Abstract
Background
Proliferative diabetic retinopathy (PDR) is a complication of diabetic retinopathy that can cause blindness. Although panretinal photocoagulation (PRP) is the treatment of choice for PDR, it has secondary effects that can affect vision. An alternative treatment such as anti‐vascular endothelial growth factor (anti‐VEGF), which produces an inhibition of vascular proliferation, could improve the vision of people with PDR.
Objectives
To assess the effectiveness and safety of anti‐VEGFs for PDR.
Search methods
We searched CENTRAL (which contains the Cochrane Eyes and Vision Group Trials Register) (2014, Issue 3), Ovid MEDLINE, Ovid MEDLINE In‐Process and Other Non‐Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to April 2014), EMBASE (January 1980 to April 2014), the metaRegister of Controlled Trials (mRCT) (www.controlled‐trials.com), ClinicalTrials.gov (www.clinicaltrials.gov) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 28 April 2014.
Selection criteria
We included randomised controlled trials (RCTs) comparing anti‐VEGFs to another active treatment, sham treatment or no treatment for people with PDR. We also included studies that assessed the combination of anti‐VEGFs with other treatments.
Data collection and analysis
Two review authors independently selected studies for inclusion, extracted data and assessed risk of bias for all included trials. We calculated the risk ratio (RR) or the mean difference (MD), and 95% confidence intervals (CI).
Main results
We included 18 RCTs with 1005 participants (1131 eyes) of whom 57% were men. The median number of participants per RCT was 40 (range 15 to 261). The studies took place in Asia (three studies), Europe (two studies), the Middle East (seven studies), North America (three studies) and South America (three studies). Eight RCTs recruited people eligible for PRP, nine RCTs enrolled people with diabetes requiring vitrectomy and one RCT recruited people undergoing cataract surgery. The median follow‐up was six months (range one to 12 months). Seven studies were at high risk of bias and the remainder were unclear risk of bias in one or more domains.
Very low quality evidence from one study of 61 people showed that people treated with bevacizumab and PRP were less likely to lose 3 or more lines of visual acuity at 12 months compared with people treated with PRP alone (RR 0.19, 95% CI 0.05 to 0.81). People treated with anti‐VEGF had an increased chance of gaining 3 or more lines of visual acuity but the effect was imprecise and compatible with no effect or being less likely to gain vision (RR 6.78, 95% CI 0.37 to 125.95). No other study reported these two outcomes. On average, people treated with anti‐VEGF (bevacizumab, pegaptanib or ranibizumab) had better visual acuity at 12 months compared with people not receiving anti‐VEGF (MD ‐0.07 logMAR, 95% CI ‐0.12 to ‐0.02; 5 RCTs, 373 participants, low quality evidence). There was some evidence to suggest a regression of PDR with smaller leakage on fluorescein angiography but it was difficult to estimate a pooled result from the two trials reporting this outcome. People receiving anti‐VEGF were less likely to have vitreous or pre‐retinal haemorrhage at 12 months (RR 0.32, 95% CI 0.16 to 0.65; 3 RCTs, 342 participants, low quality evidence). No study reported on fluorescein leakage or quality of life.
All of the nine trials of anti‐VEGF before or during vitrectomy investigated bevacizumab; most studies investigated bevacizumab before vitrectomy, one study investigated bevacizumab during surgery.
People treated with bevacizumab and vitrectomy were less likely to lose 3 or more lines of visual acuity at 12 months compared with people given vitrectomy alone but the effect was imprecise and compatible with no effect or being more likely to lose vision (RR 0.49, 95% CI 0.08 to 3.14; 3 RCTs, 94 participants, low quality evidence). People treated with bevacizumab were more likely to gain 3 or more lines of visual acuity (RR 1.62, 95% CI 1.20 to 2.17; 3 RCTs, 94 participants, low quality evidence). On average, people treated with bevacizumab had better visual acuity at 12 months compared with people not receiving bevacizumab but there was uncertainty in the estimate (the CIs included 0; i.e. were compatible with no effect, and there was considerable inconsistency between studies; MD ‐0.24 logMAR, 95% CI ‐0.50 to 0.01; 6 RCTs, 335 participants, I2 = 67%; low quality evidence). People receiving bevacizumab were less likely to have vitreous or pre‐retinal haemorrhage at 12 months (RR 0.30, 95% CI 0.18 to 0.52; 7 RCTs, 393 participants, low quality evidence). No study reported on quality of life.
Reasons for downgrading the quality of the evidence included risk of bias in included studies, imprecision of the estimates, inconsistency of effect estimates and indirectness (few studies reported at 12 months).
Adverse effects were rarely reported and there was no evidence for any increased risk with anti‐VEGF but given the relatively few studies that reported these, and the low event rate, the power of the analysis to detect any differences was low.
Authors' conclusions
There was very low or low quality evidence from RCTs for the efficacy and safety of anti‐VEGF agents when used to treat PDR over and above current standard treatments. However, the results suggest that anti‐VEGFs can reduce the risk of intraocular bleeding in people with PDR. Further carefully designed clinical trials should be able to improve this evidence.
Plain language summary
Injections of anti‐vascular endothelial growth factor for advanced diabetic retinopathy
Review question Do injections of anti‐vascular endothelial growth factor (anti‐VEGF) help people with advanced diabetic retinopathy in terms of vision and progression of the disease? Is this treatment safe?
Background Diabetic retinopathy is a problem of the back of the eye that occurs in people with diabetes. In later stages of the disease, new blood vessels grow in the back of the eye and cause problems with vision. This advanced form of the disease is known as proliferative diabetic retinopathy. Anti‐VEGF has been developed to block the growth of these new vessels. It has to be injected into the eye.
Search date We examined research published up to 28 April 2014.
Study characteristics We found 18 trials. They took place in Asia (three trials), Europe (two trials), the Middle East (seven trials), North America (three trials) and South America (three trials). A total of 1005 people took part in these trials and 1131 eyes were studied. Eight trials studied anti‐VEGF with another commonly used treatment for diabetic retinopathy (laser), nine studies looked at anti‐VEGF at the time of diabetic eye surgery (vitrectomy) and one study investigated use of anti‐VEGF in people with diabetic retinopathy having cataract surgery. Most studies followed up the participants for six months but some studies followed up for one year.
Study funding sources One study was industry funded, one study was funded by a mixture of government and industry, and three studies were funded by government and non‐government organisations. The remainder of the studies did not report a funding source.
Key results In one small study, we found that people treated with anti‐VEGF plus laser were less likely to lose some vision compared with people treated with laser alone but the estimate was imprecise: around 30% of people treated with laser lost some vision compared with 6% and 24% of people treated with anti‐VEGF plus laser.
On average, people treated with anti‐VEGF had slightly better vision than people not treated with anti‐VEGF. They were also less likely to have bleeding in the eye. None of the studies reported on quality of life. One study suggested that injection of anti‐VEGF was less painful than having laser treatment.
People treated with anti‐VEGF before or during diabetic eye surgery (vitrectomy) were less likely to lose some vision compared with people treated with surgery alone, but the estimate was uncertain and it could be that anti‐VEGF did not make a difference, or increased the risk of losing vision. On average, people receiving anti‐VEGF before or during diabetic eye surgery had slightly better vision than people not treated with anti‐VEGF, but again the estimate was uncertain. They were also less likely to have bleeding in the eye. None of the studies reported on quality of life.
Side effects were uncommon and there were not enough data to detect a difference between the two groups.
Quality of the evidence The quality of the evidence was low or very low. We judged some of the included trials to be at risk of bias because of lack of masking of treatments and problems with follow‐up. Some of the findings were based on too small a numbers of participants. Few studies followed up participants for more than six months.
Summary of findings
Background
Description of the condition
Introduction and epidemiology
Diabetic retinopathy (DR) is a vascular disorder involving the retina that is characterised by increased vascular permeability, retinal ischaemia and oedema, and formation of new vessels (neovascularisation) (Carmeliet 2004). DR produces visual impairment that can progress to blindness. It is a complication of both types of diabetes mellitus (DM), type 1 and type 2. DR may develop before a diagnosis of diabetes is made, such that one in five people with type 2 DM has retinopathy at the time of diagnosis. More than 60% of people with type 2 DM and almost all people with type 1 DM develop DR during the first 20 years of the disease (ADA 2006).
A person with diabetes has a three‐fold increased risk of blindness compared with the general population (Hayward 2002). In one study conducted by Moss et al., the incidence of blindness 10 years after the onset of DM was 1.8% in people with type 1 DM, 4.0% in people with insulin‐treated type 2 DM, and 4.8% in people with non‐insulin treated type 2 DM (Moss 1994). In the same study, the incidence of visual impairment at 10 years was 9.4% in people with type 1 DM, 37.2% in people with insulin‐treated type 2 DM, and 23.9% in people with non‐insulin treated type 2 DM. In the USA, in 2002, 17% of blindness was attributed to DR (Resnikoff 2004).
The principal risk factors for developing DR are the duration of DM and the severity of hyperglycaemia (Davis 1998; Klein 1988; UKPDSG 1998a; Van Leiden 2003). Other risk factors are age (in type 1 DM) (Klein 1984), hypertension (Klein 1989; Klein 2002a; UKPDSG 1998b), nephropathy (Mathiesen 1995), hypercholesterolaemia (Chew 1996; Klein 2002b; Van Leiden 2002), abdominal obesity and high body mass index (Van Leiden 2003), anaemia (Davis 1998), pregnancy (Klein 1990), age at onset (Kullberg 2002), smoking and ethnicity (Moss 1996).
Presentation and diagnosis
DR is clinically characterised by a progressive loss of visual acuity (acuteness or clearness of vision). The retinal damage progresses sequentially from a mild non‐proliferative stage to a severe proliferative stage. Signs of non‐proliferative diabetic retinopathy (NPDR) include presence of microaneurysms, intraretinal haemorrhages, hard exudates (lipid deposits), vascular changes (such as beading and looping or segmentation of the veins), soft exudates or cotton wool spots (which result from the closure of small retinal arterioles), intraretinal microvascular abnormalities and retinal oedema.
There are two important NPDR clinical classification systems: the Early Treatment Diabetic Retinopathy (ETDR) study research group classification (ETDRSRG 1991a; ETDRSRG 1991b; Table 5) and the International Clinical Diabetic Retinopathy Disease Severity scale (ICDRDS; Wilkinson 2003; Table 6).
1. ETDRS classification of diabetic retinopathy.
| Mild | Presence of at least 1 microaneurysm |
| Moderate | Haemorrhages or microaneurysms (or both) more than standard photo 2A, presence of soft exudates, venous beading, IRMA definitively present |
| Severe | Haemorrhages or microaneurysms (or both) more than standard photo 2A in all 4 quadrants, or venous beading in ≥ 2 quadrants, or IRMA more than standard photo 8A in at least 1 quadrant |
| Very severe | Any ≥ 2 of the changes seen in severe NPDR |
| Early PDR | Presence of new vessels |
| High‐risk PDR | Any of the following: NVD more than one‐third to one‐quarter disc diameter, NVD less than one‐third to one‐quarter disc diameter with vitreous or pre‐retinal haemorrhage, new vessels elsewhere with vitreous or pre‐retinal haemorrhage |
ETDRS: Early Treatment Diabetic Retinopathy Study; IRMA: intraretinal microaneurysm; NPDR: non‐proliferative diabetic retinopathy; NVD: new vessels at optic disc; PDR: proliferative diabetic retinopathy.
2. ICDRDS scale.
| Non‐apparent retinopathy | No abnormalities |
| Mild NPDR | Microaneurysms only |
| Moderate NPDR | More than just microaneurysms but less than severe NPDR |
| Severe NPDR | Any of the following: > 20 intraretinal haemorrhages in each of 4 quadrants; definite venous beading in 2 quadrants; prominent intraretinal microvascular abnormalities in 1 quadrant and no signs of proliferative retinopathy |
| Proliferative diabetic retinopathy | ≥ 1 of the following: neovascularisation, vitreous or pre‐retinal haemorrhage |
ICDRDS: International Clinical Diabetic Retinopathy Disease Severity scale; NPDR: non‐proliferative diabetic retinopathy.
Approximately 50% of people with very severe NPDR progress to proliferative diabetic retinopathy (PDR) within one year (ETDRSRG 1991c). PDR is characterised by neovascularisation, which starts in the retina but can grow and affect the vitreous. These new vessels are prone to bleeding, which results in vitreous haemorrhage and fibrosis, and may lead to vitreous or retinal detachments.
Description of the intervention
The treatment strategies for DR include 1. laser photocoagulation (DRSRG 1978; DRSRG 1981a; DRSRG 1981b; ETDRSRG 1985), 2. vitrectomy (DRVSRG 1985), and 3. pharmacotherapy to prevent both the retinal neovascularisation and the blood flow abnormalities affecting metabolic pathways. Generally, the drug is administered by intravitreal injection.
There are several lines of treatment including vascular endothelial growth factor (VEGF) inhibitors (anti‐VEGF). Some anti‐VEGFs are non‐selective, such as corticosteroids (Jaffe 2006; Martidis 2002; Nauck 1997), cyclo‐oxygenase inhibitors (Sennlaub 2003), and angiotensin‐converting enzyme (ACE) inhibitors (Gilbert 2000). Other anti‐VEGFs are selective, such as pegaptanib sodium (Adamis 2006; Cunningham 2005), and antibodies such as bevacizumab (Arevalo 2007; Avery 2006a; Avery 2006b; Chen 2006; Haritoglou 2006; Mason 2006; Scott 2007; Spaide 2006), and ranibizumab (Chun 2006), which cause regression of neovascularisation, macular oedema, or both.
How the intervention might work
VEGFs are present in the retinal pigment epithelium, pericytes and endothelial cells of the retina. VEGFs are released physiologically when ischaemia occurs and they stimulate the formation of new blood vessels. Hyperglycaemia induces chronic retinal hypoxia and leads to the over‐expression of VEGFs that stimulate the formation of neovascularisation (Bussolati 2001), and cause vascular disease in the retina.
Selective anti‐VEGF drugs inhibit only specific VEGF isoforms, pegaptanib (a modified oligonucleotide) inhibits only the VEGF 165 isoform. Bevacizumab and ranibizumab (a murine humanised monoclonal antibody fragment) inhibit all isoforms of VEGF‐A. Some studies showed that local intravitreal administration of these drugs may be useful in macular oedema and neovascularisation although anti‐VEGFs can produce local adverse effects (in 1.27% of cases) such as endophthalmitis (severe inflammation of the intraocular cavities usually caused by infection) (Shima 2008), and systemic adverse effects (in 1.5% of cases) such as acute elevation of systemic blood pressure or cerebrovascular accident (CVA) (Wu 2008).
Why it is important to do this review
Despite the standard of care given for the prevention and treatment of DR, it remains an important cause of vision loss. Due to this, new lines of treatment, such as with selective anti‐VEGF drugs, are being developed. Some of these anti‐VEGFs do not have authorisation to be used in DR and are prescribed as off‐label or compassionate‐use drugs, but the evidence that supports this practice has not been sufficiently determined. One Cochrane systematic review has been completed on diabetic macular oedema (DMO) (Virgili 2012). It is important to do a systematic review that clarifies the efficacy of the selective anti‐VEGFs in PDR. In addition, we examined the evidence from randomised controlled trials (RCT) on harms of such therapy.
Objectives
To assess the effectiveness and safety of anti‐VEGFs for PDR.
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs without any date or language restrictions. We excluded studies that included DMO as part of the principal inclusion from the review because this has been assessed in the Cochrane review by Virgili 2012.
Types of participants
We included trials in adults (aged 18 years and over) with proliferative DR. We included participants with DR at baseline but the criteria to be selected in the studies was not based on having DMO.
There were two different patient groups with proliferative DR: people who were eligible for laser photocoagulation and people eligible for vitrectomy due to retinal haemorrhage. We judged that these two groups were sufficiently different that it did not make clinical sense to pool the results of these studies; thus, we have considered them separately. This was a post hoc decision and was not planned in our protocol.
Types of interventions
We included studies in which selective anti‐VEGFs were compared with another active treatment, sham treatment or no treatment. We also included studies that assessed the combination of anti‐VEGFs with other treatments, for example, photocoagulation.
Two different comparisons were made: anti‐VEGFs compared with panretinal photocoagulation (PRP) and anti‐VEGFs as an adjunct to vitrectomy compared with vitrectomy alone.
Types of outcome measures
Primary outcomes
Best‐corrected visual acuity at 12 months.
We used three measures:
loss of 3 or more lines of vision on the ETDRS visual acuity charts;
gain of 3 or more lines of vision on the ETDRS visual acuity charts.
This 3‐line change is equivalent to a doubling of the visual angle. For studies that did not use the ETDRS chart, we used the measure of visual acuity reported that corresponded most closely to a doubling of the visual angle.
We also considered mean visual acuity:
corrected visual acuity measured on a continuous scale (logMAR visual acuity or ETDRS letters).
Secondary outcomes
Regression of PDR (i.e. regression of neovascularisation to an inactive stage as defined with fluorescein angiography (absence of leakage) or clinical examination (fibrotic new vessels and absence of haemorrhage from new vessels) or any validated DR staging system, such as ETDRS or ICRDS scale). We measured regression sustained at least three months after the last injection.
Presence of microaneurysms.
Presence of vitreous or pre‐retinal haemorrhage.
Need for laser photocoagulation.
Need for vitrectomy.
People with any ocular or systemic adverse outcomes.
DMO.
Quality of life measures in any validated scale.
Adverse effects.
Search methods for identification of studies
Electronic searches
We searched CENTRAL (which contains the Cochrane Eyes and Vision Group Trials Register) (2014, Issue 3), Ovid MEDLINE, Ovid MEDLINE In‐Process and Other Non‐Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to April 2014), EMBASE (January 1980 to April 2014), the metaRegister of Controlled Trials (mRCT) (www.controlled‐trials.com), ClinicalTrials.gov (www.clinicaltrials.gov) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 28 April 2014.
See: Appendices for details of search strategies for CENTRAL (Appendix 1), MEDLINE (Appendix 2), EMBASE (Appendix 3), mRCT (Appendix 4), ClinicalTrials.gov (Appendix 5) and the ICTRP (Appendix 6).
Searching other resources
We looked for other published systematic reviews in this area as a source of additional RCTs. We reviewed the reference lists of the identified clinical trials. When necessary, we contacted study authors to obtain more information regarding their published trials.
Data collection and analysis
Selection of studies
Two authors (MJM, and JAC or CHF or JRE) independently assessed the eligibility of the studies identified in the search. When there were disagreements, a third author (AMC) evaluated the study independently and discussed it with the remainder of the team.
We graded the eligible studies as included or excluded. We contacted three study authors to clarify secondary publications of the main clinical trial (Cho 2010; Ernst 2012; Ramos Filho 2011).
Data extraction and management
Two authors (MJM, and JAC or JRE) collected data independently on a previously tested standardised form. The collected information recorded the risk of bias, characteristics of participants in the study, characteristics of the intervention and control groups, and outcome characteristics of each group of participants. Two review authors (MJM and JRE) entered the data into Review Manager 5.3 (RevMan 2014).
We contacted two authors to obtain information about missing data (Farahvash 2011; Rizzo 2008).
When visual acuity was measured using the ETDRS chart but reported in letters rather than logMAR score, we converted to logMAR score using the following formula: (85‐mean letter score) * 0.02 and for the standard deviation (SD) (letter score * 0.02) (Ferris 1982).
Assessment of risk of bias in included studies
Two authors (MJM, and JAC or JRE) assessed the risk of bias of the included studies, specifically examining the randomisation method (sequence generation and allocation concealment); whether the intervention was blinded to the participants, investigators and outcome assessors; incomplete outcome data; selective outcome reporting and percentage of losses to follow‐up. We also considered whether the number of post‐randomisation losses and exclusions had been made explicit. Once this information was gathered, the authors classified each study into one of the three levels of risk of bias: low, unclear or high risk of bias. We followed the criteria specified in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Measures of treatment effect
We considered the following effect measures for each study: risk ratios (RR) for dichotomous variables and mean differences (MD) for continuous variables. We calculated 95% confidence interval (CI).
