Abstract
α-Linolenic acid (ALA) is an essential fatty acid and the precursor for long-chain n-3 PUFA. However, biosynthesis of n-3 PUFA is limited in a Western diet likely due to an overabundance of n-6 PUFA. We hypothesized that dietary reduction of n-6/n-3 PUFA ratio is sufficient to promote the biosynthesis of long-chain n-3 PUFA, leading to an attenuation of high fat (HF) diet-induced obesity and inflammation. C57BL/6J mice were fed a HF diet from ALA-enriched butter (n3Bu, n-6/n-3=1) in comparison with isocaloric HF diets from either conventional butter lacking both ALA and LA (Bu, n-6/n-3=6), or margarine containing a similar amount of ALA and abundant LA (Ma, n-6/n-3=6). Targeted lipidomic analyses revealed that n3Bu feeding promoted the bioconversion of long-chain n-3 PUFA and their oxygenated metabolites (oxylipins) derived from ALA and EPA. The n3Bu supplementation attenuated hepatic TG accumulation and adipose tissue inflammation, resulting in improved insulin sensitivity. Decreased inflammation by n3Bu feeding was attributed to the suppression of NF-κB activation and M1 macrophage polarization. Collectively, our work suggests that dietary reduction of the n-6/n-3 PUFA ratio, as well as total n-3 PUFA consumed, is a crucial determinant that facilitates n-3 PUFA biosynthesis and subsequent lipidomic modifications, thereby conferring metabolic benefits against obesity-induced inflammation and insulin resistance.
Keywords: Oxylipins, ALA, butter, macrophage polarization, insulin resistance, n-6/n-3 PUFA ratio
1. Introduction
Fatty acids (FA) are critical sources of energy production and structural components for cells, and essential regulators that control gene expressions by interacting with various transcription factors [1]. Consumption of different types of FA, as well as the total amount of fat, robustly induces metabolic changes [2, 3]. The ratio of n-6 to n-3 polyunsaturated fatty acids (PUFA) in the diet is important based on the opposing roles of these two classes of PUFA on metabolic and inflammatory processes. The n-3 PUFA, including alpha-linoleic acid (ALA) and its derivatives, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), exhibit anti-inflammatory properties [4]. In contrast, n-6 PUFA, including linoleic acid (LA) and its derivative arachidonic acid (ARA) exert pro-inflammatory effects [5]. Thus, compositional changes of membrane PUFAs could lead to systemic modulation of multiple metabolic pathways and immunological responses. These changes are mediated by the differential production of PUFA-derived lipid mediators such as oxylipins and endocannabinoids [6, 7]. Numerous clinical and epidemiological studies have demonstrated that increased consumption of n-3 PUFA decreases the risks of a wide range of inflammatory diseases, including cardiovascular disease (CVD), non-alcoholic steatohepatitis, and insulin resistance [8]. However, the typical American diet contains higher n-6 PUFA than n-3 PUFA, resulting in a significant imbalance between n-6/n-3 PUFA. Hence, there is an unmet need for a new dietary strategy that helps reduce n-6/n-3 PUFA ratio through our daily regimen.
Trans-fat free margarine (from vegetable oil) is regarded healthier than butter (milk fat) due to the reduced saturated FA (SFA) and increased total PUFA content. This traditional view has been challenged by the following recent findings; 1) SFA is not as bad as it was previously known [9, 10], and 2) LA, an essential and most abundant n-6 PUFA in nature, could be harmful [11-13]. In a meta-analysis, Ramsden et al. found that substitution of LA for SFA may decrease the serum cholesterol levels, but do not significantly reduce CVD risk [14]. In addition, there was no significant correlation between butter consumption and cardio-metabolic disease risk or mortality [15]. A growing body of evidence suggests that intake of LA promotes inflammatory responses that contribute to the prevalence of chronic diseases such as nonalcoholic steatohepatitis (NASH) and breast cancer [16, 17]. Besides, it has long been suggested that LA limits the bioconversion of ALA into n-3 PUFA at substrate levels; the prevalence of LA predominates the delta-5 and delta-6 desaturases (encoded by FADS 1 and FADS2, respectively), which favorably shifts the enzymatic reactions to the production of n-6 PUFA (i.e., ARA) over n-3 PUFA (i.e., DHA or EPA) [18, 19]. Despite extensive studies on the role of ALA-rich oils including echium or flaxseed oils in prevention from atherosclerosis [20], our current understanding is limited as to whether the modulation of dietary n-6/n-3 ratio per se poses a direct impact on bioconversion for long-chain n-3 PUFA (i.e., DHA and EPA) and subsequent PUFA-derived oxygenated metabolites.
In the current study, we hypothesized dietary reduction of LA/ALA ratio could promote the biosynthesis of long-chain n-3 PUFA, thereby inducing metabolic benefits. Our aim was to compare the differential impact of isocaloric HF diet prepared from butter, ALA-enriched butter, and margarine on intracellular PUFA balance in obesity-prone C57BL/6J mice. By taking a lipidomic approach, our study focused on investigating the impact of dietary n-6/n-3 PUFA ratio on endogenous production of long-chain n-3 PUFA and their oxygenated PUFA-metabolites as well as metabolic susceptibility to diet-induced metainflammation.
2. Materials and Methods
2.1. Diet preparation
Three different solid fats were used to prepare the experimental diets containing different n-6/n-3 ratio. A commercially available butter blend (Hiland Dairy Foods) and trans-fat free margarine (Land O’Lakes) were purchased from a local supermarket. The ALA-enriched butter (Sunseo Milk Butter™) was provided by Sunseo Omega Inc., which was made from ALA-enriched milk (1:4 Milk™, commercially available). These solid-fat products were used to prepare isocaloric diets containing 45% calorie from fat by using either conventional butter (Bu), n-3 enriched butter (n3Bu), or margarine (Ma) based on AIN 93M purified rodent formula (Supplement Table 1). The distribution of fatty acid species in each diet was analyzed by gas chromatography-mass spectrometry (GC/MS) and is detailed in Table 1.
Table 1.
Fatty acid composition of commercial and n-3 enriched butter and margarine
| Fatty acids | Conventional butter blend |
n-3 enriched butter |
Conventional margarine |
|---|---|---|---|
| 8:0 | 0.48 | 0.41 | 0.02 |
| 10:0 | 2.72 | 2.33 | 0.36 |
| 12:0 | 4.17 | 3.63 | 9.32 |
| 13:0 | 0.31 | 0.24 | N.D. |
| 14:0 | 12.02 | 11.05 | 4.38 |
| 14:1 | 1.36 | 1.29 | 0.09 |
| 15:0 | 1.48 | 1.07 | N.D. |
| 16:0 | 31.85 | 31.62 | 29.13 |
| 16:1n-7 | 2.40 | 2.35 | 0.35 |
| 17:0 | 1.12 | 0.83 | 0.14 |
| 18:0 | 11.16 | 11.02 | 3.93 |
| 18:1 T (elaidic) | N.D. | N.D. | N.D. |
| 18:1n-9 (oleic) | 23.29 | 22.89 | 23.48 |
| 18:1n-7 (vaccenic) | 2.05 | 1.92 | 0.15 |
| 18:2n-6 (LA) | 3.30 | 3.53 | 23.94 |
| 18:3n-6 | 0.15 | 0.03 | 0.13 |
| 18:3n-3 (ALA) | 0.49 | 3.88 | 4.00 |
| 20:1n-9 | 0.56 | 0.56 | 0.14 |
| 20:4n-6 (ARA) | 0.24 | 0.20 | 0.08 |
| 20:5n-3 (EPA) | 0.09 | 0.22 | N.D. |
| 22:6n-3 (DHA) | 0.07 | 0.29 | N.D. |
| 24:0 | 0.16 | 0.12 | 0.09 |
| 24:1 | 0.07 | 0.07 | 0.08 |
| ΣSFA | 65.52 | 62.50 | 47.38 |
| ΣMUFA | 27.33 | 26.73 | 23.94 |
| ΣPUFA | 7.10 | 10.72 | 28.62 |
| Σn-6 | 4.05 | 3.92 | 24.30 |
| Σn-3 | 0.65 | 4.40 | 4.26 |
| n-6/n-3 ratio | 6.27 | 0.91 | 6.08 |
2.2. Animals, body composition, and glucose tolerance test (GTT)
All animal experiments were conducted according to the protocols approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Nebraska-Lincoln. Six-week-old male C57BL/6J mice (Jackson Laboratory) were randomly assigned to one of four diet groups (n=8/group), chow, Bu, n3Bu or Ma, and fed ad libitum. After 10 weeks of dietary intervention, body composition was measured in conscious mice using the Minispec LF50 Body Composition Analyzer (Bruker Corporation) according to the manufacturer’s instruction. The values of fat tissue, lean tissue, and free fluid were obtained from the Minispec software. To measure glucose tolerance, mice were fasted overnight and intraperitoneally injected with a 10% D-glucose solution (1g/kg BW). Disposal of glucose from blood circulation was measured using a glucometer (Bayer, Contour) at time intervals. The area under the curve (AUC) was used to calculate the data. Blood was collected at 30 minutes post-glucose injection during GTT to measure insulin levels using Ultra-Sensitive Mouse Insulin ELISA Kit (Crystal Chem). The HOMA-IR (homeostasis model assessment of insulin resistance) index was calculated as [fasting plasma glucose× fasting plasma insulin/22.5] to assess insulin sensitivity.
