Abstract
Mutations in the cardiac sodium channel SCN5A can cause phenotypic overlap syndrome of long QT syndrome and Brugada syndrome. However, Brugada-type ST elevations in patients with overlap syndrome are often concealed, which creates a diagnostic challenge. A 38-year-old man was admitted due to ventricular fibrillation (VF). The 12-lead electrocardiogram showed a prolonged QT interval and saddleback-type ST elevation. Pilsicainide administration induced coved-type ST elevation and VF triggered by a single premature ventricular contraction. A genetic analysis showed an SCN5A c.5350G>A p.E1784K mutation. The present case suggests the importance of a drug administration test being performed in the clinical management of overlap syndrome.
Keywords: E1784K, overlap syndrome, Brugada syndrome, long QT syndrome, sodium channel blocker, SCN5A
Introduction
Mutations in the SCN5A gene are responsible for a spectrum of hereditary arrhythmias, such as Brugada syndrome, long QT syndrome type 3 (LQT3), sinus node dysfunction, and cardiac conduction disease (1-5). Of these, the SCN5A E1784K mutation has been reported to be associated with the phenotypic overlap of Brugada syndrome and LQT3 (6-9). However, in a fair few patients with overlap syndrome, Brugada-type ST elevations are concealed, and provocation with a sodium channel blocker is needed to unmask them (6,7).
We herein report a case of overlap syndrome with SCN5A E1784K mutation causing ventricular fibrillation. Pilsicainide administration unmasked coved-type ST elevation and induced ventricular fibrillation. This case implies the importance of performing a provocation test with a sodium channel blocker in the clinical management of overlap syndrome.
Case Report
A 38-year-old man was admitted with sudden cardiac arrest due to ventricular fibrillation (VF) that occurred when he had been resting in a car, and he was successfully resuscitated by automated external defibrillator application. He had no history of syncope and had been undergoing no medical treatment, although he had been found to have paroxysmal atrial fibrillation (AF) at a health checkup at 35 years of age.
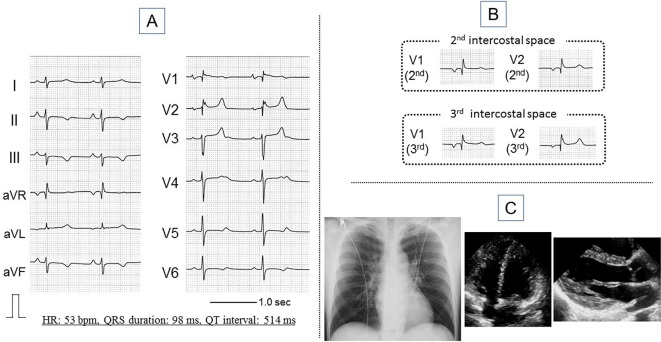
The physical findings showed a height of 173 cm, weight of 63 kg, blood pressure of 118/68 mmHg, and regular pulse of 58 beats per minute (bpm). There were no murmurs or rales in the chest. Laboratory testing, including serum levels of electrolytes, such as potassium, sodium, calcium, and magnesium, showed values within normal ranges. The surface 12-lead electrocardiogram (ECG) at rest showed sinus rhythm, a prolonged QT interval of 514 ms, prolonged QT interval corrected for heart rate of 485 ms, and saddleback-type ST elevation in lead V2 (Fig. 1A). No typical coved-type ST elevation was observed even in the second or third intercostal space (Fig. 1B). Chest X-ray, transthoracic echocardiogram (Fig. 1C), and coronary angiogram evaluations showed no abnormalities. One of his nieces had an asymptomatic ECG abnormality of a long QT interval.
Figure 1.
