Abstract
Depression affects at least 322 million people globally, or approximately 4.4% of the world’s population. While the earnestness of researchers and clinicians to understand and treat depression is not waning, the number of individuals suffering from depression continues to increase over and above the rate of global population growth. There is a sincere need for a paradigm shift. Research in the past decade is beginning to take a more holistic approach to understanding depression etiology and treatment, integrating multiple body systems into whole-body conceptualizations of this mental health affliction. Evidence supports the hypothesis that the gut microbiome, or the collective trillions of microbes inhabiting the gastrointestinal tract, is an important factor determining both the risk of development of depression and persistence of depressive symptoms. This review discusses recent advances in both rodent and human research that explore bidirectional communication between the gut microbiome and the immune, endocrine, and central nervous systems implicated in the etiology and pathophysiology of depression. Through interactions with circulating inflammatory markers and hormones, afferent and efferent neural systems, and other, more niche, pathways, the gut microbiome can affect behavior to facilitate the development of depression, exacerbate current symptoms, or contribute to treatment and resilience. While the challenge of depression may be the direst mental health crisis of our age, new discoveries in the gut microbiome, when integrated into a holistic perspective, hold great promise for the future of positive mental health.
Keywords: depression, microbiome, central nervous system, immune system, endocrine system, inflammation, microbiome-gut-brain axis, microbiota, prebiotics, probiotics
Graphical Abstract
Introduction
The English language is filled with idioms connecting the gastrointestinal tract with emotion, as is perhaps most evident from a quick scan of the microbiome literature (a few examples: Dinan & Cryan, 2017; Forsythe, Sudo, Dinan, Taylor, & Bienenstock, 2010; Foster, 2013; Mosher & Wyss-Coray, 2015). Gut feelings, gut-wrenching sadness, spilling one’s guts, to have one’s bowels in an uproar, having one’s stomach in knots: these are just a sampling of the many ways language itself reflects the strong relationship between powerful emotions and the human digestive system. In the words of celebrated English author Charles Dickens, “a damaged stomach occasions melancholy, disgust, envy, hatred and all uncharitableness” (Dickens, 1867). While modern microbiome research would perhaps substitute “stomach” for intestines,” Mr. Dickens’ point is well taken. Although not known in Dickens’ time, it is now clear that a living, thriving, microbiome, the collection of tens of trillions of microorganisms inhabiting the human gut (Ursell, Metcalf, Parfrey, & Knight, 2012), may play a role in the relationship between the gastrointestinal tract and emotional states. As the relatively new field of microbiome research continues to grow, wielding this powerful new knowledge not only has implications in our understanding of emotional dysregulation, but also in its treatment and human resilience: true intestinal fortitude. Understanding the role of the microbiome in depression is one of the most pressing psychiatric applications of this developing field.
According to the World Health Organization’s 2015 survey of global mental disorders, 322 million people are estimated to suffer from depression, which is equivalent to approximately 4.4% of the world’s population. This number increased by 18.4% in the decade from 2005 to 2015, contrasted to the global population increase of 12.9% over that same time period (World Health Organization, 2017). In the United States alone, direct costs incurred by individuals diagnosed with major depressive disorder (MDD) was almost $100 billion in 2010, and a combined cost of $210 billion with comorbid conditions (Greenberg, Fournier, Sisitsky, Pike, & Kessler, 2015). Depression consistently results in a greater decrease in health than many other chronic health conditions, such as diabetes, arthritis, and asthma, but also shares a high level of comorbidity with other chronic physical illnesses: from 9.3% to 18.1% percent for a single condition and 23% for two or more (Moussavi et al., 2007). Across the globe, depression has led to a total of 50 million years lived with disability in 2015, with the majority of the burden falling to low- and middle-income families, and it is the single largest contributor to non-fatal health loss (World Health Organization, 2017). Understanding the nature of this disorder is paramount from a global health perspective.
Adding to the urgency, our current treatment methods are only covering a fraction of those in need. According to one meta-analysis, even with combined psychotherapy and pharmacotherapy, only 46% of individuals reach remission at treatment termination (de Maat, Dekker, Schoevers, & de Jonghe, 2007). This is complicated by treatment non-adherence rates that have been estimated to range from 30% to 60% in epidemiological studies (Pampallona, Bollini, Tibaldi, Kupelnick, & Munizza, 2002). Much of this has been attributed to a lack of patient education provided by treatment providers. This often went hand in hand with a need for increased treatment provider education and understanding (Bollini, Tribaldi, Testa, & Munizza, 2004). In the face of these challenges, former leader of the National Institute of Mental Health in the United States Thomas Insel and others have called for radical changes in how basic research approaches mental health conditions. Insel has advocated for a move away from incremental advances and toward radical reimagining, and novel discovery, of the causes in hopes of identifying prevention strategies (Insel & Scolnick, 2006).
While the desire for more effective treatment and prevention of depression seems perpetually at the top of the list in terms of global health concerns, this begs the question of why this particular health concern has been so intractable. What makes this gut feeling so hard to digest? As recent research continues to discover, depression is far more multifaceted than has been historically assumed.
Depression: a multifaceted mood disorder
The history of Western medicine’s understanding of depression dates back as far as ancient Greek physician Hippocrates, whose humoral theory of pathology identified black bile as the cause of what we today refer to as depression (Hippocrates, translated 1931). The Greek term for black bile μέλαινα χολή, transliterates to melaina chole, the origin of the words melancholy and melancholia. In 1621, renaissance scholar Robert Burton published “The Anatomy of Melancholy,” an encyclopedic tome that was one of the first published works to combine history, cause, and treatment in a single volume. While Brunton’s treatments varied widely, including practices such as blood-letting, Burton did suggest changes in diet as being connected with melancholia, changes that are pertinent to the current review (Burton, 1621). By the late 19th century, British Psychiatrist Charles Mercier is credited as one of the first to propose the idea that melancholia was a brain disorder, a concept that would go on to shape the field of mental health for the next century (Lawlor, 2012).
In the tradition of viewing depressed mood as a brain disorder, psychiatrist Adolph Meyer (1866-1950) pushed for a transition from using the term “melancholia” to “depression” in medicine, partially to promote moving away from previous treatment practices in favor of developing what he termed “somatic therapies,” including early psychopharmacology and shock treatment. This new, medical perspective on depression would go on to influence the formation of the first Diagnostic and Statistical Manual for Mental Disorders (DSM-I) in 1952. While the DSM-I and its successor, the DSM-II, were still strongly grounded in the psychoanalytic approach, pioneered by Freud in the early 20th century, the DSM-III in 1980 would transform the United States’ (and the global perspective, to a large extent) perspective on mental health. The DSM-III was the first modern diagnostic guide to organize around observable symptoms, rather than theoretical constructs, creating a shared language for health care professionals. The DSM-IV (1994), and now the current DSM-5 (2013) would go on to clarify MDD as requiring a single major depressive episode with at least five depression-related symptoms (Lawlor, 2012). This common language has fueled an explosion of research that has made cross-study comparisons standardized and much simpler to carry out. The biotechnology revolution beginning in the 1980s and the development of fMRI as a technique in research psychology in the 1990s catapulted our understanding of depression etiology in the domain of the brain (Faro & Mohamed, 2010; Ogawa et al., 1992).
However, a decade into the 20th century, a completely brain-based understanding of depression was in question. The popular “chemical imbalance” theory of depression, stating that depression was related to an imbalance of specific neurotransmitters, was proving less effective both in explaining the etiology of depression and in developing novel treatments (Malenka, 2012). The current understanding of MDD has come to encompass not just changes in neurotransmitters, but shifts in neural circuits, as well as alterations in both immune and endocrine functioning (Irwin & Miller, 2007; see Fig. 1). This broadened scope is now beginning to inform a vast array of new, personalized treatments that are beginning to show great promise in a new holistic approach to depression (Henter et al., 2017).
Figure 1.
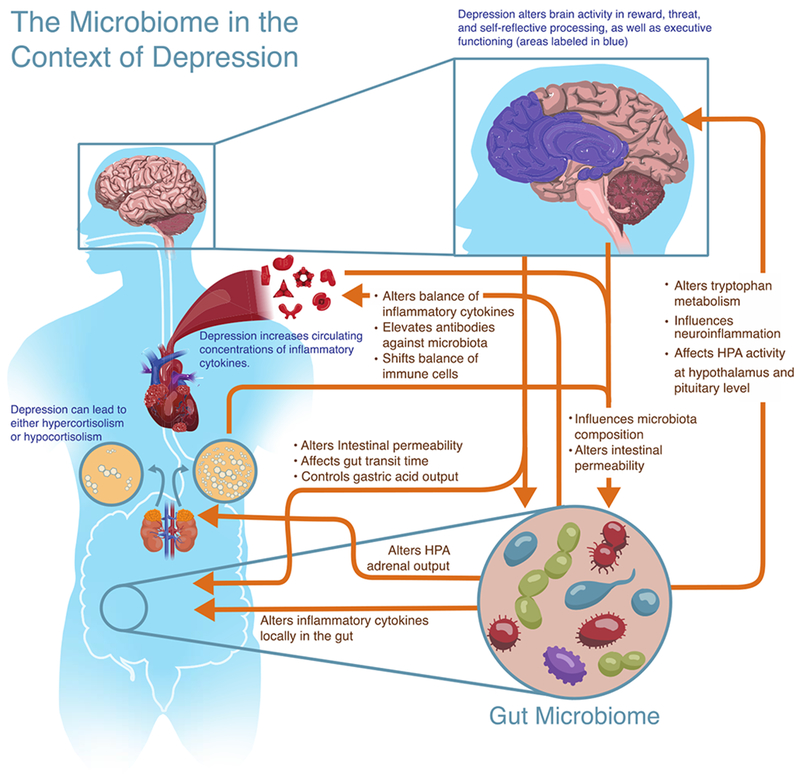
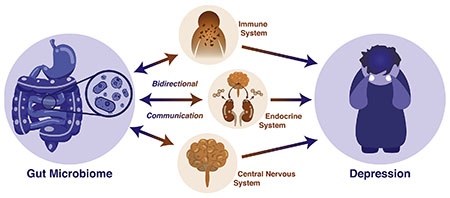
Illustration of reviewed bidirectional communication between the central nervous system, endocrine system, and immune system and the microbiome, which contribute to depression.
Depression and the central nervous system
Moving from a focus on neurotransmitters to one of the entire brain has allowed for the identification of brain regions and circuits associated with depression. The following section surveys several representative highlights. Imaging techniques have identified structural changes in the brains of individuals with a diagnosis of MDD, including decreased volume in the prefrontal cortex (PFC), the anterior cingulate cortex (ACC), the basal ganglia, thalamus, and hippocampus (Drevets, 2007; Dusi, Barlati, Vita, & Brambilla, 2015). Depression has also been associated with a number of resting state connectivity differences (Gong & He, 2015). Recent works have found distinct changes in prefrontal-limbic circuitry, such as altered connectivity between the amygdala and medial PFC in individuals who experienced early life adversity (A. T. Park et al., 2018). Others have found increased connectivity in the default mode network (DMN) connectivity in depressed adults, a brain network associated with self-referential thought and rumination, in depressed adults (Bessette et al., 2018; Korgaonkar, Fornito, Williams, & Grieve, 2014). Increased connectivity has also been found within the dorsolateral PFC, a brain region involved in executive functioning and cognitive flexibility (Murrough et al., 2016; M. K. Singh et al., 2013). In contrast, decreased connectivity has been described between areas of the salience network, a network that monitors the environment for events of personal relevance (Kaiser, Andrews-Hanna, Wager, & Pizzagalli, 2015).
Functional changes in response to experimental stimuli relevant to depression have also been identified. For example, depression is associated with increased amygdala activity in response to threat and decreased PFC activity during cognitive tasks (Kerestes, Davey, Stephanou, Whittle, & Harrison, 2014). Individuals with MDD show hyperactivation to negatively valanced emotional stimuli and hypoactivation to positively valanced emotional stimuli in brain regions that process emotions, including the amygdala, striatum, hippocampus, and ACC (Groenewold, Opmeer, de Jonge, Aleman, & Costafreda, 2013). Faulty reward processing as also been observed in MDD (Whitton, Treadway, & Pizzagalli, 2015), which has been elucidated, in part, through experimental paradigms that study brain activity in the context of monetary wins and losses (Pizzagalli et al., 2009), studies of genes related to the neurotransmission of dopamine (Bogdan, Nikolova, & Pizzagalli, 2013), and large-scale circuitry studies (Peters, Dunlop, & Downar, 2016).
This larger perspective on brain function has also enhanced the field’s understanding of depression treatment. For example, increased functional connectivity between frontal and limbic regions has been observed in response to pharmacotherapy (Dichter, Gibbs, & Smoski, 2015). Antidepressant treatment is associated with a normalization of limbic, ACC, and PFC activity in response to aversive stimuli (Wessa & Lois, 2015). Cognitive behavioral therapy (CBT) has been connected to changes in ACC activity, altered dynamics in the PFC, and shifts in activity in the amygdala and hippocampus (Anthes, 2014; Franklin, Carson, & Welch, 2016).
Depression and the endocrine system
While the understanding that stress is related to depression goes back at least as far as Burton’s “Anatomy of Melancholy” (1621), a modern understanding that the relationship between stress and depression is grounded in body systems and molecular biology is more recent. The predominant system associated with endocrine responses to stress is the hypothalamic-pituitary-adrenal (HPA) axis, which relies on a cascade of hormones that ultimately prepare the body for adaptive responses to stress. Information about threat encoded in other areas of the brain signal the hypothalamus to release corticotropin-releasing hormone (CRH), which signals the pituitary gland to release adrenocorticotropic hormone (ACTH). ACTH then travels through the bloodstream to the adrenal cortex of the adrenal glands, which are then prompted to manufacture and release the stress hormone cortisol in humans (Tafet & Nemeroff, 2016).
Early study of this system in relation to depression made use of the dexamethasone suppression test (DST). In the DST, administration of the synthetic glucocorticoid dexamethasone typically creates negative feedback for the HPA axis and will reduce cortisol output the following day. Research found that depressed individuals often had a blunted response to the DST, functionally resulting in a less robust decrease in cortisol secretion. (Lesch & Rupprecht, 1989; Rupprecht & Lesch, 1989). This response often returned to normal, following successful depression treatment (Murphy, 1991).
Subsequently, the 1990s would see an explosion of research looking at the dynamics of psychosocial stress, in part due to the development of the Trier Social Stress Test (TSST), a public speaking-based stressor (Kirschbaum, Pirke, & Hellhammer, 1993). Psychosocial stress was linked to activity of the HPA axis, and in some cases with depression as well (Foley & Kirschbaum, 2010). A recent study contrasting the cortisol response to the TSST between women with depression, panic disorder, posttraumatic stress disorder (PTSD), and typical controls found that depression showed a much higher cortisol response than the other diagnoses, but lower than controls (Wichmann, Kirschbaum, Böhme, & Petrowski, 2017). However, recent work has demonstrated that there may be gender differences in depression-induced changes in HPA axis activity. An experiment of heterosexual couples discussing an unresolved relationship conflict with each other in a laboratory setting showed distinct gender differences related to current depressive symptoms (Powers, Laurent, Gunlicks-Stoessel, Balaban, & Bent, 2016). Women experienced hypoactivation of the cortisol response to stress, including attenuated cortisol levels overall along with decreased reactivity and a flatter recovery curve. Men, on the other hand, presented with hyperactivation, including elevated levels of HPA axis activity, during the laboratory conflict.
Differences in depression-related HPA axis activity may even occur in response to different subtypes of depression, such as typical melancholic depression, anxious depression, defined as also having subsyndromal anxiety or a diagnosed anxiety disorder, and atypical depression, which is often characterized by increased mood reactivity (Fischer, Strawbridge, Vives, & Cleare, 2017; Ionescu, Niciu, Mathews, Richards, & Zarate, 2013; ten Have et al., 2016). A recent examination of the depression subtype literature speaks to the high level of heterogeneity when it comes to measures of the HPA axis in depression (Juruena, Bocharova, Agustini, & Young, 2018). Perhaps the most salient divide lies in response to the DST, with most patients with melancholic depression showing elevated non-suppression and patients with atypical depression showing a profile more consistent with suppression. Other measures, such as basal levels of cortisol or ACTH, showed a great deal of variability from individual to individual. This is consistent with the literature, which often finds sustained, elevated HPA axis activity via higher basal plasma cortisol concentration at both circadian trough and peak, increased amplitude of cortisol pulses in the context of circadian fluctuations, elevated 24-hour urinary free cortisol, and even increased adrenal size; however, studies often show heightened variation and high numbers of subjects with very different patterns of HPA axis activity (Jacobson, 2014).
This heterogeneity may also be due to the interaction of depression with stress and trauma. While increased stress typically leads to elevated cortisol secretion, ongoing stress and severe trauma are typically associated with the opposite, hypocortisolism (Heim, Ehlert, & Hellhammer, 2000). This is typical with stress-related disorders, such as PTSD, which frequently presents decreased circulating cortisol and hypersuppression in response to the DST and has even been incorporated into the development of mouse models of PTSD (Reber, Langgartner, et al., 2016; Yehuda & Seckl, 2011; Yehuda et al., 1993). Interestingly, depression in the context of a trauma history, though not necessarily a diagnosis of PTSD, is associated with a hypersuppression response to the DST (Savic, Knezevic, Damjanovic, Spiric, & Matic, 2012; Yehuda, Halligan, Golier, Grossman, & Bierer, 2004). Approximately half of people with a PTSD diagnosis also meet criteria for major depression, which may explain some of the variation in HPA axis activity among depressed individual (Flory & Yehuda, 2015).
Neuroscience and genetics studies support these systems-wide findings, and particularly tie together the central nervous and endocrine systems. Gene polymorphisms in the HPA axis are associated with increased amygdala reactivity, which is a proposed link between early life adversity, stress reactivity, and depression (Iorio et al., 2017). One study found that functioning of the HPA axis acts as a mediator between certain gene variants of the serotonin transporter and developing MDD, linking neurotransmission with endocrine activity (Ancelin et al., 2017). Recent work has proposed that elevated cortisol levels in MDD decrease hippocampal volume by interfering with neurogenesis, and that these changes may be etiological for depressive symptoms (Boku, Nakagawa, Toda, & Hishimoto, 2018). Finally, epigenetic changes in response to extended stress have been connected to depression. Specifically, heightened stress, particularly in response to early-life adversity, leads to epigenetic changes that alter glucocorticoid receptor (GR) expression and function, resulting in the prolonged and dysregulated HPA axis activity often associated with depression (Farrell & O’Keane, 2016).
Overall, an astounding 40% to 60% of depressed individuals have been found to have a dysregulated HPA axis to some degree, which can also be accompanied by abnormalities in other branches of the endocrine system, including the hypothalamic-pituitary-thyroid (HPT), and hypothalamic-pituitary-gonadal (HPG) axes (Howland, 2010). This makes the endocrine system a tantalizing target for pharmacological intervention in the context of depression. While showing theoretical promise, research beginning in the 1990s that explored antagonism of CRH receptor 1 has largely been scaled back in response to poor safety and efficacy of initial candidates, along with the unanticipated interaction of antagonists with other receptors, including CRH receptor 2 (Spierling & Zorrilla, 2017). Alternatively, glucocorticoid receptor antagonists, primarily mifepristone, have also been explored in the context of depression (Howland, 2013). Perhaps the most work has been done in Cushing’s syndrome, a disorder that results in heightened circulating cortisol that has a comorbidity with anxiety and depression that may be as high as 81% (Pivonello, De Leo, Cozzolino, & Colao, 2015). Mifepristone is typically prescribed to manage Cushing’s syndrome (Nieman et al., 1985); however, it does not typically alleviate depressive symptoms, which often persist long after treatment (Pivonello et al., 2015). When mifepristone has been used in the context of mood disorders in non-Cushing’s syndrome individuals, studies have found cognitive improvements but typically no related shifts in mood (Roat-Shumway, Wroolie, Watson, Schatzberg, & Rasgon, 2018; Young et al., 2004). However, while direct pharmacological manipulation of the endocrine system has not revealed promising treatments, many depression treatments help to regulate endocrine function indirectly, from psychopharmaceuticals (Manthey et al., 2011) to meditation (Cahn, Goodman, Peterson, Maturi, & Mills, 2017).
Depression and the immune system
In parallel to, and often in concert with, the central nervous and endocrine systems, the immune system has increasingly been found to play a large role in depression as well. The seminal Maier and Watkins paper, “Cytokines for psychologists,” (1998) was an early integration of inflammation and mood that presciently set the course of the field for the past two decades. The authors outlined the bidirectional lines of communication between the immune system and the brain, mediated by a variety of inflammatory molecules, known as cytokines, released by immune cells both centrally and peripherally. As evidence, they cited the expression of proinflammatory cytokines throughout the central nervous system, and the powerful effects sickness has on mood in general (S. F. Maier & Watkins, 1998). However, in order to reach signaling receptors on neurons and glial cells, cytokines would need to cross the blood-brain barrier (BBB), the highly selective, semipermeable barrier that separates the vascular system from the central nervous system. Due to the size of cytokines, it was initially assumed that transport across the BBB would be rare, and thus, early research focused on other paths, identifying the vagus nerve as a means of relaying signals of peripheral inflammation to the central nervous system (Konsman, Luheshi, Bluthé, & Dantzer, 2000). However, recent research has identified mechanisms through which cytokines can traverse the BBB. Distinct transport molecules present at certain locations along the BBB can actively shuttle key immune-modulating cytokines such as interleukin (IL)-1β, IL-6 and tumor necrosis factor (TNF) across this selectively permeable barrier (W. Banks, 2005; W. A. Banks, Kastin, & Broadwell, 1995). Additionally, inflammation can disrupt the BBB, making it more permeable to circulating immune molecules (Varatharaj & Galea, 2016).
These cytokine signals have been connected to a pattern of activity collectively known as “sickness behavior.” They include depressed mood, lethargy, decreased appetite, heightened sensitivity to pain, difficulty concentrating, and malaise: all hallmarks of MDD. These behavioral changes, along with fever and several other physiological changes, are adaptive strategies that evolved to fight infection, and have been directly linked to cytokine signaling (Dantzer, 2001; Konsman, Parnet, & Dantzer, 2002). Interestingly, cytokine signals associated with a proinflammatory response, and ensuing sickness behaviors, can be triggered by stress, including psychosocial stressors (Miller, Cohen, & Ritchey, 2002). From an evolutionary perspective, stress has typically been associated with the risk of physical injury and infection. Mounting a preemptive immune response, including behaviors that increase the chance of healing and recovery, has maximized the survival of the human race (Charles L. Raison, Capuron, & Miller, 2006).
In modern life, however, these past evolutionary advantages have created a very real present problem (Miller & Raison, 2016). Depressive symptoms align closely with immune-mediated sickness behaviors, and hypotheses have been generated to unify our understanding of the two. For example, the Pathogen Host Defense (PATHOS-D) hypothesis presented by Raison and Miller outlines that symptoms such as hyperthermia, conservation/withdrawal behavior, hypervigilance, and anorexia, all associated with depression, also played a role in the survival of our ancestors during pathogen defense (C. L. Raison & Miller, 2013). When measuring circulating cytokines, individuals with depression often have elevated circulating IL-1β IL-6, TNF, IL-10, IL-12, with decreased levels of interferon gamma (IFNγ) and IL-4 (Goldsmith, Rapaport, & Miller, 2016). Laboratory psychosocial stressors tend to elicit elevated levels of IL-6 and CRP in response to stress as compared to individuals without depression (Irwin & Miller, 2007). However, as with endocrine profiles in depression, there is a great deal of variation within measured samples.
Supporting the relationship between inflammation and depression, many current treatments for MDD have anti-inflammatory properties. Some antidepressants reduce endogenous production of proinflammatory cytokines, and can even modify immune reactivity in the central nervous system (Capuron, Hauser, Hinze-Selch, Miller, & Neveu, 2002; Gałecki, Mossakowska-Wójcik, & Talarowska, 2018; Nazimek et al., 2017). A recent study found that depressed individuals treated either pharmacologically or with psychotherapy for four weeks both experienced a reduction in proinflammatory cytokines; however, individuals that were treatment-resistant maintained elevated cytokine levels, suggesting that treatment-resistant depression may be related to altered responsivity to inflammatory signals (Syed et al., 2018).
Viewing depression through the lens of inflammation has opened up the possibility of using a new generation of anti-inflammatory compounds to augment current therapies, including cytokine inhibitors, non-steroidal anti-inflammatory drugs (NSAIDS), statins, and even anti-epileptics (Andrade, 2014; Cowen, 2017; Charles L Raison & Miller, 2011; Shariq et al., 2018). However, while the future of novel immune therapeutics for MDD holds great promise, the complex nature of immune communication and the heterogeneous presentation of depression itself have warranted caution, particularly since anti-inflammatory treatments can have no effect or even exacerbate depressive symptoms in some individuals (Köhler et al., 2014; Charles L. Raison et al., 2013).
Advancing modern understanding and treatment of depression necessitates a holistic view of the individual to uniquely identify and target treatments that take into account the many body systems involved. In the past 15 years, a new player has entered the field of depression. It interacts with the human body’s endocrine, immune, and central nervous systems, influencing mood and behavior from a position within the body, but uniquely separate from it. This new edition to our understanding of depression is the human gut microbiome.
Defining the microbiome
A microbiome, in general, refers to a collective population of commensal microbes living symbiotically with a multicellular organism (Turnbaugh et al., 2007). While the initial usage of the term “microbiome” referred to the collective genomes of these microbes, and the initial usage of the term “microbiota” referred to the actual microorganisms themselves, the two words have since become fairly interchangeable, with microbiome often being used as a catchall for both (Ursell et al., 2012). For clarity, this paper will generally follow the original convention, referring to the microbes as microbiota, with the exception of describing the microbiome at an organism level or in integrated systems, such as immune-microbiome interactions, or the microbiome-gut-brain axis.
Microbiomes have existed on planet earth for over a billion years, and are present at all levels of multicellular life, from plants to invertebrates and vertebrates (Berg, Rybakova, Grube, & Köberl, 2016; Ley, Lozupone, Hamady, Knight, & Gordon, 2008). Strains of stomach-associated Helicobacter pylori can even be used to trace migration patterns and human evolution (Ley et al., 2008). Interest in the human gut microbiome and its relationship to health can be dated as far back as the early 1900s, when authorities advocated for the ingestion of certain lactic acid producing bacteria as a cure for “autointoxication,” or the process by which intestinally derived toxins negatively affect systemic health (Bested, Logan, & Selhub, 2013b). However, interest in the microbiome and its relationship to the brain largely waned, and although a theory of gastrointestinal-related depression was developed almost 90 years ago in the early 20th century, it was “swept into the dustbin of history” (Bowe & Logan, 2011; Kligman, 2002; Stokes & Pillsbury, 1930). A wave of identification of potential uses for probiotics therapeutically helped to bring the microbiome and its connection to the brain back into focus, perhaps culminating in the initiation of the Human Microbiome Project (HMP) (Bested, Logan, &Selhub, 2013a; Turnbaugh et al., 2007).
The mission of the Human Microbiome Project was to characterize the nature of the human microbiome, understanding its distribution and evolution in ways that would benefit our understanding of human health and disease (Turnbaugh et al., 2007). While research efforts have predominantly focused on the bacterial inhabitants of the gastrointestinal tract, partly due to the relative ease in studying bacteria, the human microbiome is also made up of fungal flora (the mycobiome) and viruses (the virome or phageome for bacteria-infecting viruses), both of which are receiving increasing attention (Enaud et al., 2018; Mukhopadhya, Segal, Carding, Hart, & Hold, 2019). Alterations in gut fungal balance have been associated with gastrointestinal disorders, changes in cognition, and altered immune and endocrine functioning (Enaud et al., 2018). Viruses that make up the human virome have been linked to chronic fatigue syndrome, type 2 diabetes, and, potentially, mood disorders (Ma, You, Mai, Tokuyasu, & Liu, 2018; Newberry, Hsieh, Wileman, & Carding, 2018; Prusty et al., 2018). Specific viral microbiota, particularly phages, have even been linked to positive clinical outcomes in the treatment of Clostridium difficile infections via fecal-microbiota transplantation, a technique discussed further below (Broecker, Klumpp, & Moelling, 2016; Zuo et al., 2018). The prospects of greater incorporation of the mycobiome and virome into modern study of the microbiome holds great promise. However, as there are few depression-related studies in these domains, bacterial residents of the human gut microbiome will be the ensuing focus.
Study of the gut microbiome in humans typically involves fecal analysis, although this is not without limitations, as fecal samples are often more representative of microbiota inhabiting specific segments of the colon lumen and do not reflect the complex diversity of the gut mucosa and other segments of the intestines (Parthasarathy et al., 2016; Sartor, 2015). Early efforts at characterization of microbiome composition were culture-based, but these methods lacked specificity, as many microbiota strains do not grow in culture (Turnbaugh et al., 2007), and gave way to genomic techniques that have allowed for more specific identification of larger numbers of gut microbiota species than ever before (Knight et al., 2018). Recent First-wave genomic methods have focused on the 16S ribosomal RNA (rRNA) gene sequence, a gene less susceptible to horizontal transfer, or transfer of genetic information between a microbe and other microbes, or between a microbe and its multicellular host. Sequencing of 16S rRNA gene allowed for the identification of divergence between microbiota species, and thus detailed phylogenetic mapping of the gut microbiome (Zaneveld, Lozupone, Gordon, & Knight, 2010). More recently, massively parallel shotgun sequencing techniques have allowed for sequencing of broad regions of the microbiota genome, not just the 16S rRNA gene component. This allows not just for greater specificity in strain identification, but for the mapping of particular genes of interest as well (Ranjan, Rani, Metwally, McGee, & Perkins, 2016). Patterns of variation within the microbiome are typically measured via alpha and beta diversity. Measurements of alpha diversity are within a single individual or sample and include species richness (how many species are present?), generally measured by the number of different operational taxonomic units (OTUs), as well as species evenness (i.e., how evenly represented are the different species?), using measures including the Shannon index and Faith’s phylogenetic diversity. Beta diversity, on the other hand, compares individuals or samples to each other and measures how different they are from one another. Quantitative measures of beta diversity include Bray-Curtis dissimilarity and weighted UniFrac, while qualitative measures include the Jaccard distance and unweighted UniFrac (Knight et al., 2018).
Modern sequencing techniques have identified over 1000 unique bacterial species making up the human microbiome, mostly dominated by the two phyla Bacteriodetes and Firmicutes (Lloyd-Price, Abu-Ali, & Huttenhower, 2016). There is incredible variation in microbiome makeup not just between individuals, but even between body habitats on the same person (Ding & Schloss, 2014; Huttenhower et al., 2012). This deeper understanding of microbiome variation between individuals, as well as within a single person across time, has challenged the idea of what it means to have a healthy microbiome as compared to one that is unhealthy and out of balance, historically referred to as “dysbiosis” (Falony et al., 2016; Lloyd-Price et al., 2016) Efforts to identify distinct “enterotypes” that most microbiomes fit into has also proven to be more complex than initially assumed, prompting the idea that microbiome makeup may exist more on a continuum (Arumugam et al., 2011; Bäckhed et al., 2012). This has led to a view of dysbiosis grounded in individual health, not as a standard to compare all microbiomes against (Petersen & Round, 2014). Additionally, a growing interest in functional readouts of microbiome activity, such as through metabolomics, has introduced the concept that a healthy microbiome may also depend on the active metabolic pathways in which the microbiota take part (Ursell et al., 2014). This nuanced understanding of microbiome makeup and health has allowed for more nuanced connections with human health and behavior, including connections between the microbiome and depression (Fond et al., 2015).
Research techniques in microbiome research
Modern microbiome research has grown to include a vast array of techniques beyond sequencing human fecal samples (Claesson, Clooney, & O’Toole, 2017; Hamady & Knight, 2009). Rodent research has facilitated study of the relationship between the microbiome and depression at a depth not possible in humans. Rodents are well-characterized genomically, physiologically, and behaviorally, and have many physiological similarities to humans that facilitate microbiome study (Knight et al., 2018). However, as many research rodents are coprophagic, care must be taken in planning housing, environment, and experimental design (McCoy, Geuking, & Ronchi, 2017). While concerns have been raised regarding the degree of similarity of microbiome composition and metabolism between rodents and humans, they are still one of the best characterized and tractable models in the experimental study of the microbiome (Clavel, Lagkouvardos, Blaut, & Stecher, 2016; Hugenholtz & de Vos, 2018).
Experimental models
Understanding the connection between the microbiome and depression makes use of a variety of different experimental models, both human and rodent. Drawing from the use of rodents in the study of depression, microbiome research makes use of a variety of models that induce depression-like behaviors, both at long and short timescales (Bergner et al., 2010; Ménard, Hodes, & Russo, 2016). Rodent models of depression that have been used in microbiome research include: 1) olfactory bulbectomy, which induces behavioral changes with high face validity to human depression (Harkin, Kelly, & Leonard, 2003), alterations in hippocampal and amygdala function congruent with human changes in depression (Morales-Medina, Iannitti, Freeman, & Caldwell, 2017; Song & Leonard, 2005), and microbiome changes that correlate with depression-like behavior (A. J. Park et al., 2013); 2) social stress models (Toyoda, 2017), including repeated social defeat stress (Golden, Covington, Berton, & Russo, 2011) and subchronic social defeat (Goto, Kubota, & Toyoda, 2016), which have also been linked to disruption of the gut microbiome (Galley et al., 2015); 3) maternal separation models of early life adversity (Matthews & Robbins, 2003; Neumann et al., 2005; Siobhain M. O’Mahony et al., 2009); 4) repeated restraint stress models, which involve repeatedly confining rodents to a constricted space for several hours at a time (Bailey et al., 2010; Glavin, Paré, Sandbak, Bakke, & Murison, 1994); and finally, 5) diet-induced obesity, which is often used to both induce a depressed phenotype and understand the interplay of diet, the microbiome, and behavior (Agusti et al., 2018; Bridgewater et al., 2017; Bruce-Keller et al., 2015; Soto et al., 2018). In addition to the induction of depressive phenotypes, particular mouse strains are often chosen due to their susceptibility to anxious behavior, such as C57BL/6 strains, which show low anxiety-like behavior compared to other strains, but can show interindividual differences along a continuum of anxiety-like behaviors following stress exposure, the BALB/c strain, which displays an intermediate level of anxiety-like behavior, and the A/J strain, which is characterized by a high degree of anxiety-related behavior (Griebel, Belzung, Perrault, & Sanger, 2000; Van Gaalen & Steckler, 2000).
As rodents are incapable of responding to clinical interview-based methods of determining depression, depression-like behaviors are measured in several validated ways, including behavioral assessment in the tail suspension test (Can et al., 2011; Stem, Chermat, Thierry, & Simon, 1985) and the forced swim test (Slattery & Cryan, 2012). Related, and relevant to this review, anxiety-like defensive behavioral responses also have several well-validated measurements in rodents, including: exploration in the elevated plus-maze (Hogg, 1996), exploration in the open-field test (Prut & Belzung, 2003); behavior in the light/dark box (Hascoët & Bourin, 2009); and behavior in the water avoidance test, which was initially developed, in part, to study the relationship between gastrointestinal inflammation and anxiety (Bradesi et al., 2005).
