Abstract
Objective
Oryeongsan (Goreisan), a formula composed of five herbal medicines, has long been used to treat impairments of the regulation of body fluid homeostasis. Goreisan has been revealed to have anti-inflammatory actions and inhibit a water channel, the aquaporin (AQP). We herein report the therapeutic effect of Goreisan on experimental autoimmune encephalomyelitis (EAE in, an animal model of inflammatory demyelinating diseases.
Materials and Methods
EAE mice immunized with MOG35-55 peptide were divided into Goreisan- and sham-treated groups. The clinical EAE score and histopathological finding of the central nervous system (CNS) were analyzed. For the proliferation assay, prepared spleen cells from immunized mice were cultured and analyzed for the [3H]-thymidine uptake and cytokine concentrations of the culture supernatant. The relative quantification of AQP4 mRNA in the CNS of EAE mice was analyzed quantitatively.
Results
The EAE score of the Goreisan-treated mice was significantly lower than that of the sham-treated mice. The CD4-positive cell number in the CNS of Goreisan-treated mice was lower than that of sham-treated mice. In the recall response to MOG35-55 peptide, the cell proliferation did not differ markedly between the spleen cells from Goreisan- and sham-treated mice. Furthermore, Goreisan decreased the mRNA level of AQP4 in the spinal cord during EAE.
Conclusion
Goreisan prevented the disease activity of EAE by inhibiting the migration of pathogenic cells into the CNS by suppressing the AQP4 expression in the CNS. Goreisan may have a therapeutic effect on inflammatory demyelinating diseases.
Keywords: multiple sclerosis, experimental autoimmune encephalomyelitis, herbal medicine, aquaporin, Goreisan, Oryeongsan
Introduction
Kampo medicine, which is traditionally practiced in Japan, is based on ancient Chinese medicine. Oryeongsan (Goreisan) has long been used to treat impairments of the regulation of body fluid homeostasis in China (Wulingsan), Japan (Goreisan) and Korea (Oryeongsan). Goreisan is composed of five herbal medicines (1). Each of these five herbs-Alisma orientalis Juzep, Polyporus umbellatus Fries, Atractylodes chinensis, Poria cocos Wolf and Cinnamomom Cassia Presl-is frequently contained in different formulas for the treatment of dysfunctions of the body fluid balance (2). Goreisan is used as an aquaretic drug to adjust the water balance of the body. Therefore, Goreisan is prescribed for patients with oral dryness and decreased urine volume, such as in edema, nephrosis, alcoholic hangover, acute gastrointestinal catarrh, diarrhea, nausea, vomiting, dizziness, water retention in the stomach, headache, uremia, heat-stroke, and diabetes mellitus (3).
Although the pharmacological mechanism of action of Goreisan is still unclear, it has been reported to inhibit the aquaporin (AQP) channel in recent years (4,5). In the present study, we investigated whether or not Goreisan has efficacy against neuro-immunological diseases using experimental autoimmune encephalomyelitis (EAE), which is a known animal model of inflammatory demyelinating diseases.
Materials and Methods
Mice
Wild-type (WT) C57BL/6 mice were purchased from Clea Japan (Tokyo, Japan) and maintained under-specific pathogen-free conditions in an environmentally controlled clean room. All experiments were conducted according to the institutional ethics guidelines for animal experiments and safety guidelines for gene manipulation experiments. All mice used for experiments were 8 to 12 weeks old.
Peptides
Peptides 35-55 of myelin oligodendrocyte glycoprotein (MOG35-55; single-letter amino acid code: MEVGWYRSPFSRVVHLYRNGK) were synthesized by Tore Research Institute (Tokyo, Japan). The peptides were >90% pure, as determined using high-performance liquid chromatography.
Induction and evaluation of EAE
Mice were bilaterally injected subcutaneously in the flank with 200 μL inoculum containing 100 μg MOG35-55 and 0.5 mg Mycobacterium tuberculosis H37RA (Difco Laboratories, Detroit, USA) in complete Freund's adjuvant (CFA). Pertussis toxin (300 ng; Sigma-Aldrich, St. Louis, USA) was then injected intravenously at 0 and 2 days post-immunization (6). Immunized mice were examined daily and scored as follows: 0, no clinical signs; 1, partial drop tail; 2, drop tail; 3, partial hind leg paralysis; 4, total hind leg paralysis; and 5, moribund or dead. Mice were examined daily for signs of EAE by a blinded observer, and the cumulative score was obtained by summing the EAE score from days 0 to 30.
