Abstract
Mitochondrial diseases are a group of clinically and genetically heterogeneous disorders driven by oxidative phosphorylation dysfunction of the mitochondrial respiratory chain which due to pathogenic mutations of mitochondrial DNA (mtDNA) or nuclear DNA (nDNA). Recent progress in molecular genetics and biochemical methodologies has provided a better understanding of the etiology and pathogenesis of mitochondrial diseases, and this has expanded the clinical spectrum of this conditions. But the treatment of mitochondrial diseases is largely symptomatic and thus does not significantly change the course of the disease. Few clinical trials have led to the design of drugs aiming at enhancing mitochondrial function or reversing the consequences of mitochondrial dysfunction which are now used in the clinical treatment of mitochondrial diseases. Several other drugs are currently being evaluated for clinical management of patients with mitochondrial diseases. In this review, the current status of treatments for mitochondrial diseases is described systematically, and newer potential treatment strategies for mitochondrial diseases are also discussed.
Keywords: Mitochondrial diseases, drug therapy, electron transfer chain, mitochondrial biogenesis, mitochondrial dynamics
Introduction
Mitochondria play multiple functions, such as supplying energy and maintaining intracellular homeostasis, and are widely found in many eukaryotic cells. From the outside to the inside, the mitochondrial structure is divided into four functional areas: the outer membrane, the intermembrane space, the inner membrane, and the matrix. Except glycolysis which takes place in the cytoplasm, other cellular oxidation processes are carried out in mitochondria. The electron transport chain (ETC) of oxidative phosphorylation is located in the inner membrane of mitochondria and consists of five enzyme complexes (I–V), two mobile electron carriers, coenzyme Q10 (CoQ10) and Cyt C. The mitochondrial matrix contains many enzymes and mitochondrial DNA (mtDNA), which together regulates pyruvate oxidation, tricarboxylic acid cycle, and other biochemical reactions (1).
Mitochondrial diseases are a group of clinical and genetic heterogeneous diseases stemming from disputation of the oxidative phosphorylation of mitochondrial respiratory chain caused by the pathogenic mutation of mtDNA or nuclear DNA (nDNA). In addition to abnormal intracellular calcium homeostasis, increased production of reactive oxygen species (ROS), apoptosis disorder, and other cell disorders makes the mitochondria unable to produce enough energy to match needs of various organs, especially the nervous system, skeletal muscle, myocardium, kidney, liver and endocrine system. This suboptimal energy situation in different organs may lead to disruption of various processes which clinically manifest as mitochondrial diseases, including cognitive impairment, seizures, motor disorders, heart diseases, kidney disease, liver disease, and endocrine diseases (2). Because the mitochondria of fertilized eggs only come from eggs, mitochondrial diseases caused by mtDNA mutation show maternal inheritance pattern, which differs from that caused by nDNA mutation. It is worth noting that only a subset of patients with mitochondrial diseases carry mitochondrial mutations, and the the corresponding cell dysfunction and organ damage occurs only when the level of mutant mtDNA in the cells reaches a certain proportion. Moreover, the proportion of mutant and non-mutant mtDNA show significant differences even in cells from the same organ obtained from different patients, or cells from different organs of the same patient. This results in different degrees of organ involvement and hence clinical manifestations, that is to say, the clinical heterogeneity of mtDNA hereditary mitochondrial diseases is immense. Similarly, nDNA hereditary mitochondrial diseases show moderate clinical heterogeneity for some subtypes (3).
With the advancements in molecular genetics and biochemical methodologies, especially for next generation sequencing, researchers have gained substantial in-depth understanding of the etiology and pathogenesis of mitochondrial diseases, this has led to the diagnosis of more mitochondrial diseases. However, progress in the treatment of mitochondrial diseases has been slow. Most treatments for mitochondrial diseases are symptomatic, and this has led to suboptimal control of the disease progress. Clinicians often use antioxidants, vitamins, and auxiliary factors as the mainstay treatments of patients with mitochondrial diseases, among which arginine, CoQ10, L-carnitine and creatine are the most commonly prescribed drugs. Most doctors use a combination of three to six drugs, the so-called “cocktail therapy”. It is not efficient, but can partly improve symptoms. The components of this type of “cocktail therapy” are many and have not been standardized. Although the clinical application of these compounds is based on the current understanding of the pathophysiology of mitochondrial diseases, the evidence base for clinical efficacy is very limited (4).
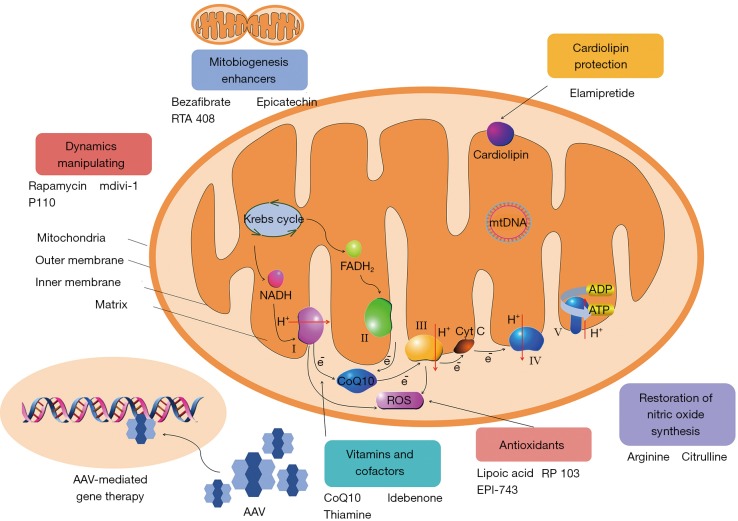
The pathological mechanisms of mitochondrial diseases include: oxidative phosphorylation deficiency, insufficient energy production, toxic effect caused by high ROS production, impaired nitric oxide (NO) synthesis in vascular endothelial cells, fission and fusion disorder, among others (5). These pathogenic mechanisms may provide effective therapeutic targets for mitochondrial diseases. Here, the potential treatment strategies for mitochondrial diseases are discussed on the basis of possible therapeutic targets (Figure 1).
