Abstract
The ureteric epithelial progenitor (UEP) population within the embryonic kidney generates the arborized epithelial network of the kidney’s collecting system and plays a critical role in the expansion and induction of the surrounding nephron progenitor pool. Adamts18 shows UEP- restricted expression in the kidney and progenitor tip-restricted expression in several other organs undergoing branching epithelial growth. Adamts18 is encoded by 23 exons. Genetic removal of genomic sequence spanning exons 1 to 3 led to a specific loss of Adamts18 expression in UEPs, suggesting this region may encode a UEP-specific enhancer. Intron 2 (3 kb) was shown to have enhancer activity driving expression of the doxycycline inducible tet-on transcriptional regulator (rtTA) in an Adamts18en-rtTA transgenic mouse strain. Crossing Adamts18en-rtTA mice to a doxycycline dependent GFP reporter mouse enabled the live imaging of embryonic kidney explants. This facilitated the analysis of ureteric epithelial branching events at the cellular level. Ablation of UEPs at the initiation of ureteric bud outgrowth through the doxycycline-mediated induction of Diphtheria Toxin A (DTA) generated a range of phenotypes from complete kidneys agenesis, to duplex kidneys with double ureters. The latter outcome points to the potential of regulative processes to restore UEPs. In contrast, overexpression of YAP prior to ureteric bud outgrowth led to a complete failure of kidney development. Elevating YAP levels at later stages retarded branching growth. A similar phenotype was observed with the overexpression of MYC within the branch-tip localized UEP population. These experiments showcase the utility of the Adamts18en-rtTA transgenic model to the investigation of cellular and molecular events specific to branch tip progenitors within the mammalian kidney complementing existing CRE-dependent genetic tools. Further, the illustrative examples point to areas where new insight may be gained into the regulation of UEP programs.
Keywords: ureteric bud tip progenitor, Adamts18, tip ablation, MYC, YAP, live imaging
INTRODUCTION
The kidney maintains homeostasis of the body’s fluids by filtering the blood to retain important nutrients while removing toxins and balancing salt, water, and pH levels. The development and patterning of this organ is elaborate due to the complexity of the internal structures [for review see McMahon, 2016]. Each mouse kidney has 12,000-16,000 nephrons, which are intricate epithelial structures with highly specialized cells distributed at specific locations [Short et al., 2014]. The plasma filtrate traffics through the epithelial network of nephrons into an independently derived collecting duct system which is connected to the bladder by the ureter.
The collecting duct system develops through a process of epithelial branching morphogenesis, a developmental routine with conserved cellular and molecular features across different mammalian organ systems and a range of metazoans [Ochoa-Espinosa and Affolter, 2012; Iber and Menshykau, 2013, Lang et al., 2018; Myllymäki and Mikkola, 2019]. Morphogenesis of the mouse kidney initiates around embryonic day 10.5 (E10.5). A subset of cells within the nephric duct at the hindlimb region, the ureteric bud (UB), evaginates to contact a surrounding population of pre-determined metanephric mesenchyme that condenses around the UB tip. The interplay between nephron and interstitial progenitor populations in the metanephric mesenchyme and ureteric epithelial progenitors (UEPs) in the UB drives kidney development [McMahon, 2016]. Ligand-mediated activation of a variety of receptor tyrosine kinase pathways in the UEP pool, most notably Gdnf/Ret and Fgf/Fgfr, coordinate growth and branching morphogenesis of the ureteric epithelial network of the collecting system [Lemmon et al., 2010; Neben et al., 2019]. A variety of approaches, including genetic dissection of key determinants of UEP actions [Costantini, 2012] and expression screens to identify branch-tip restricted gene activity [Rutledge et al., 2017], have shed light on regulatory programs within the UEP population. These studies highlight the important role of different genetic tools to visualize and modulate gene activity within the UEPs [Schuchardt et al., 1994; Majumdar et al., 2003; Chi et al., 2009a; Chi et al., 2009b; O’Brien et al., 2018].
Adamts18 encodes a secreted metalloprotease of the Adamts family that is expressed in branch tip progenitors within mammalian organ systems undergoing branching morphogenesis [Rutledge et al., 2017]. In this report, we identified an enhancer regulating UEP specific expression of Adamts18, and we co-opted this DNA sequence to generate a transgenic mouse line where expression of the reverse tetracycline-controlled transactivator (rtTA; [Gossen et al., 1995; Schönig and Bujard, 2003]) is directed to UEPs. We demonstrate the utility of this Adamts18en-rtTA (AE-rtTA) transgene through the doxycycline-dependent regulation of a number of tetracycline-responsive element (TRE) containing transgenes, modifying molecular and cellular actions within the UEP population during mouse kidney development.
RESULTS
Identification of an Adamts18 Kidney-specific Enhancer
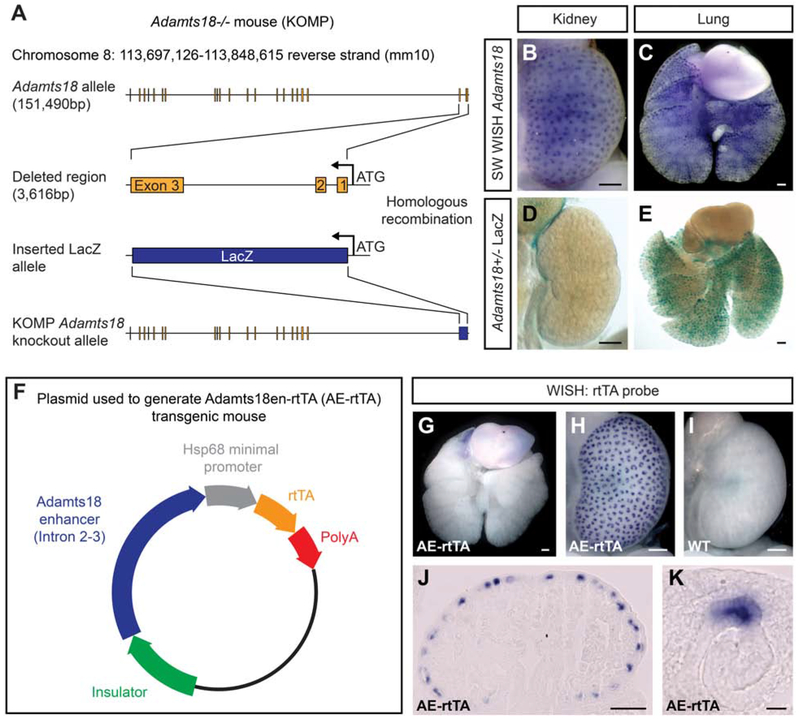
We have previously reported on an Adamts18tm1(KOMP)Vlcg knockout allele generated in the NIH Knock-Out Mouse Project [Rutledge et al., 2019]. Gene targeting at the Adamts18 locus generated a null allele by removing a 3,616 bp region directly downstream from the ATG encoding the start of translation in exon 1 to coding sequences in exon 3. This deleted genomic sequence was replacing with an in-frame fusion of an E. coli LacZ gene sequence [Fig. 1A]. Activation of Adamts18 was expected to result in LacZ-encoded β-galactosidase enzyme production in different organs where Adamts18 is specifically expressed. However, whereas whole-mount in situ hybridization (WISH) analysis of E15.5 kidneys and lungs shows an expected pattern of Adamts18 expression within the branch tip population of both organs [Fig. 1B, C], β-galactosidase activity was only detected in the lung [Fig. 1D, E].
Fig. 1: Identification of a kidney-specific Adamts18 enhancer and generation of the Adamts18en-rtTA tet-on inducible system.
A: The Adamts18−/− mouse (VG 12442) generated by the trans-NIH Knock-Out Project (KOMP). The first three exons with associated intronic regions were deleted and replaced with an E. coli LacZ sequence directly downstream of the ATG site. B, C: Whole-mount in situ hybridization (WISH) of Swiss Webster E15.5 kidney (B) and lung (C) demonstrating the tip-specific expression of an Adamts18 probe. D, E: LacZ staining of E15.5 Adamts18+/− kidney (D) and lung (E). F: The plasmid constructed to generate the Adamts18en-rtTA (AE-rtTA) transgenic mouse. The Adamts18 enhancer sequence is the entire intronic region between Exon 2 and Exon 3. G-I: WISH of E15.5 AE-rtTA lung (G) and kidneys (H, I) showcasing the tip-specific expression of rtTA only within the AE-rtTA kidney. J, K: Sectioned kidney tissue from H showing the tip-specific expression of rtTA at the cellular level. Line in B-E, G-J = 200μm; Line in K = 20μm.
Generation of Adamts18en-rtTA (AE-rtTA) Transgenic Mouse
We speculated the unexpected kidney result may reflect the loss of a kidney-specific intronic enhancer within intron 1 or 2 of the deleted region of the Adamts18 allele. Given the large size of intron 2 (3,025 bp) versus intron 1 (102 bp), we focused further analysis on intron 2. We cloned this sequence upstream of a minimal promoter driving expression of an rtTA cDNA and generated a transgenic, C57BL/6J mouse line (Adamts18en-rtTA; hereafter referred to as AE-rtTA) with this construct [Fig. 1F]. WISH demonstrated rtTA was expressed specifically within kidney UEPs with a temporal and spatial pattern closely resembling Adamts18, confirming the presence of a kidney enhancer within the tested region [Fig. 1B, G-K].