Composition of Goreisan
We used Goreisan extracted and manufactured by TSUMURA (Tokyo, Japan). Goreisan is composed of Alisma orientalis Juzep (Alisma plantago-aquatica L.), Polyporus umbellatus Fries (Polyporus umbellatus), Atractylodes chinensis (Atractylodes lancea), Poria cocos Wolf (Wolfiporia extensa) and Cinnamomom Cassia Presl (Cinnamomum cassia L. J. Presl). The compounding ratio of the extract of Goreisan is 33.0% Alisma orientalis Juzep, 19.8% Polyporus umbellatus Fries, 19.8% Atractylodes chinensis, 19.8% Poria cocos Wolf and 13.2% Cinnamomon Cassia Presl.
Administration of Goreisan
Goreisan (10 mg) was dissolved in 200 μL of phosphate-buffered saline (PBS) and administered via a nasogastric (NG) tube while the sham mice were administered PBS only. The Goreisan-treated mice were divided into two groups: one was orally administered Goreisan three times a week from the day of EAE induction while the other was administered Goreisan from day 10 when the onset of paralysis was noted. To reflect the typical human dosage, it is necessary to administer 3 mg/mouse/day of Goreisan. We attempted to administer 1, 10 or 100 mg per mouse, but many mice died of aspiration when oral daily administration was attempted. When we switched to medication three times a week, aspiration occurred only in the 100 mg administration group only. As dosage of 1 mg was judged to be small, the administration of 10 mg/mouse was selected for the present study.
Primary response of pathogenic T cells
To analyze the primary response of MOG35-55 reactive T cells, we performed a proliferation assay and detected cytokines in the culture supernatant. According to a previously described method, the mice were immunized with MOG35-55, the spleens were harvested on day 12 post-immunization (6), and the spleen cells were cultured in Rowell Park Memorial Institute GlutaMAX medium (Gibco, Grand Island, NY, USA) containing 5% heat-inactivated fetal bovine serum in 96-well plates (1×106 cells/well).
The cells were incubated with MOG35-55 in a 37°C, 5% CO2 incubator for 48 hours, and then [3H]-thymidine was added (1 μCi/well) during the final 18 hours of culture. The counts per minute (cpm) were measured using a beta-1205 counter (Pharmacia, Uppsala, Sweden). The concentrations of cytokines such as interleukin (IL)-2, IL-4, IL-6, IL-10, IL-17a, interferon (IFN)-γ and tumor necrosis factor (TNF), in the supernatant from the proliferation assay were measured using bead capture assays (BD Bioscience, San Jose, USA) according to the manufacturer's guidelines.
Pathological analyses
The spinal cord of each mouse was removed on day 20 after immunization with EAE and fixed with paraformaldehyde, and then paraffin-embedded sections were cut and stained with hematoxylin and eosin.
Analyses of infiltrating cells in the central nervous system (CNS)
Mice were anesthetized with diethyl ether on day 9 after EAE induction, and after perfusion with PBS, the brains and spinal cords were removed and homogenized. Mononuclear cells in the brain and spinal cord were isolated using Percoll gradient (Amersham Biosciences, Piscataway, USA) (7). The CD4-positive cells were isolated using flow cytometry (BD FACSCalibur) and as a control, the brains and spinal cords of naïve mice were also analyzed using the same method.
Relative quantification of AQP4 mRNA
EAE was induced in mice with MOG35-55, and their spinal cords were removed on the day of the onset of paralysis. cDNA was then generated using a reverse transcriptase reaction based on the mRNA of AQP4 extracted from the spinal cord tissues. The mRNA levels were quantified using the comparative CT method of the StepOne™ real-time polymerase chain reaction (PCR) system (Applied Biosystems). For the comparison, the spinal cords of naïve mice were similarly analyzed.
