Abstract
Background
Hereditary diffuse leukoencephalopathy with spheroid (HDLS) is an autosomal dominant white matter disease characterized by adult-onset cognitive impairment, behavioral or emotional changes, paresis, Parkinsonism, and seizures. Mutations in the gene encoding colony-stimulating factor 1 receptor (CSF1R) have been identified as the cause of HDLS.
Methods
Detail medical history, clinical features and brain imaging of a patient with adult-onset leukoencephalopathy, cognitive impairment and motor dysfunction was reviewed and next generation sequencing was performed. An extensive literature research was then performed to identify all patients with HDLS previously reported. The clinical characteristics, brain imaging and genetic features of patients with HDLS were reviewed.
Results
A novel CSF1R mutation, c.1952G>A p.G651E was identified in the patient. Extensive review showed that HDLS typically presents with broad phenotypic variability. The most common symptoms of HDLS were cognitive impairment, followed by psychiatric symptoms, Parkinsonism, gait disorder, and dysphagia. The most common brain imaging findings of HDLS were bilateral white matter lesion, mostly around the ventricles, frontal lobe, and parietal lobe. Calcifications in white matter on CT, cerebral atrophy and thinning of corpus callosum were also common features. Although HDLS demonstrates an autosomal dominant pattern, sporadic cases are not uncommon.
Conclusions
Early recognition of clinical and neuroradiographical characteristics of HDLS is key for the correct diagnosis of the disease.
Keywords: Colony-stimulating factor 1 receptor (CSF1R), hereditary diffuse leukoencephalopathy with spheroid (HDLS), clinical features, genetics
Introduction
Hereditary diffuse leukoencephalopathy with spheroids (HDLS) is an autosomal dominant disease that causes adult-onset cognitive impairment, behavioral or emotional changes, paresis, Parkinsonism, and seizures (1). In 1984, Axelsson et al. first reported a family with HDLS (2). In 2011, mutations in the colony-stimulating factor 1 receptor (CSF1R) were identified as the cause of HDLS (3). Thereafter, CSF1R mutations linked two previously separate disease entities, HDLS and pigmented orthochromatic leukodystrophy, as a single disease (4). Patients with HDLS show significant variability in phenotypes; therefore, these patients have often been misdiagnosed with other diseases. To date, more than 60 CSF1R mutations have been reported. Most patients with HDLS caused by CSF1R mutations were identified in Japanese, European, and American populations (5). Here we report a Chinese patient with HDLS due to a novel CSF1R mutation. We also review all patients with HDLS reported in the literature and summarize the clinical, brain imaging and genetic characteristics of HDLS.
Methods
Case report
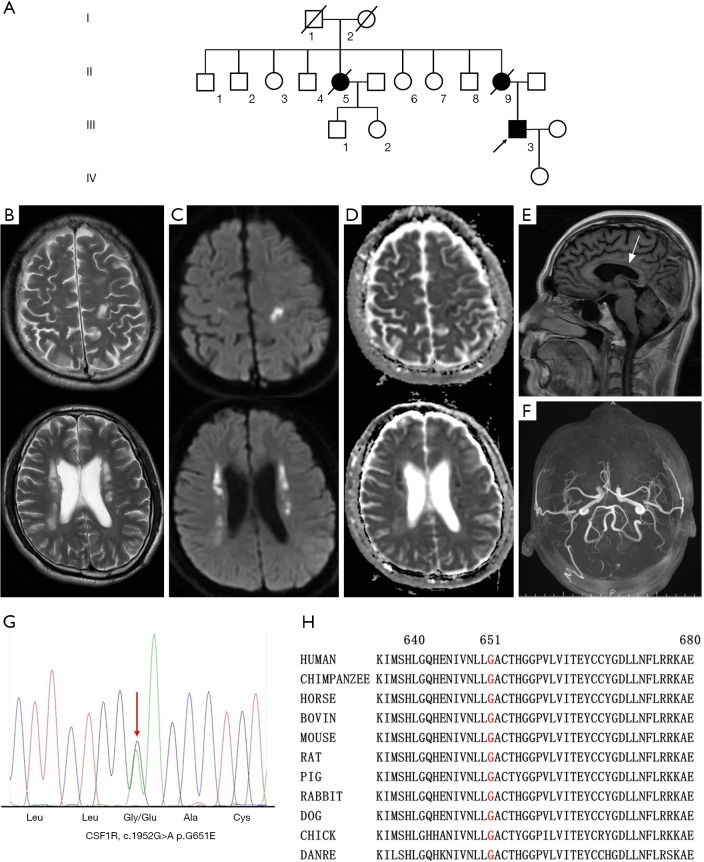