Figure 1.
The potential treatment strategies for mitochondrial diseases. AAV, Adeno-associated virus; ROS, reactive oxygen species; mtDNA, mitochondrial DNA.
The treatment strategies of mitochondrial diseases
Treatments that enhance the function of the electron transfer chain
Some mitochondrial treatments strengthen the function of the ETC by increasing the levels of its components (CoQ10, idebenone) or utilization rate of its substrate (thiamine).
CoQ10 is an important component of ETC, which transmits electrons from complexes I and II to complex III, facilitating oxidative phosphorylation in cells. Primary CoQ10 deficiency is caused by gene defects of enzymes involved in the synthesis of CoQ10 (6). CoQ10 supplementation can restore electron flow and improve the clinical symptoms related to CoQ10 deficiency (7). Some open-label studies have shown that supplementation of CoQ10 may have a beneficial effect on patients with mitochondrial diseases. However, other studies have found that supplementation of CoQ10 has a little effect on aerobic exercise ability and lactic acid after exercise, but has no influence on other clinical variables such as resting lactic acid or muscle strength (8). Accordingly, except for CoQ10 deficiency, the benefits of this supplement for other mitochondrial diseases are minimal. The dosage of CoQ10 is 5 to 30 mg/kg daily divided into two doses. Some studies indicate that the absorption effect of reduced CoQ10 is 3 folds higher than that of oxidized CoQ10, when applied at the dose of 2 to 8 mg/kg daily divided into two doses (9).
Idebenone is an analog of CoQ10, which can readily pass through the cell membrane and blood-brain barrier. It has been used in the clinical treatment of Leber hereditary optic neuropathy (LHON). A clinical study evaluated the efficacy of idebenone on LHON. The main results showed that there were no significant differences in the efficacy of idebenone and placebo in visual recovery. Furthermore, subsequent analysis showed that idebenone could prevent further visual loss, especially in patients with discordant vision (10). Elsewhere, it was reported that although treatment had been stopped for 30 months, the beneficial effects of idebenone from the previous treatment persisted (11). Another clinical trial showed that idebenone prevented color vision loss in patients with LHON, and its efficacy was most evident in individuals with discordant vision (12). The dose of idebenone used ranged from 30 to 300 mg/dose thrice daily.
Thiamine (vitamin B1) can increase the activity of pyruvate dehydrogenase, thus enhance the oxidative decomposition of pyruvate. Thiamine has been used alone or in combination with other drugs in the treatment of mitochondrial diseases. It is reported that thiamine supplementation in a family with thiamine deficiency and mitochondria myopathy, encephalopathy, lactic acidosis, and stroke-like episodes (MELAS syndrome) can improve the symptoms of myopathy and lactic acidosis (13). The combined use of CoQ10, carnitine, thiamine and vitamins C, E significantly improves the clinical symptoms of adult patients with Leigh syndrome with subacute severe brainstem encephalopathy (14). For adults, dosages of thiamine are 100–1,000 mg daily and 10 mg/kg daily for children (15). Thiamine transporter-2 deficiency is caused by SLC19A3 gene mutation. The main manifestations of this disease include neuroimaging features and lactic acidosis of Encephalopathy and Leigh syndrome. Higher dosage of thiamine (20 mg/kg daily) is required for optimal treatment and improvement of neurological and biochemical abnormalities of this disease (16).
Energy buffering
Creatine binds to phosphate in the mitochondria to form phosphocreatine, which is the main source of high energy phosphate release in the process of anaerobics metabolism. Creatine is highest in tissues with high energy demand, including muscles and brain (9). The content of phosphocreatine is decreased in both muscle tissues of patients with mitochondrial myopathy and in brain tissue of patients with mitochondrial encephalopathy (17,18). Supplementation of creatine monohydrate effectively improves the motor ability of patients with mitochondrial myopathy (19). Some studies have shown that creatine monohydrate enhances high intensity aerobic activity and anaerobic activity in individuals with mitochondrial diseases, but has no significant effect on low intensity aerobic activity (20). It is often applied in dosages in the range of 2–10 g daily for adults and 100–300 mg/kg daily for children divided into three doses (9,15).
Antioxidants
During oxidative phosphorylation in the mitochondria, a small portion of oxygen is reduced and converted to ROS. Although ROS have beneficial roles in many signaling pathways, excessive ROS is toxic to cells. In normal conditions, ROS is eliminated by the mitochondrial glutathione peroxidase and superoxide dismutase (21). In addition, abnormal ETC can also increase the production of ROS (22). ROS can irreversibly modify numerous cellular macromolecules. And excessive ROS production in mitochondrial diseases is a cause of lipid, protein and DNA damage, which further disrupts cellular processes (21). Antioxidants reduce the toxicity of excessive ROS in mitochondrial diseases and hence they can be used for the treatment of mitochondrial diseases. Vitamin C and E are sometimes combined with other drugs to manage patients with mitochondrial diseases. Some studies have, however, found that this is not very beneficial to some patients (4).