Unit of analysis issues
The unit of analysis was the eye; most studies included one eye per person. We excluded from the analysis exclusively within‐person studies (trials where the fellow eye was used as a control) (Ernst 2012; Mirshahi 2008; Preti 2014), but we included studies with a low percentage of participants with fellow eye used as a control (Ahn 2011; Cho 2010; Di Lauro 2010; Ergur 2009; Sohn 2012).
Dealing with missing data
We contacted study authors to obtain further information. Our main analysis has been an 'available‐case analysis', analysing data as provided in the individual studies.
Assessment of heterogeneity
We examined the characteristics of each study to detect clinical heterogeneity. We conducted an analysis to detect the presence of heterogeneity. We regarded an I2 statistic between 50% and 75% as substantial heterogeneity and an I2 statistic between 75% and 100% considerable statistical heterogeneity, and we studied sources of heterogeneity. When heterogeneity was more than 75%, we did not pool the studies.
Assessment of reporting biases
In accordance with Chapter 10 of the Cochrane Handbook for Systematic Reviews of Interventions (Sterne 2011), we did not assess whether the review was subject to publication bias by using a funnel plot because the number of clinical trials identified for inclusion in the meta‐analyses was fewer than 10.
Data synthesis
We determined the pooled effect estimate for each outcome through a meta‐analysis of the individual study effect measures using a random‐effects model (DerSimonian 1986), unless there were three trials or fewer in which case we used a fixed‐effect model.
We performed statistical analysis using Review Manager 5 (RevMan 2014).
Subgroup analysis and investigation of heterogeneity
We compared the effect of treatment according to type of anti‐VEGF agent, that is, pegaptanib, ranibizumab and bevacizumab.
Sensitivity analysis
We compared random‐effects models and fixed‐effect models for those analyses that had three or more trials.
We compared the results of high risk of bias trials (i.e. high risk of bias in one or more domains) and low risk trials (i.e. not high risk in any domain) for those analyses that had more than two trials contributing to the analysis and at least one trial in each high risk/low risk group.
'Summary of findings' table
We prepared two 'Summary of findings' tables, including assessment of the overall quality of the evidence for each outcome using the GRADE scheme (GRADEpro 2014).
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
Results of the search
The electronic searches yielded 3400 references (Figure 1). After removing duplicates, we screened 2774 records and obtained the full‐text reports of 52 potentially relevant publications pertaining to 42 studies. We included 18 studies (Ahmadieh 2009; Ahn 2011; Cheema 2009; Cho 2010; Di Lauro 2010; DRCR.Net 2013; El‐Batarny 2008; Ergur 2009; Ernst 2012; Farahvash 2011; González 2009; Mirshahi 2008; Modarres 2009; Preti 2014; Ramos Filho 2011; Rizzo 2008; Sohn 2012; Zaman 2013), and excluded 19 studies (Arimura 2009; Fulda 2010; Genovesi‐Ebert 2007; Gonzalez 2006; Hattori 2010; Huang 2009; Ip 2012; Jiang 2009; Jorge 2006; Lanzagorta‐Aresti 2009; López‐López 2012; Michaelides 2010; Minnella 2008; Scott 2008; Shin 2009; Stergiou 2007; Tonello 2008; Yeh 2009; Zhou 2010). We have included five ongoing studies and will assess the data when results become available.
1.
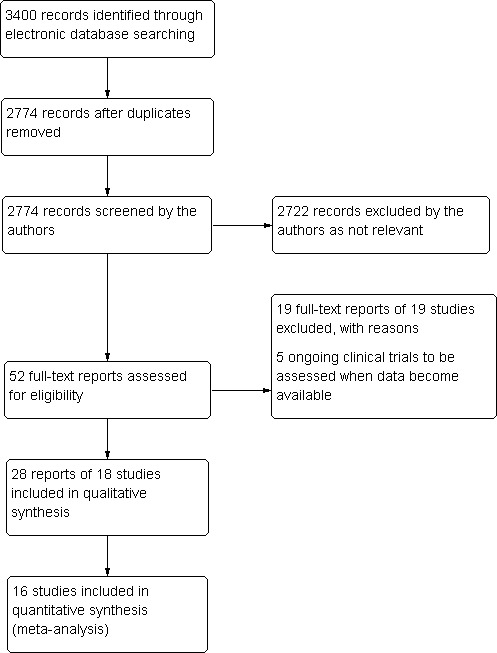
Results from searching for studies for inclusion in the review.
We contacted authors to obtain additional information (Cho 2010; Ernst 2012; Farahvash 2011; Ramos Filho 2011; Rizzo 2008). Three authors responded to our questions (Ernst 2012; Farahvash 2011; Ramos Filho 2011).
Included studies
Overall, we included data on 1005 participants from 18 RCTs in the review. Forty‐three per cent of participants were women and 57% were men, with a mean age of 56 years (range 44 to 71 years). The median number of participants per RCT was 40 (range 15 to 261).
Eight studies evaluated anti‐VEGF in people who needed PRP. In six of these studies, anti‐VEGF was combined with PRP and compared with PRP alone (Cho 2010; DRCR.Net 2013; Ergur 2009; Mirshahi 2008; Preti 2014; Ramos Filho 2011); two studies compared anti‐VEGF alone with PRP (Ernst 2012; González 2009). Five of these studies used bevacizumab (Cho 2010; Ergur 2009; Ernst 2012; Mirshahi 2008; Preti 2014); two studies used ranibizumab (DRCR.Net 2013; Ramos Filho 2011), and one study used pegaptanib (González 2009).
Nine studies evaluated anti‐VEGF as an adjunct to vitrectomy (Ahmadieh 2009; Ahn 2011; Di Lauro 2010; El‐Batarny 2008; Farahvash 2011; Modarres 2009; Rizzo 2008; Sohn 2012; Zaman 2013). All nine trials used bevacizumab.
One study evaluated bevacizumab applied during the course of cataract surgery to prevent progression of proliferative DR (Cheema 2009).
The primary outcome was visual acuity in five trials (Cho 2010; Ergur 2009; Ernst 2012; Preti 2014; Sohn 2012), incidence of vitreous haemorrhage in three trials (Ahmadieh 2009; Ahn 2011; Farahvash 2011), feasibility of the surgery in three trials (El‐Batarny 2008; Modarres 2009; Rizzo 2008), regression of PDR in two studies (González 2009; Mirshahi 2008), progression of DR and maculopathy in one trial (Cheema 2009), active neovascularisation in one trial (Ramos Filho 2011), cumulative probability of vitrectomy in one trial (DRCR.Net 2013), clearing of vitreous haemorrhage in one trial (Di Lauro 2010), severity of intraoperative bleeding in one trial (Farahvash 2011), and changes in contrast sensitivity in one trial (Preti 2014).
The median follow‐up of participants was six months (range 1 (Ahmadieh 2009) to 12 months (El‐Batarny 2008; Ernst 2012; Farahvash 2011)).
Only one trial specified the calculation of the sample size (DRCR.Net 2013). There was imbalance between groups at baseline in one trial (Sohn 2012). Participants in the control group were worse than the experimental group at baseline: two had visually significant cataract (one participant in each group), two had worsening ischaemia (control group), one had severe neovascular glaucoma (control group), and one had vitreous haemorrhage (control group).
Only five trials reported the sources of funding (DRCR.Net 2013; González 2009; Preti 2014; Ramos Filho 2011; Sohn 2012). One study was industry funded (González 2009), one study was funded by a mixture of government and industry (DRCR.Net 2013), and three studies were funded by government and non‐government organisations (Preti 2014; Ramos Filho 2011; Sohn 2012). The remaining studies did not report a funding source.
Excluded studies
We excluded 19 clinical trials (Arimura 2009; Fulda 2010; Genovesi‐Ebert 2007; Gonzalez 2006; Hattori 2010; Huang 2009; Ip 2012; Jiang 2009; Jorge 2006; Lanzagorta‐Aresti 2009; López‐López 2012; Michaelides 2010; Minnella 2008; Scott 2008; Shin 2009; Stergiou 2007; Tonello 2008; Yeh 2009; Zhou 2010). The Characteristics of excluded studies table shows the reasons for exclusion. Briefly, eight studies were prospective non‐randomised clinical trials (Fulda 2010; Genovesi‐Ebert 2007; Hattori 2010; Huang 2009; Jorge 2006; López‐López 2012; Minnella 2008; Yeh 2009), four studies were retrospective (Arimura 2009; Jiang 2009; Shin 2009; Stergiou 2007), four trials were in people with macular oedema (Gonzalez 2006; Ip 2012; Michaelides 2010; Zhou 2010), one study had methodological issues (Scott 2008), one trial was in non‐PDR (Lanzagorta‐Aresti 2009), and one trial was partially randomised (Tonello 2008).
Risk of bias in included studies
Figure 2 and Figure 3 show the risk of bias in included studies.
2.
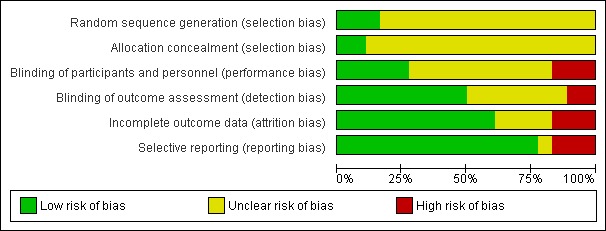
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
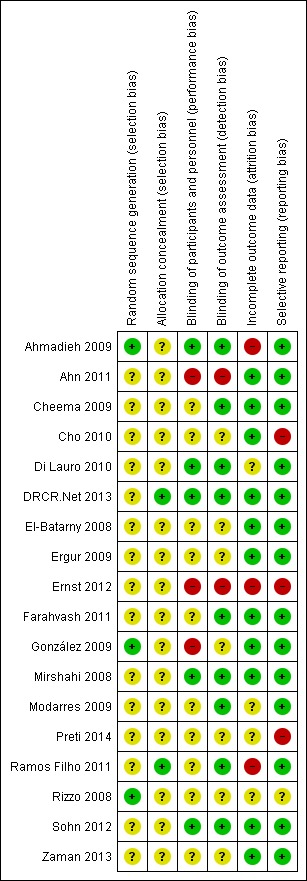
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Three studies reported methods of sequence generation that we considered were low risk of bias with mention of computer‐generated random allocation lists (Ahmadieh 2009; González 2009), and use of random number tables (Rizzo 2008). The remaining studies did not report how they generated the allocation in enough detail to enable us to judge.
Only two studies reported adequate methods of allocation concealment. One study had a central online randomisation system (DRCR.Net 2013), and one study used sealed opaque envelopes (Ramos Filho 2011). The remainder of the studies did not report allocation.
Blinding
Five studies reported blinding of participants, personnel and outcome assessors, usually by means of a sham injection or procedure (Ahmadieh 2009; Di Lauro 2010; Mirshahi 2008; Sohn 2012), but in one study, both interventions were delivered by injection and these were identified by number only (DRCR.Net 2013). A further four studies reported blinding outcome assessors only (Cheema 2009; Farahvash 2011; Modarres 2009; Ramos Filho 2011). We judged three studies to be at high risk of bias for blinding because they were not blinded (open label) and the interventions were different (Ahn 2011; Ernst 2012; González 2009).
Incomplete outcome data
Most studies did not appear to have a problem with incomplete outcome data but, for some studies, it was not clearly reported (Di Lauro 2010; Modarres 2009; Preti 2014; Rizzo 2008), and three studies had relatively high loss to follow‐up so we judged them to be at high risk of attrition bias (Ahmadieh 2009; Ernst 2012; Ramos Filho 2011).
Selective reporting
For most studies, we considered selective outcome reporting was not a problem because they reported the main outcomes expected or mentioned them in the methods section of the paper. We judged three studies to be at high risk of bias for selective reporting because the outcomes were reported incompletely (Cho 2010), or differed to those stated in the protocol (Ernst 2012), or on the trials register (Preti 2014); for one study, this information was unclear (Rizzo 2008).
Effects of interventions
Summary of findings for the main comparison. Anti‐VEGF with or without laser (panretinal photocoagulation; PRP) compared with PRP alone for proliferative diabetic retinopathy.
| Anti‐VEGF with or without laser (panretinal photocoagulation; PRP) compared with PRP alone for proliferative diabetic retinopathy | |||||
|
Patient or population: people with PDR Settings: hospital Intervention: anti‐VEGF with or without PRP Comparison: PRP | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| PRP | Anti‐VEGF with or without PRP | ||||
| Loss of ≥ 3 lines of ETDRS visual acuity Follow‐up: 12 months | 300 per 1000 | 57 per 1000 (15 to 243) | RR 0.19 (0.05 to 0.81) | 61 (1 study) | ⊕⊝⊝⊝ very low1 |
| Gain of ≥ 3 lines of ETDRS visual acuity Follow‐up: mean 12 months | 10 per 1000 | 68 per 1000 (4 to 1260) | RR 6.78 (0.37 to 125.95) | 61 (1 study) | ⊕⊕⊝⊝ very low1 |
|
Visual acuity
logMAR (logMAR scale value of 0 = 6/6 vision, higher score = worse vision) Follow‐up: 12 months |
The mean visual acuity ranged across control groups from 0.08 to 0.72 logMAR | The mean visual acuity in the intervention groups was 0.07 logMAR units lower (0.12 to 0.02 lower) | ‐ | 373 (5 studies) | ⊕⊕⊝⊝ low2 |
|
Regression of proliferative diabetic retinopathy (as measured by area of fluorescein leakage) Follow‐up: 12 months |
In 1 trial, people who received bevacizumab in addition to PRP had more regression of PDR, as measured by area of fluorescein leakage at 6 months compared with people who had PRP alone (MD ‐8.13 mm2, 95% CI ‐10.94 mm2 to ‐5.32 mm2, 19 participants). In another trial, people who received ranibizumab in addition to PRP had more regression of PDR, as measured by change in area of fluorescein leakage between baseline and 12 months compared with people who had PRP alone, however, the size of the effect was smaller and the CIs were compatible with no effect, or less regression (MD ‐1.0 mm2, 95% CI ‐5.3 mm2 to 3.3 mm2, 20 participants) | ||||
|
Presence of vitreous/pre‐retinal haemorrhage Follow‐up: 12 months |
150 per 1000 | 48 per 1000 (24 to 98) | RR 0.32 (95% CI 0.16 to 0.65) | 342 (3 studies) | ⊕⊕⊝⊝ low3 |
| Quality of life | No data reported on quality of life | ||||
| Adverse effects | Adverse effects were reported in 3 studies: 1 study of bevacizumab plus PRP compared with PRP alone and followed up to 3 months (61 participants); 1 study of ranibizumab compared with saline (both groups received PRP if indicated) and followed up to 4 months (261 participants); 1 study of ranibizumab plus PRP compared with PRP alone and followed up to 12 months (31 participants)
|
||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
CI: confidence interval; ETDRS: Early Treatment Diabetic Retinopathy Study; MD: mean difference; PDR: proliferative diabetic retinopathy; PRP: panretinal photocoagulation; RR: risk ratio; VEGF: vascular endothelial growth factor. GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
|
1 Downgraded for risk of bias (‐1) (study at high risk of selective reporting bias) imprecision (‐1) (wide CIs) and indirectness (‐1) (study reported gain/loss of ≥ 2 lines at 3 months only).
2 Downgraded for risk of bias (‐1) (3 studies at high risk of bias in ≥ 1 domains) and downgraded for indirectness (‐1) (only 1 of the studies followed up to 12 months). 3 Downgraded for risk of bias (‐1) (2 studies at high risk of bias in ≥ 1 domain) and downgraded for indirectness (‐1) (no study reported at 12 months). | |||||
Summary of findings 2. Bevacizumab before or during vitrectomy compared with vitrectomy alone.
| Bevacizumab before or during vitrectomy compared with vitrectomy alone | |||||
|
Patient or population: people undergoing vitrectomy for PDR Settings: hospital Intervention: bevacizumab before or during vitrectomy Comparison: vitrectomy alone or vitrectomy with sham injection | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Surgery | Anti‐VEGF plus surgery | ||||
|
Loss of ≥ 3 lines of ETDRS visual acuity Follow‐up: 12 months |
60 per 1000 | 29 per 1000 (5 to 188) | RR 0.49 (0.08 to 3.14) | 94 (3 studies) | ⊕⊕⊝⊝ low1 |
|
Gain of ≥ 3 lines of ETDRS visual acuity Follow‐up: 12 months |
500 per 1000 | 810 per 1000 (600 to 1000) | RR 1.62 (1.2 to 2.17) | 94 (3 studies) | ⊕⊕⊝⊝ low 1 |
|
Visual acuity logMAR (logMAR scale value of 0 = 6/6 vision, higher score = worse vision) Follow‐up: 12 months |
The mean visual acuity ranged across control groups from 0.51 to 1.46 logMAR units | The mean visual acuity in the intervention groups was 0.24 logMAR units lower (0.50 lower to 0.01 higher) | ‐ | 335 (6 studies) | ⊕⊕⊝⊝ low3 |
|
Regression of PDR (as measured by area of fluorescein leakage) Follow‐up: 12 months |
No data reported on regression of PDR | ||||
|
Presence of vitreous/pre‐retinal haemorrhage Follow‐up: 12 months |
500 per 1000 | 150 per 1000 (90 to 260) | RR 0.30 (0.18 to 0.52) | 393 (7 studies) | ⊕⊕⊝⊝ low4 |
| Quality of life | No data reported on quality of life | ||||
| Adverse effects | Neovascular glaucoma: RR 2.33 (95% CI 0.28 to 19.17; 1 RCT, 368 participants) Retinal detachment: RR 0.56 (95% CI 0.11 to 2.86; 3 RCTs, 182 participants) Cataract: RR 0.68 (95% CI 0.38 to 1.23; 2 RCTs, 137 participants) Raised intraocular pressure: RR 0.31 (95% CI 0.01 to 7.47; 1 RCT, 68 participants) Myocardial infarction: no events in 2 trials (175 participants) Cerebrovascular accident: no events in 2 trials (175 participants) Endophthalmitis: none of the studies reported endophthalmitis Arterial hypertension: none of the studies reported arterial hypertension Pain: none of the studies reported pain |
||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; ETDRS: Early Treatment Diabetic Retinopathy Study; PDR: proliferative diabetic retinopathy; RR: risk ratio; VEGF: vascular endothelial growth factor. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
|
1 Downgraded for imprecision (‐1) (wide CIs) and downgraded for indirectness (‐1) (only 1 trial reported at 12 months and only 1 (other) trial reported loss of ≥ 3 lines).
2 Downgraded for indirectness (‐1) (only 1 trial reported at 12 months and only 1 (other) trial reported gain of ≥ 3 lines) and downgraded for inconsistency (‐1) (I2 = 73%). 3Downgraded for risk of bias (‐1) (2 studies at high risk of bias in ≥ 1 domains) and downgraded for inconsistency (‐1) (I2 = 66%). 4 Downgraded for risk of bias (‐1) (2 studies at high risk of bias in ≥ 1 domains, 3 studies at unclear risk of bias in ≥ 3 domains) and downgraded for indirectness (‐1) (only 1 study reported at 12 months). | |||||
Comparison 1: anti‐vascular endothelial growth factor with or without panretinal photocoagulation versus panretinal photocoagulation alone
1.1 Loss of 3 or more lines of ETDRS visual acuity
One study reported loss of visual acuity measured as a dichotomous outcome (Cho 2010). The study reported a cut‐point of loss of 2 or more lines at three months and used intravitreal bevacizumab as an adjunct to PRP (injected one week before laser treatment) and compared with PRP alone.
Participants who received anti‐VEGF before PRP were less likely to lose visual acuity compared with participants who did not (RR 0.19, 95% CI 0.05 to 0.81; 61 participants).
1.2 Gain of 3 or more lines of ETDRS visual acuity
One study reported gain of visual acuity measured as a dichotomous outcome (Cho 2010). The study reported a cut‐point of loss of 2 or more lines at three months and used intravitreal bevacizumab as an adjunct to PRP (injected one week before laser treatment) and compared with PRP alone.
People who received anti‐VEGF were more likely to gain visual acuity but the CIs were wide and compatible with no effect (RR 6.78, 95% CI 0.37 to 125.95; 61 participants).