The index patient (III-3) (Figure 1A) was a 40-year-old male who was referred because of progressive right leg paresis and cognitive impairment. He presented with weakness of the right lower limb three months before admission. Memory decline was also noticed. Two months before admission, ambiguous and vague speech began to emerge. On neurological examination, there was limited abduction of both eyes, with decreased gap reflex. Muscle strength of the right lower limb was grade 4, muscle tone was increased in all extremities, and deep tendon reflexes were hyperactive in all limbs, with bilateral positive Babinski sign. Sensory system was normal. His Mini-Mental State Examination score was 25/30 and Montreal Cognitive Assessment score was 20/30. Notably, his mother (II-9) and aunt (II-5) had similar clinical symptoms of hemiparalysis and died at middle age (Figure 1A). Brain MRI showed bilateral white matter lesions in bi-frontoparietal and periventricular areas; hyperintense on T2-weighted (Figure 1B), FLAIR and diffusion weighted images (DWI) (Figure 1C); hypointense on T1-weighted and apparent diffusion coefficient (ADC) images (Figure 1D); and no obvious microhemorrhage in SWI images. Thinning of corpus callosum was also seen (Figure 1E). No enhanced lesions were seen in the enhancement sequence. MRA did not show obvious abnormalities (Figure 1F). The routine and biochemical results of cerebrospinal fluid were normal. Serum anti-NMO, anti-MBP, and anti-MOG antibody levels were negative. Serum ANA+ANA antibodies, ANCA, PR3 and MPO antibodies were negative. On the last follow up at one and a half year after onset, he was bedridden because of rigidity of the extremities, with dysphagia with tube feeding, severe dysarthria, and obvious dementia, and the patient could hardly communicate with his family.
Figure 1.
Imaging features and genetic analysis of the proband carrying the colony-stimulating factor 1 receptor (CSF1R) p.G651E mutation. (A) The pedigree of the proband with hereditary diffuse leukoencephalopathy with spheroid (HDLS). Brain MRI of the proband with CSF1R p.G651E mutation showed bilateral white matter lesions in bi-frontoparietal and peri-ventricular areas, which showed hyperintensity on T2-weighted images (B) and diffusion weighted images (DWI) images (C) and hypointensity on apparent diffusion coefficient (ADC) images (D). Thinning of corpus callosum (arrow) was also seen and no enhanced lesions were seen in the enhancement sequence (E). MRA did not see obvious abnormalities (F). (G) Sequencing chromatograms of the CSF1R p.G651E mutation. (H) Evolutionary conservation of the p.G651E mutation. G651 is highly conserved across species.
Next generation sequencing revealed a novel heterozygous variation, c.1952G>A (p.G651E), in the CSF1R gene (Figure 1G). In silico analysis of this variation using multiple prediction programs [PolyPhen-2 (http://genetics.bwh.havard.edu/pph2), MutationTaster (www.mutationtaster.org), MutPred (http://mutpred.org)] consistently predicted the variant as damaging. Segregation analysis of the mutation was not feasible due to deceased or unreachable relatives of the proband.
Literature review
We conducted a literature search in Medline, EMBASE, WANFANG (old.g.wanfangdata.com.cn) and CNKI (www.cnki.net) using the following keywords: “hereditary diffuse leukoencephalopathy with spheroids” or “HDLS”. Only English and Chinese language literature were included in the review. The diagnosis of HDLS was required to be based on both clinical features and genetic tests. We extracted the following information from the relevant papers: first author, year of study, population, sex, age, clinical features, brain imaging findings, genetic characteristics and family history.