2.3. Fatty acid profile of red blood cells (RBC) and other tissues
To determine FA profiles in the red blood cells (RBC), whole blood was collected by submandibular bleeding in the EDTA containing tube at 0, 2, 4 and 8 weeks post dietary intervention. After centrifugation, 50 μl of the packed volume of RBC were transferred to a fresh glass vial and total lipids were extracted as we previously described [21]. To analyze the tissue specific FA profile, total lipids were extracted from liver and several depots of adipose tissue. For preparation of visceral fat, epididymal fats (i.e., eWAT) were collected by dissecting the anatomically distinct fats around the testis and epididymis. Mesenteric fats (i.e., Mes), loosely attached to the intestine, were carefully detached from intestines and pancreas. For subcutaneous fat, we collected a pair of inguinal white adipose tissue (i.e., iWAT), which were found in the anterior to the upper segment of the hind limb. For the lipid analysis of brain (Supplement Table 5), whole brain tissue was snap-frozen in liquid nitrogen and grinded to powder to avoid a regional difference in FA composition.
Briefly, ~100 mg of tissue was minced, and total lipids were extracted and subjected to fatty acid methylation to form fatty acid methyl ester (FAME). Capillary HP-88 column (100 m × 0.25 mm × 0.2 μm film thickness, Agilent Technologies) was used, and the individual FA peak was identified by comparing its relative retention times with the commercial mixed-FA standard (NU-CHEK PREP, Inc). The area percentages for all resolved peaks were analyzed using the GC/MSD ChemStation Software (Agilent Technologies). FA profiles in tissues (liver, eWAT, iWAT, and brain) are provided in Supplemental Tables 2-5.
2.4. Plasma chemistry and liver TG analysis
Total cholesterol levels in plasma were measured using a colorimetric assay (BioVision). Plasma triglycerides (TG) were measured enzymatically according to the manufacturer’s instruction (Pointe Scientific). To determine total apolipoprotein distributions as an indication of changes in lipoprotein profiles, pooled plasma (n=4) was separated by a Superose 6 FPLC column (10/300 GL, GE Healthcare Life Sciences; flow rate 0.15 mL/min) coupled with a UV detector (Waters) for protein absorbance at 280 nm. For TG determination in the liver, total lipid was extracted from ~100 mg of tissue (n=8/group) in chloroform: methanol (2:1) solution and processed as described previously [22].
2.5. Oxylipin assay in plasma and liver
All oxylipin analyses were conducted at the USDA-Western Human Nutrition Research Center (Davis CA). Non-esterified oxylipins and endocannabinoids were isolated by methanol/acetonitrile mixture (1:1 v/v) from plasma or liver tissue, and quantified by UPLC-MS/MS [23]. Briefly, 50 μL of plasma or 50 mg of liver tissue were mixed with 5 μL BHT/EDTA (1:1 MeOH:H2O), 5 μL of 1,250 nM deuterated oxylipins and endocannabinoids surrogates in methanol. Next, plasma proteins were precipitated by addition of 185 μL of 1:1 methanol: acetonitrile. Liver tissue was homogenized by agitation with three 3mm stainless steel beads in a 2010 Geno/Grinder (SPEX Sample Prep, Metuchen, NJ) with 235 μL of 1:1 methanol:acetonitrile. Plasma extracts and tissue homogenates were centrifuged at 15,000 g × 10 minutes and supernatants were filtered (0.1 μm PVDF membrane filter) prior to UPLC separations. Residues in extracts were separated on a 2.1 mm × 150 mm, 1.7 μm BEH C18 column (Waters) and detected by electrospray ionization with multi reaction monitoring on a API 6500 QTRAP (SCIEX), and quantified against 7-9 point calibration curves of authentic standards as previously reported methods [24].
2.6. Western blot analysis
Total protein was extracted from tissue using RIPA buffer containing protease and phosphatase inhibitors (MilliporeSigma). The proteins were separated by SDS-PAGE and transferred onto a PVDF membrane. Antibodies targeting acetyl-CoA carboxylase (ACC, #3676), fatty acid synthase (FASN, #3180), stearoyl CoA desaturase (SCD-1, #2794), IκBα (#4812), CD11c (#97585), F4/80 (#70076), UCP1 (#14670), CytC (#11940), CD45 (#13917), and β-actin (#4970) were purchased from Cell Signaling Technology. Antibodies targeting ApoB (#MAC23-031) and GAPDH (sc-47724) were purchased from Meridian Life Sciences, and Santa Cruz Biotechnology, respectively. Blots were visualized with a FluorChem™E imaging system (Protein Simple).
2.7. qPCR analysis
Total RNA was extracted using TRIzol™ Reagent (ThermoFisher Scientific) from homogenized tissues. RNA was purified using DNA-free™ DNA Removal Kit (ThermoFisher Scientific), and 2 μg of RNA was converted into cDNA (iScript, BioRad) via reverse transcription. Relative gene expressions were determined using SYBR Green-based real-time qPCR (Applied Biosystems). The data was calculated based on the 2−ΔΔCT method with normalization of the raw Ct values to 36b4.
2.8. H&E staining and immunofluorescence staining
Tissue samples were fixed in 10% buffered formalin, embedded in paraffin, cut to 5 μm sections, and processed for hematoxylin and eosin (H&E) staining. From each adipose depot, five sections were prepared and stained, and 20X magnification images were captured. A total of 300 diameter measurements were made for each adipose tissue sample by using NIH Image J and Adiposoft software [25] (Fig 6C).
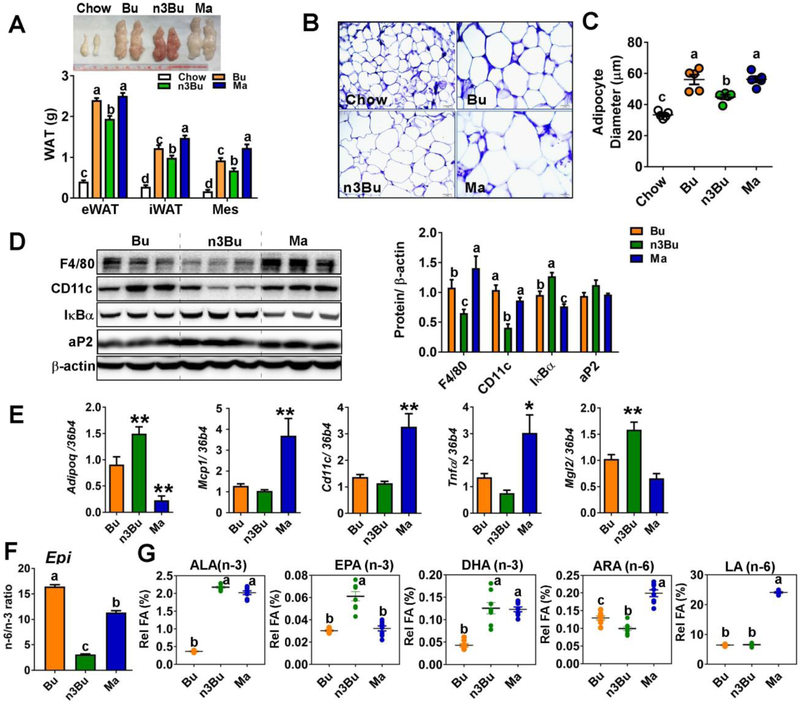
Figure 6. ALA-enriched butter increased the adipose EPA content and suppressed M1 macrophage polarization.
A. Gross images of epididymal fat (eWAT, upper) and depot-specific weight of epididymal (eWAT), inguinal (iWAT) and mesenteric (Mes) fat (lower). B. H&E staining of eWAT. C. Average adipocyte diameter (μm). Each dot represents the individual animal (n=5/group). D. Western blot analysis of macrophage related proteins of F4/80, CD11c, and IκBα. aP2 is an adipocyte marker. Relative protein intensities quantified by Image J were normalized to β-actin. E. mRNA levels of adiponectin (Adipoq), M1 macrophage markers of Mcp1, Cd11c, and Tnfα, and M2 marker of Mgl2. F. n-6/n-3 ratio in the eWAT. G. FA composition of n-3 (ALA, EPA, and DHA) and n-6 (ALA and LA) PUFA in the eWAT. All data represents as mean ± SEM. n=8/group were used except for B and C (n=5/group). Treatments with different letters are significantly different from one another (P<0.05) by one-way ANOVA. *, P<0.05, **, P<0.01, ***, P<0.001 by one-way ANOVA relative to Bu as the control.
2.9. Statistics
All data were presented as mean ± SEM. Data analyses collected at a single time point were conducted by one-way ANOVA among the isocaloric HF diet groups followed by Tukey’s multiple comparison tests. For GTT, the area under the curve was calculated for individual animals and analyzed by one-way ANOVA. For data collected over time, repeat measures two-way ANOVA with Tukey’s multiple comparison tests were used to determine the major treatment effects (diet) over time (time factor), i.e., body weight (Fig 1B), total plasma cholesterol concentration (Fig 2 A), and FA content in the RBC (Fig 2D-F)
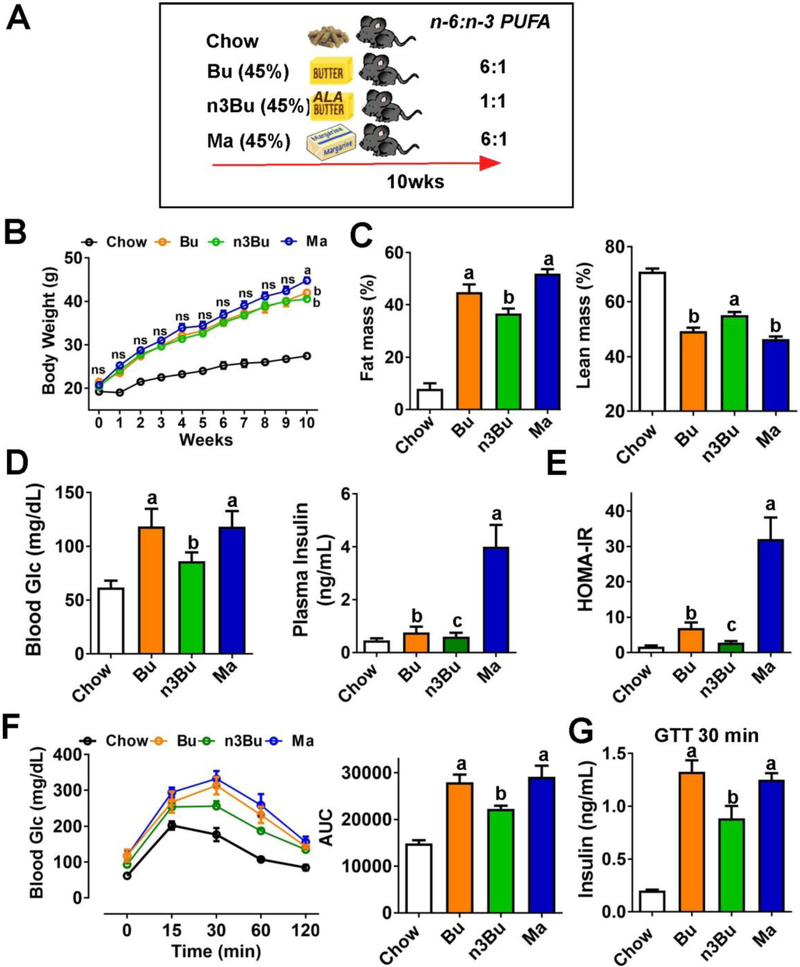
Figure 1. ALA-enriched butter attenuated adiposity and systemic insulin resistance.
