Abstract
It has become clear that sodium glucose cotransporter (SGLT)-2 inhibitors not only do not increase the incidence of cardiovascular events but they also reduce the duration of hospitalization for heart failure in type 2 diabetes mellitus (T2DM) patients. The administration of SGLT2 inhibitor in T2DM patients with hypertension and a fluid retention tendency lowers the blood pressure and mitigates fluid retention. It also reduces the heart rate in T2DM patients with a fast heart rate. As an explanation for the multifaceted effects of SGLT2 inhibitors on hemodynamics, we hypothesize that these agents act on the inter-organ communication pathway, which modulates the sympathetic nerve activity to the cardiovascular system.
Keywords: SGLT2 inhibitor, heart failure, inter-organ communication, erythropoietin, hematocrit
Heart Failure is an Overlooked Complication of Diabetes
The goal of diabetes treatment is to prevent the onset and exacerbation of complications characteristic of diabetes in order to maintain the same quality of life (QOL) and enjoy the same lifespan as an otherwise healthy person. To achieve this goal, the importance of preventing the onset and exacerbation of microangiopathy (retinopathy, nephropathy, neuropathy) and macroangiopathy (myocardial infarction, stroke, arteriosclerosis obliterans) has been emphasized. In this goal, however, heart failure is often overlooked despite it being a more frequent complication and more adversely affecting the prognosis and QOL than either myocardial infarction or stroke.
According to the CVD-REAL 2 study (1), the incidence of heart failure in Japanese real-world diabetic practice is 4-5 cases per 1,000 persons per year. This incidence is higher than myocardial infarction (1 case) and cerebral infarction (2 cases). Once a diabetic patient develops heart failure, the 5-year survival rate is only 20%, an extremely poor prognosis. In addition, because patients with heart failure undergo repeated hospital admissions until they eventually die, their QOL is significantly impaired. Therefore, diabetes treatment with heart failure risk in mind is now beginning to be demanded.
The American College of Cardiology/American Heart Association AHA classifies heart failure into four stages: Stage A, patients at risk for heart failure who have not yet developed structural heart changes (i.e., those with diabetes, those with coronary disease without prior infarct); Stage B, patients with structural heart disease (i.e., reduced ejection fraction, left ventricular hypertrophy, chamber enlargement) who have not yet developed symptoms of heart failure; Stage C, patients who have developed clinical heart failure; Stage D, patients with refractory heart failure requiring advanced intervention (i.e., biventricular pacemakers, left ventricular assist device, transplantation). The progress of the stage is unidirectional and never reverts; once symptoms develop, patients are classified as class C and never return to stage B.
Patients with a diabetes duration >10 years often have organic diseases and/or functional abnormalities in the heart. These patients can therefore be considered to be in stage B. In addition, coexisting hemodynamic overload, such as hypertension, tachycardia, and tendency to fluid retention, place continual stress on the heart. Under such circumstances, the adaptive mechanism of the cardiac pump function eventually fails; dyspnea, fatigue and edema then appear, and the exercise tolerance consequently decreases. Because the prognosis becomes as poor as or worse than with advanced cancer once heart failure develops, it is important to prevent the transition from stage B to stage C through multidisciplinary management.
Afferent Signaling from the Kidney Activates the Vasomotor Center in the Brain
Catheter-based renal denervation (RDN), which induces denervation by simultaneously cauterizing both efferent renal sympathetic nerves and afferent renal sensory nerve for intractable hypertension, has been proven safe and effective since its development (2). Hypertension has many causes, but increased sympathetic nerve activity to the cardiovascular system is considered important. The sympathetic nerve activity to cardiovascular system increases when the neurons of the rostral ventrolateral medulla (RVLM), a vasomotor center, are excited. Sympathetic signals increase the blood pressure by increasing the heart rate, constricting arteries, and-in the kidney-increasing the renin release and sodium and water retention. The kidney, in response to stress, such as ischemia, sends afferent signals to the brain, exciting the RVLM. The decrease in the muscle sympathetic nerve activity and blood pressure after catheter-based RDN are conclusive proof of the substantial contribution of afferent renal nerve signaling as input to the RVLM (3). Sympathetic nerve activity is known to be elevated in diabetic patients, as is noted in hypertensive patients. It is possible that the accumulation of stress in the kidney triggers the activation of the sympathetic nerves innervating the cardiovascular system in type 2 diabetes mellitus (T2DM).
