Abstract
We have shown that combination treatment with decaffeinated green tea extract (GTE) and voluntary exercise (Ex) reduce obesity and insulin resistance in high fat (HF)-fed mice to a greater extent than either treatment alone. Here, we investigated the effects of GTE-, Ex-, or the combination on the development of obesity-related NAFLD. Male C57BL/6J mice were treated for 16 weeks with HF diet (60% energy from fat), HF supplemented with 7.7g GTE/kg, HF plus access to a voluntary running wheel, or the combination. We found that treatment of mice with the combination mitigated the development of HF-induced NAFLD to a greater extent than either treatment alone. Combination-treated mice had lower plasma alanine aminotransferase (92% lower) and hepatic lipid accumulation (80% lower) than HF-fed controls: the effect of the single treatments were less significant. Mitigation of NAFLD was associated with higher fecal lipid and nitrogen levels. Combination treated, but not singly treated mice, had higher hepatic expression of genes related to mitochondrial biogenesis (sirtuin 1 [59%]; peroxisome proliferator-activated receptor γ coactivator 1α [42%]; nuclear respiratory factor 1 [38%]; and transcription factor B1, mitochondrial [89%]) compared to the HF-fed controls. GTE-, Ex-, and the combination-treatment groups also had higher hepatic expression of genes related to cholesterol synthesis and uptake, but the combination was not better than the single treatments. Our results suggest the combination of GTE and Ex can effectively mitigate NAFLD. Future studies should determine if the combination is additive or synergistic compared to the single treatments.
Keywords: Green tea extract, (-)-Epigallocatechin-3-gallate, Non-alcoholic fatty liver disease, Voluntary exercise
1. INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) covers a spectrum of progressive diseases characterized by abnormal lipid accumulation in the liver in the absence of excessive consumption of alcohol [1]. NAFLD is a significant health problem with a global prevalence of about 25% [2]. The incidence of NAFLD is forecasted to increase to 100.9 million by 2030 based on the prevalence of the NAFLD risk factors, obesity and type 2 diabetes (T2D) [1, 3]. There are currently no validated therapies for NAFLD although some studies have suggested that lifestyle interventions that promote weight loss may be beneficial [4].
In the United States, consumption of green tea (Camellia sinensis, Theaceae) beverage exceeded 12.6 billion servings in 2018 [5] Green tea extract-based dietary supplements are marketed for a variety of indications including weight management, and represented the 8th most popular herbal supplement in the US in 2016 [6]. Green tea is characterized by the presence of high levels of polyphenols known as catechins with (-)-epigallocatechin-3-gallate (EGCG) being the most abundant. Green tea also contains the methylxanthines, caffeine and theophylline. Both green tea beverage and dietary supplements are available in decaffeinated forms. Studies have indicated that green tea, decaffeinated green tea, EGCG, and caffeine can mitigate NAFLD [7-12].
In human subjects with NAFLD, treatment with green tea containing caffeine has been shown to reduce serum ALT and aspartate aminotransferase (AST), two markers of hepatic injury [13, 14]. The relative contribution of caffeine or catechins to these effects was not examined. A recent study by Yang et al., has shown that daily oral bolus dosing of HF-fed rats with EGCG and caffeine led to greater histological improvement in NAFLD than treatment with either compound alone [15]. Decaffeinated green tea extract has been shown to mitigate NAFLD in HF-fed mice [16]. These effects were related to activation of AMP-activated protein kinase (AMPK) via liver kinase B and downregulation of acetyl-CoA carboxylase (ACC) and fatty acid synthase (FAS). A number of studies have shown that EGCG can mitigate the development of NAFLD in animal models (reviewed in [7, 8]). For example, treatment of HF-fed mice with dietary EGCG has been shown to reduce body weight gain, hepatic triglycerides (TAG), and plasma alanine aminotransferase (ALT) levels. These effects were associated with increased fecal lipid levels, suggesting a reduction in the digestion and absorption of dietary fat [17].
While studies suggest that green tea and green tea polyphenols may mitigate NAFLD, it is likely that in humans, green tea will be used in combination with other lifestyle modifications (e.g. exercise and calorie restriction). A number of studies have looked at lifestyle interventions such as exercise for the treatment of NAFLD, however the underlying mechanisms are not well understood (reviewed in [18, 19]). Exercise has been shown to counteract liver inflammation by causing the release of anti-inflammatory mediators and activating antioxidant response systems [20]. In rodent models, aerobic exercise has been shown to activate hepatic AMPK [21], which in turns increases hepatic lipid oxidation and decreases hepatic lipid content [22]. In addition, aerobic exercise has also been shown to suppress hepatic de novo lipogenesis and TAG synthesis [23].
Previous studies in our laboratory have shown that combination treatment with decaffeinated green tea extract (GTE) and voluntary exercise (Ex) attenuates the development of obesity and insulin resistance in HF-fed mice to a greater extent than treatment with either GTE or Ex alone [24, 25]. These effects were related to increased expression of genes related to mitochondrial biogenesis in skeletal muscle and visceral adipose tissue “browning”. The effects of the combination of GTE and Ex on NAFLD have not been described.
In this study, we investigated the effect of GTE, Ex, or the combination on parameters related to NAFLD in HF-fed mice. We elected to examine decaffeinated green tea extract because (1) we were following up on our previous studies with EGCG and decaffeinated green tea extract, (2) decaffeinated products are widely commercially-available, and (3) there is a level of concern among consumers about consuming excess amounts of caffeine. We hypothesized that the combination of GTE- and Ex- would have greater NAFLD preventive effects than either GTE- or Ex- alone and that these effects would be associated with the inhibition of macronutrient digestion and the regulation of hepatic expression of genes related to mitochondrial biogenesis and lipid metabolism.