Lipoic acid is a coenzyme of pyruvate dehydrogenase and α-ketoglutarate dehydrogenase. It is also an effective antioxidant that reduces oxidative stress markers (23). Lipoic acid is usually used in conjunction with other antioxidants in patients with mitochondrial diseases (9). Some studies have shown that the combination of lipoic acid, CoQ10 and creatine monohydrate effectively reduces plasma lactic acid content and oxidative stress markers in urine, and improves the symptoms of muscle strength in patients with mitochondrial diseases (24). The frequently used dosage is 300–600 mg daily or 25 mg/kg daily (15).
Glutathione is an important intracellular antioxidant, and its synthesis mainly relies on the availability of cysteine. Because some patients with mitochondrial diseases may be deficient in glutathione levels, cysteine supplementation in such patients may help to eliminate excessive ROS production by restoring the level of glutathione (25). Cysteamine is an amino thiol mainly used in the treatment of cystine storage disease, that is a lysosome storage disease. The deficiency of the lysosomal cystine transporter results in cystine accumulation in cells. Entry of cysteamine into the lysosome destroys the disulfide bond of cystine, resulting in the generation of cysteine-cysteamine disulfide and cysteine. Cysteine exits from the lysosome with the assistance of cystine transporter. Therefore, cysteamine can increase the level of intracellular glutathione by providing the cysteine required for the synthesis of reduced glutathione (26). RP 103, cysteamine bitartrate delayed-release capsule, has been used to evaluate its efficacy, safety, tolerance, pharmacodynamics, and pharmacokinetics in children with mitochondrial diseases in an open-label, dose-escalating study. The primary outcome measure was assessed based on Newcastle Pediatric Mitochondrial Disease Scale Score. Other metabolites, including glutathion disulfide, glutathion and lactic acid, were assessed as secondary outcome measures. This study has been already completed and a follow-up information analysis is being prepared for release (27).
EPI-743 is a synthetic analog of vitamin E with better pharmacological and therapeutic effects. It can prevent excessive ROS production by affecting the redox state of intracellular glutathione (28,29). An open-label study of children with Leigh syndrome reported that EPI-743 supplements can stabilize the disease and reverse the disease process (30). Another open-label study has shown that EPI-743 can change the course of mitochondrial diseases, improving the clinical symptoms of some patients at risk of progressing to end-of-life care within 90 days (28). At present, several studies on EPI-743 are being carried out. One randomized, double-blind, placebo-controlled has completed the recruitment of children with mitochondrial diseases and is in a follow-up phase. The primary outcome measure was the effect of EPI-743 on quality of life. Secondary outcome measures included clinical manifestations, imaging, and various biochemical (https://clinicaltrials.gov/ct2/show/nct01642056).
Restoration of NO synthesis
NO, produced by vascular endothelial cells, plays an essential part in maintaining the relaxation of vascular smooth muscles and keeping small vessels unobstructed. Abnormal mitochondrial proliferation of vascular endothelial cells can lead to endothelial dysfunction and compromise endothelial NO synthesis. Besides reduced energy production, accumulating evidence indicate that NO deficiency in mitochondrial diseases plays an essential role in the pathogenesis of several complications, including myopathy, stroke-like episodes, lactic acidosis and diabetes (31,32). As precursors of NO, citrulline and arginine can be used to restore the synthesis of NO. Accordingly, citrulline and arginine have therapeutic effects on symptoms related to NO deficiency in mitochondrial diseases (31,32). So far, few clinical studies have evaluated the impact of citrulline and arginine on patients with mitochondrial diseases. These studies have shown that arginine can improve the clinical symptoms associated with stroke-like episodes and reduce the severity and frequency of these attacks in patients with MELAS syndrome (33). Therefore, it is prudent to further study the clinical effects of citrulline and arginine on different aspects of mitochondrial diseases to clarify the potential therapeutic value of these drugs. A random cross-over study evaluating the effects of citrulline and arginine supplementation on endothelial dysfunction in children suffering from mitochondrial diseases is underway. The primary outcome of the study is the change of reactive hyperemia index (reflecting endothelial function) after the supplementation of citrulline and arginine (https://clinicaltrials.gov/ct2/show/nct02809170).
Cardiolipin protection
Cardiolipin is mainly expressed in the intima of mitochondria, and it is a unique phospholipid. It plays a significant role in regulating the curvature of mitochondrial intima, forming cristae and assembling ETC complexes into supercomplexes. Cardiolipin can also anchor cytochrome c to the intima and promote the transfer of electrons from complex III to complex IV (34). In addition, when oxidized, cardiolipin participates in cell death. Cardiolipin is highly vulnerable to oxidative damage because it contains many of unsaturated fatty acids and is located close to the production site of ROS. Oxidation of cardiolipin leads to the destruction of intimal microregions and the loss of membrane curvature and cristae. It also interferes with the supercomplex, releases cytochrome c from the intima, thereby compromising oxidative phosphorylation and energy generation (35). Moreover, the synergy of oxidized cardiolipin and calcium leads to the opening of the mitochondrial permeability transition pores, resulting in the release of cytochrome c and other proapoptotic proteins into the cytoplasm, an effect that activates the caspase cascade and cell death by apoptosis (36). Elamipretide is an aromatic cationic tetrapeptide, which selectively binds to cardiolipin through hydrophobic and electrostatic interaction to protect it from oxidative damage. It also protects the cristae by inhibiting the oxidation of cardiolipin. It can inhibit the opening of mitochondrial permeability transition pores and promote oxidative phosphorylation as well as energy production. For this reason, it is used as a drug for primary mitochondrial myopathy (PMM). Studies have tested the impact of elamipretide on aging, ischemia and heart failure under pathological conditions related to mitochondrial injury (36). In a study of patients with mitochondrial myopathy, the primary endpoints of the phase 2 trial were tolerability, safety and improvement of walking distance on 6-minute walk test in the MMPOWER (A Study Investigating the Safety, Tolerability, and Efficacy of MTP-131 for the Treatment of Mitochondrial Myopathy)-1 and MMPOWER-2 studies. The results showed that elamipretide can improve the motor ability of PMM patients and resolve the symptoms related to myopathy without increasing the safety related problems (37).