Paradoxical Increase in Glucose Reabsorption in the Context of Hyperglycemia
Sodium glucose cotransporter (SGLT) 2 inhibitors have emerged as a new hypoglycemic agent. Since glucose is a valuable energy source, it is reabsorbed in the renal proximal tubule after filtration in the glomerulus and is not normally excreted through the urine. Of the glucose reabsorption in the renal proximal tubule, 90% is due to the action of SGLT2. SGLT2 inhibitors are drugs that improve hyperglycemia by inhibiting the action of SGLT2, thereby suppressing glucose reabsorption in the renal proximal tubule and inducing glucose excretion into the urine. In patients with T2DM, as the expression of SGLT2 increases, the amount of glucose reabsorption increases, reaching levels greater than in healthy individuals. Despite being in a hyperglycemic state, this phenomenon of increasing glucose reabsorption is seemingly contradictory. This contradiction can be explained in relation to the process of evolution. During past eras, humans have always been exposed to hunger. To adapt to hunger, human acquired the ability to increase glucose reabsorption in response to elevated blood sugar levels when humans finally have a meal. This positive feedback system works advantageously for ensuring that humans survive bouts of starvation. However, in the setting of relatively constant satiation, it not only exacerbates hyperglycemia but also continues to chronically load the kidneys.
SGLT2 Inhibitors Alleviate Metabolic Stress in the Proximal Renal Tubular Epithelial Cells
In order for the proximal tubular epithelial cells to resorb glucose via SGLT2 localized at the brush border membrane, the Na+/K+ pump (Na+/K+-ATPase) localized at the basolateral membrane must be driven. This means that the reabsorption of glucose is carried out through the consumption of energy.
As mentioned above, in diabetic patients, more glucose is reabsorbed than in healthy persons, and a vicious cycle keeps blood sugar levels high. Under such circumstances, mitochondria in the renal proximal tubular epithelial cells consume large amounts of oxygen in order to keep producing large amounts of ATP. Indeed, in the renal cortex of hyperglycemic rats, the oxygen consumption increases, and the tissue oxygen partial pressure decreases (4). In a hyperglycemic state, mitochondria in renal proximal tubular epithelial cells are subjected to harsh metabolic stress. If SGLT2 is blocked in this state, the oxygen consumption is reduced, and the tissue oxygen partial pressure recovers, resulting in the alleviation of the metabolic stress in the renal proximal tubular epithelial cells under chronic hyperglycemic conditions. The elevated hematocrit (Hct) value characteristically observed after the administration of SGLT2 inhibitors is believed to be a sign that metabolic stress has been released (5-8).
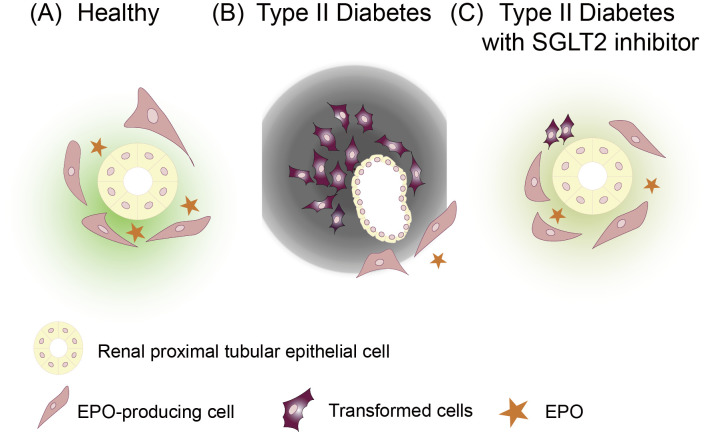
Erythropoietin (EPO) in the kidney is produced by fibroblasts around the proximal tubule. Under conditions of kidney disease, the inflammatory microenvironment transforms these EPO-producing cells. The research group of Prof. Masayuki Yamamoto found that kidney fibrosis is primarily mediated by transformed EPO-producing cells in experiments using genetically modified animals (9). Transformed EPO-producing cells lost their ability to produce EPO and instead began to secrete large amounts of extracellular matrix proteins, such as collagen. Of note, this change was found to be reversible to some extent. By improving the microenvironment, the transformed cells were able to recover to a normal state, regaining their EPO productivity. Subsequently, the research group of Prof. Motoko Yanagita reported that the development of an inflammatory microenvironment and the subsequent transformation of EPO-producing cells were induced by the selective damage of renal proximal tubular epithelial cells (10,11).