Findings of the 12-lead electrocardiogram at rest (A, B), chest X-ray, and transthoracic echocardiogram (C). The electrocardiogram shows sinus rhythm, QT prolongation (QT interval: 514 ms, QTc interval: 485 ms), and mild saddleback-type ST elevation (A). Coved-type ST elevation is not observed, even in the second and third intercostal spaces (B). The chest X-ray and transthoracic echocardiogram findings are essentially normal (C). HR: heart rate
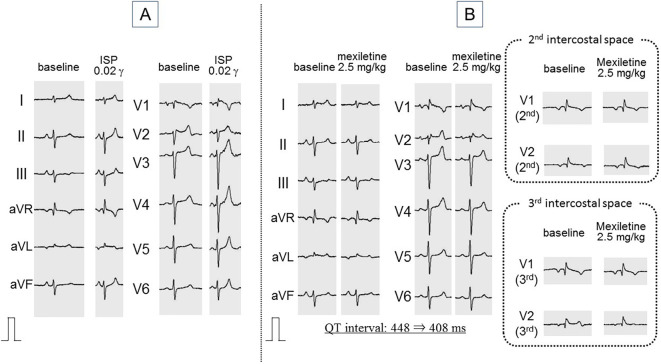
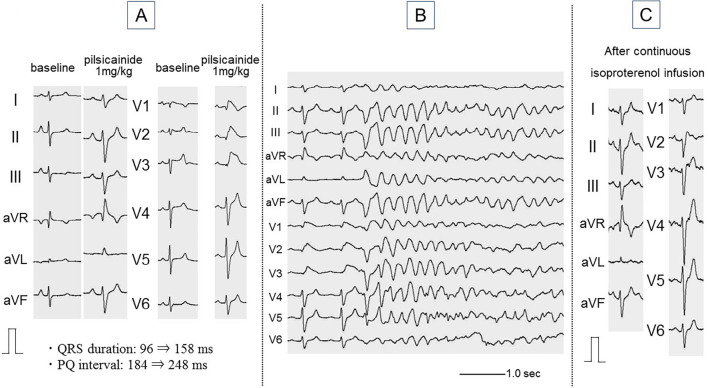
The patient was transferred to Shizuoka Saiseikai General Hospital for a further evaluation. A continuous infusion test of isoproterenol did not induce paradoxical QT prolongation (Fig. 2A). A mexiletine 2.5 mg/kg administration test shortened the QT interval from 448 to 408 ms and did not change the saddleback-type ST elevation (Fig. 2B). A pilsicainide administration 1 mg/kg test induced prominent coved-type ST elevation in leads V1-3 and prolonged QRS duration from 96 to 158 ms as well as a PQ interval from 184 ms to 248 ms. Shortly after the appearance of coved-type ST elevation, a premature ventricular contraction (PVC) induced VF that was successfully terminated by a defibrillator. The coved-type ST elevation was reversed by continuous isoproterenol infusion (Fig. 3).
Figure 2.
Isoproterenol administration test (A) and mexiletine administration test (B). Continuous infusion of isoproterenol does not induce paradoxical QT prolongation (A). Mexiletine 2.5 mg/kg administration shortens the QT interval by 40 ms and does not change the saddleback-type ST elevation, even in the second and third intercostal spaces (B). ISP: isoproterenol, γ: μg/kg/min
Figure 3.
Pilsicainide administration test. Pilsicainide administration at 1 mg/kg induces coved-type ST elevation in leads V1-3 and prolongs QRS duration from 96 to 158 ms and the PQ interval from 184 to 248 ms (A). Shortly after the appearance of coved-type ST elevation, a premature ventricular contraction induces VF (B). The coved-type ST elevation is reversed by continuous isoproterenol infusion (C).
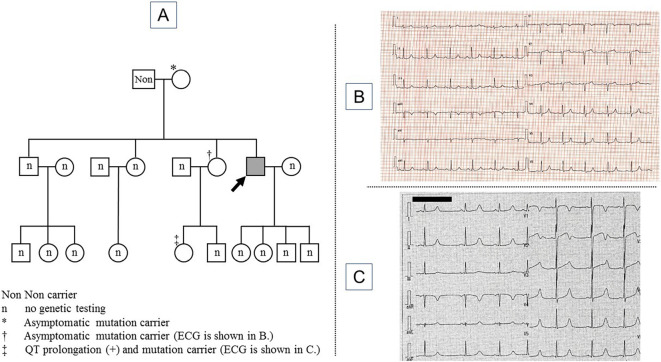
A genetic analysis showed an SCN5A c.5350G>A p.E1784K mutation. This mutation has been reported to be associated with the phenotypic overlap of LQT3 and Brugada syndrome. The patient's mother, elder sister and niece also had the same mutation; the patient's mother and elder sister did not present QT prolongation on ECG, while his niece showed a long QT interval on ECG (Fig. 4).
Figure 4.
The family pedigree (A) and 12-lead electrocardiogram findings of the patient’s elder sister (B) and niece (C). The patient is indicated by an arrow (A). The electrocardiogram of the patient’s elder sister shows normal findings (B). The electrocardiogram of the patient’s niece shows QT prolongation (C).
The diagnosis of overlap syndrome of LQT3 and Brugada syndrome was made based on the findings of pharmacological provocation tests and a genetic analysis. The patient underwent implantation of a subcutaneous-implantable cardioverter defibrillator (S-ICD) and has been followed-up by an internet-based remote monitoring system of the S-ICD and conventional hospital visits. The patient has remained free of ICD shocks to date (as of 12-month follow-up) without any antiarrhythmic drugs. Only one AF event of five minutes' duration was detected on the remote monitoring system.