1.3 Mean visual acuity
Five trials contributed to the analyses of mean visual acuity. We planned to collect data on final visual acuity at follow‐up. Two studies reported change in visual acuity from baseline and we included this in the analysis (González 2009; Ramos Filho 2011).
Two of the trials used intravitreal bevacizumab (Cho 2010; Ergur 2009), one trial used intravitreal pegaptanib (González 2009), and two trials used ranibizumab (DRCR.Net 2013; Ramos Filho 2011). Three trials used bevacizumab as an adjunct to PRP (injected at the same time or up to three weeks before PRP) compared with PRP alone (Cho 2010; Ergur 2009; Ramos Filho 2011). One trial compared pegaptanib injected every six weeks for 30 weeks with treatment with PRP (González 2009). One trial compared three injections of ranibizumab at baseline, four and eight weeks with an injection of saline; both groups also received PRP (DRCR.Net 2013).
Mean visual acuity was reported at three months (Cho 2010), four months (DRCR.Net 2013), six months (Ergur 2009), nine months (González 2009), and 12 months (Ramos Filho 2011).
People who received anti‐VEGF on average had better visual acuity at follow‐up compared with people who received PRP alone (MD ‐0.07 logMAR, 95% CI ‐0.12 to ‐0.02; 373 participants; Analysis 1.1;Figure 4).
1.1. Analysis.
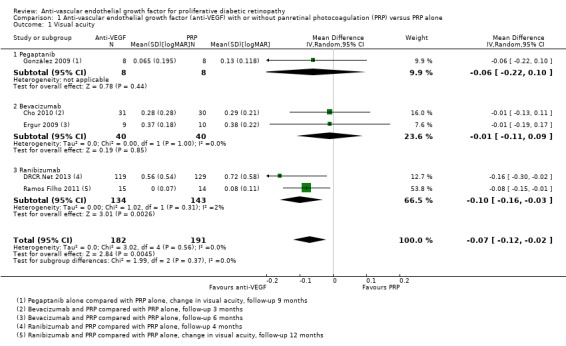
Comparison 1 Anti‐vascular endothelial growth factor (anti‐VEGF) with or without panretinal photocoagulation (PRP) versus PRP alone, Outcome 1 Visual acuity.
4.
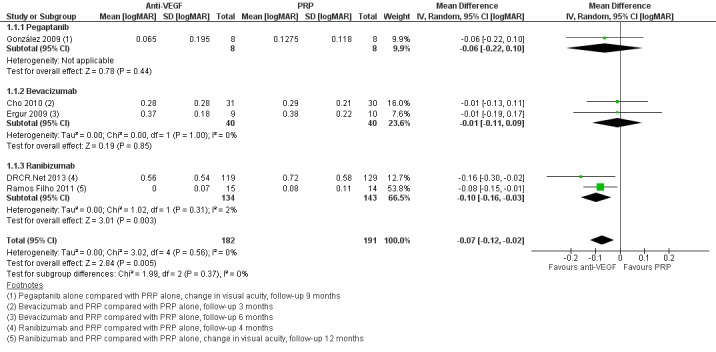
Forest plot of comparison: 1 Anti‐vascular endothelial growth factor (anti‐VEGF) versus photocoagulation, outcome: 1.3 Visual acuity [logMAR].
Overall, there was no evidence for heterogeneity (I2 = 0%) and no evidence for any difference according to type of anti‐VEGF (test for subgroup differences P value = 0.37).
1.4 Regression of proliferative diabetic retinopathy (dichotomous outcome)
None of the studies reported regression of PDR (dichotomous outcome).
1.5 Regression of proliferative diabetic retinopathy (mean area of fluorescein leakage)
People who received bevacizumab in addition to PRP had more regression of PDR, as measured by area of fluorescein leakage, at six months compared with people who had PRP alone (MD ‐8.13 mm2, 95% CI ‐10.94 to ‐5.32; 19 participants; Analysis 1.2; Ergur 2009).
1.2. Analysis.
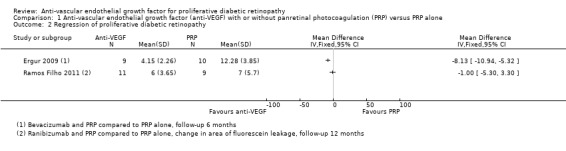
Comparison 1 Anti‐vascular endothelial growth factor (anti‐VEGF) with or without panretinal photocoagulation (PRP) versus PRP alone, Outcome 2 Regression of proliferative diabetic retinopathy.
People who received ranibizumab in addition to PRP had more regression of PDR, as measured by change in area of fluorescein leakage between baseline and 12 months, compared with people who had PRP alone; however, the size of the effect was smaller and the CIs were compatible with no effect or less regression (MD ‐1.0 mm2, 95% CI ‐5.3 to 3.3; 20 participants; Analysis 1.2; Ramos Filho 2011).
Overall, there was considerable heterogeneity (I2 = 86%) and we did not pool the data of the two studies. It was unclear whether or not the differences between the estimates reflected differences in the interventions or comparators, length of follow‐up or some other attributes of these studies. Intravitreal bevacizumab (1.25 mg) was injected 20 days before three sessions of PRP and compared with PRP alone (Ergur 2009). Ranibizumab 0.5 mg was injected 60 minutes before PRP and compared with PRP alone (Ramos Filho 2011).
1.6 Presence of microaneurysms
None of the studies reported presence of microaneurysms.
1.7 Presence of vitreous or pre‐retinal haemorrhage
Three trials reported on the presence of vitreous or pre‐retinal haemorrhage. One of these trials used intravitreal bevacizumab (Cho 2010), one trial used intravitreal pegaptanib (González 2009), and one trial used ranibizumab (DRCR.Net 2013). Bevacizumab was used as an adjunct to PRP (injected at the same time or up to one week before PRP) and compared with PRP alone (Cho 2010). Pegaptanib was injected every six weeks for 30 weeks and compared with treatment with PRP (González 2009). Three injections of ranibizumab at baseline, four and eight weeks were compared with an injection of saline; both groups also received PRP (DRCR.Net 2013).
People who received anti‐VEGF were less likely to present with vitreous or pre‐retinal haemorrhage compared with people that received PRP (overall pooled RR 0.32, 95% CI 0.16 to 0.65; 342 participants; Analysis 1.3).
1.3. Analysis.
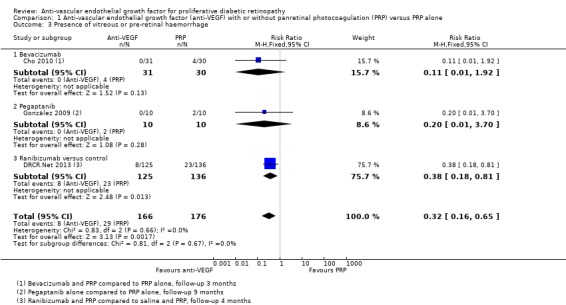
Comparison 1 Anti‐vascular endothelial growth factor (anti‐VEGF) with or without panretinal photocoagulation (PRP) versus PRP alone, Outcome 3 Presence of vitreous or pre‐retinal haemorrhage.
Overall there was no evidence for heterogeneity (I2 = 0%) and no evidence of any difference according to type of anti‐VEGF (test for subgroup differences P value = 0.67).
1.8 Need for laser photocoagulation
None of the studies reported need for laser photocoagulation.
1.9 Need for vitrectomy
We only found one relevant trial that reported need for vitrectomy (DRCR.Net 2013). Eyes with vitreous haemorrhage due to PDR that received ranibizumab were less likely to need vitrectomy by four months compared with eyes that received saline but the CIs were wide and compatible with no effect or increased risk of need for vitrectomy (RR 0.74, 95% CI 0.40 to 1.36; 261 participants).
1.10 Diabetic macular oedema
One trial reported DMO at six months (Ergur 2009). People who received bevacizumab were less likely to develop DMO but the CIs were wide and compatible with no effect or reduced risk of developing DMO (RR 0.14, 95% CI 0.01 to 2.45; 30 participants).
1.11 Quality of life
No studies reported quality of life.
1.12 Adverse effects
One study of bevacizumab (Cho 2010), and two of ranibizumab (DRCR.Net 2013; Ramos Filho 2011) reported adverse events. See Analysis 1.4.
1.4. Analysis.
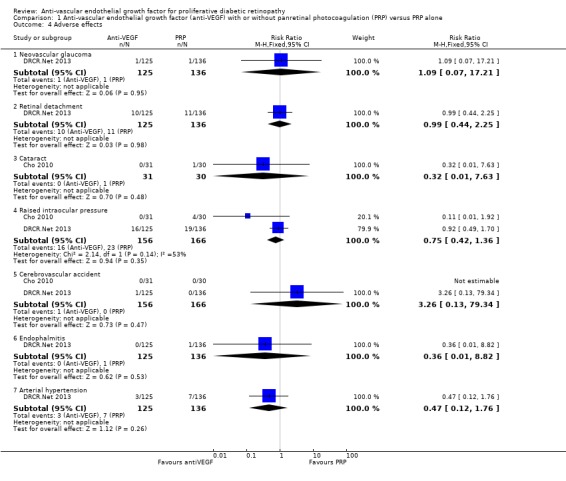
Comparison 1 Anti‐vascular endothelial growth factor (anti‐VEGF) with or without panretinal photocoagulation (PRP) versus PRP alone, Outcome 4 Adverse effects.
Neovascular glaucoma
One trial reported neovascular glaucoma (DRCR.Net 2013). One person in each arm of the study developed neovascular glaucoma (RR 1.09, 95% CI 0.07 to 17.21; 261 participants).
Retinal detachment
One trial reported retinal detachment (DRCR.Net 2013). Similar numbers of people developed retinal detachment in the ranibizumab and saline groups (10/125 with ranibizumab versus 11/136 with saline; RR 0.99, 95% CI 0.44 to 2.25; 261 participants).
Cataract
One trial reported cataract (Cho 2010). People who received anti‐VEGF were less likely to develop cataract compared with people who did not receive anti‐VEGF, but the CIs were wide and compatible with no effect or increased risk of cataract (RR 0.32, 95% CI 0.01 to 7.63; 61 participants).
Raised intraocular pressure
Two trials reported increase of intraocular pressure (IOP) (322 participants) (DRCR.Net 2013; Cho 2010).
People who received bevacizumab were less likely to have developed increased IOP at three months compared with people who did not receive anti‐VEGF, but the CIs were wide and compatible with no effect or increased risk of increased IOP (RR 0.11, 95% CI 0.01 to 1.92; 61 participants; Cho 2010).
The risk of raised IOP was similar between the eyes that received ranibizumab and eyes that received saline (RR 0.92, 95% CI 0.49 to 1.70; 261 participants; DRCR.Net 2013).
Cerebrovascular accident
Two trials reported CVA (DRCR.Net 2013; Cho 2010). The two trials reported only one case of CVA in the anti‐VEGF group in DRCR.Net 2013 (RR 3.26, 95% CI 0.13 to 79.34; 322 participants).
Endophthalmitis
One trial reported endophthalmitis (DRCR.Net 2013). There was only one case of endophthalmitis, which was in the saline group (RR 0.36, 95% CI 0.01 to 8.82; 261 participants).
Arterial hypertension
One trial reported arterial hypertension (DRCR.Net 2013). People who received anti‐VEGF were less likely to develop arterial hypertension compared with people who did not receive anti‐VEGF, but the CIs were wide and compatible with no effect or increased risk of arterial hypertension (RR 0.47, 95% CI 0.12 to 1.76; 261 participants).
Pain
One trial reported pain, which was measured on a 100‐mm visual analogue scale (Ramos Filho 2011). People receiving ranibizumab intravitreal injection reported a mean pain score of 4.7 (SD 8.4), which was much lower than people receiving PRP who reported a mean pain score of 60.8 (SD 29.2). This gave an MD of ‐56.1 (95% CI ‐71.9 to ‐40.3; 31 participants) in favour of ranibizumab intravitreal injection.
Comparison 2: anti‐vascular endothelial growth factor with vitrectomy compared with vitrectomy alone
Nine trials investigated the use of anti‐VEGF with vitrectomy. All of these studies used bevacizumab.
Three of these studies used a sham injection in addition to vitrectomy in the control group (Ahmadieh 2009; Di Lauro 2010; Sohn 2012), in the other six trials the control intervention was vitrectomy alone.
2.1 Loss of 3 or more lines of ETDRS visual acuity
Three studies reported loss of visual acuity measured as a dichotomous outcome. One of the studies used the cut‐point loss of 3 or more lines (Sohn 2012); but the other two studies reported a "deterioration", which was not defined (El‐Batarny 2008; Zaman 2013). All studies used intravitreal bevacizumab as an adjunct to vitrectomy (injected three to seven days before) and compared it with vitrectomy alone or vitrectomy plus sham injection.
People receiving bevacizumab before vitrectomy were less likely to lose vision, but the CIs were wide and compatible with no effect or increased risk of losing vision (RR 0.49, 95% CI 0.08 to 3.14; 94 participants; I2 = 0%) (Analysis 2.1).
2.1. Analysis.
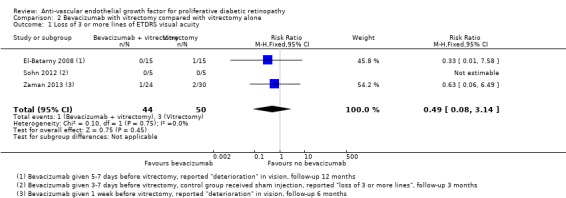
Comparison 2 Bevacizumab with vitrectomy compared with vitrectomy alone, Outcome 1 Loss of 3 or more lines of ETDRS visual acuity.
2.2 Gain of 3 or more lines of ETDRS visual acuity
Three studies reported gain of visual acuity measured as a dichotomous outcome. One of the studies used the cut‐point gain of 3 or more lines (Sohn 2012); but the other two studies reported "improvement", which was not defined (El‐Batarny 2008; Zaman 2013). All studies used intravitreal bevacizumab as an adjunct to vitrectomy (injected three to seven days before) and compared it with vitrectomy alone or vitrectomy plus sham injection.
People who received bevacizumab before vitrectomy were more likely to gain visual acuity compared with people that received vitrectomy alone (RR 1.62, 95% CI 1.20 to 2.17; 94 participants; Analysis 2.2). There was inconsistency in the results of the individual trials (I2 = 73%) with the RR varying from 1.08 to 3.0, but as all effects were in the same direction we presented a pooled estimate.
2.2. Analysis.
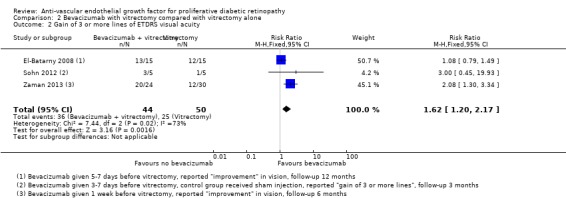
Comparison 2 Bevacizumab with vitrectomy compared with vitrectomy alone, Outcome 2 Gain of 3 or more lines of ETDRS visual acuity.
2.3 Mean visual acuity
Six trials reported mean visual acuity (Ahmadieh 2009; Ahn 2011; Di Lauro 2010; El‐Batarny 2008; Modarres 2009; Sohn 2012).
On average, people receiving bevacizumab before or during vitrectomy had better vision at follow‐up (between 2 and 3 lines better), but the CIs were wide and compatible with no effect of treatment (MD ‐0.24 logMAR, 95% CI ‐0.50 to 0.01; 335 participants; 6 studies; Analysis 2.3; Figure 5).
2.3. Analysis.
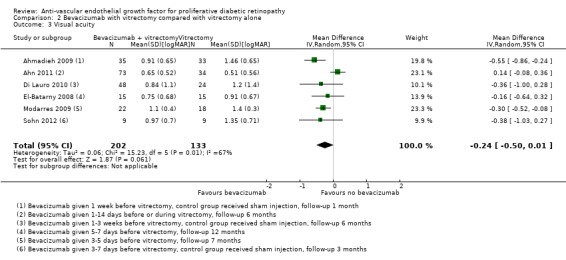
Comparison 2 Bevacizumab with vitrectomy compared with vitrectomy alone, Outcome 3 Visual acuity.
5.
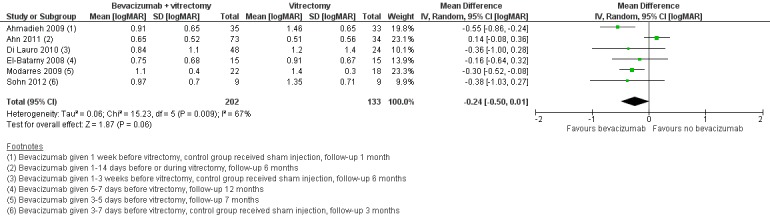
Forest plot of comparison: 2 Anti‐vascular endothelial growth factor (anti‐VEGF) plus surgery versus surgery alone or surgery plus sham or placebo, outcome: 2.3 Visual acuity [logMAR].
Overall there was substantial heterogeneity (I2 = 67%) but most of the studies found in favour of bevacizumab.
2.4 Regression of proliferative diabetic retinopathy
None of the studies reported regression of PDR.
2.5 Regression of proliferative diabetic retinopathy (mean area of fluorescein leakage)
None of the studies reported regression of PDR (mean area of fluorescein leakage).
2.6 Presence of microaneurysms
None of the studies reported presence of microaneurysms.
2.7 Presence of vitreous or pre‐retinal haemorrhage
Seven trials reported presence of vitreous or pre‐retinal haemorrhage (Ahmadieh 2009; Ahn 2011; Di Lauro 2010; El‐Batarny 2008; Modarres 2009; Rizzo 2008; Zaman 2013). All trials used intravitreal bevacizumab as an adjunct to vitrectomy (injected perioperatively or up to three weeks before, or both) and compared it with vitrectomy alone or vitrectomy plus sham injection.
People who received bevacizumab before or during vitrectomy were less likely to have vitreous or pre‐retinal haemorrhage at follow‐up compared with people who had vitrectomy alone (overall pooled RR 0.30, 95% CI 0.18 to 0.52; 393 participants; Analysis 2.4). Overall there was some heterogeneity (I2 = 47%).
2.4. Analysis.
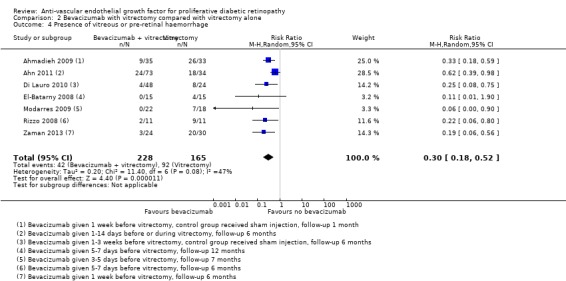
Comparison 2 Bevacizumab with vitrectomy compared with vitrectomy alone, Outcome 4 Presence of vitreous or pre‐retinal haemorrhage.
2.8 Need for laser photocoagulation
None of the studies reported need for laser photocoagulation.
2.9 Need for vitrectomy
Need for vitrectomy was not relevant, as participants had vitrectomy.
2.10 Diabetic macular oedema
None of the studies reported DMO.
2.11 Quality of life
None of the studies reported quality of life.
2.13 Adverse effects
See Analysis 2.5.
2.5. Analysis.
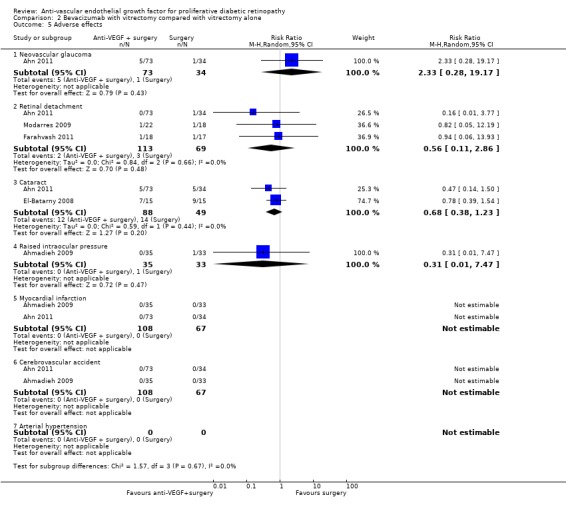
Comparison 2 Bevacizumab with vitrectomy compared with vitrectomy alone, Outcome 5 Adverse effects.