Titration of the Doxycycline Treatment for Optimal Induction of the Tet-on System
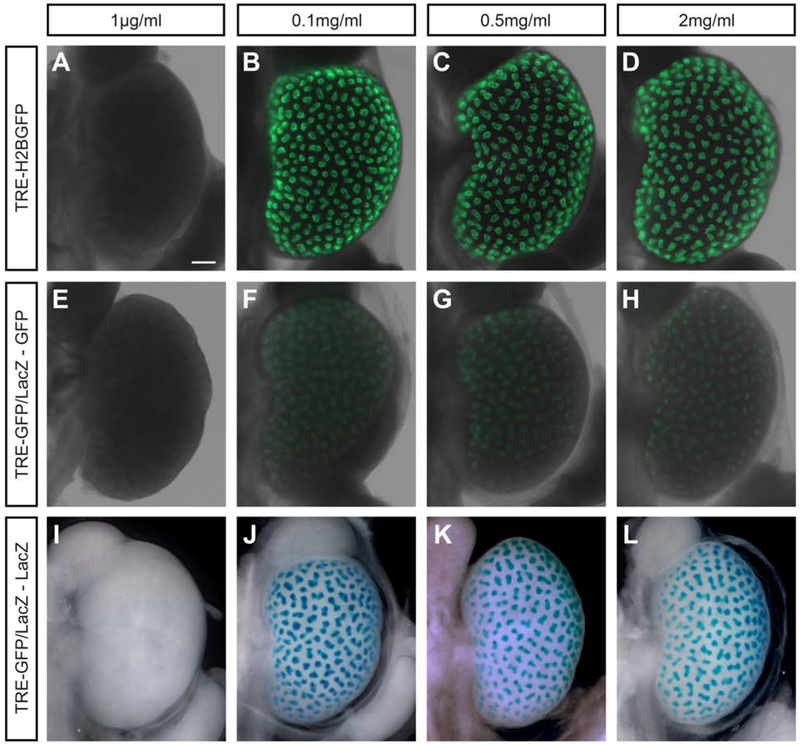
To determine an optimal concentration for doxycycline-mediated activation of target transgenes, we observed the response of TRE-H2BGFP and TRE-GFP/LacZ reporter mouse strains where the former displays GFP fluorescence and the latter displays both GFP fluorescence and β-galactosidase enzyme activity upon activation [Fig. 2E-L; Tumbar et al., 2004; Krestel et al., 2001]. Doxycycline was added to the drinking water at concentrations between 1μg/ml-2mg/ml in 5% sucrose water 24hr prior to harvesting the kidneys at E15.5. GFP activity was captured immediately following dissection. AE-rtTA;TRE-GFP/LacZ-positive kidney samples were followed up with β-galactosidase staining to visualize LacZ activity. No GFP or LacZ staining was observed at 1μg/ml doxycycline for either reporter mouse [Fig. 2A, E, I] while similar robust activation was observed with 0.1-2.0 mg/ml with a markedly stronger fluorescent signal with the TRE-H2BGFP reporter strain [Fig. 2B-D, F-H, J-L].
Fig. 2: Titration of 24 hour doxycycline treatment for Adamts18en-rtTA tet-on induction demonstrates consistency in activation through two rtTA reporter mice.
For all kidneys collected, various concentrations of doxycycline (indicted in figure) in 5% sucrose water was given to the pregnant mouse 24hr prior to dissection at E15.5. A-D: AE-rtTA;TRE-H2BGFP kidneys show GFP expression in the UB tip population starting at 0.1mg/ml. E-H: AE-rtTA;TRE-GFP/LacZ kidneys have very weak GFP expression from 0.1mg/ml to 2mg/ml. I-L: LacZ staining shows the presence of β-galactosidase in AE-rtTA;TRE-GFP/LacZ kidneys within the ureteric bud tip population that matches the GFP expression in F-H. Line = 200μm.
The Adamts18 Enhancer is Expressed within the Kidney, Limb Buds, Epidermis, and Heart of Embryonic Mice
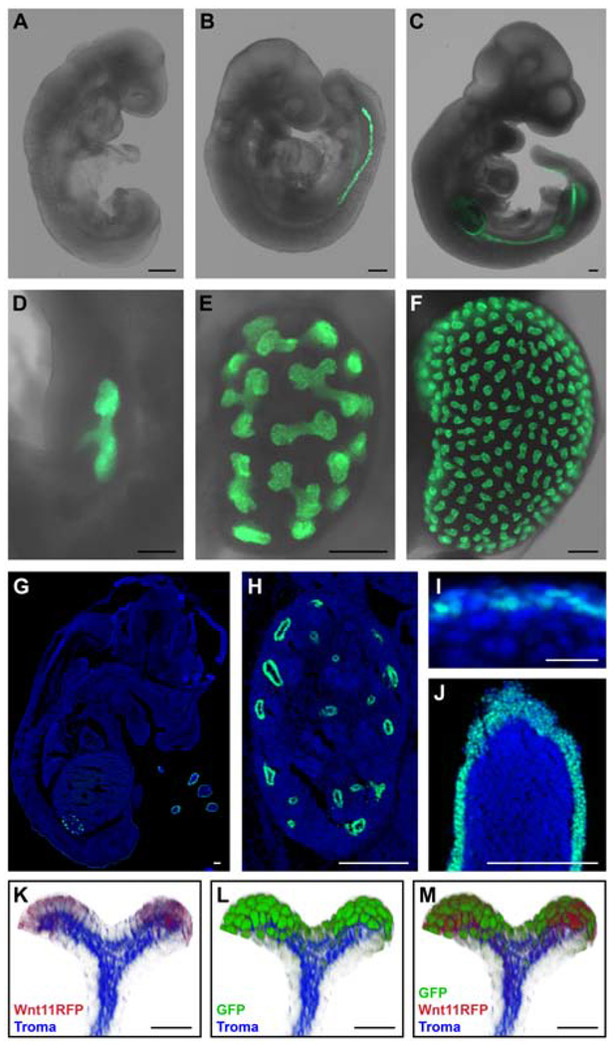
To examine Adamts18 enhancer activity in detail, AE-rtTA carrier mice were crossed to a doxycycline-dependent TRE-H2BGFP reporter mouse strain [Tumbar et al., 2004]. Doxycycline was added to the drinking water (2mg/ml) 24hrs before embryo collection and embryos were imaged to detect an endogenous GFP signal. At E8.5 there was no observable GFP expression [Fig. 3A]. By E9.5, GFP activity was visible within the nephric duct [Fig. 3B] moving caudally with the posterior elongation of the nephric duct to the level of the hind limb by E10.5 [Fig. 3C]. As kidney morphogenesis initiated, GFP activity was restricted to the ureteric epithelial branch tips during the entire period of branching morphogenesis [E10.5-P1; Fig. 3D-F, H], mimicking the native Adamts18 expression found in the kidney. At E14.5, GFP-positive cells were evident in the limb throughout the epidermal layer surrounding the digits, in sporadic epidermal cells in other body regions, and in the heart. [Fig. 3G, I, J, heart data not shown]. No GFP activity was observed in the lung at any of these stages [Fig. 3G]. Although native Adamts18 is expressed within the lung epithelial tip population, the lack of GFP expression in the lung is expected from the absence of rtTA in the lung of the AE-rtTA transgenic mouse [Fig. 1G].
Fig. 3: Embryonic mouse tissue of AE-rtTA;TRE-H2BGFP highlights the temporal and spatial expression of Adamts18en-rtTA.
For all embryos collected (except K-M), 2mg/ml doxycycline in 5% sucrose water was given to the pregnant mouse 24hr prior to dissection. A: No GFP expression observed within the E8.5 embryo. B: GFP expression is within the nephric duct at E9.5. C: At E10.5, GFP expression is present in the nephric duct and limb buds. D-F: Kidneys at E11.5 (D), E13.5 (E), and E15.5 (F) with GFP expression restricted to the distal ends of the ureteric epithelium. G-J: Sectioned E14.5 embryo stained with Hoechst 33342 and demonstrating native GFP expression pattern within the whole embryo (G), ureteric epithelium of the kidney (H), epidermis (I), and digits (J). K-M: Volume projections of ureteric bud tips from a whole-mount stained E16.5 AE-rtTA;TRE-H2BGFP;Wnt11RFP kidney that was administered 2mg/ml doxycycline 48hr before collection. Tissue was stained with RFP, GFP, and Troma (epithelial marker) antibodies. Line in A-H, J = 200μm; line in I, K-M = 20μm.
Wnt11 expression localizes to UEPs and Wnt11 signaling plays multiple roles in regulating adjacent Six2+ nephron progenitors [Pepicelli et al., 1997; O’Brien et al., 2018; Rutledge et al., 2017]. To visualize the activation of GFP in relation to the Wnt11+ tip population, we compared activity of the AE-rtTA transgene with a previously reported Wnt11-RFP∷CRE-ERT2 (hereafter, Wnt11RFP) Bac transgenic line [Harding et al., 2011]. ARrtTA;Wnt11RFP mice were crossed to the TRE-H2BGFP reporter strain. Doxycycline (2mg/ml) was added to the drinking water of pregnant females two days before embryo collection at E16.5. Volume projections of the ureteric bud tips immunostained with markers for the ureteric epithelium (Troma), RFP, and GFP demonstrate an extensive overlap between Wnt11RFP+ cells and GFP+ cells [Fig. 3K-M]. Most, if not all, Wnt11RFP+ cells were GFP+. However, GFP+/RFP- cells extended from the UEPs in the branch into proximal stalk regions [Fig. 3K-M].
