Abstract
To investigate the role of adipose tissue in reproductive function and mammary gland development and function, we have examined lipodystrophie (LD) mice. LD mice of both sexes are sterile, but fertility can be restored with leptin injections. Mammary glands from lipodystrophic mice were rudimentary and lacked terminal end buds. Leptin-injected LD mice were able to become pregnant, showed normal pregnancy-associated glandular proliferation despite a smaller glandular area, were able to produce a small amount of milk that had grossly normal content of milk proteins and neutral lipids, but could not sustain pups to weaning. In order to separate the individual requirements for 1) adipokines such as leptin, 2) estradiol, and 3) physical epithelial-adipocyte interactions, we performed a series of experiments with both lipodystrophic mice and ob (obese mice with a mutation in the lep gene encoding the adipokine leptin) mice that received either estradiol treatment or preadipocyte transplant. The resulting fat pad did not rescue the defect in mammary gland development in lipodystrophic mice. The defect also could not be rescued with estradiol pellets. Ob/ob mice, like LD mice, lack leptin and estradiol, but retain adipose tissue. Ob mice have defective mammary gland development. However, in striking contrast to what was observed in lipodystrophic mice, reconstitution of a WT fat pad in ob mice rescued the defect in mammary gland development. Estradiol treatment did not rescue mammary gland development in ob mice. Therefore direct interaction between mammary gland epithelia and adipose is a requirement for full invasion and expansion of the gland, but is not required for glandular proliferation during pregnancy and milk production.
Precis
We show that mammary gland development is defective in lipodystrophic and leptin-deficient mice. In addition to a requirement for estradiol we demonstrate requirements for leptin and mammary adipose tissue for mammary gland development.
Introduction
Mammary gland development is a complex process orchestrated by both cell-cell interactions as well as circulating hormones. The embryonic phase of mammary gland development occurs with the establishment of the milk line at E10 and the formation of placodes at E11.5. At E14 the expanded placode descends into the underlying mesenchyme (1). The bud then produces a rudimentary ductal system by a series of branching events. The epithelia then remains dormant until hormones of puberty stimulate proliferation and expansion of the mammary gland epithelium to fill the inguinal adipose tissue depot. This process is thought to be dependent on estrogen as ovariectomized mice as well as mice lacking estrogen receptor 1 have defective mammary gland development in puberty (2,3).
Once thought to be passive depots of energy rich triglyceride, adipose tissue is now known to have a multitude of endocrine and developmental functions (4,5). These additional functions of adipose tissue have been deduced, in part, from the phenotype of humans and rodents lacking adipose tissue, or carrying mutations in adipose specific gene products. Several transgenic mouse models with decreased adipose tissue have been created which share common features of hepatic steatosis, diabetes, and decreased fertility (6,7). Their phenotype can be partially attributed to the lack of adipokines such as leptin as ob/ob mice lacking leptin share several of these features despite an overabundance of adipose (8,9). Ob/ob mice have a loss of function mutation in the leptin gene and are characterized by obesity, hepatic steatosis, diabetes, and sterility. These phenotypes can be reversed with leptin administration (10,11). In addition, leptin treatment of lipodystrophic (LD) mice has been shown to rescue several metabolic abnormalities (11). In humans there are several forms of congenital lipodystrophy characterized by extreme insulin resistance and hepatic steatosis which can be rescued with leptin treatment (12). That greatly decreased adipose tissue is associated with insulin resistance is likely due to a combination of hepatic steatosis and loss of adipokines such as leptin and adiponectin (13–15). In addition to these profound effects on metabolism, adipose tissue and its products appear to play a significant role in mammary gland development. Ob mice lacking leptin have defective mammary gland development (16). While ob mice have an overabundance of adipose tissue, in addition to loss of leptin there is a secondary loss of estradiol and fertility in females. These defects can be rescued with leptin injections (10). In addition, it has been shown that various lipodystrophic mice lacking adipose tissue have defects in mammary gland development (6,17–19). However, in this setting the loss of adipose tissue is associated with: (1) the loss of adipokines, (2) downstream hormonal consequences of adipokine loss (i.e. loss of estrogen due to loss of leptin), and (3) the loss of adipocyte-epithelial cell-cell interactions. Therefore, it has been difficult to dissect out the individual effects of leptin (and other adipokines) from the effects of estradiol and the effect of cell-cell interactions between mammary epithelia and mammary gland adipocytes. Here, using a combination of experiments with LD and ob mice, we dissect the contributions of adipokines including leptin, estrogen and cell-cell interactions to better understand the role of adipocytes in mammary gland development. We use these models because the ob mouse lacks leptin, estradiol, but has intact adipose tissue, whereas the lipodystrophic mouse lacks leptin, estradiol and adipose tissue. Therefore, by analyzing these two mouse models and attempting to rescue the mammary gland phenotype by addition of estradiol or leptin, we can assess the role of these factors in mammary gland development as well as the role of adipocyte-epithelial interactions which are intact in ob mice, but absent in lipodystrophic mice. Because many previously used transgenic models of lipodystrophy do not display complete loss of adipose tissue from an early age (6,7) and remnant adipocytes could have significant biological effects, we sought to create a model with complete loss of adipose tissue in order to fully understand the role of adipose tissue and to discover additional roles of adipose physiology. We generated lipodystrophic mice by crossing adiponectin-cre (20) mice with ROSA26-DTA176 mice that carry a loxP-flanked stop cassette associated with an attenuated diphtheria toxin gene (21). In these mice, any cell that expresses the adipocyte-specific gene adiponectin will die from the diphtheria toxin transgene. We have previously shown that these mice lack both brown and white adipose tissue, lack adipokines such as adiponectin and adipsin, and have hepatic steatosis (22). Lipodystrophic mice show poor survival (3/46 pups survived to weaning when expecting the 50% Mendelian ratio) when born at room temperature, but are fully viable when born at thermoneutrality (30°C). Here we turn our attention to the role of adipose and adipokines in reproduction and mammary gland development. We specifically make use of an adipose reconstitution approach by transplanting mouse embryonic fibroblasts (MEFs) that results in an ectopic fat pad anatomically removed from the mammary glands. We have previously demonstrated that these MEF-derived adipose depots resemble endogenous white adipose depots histologically and restore secretion of adipokines (22,23). This approach allows us to dissociate humoral factors derived from adipose tissue from the cell-cell interactions between adipocytes and the mammary gland epithelium.
Material and Methods
Mice were housed either at room temperature (20°C) or thermoneutral conditions (30°C) where indicated with standard humidity and 12 hour light:dark cycles in accord with the IACUC of Washington University. Mice (adiponectin-cre, JAX strain 028020; ROSA26-DTA176, JAX strain 010527; and ob, JAX strain 000632) were obtained from Jackson Labs.
Leptin injections
Male lipodystrophic mice (8–12 weeks old) were housed with 6 week old WT female mice (2 females per male). Male mice were injected daily with leptin (2.5mg/kg subcutaneously daily, RnD) for 2 weeks and pregnancies were recorded for an additional 3 weeks.
Female lipodystrophic mice (8–12 weeks old) were housed with 12–16 week old WT male mice. Mice were injected with leptin (2.5mg/kg, RnD) daily as indicated in the experiment.
Adipose rescue experiments
Mouse Embryonic Fibroblasts (MEFs) were isolated at E14 from timed-pregnant female mice as described previously (24). Briefly, pregnant females were euthanized, and the head, liver, heart and intestine of embryos were removed. The remaining carcasses were minced with a razor and placed in 1.5ml trypsin (0.05% with EDTA) at 37°C for 45 minutes. Growth media (DMEM+high glucose+10% FBS+pen/strep) was added and the tissue fragments were triturated to a single cell suspension which was washed with growth media, centrifuged and brought to a volume of 250ul PBS which was injected into 3–4 week old LD or ob hosts subcutaneously at the sternum as described previously (25,26). MEFs from multiple embryos were pooled and the equivalent of 1 embryo’s MEFs were injected into each host.
