Abstract
Introduction
To evaluate clinical inertia in patients with type 2 diabetes mellitus (T2DM), obesity and poor glycaemic control in routine clinical practice.
Methods
This was a retrospective, observational study based on the analysis of medical records from the BIG-PAC® database. Subjects who required medical care in 2013 with the following characteristics were enrolled in the study: age ≥ 30 years, diagnosis of T2DM, glycosylated haemoglobin (HbA1c) ≥ 8%, obesity (body mass index [BMI] ≥ 30 kg/m2) and treatment with ≥ 2 oral antidiabetic drugs (OADs). Inertia was evaluated by time (days) to the first intensification during the period while HbA1c levels were ≥ 8% and percentage of patients whose treatment was not intensified at 6 months, 1, 2 and 3 years and the end of follow-up. The minimum length of follow-up was 4 years. Descriptive analyses and Kaplan–Meier survival curves were performed.
Results
A total of 13,824 patients with T2DM receiving ≥ 2 OADs were identified; of these 2709 (19.6%) had HbA1c ≥ 8% and BMI ≥ 30 kg/m2, thus fulfilling the inclusion criteria. Of these 2709 patients, the mean age was 65.5 (standard deviation [SD] 12.0) years; 54.9% were male, mean HbA1c level was 9.2% (SD 1.3%); mean BMI was 32.1 (SD 0.9) kg/m2; and mean time from diagnosis was 8.2 (SD 3.0) years. HbA1c remained ≥ 8% for a median of 440 (95% confidence interval [CI] 421–459) days. The median time to first intensification was 456 (95% CI 429–483) days. No intensification had occurred in 77.8, 59.5, 41.5, 28.1 and 22.4% of patients at 6 months, 1, 2, 3 years and the end of follow-up, respectively.
Conclusions
The patients with T2DM analysed in this study had a mean HbA1c of 9.2% at baseline, and this remained at ≥ 8% for > 1 year. The time to the first treatment intensification was longer than that recommended by guidelines. Treatment was not intensified in a large percentage of patients, with almost 60% of patients not receiving intensification at 1 year of follow-up.
Keywords: Glycaemic control, Intensification, Obese, Observational, Real-world evidence, Retrospective, Therapeutic inertia, T2DM
Key Summary Points
| Why carry out this study? |
| Therapeutic inertia, defined as the failure to initiate or intensify treatment in a timely manner in accordance with evidence-based guidelines, is a key cause of uncontrolled hyperglycaemia (together with lack of adherence to treatment) in patients with type 2 diabetes mellitus (T2DM). |
| Limited evidence is available on the extent of therapeutic inertia and treatment intensification in obese patients with poor glycaemic control. |
| What was learned from this study? |
| The T2DM patients analysed in the study had a mean glycated haemoglobin (HbA1c) of 9.2% at baseline which remained ≥ 8% over a period of > 1 year. |
| The median time to first intensification was 456 (95% confidence interval 429-483) days, wich is longer than that recommended by guidelines. |
| Therapeutic inertia occurred in 77.8, 59.5, 41.5, 28.1 and 22.4% of patients at 6 months, 1, 2, 3 years and the end of follow-up, respectively. |
Introduction
Type 2 diabetes mellitus (T2DM) is associated with a great clinical and economic burden, and its incidence is expected to grow in future decades [1, 2]. Current prevalence ranges from 6 to 15%, depending on the study and diagnostic method, reaching 20% in patients aged > 75 years [3, 4]. Obesity, the main risk factor for T2DM, is diagnosed in up to 80% of T2DM patients [1, 2].
Optimal glucose control is recommended to avoid prolonged exposure to hyperglycaemia, which may trigger micro- and macrovascular complications [2–5]. Glycosylated haemoglobin (HbA1c) level is the gold standard used to monitor the degree of metabolic control, with target levels ranging from 6.5 to 8% [1–5]. Some guidelines suggest an individualised approach, with strict HbA1c targets (≤ 6.5%) in selected persons with DM who are otherwise healthy, while others promote less strict treatment goals (≤ 7.5–8.0%) for patients with a high degree of comorbidity [1, 2, 5]. In general, it is advised that if the desired metabolic objectives are not achieved after 3–6 months of lifestyle improvements, then pharmacological treatment should be initiated [2, 5]. It is estimated that more than half of patients with T2DM do not achieve the therapeutic goal of glycaemic control with the first treatment choice, even at maximum doses, and that in such cases other oral or injectable therapies, such as glucagon-like peptide receptor type 1 analogues (GLP-1 RA) or insulinisation, need to be added to the original therapeutic regimen [1, 5].
Therapeutic inertia, defined as the failure to initiate or intensify treatment in a timely manner in accordance with evidence-based guidelines, is a key cause of uncontrolled hyperglycaemia (together with a lack of adherence to treatment) in T2DM patients [6, 7]. Clinical inertia occurs when healthcare professionals recognise the problem, i.e. unachieved glycaemic objectives, but do not act to initiate or intensify treatment [6, 8–10]. The recent consensus of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) recommends evaluating and modifying treatment regularly at intervals of 3–6 months if the HbA1c level remains above target [5].
In Spain, studies show that, depending on the study design and follow-up time, between 32.2 and 52.5% of patients with deficient glycaemic control do not receive intensification of therapy [11–14]. The most notable delays in treatment intensification occur in patients with HbA1c levels of 7 and 8% receiving ≥ 2 oral antidiabetic drugs (OADs) [6]. However, few studies have evaluated therapeutic inertia and treatment intensification in obese patients with poor glycaemic control. Obesity and diabetes are two very closely related diseases. The prevalence of obesity is increasing in patients with T2DM, resulting in an increased risk of these patients developing a cardiovascular event [1]. The aim of this study (Patient 8.30 Study) was to characterise clinical inertia in T2DM patients with deficient glycaemic control (HbA1c ≥ 8%) and obesity (body mass index [BMI] ≥ 30 kg/m2) receiving treatment with ≥ 2 OADs. We have also reported on parameters such as the time to treatment intensification, the frequency of intensification and the duration of time the patient is above therapeutic target (glycaemic burden).
Methods
Design and Study Population
This was an observational, multicentre, longitudinal, retrospective study that was based on medical records included in computerised databases of various Spanish healthcare providers and complementary data sources of seven Autonomous Communities in Spain, unified in the dissociated BIG-PAC® database (Real Life Data; http://www.encepp.eu/encepp/search.htm). The inclusion period extended from 1 January 2013 to 31 December 2013, with a minimum follow-up of 4 years per patient from the inclusion date (i.e. index date defined as first date in 2013 with HbA1c ≥ 8% recorded and eligibility criteria met) up to 31 December 2017.