In addition to rodent models of depression, rodents with carefully controlled gut environments have also been crucial to the study of the microbiome and depression, including both germ-free (GF) and specific pathogen-free (SPF) animals. Germ-free (GF) refers to an animal free of all microbes throughout its lifetime, including bacteria, viruses, fungi, protozoa, and parasites. While the concept of a GF animal dates back to Louis Pasteur in 1885, they did not become a sustainable reality until the late 1950s (Bhattarai & Kashyap, 2010). In modern research, GF animals are bred from other GF animals, via sterile hysterectomy, often from a long lineage of freedom from microbes (example white papers: Charles River Laboratories, 2018). To facilitate continued germ-free development, GF animals are bred and housed in fully isolated, HEPA-filtered cages with all food, water, and bedding fully sterilized. Animals and housing are extensively examined on a regular basis for microbial contaminants (including bacteria, fungi, viruses, parasites, and other pathogens) through DNA testing, serology techniques, and both aerobic and anaerobic culturing. While it is possible that unknown pathogens may escape this rigorous testing, GF animals are functionally treated as gnobiotic (from Greek gnostos: “known” and bios: “life”) in practice. On the other hand, SPF animals are only guaranteed to be free of specific pathogens detailed by a supplier. They are typically housed with less rigor, though generally in isolation (Al-Asmakh & Zadjali, 2015). Both GF and SPF animals provide unique tools to mechanistically understand the role of the microbiota across a variety of conditions. However, due to both the differences between humans and rodents compounded by the developmental differences introduced by lacking a functioning microbiome since before birth, comparisons to human clinical conditions must be drawn with caution (Al-Asmakh & Zadjali, 2015).
Both human and rodent studies often make use of a technique known as fecal-microbiota transplant (FMT), a method that transfers intestinal contents or stool from a donor to recipient. While the first mention of FMT dates back to fourth century China, modern usage can be traced back to the 1950s in the treatment of gastrointestinal disorders (Vindigni & Surawicz, 2017). It has since expanded in human applications (Fuentes & Vos, 2016; C. R. Kelly et al., 2015; Xu et al., 2015), and broadened the scope of GF animal research (Hansen, August, Hansen, Lundberg, & Toft, 2016). The initial gut-focused applications of FMT have since broadened to include the adoptive transfer of behaviors linked to the microbiome (S. M. Collins, Kassam, & Bercik, 2013), including depression-like behaviors (Soto et al., 2018). FMT from depressed patients to rats has even induced depression- and anxiety-like defensive behavioral responses (J. R. Kelly, Borre, et al., 2016; Zheng et al., 2016). While FMT often leads to dramatic metabolic and behavioral results, the fidelity of the transplant in terms of surviving microbial strains has been called into question, particularly when donor samples are frozen and stored for extended periods of time (Fairhurst & Travis, 2018; Gaci, Chaudhary, Tottey, Alric, & Brugère, 2017; Takahashi et al., 2019). Recent novel techniques using sequential tagging with D-amino acid-based metabolic probes (STAMP) show much promise in identifying surviving microbiota and their metabolic activity post-transplantation (W. Wang et al., 2019). Preliminary use of tagging techniques has revealed that some microbiota are more highly enriched in the recipient than in the donor (W. Wang et al., 2019). Adding to fidelity concerns is the high level of variability that comes with both donor and recipient age, donor and recipient medication use (especially antibiotics), and methodological variation in sample acquisition, such as different methods of colon preparation (Fairhurst & Travis, 2018). These concerns have raised the possibility of creating standardized donor material in research settings, or using a “super donor” of known composition and efficacy in clinical applications (Fairhurst & Travis, 2018; Moayyedi et al., 2015; W. Wang et al., 2019).
Human clinical studies often make use of both depressed and non-depressed samples, and have compared the microbial makeup of individuals experiencing symptoms of depression in cultures across the globe including Western cultures such as the United States (Kleiman et al., 2017) and Norway (Naseribafrouei et al., 2014), and non-Westem cultures, including Japan (Aizawa et al., 2016) and China (Chen et al., 2018; Y. Huang et al., 2018; Jiang et al., 2015; Lin et al., 2017). Additionally, depression research often explores comorbidity with gastrointestinal disorders to better understand the relationship between mental health and changes in microbiome composition that may be relevant to gastrointestinal health (Addolorato et al., 2008; Gradus et al., 2010). Within many human and rodent studies, manipulation of diet, ingestion of antimicrobials, or ingestion of beneficial bacteria (probiotics) are all used to understand the interrelationships between treatment, human behavior, and depression (Bruce-Keller, Salbaum,& Berthoud, 2018).
Meet the biotics: Antibiotics, probiotics, prebiotics, psychobiotics, synbiotics, postbiotics…
A cornerstone of microbiome research is direct manipulation of the gut microbiota through compounds affecting growth and activity. Chief among these biotics are antibiotics. From the initial discovery of arsphenamine by Ehrlich and Sata in 1909, to perhaps the better known discovery of penicillin by Fleming in 1928, antibiotics have transformed modern human history, due to their unique disruption of bacterial propagation, curing countless conditions that were historically intractable (Zaffiri, Gardner, & Toledo-Pereyra, 2012). In microbiome research, the microbiota-depleting effects of antibiotics are used to study the consequences of decreased microbial diversity on behavior (Ferrer, Mendez-Garcia, Rojo, Barbas, & Moya, 2016). Recent research is beginning to identify connections between frequent antibiotic exposure, particularly during development, and many serious health conditions, including autoimmunity and psychiatric illness (Blaser, 2016; Lurie, Yang, Haynes, Mamtani, & Boursi, 2015). However, the use of antibiotics in research has the potential to create several confounds. Antibiotics generally affect an organism and its microbiome (or transplanted microbiome) in three ways: depletion of resident microbiota, subsequent enrichment of antibiotic-resistant microbiota, and effects on relevant host tissues (Morgun et al., 2015). Antibiotic effects on host tissues is particularly relevant when studying the central nervous system and behavior, as some antibiotics may themselves be neuroactive or neurotoxic (Champagne-Jorgensen, Kunze, Forsythe, Bienenstock, & McVey Neufeld, 2019). This can introduce a rarely controlled for confound in many neural or behavioral studies using antibiotics to alter microbiome composition.
The ingestion of fermented foods to improve health has been present in human history for millennia, though the label “probiotic” for the beneficial microbes found in those fermented foods is more recent (Bested et al., 2013b). Probiotics have been extensively studied in nonpsychiatric populations, and have been connected to improved gastrointestinal health, decreased inflammation, and even transient improvements in cognitive abilities (Khalesi et al., 2018; Sanders et al., 2013). However, the connection between mental health, dysbiosis, and probiotics is a fairly recent development (Bested, Logan, & Selhub, 2013c). And while many studies of probiotics and depression show great promise, even in national samples (Cepeda, Katz, & Blacketer, 2017), there are still mixed messages when it comes to the efficacy of probiotics in treating depression, as is discussed in greater detail later in this review (Nadeem, Rahman, Ad-Dab’bagh, & Akhtar, 2018).
Prebiotics are nutrients that can be metabolized by gut microbiota, including complex carbohydrates and plant polysaccharides, for which humans lack the enzymes to break down (Holscher, 2017). Prebiotics affect a variety of metabolic pathways, often through their metabolic byproduct, short-chain fatty acids (SCFA; Louis, Flint, & Michel, 2016). Recent studies have connected prebiotics to alterations in neurobiology affecting behavior (Kao, Harty, & Burnet, 2016), as well as specific links to the reduction of depression- and anxiety-like defensive behavioral responses in rodents (Burokas et al., 2017).
While antibiotics, probiotics, and prebiotics have become rather canonical in the microbiome literature, newer terms appear with increasing frequency. Chief among these is the term psychobiotics, referencing probiotics (and more increasingly prebiotics as well) that have significant effects on the brain and behavior, particularly in a mental health context (Dinan, Stanton, & Cryan, 2013). Much research has focused on mechanistic explanations for these effects (Sarkar et al., 2016), though the field remains in its infancy and results from human trials are often mixed (B. Liu et al., 2018; Romijn & Rucklidge, 2015). Other terms, including synbiotics (the effective combination of a probiotic and a prebiotic) and postbiotics (non-viable bacterial products or metabolic byproducts from probiotic microorganisms that have biologic activity in the host) are also becoming increasingly common in the microbiome literature (Frei, Akdis, & O’mahony, 2015; Markowiak & Ślizewska, 2017; Mörkl et al., 2018; Patel & Denning, 2013; Tsilingiri & Rescigno, 2013). Time will tell what terminology will eventually prevail, but for the remainder of the article, the terms antibiotic, probiotic, and prebiotic will primarily be used for clarity.
The microbiome-gut-brain axis and depression: a bidirectional highway
Modern approaches to understanding the relationship between the microbiome and mental health typically consider brain-gut communication as a bidirectional information highway referred to as a variant of the “microbiome-gut-brain axis” (several examples: Cryan & O’Mahony, 2011; Dinan & Cryan, 2017a; Kelly, Clarke, Cryan, & Dinan, 2016; Petra et al., 2015). Emphasis is placed on the bidirectional nature of communication. In one direction, the central nervous system sends signals to the gut environment, which modulate microbiota composition and function. In the other direction, microbiota either interface with components of the peripheral nervous system that directly relay signals to the central nervous system, such as the vagus nerve innervating the brainstem or afferent fibers traveling in sympathetic nerve bundles and innervating the spinal cord, or do so indirectly, such as via moderation by the enteric nervous system (the mesh-like network of neurons governing gastrointestinal functioning; Furness, 2006). Evidence suggests that the microbiome can also signal to the central nervous system by way of neuroactive metabolites in the blood stream (Martin, Osadchiy, Kalani, & Mayer, 2018). However, when considering the psychiatric implications of the microbiome, neural components of the brain-gut axis are not the only bidirectional pathways involved. The mucosal immune system in particular (as well as both peripheral and central immune components) is in constant communication with gut microbiota, in both an inflammatory and immunoregulatory sense (Powell, Walker, & Talley, 2017). Finally, the endocrine system, too, communicates bidirectionally with the gut microbiota, primarily through the HPA axis (Farzi, Frohlich, & Holzer, 2018) but also through sex hormones, like androgens and estrogens (Vemuri et al., 2018), and other hormonal systems (Cussotto, Sandhu, Dinan, & Cryan, 2018). In fact, these four systems (neural, immune, endocrine, and microbiome systems) are all highly interconnected in an intricate dance affecting not only depression, but behavior more broadly (see Fig. 1). Research is only beginning to scratch the surface of these interrelationships. The following review explores each of the bidirectional pathways listed above, and the current state of the literature in both rodent and human microbiome research.
Depression, the immune system, and the gut microbiota
Gut-associated lymphoid tissue (GALT) is the largest immune organ in the human body, producing 70-80% of the body’s immune cells (Rudzki & Szulc, 2018). Peyer’s patches, specialized lymphoid tissue in the intestines, act as both a first line of defense against gastrointestinal pathogens, and a point of interface for immunoregulatory commensal microbes. Through repeated contact, the immune system and microbiota generate a sort of inflammatory stalemate that, through millions of years of coevolution, benefits both parties (Murphy & Weaver, 2017). When this system is thrown out of balance, either through dysbiosis or other perturbations of the immune system, the resulting activation of the inflammatory system can contribute to depressive symptoms and MDD (Rudzki & Szulc, 2018).
Signaling from the immune system to the microbiota
Applications to depression: Rodent studies
Exaggerated inflammatory activity in rodent models of depression has been identified for over 20 years (Song & Wang, 2011); however, studying immune to microbiome signals specifically in the context of depression can be challenging, as it generally requires specific manipulation of the body’s immune system. There is often less incentive to study these mechanisms, as interventions targeting communication from the microbiota to the immune system is often lower hanging fruit when it comes to developing novel depression treatments, or a mechanistic understanding of depression in a microbiome context. However, there is a small, but growing, literature that explores these pathways in rodents. In a 10-day social defeat stress paradigm in mice, socially stressed animals developed depression-like symptoms, as measured by the tail suspension and forced swim tests. These behavioral responses were associated with increases at the genus level of fecal Oscillospira and a decreased fecal Firmicutes Bacteroidetes ratio (J. C. Zhang et al., 2017). Intravenous treatment with an anti-mouse IL-6 receptor antibody (MR16-1) normalized depression-like behavior in the tail suspension test, significantly decreased Oscillospira levels towards pre-stressor levels and attenuated the stress-induced decrease in the fecal Firmicutes/Bacteroidetes ratio.
Recent work has targeted the effects of the inflammasome in studying the relationship between immune signaling and the microbiota (Inserra, Rogers, Licinio, & Wong, 2018). The inflammasome is an intracellular multi-protein complex that assembles in response to a variety of pathogen-associated or danger-associated molecular patterns (PAMPs and DAMPs). One of its major end products is the activation of caspase-1, which facilitates cleavage of the pro- forms of IL-1β and IL-18, allowing for the release of functional, proinflammatory cytokines from activated cells (Malik & Kanneganti, 2017). In an elegant manipulation of inflammasome end products, caspase-1 activity was blocked, either through genetic deletion of the caspase-1 gene or pharmacological inhibition with minocycline (Wong et al., 2016). Using a chronic restraint stress model, mice with either genetic deletion of the caspase-1 gene or pharmacological inhibition of caspase-1 displayed reduced depression- and anxiety-like behaviors as measured by a battery of tests, including the forced swim test and elevated plus-maze. Additionally, mice in both manipulations exhibited altered fecal microbial relative abundance including an increase in the family Lachnospiracea, and general elevations in the genera Blautia spp. and Akkermansia spp., the latter of which has been associated with decreased inflammation (Anhê et al., 2015), and a rebalancing of the gut microbiota (T. Yang et al., 2015).
Applications to depression: Human studies
There is a paucity of studies exploring immune pathways that affect the gut microbiome in the context of depression. However, one study of individuals with Crohn’s disease observed altered gut microbial diversity in individuals who relapsed following discontinuation of infliximab treatment (Rajca et al., 2014). Infliximab is a monoclonal antibody against TNF, which attenuates the inflammatory response and is commonly prescribed in Crohn’s disease (Colombel et al., 2010). The study found decreased relative abundance of bacteria belonging to the Firmicutes phylum when compared to nonrelapsers. While the study did not measure depression, decreased Firmicutes levels in the gut have been associated with depression (Y. Huang et al., 2018), and 25.8% of individuals with inflammatory bowel disease (IBD) are estimated to have suffered from depression in the previous year (Byrne et al., 2017). This aligns with recent evidence connecting microbiome composition to quality of life (Valles-Colomer et al., 2019a). This is quite a leap from the 4.4% and 6.7% 12-month depression rates globally and in the United States, respectively (World Health Organization, 2017). Future studies of a similar nature are needed to resolve the role of host immune signaling on the gut microbiome in humans.
Signaling from the microbiota to the immune system
Applications to depression: Rodent studies
Understanding the signals being sent from the microbiota to the immune system is a rapidly emerging field that is heavily dependent on rodent models (Takiishi, Fenero, & Câmara, 2017). Studies are now beginning to integrate these findings into the context of depression. In an exploration of the relationship between a stressor paradigm associated with depression behavior and immune biomarker expression, mice were exposed to a seven-day, nightly restraint stressor paradigm (Bailey et al., 2010). Stressed mice had altered cecal microbial community structure, including a reduction in relative abundance of the family Porphyromonadaceae, and increased cecal colonization by Citrobacter rodentium, a proinflammatory bacterial species associated with colitis in mice (J. W. Collins et al., 2014). Additionally, stressed mice expressed increased Tnf mRNA in colonic tissue as compared to unstressed control mice. In a study designed to attempt to treat stress-induced gut dysbiosis and inflammation using antibiotics, rats were assigned to either a 21-day chronic mild stressor group (CMS), a chronic mild stressor group with daily antibiotic treatment in drinking water (CMS+ATB), or an unstressed control group (Martín-Hemández et al., 2016). When compared to controls, the CMS condition induced depression-like behavioral responses, as measured by the forced swim test, as well as increased gut permeability and bacterial translocation across the gut epithelium. Depression-like behavior was reversed, and bacterial translocation blunted by antibiotic treatment. CMS also had effects on markers of neuroinflammation in the PFC. Specifically, CMS increased protein expression of phosphorylated (activated) p38 mitogen-activated protein kinase (MAPK), and decreased mRNA expression of phosphoinositide 3-kinase (Pik3cg) and of protein kinase B (Akt), both activators of nuclear factor (erythroid-derived 2)-like 2 (Nrf2). Nrf2 mRNA levels were also reduced, as were levels of glutathione peroxidase 1 mRNA (Gpx1), the expression of which is transcriptionally regulated by Nrf2. Functionally, these proteins represent two paths of the immune system: p38 MAPK is proinflammatory, phosphorylated in response to the binding of microbe-associated molecular patterns (MAMPs) to toll-like receptor 4 (TLR4); MAMP binding acts as an initiating event to upregulate proinflammatory cellular machinery, partially through the actions of p38 MAPK (Hotamisligil & Davis, 2016). The transcription factor Nrf2 and its associated proteins, on the other hand, play a role in the cellular antioxidant and anti-inflammatory response, in part through the actions of GPx1, which enzymatically reduces hydrogen peroxide to limit cellular damage (Ahmed, Luo, Namani, Wang, & Tang, 2017; Lubos, Loscalzo, & Handy, 2011). Antibiotic treatment reversed many of these effects. In the CMS + ATB group, animals had decreased levels of phosphorylated p38 MAPK. This was potentially due to a significant increase in mRNA of two mitogen-activated protein kinase phosphatases, Mkp1 and Mkp3, both of which are able to dephosphorylate (deactivate) p38 MAPK (Yusen Liu, Shepherd, & Nelin, 2007). Antibiotic treatment also saw a partial recovery of Gpx1 mRNA levels.
Taken together, the results from this study would suggest that one route for the microbiota to signal to the immune system is through bacterial translocation from the gut into the body. Increased gut permeability in response to chronic stress led to increased bacterial translocation, as measured by elevated levels of plasma lipopolysaccharide (LPS; a cell wall component of Gram-negative bacteria, and potent TLR4 activator). The increase in circulating MAMPs then resulted in activation of an inflammatory response, a response that was curtailed by antibiotic treatment, which likely decreased both circulating translocated bacteria, as well as the gut microbiota populations from whence they came. This study focused on the repercussions of these signals on neuroinflammation by looking at specific intracellular immune signaling components that are highly relevant to neuroinflammatory disorders, including depression (Brites & Fernandes, 2015). In the brain, phosphorylation of p38 MAPK is associated with activation of macrophages and astrocytes, glial cells that play a large role in the innate immunity in the central nervous system. Their activation often results in release of proinflammatory cytokines such as IL-1β, which perpetuate neuroinflammation and can exacerbate anxious and depressive behaviors (Dunn, 2006; Dunn, Swiergiel, & De Beaurepaire, 2005; Ransohoff & Brown, 2012; Swiergiel & Dunn, 2007). Recent research has found that ketamine reduces depression-like behaviors in mice via a decrease in the inflammatory response to stress, partly through decreased levels of p38 MAPK and circulating IL-1β concentrations (Tan, Wang, Chen, Long, & Zou, 2017). However, this direct path from stress to proinflammatory microbiota translocation to LPS inducing neuroinflammation and possibly depression still faces at least one major barrier. MAMPs like LPS have difficulty crossing the BBB to initiate the TLR4 signaling that is crucial to this pathway. In fact, a radiolabeled LPS tracking experiment in mice found that only 0.025% of intravenously injected LPS crossed the BBB and entered the CNS (W. a Banks & Robinson, 2010). There are likely other factors at play here, and new ideas will emerge as the fields’s understanding of cross-BBB immune signaling improves.
In a similar approach to using antibiotics as a treatment for stress-induced dysbiosis, Bailey and colleagues demonstrate that antibiotics can reduce circulating inflammatory markers in this context (Bailey et al., 2011). Mice were either exposed to a two-hour long social disruption stressor paradigm for six consecutive days, or a home cage control condition. Mice were euthanized for analysis immediately following the final stressor session. When compared to control mice, stressor exposed mice had significantly altered community structure of their gut microbiota, with decreased abundance of the genus Bacteroides and increased abundance of the genus Clostridium. This was accompanied by increased circulating levels of IL-6 and monocyte chemoattractant protein 1 (MCP-1). In a follow-up experiment, animals were fed an antibiotic cocktail in the morning and evening by oral gavage, beginning three days before the stressor and continuing to the end of the experiment. Antibiotic treatment prevented the increases in IL-6 and MCP-1.
In a study comparing the effects of pretreatment with either antibiotics, probiotics, or ML-7 (a myosin light chain kinase inhibitor used to attenuate the endocrine response) in a rat restraint stress model, rats were either administered L. farciminis orally for two weeks, antibiotics through the drinking water for 12 days, or given intraperitoneal injections of ML-7 or vehicle at 12, 24, and 36 hours before the stressor (Ait-Belgnaoui et al., 2012). Mice then underwent 2 hours in a restraint stress, or sham restraint, paradigm before being euthanized for analysis at five time points from 15 to 120 minutes post-stressor. When compared to sham restraint, mice receiving the stressor had elevated hepatic portal blood levels of LPS (from the gastrointestinal draining vein) and elevated mRNA expression of IL-1β, IL-6, and TNF in the hypothalamus, though no changes in circulating cytokines. Pretreatment with L. farciminis, antibiotics, and ML-7 all prevented these increases.
Contrasting the previous study’s use of antibiotics as a treatment, Guida and colleagues used them as a tool to induce gut dysbiosis, and use probiotics as subsequent treatment (Guida et al., 2018). Mice were treated for 14 days with either plain drinking water or water containing a triple antibiotic mix. Following this, both groups were gavage-fed either L. casei DG or saline for seven days. Mice who received antibiotic treatment saw elevations of IL-1β in the duodenum, and TNF in the duodenum, jejunum, ileum, and colon as compared to controls. Neither was elevated in the circulation. Probiotic treatment reduced all measured intestinal cytokines except TNF in the jejunum.
Probiotics have also been used as sole treatment of depression-like behaviors and elevated markers of inflammation in a chronic mild stress (CMS) model of depression. Mice were either subjected to a collection of stressors for 28 continuous days, or housed typically without added stress (Li et al., 2018). A subset of each group also received a three-strain probiotic blend (L. helveticus R0052, L. plantarum R1012, and B. longum R0175) throughout the protocol. In the CMS without probiotic group, mice displayed elevated anxiety- and depression-like behavioral responses, as measured by the elevated plus-maze and forced swim test, and all stressed mice had fecal microbiome α- and β-diversity significantly different from the non-stressed groups, as well as depletion of the genus Lactobacillus to varying degrees. This was accompanied by changes in hippocampal cytokine protein expression. The authors identified that IFNɣ rose significantly in response to CMS but was reduced when CMS mice were supplemented with the probiotic treatment. TNF levels were generally lower in the CMS plus probiotic condition when compared to CMS only. Additionally, a recent study of F. prausnitzii ATCC 27766 probiotic treatment in a chronic unpredictable mild stress (CUMS) model in rats found positive changes in depression- and anxiety-like defensive behavioral responses in association with anti-inflammatory effects (Hao, Wang, Guo, & Liu, 2019). The probiotic F. prausnitzii ATCC 27766 was administered via oral gavage for four weeks over the course of a CUMS protocol, resulting in decreased depression-like behaviors, as measured by the forced swim test, and decreased anxiety-like behaviors, as measured by the open-field and elevated plus-maze tests. Additionally, probiotic treatment increased circulating levels of IL-10 and attenuated stressor-induced decreases in IL-6 and C-reactive protein (CRP), an acute-phase protein frequently used as a biomarker of inflammation.
Probiotic treatment has also altered levels of cytokines, both in circulation and in a whole blood culture response to stimulation, as well as the response pattern of circulating immune cells. In a study exploring the antidepressant properties of B. infantis 35624, rats treated with the probiotic, as compared to vehicle, had lower levels of circulating IL-6 and IFNɣ following exposure to the forced swim test (Desbonnet, Garrett, Clarke, Bienenstock, & Dinan, 2008). Additionally, the probiotic treatment resulted in reductions in IL-6 and IFNɣ in response to LPS stimulation of whole blood, and reductions in IL-6 and TNF in response to Concanavalin A (Con-A) stimulation. Probiotic treatment has also been combined with diet manipulation in the exploration of depression-like behavior (Abildgaard, Elfving, Hokland, Lund, & Wegener, 2017). In a study exploring the differences between a high-fat diet and control, five weeks into the diet, rats from each group were fed either an eight-strain probiotic or vehicle control for 10 more weeks (Abildgaard, Elfving, Hokland, Lund, et al., 2017). Independent of diet, probiotic treatment decreased depression-like behavior in the forced swim test. Peripheral blood mononuclear cells (PBMCs) were isolated following the experiment and stimulated with anti-CD3/28. Independent of diet and compared to vehicle control, probiotic treatment resulted in elevated absolute levels of IL-2, IL-4, and IFNɣ, and decreases in IL-6 and TNF in an analysis of each cytokine in percent of the total amount produced. This pattern suggests that specific probiotics may selectively promote an adaptive immune phenotype, while regulating the activity of the innate immune system. This shift was also demonstrated in a study using L. rhamnosus JB-1 administration in a chronic social defeat paradigm in mice (Bharwani, Mian, Surette, Bienenstock, & Forsythe, 2017). Probiotic treatment increased the population of spleen-derived regulatory T cells (Tregs) in mice experiencing a social stressor, while also preventing the stress-induced increase in spleen-derived dendritic cells. Interestingly, recent work has found that depleting CD4+CD25+ regulatory T cells can actually inhibit the anxiolytic properties of L. rhamnosus (Yunpeng Liu, Mian, McVey Neufield, & Forsythe, 2019).
Prebiotic treatment has also resulted in robust changes to the immune system in the context of depression models. A variety of depression- and anxiety-like behaviors were reduced in mice in response to treatment with both fructo- and galacto-oligosaccharides in the context of a chronic social stress paradigm (Burokas et al., 2017). Splenocytes isolated from prebiotic-treated stressed animals released less TNF in response to Con-A stimulation than did their untreated counterparts, while there was no difference in IL-1β or IL-10. In a paradigm inducing sickness behavior using endotoxin, mice fed soluble fiber expressed fewer sickness behaviors and recovered faster from endotoxin treatment (Sherry et al., 2010). This was accompanied by basal increases in IL-4 in the ileum and spleen. Con-A-stimulated splenocytes from the fiber-fed group showed increases in IL-4 and IL-5, and decreases in IL-2, IL-12, and IFNɣ, while LPS-stimulated macrophages from the same group showed decreased release of IL-1β, TNF, IFNɣ, IL-12, and nitrate, as well as decreased release of IL-1 receptor A, arginase 1, and Ym1 (a transient marker of inflammation expressed by murine macrophages; Chang et al., 2001; Raes et al., 2005). This balance of inflammatory markers also supports the hypothesis that certain probiotics facilitate the adaptive immune system while regulating the innate immune system.
This diverse collection of studies exploring microbiota signaling to the immune system in the context of rodent depression models paints a complex and varied picture of this interplay. On the one hand, in cases where microbes with a slant towards proinflammatory signaling escape the confines of the gut lumen, antibiotics appear to be effective preventatives in reducing the resulting inflammation and could potentially act as a successful behavioral intervention. However, antibiotics reduce microbiota populations that are immunoregulatory, along with ones that are proinflammatory, leading to another form of dysbiosis. Probiotics present an exciting new possibility as microbial supplements that may be able to enrich the gut microbiome with immunoregulatory microbes. These “old friends” (Rook, Raison, & Lowry, 2014) have the potential to rebalance immune signaling and regulate depressive behaviors in ways that enriches the gut microbiota, rather than depletes it.
Applications to depression: Human studies
Studies of signaling from the microbiota to the immune system in a human depression context are more rare than rodent studies; however, the literature has identified several notable connections. Chronically depressed individuals show elevated serum IgM and IgA antibodies against LPS, indicating increased bacterial translocation (Maes, Kubera, Leunis, & Berk, 2012). The IgA response to LPS also correlated with the severity of gastrointestinal symptoms. In individuals with a depressive or anxiety disorder, but without gastrointestinal distress, Stevens and colleagues found elevated plasma levels of LPS, as well as of zonulin, a modulator of tight junctions in both the BBB and the intestinal epithelium (Fasano, 2011), and fatty acid-binding protein 2, another biomarker of increased gut permeability (Stevens et al., 2018). This was also accompanied by an overrepresentation of LPS biosynthesis genes in the fecal microbiome, suggesting elevated levels of Gram-negative gut bacteria (Stevens et al., 2018). Couples with hostile marital interactions showed elevated circulating levels of LPS binding protein (LBP), and individuals with a history of mood disorders also had elevated LBP/sCD14 ratios, a marker of bacterial translocation and heightened inflammation (Kiecolt-Glaser et al., 2018; Laugerette et al., 2014, 2012). These effects were both associated with elevated circulating concentrations of CRP. Collectively, these studies associate depression with increased bacterial translocation and an elevated inflammatory response, suggesting increased proinflammatory signaling originating from the gut microbiota, resulting in decreased mood.
Probiotics have shown mixed effects on the immune system in a human depression context. A recent meta-analysis of 20 randomized controlled probiotic trials showed that probiotics were associated with a significant reduction in circulating CRP, but not on IL-10 or TNF (Mazidi, Rezaie, Ferns, & Vatanparast, 2017), though none of the included studies were of depressed samples, or even more broadly with a mood disorder diagnosis. However, a contemporary study not included in the meta-analysis confirms this finding in a depression context. In a double-blind RCT using probiotic capsules containing L. acidophilus, L. casei, and B. bifidum (no strains provided), individuals with MDD saw a reduction in circulating CRP levels in the probiotic condition, but not placebo (Akkasheh et al., 2016). Several studies have also found alterations in immune cell makeup in response to probiotic treatment. In a study of healthy volunteers, individuals consumed a probiotic yogurt containing L. gasseri SBT2055 and B. longum SBT2928 or placebo daily for 12 weeks (Nishihira et al., 2014). While there was no alteration in depression scores, anxiety was significantly reduced, both as measured by the General Health Questionnaire-28 (GHQ-28). Additionally, individuals who received the probiotic yogurt had elevated activity of circulating natural killer (NK) cells, as measured by Chromium-51 release assay. In a study of university students during exam time, consuming milk fermented with yogurt cultures plus L. casei DN-114001 twice a day resulted in increases in absolute numbers of blood lymphocytes, compared to increases in the control group (Marcos et al., 2004). There were also changes in CD56+ cells, a marker for natural killer cells and activated T cells, with decreases in the control group, with no changes in the probiotic group. Interestingly, a study administering heat-killed L. pentosus b240 to elderly adults daily for 20 weeks did not find any changes in mental health, but did identify lower incidence rates of the common cold when compared to placebo (Shinkai et al., 2013).
On the other hand, some studies find no immune changes in response to probiotic treatment. In a study of depressed individuals, evaluated by self-report, who were not currently on medication, treatment with a probiotic preparation containing L. helveticus R0052 and B. longum R0175 resulted in no change in mood symptoms and no changes in concentrations of CRP, IL-1β, IL-6, TNF, vitamin D, or BDNF between baseline and the end of the 8-week study (Romijn, Rucklidge, Kuijer, & Frampton, 2017). In a study of patients diagnosed with MDD and recruited from an outpatient clinic, current SSRI treatment was supplemented with L. plantarum 299v. Probiotic treatment did not affect depressive symptoms and did not change concentrations of circulating TNF, IL-6, or IL-1β, although there was an increase in cognitive functioning (Rudzki et al., 2019). More research is needed to determine whether probiotics play a role in altering immune function in the context of human depression, though the few existing studies would not suggest a strong role.
Depression, the endocrine system, and the gut microbiota
Bidirectional communication between gut microbiota and the endocrine system occurs at several interfaces. In the endocrine to microbiota direction, neurohormones (e.g., epinephrine) can induce a variety of bacterial responses, including increased growth or virulence, while sex hormones (e.g., estradiol) have been connected to decreasing bacterial virulence (Neuman, Debelius, Knight, & Koren, 2015), and glucocorticoids have been implicated in stress-induced proliferation of specific pathobionts, such as Helicobacter spp (Guo et al., 2009; Langgartner, Lowry, & Reber, 2019; Langgartner et al., 2017; Reber, Langgartner, et al., 2016; Reber, Siebler, et al., 2016). In the gastrointestinal tract, enteroendocrine cells line the lumen wall and respond to microbial metabolites, such as SCFAs, which, in turn, release hormones, neurohormones, and neurotransmitters that send signals throughout the body (Furness, 2016). These pathways, and perhaps others yet to be discovered, allow commensal microbes to alter hunger, metabolism, and even lower circulating glucocorticoids and reduce anxiety (Neuman et al., 2015). Exciting new research has recently been extending our understanding of this bidirectional path in the context of depression.
Signaling from the endocrine system to the microbiota
Applications to depression: Rodent studies
Direct measurements of signaling from the endocrine system to the microbiota are both difficult and rare, although a recent study has explored the effects of treatment with finasteride, a 5-alpha-reductase inhibitor, on depression-like behavior in rats, as well as one month of treatment withdrawal (Diviccaro et al., 2019). 5-alpha-reductase is an enzyme that converts steroid hormones testosterone and progesterone into DHT and DHP, respectively. Withdrawal from finasteride was associated with elevated depression-like behavioral responses, as measured by the forced swim test. Treatment with finasteride was associated with increases in the phylum Bacteroidetes and in the family Prevotellaceae, and withdrawal was associated with decreases in the family Ruminococcaceae and the genera Oscillospira and Lachnospira.
While many of the studies in this review make connections between stressful paradigms, changes in gut microbiota, and depression-like behaviors, it is generally not possible to determine, or even speculate, on the direction of communication that is occurring between the microbiome and the endocrine system. This is complicated by the fact that the endocrine, immune, and nervous systems are all highly interconnected in their regulation of complex behaviors. Elucidating the specific contributions of endocrine signaling to the gut microbiome in depression will rely on tightly controlled studies inhibiting key hormones, or supplementing with endocrine signaling molecules or their synthetic analogues. Studies using in vitro bacterial culture work to supplement in vivo behavioral experiments also hold much promise. Regardless of the methodology, understanding how the endocrine system directly affects the microbiota will provide a deeper knowledge of the hormone-microbiota feedback loops that contribute to MDD, and provide new targets for intervention.
Applications to depression: Human studies
It is challenging to disentangle the effects of stress from other causes of depression in human studies. Equally challenging are the experimental methods required to actually do so, and in many cases, they may even be ethically irresponsible. However, while the authors were not able to find a depression study that included an endocrine manipulation, a study of CRH administration in the context of acute psychological distress elegantly demonstrates endocrine signaling affecting the microbiome environment (Vanuytsel et al., 2014). In a two-stage crossover study design, participants completed several experimental conditions and a lactulose-mannitol urinary excretion test, which acted as a measure of small intestine permeability (Camilleri et al., 2009), with each condition. The first study had four conditions: 1) an unspecified negative control condition; 2) indomethacin treatment, an NSAID that is known to increase intestinal permeability as a positive control; 3) a public speech condition; and 4) a laboratory stressor involving anticipation of a painful shock. Both self-report anxiety levels, via the State Trait Anxiety Inventory (STAI), and salivary cortisol were measured. Subjects found both the public speech and laboratory task stressful according to the STAI, but salivary cortisol only peaked for the public speech. This matched the pattern of altered intestinal permeability, with increased permeability only occurring in the speech condition. These results suggest that the HPA axis may be involved in intestinal permeability, which informed the second study. The second study tested whether exogenous CRH could induce similar changes in intestinal permeability as the speech stressor. Subjects received an intravenous bolus injection of CRH, which resulted in similarly elevated intestinal permeability, but without an elevation in subjective anxiety scores. This direct manipulation of endocrine signaling identified a possible mechanism for how psychological stress can alter the microbiota environment; however, further mechanistic studies that exogenously manipulate stress hormones and use other novel methodologies are needed to continue to understand endocrine to microbiota signaling in humans.