Results
Amelioration of EAE in Goreisan-treated mice
The disease onset was similar between Goreisan- and sham-treated mice (16.3±1.05 and 16.0±1.06 days after immunization, respectively). However, there were significant differences in their maximum clinical and cumulative scores. Goreisan-treated mice showed a maximum clinical score of 1.21±0.42 and cumulative score (day 0-30) of 13.2±4.85, whereas the corresponding values of the sham-treated mice were 2.64±0.40, and 26.7±4.88, respectively (Fig. 1A and Table).
Figure 1.
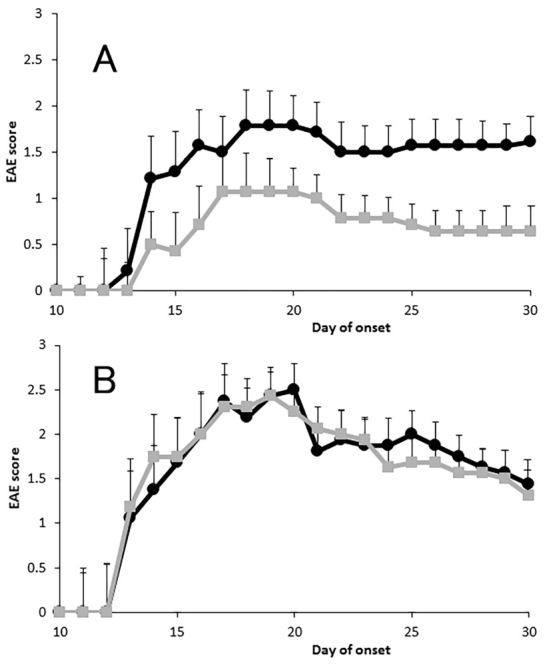
Course of experimental autoimmune encephalomyelitis (EAE). Mice were immunized with peptides 35-55 of myelin oligodendrocyte glycoprotein (MOG35-55) in complete Freund’s adjuvant and simultaneously administered Goreisan. Data are shown as the mean EAE scores of all immunized mice ±the standard error of the mean (SEM) (A). The mean EAE scores of Goreisan-treated mice were significantly lower than those of non-treated mice (■: Goreisan-treated, and ●: sham-treated mice, both n=14). Disease features are summarized in Table. Goreisan was administered from day 10, after the onset of paralytic symptoms (B). EAE score was not significantly different between the Goreisan- and non-treated mice (■: Goreisan-treated and ●: sham-treated mice, both n=8).
Table.
Statistical Analysis of Clinical EAE Scores.
| Incidence | Day of onset | Max. score | Cumulative score | |||||
|---|---|---|---|---|---|---|---|---|
| Goreisan administrated mice (n=14) | 42.9% (6/14) | 16.3±1.05 | 1.21±0.42* | 13.2±4.85* | ||||
| Sham administrated mice (n=14) | 78.6% (11/14) | 16.0±1.06 | 2.64±0.40 | 26.7±4.86 |
Each mouse was immunized EAE. The mean±SEM for the following parameters are shown: incidence of paralyzed mice among immunized mice (Incidence), the day of EAE onset, maximum score of EAE (Max. score), cumulative EAE score from 0-30 days. * p<0.05 versus sham administrated mice by Mann-Whitney U test. EAE: experimental autoimmune encepholomyelitis, SEM: standard error of the mean
A histological analysis of the spinal cord demonstrated severe monocyte infiltration and demyelination in the sham-treated mice compared with the Goreisan-treated mice (Fig. 2). In contrast, there was no significant difference in the EAE score between mice treated with Goreisan from the day of the onset of paralysis and the sham-treated mice (Fig. 1B). Therefore, the administration of Goreisan after disease onset was not deemed effective.
Figure 2.