Confidentiality of Information
The confidentiality of the records (anonymous and dissociated) was respected in accordance with current regulations on the Protection of Personal Data, Regulation (EU) 2016/679 of the European Parliament and of the Council of April 27, 2016 on Data Protection (RGPD) and Organic Law 3/2018, of December 5, regarding the Protection of Personal Data and guarantee of digital rights. The study was approved by the Ethics Committee of Ramón y Cajal University Hospital, Madrid. Due to the nature of the study (i.e. retrospective database) patient consent was not required.
Inclusion and Exclusion Criteria
Patients eligible for inclusion in this study were aged ≥ 30 years; required medical care during 2013, with a diagnosis of T2DM (International Classification of Diseases 10th Revision, Clinical Modification [ICD-10-CM) codes: E11.0–E11.9, E14 or E14.0–E14.9) at least 6 months before the index date; were treated with ≥ 2 OADs; and were in the medical prescription programme (≥ 2 health records in the computer system). Patients were excluded from the study if they transferred to other centres or other areas, were displaced/lost to follow-up or were permanently institutionalised; had a history of type 1 diabetes, gestational diabetes and/or secondary diabetes; there was no medical records on HbA1c or BMI for 2013; and they had received treatment with insulin and/or GLP-1 RAs.
Study Variables
Prevalence of the Study Population
The prevalence rate was calculated based on the number of patients with poor glycaemic control (HbA1c ≥ 8% [64 mmol/mol]) and obesity (BMI ≥ 30 kg/m2 (numerator being the first record during 2013) among subjects with T2DM treated with ≥ 2 OADs (denominator). Standardisation of the results was not necessary since there was sufficient similarity between the age pyramid of patients included in the study and the Spanish general population.
Demographic and Clinical Variables
The demographic and clinical variables collected included age, sex, BMI, time from diagnosis, history of hypertension, dyslipidaemia, asthma, chronic obstructive pulmonary disease, heart failure, osteoporosis, malignant neoplasms (all types) and liver diseases (steatosis, hepatitis, cirrhosis). Data on macrovascular complications (including ischaemic heart disease [angina, acute myocardial infarction], stroke/transient ischaemic attack and/or peripheral arterial disease) and microvascular complications (including retinopathy, nephropathy and diabetic neuropathy) were collected. As a summary variable of general comorbidity, the modified Charlson Comorbidity Index [18] was used as an approximation of severity based on the number of chronic comorbidities. These records were obtained for each patient at the index date (year 2013).
Medication Administered
Information on OADs of the following Anatomical Therapeutic Chemical Classification (ATC) [19] groups dispensed for the treatment of T2DM on the index date and used either as monotherapy or in combination therapy was obtained: (1) metformin (A10BA*), (2) insulin release stimulants (sulfonylureas [A10BB*] and glinides [A10BX*]), (3) glitazones (A10BG*), (4) alpha glycosidase inhibitors (acarbose [A10BF*] and miglitol [A10BF*]) and (5) dipeptidyl peptidase 4 inhibitors (A10BH*). Fixed dose combinations were considered as two OADs. Patients were grouped according to the number of OADs (2, 3 or 4) administered on the index date. Sodium-glucose co-transporter-2 inhibitors were not included because the first molecule of this group was not commercially available in Spain until December 2013.
Glycaemic Burden
Glycaemic burden was evaluated as the time from the index date to the end of the follow-up period that the HbA1c level was ≥ 8%.
Clinical Inertia
Clinical inertia was defined as the lack of intensification of antidiabetic treatment (addition, change and/or increase in dose) in patients with HbA1c ≥ 8% on the index date during the period in which the patient had HbA1c ≥ 8%, evaluated at 6 months, 1, 2 and 3 years and at the end of follow-up (≥ 4 and ≤ 5 years). Clinical inertia was also evaluated by the time elapsed (days) from the index date to the first intensification, the time to initiation of injectable treatment (GLP-1 RA or insulin) and the number of intensifications during the period in which the patient had HbA1c ≥ 8%.
Statistical Analysis
A descriptive statistical analysis was carried out. Categorical variables were described with absolute and relative frequencies, and quantitative data were described as means and standard deviation (SD) and median and 95% confidence intervals (95% CI). All data were based on the total number of subjects, excluding missing values. For the variables associated with the measurement of time (time for which HbA1c ≥ 8% was maintained and time until the first intervention from the first record of HbA1c ≥ 8%), Kaplan–Meier survival analysis was used. Analyses were conducted overall, in two subgroups according to age (≥ 30–64 and ≥ 65 years) and in three subgroups according to HbA1c at index (8–8.9, 9–9.9, ≥ 10%). The statistical software SPSS for Windows version 23 (IBM Corp., Armonk, NY, USA) was used.
Results
Of the 1,973,278 patients included in the BIG-PAC database, 47,492 (95% CI 7.8–8.4%) had a diagnosis of T2DM. Among those with a diagnosis of T2DM, there were 13,824 with available BMI/HbA1c data who were treated with ≥ 2 OADs, and 2709 of these (19.6%, 95% CI 19.3-19.9%) met the criteria of HbA1c ≥ 8% and BMI ≥ 30 kg/m2 and were included in the study (Fig. 1). The mean time of follow-up was 4.3 years. No deaths were registered during the follow-up period. At index, mean age was 65.5 (SD 12.0) years (55.2% were aged ≥ 65 years), 54.9% were male, the mean time from diagnosis was 8.2 (SD 3.0) years and the mean Charlson Comorbidity Index was 1.8 (SD 1.6) points (Table 1).
Fig. 1.

Study flow chart of patient recruitment. DM2 Type 2 diabetes mellitus, OAD Oral antidiabetic drug, HbA1c glycosylated haemoglobin (%), BMI body mass index (kg/m2)
Table 1.
