Abstract
Objective
To evaluate the effect of endovascular therapy (EVT) on the central blood pressure (CBP) and augmentation index (AIx) in patients with peripheral artery disease (PAD).
Methods
The CBP and AIx were assessed by radial applanation tonometry the day before and the day after EVT. We compared the differences in the therapeutic effects between the stenotic and occlusive lesions and between the iliac and superficial femoral artery (SFA) lesions.
Patients
We enrolled 60 consecutive patients with PAD who underwent EVT for de novo lesions.
Results
Both the CBP and AIx were significantly decreased after EVT (125±22 mmHg to 112±22 mmHg; p=0.002 and 84%±16% to 73%±15%; p<0.001, respectively). The effects of EVT on the CBP and AIx were equivalent, regardless of whether the target lesion was the stenotic lesion or the occlusive lesion. There were no significant differences between the iliac and SFA lesions in the effects of EVT on the CBP and AIx.
Conclusion
EVT improved the CBP and AIx in patients with PAD, regardless of the morphology or site of the lesions.
Keywords: endovascular therapy, peripheral artery disease, central blood pressure (CBP), augmentation index (AIx)
Introduction
Peripheral artery disease (PAD) is a global health burden with poor clinical outcomes in the aging societies (1). It is reported that patients with critical limb ischemia (CLI), which is the most advanced form of PAD, have a 20% mortality in the first year after presentation (2,3). PAD is considered a common manifestation of atherosclerosis and is often comorbid with cerebro-cardiovascular disease, which is the major cause of its poor prognosis (4,5).
PAD is reportedly associated with arterial stiffness, and its peripheral artery obstructions cause premature pulse wave reflections, which increase the central blood pressure (CBP) and augmentation index (AIx) (6,7). The CBP is the arterial pressure at the central level, such as the ascending aorta. The AIx is defined as the difference between the second and first systolic peaks, expressed as a percentage of the pulse pressure (8). The CBP is represented as the combination of the forward-traveling waves generated by left ventricular ejection and the backward-traveling reflected waves returning from the peripheral arteries, which is associated with the development of hypertensive target-organ damage (9,10). Elevated CBP and AIx are reportedly associated with cardiovascular disease mortality (8,11). However, the effect of endovascular revascularization on the CBP and AIx in patients with PAD remains unclear.
The aim of the present study was to assess the effect of endovascular therapy (EVT) on the CBP and AIx in patients with PAD.
Materials and Methods
Study design and patient population
This was a cross sectional, single-center study enrolling 60 consecutive patients with PAD who underwent successful EVT for de novo lesions between October 2016 and June 2018. The definition of PAD was an ankle-brachial arterial pressure index (ABI) <0.9 with symptoms of lower limb ischemia. The patients who had below-the-knee lesions, cardiac arrhythmia (e.g. atrial fibrillation), and failed revascularization were excluded from this study.
EVT was performed by experienced cardiologists who followed the Trans-Atlantic Inter-Society Consensus II (TASC II) guideline recommendation (2). CBP and AIx measurements were performed the day before and the day after EVT, using an automated radial artery applanation tonometry (HEM-9000AI; Omron Healthcare, Kyoto, Japan) (12). The peripheral BP was measured at the brachial artery with Omron's inbuilt sphygmomanometer. All measurements were performed after at least 5 minutes' rest in the sitting position in a temperature-controlled room (24℃) in the morning. The major atherosclerosis risk factors, such as hypertension, dyslipidemia, diabetes mellitus, chronic kidney disease (CKD), and smoking, were assessed. Uncontrolled hypertension is defined as a BP that remains above 140/90 mmHg despite the use of 3 antihypertensive agents of different classes (13). Smoking included both current and past smokers. The clinical data were obtained based on the medical records or a history of medical therapy, and medications were assessed at admission.
The timing of the backward-reflected waves is affected by the distance between the heart and the reflection site (14). Therefore, we compared the difference in the therapeutic impact of revascularization on the CBP and AIx between iliac and superficial femoral artery (SFA) lesions.