In part this reflects broader expression of Adamts18 in the tip [Rutledge et al., 2017, 2019]. In addition, the levels of transgene activation (doxycycline concentration dependent), and the stability of the transgenic mRNA and protein will factor into extended expression beyond the distribution transgenic protein within stalk regions.
Adamts18en-rtTA as a Live Imaging Tool
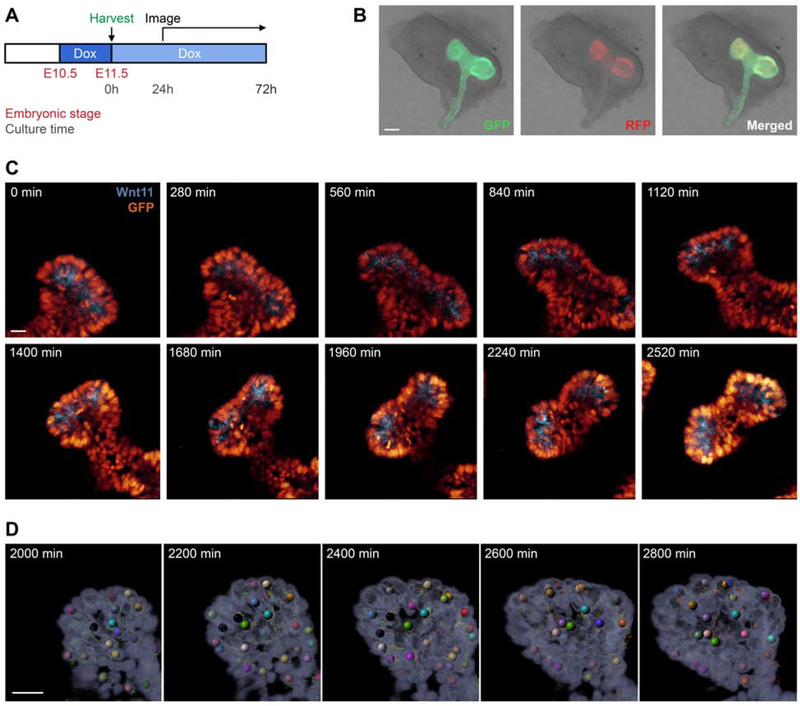
To visualize the developing ureteric epithelium at the cellular level, time-lapse imaging studies were conducted on AE-rtTA;TRE-H2BGFP;Wnt11RFP embryonic kidneys. Doxycycline was added (2mg/ml) to the drinking water of pregnant mice at day 10.5 of pregnancy, and kidneys were dissected from embryos at E11.5, cultured on a filter for various lengths of time in the presence of 1mg/ml doxycycline [Fig. 4A]. At the time of collection, ureteric branch tips were RFP+ and H2BGFP+; stable GFP activity also extended into non-branched regions as expected [Fig. 4B]. Cultures were imaged for 48 hours to capture branching events [Fig. 4C, Video S1]. The cellular resolution and bright fluorescent nuclear restricted H2BGFP labeling enabled an automatic tracking of the movements and behaviors of epithelial cells in relation to the Wnt11-positive UBT population using Imaris software [Fig. 4D]. The findings highlight a robust platform for furthering an understanding of cell behaviors and cell interactions during branching morphogenesis particularly in combination with other transgenic alleles that can modify gene activity within the UEP population (see below). This system adds a complementary live imaging tool to the current systems established for ureteric bud development [Shakya et al., 2005; Chi et al., 2009b; Leclerc and Costantini, 2016; Riccio et al., 2016].
Fig. 4: Cell tracking of AE-rtTA;TRE-H2BGFP;Wnt11RFP embryonic kidney explants through high-resolution live imaging.
A: Pregnant AE-rtTA;TRE-H2BGFP;Wnt11RFP mice were given 2mg/ml doxycycline 24hr before harvesting at E11.5. Dissected kidneys were put on a Transwell filter plate with doxycycline-treated media (1mg/ml) and incubated at 37°C. After 24hr, kidneys were imaged every 5min for 48hr with z-stacking to capture the entire structure. B: E11.5 kidney post-dissection illustrating native GFP and RFP signal. C: Time-lapse imaging of kidney explants; see also Video S1. D: Automated tracking of cells from the time-lapse imaging. Line in B = 100μm; line in C, D = 30μm.
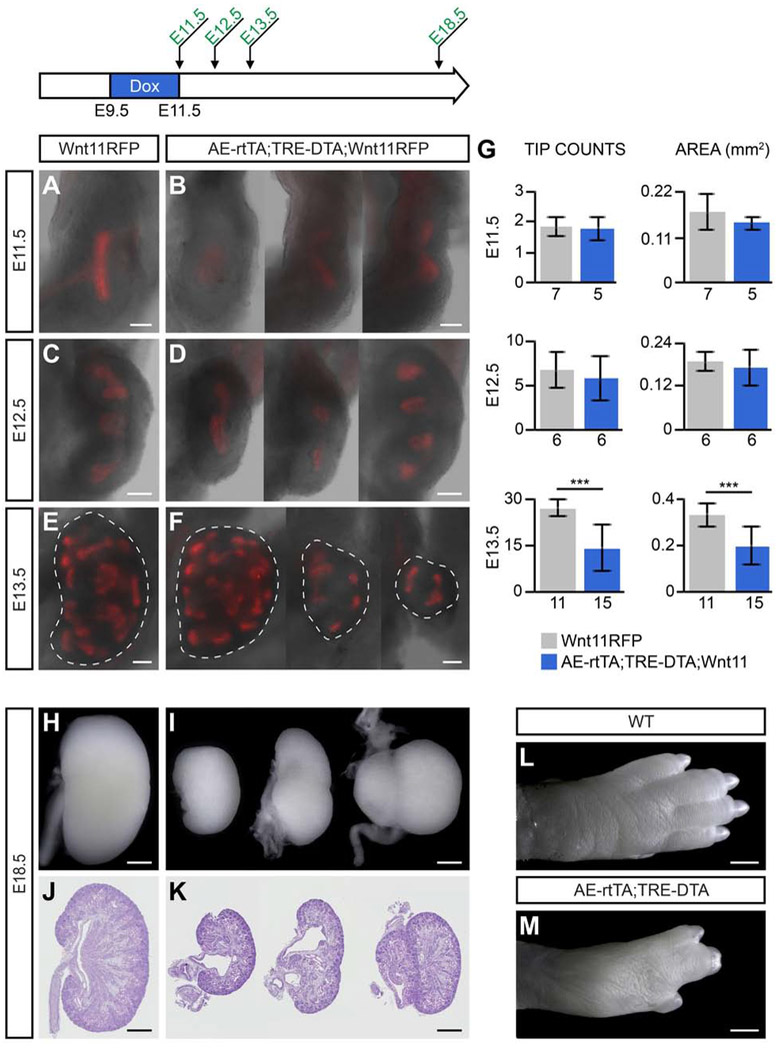
Embryonic Tip Ablation via Diphtheria Toxin Subunit A at Pre-outgrowth Stages Results in Reduced Branching Events and Smaller Kidneys
Diphtheria toxin subunit A (DTA) has been used extensively as an established method to effectively ablate specific cell lineages [Collier, 2001]. DTA inactivates eukaryotic elongation factor 2 (Eef2), inhibiting protein synthesis and resulting in rapid cell death [Breitman et al., 1987; Palmiter et al., 1987; Mateyak and Kinzy, 2013]. This ablation is highly specific to only DTA-producing cells since DTA lacks the B subunit required for cell membrane penetration, thus unaffecting cells not generating DTA [Collier, 2001].
Here, we utilize the tet-on system to induce UBT cell ablation via DTA production at specific time points during kidney development. We attempted to ablate UB tip cells through AE-rtTA and doxycycline-dependent activation of a DTA transgene derived from the TRE-DTA transgenic mouse line [Lee et al., 1998]. Production of DTA triggers rapid cell death with even low levels of active DTA [Breitman et al., 1987; Palmiter et al., 1987; Collier, 2001; Mateyak and Kinzy, 2013].