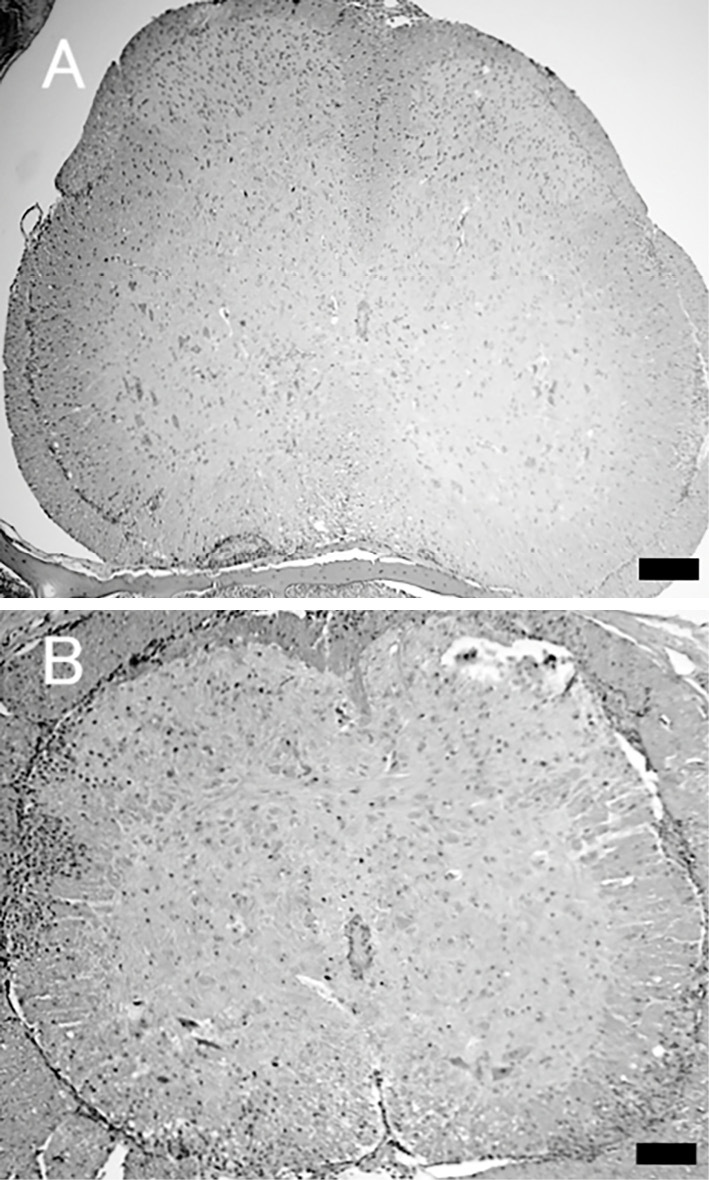
Histopathological findings in the spine. The spinal cord of each mouse (n=5, each group) was removed on day 20 after experimental autoimmune encephalomyelitis (EAE) immunization and fixed with paraformaldehyde, and paraffin sections were then prepared. Spinal tissue sections stained with Hematoxylin and Eosin staining from Goreisan-treated mice (A) showed mild cell infiltration compared with sections from sham-treated mice (B). Scale bar=100 μm.
Goreisan did not affect the primary response of pathogenic T cells
To evaluate the effect of Goreisan on antigen-specific lymphocytes, we examined the primary response of pathogenic T cells in vitro. There was no significant difference in the proliferative response of MOG35-55-reactive T cells between Goreisan- and sham-treated mice (data not shown). Furthermore, there were no significant differences in the concentrations of IFN-γ, IL-4, IL-10 or IL-17 between the culture supernatants of Goreisan- and sham-treated mice (data not shown). These results suggest that Goreisan did not affect the effector phase of EAE.
Goreisan prevented inflammatory cell infiltration into the CNS in EAE
As shown in Fig. 3, the number of CD4+ cells in the CNS of sham-treated mice was significantly greater than that in the naïve mice (3,139±691 vs. 997±469, mean±standard error; p<0.05 by the Mann-Whitney U test). However, the number of CD4+ cells in the CNS of Goreisan-treated mice (2,649±579) was not significantly different from that in naïve mice. This result suggests that the administration of Goreisan may prevent the migration of pathogenic T cells into the CNS.
Figure 3.