The study protocol was approved by institutional ethics committee and all patients provided their written informed consent.
Statistical analyses
The results are expressed as the mean ± standard deviation for continuous variables and as percentages for categorical variables. Skewed values are presented as the median and interquartile range (IQR). The predictors of improvement in the CBP and AIx were analyzed by a linear regression analysis. Correlations among the ΔCBP, ΔAIx, and ΔABI were analyzed by using Pearson's correlation coefficient. We used t-tests and chi-square tests to compare continuous and categorical variables, respectively. If the data were not normally distributed, the Mann-Whitney U-test was employed. A p value of <0.05 was considered statistically significant. All statistical analyses were performed with a standard software package (JMP version 12; SAS institute, Cary, USA).
Results
Baseline characteristics and the comparison of clinical characteristics between iliac and SFA lesions
Table 1 shows the baseline characteristics of the study population. There were 52 men and 8 women, and the mean age was 73±8 years old. There were 11 (18%) patients with CLI. Hypertension, diabetes mellitus, dyslipidemia, and CKD were identified in 49 (82%), 20 (33%), 37 (62%), and 26 (43%) patients, respectively. The SFA lesion group had significantly higher prevalence rates of CKD and hemodialysis than the iliac lesion group. There were no significant differences in the prevalence rates of hypertension, diabetes mellitus, dyslipidemia, previous ischemic heart disease, previous stroke, or medications, excluding aldosterone blockers, between the iliac and SFA lesions.
Table 1.
Baseline Characteristics and the Comparison of Clinical Characteristics between the Iliac and SFA Lesions.
| Variables | All patients n=60 | Iliac lesions n=30 | SFA lesions n=30 | p value | ||||
|---|---|---|---|---|---|---|---|---|
| Age (years old) | 73±8 | 72±7 | 74±8 | 0.315 | ||||
| Men / Women | 52 / 8 | 24 / 6 | 28 / 2 | 0.121 | ||||
| BMI (kg/m2) | 23.1±3.6 | 23.6±4.0 | 22.7±3.2 | 0.390 | ||||
| Claudication (Rutherford 2 or 3), n (%) | 49 (82) | 25 (83) | 24 (80) | 0.739 | ||||
| Critical limb ischemia (Rutherford 4-6), n (%) | 11 (18) | 5 (17) | 6 (20) | 0.739 | ||||
| pre-ABI | 0.57±0.22 | 0.57±0.24 | 0.57±0.21 | 0.991 | ||||
| post-ABI | 0.90±0.15 | 0.90±0.16 | 0.90±0.14 | 0.852 | ||||
| Hypertension, n (%) | 49 (82) | 24 (80) | 25 (83) | 0.739 | ||||
| Diabetes mellitus, n (%) | 20 (33) | 10 (33) | 10 (33) | 1.000 | ||||
| Dyslipidemia, n (%) | 37 (62) | 19 (63) | 18 (60) | 0.791 | ||||
| CKD, n (%) | 26 (43) | 9 (30) | 17 (57) | 0.036 | ||||
| Hemodialysis, n (%) | 12 (20) | 3 (10) | 9 (30) | 0.049 | ||||
