Abstract
Background:
Methamphetamine abuse and human immunodeficiency virus (HIV) are common comorbidities. HIV-associated proteins, such as the regulatory protein TAT, may contribute to brain reward dysfunction, inducing an altered sensitivity to methamphetamine reward and/or withdrawal in this population.
Objective:
These studies examined the combined effects of TAT protein expression and, chronic and binge methamphetamine regimens on brain reward function.
Methods:
Transgenic mice with inducible brain expression of the TAT protein were exposed to either saline, a chronic, or a binge methamphetamine regimen. TAT expression was induced via doxycycline treatment during the last week of methamphetamine exposure. Brain reward function was assessed daily throughout the regimens, using the intracranial self-stimulation procedure, and after a subsequent acute methamphetamine challenge.
Results:
Both methamphetamine regimens induced withdrawal-related decreases in reward function. TAT expression substantially, but not significantly increased the withdrawal associated with exposure to the binge regimen compared to the chronic regimen, but did not alter the response to acute methamphetamine challenge. TAT expression also led to persistent changes in adenosine 2B receptor expression in the caudate putamen, regardless of methamphetamine exposure. These results suggest that TAT expression may differentially affect brain reward function, dependent on the pattern of methamphetamine exposure.
Conclusion:
However, the subtle effects observed in these studies highlight that longer-term TAT expression, or its induction at earlier stages of methamphetamine exposure, may be more consequential at inducing behavioral and neurochemical effects.
Keywords: Adenosine receptor, animal model, dopamine, TAT protein, behavior, self-stimulation
1. INTRODUCTION
The disproportionately higher levels of methamphetamine dependence among HIV+ individuals compared to the general population [1] are suggestive of altered sensitivity to methamphetamine reward and/or withdrawal in this population. However, human studies offer limitations at separating cause and effect of methamphetamine exposure and HIV neurological disease on brain reward function. Animal models that replicate important aspects of the HIV infection, particularly in the brain, allow us to investigate the neurobiological mechanisms underlying methamphetamine dependence in the context of HIV, and estimate mechanisms occurring in HIV+ humans under controlled laboratory conditions.
Mesocorticolimbic reward circuits are crucial for the initiation and maintenance of drug use [2] mediated by the dopaminergic system [3] and appear particularly vulnerable to both methamphetamine and HIV disease. HIV and methamphetamine target the dopamine system in the basal ganglia in a synergistic manner [4], suggesting this may be one common neural substrate for altered reward function after combined methamphetamine dependence and HIV disease [3]. Consistent with other neuropsychiatric disorders that feature dysregulated dopamine systems [5], HIV+ patients have a heightened sensitivity to dopamine selective drugs and to psychostimulants [6]. The neurotoxic mechanisms of the HIV disease involve the participation of viral products (e.g., gp120 or TAT) [7]. The TaT protein, for instance, is the first critically produced protein, with a role in regulating viral replication. It is produced by infected cells following the formation of the proviral DNA, even in the presence of antiretroviral drugs. In addition, it has unique ability to travel along neuronal pathways [8]. The TAT protein has been found in post-mortem brain tissue of patients with HIV-1 [9, 10]. TAT plays a key role in the dysfunction of the dopamine system associated with HIV disease, and when combined with methamphetamine, has both synergistic or additive effects in dopaminergic function [7, 11]. The synergistic effects of TAT and methamphetamine on dopamine transmission suggest that subjects with dual insults may have more severe dependence.
Animal models have provided strong evidence that HIV-associated proteins lead to alterations in response to methamphetamine. In mice that express gp120, preference toward, and incentive motivation of, methamphetamine is significantly increased [12]. The combination of gp120 and methamphetamine exposure also increases cognitive impairments, similar to those seen in HIV+ and HIV+ methamphetamine-dependent individuals [13]. On the other hand, in rats that express TAT, the combined exposure to a neurotoxic methamphetamine regimen produced a greater reduction in dopamine levels in the striatum during withdrawal [14, 15]. In inducible TAT transgenic mice, brain-restricted TAT expression becomes transcriptionally active after exposure to doxycycline, a tetracycline derivative [16]. Conditional TAT exposure in mice has been shown to induce cognitive deficits in a range of behavioural tasks [17, 18] and reversal learning appears particularly susceptible to TAT-induced impairments [19, 20]. In this model, TAT expression alters dopamine levels [19, 21] as well as the expression of the dopamine transporter in the brain [20]. Together, these changes may contribute to the observed reward deficits and increased sensitivity to methamphetamine-induced reward enhancement in the context of TAT expression [21]. Furthermore, exposure to TAT enhances methamphetamine sensitization, likely by direct alterations in dopamine receptor expression and increased recruitment of dopamine neurons in the mesolimbic reward system [22]. However, the effects of TAT expression on brain reward function during chronic and binge methamphetamine regimens have not been thoroughly investigated. These regimens more accurately reflect human use patterns. Based on previously demonstrated findings that TAT expression increases sensitivity to methamphetamine reward [21], we hypothesised here that mice expressing TAT conditionally in the brain also show increased sensitivity to acute and protracted methamphetamine withdrawal, following chronic and binge exposure regimens.