2. MATERIALS AND METHODS
2.1. MATERIALS
Decaffeinated green tea extract (GTE) was a gift from Nature’s Sunshine Products, Inc. (Spanish Fork, UT, USA) and the composition is shown in Table S1. Experimental diets were purchased from Research Diets, Inc (New Brunswick, NJ, USA) and the formulations have been previously published [24]. Tri reagent was purchased from Sigma-Aldrich (St. Louis, MO). RT2 HT First Strand Kit was purchased from Qiagen (Germany). Custom primers were purchased from Integrated DNA Technologies (Coralville, IA) and SYBR® Green SuperMix was purchased from Quantabio (Beverly, MA). All other chemicals were of the highest grade commercially available.
2.2. ANIMAL EXPERIMENT
The liver and plasma samples analyzed in this study were generated as part of a larger mouse model study on the impact of GTE and Ex on metabolic syndrome in HF-fed mice. Parts of this study have been previously published [24, 25]. The original animal experiment was approved by the Institutional Animal Care and Use Committee at The Pennsylvania State University (IACUC #45380-1). In brief, male C57BL/6J (aged 5 wks, Jackson Laboratories, Bar Harbor, ME) mice were randomized to a high-fat diet (HF, 60% energy from fat), HF supplemented with decaffeinated green tea extract (7.7g GTE/kg), HF plus access to voluntary running wheel (Ex), or the combination (GTE + Ex) and treated for 16 wks. The dose of GTE (7.7g/kg diet) used in this study corresponds to human consumption of approximately 10 cups of green tea per day (assuming a 200 mL cup and 2 g of green tea leaves) based on allometric scaling [26]. This amount of green tea is achievable in Asian populations [27-31].
2.3. BLOOD AND TISSUE COLLECTION
At the end of 16 wks, mice fasted for 7 h (7:00 – 14:00) and then anesthetized and blood was collected by cardiac puncture. Plasma was isolated by centrifugation at 3200 g for 15 min and stored in −80°C until further analysis. Livers were quickly dissected, rinsed with saline, and weighed. Liver sections were fixed in 10% formalin and the remaining samples were frozen at −80°C.
2.4. ANALYSIS OF FATTY LIVER DISEASE
Liver TAGs were extracted and quantified using a colorimetric assay (Cayman Chemical, Ann Arbor, MI, USA) according to the manufacturer’s instructions. Lipid droplet size was determined histologically using a modification of our previously reported methods [32, 33]. In brief, paraffin-embedded liver samples were sectioned to 6 μm thickness, deparaffinized, and stained with hematoxylin and eosin. Blinded liver slides were viewed at 400x magnification on Olympus CX31 (Olympus Corporation, Tokyo, Japan) and captured on DSLR 80D (Canon U.S.A., Inc., Melville, NY, USA). Images of 4 random areas of each slide were collected and processed by ImageJ software (National Institutes of Health, Bethesda, MD, USA). The area occupied by lipid droplets was normalized to the total area imaged.
2.5. PLASMA MARKERS OF LIVER INJURY AND DYSLIPIDEMIA
Plasma ALT levels were determined using a commercially-available spectrophotometric assay (Catachem, Inc., Bridgeport, CT). Plasma total cholesterol and high-density lipoprotein (HDL)-cholesterol were determined using the commercially-available enzymatic assays according to the manufacturer’s protocol (Wako Diagnostics, Richmond, VA).
2.6. GENE EXPRESSION ANALYSIS
Gene expression was quantified by quantitative reverse-transcriptase polymerase chain reaction (qRT-PCR) as previously described [34]. In brief, liver RNA was extracted using TRI reagent (Sigma-Aldrich, St. Louis, MO) and quantified using a NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA). cDNA was synthesized with the RT2 HT First Strand Kit (Qiagen, Germany). After which, qRT-PCR was performed by using cDNA, custom primers (Integrated DNA Technologies, Coralville, Iowa) and SYBR® Green SuperMix (Quantabio, Beverly, MA) on a QuantStudio 12K Flex Real-Time PCR System according to the manufacturer’s protocol (Thermo Fisher Scientific, Waltham, MA). Relative quantification of PCR products was accomplished using Thermo Fisher Connect™ (Thermo Fisher Scientific, Waltham, MA). Hepatic expression of genes involved in lipolysis, cholesterol synthesis and uptake and mitochondrial biogenesis were quantified relative to the expression of glyceraldehyde 3-phosphate dehydrogenase (Gapdh) using a comparative method (2−ΔΔCT). Primer sequences are available in Table S2.
2.7. FECAL SAMPLE ANALYSIS
At week 15, 24 h fecal samples were collected and analyzed for lipid and nitrogen content. Fecal samples were grounded and dried in a vacuum oven at 60°C for 72 h. Lipid content in the fecal samples was determined via a gravimetric method as previously reported [35]. Fecal nitrogen content was used as a surrogate for fecal protein content and was determined using a LECO FP-528 nitrogen analyzer (St. Joseph, MI). EDTA was used as a standard.
2.8. STATISICAL ANALYSES
Statistical analyses were conducted using GraphPad Prism software (San Diego, CA, USA) and Minitab software (State College, PA, USA). All plots show the mean ± standard error of the mean (SEM). One-way ANOVA with Fisher’s post-test was used for statistical analysis for all data collected. Statistical significance was achieved at P < 0.05.