| Previous IHD, n (%) | 23 (38) | 11 (37) | 12 (40) | 0.791 | ||||
| Previous stroke, n (%) | 15 (25) | 7 (23) | 8 (27) | 0.766 | ||||
| Uncontrolled hypertension, n (%) | 6 (10) | 3 (10) | 3 (10) | 1.000 | ||||
| Smoking, n (%) | 55 (92) | 27 (90) | 28 (93) | 0.639 | ||||
| Number of antiplatelet agents | 1.9±0.3 | 1.9±0.4 | 1.9±0.3 | 0.703 | ||||
| Number of antihypertensive agents | 1.8±1.0 | 1.7±1.0 | 1.8±0.9 | 0.502 | ||||
| Blood examination | ||||||||
| eGFR (mL/min/1.73 m2) | 56.7±33.0 | 63.9±29.2 | 49.5±35.5 | 0.098 | ||||
| Triglycerides (mg/dL) | 132.9±79.8 | 143.2±88.1 | 122.5±70.5 | 0.327 | ||||
| LDL-C (mg/dL) | 93.7±30.0 | 97.2±25.2 | 90.1±34.1 | 0.369 | ||||
| HDL-C (mg/dL) | 51.4±16.9 | 49.2±17.4 | 53.5±16.4 | 0.339 | ||||
| HbA1c (%) | 6.3±0.8 | 6.3±0.7 | 6.3±1.0 | 0.913 | ||||
| BNP (pg/mL) | 44.4 (11.6 - 129.9) | 42.0 (9.9 - 74.2) | 53.5 (13.7 -176.5) | 0.390 | ||||
| hs-CRP (mg/dL) | 0.11 (0.05 - 0.21) | 0.11 (0.03 - 0.20) | 0.11 (0.06 - 0.30) | 0.102 | ||||
| Medications | ||||||||
| ACEIs and/or ARBs, n (%) | 47 (78) | 23 (77) | 24 (80) | 0.754 | ||||
| CCBs, n (%) | 40 (67) | 18 (60) | 22 (73) | 0.272 | ||||
| β-blockers, n (%) | 18 (30) | 9 (30) | 9 (30) | 1.000 | ||||
| Statins, n (%) | 40 (67) | 18 (60) | 22 (73) | 0.272 | ||||
| Nitrates, n (%) | 9 (15) | 3 (10) | 6 (20) | 0.274 | ||||
| Aldosterone blockers, n (%) | 7 (12) | 1 (3) | 6 (20) | 0.035 | ||||
| Aspirin, n (%) | 41 (68) | 21 (70) | 20 (67) | 0.781 | ||||
| Clopidogrel, n (%) | 55 (92) | 27 (90) | 28 (93) | 0.639 | ||||
| Cilostazol, n (%) | 15 (25) | 5 (17) | 10 (33) | 0.133 |
Data are expressed as mean±SD, number (percentage), or median (interquartile range).
ABI: ankle-brachial arterial pressure index, ACEIs: angiotensin-converting enzyme inhibitors, ARBs: angiotensin II receptor blockers, BMI: body mass index, BNP: B-type natriuretic peptide, CCBs: calcium-channel blockers, CKD: chronic kidney disease, eGFR: estimated glomerular filtration rate, HbA1c: hemoglobin A1c, HDL-C: high-density lipoprotein cholesterol, hs-CRP: high-sensitivity C-reactive protein, IHD: ischemic heart disease, LDL-C: low-density lipoprotein cholesterol, SFA: superficial femoral artery
The impact of EVT on the CBP and AIx in patients with PAD
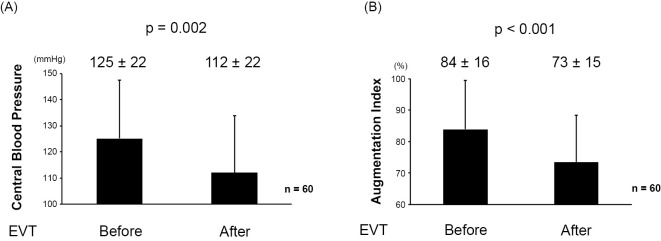
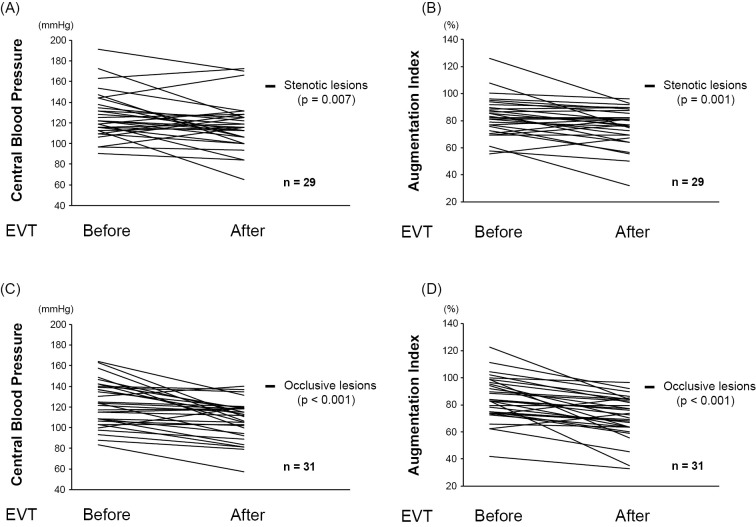
As shown in Fig. 1A, B, both the CBP and AIx were significantly improved after EVT (125±22 mmHg to 112±22 mmHg; p=0.002 and 84%±16% to 73%±15%; p<0.001, respectively). The effects of EVT on the CBP and AIx were equivalent, regardless of whether the target lesion was the stenotic lesion (Fig. 2A, B) or the occlusive lesion (Fig. 2C, D). In addition, the peripheral BP measured in the upper arm was also significantly reduced after EVT (Table 2).