Estradiol pellets
At age 3 weeks, lipodystrophic mice received estradiol pellets subcutaneously (250 mcg, 60 day pellets, Innovative Research of America). Ob mice received pellets at 4 weeks, the youngest age they could be shipped from Jackson Labs.
Estradiol measurements
Estradiol measurements were made by ELISA (Calbiotech)
Leptin measurements
Leptin measurements were performed by ELISA (performed by Eve Technologies)
Whole mount Carmine staining
Mice were euthanized. Abdominal (#4) mammary glands were dissected and spread on glass slides, fixed overnight at 4°C in Carnoy’s solution. Slides were rehydrated and stained with Carmine alum solution (StemCell) overnight. Then slides were dehydrated and destained in histoclear solution overnight. Pictures were taken using a GelDoc (Biorad) and the percent of mammary gland filled by epithelia was quantified using ImageJ.
Organoids
Mouse mammary organoids were isolated as previously described (27). Primary organoids (30–50, each ~200–1,000 cells) were cultured in 50-μl droplets of growth-factor-reduced Matrigel in the presence of 2.5 nM FGF2 in organoid media (28). Percent branching organoids were quantified.
Transplants
Mammary gland epithelium was isolated from WT or LD females aged 12 weeks as previously described (29). The epithelia was injected into female hosts in which the mammary gland epithelia had been cleared as previously described (29). 12 weeks later, the hosts were euthanized and whole mount staining of the mammary glands was performed. Control experiments were performed in which the mammary gland was cleared of epithelium and no transplant was performed, resulting in no growth.
Milk analysis
Milking experiments were performed within 48 hours of delivery of pups. Oxytocin was administered to anesthetized females as 2 IU via intraperitoneal injection (30). The skin overlying mammary gland number 4 was removed. Milk filled ducts were visualized and a large duct was cut avoiding any blood vessels. Milk was collected with a pipette within 5 minutes of oxytocin administration. An aliquot of milk was extracted using the Bligh-Dyer method (31), then run on a Thin Layer Chromatography plate using solvent hexanes:diethyl ether:acetic acid (80:20:1) to visualize major neutral lipid classes. An aliquot of milk was run on an SDS-PAGE gel and Coomassie stained to visualize major milk proteins.
Statistics
Comparison of means was performed and p-values calculated using student’s t-test Please also see the Key Resource Table.
KEY RESOURCES TABLE.
| Reagent or resource | Source | Identifier |
|---|---|---|
| Antibodies | ||
| Bacterial and Virus Strains | ||
| Biological Samples | ||
| Chemicals, Peptides, and Recombinant Proteins | ||
| Estradiol pellets | Innovative Research of America | SE-121 |
| Leptin | RnD | 498-OB |
| Critical Commercial Assays | ||
| Estradiol ELISA | Calbiotech | ES180S |
| Leptin assay | Eve Technologies | MRDMET |
| Deposited Data | ||
| Experimental Models: Cell Lines | ||
| Experimental Models: Organisms/Strains | ||
| Ob/ob mice | JAX | #000632 |
| Adiponectin-cre mice | JAX | #028020 |
| ROSA-26-DTA176 mice | JAX | #010527 |
| C57/BL6J mice | JAX | #000664 |
| Oligonucleotides | ||
| Recombinant DNA | ||
| Software and Algorithms | ||
| Other | ||
Results
Lipodystrophic mice are sterile, but can be rescued with leptin injections
We attempted to mate lipodystrophic mice to WT mice. Six male lipodystrophic mice aged 3 months were individually housed and then given young WT female mice (2 females per male) for 1 month, after which time the females were separated. No pregnancies resulted during the month of active breeding or when female mice were monitored for 3 weeks following separation from males (see Table 1 for summary). The same 6 male lipodystrophic mice (now 4 months old) were placed with new young WT females (2 females per male) and the males were injected with leptin for 14 days. 2 of the 6 cages had pups born 24 days after leptin injections were started, 2 had pups 25 days later, 1 had pups born 26 days later, and 1 cage did not result in pups. Therefore 5 of the 6 males produced litters after leptin injections. We confirmed this was not due to delayed sexual maturation of lipodystrophic mice by repeating the experiment with uninjected 4 month old lipodystrophic male mice which did not produce any litters.
Table 1:
Sterility of lipodystrophic and ob mice is rescued by mouse embryonic fibroblast injection. The denominator in the resulting pregnancies represents the number of genetically modified mice (i.e. LD or ob) as all crosses were performed with one male and two females in a cage.
| Male | Female | Resulting pregnancies |
|---|---|---|
| LD | WT | 0/6 |
| LD+leptin | WT | 5/6 |
| WT | LD | 0/6 |
| WT | LD+leptin1,2 | 6/6 |
| WT | LD+leptin2,3 | 6/6 |
| WT | LD+MEFs | 8/8 |
| WT | Ob | 0/6 |
| WT | Ob+MEFs4 | 6/6 |
Leptin was injected until delivery
Pups died within 48 hours of delivery
Leptin was administered until E14 from timed matings
Pups survived until adulthood.
No female LD females became pregnant after being housed with WT males. Next we attempted to rescue the reproductive phenotype in female lipodystrophic mice. We injected female LD mice (3 months old) with the same dose of leptin and monitored for pregnancies when they were placed with WT males (4 months old). Six out of six female lipodystrophic mice became pregnant and delivered between 25 and 30 days of daily leptin injections. Therefore, leptin was able to rescue the reproductive phenotype in lipodystrophic mice. To determine whether leptin was needed for initiation or maintenance of pregnancy, in a separate experiment we performed timed-pregnancy matings and injected females daily with leptin until day E14 of pregnancy and then stopped. Again all 6 lipodystrophic females became pregnant. Interestingly, even after cessation of leptin injections, pregnant lipodystrophic mice continued to gain weight and delivered at the expected full term point of E19.5. Pups born to lipodystrophic mothers weighed the same as WT pups born to WT mothers. However, pups born to lipodystrophic mothers did not survive past day 2 of life and did not have milk spots suggesting a lactation defect. To determine if a lactation defect was responsible for neonatal mortality we examined mammary glands of lipodystrophic females.