In the present study, we tested the effects of TAT expression on brain reward function during withdrawal from chronic and binge methamphetamine regimens. In addition, we investigated whether or not exposure to chronic and binge methamphetamine regimens subsequently altered the acute rewarding effect of methamphetamine challenge. TAT expression was induced with doxycycline administration during exposure to chronic and binge methamphetamine regimens designed to mimic methamphetamine use patterns in humans [23]. Brain reward function was assessed using the intracranial self-stimulation (ICSS) discrete trials procedure. In addition, because dopamine receptors and adenosine receptors (Adora) are co-expressed in the basal ganglia [24] and contribute to methamphetamine reward [25–28], levels of these receptors were quantified in the caudate putamen.
2. MATERIALS AND METHODS
2.1. Animals
A total of 48 male mice, with 22 mice containing the GFAP promoter-controlled Tet-binding protein (TAT−) and 26 mice containing both the GFAP promoter-controlled Tet-binding protein and the TRE promotor-TAT protein transgene (TAT+) were tested. Inducible TAT transgenic mouse colonies on a C57BL/6J background were obtained by the generation of two separate transgenic lines Teton-GFAP mice and TRE-Tat86 mice, and then cross-breeding of these two lines of transgenic mice as previously described [16]. The mice were housed in a humidity- and temperature-controlled animal facility on a 12 h/12 h reverse light/dark cycle (lights off at 7:00 AM), in groups of 2–4, and had ad libitum access to food and water. Behavioral testing was conducted during the dark phase of the light/dark cycle. All of the experiments were conducted in accordance with the guidelines of the American Association for the Accreditation of Laboratory Animal Care and National Research Council’s Guide for the Care and Use of Laboratory Animals and approved by the University of California San Diego Institutional Animal Care and Use Committee.
2.2. Methamphetamine Regimens and Doxycycline Exposure
The methamphetamine regimens have been described in detail in our prior publication [23]. Briefly, mice underwent subcutaneous injections of saline or methamphetamine (5 ml/kg, methamphetamine hydrochloride; Sigma, St. Louis, MO, USA; reported as base concentration) for four ‘cycles’ of 6 days (four injections/day; 12:00, 14:00, 16:00 and 18:00 h). The first cycle featured one week of dose-escalation followed by three repeated cycles of methamphetamine exposure. The chronic regimen cycles consisted of 6 days of moderate dosing (2 mg/kg/injection). The binge regimen cycles consisted of a two-day dose-escalation (3–5 mg/kg/injection) followed by two days of high dosing (6 mg/kg/injection). The doses for both the chronic and binge regimens were selected to fit models of total use per day/month in humans. All mice were treated with a doxycycline regimen (doxycycline hyclate; Sigma) of 100 mg/kg, intraperitoneally, once a day for 7 days. Doxycycline administration began the day before the last cycle of methamphetamine (day 22 of the regimens) and was paired with the first injection of the regimen (i.e., at 12:00).
2.3. Intracranial Self-stimulation
The discrete-trial, current-intensity threshold ICSS procedure was conducted as previously described [21, 23] (see Supplementary Methods). Each trial was initiated with a non-contingent stimulation and a response resulted in a second, identical contingent stimulation. By varying the current intensity level, we determined the minimal electrical current that elicits responding for the contingent stimulation, i.e., the reward threshold. Elevations in reward thresholds reflect decreased brain reward function (i.e., reward deficits or anhedonia). Lowering of thresholds reflect reward enhancement. The ICSS procedure also provides measures of response speed (latency), disinhibition/impulsivity (timeout responses), and vigor of responses (extra responses).
2.4. Real Time-PCR
RNA was reverse transcribed using SuperScript III Reverse Transcriptase (Invitrogen, Waltham, MA). Transcripts were evaluated with customized Qiagen RT2 Primers Assays (Valencia, CA), details can be found in Supplementary Methods. PCRs were performed using RT2 SYBR Green ROX FAST Mastermix (Qiagen), in a 7900HT Fast Real-Time PCR System with Fast 96-Well Block Module (Applied Biosystems, Foster City, CA) with a SDS Plate utility v2.2 software (Applied Biosystems). The results were normalized to the geometric mean of GAPDH and 18S housekeeping genes.