Figure 1.
(A) The impact of EVT on the CBP. (B) The impact of EVT on the AIx. AIx: augmentation index, CBP: central blood pressure, EVT: endovascular therapy
Figure 2.
(A) The impact of EVT on the CBP in stenotic lesions. (B) The impact of EVT on the AIx in stenotic lesions. (C) The impact of EVT on the CBP in occlusive lesions. (D) The impact of EVT on the AIx in occlusive lesions. AIx: augmentation index, CBP: central blood pressure, EVT: endovascular therapy
Table 2.
The Impact of EVT on Peripheral Blood Pressure in Patients with PAD.
| Variables | Pre-EVT n=60 |
Post-EVT n=60 |
p value | |||
|---|---|---|---|---|---|---|
| Peripheral SBP (mmHg) | 138±20 | 130±20 | 0.024 | |||
| Peripheral DBP (mmHg) | 67±13 | 62±12 | 0.055 | |||
| Peripheral MBP (mmHg) | 91±12 | 84±13 | 0.015 | |||
| Pulse pressure (mmHg) | 72±18 | 68±18 | 0.238 | |||
| Heart rate (bpm) | 71±18 | 72±13 | 0.818 |
Data are expressed as mean±SD.
DBP: diastolic blood pressure, EVT: endovascular therapy, MBP: mean blood pressure, PAD: peripheral artery disease, SBP: systolic blood pressure
The comparison of the effects of EVT between iliac and SFA lesions
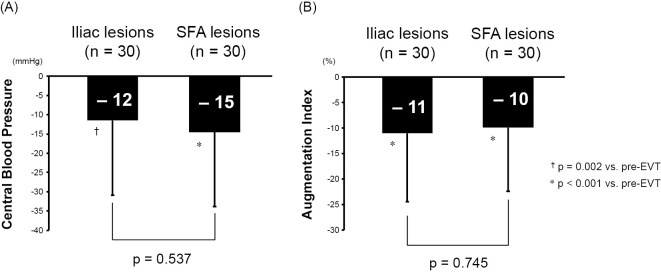
The comparison of the lesion characteristics between the iliac and SFA lesions is shown in Table 3. Chronic total occlusion was noted in 11 (37%) iliac lesions and 20 (67%) SFA lesions. There were no significant differences in TASC II category C or D, the proportion of stent use, or number of stents between the groups. The SFA lesion group had a significantly longer lesion length, whereas the iliac lesion group had a significantly larger stent size. As Fig. 3 show, the effects of the EVT on CBP and AIx were not significantly different between the iliac and SFA lesions (−12 mmHg vs. −15 mmHg; p=0.537, and −11% vs. −10%; p=0.745, respectively).
Table 3.