Discussion
The overlap syndrome of LQT3 and Brugada syndrome has been reported in SCN5A mutations (1-3,8,10). Of them, the SCN5A E1784K mutation accounts for up to 34% of LQT3 cases (6,7). Previous in vitro studies have suggested a negative shift in the steady-state sodium channel inactivation, enhanced sodium channel inactivation, and enhanced tonic block in response to sodium channel blockers as potential mechanisms for phenotypic overlap (9-12). Although this mutation is prevalent in the Okinawa region, neither this patient nor his family members are from the Okinawa region (13).
Makita reported that, of 41 cases with an SCN5A E1784K mutation, 38 (93%) had LQT3, and 9 (22%) had overlaps of LQT3 and Brugada syndrome (10). Of those nine cases, five had spontaneous Brugada-type ST elevation, and four displayed Brugada-type ST elevation during provocation testing with sodium channel blockers. In the remaining 32 patients without spontaneous Brugada-type ST elevation, provocation testing was not performed. However, Veltmann et al. reported that 13 of 15 cases with an SCN5A E1784K mutation presented with the Brugada phenotype by applying the ajmaline test to all cases (7). The difference in the prevalence of the Brugada phenotype between the studies by Makira and Veltmann implies the importance of the aggressive application of sodium channel blockers to unmask the Brugada phenotype in patients with an SCN5A E1784K mutation. The present case displayed only saddleback-type ST elevation on the baseline ECG but showed prominent coved-type ST elevation induced by pilsicainide administration. In addition, spontaneous VF was induced by a single PVC shortly after the appearance of coved-type ST elevation, implying that the Brugada phenotype had participated in the clinical VF.
In general, life-threatening events associated with Brugada syndrome occur in adults and are less frequent in infants and children. A previous in vitro study using induced pluripotent stem cells (iPSCs) showed that the sodium channel β-subunit, SCN3B, which is highly expressed in embryonic hearts and decreases with age, masks the phenotype of Brugada syndrome associated with an SCN5A E1784K mutation (9). Decreases in SCN3B levels with age might be associated with the occurrence of the first VF event in the adult patient in the present case.
Pilsicainide administration also induced PQ interval and QRS duration prolongation, implying the existence of a conduction disturbance which is common in SCN5A E1784K mutation carriers.
The manifestation of coved-type ST elevation was reversed by isoproterenol infusion without exacerbation of QT prolongation in the present case. However, isoproterenol infusion might have exacerbated QT prolongation if LQTS had been type 1 or 2 (14). In general, it takes time to obtain the result of a genetic analysis. In clinical practice, evaluating the responses to various drugs in advance might be useful in cases demonstrating electrical storm. In addition to the isoproterenol infusion test, the mexiletine administration test was performed and induced QT shortening of 40 ms. The diagnosis of LQT3 was therefore deemed feasible based on the results of the isoproterenol infusion test and the mexiletine administration test before the results of the genetic analysis became available (14,15).
The S-ICD represents an important alternative to traditional ICD therapy. The S-ICD was selected instead of a transvenous ICD (T-ICD) for several reasons. First, ICD therapy carries significant risks of complications, such as lead breakage and infection, and the risks of these complications increase as the duration after implantation increases. A previous study reported that lead-related complications and serious bloodstream infections were less common with S-ICDs than with T-ICDs (16). Given the patient's age of 38 years, avoiding these risks was prioritized. Second, the patient had a history of paroxysmal atrial fibrillation, which is a potential cause of inappropriate shock. The rate of inappropriate shock with S-ICDs has been reported to be as low as 1.5% at 1 year, which is lower than that with T-ICDs (16). Third, the major limitation associated with S-ICDs is their inability to provide pacing. Sinus bradycardia or sinus arrest was not observed before implantation. In cases of LQT1 or LQT2, β-blockers are commonly used to prevent ventricular arrhythmia. Use of β-blockers can cause bradycardia, and a combination of β-blockers and continuous cardiac pacing is a therapeutic option for LQTS (17). However, on the isoproterenol administration test, paradoxical QT prolongation was not observed, suggesting that the patient was unlikely to have LQT1 or LQT2. Therefore, the need for cardiac pacing seemed to be low. Furthermore, if the patient were to need cardiac pacing, a combination of S-ICD and a conventional pacemaker would be potential option (18).