Neovascular glaucoma
One trial reported neovascular glaucoma (Ahn 2011). People who received anti‐VEGF were more likely to develop neovascular glaucoma compared with people who did not receive anti‐VEGF, but the CIs were wide and compatible with no effect or reduced risk of neovascular glaucoma (RR 2.33, 95% CI 0.28 to 19.17; 107 participants).
Retinal detachment
Three trials reported retinal detachment (Ahn 2011; Farahvash 2011; Modarres 2009). People who received anti‐VEGF were less likely to develop retinal detachment compared with people who did not receive anti‐VEGF, but the CIs were wide and compatible with no effect or reduced risk of retinal detachment (RR 0.56, 95% CI 0.11 to 2.86; 182 participants; I2 = 0%).
Cataract
Two trials reported cataract (Ahn 2011; El‐Batarny 2008). People who received anti‐VEGF were less likely to develop cataract compared with people who did not receive anti‐VEGF, but the CIs were wide and compatible with no effect or increased risk of cataract (RR 0.68, 95% CI 0.38 to 1.23; 137 participants; I2 = 0%).
Raised intraocular pressure
One trial reported IOP (Ahmadieh 2009). People who received anti‐VEGF were less likely to develop increased IOP compared with people who did not receive anti‐VEGF, but the CIs were wide and compatible with no effect or increased risk of increased IOP (RR 0.31, 95% CI 0.01 to 7.47; 68 participants).
Myocardial infarction
Two trials reported myocardial infarction (MI) (Ahmadieh 2009; Ahn 2011). There were no events in these trials (175 participants).
Cerebrovascular accident
Two trials reported CVA (Ahmadieh 2009; Ahn 2011). There were no events (175 participants).
Endophthalmitis
None of the studies reported endophthalmitis.
Arterial hypertension
None of the studies reported arterial hypertension.
Pain
None of the studies reported pain.
Comparison 3: anti‐vascular endothelial growth factor with cataract surgery compared with cataract surgery alone
Only one trial considered the use of anti‐VEGF (bevacizumab) for PDR at the time of cataract surgery in 88 eyes with DR (Cheema 2009).
At six months after surgery, there was little difference in visual acuity. The mean logMAR acuity in the bevacizumab group was 0.57 (SD 0.47) compared with a mean visual acuity in the non‐bevacizumab group of 0.56 (SD 0.48) (MD 0.01, 95% CI ‐0.22 to 0.24). Twenty of 35 people in the bevacizumab group required further laser treatment compared with 16/33 people of the non‐bevacizumab group (RR 1.18, 95% CI 0.75 to 1.86).
None of the other outcomes was reported.
Sensitivity analysis: random‐effects models versus fixed‐effect models
Choice of model did not affect the conclusions with the exception of analysis 2.3 (mean visual acuity in trials of bevacizumab with vitrectomy). The 95% CIs of the pooled effect estimate from the fixed‐effect model did not include zero (null value).
| Analysis | Measure of effect in random‐effects models (95% CI) | Measure of effect in fixed‐effect models |
| Analysis 1.1 | MD ‐0.07 logMAR (‐0.12 to ‐0.02) | MD ‐0.07 logMAR (‐0.12 to ‐0.02) |
| Analysis 2.3 | MD ‐0.24 logMAR (‐0.50 to 0.01) | MD ‐0.19 logMAR (‐0.32 to ‐0.06) |
| Analysis 2.4 | RR 0.30 (0.18 to 0.52) | RR 0.32 (0.24 to 0.45) |
CI: confidence intervals; MD: mean difference; RR: risk ratio.
Sensitivity analysis: low risk of bias versus high risk of bias
For Analysis 1.1 and Analysis 2.3 (mean visual acuity) there was little difference between the estimates according to risk of bias in studies. For Analysis 1.3, it was difficult to interpret, as there was only one low risk of bias trial and there may be other differences between this study and the other studies. For Analysis 2.4, there was a difference between the low risk of bias and high risk of bias trials but it was not in the anticipated direction (i.e. the low risk of bias trials appeared to demonstrate a larger effect). However, with only two RCTs in the high risk of bias group, this result must be interpreted cautiously.
| Analysis | Measure of effect in studies at low or unclear risk of bias in all domains (95% CI) | Measure of effect in studies at high risk of bias in ≥ 1 domains (95% CI) |
| Analysis 1.1 | MD ‐0.10 logMAR (‐0.24 to 0.05); 2 RCTs | MD ‐0.06 logMAR (‐0.12 to ‐0.01); 3 RCTs |
| Analysis 1.3 | RR 0.38 (0.18 to 0.81); 1 RCT | RR 0.14 (0.02 to 1.08); 2 RCTs |
| Analysis 2.3 | MD ‐0.29 logMAR (‐0.47 to ‐0.11); 4 RCTs | MD ‐0.20 logMAR (‐0.87 to 0.48); 2 RCTs |
| Analysis 2.4 | RR 0.20 (0.10 to 0.37); 5 RCTs | RR 0.46 (0.25 to 0.87); 2 RCTs |
CI: confidence intervals; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio.
Discussion
Summary of main results
The aim of this review was to evaluate the effectiveness and safety of anti‐VEGF in PDR. We included 18 RCTs with 1005 participants that needed laser or surgical treatment for PDR or the complications of PDR.
People receiving anti‐VEGF in association with laser or surgical (vitrectomy) treatment for PDR were less likely to lose vision and more likely to gain vision and on average had better visual acuity at follow‐up. They were less likely to have progression of DR and less likely to experience vitreous or pre‐retinal haemorrhage. The size of the effects were of the same order of magnitude for use of anti‐VEGF associated with both laser and surgical treatment. There was only one relatively small and inconclusive trial of use of anti‐VEGF at the time of cataract surgery in people with DR.
Overall completeness and applicability of evidence
Participants included in the review presented PDR that needed PRP (eight from 18 RCTs) or complications such as vitreous haemorrhage (nine from 18 RCTs) or cataracts that needed surgery (one from 18 RCTs). The median follow‐up was six months.
Few studies have been included that assessed our primary outcome (gain or loss of 3 or more lines of ETDRS). The effects of regression of vascular proliferation were poorly reported, and quality of life was not mentioned. Furthermore, the monitoring of participants was less than one year in most studies. However, there was a sufficient number of studies that calculated visual acuity in logMAR (13 RCTs and 811 eyes) and presented data about vitreous or pre‐retinal haemorrhage (10 RCTs and 735 eyes).
The number of RCTs was variable between anti‐VEGFs, and bevacizumab (15 RCTs) was the most evaluated, followed by ranibizumab (two RCTs) and pegaptanib (one RCT). Although the level of assessment of these drugs was not the same, in the overall analysis there was no significant differences between subgroups in visual acuity and vitreous or pre‐retinal haemorrhage.
Our pre‐specified outcomes were for 12 months' follow‐up. Only two of the 18 included studies followed up to 12 months. We did not find any evidence that the size of the effect was related to length of follow‐up (data not shown) but ideally, longer follow‐up would have been available.
We found five ongoing RCTs that, in the future, may resolve doubts about the efficacy and safety of these drugs for PDR (Characteristics of ongoing studies).
Quality of the evidence
The overall quality of evidence was low or very low in this review. For the main outcome of best‐corrected visual acuity at 12 months, we downgraded the quality of the evidence to 'very low' because it was an indirect assessment. In fact, no study reported loss/gain of 3 or more lines at 12 months. Two studies reported at three months, one of these studies reported loss/gain of 2 or more lines and one study reported loss/gain of 3 or more lines; two studies reported "deterioration", which was not defined, one at six months and one at 12 months. Imprecise estimates of visual benefit were also a reason for downgrading evidence on the primary outcome expressions.
For other outcomes, we downgraded the quality of the evidence because seven RCTs had high risk of bias. The high risk of bias was due to not blinding the interventions (Ahn 2011; Ernst 2012; González 2009), attrition bias (Ahmadieh 2009; Ernst 2012; Ramos Filho 2011), and selective reporting (Ahmadieh 2009; Cho 2010; Preti 2014). Furthermore, only one trial specified the calculation of the sample size (DRCR.Net 2013), and there was imbalance between groups at baseline in one trial (Sohn 2012), and participants of the control group were worse than the participants of the experimental group at baseline.
Finally, for some outcomes, the results of the individual studies were heterogeneous and, although we provided a pooled estimate, we downgraded for inconsistency.
Potential biases in the review process
This review has methodological strengths, as it has been successful in obtaining information from trial investigators. Although not all have responded, most investigators have done so. We have also made an exhaustive search of clinical trials (including those in progress), and have assessed the risk of bias and extracted data in a duplicate way.
However, this review is limited by the quality of RCTs, which included a low number of participants and presented unclear or high risk of bias. Furthermore, three studies were not included in efficacy analysis because the fellow eye was used as a control group (Ernst 2012; Mirshahi 2008; Preti 2014).
We made some modifications to the protocol (Differences between protocol and review), but did not consider that these changes will have introduced bias.
Agreements and disagreements with other studies or reviews
As far as we know, there are no systematic reviews that have assessed overall anti‐VEGFs for PDR. We found two systematic reviews that assessed anti‐VEGF as adjuvant of vitrectomy for PDR (Zhang 2013; Smith 2011). Zhang 2013 included eight RCTs that assessed efficacy and safety of bevacizumab in the short‐term (less than one month). The pooled results showed significant benefits of bevacizumab in overall surgical time, less intraoperative bleeding and less recurrent haemorrhage within the first month. The Cochrane systematic review, Smith 2011, included four RCTs, but the results of studies were not pooled due to methodological issues. However, the authors concluded that bevacizumab may reduce the incidence of early postoperative vitreous cavity haemorrhage.
Our review has included not only studies about complications of DR that required surgery, but also those trying to treat vascular proliferation. For these reasons, our systematic review has presented a larger number of included studies and participants. The results point in the same direction as Zhang 2013. However, the quality of the evidence was low or very low and these results must be treated with caution.
Authors' conclusions
Implications for practice.
There was very low or low quality evidence from randomised controlled trials for the efficacy and safety of anti‐vascular endothelial growth factor (anti‐VEGF) drugs when used to treat proliferative diabetic retinopathy (PDR) over and above current standard treatments. However, the results suggested that anti‐VEGFs can reduce the risk of intraocular bleeding in people with PDR.
Implications for research.
There is a clear need for further adequate clinical trials to assess efficacy of anti‐VEGFs for PDR.
The unit of randomisation could be the eye, but for analysis, it is preferable that only one eye is included per participant. The calculations of sample size should be based on relevant clinical differences. The concealment of interventions and a long‐term follow‐up (at least 12 months) is necessary to improve the quality of clinical trials. Future clinical trials should report data by subgroup of PDR severity or haemorrhage at baseline, as there may be subgroups of people who benefit most.
We identified five ongoing trials registered with various trials registries. Two of these studies are evaluating anti‐VEGF (ranibizumab in one study, aflibercept in one study) combined with PRP versus PRP alone; two studies are evaluating bevacizumab as an addition to vitrectomy and one study is evaluating aflibercept in cataract surgery.
What's new
| Date | Event | Description |
|---|---|---|
| 25 November 2014 | Amended | Minor edit made to text |
Acknowledgements
The Cochrane Eyes and Vision Group (CEVG) created the strategies and ran the searches on the electronic databases. We thank Satyamurthy Anuradha for her comments on the protocol, Nigel Davies for his comments on the review, Catey Bunce and Gianni Virgili for their comments on the protocol and review. We thank Anupa Shah, Managing Editor for CEVG her assistance throughout the editorial process.
We used Covidence (www.covidence.org) to screen the studies. We would like to thank Claire Irving and Clive Adams of the Cochrane Schizophrenia Group for their help in using RevmanHAL (szg.cochrane.org/revman‐hal) to prepare the "effects of interventions" section.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Diabetic Retinopathy] explode all trees #2 diabet* near/3 retinopath* #3 proliferat* near/3 retinopath* #4 (retinopath* or retinal or intraocular or intravitreal or glaucoma) near/2 (neovascular*) #5 new blood vessel #6 #1 or #2 or #3 or #4 or #5 #7 MeSH descriptor: [Angiogenesis Inhibitors] explode all trees #8 MeSH descriptor: [Angiogenesis Inducing Agents] explode all trees #9 MeSH descriptor: [Endothelial Growth Factors] explode all trees #10 anti near/2 VEGF* #11 endothelial near/2 growth near/2 factor* #12 anti near/1 angiogen* #13 macugen* or pegaptanib* or lucentis* or rhufab* or ranibizumab* or bevacizumab* or avastin or aflibercept* #14 VEGF TRAP* #15 #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 #16 #6 and #15
Appendix 2. MEDLINE (OvidSP) search strategy
1. randomized controlled trial.pt. 2. (randomized or randomised).ab,ti. 3. placebo.ab,ti. 4. dt.fs. 5. randomly.ab,ti. 6. trial.ab,ti. 7. groups.ab,ti. 8. or/1‐7 9. exp animals/ 10. exp humans/ 11. 9 not (9 and 10) 12. 8 not 11 13. exp diabetic retinopathy/ 14. (diabet$ adj3 retinopath$).tw. 15. (proliferat$ adj3 retinopath$).tw. 16. ((retinopath$ or retinal or intraocular or intravitreal or glaucoma) adj2 neovascular$).tw. 17. new blood vessel$.tw. 18. or/13‐17 19. exp angiogenesis inhibitors/ 20. exp angiogenesis inducing agents/ 21. exp endothelial growth factors/ 22. (anti adj2 VEGF$).tw. 23. (endothelial adj2 growth adj2 factor$).tw. 24. (anti adj1 angiogen$).tw. 25. (macugen$ or pegaptanib$ or lucentis$ or rhufab$ or ranibizumab$ or bevacizumab$ or avastin or aflibercept$).tw. 26. VEGF TRAP$.tw. 27. or/19‐25 28. 18 and 27 29. 12 and 28
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville et al (Glanville 2006).
Appendix 3. EMBASE (OvidSP) search strategy
1. exp randomized controlled trial/ 2. exp randomization/ 3. exp double blind procedure/ 4. exp single blind procedure/ 5. random$.tw. 6. or/1‐5 7. (animal or animal experiment).sh. 8. human.sh. 9. 7 and 8 10. 7 not 9 11. 6 not 10 12. exp clinical trial/ 13. (clin$ adj3 trial$).tw. 14. ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. 15. exp placebo/ 16. placebo$.tw. 17. random$.tw. 18. exp experimental design/ 19. exp crossover procedure/ 20. exp control group/ 21. exp latin square design/ 22. or/12‐21 23. 22 not 10 24. 23 not 11 25. exp comparative study/ 26. exp evaluation/ 27. exp prospective study/ 28. (control$ or prospectiv$ or volunteer$).tw. 29. or/25‐28 30. 29 not 10 31. 30 not (11 or 23) 32. 11 or 24 or 31 33. exp diabetic retinopathy/ 34. (diabet$ adj3 retinopath$).tw. 35. (proliferat$ adj3 retinopath$).tw. 36. ((retinopath$ or retinal or intraocular or intravitreal or glaucoma) adj2 neovascular$).tw. 37. new blood vessel$.tw. 38. or/33‐37 39. angiogenesis/ 40. angiogenesis inhibitors/ 41. angiogenesis factor/ 42. monoclonal antibody/ 43. exp endothelial cell growth factor/ 44. vasculotropin/ 45. (anti adj2 VEGF$).tw. 46. (endothelial adj2 growth adj2 factor$).tw. 47. (anti adj1 angiogen$).tw. 48. (macugen$ or pegaptanib$ or lucentis$ or rhufab$ or ranibizumab$ or bevacizumab$ or avastin or aflibercept$).tw. 49. VEGF TRAP$.tw. 50. or/39‐49 51. 38 and 50 52. 32 and 51
Appendix 4. metaRegister of Controlled Trials search strategy
(macugen or pegaptanib or lucentis or rhufab or ranibizumab or bevacizumab or avastin or aflibercept) and (diabetic retinopathy)
Appendix 5. ClinicalTrials.gov search strategy
(Macugen OR Pegaptanib OR Lucentis OR Rhufab OR Ranibizumab OR Bevacizumab OR Avastin OR Aflibercept) AND (Diabetic Retinopathy)
Appendix 6. ICTRP search strategy
Diabetic Retinopathy = Condition AND Macugen OR Pegaptanib OR Lucentis OR Rhufab OR Ranibizumab OR Bevacizumab OR Avastin OR Aflibercept = Intervention
Data and analyses
Comparison 1. Anti‐vascular endothelial growth factor (anti‐VEGF) with or without panretinal photocoagulation (PRP) versus PRP alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Visual acuity | 5 | 373 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.12, ‐0.02] |
| 1.1 Pegaptanib | 1 | 16 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.22, 0.10] |
| 1.2 Bevacizumab | 2 | 80 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.11, 0.09] |
| 1.3 Ranibizumab | 2 | 277 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.16, ‐0.03] |
| 2 Regression of proliferative diabetic retinopathy | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Presence of vitreous or pre‐retinal haemorrhage | 3 | 342 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.16, 0.65] |
| 3.1 Bevacizumab | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 1.92] |
| 3.2 Pegaptanib | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 3.70] |
| 3.3 Ranibizumab versus control | 1 | 261 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.18, 0.81] |
| 4 Adverse effects | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Neovascular glaucoma | 1 | 261 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.07, 17.21] |
| 4.2 Retinal detachment | 1 | 261 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.44, 2.25] |
| 4.3 Cataract | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.63] |
| 4.4 Raised intraocular pressure | 2 | 322 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.42, 1.36] |
| 4.5 Cerebrovascular accident | 2 | 322 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.26 [0.13, 79.34] |
| 4.6 Endophalmitis | 1 | 261 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.01, 8.82] |
| 4.7 Arterial hypertension | 1 | 261 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.12, 1.76] |
Comparison 2. Bevacizumab with vitrectomy compared with vitrectomy alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Loss of 3 or more lines of ETDRS visual acuity | 3 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.08, 3.14] |
| 2 Gain of 3 or more lines of ETDRS visual acuity | 3 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.62 [1.20, 2.17] |
| 3 Visual acuity | 6 | 335 | Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.50, 0.01] |
| 4 Presence of vitreous or pre‐retinal haemorrhage | 7 | 393 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.18, 0.52] |
| 5 Adverse effects | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Neovascular glaucoma | 1 | 107 | Risk Ratio (M‐H, Random, 95% CI) | 2.33 [0.28, 19.17] |
| 5.2 Retinal detachment | 3 | 182 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.11, 2.86] |
| 5.3 Cataract | 2 | 137 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.38, 1.23] |
| 5.4 Raised intraocular pressure | 1 | 68 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.01, 7.47] |
| 5.5 Myocardial infarction | 2 | 175 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.6 Cerebrovascular accident | 2 | 175 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.7 Arterial hypertension | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ahmadieh 2009.
| Methods | Study design: prospective, randomised, double‐blind clinical trial of intravitreal bevacizumab for prevention of early post‐vitrectomy haemorrhage in people with diabetes Unit of randomisation: participant Unit of analyses: the eye, but 1 eye only of each person was included in the study Follow‐up: 1 week and 1 month after surgery |
|
| Participants | Country: Iran Setting: Ophthalmic Research Center, Shahid Beheshti University of Medical Sciences, Tehran Number of participants: 68 (68 eyes) Exclusions post‐randomisation: 0 Losses to follow‐up: 34 Age (mean (SD)): 53.69 (11.7) years in bevacizumab plus vitrectomy group, 56.70 (10.4) years in sham plus vitrectomy group Gender: 34 men and 34 women Inclusion criteria: indications for pars plana vitrectomia for complications of PDR existed such as non‐clearing VH, TRD involving or threatening the macula and active progressive PDR Exclusion criteria: BCVA of 20/40 or better, pregnancy, history of intravitreal bevacizumab injection, intraoperative use of long‐acting gas or silicone oil, and simultaneous intraocular surgery such as cataract extraction. Monocular participants |
|
| Interventions | Treatment: intravitreal injection of bevacizumab 1.25 mg/0.05 mL 1 week before vitrectomy Control: sham injection and vitrectomy Duration: only 1 dose |
|
| Outcomes | Primary: incidence of early (4 weeks) postoperative VH at 1 week and 1 month after vitrectomy Secondary: mean change in BCVA and any bevacizumab‐related adverse event |
|
| Notes | Funding: not reported Trial registration: NCT00524875 Date conducted: not reported Conflict of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed by random block permutation according to a computer‐generated randomization list" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Details of the series were unknown to the investigators" Comment: there was not specified the allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Subjects were masked to the treatment method" Comment: surgeons were not blinded to the interventions assessed |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Visual acuity was measured by an optometrist who was masked to the groups. All preoperative and postoperative examinations were performed by one of the authors (NS), who also was masked to the study group identification" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: there were a 50% of losses during the study |
| Selective reporting (reporting bias) | Low risk | Comment: the results of the variables were described in the methods section |
Ahn 2011.