Signaling from the microbiota to the endocrine system
Applications to depression: Rodent studies
While the modern history of research with germ-free animals goes back to the mid-1940s (Bhattarai & Kashyap, 2010), much of our modern understanding of microbiota-to-endocrine signaling can be traced back to the work of Dr. Nobuyuki Sudo and his study of HPA axis development in GF mice (Sudo et al., 2004). Sudo found that exposure to microbes early in development was necessary for the proper development of the HPA axis. Now, 15 years later, we are building on this foundation to understand the role those interactions play in depression. Much of our understanding of these interactions in rodents centers around corticosterone, the rodent analogue of cortisol and master endocrine regulator of the body. However, when it comes to the effects of microbiota-related interventions, there is a surprising amount of variability in the effects on circulating corticosterone. For example, in one study exploring the effects of open-field stress on circulating corticosterone in GF stress-sensitive rats (F344), corticosterone was elevated, as compared to SPF rats (Crumeyrolle-Arias et al., 2014). On the other hand, antibiotic-induced microbiota reduction has both prevented increases in corticosterone in a restraint stress paradigm that occur in untreated rats (Ait-Belgnaoui et al., 2012) or has led to no change in circulating corticosterone at all in response to the forced swim test, again as compared with untreated rats (Hoban, Moloney, et al., 2016). Probiotic treatment, using either L. farciminis or a combination of L. helveticus R0052 and B. longum R0175, has been shown to decrease circulating corticosterone levels that have been elevated in response to restraint stress and water avoidance stress, respectively (Ait-Belgnaoui et al., 2014, 2012). In a prebiotic study, treatment with both fructo- and galacto-oligosaccharides reduced the increase in circulating corticosterone induced by the forced swim test (Burokas et al., 2017).
Microbial signals can also affect the balance of endocrine signaling in the brain. In a study measuring hypothalamic differences between GF and SPF Kunning mice exposed to chronic restraint stress four hours per day for 21 days, GF mice displayed elevated levels of CRH, ACTH, and corticosterone, as well as elevated mRNA for corticotropin-releasing factor receptor 1, Crhr1 in the hypothalamus (Huo et al., 2017). This echoes the study of Sudo and colleagues mentioned at the beginning of this section, which found exaggerated HPA axis reactions to acute restraint stress in GF mice, but extends this to signaling in the brain (Sudo et al., 2004). GF stress-sensitive F344 mice have elevated hypothalamic levels of Crh mRNA compared to SPF F344 rats (Crumeyrolle-Arias et al., 2014). Water avoidance stress significantly decreased the expression of hypothalamic brain-derived neurotrophic factor (BDNF), a marker associated with neuroplasticity and linked to antidepressant treatment effects (Björkholm & Monteggia, 2016), which was reversed and increased in response to treatment with a dual L. helveticus R0052 and B. longum R0175 probiotic treatment (Ait-Belgnaoui et al., 2014). Restraint stress increased Crh mRNA expression and CRH-positive cells in the paraventricular nucleus of the hypothalamus, which was returned to control levels via L. farciminis treatment (Ait-Belgnaoui et al., 2012).
Elsewhere in the brain, GF stress-sensitive F344 rats have reduced glucocorticoid receptor (Nr3c1) mRNA in the hippocampus compared to SPF F344 rats (Crumeyrolle-Arias et al., 2014). Reduced Nr3c1 and Crhr1 mRNA were found in both the hippocampus and the amygdala of antibiotic-treated adult rats in the context of increased depression-like behavior (Hoban, Moloney, et al., 2016). Probiotic treatment, on the other hand, reduced HPA axis-related transcripts in the hippocampus, including Crhr1, Crhr2, and Mr (Abildgaard, Elfving, Hokland, Wegener, & Lund, 2017). Overall, a pattern emerges of stress increasing hypothalamic reactivity, while decreasing HPA axis responsiveness in the hippocampus and amygdala. Both of these patterns can be reversed through probiotic treatment.
Applications to depression: Human studies
In a study using an explicitly depressed sample, correlations between mood and stress were found with fecal levels of the isovaleric acid, an SCFA product of fiber fermentation by gut microbiota. Isovaleric acid levels were positively correlated both with depression and with an average of morning and mid-day measurements of salivary cortisol (Szczesniak, Hestad, Hanssen, & Rudi, 2016). While directionality is not known in the previous experiment, it would suggest that elevated SCFA-producing bacteria is associated with elevated cortisol. Probiotic studies have also shone a light on signals between the microbiota and endocrine system in humans. In a study exploring the relationships between probiotic treatment, mood, and stress in university students during exam time, a significant relationship was observed between probiotic consumption and morning cortisol. Specifically, university students who drank a probiotic milk beverage containing L. casei DN-11400 twice a day showed no significant changes in morning cortisol throughout the six weeks of the study. Control students, on the other hand, showed a significant increase in morning cortisol during exam time. Of note, however, there was no significant difference in anxiety between the probiotic and control groups over the course of the study (Marcos et al., 2004). In a study of mood and endocrine activity in healthy volunteers, a probiotic yogurt containing L. gasseri SBT2055 and B. longum SBT2928 administered daily for 12 weeks resulted in a decrease in circulating ACTH levels. While this was not accompanied by a decrease in depressive symptoms, anxiety was significantly reduced, as measured by the GHQ-28 (Nishihira et al., 2014). In a probiotic trial using a dual formulation of L. helveticus R0052 and B. longum R0175, treatment both decreased psychological distress, including depression symptoms, as measured by the 90-item version of the Hopkins Symptom Checklist (HSCL-90), the Hospital Anxiety and Depression Scale (HADS), and the Coping Checklist (CCL). Probiotic treatment also reduced 24-hour urinary free cortisol (Messaoudi, Lalonde, et al., 2011). Taken together, these results suggest that isovaleric acid-producing gut microbiota may play a role in increasing cortisol and depressive symptoms, while probiotic treatment may play a role in reducing cortisol levels, particularly in response to stress. This highlights the many roles gut microbiota can play along the spectrum from host health promotion to host health antagonism.
Depression, the central nervous system, and the gut microbiota
The neural component of the microbiome-gut-brain axis is perhaps the best understood bidirectional pathway in the depression axis. The brain controls gut motility and gastric acid secretion, both of which contribute to regulation of the microbiota environment (Furness, 2016). The gut microbiota regulate the levels of the neurotransmitter serotonin by engaging in tryptophan metabolism (S. M. O’Mahony, Clarke, Borre, Dinan, & Cryan, 2015), and are even able to synthesize many neuroactive molecules de novo, such as γ-aminobutyric acid (GABA), histamine, norepinephrine, and dopamine (Asano et al., 2012; Barrett, Ross, O’Toole, Fitzgerald, & Stanton, 2012; Özogul, 2011; Shishov, Kirovskaya, Kudrin, & Oleskin, 2009; Valles-Colomer et al., 2019a). While a direct role of microbiota-produced neuroactive molecules on CNS activity is still unclear, it is possible that they act directly on neurons of the enteric nervous system, on visceral afferent fibers, or even as immunomodulators (Basu & Dasgupta, 2000; Brierley & Costa, 2016; Furness, 2006; Herr, Bode, & Duerschmied, 2017; Jin, Mendu, & Birnir, 2013). Stimulation of afferent vagal fibers via microbial action, such as through the synthesis and metabolism of SCFA, contributes to mood and behavior, with many implications for psychiatric disorders (Javier A. Bravo et al., 2012; Breit, Kupferberg, Rogler, & Hasler, 2018; R. T. Liu, 2017). In fact, research has connected microbiome-central nervous system communication to schizophrenia, bipolar disorder, obsessive compulsive disorder, PTSD, anxiety, and, of course, depression (Chrobak, Nowakowski, & Dudek, 2016; Leclercq, Forsythe, & Bienenstock, 2016; Tuma, Grosman Kaplan, Anglin, & Van Ameringen, 2016; Winter, Hart, Charlesworth, & Sharpley, 2018). The following section highlights previous research with recent advances along this bidirectional line of communication in depression.
Efferent neural signaling from the CNS to the microbiota
Applications to depression: Rodent studies
CNS efferent signaling pathways have the potential to affect the gut microbiome mainly through descending fibers of the autonomic nervous system (ANS). The ANS is made up of two major pathways, the sympathetic nervous system (SNS) and the parasympathetic nervous system (PNS), which is largely made up of vagal fibers. These efferent signals synapse on the enteric nervous system (ENS) to guide gut motility, direct gut fluid balance, and control gastric acid secretion (Furness, 2016). A challenge in studying these efferent pathways arises from the difficulty in targeted ablation of descending fibers while avoiding ascending fibers (or vice versa). In fact, this is a major criticism of total vagotomy as a mechanism in identifying the direction of communication between the microbiome and the CNS (Bruce-Keller et al., 2018). While the authors were unable to find any selective vagal (or relevant spinal) deafferentation studies, several studies have explored the endpoints of these efferents.
A study using bilateral olfactory bulbectomy to induce depression-like behavior measured gut motility and microbiome diversity. Gut motility was measured by fecal output in response to a water avoidance stressor and via an in vitro assessment of colonic muscle contraction, which was removed from mice euthanized immediately following water avoidance stress. Olfactory bulbectomized mice showed both increased fecal output and elevated colonic contractility in response to water avoidance stress. This was accompanied by alterations in microbial diversity as compared to sham mice, though the authors did not elaborate on specific taxa (A. J. Park et al., 2013).
In a study of stress-induced gastric damage, a rat strain hypersensitive to cholinergic stimulation frequently used as a depression model (Flinders Sensitive Line, FSL), showed basal gastric acid output twice as high as their control counterparts (Flinders Resistant Line, FRL). Vagotomy drastically reduced acid secretion in both FSL and FRL rats, and it reversed the pattern of release, with FSL rats excreting less acid than FRL rats (Padol, Wang, & Hunt, 2012). It has been suggested that gastric acid acts as an ecological filter and plays a role in shaping the gut microbiome (Beasley, Koltz, Lambert, Fierer, & Dunn, 2015). In summary, stress paradigms in a mouse depression context are linked to increased gut motility and increased gastric acid output in a rat model of depression is drastically reduced through vagotomy, both indicating a likely role for efferent signals from the CNS. Future work making use of novel vagal deafferentation techniques (Diepenbroek et al., 2017) or selective ablation using emerging genetic methods will allow for more targeted study of efferent CNS pathways to the gut.
Applications to depression: Human studies
While techniques using experimental vagotomy or colonic muscle extraction are typically not possible in humans, selective vagotomy was a historical treatment of peptic ulcer disease, though rarely used today since the introduction of proton pump inhibitors (Blalock, 1981; Lagoo, Pappas, & Perez, 2014). Reported side effects often included vomiting, diarrhea, and dumping syndrome, likely indications of altered communication between the CNS and ENS, though mood symptoms are not typically reported (Frederiksen, Johansen, & Christiansen, 1980; McMahon, Johnston, Hill, & Goligher, 1978; Towfigh, Chandler, Hines, & McFadden, 2002). However, vagal stimulation, while likely modeling afferent pathways rather than efferent, provides a modern example of vagal intervention with mood-relevant outcomes. Developed in the 1990s to treat refractory epilepsy, vagal stimulation, both invasive and noninvasive, has also proven to have a positive effect on mood (Carreno & Frazer, 2017; Giordano, Zicca, Barba, Guerrini, & Genitori, 2017). It has been an FDA-approved treatment for treatment-resistant depression in the United States since 2005, with ongoing investigations into its application to other psychiatric disorders as well (Cimpianu, Strube, Falkai, Palm, & Hasan, 2017; Yuan & Silberstein, 2016).
Medical procedures aside, there have been several studies linking behaviors associated with efferent CNS activity and the microbiome. In a study done over 20 years ago in 21 psychiatric patients with generalized anxiety disorder (GAD) or MDD, patients with GAD had the shortest whole gut transit time, as measured by abdominal radiography, when compared to individuals with MDD or controls (Gorard, Gomborone, Libby, & Farthing, 1996). There was no significant difference in whole gut transit time between MDD patients and controls. However, whole gut transit time did correlate with both the Beck Depression Inventory score (BDI) and the Hospital Anxiety and Depression Scale (HADS). This suggests that anxious symptoms decrease gut transit time, whereas depressive symptoms may lead to an increase. The lack of difference in whole gut transit time between the MDD and control groups may reflect the high level of comorbidity between anxiety and depression (which has been estimated at between 40 to 70%; Wu & Fang, 2014). Increased gut transit time associated with anxiety and decreased gut transit time associated with depression may make measuring gut transit time challenging in comorbid individuals. Other studies have supported the connection between depression and increased gut transit time, particularly in older populations where colonic transit time correlates significantly with the severity of depressive symptoms (O’Mahony, O’Leary, & Quigley, 2002).
In another study of gut motility, 37 constipated women and 19 women without a history of gastrointestinal illness and unscreened for psychological morbidities, rectal mucosal blood flow was measured in connection with a mental health-related self-report, the General Health Questionnaire-28 (GHQ-28; Emmanuel, Mason, & Kamm, 2001). Rectal mucosal blood flow is a direct measure of extrinsic neural activity in the gut, which includes autonomic innervation (Emmanuel & Kamm, 1999), and the GHQ-28 measures psychosocial functioning in four domains: anxiety, depression, somatic symptoms, and social dysfunction. Overall, there was a negative correlation between mucosal blood flow and scores on the depression and anxiety subscales of the GHQ-28 in constipated women, indicating decreased extrinsic neuronal activity in women with elevated symptoms of depression and anxiety. Overall, these results would suggest that depression may be associated with decreased extrinsic neural activity leading to longer gut transit times.
Finally, an intriguing study connecting early childhood temperament with the community structure of the gut microbiome found links between personality traits and microbial diversity (Christian et al., 2015). Temperament was assessed through an online questionnaire filled out by the child’s mother. It was made up, primarily, of the Early Childhood Behavior Questionnaire, which contains three composite scales: Negative Affectivity, Surgency/Extraversion, and Effortful Control. Among both boys and girls, lower scores on Surgency/Extraversion, which is associated with elevated depressive symptoms, were associated with decreased phylogenetic diversity in the gut. While there is no way to know the direction along the CNS-microbiome continuum, it suggests that the interplay between the CNS and microbiome both starts early and may be related to an individual’s personality.
Afferent neural signaling from the microbiota to the CNS
Applications to depression: Rodent studies
Perhaps the largest available literature exploring afferent or efferent signals between the microbiome and any other system in the context of depression lies in rodent studies exploring afferent neural connections from the microbiota to the CNS. To consolidate, this section is arranged by common microbiome-related changes found in the literature, beginning with changes in the PFC, the amygdala, the hippocampus, and several other affected brain regions. This will be followed by a discussion of altered tryptophan metabolism and its relationship with depression and mood.
Prefrontal cortex.
Out of all mammals, the human prefrontal cortex is perhaps the most complex, and thus it would follow that its role in depression is equally complex (Koenigs & Grafman, 2009). While comparisons between humans and rodents in this particular region often come up lacking, homology between computational processes and cytoarchitecture are still a source of value, and an excellent starting point for the budding field of depression and the microbiome (Brown & Bowman, 2002). In terms of cytoarchitecture, GF mice have elevated expression of genes related to myelination and myelin plasticity, as well as hypermyelination of axons in the PFC (Hoban, Stilling, et al., 2016). This is notable, since depression has been linked to altered expression of myelin-related mRNA in prefrontal cortex white matter in MDD (Rajkowska et al., 2015). In another GF to SPF comparison study, using stress-sensitive F344 rats in this case, animals were subjected to an open-field test and then assessed for changes in neural dopamine metabolites (Crumeyrolle-Arias et al., 2014). Regardless of stress condition, when compared to SPF rats, GF rats showed strong reductions in concentrations of homovanillic acid (HVA) and dihydroxyphenylacetic acid (DOPAC), both dopamine metabolites, as well as dopamine itself. Dopamine pathways have been implicated in the pathophysiology of depression, with decreased plasma levels of HVA and DOPAC typically being associated with depressed individuals (Dunlop & Nemeroff, 2007; Mitani, Shirayama, Yamada, & Kawahara, 2006). In a study treating healthy mice with L. rhamnosus JB-1 chronically for 28 days, there was a reduction in depression-like behavioral responses, as measured by the forced swim test, as well as decreased mRNA expression for the GABAA receptor α2 subunit (GABAAα2) in the PFC, when compared to controls (J. A. Bravo et al., 2011). The GABAA receptor is the ionotropic, fast acting GABA receptor, as opposed to the slower acting, metabotropic GABAB receptor, and is a main pharmacological target of several types of anxiolytic drugs, such as benzodiazepines (GABAA: Nutt, 2006; GABAB: Tyacke, Lingford-Hughes, Reed, & Nutt, 2010; GABA receptors and depression: Kalueff & Nutt, 2007). Finally, in a study treating healthy rats chronically with B. infantis 35624 for 14 days, no behavioral changes were observed in the forced swim test; however, there was a reduction in the serotonin metabolite 5-HIAA in the frontal cortex (Desbonnet et al., 2008). Together, these studies suggest a pathway between gut dysbiosis and MDD through altered expression of myelination proteins, as well as decreased GABAergic and serotonin signaling in the PFC in response to probiotic treatment.
Amygdala.
In humans, studies have identified several amygdala changes that are associated with depression, including altered connectivity, both resting state and functional, as well changes in both gross and cellular anatomy, thus making it a relevant potential target of study in microbiome research (Cheng et al., 2018; Helm et al., 2018; Rubinow et al., 2016). In a study comparing the behavior of GF mice with SPF mice in the elevated plus-maze, GF mice exhibited decreased anxiety-like behaviors and expressed decreased levels of the N-methyl-D-aspartate (NMDA) receptor subunit NR2B mRNA (Grin2b) in the central amygdala (Neufeld, Kang, Bienenstock, & Foster, 2011). NMDA receptors are ionotropic receptors activated by the excitatory neurotransmitter glutamate, and their antagonism has been associated with reductions in anxiety-like behavior in rats (González-Castañeda et al., 2012; Kotlinska & Liljequist, 1998). The NR2 subunits play a role in the receptor’s functional diversity and sensitivity (González-Castañeda et al., 2012) with NR2B subunit specifically being implemented in amygdala synaptic plasticity, as well as learning and memory (Cull-Candy, Brickley, & Farrant, 2001). Pharmaceutical antagonists targeting the NR2B subunit have been an actively explored therapeutic target for humans (McCauley, 2005). In a study of SPF mice treated with oral antimicrobials for seven days, treated mice demonstrated decreased anxiety-like behaviors in the step-down and light/dark preference tests accompanied by changes in microbiota composition and a decrease in BDNF protein levels in the amygdala, as compared to untreated controls (Bercik, Denou, et al., 2011). When the cecal contents of the antibiotic-treated SPF mice were transferred via gavage to GF mice, however, BDNF protein levels in the amygdala were unaltered as compared to GF mice who received the cecal contents of control mice. In contrast, in a study of rats chronically treated with antibiotics for 13 weeks, treated rats sustained a depletion of gut microbiota, increased depression-like behavior as measured by the forced swim test, and an increase in amygdala Bdnf mRNA levels (Hoban, Moloney, et al., 2016). This discrepancy could be due to one of several methodological differences. The study by Bercik, Denou, and colleagues treated mice with a blend of three antimicrobials (two antibiotics, neomycin and bacitracin, and an antifungal, pimaricin) for one week, whereas the study by Hoban and colleagues treated rats with a blend of five antibiotics (ampicillin, vancomycin, ciprofloxacin HCL, imipenem, and metronidazole) for 13 weeks. The time difference may be key, as transient and chronic stress in rodent models of depression have been associated with different expression profiles of BDNF and Bdnf mRNA in the amygdala (Yu & Chen, 2011). Additionally, the relationship between BDNF and depression in humans is also complicated, with the BDNF Val66Met gene polymorphism generally being linked with an increased susceptibility to depression, but the details are highly dependent on environmental and developmental factors (Wheeler et al., 2018). Further studies that are more equivalent in methodology are necessary to further elucidate the effects of microbiota depletion on BDNF in the amygdala.
Finally, probiotic treatment has also been associated with amygdala changes. The aforementioned study by Bravo and colleagues (2011) found reductions in mRNA of GABAB receptor subunit 1b (GABAB1b) and GABAAα2 in both the central and basolateral amygdala as compared to controls (J. A. Bravo et al., 2011). Treatment of rats with B. infantis 35624 in the previously mentioned 2008 study by Desbonnet and colleagues led to decreased levels of DOPAC in the amygdaloid cortex compared to controls (Desbonnet et al., 2008). In summary, results from studying the microbiome’s effects on the amygdala in rodents indicate that reduction (or absence) of gut microbiota is associated with decreased NMDA receptor subunit NR2b and altered BDNF expression, and probiotic treatment is connected to both decreased GABAAα2 and GABAB1b receptor subunit expression and reduced dopamine metabolites. While some of these results align with the current understanding in the literature of neurobiological mechanisms of depression, others would benefit from further investigation.
Hippocampus.
In humans, one of the earliest and most cited hippocampal changes associated with MDD is decreased hippocampal volume (Sheline, Liston, & McEwen, 2019). However, many changes, from the neurobiological to the functional, are currently associated with the hippocampus in both human MDD and rodent models of depression (Helm et al., 2018; Sheline et al., 2019). Rodent microbiome research is extending these findings. The above study by Crumeyrolle-Arias and colleagues (2014) also found decreased concentrations of HVA in the hippocampus of GF rats (Crumeyrolle-Arias et al., 2014). In the previously mentioned study by Neufeld and colleagues (2011), GF mice show increased Bdnf mRNA and decreased serotonin 1A receptor (Htr1a) mRNA in the dentate gyrus of the hippocampus (Neufeld et al., 2011). Relatedly, in a comprehensive study by Guida and colleagues (2018) examining the effects of antimicrobial and subsequent probiotic treatments on mice, two weeks of antimicrobial treatment resulted in depression-like behaviors, as measured by the tail suspension test and forced swim test, accompanied by altered microbiota composition and diversity, decreased levels of BDNF and its receptor, tropomyosin receptor kinase B (TrkB), and increased phosphorylation (indicating activation) of transient receptor potential cation channel subfamily V member 1 (TrpV1), a ligand-gated cation channel involved in hippocampal synaptic plasticity that may also play a role in mood disorders (Gibson, Edwards, Page, Van Hook, & Kauer, 2008; Micale, Di Marzo, Sulcova, Wotjak, & Drago, 2013). Following a week-long treatment with L. casei DG, hippocampal BDNF protein expression was normalized, and the elevated phosphorylation of TrpV1 was reversed, though TrkB expression was not affected. In addition to these molecular changes, researchers also conducted in vivo extracellular recordings in the cornus ammonis region 3 (CA3) region of the dorsal hippocampus. Exposure to antimicrobials induced an overall decrease in neuronal firing rates in CA3, as well as a reduction of bursts and the percentage of spikes per burst, activity that is possibly associated with altered memory encoding (Rebola, Carta, & Mulle, 2017), all of which was recovered in response to probiotic treatment (Guida et al., 2018). In a study of the neuroprotective effects of L. helveticus R0052 and B. longum R0175 in mice, a one-hour water avoidance stress protocol over four consecutive days resulted in elevated c-Fos levels (an indirect marker of neuronal activity; Dragunow & Faull, 1989), in the cornus ammonis region 1 (CA1) and CA3 of the hippocampus, and decreased c-Fos levels in the dentate gyrus, as compared to sham stressor (Ait-Belgnaoui et al., 2014). This elevation was attenuated following pretreatment with the above probiotic formulation for two weeks prior to the stressor. Probiotic treatment also increased both c-Fos levels and neurogenesis, as measured by doublecortin staining (Couillard-Despres et al., 2005; Saper & Stornetta, 2014), in the dentate gyrus of the hippocampus. Our current understanding of the functions of these hippocampal regions suggest that while manifold, they play roles in learning, memory, and identifying context (see Knierim, 2015, for a succinct review). In another study examining neural growth, increasing adult hippocampal neurogenesis has been shown to be sufficient in decreasing both anxiety- and depression-like behaviors in mice (Hill, Sahay, & Hen, 2015); however, strong connections have yet to be made in humans (Schoenfeld & Cameron, 2015). Probiotic treatment with L. rhamnosus JB-1, as discussed above in a study by Bravo and colleagues (2011), was associated with decreased levels of GABAB1b mRNA expression in the dentate gyrus, CA1, and CA3, as well as increased levels of GABAAα2 mRNA expression in the dentate gyrus (J. A. Bravo et al., 2011). Finally, a three-week prebiotic treatment, consisting of fructo-oligosaccharides and galacto-oligosaccharides, resulted in changes in hippocampal gene expression consisting of elevated mRNA expression of Bdnf elevation of both GABAB1 and GABAB2 receptor mRNA, and decreased NMDA receptor subunit NR2A mRNA (Grin2a) (Burokas et al., 2017).
In summary, absence of gut microbiota in rodents is associated with overall decreased concentrations of the dopamine metabolite HVA, elevated Bdnf mRNA and decreased 5-HT1A receptors in the dentate gyrus, while microbiota depletion is associated with decreased hippocampal BDNF and its receptor, TrkB, elevated phosphorylation of TrpVl, and reduced firing rates and bursting in CA3. These effects were recovered by probiotic treatment, except for TrkB expression, which remained reduced. In response to a water avoidance stressor, c-Fos expression was elevated in CA1 and CA3, and expression was decreased in the dentate gyrus. These changes were all attenuated by probiotic treatment. The same water avoidance stressor resulted in decreased neurogenesis in the dentate gyrus, which was also rescued by probiotic treatment. Probiotic treatment was connected with decreased expression of GABAB1b mRNA in the dentate gyrus, CA1, and CA3, as well as increased expression of GABAAα2 mRNA in the dentate gyrus. Finally, prebiotic treatment led to increased hippocampal expression of Bdnf, GABAB1 and GABAB2 mRNA and decreased Grin2a mRNA. While a pattern from these few studies with highly varied methodology is difficult to draw, it would appear that both stress and microbiota depletion alter (decrease in several regions) activity in the hippocampus, which can be rescued by probiotic treatment. This lays an exciting foundation for future microbiome-depression-hippocampus research to come.
Other brain regions.
Studies of the effects of the microbiome on the brain often include a wide array of brain regions when exploring pathways from gut microbiota to the central nervous system. For example, in the paradigm tested above by Crumeyrolle-Arias and colleagues (2014), GF rats showed a two- to three-fold reduction in HVA concentration in the striatum when compared to SPF rats. In terms of probiotic effects, in the study discussed above by Bravo and colleagues (2011), chronic treatment with L. rhamnosus JB-1 resulted in reduced expression of GABAB1b mRNA in the locus coeruleus, a key regulator of attentional control (Aston-Jones & Cohen, 2005), and elevated expression of GABAB1b mRNA in the cingulate area 1 (Cg1) and prelimbic (PrL) cortices that may play a role in gut motility and in behavioral response to rectal discomfort in IBD (Gao, Wu, Owyang, & Li, 2006; Saper & Stometta, 2014). The aforementioned study by Ait-Belgnaoui and colleagues (2014) found that while the water avoidance stressor significantly decreased Bdnf mRNA levels in the hypothalamus, these levels were restored and increased after probiotic treatment with L. helveticus R0052 and B. longum R0175. This was also accompanied by decreased markers of cytoskeleton organization, microglial activation, synaptogenesis, and cell adhesion (Ait-Belgnaoui et al., 2014). In a rat maternal separation (MS) model, MS adult rats were treated with B. infantis 35624 chronically for 45 days (Desbonnet et al., 2010). In addition to reversing depression-like behavioral deficits, as measured by the forced swim test, the treatment also restored norepinephrine levels in the brainstem that had been reduced due to MS. Finally, in a model of chemical colitis, mice who received dextran sodium sulfate in their drinking water during three, 1-week cycles displayed elevated anxiety-like behaviors (Bercik, Park, et al., 2011). However, these behaviors were absent in mice receiving a subdiaphragmatic vagotomy or normalized via treatment with B. longum NCC3001.
Tryptophan metabolism.
Finally, an active area of investigation in the microbiome literature is tryptophan metabolism. Tryptophan is the precursor to many neuroactive molecules, including the neurotransmitter serotonin, the neuroprotective molecule kynurenic acid, the neurotoxic molecule 3-hydroxykynurenine (3-HK), and the neurotoxic breakdown product of 3-HK, quinolinic acid (S. M. O’Mahony et al., 2015). Of note, the initial breakdown of tryptophan to kynurenine is accomplished by the enzyme indoleamine-2,3-dioxygenase 1 (IDO1). IDO1 can be induced via inflammatory pathways, most potently by IFNɣ (Ruddick et al., 2006), and may play a role in both intestinal immunity and gut microbiota balance, providing a potential link between CNS-microbiome and immune-microbiome pathways (Harrington et al., 2008; Laurans et al., 2018; Vujkovic-Cvijin et al., 2015; see Fig. 4). Tryptophan metabolites have the potential to create a large impact on neural function, and, thus, commensal bacteria that metabolize tryptophan play a large role in modulating the central nervous system. Since tryptophan is an essential amino acid in humans, meaning we cannot synthesize it on our own, all of the tryptophan we need must come from our diet. This results in competition between the microbiota and the amino-acid transporters that absorb free tryptophan from the gut. The dynamics of this competition are a major determinant of free tryptophan in the circulation. This is important in terms of the central nervous system, as tryptophan is a precursor to the neurotransmitter serotonin, along with several other molecules with neuroregulatory properties (S. M. O’Mahony et al., 2015). The study by Hoban and colleagues (2016) mentioned above found that, following 13 weeks of antibiotic treatment, circulating levels of tryptophan had increased (Hoban, Moloney, et al., 2016). Probiotics also have been shown to have an effect on tryptophan and its metabolites in depression contexts. In the previously mentioned 2008 study by Desbonnet and colleagues, treatment with B. infantis 35624 resulted in elevated circulating levels of both tryptophan and kynurenic acid (Desbonnet et al., 2008). However, in the abovementioned 2010 study by Desbonnet and colleagues, which added a maternal separation paradigm involving treatment with B. infantis 35624, no changes in tryptophan, kynurenine, or kynurenic acid were observed between treatment groups (Desbonnet et al., 2010). In a study exploring the effects of a five-week chronic mild stress paradigm on mouse behavior and microbiota composition, chronic stress was connected with increased depression-like behavior (as measured by the forced swim test), reduced Lactobacillus spp. in the fecal microbiome, as well as increased circulating levels of kynurenine, a direct metabolite of tryptophan via the enzyme IDO1 (Marin et al., 2017). The investigators then supplemented the diet of the mice with L. reuteri ATCC 23272 for an additional 4 weeks, which was sufficient to reverse behavior and kynurenine concentrations. The reversal of depression-like behaviors was diminished if kynurenine levels were artificially elevated during probiotic supplementation, suggesting a link between kynurenine signaling and depression-like behavior. Through a series of elegant mechanistic follow-up experiments, the researchers identified that L. reuteri ATCC 23272 produced peroxide in vitro, as peroxide is an inhibitor of IDO1, which is sensitive to reactive oxygen species. They then identified that fecal peroxide levels were decreased in stressed mice but elevated in mice treated with L. reuteri ATCC 23272. This was supported by increased ido1 mRNA in the intestines after stress, which then decreased following probiotic treatment (Marin et al., 2017). Finally, prebiotics have also been shown to decrease circulating tryptophan levels in a depression-like context, which was observed in the aforementioned study by Burokas and colleagues (Burokas et al., 2017). These patterns suggest that depleting the gut microbiota increases available tryptophan in the circulation, as it is not being metabolized as frequently by commensal microbes. On the other hand, probiotics and prebiotics help support the growth of healthful microbiota, which would lead to increased tryptophan competition, and lower levels in circulation. Additionally, gut microbiota have some regulatory control over tryptophan metabolism, in part due to the creation of regulatory reactive oxygen species.
Figure 4.
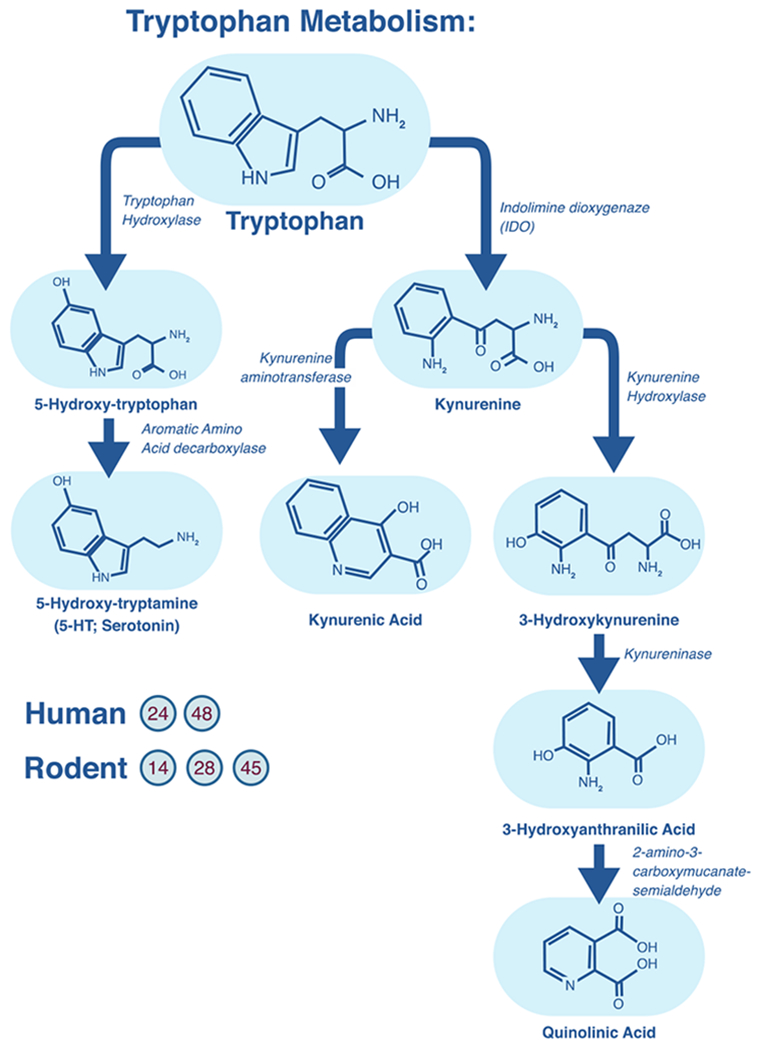
Relevant aspects of tryptophan metabolism to articles reviewed. Five articles (numbers listed in the figure correspond to Table 1) explicitly looked at different aspects of tryptophan metabolism.
Applications to depression: Human studies
Several studies have explored the makeup of the microbiome in connection with gastrointestinal dysbiosis. A study in individuals with IBD found that 65% of patients showed elevated psychological distress, with 21% of patients presenting with symptoms of depression as measured by the HADS (Peter et al., 2018). In a study of women who were exposed to antibiotics from the onset of labor to 14 days postpartum, antibiotic exposure was independently predictive of depressive symptoms at 1 month postpartum, though not at 3 or 6 months (Murphy, Paul, Dunlop, & Corwin, 2018). Additionally, levels of fecal isovaleric acid have been found to correlate with depressive symptoms (Szczesniak et al., 2016). Isovaleric acid is a SCFA that can act as an inverse agonist of the adenosine A1 receptor, potentially interfering with neuroinflammation and natural sleep promotion (Dunwiddie & Masino, 2001; Ingiosi, Opp, & Krueger, 2013; Sichardt, Müller, Lacher, Nieber, & Mayer, 2006). Of note, isovaleric acid also correlated with several taxa associated with depression, including Faecalibacterium, Alistipes, Ruminococcus, and Oscillobacter.