Results
Clinical features
Overall, 137 patients with HDLS from 102 families were identified (http://cdn.amegroups.cn/static/application/81246daeff6dfc2975d183fab093c632/10.21037atm.2019.12.17-1.pdf). The main clinical features of these patients are summarized in Table 1. The average age of onset was 43.3 years (range, 10–71 years). There was no sex difference in age of onset (43.3 years for female vs. 43.2 years for male). Patients were mainly European (36 families), American (26 families) and Japanese (23 families); only 10 families were Chinese (http://cdn.amegroups.cn/static/application/81246daeff6dfc2975d183fab093c632/10.21037atm.2019.12.17-1.pdf). The most common symptoms of HDLS were cognitive impairment (87%), followed by psychiatric symptoms (55%), Parkinsonism (41%), gait disorder (34%), and dysphagia (34%) (Table 1). Other symptoms included hemiplegia, seizures, speech disorder, apraxia, urinary incontinence, dysarthria, tremor, and nystagmus. Psychiatric symptoms such as anxiety, depression, apathy, indifference, abulia, irritability, disinhibition, distraction and other behavioral and personality changes were also reported (http://cdn.amegroups.cn/static/application/81246daeff6dfc2975d183fab093c632/10.21037atm.2019.12.17-1.pdf). The mean survival was 6.2 years (range, 1–29 years), with no difference in survival according to sex of patients (5.9 years for female vs. 5.5 years for male).
Table 1. Summary of the main clinical features of patients with hereditary diffuse leukoencephalopathy with spheroid (HDLS).
| Clinical features | Value |
|---|---|
| Age of onset, years, mean [range] | 43 [18–71] |
| Disease duration, years, mean [range] | 6.2 [1–29] |
| Cognitive impairment, n [%] | 119 [87] |
| Psychiatric symptoms, n [%] | 75 [55] |
| Parkinsonism, n [%] | 56 [41] |
| Gait disorder, n [%] | 46 [34] |
| Dysphagia, n [%] | 46 [34] |
| Hemiplegia, n [%] | 40 [29] |
| Seizures, n [%] | 38 [28] |
| Speech disorder, n [%] | 22 [16] |
| Apraxia, n [%] | 21[15] |
| Urinary incontinence, n [%] | 16 [12] |
| Dysarthria, n [%] | 10 [7] |
| Tremor, n [%] | 8 [6] |
| Nystagmus, n [%] | 5 [4] |
Brain imaging features
The brain images of 103 out of 137 patients with HDLS were available. The main features of brain images of these patients are summarized in Table 2. The most common brain imaging findings of HDLS were bilateral white matter lesion (90%), mostly around the ventricles (67%), frontal lobe (29%), and parietal lobe (27%). Calcifications in white matter on CT (64%), cerebral atrophy (37%) and thinning of corpus callosum (35%) were also common features. Other findings include dilation of the lateral ventricles and abnormal signals in the corpus callosum, brainstem and spinal cord.
Table 2. Summary of the main features of brain imaging of patients with hereditary diffuse leukoencephalopathy with spheroid (HDLS).
| Brain imaging features | n [%] |
|---|---|
| Bilateral white matter lesions | 93 [90] |
| Abnormal signals in periventricular areas | 69 [67] |
| Calcifications in white matter on CT | 23 [64] |
| Cerebral atrophy | 38 [37] |
| Thinning of corpus callosum | 36 [35] |
| Abnormal signals in frontal lobe | 27 [29] |
| Abnormal signals in parietal lobe | 25 [27] |
| Dilation of the lateral ventricles | 14 [14] |
| Abnormal signals in temporal lobe | 8 [9] |
| Abnormal signals in the corpus callosum | 4 [4] |
| Abnormal signals in Brainstem | 1 [1] |
| Abnormal signals in spinal cord | 1 [1] |
Genetic features
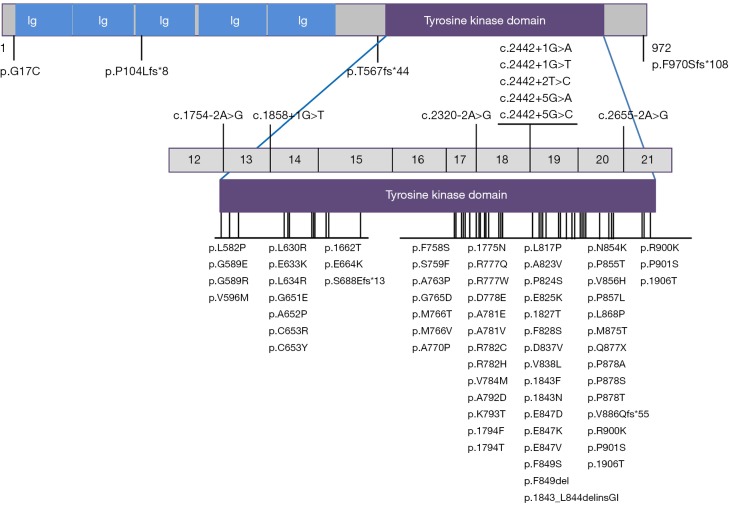
Among the total HDLS cases, 101 cases (65% of all families) had a definite family history. A total of 80 mutations in CSF1R have been reported (Figure 2), including 63 missense mutations, one nonsense mutation, two insertion/deletion mutations, five frameshift mutations, and nine splicing mutations. All mutations except for a missense mutation p.G17C and three frameshift mutations, p.P104Lfs*8, p.T567fs*44 and p.970Sfs*108, are located in the tyrosine kinase domain (TKD) of CSF1R protein, encoded by exons 12–21 of the gene. Mutations were more frequent in the distal TKD region encoded by exons 17–21 (53 mutations) than in the proximal TKD region encoded by exons 12–15 (14 mutations). Mutations hotspots were observed in exons 18–20, where 43 CSF1R mutations are located (Figure 2).