In diabetic patients with hyperglycemia, the blood EPO concentration tends to decrease (12). The higher the HbA1c level, the lower the EPO concentration. When SGLT2 inhibitors are administered to diabetic patients, the EPO concentration rises, and erythropoiesis is enhanced (13). Given the above findings, several hypotheses can be made (Fig. 1). In the chronic hyperglycemic state of diabetic patients, metabolic stress is loaded to renal proximal tubular epithelial cells. Stressed tubular epithelial cells then secrete proinflammatory cytokines, deteriorating the microenvironment of the interstitium and thereby resulting in the transformation of EPO-producing cells and reduction in their EPO productivity. SGLT2 inhibitor improves the microenvironment by alleviating metabolic stress in renal proximal tubular epithelial cells. Consequently, the transformed cells are reverse-transformed, regaining the phenotype of a normal EPO-producing cell (5,8). In the clinical setting, we can observe this phenomenon as the increase in the Hct value after the administration of an SGLT2 inhibitor.
Figure 1.
Transformed cells in T2DM patients are reverse-transformed by SGLT2 inhibitors, subsequently regaining the phenotype of normal EPO-producing cells. (A) EPO-producing cells are localized around the proximal tubule in the renal cortex. (B) The renal proximal tubular epithelial cells in T2DM patients are exhausted by the task of relentless excess sugar reabsorption. With the increase in the oxygen consumption by renal proximal tubular epithelial cells, the stroma becomes hypoxic, and an inflammatory microenvironment sets in. The EPO-producing cells undergo transformation, lose their ability to produce EPO, and instead secrete a large amount of extracellular matrix proteins, such as collagen. (C) SGLT2 inhibitors improve the interstitial microenvironment by reducing the oxygen consumption of renal proximal tubular epithelial cells. Transformed cells in T2DM patients are reverse-transformed by SGLT2 inhibitors, subsequently regaining their EPO-producing ability. T2DM: type 2 diabetes mellitus, SGLT2: sodium glucose cotransporter 2, EPO: erythropoietin
Inter-organ Communication Pathway Created by Non-physiological Stress to the Kidney in Type II Diabetic Patients
Patients with T2DM often have hemodynamic abnormalities such as hypertension, tachycardia, and excessive fluid retention. Since the point of hemodynamic homeostasis is altered in T2DM patients compared with healthy persons, it is postulated that there is an organ interaction mechanism that manifests as a result of unexpected biological distortion created by a sustained hyperglycemic state. These changes in hemodynamics continue to exert hemodynamic overload on the heart of T2DM patients, consequently increasing the risk of developing heart failure. Interestingly, SGLT2 inhibitors counteract high blood pressure, tachycardia, and fluid retention. Consistent with this, large-scale clinical trials have shown that SGLT2 inhibitors suppress hospitalization for heart failure (14-16). Based on these observations, SGLT2 inhibitor is thought to return the set point of hemodynamic homeostasis to the normal state by modifying the inter-organ communication pathway.
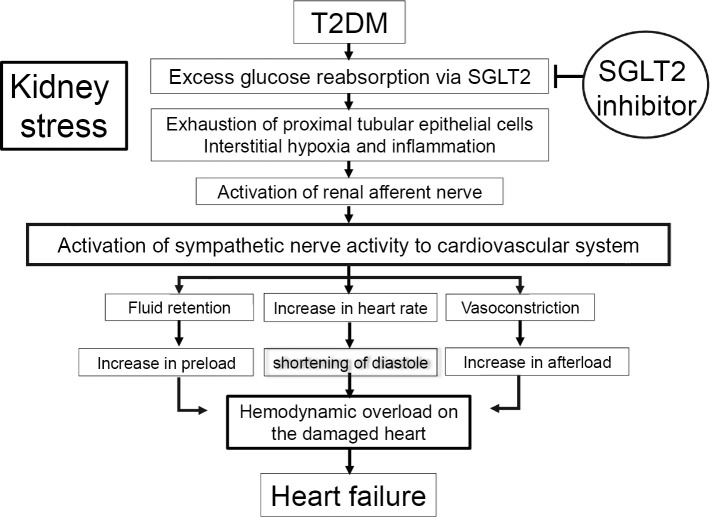
Metabolic stress due to excessive reabsorption of glucose in the kidney is transmitted to the vasomotor centers of the brain via renal afferent sensory neurons, resulting in an increased sympathetic output to the cardiovascular system (Fig. 2). This triggers a change in the hemodynamic setpoint (hypertension, tachycardia, excessive fluid retention). SGLT2 inhibitors ameliorate excessive sympathetic nervous activity by suppressing afferent signaling from renal sensory nerves through the improvement of metabolic stress in the kidney. As evidence, SGLT2 inhibitors reduce the heart rate in T2DM patients with a fast heart rate (17).
Figure 2.