Mammary glands of LD mice
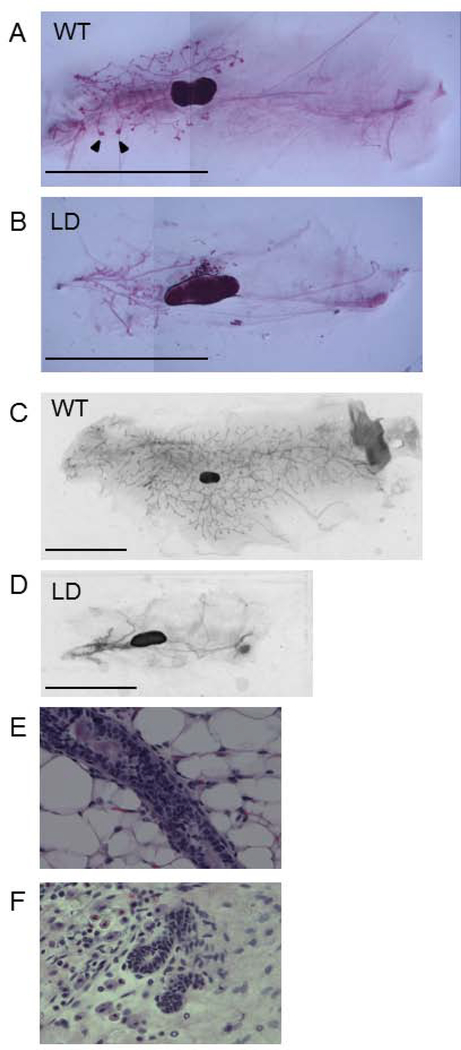
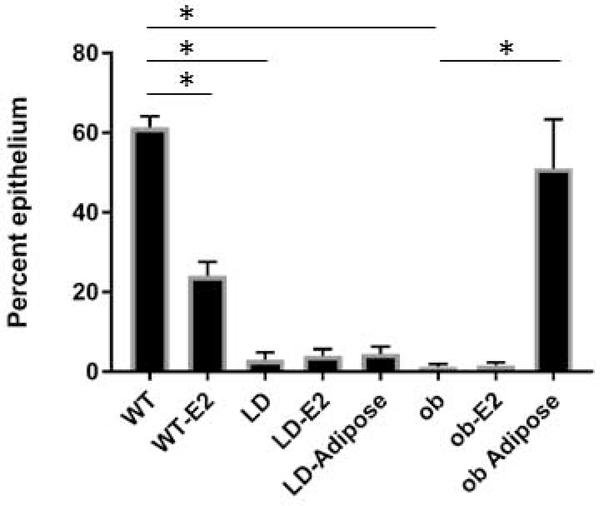
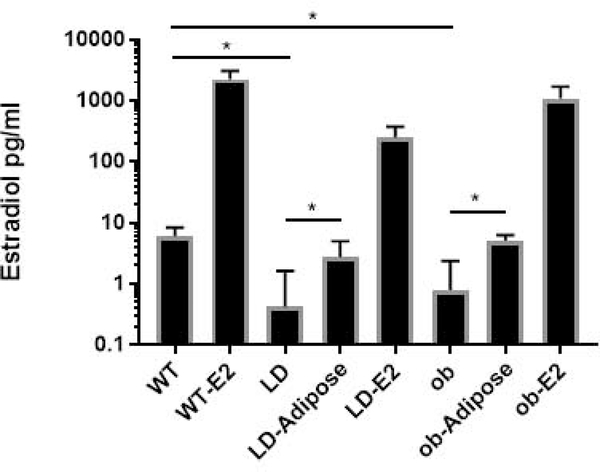
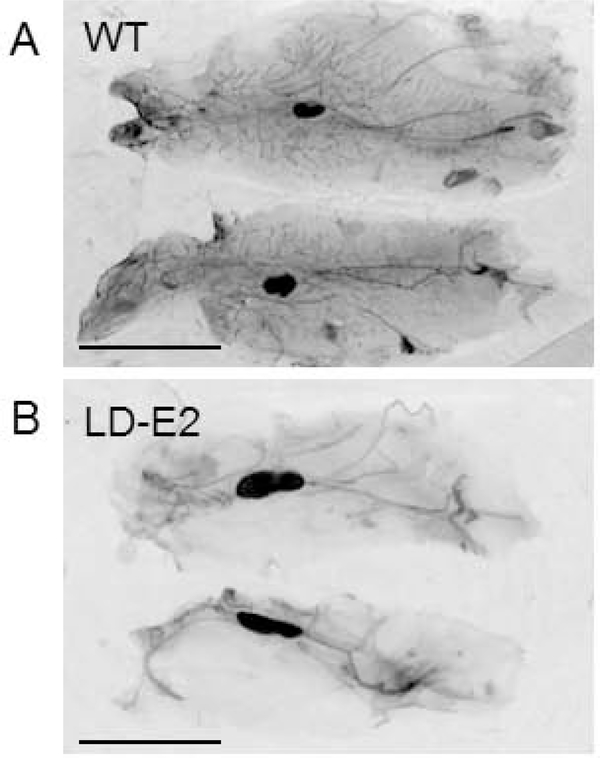
We examined mammary glands of WT and LD 5 week old virgin females. This revealed that the MG of LD mice were undeveloped with a striking absence of terminal end buds. (Figure 1, A, B). We also observed decreased mammary gland development in mature 6 month old mice (Figure 1, C, D). Specifically, in mature mice, 3.0 ± 1.9 percent of the inguinal area was occupied by mammary gland epithelium in lipodystrophic mice compared to 61.4 ± 2.8 % in wildtype mice (Figure 2, n=8, p<0.05). Histological examination of WT and LD 6 month old mammary gland (Figure 1, E, F) confirmed the findings of carmine staining. One potential explanation for this defective mammary gland development is low estrogen levels seen in mice and humans with lipodystrophy as leptin is required for a functional hypothalamic pituitary gonadal axis (10). Indeed, measurement of estradiol in mice showed very low levels in LD mice compared to WT mice (0.4 ± 1.2 pg/ml vs 6.1 ± 2.3 pg/ml, p<0.05, n=8, Figure 3). In fact only one of eight lipodystrophic samples had an estradiol concentration above the lowest standard 3 pg/ml. To determine if mammary gland development in LD mice could be rescued by estradiol, we implanted estradiol pellets into LD mice at age 3–4 weeks (prior to the pubertal expansion of mammary gland epithelia) and performed whole mount staining of mammary glands at the end of the 60-day release period for the pellets (age 13–14 weeks). This revealed that estradiol pellets did not rescue the mammary gland defect in LD mice (Figure 4) as the percent area of the mammary gland occupied by epithelia in estradiol treated mice was only 4.0 ± 1.7 percent (Figure 2, n=6, p<0.05). Therefore estradiol was not sufficient to induce mammary gland development in the absence of leptin and mammary adipose tissue. We also treated WT female mice with estradiol. While there was significant mammary gland development in these mice, it was reduced (24.1 ± 3.5 %) compared to untreated WT animals (Figure 2, p<0.05, n=6). We confirmed proper function of estradiol pellets by measuring plasma estradiol in mice that had been implanted with estradiol pellets which was elevated (Figure 3). We also confirmed biological activity of the estradiol pellets by examining the uteri of LD mice treated with estradiol. These uteri confirmed estradiol exposure as evidenced by increased uterine weight (data not shown).
Figure 1.
Lipodystrophie mice have defective mammary gland development. A) 5 week old WT and B) LD mammary glands. C) 6 month old WT and D) LD mammary glands. Note terminal end buds (arrowhead) are missing in LD mammary glands. Seale bar, 1 cm. H&E staining of 6 month old WT (E) and LD (F) mammary gland.
Figure 2.
Mammary gland development in adult mice. Shown is the percent epithelium (epithelial area/fat pad area) in various groups used in this study. E2, estradiol. Adipose refers to mice having received a transplant of mouse embryonic fibroblasts that develop into an adipose depot. n=4–8. *, p<0.05.
Figure 3.
Estradiol levels in mice. Shown are various groups used in this study. WT, wildtype; E2, estradiol treatment; LD, lipodystrophic; Adipose, mice that received preadipocyte transplant resulting in formation of a fat pad distant from the mammary gland. *, p<0.05. n=4–8.
Figure 4.
Estradiol does not rescue mammary gland development in lipodystrophic mice. Mammary glands were examined at age 4 months. A) Mammary gland of a control wild type mouse B) Lipodystrophic mice treated with 60 day estradiol pellet at age 3 weeks. Scale bar, 1 cm.
