Abstract
Background
The objective of this study was to investigate whether androgen deprivation therapy (ADT) with gonadotropin-releasing hormone agonist (GnRHa) in prostate cancer (Pca) patients is associated with cardiovascular disease in the cohort based from the entire Korean population.
Methods
Using the Korean National Health Insurance database, we conducted an observational study of 579,377 men who sought treatment for Pca between January 1, 2012 and December 31, 2016. After excluding patients with previously diagnosed cardiovascular disease or who had undergone chemotherapy, we extracted the data from 2,053 patients who started GnRHa (GnRHa users) and 2,654 men who were newly diagnosed with Pca (GnRHa nonusers) between July 1, 2012, and December 31, 2012, with follow-up through December 31, 2016. The primary outcomes were cerebrovascular attack (CVA) and ischemic heart disease (IHD).
Results
GnRHa users were older, were more likely to reside in rural areas, had lower socioeconomic status, and had more comorbidities than nonusers (all P < 0.050). Although GnRHa users had an increased incidence of CVA and IHD (P = 0.013 and 0.048, respectively) in univariate analysis, GnRHa use was not associated with the outcomes in multivariate analysis. Furthermore, the cumulative duration of ADT was not associated with the outcomes whereas the associations between age at diagnosis with all diseases were significant.
Conclusion
Our complete enumeration of the Korean Pca population shows that ADT is not associated with increased risks of cardiovascular disease.
Keywords: Antineoplastic Agents, Prostatic Neoplasm, Adverse Effects, Cardiovascular Diseases
Graphical Abstract
INTRODUCTION
Due to the remarkable advances in the treatment of prostate cancer (Pca), a large proportion of patients are diagnosed with loco-regional disease and have excellent cancer-related survival.1 Men with Pca, however, have higher non cancer mortality rates than the general population, and some of these excess deaths may be related to treatment.2 There are many reports of the adverse effects of Pca treatment, especially androgen deprivation therapy (ADT), that could potentially impact overall health status and affect the mortality rate.
Although ADT has been a treatment of choice for patients with metastatic disease since it was introduced in the 1940s, evidence from randomized trials supports the use of ADT in combination with external-beam radiation therapy for locally advanced Pca with high-risk features.3,4 Even localized disease without adverse pathologic features (pT stage T2b or less) has been shown to benefit from combination therapy with ADT,5 accounting for a dramatic increase in ADT use.6 Despite its excellent treatment effects, ADT is associated with numerous adverse effects, including vasomotor flushing, osteoporosis, fatigue, loss of libido, and gynecomastia.7,8 Patients may develop more serious events, including cardiovascular disease, and diabetes mellitus (DM), which in turn can lead to significant decrease in quality of life or even life-threatening consequences.
There are some controversial reports about these notable adverse effects. Several previous studies reported that ADT was associated with increased risks of myocardial infarction (MI) along with earlier onset of fatal MI.1,9,10 In contrast to these findings, there are many reports that suggest that the association between ADT use and these serious events is questionable. A report from the Radiation Therapy Oncology Group protocol 86-10 found that neoadjuvant ADT was not associated with an increased risk of cardiac events.11 Adjuvant ADT was also not associated with increased risk of cardiovascular mortality.12 Taken together, these results have collectively led to significant uncertainty in clinical practice. Because ADT with a gonadotropin-releasing hormone agonist (GnRHa) continues to be a crucial component of the treatment of Pca, identifying potential risks related to GnRHa use is important. We conducted this population-based study to investigate whether GnRHa use in patients with Pca was associated with increased incidences of cardiovascular diseases.
METHODS
Data sources and study cohort
We obtained the records of 579,377 men with code C61, which indicates Pca according to the International Classification of Diseases, 10th edition (ICD-10), who were diagnosed between January 1, 2012, and December 31, 2016, from the database provided by the Korean Health Insurance Review and Assessment Service (HIRA) (Supplementary Table 1). After excluding patients (Supplementary Table 2), a total of 4,707 men who received a diagnosis of Pca during 6 months of the aforementioned period and those who had claim records through December 31, 2016 (indicating patients with follow-up periods of 48 to 54 months) were finally included. GnRHa users were defined as patients who first used GnRHa between July 1, 2012, and December 31, 2012, to eliminate confounding effects due to use of GnRHa before the enrollment period. We used claim data to ascertain receipt of GnRHa to identify 2,053 men as users and 2,654 men without claim data for receipt of GnRHa throughout the study period as nonusers (Supplementary Table 3). The duration of GnRHa exposure was calculated by summing the number of 1-month equivalent doses.
Ascertainment of primary outcomes and other covariates
The primary outcome was the development of cardiovascular disease; cerebrovascular attack (CVA) or ischemic heart disease (IHD). Outcomes were ascertained using ICD-10 diagnosis codes associated with hospital admission and/or outpatient clinic visits from the database (Supplementary Table 4). In order not to identify cardiovascular disease diagnosed before the diagnosis of Pca, we defined prevalent cardiovascular disease as either condition diagnosed between January 1, 2012 and December 31, 2012. We defined incident CVA and IHD when the condition was identified after the enrollment period (after January 1, 2013).