| Methods | Study design: prospective, randomised, clinical trial of intravitreal bevacizumab for preventing postvitrectomy haemorrhage in PDR Unit of randomisation: participant Unit of analyses: the eye, but 1 eye of each participant was included in the study. However, if the study eye completed 6 months of follow‐up, the contralateral eye requiring vitrectomy also was allowed to enrol in this study. A total of 107 eyes of 91 participants, of which there were 16 bilateral participants, were included for analysis Follow‐up: 1 day, 1 week, 1, 3 and 6 months after surgery |
|
| Participants | Country: Korea Setting: Department of Ophthalmology, Seoul National University College of Medicine, Seoul, Korea Number of participants: 91 (107 eyes) Exclusions post‐randomisation: 2 Losses to follow‐up: 17 Age (mean (SD)): 51.0 (9.5) years in preoperative bevacizumab group, 55.6 (SD 10.3) years in intraoperative bevacizumab group, 55.0 (11.4) years in control group Gender: 60 men and 47 women Inclusion criteria: people that needed pars plana vitrectomy due to PDR‐related complications such as non‐clearing VH, macula‐involving or macula‐threatening TRD or fibrovascular proliferation with vitreoretinal adhesions Exclusion criteria: follow‐up period of < 6 months, intraoperative use of long‐acting gas or silicone oil, repeat vitrectomy after first vitrectomy for retinal diseases other than VH, previous history of vitrectomy, uncontrolled hypertension, medical history of blood coagulopathy, interval between bevacizumab injection and pars plana vitrectomy > 2 weeks, or < 3 months of bevacizumab treatment |
|
| Interventions | Treatment group 1 ‐ preoperative bevacizumab: intravitreal bevacizumab 1.25 mg/0.05 mL injection 1‐14 days before postoperative VH Treatment group 2 ‐ intraoperative bevacizumab: intravitreal bevacizumab 1.25 mg/0.05 mL injection at the end of postoperative VH Control: no injection and vitrectomy Duration: only 1 dose |
|
| Outcomes | Primary: incidence of early (4 weeks) and late (4 weeks) recurrent VH Secondary: initial time of vitreous clearing, BCVA at 6 months after surgery and adverse events |
|
| Notes | Funding: not reported Trial registration: NTC00745498 Date conducted: not reported Conflict of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomization was carried out using permuted block randomization with equal allocation ratio" |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "the lack of double‐masking, leaving room for possible bias" Comment: the authors say the study was not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "the lack of double‐masking, leaving room for possible bias" Comment: the authors say the study was not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were 0 losses |
| Selective reporting (reporting bias) | Low risk | Comment: the results of the variables were described in the methods section |
Cheema 2009.
| Methods | Study design: prospective, randomised, clinical trial of intravitreal bevacizumab in cataract surgery for preventing progression of diabetic retinopathy Unit of randomisation: participant Unit of analyses: the eye, but 1 eye of each participant was included in the study Follow‐up: 1 day; 1, 2 and 4 weeks and then at monthly intervals for 6 months |
|
| Participants | Country: Saudi Arabia Setting: hospital, Dhahran, Kingdom of Saudi Arabia Number of participants: 68 (68 eyes) Exclusions post‐randomisation: 0 Losses to follow‐up: 0 Age (mean): 66.14 years in bevacizumab group, 64.5 years in control group Gender: 43 men and 25 women Inclusion criteria: cataract in people with diabetes with poor fundus view with 1. the presence of clinically significant macular oedema, 2. mild, moderate, severe or very severe non‐PDR or PDR or 3. a combination of 1 and 2; people with previous focal or grid laser photocoagulation for macular oedema Exclusion criteria: eyes with glaucoma, uveitis and age‐related macular degeneration or a history of trauma or ocular surgery; people with previous panretinal laser photocoagulation |
|
| Interventions | Treatment: phacoemulsification with intraocular lens implantation and intravitreal bevacizumab 1.25 mg at the end of surgery Control: phacoemulsification with intraocular lens implantation alone Duration: only 1 dose |
|
| Outcomes | Primary: progression of postoperative diabetic retinopathy and diabetic maculopathy during a 6‐month follow‐up Secondary: change in BCVA, changes in central macular thickness and macular thickness determined by optical coherence tomography, postoperative laser therapy, progression to neovascular glaucoma |
|
| Notes | Funding: not reported Trial registration: not reported Date conducted: the participants were recruited between February and December 2007 Conflict of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were randomized to a standardized procedure of phacoemulsification with IOL [intraocular lens] implantation alone (control group) or to receive 1.25 mg intravitreal bevacizumab (Avastin) at the end of surgery (intervention group)" Comment: not described how it was generated the random |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Progression of DR [diabetic retinopathy] was based on assessment in a masked fashion by 2 retina specialists (R.A.C., Y.M.A.)" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were 0 losses |
| Selective reporting (reporting bias) | Low risk | Comment: the results of the variables were described in the methods section |
Cho 2010.
| Methods | Study design: prospective, randomised, clinical trial of intravitreal bevacizumab and intravitreal triamcinolone as adjunctive treatments to PRP in diabetic retinopathy Unit of randomisation: eye Unit of analyses: eye Follow‐up: 1 day, 1 week, 1 and 3 months |
|
| Participants | Country: Korea Setting: Department of Ophthalmology, Konkuk University Medical Center, Konkuk University School of Medicine, Seoul, Republic of Korea Number of participants: 76 (91 eyes) Exclusions post‐randomisation: 0 Losses to follow‐up: 0 Age (mean (SD)): 50.96 (46.0) years in bevacizumab group, 51.06 (26.0) years in triamcinolone group Gender: 55 men and 21 women Inclusion criteria: aged ≥ 18 years, very severe non‐PDR to high‐risk PDR, Snellen BCVA of ≥ 3 Exclusion criteria: blood pressure > 180 mmHg (systolic) and > 110 mmHg (diastolic), glycated haemoglobin levels > 9.5%, chronic renal failure, major surgery within 1 month, or previous systemic steroids or anti‐VEGF treatment. Ocular conditions other than diabetic retinopathy (e.g. retinal vein occlusion, uveitis or other ocular inflammatory disease, neovascular glaucoma, etc.). History of treatment for diabetic macular oedema, PRP or focal/grid laser photocoagulation, or previous intraocular surgery, or uncontrolled glaucoma in the last 3 months |
|
| Interventions | Treatment group 1: intravitreal bevacizumab 1.25 mg/0.05 mL, 1 week before PRP Treatment group 2: intravitreal triamcinolone 4 mg/0.1 mL, 1 day after PRP Control: PRP Duration: only 1 dose |
|
| Outcomes | Primary: changes in BCVA and central macular thickness at 1 and 3 months Secondary: proportion of visual gain or loss, decreased or increased central macular thickness, adverse events |
|
| Notes | Funding: no financial interest of the authors Trial registration: not reported Date conducted: March 2007 to August 2008 Conflict of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses |
| Selective reporting (reporting bias) | High risk | Comment: incomplete results of the principal variable were described in the methods section |
Di Lauro 2010.
| Methods | Study design: prospective, randomised, clinical trial of intravitreal bevacizumab for surgical treatment of severe PDR Unit of randomisation: participant Unit of analyses: eye/participant Follow‐up: 1, 6, 12 and 24 weeks after the surgery |
|
| Participants | Country: Italy Setting: Department of Ophthalmology, Hospital C.T.O. of Naples, Naples, Italy Number of participants: 68 (72 eyes) Exclusions post‐randomisation: 3 (regression of the haemorrhage in a bevacizumab group) Losses to follow‐up: 0 Age: not reported Gender: not reported Inclusion criteria: people affected by VH and TRD consequent to active PDR Exclusion criteria: people with neovascular glaucoma or cataract (or both) and cases of combined traction and rhegmatogenous retinal diabetes (diagnosed either before or during the surgery) |
|
| Interventions | Treatment group 1: intravitreal bevacizumab 1.25 mg/0.05 mL, 7 days before vitrectomy Treatment group 2: intravitreal bevacizumab 1.25 mg/0.05 mL, 20 days before vitrectomy Control: sham injection 20 days before vitrectomy Duration: only 1 dose |
|
| Outcomes | Primary: clearing of VH, incidence of adverse effects and the need of other procedures during the surgery Secondary: change in BCVA and duration of surgery |
|
| Notes | Funding: not reported Trial registration: NCT01025934 Date conducted: October 2005 to May 2007 Conflict of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Patients in group A [control] were given a subconjunctival injection of 0.05 ml of BSS (Blood saline serum) 3 weeks before the vitrectomy" Comment: control received a sham intervention. The participant was blind to the treatment received. However, it is possible that the personnel that administered the sham were aware of treatment because the site of application was subconjunctival and not intravitreal as with bevacizumab |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Patients in group A [control] were given a subconjunctival injection of 0.05 ml of BSS (Blood saline serum) 3 weeks before the vitrectomy" Comment: control received a sham intervention. The outcome assessor was blinded to the treatment administered |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There were 3 losses post‐randomisation, but losses during follow‐up were not noted |
| Selective reporting (reporting bias) | Low risk | Comment: the results of the variables were reported in the methods section |
DRCR.Net 2013.
| Methods | Study design: phase 3, double‐blind, randomised, multicentre clinical trial of intravitreal ranibizumab for VH from PDR Unit of randomisation: eye (1 eye per participant) Unit of analyses: eye Follow‐up: at 4, 8, 12 and 16 weeks |
|
| Participants | Country: USA Setting: community‐based and academic‐based ophthalmology practices specialising in retinal diseases (61 centres) Number of participants: 261 (261 eyes) Exclusions post‐randomisation: 10 (3 in ranibizumab group and 7 in the control group) Losses to follow‐up: 4 (2 in each group) Age (mean (SD)): 58 (12) years Gender: 52% women Inclusion criteria: ≥ 18 years of age with type 1 or type 2 diabetes. Eyes with VH associated to PDR, causing vision impairment and precluding completion of PRP Exclusion criteria: eyes requiring immediate vitrectomy for reasons such as rhegmatogenous or traction retinal detachment; vision of no light perception, neovascular glaucoma, active iris neovascularisation judged or angle neovascularisation; history of intravitreal anti‐VEGF treatment for VH |
|
| Interventions | Treatment: intravitreal ranibizumab 0.5 mg at baseline and 4 and 8 weeks Control: intravitreal saline at baseline and 4 and 8 weeks Both groups received PRP as soon as possible after the first injection Duration: 3 doses |
|
| Outcomes | Primary: cumulative probability of vitrectomy performed within 16 weeks Secondary: the proportion of eyes with "complete" PRP by 16 weeks in the absence of vitrectomy; improvement in visual acuity from baseline to the 12‐week follow‐up visit; extent of VH measured by optical coherence tomography signal strength; systemic and ocular adverse events |
|
| Notes | Funding: co‐operative agreements EY14231 and EY18817 from the National Eye Institute and the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Department of Health and Human Services (USA). Genentech provided the ranibizumab for the study and provided funds to DRCR.net Trial registration: NCT00996437 Date conducted: June 2010 to March 2012 Conflict of interest: Genentech provided the ranibizumab for the study and provided funds to DRCR.net to defray the study's clinical site costs. DRCR.net had complete control over the design of the protocol, conduct, and reporting of the research and retained ownership of the data |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: it was not specified how the random sequence was generated. Only specified that used a permuted block design stratified by site |
| Allocation concealment (selection bias) | Low risk | Quote: "randomly assigned on the DRCR.net website" Comment: the randomisation was centralised and the investigator were blinded to the random sequence |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "eyes received an injection of saline or 0.5‐mg ranibizumab at randomization, 4 weeks, and 8 weeks using a masked vial provided by the Coordinating Center that was identified by number only" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "eyes received an injection of saline or 0.5‐mg ranibizumab at randomization, 4 weeks, and 8 weeks using a masked vial provided by the Coordinating Center that was identified by number only" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: the analyses were by intention to treat, and there were 4 losses of follow‐up (2 in each group) |
| Selective reporting (reporting bias) | Low risk | Comment: the results of the outcomes were specified in the methods section |
El‐Batarny 2008.
| Methods | Study design: prospective, randomised trial of intravitreal bevacizumab as an adjunctive treatment before diabetic vitrectomy Unit of randomisation: participant Unit of analyses: eye/participant Follow‐up: 1 day, 1 week, 2 weeks, 1 month after surgery and monthly up to the end of the follow‐up (mean 12 months; range 7‐18 months) |
|
| Participants | Country: Sultanate of Oman Setting: Magrabi Eye and Ear Hospital, Muscat, Sultanate of Oman Number of participants: 30 (30 eyes) Exclusions post‐randomisation: 0 Losses to follow‐up: 0 Age (mean (SD)): 44 (11) years in bevacizumab plus vitrectomy group, 46 (12) years in vitrectomy alone group Gender: not reported Inclusion criteria: people with indications for vitrectomia for complications of PDR existed such as TRD involving or treating the macula, not resolving VH, pre‐retinal subhyaloid bleeding Exclusion criteria: not reported |
|
| Interventions | Treatment: intravitreal injection of bevacizumab 1.25 mg/0.05 mL, 5‐7 days before vitrectomy Control: vitrectomy alone Duration: only 1 dose |
|
| Outcomes | Primary: feasibility of the surgery and postoperative complications Secondary: visual acuity at 6 months of follow‐up, any bevacizumab‐related adverse event |
|
| Notes | Funding: not reported Trial registration: not reported Date conducted: not reported Conflict of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were 0 losses |
| Selective reporting (reporting bias) | Low risk | Comment: the results of the variables were described in the methods section |
Ergur 2009.
| Methods | Study design: prospective, randomised clinical trial of intravitreal bevacizumab for PDR Unit of randomisation: participant Unit of analyses: eye Follow‐up: 1 day, 1 week, 1 and 6 months |
|
| Participants | Country: Turkey Setting: M.D., Ministry of Health Atatürk Research and Training Hospital 2st Eye Clinic Ankara, Turkey Number of participants: 16 (19 eyes) Exclusions post‐randomisation: 0 Losses to follow‐up: 0 Age (mean (SD)): 71.4 (4.6) years in bevacizumab plus PRP group, 68.3 (3.4) years in PRP group Gender: 9 men and 7 women Inclusion criteria: people with PDR Exclusion criteria: people with history of cataract surgery or thromboembolic ictus |
|
| Interventions | Treatment: intravitreal bevacizumab 1.25 mg/0.05 mL, 20 days before PRP, 3 sessions Control: PRP/week/3 weeks, 3 sessions |
|
| Outcomes | Primary: BCVA, intraocular pressure, biomicroscopic examination, fundus examination, colour fundus photography, fluorescein leakage areas | |
| Notes | Funding: not reported Trial registration: not reported Date conducted: not reported Conflict of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were 0 losses |
| Selective reporting (reporting bias) | Low risk | Comment: the results of the variables were described in the methods section |
Ernst 2012.
| Methods | Study design: prospective, randomised, clinical trial of intravitreal bevacizumab for treatment of naive PDR and severe non‐PDR Unit of randomisation: eye Unit of analyses: eye Follow‐up: 1, 2, 6 and 12 months |
|
| Participants | Country: Mexico Setting: Asociación para Evitar la Ceguera en México Number of participants: 15 (20 eyes) Exclusions post‐randomisation: 0 Losses to follow‐up: 5 Age (mean (SD)): 53.3 (9) years Gender: 4 men and 6 women Inclusion criteria: people with type 2 diabetes mellitus and symmetric untreated severe naive PDR or PDR without macular oedema or prior intraocular surgery Exclusion criteria: people with history of myocardial infarction or cerebrovascular accident, retinal detachment, VH, previous treatment for diabetic retinopathy, media opacities that precluded visualisation of the fundus, pregnancy and inability to understands the implications of the protocol |
|
| Interventions | Treatment: intravitreal bevacizumab 2.5 mg/0.1 mL every 2 months for 12 months (6 injections in total) Control: PRP, 2 sessions. A third session was administered if there was neovascularisation |
|
| Outcomes | Primary: BCVA, macular thickness, median deviation in visual fields at 1 year, and score on a participant satisfaction scale at 6 months and 1 year Secondary: complications associated to the treatments |
|
| Notes | Funding: not reported Trial registration: NCT00347698 Date conducted: March 2006 to August 2007 Conflict of interest: none reported This study was designed using both treatments in the same participant: intravitreal bevacizumab in 1 eye compared with PRP in the contralateral eye |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "the right eye was randomly assigned to treatment with PRP or intravitreal bevacizumab, and the left eye received the other treatment" Comment: not reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The initial number of participants was 30, but only 15 participants were included and there was 5 losses |
| Selective reporting (reporting bias) | High risk | Some results of variables specified in the published protocol were not reported: median deviation in visual fields at 1 year, and score on a participant satisfaction scale at 6 months and 1 year |
Farahvash 2011.
| Methods | Study design: randomised, clinical trial in people with diabetes with indication for vitrectomy Unit of randomisation: participant Unit of analyses: participant/eye Follow‐up: first day, first week, first month, and then every 3 months until the last visit. Median: 8 months (range 3‐15 months) |
|
| Participants | Country: Iran Setting: hospital Number of participants: 35 (35 eyes) Exclusions post‐randomisation: 0 Losses to follow‐up: 0 Age (mean (range)): 58 (37‐73) years Gender: 18 men and 17 women Inclusion criteria: people with indications for vitrectomy. The indications were "persistent vitreous hemorrhage >1 month in a patient with no history of PRP, nonclearing vitreous hemorrhage in a patient with history of complete PRP, vitreous hemorrhage with neovascularization of iris, vitreous hemorrhage with glaucoma, and vitreous hemorrhage with retinal detachment (based on the echography)" Exclusion criteria: "history of vitrectomy or any intraocular injection in the study eye or history of IVB [intravitreal bevacizumab injection] in either eye, previous myocardial infarction, cerebrovascular accident or thromboembolic event, uncontrolled hypertension, coagulation abnormalities, or current use of any anticoagulants but aspirin (aspirin was discontinued 1 week before injection) and those with unstable medical conditions" |
|
| Interventions | Treatment: intravitreal injection bevacizumab 1.25 mg 7 days prior to surgery Control: no treatment before surgery and vitrectomy Duration: only 1 dose |
|
| Outcomes | Primary: severity of intraoperative bleeding and break formation (based in surgeons observation) Secondary: visual acuity, complete attachment of the retina, complications |
|
| Notes | Funding: not reported Trial registration: not reported Date conducted: January 2008 to January 2009 Conflict of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "in each subgroup, the patients were randomly assigned to injection of bevacizumab preoperatively (injection group) or not (control group) Comment: not described the method of randomization |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "the surgeons were masked regarding patient groups and subgroup" Comment: not clear if the participants were blinded to the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "the surgeons were masked regarding patient groups and subgroup" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no losses for the main outcome |
| Selective reporting (reporting bias) | Low risk | Comment: the results of the variables were described in the methods section. SD of the BCVA after intervention were missing |
González 2009.