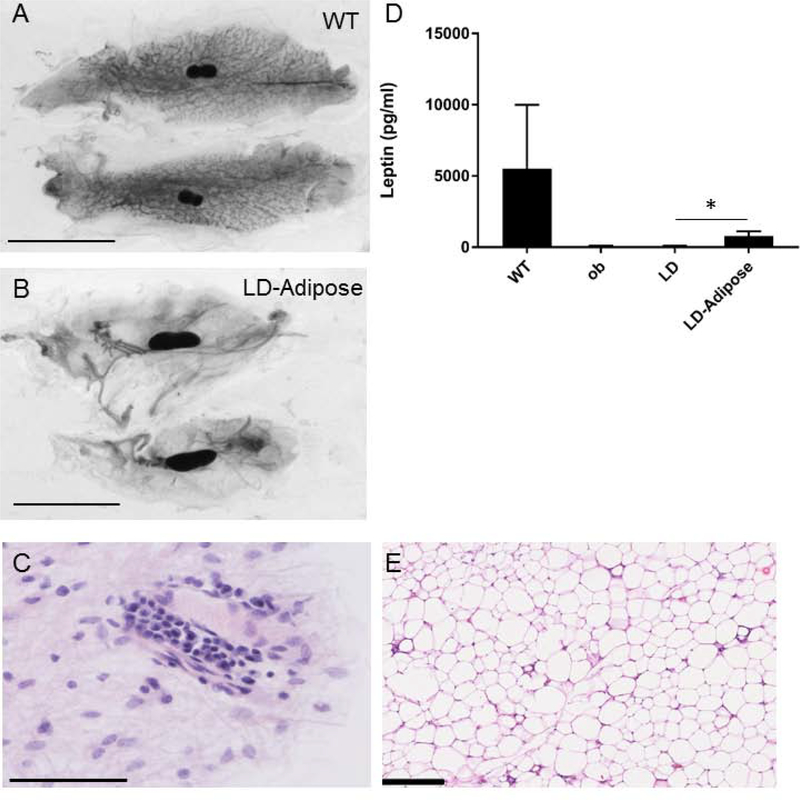
To determine if mammary gland development can be rescued by adipokines, we performed adipose rescue experiments on lipodystrophic female mice. Given the prolonged time required for development of mammary glands it was not practical to treat lipodystrophic mice with leptin injections for the entire duration of mammary gland development. We therefore used a mouse embryonic fibroblast transplant model (25,26) that results in an ectopic adipose depot in lipodystrophic mice and reconstitutes adipokine secretion (22,23). We have previously shown that the adipose depot resulting from MEF transplantation histologically resembles white adipose tissue and expresses adipocyte-specific genes (32). At 3 weeks of age, LD females received an injection of WT MEFs subcutaneously distant from the mammary glands to be studied. This results in the formation of an ectopic fat pad and restores most metabolic abnormalities of LD mice including hepatic steatosis (22). Despite rescue of the hepatic steatosis by the ectopic fat pad, there was no rescue of mammary gland development as adipose-rescued LD mice had an epithelia/adipose percentage of 4.6 ± 1.7 percent compared to the LD 3.0 ± 1.9 percent (Figure 2 and 5A, 5B, n=8, not significant). Histological examination showed underdeveloped epithelia in these mice (Figure 5C). LD mice with adipose rescue had circulating leptin levels of 799 ± 315 pg/ml or 15% of WT levels (Figure 5D). This was significantly greater than the leptin levels in untreated LD mice 57.0 ± 43.2 pg/ml. In fact the leptin levels observed in LD mice likely represent background from the assay as similar low, but detectable levels were measured in ob mice (84.8 ± 20.0 pg/ml). We previously measured leptin levels in ob mice rescued with adipose which were similar to leptin levels in LD mice rescued with adipose (412.8 ± 27.5 pg/ml (23)).
Figure 5.
Ectopic fat pad does not rescue mammary gland development in lipodystrophic mice. Lipodystrophic mice were injected with MEF preadipocytes at 3 weeks of age and mammary glands examined at age 4 months. A) Mammary gland of wild type control mouse B) Mammary glands of lipodystrophic mouse with ectopic fat pad. Scale bar, 1 cm. C) H&E staining of mammary gland from lipodystrophic mouse with ectopic fat pad. Scale bar 50 μm D) Leptin levels in wildtype (WT), ob, lipodystrophic (LD), and lipodystrophic mice receiving MEF transplant (LD-Adipose) n=4–5; *, p<0.05. E) H&E of MEF-derived ectopic fat pad from a rescued lipodystrophic mouse. Scale bar 250 μm.
Importantly estradiol levels in adipose-rescued LD mice were increased to 2.8 ± 2.2 pg/ml compared to LD mice estradiol levels of 0.4 ± 1.2 pg/ml (Figure 3, p<0.05). Of the eight lipodystrophic mice that received MEF transplant 5 had estradiol levels above 3 pg/ml. In addition, LD mice rescued with ectopic adipose tissue were fertile (Table 1), indicating proper ovarian function that requires physiological levels of estradiol. This scenario represents a situation in which mice have leptin and estradiol, but no mammary gland adipose tissue. In conclusion, leptin and estradiol are not sufficient to induce mammary gland development, but are sufficient for fertility in the absence of mammary adipose tissue.
Mammary glands from LD mice can develop in the proper environment
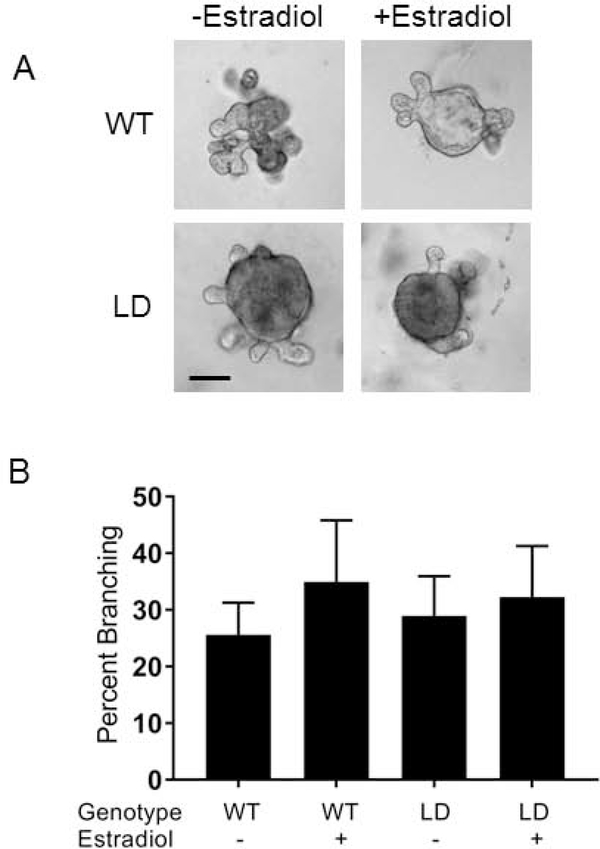
To determine if there was an inherent developmental defect in mammary glands of LD mice or if they just lacked the proper environment to grow in, we isolated mammary gland epithelium from WT and LD mice and performed the ex vivo organoid assay. LD organoids branched in similar numbers to WT organoids and had similar branching patterns. Specifically, WT organoids had 26 ± 6% branching and LD organoids had 29 ± 7% branching (Figure 6, not statistically significant, n=4). We also performed the organoid assay in the presence of 1 micromolar estradiol. Estradiol did not substantially augment branching under these conditions.
Figure 6.
Lipodystrophic epithelium is competent to branch in vitro A) Mammary organoids from WT and Lipodystrophic mice were grown in vitro in the absence or presence of estradiol B) Quantification of A. No significant differences between groups. Scale bar, 100 μm.
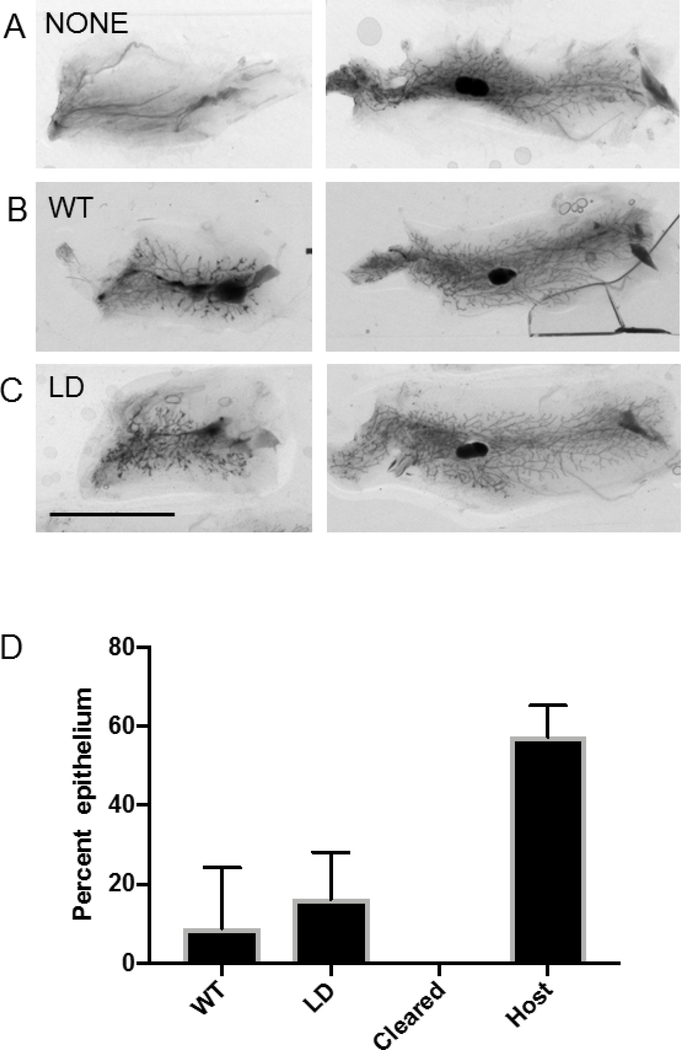
WT+Estradiol displayed 35 ± 11 % branching and LD + estradiol displayed 32 ± 9 % branching (Figure 6, no significant differences between groups). In order to see if LD epithelia could grow in a wild-type stroma with intact adipose tissue, we transplanted WT or LD mammary epithelia into WT mice with mammary glands that had been cleared of endogenous mammary epithelia. Twelve out of thirteen LD transplants took while seven out of twelve wildtype transplants took (p<0.01 by chi-squared test). LD mammary epithelia were able to branch and develop in the adipose containing WT host in a manner similar to WT epithelia transplanted into WT cleared mammary gland (Figure 7D). These experiments indicate there is no inherent defect in lipodystrophic mammary gland epithelia, but it lacks the proper environment in which to develop.