The vast majority of human studies exploring afferent signaling from the microbiome to the CNS in depression, however, have explored the effects of probiotic treatment. To date, there have been two meta-analyses and one review article evaluating the use of probiotics to treat depressive symptoms in humans. The first meta-analysis, from 2016, evaluated 5 articles and concluded that probiotics significantly decrease depression scores, both in the healthy population and in those with MDD, though not in individuals over 65 years of age (R. Huang, Wang, & Hu, 2016). The second, published a year later and using somewhat looser criteria, included 10 articles. The authors found no significant effects of probiotic supplementation on mood; however, they recognized limitations in the variability of probiotic strains used, and low numbers of included subjects with an MDD diagnosis (Ho, Lim, Peters, Yeo, & Ng, 2017). Finally, the review article, also from 2017, evaluated 10 studies that met their criteria, and found evidence that probiotics alleviate depressive symptoms, but advised that more double-blind randomized controlled trials using patients with clinical MDD are needed (Wallace & Milev, 2017). Studies from the previous 3 articles that are directly relevant to depression, along with several recent additions, are discussed below.
Out of 16 articles (15 RCTs) identified that explore the relationship between probiotic treatment and depressive (or closely related) symptoms, only five actually studied a depressed sample. Two of those four mentioned using DSM-IV diagnostic criteria and sourced participants from hospitals (Akkasheh et al., 2016; Rudzki et al., 2019), one used “mild to moderate major depressed patients” referred by a hospital psychiatrist (Kazemi, Noorbala, Azam, Eskandari, & Djafarian, 2018), and the final two relied on self-report using either “mild” or “moderate” symptom cut-offs (Pinto-Sanchez et al., 2017; Romijn et al., 2017). The remaining 10 RCTs were in a mix of either subjects free of mental health conditions (Chung et al., 2014; Messaoudi, Lalonde, et al., 2011; Messaoudi, Violle, et al., 2011; Nishihira et al., 2014; Östlund-Lagerström et al., 2015; Shinkai et al., 2013; Slykerman et al., 2017; Steenbergen, Sellaro, van Hemert, Bosch, & Colzato, 2015), or subjects sampled from the general population (Benton, Williams, & Brown, 2007; Mohammadi et al., 2016), with the exception of one study of individuals with chronic fatigue syndrome (Rao et al., 2009). Out of the 15 articles, six showed significant decreases in depressive symptoms.
Of the six studies showing a decrease in depressive symptoms, three did so in an MDD sample. In the first study, subjects were randomly assigned to an 8-week treatment of either placebo or a probiotic capsule containing freeze-dried L. acidophilus, L. casei, and B. bifidum (no strains provided). Subjects in the probiotic condition showed a significant reduction in depression scores, as measured by the BDI (Akkasheh et al., 2016). The second study administered an 8-week, 3-arm treatment to individuals with mild to moderate major depression who were currently taking antidepressant medication for 3 months prior to the study (Kazemi et al., 2018). The arms consisted of a probiotic condition, including a dual formulation of L. helveticus R0052 and B. longum R0175, a condition using the prebiotic galacto-oligosaccharide, or placebo. The probiotic group, as compared to placebo and the prebiotic group, showed a significant decrease in depressive symptoms, as measured by the BDI. Of a mechanistic note, this study also found markers of altered tryptophan metabolism in the probiotic group as compared to the placebo group. After adjusting for circulating isoleucine (which competes with tryptophan when being transported across barriers, such as the BBB) there was a decreased kynurenine/tryptophan ratio. This would be indicative of greater amounts of available tryptophan in the body, which could be used in serotonin synthesis centrally. Although the previous study did not find an association between prebiotics and depression, a correlational study in a general population sample found an association between dietary fiber intake and decreased depressive symptoms (Miki et al., 2016). One pilot RCT required a mild to moderate anxiety and/or depression score on the HADS and comorbid IBD as inclusion criteria (Pinto-Sanchez et al., 2017). This study randomized participants either into a probiotic group, using the single strain B. longum NCC3001, or a placebo group and treated for six weeks. At the end of treatment, subjects in the probiotic group showed significant reduction in the depression subscale of the HADS, but not in anxiety or IBD symptoms. This was sustained at follow-up on week 10. Unique to this study was the incorporation of fMRI measurement at baseline and at week 6. Probiotic treatment reduced responses to negative emotional stimuli in the amygdala and fronto-limbic regions when compared to placebo.
Of the remaining three studies showing a decrease in depressive symptoms, one provided petrochemical workers with either a probiotic capsule, probiotic yogurt, or conventional yogurt control, with both probiotic conditions resulting in a reduction of depression symptoms on the GHQ-28 as compared to control (Mohammadi et al., 2016). The final two studies were both part of the same RCT, which explored the effects of a dual probiotic formulation containing L. helveticus R0052 and B. longum R0175 on stress and psychiatric symptoms in healthy volunteers. The first study found a reduction in psychological distress, as measured by the HSCL-90, including a decrease in depressive symptoms (Messaoudi, Lalonde, et al., 2011). The second study re-analyzed the previous experiment using a 25-subject subset with baseline urinary free cortisol levels below 50 ng/mL. The conclusions from the original analysis remained unchanged, which the authors interpreted to mean that the mental health benefits of probiotics contribute to individuals low in stress, and that probiotics could have possible prophylactic uses against stress-related disorders (Messaoudi, Violle, et al., 2011). Of the five independent probiotic formulations used in these studies, strains of B. longum were used in four, strains of either L. helveticus or L. acidophilus were used in three, and strains of L. casei were used in two, suggesting possible targets of future research.
Another promising study of depression was not in a sample of depressed individuals, but in a sample at risk of developing depression in the near future. L. rhamnosus HN001 was evaluated in a randomized, double-blind, placebo-controlled trial as a prophylactic treatment to prevent postpartum depression (Slykerman et al., 2017). Pregnant women were recruited between 14-16 weeks gestation and received probiotic treatment up to 6 months postpartum if breastfeeding. Mothers in the probiotic treatment group showed significantly lower depression and anxiety scores on the Edinburgh Postnatal Depression Scale and STAI 6-item version, respectively, as compared to placebo.
Two studies showed mood improvement, but depressive symptoms were not directly measured. In one RCT, a probiotic milk drink containing L. casei Shirota or placebo was provided to a general population sample for three weeks. Initial analysis of the data showed no change between the probiotic and placebo groups in mood, as measured by the Profile of Mood States (POMS). However, a secondary analysis of subjects in the bottom third of the depressed/elated POMS dimension showed significant increase in mood in response to probiotic treatment. The authors suggest that the benefits of probiotic treatment on mood may be strongest for those whose mood is initially poor (Benton et al., 2007). Another study explored the effects of a multi-strain probiotic on cognitive reactivity in a non-depressed sample. The authors found that, compared to placebo, a 4-week probiotic supplementation resulted in decreased cognitive reactivity to sad mood, as measured by the Leiden index of depression sensitivity scale (Steenbergen et al., 2015).
While the previous studies show promising results towards the use of probiotics in the treatment of depressed mood, not all studies that have set out to identify changes in depression through probiotics have done so. In a study exploring the efficacy of probiotics in treating chronic fatigue syndrome, subjects were treated with either L. casei Shirota or placebo daily for two months. While no changes were found in depression symptoms, as measured by the BDI, there was a significant improvement in anxiety symptoms, as measured by the Beck Anxiety Inventory (Rao et al., 2009). Improvements in anxiety, but not depression, were found in another probiotic study, which used a probiotic yogurt containing L. gasseri SBT2055 and B. longum SBT2928. The yogurt was administered daily for 12 weeks and resulted in a significant reduction in the anxiety subscale of the GHQ-28, which was not present in subjects who ate the placebo yogurt (Nishihira et al., 2014). In a study of adults over age 65, participants were either randomized into a probiotic treatment group, which included tablets composed of heat-killed L. pentosus b240, or placebo. While there were no significant changes in anxiety or depression as measured by the POMS, there was a significant elevation in general health perception as measured by the 36-Item Short Form Health Survey (SF-36) in the probiotic group, as compared to placebo (Shinkai et al., 2013).
Several studies that found no measurable differences in depressive symptoms did find improvements in cognitive function. A 12-week RCT investigated the effects of L. helveticus IDCC3801 in fermented milk on mood and cognitive functioning in participants between 60 and 75 years of age. While there were no significant changes on the Geriatric Depression Scale Short Form or the Perceived Stress Scale, participants in the probiotic group demonstrated improvements on a battery of neuropsychological tests as compared to placebo (Chung et al., 2014). Cognitive benefits, but not benefits in depressive symptoms were also seen in the only other study to use a sample with confirmed MDD diagnosis. In a double-blind RCT of adjunct placebo therapy with SSRI treatment, either L. plantarum 299v or placebo was administered for eight weeks to patients with MDD. While the probiotic group displayed no changes in depressive or anxious symptoms, as measured by the Hamilton Depression Rating Scale and the Symptom Checklist 90, there were improvements on several neuropsychological tests of cognitive function, when compared with placebo. However, this study did find altered markers of tryptophan metabolism in the probiotic group, as compared to placebo. They observed a significant decrease in kynurenine, as well as an increase in the 3-hydroxykynurenine (3-HK):kynurenine ratio, but no significant changes in circulating 3-HK. This is perplexing, as on the surface this would suggest a shift in kynurenine breakdown away from the neuroprotective kynurenic acid, towards the neurotoxic 3-HK, which would argue against the cognitive enhancement observed in the study. While the authors discuss several alternative hypotheses for why this may be the case, it appears evident that more human studies with larger sample sizes are necessary to tease apart whether this is a true finding, or artifact from a complex study (Rudzki et al., 2019).
The final two probiotic RCTs that examined depression found no significant effects in all relevant domains tested. One was in healthy older adults, while the second was in individuals who scored in the moderate range or higher on a self-report measure of depression. In the first study, individuals older than 65 but still living in their own home and who were free of gastrointestinal disease were recruited into an RCT evaluating the health properties of L. reuteri DSM17938 (Östlund-Lagerström et al., 2015). Either probiotic or placebo was taken twice a day for 12 weeks. The trial failed to show any improvements in digestive health, general wellbeing, stress, anxiety, or depression when comparing the probiotic group to placebo. In the final RCT, participants were self-ref erred to the study and screened via an online survey (Romijn et al., 2017). Inclusion criteria included scoring in the “moderate” or higher range on either of two self-report depression scales (including the Quick Inventory of Depressive Symptomatology or the DASS-42), as well as being free of psychiatric medication for at least four weeks prior to the trial. Subjects were randomized into either the probiotic condition, which administered a combined preparation of L. helveticus R0052 and B. longum R0175, or placebo. There were no significant differences between groups in any measured psychological outcomes, including depressive symptoms.
Depression treatment and the microbiome
Several depression treatments have been found to have anti-microbial properties or to influence the human microbiome. Older antidepressants, like the tricyclic desipramine, reduce vulnerability to induced colitis in a mouse maternal separation model (Varghese et al., 2006). Some studies have found that antidepressants increase your risk for intestinal infections (Rogers et al., 2013). Still, other studies found that several classes of antidepressants have anti-microbial properties against pathogenic bacteria (Macedo et al., 2017). A comprehensive screen of over a thousand marketed drugs on 40 representative gut bacteria found that 24% of drugs with human targets (over 200) had antimicrobial effects (L. Maier et al., 2018). Among these were many antipsychotics, which can be prescribed to treat depression (P. Wang & Si, 2013). Finally, a recent study of microbiome composition in mice found that the antidepressant fluoxetine alters microbiome makeup, particularly enriching species associated with regulation of body mass (Lyte, Daniels, & Schmitz-Esser, 2019).
Next-generation depression treatment ketamine appears to play a role in regulating intestinal microbial diversity. Two enantiomers of ketamine attenuated chronic social defeat stress-induced changes in microbial diversity in mice (C. Yang et al., 2017). In the rat fecal microbiome, ketamine increases the genera Lactobacillus, Turicibacter, and Sarcina, while decreasing opportunistic pathogens Mucispirillum and Ruminococcus, subsequently reducing the prevalence of opportunistic infections (Getachew et al., 2018).
Finally, depression interventions targeting daily behaviors, such as exercise and nutritional psychiatry, are also closely intertwined with the microbiome. While the field of nutritional psychiatry may still be in its infancy (Firth et al., 2019), the basic concept that diet and mental health are related dates back at least 4000 years, since the ancient Chinese supplemented their diet with garlic to treat sadness and depression (Jacka, 2017; Rivlin, 2001). The American Gut Project has observed that increased variety of plants in a person’s diet is a major determinant of alpha diversity in the gut microbiome (McDonald et al., 2018). In fact, dietary alterations can lead to dramatic, temporary shifts in microbiome composition within a single day (R. K. Singh et al., 2017). These diet-induced shifts are rapidly being linked to psychiatric outcomes, with strong implications for depression and anxiety (Sandhu et al., 2017). Additionally, adding exercise, a depression intervention that already has a positive track record, to shifts in diet has led to even greater improvements in depression and anxiety when combined (Firth et al., 2019; Hiles, Lamers, Milaneschi, & Penninx, 2017). Exercise is believed to benefit mood by acting on the central nervous system, the endocrine system, and the immune system in ways that promote health and well-being (Mikkelsen, Stojanovska, Polenakovic, Bosevski, & Apostolopoulos, 2017). In harmony with this, the gut microbiomes of professional athletes differ significantly from those of more sedentary control subjects (Barton et al., 2018), and exercise has been linked with increasing gut microbial diversity, potentially through anti-inflammatory mechanisms (Codella, Luzi, & Terruzzi, 2018). Depression treatments across many modalities may all be influencing the gut microbiome in unique ways.
Moving towards the future: a holistic synthesis of depression
From the health and societal burden alone (World Health Organization, 2017), it is obvious that there is a need for a paradigm shift in both the understanding, prevention, and treatment of major depressive disorder on a global scale. Integrative approaches to understanding various body systems and their role in MDD have appeared at an ever-increasing rate in the literature in the past five years. Reviews integrating stress aspects of the endocrine system and inflammation in a depression context have become commonplace (Chiriţă, Gheorman, Bondari,& Rogoveanu, 2015; Gold, Machado-Vieira, & Pavlatou, 2015; Miller & Raison, 2016; Strain, 2018; Wohleb, Franklin, Iwata, & Duman, 2016). Reviews synthesizing these systems into a microbiome context from general health, mental health, and depression-specific perspectives are also appearing with increased frequency (Bruce-Keller et al., 2018; Koopman, Daniels, Spitzer, Lampe, & El Aidy, 2017; Moloney, Desbonnet, Clarke, Dinan, & Cryan, 2014). This begs the question, however: where do we go from here?
While individual variability in the presentation of depressive symptoms has historically been high (Lawlor, 2012), the need for standardization of treatment and reliance on symptoms-based disorder categorization has homogenized depressive symptoms into the Major Depressive Disorder label we make use of today, both clinically and academically. But it is estimated that 50% of depressed individuals are still not receiving adequate treatment, even with greater awareness and standardization of care (Akil et al., 2018). Combined efforts between behavioral and neurobiological research have identified subtypes of depression based on specific biological signatures, which may have broader implications for treatment (Carboni et al., 2014; Juruena et al., 2018; ten Have et al., 2016). For example, elevated inflammatory markers may predict whether a depressed person will respond favorably to anti-inflammatory treatment (Kohler, Krogh, Mors, & Eriksen Benros, 2016; Miller & Raison, 2015; Raison et al., 2013).
Identifying microbiome signatures related to the time course of depression and its treatment will necessitate further advancement in technique and analysis. While 16S ribosomal RNA gene sequencing has previously prevailed as the dominant identification method of microbiota species, shotgun whole genome sequencing (WGS) has become much cheaper and more widely available (Ranjan et al., 2016). Shotgun WGS improves detection and accuracy of microbiome composition and is increasingly becoming the field’s new gold standard. Branching out beyond just microbiota identification and towards more functional readouts is also crucial. This expansion will provide a greater wealth of information and paint a fuller picture of microbiome-related shifts that accompany depression. Also, an increased focus on metabolomics may also be useful (Ursell et al., 2014). Recent novel methodologies such as the use of mass spectrometry (Melnik et al., 2017) and functional imaging of glucose metabolism in the colon (Boursi et al., 2018), are paving the way to increased integration of measures of microbiome activity.
Additionally, standardizing collection, storage, and processing methods, or clearly communicating how procedures vary, will be crucial to global depression research as larger data sets are collaborated on more frequently (Falony et al., 2016; He et al., 2018; Vandeputte, Tito, Vanleeuwen, Falony, & Raes, 2017). This includes creating at-home collection methods that are simple and robust. For example, a recent proof of concept study has demonstrated that analyzing soiled toilet tissue stored at room temperature for seven days can be comparable to using an immediately frozen fecal sample for some purposes (Al, Bisanz, Gloor, Reid, & Burton, 2018). In fact, a variety of storage systems that allow for longer fecal sample stability are proving comparable to the immediate freezing without preservation gold standard (Bassis et al., 2017; Z. Wang et al., 2018). However, while collection and storage of fecal samples becomes streamlined, future work should take note of other forms of variability associated with collection that are often overlooked in microbiome analysis. For example, various forms of bowel preparation can have significant effects on microbiome composition, and may take days to make a full recovery (Nagata et al., 2019). Temporal stability, medication use, and eating behaviors may also play a role in microbiome variability. While fecal microbiome samples have been found to be individually stable over many months, a single course of antibiotic treatment may shift microbial diversity for over a year (Voigt et al., 2015). On a shorter timescale, one study found that 35% of bacterial OTUs measured via fecal samples varied by time of day, as did several prominent metabolites, and that both were highly tied to eating behaviors, such as feeding times and diet composition (Kaczmarek, Musaad, & Holscher, 2017). Depression is often accompanied by circadian disruption and shifts in eating habits, making special attention to these variables at time of collection critical for future research (Quirk et al., 2013; Wichniak, Wierzbicka, & Jernajczyk, 2013).
However, sole reliance on fecal sampling, while convenient in humans, may only reveal a subset of depression-relevant data that the microbiome has to offer. Most relevant to this is that the gut lumen and mucosa have individually distinct microbial compositions (Parthasarathy et al., 2016; Sartor, 2015). Fecal samples typically represent microbiota from the gut lumen, but it is microbial residents of the gut mucosa that are often more representative of species engaging directly with the intestinal epithelium, and, thus, playing a more intimate role in metabolism and immunoregulation (Rook, 2019). Additionally, variation also exists when comparing regions of the colon, which can be seen in terms of absolute OTUs, diversity, and even distinct levels of protein expression (Hu et al., 2010; Lavelle et al., 2015; Lyra et al., 2012). Novel sampling strategies to increase the coverage of an individual’s microbiome will be crucial to understanding the relationship between the microbiome and depression moving forward. These strategies may include methods completed independently by subjects, such as the use of rectal swabs (Bassis et al., 2017), or using more invasive sampling methods conducted in combination with a colonoscopy, such as using a protected specimen brush (Lavelle et al., 2015) or laser capture microdissection of the mucosa (Rowan et al., 2010; S. Zhang, Cao, & Huang, 2017). As a more extreme sampling tool, surgical removal of the rectum or colon through partial colectomy or proctocolectomy preserves tissue architecture and microbiota composition, although this technique is typically reserved for individuals with severe IBD (S. Zhang et al., 2017). Expanding the methodology used in depression-based microbiome research will further allow for the characterization of mental health at an individual level and could one day contribute to the generation of individualized treatment plans based on a person’s unique biological signature of depression.
While the goal of personalized medicine is tantalizing, this level of individualization also paves the way for a far loftier goal: promoting resiliency based on the unique needs of the individual. The focus of medicine is often, by necessity, more reactive than proactive, but with the microbiome particularly, there is opportunity to use health information to encourage wellness. While resiliency is often thought about in behavioral terms (Waugh & Koster, 2015), maintaining positive health habits unique to a person’s biochemistry, neurobiology, and microbiome make-up could stop depression in its tracks, before it interferes with daily life. Systems-wide approaches that integrate data across fields in the interest of reducing depression and promoting resilience and holistic health are still in their infancy, but are a necessity in furthering global wellness (Akil et al., 2018).
The market may be pushing the academic world in this direction regardless. While large scale academic microbiome characterization projects, like the American Gut Project (McDonald et al., 2018), and the Flemish Gut Flora Project (Valles-Colomer et al., 2019b) are rapidly advancing our global understanding of gut microbiota and health, many companies with access to affordable sequencing technologies have brought the microbiome into the home of consumers (Staley, Kaiser, & Khoruts, 2018). The prospects of individualized medicine are both exciting and tantalizing; however, caution must be exercised when implementing basic research data in a clinical setting. Challenges not only exist in translating the sparse number of human clinical trials into clinical practice, but also in the knowledge gap between the field and primary care providers due to the recent rapid pace of advancement (Staley et al., 2018). As researchers and clinicians, it is our responsibility to communicate microbiome discoveries in a way that reflects the immense hope they hold for depression treatment, while also recognizing current limitations.
Conclusions
The role of the microbiome in depression lies at the intersection of the immune, endocrine, and neural systems. The pace of microbiome research in a depression context has accelerated in the past five years and continues to deliver novel mechanisms with potentially broad clinical implications. Understanding these mechanisms hinges on the awareness of bidirectional intercommunication between these four body systems, and the holistic implications those relationships have for an organism. Recent research has identified novel therapeutics, in the form of prebiotics and probiotics (among other biotics), that hold promise in both the treatment of depression and in mental health resiliency at large. Developing a broader understanding of depression, with the inclusion of the microbiome, promotes increases in global health and brings clinical science closer to the vision of individualized medicine.
Figure 2.
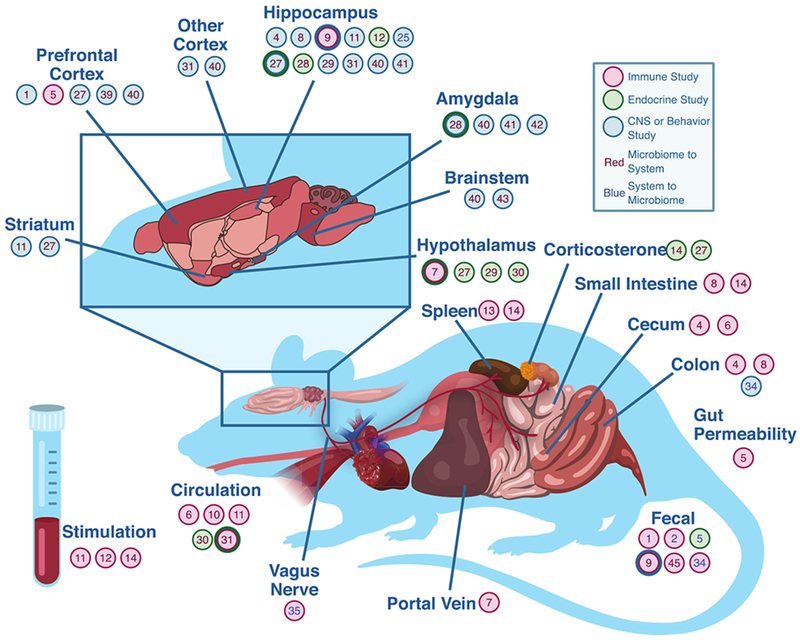
Diagram showing where in the rodent body each reviewed article focused. Red circles indicate an immune study, green circles indicate an endocrine study, and blue circles indicate a study involving the central nervous system. Circles with two different colored rings indicate measurements from both systems represented by those colors. Red text indicates that the direction measured was from the microbiome to the system, and blue text indicates that the direction was from the system to the microbiome. Numbers listed in the figure correspond to Table 1.
Figure 3.
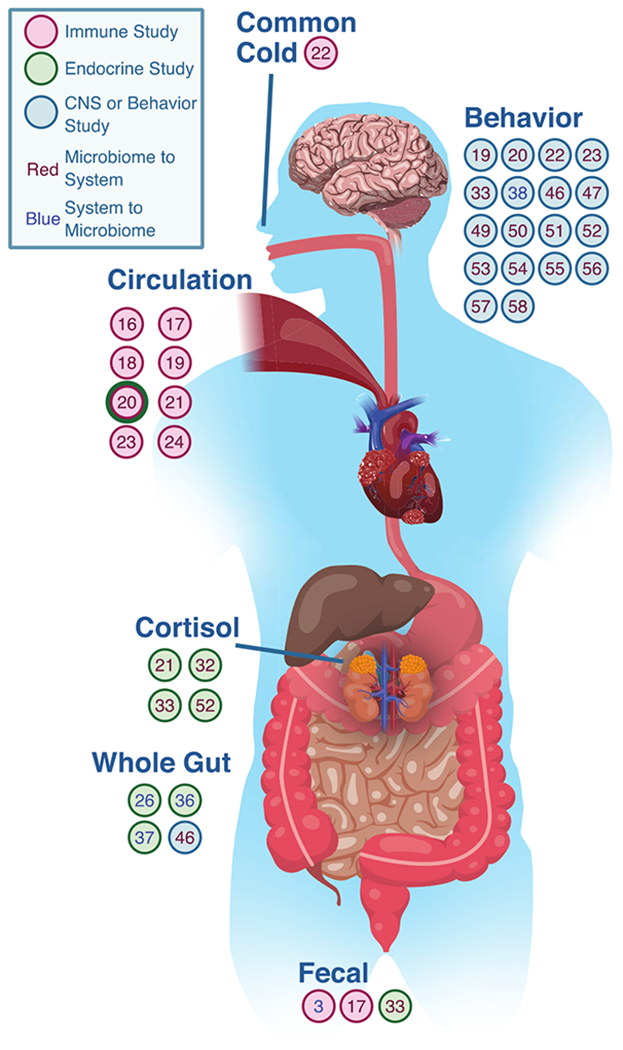
Diagram showing where in the human body each reviewed article focused. Red circles indicate an immune study, green circles indicate an endocrine study, and blue circles indicate a study involving the central nervous system. Circles with two different colored rings indicate measurements from both systems represented by those colors. Red text indicates that the direction measured was from the microbiome to the system, and blue text indicates that the direction was from the system to the microbiome. Numbers listed in the figure correspond to Table 1.
Table 1.
Afferent and efferent signaling along the microbiome-gut-brain axis, preclinical studies
| Reference | Direction | System | Dep. Induction/Dx | Treatment | Measurement | Symptoms/Behavior | Relevant Effects | |
|---|---|---|---|---|---|---|---|---|
| 1 | (Zhang, Yao, et al., 2017) | Efferent (host systems to microbiota) | Mouse | Social defeat stress | Anti-mouse IL-6 receptor antibody | Forced swim test, Tail suspension | Antibody treatment decreased depression-like behaviors induced by stressor | Immune System (IS): Antibody treatment reversed alterations in fecal microbiota abundance induced by stressor (↑ Oscillospera spp., ↓ phylum Firmicutes/Bacteroidetes ratio). |
| Endocrine System (ES): None Reported | ||||||||
| Central Nervous System (CNS): None Reported | ||||||||
| 2 | (Wong et al., 2016) | Efferent (system to microbiota) | Mouse | Chronic restraint stress | Mice genetically deficient in caspase-1 or pharmacological inhibition of caspase-1 activity | Forced swim test, Elevated plus-maze | Both genetic manipulation and treatment decreased depression-like behaviors induced by stressor | IS: Genetic modification and pharmacological inhibition of caspase-1 activity reversed stressor-induced changes in fecal microbiota relative abundance (↑ family Lachnospiracea, ↑ Blautia spp. And ↑ Akkermansia spp.). |
| ES: None Reported | ||||||||
| CNS: None Reported | ||||||||
| 3 | (Bailey et al., 2010) | Afferent (microbiota to system) | Mouse | Chronic restraint stress | None | None | NA | IS: Stress induced increased colonic Tnf mRNA and altered cecal microbiota richness, diversity, and relative abundance (↑ anaerobic microbiota, ↓ family Porphyromonadaceae). |
| ES: None Reported | ||||||||
| CNS: None Reported | ||||||||
| 4 | (Martín-Hemández et al., 2016) | Afferent (microbiota to system) | Mouse | Chronic mild stress | Antibiotics | Forced swim test | Stressor increased depression-like behavior | IS: Stress ↑ gut permeability, ↑ bacterial translocation and altered markers of neuroinflammation in the prefrontal cortex (PFC), including ↑ p38, MAPK protein expression, and ↓ mRNA levels of Pik3cg, Akt, Nrf2, and Gpx1. Gut permeability, bacterial translocation, and levels of p38 MAPK and Gpx1 were partially or Hilly restored following antibiotic treatment. |
| ES: None Reported | ||||||||
| CNS: None Reported | ||||||||
| 5 | (Bailey et al., 2011) | Afferent (microbiota to system) | Mouse | Social disruption stress | Antibiotics | None | NA | IS: Stress induced alterations in cecal relative abundance (↓ Bacteroides and ↑ Clostridium), along with increased circulating IL-6 and MCP-1. Antibiotic treatment prevented the increase in Il-6 and MCP-1. |
| ES: None Reported | ||||||||
| CNS: None Reported | ||||||||
| 6 | (Ait-Belgnaoui et al., 2012) | Afferent (microbiota to system) | Mouse | Chronic restraint stress | L. farciminis, antibiotics, or ML-7 | None | NA | IS: Stress ↑ portal blood LPS, and ↑ mRNA expression of Il1b, Il6, and Tnf in the hypothalamus, all of which was prevented by probiotic, antibiotic, and ML-7 treatment. |
| ES: Stress ↑ Crh mRNA and ↑ CRH-positive cells in the paraventricular nucleus of the hypothalamus, which was normalized by probiotic treatment | ||||||||
| CNS: None Reported | ||||||||
| 7 | (Guida et al., 2018) | Afferent (microbiota to system) | Mouse | Antibiotic treatment | L. casei DG | Forced swim test, Tail suspension | Probiotic treatment decreased depression-like behaviors induced by antibiotic treatment | IS: Antibiotic treatment ↑ IL-1β in the duodenum and ↑ TNF in the small intestine and colon. Probiotic treatment reduced these concentrations except TNF in the jejunum. |
| ES: None Reported | ||||||||
| CNS: Antibiotic treatment ↓ BDNF, ↓ the BDNF receptor, ↓ TrkB, and ↑ phosphorylation of TrpV1 in the hippocampus. Probiotic treatment normalized BDNF protein expression and reversed the elevated phosphorylation of TrpV1. | ||||||||
| 8 | (Li et al., 2018). | Afferent (microbiota to system) | Mouse | Chronic mild stress | Three-strain probiotic blend (L. helveticus R0052, L. plantarum R1012, and B. longum R0175) | Elevated plus-maze, Forced swim test | Probiotic treatment decreased depression-like behaviors induced by stressor | IS: Stress altered fecal α- and β-diversity, including ↓ Lactobacillus, and ↑ IFNγ in the hippocampus. Probiotic treatment reduced IFNγ and TNF concentrations in the hippocampus. |
| ES: None Reported | ||||||||
| CNS: Stress ↑ IDO in the hippocampus, which was reversed by probiotic treatment. | ||||||||
| 9 | (Hao, et al., 2019) | Afferent (microbiota to system) | Rat | Chronic mild stress | F. prausnitzii ATCC 27766 | Elevated plus-maze, Forced swim test, Open-field test | Probiotic treatment decreased depression-like behaviors induced by stressor | IS: Probiotic treatment ↓ depression-like behaviors, ↑ circulating levels of IL-10, and attenuated stressor-induced ↓ in IL-6 and ↓ CRP. |
| ES: None Reported | ||||||||
| CNS: None Reported | ||||||||
| 10 | (Desbonnet, et al., 2008) | Afferent (microbiota to system) | Rat | None | B. infantis 35624 | Forced swim test | Probiotic treatment had no effect on depression-like behaviors | IS: Probiotic treatment ↓ circulating IL-6 and ↓ IFNγ following the forced swim test, and ↓ IL-6 and ↓ IFNγ in response to LPS and Con-A stimulation of whole blood. |
| ES: None Reported | ||||||||
| CNS: Probiotic treatment ↓ 5-HIAA in the frontal cortex, ↓ DOPAC in the amygdaloid cortex, and ↑ circulating levels of tryptophan and kynurenic acid. No changes were found in the striatum or brainstem. | ||||||||
| 11 | (Abildgaard, Elfving, Hokland, Lund, & Wegener, 2017) | Afferent (microbiota to system) | Rat | High fat diet | 8-strain probiotic | Forced swim test | Probiotic treatment decreased depression-like behaviors induced by high fat diet | IS: Probiotic treatment ↓ depression-like behaviors, and altered the following cytokines produced by CD3/28 stimulation of PBMCs: ↑ IL-2, ↑ IL-4, and ↑ IFNγ and led to a percent change of: ↓ IL-6 and ↓ TNF. |
| ES: Probiotic treatment altered hippocampal mRNA levels: ↓ Crhr1, ↓ Crhr2, and ↓ Mr | ||||||||
| CNS: None Reported | ||||||||
| 12 | (Bharwani, et al., 2017) | Afferent (microbiota to system) | Mouse | Chronic social defeat | L. rhamnosus JB-1 | Open-field test, Light-dark box | Probiotic treatment decreased anxiety-like behaviors induced by stressor | IS: Probiotic treatment ↑ spleen-derived Tregs in stressed mice and prevented the stress-induced ↑ in spleen-derived dendritic cells. |
| ES: None Reported | ||||||||
| CNS: None Reported | ||||||||
| 13 | (Burokas et al., 2017) | Afferent (microbiota to system) | Mouse | Chronic social stress | Fructo- and galacto-oligosaccharides | Forced swim test, Tail suspension test | Prebiotic treatment decreased depression-like behaviors induced by stressor | IS: Prebiotic treatment ↓ TNF release from Con-A stimulated splenocytes. |
| ES: Prebiotic treatment attenuated the ↑ in circulating corticosterone induced by the forced swim test | ||||||||
| CNS: Prebiotic treatment resulted in altered hippocampal mRNA levels: ↑ Bdnf, ↑ Gabbr1 and ↑ Gabbr2, and ↓ Grin2a; as well as decreased circulating tryptophan | ||||||||
| 14 | (Sherry et al., 2010) | Afferent (microbiota to system) | Mouse | Endotoxin-induced sickness syndrome | Soluble fiber | Quantified sickness behaviors and symptoms (i.e. social withdrawal, fever, food intake, weight) | Fiber treatment decreased sickness syndrome symptoms and shortened recovery time | IS: Prebiotic treatment ↑ IL-4 in the ileum and spleen; altered output of Con-A-stimulated splenocytes: ↑ IL-4, ↑ IL-5, ↓ IL-2, ↓ IL-12, and ↓ IFNγ; altered output of LPS-stimulated macrophages: ↓ IL-1β, ↓ TNF, ↓ IFNɣ, ↓ IL-12, ↓ nitrate. ↑ IL-1RA, ↑ arginase 1, and ↑ Ym1. |
| ES: None Reported | ||||||||
| CNS: None Reported | ||||||||
| 15 | (Diviccaro et al., 2019) | Efferent (system to microbiota) | Rat | 20-day finestrade treatment 1-month withdrawal | Finasteride (5-alpha-reductase inhibitor) | Forced swim test | Depression-like behaviors were increased during 1-month withdrawal period | IS: None Reported |
| ES: Immediately following treatment fecal microbiota: ↑ phylum Bacteroidetes, and ↑ family Prevotellaceae; following withdrawal fecal microbiota: ↓ family Ruminococcaceae and ↓ Oscillospira and ↓ Lachnospira. | ||||||||
| CNS: Immediately following treatment: ↑ pH3-positive cells in the subgranular zone of the dentate gyrus, ↑ hippocampal Tnf mRNA; Following withdrawal ↓ pH3-immunoreactive cells in the subgranular zone of the dentate gyrus, ↓ in granule cell density in the granule cell layer and ↑ GFAP positive astrocytes in the dentate gyrus. | ||||||||
| 16 | (Crumeyrolle-Arias et al., 2014) | Afferent (microbiota to system) | Rat | None | None | Open-field test, Social interaction test | GF rats presented with elevated anxiety- and depression-like behaviors | IS: None Reported |
| ES: GF rats had ↑ circulating corticosterone in response to open-field stress, ↑ Crh mRNA in the hypothalamus and ↓ glucocorticoid receptor mRNA in the hippocampus. | ||||||||
| CNS: GF rats had decreased turnover of dopamine: ↓ frontal cortex ↓ hippocampus, and ↓ striatum in response to the open-field test. | ||||||||
| 17 | (Hoban, Moloney, et al., 2016) | Afferent (microbiota to system) | Rat | 10-week antibiotic treatment | Antibiotics | Forced swim test | Antibiotic treatment increased depression-like behaviors | IS: None Reported |
| ES: Antibiotic treatment resulted in no change in corticosterone following the forced swim test but altered mRNA levels in the hippocampus and amygdala: ↑ Nr3c1 and ↑ Crhr1. | ||||||||
| CNS: Antibiotic treatment ↑ Bdnf mRNA in the amygdala, and ↑ circulating tryptophan | ||||||||
| 18 | (Ait-Belgnaoui et al., 2014) | Afferent (microbiota to system) | Mouse | Water avoidance stress | L. helveticus R0052 and B. longum R0175 | None | NA | IS: None Reported |
| ES: Stressor ↓ BDNF in the hypothalamus, which was reversed in response to probiotic treatment | ||||||||
| CNS: Probiotic treatment ↑ c-Fos in the dentate gyrus of the hippocampus, ↑ neurogenesis in the dentate gyrus, and reversed the ↑ c-Fos in CA1 and CA3 of the hippocampus in response to stress. | ||||||||
| 19 | (Huo et al., 2017) | Afferent (microbiota to system) | Mouse | Chronic restraint stress | None | Open field test | GF mice exhibited decreased anxiety-like behavior as compared to SPF mice | IS: None Reported |
| ES: GF mice displayed altered circulating levels of: ↑ CRH, ↑ ACTH, and ↑ corticosterone; and ↑ Crhr1 mRNA in the hypothalamus in response to stress. | ||||||||
| CNS: None Reported | ||||||||
| 20 | (Sudo et al., 2004) | Afferent (microbiota to system) | Mouse | Acute restraint stress | B. infantis, E. coli, E. coli lacking the translocated intimin receptor (Tir) gene, FMT: SPF to GF | None | NA | IS: E. coli inoculation in GF altered plasma concentrations 12 hours following: ↑ IL-1β and ↑ IL-6. Tir-negative E. coli or B. infantis inoculation led to only a small ↑ in IL-6. |
| ES: Stress induced ↑ in circulating ACTH and corticosterone were reversed by treatment with B. infantis, enhanced by treatment with E. coli, and partially reversed by FMT from SPF to GF mice at 9 weeks of age, but not 17 weeks. | ||||||||
| CNS: GF mice had altered BDNF levels: ↓ cortex and ↓ hippocampus | ||||||||
| 21 | (Park et al., 2013) | Efferent (system to microbiota) | Mouse | Bilateral olfactory bulbectomy. Water avoidance stressor | None | Open-field test, Step-down test, Tail suspension test | Olfactory bulbectomy increased depression-like behavior induced by stressor | IS: None Reported |
| ES: None Reported | ||||||||
| CNS: Olfactory bulbectomized mice showed both ↑ fecal output and ↑ colonic contractility in response to water avoidance stress | ||||||||
| 22 | (Padol, et al., 2012) | Efferent (system to microbiota) | Rat | Flinders Sensitive Line (FSL) rats with induced gastric damage | Vagotomy | None | NA | IS: None Reported |
| ES: None Reported | ||||||||
| CNS: FSL rats exhibited basal gastric acid output twice as high as controls. Vagotomy dramatically reduced gastric acid output in both groups, but to a greater extent in FSL rats. | ||||||||
| 23 | (Hoban, Stilling, et al., 2016) | Afferent (microbiota to system) | Mouse | GF mice | None | None | NA | IS: None Reported |
| ES: None Reported | ||||||||
| CNS: GF mice have elevated expression of genes related to myelination and myelin plasticity, as well as hypermyelination of axons, all in the PFC | ||||||||
| 24 | (Bravo et al., 2011) | Afferent (microbiota to system) | Mouse | none | L. rhamnosus JB-1 | Forced swim test | Probiotic treatment decreased depression-like behaviors induced by the forced swim test | IS: None Reported |
| ES: None Reported | ||||||||
| CNS: Probiotic treatment ↓ Gabra2 mRNA in the PFC; ↓ Gabbr1 and ↓ Gabra2 mRNA in the amygdala; ↓ Gabbr1 mRNA in CA1, CA3, and the dentate gyrus of the hippocampus; ↑ Gabra2 mRNA in the hippocampal dentate gyrus; ↓ Gabbr1b in locus coeruleus; ↑ Gabbr1b in cingulate area 1; and ↑ Gabbr1b in prelimbic cortex | ||||||||
| 25 | (Neufeld, et al., 2011) | Afferent (microbiota to system) | Mouse | None | None | Elevated plus-maze | GF mice exhibited decreased anxiety-like behavior as compared to SPF mice | IS: None Reported |
| ES: None Reported | ||||||||
| CNS: GF mice had ↓ Grin2b mRNA in the central amygdala; and ↑ Bdnf mRNA and ↓ Htr1a mRNA in the hippocampal dentate gyrus | ||||||||
| 26 | (Bercik, Denou, et al., 2011) | Afferent (microbiota to system) | Mouse | 7-day antibiotic treatment FMT | Antibiotics, FMT | Light/dark preference test, Step-down test | Antibiotic treatment decreased anxiety-like behaviors induced by antibiotics | IS: None Reported |
| ES: None Reported | ||||||||
| CNS: Antibiotic treatment ↓ BDNF levels in the amygdala, which was reversed by FMT | ||||||||
| 27 | (Desbonnet et al., 2010) | Afferent (microbiota to system) | Rat | Maternal separation model | B. infantis 35624 | Forced swim test | Probiotic treatment reversed depression-like symptoms induced by maternal separation | IS: None Reported |
| ES: None Reported | ||||||||
| CNS: Probiotic treatment attenuated a maternal separation-induced ↓ in norepinephrine levels in the brainstem, but did not affect circulating tryptophan, kynurenine, or kynurenic acid concentrations | ||||||||
| 28 | (Bercik, Park, et al., 2011) | Afferent (microbiota to system) | Mouse | Dextran sodium sulfate (DSS)-induced chemical colitis | B. longum NCC3001, subdiaphragmatic vagotomy | Step-down test | Vagotomy and probiotic treatment either prevented or normalized anxiety-like behaviors induced by DSS | IS: None Reported |
| ES: None Reported | ||||||||
| CNS: Probiotic treatment did not affect Bdnf mRNA expression in cultured human SH-SY5Y neuroblastoma cells treated with serum of probiotic-treated mice | ||||||||
| 29 | (Marin et al., 2017) | Afferent (microbiota to system) | Mouse | Chronic mild stress | L. reuteri ATCC 23272 | Forced swim test | Probiotic treatment reversed depression-like behavior induced by stressor | IS: None Reported |
| ES: None Reported | ||||||||
| CNS: Stress ↓ fecal Lactobacillus spp., ↑ circulating kynurenine, and ↑ fecal Ido1 mRNA. Subsequent probiotic treatment restored kynurenine levels and ↓ fecal Ido1 mRNA. Artificial elevation of kynurenine prevented the probiotic-induced reversals. |
Table 2.