| Methods | Study design: randomised, prospective, open‐label direct comparison of pegaptanib alone with PRP alone in people with PDR Unit of randomisation: eyes (Quote: "for subjects in whom both eyes were eligible, one eye was selected randomly as the study eye. Fellow eyes of these subjects were treated according to standard clinical guidelines established") Unit of analyses: eye Follow‐up: 30 weeks |
|
| Participants | Country: USA Setting: Valley Retina Institute Number of participants: 20 (20 eyes) Exclusions post‐randomisation: 1 Losses to follow‐up: 3 Age (mean): 56.2 years in intravitreal pentaganib group, 59 years in PRP group Gender: 13 men and 7 women Inclusion criteria: active PDR, in 1 or both eyes, with at least 1 of the following high‐risk characteristics as defined by the Diabetic Retinopathy Study: 1. new vessels within 1 disc diameter of the optic nerve head that were larger than one‐third of the disc area; 2. VH or pre‐retinal haemorrhage associated with either less extensive new vessels at the optic disc, or with new vessels elsewhere half the disc area or larger; or both 1. and 2. Exclusion criteria: haemorrhage or media opacity obscuring visualisation of the macula and optic nerve; epiretinal membranes involving the macula; proliferative diabetic membranes along the major retinal arcades sufficiently extensive to cause either significant vitreomacular traction or significant impairment in BCVA; any TRD; severe ischaemia involving the foveal avascular zone; neovascular glaucoma; study eye treated with intravitreal steroid injections within 6 months prior to baseline or PRP treatment within 90 days of baseline (or both) |
|
| Interventions | Treatment: intravitreal pentaganib 0.3 mg every 6 weeks for 30 weeks Control: PRP laser every 6 weeks for 30 weeks |
|
| Outcomes | Primary: regression of PDR from baseline to week 36, defined as regression of neovascularisation of the optic disc , neovascularisation elsewhere, or both Secondary: BCVA assessed by ETDRS letter score, as well as changes in optical coherence tomography assessments of central macular thickness and macular volume |
|
| Notes | Funding: grant from Pfizer, New York and (OSI) Eyetech, New York Trial registration: not reported Date conducted: not reported Conflict of interest: first author was a paid consultant and speaker for (OSI) Eyetech Pharmaceuticals |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "eligible eyes were randomly assigned (1:1) to either pegaptanib alone or PRP alone based on a sequence generated by the random number function in Microsoft Excel (Microsoft Corporation, Seattle, Washington)" |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "prospective, randomised, controlled, open‐label, exploratory study" Comment: the participants and personnel were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "prospective, randomised, controlled, open‐label, exploratory study" Comment: the outcome assessor was not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were 4 losses (2 in each group) |
| Selective reporting (reporting bias) | Low risk | Comment: the results of the variables were described in the methods section |
Mirshahi 2008.
| Methods | Study design: prospective, randomised, double‐blind clinical trial of intravitreal bevacizumab in PDR Unit of randomisation: eye Unit of analyses: eye Follow‐up: 6 and 16 weeks |
|
| Participants | Country: Iran Setting: Eye Research Center, Farabi Eye Hospital, Medical Sciences/University of Tehran Number of participants: 40 (80 eyes) Exclusions post‐randomisation: 0 Losses to follow‐up: 0 Age (median (range)): 52 (39‐68) years Gender: 12 men and 28 women Inclusion criteria: people with high‐risk characteristics identified by Diabetic Retinopathy Study criteria: neovascularisation of the disc ≥ one‐quarter to one/third disc area, any amount of disc neovascularisation with VH or pre‐retinal haemorrhage, or neovascularisation elsewhere ≥ one‐half disc area with VH or pre‐retinal haemorrhage (with or without macular oedema) Exclusion criteria: people with uncontrolled hypertension, recent (in the past 6 months) myocardial infarction or cerebrovascular accident, uncontrolled glaucoma, a history of any type of retinal photocoagulation, a diagnosis of TRD |
|
| Interventions | Treatment: intravitreal injection bevacizumab 1.25 mg/0.05 mL at the first session of laser photocoagulation and 3 sessions of laser photocoagulation (1 week apart) Control: sham injection in the fellow eye at the first session of laser photocoagulation and 3 sessions of laser photocoagulation (1 week apart) Duration: only 1 dose |
|
| Outcomes | Primary: regression response was defined angiographically Secondary: recurrence of PDR and complications of treatment |
|
| Notes | Funding: not reported Trial registration: not reported Date conducted: December 2005 to September 2006 Conflict of interest: none reported This study was designed using both treatments in the same participant: intravitreal bevacizumab in 1 eye compared with PRP in the contralateral eye |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "fellow eyes of each case were randomly assigned to receive Avastin [bevacizumab] or sham" Comment: not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "fellow eye injection was mimicked with a needleless syringe" Comment: personnel were not blinded, but the participants were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "this assessment was carried out by two independent masked observers; in case of conflict it was resolved through discussion" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were 0 losses |
| Selective reporting (reporting bias) | Low risk | Comment: the results of the variables were described in the methods section |
Modarres 2009.
| Methods | Study design: prospective surgeon‐blinded randomised clinical trial in people undergoing pars plana vitrectomy for complications of PDR Unit of randomisation: eye Unit of analyses: eye Follow‐up: mean (SD) 7 (3.6) months |
|
| Participants | Country: Iran Setting: Department of Ophthalmology Number of participants: 40 (40 eyes) Exclusions post‐randomisation: 0 Losses to follow‐up: 0 Age (mean (SD)): 55.8 (11.3) years in bevacizumab group, 53.2 (SD 11.7) years in control group Gender: not reported Inclusion criteria: people with diabetes who were candidates for vitrectomy with complexity scores of 4‐8 Exclusion criteria: presence of significant cataract that caused impairment of vision, previous vitreoretinal surgery, previous intravitreal bevacizumab injection and the presence of any other vitreoretinal pathology |
|
| Interventions | Treatment: intravitreal bevacizumab 2.5 mg 3‐5 days before operation Control: no preoperative injection was performed Duration: only 1 dose |
|
| Outcomes | Primary: facilitation of the surgery (number of endodiathermy applications, backflush needle applications, duration of surgery, type of tamponade) and decrease of complications (postoperative VH) Secondary: anatomic and visual outcomes (3‐month postoperative BCVA as well as visual acuity at the last follow‐up) |
|
| Notes | Funding: not reported Trial registration: not reported Date conducted: not reported Conflict of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "prospective surgeon‐masked randomized clinical trial. The surgeons (MM, MH, MN, and MMP) were masked as to injection. During each operation, the number of endodiathermy applications, backflush needle applications, and the duration of surgery were recorded by an independent observer" Comment: the blinding of the participants was not mentioned. The participants were either given an injection or not of bevacizumab. Therefore, they would know which group they were in |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "prospective surgeon‐masked randomized clinical trial. The surgeons (MM, MH, MN, and MMP) were masked as to injection. During each operation, the number of endodiathermy applications, backflush needle applications, and the duration of surgery were recorded by an independent observer" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Losses during follow‐up were not reported |
| Selective reporting (reporting bias) | Low risk | Comment: the results of the variables were described in the methods section |
Preti 2014.
| Methods | Study design: prospective, randomised, blinded, controlled trial comparing of PRP with intravitreal bevacizumab injections versus PRP alone in high‐risk PDR Unit of randomisation: eye, within‐person study Unit of analyses: eye but not pair‐matched analysis Follow‐up: 6 months |
|
| Participants | Country: Brazil Setting: Department of Ophthalmology, University of Sap Paulo Medical School Number of participants: 42 (84 eyes) Exclusions post‐randomisation: 7 people with VH Losses to follow‐up: 0 Age (mean (range)): 56 (43‐73) years Gender: 28 men and 14 women Inclusion criteria: aged ≥ 18 years, high‐risk PDR with or without diabetic macular oedema; visual acuity ≥ 20/200 Exclusion criteria: pretreatment for diabetic retinopathy (laser, intraocular medications and surgeries); pre‐retinal haemorrhage and VH; presence of changes in the vitreous‐retinal interface (epiretinal membrane, macular hole and vitreoretinal traction syndrome); evidence of active external eye infection such as blepharitis; prior thromboembolic events, including myocardial infarction, stroke and deep vein thrombosis; systolic blood pressure > 180 mm Hg and diastolic blood pressure > 110 mm Hg; glycated haemoglobin levels > 15%; chronic renal failure; major surgery within 1 month; previous systemic anti‐VEGF |
|
| Interventions | Treatment: 2 intravitreal bevacizumab injections 1.25 mg/0.05 mL, 1 dose 1 week before the PRP, and the other dose after the last session of PRP. The PRP was performed weekly over 3 weeks Control: PRP performed weekly over 3 weeks Duration: 4 weeks |
|
| Outcomes | Primary: changes in contrast sensitivity measured with Vistech Consultants Incorporation® (VCTS) at 1, 3 and 6 months between the groups with and without diabetic macular oedema Secondary: changes in VCTS within each group with and without diabetic macular oedema; ocular safety (ocular hypertension, lens opacity progression and anterior chamber reaction arterial); systemic safety (thromboembolic events) |
|
| Notes | Funding: study was supported by the Sao Paulo Research Foundation (FAPESP) No 2009/08895‐1 Trial registration: NCT01389505 Date conducted: February 2011 to June 2012 Conflict of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: blinding not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: blinding not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 7 post‐randomisation losses, not specified by group |
| Selective reporting (reporting bias) | High risk | Comments: outcome measures on clinical trials.gov were different to those reported in the paper: Primary outcome measures: functional macular evaluation [timeframe: 24 weeks] [designated as safety issue: yes]; during this 24 weeks of follow‐up the visual acuity (ETDRS), contrast vision will be measured at baseline, 4, 12 and finally at 24 weeks. Secondary outcome measures: structural macular evaluation [timeframe: 24 weeks] [designated as safety issue: yes]; during the 24 weeks of follow‐up the following measured will be made: optical coherence tomography |
Ramos Filho 2011.
| Methods | Study design: randomised, clinical trial that assessed efficacy of ranibizumab in people with high‐risk PDR Unit of randomisation: participant Unit of analyses: participant/eye Follow‐up: 16, 32 and 48 weeks |
|
| Participants | Country: Brazil Setting: Department of Ophthalmology, School of Medicine Number of participants: 40 (40 eyes) Exclusions post‐randomisation: 1 Losses to follow‐up: 10 Age (mean): 50.5 years in ranibizumab plus PRP group, 63.3 years in PRP alone group Gender: 18 men and 11 women Inclusion criteria: people with high‐risk PDR, which was defined according to the guidelines set forth by the ETDRS: 1. presence of neovascularisation at the disc > ETDRS standard photograph 10A, 2. presence of neovascularisation at the disc associated with VH or pre‐retinal haemorrhage or 3. neovascularisation elsewhere with more than one‐half disk area associated with VH or pre‐retinal haemorrhage Exclusion criteria: 1. history of prior laser treatment or vitrectomy in the study eye; 2. history of thromboembolic event, 3. major surgery within the prior 6 months or planned within the next 28 days; 4. uncontrolled hypertension, 5. known coagulation abnormalities or current use of anticoagulative medication other than aspirin or 6. any condition affecting documentation |
|
| Interventions | Treatment: intravitreal ranibizumab 0.5 mg, 60 minutes after the completion of PRP Control: PRP Duration: only 1 dose |
|
| Outcomes | Primary: total area (mm2) of fluorescein leakage from active neovascularisation Secondary: BCVA (logMAR) and the central subfield macular thickness |
|
| Notes | Funding: Fundacao de Amparo a Pesquisa do Estado de Sao Paulo (FAPESP). Grant number: 2009 ⁄ 01036‐3 Trial registration: NCT01988246 Trial registration: not reported Date conducted: February 2009 to December 2009 Conflict of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The technician was asked to pick up one of two identical opaque envelopes; one contained the designation for PRP, and the other contained the designation for PRP plus treatment" Comment: the method of randomisation was not described. There was an imbalance between groups in the age of the participants (mean (SD): 63.3 (2.5) with intravitreal ranibizumab + PRP vs. 50.5 (3.0) with PRP alone; P value = 0.0036)), which suggest doubts about if they were correctly randomised |
| Allocation concealment (selection bias) | Low risk | Quote: "the technician was asked to pick up one of two identical opaque envelopes; one contained the designation for PRP, and the other contained the designation for PRP plus treatment" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: blinding of participants and personnel were not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "a single masked certified examiner performed Early Treatment Diabetic Retinopathy Study (ETDRS) best‐corrected visual acuity (BCVA) measurements prior to any other study procedure. A single retinal specialist performed the ophthalmic evaluations (JARF) and the stereoscopic fundus photography (FPPA). Study data were analysed and interpreted by AM, RAC, IUS, JASR, RJ" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "twenty‐nine of 40 patients initially included in this trial completed the 48‐week follow‐up evaluation" Comment: there were 11 losses (27.5%) |
| Selective reporting (reporting bias) | Low risk | Comment: the results of the variables were described in the methods section |
Rizzo 2008.
| Methods | Study design: randomised clinical trial in people undergoing pars plana vitrectomy for retinal detachment Unit of randomisation: participant Unit of analyses: participant/eye Follow‐up: 6 months |
|
| Participants | Country: Italy Setting: Eye Surgery Clinic Number of participants: 22 (22 eyes) Exclusions post‐randomisation: 0 Losses to follow‐up: 0 Age (mean (range)): 52 (24‐63) years Gender: not described Inclusion criteria: TRD, tractional‐rhegmatogenous retinal detachment or tractional detachment complicated with VH Exclusion criteria: history of vitrectomy in the study eye, thromboembolic events, major surgery within the previous 3 months or planned within the next 28 days, uncontrolled hypertension, known coagulation abnormalities or current use of anticoagulative medication other than aspirin |
|
| Interventions | Treatment: intravitreal bevacizumab 1.25 mg/0.05 mL, 5‐7 days before surgery Control: no preoperative injection Duration: only 1 dose |
|
| Outcomes | Primary: feasibility of the surgery Secondary: visual and anatomic outcome at 6 months |
|
| Notes | Funding: not reported Trial registration: not reported Date conducted: not reported Conflict of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "we used a table of random numbers in order to assign each study participant to group 1 or 2" |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: there were 0 losses |
| Selective reporting (reporting bias) | Unclear risk | Comment: there was no complete data for BCVA (SD) |
Sohn 2012.
| Methods | Study design: randomised double‐blind clinical trial Unit of randomisation: eye Unit of analyses: eye Follow‐up: 3 months |
|
| Participants | Country: USA Setting: Department of Ophthalmology Number of participants: 19 (20 eyes) Exclusions post‐randomisation: 0 Losses to follow‐up: 2 Age (mean (range)): 52 (31‐64) years Gender: 12 men and 7 women Inclusion criteria: people with TRD or combined TRD/rhegmatogenous retinal detachment secondary to PDR who were given anaesthesia clearance for pars plana vitrectomy. Indications for pars plana vitrectomy included TRD involving the macula, TRD/rhegmatogenous retinal detachment and non‐clearing or recurrent VH precluding complete PRP with TRD not necessarily involving the macula Exclusion criteria: history of pars plana vitrectomy; dense VH preventing preoperative grading of fibrovascular membranes; an inability to return for pars plana vitrectomy within 3‐7 days after randomisation; a history of cerebrovascular accident, thromboembolic event or myocardial infarction within 6 months; aged < 18 years and pregnancy |
|
| Interventions | Treatment: intravitreal bevacizumab injection 1.25 mg/0.05 mL, 3‐6 days before surgery Control: sham injection (1 syringe without a needle placed to simulate intravitreal injection) Duration: only 1 dose |
|
| Outcomes | Primary: visual acuity at 3 months of follow‐up, vitreous levels of VEGF Secondary: amount of intraoperative bleeding |
|
| Notes | Funding: supported by: the Eugene de Juan Jr Award for Innovation (Dr Sohn); the Heed Foundation (Drs Kim and Javaheri); grant K12‐EY16335 from the National Eye Institute, National Institutes of Health (Dr Kim); The Arnold and Mabel Beckman Foundation (Dr Hinton); Research to Prevent Blindness (Department of Ophthalmology, University of Iowa Hospitals and Clinics); and core grant EY03040 from the National Eye Institute (Doheny Eye Institute) Trial registration: not reported Date conducted: not reported Conflict of interest: Dr Hinton served as a consultant to FibroGen, Inc. Dr Eliott served as an ad hoc consultant to Genentech Other comments: participants of the control group had more severe symptoms than the bevacizumab group at baseline: 2 had visually significant cataract (1 participant in each group), 2 had worsening ischaemia (in control group), 1 had severe neovascular glaucoma (in control group) and 1 had VH (in control group) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "the patient and surgeon were masked to the patients' randomization group" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "the patient and surgeon were masked to the patients' randomization group" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: only 2 participants (1 in each group) were lost during the follow‐up |
| Selective reporting (reporting bias) | Low risk | Comment: the results of the variables were described in the methods section |
Zaman 2013.
| Methods | Study design: randomised, controlled trial comparing intravitreal bevacizumab injection 5‐7 days prior to pars plana vitrectomy versus pars plana vitrectomy alone Unit of randomisation: participant Unit of analyses: participant Follow‐up: 6 months |
|
| Participants | Country: Pakistan Setting: Al‐Ibrahim Eye Hospital Number of participants: 54 (54 eyes) Exclusions post‐randomisation: 0 Losses to follow‐up: 0 Age (mean (range)): 52 (39‐67) years Gender: 32 men and 22 women Inclusion criteria: non‐clearing VH of at least 1 month; TRD involving or threatening the macula; pre‐retinal subhyaloid bleeding covering the macula Exclusion criteria: not reported |
|
| Interventions | Treatment: intravitreal bevacizumab 1.25 mg/0.05 mL (Avastin, Genentech), 5‐7 days before PPV. Topical antibiotic (moxifloxacin) was started 1 day before the procedure and was continued for 3 days post injection Control: PPV alone Duration: only 1 dose |
|
| Outcomes | Primary: improvement of BCVA after surgery, postoperative complications, hyphema, rubeosis, frequency of VH. Early postoperative VH was taken as VH occurring within 4 weeks after surgery. Later postoperative VH was taken as VH occurring within 5 weeks and 6 months | |
| Notes | Funding: not reported Trial registration: not reported Date conducted: September 2010 to August 2011 Conflict of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: blinding not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: blinding not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "all cases completed a minimum follow up of 6 months" Comment: there were no losses |
| Selective reporting (reporting bias) | Low risk | Comment: in the paper the results of outcomes were specified in the methods section, but we have not access to the protocol to check if all outcomes were reported |
BCVA: best‐corrected visual acuity; ETDRS: Early Treatment Diabetic Retinopathy Study; PDR: proliferative diabetic retinopathy; PRP: panretinal photocoagulation; SD: standard deviation; TRD: tractional retinal detachment; VEGF: vascular endothelial growth factor; VH: vitreous haemorrhage.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Arimura 2009 | Retrospective, comparative study |
| Fulda 2010 | Not a randomised clinical trial. Each participant received the 2 evaluated interventions. The right eye received intravitreal bevacizumab and 1 session of 800 scattered laser spots. The left eye underwent a full 1600 laser panretinal photocoagulation |
| Genovesi‐Ebert 2007 | Not a randomised clinical trial |
| Gonzalez 2006 | RCT assessed the efficacy and safety of pegaptanib in treating diabetic macular oedema and diabetic retinopathy. The publication was an abstract and there was insufficient information to include the study. The principal focus is of participants with macular oedema |
| Hattori 2010 | Not a randomised clinical trial |
| Huang 2009 | Compared with historical controls. Not randomised |
| Ip 2012 | 2 years of follow‐up to evaluate effects of intravitreal ranibizumab on diabetic retinopathy severity over time in 2 phase 3 clinical trials (RIDE, NCT00473382; RISE, NCT00473330) for diabetic macular oedema |
| Jiang 2009 | Retrospective study |
| Jorge 2006 | Non‐randomised study |
| Lanzagorta‐Aresti 2009 | The included participants did not have proliferative diabetic retinopathy. The outcomes measured were central macular thickness and visual acuity in participants with a moderate retinopathy not proliferative that needed a cataract surgery |
| López‐López 2012 | Anti‐VEGF group was not randomised |
| Michaelides 2010 | Focus of the clinical trial was diabetic macular oedema |
| Minnella 2008 | Non‐controlled clinical trial |
| Scott 2008 | Study evaluated agreement in diabetic retinopathy severity classification by retina specialists performing ophthalmoscopy vs. reading centre grading of 7‐field stereoscopic fundus photographs in a phase 2 clinical trial of intravitreal bevacizumab for centre‐involved diabetic macular oedema |
| Shin 2009 | Data were collected retrospectively |
| Stergiou 2007 | Retrospective case series |
| Tonello 2008 | Quote: "for patients (n= 8) presenting with high‐risk PDR [proliferative diabetic retinopathy] in both eyes, the eye with worse BCVA [best‐corrected visual acuity] was selected to receive PRP [panretinal photocoagulation] plus intravitreal bevacizumab (eight eyes) and the fellow eye was treated with PRP alone (eight eyes)" Comment: clinical trial partially randomised |
| Yeh 2009 | Not a randomised study. The treatment assignment was alternative |
| Zhou 2010 | Focus of the clinical trial is diabetic macular oedema |
Characteristics of ongoing studies [ordered by study ID]
EUCTR2013‐003272‐12‐GB.