Figure 7.
Lipodystrophic mammary epithelium is competent to branch when transplanted to wildtype host. For each panel the left mammary gland was cleared and received A) No cells B) WT mammary gland epithelium C) Lipodystrophic mammary gland epithelium. The right mammary gland in each panel is the intact contralateral gland. D) Quantification of A through C. Scale bar, 1 cm. n=12 wildtype and 13 lipodystrophic.
Mammary gland development in ob mice
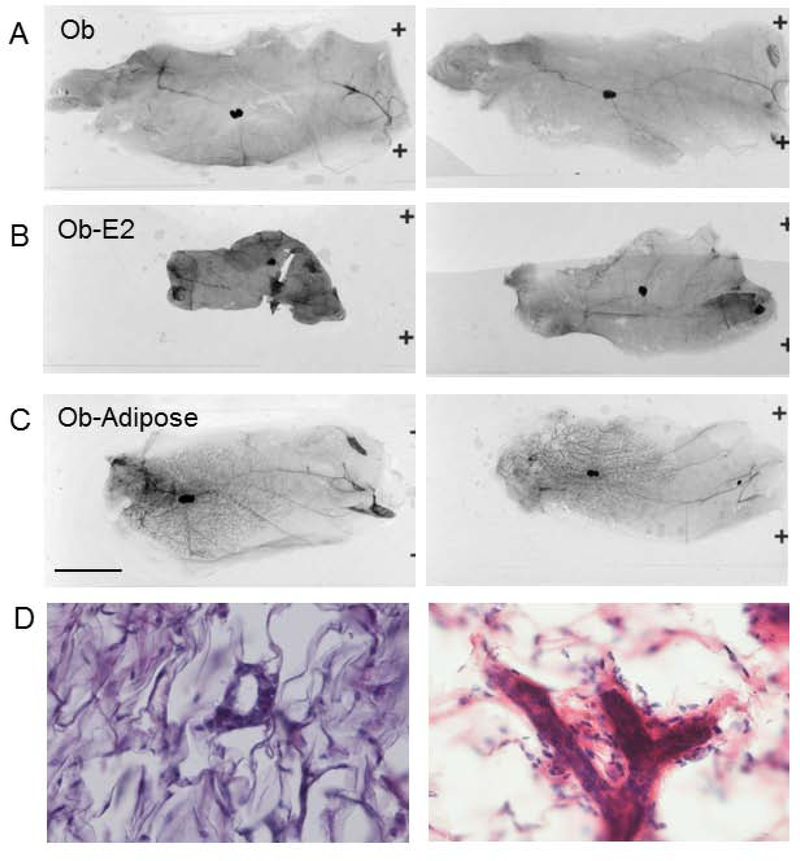
We were able to confirm prior studies showing defective mammary gland development in ob mice. We attempted to rescue this defect in ob mice by transplantation of WT preadipocytes/MEFs. This resulted in an ectopic fat pad distant from the mammary gland. Remarkably, ob female mice rescued with ectopic adipose had normal mammary gland development (ob+Adipose 51.1 ± 12.2 % vs WT 61.4 ± 2.8 %, not-significant, n=4, Figures 2, 8). Histological examination of mammary glands confirmed that ob mammary gland was primitive and this was rescued in ob mice with an ectopic MEF-derived fat pad (Figure 8D). In addition, ob female mice rescued with WT fat pad were fertile and were able to lactate their pups (Table 1). Reconstitution of ob mice with WT ectopic adipose results in a setting in which mice have restored leptin (23), estradiol (Figure 3) and also have mammary adipose tissue, though it is leptin deficient. To determine if leptin is directly required for mammary gland development or whether leptin treatment restores mammary gland development indirectly via restoration of the hypothalamic-pituitary-gonadal axis and estradiol we treated ob mice with estradiol pellets. In this situation mice have mammary adipose tissue and estradiol, but not leptin. Estradiol treated ob mice showed a persistent defect in mammary gland development (1.6 ± 0.7 % epithelial area, p<0.05 vs WT, n=6, Figures 2, 8) indicating the combination of estradiol and adipose mammary adipose tissue were not sufficient to restore mammary gland development in the absence of leptin.
Figure 8.
Preadipocyte transplant, but not estradiol rescues mammary gland development in ob mice. A) Mammary gland from untreated ob mouse. B) Mammary gland from estradiol treated ob mouse C) Mammary gland from preadipocyte (MEF) treated mouse. D, left) H&E stain of ob mammary gland and right) H&E stain of mammary gland from ob mouse with ectopic fat pad.
Adipose tissue is not required for milk production
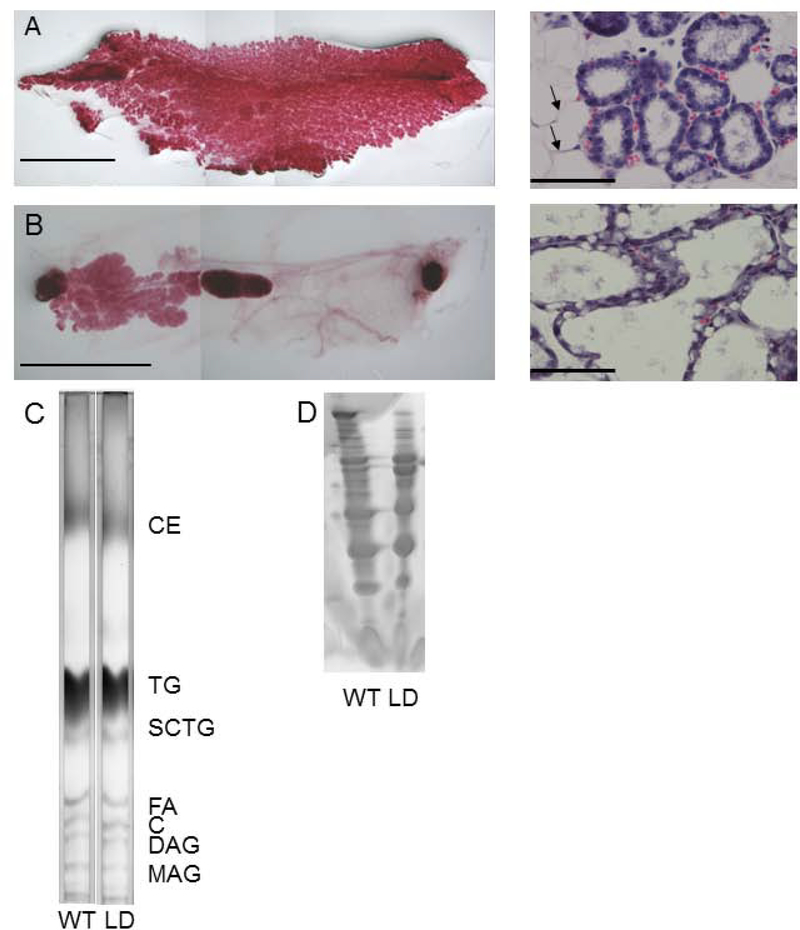
The milk producing mammary gland epithelium is buried in adipose tissue which can serve as a reservoir for the production of milk lipids. Whole mount staining of mammary glands from lipodystrophic mice revealed epithelium with decreased branching and absent terminal end buds in virgin females. Fertility of LD mice could be rescued with either leptin injection or MEF transplant (Table 1). Mammary glands of leptin-treated pregnant lipodystrophic females underwent normal proliferation and expansion of a smaller mammary gland (compare Figure 1 to Figure 9A, B). To determine if adipose tissue is required for forming milk of proper composition, we harvested milk from WT and lipodystrophic females. Thin-layer chromatography analysis of milk revealed grossly equal concentrations of all lipids including long chain triglycerides (TGs), short chain TG, cholesterol ester, diacylglycerol and monoacylglycerol (Figure 9C). We also examined the protein content of the milk which showed identical pattern in milk from lipodystrophic and WT females (Figure 9D).