A. Schematic representation of study design. C57BL/6J mice were fed an isocaloric HF diet (45% calorie from fat) either from conventional butter (Bu), ALA-enriched butter (n3Bu) or margarine for 10 weeks. B. Changes of body weight (interaction P<0.05, time P<0.001, and treatment P=0.12). C. Body composition by Bruker NMR minispec. D. Fasting blood glucose (left) and insulin (right) concentration. E. HOMA-IR. F. Glucose tolerance test (GTT) and area under the curve (AUC) during GTT. G. Insulin levels measured by ELISA at 30 minutes during GTT. All values are represented as the mean ± SEM (n=8/group). In A, two-way repeated measures ANOVA was performed among HF-fed groups with Tukey’s multiple comparison in each time point, Body weights annotated by different letters are different between diets at the indicated time point. In C-F, treatments with different letters are significantly different from one another (P<0.05) by one-way ANOVA among the HF fed groups. ns: not significant.
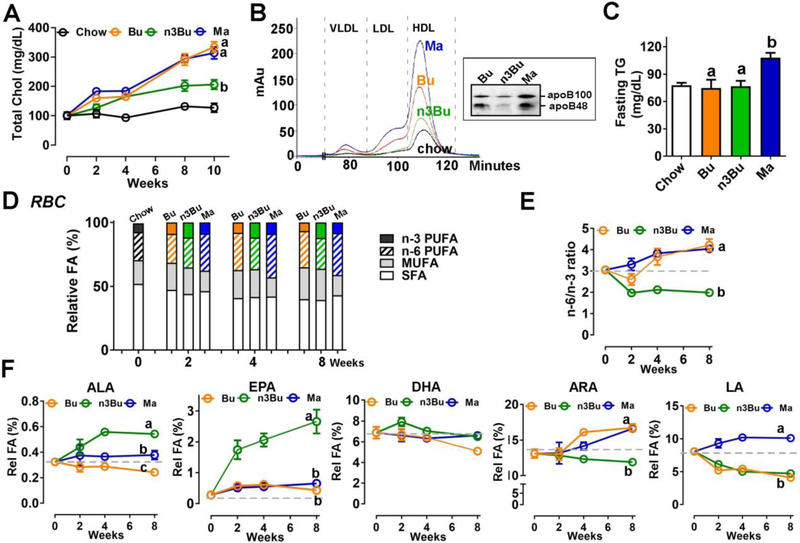
Figure 2. ALA-enriched butter altered plasma lipid profile and decreased n-6/n-3 PUFA ratio in red blood cells.
A. Changes in plasma cholesterol levels (interaction P<0.001, time P<0.001, and treatment P<0.001). B. FPLC apolipoprotein profile of pooled plasma of chow-, Bu-, n3Bu- and Ma-fed mice (n=4). Inset, ApoB protein levels by Western blot analysis. C. Plasma triglyceride (TG) concentration. D. Relative distribution of SFA, MUFA and PUFA (n-3 and n-6) in the red blood cells (Detailed statistical analysis is found in Supplement Table 6). E. n-6/n-3 PUFA ratio in RBC (interaction P<0.001, time P<0.001, and treatment P<0.001). F. Kinetic changes of ALA (interaction P<0.01, time P<0.05, and treatment P<0.001), EPA (interaction P<0.001, time P<0.001, and treatment P<0.001), DHA (interaction p=0.09, time p<0.01, and treatment p=0.14), ARA (interaction p<0.001, time p<0.001, and treatment p<0.05), and LA (interaction p<0.001, time p<0.001, and treatment p<0.001) in RBC. All values are represented as the mean ± SEM (n=8/group). In A, E and F, two-way repeated ANOVA was conducted among HF-fed groups with Tukey’s multiple comparison on main treatment effect. Treatments with different letters are significantly different from one another (P<0.05) over the treatment period. In C, P<0.05 by one-way ANOVA among HF-fed groups.
The preceding tests were performed in Prism 7 (GraphPad Software). To reduce oxylipin data dimensionality, variables were clustered with the principle components analysis (PCA) clustering algorithm in JMP Pro v14.0 (SAS Institute Inc). Plasma and liver data were clustered independently. Variables within each cluster were collapsed into single variables (i.e. cluster components) and cluster component differences between treatment groups were evaluated by ANOVA with the Tukey’s honest significant difference test. The principal component analysis (PCA) and heatmaps were performed with JMP Pro v14.0. Plasma oxylipins were auto-scaled [26] prior to multivariate analysis and the calculation of relative abundances used for heatmap generation.
3. Results
3.1. ALA-enriched butter attenuated adiposity and systemic insulin resistance
The FA profile of ALA-enriched butter showed a similar pattern (high SFA and low LA) with butter except for 4 % of ALA content, resulting in a 6-fold decrease of n-6/n-3 ratio (i.e., n-6/n-3 ratio=6 for Bu and n-6/n-3 PUFA=1 for n3Bu). Made from mixed vegetable oil, margarine contained a higher content of LA than butter but a similar content of ALA, resulting in n-6/n-3 PUFA ratio identical with conventional butter (i.e., n-6/n-3 ratio=6 for Ma) (Table 1). After 10 weeks of isocaloric high fat (45%) diets (Fig 1A), mice in the Ma group showed a slight but significant weight gain compared to butter-based diet groups of Bu or n3Bu (Fig 1B). Despite no differences in body weight between Bu and n3Bu, n3Bu mice had significantly lower fat mass and higher lean mass compared to Bu (Fig 1C). Consistent with the reduced fat mass, fasting blood glucose and insulin levels were significantly lower in n3Bu fed mice than Bu or Ma fed mice (Fig 1D). Based on these results, the HOMA-IR value, an indicator of insulin resistance, was the lowest in Bu fed mice among the HF fed mice, comparable to chow fed mice (Fig 1E). During glucose tolerance test (GTT), glucose disposal rate was faster in n3Bu fed mice than Bu or Ma fed mice (Fig 1F). Insulin levels at 30 minutes post-glucose injection were significantly lower in n3Bu fed mice than Bu or Ma fed mice (Fig 1G). These results suggest that reduction of dietary LA/ALA ratio may promote insulin sensitivity in mice.
3.2. ALA-enriched butter increased EPA content in RBC and its corresponding oxylipins in plasma
To examine the impact of dietary n-6/n-3 PUFA ratio on plasma lipid profile, we collected blood biweekly. All three HF diets elevated total cholesterol levels compared to the chow diet with the rank order of Bu and Ma > n3Bu > chow (Fig 2A). The lipoprotein distribution profiles by FPLC revealed that Bu and Ma diets markedly increased LDL as well as HDL, while n3Bu showed a negligible impact on LDL as well as ApoB levels (Fig 2B). The plasma triglyceride (TG) levels were significantly higher in Ma group, while there were no significant differences between chow and butter-based diet groups (Fig 2C).
Next, we analyzed the FA composition of red blood cells (RBC), which reflects dietary fat intake. The increase of n-3 PUFA percentage by n3Bu was evident in RBC as early as two weeks after the dietary intervention compared to chow or other isocaloric diets of Bu and Ma. Similarly, Ma feeding significantly augmented n-6 PUFA composition compared to chow or butter-based diets of Bu or n3Bu (Fig 2D). Consequently, the n3Bu diet induced a substantial reduction of n-6/n-3 ratio in the RBC compared to baseline, which occurred approximately two weeks after n3Bu diet introduction (Fig 2E). Notably, EPA and ALA composition in RBC was significantly increased with n3Bu feeding, while DHA composition in RBC remained relatively stable regardless of dietary intervention (Fig 2F). Reflecting the low LA content in butter (Table 1), Bu and n3Bu decreased the LA content compared to the baseline, while Ma increased the LA composition in the RBC. The ARA levels were elevated with Bu and Ma feeding, but not with n3Bu feeding in the RBC (Fig 2F). Oxylipins are oxygenated products of PUFA that play crucial roles as lipid mediators of PUFA effects in systemic levels [27]. Next, we examined the impact of dietary n-6/n-3 ratio on modulating the oxylipin profiles in plasma. Approximately 50 of 80 measured oxylipins were identified by UPLC-MS/MS analysis. The principal components analysis (PCA) of oxylipin composition showed a pronounced separation of n3Bu from Bu and Ma (Fig 3A). The heatmap visualization of the oxylipin species distribution highlights that n3Bu decreased the ARA-derived oxylipins compared with Bu or Ma. In contrast, the LA-derived oxylipins were higher in Ma than Bu and n3Bu, reflecting higher LA content in Ma. Despite the similar amount of total ALA content in n3Bu and Ma, EPA-derived oxylipins were higher in n3Bu than Ma. However, there was no significant difference in DHA-derived oxylipins among the groups (Fig 3B).