| Trial name or title | EUCTR2013‐003272‐12‐GB |
| Methods | Prospective, randomised, controlled, single‐masked study |
| Participants | 220 participants with proliferative diabetic retinopathy |
| Interventions | Aflibercept versus PRP laser treatment |
| Outcomes | Primary:
Secondary:
|
| Starting date | 8 April 2014 |
| Contact information | Natasha Ajraam. Moorfields Eye Hospital, London, UK e‐mail: natasha.ajraam@moorfields.nhs.uk |
| Notes | Funding: Bayer PLC and NIHR MRC ‐ EME grant |
NCT01854593.
| Trial name or title | NCT01854593 |
| Methods | Prospective, randomised, controlled, double‐masked (participant and carer) study |
| Participants | People with proliferative diabetic retinopathy and indication for primary vitrectomy |
| Interventions | Intravitreal bevacizumab 0.16 mg versus sham injection |
| Outcomes | VEGF concentration in vitreous after intravitreal bevacizumab injection at 1 year Early (within 4 weeks) postoperative vitreous haemorrhage. Re‐operation due to vitreous haemorrhage |
| Starting date | May 2012 |
| Contact information | Ayumu Manabe. Nihon University, Japan |
| Notes |
NCT01941329 (PROTEUS).
| Trial name or title | PROTEUS study |
| Methods | Prospective, randomised, multicentre, open‐label, phase II‐III study |
| Participants | People with high‐risk proliferative diabetic retinopathy. Number: 94 |
| Interventions | Intravitreal injection ranibizumab 0.5 mg plus PRP (group 1) vs. PRP alone (group 2) Group 1: 3 x intravitreal injections of ranibizumab combined with standard PRP (mean 2 (standard deviation 1) weeks after injection), at month 0, month 1 and month 2 that can be repeated after month 3, with always at least a 1‐month interval between injections Group 2: PRP between month 0 and month 2, with 1 mandatory laser session in month 0 and more laser sessions as needed until month 2 to complete the PRP treatment After completing the PRP treatment, PRP sessions can be repeated from month 3 to month 11 |
| Outcomes | Primary:
Secondary:
|
| Starting date | April 2014 |
| Contact information | José Cunha‐Vaz, MD, PhD; mail: 4c@aibili.pt |
| Notes | NCT01941329 |
NCT01976923 (PACORES).
| Trial name or title | PACORES study |
| Methods | Prospective, randomised, active‐controlled study |
| Participants | Participants with tractional retinal detachment secondary to proliferative diabetic retinopathy and indication for vitrectomy. Number: 374 |
| Interventions | Intravitreal bevacizumab 1.25 mg/0.05 mL versus small‐gauge pars plana vitrectomy |
| Outcomes | Primary:
Secondary:
|
| Starting date | November 2013 |
| Contact information | J. Fernando Arevalo, MD, FACS; mail: arevalojf@jhmi.edu Igor Kozak, MD; mail: ikozak@kkesh.med.sa |
| Notes | NCT01976923 |
NCT01988246.
| Trial name or title | PROMISE |
| Methods | Prospective, randomised, controlled, single‐masked (participant) study |
| Participants | Prevention of macular oedema in participants with diabetic retinopathy undergoing cataract surgery |
| Interventions | Aflibercept 2 mg intravitreal injection (0.05 mL or 50 μL) administered at time of surgery (post cataract excision) versus sham injection |
| Outcomes | Primary:
Secondary:
|
| Starting date | December 2013 |
| Contact information | Rishi Singh, M.D.; mail: singhr@ccf.org Gail Kolin, BSN RN; mail: koling@ccf.org |
| Notes | There will be participants with non‐proliferative diabetic retinopathy |
BCVA: best‐corrected visual acuity; ETDRS: Early Treatment Diabetic Retinopathy Study; PRP: panretinal photocoagulation; VEGF: vascular endothelial growth factor.
Differences between protocol and review
We made the following amendments to the protocol (Martinez‐Zapata 2010).
In the protocol, we had not considered that anti‐VEGFs would be used in different patient groups with PDR (i.e. people eligible for laser treatment, people eligible for vitrectomy and people undergoing cataract surgery. We felt that clinically it did not make sense to combine these different patient groups and so have presented the results separately.
In the protocol, the primary outcome was regression of proliferative retinopathy and visual acuity was a secondary outcome. On reflection, we felt this was the wrong emphasis and considered that the effect on visual acuity was more relevant for the person than checking if anti‐VEGFs could produce regression of new vessels. We have changed visual acuity to the primary outcome and considered regression of proliferative retinopathy as a secondary outcome.
In the protocol, we planned to exclude from the analysis studies where the fellow eye was used as a control (i.e. the within‐person studies). However, some studies had a parallel group design but included a low percentage of participants with the fellow eye used as a control. We included these studies in the analysis.
We did not calculate the number needed to treat for an additional beneficial outcome (NNTB) and the number needed to treat for an additional harmful outcome (NNTH) due to the low quality of the evidence.
In the protocol, we planned to do a sensitivity analysis by intention‐to‐treat considering the "worst‐case scenario". In the event, we did not do this, partly due to the characteristics of the majority of studies and partly because, on reflection, we felt that this analysis was too extreme and unlikely to be informative.
We planned to do a sensitivity analysis excluding unpublished studies but did not have any data on unpublished studies to do this.
Contributions of authors
Conceiving the review: MJM. Designing the review: MJM, AM. Co‐ordinating the review: MJM. Designing electronic search strategy: Cochrane Eyes and Vision Group editorial base. Screening search results: MJM, ChF, JAC, JRE. Obtaining copies of trials: IS, MJM, ChF, JRE. Appraising quality of papers: MJM, ChF, JAC, JRE. Abstracting data from papers: MJM, JAC, JRE. Data management for the review: MJM. Entering data into Review Manager 5: MJM, JRE. Analysis of data: MJM. Interpretation of data: all authors. Writing the review: MJM, JRE. Draft the final review: all authors. Guarantor for the review: MJM.
Sources of support
Internal sources
CIBER de Epidemiología y Salud Pública (CIBERESP), Spain.
External sources
Grant of the Spanish Ministry of Health (2006‐2008); PI0690322, Spain.
-
National Institute for Health Research (NIHR), UK.
- Richard Wormald, Co‐ordinating Editor for the Cochrane Eyes and Vision Group (CEVG) acknowledges financial support for his CEVG research sessions from the Department of Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology.
- The NIHR also funds the CEVG Editorial Base in London.
- The Cochrane Review Incentive Scheme provided funding for Jennifer Evans to assist with completing this review.
The views expressed in this publication are those of the authors and not necessarily those of the NIHR, NHS, or the Department of Health.
Declarations of interest
None.
Edited (no change to conclusions)
References
References to studies included in this review
Ahmadieh 2009 {published data only}
- Ahmadieh H, Shoeibi N, Entezari M, Monshizadeh R. Intravitreal bevacizumab for prevention of early postvitrectomy hemorrhage in diabetic patients: a randomized clinical trial. Ophthalmology 2009;116(10):1943‐8. [DOI] [PubMed] [Google Scholar]
Ahn 2011 {published data only}
- Ahn J, Woo SJ, Chung H, Park KH. The effect of adjunctive intravitreal bevacizumab for preventing postvitrectomy hemorrhage in proliferative diabetic retinopathy. Ophthalmology 2011;118(11):2218‐26. [DOI] [PubMed] [Google Scholar]
Cheema 2009 {published data only}
- Cheema RA, Al‐Mubarak MM, Amin YM, Cheema MA. Role of combined cataract surgery and intravitreal bevacizumab injection in preventing progression of diabetic retinopathy: prospective randomized study. Journal of Cataract and Refractive Surgery 2009;35(1):18‐25. [DOI] [PubMed] [Google Scholar]
Cho 2010 {published data only}
- Cho WB, Moon JW, Kim HC. Intravitreal triamcinolone and bevacizumab as adjunctive treatments to panretinal photocoagulation in diabetic retinopathy. British Journal of Ophthalmology 2010;94(7):858‐63. [DOI] [PubMed] [Google Scholar]
- Cho WB, Oh SB, Moon JW, Kim HC. Panretinal photocoagulation combined with intravitreal bevacizumab in high‐risk proliferative diabetic retinopathy. Retina 2009;29(4):516‐22. [DOI] [PubMed] [Google Scholar]
Di Lauro 2010 {published data only}
- Lauro R, Ruggiero P, Lauro MT, Romano MR. Intravitreal bevacizumab for surgical treatment of severe proliferative diabetic retinopathy. Graefe's Archive for Clinical and Experimental Ophthalmology 2010;248(6):785‐91. [DOI] [PubMed] [Google Scholar]
DRCR.Net 2013 {published data only}
- Diabetic Retinopathy Clinical Research Network. Randomized clinical trial evaluating intravitreal ranibizumab or saline for vitreous hemorrhage from proliferative diabetic retinopathy. JAMA Ophthalmology 2013;131(3):283‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
El‐Batarny 2008 {published data only}
- El‐Batarny AM. Intravitreal bevacizumab as an adjunctive therapy before diabetic vitrectomy. Clinical Ophthalmology 2008;2(4):709‐16. [PMC free article] [PubMed] [Google Scholar]
Ergur 2009 {published data only}
- Ergur O, Bayhan HA, Kurkcuoglu P, Takmaz T, Gurdal C, Can I. Comparison of panretinal photocoagulation (PRP) with PRP plus intravitreal bevacizumab in the treatment of proliferative diabetic retinopathy [Proliferatif diyabetik retinopati tedavisinde tek basina panretinal fotokoagulasyon (PRF) ile PRF ve intravitreal bevacizumab kombinasyonunun karsilastirilmasi]. Retina‐Vitreus 2009;17(4):273‐7. [Google Scholar]
Ernst 2012 {published data only}
- Ernst BJ, García‐Aguirre G, Oliver SC, Olson JL, Mandava N, Quiroz‐Mercado H. Intravitreal bevacizumab versus panretinal photocoagulation for treatment‐naïve proliferative and severe nonproliferative diabetic retinopathy. Acta Ophthalmologica 2012;90(7):e573‐4. [DOI] [PubMed] [Google Scholar]
- Garcia‐Aguirre G, Reyna‐Castelan E, Torres M, Kon‐Jara V, Quiroz‐Mercado H. Intravitreal bevacizumab vs. panretinal photocoagulation for the treatment of proliferative and severe non‐proliferative diabetic retinopathy: a contralateral eye study. Investigative Ophthalmology and Visual Science 2007;48:ARVO E‐abstract 5041. [Google Scholar]
- Garcia‐Aguirre G, Reyna‐Castelán E, Torres‐Soriano M, Kon‐Jara V, Quiroz‐Mercado H. Bevacizumab vs. panretinal photocoagulation for the treatment of proliferative and severe non‐proliferative diabetic retinopathy: a contralateral eye study. Investigative Ophthalmology and Visual Science 2008;48:ARVO E‐abstract 2750. [Google Scholar]
Farahvash 2011 {published data only}
- Farahvash MS, Majidi AR, Roohipoor R, Ghassemi F. Preoperative injection of intravitreal bevacizumab in dense diabetic vitreous hemorrhage. Retina 2011;31(7):1254–60. [DOI] [PubMed] [Google Scholar]
González 2009 {published data only}
- Gonzalez VH, Vann VR. Treatment of proliferative diabetic retinopathy with intravitreal pegaptanib vs. panretinal photocoagulation. American Academy of Ophthalmology 2007:265. [Google Scholar]
- Gonzalez VH, Vann VR, Banda RM, Giuliari GP, Guel DA. Pegaptanib sodium (Macugen®) versus panretinal photocoagulation (PRP) for the regression of proliferative diabetic retinopathy. Investigative Ophthalmology and Visual Science 2007;48:ARVO E‐abstract 4030. [Google Scholar]
- Gonzalez VH, Vann VR, Banda‐Gonzales RM. Selective VEGF Inhibition: effectiveness in modifying the progression of proliferative diabetic retinopathy. American Academy of Ophthalmology 2006:279. [Google Scholar]
- González VH, Giuliari GP, Banda RM, Guel DA. Intravitreal injection of pegaptanib sodium for proliferative diabetic retinopathy. British Journal of Ophthalmology 2009;93(11):1474‐8. [DOI] [PubMed] [Google Scholar]
Mirshahi 2008 {published data only}
- Mirshahi A, Roohipoor R, Lashay A, Mohammadi SF, Abdoallahi A, Faghihi H. Bevacizumab‐augmented retinal laser photocoagulation in proliferative diabetic retinopathy: a randomized double‐masked clinical trial. European Journal of Ophthalmology 2008;18(2):263‐9. [DOI] [PubMed] [Google Scholar]
Modarres 2009 {published data only}
- Modarres M, Nazari H. Intravitreal injection of bevacizumab before vitrectomy for proliferative diabetic retinopathy. American Academy of Ophthalmology 2007:199. [DOI] [PubMed] [Google Scholar]
- Modarres M, Nazari H, Falavarjani KG, Naseripour M, Hashemi M, Parvaresh MM. Intravitreal injection of bevacizumab before vitrectomy for proliferative diabetic retinopathy. European Journal of Ophthalmology 2009;19(5):848‐52. [DOI] [PubMed] [Google Scholar]
Preti 2014 {published data only}
- Preti RC, Vasquez Ramirez LM, Ribeiro Monteiro ML, Pelayes DE, Takahashi WY. Structural and functional assessment of macula in patients with high‐risk proliferative diabetic retinopathy submitted to panretinal photocoagulation and associated intravitreal bevacizumab injections: a comparative, randomised, controlled trial. Ophthalmologica 2013;230(1):1‐8. [DOI] [PubMed] [Google Scholar]
- Preti RC, Vazquez L, Ribeiro M, Kehdi M, Pelayes DE, Yukihiko W. Contrast sensitivity evaluation in high risk proliferative diabetic retinopathy treated with panretinal photocoagulation associated or not with intravitreal bevacizumab injections: a randomised clinical trial. British Journal of Ophthalmology 2014;97(7):885‐9. [DOI] [PubMed] [Google Scholar]
Ramos Filho 2011 {published data only}
- Lucena CR, Ramos Filho JA, Messias AM, Silva JA, Almeida FP, Scott IU, et al. Panretinal photocoagulation versus intravitreal injection retreatment pain in high‐risk proliferative diabetic retinopathy. Arquivos Brasileiros de Oftalmologia 2013;76(1):18‐20. [DOI] [PubMed] [Google Scholar]
- Messias A, Ramos Filho JA, Messias K, Almeida FP, Costa RA, Scott IU, et al. Electroretinographic findings associated with panretinal photocoagulation (PRP) versus PRP plus intravitreal ranibizumab treatment for high‐risk proliferative diabetic retinopathy. Documenta Ophthalmologica 2012;124(3):225‐36. [DOI] [PubMed] [Google Scholar]
- Ramos Filho JA, Messias A, Almeida FP, Ribeiro JA, Costa RA, Scott IU, et al. Panretinal photocoagulation (PRP) versus PRP plus intravitreal ranibizumab for high‐risk proliferative diabetic retinopathy. Acta Ophthalmologica 2011;89(7):e567‐72. [DOI] [PubMed] [Google Scholar]
Rizzo 2008 {published data only}
- Rizzo S, Genovesi‐Ebert F, Bartolo E, Vento A, Miniaci S, Williams G. Injection of intravitreal bevacizumab (Avastin) as a preoperative adjunct before vitrectomy surgery in the treatment of severe proliferative diabetic retinopathy (PDR). Graefe's Archive for Clinical and Experimental Ophthalmology 2008;246(6):837‐42. [DOI] [PubMed] [Google Scholar]
Sohn 2012 {published data only}
- Sohn EH, He S, Kim LA, Salehi‐Had H, Javaheri M, Spee C, et al. Angiofibrotic response to vascular endothelial growth factor Inhibition in diabetic retinal detachment. Archives of Ophthalmology 2012;130(9):1127‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zaman 2013 {published data only}
- Zaman Y, Rehman A, Fattah M. Intravitreal Avastin as an adjunct in patients with proliferative retinopathy undergoing pars plana vitrectomy. Pakistan Journal of Medical Sciences 2013;29(2):590‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Arimura 2009 {published data only}
- Arimura N, Otsuka H, Yamakiri K, Sonoda Y, Nakao S, Noda Y, et al. Vitreous mediators after intravitreal bevacizumab or triamcinolone acetonide in eyes with proliferative diabetic retinopathy. Ophthalmology 2009;116(5):921‐6. [DOI] [PubMed] [Google Scholar]
Fulda 2010 {published data only}
- Fulda E, Ariza E, Lopez A, Graue F. Intravitreal bevacizumab and panretinal photocoagulation as combined treatment in proliferative diabetic retinopathy. Retina‐Vitreus 2010;18(1):52‐5. [Google Scholar]
Genovesi‐Ebert 2007 {published data only}
- Genovesi‐Ebert F, Rizzo S, Bartolo E, Miniaci S, Vento A, Palla M, et al. Injection of intravitreal Avastin before vitrectomy surgery in the treatment of severe proliferative diabetic retinopathy. Investigative Ophthalmology and Visual Science 2007;48:ARVO E‐abstract 5044. [Google Scholar]
Gonzalez 2006 {published data only}
- Gonzalez VH, Macugen Diabetic Retinopathy Study Group. Pegaptanib in diabetic retinopathy: improvements in diabetic macular edema, retinal neovascularization, and diabetic retinopathy severity. American Academy of Ophthalmology 2006:192. [Google Scholar]
Hattori 2010 {published data only}
- Hattori T, Shimada H, Nakashizuka H, Mizutani Y, Mori R, Yuzawa M. Dose of intravitreal bevacizumab (Avastin) used as preoperative adjunct therapy for proliferative diabetic retinopathy. Retina 2010;30(5):761‐4. [DOI] [PubMed] [Google Scholar]
Huang 2009 {published data only}
- Huang YH, Yeh PT, Chen MS, Yang CH, Yang CM. Intravitreal bevacizumab and panretinal photocoagulation for proliferative diabetic retinopathy associated with vitreous hemorrhage. Retina 2009;29(8):1134‐40. [DOI] [PubMed] [Google Scholar]
Ip 2012 {published data only}
- Ip MS, Domalpally A, Hopkins JH, Wong P, Ehrlich JS. Long‐term effects of ranibizumab on diabetic retinopathy severity and progression. Archives of Ophthalmology 2012;130(9):1145‐52. [DOI] [PubMed] [Google Scholar]
Jiang 2009 {published data only}
- Jiang Y, Liang X, Li X, Tao Y, Wang K. Analysis of the clinical efficacy of intravitreal bevacizumab in the treatment of iris neovascularization caused by proliferative diabetic retinopathy. Acta Ophthalmologica 2009;87(7):736‐40. [DOI] [PubMed] [Google Scholar]
Jorge 2006 {published data only}
- Jorge R, Costa RA, Calucci D, Cintra LP, Scott IU. Intravitreal bevacizumab (Avastin) for persistent new vessels in diabetic retinopathy (IBEPE study). Retina 2006;26(9):1006‐13. [DOI] [PubMed] [Google Scholar]
Lanzagorta‐Aresti 2009 {published data only}
- Lanzagorta‐Aresti A, Palacios‐Pozo E, Menezo Rozalen JL, Navea‐Tejerina A. Prevention of vision loss after cataract surgery in diabetic macular edema with intravitreal bevacizumab: a pilot study. Retina 2009;29(4):530‐5. [DOI] [PubMed] [Google Scholar]
López‐López 2012 {published data only}
- López‐López F, Gómez‐Ulla F, Rodriguez‐Cid MJ, Arias L. Triamcinolone and bevacizumab as adjunctive therapies to panretinal photocoagulation for proliferative diabetic retinopathy. ISRN Ophthalmology 2012;2012:Article ID 267643. [DOI] [PMC free article] [PubMed] [Google Scholar]
Michaelides 2010 {published data only}
- Michaelides M, Kaines A, Hamilton RD, Fraser‐Bell S, Rajendram R, Quhill F, et al. A prospective randomized trial of intravitreal bevacizumab or laser therapy in the management of diabetic macular edema (BOLT study) 12‐month data: report 2. Ophthalmology 2010;117(6):1078‐86. [DOI] [PubMed] [Google Scholar]
Minnella 2008 {published data only}
- Minnella AM, Savastano CM, Ziccardi L, Scupola A, Falsini B, Balestrazzi E. Intravitreal bevacizumab (Avastin) in proliferative diabetic retinopathy. Acta Opthalmologica 2008;86(6):683‐7. [DOI] [PubMed] [Google Scholar]
Scott 2008 {published data only}
- Scott IU, Bressler NM, Bressler SB, Browning DJ, Chan CK, Danis RP, et al. Agreement between clinician and reading center gradings of diabetic retinopathy severity level at baseline in a phase 2 study of intravitreal bevacizumab for diabetic macular edema. Retina 2008;28(1):36‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shin 2009 {published data only}
- Shin YW, Lee YJ, Lee BR, Cho HY. Effects of an intravitreal bevacizumab injection combined with panretinal photocoagulation on high‐risk proliferative diabetic retinopathy. Korean Journal of Ophthalmology 2009;23(4):266‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stergiou 2007 {published data only}
- Stergiou P, Kokkinou D, Malamos K, Felekidis A, Kailari S. Varying doses of intravitreal bevacizumab (Avastin) for the treatment of proliferative diabetic retinopathy. Investigative Ophthalmology and Visual Science 2007;48:ARVO E‐abstract 1395. [Google Scholar]
Tonello 2008 {published data only}
- Tonello M, Costa RA, Almeida FP, Barbosa JC, Scott IU, Jorge R. Panretinal photocoagulation versus PRP plus intravitreal bevacizumab for high‐risk proliferative diabetic retinopathy (IBeHi study). Acta Ophthalmologica 2008;86(4):385‐9. [DOI] [PubMed] [Google Scholar]
Yeh 2009 {published data only}
- Yeh PT, Yang CM, Lin YC, Chen MS, Yang CH. Bevacizumab pretreatment in vitrectomy with silicone oil for severe diabetic retinopathy. Retina 2009;29(6):768‐74. [DOI] [PubMed] [Google Scholar]
Zhou 2010 {published data only}
- Zhou YY, Zhang RJ. Avastin combined with vitreous cavity injection of triamcinolone acetonide in treatment of diabetic retinopathy with macular edema. International Journal of Ophthalmology 2010;10(3):475‐6. [Google Scholar]
References to ongoing studies
EUCTR2013‐003272‐12‐GB {published data only}
- EUCTR2013‐003272‐12‐GB. Clinical efficacy and mechanistic evaluation of aflibercept for proliferative diabetic retinopathy. www.clinicaltrialsregister.eu/ctr‐search/trial/2013‐003272‐12/GB (accessed 2 November 2014).