Afferent and efferent signaling along the microbiome-gut-brain axis, clinical studies
| Reference | Direction | Dep. Induction/Dx | Treatment | Measurement | Relevant Effects | |
|---|---|---|---|---|---|---|
| 1 | (Rajca et al., 2014) | Efferent (host system to microbiota) | Mood symptoms not reported | None | None | Immune System (IS): Individuals who relapsed with Crohn’s disease symptoms after discontinuing infliximab treatment had decreased fecal microbiota relative abundance (↑phylum Firmicutes). |
| Endocrine System (ES): None Reported | ||||||
| Central Nervous System (CNS): None Reported | ||||||
| 2 | (Maes, et al., 2012) | Afferent (microbiota to host system) | DSM-IV-R diagnosis of major depression with or without melancholia | None | Fibromyalgia and Chronic Fatigue Syndrome Rating Scale (FF), Hamilton Depression Rating Scale (HDRS) | IS: Depressed individuals had ↑ circulating IgM and ↑ IgA antibodies against LPS, indicating increased bacterial translocation. IgA concentration correlated with severity of gastrointestinal symptoms. |
| ES: None Reported | ||||||
| CNS: None Reported | ||||||
| 3 | (Stevens et al., 2018) | Afferent (microbiota to host system) | DSM-5 criteria for a depressive or anxiety disorder | None | None | IS: Individuals with depressive and/or anxiety disorders had altered plasma levels of: ↑ LPS, ↑ zonulin, and ↑ fatty acid-binding protein 2, as well as an overrepresentation of LPS biosynthesis genes in the fecal microbiome. |
| ES: None Reported | ||||||
| CNS: None Reported | ||||||
| 4 | (Kiecolt-Glaser et al., 2018) | Afferent (microbiota to host system) | No diagnostic inclusion criteria | None | Couples Satisfaction Index (CSI), Behavioral coding of exchanges via the Rapid Marital Interaction Coding System (RMICS), Structured Clinical Interview for the DSM-IV, nonpatient version (SCID-IV-NP) | IS: Couples with hostile marital interactions had altered circulating: ↑ LPS binding protein (LBP), and ↑ CRP, and individuals with a history of mood disorders had altered circulating levels: ↑ ratio LBP/sCD14 and ↑ CRP |
| ES: None Reported | ||||||
| CNS: None Reported | ||||||
| 5 | (Akkasheh et al., 2016) | Afferent (microbiota to host system) | DSM-IV diagnosis of major depressive disorder | Three-strain probiotic blend (L. acidophilus, L. casei, and B. bifidum) | Beck Depression Inventory (BDI) | IS: Depressed individuals receiving the probiotic had ↓ circulating CRP. |
| None Reported | ||||||
| CNS: Probiotic treatment decreased depression symptoms | ||||||
| 6 | (Nishihira et al., 2014) | Afferent (microbiota to host system) | Healthy subjects | Probiotic yogurt containing L. gasseri SBT2055 and B. longum SBT2928 | General Health Questionnaire-28 (GHQ-28) | IS: Probiotic treatment resulted in ↑ activity of circulating NK cells. |
| ES: Probiotic treatment ↓ circulating ACTH | ||||||
| CNS: Probiotic treatment decreased anxiety, but not depression, symptoms | ||||||
| 7 | (Marcos et al., 2004) | Afferent (microbiota to host system) | Healthy subjects | Probiotic milk beverage containing L. casei DN-11400 | None | IS: Twice daily probiotic consumption resulted in ↑ blood lymphocytes and rescued ↓ CD56+ cells in response to stress. |
| ES: Probiotic treatment prevented the ↑ in morning cortisol due to exam stress. | ||||||
| CNS: Probiotic treatment had no effect on anxiety symptoms | ||||||
| 8 | (Shinkai et al., 2013) | Afferent (microbiota to host system) | Healthy subjects over 65 years of age | L. pentosus b240 | Profile of Mood States Questionnaire (POMS), 36-Item Short Form Survey (SF-36) | IS: Probiotic treatment ↓ incidence of the common cold. |
| ES: None Reported | ||||||
| CNS: Probiotic treatment had no effect on depression or anxiety symptoms, but did improve general health perception | ||||||
| 9 | (Romijn, et al., 2017) | Afferent (microbiota to host system) | Self-reported depression | L. helveticus R0052 and B. longum R0175 | Depression, Anxiety and Stress Scales (DASS-42), Global Assessment of Functioning (GAF), Improved Clinical Global Impressions scale (iCGI), Montgomery–Åsberg Depression Rating Scale (MADRS), Quick Inventory of Depressive Symptomatology-Self-Report-16 (QIDS-SR-16) | IS: Probiotic treatment resulted in no change to measured circulating immune markers. |
| ES: None Reported | ||||||
| CNS: Probiotic treatment had no effect on depression or anxiety symptoms | ||||||
| 10 | (Rudzki et al., 2019) | Afferent (microbiota to host system) | DSM-IV-R diagnosis of major depression | L. plantarum 299v | Attention and Perceptivity Test (APT), California Verbal Learning Test (CVLT), HAMD, Symptom Checklist-90 (SC-90), Perceived Stress Scale (PSS) | IS: Probiotic treatment resulted in no change to measured circulating immune markers. |
| ES: None Reported | ||||||
| CNS: Probiotic treatment had no effect on depression or anxiety symptoms, however it did increase cognitive functioning on the APT and CVLT, as well as decrease circulating kynurenine and increased the 3-HK:kynurenine ratio. | ||||||
| 16 | (Vanuytsel et al., 2014) | Efferent (system to microbiota) | Healthy subjects | Intravenous bolus injection of CRH | State Trait Anxiety Inventory (STAI) | IS: None Reported |
| ES: Both the public speech condition and the CRH injection resulted in elevated intestinal permeability as measured by a lactulose-mannitol urinary excretion test. | ||||||
| CNS: The public speech condition increased anxiety scores, while the injection of CRH did not. | ||||||
| 22 | (Szczesniak, et al., 2016) | Afferent (microbiota to system) | ICD-10 diagnosis of depression | None | MADRS | IS: None Reported |
| ES: Fecal levels of isovaleric acid positively correlated with an average of morning and mid-day salivary cortisol measurements. | ||||||
| CNS: Depression symptoms positively correlated with fecal levels of isovaleric acid | ||||||
| 23 | (Messaoudi, Lalonde, et al., 2011) | Afferent (microbiota to system) | Healthy subjects | L. helveticus R0052 and B. longum R0175 | 90-item Hopkins Symptom Checklist (HSCL-90), Coping Checklist (CCL), HADS | IS: None Reported |
| ES: Probiotic treatment reduced 24-hour urinary free cortisol | ||||||
| CNS: Probiotic treatment decreased psychological distress, including depression symptoms | ||||||
| 11 | (Gorard, et al., 1996) | Efferent (host system to microbiota) | DSM-III-R criteria for major depression and or generalized anxiety disorder | None | BDI, HADS | IS: None Reported |
| ES: None Reported | ||||||
| CNS: Individuals with GAD had the shortest whole-gut transit time when compared to individuals with MDD or controls. Whole gut transit time positively correlated with the BDI and HADS | ||||||
| 12 | (Emmanuel, et al., 2001) | Efferent (host system to microbiota) | Women with idiopathic constipation | None | GHQ-28 | IS: None Reported |
| ES: None Reported | ||||||
| CNS: Mucosal blood flow correlated negatively with scores of depression and anxiety | ||||||
| 13 | (Christian et al., 2015) | Efferent (host system to microbiota) | Healthy children | None | Early Childhood Behavior Questionnaire | IS: None Reported |
| ES: None Reported | ||||||
| CNS: Lower scores on Surgency/Extraversion were associated with decreased phylogenetic diversity in the gut | ||||||
| 14 | (Peter et al., 2018) | Afferent (microbiota to host system) | Subjects with IBD | None | HADS | IS: None Reported |
| ES: None Reported | ||||||
| CNS: 65% of individuals with IBD exhibited elevated psychological distress and 21% exhibited symptoms of depression | ||||||
| 15 | (Murphy, et al., 2018) | Afferent (microbiota to host system) | Women exposed to antibiotics peripartum | None | Edinburgh Postnatal Depression Scale (EPDS), PSS | IS: None Reported |
| ES: None Reported | ||||||
| CNS: Antibiotic exposure predicted depressive symptoms at 1 month, but not at 3 or 6 months, postpartum | ||||||
| 16 | (Kazemi et al., 2018) | Afferent (microbiota to host system) | Mild to moderate major depression | L. helveticus R0052 and B. longum R0175 or galacto-oligosaccharide | BDI, HADS | IS: None Reported |
| ES: None Reported | ||||||
| CNS: Probiotic treatment, but not prebiotic treatment, decreased depressive symptoms. Probiotic treatment resulted in ↓ circulating kynurenine/tryptophan ratio, when adjusting for isoleucine | ||||||
| 17 | (Miki et al., 2016) | Afferent (microbiota to host system) | Healthy subjects | None | Center for Epidemiologic Studies Depression scale (CES-D) | IS: None Reported |
| ES: None Reported | ||||||
| CNS: Dietary fiber intake negatively correlated with symptoms of depression | ||||||
| 18 | (Pinto-Sanchez et al., 2017) | Afferent (microbiota to host system) | Mild to moderate anxiety and/or depression scores on the HADS | B. longum NCC3001 | HADS | IS: None Reported |
| ES: None Reported | ||||||
| CNS: Probiotic treatment showed sustained reduction in depression symptoms, but not anxiety symptoms | ||||||
| 19 | (Mohammadi et al., 2016) | Afferent (microbiota to host system) | Healthy subjects | Probiotic capsule, probiotic yogurt, or conventional yogurt | GHQ-28 | IS: None Reported |
| ES: None Reported | ||||||
| CNS: Both probiotic treatment conditions decreased depression symptoms | ||||||
| 20 | (Messaoudi, Violle, et al., 2011) | Afferent (microbiota to host system) | Healthy subjects | L. helveticus R0052 and B. longum R0175 | HSCL-90 | IS: None Reported |
| ES: Individuals who are low in urinary free cortisol still benefit from probiotic treatment | ||||||
| CNS: Probiotic treatment decreased psychological distress, including depression symptoms | ||||||
| 21 | (Slykerman et al., 2017) | Afferent (microbiota to host system) | Pregnant woman (due to risk for postpartum depression) | L. rhamnosus HN001 | EPDS, STAI | IS: None Reported |
| ES: None Reported | ||||||
| CNS: Probiotic treatment decreased depression and anxiety symptoms | ||||||
| 22 | (Benton et al., 2007) | Afferent (microbiota to host system) | Healthy subjects | Milk drink containing L. casei Shirota | POMS | IS: None Reported |
| ES: None Reported | ||||||
| CNS: Probiotic treatment decreased depression symptoms for subjects who initially began the study in the bottom third of the depressed/elated POMS dimension | ||||||
| 23 | (Steenbergen et al., 2015) | Afferent (microbiota to host system) | Healthy subjects | Multi-strain probiotic | Leiden Index of Depression Sensitivity Scale | IS: None Reported |
| ES: None Reported | ||||||
| CNS: Probiotic treatment decreased cognitive reactivity to sad mood | ||||||
| 24 | (Rao et al., 2009) | Afferent (microbiota to host system) | Subjects with chronic fatigue syndrome | L. casei Shirota | Beck Anxiety Inventory (BAI), BDI | IS: None Reported |
| ES: None Reported | ||||||
| CNS: Probiotic treatment decreased anxiety, but not depression, symptoms | ||||||
| 25 | (Chung et al., 2014) | Afferent (microbiota to host system) | Healthy subjects between 60 and 75 years of age | Fermented milk containing L. helveticus IDCC3801 | Geriatric Depression Scale Short Form, PSS, Neuropsychological test battery | IS: None Reported |
| ES: None Reported | ||||||
| CNS: Probiotic treatment had no effect on depression or anxiety symptoms, but did↑ cognitive functioning on a battery of neuropsychological tests | ||||||
| 26 | (Östlund-Lagerström et al., 2015) | Afferent (microbiota to host system) | Healthy subjects over 65 years of age | L. reuteri DSM17938 | HADS, EuroQuol, PSS | IS: None Reported |
| ES: None Reported | ||||||
| CNS: Probiotic treatment had no effect on symptoms of depression, anxiety, or well-being |
Acknowledgements
We gratefully acknowledge Zachary D. Barger for proofreading the manuscript. Christopher A. Lowry is supported by the National Institute of Mental Health (grant number 1R21MH116263), Department of the Navy, Office of Naval Research Multidisciplinary University Research Initiative (MURI) Award (grant number N00014-15-1-2809), Department of Veterans Affairs Office of Research and Development (VA-ORD) RR&D Small Projects in Rehabilitation Research (SPiRE) (I21) (grant number 1 I21 RX002232-01), the Colorado Department of Public Health and Environment (CDPHE; grant number DCEED-3510), and the Alfred P. Sloan Foundation (grant number, G-2016-7077). Christopher A. Lowry serves on the Scientific Advisory Board of Immodulon Therapeutics Ltd.
Abbreviations
- ACC
anterior cingulate cortex
- ACTH
adrenocorticotropic hormone
- ANS
autonomic nervous system
- BBB
blood-brain barrier
- BDI
Beck Depression Inventory
- BDNF
brain-derived neurotrophic factor
- CNS
central nervous system
- Con-A
Concanavalin A
- CA1
cornus ammonis region 1
- CA3
cornus ammonis region 3
- Cg1
cingulate cortex area 1
- CMS
chronic mild stress
- CRH
corticotropin-releasing hormone; corticotropin-releasing factor
- DAMP
danger-associated molecular pattern
- DOPAC
3,4-dihydroxyphenylacetic acid
- FMT
fecal microbiota transplant
- FRL
Flinders Resistant Line
- FSL
Flinders Sensitive Line
- GABA
gamma-aminobutyric acid
- GAD
generalized anxiety disorder
- GALT
gut-associated lymphoid tissue
- GF
germ-free
- GHQ-28
General Health Questionnaire-28
- HADS
Hospital Anxiety and Depression Scale
- HPA
hypothalamic-pituitary-adrenal
- HSCL-90
90-item version of the Hopkins Symptom Checklist
- HVA
homovanillic acid
- IBD
inflammatory bowel disease
- IDO1
indoleamine 2,3-dioxygenase 1
- IFNɣ
interferon gamma
- IL-1β
interleukin 1 beta
- IL-6
interleukin 6
- LBP
LPS binding protein
- LPS
lipopolysaccharide
- MCP-1
monocyte chemoattractant protein 1
- MDD
major depressive disorder
- MAMP
microbe-associated molecular pattern
- MGB
microbiome-gut-brain
- MS
maternal separation model
- MAPK
mitogen-activated protein kinase
- NMDA
N-methyl-D-aspartate
- Nrf2
nuclear factor (erythroid-derived)-2
- OTU
operational taxonomic unit
- PAMP
pathogen-associated molecular pattern
- PATHOS-D
pathogen host defense
- PFC
prefrontal cortex
- PNS
parasympathetic nervous system
- POMS
Profile of Mood States
- PrL
prelimbic cortex
- RCT
randomized controlled trial
- SCFA
short-chain fatty acid
- SPF
specific pathogen-free
- Spp
species, referencing all species of a given genus
- SNS
sympathetic nervous system
- SSRI
selective serotonin reuptake inhibitor
- STAMP
sequential tagging with D-amino acid-based metabolic probes
- TLR
toll-like receptor
- TNF
tumor necrosis factor
- Treg
regulatory T cell
- TrpV1
transient receptor potential cation channel subfamily V member 1
- TrkB
tropomyosin receptor kinase B
- WGS
whole genome sequencing
- 3-HK
3-hydroxykynurenine
- 5-HT
5-hydroxytryptamine, serotonin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abildgaard A, Elfving B, Hokland M, Lund S, & Wegener G (2017). Probiotic treatment protects against the pro-depressant-like effect of high-fat diet in Flinders Sensitive Line rats. Brain, Behavior, and Immunity, 65, 33–42. 10.1016/j.bbi.2017.04.017 [DOI] [PubMed] [Google Scholar]
- Abildgaard A, Elfving B, Hokland M, Wegener G, & Lund S (2017). Probiotic treatment reduces depressive-like behaviour in rats independently of diet. Psychoneuroendocrinology, 79, 40–48. 10.1016/j.psyneuen.2017.02.014 [DOI] [PubMed] [Google Scholar]
- Addolorato G, Mirijello A, D’Angelo C, Leggio L, Ferrulli A, Abenavoli L, … Gasbarrini G (2008). State and trait anxiety and depression in patients affected by gastrointestinal diseases: Psychometric evaluation of 1641 patients referred to an internal medicine outpatient setting. International Journal of Clinical Practice, 62(1), 1063–1069. 10.1111/j.1742-1241.2008.01763.x [DOI] [PubMed] [Google Scholar]
- Agusti A, Moya-Pérez A, Campillo I, Montserrat-de laPaz S, Cerrudo V, Perez-Villalba A , & Sanz Y (2018). Bifidobacterium pseudocatenulatum CECT 7765 Ameliorates Neuroendocrine Alterations Associated with an Exaggerated Stress Response and Anhedonia in Obese Mice. Molecular Neurobiology, 55(6), 5337–5352. 10.1007/sl2035-017-0768-z [DOI] [PubMed] [Google Scholar]
- Ahmed SMU, Luo L, Namani A, Wang XJ, & Tang X (2017). Nrf2 signaling pathway: Pivotal roles in inflammation. Biochimica et Biophysica Acta (BBA) - Mole cidar Basis of Disease, 1863(2), 585–597. 10.1016/j.bbadis.2016.ll.005 [DOI] [PubMed] [Google Scholar]
- Ait-Belgnaoui A, Colom A, Braniste V, Ramalho L, Marrot A, Cartier C, … Tompkins T (2014). Probiotic gut effect prevents the chronic psychological stress-induced brain activity abnormality in mice. Neurogastroenterology and Motility, 26(4), 510–520. 10.1111/nmo.12295 [DOI] [PubMed] [Google Scholar]
- Ait-Belgnaoui A, Durand H, Cartier C, Chaumaz G, Eutamene H, Ferrier L, … Theodorou V (2012). Prevention of gut leakiness by a probiotic treatment leads to attenuated HPA response to an acute psychological stress in rats. Psychoneuroendocrinology, 57(11), 1885–1895. 10.1016/j.psyneuen.2012.03.024 [DOI] [PubMed] [Google Scholar]
- Aizawa E, Tsuji EL, Asahara T, Takahashi T, Teraishi T, Yoshida S, … Kunugi H (2016). Possible association of Bifidobacterium and Lactobacillus in the gut microbiota of patients with major depressive disorder. Journal of Affective Disorders, 202, 254–257. 10.1016/j.jad.2016.05.038 [DOI] [PubMed] [Google Scholar]
- Akil EL, Gordon J, Hen R, Javitch J, Mayberg H, McEwen B, … Nestler EJ (2018). Treatment resistant depression: A multi-scale, systems biology approach. Neuroscience & BiobehavioralReviews, 84(2), 272–288. 10.1016/j.neubiorev.2017.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akkasheh G, Kashani-Poor Z, Tajabadi-Ebrahimi M, Jafari P, Akbari H, Taghizadeh M, … Esmaillzadeh A (2016). Clinical and metabolic response to probiotic administration in patients with major depressive disorder: A randomized, double-blind, placebo-controlled trial. Nutrition, 32(3), 315–320. 10.1016/j.nut.2015.09.003 [DOI] [PubMed] [Google Scholar]
- Al-Asmakh M, & Zadjali F (2015). Use of germ-free animal models in microbiota-related research. Journal of Microbiology and Biotechnology, 25(10), 1583–1588. 10.4014/jmb.1501.01039 [DOI] [PubMed] [Google Scholar]
- Al KF, Bisanz JE, Gloor GB, Reid G, & Burton JP (2018). Evaluation of sampling and storage procedures on preserving the community structure of stool microbiota: A simple at-home toilet-paper collection method. Journal of Microbiological Methods, 144 (September 2017), 117–121. 10.1016/j.mimet.2017.11.014 [DOI] [PubMed] [Google Scholar]
- Ancelin ML, Scab J, Norton J, Ritchie K, Dupuy AM, Chaudieu L, & Ryan J (2017). Heterogeneity in HPA axis dysregulation and serotonergic vulnerability to depression. Psychoneuroendocrinology, 77, 90–94. 10.1016/j.psyneuen.2016.11.016 [DOI] [PubMed] [Google Scholar]
- Andrade C (2014). Antidepressant augmentation with anti-inflammatory agents. Journal of Clinical Psychiatry, 75(9), 975–977. 10.4088/JCP.14f09432 [DOI] [PubMed] [Google Scholar]
- Anhê FF, Roy D, Pilon G, Dudonné S, Matamoros S, Varin TV, … Marette A (2015). A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut, 64(6), 872–883. 10.1136/gutjnl-2014-307142 [DOI] [PubMed] [Google Scholar]
- Anthes E (2014). Depression: a change of mind. Nature, 575(7526), 185–187. 10.1038/515185a [DOI] [PubMed] [Google Scholar]
- Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, … Bork P (2011). Enterotypes of the human gut microbiome. Nature, 473(7346), 174–180. 10.1038/nature09944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano Y, Hiramoto T, Nishino R, Aiba Y, Kimura T, Yoshihara K, … Sudo N (2012). Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. American Journal of Physiology-Gastrointestinal and Liver Physiology, 303(11), G1288–G1295. 10.1152/ajpgi.00341.2012 [DOI] [PubMed] [Google Scholar]
- Association American Psychiatric. (2013). Diagnostic and Statistical Manual of Mental Disorders (5th ed.). Washington, DC: American Psychiatric Publishing; 10.1176/appi.books.9780890425596.744053 [DOI] [Google Scholar]
- Aston-Jones G, & Cohen JD (2005). An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annual Review of Neuroscience, 25(1), 403–450. 10.1146/annurev.neuro.28.061604.135709 [DOI] [PubMed] [Google Scholar]
- Bäckhed F, Fraser CM, Ringel Y, Sanders ME, Sartor RB, Sherman PM, … Finlay BB. (2012). Defining a healthy human gut microbiome: Current concepts, future directions, and clinical applications. Cell Host and Microbe, 72(5), 611–622. 10.1016/j.chom.2012.10.012 [DOI] [PubMed] [Google Scholar]
- Bailey MT, Dowd SE, Galley JD, Hufnagle AR, Allen RG, & Lyte M (2011). Exposure to a social stressor alters the structure of the intestinal microbiota: Implications for stressor-induced immunomodulation. Brain, Behavior, and Immunity, 25(3), 397–407. 10.1016/j.bbi.2010.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey MT, Dowd SE, Parry NMA, Galley JD, Schauer DB, & Lyte M (2010). Stressor exposure disrupts commensal microbial populations in the intestines and leads to increased colonization by Citrobacter rodentium. Infection and Immunity, 75(4), 1509–1519. 10.1128/IAI.00862-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks W (2005). Blood-Brain Barrier Transport of Cytokines: A Mechanism for Neuropathology. Current Pharmaceutical Design, 77(8), 973–984. 10.2174/1381612053381684 [DOI] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ, & Broadwell RD (1995). Passage of Cytokines across the Blood-Brain Barrier. Neuroimmunomodulation, 2(4), 241–248. 10.1159/000097202 [DOI] [PubMed] [Google Scholar]
- Banks W. a, & Robinson SM (2010). Minimal penetration of lipopolysaccharide across the murine blood-brain barrier. Brain, Behavior, and Immunity, 24{ 1), 102–109. 10.1016/j.bbi.2009.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett E, Ross RP, O’Toole PW, Fitzgerald GF, & Stanton C (2012). γ-Aminobutyric acid production by culturable bacteria from the human intestine. Journal of Applied Microbiology, 775(2), 411–417. 10.1111/j.1365-2672.2012.05344.x [DOI] [PubMed] [Google Scholar]
- Barton W, Penney NC, Cronin O, Garcia-Perez F, Molloy MG, Holmes E, … O’Sullivan O. (2018). The microbiome of professional athletes differs from that of more sedentary subjects in composition and particularly at the functional metabolic level. Gut, 67(4), 625–633. 10.1136/gutjnl-2016-313627 [DOI] [PubMed] [Google Scholar]
- Bassis CM, Moore NM, Lolans K, Seekatz AM, Weinstein RA, Young VB, & Hayden MK (2017). Comparison of stool versus rectal swab samples and storage conditions on bacterial community profiles. BMC Microbiology, 77(1), 1–7. 10.1186/sl2866-017-0983-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S, & Dasgupta PS (2000). Dopamine, a neurotransmitter, influences the immune system. Journal of Neuroimmunology, 102(2), 113–124. 10.1016/S0165-5728(99)00176-9 [DOI] [PubMed] [Google Scholar]
- Beasley DE, Koltz AM, Lambert JE, Fierer N, & Dunn RR (2015). The evolution of stomach acidity and its relevance to the human microbiome. PLoS ONE, 10(1), 1–12. 10.1371/joumal.pone.0134116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton D, Williams C, & Brown A (2007). Impact of consuming a milk drink containing a probiotic on mood and cognition. European Journal of Clinical Nutrition, 67(3), 355–361. 10.1038/sj.ejcn.1602546 [DOI] [PubMed] [Google Scholar]
- Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J, … Collins SM. (2011). The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology, 141(2), 599–609. 10.1053/j.gastro.2011.04.052 [DOI] [PubMed] [Google Scholar]
- Bercik P, Park AJ, Sinclair D, Khoshdel A, Lu J, Huang X, … Verdu EF. (2011). The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterology and Motility, 23(12), 1132–1139. 10.1111/j.1365-2982.2011.01796.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg G, Rybakova D, Grube M, & Köberl M (2016). The plant microbiome explored: implications for experimental botany. Journal of Experimental Botany, 67(4), 995–1002. 10.1093/jxb/erv466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergner CL, Smolinsky AN, Hart PC, Dufour BD, Egan RJ, Laporte JL, & Kalueff AV (2010). Mouse Models for Studying Depression-Like States and Antidepressant Drugs In Proetzel G & Wiles MV (Eds), Mouse Models for Drug Discovery (2nd Editio, Vol. 602, pp. 255–269). Totowa, NJ: Humana Press; 10.1007/978-l-60761-058-8 [DOI] [Google Scholar]
- Bessette KL, Jenkins LM, Skerrett KA, Gowins JR, Deldonno SR, Zubieta J, … Langenecker SA (2018). Reliability , Convergent Validity and Time Invariance of Default Mode Network Deviations in Early Adult Major Depressive Disorder, 9(June), 1–15. 10.3389/fpsyt.2018.00244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bested AC, Logan AC, & Selhub EM (2013a). Intestinal microbiota, probiotics and mental health: From Metchnikoff to modern advances: Part I - Autointoxication revisited. Gut Pathogens, 5(1), 1 10.1186/1757-4749-5-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bested AC, Logan AC, & Selhub EM (2013b). Intestinal microbiota, probiotics and mental health: from Metchnikoff to modern advances: Part II - contemporary contextual research. Gut Pathogens, 5(1), 3 10.1186/1757-4749-5-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bested AC, Logan AC, & Selhub EM (2013c). Intestinal microbiota, probiotics and mental health: From Metchnikoff to modern advances: Part III - Convergence toward clinical trials. Gut Pathogens, 5(1), 1 10.1186/1757-4749-5-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharwani A, Mian MF, Surette MG, Bienenstock J, & Forsythe P (2017). Oral treatment with Lactobacillus rhamnosus attenuates behavioural deficits and immune changes in chronic social stress. BMC Medicine, 75(1), 1–14. 10.1186/sl2916-016-0771-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattarai Y, & Kashyap PC (2010). Germ-Free Mice Model for Studying Host-Microbial Interactions In Proetzel G & Wiles MV (Eds.), Mouse Models for Drug Discovery (2nd Editio, Vol. 602, pp. 123–135). Totowa, NJ: Humana Press, 10.1007/978-1-60761-058-8 [DOI] [PubMed] [Google Scholar]
- Björkholm C, & Monteggia LM (2016). BDNF – a key transducer of antidepressant effects. Neuropharmacology, 102, 72–79. 10.1016/j.neuropharm.2015.10.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blalock JB (1981). History and evolution of peptic ulcer surgery. The American Journal of Surgery, 141(3), 317–322. 10.1016/0002-9610(81)90187-2 [DOI] [PubMed] [Google Scholar]
- Blaser MJ. (2016). Antibiotic use and its consequences for the normal microbiome. Science, 352(6285), 544–545. 10.1126/science.aad9358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdan R, Nikolova YS, & Pizzagalli DA (2013). Neurogenetics of depression: A focus on reward processing and stress sensitivity. Neurobiology of Disease, 52(3), 12–23. 10.1016/j.nbd.2012.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boku S, Nakagawa S, Toda H, & Hishimoto A (2018). Neural basis of major depressive disorder: Beyond monoamine hypothesis. Computer Graphics Forum, 37(2), 3–12. 10.1111/pcn.12604 [DOI] [PubMed] [Google Scholar]
- Bollini P, Tribaldi G, Testa C, & Munizza C (2004). Understanding treatment adherence in affective disorders : a qualitative study. Journal of Psychiatric and Mental Health Nursing, 11, 668–674. [DOI] [PubMed] [Google Scholar]
- Boursi B, Werner TJ, Gholami S, Houshmand S, Mamtani R, Lewis JD, … Yang YX. (2018). Functional imaging of the interaction between gut microbiota and the human host: A proof-of-concept clinical study evaluating novel use for 18F-FDG PET-CT. PLoS ONE, 13(2), 1–9. 10.1371/journal.pone.0192747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowe WP, & Logan AC (2011). Acne vulgaris, probiotics and the gut-brain-skin axis - Back to the future? Gut Pathogens, 5(1), 1–11. 10.1186/1757-4749-3-l [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradesi S, McRoberts JA, Ohning G, Schwetz I, Pothoulakis C, Fanselow M, … Lamy CMR (2005). Repeated exposure to water avoidance stress in rats: a new model for sustained visceral hyperalgesia. American Journal of Physiology-Gastrointestinal and Liver Physiology, 259(1), G42–G53. 10.1152/ajpgi.00500.2004 [DOI] [PubMed] [Google Scholar]
- Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, … Cryan JF. (2011). Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proceedings of the National Academy of Sciences, 705(38), 16050–16055. 10.1073/pnas.1102999108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo Javier A., Julio-Pieper M, Forsythe P, Kunze W, Dinan TG, Bienenstock J, & Cryan JF (2012). Communication between gastrointestinal bacteria and the nervous system. Current Opinion in Pharmacology, 12(6), 667–672. 10.1016/j.coph.2012.09.010 [DOI] [PubMed] [Google Scholar]
- Breit S, Kupferberg A, Rogler G, & Hasler G (2018). Vagus nerve as modulator of the brain-gut axis in psychiatric and inflammatory disorders. Frontiers in Psychiatry, 9(March). 10.3389/fpsyt.2018.00044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridgewater LC, Zhang C, Wu Y, Hu W, Zhang Q, Wang J, … Zhao L (2017). Gender-based differences in host behavior and gut microbiota composition in response to high fat diet and stress in a mouse model. Scientific Reports, 7(1), 1–12. 10.1038/s41598-017-11069-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brierley S, & Costa M (Eds.). (2016). The Enteric Nervous System: 30 Years Later (Vol. 891). Switzerland: Springer International Publishing, 10.1007/978-3-319-27592-5 [DOI] [Google Scholar]
- Brites D, & Fernandes A (2015). Neuroinflammation and Depression: Microglia Activation, Extracellular Microvesicles and microRNA Dysregulation. Frontiers in Cellular Neuroscience, 9(December), 1–20. 10.3389/fncel.2015.00476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broecker F, Klumpp J, & Moelling K (2016). Long-term microbiota and virome in a Zurich patient after fecal transplantation against Clostridium difficile infection. Annals of the New York Academy of Sciences, 1372(1), 29–41. 10.1111/nyas.13100 [DOI] [PubMed] [Google Scholar]
- Brown VJ, & Bowman EM (2002). Rodent models of prefrontal cortical function. Trends in Neurosciences, 25(7), 340–343. 10.1016/S0166-2236(02)02164-1 [DOI] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Salbaum JM, & Berthoud HR (2018). Harnessing Gut Microbes for Mental Health: Getting From Here to There. Biological Psychiatry, 55(3), 214–223. 10.1016/j.biopsych.2017.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Salbaum JM, Luo M, Blanchard E, Taylor CM, Welsh DA, & Berthoud H (2015). Obese-type Gut Microbiota Induce Neurobehavioral Changes in the Absence of Obesity. Biological Psychiatry, 77(1), 607–615. 10.1016/j.biopsych.2014.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burokas A, Arboleya S, Moloney RD, Peterson VL, Murphy K, Clarke G, … Cryan JF (2017). Targeting the Microbiota-Gut-Brain Axis: Prebiotics Have Anxiolytic and Antidepressant-like Effects and Reverse the Impact of Chronic Stress in Mice. Biological Psychiatry, 52(7), 472–487. 10.1016/j.biopsych.2016.12.031 [DOI] [PubMed] [Google Scholar]
- Burton R (1621). The Anatomy of Melancholy. [Google Scholar]