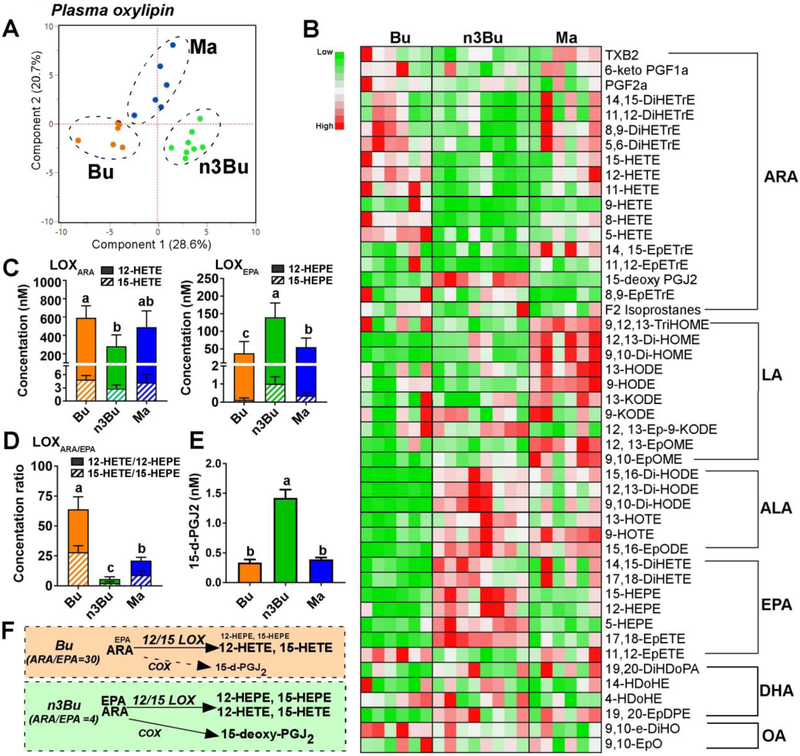
Figure 3. Plasma ALA and EPA-derived oxylipins are elevated by ALA-enriched butter.
A. Principal component analysis (PCA) score plot. B. Relative abundances heatmap by metabolite (low to high = green to red). C. Clusters of 12/15-lipooxygenase (LOX)-dependent oxylipins derived from ARA (12-HETE and 15-HETE, left) and EPA (12-HEPE and 15-HEPE, right). D. Ratio of ARA- to EPA-derived LOX-metabolites. E. 15-deoxy-PGJ2 levels. F. Schematic representation of 20-carbon fatty acid substrate (i.e., ARA and EPA) abundance and oxylipin relative production via LOX and cyclooxygenase (COX): font size indicates relative abundance. Data are mean ± SEM for Bu (n=6), Ma (n=6) and n3Bu (n=8). Treatments with different letters are significantly different from one another (P<0.05) by one-way ANOVA. Abbreviations used: DiHDoPE, dihydroxydocosapentaenoic acid; DiHETE, dihydroxy-eicosatetraenoic acid; DiHETrE, dihydroxy-eicosatrienoic acid; DiHODE, dihydroxy-octadeca(di)enoic acid; DiHOME, dihydroxy-octadeca(mono)enoic acid; HDoPA, hydroxyl-docosapentenoic acid; EpDPE, epoxyedocosapentaenoic acid; EpETE, epoxyeicosatetraenoic acid; EpETrE, epoxyeicosatrienoic acid; EpODE, epoxyoctadecadienoic acid; EpOME, epoxyoctadecamonoenoic acid; HDoHE, hydroxydocosahexaenoic acid; HEPE, hydroxyeicosapentaenoic acid; HETE, hydroxyeicosatetraenoic acid; HETrE, hydroxyeicosatrienoic acid; HODE, hydroxyl-octadecadienoic acid; HOTE, hydroxyl-octadecatrienoic acid; KETE (i.e., oxo-ETE), ketoeicosatetraenoic acid; KODE (i.e., oxo-ODE), ketooctadecadienoic acid; KOTE, ketooctadecadienoic acid; PG, prostaglandin; TriHOME, trihydroxyoctadecenoic acid; TX, thromboxane.
Similar to the oxylipin pattern found in human plasma [28], lipoxygenase (LOX)-dependent alcohols were the predominant oxylipin species in mouse plasma, including hydroxyeicosatetraenoic acids (HETE) and hydroxyeicosapentaenoic acids (HEPE). To understand the plasma oxylipin formation in the context of substrate availability of 12/15-LOXs, we clustered the LOX-dependent hydroxyl oxylipins into either HETEs as ARA-derived metabolites (LOXARA, i.e., 12-HETE and 15-HETE) or HEPEs as EPA-derived metabolites (LOXEPA, i.e., 12-HEPE and 15-HEPE). In response to the n3Bu feeding, there was a substantial decrease in HETE concentration with a concomitant increase in HEPE level (Fig 3C). Consequently, n3Bu feeding resulted in a huge reduction in the ratio of HETE/HEPE (LOXARA/EPA) in a rank order of Bu ≥ Ma > n3Bu (Fig 3D). It is also worthy to note that the levels of 15-deoxy-PGJ2, an ARA-derived oxylipin by cyclooxygenase (COX), were 3-fold higher in n3Bu than Bu or Ma (Fig 3E).
Collectively, these results suggest that dietary reduction of n-6/n-3 FA ratio is effective in reducing systemic levels of n-6/n-3 ratio, promoting biosynthesis of long chain n-3 PUFA and generation of both ALA and EPA-derived oxylipins.
3.3. ALA-enriched butter promoted bioconversion of long-chain n-3 PUFA, modulated oxylipin profile, and attenuated TG accumulation in the liver
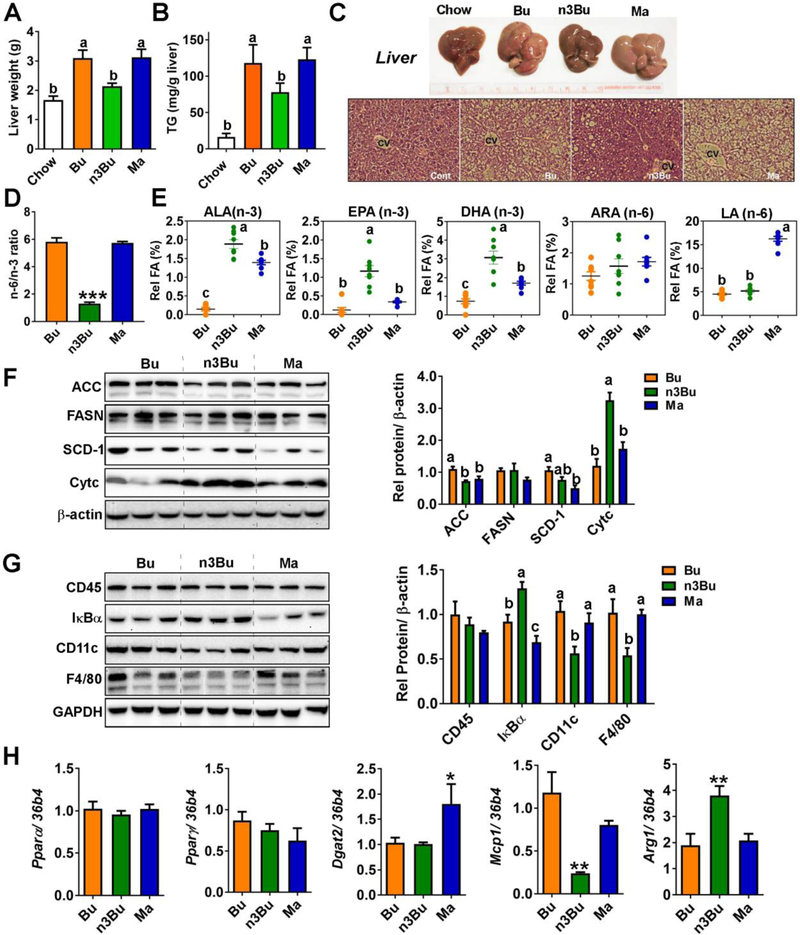
We evaluated the impact of dietary n-6/n-3 PUFA ratio on hepatic lipid metabolism. Liver weight (Fig 4A) and TG accumulation (Fig 4B) is reduced in n3Bu relative to Bu and Ma. Gross inspection and liver histology indicated that n3Bu diet showed less hepatic lipid accumulation than other isocaloric HF diets and were comparable to chow feeding (Fig 4C). The n-6/n-3 PUFA ratio was ~1 in the n3Bu fed liver, while it was close to 6 in Bu or Ma fed liver (Fig 4D). The hepatic PUFA profile was slightly different from plasma. The n3Bu feeding increased the three major n-3 PUFA of ALA, EPA and DHA, while Ma feeding increased the LA content >3-fold compared to Bu or Ma. No differences in liver ARA content were seen among diet groups (Fig 4E). The expression levels of key FA synthesis enzymes including acetyl CoA carboxylase (ACC) and stearoyl CoA desaturase-1 (SCD-1), but not fatty acid synthase (FASN), were significantly higher in the Bu-fed liver compared to Ma feeding (Fig 4F). Intriguingly, protein expression levels of FASN and SCD-1 were not different between Bu and n3Bu fed liver, while ACC levels were significantly lower in the n3Bu fed liver than Bu fed liver (Fig 4F). Unexpectedly, there were no significant differences in the expression levels of peroxisome proliferator-activated receptors, Pparα or Pparγ, among the HF-fed groups. In contrast, Ma fed mice increased the mRNA levels of diacylglycerol acyltransferase (Dgat2), a crucial enzyme for TG esterification (Fig 4H).