Characteristics of patients who met the inclusion criteria on the index date by total study population and by age group
| Patient characteristics | Age subgroups | Total patient population (N = 2709; 100%) | |
|---|---|---|---|
| ≥ 30–64 years (n = 1214; 44.8%) | ≥ 65 years (n = 1495; 55.2%) | ||
| Demographic characteristics | |||
| Mean age, years (SD; 95% CI) | 54.7 (7.6; 54.3–55.1) | 74.3 (6.6; 74.0–74.6) | 65.5 (12.0; 65.0–66.0) |
| Sex, % (male; 95% CI) | 62.5% (59.8–65.2%) | 48.7% (46.2–51.2%) | 54.9% (53.0–56.8%) |
| Time from diagnosis, years (SD; 95% CI) | 6.1 (2.0; 6.0–6.2) | 9.9 (2.6; 9.8–10.0) | 8.2 (3.0; 8.1–8.3) |
| General comorbidity | |||
| Mean Charlson Comoridity Index (SD; 95% CI) | 1.3 (1.2; 1.2–1.4) | 2.3 (1.7; 2.2–2.4) | 1.8 (1.6; 1.7–1.9) |
| Mean comorbidities, n (SD; 95% CI) | 2.8 (1.3; 2.7–2.9) | 3.5 (1.6; 3.4–3.6) | 3.2 (1.5; 3.1–3.3) |
| Mean comorbidies, % (95% CI) | |||
| 1 | 13.3% (11.4–15.2) | 5.8% (4.6–7.0%) | 9.2% (9.1–9.3%) |
| 2 | 30.9% (28.3–33.5%) | 17.9% (16.0–19.8%) | 23.7% (22.6–24.8%) |
| 3 | 28.3% (25.8–30.8%) | 28.9% (26.6–31.2%) | 28.6% (27.1–29.3%) |
| 4 | 15.7% (13.7–17.7%) | 24.7% (22.5–26.9%) | 20.6% (20.0–25.2%) |
| 5+ | 11.9% (10.1–13.7%) | 22.7% (20.6–24.8%) | 17.9% (16.9–18.9%) |
| Specific comorbidity, % (95% CI) | |||
| Hypertension | 64.0% (61.3–66.7%) | 79.6% (77.6–81.6%) | 72.6% (70.9–74.3%) |
| Dyslipidaemia | 59.5% (56.7–62.3%) | 56.9% (54.4–59.4%) | 58.0% (56.1–59.9%) |
| Smoking | 15.6% (13.6–17.6%) | 6.8% (5.5–8.1%) | 10.7% (9.5–11.9%) |
| Bronchial asthma | 5.5% (4.2–6.8%) | 8.8% (7.4–10.2%) | 7.3% (6.3–8.3%) |
| COPD | 6.6% (5.2–8.0%) | 13.0% (11.3–14.7%) | 10.1% (9.0 -11.2%) |
| Neoplasms | 8.6% (7.0–10.2%) | 10.2% (8.7–11.7%) | 9.5% (8.4–10.6%) |
| Osteoporosis | 5.3% (4.0–6.6%) | 10.6% (9.0–12.2%) | 8.2% (7.2 - 9.2%) |
| Heart failure | 3.8% (2.7–4.9%) | 18.6% (16.6–20.6%) | 12.0% (10.9–13.9%) |
| Liver diseases | 37.2 (34.5–39.9%) | 45.8 (43.3–48.3%) | 41.5 (39.6–43.4%) |
| Macrovascular complications, % (95% CI) | |||
| Ischaemic heart disease | 9.5% (7.9–11.1%) | 18.4% (16.4–20.4%) | 14.4% (13.1–15.7%) |
| Stroke | 2.6% (1.7–3.5%) | 10.2% (8.7–11.7%) | 6.8% (5.9–7.7%) |
| Peripheral arteriopathy | 4.3% (3.2–5.4%) | 9.0% (7.5–10.5%) | 6.9% (5.9–7.9%) |
| Microvascular complications, % (95% CI) | |||
| Diabetic neuropathy | 5.5% (4.0–6.6%) | 8.1% (6.7–9.5%) | 6.9% (5.9–7.9%) |
| Diabetic nephropathy | 3.6% (2.6–4.6%) | 11.0% (9.4–12.6%) | 7.8% (6.8–8.8%) |
| Diabetic retinopathy | 25.4% (23.0–27.6%) | 27.6% (25.3–29.9%) | 26.6% (24.9–28.3%) |
Inclusion criteria: age ≥ 30 years; diagnosis of type 2 diabetes (T2DM) at least 6 months before index date; deficient glycaemic control (glycosylated haemoglobin [HbA1c] ≥ 8%); obesity (body mass index [BMI] ≥ 30 kg/m2); receiving treatment with ≥ 2 oral antidiabetics (OADs)
CI Confidence interval, COPD chronic obstructive pulmonary disease, SD standard deviation,
At index, mean HbA1c was 9.2% (SD 1.3%), with 57.2% of patients having levels between 8 and 8.9%, mean BMI was 32.1 (SD 0.9) kg/m2 and 76.8% of patients were receiving 2 OADs, with the combination of metformin + sulfonylureas being the most frequent (63.3%). Patients aged ≥ 65 years showed better glycaemic control (mean HbAc1 9.0%; 95% CI 8.9–9.1) than those aged < 65 years (mean HbAc1 9.5%; 95% CI 9.4-9.6), but had a higher mean OAD use (2.4 [95% CI 2.3–2.5] vs. 2.2 [95% CI 2.1–2.3]) (Table 2).
Table 2.