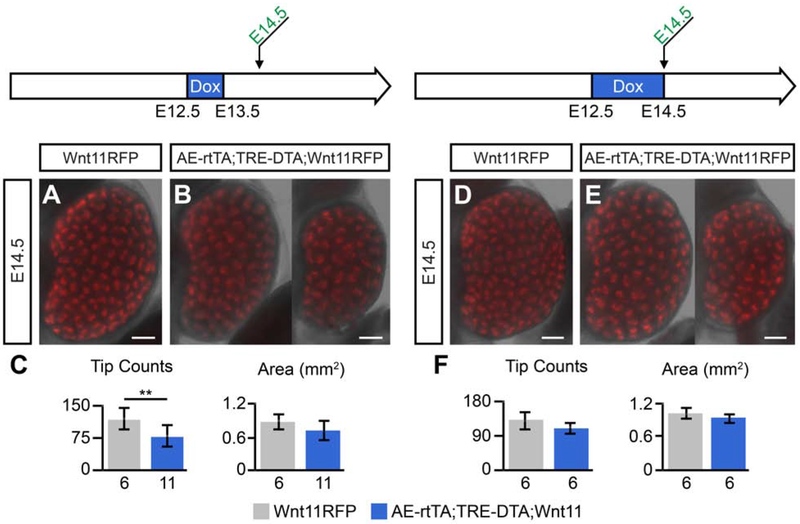
Surprisingly, given results with the reporter mice, when 2mg/ml doxycycline was administered to the drinking water of pregnant females over a period of embryonic development encompassing pre-ureteric outgrowth stages of nephric duct development to ureteric bud formation and initiation of the first branching event (E9.5-E11.5), kidney development was initiated and RFP-producing cells were evident in emerging UB branch tips, although branch tips were markedly reduced in size [Fig. 5A, B, G]. At E12.5 and E13.5 (24hr and 48hr after removal of doxycycline from dams), some kidneys showed retarded development while others appeared similar to wildtype [Fig. 5C-F]. Quantification at E13.5 showed a significant reduction in the number of tips and the area of the kidney in doxycycline-activated embryos [Fig. 5G]. By E18.5, kidneys exhibited a wide range of phenotypes including agenesis, duplicated ureters and duplicated kidneys [Fig. 5H-K]. Notable kidney duplications were observed along the anterior posterior axis as is frequently observed when extra ureteric outgrowths occur [Rutledge et al., 2019] but also along the medial-lateral axis suggesting a more dramatic perturbation in epithelial outgrowth [Fig. 5H-K]. In parallel, embryos displayed marked digit fusion consistent with loss of cells within the apical ectoderm ridge, a critical structure in outgrowth and patterning of the vertebrate limb [Fig. 5L, M; Verheyden and Sun, 2017]. Doxycycline administration following ureteric bud outgrowth at E12.5 for 24-48 hours had no pronounced effect on kidney development [Fig. 6A-F].
Fig. 5: Tip ablation through diphtheria toxin subunit A (DTA) at pre-outgrowth of the ureteric bud results in a range of structural abnormalities.
AE-rtTA;Wnt11RFP mice were crossed to TRE-DTA mice, and the pregnant females were given 2mg/ml doxycycline from E9.5-E11.5. Kidneys were harvested at various timepoints post-doxycycline treatment to determine the effects of tip ablation. A, B: E11.5 kidneys harvested with no doxycycline recovery time. C-D: E12.5 kidneys (24hr recovery time) show slight variations in phenotype. E-F: E13.5 kidneys (48hr recovery time) with dashed lines around the perimeter of the organ. G: Quantification of average tip counts and average area from kidneys harvested at E11.5, E12.5 and E13.5. Sample sizes are listed on x-axis. Error bars are SD and significance was determined by a two-tailed t-test (*** = p ≤ 0.001). H-M: At E18.5, kidneys with DTA have a range of phenotypes including kidney agenesis, duplex kidneys, and double ureters compared to wildtype (H, I - whole kidney, J, K - sectioned H&E). The digits are malformed in tip-ablated embryos (M) compared to wildtype (L). Line in A-F = 100μm, line in H-M = 500μm.
Fig. 6: DTA tip ablation at ureteric bud post-outgrowth stages has little to no effect on kidney development.
AE-rtTA;Wnt11RFP mice were crossed to TRE-DTA mice and the resulting pregnant mice were administered doxycycline post-outgrowth of the ureteric bud to analyze the effect of tip ablation. A-C: Kidneys harvested at E14.5 that were given 2mg/ml doxycycline from E12.5-E13.5. D-E: Pregnant mice were administered 2mg/ml doxycycline from E12.5-E14.5 and kidneys were collected at E14.5. Sample sizes are listed on x-axis of quantifications for tip count and area. Error bars are SD and significance was determined by a two-tailed t-test (** = p ≤ 0.01). Line = 200μm.
The simplest interpretation of these results is a marked mosaicism in effective activation of the TRE-DTA transgene that was not observed with the reporter strains. Given the negative outcomes of any spontaneous activity of the transgene, a strong selection is expected for transcriptional silencing that may impact responsiveness to doxycycline-mediated activation. For example, epigenetic controls at the site of transgene insertion may attenuate access to rtTA or other essential transcriptional components. Unfortunately, these technical limitations preclude the use of this approach to completely ablate the UEP pool to examine the potential or regenerative plasticity in the ureteric bud outgrowth to the macro-organization of the kidney.
YAP Overexpression Results in Kidney Agenesis with Abnormalities in the Branched Network
The Hippo pathway is a conserved core kinase cascade that modulates tissue growth [Pan, 2010; Meng et al., 2016]. A variety of environmental cues typically linked (but not restricted) to cell adhesion, mechano-sensation, and cell polarity modulate Hippo pathway signaling. Pathway activation leads to phosphorylation-dependent degradation of the transcriptional co-activators Yap and Taz and transcriptional silencing [Varelas et al., 2010; Yu et al., 2010; Yin et al., 2013]. In contrast, pathway inhibition enables unphosphorylated Yap or Taz to translocate into the nucleus where they directly interact with Tead DNA binding transcriptional regulators promoting transcription of target genes which includes genes promoting cell proliferation and blocking apoptosis [Zheng and Pan, 2019]. Remarkably, downregulated Yap activity can dramatically increase mammalian organ size through the expansion of progenitor cell types [Dong et al., 2007]. In the developing kidney, organ size is linked to expansion of the ureteric tree as extra early ureteric branches lead to larger kidneys with more nephrons [Rutledge et al., 2019]. The ureteric bud tips are highly proliferative. Further, branch tips are likely experiencing significant changes in mechanical forces in conjunction with matrix interactions and epithelial rearrangements essential to branching morphogenesis. Consequently, the Hippo pathway is an interesting candidate for regulating tip programs and Yap/Taz activity within UEPs is unclear. Kidney-specific loss of Nf2 or Latx1/2, key proteins in the kinase cascade leading to Yap/Taz turnover, leads to a reduction of branching growth, but it is not clear whether this phenotype reflects a direct action of Yap or Taz in UEPs [Reginensi et al., 2016].
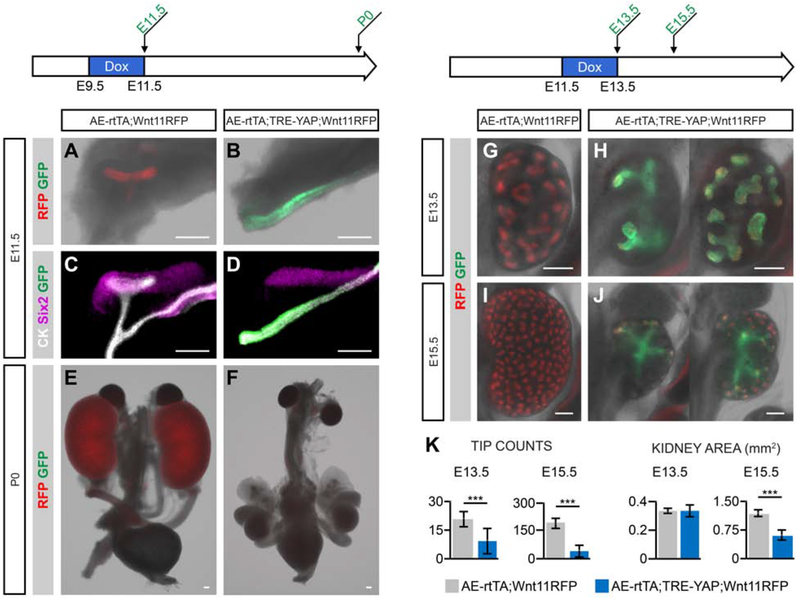
To examine the effects of ectopic Yap activity in the developing ureteric system, AE-rtTA;Wnt11RFP mice were crossed to a TRE-YAPGFP transgenic strain [Gao et al., 2013]. The TRE-YAP transgene encodes a YAP-GFP fusion protein in which a serine to alanine amino acid substitution at the 112 position in YAP1 protein prevents Hippo pathway turnover and GFP facilitates the visualization of this activated YAP protein [Gao et al., 2013]. The amino acid substitution is reported to promote the translocation of YAP into the nucleus [Dong et al., 2007; Camargo et al., 2007].
Doxycycline administration (2mg/ml) to pregnant females between E9.5-E11.5 resulted in strong GFP activation within the nephric duct of AE-rtTA;TRE-YAP;Wnt11RFP embryos [Fig. 7B, D]. In contrast, control kidneys at E11.5 lack GFP expression and have a ureteric bud that has branched to a T-shaped epithelial structure in contact with the adjacent Six2+ mesenchymal nephron progenitors [Fig. 7A, C]. Remarkably, ectopic YAP completely suppressed ureteric bud outgrowth (20/20 AE-rtTA;TRE-YAP;Wnt11RFP embryos) [Fig. 7B, D]. A GFP+ thickening was observed in the nephric duct facing the metanephric mesenchyme where outgrowth was expected to initiate, suggesting UEP precursor cells may be present, but unable to undergo epithelial morphogenesis [Fig. 7C, D]. Removal of doxycycline from the drinking water at E11.5 failed to rescue kidney development [Fig. 7E, F; 8/8 AE-rtTA;TRE-YAP;Wnt11RFP P0 neonates].