Figure 4. ALA-enriched butter promoted biosynthesis of long-chain n-3 PUFA, reduced TG accumulation and attenuated inflammation in the liver.
A. Liver weight. B. Hepatic TG content. C. Gross images (upper) and H&E staining (lower) of liver after 10 weeks HF feeding. D. Hepatic n-6/n-3 PUFA ratio at 10 weeks. E. Composition of n-3 (ALA, EPA, and DHA) and n-6 (ARA and LA) PUFA in the liver. F. Western blot analysis of de novo lipogenesis-related proteins of acetyl CoA carboxylase (ACC), fatty acid synthase (FASN), stearoyl-CoA desaturase 1 (SCD-1), and cytochrome C (Cytc). G. Western blot analysis of macrophage related proteins of CD45, CD11c, F4/80, and IκBα, the NFκB inhibitory protein. Relative protein intensities quantified by ImageJ were normalized to β-actin. In F and G, each lane represents individual animals. H. mRNA levels of Pparα, Pparγ, Dgat2, Mcp1/Ccl2, and arginase 1 (Arg1). All data represented as mean±SEM (n=8/group). Treatments with different letters are significantly different from one another (P<0.05) by one-way ANOVA (A-G). In H, *, P<0.05 and **, P<0.01 by one-way ANOVA relative to Bu as the control.
The reduction of hepatic TG levels by n3Bu supplementation was associated with a change in macrophage (Mφ) activation status in the liver. Feeding with n3Bu decreased the expression levels of 1) F4/80, a major macrophage marker without changes of total leukocyte common antigen (CD45), 2) IκBα degradation, an inhibitory protein for NF-κB activation, and 3) CD11c, a marker for pro-inflammatory M1 Mφ activation compared to isocaloric diet groups of Bu or Ma (Fig 4G). Moreover, there was a significant reduction in monocyte chemo-attractive protein-1(Mcp1/Ccl2) and an increase in the M2 macrophage maker of arginase 1 (Arg1) in n3Bu fed liver compared to Bu or Ma fed liver (Fig 4H).
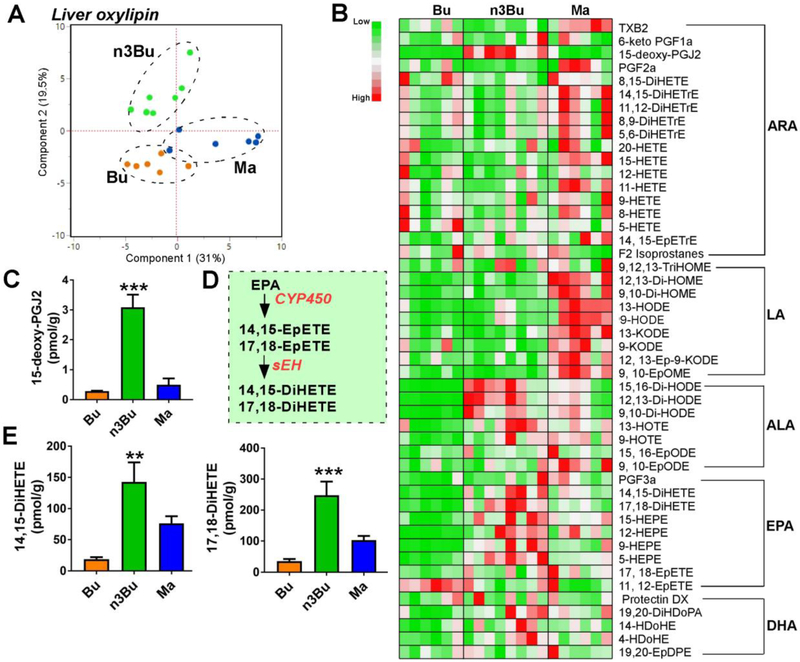
Next, we examined the modulation of hepatic oxylipins by n3Bu feeding. The hepatic oxylipin profiles showed a distinct separation among Bu, n3Bu, and Ma by PCA analysis (Fig 5A). The heatmap distribution of hepatic oxylipins revealed that 1) the content of oxylipins derived from LA was higher in Ma-fed liver than the Bu- or n3Bu-fed liver, and 2) n3Bu intake showed a trend towards increased oxylipins produced from ALA and EPA, but not DHA, compared to Bu or Ma fed liver (Fig 5B). Similar to the plasma oxylipin profile, 15-deoxy-PGJ2, a natural ligand for PPARγ, increased by ~10-fold in the n3Bu fed liver compared with the Bu or Ma fed liver (Fig 5C). The two most abundant EPA-derived oxylipins in the liver were 14,15-DiHETE and 17,18-DiHETE, formed by cytochrome P450-dependent epoxidation followed by soluble epoxide hydroxylase (sEH)-dependent conversion to 1,2- or vicinal-diols [28] (Fig 5D). Consistent with the increased EPA levels, the n3Bu fed liver showed a marked increase in 14, 15-DiHETE and 17, 18-DiHETE levels compared to Bu or Ma-fed liver (Fig 5E).
Fgure 5. Liver oxylipin production is altered by ALA-enriched butter.
A. Principal component analysis (PCA) scores plot. B. Relative abundance heatmap by metabolite (low to high = green to red). C. 15-deoxy-PGJ2 levels. D. Schematic representation of cytochrome P450-dependent EPA oxylipin production. E. 14,15-DiHETE and 17,18-DiHETE levels. Data are mean ± SEM for Bu (n=6), Ma (n=6) and n3Bu (n =8). Mean differences by one-way ANOVA relative to Bu are indicated at **, P <0.01 and ***, P<0.001. Abbreviations used: DiHDoPE, dihydroxydocosapentaenoic acid; DiHETE, dihydroxy-eicosatetraenoic acid; DiHETrE, dihydroxy-eicosatrienoic acid; DiHODE, dihydroxy-octadeca(di)enoic acid; DiHOME, dihydroxy-octadeca(mono)enoic acid; HDoPA, hydroxyl-docosapentenoic acid; EpDPE, epoxyedocosapentaenoic acid; EpETE, epoxyeicosatetraenoic acid; EpETrE, epoxyeicosatrienoic acid; EpODE, epoxyoctadecadienoic acid; EpOME, epoxyoctadecamonoenoic acid; HDoHE, hydroxydocosahexaenoic acid; HEPE, hydroxyeicosapentaenoic acid; HETE, hydroxyeicosatetraenoic acid; HETrE, hydroxyeicosatrienoic acid; HODE, hydroxyl-octadecadienoic acid; HOTE, hydroxyl-octadecatrienoic acid; KETE (i.e., oxo-ETE), ketoeicosatetraenoic acid; KODE (i.e., oxo-ODE), ketooctadecadienoic acid; KOTE, ketooctadecadienoic acid; PG, prostaglandin; TriHOME, trihydroxyoctadecenoic acid; TX, thromboxane.
3.4. ALA-enriched butter attenuated adipose tissue inflammation
To investigate the impact of lowered n-6/n-3 ratio on adipose tissue metabolism, we collected the different depots of adipose tissue including epididymal (eWAT, visceral), inguinal (iWAT, subcutaneous), and mesenteric (Mes, peri-intestinal, and visceral) fat. Consistent with the reduced fat composition (Fig 1C), n3Bu diet significantly reduced both visceral and subcutaneous fat mass compared with the corresponding depots from isocaloric diets of Bu and Ma (Fig 6A). The H&E staining of eWAT revealed that n3Bu decreased HF-diet induced adipocyte hypertrophy (Fig 6B,C). Western blot analysis revealed that n3Bu significantly reduced F4/80 and CD11c expression in the eWAT compared to Bu or Ma feeding (Fig 6D). Consistently, immunostaining of F4/80 revealed that macrophage infiltration is reduced in the n3Bu fed eWAT compared with Bu or Ma-fed eWAT (Supplemental Fig 4). It is also noticeable that the rank order of IκBα degradation in the eWAT was Ma > Bu > n3Bu (Fig 6D). The same relative pattern was observed in iWAT (data not shown).Consistent with reduced NF-κB activation and smaller adipocytes, adiponectin (Adipoq) gene levels were significantly higher in n3Bu compared to Bu or Ma, while the proinflammatory mRNA expressions of Mcp1, Cd11c, and Tnfα were the highest in the Ma group (Fig 6E). Similar to RBC, and liver, the n-6/n-3 FA ratio was decreased in n3Bu by 5-fold compared to Bu, and 3-fold compared to Ma (Fig 6F). There was a striking resemblance in FA profile between eWAT, and iWAT (Fig 6G and Supplemental Fig 2A and see Supplement Table 3 and 4). Collectively, dietary reduction of n-6/n-3 PUFA ratio effectively increases the LC n-3 PUFA content in the eWAT, which contributes to the attenuation of HF-diet-induced inflammation in adipose tissue.
4. Discussion
Dietary supplementation with n-3 PUFA has been demonstrated to attenuate the risk of metabolic diseases, including obesity, insulin resistance, and non-alcoholic fatty liver disease. It is likely due to the divergent effects of n-6 and n-3 PUFA on immune response and lipid metabolism. However, the relevance of dietary n-6/n-3 ratio has not been thoroughly evaluated in terms of endogenous production of long-chain n-3 PUFA and their oxygenated metabolites. Here, we aimed to assess the impacts of commonly consumed solid fats (i.e., butter, ALA-enriched butter, and margarine) on adiposity control and insulin sensitivity, and interpret the results with respect to intracellular PUFA balance. Our work underscores the metabolic significance of dietary n-6/n-3 PUFA ratio as a predictor for 1) intracellular biosynthesis of long-chain n-3 PUFA, 2) subsequent modulation in oxygenated PUFA metabolites, and 3) susceptibility to diet-induced metainflammation.