Figure 2.
Schematic graph of the CSF1R protein and overview of the CSF1R mutations identified in HDLS. CSF1R, colony-stimulating factor 1 receptor; Ig, immunoglobulin domain; HDLS, hereditary diffuse leukoencephalopathy with spheroid.
Discussion
In this study, we identified a novel missense mutation p.G651E in the CSF1R gene in a patient presenting with adult-onset leukoencephalopathy, cognitive impairment and motor dysfunction. G651 is highly conserved across species (Figure 1H) and lies in the TKD domain, the critical domain of CSF1R (PM1). The p.G651H variant was absent from the control databases (gnomAD, 1000 Genomes Project, ClinVar) (PM2). In silico tools indicated the variant was deleterious (PP3), and the patient’s phenotype and family history are highly specific for a disease with a single genetic etiology (PP4). Therefore, according to the guidelines of the American College of Medical Genetics and Genomics for sequence variant interpretation, the p.G651E variant was interpreted as likely pathogenic (6). The patient had the core features of HDLS: age at onset ≤60 years, cognitive impairment and pyramidal signs, autosomal dominant inheritance, bilateral cerebral white matter lesions and thinning of the corpus callosum in brain MRI images. He also carried a CSF1R gene mutation. Therefore, a diagnosis of definite HDLS can be made according to the diagnostic criteria (7).
Our review showed that average age of onset of patients with HDLS is 43 years; however, the onset age can vary from 10 to 71 years. This disease is clinically characterized by two groups of symptoms: neuropsychiatric and motor symptoms (8). The neuropsychiatric symptoms include memory impairment, progressive cognitive decline, depression, apathy, anxiety, and other behavioral or personality changes. Motor symptoms include Parkinsonism, pyramidal signs, dysarthria, dysphagia, and ataxia (8). The most common clinical characteristic of patients with HDLS is cognitive impairment (84%), followed by psychiatric symptoms, Parkinsonism, gait disorders, and dysphagia.
The neuroradiographic characteristics of patients with HDLS are bilateral but asymmetric T2-weighted and FLAIR hyperintensities in the deep and subcortical white matter, predominantly in the frontal, frontoparietal, and periventricular areas (Table 2) (5,8). Early lesions are patchy and focal, but with time spread around and become confluent. The preferential involvement of the frontal white matter may account for the predominant cognitive impairment and psychiatric symptoms in patients with HDLS. Diffusion-restricted lesions with reduced ADC can be observed in the white matter and can be persistent for several months or more, which can be differentiated from stroke. There was no enhancement and microbleeding. Thinning of the corpus callosum, cerebral atrophy and dilation of the lateral ventricles is typical, even in the early phases of the disease. Calcifications in the white matter on CT scan are characteristic imaging features of HDLS and demonstrate a “stepping stone appearance” in the frontal pericallosal area and punctate appearance in the frontal white matter adjacent to the anterior horns of the lateral ventricles (8). It should be noted that neuroradiographical abnormalities could precede the presence of clinical symptoms. At least three asymptomatic CSF1R mutation carriers with subtle T2 hyperintensities or bilateral white matter lesion, abnormal signals in lateral ventricle and frontal lobe have been reported (9,10).