Fig. 7: YAP overexpression in embryonic kidney UBTs leads to a decrease in branching morphogenesis with less tips and smaller organs.
AE-rtTA;Wnt11RFP mice were crossed to TRE-YAP mice and given doxycycline at various stages during the pregnancy. YAP activation is visualized with the expression of GFP. A-D: E11.5 AE-rtTA;TRE-YAP;Wnt11RFP kidneys were harvested after a doxycycline treatment (2mg/ml) from E9.5-E11.5. Native fluorescent signal post-dissection (A, B) and antibody staining (C, D) illustrate the absence of the UB in YAP-activated tissue. E-I: Kidneys collected at E13.5 (E, F) or E15.5 (G, H) that were given 0.5ml/ml Dox to the pregnant mouse from E11.5-E13.5. At both collection stages, kidneys are significantly smaller with less branching and number of tips (I). Sample size numbers for quantifications are listed on the x-axis. Error bars are SD and significance was determined by a two-tailed t-test (** = p ≤ 0.01, *** = p ≤ 0.001). Line = 200μm.
When doxycycline administration was shifted to the E11.5-E13.5 period of kidney development, the number of ureteric epithelial branch tips were reduced at E13.5 and kidneys were correspondingly smaller [Fig. 7G, H, K]. Further, the morphology of branching structures was irregular and the loss of Wnt11RFP activity within some tips suggested a reduction of UEPs [Fig. 7G, H]. Analysis of E15.5 embryos 48 hours after withdrawal of doxycycline suggested a heterogeneity in recovery responses. Some kidneys appeared to exhibit a partial recovery with new branching events while other kidneys showed some growth, but no additional branching [Fig. 7I, J]. However, even these showed a population of Wnt11RFP+ ureteric progenitors at the tips of remaining branches. Regardless of the kidney’s ability to recover post-doxycycline treatment, these kidneys still had a significant reduction in tip numbers and kidney area with YAP activation compared to wildtype, suggesting a deficiency to recover fully [Fig. 7K].
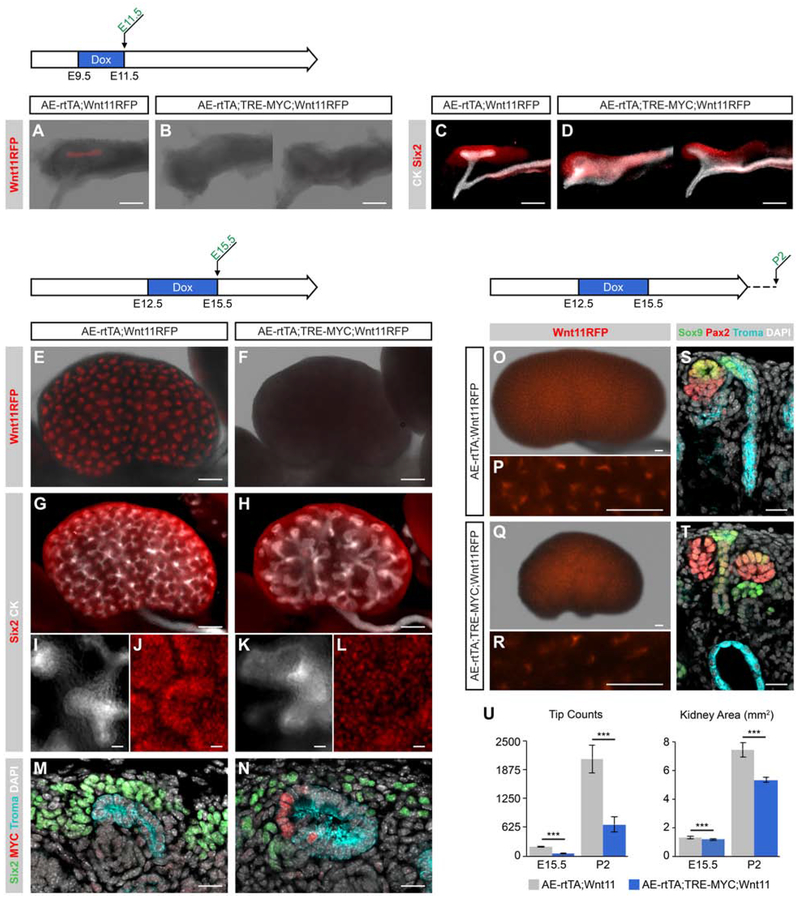
Kidneys with MYC Overexpression are Smaller with Reduced Wnt11-positive Tip Progenitor Population
Myc family members play a critical role promoting the proliferative expansion of stem/progenitor populations and regulating normal and neoplastic organ growth [Meyer and Penn, 2008; Dang, 2013; Bretones et al., 2015]. In kidney development, Myc is active within the nephron progenitor cells (NPCs) and developing nephron structures [Zimmerman et al., 1986; Schmid et al., 1989; Semsei et al., 1989; Mugrauer et al., 1991]. Conditional deletion of Myc within the metanephric mesenchyme leads to renal hypoplasia [Couillard and Trudel, 2009]. Additionally, Myc has been shown to interact with beta-catenin promoting the proliferation of the NPCs during development [Pan et al., 2017]. Mycn levels are particularly high within the UEP population; there is an 11-fold higher level of Mycn in the ureteric tip to ureteric stalk region (RPKM = 16.01, FC = 11.1; Rutledge et al., 2017). These findings are consistent with Myc-family regulation of progenitor growth in both nephron and UEP pools.
To examine Myc action in kidney growth, we crossed AE-rtTA;Wnt11RFP mice to a TRE-MYC transgenic strain enabling doxycycline-dependent induction of the human MYC gene in UEPs [Felsher and Bishop, 1999]. Doxycycline (2mg/ml) administration to pregnant females over the E9.5-E11.5 period lead to a loss of Wnt11RFP expression and abnormal outgrowth of the ureteric bud at E11.5 [Fig. 8A-D]. When doxycycline was administered post-outgrowth from E12.5-E15.5, the AE-rtTA;TRE-MYC;Wnt11RFP kidneys were markedly smaller and displayed a loss of Wnt11RFP expression at E15.5, suggesting a MYC-dependent loss of UEPs [Fig 8E, F]. Overall, these kidneys exhibited fewer branches, and the epithelium at the tips was thicker [Fig. 8G, H] and tips were expanded [Fig. 8I, K]. The ureteric tip cell population maintains adjacent nephron progenitor cells. Consequently, the marked reduction in Six2+ nephron progenitors around abnormal branch tips likely reflects the loss of UEP-derived signals essential to the nephron progenitor pool [Fig. 8J, L]. An analysis of kidney sections confirmed observations from whole-mount analysis, in addition to demonstrating MYC activation in the ureteric tip region [Fig. 8M, N].
Fig. 8: The overexpression of MYC within embryonic kidney UBTs results in reduced kidney size and number of tips.
AE-rtTA;Wnt11RFP mice crossed to TRE-MYC mice and given 2mg/ml doxycycline at various stages and collected throughout development. A-D: Doxycycline was administered from E9.5-E11.5 and kidneys were harvested at E11.5. Native Wnt11RFP is observed in AE-rtTA;Wnt11RFP (A) while absent in AER-rtTA;TRE-MYC;Wnt11RFP (B). Whole-mount immunostaining demonstrates the abnormalities in the nephric duct and ureteric bud (C, D). E, F: Native Wnt11RFP signal post-dissection at E15.5. G-L: Whole-mount immunostaining of E15.5 organs with Six2 (NPC marker) and CK (ureteric epithelial marker). High magnification of G and F shown in I-L as single channels. M, N: E15.5 kidney cryosections stained with MYC, Six2, and Troma (epithelial marker) demonstrate the enlarged tip morphology and loose distribution of NPCs compared to non-MYC activated samples. O-R: AE-rtTA;TRE-MYC;Wnt11RFP kidneys harvested at P2. S, T: Cryosections from P2 AE-rtTA;TRE-MYC;Wnt11RFP samples reveal cyst-like structures within the ureteric epithelium that are not present in non-MYC activated samples. U: Quantification of tip counts and kidney area at E15.5 and P2. Sample sizes for quantifications are 6 kidneys except for AE-rtTA;TRE-MYC;Wnt11RFP P2 which had 4 kidneys. Error bars are SD and significance was determined by a two-tailed t-test (*** = p ≤ 0.001). Line in A-H, O-R = 200μm; line in I-N, S, T = 20μm.
When doxycycline treated kidneys (E12.5-E15.5) were examined at post-natal day 2 (P2) several days after doxycycline removal, AE-rtTA;TRE-MYC;Wnt11RFP kidneys were significantly smaller in size with a marked reduction in ureteric tips compared with control kidneys [Fig. 8U], though the presence of a few sporadic patches of Wnt11RFP tips indicate a potential recovery of UEPs [Fig. 8O-R]. Notably, some tips terminated in large cystic structures some distance beneath the kidney capsule, suggesting an earlier loss of the ureteric progenitor population whereas other tips retaining UEPs continue to grow outward with cortical expansion [Fig. 8S, T].