A debate continues regarding whether the total PUFA vs. total SFA content and/or the dietary ratio of n-6/n-3 PUFA confers metabolic advantages [29-31]. In our experimental setting, a margarine-based diet (Ma) did not exert metabolic benefits compared to a butter-based isocaloric diet (Bu), despite lower SFA and higher PUFA. In contrast, an ALA-enriched butter diet (n3Bu) reduced inflammatory responses and improved insulin sensitivity. Given that n3Bu contains a similar n-3 PUFA content with Ma, less total n-6 PUFA, and higher SFA than Ma, these results suggest that the n-6/n-3 PUFA ratio is a more critical metabolic determinant than the total dietary PUFA or SFA content. These results are well-supported by the literature. Duivenvoorde et al. assessed the metabolic flexibility between high-fat diets with different FA compositions but similar n-6/n-3 ratio [32]. Consistent with our results, this study failed to find differences in adiposity control, adipokine levels, or whole body energy balance [32]. By comparing the metabolic effects of corn oil (high n-6 PUFA) and lard (high SFA), Pavlisova et al. found that HF induces similar weight gain and insulin resistance in mice regardless of fat whether it is derived from SFA or n-6 PUFA [33]. Intriguingly, supplementing the lard-based HF diet with fish oil, but not with corn oil-based isocaloric diet, restored insulin sensitivity, implicating a more significant reduction in n-6/n-3 PUFA ratio by lard-based diet than in corn oil-based diet [33]. Our results further reinforce the idea that lowering dietary n-6/n-3 PUFA ratio, but not FA composition, specifically mediates metabolic improvements.
Since mammals cannot synthesize LA and ALA de novo, the dietary intake of LA and ALA is essential [34]. The primary focus of this study was to evaluate the role of dietary n-6/n-3 PUFA ratio in modulating the efficiency of biosynthesis of long-chain n-3 PUFA from dietary LA, and we assumed that intestinal uptake of n-3 PUFA would be similar regardless of butter- or margarine-based HF diet. This assumption is based on the study by Dias et al. showing that the kinetics of postprandial incorporation of n-3 PUFA into plasma lipids is identical, irrespective of co-administered fats either from SFA or n-6 PUFA [35]. Therefore, competition between n-3 and n-6 PUFA at the absorption level is unlikely, and thus the substrate competition at the enzymatic level must be responsible for their metabolic differences. Our results support that long chain (LC) n-3 PUFA synthesis is regulated by substrate competition for existing enzymes. Despite the similar ALA content between n3Bu and Ma diet, feeding with n3Bu induced a substantial increase of intracellular EPA compared to feeding with Ma in the RBC occurring as early as 2 weeks after dietary intervention, and similar FA profile changes were observed in the liver and adipose tissue. These findings implicate that availability of ALA upon n3Bu feeding shifts the desaturase reactions to favorably produce n-3 PUFA, while the abundance of LA upon Ma feeding dominates desaturases to generate ARA, limiting the access of ALA for long-chain n-3 PUFA production. Supporting this notion, Tu et al. showed that availability of ALA is an independent factor for promoting LC n-3 PUFA synthesis rather than transcriptional levels of FA desaturases [36]. Furthermore, consistent with our results, the Fat-1 transgenic mouse, containing genetic overexpression of n-3 desaturases derived from C. elegans, significantly promotes bioconversion of ALA especially into EPA compared with its wild-type counterpart [37].
It is important to note that the dietary strategy to reduce n-6/n-3 PUFA ratio by limiting dietary LA and enriching ALA in butter, is effective in boosting intracellular EPA production comparable to the degree of the genetic introduction of n-3 FA desaturase in Fat-1 mice [37]. It is also supported by a recent study showing that replacing LA with ALA or long-chain n-3 PUFA (reduction of n-6/n-3 ratio from 200 to 2~5) prevents nonalcoholic steatohepatitis in rats [38].
Oxylipins are oxygenated PUFA metabolites known to mediate peripheral immune responses to the systemic levels. Our results showed that the changes in PUFA composition by dietary challenges with Bu, n3Bu and Ma diet were correlated with the oxylipin patterns in plasma (Fig 3) as well as in the liver (Fig 5). In response to n3Bu feeding, the most substantial increases were found in EPA-derived oxylipins, while DHA-derived oxylipins were heterogeneous, which is consistent with oxylipin responses to n-3 PUFA intervention [39]. The oxylipins generated by lipoxygenase (LOX) are predominant in the plasma, while cytochrome P450 pathway-dependent oxylipins are most abundant in the liver. Compared to Bu and Ma feeding, n3Bu supplementation induced a major oxylipin shift from HETE to HEPE in plasma (Fig 3C), suggesting a switch of the primary substrate of LOX from ARA to EPA in response to a rise of ALA availability (Fig 3F). These results coincide with the oxylipin pattern found in Fat-1 transgenic mice compared with wild-type mice [37]. On the other hand, 12-HETE could be generated by platelet-derived 12-LOX during the platelet activation upon chronic high-fat diet [40]. Given the implication of 12-HETE to the pathophysiological progression of various disease risk [41], a three-fold decrease in 12-HETE in n3Bu fed plasma (Fig 3C) may contribute to the metabolic benefits found in this work.
Unexpectedly, we identified a small yet significant increase of 15d-PGJ2 in response to n3Bu feeding. The synthesis of prostanoids initiated by COX production of PGH2, results in an array of metabolites whose balance depends on the expression of terminal synthetases [42, 43]. To date, no specific 15d-PGJ2 synthase has been identified, rather being formed by the non-enzymatic decomposition of PGD2 [44]. Moreover, some authors suggest that 15d-PGJ2 and PGD2 function in inflammatory resolution, quench production and inhibiting pro-inflammatory PGE2 signaling [42]. Therefore, if COX is specifically coupled to PGD2 synthesis in this system, it could explain the observed increase in small amounts of 15d-PGJ2 without measurable changes in other COX products, and may confer metabolic advantages against HF diet-mediated metabolic insults.
15d-PGJ2 is one of the best-studied anti-inflammatory PGD2 inhibiting hepatic apoptosis [45], cancer cell proliferation [46], and endothelial inflammation [47]. In addition, EPA induced antiinflammatory effects against toll-like receptor 3 activation by poly (I:C), has been associated to the upregulation of 15d-PGJ2 production [48]. Similarly, we observed a significant decrease of NFκB activation in animals fed with n3Bu along with elevation of 15d-PGJ2 levels in plasma (Fig 3E) and the liver (Fig 5C). Importantly, we observed the consistent suppression of M1 Mφ activation and an increase in M2 Mφ phenotypes (Fig 4G, H and Fig 6D, E), which is reminiscent of the role of 15d-PGJ2 on alternative Mφ polarization [49]. Given that 15d-PGJ2 is a natural PPARγ agonist, we examined whether an increase of 15d-PGJ2 in n3Bu feeding stimulates PPARγ activation. However, we were unable to find the direct relationship between PPARγ expression and 15d-PGJ2, suggesting that 15d-PGJ2 may exhibit the anti-inflammatory effects in PPARγ-independent mechanisms as previously reported [50, 51].
Another notable impact on the oxylipin profiles was a dramatic increase of LA-derived oxylipins in response to Ma feeding (Fig 3B). Increasing evidence suggests that LA impacts systemic and peripheral tissue oxylipin levels [52, 53]. Therefore, future studies should investigate whether augmented levels of LA-derived oxylipins directly contribute to the pathogenic progression of insulin resistance. In addition to the modulation of oxylipins, Bu feeding increased the ARA-derived endocannabinoids such as arachidonoyl-ethanolamide (AEA), while n3Bu increased DHA-derived docosahexenoylethanolamide (DHEA) (Supplemental Fig 3). Therefore, the potential for endocannabinoid-dependent physiological benefits of n-3 PUFA cannot be ignored [54].
Development of hepatic steatosis is attributable to the confounding effects of 1) increased dietary FA influx, 2) reduced FA oxidation, 3) increased de novo lipogenesis, or 4) augmented FA influx from the adipose tissue [55]. The animals with n3Bu feeding remarkably reduced the HF diet-induced adipose inflammation and hypertrophy (Fig 6), while the de novo FA synthesis or β-oxidation by n3Bu were not directly correlated with the hepatic TG content (Fig 4). These results are consistent with a reduction in adipose inflammation and subsequent adipose lipolysis, which may explain the reduced HF-feeding hepatic steatosis associated with the n3Bu feeding.
The 2015-2020 Dietary Guidelines for Americans recommends the consumption of ~8 ounces of a variety of seafood per week provides an average consumption of 250 mg per day of EPA and DHA [56]. However, the average intake of n-3 PUFA in Americans remains dismally below the recommendation, mainly attributed to low fish consumption and preferred use of solid fat such as butter which contains <3% of PUFA [57]. Despite metabolic relevance of dietary n-6/n-3 PUFA ratio in foods, research has hitherto focused on enhancing the consumption of n-3 PUFA, either from fish oil [58] or ALA-rich plant oils such as flaxseed, echium, or borage oil [59], rather than decreasing the co-existing n-6 PUFA content in the diet. There is growing evidence that the reduction of the n-6/n-3 PUFA ratio can be achieved in humans by reducing LA-containing food by replacing LA with oleic acid [60]. As butter is almost scarce of both n-3 and n-3 PUFA, enriching the ALA in butter can exhibit dual benefits by elevating n-3 PUFA precursors and promptly reducing n-6/n-3 PUFA ratio. Given that butter is the most preferred solid fat in American diets and butter consumption continuously increases, enriching ALA in butter may pose a significant impact on public health perspective. Consistent with this idea, consumption of fish-oil incorporated butter blend [61] or fortified fish oil products [62] have shown to be effective in elevating n-3 PUFA content in the metabolic cells and plasma, although adding fish oil seems to be unrealistic due to the difficulty in separating unpleasant fish odor from the fortified products.