3. RESULTS
3.1. NAFLD IN HF-FED MICE
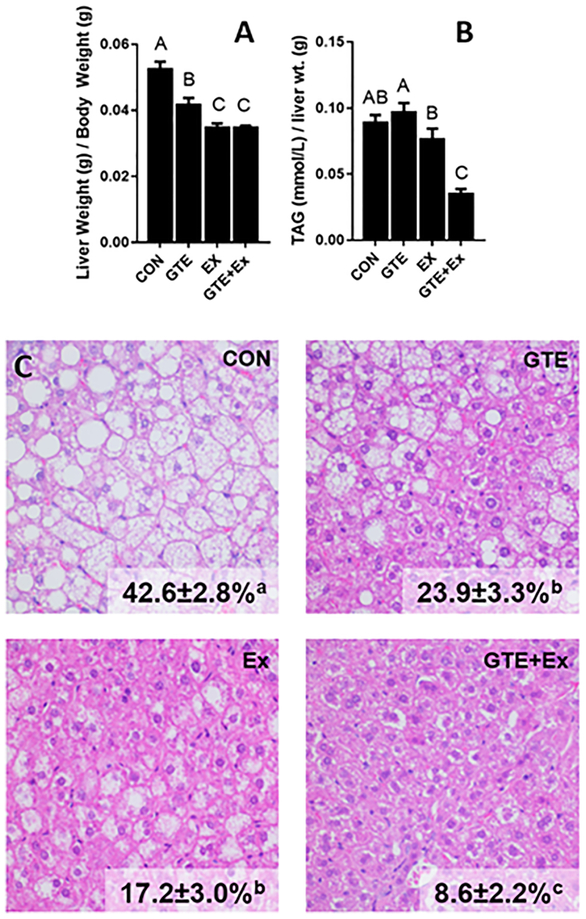
Relative liver weights were 20.7% and 45% lower in GTE- and Ex-treated mice compared to HF-fed controls (“CON”, Fig 1A). Treatment with the combination was not better than Ex-treated mice (Fig 1A). Hepatic TAG levels in the GTE- or Ex-treated groups were not different from the HF-fed control, whereas levels of hepatic TAG levels in the combination treatment were 60.9% lower compared to those in HF-fed mice (Fig 1B). Histological analysis showed that the relative area of hepatic lipid accumulation was 79.8% lower in the combination-treated mice compared to the HF-fed mice (Fig 1C). GTE- and Ex-treated mice had 43.9% and 59.6% lower hepatic lipid accumulation respectively compared to control HF-fed mice.
Figure 1.
Effect of GTE-, Ex-, or the combination on hepatic lipidosis in HF-fed male C57BL/6J mice. (A) Relative liver weight, (B) hepatic triglycerides (determined biochemically), and (C) hepatic lipidosis (measured histologically) were determined at euthanasia. Triglycerides were normalized to liver wet weight. Data represent the mean ± SEM (n = 22). Hepatic lipid accumulation (%) was determined histologically using H&E stained liver sections. Data represent the mean ± SEM (n = 10). Values that do not share a common superscript letter are different (p < 0.05) by one-way ANOVA with Fisher’s post-test.
3.2. PLASMA MARKERS OF LIVER INJURY AND DYSLIPIDEMIA
Plasma ALT levels, a marker of liver injury, were 92% lower in combination- treated mice compared to HF-fed mice (Table 1). Although the plasma ALT levels in GTE- and EX-treated groups appeared lower than control group, the variation in the data was large and the differences were not statistically significant (Table 1). Plasma total cholesterol levels were 19.4% and 16% lower in the Ex- treated and combination-treated mice, respectively, compared to HF-fed control mice (Table 1). Levels in GTE-treated mice were not different from those in the HF-fed control mice. LDL-cholesterol levels were 24% lower in EX mice compared to HF-fed controls. The levels in the GTE- and combination groups were not different from the HF-fed controls. Plasma HDL-cholesterol levels were 22% lower in the combination- treated mice compared to HF-fed control mice (Table 1). Neither the GTE- nor the Ex-treated mice had plasma HDL-cholesterol levels that were different from the HF-fed controls.
Table 1.
Plasma markers of liver injury and dyslipidemia1
| CON | GTE | Ex | GTE+Ex | |
|---|---|---|---|---|
| ALT (U/L) | 431±162a | 209±120ab | 149±100ab | 34.0±24.3b |
| Total cholesterol (mM) | 6.2±0.2a | 5.9±0.1a | 5.0±0.2b | 5.2±0.1b |
| HDL-cholesterol (mM) | 2.3±0.1a | 2.1±0.1a | 2.0±0.1a | 1,7±0.1b |
| LDL-cholesterol (mM) | 3.8±0.2a | 3.9±0.3a | 2.9±0.2b | 3.5±0.1a,b |
Data represent as mean ± SEM (n=22). Different superscript letters indicate p < 0.05 by one-way ANOVA with Fisher’s post-test.
3.3. FECAL LIPID AND NITROGEN LEVELS
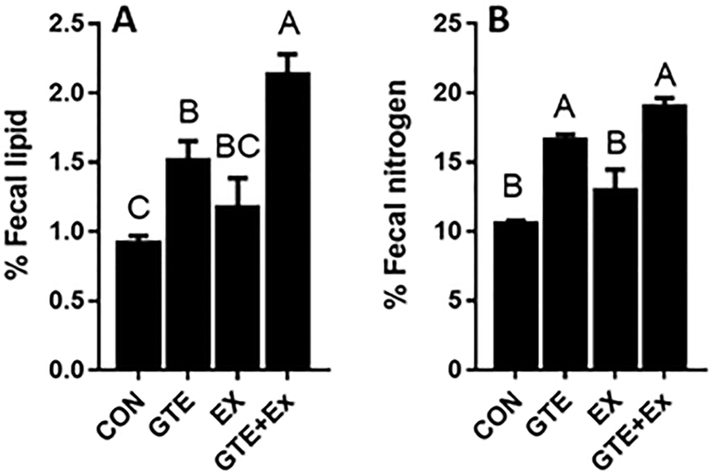
Analysis of fecal macronutrients showed that the mice treated with the combination of GTE and Ex had 1.3-fold higher fecal lipid content than the HF-fed control mice (Fig 2). GTE-treated mice, but not Ex-treated mice, also had higher fecal lipid levels but the difference was not as great as that seen with the combination. Fecal nitrogen levels were higher in both GTE-treated mice and combination-treated mice (70 – 80%) compared to HF-fed control mice (Fig 2). Fecal nitrogen levels in Ex-treated mice were not significantly different from those in HF-fed control mice.