- Byrne G, Rosenfeld G, Leung Y, Qian H, Raudzus J, Nunez C, & Bressler B (2017). Prevalence of Anxiety and Depression in Patients with Inflammatory Bowel Disease. Canadian Journal of Gastroenterology and Hepatology, 2017, 1–6. 10.1155/2017/6496727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahn BR, Goodman MS, Peterson CT, Maturi R, & Mills PJ (2017). Yoga, Meditation and Mind-Body Health: Increased BDNF, Cortisol Awakening Response, and Altered Inflammatory Marker Expression after a 3-Month Yoga and Meditation Retreat. Frontiers in Human Neuroscience, 77(June), 1–13. 10.3389/fnhum.2017.00315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camilleri M, Nadeau A, Lamsam J, linker nord S, Ryks M, Burton D, … Singh R (2009). Understanding measurements of intestinal permeability in healthy humans with urine lactulose and mannitol excretion. Neurogastroenterology & Motility, 22(1), 1–22. 10.1111/j.1365-2982.2009.01361.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Can A, Dao DT, Terrillion CE, Piantadosi SC, Bhat S, & Gould TD (2011). The Tail Suspension Test. Journal of Visualized Experiments, (58), 3–7. 10.3791/3769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Hauser P, Hinze-Selch D, Miller AH, & Neveu PJ (2002). Treatment of cytokine-induced depression. Brain Behavior and Immunity, 16(5), 575–580. 10.1016/s0889-1591(02)00007-7 [DOI] [PubMed] [Google Scholar]
- Carboni L, Lourdusamy A, Keers R, McGuffin P, Schalkwyk LC, Uher R, … Domenici E (2014). The endogenous and reactive depression subtypes revisited: integrative animal and human studies implicate multiple distinct molecular mechanisms underlying major depressive disorder. BMC Medicine, 72(1), 1–14. 10.1186/1741-7015-12-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreno FR, & Frazer A (2017). Vagal Nerve Stimulation for Treatment-Resistant Depression. Neurotherapeutics, 14(3), 716–727. 10.1007/sl3311-017-0537-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda MS, Katz EG, & Blacketer C (2017). Microbiome-Gut-Brain Axis: Probiotics and Their Association With Depression. The Journal of Neuropsychiatry and Clinical Neurosciences, 29(1), 39–44. 10.1176/appi.neuropsych.15120410 [DOI] [PubMed] [Google Scholar]
- Champagne-Jorgensen K, Kunze WA, Forsythe P, Bienenstock J, & McVey Neufeld KA (2019). Antibiotics and the nervous system: More than just the microbes? Brain, Behavior, and Immunity, 77(September 2018), 7–15. 10.1016/j.bbi.2018.12.014 [DOI] [PubMed] [Google Scholar]
- Chang N-CA, Hung S-I, Hwa K-Y, Kato T, Chen J-E, Liu C-H, & Chang AC (2001). A Macrophage Protein, Yml, Transiently Expressed during Inflammation Is a Novel Mammalian Lectin. Journal of Biological Chemistry, 276(20), 17497–17506. 10.1074/jbc.M010417200 [DOI] [PubMed] [Google Scholar]
- Charles River Laboratories. (2018). C57BL/6NCrl (B6N) Germ-Free Mice. Wilmington, MA. [Google Scholar]
- Chen JJ, Zheng P, Liu YY, Zhong XG, Wang HY, Guo YJ, & Xie P (2018). Sex differences in gut microbiota in patients with major depressive disorder. Neuropsychiatric Disease and Treatment, 14, 647–655. 10.2147/NDT.S159322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng W, Rolls ET, Qiu J, Xie X, Lyu W, Li Y, … Feng J (2018). Functional connectivity of the human amygdala in health and in depression. Social Cognitive and Affective Neuroscience, 13(6), 557–568. 10.1093/scan/nsy032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiriţă AL, Gheorman V, Bondari D, & Rogoveanu I (2015). Current understanding of the neurobiology of major depressive disorder. Romanian Journal of Morphology and Embryology, 56(2), 651–658. 10.1093/nar/gks945 [DOI] [PubMed] [Google Scholar]
- Christian LM, Galley JD, Hade EM, Schoppe-Sullivan S, Kamp Dush C, & Bailey MT (2015). Gut microbiome composition is associated with temperament during early childhood. Brain, Behavior, and Immunity, 45, 118–127. 10.1016/j.bbi.2014.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrobak A, Nowakowski J, & Dudek D (2016). Interactions between the gut microbiome and the central nervous system and their role in schizophrenia, bipolar disorder and depression. Archives of Psychiatry and Psychotherapy, 18(2), 5–11. 10.12740/APP/62962 [DOI] [Google Scholar]
- Chung YC, Jin H-MM, Cui Y, Kim DS, Jung JM, Park J-I Il, … Chae S-WW (2014). Fermented milk of Lactobacillus helveticus IDCC3801 improves cognitive functioning during cognitive fatigue tests in healthy older adults. Journal of Functional Foods, 10, 465–474. 10.1016/j.jff.2014.07.007 [DOI] [Google Scholar]
- Cimpianu CL, Strube W, Falkai P, Palm U, & Hasan A (2017). Vagus nerve stimulation in psychiatry: a systematic review of the available evidence. Journal of Neural Transmission, 124(1), 145–158. 10.1007/s00702-016-1642-2 [DOI] [PubMed] [Google Scholar]
- Claesson MJ, Clooney AG, & O’Toole PW (2017). A clinician’s guide to microbiome analysis. Nature Reviews Gastroenterology and Hepatology, 14(10), 585–595. 10.1038/nrgastro.2017.97 [DOI] [PubMed] [Google Scholar]
- Clavel T, Lagkouvardos I, Blaut M, & Stecher B (2016). The mouse gut microbiome revisited: From complex diversity to model ecosystems. International Journal of Medical Microbiology, 306(5), 316–327. 10.1016/j.ijmm.2016.03.002 [DOI] [PubMed] [Google Scholar]
- Codella R, Luzi L, & Terruzzi I (2018). Exercise has the guts: How physical activity may positively modulate gut microbiota in chronic and immune-based diseases. Digestive and Liver Disease, 50(4), 331–341. 10.1016/j.dld.2017.11.016 [DOI] [PubMed] [Google Scholar]
- Collins JW, Keeney KM, Crepin VF, Rathinam VAK, Fitzgerald KA, Finlay BB, & Frankel G (2014). Citrobacter rodentium: infection, inflammation and the microbiota. Nature Reviews Microbiology, 12(9), 612–623. 10.1038/nrmicro3315 [DOI] [PubMed] [Google Scholar]
- Collins SM, Kassam Z, & Bercik P (2013). The adoptive transfer of behavioral phenotype via the intestinal microbiota: Experimental evidence and clinical implications. Current Opinion in Microbiology, 16(3), 240–245. 10.1016/j.mib.2013.06.004 [DOI] [PubMed] [Google Scholar]
- Colombel JF, Sandbom WJ, Reinisch W, Mantzaris GJ, Kombluth A, Rachmilewitz D, … Rutgeerts P (2010). Infliximab, Azathioprine, or Combination Therapy for Crohn’s Disease. New England Journal of Medicine, 362(15), 1383–1395. 10.1056/NEJMoa0904492 [DOI] [PubMed] [Google Scholar]
- Couillard-Despres S, Winner B, Schaubeck S, Aigner R, Vroemen M, Weidner N, … Aigner L (2005). Doublecortin expression levels in adult brain reflect neurogenesis. European Journal of Neuroscience, 21(1), 1–14. 10.1111/j.1460-9568.2004.03813.x [DOI] [PubMed] [Google Scholar]
- Cowen PJ (2017). Backing into the future: pharmacological approaches to the management of resistant depression. Psychological Medicine, 47(15), 2569–2577. 10.1017/s003329171700068x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crumeyrolle-Arias M, Jaglin M, Bruneau A, Vancassel S, Cardona A, Daugé V, … Rabot S (2014). Absence of the gut microbiota enhances anxiety-like behavior and neuroendocrine response to acute stress in rats. Psychoneuroendocrinology, 42, 207–217. 10.1016/j.psyneuen.2014.01.014 [DOI] [PubMed] [Google Scholar]
- Cryan JF, & O’Mahony SM (2011). The microbiome-gut-brain axis: From bowel to behavior. Neurogastroenterology and Motility, 23(3), 187–192. 10.1111/j.1365-2982.2010.01664.X [DOI] [PubMed] [Google Scholar]
- Cull-Candy S, Brickley S, & Farrant M (2001). NMDA receptor subunits: diversity, development and disease. Current Opinion in Neurobiology, 11(3), 327–335. 10.1016/S0959-4388(00)00215-4 [DOI] [PubMed] [Google Scholar]
- Cussotto S, Sandhu KV, Dinan TG, & Cryan JF (2018). The Neuroendocrinology of the Microbiota-Gut-Brain Axis: A Behavioural Perspective. Frontiers in Neuroendocrinology, 57(May), 80–101. 10.1016/j.yfme.2018.04.002 [DOI] [PubMed] [Google Scholar]
- Dantzer R (2001). Cytokine-induced sickness behavior: Where do we stand? Brain, Behavior, and Immunity, 75(1), 7–24. 10.1006/brbi.2000.0613 [DOI] [PubMed] [Google Scholar]
- de Maat SM, Dekker J, Schoevers RA, & de Jonghe F (2007). Relative efficacy of psychotherapy and combined therapy in the treatment of depression: A meta-analysis. European Psychiatry, 22(1), 1–8. 10.1016/j.eurpsy.2006.10.008 [DOI] [PubMed] [Google Scholar]
- Desbonnet L, Garrett L, Clarke G, Bienenstock J, & Dinan TG (2008). The probiotic Bifidobacteria infantis: An assessment of potential antidepressant properties in the rat. Journal of Psychiatric Research, 43(2), 164–174. 10.1016/j.jpsychires.2008.03.009 [DOI] [PubMed] [Google Scholar]
- Desbonnet L, Garrett L, Clarke G, Kiely B, Cryan JF, & Dinan TG (2010). Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience, 170(4), 1179–1188. 10.1016/j.neuroscience.2010.08.005 [DOI] [PubMed] [Google Scholar]
- Dichter GS, Gibbs D, & Smoski MJ (2015). A systematic review of relations between resting-state functional-MRI and treatment response in major depressive disorder. Journal of Affective Disorders, 172(4 PART 2), 8–17. 10.1016/j.jad.2014.09.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickens C (1867). Stomach and Heart. All the Year Round, 77(419), 438–440. [Google Scholar]
- Diepenbroek C, Stephens R, Pan A, Anderson S, de Lartigue G, Quinn D, & Zollinger B (2017). Validation and characterization of a novel method for selective vagal deafferentation of the gut. American Journal of Physiology-Gastrointestinal and Liver Physiology, 212(4), G342–G352. 10.1152/ajpgi.00095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinan TG, & Cryan JF (2017a). Brain-Gut-Microbiota Axis and Mental Health. Psychosomatic Medicine, 79(8), 920–926. 10.1097/PSY.0000000000000519 [DOI] [PubMed] [Google Scholar]
- Dinan TG, & Cryan JF (2017b). Gut instincts: microbiota as a key regulator of brain development, ageing and neurodegeneration. Journal of Physiology, 595(2), 489–503. 10.1113/JP273106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinan TG, Stanton C, & Cryan JF (2013). Psychobiotics: A novel class of psychotropic. Biological Psychiatry, 74(10), 720–726. 10.1016/j.biopsych.2013.05.001 [DOI] [PubMed] [Google Scholar]
- Ding T, & Schloss PD (2014). Dynamics and associations of microbial community types across the human body. Nature, 509(1500), 357–360. 10.1038/naturel3178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diviccaro S, Giatti S, Borgo F, Barcella M, Borghi E, Trejo JL, … Melcangi RC (2019). Treatment of male rats with finasteride, an inhibitor of 5alpha-reductase enzyme, induces long-lasting effects on depressive-like behavior, hippocampal neurogenesis, neuroinflammation and gut microbiota composition. Psychoneuroendocrinology, 99(September 2018), 206–215. 10.1016/j.psyneuen.2018.09.021 [DOI] [PubMed] [Google Scholar]
- Dragunow M, & Faull R (1989). The use of c-fos as a metabolic marker in neuronal pathway tracing. Journal of Neuroscience Methods, 29(3), 261–265. 10.1016/0165-0270(89)90150-7 [DOI] [PubMed] [Google Scholar]
- Drevets WC (2007). Orbitofrontal cortex function and structure in depression. Annals of the New York Academy of Sciences, 1121, 499–527. [DOI] [PubMed] [Google Scholar]
- Dunlop BW, & Nemeroff CB (2007). The Role of Dopamine in the Pathophysiology of Depression. Archives of General Psychiatry, 64(3), 327 10.1001/archpsyc.64.3.327 [DOI] [PubMed] [Google Scholar]
- Dunn AJ (2006). Effects of cytokines and infections on brain neurochemistry. Clinical Neuroscience Research, 6(1–2), 52–68. 10.1016/j.cnr.2006.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn AJ, Swiergiel AEL, & De Beaurepaire R (2005). Cytokines as mediators of depression: What can we learn from animal studies? Neuroscience and Biobehavioral Reviews, 29(4–5), 891–909. 10.1016/j.neubiorev.2005.03.023 [DOI] [PubMed] [Google Scholar]
- Dunwiddie TV, & Masino SA (2001). The role and regulation of adenosine in the central nervous system. Annual Review of Neuroscience, 24(1), 31–55. 10.1146/annurev.neuro.24.1.31 [DOI] [PubMed] [Google Scholar]
- Dusi N, Barlati S, Vita A, & Brambilla P (2015). Brain Structural Effects of Antidepressant Treatment in Major Depression. Current Neuropharmacology, 13(4), 458–465. 10.2174/1570159X1304150831121909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmanuel AV, & Kamm MA (1999). Laser Doppler measurement of rectal mucosal blood flow. Gut, 45(1), 64–69. 10.1136/gut.45.T64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmanuel AV, Mason HJ, & Kamm MA (2001). Relationship between psychological state and level of activity of extrinsic gut innervation in patients with a functional gut disorder. Gut, 49(2), 209–213. 10.1136/gut.49.2.209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enaud R, Vandenborght L-E, Coron N, Bazin T, Prevel R, Schaeverbeke T, … Delhaes L (2018). The Mycobiome: A Neglected Component in the Microbiota-Gut-Brain Axis. Microorganisms, 6(1), 22 10.3390/microorganisms6010022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairhurst NG, & Travis SPL (2018). Why is it so difficult to evaluate faecal microbiota transplantation as a treatment for ulcerative colitis? Intestinal Research, 16(2), 209 10.5217/ir.2018.16.2.209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falony G, Joossens M, Vieira-Silva S, Wang J, Darzi Y, Faust K, … Raes J (2016). Population-level analysis of gut microbiome variation. Science. 10.1126/science.aad3503 [DOI] [PubMed] [Google Scholar]
- Faro SFL, & Mohamed FB (Eds.). (2010). BOLD fMRI: A Guide to Functional Imaging for Neuroscientists (Vol. 91). New York, NY: Springer New York, 10.1007/978-1-4419-1329-6 [DOI] [Google Scholar]
- Farrell C, & O’Keane V (2016). Epigenetics and the glucocorticoid receptor: A review of the implications in depression. Psychiatry Research, 242, 349–356. 10.1016/j.psychres.2016.06.022 [DOI] [PubMed] [Google Scholar]
- Farzi A, Fröhlich EE, & Holzer P (2018). Gut Microbiota and the Neuroendocrine System. Neurotherapeutics, 75(1), 5–22. 10.1007/sl3311-017-0600-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano A (2011). Zonulin and Its Regulation of Intestinal Barrier Function: The Biological Door to Inflammation, Autoimmunity, and Cancer. Physiological Reviews, 91(1), 151–175. 10.1152/physrev.00003.2008 [DOI] [PubMed] [Google Scholar]
- Ferrer M, Mendez-Garcia C, Rojo D, Barbas C, & Moya A (2016). Antibiotic use and microbiome function. Biochemical Pharmacology (Amsterdam, Netherlands), 134, Ahead of Print. 10.1016/j.bcp.2016.09.007 [DOI] [PubMed] [Google Scholar]
- Firth J, Marx W, Dash S, Carney R, Teasdale SB, Solmi M, … Sarris J (2019). The effects of dietary improvement on symptoms of depression and anxiety. Psychosomatic Medicine, O(April), 1 10.1097/PSY.0000000000000673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer S, Strawbridge R, Vives AFL, & Cleare AJ (2017). Cortisol as a predictor of psychological therapy response in depressive disorders: Systematic review and meta-analysis. British Journal of Psychiatry, 210(2), 105–109. 10.1192/bjp.bp.115.180653 [DOI] [PubMed] [Google Scholar]
- Flory JD, & Yehuda R (2015). Comorbidity between post-traumatic stress disorder and major depressive disorder: alternative explanations and treatment considerations. Dialogues in Clinical Neuroscience, 17(2), 141–150. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/26246789%0Ahttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC4518698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley P, & Kirschbaum C (2010). Human hypothalamus-pituitary-adrenal axis responses to acute psychosocial stress in laboratory settings. Neuroscience and Biobehavioral Reviews, 55(1), 91–96. 10.1016/j.neubiorev.2010.01.010 [DOI] [PubMed] [Google Scholar]
- Fond G, Boukouaci W, Chevalier G, Regnault A, Eberl G, Hamdani N, … Leboyer M (2015). The “psychomicrobiotic”: Targeting microbiota in major psychiatric disorders: A systematic review. Pathologie Biologie, 63(1), 35–42. 10.1016/j.patbio.2014.10.003 [DOI] [PubMed] [Google Scholar]
- Forsythe P, Sudo N, Dinan T, Taylor VH, & Bienenstock J (2010). Mood and gut feelings. Brain, Behavior, and Immunity, 24(1), 9–16. 10.1016/j.bbi.2009.05.058 [DOI] [PubMed] [Google Scholar]
- Foster JA (2013). Gut Feelings : Bacteria and the Brain. Cerebrum, (July), 1–14. [PMC free article] [PubMed] [Google Scholar]
- Franklin G, Carson AJ, & Welch KA (2016). Cognitive behavioural therapy for depression: systematic review of imaging studies. Acta Neuropsychiatrica, 28(02), 61–74. 10.1017/neu.2015.41 [DOI] [PubMed] [Google Scholar]
- Frederiksen HJB, Johansen TS, & Christiansen PM (1980). Postvagotomy diarrhoea and dumping treated with reconstruction of the pylorus. Scandinavian Journal of Gastroenterology, 15(2), 245–248. 10.3109/00365528009181463 [DOI] [PubMed] [Google Scholar]
- Frei R, Akdis M, & O’mahony L (2015). Prebiotics, probiotics, synbiotics, and the immune system: Experimental data and clinical evidence. Current Opinion in Gastroenterology, 31(2), 153–158. 10.1097/MOG.0000000000000151 [DOI] [PubMed] [Google Scholar]
- Fuentes S, & Vos W. M. De. (2016). How to Manipulate the Microbiota: Fecal Microbiota Transplantation In Schwiertz A (Ed.), Microbiota of the Human Body (Vol. 902, pp. 143–153). Cham: Springer International Publishing, 10.1007/978-3-319-31248-4 [DOI] [PubMed] [Google Scholar]
- Furness JB (2006). The Enteric Nervous System. Malden, Massachusetts, USA: Blackwell Publishing, 10.1002/9780470988756 [DOI] [Google Scholar]
- Furness JB (2016). Integrated Neural and Endocrine Control of Gastrointestinal Function In Brierley S & Costa M (Eds.), The Enteric Nervous System: 30 Years Later (Vol. 891, pp. 159–173). Switzerland: Springer International Publishing, 10.1007/978-3-319-27592-5 [DOI] [PubMed] [Google Scholar]
- Gaci N, Chaudhary PP, Tottey W, Alric M, & Brugere J-F (2017). Functional amplification and preservation of human gut microbiota. Microbial Ecology in Health and Disease, 28(1), 1308070 10.1080/16512235.2017.1308070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gałecki P, Mossakowska-Wójcik J, & Talarowska M (2018). The anti-inflammatory mechanism of antidepressants – SSRIs, SNRIs. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 50(March 2017), 291–294. 10.1016/j.pnpbp.2017.03.016 [DOI] [PubMed] [Google Scholar]
- Galley JD, Yu Z, Kumar P, Dowd SE, Lyte M, & Bailey MT (2015). The structures of the colonic mucosa-associated and luminal microbial communities are distinct and differentially affected by a prolonged murine stressor. Gut Microbes, 5(6), 748–760. 10.4161/19490976.2014.972241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Wu X, Owyang C, & Li Y (2006). Enhanced responses of the anterior cingulate cortex neurones to colonic distension in viscerally hypersensitive rats. The Journal of Physiology, 570(1), 169–183. 10.1113/jphysiol.2005.096073 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Getachew B, Aubee JI, Schottenfeld RS, Csoka AB, Thompson KM, & Tizabi Y (2018). Ketamine interactions with gut-microbiota in rats: relevance to its antidepressant and anti-inflammatory properties. BMC Microbiology, 75(1), 222 10.1186/s12866-018-1373-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson HE, Edwards JG, Page RS, Van Hook MJ, & Kauer JA (2008). TRPV1 Channels Mediate Long-Term Depression at Synapses on Hippocampal Interneurons. Neuron, 57(5), 746–759. 10.1016/j.neuron.2007.12.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano F, Zicca A, Barba C, Guerrini R, & Genitori L (2017). Vagus nerve stimulation: Surgical technique of implantation and revision and related morbidity. Epilepsia, 58, 85–90. 10.1111/epi.13678 [DOI] [PubMed] [Google Scholar]
- Glavin GB, Pare WP, Sandbak T, Bakke H-K, & Murison R (1994). Restraint stress in biomedical research: An update. Neuroscience & Biobehavioral Reviews, 75(2), 223–249. 10.1016/0149-7634(94)90027-2 [DOI] [PubMed] [Google Scholar]
- Gold PW, Machado-Vieira R, & Pavlatou MG (2015). Clinical and Biochemical Manifestations of Depression: Relation to the Neurobiology of Stress. Neural Plasticity, 2015, 1–11. 10.1155/2015/581976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden SA, Covington HE, Berton O, & Russo SJ (2011). A standardized protocol for repeated social defeat stress in mice. Nature Protocols, 6(8), 1183–1191. 10.1038/nprot.2011.361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith DR, Rapaport MH, & Miller BJ (2016). A meta-analysis of blood cytokine network alterations in psychiatric patients: Comparisons between schizophrenia, bipolar disorder and depression. Molecular Psychiatry, 27(12), 1696–1709. 10.1038/mp.2016.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Q, & He Y (2015). Depression, neuroimaging and connectomics: A selective overview. Biological Psychiatry, 77(3), 223–235. 10.1016/j.biopsych.2014.08.009 [DOI] [PubMed] [Google Scholar]
- González-Castañeda RE, Flores-Soto ME, Escoto-Delgadillo M, Beas-Zarate C, Chaparro-Huerta V, & Vazquez-Vails E (2012). Structure and function of NMDA-type glutamate receptor subunits. Neurología (English Edition), 27(5), 301–310. 10.1016/j.nrleng.2011.10.003 [DOI] [PubMed] [Google Scholar]
- Gorard DA, Gomborone JE, Libby GW, & Farthing MJG (1996). Intestinal transit in anxiety and depression. Gut, 39(4), 551–555. 10.1136/gut.39.4.551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto T, Kubota Y, & Toyoda A (2016). Effects of diet quality on vulnerability to mild subchronic social defeat stress in mice. Nutritional Neuroscience, 19(7), 284–289. 10.1179/1476830515Y.0000000017 [DOI] [PubMed] [Google Scholar]
- Gradus JL, Qin P, Lincoln AK, Miller M, Lawler E, Sorensen HT, & Lash TL (2010). Inflammatory bowel disease and completed suicide in Danish adults. Inflammatory Bowel Diseases, 7(5(12), 2158–2161. 10.1002/ibd.21298 [DOI] [PubMed] [Google Scholar]
- Greenberg PE, Fournier AA, Sisitsky T, Pike CT, & Kessler RC (2015). The economic burden of adults with major depressive disorder in the United States (2005 and 2010). Journal of Clinical Psychiatry, 76(2), 155–162. 10.4088/JCP.14m09298 [DOI] [PubMed] [Google Scholar]
- Griebel G, Belzung C, Perrault G, & Sanger DJ (2000). Differences in anxiety-related behaviours and in sensitivity to diazepam in inbred and outbred strains of mice. Psychopharmacology, 148(2), 164–170. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10663431 [DOI] [PubMed] [Google Scholar]
- Groenewold NA, Opmeer EM, de Jonge P, Aleman A, & Costafreda SG (2013). Emotional valence modulates brain functional abnormalities in depression: Evidence from a meta-analysis of fMRI studies. Neuroscience and Biobehavioral Reviews, 37(2), 152–163. 10.1016/j.neubiorev.2012.11.015 [DOI] [PubMed] [Google Scholar]
- Guida F, Turco F, Iannotta M, De Gregorio D, Palumbo I, Sarnelli G, … Maione S (2018). Antibiotic-induced microbiota perturbation causes gut endocannabinoidome changes, hippocampal neuroglial reorganization and depression in mice. Brain, Behavior, and Immunity, 67, 230–245. 10.1016/j.bbi.2017.09.001 [DOI] [PubMed] [Google Scholar]
- Guo G, Jia K-R, Shi Y, Liu X-F, Liu K-Y, Qi W, … Zou Q-M. (2009). Psychological stress enhances the colonization of the stomach by Helicobacter pylori in the BALB/c mouse. Stress, 12(6), 478–485. 10.3109/10253890802642188 [DOI] [PubMed] [Google Scholar]
- Hamady M, & Knight R (2009). Microbial community profiling for human microbiome projects: Tools, techniques, and challenges. Genome Research, 19(1), 1141–1152. 10.1101/gr.085464.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen AK, August B, Hansen CHF, Lundberg R, & Toft MF (2016). Antibiotic-treated versus germ-free rodents for microbiota transplantation studies. Gut Microbes, 7(1), 68–74. 10.1080/19490976.2015.1127463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Z, Wang W, Guo R, & Liu H (2019). Faecalibacterium prausnitzii (ATCC 27766) has preventive and therapeutic effects on chronic unpredictable mild stress-induced depression-like and anxiety-like behavior in rats. Psychoneuroendocrinology, (Atcc 27766). 10.1016/j.psyneuen.2019.02.025 [DOI] [PubMed] [Google Scholar]
- Harkin A, Kelly JP, & Leonard BE (2003). A review of the relevance and validity of olfactory bulbectomy as a model of depression. Clinical Neuroscience Research, 5(4–5), 253–262. 10.1016/S1566-2772(03)00087-2 [DOI] [Google Scholar]
- Harrington L, Srikanth CV, Antony R, Rhee SJ, Mellor AL, Hai NS, & Cherayil BJ (2008). Deficiency of indoleamine 2,3-dioxygenase enhances commensal-induced antibody responses and protects against Citrobacter rodentium-induced colitis. Infection and Immunity, 76(7), 3045–3053. 10.1128/IAI.00193-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hascoët M, & Bourin M (2009). The mouse light-dark box test. Neuromethods, 42, 197–223. 10.1007/978-1-60761-303-9-11 [DOI] [Google Scholar]
- He Y, Wu W, Zheng HM, Li P, McDonald D, Sheng HF, … Zhou HW (2018). Regional variation limits applications of healthy gut microbiome reference ranges and disease models. Nature Medicine, 24(10), 1532–1535. 10.1038/s41591-018-0164-x [DOI] [PubMed] [Google Scholar]
- Heim C, Ehlert U, & Hellhammer DH (2000). The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology, 25(1), 1–35. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10633533 [DOI] [PubMed] [Google Scholar]
- Helm K, Viol K, Weiger TM, Tass ΡA, Grefkes C, del Monte D, & Schiepek G (2018). Neuronal connectivity in major depressive disorder: a systematic review. Neuropsychiatric Disease and Treatment, Volume 14, 2715–2737. 10.2147/NDT.S170989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henter ID, de Sousa RT, Gold PW, Brunoni AR, Zarate CA, & Machado-Vieira R (2017). Mood Therapeutics: Novel Pharmacological Approaches for Treating Depression. Expert Review of Clinical Pharmacology, 70(2), 153–166. 10.1080/17512433.2017.1253472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr N, Bode C, & Duerschmied D (2017). The Effects of Serotonin in Immune Cells. Frontiers in Cardiovascular Medicine, 4(July), 1–11. 10.3389/fcvm.2017.00048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiles SA, Lamers F, Milaneschi Y, & Penninx BWJH (2017). Sit, step, sweat: Longitudinal associations between physical activity patterns, anxiety and depression. Psychological Medicine, 47(8), 1466–1477. 10.1017/S0033291716003548 [DOI] [PubMed] [Google Scholar]
- Hill AS, Sahay A, & Hen R (2015). Increasing Adult Hippocampal Neurogenesis is Sufficient to Reduce Anxiety and Depression-Like Behaviors. Neuropsychopharmacology, 40(10), 2368–2378. 10.1038/npp.2015.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hippocrates. (1931). Works of Hippocrates. (Jones W & Withington E, Eds.). Cambridge, MA: Harvard University Press. [Google Scholar]
- Ho CYX, Lim DY, Peters C, Yeo W-S, & Ng QX (2017). A meta-analysis of the use of probiotics to alleviate depressive symptoms. Journal of Affective Disorders, 22S(September 2017), 13–19. 10.1016/j.jad.2017.11.063 [DOI] [PubMed] [Google Scholar]
- Hoban AE, Moloney RD, Golubeva AV, McVey Neufeld KA, O’Sullivan O, Patterson E, … Cryan JF. (2016). Behavioural and neurochemical consequences of chronic gut microbiota depletion during adulthood in the rat. Neuroscience, 339, 463–477. 10.1016/j.neuroscience.2016.10.003 [DOI] [PubMed] [Google Scholar]
- Hoban AE, Stilling RM, Ryan FJ, Shanahan F, Dinan TG, Claesson MJ, … Cryan JF. (2016). Regulation of prefrontal cortex myelination by the microbiota. Translational Psychiatry, 6(4), e774–9. 10.1038/tp.2016.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg S (1996). A review of the validity and variability of the elevated plus-maze as an animal model of anxiety. Pharmacology Biochemistry and Behavior, 54(l), 21–30. 10.1016/0091-3057(95)02126-4 [DOI] [PubMed] [Google Scholar]
- Holscher HD (2017). Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes, 8(2), 172–184. 10.1080/19490976.2017.1290756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS, & Davis RJ (2016). Cell Signaling and Stress Responses. Cold Spring Harbor Perspectives in Biology, 5(10), a006072 10.1101/cshperspect.a006072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howland RH (2010). Use of Endocrine Hormones for Treating Depression. Journal of Psychosocial Nursing and Mental Health Services, 48(12), 13–16. 10.3928/02793695-20101105-01 [DOI] [PubMed] [Google Scholar]
- Howland RH (2013). Mifepristone as a therapeutic agent in psychiatry. Journal of Psychosocial Nursing and Mental Health Services, 51(6), 11–14. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/23814820 [DOI] [PubMed] [Google Scholar]
- Hu S, Wang Y, Lichtenstein L, Tao Y, Musch MW, Jabri B, … Chang EB (2010). Regional differences in colonic mucosa-associated microbiota determine the physiological expression of host heat shock proteins. American Journal of Physiology-Gastrointestinal and Liver Physiology, 299(6), G1266–G1275. 10.1152/ajpgi.00357.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang R, Wang K, & Hu J (2016). Effect of probiotics on depression: A systematic review and meta-analysis of randomized controlled trials. Nutrients, 5(8). 10.3390/nu8080483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Shi X, Li Z, Shen Y, Shi X, Wang L, … Liang Y (2018). Possible association of Firmicutes in the gut microbiota of patients with major depressive disorder. Neuropsychiatric Disease and Treatment, Volume 14, 3329–3337. 10.2147/NDT.S188340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugenholtz F, & de Vos WM (2018). Mouse models for human intestinal microbiota research: a critical evaluation. Cellular and Molecular Life Sciences, 75(1), 149–160. 10.1007/s00018-017-2693-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo R, Zeng B, Zeng L, Cheng K, Li B, Luo Y, … Xie P. (2017). Microbiota Modulate Anxiety-Like Behavior and Endocrine Abnormalities in Hypothalamic-Pituitary-Adrenal Axis. Frontiers in Cellular and Infection Microbiology, 7(November), 1–9. 10.3389/fcimb.2017.00489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenhower C, Gevers D, Knight R, Abubucker S, Badger JH, Chinwalla AT, … White O (2012). Structure, function and diversity of the healthy human microbiome. Nature, 456(7402), 207–214. 10.1038/nature11234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingiosi AM, Opp MR, & Krueger JM (2013). Sleep and immune function: Glial contributions and consequences of aging. Current Opinion in Neurobiology, 23(5), 806–811. 10.1016/j.conb.2013.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, & Scolnick EM (2006). Cure therapeutics and strategic prevention: Raising the bar for mental health research. Molecidar Psychiatry, 11(1), 11–17. 10.1038/sj.mp.4001777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inserra A, Rogers GB, Licinio J, & Wong ML (2018). The Microbiota-Inflammasome Hypothesis of Major Depression. BioEssays, 40(9), 1–11. 10.1002/bies.201800027 [DOI] [PubMed] [Google Scholar]
- Ionescu DF, Niciu MJ, Mathews DC, Richards EM, & Zarate CA (2013). Neurobiology of Anxious Depression: A Review. Depression and Anxiety, 30(4), 374–385. 10.1002/da.22095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iorio CRD, Carey CE, Michalski LJ, Corral-Frias NS, Conley ED, Hariri AR, & Bogdan R (2017). Hypothalamic-pituitary-adrenal axis genetic variation and early stress moderates amygdala function. Psychoneuroendocrinology, 80, 170–178. 10.1016/j.psyneuen.2017.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, & Miller AH (2007). Depressive disorders and immunity: 20 years of progress and discovery. Brain, Behavior, and Immunity, 21(4), 374–383. [DOI] [PubMed] [Google Scholar]