Clinical variables and use of oral antidiabetics at index date in total patient population and by age subgroup
| Clinical variables and OAD use | Age subgroups | Total patient population (N = 2709; 100%) | |
|---|---|---|---|
| 30–64 years (n = 1214; 44.8%) | ≥ 65 years (n = 1495; 55.2%) | ||
| HbA1c, % | |||
| Mean level (SD) | 9.5 (1.5) | 9.0 (1.1) | 9.2 (1.3) |
| Median level (95% CI) | 9.0 (9.4–9.6) | 8.7 (8.9–9.1) | 8.8 (9.1–9.3) |
| Percentage of patients with HbA1c of: | |||
| 8.0–8.9% (95% CI) | 49.3% (46.5–52.1%) | 63.6% (61.2–66.0%) | 57.2% (55.3–59.1%) |
| 9.0–9.9% (95% CI) | 25.3% (22.9–27.7%) | 23.7% (21.5–25.7%) | 24.4% (22.8–26.0%) |
| ≥ 10.0% (95% CI) | 25.4% (23.0–27.8%) | 12.7% (11.0–14.4%) | 18.4% (16.9–19.9%) |
| BMI, kg/m2 (SD; 95% CI) | 32.1 (0.9; 32.0–32.2) | 32.0 (1.0; 31.9–32.1) | 32.1 (0.9; 32.0–32.2) |
| OADs | |||
| Mean, n (SD) | 2.2 (0.4) | 2.4 (0.6) | 2.3 (0.5) |
| Median, n (95% CI) | 2.0 (2.1–2.3) | 2.0 (2.3–2.5) | 2.0 (2.2–2.4) |
| Percentage of patients with 2 OADs (95% CI) | 85.6% (83.6–87.6%) | 69.7% (67.4–72.0%) | 76.8% (75.2–78.4%) |
| Met + SU | 72.9% (70.4–75.4%) | 55.5% (53.0–58.0%) | 63.3% (61.4–65.0%) |
| Met + IDPP4 | 7.4% (5.9–8.9%) | 10.1% (8.6–11.6%) | 8.9% (7.8–10.0%) |
| Other combinations | 5.3% (4.0–6.6%) | 4.1% (3.1–5.1%) | 4.6% (3.8–5.4%) |
| Percentage of patients with 3 OADs (95% CI) | 13.3% (11.4–15.2%) | 24.9% (22.7–27.1%) | 19.7% (18.2–21.2%) |
| Met + SU + IDPP4 | 7.2% (5.7–8.7%) | 14.4% (12.6–16.2%) | 11.2% (10.0–12.4%) |
| Met + SU + glitazones | 5.7% (4.4–7.0%) | 10.2% (8.7–11.7%) | 8.2% (7.2–9.2%) |
| Other | 0.3% (0.0–0.6%) | 0.3% (0.0–0.5%) | 0.3% (0.1–0.5%) |
| Percentage of patients with 4 OADs (95%CI) | 1.2% (0.6–1.8) | 5.4% (4.3–6.5%) | 3.3% (2.9–4.3%) |
| Met + SU + glitazones + IDPP4 | 1.0% (0.4–1.6%) | 4.0% (3.0–5.0%) | 2.3% (1.7–2.9%) |
| Other combinations | 0.2% (0.0–0.6%) | 1.4% (0.8–2.0%) | 1.2% (0.8–1.6%) |
OAD oral antidiabetics, HbA1c glycosylated haemoglobin (%), BMI body mass index (kg/m2)
Met Metformin, SU sulfonylureas, IDDP4 dipeptyl peptidase 4 inhibitors
Values expressed as percentages, mean (SD: standard deviation), 95% CI; confidence interval
Glycaemic Burden
The HbA1c level remained ≥ 8% for a median of 440 (95% CI 421–459) days; there were no differences between patients aged ≥ 65 years and those aged < 65 years. The median time at HbA1c ≥ 8% increased as a function of the index HbA1c value, being 390 (95% CI 372–408) days at an index HbA1c of 8–8.9%, 529 (95% CI 510–598) days at an index HbA1c of 9–9.9% and 578 (95% CI 560–598) days at an index HbA1c of ≥ 10.0%. The mean HbA1c prior to the last intensification was 8.5% (SD 1.8), with no differences found between patients aged ≥ 65 years and those aged < 65 years (Table 3; Fig. 2).
Table 3.
Duration of time glycated haemoglobin level remained ≥ 8% in total patient population and by age subgroup
| HbAc1 level | Age subgroups | Total patient population (N = 2709; 100%) | |
|---|---|---|---|
| ≥ 30–64 years (n = 1214; 44.8%) | ≥ 65 years (n = 1495; 55.2%) | ||
| Length of time HbA1c was maintained at ≥ 8%, days | |||
| Mean (SD) | 596.4 (464.8) | 598.8 (454.9) | 597.7 (459.3) |
| Median (95% CI) | 428 (400–456) | 449 (423–475) | 440 (421–459) |
| HbA1c 8.0–8.9%, median (95% CI) | 380 (353–407) | 404 (378–430) | 390 (372–408) |
| HbA1c 9.0–9.9%, median (95% CI) | 489 (461–517) | 582 (556–608) | 529 (510–548) |
| HbA1c ≥ 10.0%, median (95% CI) | 524 (496–552) | 653 (628–678) | 578 (560–598) |
| HbA1c value before last intensification, % | |||
| Mean (SD) | 8.5 (1.6) | 8.6 (1.9) | 8.5 (1.8) |
| Median (95% CI) | 8.3 (8.2–8.5)) | 8.4 (8.3–8.6) | 8.4 (8.3–8.6) |
| Treatment addition (95% CI) | 8.2 (8.1–8.3) | 8.1 (8.0–8.2) | 8.1 (8.0–8.2) |
| Treatment change (95% CI) | 8.2 (8.1–8.3) | 8.1 (8.0–8.2) | 8.2 (8.1–8.3) |
| Increased dose (95% CI) | 8.3 (8.2–8.4) | 8.2 (8.1–8.3) | 8.2 (8.1–8.3) |
HbA1c glycosylated haemoglobin (%)
Values expressed as percentages, mean (SD: standard deviation), 95% CI: confidence interval
Fig. 2.

Time HbA1c remained ≥ 8% according to the baseline HbA1c (a) and age groups (b). Kaplan–Meier curve: LOG RANK procedure (Mantel–Cox)
Treatment Intensification
At the end of the follow-up, 22.4% (n = 606) of patients overall were in clinical inertia (without treatment intensification), with a higher frequency of clinical inertia in patients aged ≥ 65 years than in those aged < 65 years (24.1 vs. 20.2%). Among these 606 patients, 77.8% were in clinical inertia at 6 months, 59.5% at 1 year, 41.5% at 2 years and 28.1% at 3 years (Fig. 3). The percentage of patients in clinical inertia at the end of the follow-up according to index HbA1c was 24.6% for those with HbA1c 8–8.9%, 21.1% for those with HbA1c 9–9.9% and 17.1% for those with HbA1c ≥ 10.0% (Fig. 4a).
Fig. 3.
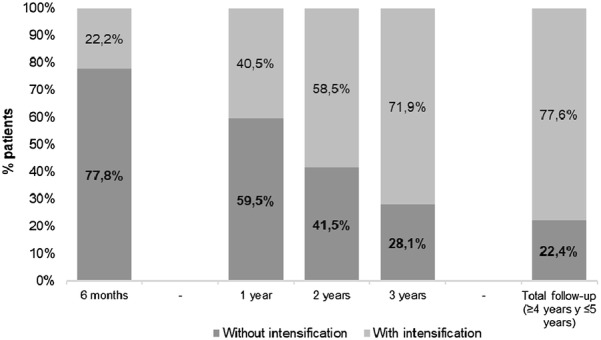
Treatment intensification from index to end of follow-up (≥ 4 years and ≤ 5 years)
Fig. 4.