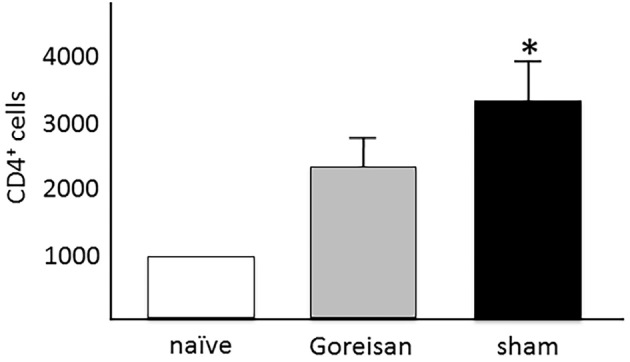
CD4+ cells in the central nervous system (CNS). The number of CD4+ cells in the CNS of sham-treated mice was significantly greater than that in naïve mice [both n=6, 3,139±691 vs. 997±469, mean±standard error of the mean (SEM); p<0.05 by the Mann-Whitney U test]. However, the number of CD4+ cells in the CNS of Goreisan-treated mice (n=5, 2,649±579) was not significantly different from that in the CNS of naïve mice.
Goreisan decreased the expression of AQP4 in the spinal cord during EAE
To confirm whether or not AQP4 is upregulated in the CNS during EAE and whether or not Goreisan modulates the expression of AQP4, we analyzed the relative mRNA levels of AQP4. Fig. 4 shows that AQP4 mRNA was upregulated in spinal cord tissue from EAE mice compared with that from naïve mice. Goreisan-treated mice showed a lower expression of AQP4 mRNA in the spinal cord than sham-treated mice (1.39±0.14 vs. 2.41±0.42, mean±standard error; p<0.05 by the Mann-Whitney U test).
Figure 4.
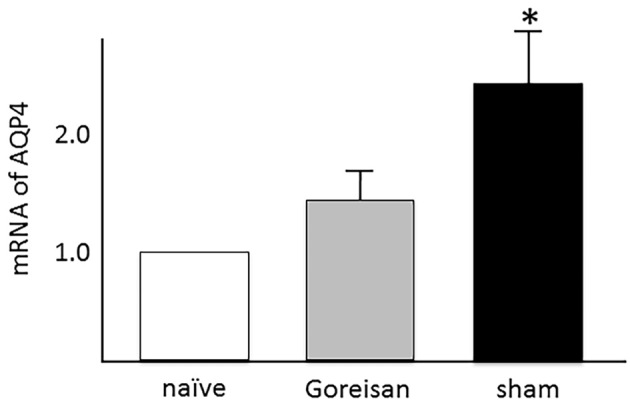
mRNA expression of aquaporin 4 (AQP4). The mRNA of AQP4 was upregulated in spinal cord tissue from experimental autoimmune encephalomyelitis (EAE) mice compared with naïve mice (non-EAE mice, n=5). Values are relative to the mRNA value of naïve mice, which was set as 1. In Goreisan-treated mice (n=9), the expression of mRNA of AQP4 in spinal cord tissue was decreased compared to that from sham-treated mice [n=10, 1.39±0.14 vs. 2.41±0.42, mean±standard error of the mean (SEM); p<0.05 by the Mann-Whitney U test].
Discussion
In the present study, the administration of Goreisan ameliorated EAE. Since there was no significant difference in the primary response of MOG35-55-specific lymphocytes, Goreisan did not affect the induction phase of EAE. Goreisan was ineffective when administered after the onset of EAE, making it highly likely that it did not affect the effector phase of EAE. This finding indicates that the effect of Goreisan in the effector phase was insufficient. Fewer infiltrating cells were observed in the CNS of mice administered with Goreisan than in sham-treated mice. These results suggested that Goreisan may prevent the progress of EAE by inhibiting inflammatory cell infiltration through the blood-brain-barrier (BBB).
We showed that Goreisan suppressed AQP4 expression in the CNS of EAE mice. AQP is a family of water channel proteins expressed on the plasma membrane as water channels. Thirteen subtypes have been identified, and subtypes AQP1, 4, and 9 have been confirmed in the CNS. AQP4 is one of the most strongly expressed AQPs in the CNS (8,9). Although AQP4 is mainly expressed on astrocytes, it is also expressed on ependymal cells around the ventricle and is thought to be involved in cerebrospinal fluid circulation. Jung et al. showed that AQP4 mRNA in rat brain is localized to ependymal cells lining the aqueduct, glial cells forming the edge of the cerebral cortex and brainstem, vasopressin-secretory neurons in supra-optic and paraventricular nuclei of hypothalamus and Purkinje cells of the cerebellum (8). More interestingly, AQP4 has also been reported to be involved in cell adhesion in addition to its function as a water channel (10).