- Jacka FN (2017). Nutritional Psychiatry: Where to Next? EBioMedicine, 17, 24–29. 10.1016/j.ebiom.2017.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson L (2014). Hypothalamic-pituitary-adrenocortical axis: Neuropsychiatric aspects. Comprehensive Physiology, 4(2), 715–738. 10.1002/cphy.c130036 [DOI] [PubMed] [Google Scholar]
- Jiang H, Ling Z, Zhang Y, Mao H, Ma Z, Yin Y, … Ruan B (2015). Altered fecal microbiota composition in patients with major depressive disorder. Brain, Behavior, and Immunity, 48, 186–194. 10.1016/j.bbi.2015.03.016 [DOI] [PubMed] [Google Scholar]
- Jin Z, Mendu SK, & Birnir B (2013). GABA is an effective immunomodulatory molecule. Amino Acids, 45(1), 87–94. 10.1007/s00726-011-1193-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juruena MF, Bocharova M, Agustini B, & Young AH (2018). Atypical depression and non-atypical depression: Is HPA axis function a biomarker? A systematic review. Journal of Affective Disorders, 233(July 2017), 45–67. 10.1016/j.jad.2017.09.052 [DOI] [PubMed] [Google Scholar]
- Kaczmarek JL, Musaad SMA, & Holscher HD (2017). Time of day and eating behaviors are associated with the composition and function of the human gastrointestinal microbiota. American Journal of Clinical Nutrition, 106(5), 1220–1231. 10.3945/ajcn.117.156380 [DOI] [PubMed] [Google Scholar]
- Kaiser RH, Andrews-Hanna JR, Wager TD, & Pizzagalli DA (2015). Large-Scale Network Dysfunction in Major Depressive Disorder. JAMA Psychiatry, 72(6), 603 10.1001/jamapsychiatry.2015.0071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalueff AV, & Nutt DJ (2007). Role of GABA in anxiety and depression. Depression and Anxiety, 24(1), 495–517. 10.1002/da.20262 [DOI] [PubMed] [Google Scholar]
- Kao ACC, Harty S, & Burnet PWJ (2016). The Influence of Prebiotics on Neurobiology and Behavior International Review of Neurobiology (1st ed., Vol. 131). Elsevier Inc; 10.1016/bs.irn.2016.08.007 [DOI] [PubMed] [Google Scholar]
- Kazemi A, Noorbala AA, Azam K, Eskandari MH, & Djafarian K (2018). Effect of probiotic and prebiotic vs placebo on psychological outcomes in patients with major depressive disorder: A randomized clinical trial. Clinical Nutrition, (April), 1–7. 10.1016/j.clnu.2018.04.010 [DOI] [PubMed] [Google Scholar]
- Kelly CR, Kahn S, Kashyap P, Laine L, Rubin D, Atreja A, … Wu G (2015). Update on Fecal Microbiota Transplantation 2015: Indications, Methodologies, Mechanisms, and Outlook. Gastroenterology, 149(\), 223–237. 10.1053/j.gastro.2015.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly JR, Borre Y, O’ Brien C, Patterson E, El Aidy S, Deane J, … Dinan TG. (2016). Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. Journal of Psychiatric Research, 82, 109–118. 10.1016/j.jpsychires.2016.07.019 [DOI] [PubMed] [Google Scholar]
- Kelly JR, Clarke G, Cryan JF, & Dinan TG (2016). Brain-gut-microbiota axis: challenges for translation in psychiatry. Annals of Epidemiology, 26(5), 366–372. 10.1016/j.annepidem.2016.02.008 [DOI] [PubMed] [Google Scholar]
- Kerestes R, Davey CG, Stephanou K, Whittle S, & Harrison BJ (2014). Functional brain imaging studies of youth depression: A systematic review. Neuroimage: Clinical, 4, 209–231. 10.1016/j.nicl.2013.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalesi S, Bellissimo N, Vandelanotte C, Williams S, Stanley D, & Irwin C (2018). A review of probiotic supplementation in healthy adults: helpful or hype? European Journal of Clinical Nutrition, 1–14. 10.1038/s41430-018-0135-9 [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Wilson SJ, Bailey ML, Andridge R, Peng J, Jaremka LM, … Belury MA (2018). Marital distress, depression, and a leaky gut: Translocation of bacterial endotoxin as a pathway to inflammation. Psychoneuroendocrinology, 98(August), 52–60. 10.1016/j.psyneuen.2018.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, & Hellhammer DH (1993). The ‘Trier Social Stress Test’--a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. https://doi.org/119004 [DOI] [PubMed] [Google Scholar]
- Kleiman SC, Bulik-Sullivan EC, Glenny EM, Zerwas SC, Huh EY, Tsilimigras MCB, … Carroll IM (2017). The gut-brain axis in healthy females: Lack of significant association between microbial composition and diversity with psychiatric measures. PLoS ONE, 12(1), 1–14. 10.1371/journal.pone.0170208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kligman AM (2002). Origin of the annual symposium on the biology of skin. Journal of Investigative Dermatology Symposium Proceedings, 7(1), 1–3. 10.1046/j.1523-1747.2002.19641.x [DOI] [PubMed] [Google Scholar]
- Knierim JJ (2015). The hippocampus. Current Biology, 25(23), R1116–R1121. 10.1016/j.cub.2015.10.049 [DOI] [PubMed] [Google Scholar]
- Knight R, Vrbanac A, Taylor BC, Aksenov A, Callewaert C, Debelius J, … Dorrestein PC (2018). Best practices for analysing microbiomes. Nature Reviews Microbiology, 16(7), 410–422. 10.1038/s41579-018-0029-9 [DOI] [PubMed] [Google Scholar]
- Koenigs M, & Grafman J (2009). The functional neuroanatomy of depression: Distinct roles for ventromedial and dorsolateral prefrontal cortex. Behavioural Brain Research, 201(2), 239–243. 10.1016/j.bbr.2009.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler O, Benros ME, Nordentoft M, Farkouh ME, Iyengar RL, Mors O, & Krogh J (2014). Effect of Anti-inflammatory Treatment on Depression, Depressive Symptoms, and Adverse Effects. JAMA Psychiatry, 77(12), 1311–1381. 10.1001/jamapsychiatry.2014.1611 [DOI] [PubMed] [Google Scholar]
- Kohler O, Krogh J, Mors O, & Eriksen Benros M (2016). Inflammation in Depression and the Potential for Anti-Inflammatory Treatment. Current Neuropharmacology, 14(1), 732–742. 10.2174/1570159X14666151208113700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konsman JP, Luheshi GN, Bluthe RM, & Dantzer R (2000). The vagus nerve mediates behavioural depression, but not fever, in response to peripheral immune signals; a functional anatomical analysis. The European Journal of Neuroscience, 72(12), 4434–4446. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11122354 [DOI] [PubMed] [Google Scholar]
- Konsman JP, Parnet P, & Dantzer R (2002). Cytokine-induced sickness behaviour: Mechanisms and implications. Trends in Neurosciences, 25(3), 154–159. 10.1016/S0166-2236(00)02088-9 [DOI] [PubMed] [Google Scholar]
- Koopman M, Daniels JK, Spitzer C, Lampe A, & El Aidy S (2017). Depressed gut? the microbiota-diet-inflammation trialogue in depression. Current Opinion in Psychiatry, 30(5), 369–377. 10.1097/YCO.0000000000000350 [DOI] [PubMed] [Google Scholar]
- Korgaonkar MS, Fomito A, Williams LM, & Grieve SM (2014). Abnormal structural networks characterize major depressive disorder: A connectome analysis. Biological Psychiatry, 76(1), 567–574. 10.1016/j.biopsych.2014.02.018 [DOI] [PubMed] [Google Scholar]
- Kotlinska J, & Liljequist S (1998). A characterization of anxiolytic-like actions induced by the novel NMD A/glycine site antagonist, L-701,324. Psychopharmacology, 135(2), 175–181. 10.1007/s002130050499 [DOI] [PubMed] [Google Scholar]
- Lagoo J, Pappas TN, & Perez A (2014). A relic or still relevant: The narrowing role for vagotomy in the treatment of peptic ulcer disease. American Journal of Surgery, 207(1), 120–126. 10.1016/j.amjsurg.2013.02.012 [DOI] [PubMed] [Google Scholar]
- Langgartner D, Lowry CA, & Reber SO (2019). Old Friends, immunoregulation, and stress resilience. Pflügers Archiv - European Journal of Physiology, 471(2), 237–269. 10.1007/s00424-018-2228-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langgartner D, Peterlik D, Foertsch S, Füchsl AM, Brokmann P, Flor PJ, … Reber SO (2017). Individual differences in stress vulnerability: The role of gut pathobionts in stress-induced colitis. Brain, Behavior, and Immunity, 64, 23–32. 10.1016/j.bbi.2016.12.019 [DOI] [PubMed] [Google Scholar]
- Laugerette F, Alligier M, Bastard J-P, Drai J, Chanséaume E, Lambert-Porcheron S, … Michalski M-C (2014). Overfeeding increases postprandial endotoxemia in men: Inflammatory outcome may depend on LPS transporters LBP and sCD14. Molecular Nutrition & Food Research, 55(7), 1513–1518. 10.1002/mnfr.201400044 [DOI] [PubMed] [Google Scholar]
- Laugerette F, Furet J-P, Debard C, Daira P, Loizon E, Géloën A, … Michalski M-C (2012). Oil composition of high-fat diet affects metabolic inflammation differently in connection with endotoxin receptors in mice. American Journal of Physiology-Endocrinology and Metabolism, 302(3), E374–E386. 10.1152/ajpendo.00314.2011 [DOI] [PubMed] [Google Scholar]
- Laurans L, Venteclef N, Haddad Y, Chajadine M, Alzaid F, Metghalchi S, … Taleb S (2018). Genetic deficiency of indoleamine 2,3-dioxygenase promotes gut microbiota-mediated metabolic health. Nature Medicine, 24(8), 1113–1120. 10.1038/s41591-018-0060-4 [DOI] [PubMed] [Google Scholar]
- Lavelle A, Lennon G, O’Sullivan O, Docherty N, Balfe A, Maguire A, … O’Connell PR (2015). Spatial variation of the colonic microbiota in patients with ulcerative colitis and control volunteers. Gut, 64(10), 1553–1561. 10.1136/gutjnl-2014-307873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor C (2012). From Melancholia to Prozac. Oxford, UK: Oxford University Press. [Google Scholar]
- Leclercq S, Forsythe P, & Bienenstock J (2016). Posttraumatic stress disorder: Does the gut microbiome hold the key? Canadian Journal of Psychiatry, 61(4), 204–213. 10.1177/0706743716635535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesch KP, & Rupprecht R (1989). Psychoneuroendocrine research in depression. II. Hormonal responses to releasing hormones as a probe for hypothalamic-pituitary-endorgan dysfunction, .Journal of Neural Transmission, 75(3), 179–194. 10.4135/9781412984386 [DOI] [PubMed] [Google Scholar]
- Ley RE, Lozupone CA, Hamady M, Knight R, & Gordon JI (2008). Worlds within worlds: evolution of the vertebrate gut microbiota. Nature Reviews. Microbiology, 6(10), 776–788. 10.1038/nrmicro1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Wang Q, Wang Y, Sun A, Lin Y, Jin Y, & Li X (2018). Oral Probiotics Ameliorate the Behavioral Deficits Induced by Chronic Mild Stress in Mice via the Gut Microbiota-Inflammation Axis. Frontiers in Behavioral Neuroscience, 72(November), 266 10.3389/fnbeh.2018.00266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin P, Ding B, Feng C, Yin S, Zhang T, Qi X, … Li Q (2017). Prevotella and Klebsiella proportions in fecal microbial communities are potential characteristic parameters for patients with major depressive disorder. Journal of Affective Disorders, 207(April 2016), 300–304. 10.1016/j.jad.2016.09.051 [DOI] [PubMed] [Google Scholar]
- Liu B, He Y, Wang M, Liu J, Ju Y, Zhang Y, … Li Q. (2018). Efficacy of probiotics on anxiety—A meta-analysis of randomized controlled trials. Depression and Anxiety, 55(10), 935–945. 10.1002/da.22811 [DOI] [PubMed] [Google Scholar]
- Liu RT (2017). The microbiome as a novel paradigm in studying stress and mental health. American Psychologist, 72(1), 655–667. 10.1037/amp0000058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Yunpeng, Mian F, McVey Neufield K-A, & Forsythe P (2019). S20. Depletion of CD4+CD25+ Regulatory T Cells Inhibits the Anxiolytic Effects of Lactobacillus Rhamnosus (JB-1). Biological Psychiatry, 55(10), S304 10.1016/j.biopsych.2019.03.771 [DOI] [Google Scholar]
- Liu Yusen, Shepherd EG, & Nelin LD. (2007). MAPK phosphatases — regulating the immune response. Nature Reviews Immunology, 7(3), 202–212. 10.1038/nri2035 [DOI] [PubMed] [Google Scholar]
- Lloyd-Price J, Abu-Ali G, & Huttenhower C (2016). The healthy human microbiome. Genome Medicine, 5(1), 51 10.1186/s13073-016-0307-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis P., Flint HJ, & Michel C (2016). How to Manipulate the Microbiota: Prebiotics. Microbiota of the Human Body, 902, 119–142. 10.1007/978-3-319-31248-4 [DOI] [PubMed] [Google Scholar]
- Lubos E, Loscalzo J, & Handy DE (2011). Glutathione Peroxidase-1 in Health and Disease: From Molecular Mechanisms to Therapeutic Opportunities. Antioxidants & Redox Signaling, 75(7), 1957–1997. 10.1089/ars.2010.3586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lurie F, Yang YX, Haynes K, Mamtani R, & Boursi B (2015). Antibiotic exposure and the risk for depression, anxiety, or psychosis: A nested case-control study. Journal of Clinical Psychiatry, 76(11), 1522–1528. 10.4088/JCP.15m09961 [DOI] [PubMed] [Google Scholar]
- Lyra A, Forssten S, Rolny P, Wettergren Y, Lahtinen SJ, Salli K, … Ouwehand AC (2012). Comparison of bacterial quantities in left and right colon biopsies and faeces. World Journal of Gastroenterology, 75(32), 4404–4411. 10.3748/wjg.v18.i32.4404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyte M, Daniels KM, & Schmitz-Esser S (2019). Fluoxetine-induced alteration of murine gut microbial community structure: evidence for a microbial endocrinology-based mechanism of action responsible for fluoxetine-induced side effects. PeerJ, 7, e6199 10.7717/peeij.6199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, You X, Mai G, Tokuyasu T, & Liu C (2018). A human gut phage catalog correlates the gut phageome with type 2 diabetes. Microbiome, 6(1), 1–12. 10.1186/s40168-018-0410-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macedo D, Filho AJMC, Soares de Sousa CN, Quevedo J, Barichello T, Jdnior HVN, & Freitas de Lucena D (2017). Antidepressants, antimicrobials or both? Gut microbiota dysbiosis in depression and possible implications of the antimicrobial effects of antidepressant drugs for antidepressant effectiveness. Journal of Affective Disorders, 208(May 2016), 22–32. 10.1016/j.jad.2016.09.012 [DOI] [PubMed] [Google Scholar]
- Maes M, Kubera M, Leunis JC, & Berk M (2012). Increased IgA and IgM responses against gut commensals in chronic depression: Further evidence for increased bacterial translocation or leaky gut. Journal of Affective Disorders, 141(1), 55–62. 10.1016/j.jad.2012.02.023 [DOI] [PubMed] [Google Scholar]
- Maier L, Pruteanu M, Kuhn M, Zeller G, Telzerow A, Anderson E, … Typas A (2018). Extensive impact of non-antibiotic drugs on human gut bacteria Europe PMC Funders Group. Nature, 555(7698), 623–628. 10.6084/m9.figshare.4813882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier SF, & Watkins LR (1998). Cytokines for psychologists - Implications of bidirectional immune-to-brain communication for understanding behaviour, mood and cognition. Physiol. Rev, 705(1), 83–107. [DOI] [PubMed] [Google Scholar]
- Malenka RC (2012). Moving Beyond ‘Chemical Imbalance’ Theory of Depression. Brain and Behavior Magazine. Retrieved from https://www.bbrfoundation.org/content/moving-beyond-chemical-imbalance-theory-depression [Google Scholar]
- Malik A, & Kanneganti T-D (2017). Inflammasome activation and assembly at a glance. Journal of Cell Science, 130(23), 3955–3963. 10.1242/jcs.207365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manthey L, Leeds C, Giltay EJ, van Veen T, Vreeburg SA, Penninx BWJH, & Zitman FG (2011). Antidepressant use and salivary cortisol in depressive and anxiety disorders. European Neuropsychopharmacology, 21(9), 691–699. 10.1016/j.euroneuro.2011.03.002 [DOI] [PubMed] [Google Scholar]
- Marcos A, Wamberg J, Nova E, Gomez S, Alvarez A, Alvarez R, … Cobo JM (2004). The effect of milk fermented by yogurt cultures plus Lactobacillus casei DN-114001 on the immune response of subjects under academic examination stress. European Journal of Nutrition, 43(6), 381–389. 10.1007/s00394-004-0517-8 [DOI] [PubMed] [Google Scholar]
- Marin IA, Goertz JE, Ren T, Rich SS, Onengut-Gumuscu S, Farber E, … Gaultier A (2017). Microbiota alteration is associated with the development of stress-induced despair behavior. Scientific Reports, 7, 1–10. 10.1038/srep43859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowiak P, & Ślizewska K (2017). Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients, 9(9). 10.3390/nu9091021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín-Hemández D, Caso JR, Bris ÁG, Maus SR, Madrigal JLM, García-Bueno B, … Leza JC (2016). Bacterial translocation affects intracellular neuroinflammatory pathways in a depression-like model in rats. Neuropharmacology, 103, 122–133. 10.1016/j.neuropharm.2015.12.003 [DOI] [PubMed] [Google Scholar]
- Martin CR, Osadchiy V, Kalani A, & Mayer EA (2018). The Brain-Gut-Microbiome Axis. Cellular and Molecular Gastroenteroogy and Hepatology, 6(2), 133–148. 10.1016/j.jcmgh.2018.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews K, & Robbins TW (2003). Early experience as a determinant of adult behavioural responses to reward: The effects of repeated maternal separation in the rat. Neuroscience and Biobehavioral Reviews, 27(1–2), 45–55. 10.1016/S0149-7634(03)00008-3 [DOI] [PubMed] [Google Scholar]
- Mazidi M, Rezaie P, Ferns GA, & Vatanparast H (2017). Impact of probiotic administration on serum C-reactive protein concentrations: Systematic review and meta-analysis of randomized control trials. Nutrients, 9(1). 10.3390/nu9010020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCauley JA (2005). NR2B subtype-selective NMDA receptor antagonists: 2001 – 2004. Expert Opinion on Therapeutic Patents, 75(4), 389–407. 10.1517/13543776.15.4.389 [DOI] [Google Scholar]
- McCoy KD, Geuking MB, & Ronchi F (2017). Gut microbiome standardization in control and experimental mice. Current Protocols in Immunology, 2077(April), 23.1.1–23.1.13. 10.1002/cpim.25 [DOI] [PubMed] [Google Scholar]
- McDonald D, Hyde E, Debelius JW, Morton JT, Gonzalez A, Ackermann G, … Gunderson B (2018). American Gut: an Open Platform for Citizen Science Microbiome Research. MSystems, 5(3), 1–28. 10.1128/mSystems.00031-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon MJ, Johnston D, Hill GL, & Goligher JC (1978). Treatment of severe side effects after vagotomy and gastroenterostomy by closure of gastroenterostomy without pyloroplasty. British Medical Journal, 7(6104), 7–8. 10.1136/bmj.1.6104.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnik AV, Da Silva RR, Hyde ER, Aksenov AA, Vargas F, Bouslimani A, … Dorrestein PC (2017). Coupling Targeted and Untargeted Mass Spectrometry for Metabolome-Microbiome-Wide Association Studies of Human Fecal Samples. Analytical Chemistry, 59(14), 7549–7559. 10.1021/acs.analchem.7b01381 [DOI] [PubMed] [Google Scholar]
- Ménard C, Hodes GE, & Russo SJ (2016). Pathogenesis of depression: Insights from human and rodent studies. Neuroscience, 321(6), 138–162. 10.1016/j.neuroscience.2015.05.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messaoudi M, Lalonde R, Violle N, Javelot H, Desor D, Nejdi A, … Cazaubiel JM (2011). Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. British Journal of Nutrition, 105(5), 755–764. 10.1017/S0007114510004319 [DOI] [PubMed] [Google Scholar]
- Messaoudi M, Violle N, Bisson JF, Desor D, Javelot H, & Rougeot C (2011). Beneficial psychological effects of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in healthy human volunteers. Gut Microbes, 2(4). 10.4161/gmic.2.4.16108 [DOI] [PubMed] [Google Scholar]
- Micale V, Di Marzo V, Sulcova A, Wotjak CT, & Drago F (2013). Endocannabinoid system and mood disorders: Priming a target for new therapies. Pharmacology and Therapeutics, 755(1), 18–37. 10.1016/j.pharmthera.2012.12.002 [DOI] [PubMed] [Google Scholar]
- Miki T, Eguchi M, Kurotani K, Kochi T, Kuwahara K, Ito R, … Mizoue T (2016). Dietary fiber intake and depressive symptoms in Japanese employees: The Furukawa Nutrition and Health Study. Nutrition, 32(5), 584–589. 10.1016/j.nut.2015.11.014 [DOI] [PubMed] [Google Scholar]
- Mikkelsen K, Stojanovska L, Polenakovic M, Bosevski M, & Apostolopoulos V (2017). Exercise and mental health. Maturitas, 106(September), 48–56. 10.1016/j.maturitas.2017.09.003 [DOI] [PubMed] [Google Scholar]
- Miller AH, & Raison CL (2015). Are Anti-inflammatory Therapies Viable Treatments for Psychiatric Disorders? JAMA Psychiatry, 72(6), 527 10.1001/jamapsychiatry.2015.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, & Raison CL (2016). The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nature Reviews Immunology, 76(1), 22–34. 10.1038/nri.2015.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Cohen S, & Ritchey AK (2002). Chronic psychological stress and the regulation of pro-inflammatory cytokines: A glucocorticoid-resistance model. Health Psychology, 27(6), 531–541. [DOI] [PubMed] [Google Scholar]
- Mitani H, Shirayama Y, Yamada T, & Kawahara R (2006). Plasma levels of homovanillic acid, 5-hydroxyindoleacetic acid and cortisol, and serotonin turnover in depressed patients. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 30(3), 531–534. 10.1016/j.pnpbp.2005.11.021 [DOI] [PubMed] [Google Scholar]
- Moayyedi P, Surette MG, Kim PT, Libertucci J, Wolfe M, Onischi C, … Lee CH (2015). Fecal Microbiota Transplantation Induces Remission in Patients With Active Ulcerative Colitis in a Randomized Controlled Trial. Gastroenterology, 7-/9(l), 102–109.e6. 10.1053/j.gastro.2015.04.001 [DOI] [PubMed] [Google Scholar]
- Mohammadi AA, Jazayeri S, Khosravi-Darani K, Solati Z, Mohammadpour N, Asemi Z, … Eghtesadi S (2016). The effects of probiotics on mental health and hypothalamic-pituitary-adrenal axis: A randomized, double-blind, placebo-controlled trial in petrochemical workers. Nutritional Neuroscience, 79(9), 387–395. 10.1179/1476830515Y.0000000023 [DOI] [PubMed] [Google Scholar]
- Moloney RD, Desbonnet L, Clarke G, Dinan TG, & Cryan JF (2014). The microbiome: Stress, health and disease. Mammalian Genome, 25(1–2), 49–74. 10.1007/s00335-013-9488-5 [DOI] [PubMed] [Google Scholar]
- Morales-Medina JC, Iannitti T, Freeman A, & Caldwell HK (2017). The olfactory bulbectomized rat as a model of depression: The hippocampal pathway. Behavioural Brain Research, 377, 562–575. 10.1016/j.bbr.2016.09.029 [DOI] [PubMed] [Google Scholar]
- Morgun A, Dzutsev A, Dong X, Greer RL, Sexton DJ, Ravel J, … Shulzhenko N (2015). Uncovering effects of antibiotics on the host and microbiota using transkingdom gene networks. Gut, 64(11), 1732–1743. 10.1136/gutjnl-2014-308820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mörkl S, Wagner-Skacel J, Lahousen T, Lackner S, Holasek SJ, Bengesser SA, … Reininghaus E (2018). The Role of Nutrition and the Gut-Brain Axis in Psychiatry: A Review of the Literature. Neuropsychobiology, 1–9. 10.1159/000492834 [DOI] [PubMed] [Google Scholar]
- Mosher KI, & Wyss-Coray T (2015). Go with your gut: microbiota meet microglia. Nature Neuroscience, 75(7), 930–931. 10.1038/nn.4051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, & Ustun B (2007). Depression, chronic diseases, and decrements in health: results from the World Health Surveys. The Lancet, 370(9590), 851–858. 10.1016/S0140-6736(07)61415-9 [DOI] [PubMed] [Google Scholar]
- Mukhopadhya I, Segal JP, Carding SR, Hart AL, & Hold GL (2019). The gut virome the ‘missing link’ between gut bacteria and host immunity? Therapeutic Advances in Gastroenterology, 12(2%), 175628481983662. 10.1177/1756284819836620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy BEP (1991). General Review Steroids and Depression. J. Steroid Biochem. Molec. Biol, 55(5), 537–559. [DOI] [PubMed] [Google Scholar]
- Murphy JR, Paul S, Dunlop AL, & Corwin EJ (2018). Maternal peripartum antibiotic exposure and the risk of postpartum depression. Research in Nursing and Health, 41(A), 369–377. 10.1002/nur.21881 [DOI] [PubMed] [Google Scholar]
- Murphy K, & Weaver C (2017). The mucosal immune system In Janeway’s Immunobiologyy (9th Editio, pp. 493–531). New York, NY: Garland Science, https://doi.org/632 [pii] [Google Scholar]
- Murrough JW, Abdallah CG, Anticevic A, Collins KA, Geha P, Averill LA, … Chamey DS (2016). Reduced global functional connectivity of the medial prefrontal cortex in major depressive disorder. Human Brain Mapping, 37(9), 3214–3223. 10.1002/hbm.23235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeem I, Rahman MZ, Ad-Dab’bagh Y, & Akhtar M (2018). Effect of probiotic interventions on depressive symptoms: A narrative review evaluating systematic reviews. Psychiatry and Clinical Neurosciences, 1–9. 10.1111/pcn.12804 [DOI] [PubMed] [Google Scholar]
- Nagata N, Tohya M, Fukuda S, Suda W, Nishijima S, Takeuchi F, … Hattori M (2019). Effects of bowel preparation on the human gut microbiome and metabolome. Scientific Reports, 9(1), 1–8. 10.1038/s41598-019-40182-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naseribafrouei A, Hestad K, Avershina E, Sekelja M, Li nl ok ken A, Wilson R, & Rudi K (2014). Correlation between the human fecal microbiota and depression. Neurogastroenterology and Motility, 26(8), 1155–1162. 10.1111/nmo.12378 [DOI] [PubMed] [Google Scholar]
- Nazimek K, Strobel S, Bryniarski P, Kozlowski M, Filipczak-Bryniarska I, & Bryniarski K (2017). The role of macrophages in anti-inflammatory activity of antidepressant drugs. Immunobiology, 222(6), 823–830. 10.1016/j.imbio.2016.07.001 [DOI] [PubMed] [Google Scholar]
- Neufeld KM, Kang N, Bienenstock I, & Foster JA (2011). Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterology and Motility, 23(3), 255–265. 10.1111/j.1365-2982.2010.01620.x [DOI] [PubMed] [Google Scholar]
- Neuman FL, Debelius JW, Knight R, & Koren O (2015). Microbial endocrinology: The interplay between the microbiota and the endocrine system. FEMS Microbiology Reviews, 59(4), 509–521. 10.1093/femsre/fuu010 [DOI] [PubMed] [Google Scholar]
- Neumann ID, Wigger A, Kromer S, Frank E, Landgraf R, & Bosch OJ (2005). Differential effects of periodic maternal separation on adult stress coping in a rat model of extremes in trait anxiety. Neuroscience, 132(3), 867–877. 10.1016/j.neuroscience.2005.01.034 [DOI] [PubMed] [Google Scholar]
- Newberry F, Hsieh S-Y, Wileman T, & Carding SR (2018). Does the microbiome and virome contribute to myalgic encephalomyelitis/chronic fatigue syndrome? Clinical Science, 132(5), 523–542. 10.1042/cs20171330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieman LK, Chrousos GP, Kellner C, Spitz IM, Nisula BC, Cutler GB, … Loriaux DL (1985). Successful Treatment of Cushing’s Syndrome with the Glucocorticoid Antagonist RU 486*. The Journal of Clinical Endocrinology & Metabolism, 61(3), 536–540. 10.1210/jcem-61-3-536 [DOI] [PubMed] [Google Scholar]
- Nishihira J, Kagami-Katsuyama H, Tanaka A, Nishimura M, Kobayashi T, & Kawasaki Y (2014). Elevation of natural killer cell activity and alleviation of mental stress by the consumption of yogurt containing Lactobacillus gasseri SBT2055 and Bifidobacterium longum SBT2928 in a double-blind, placebo-controlled clinical trial. Journal of Functional Foods, If 261–268. 10.1016/j.jff.2014.09.002 [DOI] [Google Scholar]
- Nutt D (2006). GABAA receptors: Subtypes, regional distribution, and function. Journal of Clinical Sleep Medicine, 2(2). [PubMed] [Google Scholar]
- O’Mahony D, O’Leary P, & Quigley EMM (2002). Aging and intestinal motility: A review of factors that affect intestinal motility in the aged. Drugs and Aging, 19(1), 515–527. 10.2165/00002512-200219070-00005 [DOI] [PubMed] [Google Scholar]
- O’Mahony SM, Clarke G, Borre YE, Dinan TG, & Cryan JF (2015). Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behavioural Brain Research, 277, 32–48. 10.1016/j.bbr.2014.07.027 [DOI] [PubMed] [Google Scholar]
- O’Mahony Siobhain M., Marchesi JR, Scully P, Codling, Ceolho AM, Quigley EMM, … Dinan TG (2009). Early Life Stress Alters Behavior, Immunity, and Microbiota in Rats: Implications for Irritable Bowel Syndrome and Psychiatric Illnesses. Biological Psychiatry, 65(2), 263–267. 10.1016/j.biopsych.2008.06.026 [DOI] [PubMed] [Google Scholar]
- Ogawa S, T. DW, M RS,R, E. JM, S. G,K, M. H, & U K (1992). Intrinsic Signal Changes Accompanying Sensory Stimulation: Functional Brain Mapping with Magnetic Resonance Imaging. Proceedings of the National Academy of Sciences, 59(13), 5951–5955. 10.1073/pnas.89.13.5951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Östlund-Lagerström L, Kihlgren A, Repsilber D, Björkstén B, Brummer RJ, & Schoultz I (2015). Probiotic administration among free-living older adults: a double blinded, randomized, placebo-controlled clinical trial. Nutrition Journal, 75(1), 80 10.1186/s12937-016-0198-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özogul F (2011). Effects of specific lactic acid bacteria species on biogenic amine production by foodborne pathogen. International Journal of Food Science and Technology, 76(3), 478–484. 10.1111/j.1365-2621.2010.02511.x [DOI] [Google Scholar]
- Padol IT, Wang C, & Hunt RH (2012). Altered physiology of acid secretion in depression-prone Flinders rats results in exacerbated NS AID and stress-induced gastric damage. Neurogastroenterology and Motility, 24(2), 154–164. 10.1111/j.1365-2982.2011.01811.x [DOI] [PubMed] [Google Scholar]
- Pampallona S, Bollini P, Tibaldi G, Kupelnick B, & Munizza C (2002). Patient adherence in the treatment of depression. British Journal of Psychiatry, 180(02), 104–109. 10.1192/bjp.180.2.104 [DOI] [PubMed] [Google Scholar]
- Park AJ, Collins J, Blennerhassett PA, Ghia JE, Verdu EF, Bercik P, & Collins SM (2013). Altered colonic function and microbiota profile in a mouse model of chronic depression. Neurogastroenterology and Motility, 25(9). 10.1111/nmo.12153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park AT, Leonard JA, Saxler PK, Cyr AB, Gabrieli JDE, & Mackey AP (2018). Amygdala-medial prefrontal cortex connectivity relates to stress and mental health in early childhood. Social Cognitive and Affective Neuroscience, 73(4), 430–439. 10.1093/scan/nsy017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy G, Chen J, Chen X, Chia N, O’Connor HM, Wolf PG, … Bharucha AE (2016). Relationship Between Microbiota of the Colonic Mucosa vs Feces and Symptoms, Colonic Transit, and Methane Production in Female Patients With Chronic Constipation. Gastroenterology, 150(2), 367–379.e1. 10.1053/j.gastro.2015.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel RM, & Denning PW (2013). Therapeutic Use of Prebiotics, Probiotics, and Postbiotics to Prevent Necrotizing Enterocolitis. Clinics in Perinatology, 40(1), 11–25. 10.1016/j.clp.2012.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter J, Fournier C, Durdevic M, Knoblich L, Keip B, Dejaco C, … Moser G (2018). A microbial signature of psychological distress in irritable bowel syndrome. Psychosomatic Medicine, 80(8), 698–709. 10.1097/PSY.0000000000000630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters SK, Dunlop K, & Downar J (2016). Cortico-Striatal-Thalamic Loop Circuits of the Salience Network: A Central Pathway in Psychiatric Disease and Treatment. Frontiers in Systems Neuroscience, 10(December), 1–23. 10.3389/fnsys.2016.00104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen C, & Round JL (2014). Defining dysbiosis and its influence on host immunity and disease. Cellular Microbiology, 16(1), 1024–1033. 10.1111/cmi.12308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petra AI, Panagiotidou S, Hatziagelaki E, Stewart JM, Conti P, & Theoharides TC (2015). Gut-Microbiota-Brain Axis and Its Effect on Neuropsychiatric Disorders With Suspected Immune Dysregulation. Clinical Therapeutics, 57(5), 984–995. 10.1016/j.clinthera.2015.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto-Sanchez MI, Hall GB, Ghajar K, Nardelli A, Bolino C, Lau JT, … Bercik P. (2017). Probiotic Bifidobacterium longum NCC3001 Reduces Depression Scores and Alters Brain Activity: A Pilot Study in Patients With Irritable Bowel Syndrome. Gastroenterology, 153(2), 448–459.e8. 10.1053/j.gastro.2017.05.003 [DOI] [PubMed] [Google Scholar]