So far, there have been no obvious genotype-phenotype correlations regarding HDLS, with some family members showing significant differences in disease presentation and course within the same family. Therefore, the clinical symptoms of patients with HDLS are variable and easily misdiagnosed with other diseases. Patients with HDLS presented with cognitive decline and personality changes in midlife with a progressive course, and evident white matter lesions on MRI should be differentiated with other leukoencephalopathy, such as adult-onset autosomal dominant leukodystrophy, Alexander disease, or cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Patients with predominant motor symptoms are easily misdiagnosed as multiple sclerosis, especially during the earliest phases of the disease (1). Recently, Konno et al proposed a diagnostic criterion for CSF1R-related leukoencephalopathy. The criteria yield high sensitivity (96%) and can successfully exclude other leukoencephalopathy. If a patient fulfills the probable criteria, genetic testing for CSF1R should be performed (7).
The mean disease duration for patients with HDLS was 6.2 years (Table 1). However, the rate of progression varies among individuals and patients may develop a rapid progression or a very slow progression (http://cdn.amegroups.cn/static/application/81246daeff6dfc2975d183fab093c632/10.21037atm.2019.12.17-1.pdf). Indicators of rapid disease progression of patients with HDLS were symptomatic disease onset before 45 years, female, white matter lesions extending beyond the frontal regions, an MRI severity score based on a point system [0–57] greater than 15 points, and mutation type of deletion (11).
Although HDLS is usually inherited in an autosomal dominant pattern, 36% of HDLS cases were apparent sporadic cases (http://cdn.amegroups.cn/static/application/81246daeff6dfc2975d183fab093c632/10.21037atm.2019.12.17-1.pdf). However, whether these sporadic cases reflect incomplete or non-penetrance or were caused by de novo mutations in the CSF1R gene remains unknown. The HDLS pedigrees reported so far showed that mutations in CSF1R have a high penetrance, but in one family carrying the CSF1R p.Q877X mutation and another p.V784M mutated pedigree, the index patient was severely affected since the age of 28 years whereas their parents who carried the same CSF1R mutation still had no neurological symptoms at 69 years and 79 years, suggesting incomplete penetrance in HDLS (9,12). In addition, three confirmed de novo CSF1R mutations (3,9) and two apparent (without paternity confirmation) de novo CSF1R mutations (13,14) have been reported in patients with HDLS, suggesting at least some patients with HDLS are true sporadic and caused by de novo mutations in the CSF1R gene.
CSF1R is an essential factor for development and maintenance of microglia. Approximately 95% of CSF1R mutations in HDLS are located within the TKD (Figure 2), suggesting that loss of tyrosine kinase activity may be necessary for the development of HDLS. Reduced expression of CSF1R was also observed in brains of patients with missense and splice-site mutations, indicating that any type of CSF1R mutation may cause HDLS by haploinsufficiency (4,15) (Figure 2). A mutant CSF1R mouse strain with a haploinsufficient allele developed HDLS-like symptoms, including cognitive decline, behavioral changes, and motor symptoms. White matter abnormalities, enlargement of the lateral ventricles, and thinning of the corpus callosum were also evident on MRI. The mouse model provides strong evidence that CSF1R haploinsufficiency is enough to cause white matter degeneration (16). However, marginally elevated cell surface CSF1 receptor levels with increased Tyr723 autophosphorylation was observed in a HDLS patient with CSF1R p.I843_L844delinsGI mutation, suggesting a mutation-related CSF1R gain-of-function (17). Notably, mutations tend to occur more frequently in the distal part of the TKD than the proximal part (5), and exons 18–20 of CSF1R gene are mutation hotspots where 63% of CSF1R mutations exist (Figure 2). Further functional experiments are needed to elucidate the pathogenesis of CSF1R mutations.
In conclusion, HDLS typically presents with broad phenotypic variability, and although it demonstrates an autosomal dominant pattern, sporadic cases are not uncommon. Early recognition of clinical and neuroradiographical characteristics of HDLS is key for the correct diagnosis of the disease, given the poor prognosis, rapid course, and genetic testing implications for family members.