2.5. Experimental Design
Mice with bipolar stainless steel electrodes were trained in the ICSS procedure until stable reward thresholds were achieved (<10% standard deviation over three days; minimum 14 days of baseline testing). Mice were tested daily in the ICSS procedure (07:30–11:30h) prior to saline/methamphetamine administration (i.e., 12 h acute withdrawal). Then, mice were exposed to chronic or binge methamphetamine regiments. There were six experimental groups: TAT− saline (n=8), TAT− chronic (n=7) and TAT− binge (n=7), and TaT+ saline (n=9), TAT+ chronic (n=8) and TAT+ binge (n=9). After completion of methamphetamine regimens, mice underwent a six-day protracted withdrawal. Then, a dose-response function for acute methamphetamine (0, 0.2, 0.4 and 0.8 mg/kg, intraperitoneally, 10 ml/kg, 20 min before testing, three days apart) was assessed using a within-subject Latin square design (12 days). Six days after the final acute methamphetamine challenge (22 days after the final doxycycline treatment), brain samples were collected for real time-PCR (TAT− saline [n=6], TAT− chronic [n=7] and TAT− binge [n=7], and TAT+ saline [n=9], TAT+ chronic [n=7] and TAT+ binge [n=9]).
2.6. Statistical Analyses
All analyses were performed with IBM SPSS Statistics 20 (Armonk, NY, USA). Data were analyzed using the analysis of variance (ANOVA), with TAT and Regimen as the between-subject factors. Repeated-measures ANOVAs were used when within-subject factors were present. Data not meeting the assumption of homogeneity of variance were analyzed using Greenhouse-Geiser adjusted degrees of freedom. When appropriate, post hoc comparisons were performed using Least Significant Difference (LSD) analyses. Results are expressed as mean±SEM. Differences were considered statistically significant at p<0.05.
3. RESULTS
3.1. Daily Withdrawal Assessments
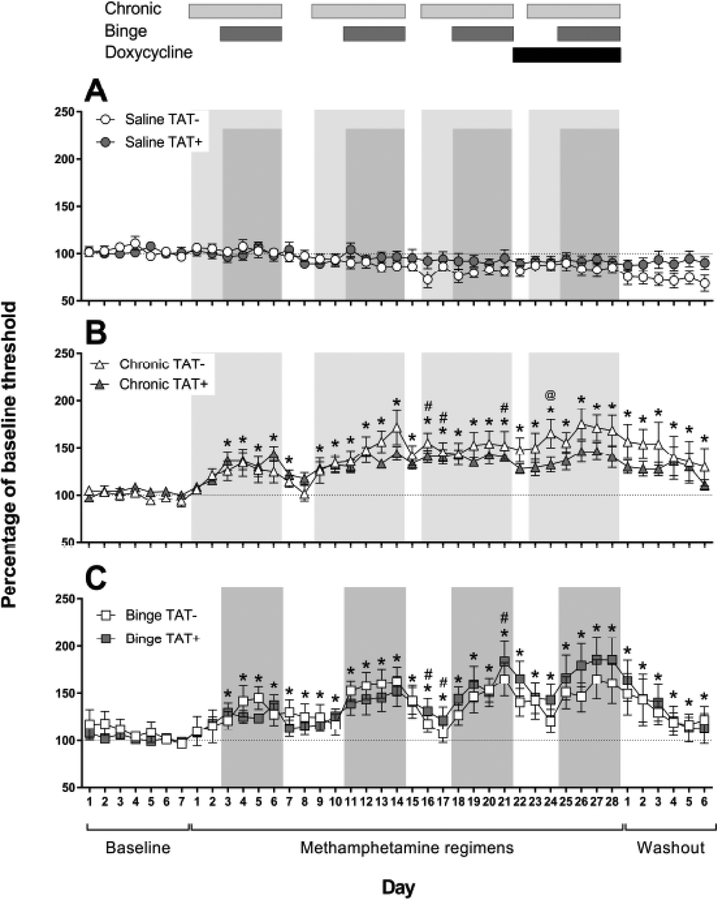
Reward thresholds data during the baseline, throughout the methamphetamine regimens and during protracted withdrawal (41 days in total) are presented in Fig. (1). Analyses revealed significant main effects of Day (F40,1160=8.9, p<0.001), Regimen (F2,29=9.7, p<0.001) and a significant interaction of Day × Regimen (F80,1160=3.6, p<0.001).
Fig. (1).
Reward thresholds in the intracranial self-stimulation procedure in TAT− and TAT+ mice administered saline (A), or chronic (B) and binge methamphetamine regimens (C). Reward thresholds are presented as a percentage of baseline threshold average (dotted lines) over the 5-days prior to beginning the methamphetamine regimens. Time points assessed 12 h after a day of methamphetamine administration are represented by the shaded regions with lighter gray for the chronic regimen and darker gray for the binge regimen. The days of doxycycline administration are shown by the black bar (top). Data are expressed as mean ± SEM. *p<0.05 compared with saline, #p<0.05 between chronic and binge regimens, @p<0.05 between TAT− and TAT+ mice.