The Comparison of Lesion Characteristics between the Iliac and SFA Lesions.
| Variables | All patients n=60 |
Iliac lesions n=30 |
SFA lesions n=30 |
p value | ||||
|---|---|---|---|---|---|---|---|---|
| Chronic total occlusion, n (%) | 31 (52) | 11 (37) | 20 (67) | 0.019 | ||||
| Lesion length (mm) | 121±88 | 90±83 | 151±85 | 0.007 | ||||
| TASC II category C/D, n (%) | 27 (45) | 12 (40) | 15 (50) | 0.436 | ||||
| Stent use, n (%) | 58 (97) | 30 (100) | 28 (93) | 0.092 | ||||
| Mean stent diameter (mm) | 7.4±1.3 | 8.3±1.2 | 6.5±0.6 | <0.001 | ||||
| Number of stents | 1.7±0.8 | 1.7±0.7 | 1.7±0.9 | 1.000 |
Data are expressed as mean±SD, number (percentage).
SFA: superficial femoral artery
Figure 3.
(A) The comparison of the effects of EVT on the CBP between the iliac and SFA lesions. (B) The comparison of the effects of EVT on the AIx between the iliac and SFA lesions. AIx: augmentation index, CBP: central blood pressure, EVT: endovascular therapy, SFA: superficial femoral artery
The factors associated with improvement in the CBP and AIx by EVT
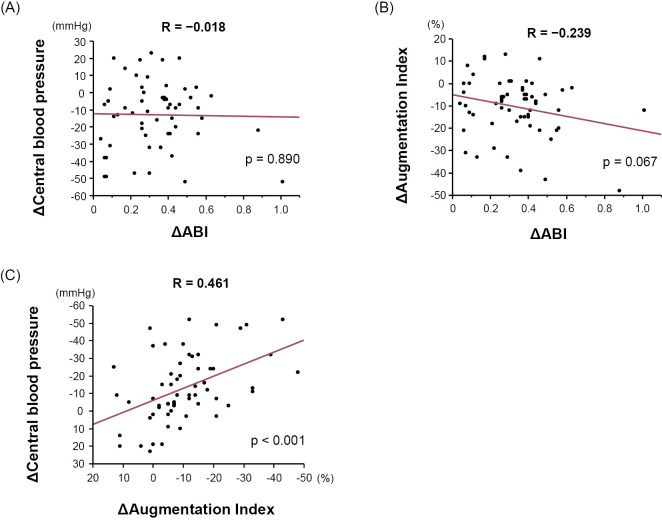
The factors associated with improvement of the CBP and AIx are detailed in Table 4. A higher pre-EVT CBP was associated with a greater decrease in the CBP after EVT (β=−0.454; p<0.001). Similarly, a higher pre-EVT AIx was associated with a greater decrease in AIx after EVT (β=−0.462; p<0.001). TASC II category C or D was associated with a greater decrease in AIx after EVT (β=−0.279; p=0.031). As shown in Fig. 4, the patients with TASC II category C or D had significantly greater decrease in AIx after EVT than those with TASC II category A or B (−7 mmHg vs. −14 mmHg; p=0.031). Fig. 5A and B show the correlations among ΔCBP, ΔAIx, and ΔABI. Intriguingly, there was no significant correlation between ΔCBP and ΔABI, while ΔAIx tended to be correlated with ΔABI, although the correlation did not reach statistical significance. As expected, there was a significant positive correlation between ΔCBP and ΔAIx (Fig. 5C).
Table 4.
The Factors Associated with Improvement in the CBP and AIx.