Enhancing mitochondrial biogenesis
As the energy demand increases, more biogenic mitochondrial cells are produced, and this process is driven by the activation of PGC-1α, a transcriptional co-activator known as the master regulator of mitochondrial biogenesis. PGC-1α is activated by factors such as declining ATP production and increased NAD+. The expression and activity of PGC-1α are also regulated by the peroxi-some proliferative-activated receptors (PPARs). Activation leads to transcription of nuclear-encoded mitochondrial gene and consequently mitochondrial biogenesis, increasing the amount of ATP production (38,39). Given that activation of PCG-1α can potentially alleviate the lack of ATP in patients with mitochondrial diseases, development of drugs to promote mitochondrial biogenesis presents an active research field in the treatment of mitochondrial diseases.
Bezafibrate is a PPAR activator that can activate PPAR-PCG-1α pathway to enhance mitochondrial biogenesis. It is a common drug for the treatment of dyslipidemia. A mouse model of cytochrome c oxidase deficiency treated with bezafibrate showed increased oxidative phosphorylation due to activation of mitochondrial biogenesis, an effect that increased energy production and improved the disease phenotype (40). A study is under way to assess the role of bezafibrate on adult mitochondrial myopathy. The main assessment results are changes in the activity of ETC, and a variety of clinical indicators and other biochemical variables are secondary outcomes (https://clinicaltrials.gov/ct2/show/nct02398201) (27).
Epicatechin is an isoflavone derived from cocoa, and it has the biological characteristics of mitochondria. It has been reported that mice fed with epicatechin show improved motor ability and anti-fatigue, and this is accompanied with enhanced biogenesis of mitochondria, and increased ETC proteins, porin, mitofilin, mitochondrial volume, mitochondrial transcription factor A (Tfam), and cristae abundance (41). An in vitro study on bovine coronary artery endothelial cells showed that epicatechin enhanced the function of mitochondria by increasing the activity of citric acid synthase and inducing changes in the structure of mitochondria and the level of oxidative phosphorylation protein (42). An open-label study is under way to assess the safety and effectiveness of epicatechin in individuals with Friedreich ataxia. The primary outcome measures are the changes in the comprehensive score of a clinical score scale and the changes in ventricular hypertrophy from baseline assessed by cardiac MRI (https://clinicaltrials.gov/ct2/show/nct02660112).
RTA 408 is a synthetic isoprenoid that increases the activity of the nuclear respiratory factor 2 (Nrf 2), a downstream effector of PGC-1α and an activator of mitochondrial biogenesis (39). A study based on mouse models of amyotrophic lateral sclerosis (ALS), RTA 408 increased the levels of glutathione and promoted mitochondrial biogenesis (43). A MOTOR study is underway which has two parts. The purpose of the first part is to assess the safety of different doses of RTA 408 in patients with mitochondrial myopathy. The second part is to assess the efficacy, safety, and pha0rmacokinetics of RTA 408 in patients with mitochondrial myopathy when the dose level is no more than double. The primary outcome is the change of the peak value of exercise volume during the exercise test, and the secondary outcome is the change of walking distance in the 6-minute walking test (https://clinicaltrials.gov/ct2/show/nct02255422) (27).
Manipulating mitochondrial dynamics
As a dynamic organelle, mitochondrion often undergoes fusion and fission to adjust to the changes in the cellular environment. In physiological conditions, the fission and fusion of mitochondria occur in a consistent and balanced manner to adapt to the morphological mitochondrial network of the metabolic needs of the cell (44). Mitochondrial autophagy is a cell recycling process in which damaged or dysfunctional mitochondria are selectively targeted by autophagosomes which deliver them to lysosomes. In addition, mitophagy may play an important role in diseases caused by mtDNA mutations by blocking the expansion of heteroplasmy (45). Manipulation of mitochondrial dynamics may be a potential method of treating mitochondrial diseases because the balance between fission and fusion processes controls the repair of damaged mitochondria through mitochondrial fusion, or the selective elimination of abnormal mitochondria through mitochondrial autophagy.
In recent years, drugs to treat mitochondrial diseases by promoting mitochondrial autophagy have been put on the market. Rapamycin and its derivatives are the most studied studies drugs. Rapamycin promotes autophagy by competitively inhibiting mTOR (a target of rapamycin) complex. In a mouse model of Leigh syndrome, rapamycin treatment delayed the onset and progression of neurological symptoms in Ndufs4−/− model by inhibiting mTOR, reducing neuroinflammation which effectively prolonged the survival time of mice (46). In another study, rapamycin improved the exercise endurance of specific Cox15 knockout mice (Cox15sm/sm) by coordinated activation of autophagy and lysosome biogenesis, correction of morphological abnormalities of muscles, and increasing the activity of cytochrome c oxidase (Cox) in muscles (47).