Figure 2.
Effect of GTE-, Ex-, or the combination on lipid and nitrogen content in HF-fed male C57BL/6J mice feces. (A) Fecal lipid content was determined gravimetrically following Folch extraction. (B) Fecal nitrogen content was determined by Dumas method. Data represent the mean ± SEM (n = 3). Values that do not share a common superscript letter are different (p < 0.05) by one-way ANOVA with Fisher’s post-test.
3.4. GENES RELATED TO HEPATIC MITOCHONDRIAL BIOGENESIS
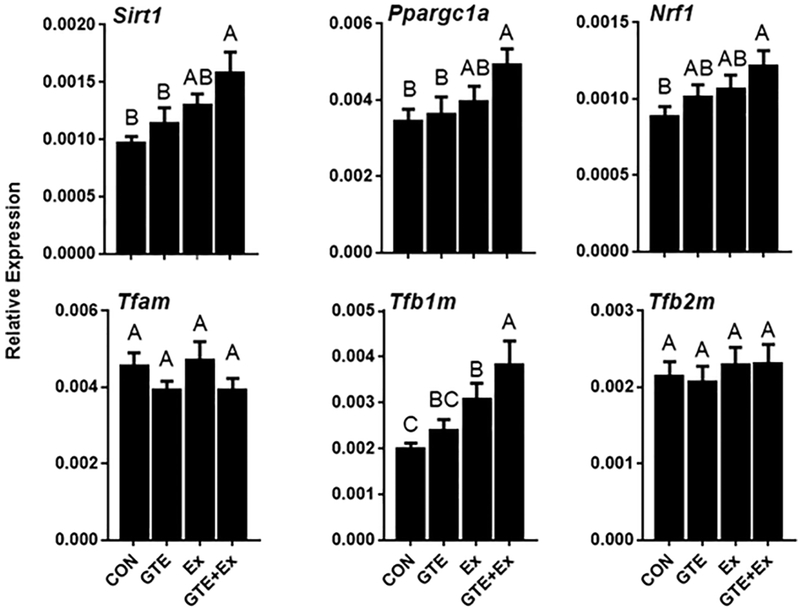
The effect of GTE and Ex on the expression of hepatic genes related to mitochondrial biogenesis was analyzed by qRT-PCR. Mice that were treated with the combination of GTE and Ex had greater hepatic expression sirtuin 1 (Sirt1, encodes SIRT1 protein, 63.9% higher), Ppargc1a (42.4% higher), nuclear respiratory factor 1 (Nrf1, encodes NRF1 protein, 37.6% higher), and mitochondrial transcription factor B1 (Tfb1m, encodes TFB1M protein, 92.2% higher) compared to HF-fed control mice (Fig 3). The effects in mice treated with GTE- or Ex- alone were less dramatic or not statistically significant. Hepatic mRNA levels of mitochondrial transcription factor A (Tfam) and mitochondrial transcription factor (Tfb2m) were not different among the treatment groups.
Figure 3.
Effect of GTE-, Ex-, or the combination on the hepatic expression of genes related to mitochondrial biogenesis in HF-fed male C57BL/6J mice. Gene expression was determined by qRT-PCR and normalized to Gapdh. Data represent the mean ± SEM (n = 16). Values that do not share a common superscript letter are different (p < 0.05) by one-way ANOVA with Fisher’s post-test.
3.5. GENES EXPRESSION RELATED TO LIPOLYSIS
The effect of GTE and Ex on hepatic gene expressions relating to lipolysis was determined by qRT-PCR. Hepatic expression of hormone-sensitive lipase (Lipe) and adipose triglyceride lipase (Pnpla2), were not different in GTE-, Ex-, or the combination-treated mice compared to HF-fed controls (Fig S1).
3.6. GENES EXPRESSION RELATED TO CHOLESTEROL BIOSYNTHESIS AND TRANSPORT
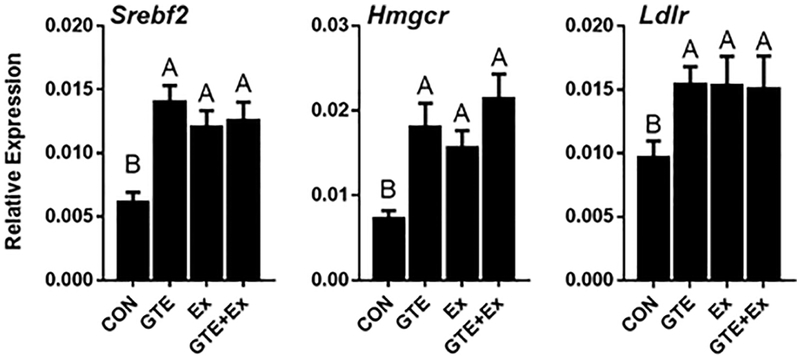
Hepatic gene expression of sterol regulatory element-binding protein 2 (SREBP2, encoded by Srebf2 gene), 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase (HMGCR, encoded by Hmgcr gene) and low-density lipoprotein receptor (LDLR, encoded by Ldlr gene) were significantly higher in the GTE-, Ex-, and the combination-treated mice compared to the HF-fed controls (Fig 4). No difference was observed between the individual treatments and the combination.