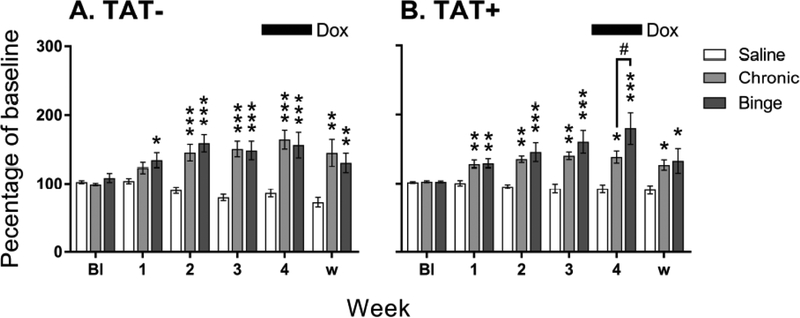
To account for a different number of days of methamphetamine administration in each regimen, we compared the average reward thresholds at the 12 h withdrawal time points during each methamphetamine cycle (4-day average for binge and 6-day average for chronic regimen; Fig. 2). There were significant main effects of Week (F5,210=17.5, p<0.001), Regimen (F2,42=21. 1, p<0.001) and a significant interaction of Week × Regimen (F10,210=10.6, p<0.001).
Fig. (2).
Reward thresholds during each cycle of methamphetamine exposure (average of 12 h withdrawal time points) in TAT− (A) and TAT+ (B) mice administered saline, or chronic and binge methamphetamine regimens. Both regimens increased reward thresholds compared with saline. During doxycycline treatment, TAT+ mice exposed to the binge regimen had higher reward thresholds than those exposed to the chronic regimen. Reward thresholds are presented as a percentage of baseline threshold average over the 5-days prior to beginning the methamphetamine regimens. The period of doxycycline (Dox) administration is shown by the black bar (top). Data are expressed as mean ± SEM. Bl, baseline; w, protracted withdrawal. *p<0.05, **p<0.01, ***p<0.001 compared with saline, #p<0.05 between chronic and binge regimens.
Post hoc comparisons after both analyses revealed that chronic (p<0.001) and binge (p<0.001) regimens led to significantly higher reward thresholds than saline exposure with a greater magnitude of threshold elevations during periods of acute withdrawal and protracted withdrawal (Fig. 2A, B). There was also a trend for the interaction of Week × Regimen × TAT (F10,210=1.9, p=0.051). Post hoc analyses indicated that this was likely due to a significant difference in threshold elevation between the chronic and binge regimen in TAT+ mice (Fig. 2B; p<0.05). During doxycycline treatment, TAT+ mice on the binge regimen had higher reward thresholds than those on the chronic regimen. There were no significant effects of TAT expression on response latency (Fig. S1), timeout responses (Fig. S2) or extra responses (Fig. S3).
3.2. Acute Methamphetamine Challenge
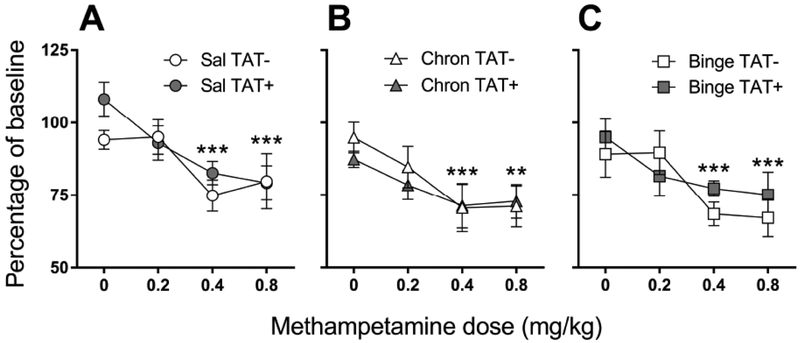
For reward thresholds after acute methamphetamine challenge (Fig. 3), there was a significant main effect of Dose (F3,126=20.6, p<0.001) with acute methamphetamine challenge lowering reward thresholds, as expected. Post hoc analyses revealed that reward thresholds after 0.2 mg/kg methamphetamine dose were significantly lower than 0 mg/kg methamphetamine (p<0.05). Moreover, both 0.4 and 0.8 mg/kg methamphetamine doses were significantly lower than both 0 and 0.2 mg/kg methamphetamine (p<0.001). There was a trend toward a main effect of Regimen (F2,42=3.2, p=0.051), with larger reward thresholds lowering after the chronic (p<0.05) and binge (p=0.054) regimens compared with saline exposure. For reward thresholds, response latency, extra responses and timeout responses, there were no significant main effects or interactions with TAT.
Fig. (3).
Reward thresholds in response to acute methamphetamine challenge in TAT− and TAT+ mice exposed to saline (A), or chronic (B) and binge (C) methamphetamine regimens. Reward thresholds are presented as a percentage of baseline threshold average over the 2-days prior to each day of methamphetamine treatment. Data are expressed as mean ± SEM. **p<0.01, ***p<0.001 compared with 0 mg/kg methamphetamine.