Another strategy for treating mitochondrial diseases is the use of agents which inhibit mitochondrial fission such as mdivi-1 (mitochondrial division inhibitor 1), an inhibitor of Drp 1 that selectively blocks mitochondrial fission (48). It has been reported that mdivi-1 corrects morphological and functional defects of mitochondria caused by PINK1 mutation (49). The selective peptide inhibitor P110, another inhibitor of the mitochondrial fission protein Drp1, may be beneficial as it reduces abnormal mitochondrial fission in these diseases (50).
Other potential treatment
MtDNA replacement therapy: due to the congenital mutation related mitochondrial diseases, significant progress has been made in the study of mtDNA genetics in recent years, as evidenced by the developments in mitochondrial replacement therapy (MRT) (51). MRT prevents the inheritance of mutated mtDNA from mother to offspring through in vitro fertilisation. In this process, the nuclear genome of the individual’s egg is transferred to the enucleated healthy egg before normal fertilization. In this manner, mitochondrial diseases induced by mtDNA mutation can be prevented. Nevertheless, there are uncertainties about the long-term application prospects of this technology. MRT is not suitable for mitochondrial diseases caused by nDNA mutations that encode most of the proteins, rather only for mtDNA mutations. However the proteins encoded by mtDNA account for only 13 out of the 1,000 proteins expressed in mitochondria. As a result, the proportion of suitable patients is quite small. In addition, MRT can only prevent the occurrence of mitochondrial diseases in the offspring of mutant carriers, but can not treat the secondary mitochondrial dysfunction induced by environmental stress or the existing mitochondrial diseases. Social ethical disputes have also limited the development and application of this technology. As a result, this technology can be a potential complement but cannot be regarded as a mainstay treatment.
Hypoxia therapy: chronic hypoxia has been found to significantly improve the survival rate, body temperature, body weight, behavior, disease biomarkers and neuropathology in mice, which are the most common manifestations of mitochondrial diseases in children. In a study of Leigh syndrome mice with Ndufs4 gene disruption, the results showed that chronic hypoxia (11% O2) prolonged the lifespan of mice and alleviated the disease phenotype of model mice, while chronic hyperoxia (55% O2) aggravated the disease phenotype. These findings suggest that hypoxia may not only trigger innate adaptation procedures, but also limit the accumulation of toxic oxygen substrate, thus it may be a natural solution to overcome the pathology of mitochondrial diseases. Further preclinical studies are required to assess whether hypoxia exposure can be a safe and effective treatment for mitochondrial diseases (52).
Gene therapy: neither metabolic pathway nor symptomatic treatment can fundamentally correct mitochondrial diseases given their genetic basis. Thus, gene therapy is the ideal approach. Up to now, most advances in gene therapy for mitochondrial diseases are aimed at LHON. About 70% of individuals with LHON carry pathogenic variations of mtDNA encoding complex IV subunit 4 (MT-ND4). The adeno-associated virus (AAV) can be used as the vehicle of mtDNA, and its capsid VP2 can fuse with plasmid targeting sequence, which reexpresses ND4. The expression of wild type ND4 in ND4 mutant cells can restore ATP synthesis defects. Some studies have shown that when human MT-ND4 DNA is injected into rodent eyes, its level in mitochondria can reach 80% of that of mouse homologues. It can also be expressed in most retinal neurons where it inhibits optic atrophy and visual loss caused by mutant ND4 homologue (53). Five patients with LHON carrying m.11778G>A mutation were treated with unilateral intravitreal injection of AAV vector. Three cases showed no significant improvement in visual acuity, two cases exhibited increased visual acuity, without any serious adverse events (54). The results of subsequent trials after this study showed that the average visual acuity of 12 patients with bilateral visual impairment after unilateral intravitreous injection was improved to some extent. The study confirmed the safety of allogeneic therapy for LHON (55). Recently, two teams used the classic gene editing techniques ZFN and TALENs to eliminate the mutation mtDNA in animals which leads to mitochondrial diseases. This is the first time gene editing technology was used to edit mtDNA in vivo, pointing to the possibility of curing mitochondrial diseases using gene editing strategies (56,57). Although research on its application is at an early stage, it does show great promise.
Symptomatic treatment, exercise and diet treatment of mitochondrial diseases
The symptomatic treatment of mitochondrial diseases includes physiotherapy for muscle strength loss and motor retardation, cochlear implantation or hearing aid for hearing loss, cardiac pacemaker for arrhythmia, slow injection of sodium bicarbonate during acute aggravation of lactic acidosis, surgical treatment of blepharoptosis, use of trypsin to treat pancreatic exocrine dysfunction, diet, sulfonylurea and insulin in the treatment of diabetes.
Exercise is beneficial to patients with mitochondrial diseases. Indeed, the lack of exercise in healthy individuals may lead to the decline of mitochondrial ETC activity, whereas the activity of ETC can be improved by endurance training. Resistance training triggers the transfer of normal mtDNA templates from satellite cells to mature muscles, thus reducing the heterogeneity of mutations, because there are fewer mitochondria containing mutant mtDNA in satellite cells. In addition, exercise can promote the biogenesis of mitochondria by activating PCG-1α (58,59).
There are no specific dietary treatments that show clear benefits for patients with mitochondrial diseases. Because optimizing the quantity and quality of calorie intake can improve the oxidative phosphorylation ability of patients with mitochondrial diseases, it is imperative to conduct comprehensive nutritional evaluation and support for patients with mitochondrial diseases (60,61). A high-fat and low-carbohydrate diet is recommended because aerobic oxidation is the main process of glucose oxidation, while a high-carbohydrate diet may confer metabolic difficulties in patients with dysfunctional oxidative phosphorylation. Ketogenic diet is beneficial to patients with pyruvate dehydrogenase deficiency. Some studies have shown that implementation of long-term ketogenic diet at early stage or the strict restriction of carbohydrate intake after onset of pyruvate dehydrogenase deficiency, prolonges the life expectancy to a certain extent, and improves intellectual development to some extent (62).