In summary, we demonstrated that reducing the n-6/n-3 PUFA ratio by enriching ALA in butter is effective in attenuating the HF diet-driven adiposity, inflammation, and insulin resistance in addition to the previous known cardio-protective effects. We highlighted the impact of dietary n-6/n-3 ratio on endogenous biosynthesis of long-chain n-3 PUFA and the subsequent lipidomic switch in oxylipins against inflammation. Our present study reinforces the notion that metabolic regulation relies on n-6/n-3 PUFA ratio rather than compositional changes in PUFA or SFA. This hypothesis requires a thorough evaluation in humans by testing the ALA-enriched agricultural products, some of which have already launched in the marketplace in the form of ALA-enriched eggs, milk, and butter [63]. By conducting a pilot experiment, our work also proposes that limiting LA may be an alternative dietary strategy to circumvent obesity-mediated metabolic complications, leading to a reduction of n-6/n-3 PUFA ratio and subsequently modulating the oxygenated PUFA metabolites.
Supplementary Material
Acknowledgement / Grant support:
This work was supported in part by grant from NIH 1R21HD094273 awarded to S.C., and a USDA-Hatch grant (NEB-36-085) at the University of Nebraska-Lincoln awarded to S.C. Additional support was provided by USDA Projects 2032-51530-022-00D, 2032-51530-025-00D, and NIH U24 DK097154 awarded to the West Coast Central Comprehensive Metabolomics Resource Core. A research innovation grant (319045-3) from the Ministry of Agriculture, Food and Rural Affairs of South Korea was awarded to S.S. and Sunseo Omega 3 Inc. The content is the sole responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations used:
- ALA
α-linolenic acid
- ARA
arachidonic acid
- BAT
brown adipose tissue
- Bu: DHA
docosahexaenoic acid
- 15-d-PGJ2
15-deoxy prostaglandin J2
- EPA
eicosapentaenoic acid
- eWAT
epididymal white adipose tissue
- HETE
hydroxyeicosatetraenoic acid
- HEPE
hydroxyeicosapentaenoic acids
- iWAT
inguinal white adipose tissue
- LA
linoleic acids
- LC n-3 PUFA
long-chain omega 3 polyunsaturated fatty acids
- Ma
HF diet made with conventional margarine
- n3Bu
HF diet made with n-3 enriched butter
Footnotes
Competing interests
The authors have no competing interests to declare.
REFERENCES
- [1].Jump DB, Botolin D, Wang Y, Xu J, Christian B, Demeure O. Fatty acid regulation of hepatic gene transcription. J Nutr. 2005;135:2503–6. [DOI] [PubMed] [Google Scholar]
- [2].Hu FB, Manson JE, Willett WC. Types of dietary fat and risk of coronary heart disease: a critical review. J Am Coll Nutr. 2001;20:5–19. [DOI] [PubMed] [Google Scholar]
- [3].Silva Figueiredo P, Carla Inada A, Marcelino G, Maiara Lopes Cardozo C, de Cassia Freitas K, de Cassia Avellaneda Guimaraes R, et al. Fatty Acids Consumption: The Role Metabolic Aspects Involved in Obesity and Its Associated Disorders. Nutrients. 2017;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Calder PC. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006;83:1505S–19S. [DOI] [PubMed] [Google Scholar]
- [5].Innes JK, Calder PC. Omega-6 fatty acids and inflammation. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2018;132:41–8. [DOI] [PubMed] [Google Scholar]
- [6].Turk HF, Chapkin RS. Membrane lipid raft organization is uniquely modified by n-3 polyunsaturated fatty acids. Prostaglandins, Leukotrienes and Essential Fatty Acids (PLEFA). 2013;88:43–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Balvers MG, Verhoeckx KC, Bijlsma S, Rubingh CM, Meijerink J, Wortelboer HM, et al. Fish oil and inflammatory status alter the n-3 to n-6 balance of the endocannabinoid and oxylipin metabolomes in mouse plasma and tissues. Metabolomics. 2012;8:1130–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Siriwardhana N, Kalupahana NS, Moustaid-Moussa N. Health benefits of n-3 polyunsaturated fatty acids: eicosapentaenoic acid and docosahexaenoic acid Advances in food and nutrition research: Elsevier; 2012. p. 211–22. [DOI] [PubMed] [Google Scholar]
- [9].Szajewska H, Szajewski T. Saturated Fat Controversy: Importance of Systematic Reviews and Metaanalyses. Crit Rev Food Sci Nutr. 2016;56:1947–51. [DOI] [PubMed] [Google Scholar]
- [10].Malhotra A. Saturated fat is not the major issue. BMJ. 2013;347:f6340. [DOI] [PubMed] [Google Scholar]
- [11].Schuster S, Johnson CD, Hennebelle M, Holtmann T, Taha AY, Kirpich IA, et al. Oxidized linoleic acid metabolites induce liver mitochondrial dysfunction, apoptosis, and NLRP3 activation in mice. J Lipid Res. 2018;59:1597–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ramsden CE, Hennebelle M, Schuster S, Keyes GS, Johnson CD, Kirpich IA, et al. Effects of diets enriched in linoleic acid and its peroxidation products on brain fatty acids, oxylipins, and aldehydes in mice. Biochim Biophys Acta Mol Cell Biol Lipids. 2018;1863:1206–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Naughton SS, Mathai ML, Hryciw DH, McAinch AJ. Linoleic acid and the pathogenesis of obesity. Prostaglandins Other Lipid Mediat. 2016;125:90–9. [DOI] [PubMed] [Google Scholar]
- [14].Ramsden CE, Zamora D, Majchrzak-Hong S, Faurot KR, Broste SK, Frantz RP, et al. Re-evaluation of the traditional diet-heart hypothesis: analysis of recovered data from Minnesota Coronary Experiment (1968-73). BMJ. 2016;353:i1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Pimpin L, Wu JH, Haskelberg H, Del Gobbo L, Mozaffarian D. Is Butter Back? A Systematic Review and Meta-Analysis of Butter Consumption and Risk of Cardiovascular Disease, Diabetes, and Total Mortality. PLoS One. 2016;11:e0158118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Serna-Marquez N, Diaz-Aragon R, Reyes-Uribe E, Cortes-Reynosa P, Salazar EP. Linoleic acid induces migration and invasion through FFAR4- and PI3K-/Akt-dependent pathway in MDA-MB-231 breast cancer cells. Med Oncol. 2017;34:111. [DOI] [PubMed] [Google Scholar]
- [17].Vangaveti VN, Jansen H, Kennedy RL, Malabu UH. Hydroxyoctadecadienoic acids: Oxidised derivatives of linoleic acid and their role in inflammation associated with metabolic syndrome and cancer. Eur J Pharmacol. 2016;785:70–6. [DOI] [PubMed] [Google Scholar]
- [18].Domenichiello AF, Kitson AP, Chen CT, Trépanier M-O, Stavro PM, Bazinet RP. The effect of linoleic acid on the whole body synthesis rates of polyunsaturated fatty acids from α-linolenic acid and linoleic acid in free-living rats. The Journal of nutritional biochemistry. 2016;30:167–76. [DOI] [PubMed] [Google Scholar]
- [19].Sprecher H, Luthria DL, Mohammed BS, Baykousheva SP. Reevaluation of the pathways for the biosynthesis of polyunsaturated fatty acids. J Lipid Res. 1995;36:2471–7. [PubMed] [Google Scholar]
- [20].Shewale SV, Boudyguina E, Zhu X, Shen L, Hutchins PM, Barkley RM, et al. Botanical oils enriched in n-6 and n-3 FADS2 products are equally effective in preventing atherosclerosis and fatty liver. J Lipid Res. 2015;56:1191–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Fan R, Toney AM, Jang Y, Ro SH, Chung S. Maternal n-3 PUFA supplementation promotes fetal brown adipose tissue development through epigenetic modifications in C57BL/6 mice. Biochim Biophys Acta Mol Cell Biol Lipids. 2018;1863:1488–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kim Y, Natarajan SK, Chung S. Gamma-Tocotrienol Attenuates the Hepatic Inflammation and Fibrosis by Suppressing Endoplasmic Reticulum Stress in Mice. Mol Nutr Food Res. 2018;62:e1800519. [DOI] [PubMed] [Google Scholar]
- [23].Borkowski K, Yim SJ, Holt RR, Hackman RM, Keen CL, Newman JW, et al. Walnuts change lipoprotein composition suppressing TNFalpha-stimulated cytokine production by diabetic adipocyte. J Nutr Biochem. 2019;68:51–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Pedersen TL, Newman JW. Establishing and Performing Targeted Multi-residue Analysis for Lipid Mediators and Fatty Acids in Small Clinical Plasma Samples. Methods Mol Biol. 2018;1730:175–212. [DOI] [PubMed] [Google Scholar]
