am. 2016;100(3):535–550. [DOI] [PubMed] [Google Scholar]
- 19.Yadav AD, Chang Y- H, Aqel BA, et al. New Onset Diabetes Mellitus in Living Donor versus Deceased Donor Liver Transplant Recipients: Analysis of the UNOS/OPTN Database. J Transplant. 2013;2013:269096. doi: 10.1155/2013/269096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.American Diabetes Association. 2. Classification and Diagnosis of Diabetes. Diabetes Care. 2017;40(Suppl 1):S11–S24. doi: 10.2337/dc17-S005. [DOI] [PubMed] [Google Scholar]
- 21.Paka P, Lieber SR, Lee R- A, Desai CS, Dupuis RE, Barritt AS. Perioperative glucose management and outcomes in liver transplant recipients: A qualitative systematic review. World J Transplant. 2018;8(3):75–83. doi: 10.5500/wjt.v8.i3.75. [DOI] [PMC free article] [PubMed] [Google Scholar]