Summary and prospect
Some of the drugs intended to enhance mitochondrial function or to treat the consequences of mitochondrial dysfunction have been applied in the clinical treatment of mitochondrial diseases, but the application of these drugs is based on limited clinical trials and are only successful in certain mitochondrial diseases. Consequently, the treatment of mitochondrial diseases is largely symptomatic and this does not significantly change the course of such diseases. Given the lack of effective treatments for many mitochondrial diseases, several clinical studies have been designed to investigate different aspects of mitochondrial diseases. These studies have shown great promise and are expected to provide more effective treatment strategies for mitochondrial diseases. Some of the drugs that are currently being assessed include mitochondrial biogenesis enhancers (bezafibrate, epicatechin, and RTA 408), antioxidants (RP 103 and EPI-743), and cardiolipin protector (elamipretide). Gene therapy has shown positive results in the treatment of LHON, and the first successful gene therapy based on editing of the mtDNA in vivo has brought hope of curing mitochondrial diseases. Moreover other potential therapeutic methods are expected to provide more treatment options of mitochondrial diseases.
Acknowledgments
None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Qiu WS, Chen YJ. The structure and function of mitochondria and its usual research methods. Int J Anesth Resus 2007;28:282-5. [Google Scholar]
- 2.El-Hattab AW, Scaglia F. Mitochondrial cytopathies. Cell Calcium 2016;60:199-206. 10.1016/j.ceca.2016.03.003 [DOI] [PubMed] [Google Scholar]
- 3.Stewart JB, Chinnery PF. The dynamics of mitochondrial DNA heteroplasmy: implications for human health and disease. Nat Rev Genet 2015;16:530-42. 10.1038/nrg3966 [DOI] [PubMed] [Google Scholar]
- 4.Enns GM. Treatment of Mitochondrial Disorders. J Child Neurol 2014;29:1235-40. 10.1177/0883073814538509 [DOI] [PubMed] [Google Scholar]
- 5.Rahman J, Rahman S. Mitochondrial medicine in the omics era. Lancet 2018;391:2560-74. 10.1016/S0140-6736(18)30727-X [DOI] [PubMed] [Google Scholar]
- 6.Potgieter M, Pretorius E, Pepper MS. Primary and secondary CoQ10 deficiency: the role of therapeutic supplementation. Nutr Rev 2013;71:180-8. 10.1111/nure.12011 [DOI] [PubMed] [Google Scholar]
- 7.Horvath R. Update on clinical aspects and treatment of selected vitamin-responsive disorders II (riboflavin and CoQ10). J Inherit Metab Dis 2012;35:679-87. 10.1007/s10545-011-9434-1 [DOI] [PubMed] [Google Scholar]
- 8.Glover EI, Martin J, Maher A, et al. A randomized trial of CoQ10 in mitochondrial disorders. Muscle Nerve 2010;42:739-48. 10.1002/mus.21758 [DOI] [PubMed] [Google Scholar]
- 9.Parikh S, Saneto R, Falk MJ, et al. A modern approach to the treatment of mitochondrial diseases. Curr Treat Options Neurol 2009;11:414-30. 10.1007/s11940-009-0046-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klopstock T, Yu-Wai-Man P, Dimitriadis K, et al. A randomized placebo-controlled trial of idebenone in Leber’s hereditary optic neuropathy. Brain 2011;134:2677-86. 10.1093/brain/awr170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klopstock T, Metz G, Yu-Wai-Man P, et al. Persistence of the treatment effect of idebenone in Leber’s hereditary optic neuropathy. Brain 2013;136:e230. 10.1093/brain/aws279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rudolph G, Dimitriadis K, Buchner B, et al. Effects of idebenone on color vision in patients with leber hereditary optic neuropathy. J Neuroophthalmol 2013;33:30-6. 10.1097/WNO.0b013e318272c643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sato Y, Nakagawa M, Higuchi I, et al. Mitochondrial myopathy and familial thiamine deficiency. Muscle Nerve 2000;23:1069-75. [DOI] [PubMed] [Google Scholar]
- 14.Mermigkis C, Bouloukaki I, Mastorodemos V, et al. Medical treatment with thiamine, coenzyme Q, vitamins E and C, and carnitine improved obstructive sleep apnea in an adult case of Leigh disease. Sleep Breath 2013;17:1129-35. 10.1007/s11325-013-0816-5 [DOI] [PubMed] [Google Scholar]
- 15.Alfadhel M, Al-Thihli K, Moubayed H, et al. Drug treatment of inborn errors of metabolism: a systematic review. Arch Dis Child 2013;98:454-61. 10.1136/archdischild-2012-303131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pérez-Dueñas B, Serrano M, Rebollo M, et al. Reversible lactic acidosis in a newborn with thiamine transporter-2 deficiency. Pediatrics 2013;131:e1670-5. 10.1542/peds.2012-2988 [DOI] [PubMed] [Google Scholar]
- 17.Moroni I, Bugiani M, Bizzi A, et al. Cerebral white matter involvement in children with mitochondrial encephalopathies. Neuropediatrics 2002;33:79. 10.1055/s-2002-32372 [DOI] [PubMed] [Google Scholar]
- 18.Tarnopolsky MA, Parise G. Direct measurement of high-energy phosphate compounds in patients with neuromuscular disease. Muscle Nerve 1999;22:1228-33. [DOI] [PubMed] [Google Scholar]