Mechanism of heart failure onset in diabetes. The risk of heart failure in T2DM patients is high because of organic and functional abnormalities in the heart. The chronic hemodynamic load on the damaged heart induces heart failure. In the short term, SGLT2 inhibitors reduce the hemodynamic load imposed on the heart by suppressing excessive activation of the sympathetic nervous system, thereby suppressing the onset of heart failure. T2DM: type 2 diabetes mellitus, SGLT2: sodium glucose cotransporter 2
Based on these observations, I propose that the cardiovascular protective effect of SGLT2 inhibitors is exerted by a mechanism common to RDN. It may also be superior to RDN, as the suppression of both efferent renal sympathetic nerves and afferent renal sensory nerves is reversible.
Author's disclosure of potential Conflicts of Interest (COI).
Motoaki Sano: Honoraria, Boehringer Ingelheim, Mitsubishi Tanabe, Daiichi Sankyo, AstraZeneca, Ono, Taisho Toyama, Novartis, Astellas, MSD and Kowa.
References
- 1.Kosiborod M, Lam CSP, Kohsaka S, et al. ; CVD-REAL Investigators and Study Group. Cardiovascular events associated with SGLT-2 inhibitors versus other glucose-lowering drugs: the CVD-REAL 2 study. J Am Coll Cardiol 71: 2628-2639, 2018. [DOI] [PubMed] [Google Scholar]
- 2.Townsend RR, Mahfoud F, Kandzari DE, et al. ; SPYRAL HTN-OFF MED trial investigators. Catheter-based renal denervation in patients with uncontrolled hypertension in the absence of antihypertensive medications (SPYRAL HTN-OFF MED): a randomised, sham-controlled, proof-of-concept trial. Lancet 390: 2160-2170, 2017. [DOI] [PubMed] [Google Scholar]
- 3.Hering D, Marusic P, Walton AS, et al. . Sustained sympathetic and blood pressure reduction 1 year after renal denervation in patients with resistant hypertension. Hypertension 64: 118-124, 2014. [DOI] [PubMed] [Google Scholar]
- 4.O'Neill J, Fasching A, Pihl L, Patinha D, Franzén S, Palm F. Acute SGLT inhibition normalizes O2 tension in the renal cortex but causes hypoxia in the renal medulla in anaesthetized control and diabetic rats. Am J Physiol Renal Physiol 309: F227-F234, 2015. [DOI] [PubMed] [Google Scholar]
- 5.Sano M, Takei M, Shiraishi Y, Suzuki Y. Increased Hematocrit During Sodium-Glucose Cotransporter 2 Inhibitor Therapy Indicates Recovery of Tubulointerstitial Function in Diabetic Kidneys. J Clin Med Res 8: 844-847, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sano M. Hemodynamic effects of sodium-glucose cotransporter 2 inhibitors. J Clin Med Res 9: 457-460, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sano M. A new class of drugs for heart failure: SGLT2 inhibitors reduce sympathetic overactivity. J Cardiol 71: 471-476, 2018. [DOI] [PubMed] [Google Scholar]
- 8.Sano M, Goto S. Circulation (in press). [Google Scholar]
- 9.Souma T, Yamazaki S, Moriguchi T, et al. . Plasticity of renal erythropoietin-producing cells governs fibrosis. J Am Soc Nephrol 24: 1599-1616, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asada N, Takase M, Nakamura J, et al. . Dysfunction of fibroblasts of extrarenal origin underlies renal fibrosis and renal anemia in mice. J Clin Invest 121: 3981-3990, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takaori K, Nakamura J, Yamamoto S, et al. . Severity and frequency of proximal tubule injury determines renal prognosis. J Am Soc Nephrol 27: 2393-2406, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Symeonidis A, Kouraklis-Symeonidis A, Psiroyiannis A, et al. . Inappropriately low erythropoietin response for the degree of anemia in patients with noninsulin-dependent diabetes mellitus. Ann Hematol 85: 79-85, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Lambers Heerspink HJ, de Zeeuw D, Wie L, Leslie B, List J. Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab 15: 853-862, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zinman B, Wanner C, Lachin JM, et al. ; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 373: 2117-2128, 2015. [DOI] [PubMed] [Google Scholar]
- 15.Neal B, Perkovic V, Mahaffey KW, et al. ; CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 377: 644-657, 2017. [DOI] [PubMed] [Google Scholar]
- 16.Wiviott SD, Raz I, Bonaca MP, et al. ; DECLARE-TIMI 58 Investigators. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 380: 347-357, 2019. [DOI] [PubMed] [Google Scholar]
- 17.Sano M, Chen S, Imazeki H, Ochiai H, Seino Y. Changes in heart rate in patients with type 2 diabetes mellitus after treatment with luseogliflozin: subanalysis of placebo-controlled, double-blind clinical trials. J Diabetes Investig 9: 638-641, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]