| CBP | AIx | ||||||
|---|---|---|---|---|---|---|---|
| Standardized Coefficient (beta) | R2 | p value | Standardized Coefficient (beta) | R2 | p value | ||
| Age | 0.072 | 0.005 | 0.586 | -0.010 | <0.001 | 0.942 | |
| Male gender | -0.132 | 0.017 | 0.315 | -0.048 | 0.002 | 0.715 | |
| BMI | -0.214 | 0.046 | 0.104 | -0.212 | 0.045 | 0.106 | |
| CLI | -0.231 | 0.053 | 0.076 | -0.229 | 0.052 | 0.079 | |
| Pre-EVT ABI | -0.058 | 0.003 | 0.660 | 0.102 | 0.010 | 0.438 | |
| Iliac lesion | 0.081 | 0.007 | 0.537 | -0.043 | 0.002 | 0.745 | |
| Chronic total occlusion | -0.151 | 0.023 | 0.249 | -0.224 | 0.050 | 0.086 | |
| Lesion length | -0.069 | 0.005 | 0.599 | -0.124 | 0.015 | 0.345 | |
| TASC II category C/D | -0.128 | 0.016 | 0.332 | -0.279 | 0.078 | 0.031 | |
| Stent use | 0.082 | 0.007 | 0.532 | -0.188 | 0.035 | 0.151 | |
| Mean stent diameter | 0.109 | 0.012 | 0.417 | 0.049 | 0.002 | 0.714 | |
| Number of stents | -0.062 | 0.004 | 0.639 | 0.158 | 0.025 | 0.229 | |
| Pre-EVT CBP | -0.454 | 0.206 | <0.001 | -0.202 | 0.041 | 0.122 | |
| Pre-EVT AIx | -0.218 | 0.047 | 0.095 | -0.462 | 0.214 | <0.001 | |
ABI: ankle-brachial arterial pressure index, AIx: augmentation index, BMI: body mass index, CBP: central blood pressure, CLI: critical limb ischemia, EVT: endovascular therapy
Figure 4.
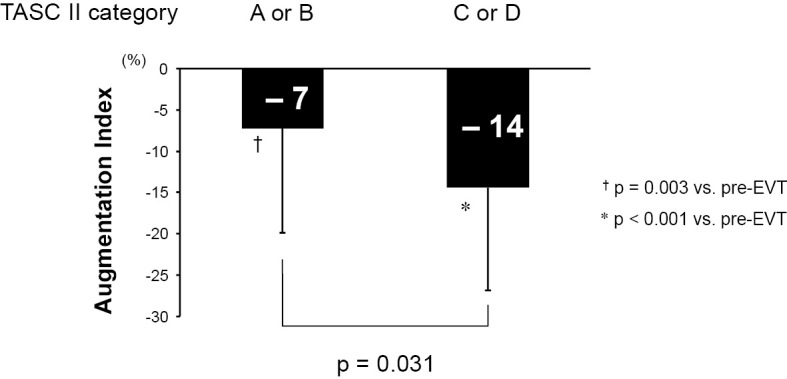
The comparison of the effects of EVT on the AIx between TASC II category A/B and C/D. AIx: augmentation index, EVT: endovascular therapy
Figure 5.
(A) The correlation between the ΔCBP and ΔABI. (B) The correlation between ΔAIx and ΔABI. (C) The correlation between ΔCBP and ΔAIx. ABI: ankle-brachial pressure index, AIx: augmentation index, CBP: central blood pressure
Discussion
Main findings
The main findings of this study were as follows: 1) Both the CBP and AIx were significantly improved after EVT, regardless of whether the target lesion was the stenotic lesion or the occlusive lesion; 2) There were no significant differences in the effects of EVT on the CBP or AIx between the iliac and SFA lesions; 3) Patients with a higher CBP were more likely to enjoy an improvement in their CBP, and patients with a higher AIx were similarly more likely to enjoy an improvement in their AIx after EVT. Furthermore, patients with TASC II category C or D were more likely to enjoy an improvement in their AIx than those with TASC II category A or B after EVT.
The effect of EVT on the CBP and AIx
It was previously reported that EVT was associated with a reduction in the AIx at three months after revascularization, which was caused by an increase in the physical activity due to reducing claudication symptoms (15,16). However, our results showed that EVT induced an immediate reduction in both the CBP and AIx, surprisingly on the day after EVT.
Several potential mechanisms whereby endovascular reperfusion was associated with the improvement in both the CBP and AIx may be speculated. The presence of lower limb arterial stenoses and obstructions can alter the timing and amplitude of the backward-reflected waves (10). Consequently, the premature reflection pulse waves during late systole can increase the central pressure augmentation. Although we cannot measure the precise timing of backward-reflected waves directly, the AIx decreased immediately after EVT. Therefore, it is reasonable to conclude that EVT ameliorated the premature pulse wave reflections by improving the impairment of lower limb perfusion. In addition, EVT can increase the vascular bed capacity, which is decreased by peripheral artery obstructions in patients with PAD. The increase in the vascular capacity due to EVT can contribute to a decrease in the total systemic resistance and a subsequent decrease in the CBP.