Time to intensification according to baseline HbA1c (a) and age group (b). Kaplan–Meier curve: LOG RANK procedure (Mantel–Cox)
The mean number of treatment intensifications per patient during the period in which HbA1c remained ≥ 8% was 1.4 (SD 0.6). There were 2945 intensifications in 2103 in patients whose treatment was intensified during the follow-up. Specifically, the first intensifications (n = 2103) were mostly treatment changes (65.3%), followed by additions to treatment (33.8%) and dose increases (1.0%). The results were similar in the age groups (Fig. 4a).
Time to Treatment Intensification
The median time to the first treatment intensification was 456 (95% CI 429–483) days. The time to first intensification was longer in patients aged ≥ 65 years than in patients aged 30–64 years (485 vs. 422 days). The median time to the first treatment intensification by different index HbA1c range is shown in Fig. 4b.
The median time from the index date to the start of injectable treatment (GLP-1 RA or insulin) was 854 (95% CI 794–909) days for overall population, 783 days for the subgroup of patients aged < 65 years and 1055 days for the subgroup of patients aged ≥ 65 years. The median time decreased numerically with increasing baseline HbA1c. In the overall patient population, 34.8% of patients received GLP-1 RA or insulin; in the age subgroups of ≥ 65 years and < 65 years, these values were 38.3 and 31.6%, respectively) (Table 4).
Table 4.
Clinical inertia in total patient population and by age subgroup
| Clinical inertia | Age subgroups | Total patient population (N = 2709; 100%) | |
|---|---|---|---|
| 30–64 years (n = 1214; 44.8%) | ≥ 65 years (n = 1495; 55.2%) | ||
| Patients without intensification at the end of follow-up (n = 606) according to HbAc1 range, % (95% CI) | 20.2% (17.9–22.5%) | 24.1% (21.8–26.4%) | 22.4% (20.8–24.0%) |
| HbA1c 8.0–8.9% | 23.4% (20.0–25.8%) | 25.8% (23.3–28.3%) | 24.6% (23.0–26.2%) |
| HbA1c 9.0–9.9% | 20.5% (18.2–22.8%) | 21.7% (19.4–24.0%) | 21.1% (19.6–22.6%) |
| HbA1c ≥ 10.0% | 13.6% (11.7–15.5%) | 20.5% (18.4–22.6%) | 17.1% (15.7–18.5%) |
| Patients without intensification at various time points during follow-up, % (95% CI) | |||
| 6 months | 78.4% (76.1–80.7%) | 77.4% (75.3–79.5%) | 77.8% (76.2–79.4%) |
| 1 year | 58.3% (55.5–61.1%) | 60.6% (58.1–63.1%) | 59.5% (57.6–61.4%) |
| 2 years | 41.3% (38.5–44.1%) | 41.7% (39.2–44.2%) | 41.5% (39.6–43.4%) |
| 3 years | 26.1% (23.6–28.6%) | 29.7% (27.4–32.0%) | 28.1% (26.4–29.8%) |
| Time to first intensification, days | |||
| Mean (SD) | 460.8 (394.2) | 516.1 (446.0) | 490.6 (423.7) |
| Median (95% CI) | 422 (394–450) | 485 (460–510) | 456 (429–483) |
| HbA1c 8.0–8.9%, median (95% CI) | 436 (408–464) | 493 (468–518) | 495 (480–518) |
| HbA1c 9.0–9.9%, median (95% CI) | 427 (399–455) | 484 (459–509) | 457 (438–476) |
| HbA1c ≥ 10.0%, median (95% CI) | 403 (375–431) | 472 (447–497) | 388 (370–406) |
| Time to administration of GLP-1 RA/insulin, days | |||
| Mean (SD) | 878.6 (630.3) | 1082.8 (631.5) | 962.2 (637.7) |
| Median (95% CI) | 783 (761–805) | 1055 (1029–1081) | 854 (827–881) |
| HbA1c 8.0–8.9%, median (95% CI) | 840 (820–860) | 1178 (1152–1204) | 912 (885–939) |
| HbA1c 9.0–9.9%, median (95% CI) | 648 (622–674) | 991 (965–1017) | 766 (739–793) |
| HbA1c ≥ 10.0%, median (95% CI) | 610 (583–637) | 905 (879–931) | 730 (703–757) |
| Patients administered GLP-1 RA/insulin, % (95% CI) | 31.6% (29.0–34.2%) | 38.3% (35.8–40.8%) | 34.8% (33.0–36.6%) |
| HbA1c 8.0–8.9% | 16.5% (14.4–18.6%) | 11.5% (9.9–13.1%) | 14.2% (12.9–15.5%) |
| HbA1c 9.0–9.9% | 38.3% (35.6–41.0%) | 40.2% (37.7–42.7%) | 39.2% (37.4–41.0%) |
| HbA1c ≥ 10.0% | 45.2% (42.4–48.0%) | 48.3% (45.8–50.8%) | 46.6% (44.7–48.5%) |
| Mean number of intensifications per patient during study period (N = 2103), HbA1c ≥ 8% | 1.4 (0.6; 1.3–1.5) | 1.5 (0.6; 1.4–1.6) | 1.4 (0.6; 1.3–1.5) |
| HbA1c 8.0–8.9% (SD; 95% CI) | 1.1 (0.3; 1.0–1.2) | 1.2 (0.5; 1.1–1.3) | 1.2 (0.4; 1.1–1.3) |
| HbA1c 9.0–9.9% (SD; 95% CI) | 1.3 (0.5; 1.2–1.4) | 1.4 (0.5; 1.3–1.5) | 1.4 (0.5; 1.3–1.5) |
| HbA1c ≥ 10.0% (SD; 95% CI) | 1.8 (0.8; 1.7–1.9) | 2.0 (0.8; 1.9–2.1) | 1.9 (0.8; 1.8–2.0) |
| Treatment addition* (95% CI) | 33.0% (30.4–35.6%) | 34.4% (32.0–36.8%) | 33.8% (32.0–35.6) |
| Treatment change* (95% CI) | 65.7% (63.0–68.4) | 64.9% (62.5–67.3%) | 65.3% (63.5–67.1%) |
| Increased dose* (95% CI) | 1.2% (0.6–1.8%) | 0.7% (0.3–1.1%) | 1.0% (0.8–1.3%) |
HbA1c glycosylated haemoglobin (%), GLP-1 glucagon-like peptide type 1
Values expressed as percentages, mean (SD: standard deviation), 95%CI: confidence interval
p statistical significance* percentages refer to total intensifications
Discussion
The results of this study show that in T2DM patients with poor glycaemic control and obesity on concomitant treatment with ≥ 2 OADs, HbA1c levels remained at ≥ 8% for a long time (median 1.2 years and up to a median 1.6 years in patients with the worst baseline control). Similarly, a high percentage of patients were in clinical inertia, ranging from almost 60% at 1 year of follow-up to 22% at the end of the study (≥ 4 years and ≤ 5 years). These findings show a delay in treatment intensification with antidiabetic therapy in routine clinical practice.