- 19.Tarnopolsky MA. Creatine as a therapeutic strategy for myopathies. Amino Acids 2011;40:1397-1407. 10.1007/s00726-011-0876-4 [DOI] [PubMed] [Google Scholar]
- 20.Tarnopolsky MA, Roy BD, MacDonald JR. A randomized, controlled trial of creatine monohydrate in patients with mitochondrial cytopathies. Muscle Nerve 1997;20:1502-9. [DOI] [PubMed] [Google Scholar]
- 21.Balaban RS, Nemoto S, Finkel T. Mitochondria, Oxidants, and Aging. Cell 2005;120:483-95. 10.1016/j.cell.2005.02.001 [DOI] [PubMed] [Google Scholar]
- 22.Smeitink JA, Zeviani M, Turnbull DM, et al. Mitochondrial medicine: A metabolic perspective on the pathology of oxidative phosphorylation disorders. Cell Metab 2006;3:9-13. 10.1016/j.cmet.2005.12.001 [DOI] [PubMed] [Google Scholar]
- 23.Marangon K, Devaraj S, Tirosh O, et al. Comparison of the effect of alpha-lipoic acid and alpha-tocopherol supplementation on measures of oxidative stress. Free Radic Biol Med 1999;27:1114-21. 10.1016/S0891-5849(99)00155-0 [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez MC, MacDonald JR, Mahoney DJ, et al. Beneficial effects of creatine, CoQ10, and lipoic acid in mitochondrial disorders. Muscle Nerve 2007;35:235-42. 10.1002/mus.20688 [DOI] [PubMed] [Google Scholar]
- 25.Avula S, Parikh S, Demarest S, et al. Treatment of Mitochondrial Disorders. Curr Treat Options Neurol 2014;16:292. 10.1007/s11940-014-0292-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Besouw M, Masereeuw R, van den Heuvel L, et al. Cysteamine: an old drug with new potential. Drug Discov Today 2013;18:785-92. 10.1016/j.drudis.2013.02.003 [DOI] [PubMed] [Google Scholar]
- 27.El-Hattab AW, Zarante AM, Almannai M, et al. Therapies for mitochondrial diseases and current clinical trials. Mol Genet Metab 2017;122:1-9. 10.1016/j.ymgme.2017.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Enns GM, Kinsman SL, Perlman SL, et al. Initial experience in the treatment of inherited mitochondrial diseases with EPI-743. Mol Genet Metab 2012;105:91-102. 10.1016/j.ymgme.2011.10.009 [DOI] [PubMed] [Google Scholar]
- 29.Shrader WD, Amagata A, Barnes A, et al. α-Tocotrienol quinone modulates oxidative stress response and the biochemistry of aging. Bioorg Med Chem Lett 2011;21:3693-8. 10.1016/j.bmcl.2011.04.085 [DOI] [PubMed] [Google Scholar]
- 30.Martinelli D, Catteruccia M, Piemonte F, et al. EPI-743 reverses the progression of the pediatric mitochondrial diseases—Genetically defined Leigh syndrome. Mol Genet Metab 2012;107:383-8. 10.1016/j.ymgme.2012.09.007 [DOI] [PubMed] [Google Scholar]
- 31.El-Hattab AW, Emrick LT, Hsu JW, et al. Impaired nitric oxide production in children with MELAS syndrome and the effect of arginine and citrulline supplementation. Mol Genet Metab 2016;117:407-12. 10.1016/j.ymgme.2016.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.El-Hattab AW, Hsu JW, Emrick LT, et al. Restoration of impaired nitric oxide production in MELAS syndrome with citrulline and arginine supplementation. Mol Genet Metab 2016;117:407-12. 10.1016/j.ymgme.2016.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koga Y, Povalko N, Inoue E, et al. Therapeutic regimen of L-arginine for MELAS: 9-year, prospective, multicenter, clinical research. J Neurol 2018;265:2861-74. 10.1007/s00415-018-9057-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schlame M, Ren M. The role of cardiolipin in the structural organization of mitochondrial membranes. Biochim Biophys Acta 2009;1788:2080-3. 10.1016/j.bbamem.2009.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mancuso M, Orsucci D, LoGerfo A, et al. Oxidative stress biomarkers in mitochondrial myopathies, basally and after cystine donor supplementation. J Neurol 2010;257:774-81. 10.1007/s00415-009-5409-7 [DOI] [PubMed] [Google Scholar]
- 36.Szeto HH. First-in-class cardiolipin-protective compound as a therapeutic agent to restore mitochondrial bioenergetics. Br J Pharmacol 2014;171:2029-50. 10.1111/bph.12461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karaa A, Haas R, Goldstein A, et al. Randomized dose-escalation trial of elamipretide in adults with primary mitochondrial myopathy. Neurology 2018;90:e1212-21. 10.1212/WNL.0000000000005255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liang H, Ward WF. PGC-1α: a key regulator of energy metabolism. Adv Physiol Educ 2006;30:145-51. 10.1152/advan.00052.2006 [DOI] [PubMed] [Google Scholar]
- 39.Rai PK, Russell OM, Lightowlers RN, et al. Potential compounds for the treatment of mitochondrial diseases. Br Med Bull 2015;116:5-18. [DOI] [PubMed] [Google Scholar]