The upregulation of AQP4 in the CNS has been reported in inflammatory diseases of the CNS. The AQP4 mRNA and protein levels were significantly increased in the optic nerves of EAE mice (11). AQP4-mRNA expression in the brain and spinal cord of mice is elevated in the acute phase of EAE. AQP4 is expressed on astrocytes, suggesting that an increase in the AQP4 expression may be involved in the development of inflammation with astrocytic activation (12). Therefore, AQP4 may be a pathophysiological factor in EAE.
Goreisan is known to inhibit cell membrane water permeability in a concentration-dependent manner, suppressing only the movement of water passing through aquaporin without changing the cell membrane potential. However, many points concerning its pharmacological action remain unclear. It has been reported that a component containing manganese, which is a constituent of Goreisan, exerts AQP inhibitory action (5,13). Isohama et al. reported in their study using MLE-12 cells of a mouse lung epithelial cell line that the inhibitory effect on cell membrane water permeability is due to the manganese ion contained in the herbal medicine component (14).
Goreisan also inhibits the chemokine expression in cells expressing AQP at high levels. The underlying mechanism is presumed to be the suppression of signaling molecules, such as extracellular signal-regulated kinase and the c-Jun N-terminal kinase pathway (15,16).
As AQP 3, 4 and 5 enhance chemokine production (17), Goreisan may inhibit chemokine production via AQP suppression as another mechanism. The inhibition of chemokines ameliorates EAE (18). Chemokines play an important role in cell adhesion when pathogenic T cells pass through the BBB, and EAE is ameliorated when chemokines and adhesion molecules are suppressed (7,19).
We therefore speculated that Goreisan ameliorated EAE by preventing the migration of pathogenic cells into the CNS through the suppression of adhesion. Since Goreisan decreased the expression of chemokines and AQP4, which are related to adhesion, we thought that these pathways might act in combination to exert an effect. Regarding the reduction in AQP4 in the CNS noted in our study, we must consider the influence of the reduction in the number of microglia expressing AQP4. However, because AQP4 is also expressed in many tissues other than microglia, we consider our data to be significant.
Conclusion
Goreisan prevented the disease activity of EAE by inhibiting the migration of pathogenic cells into the CNS through the suppression of chemokines and AQP4 expression in the CNS. Goreisan may have a therapeutic effect on inflammatory demyelinating diseases. In conclusion, Goreisan may be a viable new therapeutic agent for neuroimmunological diseases.
The authors state that they have no Conflict of Interest (COI).
Financial Support
This work was supported, in part, by a grant for research on intractable diseases from the Ministry of Health, Labor, and Welfare of Japan and by Grants-in-Aid for Scientific Research on Innovative Areas (24110518 and 26110721) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- 1. Orita M. The effects of Gorei-san on water distribution in the body (long term administration of Gorei-san compared to short term administration). Wakan Iyakugaku Zasshi (J Trad Med) 16: 32-37, 1999(in Japanese, Abstract in English). [Google Scholar]
- 2. Ahn YM, Cho KW, Kang DG, Lee HS. Oryeongsan (Wulingsan), a traditional Chinese herbal medicine, induces natriuresis and diuresis along with an inhibition of the renin-angiotensin-aldosterone system in rats. J Ethnopharmacol 141: 780-785, 2012. [DOI] [PubMed] [Google Scholar]