The mean length of time patients had a HbA1c ≥ 8% (glycaemic burden) in the present study is within the range reported by a number of other studies, but it closer to the lower end of the range reported in one study [7] and considerably lower than that reported by Khunti et at. [15] who found a mean time of up to 7 years at a HbA1c ≥ 8%. We found, unlike Brown et al. [20], that this period lengthened with increasing baseline HbA1c. However, the comparability of the data between these studies is limited due to the selection of the HbA1c cutoff points. Brown et al. [20] used > 7% and > 8% as cutoffs while our study used ≥ 8% (8–8.9%, 9–9.9% and ≥ 10%). We found a low mean number of intensifications during the time patients had HbA1c ≥ 8%. Despite the intensifications, the HbA1c value before the last intensification continued to be > 8%. These results suggest limited adherence to current recommendations to intensify treatment every 3–6 months when the patient achieves/does not achieve the objectives established [5]. Studies have found that a high glycaemic burden is associated with an increased risk of myocardial infarction, stroke and heart failure and, therefore, reducing the time during which the patient is not within target range is important [21, 22].
In our study, clinical inertia was identified in 22% of patients at the end of the follow-up and ranged from 78% at 6 months to 28% at 3 years. Other studies in Spain have found rates that vary from 18.1% [6] to 32.2–52.5% of patients (years of studies 2012–2016) [11–14]. This variability between studies may be attributed to several factors, including the type of care setting, the population studied, the duration of diabetes, the definition of intensification (addition of a new OAD, change to insulin, dose increase, etc.), the HbA1c threshold chosen and, to a large extent, the length of follow-up. The review by Khunti et al. [7] found that in most studies more than 50% of patients did not receive treatment intensification within 1 year; these results are consistent with our data at 6 months (78% inertia) and 12 months (almost 60% inertia). Likewise, as in other studies [6, 12, 23–25], we found that clinical inertia was lower when there was worse glycaemic control and that it was higher in older patients. The greater inertia in patients aged ≥ 65 years may be related to less strict control criteria recommendations in this age group [5].
We found that there was a delay in treatment intensification of > 1 year in patients with HbA1c ≥ 8%, which was also observed in the sub-group of patients with HbA1c ≥ 10% at index. This delay is shorter than that reported by Mata-Cases et al. (22.6 months) [6]. However, Mata-Cases et al. [6] did not include treatment change or dose increase in the definition of intensification, which we did. Our results are in line with those from other studies that included patients treated with ≥ 2 OADs, in which the time to treatment intensification ranged from 1.3 to 4.9 years [23, 26–29].
Our results show a median time to initiation of injectable therapy (GLP-1 RA or insulin) of 2.3 years in the overall patient sample, which is similar to that found in the subgroup of patients with HbA1c ≥ 10% (2 years). Of those patients with HbA1c ≥ 10%, only 46.6% received injectable therapy despite it being recommended by the ADA and EASD [5]. In our study, patients aged < 65 years had worse control than patients aged ≥ 65 years (with > 50% of patients aged < 65 years having a baseline HbA1c of ≥ 9%). However, patients aged < 65 years less frequently received treatment intensification with injectable drugs, even though they were the ones who could benefit most from intensifications with high-efficacy drugs [5]. These findings suggest a rejection of as well as a delay in intensification with injectable therapy, which is in line with the findings of other published studies [27]. Patients tend to be reluctant to use insulin due to the injections, while physicians reject the use of insulins due to the risks for weight gain and hypoglycaemia, lack of training in using injectable therapies or lack of educational resources, among others [30, 31]. However, injectable therapy with GLP-1 RA is not associated with an increased risk of hypoglycaemia and is associated with weight loss; consequently, it should be considered as the first injectable therapy, before insulin [5]. These data are relevant given that it is known that delays in intensification are related to macro- and microvascular complications that will jeopardise the probability of achieving glycaemic control objectives [21, 22, 24]. Therefore, strategies that help reduce the time to intensification are required.
Limitations inherent to the nature of the study design (i.e. retrospective database) include underrecording, possible inaccuracies in the coding of diagnosis and the absence of certain clinical variables. As such, lifestyle changes, perceived education, patient preferences, risk/history of hypoglycaemia, patient’s cognitive function as well as other factors, any of which may be potential factors influencing healthcare proxy decisions with respect to treatment intensification, were not available for analysis [32, 33].
The HbA1c value of > 8% was set as the cutoff point to define clinical inertia in this study. This value, which is above the 7% generally used for glycaemic control, was selected to minimise bias toward overestimation of clinical inertia. There may be interindividual differences in the glycation process and HbA1c targets not accounted for in this definition. However, at the population level, HbA1c > 8% seems to be an adequate threshold at which, in the absence of individualised goals, most adult patients are considered to have poor glycaemic control [15, 16].
Time to the start of injectable therapy with a GLP-1 RA or insulin was not studied separately by treatment type, although based on previous literature [34], it is expected that the majority of injectable treatments initiated in the follow-up would be insulin based.
The strengths of the study are that it was conducted in a real-world setting, the follow-up was longer than in other studies and clinical inertia was assessed broadly, using several parameters. The study provides additional evidence on clinical inertia in patients with ≥ 2 OADs [6, 7]. Specifically, it provides new data on treatment inertia in patients with T2DM and obesity that are relevant both clinically and economically as approximately half the patients with T2DM are obese [35]. Our results supplement specific therapeutic recommendations [5] and findings that both obesity and poor glycaemic control have a significant impact on health costs [17].
Clinical inertia is dependent on the overall patient care process, involving the physician, the patient and the healthcare system. It is hypothesised that the physician’s contribution to clinical inertia is 50%, the patient’s 30% and that of the healthcare system 20% [36]. These factors need to be taken into account when introducing improvement strategies.
Conclusions
The results of this study, carried out in real-life patients with poorly controlled T2DM and obesity and receiving treatment with ≥ 2 OADs, show that these patients remained for > 1 year with poor glycaemic control (HbA1c ≥ 8%). Clinical inertia was present in almost 60% of patients in the first year of monitoring and persisted in 28% after 3 years of monitoring, reflecting treatment delays and a lack of intensification. Avoiding or minimising clinical inertia should be a key objective that can be facilitated by effectively incorporating the recommendations of the guidelines into routine practice, with the ultimate goal of improving the prognosis of the patient.