Furthermore, it has been reported that recanalization of an occluded infarct artery triggered inflammatory responses by releasing cytokines, such as tumor necrosis factor (TNF)-α and interleukin (IL)-1, which caused reperfusion injury (17). Inflammatory cytokines can produce inducible nitric oxide synthase (iNOS), generating large amounts of nitric oxide (NO) (18). In addition to this, inflammatory mediators, such as prostaglandins and bradykinins, are associated with vascular permeability and vasodilation (19). We can therefore assume that these vasodilator substances reduce arterial stiffness and subsequently the AIx, which might lead to the reduction in the CBP.
Although the distance between the heart and the reflection site was longer in the iliac lesion group than in the SFA lesion group, our results showed that the reductions in the CBP and AIx were not significantly different between the iliac and SFA lesions. It has been shown that the backward-reflected waves are generated by not only the bifurcation of the large artery but also the myriad terminations of low-resistance arteries into high-resistance arterioles (20). This arterial conducting mechanism may thus explain how EVT improved both the CBP and AIx, regardless of whether the target lesion was an iliac lesion or SFA lesion, in the present study.
The predictors of the effects of EVT on CBP and AIx
The patients who showed an improvement in the CBP after EVT had a significantly higher pre-EVT CBP than those who had a lower pre-EVT CBP, and similarly, the patients who showed an improvement in the AIx after EVT had a significantly higher pre-EVT AIx than those who had a lower pre-EVT AIx, suggesting that the patients with higher CBP or AIx values may benefit more from EVT than those with lower values. In addition, the patients with TASC II category C or D showed a greater improvement in the AIx than those with TASC II category A or B. Since TASC II category C or D lesions were longer than those in TASC II category A or B, the timing of the backward-reflected waves may have been more delayed after EVT. In the present study population, the patients with TASC II category C or D had a significantly higher prevalence of CLI than those with TASC II category A or B (33% vs. 9%; p=0.006). Previous studies have shown that CLI is associated with microvascular dysfunction as well as macrovascular dysfunction (21), which can lead to an increase in the AIx and subsequent greater efficacy of EVT.
Clinical implications
There is no certain evidence proving the prognostic value of EVT, even in the patients with CLI (22). However, it has been reported that PAD is associated with an increase in the CBP and AIx and an increased risk of cardiovascular events (7,23). Furthermore, previous studies showed that the CBP was more strongly associated with cardiovascular events than the brachial BP (24,25). Given these findings, EVT may help improve cardiovascular disease mortality and the prognosis of patients with PAD by reducing the CBP and AIx.
Limitations
Several limitations associated with the present study warrant mention. First, this study was a single-center observational study. Second, the study population was relatively small in the present investigation. Third, since most of this study subjects were men, we could not evaluate the gender differences in the AIx. Finally, the CBP and AIx values after EVT were only measured on the day after EVT.