Figure 4.
Effect of GTE-, Ex-, or the combination on hepatic expression of genes related to cholesterol synthesis and uptake in HF-fed male C57BL/6J mice. Gene expression was determined by qRT-PCR, normalized to Gapdh. Data represent the mean ± SEM (n = 16). Values that do not share a common superscript letter are different (p < 0.05) by one-way ANOVA with Fisher’s post-test.
4. DISCUSSION
The objective of this study was to characterize the effects of decaffeinated green tea extract (GTE), voluntary exercise (Ex), or the combination on NAFLD in HF-fed mice. We have previously shown that the combination of GTE and Ex can mitigate increases in body weight and visceral adiposity, as well as the development of insulin resistance, in this model [24]. In this study, we report for the first time, that mice treated with the combination of GTE and Ex had significantly less severe NAFLD than the HF-fed control mice. These differences were greater than those observed in mice treated with either GTE- or Ex- alone. Mitigation of NAFLD was associated with increased fecal lipid and nitrogen levels and higher hepatic expression of several genes related to mitochondrial biogenesis. Interestingly, we found that GTE-, Ex-, and combination-treated mice had a higher hepatic expression of genes related to cholesterol biosynthesis and uptake, but there were no significant differences between the individual treatments and the combination.
The prevention of NAFLD by tea polyphenols, specifically EGCG, has previously been demonstrated in mice fed with a HF diet [7, 8]. These effects have been ascribed to two general modes of actions. First, tea polyphenols could modulate the digestion and absorption of dietary macronutrients from the digestive tract resulting in decreased energy absorption. Second, systemically-available tea polyphenols may modulate metabolism in the liver and other tissues promoting catabolic processes. Whether these mechanisms are independent or related remains unclear.
Consistent with previous studies on tea polyphenols, we found that GTE treatment increased fecal lipids. Green tea and EGCG were reported by us and others to inhibit pancreatic lipase [35, 36]. EGCG has also been reported to modulate lipid digestion/absorption by disrupting the structure of micelles in the digestive tract [37, 38] and inhibiting lipid transporters such as apical sodium-dependent bile salt transport [39]. We also found that GTE and the combination increased fecal nitrogen levels. Previous studies have shown that green tea and EGCG can inhibit digestive proteases [40], and tea polyphenols have also been shown to form insoluble complexes with protein, which can reduce their digestibility [41].
Interestingly, we found that mice treated with the combination of GTE and Ex had significantly higher fecal lipids than mice treated either GTE or Ex alone (Fig 2). Voluntary exercise in mice has been shown to decrease cholesterol absorption by decreasing expression of the hepatic/intestinal cholesterol transport protein, Niemann-Pick C1 like 1, thus increasing fecal neutral sterol output which contributes to the fecal lipid content [42]. Our results suggest that combination GTE and Ex treatment modulates different routes of lipid and cholesterol absorption, providing a greater effect than either GTE- or Ex- alone.
Mitochondrial biogenesis can be stimulated by exercise, cold exposure, environmental effects, drugs, oxidative stress, and starvation/caloric restriction [43]. PGC1α, a transcriptional coactivator can, in combination with the transcription factors NRF1 and NRF2, stimulate mitochondrial biogenesis [43]. Previous studies have shown that EGCG can improve mitochondrial respiratory capacity in livers of HF-fed mice by increasing activities of mitochondrial complex I and IV [16]. The effects of GTE or EGCG on the expression of genes related to hepatic mitochondrial biogenesis in the context of NAFLD have not been reported. Exercise has been shown to improve hepatic mitochondrial metabolism and biogenesis through the activation of hepatic PGC1α [44]. Our finding that the combination of GTE and Ex increased the mRNA expression of SIRT1, PGC1α, NRF1, and TFB1M (Fig 3) suggests that the combination treatment can stimulate mitochondrial biogenesis. Further studies using electron microscopy and biochemical measurements of mitochondrial function are needed to confirm hepatic mitochondrial biogenesis.
Hepatic steatosis in the liver reflects net retention of lipid that results may result from a multi-faceted imbalance of fatty acid import/export, catabolism, and de novo lipogenesis [45]. Previously, we have reported that mice treated with the combination of GTE and Ex have greater hepatic mRNA expressions of peroxisome proliferator activated receptor alpha and carnitine palmitoyltransferase which are related to fatty acid oxidation and lower mRNA expression of stearoyl-CoA desaturase-1 which is related to de novo lipogenesis [24]. Here, we investigated the impact of the treatments on the expression of genes related to the lipolysis and found no differences (Fig S1). This is in contrast to our previous studies in adipose tissues where Pnpla2 and Lipe were elevated in the combination treatment [25]. This difference could be due to tissue-specific mobilization of lipids during treatment.
Evidence from animal studies has shown that green tea or its catechins lowered blood levels of cholesterol in cholesterol-fed diets and plasma levels of TAG from a high-fat diet [7, 8]. We found that plasma total cholesterol was lowered in Ex-, and combination-treated mice (Table 1). By contrast, when we looked at the expression of hepatic genes related to cholesterol biosynthesis and uptake (Srebf2, Hmgcr, Ldlr), we found that these genes were expressed at higher levels in mice treated with GTE, Ex, or the combination (Fig 4). This is consistent with previous work by Huang et al., (2018) who reported similar mRNA changes in high fat-fed mice supplemented with EGCG [46].