Acknowledgements
Funding
This study and the journal’s Rapid Service Fee were sponsored by Lilly SA.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
The conception, design, interpretation of the results, writing, review and approval of the submitted manuscript were made by all authors. Data collection and statistical analysis were made by A. Sicras-Mainar.
Disclosures
Antoni Sicras-Mainar is an independent consultant with respect to this study. Irene Romera is an employee of Lilly SA. F. Silvia Diaz is an employee of Lilly SA. F. Tatiana Dilla is an employee of Lilly SA. F. Esther Artime is an employee of Lilly SA. F. Jesús Reviriego is an employee of Lilly SA. F. Flora Lopez-Simarro has received honoraria as a speaker from: Lilly, MSD, Astra Zéneca, Mundipharma, Sanofi, Boehringer Ingelheim, Almirrall, Ferrer.
Compliance with Ethics Guidelines
The study was approved by the Ethics Committee of Ramón y Cajal University Hospital, Madrid. Due to the nature of the study (i.e. retrospective database) patients’ consent was not required.
Data Availability
The datasets generated during and/or analysed during the current study are available in the BIG-PAC® repository [http://www.rlifedata.com].
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced Digital Features
To view enhanced digital features for this article go to 10.6084/m9.figshare.11323757.
References
- 1.Qaseem Amir, Barry Michael J., Humphrey Linda L., Forciea Mary Ann. Oral Pharmacologic Treatment of Type 2 Diabetes Mellitus: A Clinical Practice Guideline Update From the American College of Physicians. Annals of Internal Medicine. 2017;166(4):279. doi: 10.7326/M16-1860. [DOI] [PubMed] [Google Scholar]
- 2.Arrieta F, Iglesias P, Pedro-Botet J, et al. Diabetes mellitus and cardiovascular risk: Working group recommendations of Diabetes and Cardiovascular Disease of the Spanish Society of Diabetes (SED, 2015). Aten Primaria. 2016;48:325–36. [DOI] [PMC free article] [PubMed]
- 3.Soriguer F., Goday A., Bosch-Comas A., Bordiú E., Calle-Pascual A., Carmena R., Casamitjana R., Castaño L., Castell C., Catalá M., Delgado E., Franch J., Gaztambide S., Girbés J., Gomis R., Gutiérrez G., López-Alba A., Martínez-Larrad M. T., Menéndez E., Mora-Peces I., Ortega E., Pascual-Manich G., Rojo-Martínez G., Serrano-Rios M., Valdés S., Vázquez J. A., Vendrell J. Prevalence of diabetes mellitus and impaired glucose regulation in Spain: the Di@bet.es Study. Diabetologia. 2011;55(1):88–93. doi: 10.1007/s00125-011-2336-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aguayo A., Urrutia I., González-Frutos T., Martínez R., Martínez-Indart L., Castaño L., Gaztambide S. Prevalence of diabetes mellitus and impaired glucose metabolism in the adult population of the Basque Country, Spain. Diabetic Medicine. 2016;34(5):662–666. doi: 10.1111/dme.13181. [DOI] [PubMed] [Google Scholar]
- 5.Davies MJ, D’Alessio DA, Fradkin J, et al. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetologia. 2018;2018(61):2461–2498. doi: 10.1007/s00125-018-4729-5. [DOI] [PubMed] [Google Scholar]
- 6.Mata-Cases M, Franch-Nadal J, Real J, et al. Therapeutic inertia in patients treated with two or more antidiabetics in primary care: factors predicting intensification of treatment. Diabetes Obes Metab. 2018;20:103–112. doi: 10.1111/dom.13045. [DOI] [PubMed] [Google Scholar]
- 7.Khunti K, Gomes MB, Pocock S, et al. Therapeutic inertia in the treatment of hyperglycaemia in patients with type 2 diabetes: a systematic review. Diabetes Obes Metab. 2018;20:427–437. doi: 10.1111/dom.13088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mata-Cases M. Inercia terapéutica en el tratamiento de la diabetes tipo 2. Diabet Práctica 2017;8[Suppl 1]:1–20.
- 9.Dunkley AJ, Bodicoat DH, Greaves CJ, et al. Diabetes prevention in the real world: effectiveness of pragmatic lifestyle interventions for the prevention of type 2 diabetes and of the impact of adherence to guideline recommendations: a systematic review and meta-analysis. Diabetes Care. 2014;37:922–933. doi: 10.2337/dc13-2195. [DOI] [PubMed] [Google Scholar]
- 10.Bailey CJ. Under-treatment of type 2 diabetes: causes and outcomes of clinical inertia. Int J Clin Pract. 2016;70:988–995. doi: 10.1111/ijcp.12906. [DOI] [PubMed] [Google Scholar]
- 11.Mata-Cases Manel, Benito-Badorrey Belén, Roura-Olmeda Pilar, Franch-Nadal Josep, Pepió-Vilaubí Josep Maria, Saez Marc, Coll-de-Tuero Gabriel. Clinical inertia in the treatment of hyperglycemia in type 2 diabetes patients in primary care. Current Medical Research and Opinion. 2013;29(11):1495–1502. doi: 10.1185/03007995.2013.833089. [DOI] [PubMed] [Google Scholar]
- 12.González-Clemente JM, Font B, Lahoz R, Llauradó G, Gambús G; Grupo de investigadores del Estudio INERCIA. INERTIA study: Clinical inertia in non-insulinized patients on oral hypoglycemic treatment. A study in Spanish primary and specialty care settings. Med Clin (Barc). 2014;142:478–84 (in Spanish). [DOI] [PubMed]
- 13.Vernet Vernet M, Sender Palacios MJ, Bautista Galí L, Larrosa Sàez P, Vargas SJ. Therapeutic inertia in the management of type 2 diabetic patients in primary health care. Semergen. 2016;42:152–157. doi: 10.1016/j.semerg.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 14.López Simarro F, Brotons Cuixart C, Moral I, et al. Inercia y cumplimiento terapéutico en pacientes con diabetes mellitus tipo 2 en atención primaria. Med Clin (Barc). 2012;138:377–384. doi: 10.1016/j.medcli.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 15.Khunti K, Wolden ML, Thorsted BL, Andersen M, Davies MJ. Clinical inertia in people with type 2 diabetes: a retrospective cohort study of more than 80,000 people. Diabetes Care. 2013;36:3411–3417. doi: 10.2337/dc13-0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valdés Sergio, García-Torres Francisca, Maldonado-Araque Cristina, Goday Albert, Calle-Pascual Alfonso, Soriguer Federico, Castaño Luis, Catalá Miguel, Gomis Ramon, Rojo-Martínez Gemma. Prevalence of Obesity, Diabetes and Other Cardiovascular Risk Factors in Andalusia (Southern Spain). Comparison With National Prevalence Data. The Di@bet.es Study. Revista Española de Cardiología (English Edition) 2014;67(6):442–448. doi: 10.1016/j.rec.2013.09.029. [DOI] [PubMed] [Google Scholar]
- 17.Dilla Tatiana, Valladares Amparo, Nicolay Claudia, Salvador Javier, Reviriego Jesús, Costi María. Healthcare Costs Associated with Change in Body Mass Index in Patients with Type 2 Diabetes Mellitus in Spain. Applied Health Economics and Health Policy. 2012;10(6):417–430. doi: 10.1007/BF03261876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Charlson ME, Pompei P, Ales KL, Mackenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organisation. The anatomical therapeutic chemical classification system with defined daily doses (ATC/DDD). Geneva: World Health Organization. Available at: http://www.who.int/classifications/atcddd/en/. Accessed April 2018.