Conclusion
EVT improved the CBP and AIx in patients with PAD, regardless of the morphology or site of the lesions.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Fowkes FG, Rudan D, Rudan I, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet (London, England) 382: 1329-1340, 2013. [DOI] [PubMed] [Google Scholar]
- 2. Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG. Inter-society consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg 45 (Suppl S): S5-S67, 2007. [DOI] [PubMed] [Google Scholar]
- 3. Bertele V, Roncaglioni MC, Pangrazzi J, Terzian E, Tognoni EG. Clinical outcome and its predictors in 1560 patients with critical leg ischaemia. Chronic Critical Leg Ischaemia Group. Eur Vasc Endovasc Surg 18: 401-410, 1999. [DOI] [PubMed] [Google Scholar]
- 4. Bhatt DL, Steg PG, Ohman EM, et al. International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA 295: 180-189, 2006. [DOI] [PubMed] [Google Scholar]
- 5. Fowkes FG, Murray GD, Butcher I, et al. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta-analysis. JAMA 300: 197-208, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mosimann K, Jacomella V, Thalhammer C, et al. Severity of peripheral arterial disease is associated with aortic pressure augmentation and subendocardial viability ratio. Journal of clinical hypertension (Greenwich, Conn) 14: 855-860, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Catalano M, Scandale G, Carzaniga G, et al. Aortic augmentation index in patients with peripheral arterial disease. J Clin Hypertens (Greenwich) 16: 782-787, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC practice guidelines for the management of arterial hypertension. Blood Press 23: 3-16, 2014. [DOI] [PubMed] [Google Scholar]
- 9. Kollias A, Lagou S, Zeniodi ME, Boubouchairopoulou N, Stergiou GS. Association of central versus brachial blood pressure with target-organ damage: systematic review and meta-analysis. Hypertension 67: 183-190, 2016. [DOI] [PubMed] [Google Scholar]
- 10. Torjesen AA, Wang N, Larson MG, et al. Forward and backward wave morphology and central pressure augmentation in men and women in the Framingham Heart Study. Hypertension 64: 259-265, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vlachopoulos C, Aznaouridis K, O'Rourke MF, Safar ME, Baou K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with central haemodynamics: a systematic review and meta-analysis. Eur Heart J 31: 1865-1871, 2010. [DOI] [PubMed] [Google Scholar]
- 12. Takazawa K, Kobayashi H, Shindo N, Tanaka N, Yamashina A. Relationship between radial and central arterial pulse wave and evaluation of central aortic pressure using the radial arterial pulse wave. Hypertens Res 30: 219-228, 2007. [DOI] [PubMed] [Google Scholar]
- 13. Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation 117: e510-e526, 2008. [DOI] [PubMed] [Google Scholar]
- 14. Ahn KT, Park KI, Kim MJ, et al. Height and sex is strongly associated with radial augmentation index in Korean patients with never-treated hypertension. Clin Interv Aging 11: 415-422, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jacomella V, Shenoy A, Mosimann K, Kohler MK, Amann-Vesti B, Husmann M. The impact of endovascular lower-limb revascularisation on the aortic augmentation index in patients with peripheral arterial disease. Eur J Vasc Endovasc Surg 45: 497-501, 2013. [DOI] [PubMed] [Google Scholar]
- 16. Tanaka H, Safar ME. Influence of lifestyle modification on arterial stiffness and wave reflections. Am J Hypertens 18: 137-144, 2005. [DOI] [PubMed] [Google Scholar]
- 17. Blancke F, Claeys MJ, Jorens P, et al. Systemic inflammation and reperfusion injury in patients with acute myocardial infarction. Mediators Inflamm 2005: 385-389, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sprague AH, Khalil RA. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem Pharmacol 78: 539-552, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol 31: 986-1000, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. O'Rourke MF, Hashimoto J. Mechanical factors in arterial aging: a clinical perspective. J Am Coll Cardiol 50: 1-13, 2007. [DOI] [PubMed] [Google Scholar]
- 21. Gulati A, Botnaru I, Garcia LA. Critical limb ischemia and its treatments: a review. Cardiovasc Surg 56: 775-785, 2015. [PubMed] [Google Scholar]
- 22. Iida O, Takahara M, Soga Y, Azuma N, Nanto S, Uematsu M. Prognostic impact of revascularization in poor-risk patients with critical limb ischemia: The PRIORITY registry (poor-risk patients with and without revascularization therapy for critical limb ischemia). JACC Cardiovasc Interv 10: 1147-1157, 2017. [DOI] [PubMed] [Google Scholar]
- 23. Aronow WS, Ahmed MI, Ekundayo OJ, Allman RM, Ahmed A. A propensity-matched study of the association of peripheral arterial disease with cardiovascular outcomes in community-dwelling older adults. Am J Cardiol 103: 130-135, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Roman MJ, Devereux RB, Kizer JR, et al. Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: the Strong Heart Study. Hypertension 50: 197-203, 2007. [DOI] [PubMed] [Google Scholar]
- 25. Williams B, Lacy PS, Thom SM, et al. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation 113: 1213-1225, 2006. [DOI] [PubMed] [Google Scholar]