Figure 9.
Leptin treatment of LD mice confers fertility and milk production yielding milk grossly normal in protein and lipid content A) Left, Mammary gland of wildtype P0 mouse. Scale bar, 1 cm; Right, H&E section of same. Scale bar 50 μm B) Mammary gland of P0 lipodystrophic mouse having received leptin injections during pregnancy. Scale bar, 1 cm; Right, H&E section of same. Scale bar 50 μm C) Thin-layer chromatography of milk from WT and LD mice showed comparable amounts of major milk lipids. D) Coomassie stain of WT and LD milk showing comparable profiles in WT and LD milk. C, cholesterol; CE, cholesterol ester; DAG, diacylglycerol, FA, fatty acid; MAG, monoacylglycerol; SCTG, short-chain triglyceride; TG, triglyceride.
Discussion
Lipodystrophic mice are sterile, but can be rescued with leptin injections
Previously, Chehab’s group showed that sterility could be rescued in ob/ob mice with injections of leptin (10). However, these mice had normal to excess adipose mass depending on duration of leptin injections prior to breeding and so the requirement for adipose per se was not determined. Another lipodystrophic mouse aZIP was rescued of steatosis and diabetes when crossed to a leptin-transgenic mouse resulting in elevated leptin levels (33). However, the reproductive phenotype of these mice was not reported. To determine if leptin was the only requirement for fertility in lipodystrophic mice we injected female mice with leptin and observed successful delivery of pups. It is remarkable that given the 19 day gestation for mice, the lipodystrophic female mice were able to become pregnant 6–11 days after starting leptin injections. Male mice had fertility restored with a similar time-course. Our experiments also show that although leptin is necessary to become pregnant, it is not necessary to maintain pregnancy or for mammary gland milk production.
Mammary gland development in LD and ob mice
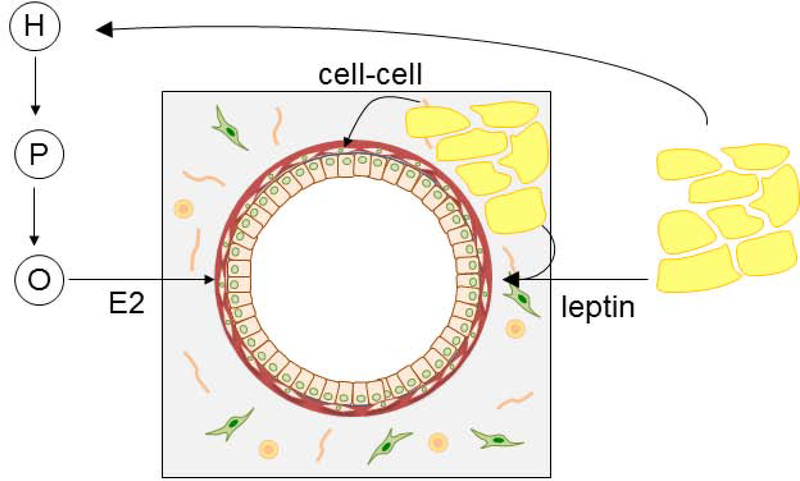
Mammary gland development was defective in LD mice. A similar phenotype of impaired mammary gland development was observed in another lipodystrophic mouse, the adiponectin-cre PPAR-γ flox mice (34). In our LD mice, the mammary gland phenotype was not due to an inherent defect in the mammary gland epithelia as the lipodystrophic epithelia was able to grow when transplanted into a WT host environment. In fact, the lipodystrophic epithelia had a better “take” when transplanted compared to wildtype epithelium. One possible reason for this is that we transplanted equal numbers of cells from the preparations of mammary gland epithelium. When isolating the epithelium from wildtype mice it is likely there were more stromal cells that were co-injected given that a vital component of the stroma (adipose) is missing in the lipodystrophic mice. Furthermore, LD mammary epithelia was also able to branch properly in an ex vivo organoid culture system. This pointed to a defect in the environment of the LD mice preventing proper mammary gland development. To dissect some of the potential variables of this complex environment, we performed a series of experiments examining the role of adipokines, estradiol, and mammary adipose. To dissect the role of local adipose tissue we performed these experiments on LD mice lacking adipose tissue and ob mice with abundant adipose tissue, but lacking leptin and estradiol. The mammary gland phenotype of LD mice could not be rescued by estradiol pellets. These results suggest estradiol is not sufficient to promote development of mammary gland epithelia alone. We initiated estradiol treatment in prepubertal animals to mimic the increased estradiol levels of mice in the pubertal period, a period of time characterized by proliferation and expansion of the mammary gland epithelia. Sufficient exposure of the animals to estradiol was evident as uteri showed classic changes seen with estrogen exposure including increased uterine mass. Estradiol exposure was also shown by direct measurements of estradiol in these mice. In fact estradiol levels were supraphysiological under these conditions and this resulted in suboptimal development of mammary gland development in WT mice. This suboptimal development with high estradiol levels has been seen previously (35). It could potentially be due to loss of the cyclic secretion of estradiol or possibly down regulation of estrogen receptors due to high ligand concentrations (36). Therefore, one limitation of our study is that we employed monotonic estradiol supplementation and not the cyclic estradiol patterns seen in vivo with the estrus cycle. However, even with supraphysiological estradiol levels wildtype mammary gland branching was more extensive than that seen in ob and LD mice. Estradiol treatment of ob mice did not rescue mammary gland development indicating a primary requirement for leptin in addition to estradiol and mammary gland adipose tissue. The site of action of leptin is unknown as it could potentially act directly on mammary gland epithelia or its actions could be secondary to another site (Figure 10).
Figure 10.
Schematic of requirements for mammary gland development. Adipose derived leptin stimulates the hypothalamic-pituitary-ovarian (H, P, O respectively) axis to produce estradiol (E2) that acts on the mammary gland epithelium. Leptin appears to have estradiol independent effects (leptin) as adipose and estradiol was not sufficient to induce mammary gland branching. Direct contact between adipose and epithelium (cell-cell) is required for branching.