SREBP2 is a transcription factor that regulates sterol synthesis by increasing the gene expression of HMGCR, rate-limiting enzyme in the production of cholesterol, and by activating gene expression of a cell-surface receptor, LDLR which facilitate cholesterol uptake [47]. Our results suggest that GTE- or Ex-, can stimulate hepatic de novo cholesterol synthesis to produce cholesterol for important biological uses (e.g. bile acid synthesis). Given that GTE and the combination increased fecal lipid levels, this increased expression could be a compensatory change to off-set cholesterol loss in the feces. In addition, EGCG [46, 48] and voluntary exercise [42, 49] have been shown to enhance de novo synthesis of bile acids. As bile acids exclusively use cholesterol as a substrate for synthesis, this process has been shown to increase hepatic cholesterol clearance with reduced lipid accumulation. Future studies are needed to look at how GTE and Ex affects bile acid synthesis as well as cholesterol and bile acid levels in the feces.
The current study has several strengths including: use of a well-established model of obesity-related NAFLD, use of physiologically relevant doses of a commercially available and well-defined GTE, and the use of voluntary rather than forced exercise. In addition to these strengths, the study has some weaknesses. Although the GTE used is considered decaffeinated, there is a low level of residual caffeine in the extract (7.6 mg caffeine/g GTE). Based on the mean food intake of the mice and the dose of GTE used in the diet, the estimated dose of caffeine delivered to each mouse is approximately 73 mg/kg, bw compared to approximately 300 mg/kg bw EGCG. Previous studies in HF-fed rodent models have examined the impact of caffeine on NAFLD and found that caffeine can mitigate NAFLD [11, 15, 50]. These studies examined different mechanistic targets from our study. In addition, it is difficult to compare our results to these previous studies since those studies used oral gavage and we used a dietary route of administration. These different routes result in significantly different peak liver levels of caffeine. Additionally, because we had no caffeine-only treatment group, we were unable to assess the role of caffeine in the overall effect of our GTE. Another weakness of our study is that we examined mRNA, but not protein levels for the molecular targets of interest. Given that protein expression and activity are can be regulated post-transcriptionally, future studies are needed to extend the results of the present study to look at protein expression.
In conclusion, our results demonstrate that the combination of GTE- and Ex- prevented NAFLD in high-fat fed mice compared to either GTE- or Ex- separately. This beneficial effect was related to decreased macronutrient digestion and energy absorption, altered hepatic lipid metabolism, and increased expression of markers related to mitochondrial biogenesis. Our results on gene expression provided evidence on these mechanistic pathways. The use of GTE- and Ex- on the treatment of NAFLD warrants further investigation.
Supplementary Material
Highlights:
HF-diet induces NAFLD in male C57/BL6J mice
Decaffeinated green tea extract (GTE) and voluntary exercise (EX) reduced NAFLD
Combo-treated mice had higher fecal lipid and nitrogen levels
Combo-treated mice had higher hepatic expression of mitochondrial biogenesis genes
GTE and EX led to higher hepatic expression of cholesterol synthesis genes
ACKNOWLEDGEMENTS
We thank the staff at PSU Genomics Core Facility for their support and patience during gene expression analysis. This work was supported by funding from the National Institutes of Health (NIH AT004678 to JDL) and the United States Department of Agriculture (USDA Hatch Project No. 4565 to JDL). The NIH and USDA had no role in the study design and execution.
Abbreviations:
- ALT
Alanine aminotransferase
- AMPK
5' AMP-activated protein kinase
- ARG1
Arginase-1
- AST
Aspartate aminotransferase
- PNPLA2
Adipose triglyceride lipase
- CON
Control
- EGCG
(-)-epigallocatechin gallate
- Ex
Voluntary exercise
- GTE
Decaffeinated green tea extract
- HDL
High-density lipoprotein
- HF
High fat
- HMGCR
3-hydroxy-3-methyl-glutaryl-coenzyme A reductase
- LIPE
Hormone-sensitive lipase
- IL
Interleukin
- iNOS
Inducible nitric oxide synthase
- LDL
Low-density lipoprotein
- LDLR
LDL receptor
- MCP-1
Monocyte chemoattractant protein 1
- NAFLD
Non-alcoholic fatty liver disease
- NASH
Non-alcoholic steatohepatitis
- NF-κB
Nuclear factor kappa-light-chain-enhancer of activated B cells
- NRF
Nuclear respiratory factor
- PGC1α
Peroxisome proliferator-activated receptor gamma coactivator 1-alpha
- qRT-PCR
Quantitative reverse-transcriptase polymerase chain reaction
- SEM
Standard error of the mean
- SIRT1
Sirtuin 1
- SREBP2
Sterol regulatory element-binding protein 2
- T2DM
Type 2 diabetes mellitus
- TAG
Triglycerides
- TFAM
Mitochondrial transcription factor A
- TFB1M
Mitchondrial transcription factor B1
- TFB2M
Mitochondrial transcription factor B2
- TNFα
Tumor necrosis factor-alpha
Footnotes
Declaration of interest: The other authors have no interests to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Byrne CD, Targher G. NAFLD: A multisystem disease. Journal of Hepatology.62:S47–S64. [DOI] [PubMed] [Google Scholar]
- [2].Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatol. 2016;64:73–84. [DOI] [PubMed] [Google Scholar]