Acknowledgments
The authors sincerely appreciate the participants for their help and willingness to participate in this study.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The patient gave an informed consent before taking part.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Sundal C, Wszolek ZK. CSF1R-Related Adult-Onset Leukoencephalopathy with Axonal Spheroids and Pigmented Glia. In: Adam MP, Ardinger HH, Pagon RA, et al. editors. GeneReviews((R)). Seattle (WA): University of Washington, Seattle University of Washington, Seattle. GeneReviews is a registered trademark of the University of Washington, Seattle.; 1993. [Google Scholar]
- 2.Axelsson R, Roytta M, Sourander P, et al. Hereditary diffuse leucoencephalopathy with spheroids. Acta Psychiatr Scand Suppl 1984;314:1-65. [PubMed] [Google Scholar]
- 3.Rademakers R, Baker M, Nicholson AM, et al. Mutations in the colony stimulating factor 1 receptor (CSF1R) gene cause hereditary diffuse leukoencephalopathy with spheroids. Nat Genet 2011;44:200-5. 10.1038/ng.1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nicholson AM, Baker MC, Finch NA, et al. CSF1R mutations link POLD and HDLS as a single disease entity. Neurology 2013;80:1033-40. 10.1212/WNL.0b013e31828726a7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Konno T, Yoshida K, Mizuno T, et al. Clinical and genetic characterization of adult-onset leukoencephalopathy with axonal spheroids and pigmented glia associated with CSF1R mutation. Eur J Neurol 2017;24:37-45. 10.1111/ene.13125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015;17:405-24. 10.1038/gim.2015.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Konno T, Yoshida K, Mizuta I, et al. Diagnostic criteria for adult-onset leukoencephalopathy with axonal spheroids and pigmented glia due to CSF1R mutation. Eur J Neurol 2018;25:142-7. 10.1111/ene.13464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Konno T, Kasanuki K, Ikeuchi T, et al. CSF1R-related leukoencephalopathy: A major player in primary microgliopathies. Neurology 2018;91:1092-104. 10.1212/WNL.0000000000006642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karle KN, Biskup S, Schule R, et al. De novo mutations in hereditary diffuse leukoencephalopathy with axonal spheroids (HDLS). Neurology 2013;81:2039-44. 10.1212/01.wnl.0000436945.01023.ac [DOI] [PubMed] [Google Scholar]
- 10.Bai Y, Lu L, Cui Y, et al. Analysis of clinical and neuroimaging features in a Chinese family with hereditary diffuse leukoencephalopathy with neuroaxonal spheroids. Chin J Neurol 2018;51:877-81. [Google Scholar]
- 11.Sundal C, Van Gerpen JA, Nicholson AM, et al. MRI characteristics and scoring in HDLS due to CSF1R gene mutations. Neurology 2012;79:566-74. 10.1212/WNL.0b013e318263575a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.La Piana R, Webber A, Guiot MC, et al. A novel mutation in the CSF1R gene causes a variable leukoencephalopathy with spheroids. Neurogenetics 2014;15:289-94. 10.1007/s10048-014-0413-1 [DOI] [PubMed] [Google Scholar]
- 13.Kondo Y, Kinoshita M, Fukushima K, et al. Early involvement of the corpus callosum in a patient with hereditary diffuse leukoencephalopathy with spheroids carrying the de novo K793T mutation of CSF1R. Intern Med 2013;52:503-6. 10.2169/internalmedicine.52.8879 [DOI] [PubMed] [Google Scholar]
- 14.Saitoh BY, Yamasaki R, Hayashi S, et al. A case of hereditary diffuse leukoencephalopathy with axonal spheroids caused by a de novo mutation in CSF1R masquerading as primary progressive multiple sclerosis. Mult Scler 2013;19:1367-70. 10.1177/1352458513489854 [DOI] [PubMed] [Google Scholar]
- 15.Konno T, Tada M, Tada M, et al. Haploinsufficiency of CSF-1R and clinicopathologic characterization in patients with HDLS. Neurology 2014;82:139-48. 10.1212/WNL.0000000000000046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chitu V, Gokhan S, Gulinello M, et al. Phenotypic characterization of a Csf1r haploinsufficient mouse model of adult-onset leukodystrophy with axonal spheroids and pigmented glia (ALSP). Neurobiol Dis 2015;74:219-28. 10.1016/j.nbd.2014.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kraya T, Quandt D, Pfirrmann T, et al. Functional characterization of a novel CSF1R mutation causing hereditary diffuse leukoencephalopathy with spheroids. Mol Genet Genomic Med 2019;7:e00595. 10.1002/mgg3.595 [DOI] [PMC free article] [PubMed] [Google Scholar]