NCT01854593 {published data only}
- NCT01854593. Prospective randomized controlled study of intravitreal injection of 0.16 mg bevacizumab one day before surgery for proliferative diabetic retinopathy. clinicaltrials.gov/show/NCT01854593 (accessed 2 November 2014).
NCT01941329 (PROTEUS) {published data only}
- NCT01941329. Prospective, randomized, multicentre, open‐label, phase II/III study to assess efficacy and safety of ranibizumab 0.5 mg intravitreal injections plus panretinal photocoagulation (PRP) versus PRP in monotherapy in the treatment of subjects with high risk proliferative diabetic retinopathy (PROTEUS). clinicaltrials.gov/show/NCT01941329 (accessed 2 November 2014).
NCT01976923 (PACORES) {published data only}
- NCT01976923. Pre‐operative intravitreal bevacizumab for tractional retinal detachment secondary to proliferative diabetic retinopathy: results of the Pan‐American Collaborative Retina Study (PACORES) Group. clinicaltrials.gov/show/NCT01976923 (accessed 2 November 2014).
NCT01988246 {published data only}
- NCT01988246. Prevention of macular edema In patients with diabetic retinopathy undergoing cataract surgery. clinicaltrials.gov/show/NCT01988246 (accessed 2 November 2014).
Additional references
ADA 2006
- Fong DS, Aiello L, Gardner TW, King GL, Blankenship G, Cavallerano JD, et al. Retinopathy in diabetes. Diabetes Care 2006;27 Suppl 1:84‐7. [DOI] [PubMed] [Google Scholar]
Adamis 2006
- Adamis AP, Altaweel M, Bressler NM, Cunningham ET Jr, Davis MD, Goldbaum M, et al. Changes in retinal neovascularization after pegaptanib (Macugen) therapy in diabetic individuals. Ophthalmology 2006;113(1):23‐8. [DOI] [PubMed] [Google Scholar]
Arevalo 2007
- Arevalo JF, Fromow‐Guerra J, Quiroz‐Mercado H, Sanchez JG, Wu L, Maia M, et al. Primary intravitreal bevacizumab (Avastin) for diabetic macular edema: results from the Pan‐American Collaborative Retina Study Group at 6‐month follow‐up. Ophthalmology 2007;114(4):743‐50. [DOI] [PubMed] [Google Scholar]
Avery 2006a
- Avery RL, Pearlman J, Pieramici DJ, Rabena MD, Castellarin AA, Nasir MA, et al. Intravitreal bevacizumab (Avastin) in the treatment of proliferative diabetic retinopathy. Ophthalmology 2006;113(10):1695.e1‐15. [DOI] [PubMed] [Google Scholar]
Avery 2006b
- Avery RL. Regression of retinal and iris neovascularization after intravitreal bevacizumab (Avastin) treatment. Retina 2006;26(3):352‐4. [DOI] [PubMed] [Google Scholar]
Bussolati 2001
- Bussolati B, Dunk C, Grohman M, Kontos CD, Mason J, Ahmed A. Vascular endothelial growth factor receptor‐1 modulates vascular endothelial growth factor‐mediated angiogenesis via nitric oxide. American Journal of Pathology 2001;159(3):993‐1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carmeliet 2004
- Carmeliet P. Manipulating angiogenesis in medicine. Journal of Internal Medicine 2004;255(5):538‐61. [DOI] [PubMed] [Google Scholar]
Chen 2006
- Chen E, Park CH. Use of intravitreal bevacizumab as a preoperative adjunct for tractional retinal detachment repair in severe proliferative diabetic retinopathy. Retina 2006;26(6):699‐700. [DOI] [PubMed] [Google Scholar]
Chew 1996
- Chew EY, Klein ML, Ferris FL 3rd, Remaley NA, Murphy RP, Chantry K, et al. Association of elevated serum lipid levels with retinal hard exudate in diabetic retinopathy. Early Treatment Diabetic Retinopathy Study (ETDRS) Report 22. Archives of Ophthalmology 1996;114(9):1079‐84. [DOI] [PubMed] [Google Scholar]
Chun 2006
- Chun DW, Heier JS, Topping TM, Duker JS, Bankert JM. A pilot study of multiple intravitreal injections of ranibizumab in patients with center‐involving clinically significant diabetic macular edema. Ophthalmology 2006;113(10):1706‐12. [DOI] [PubMed] [Google Scholar]
Cunningham 2005
- Cunningham ET Jr, Adamis AP, Altaweel M, Aiello LP, Bressler NM, D'Amico DJ, et al. A phase II randomized double‐masked trial of pegaptanib, an anti‐vascular endothelial growth factor aptamer, for diabetic macular edema. Ophthalmology 2005;112(10):1747‐57. [DOI] [PubMed] [Google Scholar]
Davis 1998
- Davis MD, Fisher MR, Gagnon RE. Risk factors for high‐risk proliferative diabetic retinopathy and severe visual loss: Early Treatment Diabetic Retinopathy Study report #18. Investigative Ophthalmology & Visual Science 1998;39(2):233‐52. [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
DRSRG 1978
- Anonymous. Photocoagulation treatment of proliferative diabetic retinopathy: the second report of Diabetic Retinopathy Study findings. Ophthalmology 1978;85(1):82‐106. [DOI] [PubMed] [Google Scholar]
DRSRG 1981a
- Anonymous. Photocoagulation treatment of proliferative diabetic retinopathy: relationship of adverse treatment effects to retinopathy severity. Diabetic Retinopathy Study report no. 5. Developments in Ophthalmology 1981;2:248‐56. [PubMed] [Google Scholar]
DRSRG 1981b
- Anonymous. Photocoagulation treatment of proliferative diabetic retinopathy. Clinical application of Diabetic Retinopathy Study (DRS) findings, DRS report number 8. The Diabetic Retinopathy Study Research Group. Ophthalmology 1981;88(7):583‐600. [PubMed] [Google Scholar]
DRVSRG 1985
- Anonymous. Early vitrectomy for severe vitreous hemorrhage in diabetic retinopathy. Two‐year results of a randomized trial. Diabetic Retinopathy Vitrectomy Study report 2. The Diabetic Retinopathy Vitrectomy Study Research Group. Archives of Ophthalmology 1985;103(11):1644‐52. [PubMed] [Google Scholar]
ETDRSRG 1985
- Anonymous. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Early Treatment Diabetic Retinopathy Study research group. Archives of Ophthalmology 1985;103(12):1796‐806. [PubMed] [Google Scholar]
ETDRSRG 1991a
- Anonymous. Grading diabetic retinopathy from stereoscopic color fundus photographs‐‐an extension of the modified Airlie House classification. ETDRS report number 10. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology 1991;98 Suppl(5):786‐806. [PubMed] [Google Scholar]
ETDRSRG 1991b
- Anonymous. Fundus photographic risk factors for progression of diabetic retinopathy. ETDRS report number 12. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology 1991;98 Suppl(5):823‐33. [PubMed] [Google Scholar]
ETDRSRG 1991c
- Anonymous. Early photocoagulation for diabetic retinopathy. ETDRS report number 9. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology 1991;98 Suppl(5):766‐85. [PubMed] [Google Scholar]
Ferris 1982
- Ferris FL 3rd, Kassoff A, Bresnick GH, Bailey I. New visual acuity charts for clinical research. American Journal of Ophthalmology 1982;94(1):91‐6. [PubMed] [Google Scholar]
Gilbert 2000
- Gilbert R, Kelly D, Cox A. Angiotensin converting enzyme inhibition reduces retinal overexpression of vascular endothelial growth factor and hyperpermeability in experimental diabetes. Diabetologia 2000;43(11):1360‐7. [DOI] [PubMed] [Google Scholar]
Glanville 2006
- Glanville JM, Lefebvre C, Miles JN, Camosso‐Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association 2006;94(2):130‐6. [PMC free article] [PubMed] [Google Scholar]
GRADEpro 2014 [Computer program]
- McMaster University. GRADEpro. Version 15 April 2014. McMaster University, 2014.
Haritoglou 2006
- Haritoglou C, Kook D, Neibauer A, Wolf A, Priglinger S, Strauss R, et al. Intravitreal bevacizumab (Avastin) therapy for persistent diffuse diabetic macular edema. Retina 2006;26(9):999‐1005. [DOI] [PubMed] [Google Scholar]
Hayward 2002
- Hayward LM, Burden ML, Burden AC, Blackledge H, Raymond NT, Botha JL, et al. What is the prevalence of visual impairment in the general and diabetic populations: are there ethnic and gender differences?. Diabetic Medicine 2002;19(1):27‐3. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jaffe 2006
- Jaffe GJ, Martin D, Callanan D, Pearson PA, Levy B, Comstock T, et al. Fluocinolone acetonide implant (Retisert) for noninfectious posterior uveitis: thirty‐four‐week results of a multicenter randomized clinical study. Ophthalmology 2006;113(6):1020‐7. [DOI] [PubMed] [Google Scholar]
Klein 1984
- Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin epidemiologic study of diabetic retinopathy. II. Prevalence and risk of diabetic retinopathy when age at diagnosis is less than 30 years. Archives of Ophthalmology 1984;102(4):520‐6. [DOI] [PubMed] [Google Scholar]
Klein 1988
- Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. Glycosylated hemoglobin predicts the incidence and progression of diabetic retinopathy. JAMA 1988;260(19):2864‐71. [PubMed] [Google Scholar]
Klein 1989
- Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. Is blood pressure a predictor of the incidence or progression of diabetic retinopathy?. Archives of Internal Medicine 1989;149(11):2427‐32. [PubMed] [Google Scholar]
Klein 1990
- Klein BE, Moss SE, Klein R. Effect of pregnancy on progression of diabetic retinopathy. Diabetes Care 1990;13(1):34‐40. [DOI] [PubMed] [Google Scholar]
Klein 2002a
- Klein R, Klein BE. Blood pressure control and diabetic retinopathy. British Journal of Ophthalmology 2002;86(4):365‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Klein 2002b
- Klein R, Sharrett AR, Klein BE, Moss SE, Folsom AR, Wong TY, et al. The association of atherosclerosis, vascular risk factors, and retinopathy in adults with diabetes: the atherosclerosis risk in communities study. Ophthalmology 2002;109(7):1225‐34. [DOI] [PubMed] [Google Scholar]
Kullberg 2002
- Kullberg CE, Abrahamsson M, Arnqvist HJ, Finnstrom K, Ludvigsson J. VISS Study Group. Prevalence of retinopathy differs with age at onset of diabetes in a population of patients with Type 1 diabetes. Diabetes Medicine 2002;19(11):924‐31. [DOI] [PubMed] [Google Scholar]
Martidis 2002
- Martidis A, Duker JS, Greenberg PB, Rogers AH, Puliafito CA, Reichel E, et al. Intravitreal triamcinolone for refractory diabetic macular edema. Ophthalmology 2002;109(5):920‐7. [DOI] [PubMed] [Google Scholar]
Mason 2006
- Mason JO 3rd, Nixon PA, White MF. Intravitreal injection of bevacizumab (Avastin) as adjunctive treatment of proliferative diabetic retinopathy. American Journal of Ophthalmology 2006;142(4):685‐8. [DOI] [PubMed] [Google Scholar]
Mathiesen 1995
- Mathiesen ER, Ronn B, Storm B, Foght H, Deckert T. The natural course of microalbuminuria in insulin‐dependent diabetes: a 10‐year prospective study. Diabetic Medicine 1995;12(6):482‐7. [DOI] [PubMed] [Google Scholar]
Moss 1994
- Moss SE, Klein R, Klein B. Ten‐years incidence of visual loss in a diabetic population. Ophthalmology 1994;101(6):1061‐70. [DOI] [PubMed] [Google Scholar]
Moss 1996
- Moss S, Klein R, Klein BE. Cigarette smoking and ten‐year progression in diabetic retinopathy. Ophthalmology 1996;103(9):1438‐42. [DOI] [PubMed] [Google Scholar]
Nauck 1997
- Nauck M, Roth M, Tamm M, Eickleberg O, Weiland H, Stulz P, et al. Induction of vascular endothelial growth factor by platelet‐activating factor and platelet‐derived growth factor is downregulated by corticosteroids. American Journal of Respiratory Cell and Molecular Biology 1997;16(4):398‐406. [DOI] [PubMed] [Google Scholar]
Resnikoff 2004
- Resnikoff S, Pascolini D, Etya'ale D, Kocur I, Pararajasegaram R, Pokharel GP, et al. Global data on visual impairment in the year 2002. Bulletin of the World Health Organization 2004;82(11):844‐51. [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Scott 2007
- Scott IU, Edwards AR, Beck RW, Bressler NM, Chan CK, Elman MJ, et al. Diabetic Retinopathy Clinical Research Network. A phase II randomized clinical trial of intravitreal bevacizumab for diabetic macular edema. Ophthalmology 2007;114(10):1860‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sennlaub 2003
- Sennlaub F, Valamanesh F, Vazquez‐Tello A, El‐Asrar AM, Checchin D, Brault S, et al. Cyclooxygenase‐2 in human and experimental ischemic proliferative retinopathy. Circulation 2003;108(2):198‐204. [DOI] [PubMed] [Google Scholar]
Shima 2008
- Shima C, Sakaguchi H, Gomi F, Kamei M, Ikuno Y, Oshima Y, et al. Complications in patients after intravitreal injection of bevacizumab. Acta Ophthalmologica 2008;86(4):372‐6. [DOI] [PubMed] [Google Scholar]
Smith 2011
- Smith JM, Steel DHW. Anti‐vascular endothelial growth factor for prevention of postoperative vitreous cavity haemorrhage after vitrectomy for proliferative diabetic retinopathy. Cochrane Database of Systematic Reviews 2011, Issue 5. [DOI: 10.1002/14651858.CD008214.pub2] [DOI] [PubMed] [Google Scholar]
Spaide 2006
- Spaide RF, Fisher YL. Intravitreal bevacizumab (Avastin) treatment of proliferative diabetic retinopathy complicated by vitreous hemorrhage. Retina 2006;26(3):275‐8. [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D. Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
UKPDSG 1998a
- Anonymous. Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998;352(9131):837‐53. [PubMed] [Google Scholar]
UKPDSG 1998b
- Anonymous. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ 1998;317(7160):703‐13. [PMC free article] [PubMed] [Google Scholar]
Van Leiden 2002
- Leiden HA, Dekker JM, Moll AC, Nijpels G, Heine RJ, Bouter LM, et al. Blood pressure, lipids, and obesity are associated with retinopathy: the Hoorn study. Diabetes Care 2002;25(8):1320‐5. [DOI] [PubMed] [Google Scholar]
Van Leiden 2003
- Leiden HA, Dekker JM, Moll AC, Nijpels G, Heine RJ, Bouter LM, et al. Risk factors for incident retinopathy in a diabetic and nondiabetic population: the Hoorn study. Archives of Ophthalmology 2003;121(2):245‐51. [DOI] [PubMed] [Google Scholar]
Virgili 2012
- Virgili G, Parravano M, Menchini F, Brunetti M. Antiangiogenic therapy with anti‐vascular endothelial growth factor modalities for diabetic macular oedema. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.CD007419.pub3] [DOI] [PubMed] [Google Scholar]
Wilkinson 2003
- Wilkinson CP, Ferris FL 3rd, Klein RE, Lee PP, Agardh CD, Davis M, et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 2003;110(9):1677‐82. [DOI] [PubMed] [Google Scholar]
Wu 2008
- Wu L, Martínez‐Castellanos MA, Quiroz‐Mercado H, Arevalo JF, Berrocal MH, Farah ME, et al. Twelve‐month safety of intravitreal injections of bevacizumab (Avastin): results of the Pan‐American Collaborative Retina Study Group (PACORES). Graefe's Archive for Clinical and Experimental Ophthalmology 2008;246(1):81‐7. [DOI] [PubMed] [Google Scholar]
Zhang 2013
- Zhang ZH, Liu HY, Hernandez‐Da Mota SE, Romano MR, Falavarjani KG, Ahmadieh H, et al. Vitrectomy with or without preoperative intravitreal bevacizumab for proliferative diabetic retinopathy: a meta‐analysis of randomized controlled trials. American Journal of Ophthalmology 2013;156(1):106‐15. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Martinez‐Zapata 2010
- Martinez‐Zapata MJ, Martí‐Carvajal AJ, Solà I, Pijoán JI, Buil‐Calvo JA. Anti‐vascular endothelial growth factor for proliferative diabetic retinopathy (Protocol). Cochrane Database of Systematic Reviews 2010, Issue 9. [DOI: 10.1002/14651858.CD008721] [DOI] [PMC free article] [PubMed] [Google Scholar]