- [3].Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatol. 2018;67:123–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Federico A, Zulli C, de Sio I, Del Prete A, Dallio M, Masarone M, et al. Focus on emerging drugs for the treatment of patients with non-alcoholic fatty liver disease. World J Gastroenterol. 2014;20:16841–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Tea Association of the USA Inc. Tea Fact Sheet – 2018-2019. About Tea. Tea Association of the USA Inc. [Google Scholar]
- [6].Basu A, Betts NM, Mulugeta A, Tong C, Newman E, Lyons TJ. Green tea supplementation increases glutathione and plasma antioxidant capacity in adults with the metabolic syndrome. Nutr Res. 2013;33:180–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Masterjohn C, Bruno RS. Therapeutic potential of green tea in nonalcoholic fatty liver disease. Nutrition Rev. 2012;70:41–56. [DOI] [PubMed] [Google Scholar]
- [8].Chen C, Liu Q, Liu L, Hu YY, Feng Q. Potential biological effects of (-)-Epigallocatechin-3-gallate on the treatment of nonalcoholic fatty liver disease. Mol Nutr Food Res. 2018;62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Shen H, Rodriguez AC, Shiani A, Lipka S, Shahzad G, Kumar A, et al. Association between caffeine consumption and nonalcoholic fatty liver disease: a systemic review and meta-analysis. Therapeutic Adv Gastroenterol. 2016;9:113–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Birerdinc A, Stepanova M, Pawloski L, Younossi ZM. Caffeine is protective in patients with non-alcoholic fatty liver disease. Alimentary Pharmacol Therap. 2012;35:76–82. [DOI] [PubMed] [Google Scholar]
- [11].Zhang SJ, Li YF, Wang GE, Tan RR, Tsoi B, Mao GW, et al. Caffeine ameliorates high energy diet-induced hepatic steatosis: sirtuin 3 acts as a bridge in the lipid metabolism pathway. Food Funct. 2015;6:2578–87. [DOI] [PubMed] [Google Scholar]
- [12].Sinha RA, Farah BL, Singh BK, Siddique MM, Li Y, Wu Y, et al. Caffeine stimulates hepatic lipid metabolism by the autophagy-lysosomal pathway in mice. Hepatol. 2014;59:1366–80. [DOI] [PubMed] [Google Scholar]
- [13].Pezeshki A, Safi S, Feizi A, Askari G, Karami F. The effect of green tea extract supplementation on liver enzymes in patients with nonalcoholic fatty liver disease. Int J Prev Med. 2016;7:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sakata R, Nakamura T, Torimura T, Ueno T, Sata M. Green tea with high-density catechins improves liver function and fat infiltration in non-alcoholic fatty liver disease (NAFLD) patients: a double-blind placebo-controlled study. Int J Mol Med. 2013;32:989–94. [DOI] [PubMed] [Google Scholar]
- [15].Yang Z, Zhu MZ, Zhang YB, Wen BB, An HM, Ou XC, et al. Coadministration of epigallocatechin-3-gallate (EGCG) and caffeine in low dose ameliorates obesity and nonalcoholic fatty liver disease in obese rats. Phytother Res. 2019;33:1019–26. [DOI] [PubMed] [Google Scholar]
- [16].Santamarina AB, Oliveira JL, Silva FP, Carnier J, Mennitti LV, Santana AA, et al. Green tea extract rich in epigallocatechin-3-gallate prevents fatty liver by AMPK activation via LKB1 in mice fed a high-fat diet. PLoS One. 2015;10:e0141227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bose M, Lambert JD, Ju J, Reuhl KR, Shapses SA, Yang CS. The major green tea polyphenol, (-)-epigallocatechin-3-gallate, inhibits obesity, metabolic syndrome, and fatty liver disease in high-fat-fed mice. J Nutr. 2008;138:1677–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kenneally S, Sier JH, Moore JB. Efficacy of dietary and physical activity intervention in non-alcoholic fatty liver disease: a systematic review. BMJ Open Gastroenterol. 2017;4:e000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Petridou A, Siopi A, Mougios V. Exercise in the management of obesity. Metabolism. 2018. [DOI] [PubMed] [Google Scholar]
- [20].Schon HT, Weiskirchen R. Exercise-induced release of pharmacologically active substances and their relevance for therapy of hepatic injury. Front Pharmacol. 2016;7:283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Park H, Kaushik VK, Constant S, Prentki M, Przybytkowski E, Ruderman NB, et al. Coordinate regulation of malonyl-CoA decarboxylase, sn-glycerol-3-phosphate acyltransferase, and acetyl-CoA carboxylase by AMP-activated protein kinase in rat tissues in response to exercise. J Biol Chem. 2002;277:32571–7. [DOI] [PubMed] [Google Scholar]
- [22].Foretz M, Even PC, Viollet B. AMPK activation reduces hepatic lipid content by increasing fat oxidation in vivo. Int J Mol Sci. 2018; 19:2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Cho J, Lee I, Kim D, Koh Y, Kong J, Lee S, et al. Effect of aerobic exercise training on non-alcoholic fatty liver disease induced by a high fat diet in C57BL/6 mice. J Exerc Nutr Biochemi. 2014;18:339–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sae-Tan S, Rogers CJ, Lambert JD. Voluntary exercise and green tea enhance the expression of genes related to energy utilization and attenuate metabolic syndrome in high fat fed mice. Mol Nutr Food Res. 2014;58:1156–9. [DOI] [PubMed] [Google Scholar]
- [25].Sae-Tan S, Rogers CJ, Lambert JD. Decaffeinated green tea and voluntary exercise induce gene changes related to beige adipocyte formation in high fat-fed obese mice. J Funct Foods. 2015;14:210–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Schneider K, Oltmanns J, Hassauer M. Allometric principles for interspecies extrapolation in toxicological risk assessment—empirical investigations. Regul Toxicol Pharmacol. 2004;39:334–47. [DOI] [PubMed] [Google Scholar]