Furthermore, preadipocyte rescue was also insufficient to restore mammary gland development in LD mice, as LD mice that formed an ectopic fat pad following MEF transplant showed poor mammary gland development. The fat pad restores adipokine secretion and results in the activation of the hypothalamic-pituitary-gonadal (HPG) axis as evidenced by the ability of the transplant to restore fertility and estradiol levels to that comparable to WT mice. However, in this state the ectopic fat pad is located distant to the mammary gland so that local adipose tissue and cell-cell interactions between mammary gland epithelia and adipocytes are still absent. One limitation of this approach is that some period of time is required for the MEFs to undergo differentiation once injected into a host. Our previous work showed that obese ob mice receiving MEF transplant start to lose weight between 2–3 week post-injection (23). Thus, we believe leptin secretion begins approximately 2 weeks after MEF injection. Given MEFs were injected into lipodystrophic mice at age 3–4 weeks leptin secretion would begin at age 5–6 weeks which is the normal onset of mammary gland development. In contrast, ob mice rescued with WT MEF derived adipose transplant had normal mammary gland development. This is a further argument that the MEFs begin adipokine secretion at a time that is consistent with rescuing mammary gland development. These mice have leptin, estradiol and adipose tissue. Therefore, adipose tissue is required, but not sufficient for mammary gland development. The exact molecules mediating the interaction and signaling between epithelia and adipocytes are unknown and represent an important future avenue of research. These studies also show that direct leptin signaling between mammary adipocytes and epithelia is not required for mammary gland development. That is leptin signaling by mammary adipocytes to adjacent epithelial cells is not required for mammary gland development, since circulating leptin from a distant adipose depot was able to rescue mammary gland development in ob mice. Previous studies have shown defective mammary gland development in lipodystrophic mice (18,19), but ours is the first to dissect the requirements for estradiol, leptin, and cell-cell interactions. A striking difference was seen in the ability of an ectopic fat pad (derived from mouse embryonic fibroblasts injected distant to the mammary gland) to rescue mammary gland development in ob mice vs the inability of an ectopic fat pad to rescue mammary gland development in lipodystrophic mice. This indicates a requirement for direct cell-cell contacts as well as leptin and estradiol for mammary gland development (Figure 10). These results are summarized in Table 2. It is worth mentioning that MEF transplant does not restore adipokine levels to normal levels. We have previously shown that formation of ectopic fat pads in ob mice results in leptin levels ~7% of wildtype levels (23) and here we show LD mice rescued with MEF transplant results in leptin levels 15% of the WT level. However, we do not think the failure of MEFs to rescue mammary gland development in LD mice is due to insufficient adipokine rescue as the transplant rescued several other features of lipodystrophy including the sterility of female and male mice, as well as the metabolic complications of lipodystrophy including diabetes and fatty liver (22). In addition MEF transplant did rescue mammary gland development in ob mice indicating sufficient adipokine secretion.
Table 2:
summary of findings with respect to requirements for mammary gland development in mice. WT, wildtype; OVX, ovariectomized (not from this study); LD, lipodystrophic; E2, estradiol; Adipose refers to rescue with preadipocytes resulting in an ectopic adipose depot. ND, not determined.
| Condition | Leptin | Estradiol | Mammary adipose | Mammary gland development | Pregnancy |
|---|---|---|---|---|---|
| WT | + | + | + | + | + |
| OVX | (+) | (−) | (+) | (−) | (−) |
| LD | − | − | − | − | − |
| LD+Adipose | + | + | − | − | + |
| LD+E2 | − | + | − | − | ND |
| Ob | − | − | + | − | − |
| Ob+Adipose | + | + | + | + | + |
| Ob+E2 | − | + | + | − | ND |
Adipose tissue is not required for milk production
The mammary gland epithelium is buried in and invades adipose during development and during pregnancy and lactation. A complex relationship exists between triglyceride synthesis and milk production. Triglycerides are synthesized in the Kennedy pathway by the sequential acylation (catalyzed by GPAT, AGPAT, and DGAT enzymes) and then dephosphorylation (catalyzed by PAP or lipin) of a glycerol-3 phosphate back bone (37). Despite having normal adipose tissue mass and TG levels, DGAT1-null mice are unable to lactate (38). GPAT4-null mice have a partial lipodystrophy and are also unable to lactate (39). Lipin1 mutant (fld) mice have a partial lipodystrophy, but are able to lactate and nurse young (40). Our findings demonstrate that adipose tissue is not required for the production of milk. Dietary fat is packaged into chylomicrons in the intestine and TGs deposited in adipose tissues by LPL. However, this deposition of chylomicron-TG into adipose is not an absolute necessity for milk lipid production as milk lipids were still synthesized in lipodystrophic mice. Several possible explanations for the source of milk lipid in lipodystrophic mice include epithelial de novo lipogenesis or extraction of lipids from chylomicrons directly by epithelium. Tissue specific deletion of fatty acid synthase, the rate-limiting enzyme in fatty acid synthesis, in epithelia results in a reduction but not elimination of milk lipids (41). Although lipodystrophic mice produced a smaller quantity of milk, it had grossly the same protein and lipid content as milk from wildtype mice. Although we did not identify the milk proteins, the pattern was identical in WT and lipodystrophic milk and similar to the published reports showing six major milk proteins: lactoferrin, albumin, α-casein, β-casein, γ-casein, and whey acidic protein. The impairment in milk quantity likely stemmed from the impaired development of mammary gland epithelium in the absence of an adipose environment. Patients with congenital generalized lipodystrophy have improvement in their metabolic profile when treated with recombinant leptin (12). There is at least one case report of a female CGL patient who became pregnant after receiving leptin therapy and delivered a baby (42). This patients was able to lactate (42). One possibility for this difference is that mouse litters contain ~7 pups whereas the human pregnancies were singleton. Therefore, the small amount of milk made by lipodystrophic mice was insufficient for the litter.
In conclusion, we find epithelial-adipocyte interaction is required, in addition to estradiol and leptin, for proper mammary gland development. Future work will be directed at the identification of molecules that mediate this interaction.
Mammary gland (MG) development is defective in lipodystrophic (LD) and ob mice
MG development of ob, but not LD mice can be rescued by an ectopic adipose depot
Leptin,E2 and adipocyte-epithelial interactions are all required for MG development
Adipose tissue independent of leptin is not required for fertility
Acknowledgements
This work was supported by NIH grant DK106083. We thank the hormone core at UVA for some of the estradiol measurements. We thank Greg Longmore for support.
Footnotes
Disclosure Summary: The authors have no disclosures.