- 20.Brown JB, Nichols GA, Perry A. The burden of treatment failure in type 2 diabetes. Diabetes Care. 2004;27:1535–1540. doi: 10.2337/diacare.27.7.1535. [DOI] [PubMed] [Google Scholar]
- 21.Paul SK, Klein K, Thorsted BL, Wolden ML, Khunti K. Delay in treatment intensification increases the risks of cardiovascular events in patients with type 2 diabetes. Cardiovasc Diabetol. 2015;14:100. doi: 10.1186/s12933-015-0260-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Folse HJ, Mukherjee J, Sheehan JJ, et al. Delays in treatment intensification with oral antidiabetic drugs and risk of microvascular and macrovascular events in patients with poor glycaemic control: an individual patient simulation study. Diabetes Obes Metab. 2017;19:1006–1013. doi: 10.1111/dom.12913. [DOI] [PubMed] [Google Scholar]
- 23.Lin Jay, Zhou Steve, Wei Wenhui, Pan Chunshen, Lingohr-Smith Melissa, Levin Philip. DOES CLINICAL INERTIA VARY BY PERSONALIZED A1C GOAL? A STUDY OF PREDICTORS AND PREVALENCE OF CLINICAL INERTIA IN A U.S. MANAGED-CARE SETTING. Endocrine Practice. 2016;22(2):151–161. doi: 10.4158/EP15868.OR. [DOI] [PubMed] [Google Scholar]
- 24.Watson L, Das R, Farquhar R, Langerman H, Barnett AH. Consequences of delaying treatment intensification in type 2 diabetes: evidence from a UK database. Curr Med Res Opin. 2016;32:1465–1475. doi: 10.1185/03007995.2016.1157462. [DOI] [PubMed] [Google Scholar]
- 25.Fu AZ, Sheehan JJ. Change in HbA1c associated with treatment intensification among patients with type 2 diabetes and poor glycemic control. Curr Med Res Opin. 2017;33:853–858. doi: 10.1080/03007995.2017.1292231. [DOI] [PubMed] [Google Scholar]
- 26.Ajmera M, Raval A, Zhou S, et al. A real-world observational study of time to treatment intensification among elderly patients with inadequately controlled type 2 diabetes mellitus. J Manag Care Spec Pharm. 2015;21:1184–1193. doi: 10.18553/jmcp.2015.21.12.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rubino A, McQuay LJ, Gough SC, Kvasz M, Tennis P. Delayed initiation of subcutaneous insulin therapy after failure of oral glucose lowering agents in patients with type 2 diabetes: a population-based analysis in the UK. Diabets Med. 2007;24:1412–1418. doi: 10.1111/j.1464-5491.2007.02279.x. [DOI] [PubMed] [Google Scholar]
- 28.Schmittdiel JA, Uratsu CS, Karter AJ, et al. Why don’t diabetes patients achieve recommended risk factor targets? Poor adherence versus lack of treatment intensification. J Gen Intern Med. 2008;23:588–594. doi: 10.1007/s11606-008-0554-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwab P, Saundankar V, Bouchard J, et al. Early treatment revisions by addition or switch for type 2 diabetes: impact on glycemic control, diabetic complications, and healthcare costs. BMJ Open Diabetes Res Care. 2016;4:e000099. doi: 10.1136/bmjdrc-2015-000099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mauricio D, Hramiak I. Second-generation insulin analogues—a review of recent real-world data and forthcoming head-to-head comparisons. Eur Endocrinol. 2018;14(Suppl 1):2–9. doi: 10.17925/EE.2018.14supp1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Escalada J, Orozco-Beltran D, Morillas C, et al. Attitudes towards insulin initiation in type 2 diabetes patients among healthcare providers: a survey research. Diabetes Res Clin Pract. 2016;122:46–53. doi: 10.1016/j.diabres.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 32.Kulzer B. Adherence problems and clinical inertia. 11 strategies keeping patients with diabetes onboard. MMW Fortschr Med. 2017;159:67–72. [DOI] [PubMed]
- 33.Schwartz SS, Kohl BA. Glycemic control and weight reduction without causing hypoglycemia: the case for continued safe aggressive care of patients with type 2 diabetes mellitus and avoidance of therapeutic inertia. Mayo Clin Proc. 2010;85(Suppl):S15–S26. doi: 10.4065/mcp.2010.0468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mata-Cases Manel, Franch-Nadal Josep, Ortega Emilio, Real Jordi, Gratacòs Mònica, Vlacho Bogdan, Mauricio Dídac. Glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes:real-world evidence from a Mediterranean area. Current Medical Research and Opinion. 2019;35(10):1735–1744. doi: 10.1080/03007995.2019.1618806. [DOI] [PubMed] [Google Scholar]
- 35.Vinagre I, Mata-Cases M, Hermosilla E, et al. Control of glycemia and cardiovascular risk factors in patients with type 2 diabetes in primary care in Catalonia (Spain) Diabetes Care. 2012;35:774–779. doi: 10.2337/dc11-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Connor PJ, Sperl-Hillen JAM, Johnson PE, Rush WA, Biltz G. Clinical inertia and outpatient medical errors. In: Henriksen K, Battles JB, Marks ES, Lewin DI, eds. Advances in patient safety: from research to implementation (vol. 2: Concepts and methodology). Rockville: Agency for Healthcare Research and Quality (US); 2005. p. 293–308. [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available in the BIG-PAC® repository [http://www.rlifedata.com].