- [27].Mineharu Y, Koizumi A, Wada Y, Iso H, Watanabe Y, Date C, et al. Coffee, green tea, black tea and oolong tea consumption and risk of mortality from cardiovascular disease in Japanese men and women. J Epidemiol Comm Health. 2011;65:230–40. [DOI] [PubMed] [Google Scholar]
- [28].Liu J, Liu S, Zhou H, Hanson T, Yang L, Chen Z, et al. Association of green tea consumption with mortality from all-cause, cardiovascular disease and cancer in a Chinese cohort of 165,000 adult men. Eur J Epidemiol. 2016;31:853–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Li X, Qiao Y, Yu C, Guo Y, Bian Z, Yang L, et al. Tea consumption and bone health in Chinese adults: a population-based study. Osteoporos Int. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Shen Q, Yu C, Guo Y, Bian Z, Zhu N, Yang L, et al. Habitual tea consumption and risk of fracture in 0.5 million Chinese adults: A prospective cohort study. Nutrients. 2018;10:1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Inoue M, Kurahashi N, Iwasaki M, Shimazu T, Tanaka Y, Mizokami M, et al. Effect of coffee and green tea consumption on the risk of liver cancer: cohort analysis by hepatitis virus infection status. Cancer Epidemiol Biomark Prev. 2009;18:1746–53. [DOI] [PubMed] [Google Scholar]
- [32].Gu Y, Yu S, Park JY, Harvatine K, Lambert JD. Dietary cocoa reduces metabolic endotoxemia and adipose tissue inflammation in high-fat fed mice. J Nutr Biochem. 2014;25:439–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Glisan SL, Ryan C, Neilson AP, Lambert JD. Cranberry extract attenuates hepatic inflammation in high-fat-fed obese mice. J Nutr Biochem. 2016;37:60–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Sae-Tan S, Grove KA, Kennett MJ, Lambert JD. (-)-Epigallocatechin-3-gallate increases the expression of genes related to fat oxidation in the skeletal muscle of high fat-fed mice. Food Funct. 2011;2:111–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Grove KA, Sae-tan S, Kennett MJ, Lambert JD. (-)-Epigallocatechin-3-gallate inhibits pancreatic lipase and reduces body weight gain in high fat-fed obese mice. Obesity (Silver Spring). 2012;20:2311–3. [DOI] [PubMed] [Google Scholar]
- [36].Cha KH, Song DG, Kim SM, Pan CH. Inhibition of gastrointestinal lipolysis by green tea, coffee, and gomchui (Ligularia fischeri) tea polyphenols during simulated digestion. J Agric Food Chem. 2012;60:7152–7. [DOI] [PubMed] [Google Scholar]
- [37].Raederstorff DG, Schlachter MF, Elste V, Weber P. Effect of EGCG on lipid absorption and plasma lipid levels in rats. J Nutr Biochem. 2003;14:326–32. [DOI] [PubMed] [Google Scholar]
- [38].Ogawa K, Hirose S, Nagaoka S, Yanase E. Interaction between tea polyphenols and bile acid inhibits micellar cholesterol solubility. J Agric Food Chem. 2016;64:204–9. [DOI] [PubMed] [Google Scholar]
- [39].Annaba F, Kumar P, Dudeja AK, Saksena S, Gill RK, Alrefai WA. Green tea catechin EGCG inhibits ileal apical sodium bile acid transporter ASBT. Amer J Physiol Gastrointest Liver Physiol. 2010;298:G467–G73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Van Buiten CB, Lambert JD, Elias RJ. Green tea polyphenols mitigate gliadin-mediated inflammation and permeability in vitro. Mol Nutr Food Res. 2018;62:1700879. [DOI] [PubMed] [Google Scholar]
- [41].Papadopoulou A, Frazier RA. Characterization of protein-polyphenol interactions. Trends in Food Sci Technol. 2004;15:186–90. [Google Scholar]
- [42].Meissner M, Havinga R, Boverhof R, Kema I, Groen AK, Kuipers F. Exercise enhances whole-body cholesterol turnover in mice. Med Sci Sports Exerc. 2010;42:1460–8. [DOI] [PubMed] [Google Scholar]
- [43].Jornayvaz FR, Shulman GI. Regulation of mitochondrial biogenesis. Essays Biochem. 2010;47: 10.1042/bse0470069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Haase TN, Ringholm S, Leick L, Bienso RS, Kiilerich K, Johansen S, et al. Role of PGC-1alpha in exercise and fasting-induced adaptations in mouse liver. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1501–9. [DOI] [PubMed] [Google Scholar]
- [45].Tolman KG, Dalpiaz AS. Treatment of non-alcoholic fatty liver disease. Therap Clin risk Management. 2007;3:1153–63. [PMC free article] [PubMed] [Google Scholar]
- [46].Huang J, Feng S, Liu A, Dai Z, Wang H, Reuhl K, et al. Green tea polyphenol EGCG alleviates metabolic abnormality and fatty liver by decreasing bile acid and lipid absorption in mice. Mol Nutr Food Res. 2018;62:1700696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Madison BB. Srebp2: A master regulator of sterol and fatty acid synthesis. J Lipid Res. 2016;57:333–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Zhang G, Miura Y, Yagasaki K. Effects of dietary powdered green tea and theanine on tumor growth and endogenous hyperlipidemia in hepatoma-bearing rats. Biosci Biotechnol Biochem. 2002;66:711–6. [DOI] [PubMed] [Google Scholar]
- [49].Meissner M, Lombardo E, Havinga R, Tietge UJF, Kuipers F, Groen AK. Voluntary wheel running increases bile acid as well as cholesterol excretion and decreases atherosclerosis in hypercholesterolemic mice. Atherosclerosis. 2011;218:323–9. [DOI] [PubMed] [Google Scholar]
- [50].Helal MG, Ayoub SE, Elkashefand WF, Ibrahim TM. Caffeine affects HFD-induced hepatic steatosis by multifactorial intervention. Hum Exp Toxicol. 2018;37:983–90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.