References
- 1.Macias H, and Hinck L (2012) Mammary gland development. Wiley Interdiscip Rev Dev Biol 1, 533–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, and Smithies O (1993) Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci U S A 90, 11162–11166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith RS, and Bern HA (1958) Effect of age at ovariectomy on mammary gland development in C3H/He Crgl mice. Proc Soc Exp Biol Med 99, 95–99 [DOI] [PubMed] [Google Scholar]
- 4.Booth A, Magnuson A, Fouts J, and Foster MT (2016) Adipose tissue: an endocrine organ playing a role in metabolic regulation. Horm Mol Biol Clin Investig 26, 25–42 [DOI] [PubMed] [Google Scholar]
- 5.Zwick RK, Guerrero-Juarez CF, Horsley V, and Plikus MV (2018) Anatomical, Physiological, and Functional Diversity of Adipose Tissue. Cell Metab 27, 68–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moitra J, Mason MM, Olive M, Krylov D, Gavrilova O, Marcus-Samuels B, Feigenbaum L, Lee E, Aoyama T, Eckhaus M, Reitman ML, and Vinson C (1998) Life without white fat: a transgenic mouse. Genes & development 12, 3168–3181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shimomura I, Hammer RE, Richardson JA, Ikemoto S, Bashmakov Y, Goldstein JL, and Brown MS (1998) Insulin resistance and diabetes mellitus in transgenic mice expressing nuclear SREBP-1c in adipose tissue: model for congenital generalized lipodystrophy. Genes & development 12, 3182–3194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ingalls AM, Dickie MM, and Snell GD (1950) Obese, a new mutation in the house mouse. J Hered 41, 317–318 [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, and Friedman JM (1994) Positional cloning of the mouse obese gene and its human homologue. Nature 372, 425–432 [DOI] [PubMed] [Google Scholar]
- 10.Chehab FF, Lim ME, and Lu R (1996) Correction of the sterility defect in homozygous obese female mice by treatment with the human recombinant leptin. Nat Genet 12, 318–320 [DOI] [PubMed] [Google Scholar]
- 11.Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, and Friedman JM (1995) Weight-reducing effects of the plasma protein encoded by the obese gene. Science 269, 543–546 [DOI] [PubMed] [Google Scholar]
- 12.Brown RJ, Oral EA, Cochran E, Araujo-Vilar D, Savage DB, Long A, Fine G, Salinardi T, and Gorden P (2018) Long-term effectiveness and safety of metreleptin in the treatment of patients with generalized lipodystrophy. Endocrine 60, 479–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shimomura I, Hammer RE, Ikemoto S, Brown MS, and Goldstein JL (1999) Leptin reverses insulin resistance and diabetes mellitus in mice with congenital lipodystrophy. Nature 401, 73–76 [DOI] [PubMed] [Google Scholar]
- 14.Petersen KF, Oral EA, Dufour S, Befroy D, Ariyan C, Yu C, Cline GW, DePaoli AM, Taylor SI, Gorden P, and Shulman GI (2002) Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. The Journal of clinical investigation 109, 1345–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asilmaz E, Cohen P, Miyazaki M, Dobrzyn P, Ueki K, Fayzikhodjaeva G, Soukas AA, Kahn CR, Ntambi JM, Socci ND, and Friedman JM (2004) Site and mechanism of leptin action in a rodent form of congenital lipodystrophy. The Journal of clinical investigation 113, 414–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu X, Juneja SC, Maihle NJ, and Cleary MP (2002) Leptin--a growth factor in normal and malignant breast cells and for normal mammary gland development. J Natl Cancer Inst 94, 1704–1711 [DOI] [PubMed] [Google Scholar]
- 17.Pajvani UB, Trujillo ME, Combs TP, Iyengar P, Jelicks L, Roth KA, Kitsis RN, and Scherer PE (2005) Fat apoptosis through targeted activation of caspase 8: a new mouse model of inducible and reversible lipoatrophy. Nat Med 11, 797–803 [DOI] [PubMed] [Google Scholar]
- 18.Couldrey C, Moitra J, Vinson C, Anver M, Nagashima K, and Green J (2002) Adipose tissue: a vital in vivo role in mammary gland development but not differentiation. Dev Dyn 223, 459–468 [DOI] [PubMed] [Google Scholar]
- 19.Landskroner-Eiger S, Park J, Israel D, Pollard JW, and Scherer PE (2010) Morphogenesis of the developing mammary gland: stage-dependent impact of adipocytes. Dev Biol 344, 968–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eguchi J, Wang X, Yu S, Kershaw EE, Chiu PC, Dushay J, Estall JL, Klein U, Maratos-Flier E, and Rosen ED (2011) Transcriptional control of adipose lipid handling by IRF4. Cell Metab 13, 249–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu S, Wu Y, and Capecchi MR (2006) Motoneurons and oligodendrocytes are sequentially generated from neural stem cells but do not appear to share common lineage-restricted progenitors in vivo. Development 133, 581–590 [DOI] [PubMed] [Google Scholar]
- 22.Wu X, Hutson I, Akk AM, Mascharak S, Pham CTN, Hourcade DE, Brown R, Atkinson JP, and Harris CA (2018) Contribution of Adipose-Derived Factor D/Adipsin to Complement Alternative Pathway Activation: Lessons from Lipodystrophy. J Immunol 200, 2786–2797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferguson D, Blenden M, Hutson I, Du Y, and Harris CA (2018) Mouse Embryonic Fibroblasts Protect ob/ob Mice From Obesity and Metabolic Complications. Endocrinology 159, 3275–3286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harris CA, Haas JT, Streeper RS, Stone SJ, Kumari M, Yang K, Han X, Brownell N, Gross RW, Zechner R, and Farese RV Jr. (2011) DGAT enzymes are required for triacylglycerol synthesis and lipid droplets in adipocytes. J Lipid Res 52, 657–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bauerle KT, Hutson I, Scheller EL, and Harris CA (2018) Glucocorticoid Receptor Signaling Is Not Required for In Vivo Adipogenesis. Endocrinology 159, 2050–2061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walkey CJ, and Spiegelman BM (2008) A functional peroxisome proliferator-activated receptor-gamma ligand-binding domain is not required for adipogenesis. The Journal of biological chemistry 283, 24290–24294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen-Ngoc KV, Shamir ER, Huebner RJ, Beck JN, Cheung KJ, and Ewald AJ (2015) 3D culture assays of murine mammary branching morphogenesis and epithelial invasion. Methods Mol Biol 1189, 135–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ewald AJ, Brenot A, Duong M, Chan BS, and Werb Z (2008) Collective epithelial migration and cell rearrangements drive mammary branching morphogenesis. Dev Cell 14, 570–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Plaks V, Brenot A, Lawson DA, Linnemann JR, Van Kappel EC, Wong KC, de Sauvage F, Klein OD, and Werb Z (2013) Lgr5-expressing cells are sufficient and necessary for postnatal mammary gland organogenesis. Cell Rep 3, 70–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DePeters EJ, and Hovey RC (2009) Methods for collecting milk from mice. Journal of mammary gland biology and neoplasia 14, 397–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bligh EG, and Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37, 911–917 [DOI] [PubMed] [Google Scholar]
- 32.Bauerle KT, Hutson I, Scheller EL, and Harris CA (2018) Glucocorticoid Receptor Signaling is Not Required for In Vivo Adipogenesis. Endocrinology [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ebihara K, Ogawa Y, Masuzaki H, Shintani M, Miyanaga F, Aizawa-Abe M, Hayashi T, Hosoda K, Inoue G, Yoshimasa Y, Gavrilova O, Reitman ML, and Nakao K (2001) Transgenic overexpression of leptin rescues insulin resistance and diabetes in a mouse model of lipoatrophic diabetes. Diabetes 50, 1440–1448 [DOI] [PubMed] [Google Scholar]
- 34.Wang F, Mullican SE, DiSpirito JR, Peed LC, and Lazar MA (2013) Lipoatrophy and severe metabolic disturbance in mice with fat-specific deletion of PPARgamma. Proc Natl Acad Sci U S A 110, 18656–18661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vandenberg LN, Wadia PR, Schaeberle CM, Rubin BS, Sonnenschein C, and Soto AM (2006) The mammary gland response to estradiol: monotonic at the cellular level, non-monotonic at the tissue-level of organization? J Steroid Biochem Mol Biol 101, 263–274 [DOI] [PubMed] [Google Scholar]
- 36.Nardulli AM, and Katzenellenbogen BS (1986) Dynamics of estrogen receptor turnover in uterine cells in vitro and in uteri in vivo. Endocrinology 119, 2038–2046 [DOI] [PubMed] [Google Scholar]
- 37.Yen CL, Stone SJ, Koliwad S, Harris C, and Farese RV Jr. (2008) Thematic review series: glycerolipids. DGAT enzymes and triacylglycerol biosynthesis. J Lipid Res 49, 2283–2301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cases S, Zhou P, Shillingford JM, Wiseman BS, Fish JD, Angle CS, Hennighausen L, Werb Z, and Farese RV Jr. (2004) Development of the mammary gland requires DGAT1 expression in stromal and epithelial tissues. Development 131, 3047–3055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beigneux AP, Vergnes L, Qiao X, Quatela S, Davis R, Watkins SM, Coleman RA, Walzem RL, Philips M, Reue K, and Young SG (2006) Agpat6--a novel lipid biosynthetic gene required for triacylglycerol production in mammary epithelium. J Lipid Res 47, 734–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reue K, and Doolittle MH (1996) Naturally occurring mutations in mice affecting lipid transport and metabolism. J Lipid Res 37, 1387–1405 [PubMed] [Google Scholar]
- 41.Suburu J, Shi L, Wu J, Wang S, Samuel M, Thomas MJ, Kock ND, Yang G, Kridel S, and Chen YQ (2014) Fatty acid synthase is required for mammary gland development and milk production during lactation. Am J Physiol Endocrinol Metab 306, E1132–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maguire M, Lungu A, Gorden P, Cochran E, and Stratton P (2012) Pregnancy in a woman with congenital generalized lipodystrophy: leptin’s vital role in reproduction. Obstet Gynecol 119, 452–455 [DOI] [PMC free article] [PubMed] [Google Scholar]