DISCUSSION
We describe a novel enhancer driven transgenic mouse line that will facilitate genetic analysis of branching morphogenesis, a developmental program shared by a variety of mammalian organ systems, specifically within the mammalian kidney. Using a variety of genetic approaches, we characterized and validated a new genetic tool, an AE-rtTA transgenic mouse line, for molecular and cellular analysis of mammalian kidney development.
Our goal was to use several different genetic models as proof-of-principle for developmental insight, rather than analyzing any one system in depth, to facilitate community awareness and access to a powerful transgenic mouse line.
While there are CRE-based genetic tools to facilitate the study of UEPs including the Wnt11RFP BAC transgenic line [Schuchardt et al., 1994; Majumdar et al., 2003; Chi et al., 2009a; Chi et al., 200b; O’Brien et al., 2018], all descendants of the cell undergoing CRE-mediated recombination inherit the genetic event, a particular problem when trying to distinguish the action of genes in stem/progenitor populations from their committed cell derivatives. The doxycycline inducible AE-rtTA system reported here enables UEP restricted genetic analysis coupled with a wide range of TRE-driven transgenic lines and the combinatorial use of other conditional genetic strategies (CRE, FLP, etc) or reporter systems.
Surprisingly, ectopic activation of two oncogenic growth promoting activities – YAP and MYC – in UEPs markedly reduced kidney development, though given the distinct phenotypes, through what are likely different mechanisms. Early YAP activity completely suppressed outgrowth of the ureteric bud leading to complete renal agenesis. This suggests YAP activity may prevent early epithelial remodeling or the response of nephric duct cells to outgrowth-promoting signaling. Interestingly, the Hippo pathway responds to mechanosensitive signals. For instance, in low cell tension states the pathway is activated and in high cell tension states it is inhibited with Yap/Taz transcriptional activity [Li et al., 2018; Meng et al., 2018]. Formation of the ureteric bud is likely to require a reduction in tension within the epithelium to enable movement of the epithelial sheet. Ectopic YAP would be predicted to provide a constitutive high tension-like state overriding Hippo pathway activity. Reducing tension may continue to play a role in regulating branching growth at ureteric branch tips at later stages leading to an arrest of branching as observed. A similar observation comes from overexpressing YAP through the entire ureteric epithelium [Reginensi et al., 2016] suggesting that the key YAP-sensing population is likely within UEPs. Interestingly, the presence of Wnt11RFP in ureteric epithelial tips suggests YAP does not inhibit branching by suppressing programs regulating a UEP state. The preliminary data supports further analysis.
Myc and Mycn are normally elevated in UEPs relative to committed cell derivatives in stalk regions, although Mycn levels are much higher than Myc [Rutledge et al., 2017]. The MYC overexpression phenotypes at different stages resulting in a loss of Wnt11RFP suggest a premature loss of UEPs with a resulting retardation of outgrowth at the initiation of ureteric bud formation and reduced branching and premature cessation of outgrowth leading to blind ending swollen ampullae deep in the kidney cortex. Given normal expression enriched within UEPs, it is not clear why elevating MYC levels might counter UEP regulatory programs though there are several scenarios that cannot be distinguished at this time.
First, high levels of MYC may squelch normal Mycn/Myc actions. Second, Mycn expression is highly mosaic in the ureteric progenitor pool. Whether stochastic or cyclic (such as cell cycle-linked), if variable levels of Mycn are critical for expansion of the UEP state, then uniform levels of MYC may favor an exit from this state. Third, although Myc family members are thought to have similar actions, if Myc and Mycn play different roles in UEPs, ectopic MYC may interfere in the dominant role played by the more highly expressed Mycn in UEPs. Interestingly, the loss of Wnt11RFP in ureteric branch tips at E15.5 at the end of the 72-hour period of doxycycline-mediated MYC expression and recovery of Wnt11RFP populations at P2 hints at a possible loss and reformation of a ureteric branch tip progenitor pool.
MATERIALS AND METHODS
Mouse Strains
The NIH Knock-Out Project (KOMP) generated the Adamts18tm1(KOMP)VlC9 mouse knockout (VG 12442) and was obtained from the KOMP Repository (www.komp.org). The Adamts18en-rtTA (AE-rtTA) transgenic mouse was generated at the Harvard Genome Modification Facility by injecting a plasmid produced in the lab into C57BL/6J fertilized eggs. This construct contained the intronic region between exons 2 and 3 of the Adamts18 gene (113,848,193-113,845,196, mouse assembly mm10) upstream of a minimal promoter driving expression of the rtTA gene [Gossen et al., 1995; Schönig and Bujard, 2003; Fig. 1A, F]. Transgenic mice were identified by a diagnostic PCR reaction. The AE-rtTA mouse line will be available from The Jackson Laboratory (Strain #034205). The Wnt11RFP mouse was previously generated [Harding et al., 2011; B6;D-Tg(Wnt11-TagRFP/cre/ERT2)28Amc/J; Jackson Laboratory stock 018683]. The mice with the tet-responsive element (TRE) utilized for the tet-on experiments are listed in the table below. Mouse handling, husbandry, and procedures were implemented according to the guidelines created by the Institutional Animal Care and Use Committees (IACUC) at the University of Southern California.
| Mouse Name | Full Strain Name | Received From |
|---|---|---|
| TRE-H2BGFP | Tg(tetO-HIST1H2BJ/GFP)47Efu/J | Jackson Laboratory (005104) |
| TRE-GFP/LacZ | B6N.Cg-Tg(tetO-GFP,-lacZ)G3Rsp/J | Jackson Laboratory (018913) |
| TRE-DTA | B6.Cg-Tg (tetO-DTA)1Gfi/J | Jackson Laboratory (008468) |
| TRE-MYC | FVB/N-Tg(tetO-MYC)36aBop/J | Jackson Laboratory (019376) |
| TRE-YAP | B6.Cg-Gt(ROSA)26Sortm1(tetO-YAP1*,GFP*)Stang/J | Dr. Ben Stanger; available at Jackson Laboratory (031279) |
Whole-mount in situ Hybridization
Whole-mount in situ hybridization (WISH) was performed based on our previously reported procedure [Yu et al., 2012]. Briefly, tissue was dissected and incubated overnight in 4% paraformaldehyde (PFA), dehydrated in a methanol series, and stored in methanol at −20°C. Samples were rehydrated and bleached with 6% hydrogen peroxide, incubated in 10μg/ml proteinase K, fixed in 4% PFA, pre-hybridized in hybridization buffer for several hours, and hybridized with RNA probes at 70°C. Subsequent formamide washes, antibody incubation, and MBST [100mM maleic acid, 150mM NaCl, 0.1% Tween-20 (pH 7.5)] washes were performed on the BioLane HTI platform. To detect an in situ hybridization signal, samples were incubated with BM Purple for up to 48hr, fixed in 4% PFA, and stored in 80% glycerol/PBS. Images were taken on an AxioZoom.V16 stereozoom microscope (Zeiss).
Whole-mount LacZ Staining
Embryonic mouse samples were harvested and fixed in 4% PFA briefly. Whole-mount tissues were permeabilized in 0.02% NP-40 temporarily and incubated at 37°C for several hours in LacZ stain solution (5mM K3Fe(CN)6, 5mM K4Fe(CN)6, 2mM MgCl2, 0.01% Na deoxycholate, 0.02% NP-40, 1mg/ml X-gal). Tissue was incubated in 4% PFA overnight at 4°C for post-fixation and stored in 80% glycerol/PBS. Tissue was imaged on an AxioZoom.V16 stereozoom microscope (Zeiss).
Tetracycline-on Induction via Doxycycline Administration
AE-rtTA;Wnt11RFP mice were crossed to a mouse with a TRE sequence upstream of a gene of interest (listed under Mouse Strains). To activate the tet-on system, doxycycline (Sigma D9891) was given as a tetracycline analog. Doxycycline was administered ad libitum in the drinking water: 1μg/ml - 2mg/ml in 5% sucrose water. Doxycycline-treated water was provided to pregnant mice as specified prior to euthanization for collection of embryonic or postnatal ptissue.
Antibody Immunofluorescent Staining
Embryonic tissue was dissected and treated to a short fixation in 4% PFA. Samples for whole-mount staining were incubated in blocking solution (10% sheep serum, 0.1% Triton, PBS) for 1hr and incubated in primary antibodies for 24-48hr at 4°C. Antibodies used were cytokeratin (1:500, Sigma C2562 and Novus Biologicals NB120-11213), Six2 (1:500, Proteintech 11562-1-AP), Pax2 (1:1000, R&D AF3364), Sox9 (1:1000, Abcam ab185230), Troma-1 (1:100, DSHB troma-1), MYC (1:1000, Abcam ab32072), RFP (1:500, Thermo Fisher Scientific MA5-15257), and GFP (1:500, Aves Labs GFP-1020). Tissue was washed in PBST (0.1%Triton, PBS) for several hours, incubated in the corresponding secondary antibodies for 24-48hr at 4°C, and then washed in PBST for several hours. Tissue was imaged on an AxioZoom.V16 stereozoom microscope (Zeiss).