3.3. Caudate Putamen Dopamine and Adenosine Receptor Expression
There were no significant effects of methamphetamine regimen on dopamine receptors and Adora 1 or 2A receptors (Table 1). TAT expression significantly decreased Adora 2B receptors regardless of methamphetamine exposure (F1,39=5.6, p<0.05).
Table 1.
Dopamine and adenosine receptor expression (ddCT).
| Saline | Chronic | Binge | |||||
|---|---|---|---|---|---|---|---|
| Genotype | Mean | SEM | Mean | SEM | Mean | SEM | |
| Dopamine | |||||||
| DRD1 | TAT− | 11215 | 4353 | 8218 | 3947 | 12616 | 3459 |
| TAT+ | 9155 | 2410 | 8598 | 3095 | 7760 | 1734 | |
| DRD2 | TAT− | 13841 | 4239 | 8453 | 3085 | 13772 | 3919 |
| TAT+ | 9199 | 1881 | 8959 | 3319 | 10140 | 2157 | |
| DRD4 | TAT− | 12337 | 5112 | 6145 | 3367 | 11400 | 4275 |
| TAT+ | 6238 | 2800 | 10941 | 7099 | 9543 | 2663 | |
| DRD5 | TAT− | 5209 | 2357 | 3471 | 1674 | 5982 | 2139 |
| TAT+ | 3262 | 1295 | 3081 | 1464 | 3659 | 994 | |
| Adora | |||||||
| Adora1 | TAT− | 387298 | 58193 | 438305 | 69251 | 364586 | 84148 |
| TAT+ | 412789 | 56186 | 328508 | 68619 | 318285 | 56477 | |
| Adora2A | TAT− | 298384 | 92072 | 208370 | 57556 | 337294 | 117657 |
| TAT+ | 217097 | 35786 | 175077 | 58387 | 182760 | 27712 | |
| Adora2B* | TAT− | 139162 | 51397 | 77609 | 39191 | 144785 | 51227 |
| TAT+ | 73319 | 21380 | 41309 | 19102 | 56025 | 9990 | |
DRD, dopamine receptor; Adora, adenosine receptor.
significant main effect of TAT expression in the ANOVA.
4. DISCUSSION
The current study assessed the effects of chronic and binge methamphetamine regimens and their interaction with the HIV TAT protein on brain reward function in transgenic mice. Chronic and binge exposure to methamphetamine significantly elevated reward thresholds during acute (12h) and protracted withdrawal, regardless of TAT expression. Acute methamphetamine challenge lowered reward threshold in all mice, however, a trend toward a larger threshold lowering was observed in mice exposed to chronic or binge methamphetamine regimens. TAT expression during the final cycle of methamphetamine exposure did not impact brain reward function during withdrawal. Nevertheless, there was a trend that TAT expression may contribute to an increased severity of withdrawal associated with exposure to the binge regimen compared with the chronic regimen. TAT expression also led to persistent changes in ADORA 2B receptor expression in the caudate putamen. Taken together, these data suggest a subtle effect of TAT expression on the response to methamphetamine.
We designed chronic and binge regimens to reflect methamphetamine use patterns commonly observed in methamphetamine-dependent individuals from reports in clinical studies [23, 29–34]. We previously demonstrated that both regimens increased reward thresholds in mice, but that the binge regimen led to a larger increase in reward thresholds when compared to the chronic regimen, particularly during the first cycle of administration [23]. These previously reported results were replicated in TAT− mice, with a slightly larger increase in reward threshold elevation during the first cycle of the binge regimen, compared with the chronic regimen. The data replicability indicate that both the chronic and binge methamphetamine regimens produce stable and consistent effects on brain reward function in mice.
In the present experimental protocol, the effects of TAT expression were subtle. We have previously shown that TAT alone elevated brain reward thresholds during induction by doxycycline administration, and for at least one week afterwards [21]. In the present study, we did not observe this effect. However, the regimen of administration in that study differed, due to extensive handling of mice and the administration of 72 injections over three weeks, prior to doxycycline induction. Considering that behavioral phenotypes are very susceptible to handling conditions [35], one possible explanation for the difference is that injection-associated stress exacerbated TAT-induced elevations in reward thresholds in our prior study. There is evidence supporting an interaction between stress and HIV on brain function. For example, in people with HIV, greater impairments in cognitive function have been associated with increased stress, and increased stress was associated with greater declines in activities of daily living in HIV+ individuals but not in those without HIV [36]. Interestingly, we did observe a differential effect of chronic and binge methamphetamine regimens on reward thresholds in TAT+ mice during doxycycline administration. Specifically, TAT+ mice exposed to the binge regimen had greater reward threshold elevations compared to TAT+ mice exposed to the chronic regimen, whereas there was no effect of methamphetamine in TAT− mice. This differential effect may be attributed to a combined decrease in mesolimbic dopamine levels induced by the binge regimen [23] and TAT expression [21]. During the protracted withdrawal, reward thresholds were similar in TAT+ mice exposed to either methamphetamine regimen. The absence of persisting effects is most likely attributed to TAT-induced deficits in mesolimbic dopamine levels returning to baseline after cessation of doxycycline administration [21]. These temporally restricted effects of TAT expression may also explain the absence of effects on reward thresholds after acute methamphetamine challenge, observed in the present study.