In conclusion, a case of phenotypic overlap syndrome of LQT3 and Brugada syndrome due to an SCN5A E1784K mutation was presented. Provocation tests using antiarrhythmic drugs provided useful information for the diagnosis and clinical management in the present case and should be aggressively applied in the management of patients with hereditary arrhythmias.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Hayashi H, Sumiyoshi M, Nakazato Y, Daida H. Brugada syndrome and sinus node dysfunction. J Arrhythm 34: 216-221, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blich M, Efrati E, Marai I, Suleiman M, Gepstein L, Boulus M. Novel clinical manifestation of the known SCN5A D1790G mutation. Cardiology 132: 228-232, 2015. [DOI] [PubMed] [Google Scholar]
- 3.Kusano K, Taniyama M, Nakamura K, et al. . Atrial fibrillation in patients with Brugada syndrome. J Am Coll Cardiol 51: 1169-1175, 2008. [DOI] [PubMed] [Google Scholar]
- 4.Khan IA, Nair CK. Brugada and long QT-3 syndromes: two phenotypes of the sodium channel disease. Ann Noninvasive Electrocardiol 9: 280-289, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yokokawa M, Noda T, Okamura H, et al. . Comparison of long-term follow-up of electrocardiographic features in Brugada syndrome between the SCN5A-positive probands and the SCN5A-negative probands. Am J Cardiol 100: 649-655, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Makita N, Behr E, Shimizu W, et al. . The E1784K mutation in SCN5A is associated with mixed clinical phenotype of type 3 long QT syndrome. J Clin Invest 118: 2219-2229, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Veltmann C, Barajas-Martinez H, Wolpert C, et al. . Further insights in the most common SCN5A mutation causing overlapping phenotype of long QT syndrome, Brugada syndrome, and conduction defect. J Am Heart Assoc 5: e003379, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakajima T, Kaneko Y, Saito A, et al. . Identification of six novel SCN5A mutations in Japanese patients with Brugada syndrome. Int Heart 52: 27-31, 2011. [DOI] [PubMed] [Google Scholar]
- 9.Okata S, Yuasa S, Suzuki T, et al. . Embryonic type Na+ channel β-subunit, SCN3B masks the disease phenotype of Brugada syndrome. Sci Rep 6: 34198, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Makita N. Phenotypic overlap of cardiac sodium channelopathies: Individual-specific or mutation-specific? Circ J 73: 810-817, 2009. [DOI] [PubMed] [Google Scholar]
- 11.Deschênes I, Baroudi G, Berthet M, et al. . Electrophysiological characterization of SCN5A mutations causing long QT (E1784K) and Brugada (R1512W and R1432G) syndromes. Cardiovasc Res 46: 55-65, 2000. [DOI] [PubMed] [Google Scholar]
- 12.Peters CH, Yu A, Zhu W, Silva JR, Ruben PC. Depolarization of the conductance-voltage relationship in the Nav 1.5 mutant, E1784K, is due to altered fast activation. PLoS One 12: e0184605, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takahashi K, Shimizu W, Miyake A, Nabeshima T, Nakayashiro M, Ganaha H. High prevalence of the SCN5A E1784K mutation in school children with long QT syndrome living on the Okinawa islands. Circ J 78: 1974-1979, 2014. [DOI] [PubMed] [Google Scholar]
- 14.Mizusawa Y, Horie M, Wilde AAM. Genetic and clinical advances in congenital long QT syndrome. Circ J 78: 2827-2833, 2014. [DOI] [PubMed] [Google Scholar]
- 15.Anderson HN, Bos JM, Kapplinger JD, Meskill JM, Ye D, Ackerman MJ. Lidocaine attenuation testing: an in vivo investigation of putative LQT3-associated variants in the SCN5A-encoded sodium channel. Heart Rhythm 14: 1173-1179, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boersma L, Barr C, Knops R, et al. . Implant and midterm outcomes of the subcutaneous implantable cardioverter-defibrillator registry. J Am Coll Cardiol 70: 830-841, 2017. [DOI] [PubMed] [Google Scholar]
- 17.Dorostkar PC, Eldar M, Belhassen B, Scheinman MM. Long-term follow-up of patients with long-QT syndrome treated with β-blockers and continuous pacing. Circulation 100: 2431-2436, 1999. [DOI] [PubMed] [Google Scholar]
- 18.Porterfield C, DiMarco JP, Mason PK. Effective of implantation of a subcutaneous implantable cardioverter-defibrillator in a patient with complete heart block and a pacemaker. Am J Cardiol 115: 276-278, 2015. [DOI] [PubMed] [Google Scholar]