- Pivonello R, De Leo M, Cozzolino A, & Colao A (2015). The Treatment of Cushing’s Disease. Endocrine Reviews, 36(4), 385–486. 10.1210/er.2013-1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Ph D, Holmes AJ, Dillon DG, Goetz EL, Birk JL, … Fava M (2009). Reduced Caudate and Nucleus Accumbens Response to Rewards in Unmedicated Subjects with Major Depressive Disorder. American Journal of Psychiatry, 166(6), 702–710. 10.1176/appi.ajp.2008.08081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reduced Powell N, Walker MM, & Talley NJ (2017). The mucosal immune system: Master regulator of bidirectional gut-brain communications. Nature Reviews Gastroenterology and Hepatology, 77(3), 143–159. 10.1038/nrgastro.2016.191 [DOI] [PubMed] [Google Scholar]
- Powers SI, Laurent HK, Gunlicks-Stoessel M, Balaban S, & Bent E (2016). Depression and anxiety predict sex-specific cortisol responses to interpersonal stress. Psychoneuroendocrinology, 69, 172–179. 10.1016/j.psyneuen.2016.04.007 [DOI] [PubMed] [Google Scholar]
- Prusty BK, Gulve N, Govind S, Krueger GRF, Feichtinger J, Larcombe L, … Toro CT. (2018). Active HHV-6 infection of cerebellar Purkinje cells in mood disorders. Frontiers in Microbiology, 9(August), 1–12. 10.3389/fmicb.2018.01955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prut L, & Belzung C (2003). The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: A review. European Journal of Pharmacology, 765(1–3), 3–33. 10.1016/S0014-2999(03)01272-X [DOI] [PubMed] [Google Scholar]
- Quirk SE, Williams LJ, O’Neil A, Pasco JA, Jacka FN, Housden S, … Brennan SL. (2013). The association between diet quality, dietary patterns and depression in adults: A systematic review. BMC Psychiatry, 73(1), 1 10.1186/1471-244X-13-175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raes G, Van den Bergh R, De Baetselier P, Ghassabeh GH, Scotton C, Locati M, … Sozzani S (2005). Arginase-1 and Ym1 Are Markers for Murine, but Not Human, Alternatively Activated Myeloid Cells. The Journal of Immunology, 777(11), 6561–6562. 10.4049/jimmunol.174.11.6561 [DOI] [PubMed] [Google Scholar]
- Raison CL, & Miller AH (2013). The evolutionary significance of depression in Pathogen Host Defense (PATHOS-D). Molecular Psychiatry, 75(1), 15–37. 10.1038/mp.2012.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison Charles L., Capuron L, & Miller AH (2006). Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends in Immunology, 27(1), 24–31. 10.1016/j.it.2005.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison Charles L., Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, … Miller AH (2013). A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: The role of baseline inflammatory biomarkers. Archives of General Psychiatry, 70(1), 31–41. 10.1001/2013.jamapsychiatry.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison Charles L, & Miller AH (2011). Is Depression an Inflammatory Disorder? Current Psychiatry Reports, 75(6), 467–475. 10.1007/s11920-011-0232-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison Charles L, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, … Miller AH (2013). A Randomized Controlled Trial of the Tumor Necrosis Factor Antagonist Infliximab for Treatment-Resistant Depression. JAMA Psychiatry, 70(1), 31 10.1001/2013.jamapsychiatry.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajca S, Grondin V, Louis E, Vernier-Massouille G, Grimaud J-C, Bouhnik Y, … Seksik P. (2014). Alterations in the Intestinal Microbiome (Dysbiosis) as a Predictor of Relapse After Infliximab Withdrawal in Crohn’s Disease. Inflammatory Bowel Diseases, 20(6), 1 10.1097/MIB.0000000000000036 [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Mahajan G, Maciag D, Sathyanesan M, Iyo AH, Moulana M, … Newton SS. (2015). Oligodendrocyte morphometry and expression of myelin – Related mRNA in ventral prefrontal white matter in major depressive disorder. Journal of Psychiatric Research, 65, 53–62. 10.1016/j.jpsychires.2015.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjan R, Rani A, Metwally A, McGee HS, & Perkins DL (2016). Analysis of the microbiome: Advantages of whole genome shotgun versus 16S amplicon sequencing. Biochemical and Biophysical Research Communications, 469(4), 967–977. 10.1016/j.bbrc.2015.12.083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM, & Brown MA (2012). Innate immunity in the central nervous system. Journal of Clinical Investigation, 122(4), 1164–1171. 10.1172/JCI58644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao AV, Bested AC, Beaulne TM, Katzman MA, Iorio C, Berardi JM, & Logan AC (2009). A randomized, double-blind, placebo-controlled pilot study of a probiotic in emotional symptoms of chronic fatigue syndrome. Gut Pathogens, 7(1), 6 10.1186/1757-4749-1-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reber SO, Langgartner D, Foertsch S, Postolache TT, Brenner LA, Guendel H, & Lowry CA (2016). Chronic subordinate colony housing paradigm: A mouse model for mechanisms of PTSD vulnerability, targeted prevention, and treatment—2016 Curt Richter Award Paper. Psychoneuroendocrinology, 74, 221–230. 10.1016/j.psyneuen.2016.08.031 [DOI] [PubMed] [Google Scholar]
- Reber SO, Siebler PH, Donner NC, Morton JT, Smith DG, Kopelman JM, … Lowry CA. (2016). Immunization with a heat-killed preparation of the environmental bacterium Mycobacterium vaccae promotes stress resilience in mice. Proceedings of the National Academy of Sciences, 113(22), E3130–E3139. 10.1073/pnas.1600324113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebola N, Carta M, & Mulle C (2017). Operation and plasticity of hippocampal CA3 circuits: implications for memory encoding. Nature Reviews Neuroscience, 75(4), 208–220. 10.1038/nrn.2017.10 [DOI] [PubMed] [Google Scholar]
- Rivlin RS (2001). Historical Perspective on the Use of Garlic. The Journal of Nutrition, 131(3), 951S–954S. 10.1093/jn/13L3.951S [DOI] [PubMed] [Google Scholar]
- Roat-Shumway S, Wroolie TE, Watson K, Schatzberg AF, & Rasgon NL (2018). Cognitive effects of mifepristone in overweight, euthymic adults with depressive disorders. Journal of Affective Disorders, 239(April), 242–246. 10.1016/j.jad.2018.07.014 [DOI] [PubMed] [Google Scholar]
- Rogers MAM, Greene MT, Young VB, Saint S, Langa KM, Kao JY, & Aronoff DM (2013). Depression, antidepressant medications, and risk of Clostridium difficile infection. BMC Medicine, 77(1). 10.1186/1741-7015-11-121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romijn AR, & Rucklidge JJ (2015). Systematic review of evidence to support the theory of psychobiotics. Nutrition Reviews, 75(10), 675–693. 10.1093/nutrit/nuv025 [DOI] [PubMed] [Google Scholar]
- Romijn AR, Rucklidge JJ, Kuijer RG, & Frampton C (2017). A double-blind, randomized, placebo-controlled trial of Lactobacillus helveticus and Bifidobacterium longum for the symptoms of depression. Australian & New Zealand Journal of Psychiatry, 51(8), 810–821. 10.1177/0004867416686694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook GAW (2019). Immune System In Briine M & Schiefenhovel W (Eds.), Oxford Handbook of Evolutionary Medicine (First Edit). Oxford, UK: Oxford University Press; 10.1093/oxfordhb/9780198789666.013.10 [DOI] [Google Scholar]
- Rook GAW, Raison CL, & Lowry CA (2014). Microbiota, Immunoregulatory Old Friends and Psychiatric Disorders. In Microbial Endocrinology: The Microbiota-Gut-Brain Axis in Health and Disease (pp. 319–356). New York, NY: Springer New York. [DOI] [PubMed] [Google Scholar]
- Rowan F, Docherty NG, Murphy M, Murphy B, Coffey JC, & O’Connell PR (2010). Desulfovibrio Bacterial Species Are Increased in Ulcerative Colitis. Diseases of the Colon and Rectum, 55(11), 1530–1536. 10.1007/DCR.0b013e3181fle620 [DOI] [PubMed] [Google Scholar]
- Rubinow MJ, Mahajan G, May W, Overholser JC, Juijus GJ, Dieter L, … Stockmeier CA. (2016). Basolateral amygdala volume and cell numbers in major depressive disorder: a postmortem stereological study. Brain Structure and Function, 221(1), 171–184. 10.1007/s00429-014-0900-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruddick JP, Evans AK, Nutt DJ, Lightman SL, Rook GAW, & Lowry CA (2006). Tryptophan metabolism in the central nervous system: medical implications. Expert Reviews in Molecular Medicine, 5(20), 1–27. 10.1017/s1462399406000068 [DOI] [PubMed] [Google Scholar]
- Rudzki L, Ostrowska L, Pawlak D, Malus A, Pawlak K, Waszkiewicz N, & Szulc A (2019). Probiotic Lactobacillus Plantarum 299v decreases kynurenine concentration and improves cognitive functions in patients with major depression: A double-blind, randomized, placebo controlled study. Psychoneuroendocrinology, (October 2018), 213–222. 10.1016/j.psyneuen.2018.10.010 [DOI] [PubMed] [Google Scholar]
- Rudzki L, & Szulc A (2018). “Immune Gate” of psychopathology-The role of gut derived immune activation in major psychiatric disorders. Frontiers in Psychiatry, 9(May). 10.3389/fpsyt.2018.00205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupprecht R, & Lesch KP (1989). Psychoneuroendocrine research in depression. I. Hormone levels of different neuroendocrine axes and the dexamethasone suppression test. Journal of Neural Transmission, 75(3), 167–178. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/2538556 [DOI] [PubMed] [Google Scholar]
- Sanders ME, Guarner F, Guerrant R, Holt PR, Quigley EMM, Sartor RB, … Mayer EA. (2013). An update on the use and investigation of probiotics in health and disease. Gut, 62(5), 787–796. 10.1136/gutjnl-2012-302504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhu KV, Sherwin E, Schellekens EL, Stanton C, Dinan TG, & Cryan JF (2017). Feeding the microbiota-gut-brain axis: diet, microbiome, and neuropsychiatry. Translational Research, 179, 223–244. 10.1016/j.trsl.2016.10.002 [DOI] [PubMed] [Google Scholar]
- Saper CB, & Stometta RL (2014). Central Autonomic System In Paxinos G (Ed.), The Rat Nervous System (Fourth Edi, Vol. 467, pp. 629–673). Academic Press; 10.1016/B978-0-12-374245-2.00023-l [DOI] [Google Scholar]
- Sarkar A, Lehto SM, Harty S, Dinan TG, Cryan JF, & Burnet PWJ (2016). Psychobiotics and the Manipulation of Bacteria-Gut-Brain Signals. Trends in Neurosciences, 59(11), 763–781. 10.1016/j.tins.2016.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor RB (2015). Gut microbiota: Optimal sampling of the intestinal microbiota for research. Nature Reviews Gastroenterology and Hepatology, 12(5), 253–254. 10.1038/nrgastro.2015.46 [DOI] [PubMed] [Google Scholar]
- Savic D, Knezevic G, Damjanovic S, Spiric Z, & Matic G (2012). Is there a biological difference between trauma-related depression and PTSD? DST says “NO.” Psychoneuroendocrinology, 37(9), 1516–1520. 10.1016/j.psyneuen.2012.02.005 [DOI] [PubMed] [Google Scholar]
- Schoenfeld TJ, & Cameron HA (2015). Adult Neurogenesis and Mental Illness. Neuropsychopharmacology, 40(1), 113–128. 10.1038/npp.2014.230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shariq AS, Brietzke E, Rosenblat JD, Barendra V, Pan Z, & McIntyre RS (2018). Targeting cytokines in reduction of depressive symptoms: A comprehensive review. Progress in Neuro-Psychopharmacology and Biological Psychiatry, S3(October 2017), 86–91. 10.1016/j.pnpbp.2018.01.003 [DOI] [PubMed] [Google Scholar]
- Sheline YI, Liston C, & McEwen BS (2019). Parsing the Hippocampus in Depression: Chronic Stress, Hippocampal Volume, and Major Depressive Disorder. Biological Psychiatry, 55(6), 436–438. 10.1016/j.biopsych.2019.01.011 [DOI] [PubMed] [Google Scholar]
- Sherry CL, Kim SS, Dilger RN, Bauer LL, Moon ML, Tapping RI, … Freund GG (2010). Sickness behavior induced by endotoxin can be mitigated by the dietary soluble fiber, pectin, through up-regulation of IL-4 and Th2 polarization. Brain, Behavior, and Immunity, 24(4), 631–640. 10.1016/j.bbi.2010.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinkai S, Toba M, Saito T, Sato I, Tsubouchi M, Taira K, … Kohno S. (2013). Immunoprotective effects of oral intake of heat-killed Lactobacillus pentosus strain b240 in elderly adults: a randomised, double-blind, placebo-controlled trial. British Journal of Nutrition, 109(10), 1856–1865. 10.1017/S0007114512003753 [DOI] [PubMed] [Google Scholar]
- Shishov VA, Kirovskaya TA, Kudrin VS, & Oleskin AV (2009). Amine neuromediators, their precursors, and oxidation products in the culture of Escherichia coli K-12. Applied Biochemistry and Microbiology, 45(5), 494–497. 10.1134/s0003683809050068 [DOI] [PubMed] [Google Scholar]
- Sichardt K, Müller CE, Lacher SK, Nieber K, & Mayer R (2006). Interaction of valerian extracts of different polarity with adenosine receptors: Identification of isovaltrate as an inverse agonist at A1 receptors. Biochemical Pharmacology, 73(2), 248–258. 10.1016/j.bcp.2006.09.029 [DOI] [PubMed] [Google Scholar]
- Singh MK, Kesler SR, Hadi Hosseini SM, Kelley RG, Amatya D, Hamilton JP, … Gotlib IH. (2013). Anomalous gray matter structural networks in major depressive disorder. Biological Psychiatry, 74(10), 777–785. 10.1016/j.biopsych.2013.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh RK, Chang H-W, Yan D, Lee KM, Ucmak D, Wong K, … Liao W. (2017). Influence of diet on the gut microbiome and implications for human health. Journal of Translational Medicine, 75(1), 73 10.1186/s12967-017-1175-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattery DA, & Cryan JF (2012). Using the rat forced swim test to assess antidepressant-like activity in rodents. Nature Protocols, 7(6), 1009–1014. 10.1038/nprot.2012.044 [DOI] [PubMed] [Google Scholar]
- Slykerman RF, Hood F, Wickens K, Thompson JMD, Barthow C, Murphy R, … Mitchell EA (2017). Effect of Lactobacillus rhamnosus HN001 in Pregnancy on Postpartum Symptoms of Depression and Anxiety: A Randomised Double-blind Placebo-controlled Trial. EBioMedicine, 24, 159–165. 10.1016/j.ebiom.2017.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C, & Leonard BE (2005). The olfactory bulbectomised rat as a model of depression. Neuroscience and Biobehavioral Reviews, 29(4–5), 627–647. 10.1016/j.neubiorev.2005.03.010 [DOI] [PubMed] [Google Scholar]
- Song C, & Wang H (2011). Cytokines mediated inflammation and decreased neurogenesis in animal models of depression. Process in Neuro-Psychopharmacology and Biological Psychiatry, 35(3), 760–768. 10.1016/j.pnpbp.2010.06.020 [DOI] [PubMed] [Google Scholar]
- Soto M, Herzog C, Pacheco JA, Fujisaka S, Bullock K, Clish CB, & Kahn CR (2018). Gut microbiota modulate neurobehavior through changes in brain insulin sensitivity and metabolism. Molecular Psychiatry, 23(12), 2287–2301. 10.1038/s41380-018-0086-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spierling SR, & Zorrilla EP (2017). Don’t stress about CRF: assessing the translational failures of CRF 1 antagonists. Psychopharmacology, 234(9–10), 1467–1481. 10.1007/s00213-017-4556-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley C, Kaiser T, & Khoruts A (2018). Clinician Guide to Microbiome Testing. Digestive Diseases and Sciences, 63(12), 3167–3177. 10.1007/s10620-018-5299-6 [DOI] [PubMed] [Google Scholar]
- Steenbergen L, Sellaro R, van Hemert S, Bosch JA, & Colzato LS (2015). A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain, Behavior, and Immunity, 48(April), 258–264. 10.1016/j.bbi.2015.04.003 [DOI] [PubMed] [Google Scholar]
- Stem L, Chermat R, Thierry B, & Simon P (1985). The tail suspension test: A new method for screening antidepressants in mice. Psychopharmacology, 55(3), 367–370. 10.1007/BF00428203 [DOI] [PubMed] [Google Scholar]
- Stevens BR, Goel R, Seungbum K, Richards EM, Holbert RC, Pepine CJ, & Raizada MK (2018). Increased human intestinal barrier permeability plasma biomarkers zonulin and FABP2 correlated with plasma LPS and altered gut microbiome in anxiety or depression. Gut, 67(8), 1555–1557. 10.1136/gutjnl-2017-314759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes JH, & Pillsbury DM (1930). The Effect on the Skin of Emotional and Nervous States: Theoretical and Practical Consideration of a Gastro-intestinal Mechanism. Archives of Dermatology and Syphilology, 22(6), 962 10.1001/archderm.1930.01440180008002 [DOI] [Google Scholar]
- Strain JJ (2018). The psychobiology of stress, depression, adjustment disorders and resilience. World Journal of Biological Psychiatry, 79(supl), S14–S20. 10.1080/15622975.2018.1459049 [DOI] [PubMed] [Google Scholar]
- Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu XN, … Koga Y. (2004). Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. Journal of Physiology, 555(1), 263–275. 10.1113/jphysiol.2004.063388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiergiel AH, & Dunn AJ (2007). Effects of interleukin-1β and lipopolysaccharide on behavior of mice in the elevated plus-maze and open field tests. Pharmacology Biochemistry and Behavior, 56(4), 651–659. 10.1016/j.pbb.2007.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed SA, Beurel E, Loewenstein DA, Lowell JA, Craighead WE, Dunlop BW, … Nemeroff CB. (2018). Defective Inflammatory Pathways in Never-Treated Depressed Patients Are Associated with Poor Treatment Response. Neuron, 99(5), 914–924.e3. 10.1016/j.neuron.2018.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczesniak O, Hestad KA, Hanssen JF, & Rudi K (2016). Isovaleric acid in stool correlates with human depression. Nutritional Neuroscience, 19(1), 279–283. 10.1179/1476830515Y.0000000007 [DOI] [PubMed] [Google Scholar]
- Tafet GE, & Nemeroff CB (2016). The Links Between Stress and Depression: Psychoneuroendocrinological, Genetic, and Environmental Interactions. The Journal of Neuropsychiatry and Clinical Neurosciences, 28(2), 77–88. 10.1176/appi.neuropsych.15030053 [DOI] [PubMed] [Google Scholar]
- Takahashi M, Ishikawa D, Sasaki T, Lu YJ, Kuwahara-Arai K, Kamei M, … Nagahara A. (2019). Faecal freezing preservation period influences colonization ability for faecal microbiota transplantation. Journal of Applied Microbiology, 126(3), 973–984. https://doi.org/10.1111/jam.14167 [DOI] [PubMed] [Google Scholar]
- Takiishi T, Fenero CIM, & Camara NOS (2017). Intestinal barrier and gut microbiota: Shaping our immune responses throughout life. Tissue Barriers, 5(4), el373208 10.1080/21688370.2017.1373208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan S, Wang Y, Chen K, Long Z, & Zou J (2017). Ketamine Alleviates Depressive-Like Behaviors via Down-Regulating Inflammatory Cytokines Induced by Chronic Restraint Stress in Mice. Biological & Pharmaceutical Bulletin, 70(8), 1260–1267. 10.1248/bpb.b17-00131 [DOI] [PubMed] [Google Scholar]
- ten Have M, Lamers F, Wardenaar K, Beekman A, de Jonge P, van Dorsselaer S, … de Graaf R. (2016). The identification of symptom-based subtypes of depression: A nationally representative cohort study. Journal of Affective Disorders, 190, 395–406. 10.1016/j.jad.2015.10.040 [DOI] [PubMed] [Google Scholar]
- Towfigh S, Chandler C, Hines OJ, & McFadden DW (2002). Outcomes from peptic ulcer surgery have not benefited from advances in medical therapy. American Surgeon, 68(4), 385–389. [PubMed] [Google Scholar]
- Toyoda A (2017). Social defeat models in animal science: What we have learned from rodent models. Animal Science Journal, 88(1), 944–952. 10.1111/asj.12809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsilingiri K, & Rescigno M (2013). Postbiotics: What else? Beneficial Microbes, 7(1), 101–107. 10.3920/BM2012.0046 [DOI] [PubMed] [Google Scholar]
- Tuma J, Grosman Kaplan K, Anglin R, & Van Ameringen M (2016). “WHAT’S BUGGING THE GUT IN OCD?” A REVIEW OF THE GUT MICROBIOME IN OBSESSIVE-COMPULSIVE DISORDER. Depression and Anxiety, 55(3), 171–178. 10.1002/da.22454 [DOI] [PubMed] [Google Scholar]
- Tumbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, & Gordon JI (2007). The Human Microbiome Project. Nature, 779(7164), 804–810. 10.1109/SAINT.2010.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyacke RJ, Lingford-Hughes A, Reed LJ, & Nutt DJ (2010). GABAB Receptors in Addiction and Its Treatment. In Advances in Pharmacology (Vol. 58, pp. 373–396). 10.1016/S1054-3589(10)58014-1 [DOI] [PubMed] [Google Scholar]
- Ursell LK, Haiser HJ, Van Treuren W, Garg N, Reddivari L, Vanamala J, … Knight R. (2014). The intestinal metabolome: An intersection between microbiota and host. Gastroenterology, 146(6), 1470–1476. 10.1053/j.gastro.2014.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursell LK, Metcalf JL, Parfrey LW, & Knight R (2012). Defining the human microbiome. Nutrition Reviews, 70(SUPPL. 1). 10.1111/j.1753-4887.2012.00493.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valles-Colomer M, Falony G, Darzi Y, Tigchelaar EF, Wang J, Tito RY, … Raes J. (2019a). The neuroactive potential of the human gut microbiota in quality of life and depression. Nature Microbiology, 10.1038/s41564-018-0337-x [DOI] [PubMed] [Google Scholar]
- Valles-Colomer M, Falony G, Darzi Y, Tigchelaar EF, Wang J, Tito RY, … Raes J. (2019b). The neuroactive potential of the human gut microbiota in quality of life and depression. Nature Microbiology, 10.1038/s41564-018-0337-x [DOI] [PubMed] [Google Scholar]
- Van Gaalen MM, & Steckler T (2000). Behavioural analysis of four mouse strains in an anxiety test battery. Behavioural Brain Research, 115(1), 95–106. 10.1016/S0166-4328(00)00240-0 [DOI] [PubMed] [Google Scholar]
- Vandeputte D, Tito RY, Vanleeuwen R, Falony G, & Raes J (2017). Practical considerations for large-scale gut microbiome studies. FEMSMicrobiology Reviews, 41(1), S154–S167. 10.1093/femsre/fux027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanuytsel T, Van Wanrooy S, Vanheel H, Vanormelingen C, Verschueren S, Houben E, … Tack J. (2014). Psychological stress and corticotropin-releasing hormone increase intestinal permeability in humans by a mast cell-dependent mechanism. Gut, 65(8), 1293–1299. 10.1136/gutjnl-2013-305690 [DOI] [PubMed] [Google Scholar]
- Varatharaj A, & Galea I (2016). The blood-brain barrier in systemic inflammation. Brain, Behavior, and Immunity, 60, 1–12. 10.1016/j.bbi.2016.03.010 [DOI] [PubMed] [Google Scholar]
- Varghese AK, Verdú EF, Bercik P, Khan WI, Blennerhassett PA, Szechtman H, & Collins SM (2006). Antidepressants Attenuate Increased Susceptibility to Colitis in a Murine Model of Depression. Gastroenterology, 130(6), 1743–1753. 10.1053/j.gastro.2006.02.007 [DOI] [PubMed] [Google Scholar]
- Vemuri R, Sylvia KE, Klein SL, Forster SC, Plebanski M, Eri R, & Flanagan KL (2018). The microgenderome revealed: sex differences in bidirectional interactions between the microbiota, hormones, immunity and disease susceptibility. Seminars in Immunopathology. 10.1007/s00281-018-0716-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vindigni SM, & Surawicz CM (2017). Fecal Microbiota Transplantation. Gastroenterology Clinics of North America, 46(1), 171–185. 10.1016/j.gtc.2016.09.012 [DOI] [PubMed] [Google Scholar]
- Voigt AY, Costea PT, Kultima JR, Li SS, Zeller G, Sunagawa S, & Bork P (2015). Temporal and technical variability of human gut metagenomes. Genome Biology, 16(1), 1–12. 10.1186/s13059-015-0639-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vujkovic-Cvijin I, Swainson LA, Chu SN, Ortiz AM, Santee CA, Petriello A, … McCune JM. (2015). Gut-Resident Lactobacillus Abundance Associates with IDO1 Inhibition and Th17 Dynamics in SIV-Infected Macaques. Cell Reports, 75(8), 1589–1597. 10.1016/j.celrep.2015.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace CJK, & Milev R (2017). The effects of probiotics on depressive symptoms in humans: A systematic review. Annals of General Psychiatry, 16(1), 1–10. 10.1186/s12991-017-0138-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, & Si T (2013). Use of antipsychotics in the treatment of depressive disorders. Shanghai Archives of Psychiatry, 25(3), 134–140. 10.3969/j.issn.1002-0829.2013.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Lin L, Du Y, Song Y, Peng X, Chen X, & Yang CJ (2019). Assessing the viability of transplanted gut microbiota by sequential tagging with D-amino acid-based metabolic probes. Nature Communications, 70(1), 1317 10.1038/s41467-019-09267-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Zolnik CP, Qiu Y, Usyk M, Wang T, Strickler HD, … Burk RD. (2018). Comparison of Fecal Collection Methods for Microbiome and Metabolomics Studies. Frontiers in Cellular and Infection Microbiology, 8(August), 1–10. 10.3389/fcimb.2018.00301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waugh CE, & Koster EHW (2015). A resilience framework for promoting stable remission from depression. Clinical Psychology Review, 41, 49–60. 10.1016/j.cpr.2014.05.004 [DOI] [PubMed] [Google Scholar]
- Wessa M, & Lois G (2015). Brain Functional Effects of Psychopharmacological Treatment in Major Depression: a Focus on Neural Circuitry of Affective Processing. Current Neuropharmacology, 73(4), 466–479. 10.2174/1570159X13666150416224801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler AL, Felsky D, Viviano JD, Stojanovski S, Ameis SFL, Szatmari P., … Voineskos AN. (2018). BDNF-Dependent Effects on Amygdala-Cortical Circuitry and Depression Risk in Children and Youth. Cerebral Cortex, 28(5), 1760–1770. 10.1093/cercor/bhx086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitton AE, Treadway MT, & Pizzagalli DA (2015). Reward processing dysfunction in major depression, bipolar disorder and schizophrenia. Current Opinion in Psychiatry, 25(1), 7–12. 10.1097/YCO.0000000000000122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichmann S, Kirschbaum C, Bohme C, & Petrowski K (2017). Cortisol stress response in post-traumatic stress disorder, panic disorder, and major depressive disorder patients. Psychoneuroendocrinology, 53(January), 135–141. 10.1016/j.psyneuen.2017.06.005 [DOI] [PubMed] [Google Scholar]
- Wichniak A, Wierzbicka A, & Jernajczyk W (2013). Sleep as a biomarker for depression. International Review of Psychiatry, 25(5), 632–645. 10.3109/09540261.2013.812067 [DOI] [PubMed] [Google Scholar]
- Winter G, Hart RA, Charlesworth RPG, & Sharpley CF (2018). Gut microbiome and depression: What we know and what we need to know. Reviews in the Neurosciences, 29(6), 629–643. 10.1515/revneuro-2017-0072 [DOI] [PubMed] [Google Scholar]
- Wohleb ES, Franklin T, Iwata M, & Duman RS (2016). Integrating neuroimmune systems in the neurobiology of depression. Nature Reviews Neuroscience, 17(8), 497–511. 10.1038/nrn.2016.69 [DOI] [PubMed] [Google Scholar]
- Wong ML, Inserra A, Lewis MD, Mastronardi CA, Leong L, Choo J, … Licinio J. (2016). Inflammasome signaling affects anxiety- and depressive-like behavior and gut microbiome composition. Molecular Psychiatry, 21(6), 797–805. 10.1038/mp.2016.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. (2017). Depression and Other Common Mental Disorders. Who, 24 https://doi.org/CCBY-NC-SA3.0IGO [Google Scholar]
- Wu Z, & Fang Y (2014). Comorbidity of depressive and anxiety disorders: challenges in diagnosis and assessment. Shanghai Archives of Psychiatry, 26(4), 227–231. 10.3969/j.issn.1002-0829.2014.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu MQ, Cao HL, Wang WQ, Wang S, Cao XC, Yan F, & Wang BM (2015). Fecal microbiota transplantation broadening its application beyond intestinal disorders. World Journal of Gastroenterology, 27(1), 102–111. 10.3748/wjg.v21.11.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Qu Y, Fujita Y, Ren Q, Ma M, Dong C, & Hashimoto K (2017). Possible role of the gut microbiota-brain axis in the antidepressant effects of (R)-ketamine in a social defeat stress model. Translational Psychiatry, 7(12). 10.1038/s41398-017-0031-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Santisteban MM, Rodriguez V, Li E, Ahmari N, Carvajal JM, … Mohamadzadeh M. (2015). Gut Dysbiosis Is Linked to Hypertension. Hypertension, 65(6), 1331–1340. 10.1161/HYPERTENSIONAHA.115.05315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, Halligan SL, Golier JA, Grossman R, & Bierer LM (2004). Effects of trauma exposure on the cortisol response to dexamethasone administration in PTSD and major depressive disorder. Psychoneuroendocrinology, 29(3), 389–404. 10.1016/S0306-4530(03)00052-0 [DOI] [PubMed] [Google Scholar]
- Yehuda R, & Seckl J (2011). Minireview: Stress-related psychiatric disorders with low cortisol levels: A metabolic hypothesis. Endocrinology, 152(12), 4496–4503. 10.1210/en.2011-1218 [DOI] [PubMed] [Google Scholar]
- Yehuda R, Southwick SM, Krystal JH, Bremner D, Charney DS, & Mason JW (1993). Enhanced suppression of cortisol following dexamethasone administration in posttraumatic stress disorder. American Journal of Psychiatry, 750(1), 83–86. 10.1176/ajp.150.L83 [DOI] [PubMed] [Google Scholar]
- Young AH, Gallagher P, Watson S, Del-Estal D, Owen BM, & Ferrier IN (2004). Improvements in neurocognitive function and mood following adjunctive treatment with mifepristone (RU-486) in bipolar disorder. Neuropsychopharmacology, 29(8), 1538–1545. 10.1038/sj.npp.1300471 [DOI] [PubMed] [Google Scholar]
- Yu H, & Chen ZY (2011). The role of BDNF in depression on the basis of its location in the neural circuitry. Acta Pharmacologica Sinica, 32(1), 3–11. 10.1038/aps.2010.184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H, & Silberstein SD (2016). Vagus Nerve and Vagus Nerve Stimulation, a Comprehensive Review: Part II. Headache, 56(2), 259–266. 10.1111/head.12650 [DOI] [PubMed] [Google Scholar]
- Zaffiri L, Gardner J, & Toledo-Pereyra LH (2012). History of antibiotics, from salvarsan to cephalosporins. Journal of Investigative Surgery, 25(2), 67–77. 10.3109/08941939.2012.664099 [DOI] [PubMed] [Google Scholar]
- Zaneveld JR, Lozupone C, Gordon JI, & Knight R (2010). Ribosomal RNA diversity predicts genome diversity in gut bacteria and their relatives. Nucleic Acids Research, 38(12), 3869–3879. 10.1093/nar/gkq066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JC, Yao W, Dong C, Yang C, Ren Q, Ma M, & Hashimoto K (2017). Blockade of interleukin-6 receptor in the periphery promotes rapid and sustained antidepressant actions: a possible role of gut-microbiota-brain axis. Translational Psychiatry, 7(5), e1138 10.1038/tp.2017.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Cao X, & Huang H (2017). Sampling Strategies for Three-Dimensional Spatial Community Structures in IBD Microbiota Research. Frontiers in Cellular and Infection Microbiology, 7(February). 10.3389/fcimb.2017.00051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng P, Zeng B, Zhou C, Liu M, Fang Z, Xu X, … Xie P. (2016). Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Molecular Psychiatry, 21(6), 786–796. 10.1038/mp.2016.44 [DOI] [PubMed] [Google Scholar]
- Zuo T, Wong SH, Lam K, Lui R, Cheung K, Tang W, … Ng SC. (2018). Bacteriophage transfer during faecal microbiota transplantation in Clostridium difficile infection is associated with treatment outcome. Gut, 67(4), 634–643. 10.1136/gutjnl-2017-313952 [DOI] [PMC free article] [PubMed] [Google Scholar]