Overall, adenosine receptor expression in the caudate putamen was largely unaffected by the methamphetamine regimens and/or TAT expression. However, there was a significant decrease in Adora 2B receptors associated with TAT expression. The role of the Adora 2B receptor in the brain is not well understood [37] but it has been shown that it plays an important role in innate immunity [38]. Thus, TAT-induced decreases in Adora 2B receptors may reflect altered immunomodulatory responses to TAT expression. Our previous work in the caudate putamen found no consistent change in Adora 2B gene expression attributed to TAT expression, although this was assessed using a sensitization paradigm, and one week after doxycycline administration [22]. The differences between these studies may reflect a delayed effect on Adora receptor expression after cessation of TAT expression.
We have also previously found decreases in dopamine receptor protein levels in the caudate putamen one week after doxycycline administration [22]. It is important to note that TAT expression in this mouse model persists for up to 2 weeks after the last doxycycline injection [39]. The present study assessed the transcription of dopamine receptor subtypes after three weeks following the completion of doxycycline administration. Therefore, changes in dopamine receptor expression may result from the TAT protein expression and direct effects induced by TAT on molecules of the dopamine system, including alterations in the dopamine transporter and vesicular monoamine transporter-2 [20, 40, 41].
CONCLUSION
TAT exposure, induced after methamphetamine exposure, had little effect on reward function or on the subsequent response to acute methamphetamine challenge. Initiating TAT protein expression at different stages of the regimens (i.e., during the first or second cycle) may induce greater behavioural and neurochemical outcomes. Future studies should also consider prolonged doxycycline administration during methamphetamine regimens, perhaps via dietary administration.
Supplementary Material
FUNDING
JPK was supported by an Advance Queensland Research Fellowship (AQRF04115-16RD1) and by the Peter F. McManus Charitable Trust research grant for Drug Abuse. MCGM was supported by NIH NIDA R01DA036164 and R21AG054240. JPK and SS were also supported by the Translational Methamphetamine AIDS Research Center funded by the National Institute on Drug Abuse (P50DA26306).
Footnotes
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the University of California San Diego Institutional Animal Care and Use Committee.
HUMAN AND ANIMAL RIGHTS
No humans were used in this research. All animals research procedures were in accordance with the guidelines of the American Association for the Accreditation of Laboratory Animal Care and National Research Council’s Guide for the Care and Use of Laboratory Animals and approved by the University of California San Diego Institutional Animal Care and Use Committee.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The dataset that support the results and findings of this research are available from JPK, upon request.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publisher’s web site along with the published article.
Publisher's Disclaimer: DISCLAIMER: The above article has been published in Epub (ahead of print) on the basis of the materials provided by the author. The Editorial Department reserves the right to make minor modifications for further improvement of the manuscript.
REFERENCES
- [1].Soontornniyomkij V, Kesby JP, Morgan EE, et al. Effects of HIV and Methamphetamine on Brain and Behavior: Evidence from Human Studies and Animal Models. J Neuroimmune Pharmacol 2016; 11(3): 495–510. [ 10.1007/s11481-016-9699-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology 2010; 35(1): 217–38. [ 10.1038/npp.2009.110] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wise RA. Dopamine and reward: the anhedonia hypothesis 30 years on. Neurotox Res 2008; 14(2–3): 169–83. [ 10.1007/BF03033808] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Theodore S, Cass WA, Nath A, Maragos WF. Progress in understanding basal ganglia dysfunction as a common target for methamphetamine abuse and HIV-1 neurodegeneration. Curr HIV Res 2007; 5(3): 301–13. [ 10.2174/157016207780636515] [DOI] [PubMed] [Google Scholar]
- [5].Kesby JP, Eyles DW, McGrath JJ, Scott JG. Dopamine, psychosis and schizophrenia: the widening gap between basic and clinical neuroscience. Transl Psychiatry 2018; 8(1): 30 [ 10.1038/s41398-017-0071-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Nath A, Anderson C, Jones M, et al. Neurotoxicity and dysfunction of dopaminergic systems associated with AIDS dementia. J Psychopharmacol (Oxford) 2000; 14(3): 222–7. [ 10.1177/026988110001400305] [DOI] [PubMed] [Google Scholar]