- 40.Noe N, Dillon L, Lellek V, et al. RETRACTED: Bezafibrate improves mitochondrial function in the CNS of a mouse model of mitochondrial encephalopathy. Mitochondrion 2013;13:417-26. 10.1016/j.mito.2012.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nogueira L, Ramirez-Sanchez I, Perkins GA, et al. (-)-Epicatechin enhances fatigue resistance and oxidative capacity in mouse muscle. J Physiol 2011;589:4615-31. 10.1113/jphysiol.2011.209924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moreno-Ulloa A, Cid A, Rubio-Gayosso I, et al. Effects of (−)-epicatechin and derivatives on nitric oxide mediated induction of mitochondrial proteins. Bioorg Med Chem Lett 2013;23:4441-6. 10.1016/j.bmcl.2013.05.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neymotin A, Calingasan NY, Wille E, et al. Neuroprotective effect of Nrf2/ARE activators, CDDO ethylamide and CDDO trifluoroethylamide, in a mouse model of amyotrophic lateral scleROSis. Free Radic Biol Med 2011;51:88-96. 10.1016/j.freeradbiomed.2011.03.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suen DF, Norris KL, Youle RJ. Mitochondrial dynamics and apoptosis. Gene Dev 2008;22:1577-90. 10.1101/gad.1658508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.East DA, Campanella M. Mitophagy and the therapeutic clearance of damaged mitochondria for neuroprotection. Int J Biochem Cell Biol 2016;79:382-7. 10.1016/j.biocel.2016.08.019 [DOI] [PubMed] [Google Scholar]
- 46.Johnson SC, Yanos ME, Kayser E, et al. mTOR Inhibition Alleviates Mitochondrial diseases in a Mouse Model of Leigh syndrome. Science 2013;342:1524-8. 10.1126/science.1244360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Civiletto G, Dogan SA, Cerutti R, et al. Rapamycin rescues mitochondrial myopathy via coordinated activation of autophagy and lysosomal biogenesis. EMBO Mol Med 2018. doi: . 10.15252/emmm.201708799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cassidy-Stone A, Chipuk JE, Ingerman E, et al. Chemical Inhibition of the Mitochondrial Division Dynamin Reveals Its Role in Bax/Bak-Dependent Mitochondrial Outer Membrane Permeabilization. Dev Cell 2008;14:193-204. 10.1016/j.devcel.2007.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qi X, Qvit N, Su Y, et al. A novel Drp1 inhibitor diminishes aberrant mitochondrial fission and neurotoxicity. J Cell Sci 2013;126:789-802. 10.1242/jcs.114439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Malena A, Loro E, Di Re M, et al. Inhibition of mitochondrial fission favours mutant over wild-type mitochondrial DNA. Hum Mol Genet 2009;18:3407-16. 10.1093/hmg/ddp281 [DOI] [PubMed] [Google Scholar]
- 51.Paull D, Emmanuele V, Weiss KA, et al. Nuclear genome transfer in human oocytes eliminates mitochondrial DNA variants. Nature 2013;493:632-7. 10.1038/nature11800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jain IH, Zazzeron L, Goli R, et al. Hypoxia as a therapy for mitochondrial diseases. Science 2016;352:54-61. 10.1126/science.aad9642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu H, Koilkonda RD, Chou TH, et al. Gene delivery to mitochondria by targeting modified adenoassociated virus suppresses Leber's hereditary optic neuropathy in a mouse model. Proc Natl Acad Sci U S A 2012;109:E1238-47. 10.1073/pnas.1119577109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Feuer WJ, Schiffman JC, Davis JL, et al. Gene Therapy for Leber Hereditary Optic Neuropathy. Ophthalmology 2016;123:558-70. 10.1016/j.ophtha.2015.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guy J, Feuer WJ, Davis JL, et al. Gene Therapy for Leber Hereditary Optic Neuropathy. Ophthalmology 2017;124:1621-34. 10.1016/j.ophtha.2017.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gammage PA, Viscomi C, Simard M, et al. Genome editing in mitochondria corrects a pathogenic mtDNA mutation in vivo. Nat Med 2018;24:1691-5. 10.1038/s41591-018-0165-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bacman SR, Kauppila JHK, Pereira CV, et al. MitoTALEN reduces mutant mtDNA load and restores tRNAAla levels in a mouse model of heteroplasmic mtDNA mutation. Nat Med 2018;24:1696-700. 10.1038/s41591-018-0166-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Taivassalo T, Shoubridge EA, Chen J, et al. Aerobic conditioning in patients with mitochondrial myopathies: physiological, biochemical, and genetic effects. Ann Neurol 2001;50:133-41. 10.1002/ana.1050 [DOI] [PubMed] [Google Scholar]
- 59.Kang C, Li Ji L. Role of PGC-1α signaling in skeletal muscle health and disease. Ann Ny Acad Sci 2012;1271:110-7. 10.1111/j.1749-6632.2012.06738.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morava E, Rodenburg R, van Essen HZ, et al. Dietary intervention and oxidative phosphorylation capacity. J Inherit Metab Dis 2006;29:589. 10.1007/s10545-006-0227-x [DOI] [PubMed] [Google Scholar]
- 61.Wortmann SB, Zweers-van EH, Rodenburg RJ, et al. Mitochondrial energy production correlates with the age-related BMI. Pediatr Res 2009;65:103-8. 10.1203/PDR.0b013e31818d1c8a [DOI] [PubMed] [Google Scholar]
- 62.Wexler ID, Hemalatha SG, McConnell J, et al. Outcome of pyruvate dehydrogenase deficiency treated with ketogenic diets. Studies in patients with identical mutations. Neurology 1997;49:1655-61. 10.1212/WNL.49.6.1655 [DOI] [PubMed] [Google Scholar]