- 3. Katayama K, Matsuda N, Kakuta K, et al. . The effect of Goreisan on the prevention of chronic subdural hematoma recurrence: multi-center randomized controlled study. J Neurotrauma 35: 1537-1542, 2018. [DOI] [PubMed] [Google Scholar]
- 4. Goto S, Kato K, Yamamoto T, Shimato S, Ohshima T, Nishizawa T. Effectiveness of Goreisan in preventing recurrence of chronic subdural hematoma. Asian J Neurosurg 13: 370-374, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nakano T, Nishigami C, Irie K, et al. . Goreisan prevents brain edema after cerebral ischemic stroke by inhibiting aquaporin 4 upregulation in mice. J Stroke Cerebrovasc Dis 27: 758-763, 2018. [DOI] [PubMed] [Google Scholar]
- 6. Miyamoto K, Miyake S, Yamamura T. A synthetic glycolipid prevents autoimmune encephalomyelitis by inducing TH2 bias of natural killer T cells. Nature 413: 531-534, 2001. [DOI] [PubMed] [Google Scholar]
- 7. Miyamoto K, Miyake S, Mizuno M, Oka N, Kusunoki S, Yamamura T. Selective COX-2 inhibitor celecoxib prevents experimental autoimmune encephalomyelitis through COX-2-independent pathway. Brain 129: 1984-1992, 2006. [DOI] [PubMed] [Google Scholar]
- 8. Jung JS, Bhat RV, Preston GM, Guggino WB, Baraban JM, Agre P. Molecular characterization of an aquaporin cDNA from brain: candidate osmoreceptor and regulator of water balance. Proc Nat Acad Sci U S A 91: 13052-13056, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Papadopoulos MC, Manley GT, Krishna S, Verkman AS. Aquaporin-4 facilitates reabsorption of excess fluid in vasogenic brain edema. FASEB J 18: 1291-1293, 2004. [DOI] [PubMed] [Google Scholar]
- 10. Hiroaki Y, Tani K, Kamegawa A, et al. . Implications of the aquaporin-4 structure on array formation and cell adhesion. J Mol Biol 355: 628-630, 2006. [DOI] [PubMed] [Google Scholar]
- 11. Sun L, Weng H, Li Z. Elevation of AQP4 and selective cytokines in experimental autoimmune encephalitis mice provides some potential biomarkers in optic neuritis and demyelinating diseases. Int J Clin Exp Pathol 8: 15749-15758, 2015. [PMC free article] [PubMed] [Google Scholar]
- 12. Miyamoto K, Nagaosa N, Motoyama M, Kataoka K, Kusunoki S. Upregulation of water channel aquaporin-4 in experimental autoimmune encephalomyeritis. J Neurol Sci 276: 103-107, 2009. [DOI] [PubMed] [Google Scholar]
- 13. Kurita T, Nakamura K, Tabuchi M, Orita M, Ooshima K, Higashino H. Effects of Goreisan: a traditional Japanese kampo medicine, on aquaporin 1, 2, 3, 4 and V2R mRNA expression in rat kidney and forebrain. J Med Sci 11: 30-38, 2011. [Google Scholar]
- 14. Isohama Y. Aquaporin modification: A new molecular mechanism to concern. J Pharm Soc Jpn 126: 70-73, 2006. [Google Scholar]
- 15. Chu H, Tang Y, Dong Q. Protection of vascular endothelial growth factor to brain edema one. PLoS One 8: e66051, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chu H, Tang Y, Dong Q. Protection of granulocyte-colony stimulating factor to hemorrhagic brain injuries and its involved mechanisms: effects of vascular endothelial growth factor and aquaporin-4. Neuroscience 260: 59-72, 2014. [DOI] [PubMed] [Google Scholar]
- 17. Sakamoto Y, Hisatsune A, Katsuki H, Horie I, Isohama Y. Aquaporin 5 increases keratinocyte-derived chemokine expression and NF-kappaB activity through ERK activation. Biochem Biophys Res Commun 448: 355-360, 2014. [DOI] [PubMed] [Google Scholar]
- 18. Moriguchi K, Miyamoto K, Tanaka N, Yoshie O, Kusunoki S. The importance of CCR4 and CCR6 in experimental autoimmune encephalomyelitis. J Neuroimmunol 257: 53-58, 2013. [DOI] [PubMed] [Google Scholar]
- 19. Kureshiro J, Miyamoto K, Tanaka N, kusunoki S. Selective phosphodiesterase-3 inhibitor cilostazol ameliorates experimental autoimmune encephalomyelitis. Neuroreport 20: 718-722, 2009. [DOI] [PubMed] [Google Scholar]