- [25].Galarraga M, Campion J, Munoz-Barrutia A, Boque N, Moreno H, Martinez JA, et al. Adiposoft: automated software for the analysis of white adipose tissue cellularity in histological sections. J Lipid Res. 2012;53:2791–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].van den Berg RA, Hoefsloot HC, Westerhuis JA, Smilde AK, van der Werf MJ. Centering, scaling, and transformations: improving the biological information content of metabolomics data. BMC Genomics. 2006;7:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gabbs M, Leng S, Devassy JG, Monirujjaman M, Aukema HM. Advances in Our Understanding of Oxylipins Derived from Dietary PUFAs. Adv Nutr. 2015;6:513–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Shearer GC, Harris WS, Pedersen TL, Newman JW. Detection of omega-3 oxylipins in human plasma and response to treatment with omega-3 acid ethyl esters. J Lipid Res. 2010;51:2074–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Farvid MS, Ding M, Pan A, Sun Q, Chiuve SE, Steffen LM, et al. Dietary linoleic acid and risk of coronary heart disease: a systematic review and meta-analysis of prospective cohort studies. Circulation. 2014;130:1568–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hamley S. The effect of replacing saturated fat with mostly n-6 polyunsaturated fat on coronary heart disease: a meta-analysis of randomised controlled trials. Nutr J. 2017;16:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Jang H, Park K. Omega-3 and omega-6 polyunsaturated fatty acids and metabolic syndrome: A systematic review and meta-analysis. Clin Nutr. 2019; 10.1016/j.clnu.2019.03.032. [DOI] [PubMed] [Google Scholar]
- [32].Duivenvoorde LP, van Schothorst EM, Swarts HM, Kuda O, Steenbergh E, Termeulen S, et al. A Difference in Fatty Acid Composition of Isocaloric High-Fat Diets Alters Metabolic Flexibility in Male C57BL/6JOlaHsd Mice. PLoS One. 2015;10:e0128515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Pavlisova J, Bardova K, Stankova B, Tvrzicka E, Kopecky J, Rossmeisl M. Corn oil versus lard: Metabolic effects of omega-3 fatty acids in mice fed obesogenic diets with different fatty acid composition. Biochimie. 2016;124:150–62. [DOI] [PubMed] [Google Scholar]
- [34].Das UN. Essential Fatty acids - a review. Curr Pharm Biotechnol. 2006;7:467–82. [DOI] [PubMed] [Google Scholar]
- [35].Dias CB, Wood LG, Phang M, Garg ML. Kinetics of omega-3 polyunsaturated fatty acids when co-administered with saturated or omega-6 fats. Metabolism. 2015;64:1658–66. [DOI] [PubMed] [Google Scholar]
- [36].Tu WC, Cook-Johnson RJ, James MJ, Muhlhausler BS, Gibson RA. Omega-3 long chain fatty acid synthesis is regulated more by substrate levels than gene expression. Prostaglandins Leukot Essent Fatty Acids. 2010;83:61–8. [DOI] [PubMed] [Google Scholar]
- [37].AI Ostermann, Waindok P, Schmidt MJ, Chiu CY, Smyl C, Rohwer N, et al. Modulation of the endogenous omega-3 fatty acid and oxylipin profile in vivo-A comparison of the fat-1 transgenic mouse with C57BL/6 wildtype mice on an omega-3 fatty acid enriched diet. PLoS One. 2017;12:e0184470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Jeyapal S, Kona SR, Mullapudi SV, Putcha UK, Gurumurthy P, Ibrahim A. Substitution of linoleic acid with alpha-linolenic acid or long chain n-3 polyunsaturated fatty acid prevents Western diet induced nonalcoholic steatohepatitis. Sci Rep. 2018;8:10953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ostermann AI, Schebb NH. Effects of omega-3 fatty acid supplementation on the pattern of oxylipins: a short review about the modulation of hydroxy-, dihydroxy-, and epoxy-fatty acids. Food Funct. 2017;8:2355–67. [DOI] [PubMed] [Google Scholar]
- [40].Aoki R, Ikarugi H, Naemura A, Ijiri Y, Yamashita T, Yamamoto J. Endothelial dysfunction precedes atherosclerotic lesions and platelet activation in high fat diet-induced prothrombotic state. Thromb Res. 2006;117:529–35. [DOI] [PubMed] [Google Scholar]
- [41].Porro B, Songia P, Squellerio I, Tremoli E, Cavalca V. Analysis, physiological and clinical significance of 12-HETE: a neglected platelet-derived 12-lipoxygenase product. J Chromatogr B Analyt Technol Biomed Life Sci. 2014;964:26–40. [DOI] [PubMed] [Google Scholar]
- [42].Scher JU, Pillinger MH. 15d-PGJ2: the anti-inflammatory prostaglandin? Clin Immunol. 2005;114:100–9. [DOI] [PubMed] [Google Scholar]
- [43].Yu R, Xiao L, Zhao G, Christman JW, van Breemen RB. Competitive enzymatic interactions determine the relative amounts of prostaglandins E2 and D2. J Pharmacol Exp Ther. 2011;339:716–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Joo M, Sadikot RT. PGD synthase and PGD2 in immune resposne. Mediators Inflamm. 2012;2012:503128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Chen K, Li JJ, Li SN, Feng J, Liu T, Wang F, et al. 15-Deoxy-Delta(12,14)-prostaglandin J2 alleviates hepatic ischemia-reperfusion injury in mice via inducing antioxidant response and inhibiting apoptosis and autophagy. Acta Pharmacol Sin. 2017;38:672–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Hsu HT, Chi CW. Emerging role of the peroxisome proliferator-activated receptor-gamma in hepatocellular carcinoma. J Hepatocell Carcinoma. 2014;1:127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Marcone S, Evans P, Fitzgerald DJ. 15-Deoxy-Delta(12,14)-Prostaglandin J2 Modifies Components of the Proteasome and Inhibits Inflammatory Responses in Human Endothelial Cells. Front Immunol. 2016;7:459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Davidson J, Higgs W, Rotondo D. Eicosapentaenoic acid suppression of systemic inflammatory responses and inverse up-regulation of 15-deoxyDelta(12,14) prostaglandin J2 production. Br J Pharmacol. 2013;169:1130–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Bouhlel MA, Derudas B, Rigamonti E, Dievart R, Brozek J, Haulon S, et al. PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab. 2007;6:137–43. [DOI] [PubMed] [Google Scholar]
- [50].Giri S, Rattan R, Singh AK, Singh I. The 15-deoxy-delta12,14-prostaglandin J2 inhibits the inflammatory response in primary rat astrocytes via down-regulating multiple steps in phosphatidylinositol 3-kinase-Akt-NF-kappaB-p300 pathway independent of peroxisome proliferator-activated receptor gamma. J Immunol. 2004;173:5196–208. [DOI] [PubMed] [Google Scholar]
- [51].Straus DS, Pascual G, Li M, Welch JS, Ricote M, Hsiang CH, et al. 15-deoxy-delta 12,14-prostaglandin J2 inhibits multiple steps in the NF-kappa B signaling pathway. Proc Natl Acad Sci U S A. 2000;97:4844–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Leng S, Winter T, Aukema HM. Dietary LA and sex effects on oxylipin profiles in rat kidney, liver, and serum differ from their effects on PUFAs. J Lipid Res. 2017;58:1702–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Cayer LGJ, Mendonca AM, Pauls SD, Winter T, Leng S, Taylor CG, et al. Adipose tissue oxylipin profiles vary by anatomical site and are altered by dietary linoleic acid in rats. Prostaglandins Leukot Essent Fatty Acids. 2019;141:24–32. [DOI] [PubMed] [Google Scholar]
- [54].Watkins BA, Kim J, Kenny A, Pedersen TL, Pappan KL, Newman JW. Circulating levels of endocannabinoids and oxylipins altered by dietary lipids in older women are likely associated with previously identified gene targets. Biochim Biophys Acta. 2016;1861:1693–704. [DOI] [PubMed] [Google Scholar]
- [55].Birkenfeld AL, Shulman GI. Nonalcoholic fatty liver disease, hepatic insulin resistance, and type 2 diabetes. Hepatology. 2014;59:713–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].U.S. Department of Health and Human Services and U.S. Department of Agriculture. 2015-2020 Dietary Guidelines for Americans 8th Edition. 8 ed. [Google Scholar]
- [57].Botta A, Ghosh S. Exploring the Impact of n-6 PUFA-rich Oilseed Production on Commercial Butter Compositions Worldwide. J Agric Food Chem. 2016;64:8026–34. [DOI] [PubMed] [Google Scholar]
- [58].Wall R, Ross RP, Fitzgerald GF, Stanton C. Fatty acids from fish: the anti-inflammatory potential of long-chain omega-3 fatty acids. Nutrition reviews. 2010;68:280–9. [DOI] [PubMed] [Google Scholar]
- [59].Chilton FH, Rudel LL, Parks JS, Arm JP, Seeds MC. Mechanisms by which botanical lipids affect inflammatory disorders. Am J Clin Nutr. 2008;87:498S–503S. [DOI] [PubMed] [Google Scholar]
- [60].Young AJ, Marriott BP, Champagne CM, Hawes MR, Montain SJ, Johannsen NM, et al. Blood fatty acid changes in healthy young Americans in response to a 10-week diet that increased n-3 and reduced n-6 fatty acid consumption: a randomised controlled trial. Br J Nutr. 2017;117:1257–69. [DOI] [PubMed] [Google Scholar]
- [61].Porsgaard T, Overgaard J, Krogh AL, Jensen MB, Guo Z, Mu H. Butter blend containing fish oil improves the level of n-3 fatty acids in biological tissues of hamster. J Agric Food Chem. 2007;55:7615–9. [DOI] [PubMed] [Google Scholar]
- [62].Metcalf RG, James MJ, Mantzioris E, Cleland LG. A practical approach to increasing intakes of n-3 polyunsaturated fatty acids: use of novel foods enriched with n-3 fats. Eur J Clin Nutr. 2003;57:1605–12. [DOI] [PubMed] [Google Scholar]
- [63].Ganesan B, Brothersen C, McMahon DJ. Fortification of foods with omega-3 polyunsaturated fatty acids. Crit Rev Food Sci Nutr. 2014;54:98–114. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.