Samples for section staining were harvested, fixed in 4% PFA briefly, incubated in 30% sucrose at 4°C overnight, and embedded in OCT in a dry ice/100% EtOH bath. Cryoblocks were sectioned at 12μm (Zeiss Microm HM550 cryostat), placed on glass slides (VWR Superfrost Plus Micro Slide), and stored at −80°C. To stain, slides were washed in PBS to remove the OCT, incubated at room temperature in blocking solution (1.5% seablock in PBST) for 1hr, then incubated in primary antibody overnight at 4°C. After primary antibodies were washed off with PBST, tissue was incubated in secondary antibodies for 1hr at room temperature, washed in PBST, and briefly incubated in Hoechst 33342. Slides were coverslipped and imaged on a SP8 confocal microscope (Leica) or an AxioScan.Z1 (Zeiss).
Volume Projections
Z-stack images of whole-mount immunostained tissue was captured on an SP8 confocal microscope (Leica). To isolate the ureteric bud structure, z-stack images were manually segmented and masked with Amira Software (Thermo Scientific) using antibody staining as a guide. A volume projection of the resulting image was generated in Amira.
Time-lapse Imaging and Cell Tracking of Kidney Explants
Pregnant mice received 2mg/ml doxycycline ad libitum from E10.5 post coitum to kidney harvesting of E11.5 embryos. The tissue was placed on a Transwell filter (Corning 3450) and incubated at 37°C in FlouroBrite DMEM (Life Technologies A18967-01) supplemented with 1mg/ml doxycycline, 10% fetal calf serum, 1% penicillin and streptomycin, and 1X Glutamax (Thermo Fisher). Twenty-four hours later, filter inserts were transferred to customized holders with a 35mm MatTek glass bottom dish and imaged for 48hr with a 25x HC FLUOTAR L 25x/0.95 water immersion objective (Leica SP8). To maintain water immersion throughout imaging, a Leica water cap with a modified water and drainage system was established for continuous water flow. Cells were tracked in Imaris v9.2.0 using the Spot function applied to the nuclear GFP channel (no filtering was applied). Spots/nuclei of a 5um diameter were sought and tracked using an autoregressive motion algorithm with a maximum distance of 5um movement between frames and an expected gap size of 5μm. Tracked cells were filtered for distance of movement greater than 4.5μm.
H&E Staining of Paraffin Tissue Sections
Dissected tissue was fixed in 4% PFA overnight at 4°C, washed in PBS, saline, and stored in 70% EtOH/saline. For embedding, samples were washed for 30min each in 85% EtOH/saline, 95% EtOH/saline, 100% EtOH, 100% EtOH, 1:1 100% EtOH:xylene, xylene, and xylene. Tissue samples were washed in wax at 80°C multiple times to remove xylene and then embedded in fresh wax in a metal mold. Once the wax hardened, the block was sectioned (5μm thick) and placed on a glass slide. For staining, the slides were washed in xylene to remove the wax, multiple ethanol washes, water, then stained with hematoxylin for 30s. Excess hematoxylin was rinsed off with running water, then slides were washed in ethanol before eosin staining for 15s. Slides were quickly washed in a series of ethanol and xylene, mounted with Permount, and imaged on an AxioScan.Z1 (Zeiss).
Kidney Tip Counts and Size Quantification
The number of tips and kidney area from whole-mount images were quantified manually with the ImageJ program. For area, the perimeter of each kidney was drawn using the freehand selection tool and the area was quantified with the measure tool. The number of tips was counted by hand with the point tool. These are not total tip numbers but instead the number of tips that can be observed from kidney images (roughly ½ of a kidney’s total tip number). The kidneys were all imaged in the same orientation and magnification to enable comparison.
Supplementary Material
KEY RESOURCES TABLE
| Reagent or resource | Source | Identifier |
|---|---|---|
| Antibodies | ||
| Ckytokeratin (pan) | Sigma | C2562 |
| Ckytokeratin (pan) | Novus Biologicals | NB120-11213 |
| Six2 | Proteintech | 11562-1-AP |
| Troma-1 | DSHB | troma-1 |
| Pax2 | R&D Systems | AF3364 |
| Sox9 | Abcam | Ab185230 |
| MYC | Abcam | Ab32072 |
| RFP | Thermo Fisher Scientific | MA5-15257 |
| GFP | Aves Lab | GFP-1020 |
| Bacterial and Virus Strains | ||
| Biological Samples | ||
| Chemicals, Peptides, and Recombinant Proteins | ||
| Doxycycline | Sigma | D9891 |
| Critical Commercial Assays | ||
| Deposited Data | ||
| Experimental Models: Cell Lines | ||
| Experimental Models: Organisms/Strains | ||
| Mouse: B6;D-Tg(Wnt11-TagRFP/cre/ERT2)28Amc/J | McMahon Lab, The Jackson Laboratory | 018683 |
| Mouse: Adamts18−/− | Knock-Out Mouse Project | VG 12442 |
| Mouse: Tg(tetO-HIST1H2BJ/GFP)47Efu/J | The Jackson Laboratory | 005104 |
| Mouse: B6N.Cg-Tg(tetO-GFP,-lacZ)G3Rsp/J | The Jackson Laboratory | 018913 |
| Mouse: B6.Cg-Tg(tetO-DTA)1Gfi/J | The Jackson Laboratory | 008468 |
| Mouse: FVB/N-Tg(tetO-MYC)36aBop/J | The Jackson Laboratory | 019376 |
| Mouse: B6.Cg-Gt(ROSA)26Sortm1(tetO-YAP1*,GFP*)Stang/J | Stanger Lab, The Jackson Laboratory | 031279 |
| Oligonucleotides | ||
| Adamts18 in situ probe primers: Forward: acccttgtcctgagaatagcttg Reverse: TAATACGACTCACTATAGGGgaggaattgacttgggtgtgttg |
Rutledge et al., 2017 | N/A |
| rtTA in situ probe primers: Forward: GCGCTCTGGAATTACTCAATGGA Reverse: TAATACGACTCACTATAGGGTAGAATCGGTGGTAGGTGTCTCT |
This paper | N/A |
| Recombinant DNA | ||
| Software and Algorithms | ||
| Amira | Thermo Scientific | N/A |
| Imaris v9.2.0 | Oxford Instruments/Bitplane | N/A |
| ImageJ | National Institutes of Health | N/A |
| Other | ||
Highlights.
Adamts18 expression demarcates epithelial branch tip progenitors for several organs
Genetic analysis identified an Adamts18 enhancer specific to ureteric progenitors
A tet-on inducible transgenic mouse line enabled genetic modulation in these cells
The novel line facilitates dynamic high-resolution imaging of ureteric branching
Tip ablation and YAP/MYC overexpression studies give insights into tip cell actions
ACKNOWLEDGEMENTS
We would like to be thank Dr. Ben Stanger for graciously providing the TRE-YAP mice. We thank Seth Ruffins for his assistance with microscopy and imaging analysis programs. We would like to thank Gohar Saribekyan and Junji Watanabe for paraffin sectioning, and Richard Lopez and Himmat Sahi for their assistance with data collection. We would like to thank Riana Parvez for generating the volume projections.