- [7].Ferris MJ, Mactutus CF, Booze RM. Neurotoxic profiles of HIV, psychostimulant drugs of abuse, and their concerted effect on the brain: current status of dopamine system vulnerability in NeuroAIDS. Neurosci Biobehav Rev 2008; 32(5): 883–909. [ 10.1016/j.neubiorev.2008.01.004] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Li W, Li G, Steiner J, Nath A. Role of Tat protein in HIV neuropathogenesis. Neurotox Res 2009; 16(3): 205–20. [ 10.1007/s12640-009-9047-8] [DOI] [PubMed] [Google Scholar]
- [9].Hudson L, Liu J, Nath A, et al. Detection of the human immunodeficiency virus regulatory protein tat in CNS tissues. J Neurovirol 2000; 6(2): 145–55. [ 10.3109/13550280009013158] [DOI] [PubMed] [Google Scholar]
- [10].Parmentier HK, van Wichen DF, Meyling FH, Goudsmit J, Schuurman HJ. Epitopes of human immunodeficiency virus regulatory proteins tat, nef, and rev are expressed in normal human tissue. Am J Pathol 1992; 141(5): 1209–16. [PMC free article] [PubMed] [Google Scholar]
- [11].Silverstein PS, Shah A, Gupte R, et al. Methamphetamine toxicity and its implications during HIV-1 infection. J Neurovirol 2011; 17(5): 401–15. [ 10.1007/s13365-011-0043-4] [PMID: 21786077] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kesby JP, Hubbard DT, Markou A, Semenova S. Expression of HIV gp120 protein increases sensitivity to the rewarding properties of methamphetamine in mice. Addict Biol 2014; 19(4): 593–605. [ 10.1111/adb.12023] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kesby JP, Heaton RK, Young JW, et al. Methamphetamine Exposure Combined with HIV-1 Disease or gp120 Expression: Comparison of Learning and Executive Functions in Humans and Mice. Neuropsychopharmacology 2015; 40(8): 1899–909. [ 10.1038/npp.2015.39] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Maragos WF, Young KL, Turchan JT, et al. Human immunodeficiency virus-1 Tat protein and methamphetamine interact synergistically to impair striatal dopaminergic function. J Neurochem 2002; 83(4): 955–63. [ 10.1046/j.1471-4159.2002.01212.x] [DOI] [PubMed] [Google Scholar]
- [15].Theodore S, Cass WA, Maragos WF. Methamphetamine and human immunodeficiency virus protein Tat synergize to destroy dopaminergic terminals in the rat striatum. Neuroscience 2006; 137(3): 925–35. [ 10.1016/j.neuroscience.2005.10.056] [DOI] [PubMed] [Google Scholar]
- [16].Kim BO, Liu Y, Ruan Y, Xu ZC, Schantz L, He JJ. Neuropathologies in transgenic mice expressing human immunodeficiency virus type 1 Tat protein under the regulation of the astrocyte-specific glial fibrillary acidic protein promoter and doxycycline. Am J Pathol 2003; 162(5): 1693–707. [ 10.1016/S0002-9440(10)64304-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Carey AN, Sypek EI, Singh HD, Kaufman MJ, McLaughlin JP. Expression of HIV-Tat protein is associated with learning and memory deficits in the mouse. Behav Brain Res 2012; 229(1): 48–56. [ 10.1016/j.bbr.2011.12.019] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Fitting S, Ignatowska-Jankowska BM, Bull C, et al. Synaptic dysfunction in the hippocampus accompanies learning and memory deficits in human immunodeficiency virus type-1 Tat transgenic mice. Biol Psychiatry 2013; 73(5): 443–53. [ 10.1016/j.biopsych.2012.09.026] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kesby JP, Markou A, Semenova S. Effects of HIV/TAT protein expression and chronic selegiline treatment on spatial memory, reversal learning and neurotransmitter levels in mice. Behav Brain Res 2016; 311: 131–40. [ 10.1016/j.bbr.2016.05.034] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kesby JP, Fields JA, Chang A, Coban H, Achim CL, Semenova S. Effects of HIV-1 TAT protein and methamphetamine exposure on visual discrimination and executive function in mice. Behav Brain Res 2018; 349: 73–9. [http://dx.doi.org/10.1016Zj.bbr.2018.04.046] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kesby JP, Markou A, Semenova S. The effects of HIV-1 regulatory TAT protein expression on brain reward function, response to psychostimulants and delay-dependent memory in mice. Neuropharmacology 2016; 109: 205–15. [ 10.1016/j.neuropharm.2016.06.011] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kesby JP, Najera JA, Romoli B, et al. HIV-1 TAT protein enhances sensitization to methamphetamine by affecting dopaminergic function. Brain Behav Immun 2017; 65(65): 210–21. [ 10.1016/j.bbi.2017.05.004] [PMID: 28495611] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kesby JP, Chang A, Markou A, Semenova S. Modeling human methamphetamine use patterns in mice: chronic and binge methamphetamine exposure, reward function and neurochemistry. Addict Biol 2018; 23(1): 206–18. [ 10.1111/adb.12502] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ferré S, Fredholm BB, Morelli M, Popoli P, Fuxe K. Adenosine-dopamine receptor-receptor interactions as an integrative mechanism in the basal ganglia. Trends Neurosci 1997; 20(10): 482–7. [ 10.1016/S0166-2236(97)01096-5] [DOI] [PubMed] [Google Scholar]