FUNDING
Research in the A.P.M. laboratory was funded by a grant from the National Institutes of Health [DK054364]. E.A.R. was supported by a graduate student training fellowship from the National Institutes of Health [5T32HD060549] and by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health [F31DK107216]. These sources of funding were not involved in the study design, data collection, data analysis, writing the report, or the decision to submit for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: none
REFERENCES
- Breitman ML, Clapoff S, Rossant J, Tsui LC, Glode LM, Maxwell IH, and Bernstein A (1987). Genetic ablation: targeted expression of a toxin gene causes microphthalmia in transgenic mice. Science 238, 1563–5. [DOI] [PubMed] [Google Scholar]
- Bretones G, Delgado MD, and Leon J (2015). Myc and cell cycle control. Biochim Biophys Acta 1849, 506–16. [DOI] [PubMed] [Google Scholar]
- Camargo FD, Gokhale S, Johnnidis JB, Fu D, Bell GW, Jaenisch R, and Brummelkamp TR (2007). YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol 17, 2054–60. [DOI] [PubMed] [Google Scholar]
- Chi X, Hadjantonakis AK, Wu Z, Hyink D, and Costantini F (2009a). A transgenic mouse that reveals cell shape and arrangement during ureteric bud branching. Genesis 47, 61–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi X, Michos O, Shakya R, Riccio P, Enomoto H, Licht JD, Asai N, Takahashi M, Ohgami N, Kato M, Mendelsohn C, and Costantini F (2009b). Ret-dependent cell rearrangements in the Wolffian duct epithelium initiate ureteric bud morphogenesis. Dev Cell 17, 199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier RJ (2001). Understanding the mode of action of diphtheria toxin: a perspective on progress during the 20th century. Toxicon 39, 1793–803. [DOI] [PubMed] [Google Scholar]
- Costantini F (2012). Genetic controls and cellular behaviors in branching morphogenesis of the renal collecting system. Wiley Interdiscip Rev Dev Biol 1, 693–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couillard M, and Trudel M (2009). C-myc as a modulator of renal stem/progenitor cell population. Dev Dyn 238, 405–14. [DOI] [PubMed] [Google Scholar]
- Dang CV (2013). MYC, metabolism, cell growth, and tumorigenesis. Cold Spring Harb Perspect Med 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, Feldmann G, Huang J, Wu S, Zhang N, Comerford SA, Gayyed MF, Anders RA, Maitra A, and Pan D (2007). Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell 130, 1120–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsher DW, and Bishop JM (1999). Reversible tumorigenesis by MYC in hematopoietic lineages. Mol Cell 4, 199–207. [DOI] [PubMed] [Google Scholar]
- Gao T, Zhou D, Yang C, Singh T, Penzo-Mendez A, Maddipati R, Tzatsos A, Bardeesy N, Avruch J, and Stanger BZ (2013). Hippo signaling regulates differentiation and maintenance in the exocrine pancreas. Gastroenterology 144, 1543–53, 1553.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossen M, Freundlieb S, Bender G, Muller G, Hillen W, and Bujard H (1995). Transcriptional activation by tetracyclines in mammalian cells. Science 268, 1766–9. [DOI] [PubMed] [Google Scholar]
- Harding SD, Armit C, Armstrong J, Brennan J, Cheng Y, Haggarty B, Houghton D, Lloyd-MacGilp S, Pi X, Roochun Y, Sharghi M, Tindal C, McMahon AP, Gottesman B, Little MH, Georgas K, Aronow BJ, Potter SS, Brunskill EW, Southard-Smith EM, Mendelsohn C, Baldock RA, Davies JA, and Davidson D (2011). The GUDMAP database--an online resource for genitourinary research. Development 138, 2845–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iber D, and Menshykau D (2013). The control of branching morphogenesis In "Open Biol", Vol. 3, pp. 130088, England. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krestel HE, Mayford M, Seeburg PH, and Sprengel R (2001). A GFP-equipped bidirectional expression module well suited for monitoring tetracycline-regulated gene expression in mouse. Nucleic Acids Res 29, E39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang C, Conrad L, and Michos O (2018). Mathematical Approaches of Branching Morphogenesis. Front Genet 9, 673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclerc K, and Costantini F (2016). Mosaic analysis of cell rearrangements during ureteric bud branching in dissociated/reaggregated kidney cultures and in vivo. Dev Dyn 245, 483–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P, Morley G, Huang Q, Fischer A, Seiler S, Horner JW, Factor S, Vaidya D, Jalife J, and Fishman GI (1998). Conditional lineage ablation to model human diseases. Proc Natl Acad Sci U S A 95, 11371–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmon MA, and Schlessinger J (2010). Cell signaling by receptor tyrosine kinases. Cell 141, 1117–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Nirala NK, Nie Y, Chen HJ, Ostroff G, Mao J, Wang Q, Xu L, and Ip YT (2018). Ingestion of Food Particles Regulates the Mechanosensing Misshapen-Yorkie Pathway in Drosophila Intestinal Growth. Dev Cell 45, 433–449.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar A, Vainio S, Kispert A, McMahon J, and McMahon AP (2003). Wnt11 and Ret/Gdnf pathways cooperate in regulating ureteric branching during metanephric kidney development. Development 130, 3175–85. [DOI] [PubMed] [Google Scholar]
- Mateyak MK, and Kinzy TG (2013). ADP-ribosylation of translation elongation factor 2 by diphtheria toxin in yeast inhibits translation and cell separation. J Biol Chem 288, 24647–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon AP (2016). Development of the Mammalian Kidney. Curr Top Dev Biol 117, 31–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Z, Moroishi T, and Guan KL (2016). Mechanisms of Hippo pathway regulation. Genes Dev 30, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Z, Qiu Y, Lin KC, Kumar A, Placone JK, Fang C, Wang KC, Lu S, Pan M, Hong AW, Moroishi T, Luo M, Plouffe SW, Diao Y, Ye Z, Park HW, Wang X, Yu FX, Chien S, Wang CY, Ren B, Engler AJ, and Guan KL (2018). RAP2 mediates mechanoresponses of the Hippo pathway. Nature 560, 655–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer N, and Penn LZ (2008). Reflecting on 25 years with MYC. Nat Rev Cancer 8, 976–90. [DOI] [PubMed] [Google Scholar]
- Mugrauer G, and Ekblom P (1991). Contrasting expression patterns of three members of the myc family of protooncogenes in the developing and adult mouse kidney. J Cell Biol 112, 13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myllymaki SM, and Mikkola ML (2019). Inductive signals in branching morphogenesis - lessons from mammary and salivary glands. Curr Opin Cell Biol 61, 72–78. [DOI] [PubMed] [Google Scholar]
- Neben CL, Lo M, Jura N, and Klein OD (2019). Feedback regulation of RTK signaling in development. Dev Biol 447, 71–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien LL, Combes AN, Short KM, Lindstrom NO, Whitney PH, Cullen-McEwen LA, Ju A, Abdelhalim A, Michos O, Bertram JF, Smyth IM, Little MH, and McMahon AP (2018). Wnt11 directs nephron progenitor polarity and motile behavior ultimately determining nephron endowment. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa-Espinosa A, and Affolter M (2012). Branching morphogenesis: from cells to organs and back. Cold Spring Harb Perspect Biol 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter RD, Behringer RR, Quaife CJ, Maxwell F, Maxwell IH, and Brinster RL (1987). Cell lineage ablation in transgenic mice by cell-specific expression of a toxin gene. Cell 50, 435–43. [DOI] [PubMed] [Google Scholar]
- Pan D (2010). The hippo signaling pathway in development and cancer. Dev Cell 19, 491–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X, Karner CM, and Carroll TJ (2017). Myc cooperates with beta-catenin to drive gene expression in nephron progenitor cells. Development 144, 4173–4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepicelli CV, Kispert A, Rowitch DH, and McMahon AP (1997). GDNF induces branching and increased cell proliferation in the ureter of the mouse. Dev Biol 192, 193–8. [DOI] [PubMed] [Google Scholar]
- Reginensi A, Enderle L, Gregorieff A, Johnson RL, Wrana JL, and McNeill H (2016). A critical role for NF2 and the Hippo pathway in branching morphogenesis. Nat Commun 7, 12309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutledge EA, Benazet JD, and McMahon AP (2017). Cellular heterogeneity in the ureteric progenitor niche and distinct profiles of branching morphogenesis in organ development. Development 144, 3177–3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutledge EA, Parvez RK, Short KM, Smyth IM, and McMahon AP (2019). Morphogenesis of the kidney and lung requires branch-tip directed activity of the Adamts18 metalloprotease. Dev Biol 454, 156–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid P, Schulz WA, and Hameister H (1989). Dynamic expression pattern of the myc protooncogene in midgestation mouse embryos. Science 243, 226–9. [DOI] [PubMed] [Google Scholar]
- Schönig K, and Bujard H (2003). Generating conditional mouse mutants via tetracycline-controlled gene expression. Methods Mol Biol 209, 69–104. [DOI] [PubMed] [Google Scholar]
- Schuchardt A, D’Agati V, Larsson-Blomberg L, Costantini F, and Pachnis V (1994). Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature 367, 380–3. [DOI] [PubMed] [Google Scholar]
- Semsei I, Ma SY, and Cutler RG (1989). Tissue and age specific expression of the myc proto-oncogene family throughout the life span of the C57BL/6J mouse strain. Oncogene 4, 465–71. [PubMed] [Google Scholar]
- Short KM, Combes AN, Lefevre J, Ju AL, Georgas KM, Lamberton T, Cairncross O, Rumballe BA, McMahon AP, Hamilton NA, Smyth IM, and Little MH (2014). Global quantification of tissue dynamics in the developing mouse kidney. Dev Cell 29, 188–202. [DOI] [PubMed] [Google Scholar]
- Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, and Fuchs E (2004). Defining the epithelial stem cell niche in skin. Science 303, 359–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varelas X, Samavarchi-Tehrani P, Narimatsu M, Weiss A, Cockburn K, Larsen BG, Rossant J, and Wrana JL (2010). The Crumbs complex couples cell density sensing to Hippo-dependent control of the TGF-beta-SMAD pathway. Dev Cell 19, 831–44. [DOI] [PubMed] [Google Scholar]
- Verheyden JM, and Sun X (2017). Embryology meets molecular biology: Deciphering the apical ectodermal ridge. Dev Biol 429, 387–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin F, Yu J, Zheng Y, Chen Q, Zhang N, and Pan D (2013). Spatial organization of Hippo signaling at the plasma membrane mediated by the tumor suppressor Merlin/NF2. Cell 154, 1342–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Valerius MT, Duah M, Staser K, Hansard JK, Guo JJ, McMahon J, Vaughan J, Faria D, Georgas K, Rumballe B, Ren Q, Krautzberger AM, Junker JP, Thiagarajan RD, Machanick P, Gray PA, van Oudenaarden A, Rowitch DH, Stiles CD, Ma Q, Grimmond SM, Bailey TL, Little MH, and McMahon AP (2012). Identification of molecular compartments and genetic circuitry in the developing mammalian kidney. Development 139, 1863–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Zheng Y, Dong J, Klusza S, Deng WM, and Pan D (2010). Kibra functions as a tumor suppressor protein that regulates Hippo signaling in conjunction with Merlin and Expanded. Dev Cell 18, 288–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, and Pan D (2019). The Hippo Signaling Pathway in Development and Disease. Dev Cell 50, 264–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman KA, Yancopoulos GD, Collum RG, Smith RK, Kohl NE, Denis KA, Nau MM, Witte ON, Toran-Allerand D, Gee CE, and et al. (1986). Differential expression of myc family genes during murine development. Nature 319, 780–3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.