- [25].Chesworth R, Brown RM, Kim JH, Ledent C, Lawrence AJ. Adenosine 2A receptors modulate reward behaviours for methamphetamine. Addict Biol 2016; 21(2): 407–21. [ 10.1111/adb.12225] [DOI] [PubMed] [Google Scholar]
- [26].Kavanagh KA, Schreiner DC, Levis SC, O’Neill CE, Bachtell RK. Role of adenosine receptor subtypes in methamphetamine reward and reinforcement. Neuropharmacology 2015; 89: 265–73. [ 10.1016/j.neuropharm.2014.09.030] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Shimazoe T, Yoshimatsu A, Kawashimo A, Watanabe S. Roles of adenosine A(1) and A(2A) receptors in the expression and development of methamphetamine-induced sensitization. Eur J Pharmacol 2000; 388(3): 249–54. [ 10.1016/S0014-2999(99)00899-7] [DOI] [PubMed] [Google Scholar]
- [28].Pierce RC, Kalivas PW. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Brain Res Rev 1997; 25(2): 192–216. [ 10.1016/S0165-0173(97)00021-0] [DOI] [PubMed] [Google Scholar]
- [29].Brecht ML, O’Brien A, von Mayrhauser C, Anglin MD. Methamphetamine use behaviors and gender differences. Addict Behav 2004; 29(1): 89–106. [ 10.1016/S0306-4603(03)00082-0] [DOI] [PubMed] [Google Scholar]
- [30].Cheng WS, Garfein RS, Semple SJ, Strathdee SA, Zians JK, Patterson TL. Binge use and sex and drug use behaviors among HIV(−), heterosexual methamphetamine users in San Diego. Subst Use Misuse 2010; 45(1–2): 116–33. [ 10.3109/10826080902869620] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Cho AK, Melega WP. Patterns of methamphetamine abuse and their consequences. J Addict Dis 2002; 21(1): 21–34. [ 10.1300/J069v21n01_03] [DOI] [PubMed] [Google Scholar]
- [32].Semple SJ, Patterson TL, Grant I. Binge use of methamphetamine among HIV-positive men who have sex with men: pilot data and HIV prevention implications. AIDS Educ Prev 2003; 15(2): 133–47. [ 10.1521/aeap.153.133.23835] [DOI] [PubMed] [Google Scholar]
- [33].Simon SL, Richardson K, Dacey J, et al. A comparison of patterns of methamphetamine and cocaine use. J Addict Dis 2002; 21(1): 35–44. [ 10.1300/J069v21n01_04] [DOI] [PubMed] [Google Scholar]
- [34].Sommers I, Baskin D, Baskin-Sommers A. Methamphetamine use among young adults: health and social consequences. Addict Behav 2006; 31(8): 1469–76. [ 10.1016/j.addbeh.2005.10.004] [DOI] [PubMed] [Google Scholar]
- [35].Burne TH, O’Loan J, McGrath JJ, Eyles DW. Hyperlocomotion associated with transient prenatal vitamin D deficiency is ameliorated by acute restraint. Behav Brain Res 2006; 174(1): 119–24. [ 10.1016/j.bbr.2006.07.015] [DOI] [PubMed] [Google Scholar]
- [36].Watson CW, Sundermann EE, Hussain MA, et al. Effects of trauma, economic hardship, and stress on neurocognition and everyday function in HIV. Health Psychol 2019; 38(1): 33–42. [ 10.1037/hea0000688] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ballesteros-Yáñez I, Castillo CA, Merighi S, Gessi S. The Role of Adenosine Receptors in Psychostimulant Addiction. Front Pharmacol 2018; 8: 985 [ 10.3389/fphar.2017.00985] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kumar V, Sharma A. Adenosine: an endogenous modulator of innate immune system with therapeutic potential. Eur J Pharmacol 2009; 616(1–3): 7–15. [ 10.1016/j.ejphar.2009.05.005] [DOI] [PubMed] [Google Scholar]
- [39].Paris JJ, Singh HD, Ganno ML, Jackson P, McLaughlin JP. Anxiety-like behavior of mice produced by conditional central expression of the HIV-1 regulatory protein, Tat. Psychopharmacology (Berl) 2014; 231(11): 2349–60. [ 10.1007/s00213-013-3385-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Theodore S, Cass WA, Dwoskin LP, Maragos WF. HIV-1 protein Tat inhibits vesicular monoamine transporter-2 activity in rat striatum. Synapse 2012; 66(8): 755–7. [ 10.1002/syn.21564] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Midde NM, Gomez AM, Zhu J. HIV-1 Tat protein decreases dopamine transporter cell surface expression and vesicular monoamine transporter-2 function in rat striatal synaptosomes. J Neuroimmune Pharmacol 2012; 7(3): 629–39. [ 10.1007/s11481-012-9369-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.