We abstracted demographic data, such as age at diagnosis, urban or suburban/rural residence, type of insurance, prior medication use (statin, antihypertensive, anticoagulants, and antiplatelet therapy), use of antiandrogen, medical history (hypertension, liver disease, other cancer, chronic kidney disease, chronic obstructive pulmonary disease, asthma, and peripheral vascular disease), and Charlson comorbidity index (CCI) score. There are two types of insurance in Korea: National Health Insurance and Medicaid, which can indirectly reflect the socioeconomic status (SES) of the patient. CCI was calculated to assess comorbidity according to established techniques.13 Drug prescription data and medical histories of interest, including those used to calculate the comorbidity index, were ascertained using ICD-10 diagnosis codes, and HIRA reimbursement codes (Supplementary Tables 3 and 4).
Statistical analysis
We first described patient characteristics and comorbid illnesses according to use of GnRHa. The χ2 test was used to assess whether these covariates differed between GnRH users and nonusers. Next, Kaplan-Meier estimation was used to compare the proportions of GnRHa users and nonusers who experienced the primary outcome (CVA and IHD). Cox proportional hazards models were used to assess the direct effect of GnRHa use on the time to developing each outcome. The covariates in the Cox proportional hazards models included demographics, drug prescriptions, and comorbidities. To investigate the impact of the duration of GnRHa use on the outcomes, we created a version of this model that included duration. We defined duration of GnRHa use as 12 months or less, 13 to 24 months, 25 to 36 months, and 37 months or more. All statistical analyses were performed using SAS® Software 9.3 (SAS Institute, Cary, NC, USA) and a P value < 0.05 was considered to indicate statistical significance.
Ethics statement
The study protocol was approved by the Institutional Review Board of Hallym University Sacred Heart Hospital (No. 2017-I106), and informed consent was waived by the board because the data did not include personal identifiers.
RESULTS
During the follow-up, 441 (9.4%) and 151 (3.2%) patients were newly diagnosed with CVA and IHD, respectively. The mean age of the cohort at diagnosis was 69.3 ± 9.2 years and the mean duration of GnRHa use was 11.1 ± 17.4 months. Compared with GnRHa users, nonusers were significantly younger (users, 72.6 ± 8.3, and nonusers, 66.8 ± 9.0 years; P < 0.001), more likely to reside in the urban area (users, 75.6% and nonusers, 79.7%; P = 0.002), and more likely to have National Health Insurance rather than be supported by the Medicaid system (users, 94.2%, and nonusers, 96.9%; P < 0.001). The two groups did not differ significantly with regard to previous medical histories that might have an impact on the development of cardiovascular disease (Table 1). However, the rate of exposure to related medications was significantly higher in nonusers than in users, except for antiplatelet therapy. Furthermore, although there were no differences between the groups in previous medical conditions that was included in the analysis, the calculated comorbidity score using the CCI scoring system showed a significant difference in favor of nonusers (Table 1).
Table 1. Basic characteristics of GnRHa users and nonusers.
| Characteristics | GnRHa users (n = 2,053) | GnRHa nonusers (n = 2,654) | P value | |
|---|---|---|---|---|
| Age at diagnosis, yr | < 0.001 | |||
| < 55 | 53 (2.6) | 246 (9.3) | ||
| 55–64 | 277 (13.5) | 785 (29.6) | ||
| 65–74 | 806 (39.2) | 1,107 (41.7) | ||
| ≥ 75 | 917 (44.7) | 516 (19.4) | ||
| Residence | 0.002 | |||
| Urban | 1,552 (75.6) | 2,116 (79.7) | ||
| Suburban/rural | 501 (24.4) | 538 (20.3) | ||
| Insurance type | < 0.001 | |||
| NHI | 1,933 (94.2) | 2,571 (96.9) | ||
| Medicaid | 120 (5.8) | 83 (3.1) | ||
| Prior medication use | ||||
| Statin | 140 (6.8) | 219 (8.3) | 0.035 | |
| Antihypertensive | 203 (9.9) | 318 (12.0) | 0.017 | |
| Anticoagulant | 185 (9.0) | 348 (13.1) | < 0.001 | |
| Antiplatelet | 91 (4.4) | 101 (3.8) | 0.457 | |
| Prior antiandrogen use | 330 (16.0) | 0 (0.0) | - | |
| Medical history | ||||
| Hypertension | 896 (43.6) | 1,126 (42.4) | 0.344 | |
| DM | 454 (22.1) | 592 (22.3) | 0.420 | |
| Liver disease | 56 (2.7) | 89 (3.4) | 0.112 | |
| Other cancer | 243 (11.8) | 280 (10.6) | 0.211 | |
| Chronic kidney disease | 52 (2.5) | 48 (1.8) | 0.079 | |
| COPD | 188 (9.2) | 252 (9.4) | 0.767 | |
| Asthma | 158 (7.7) | 176 (6.6) | 0.135 | |
| Peripheral vascular disease | 220 (10.7) | 277 (10.4) | 0.679 | |
| Other treatment | < 0.001 | |||
| Radical prostatectomy | 0 (0.0) | 1,603 (60.4) | ||
| Radiotherapy | 458 (22.3) | 517 (19.5) | ||
| Charlson comorbidity index | < 0.001 | |||
| 0, 1 | 116 (5.7) | 414 (15.6) | ||
| 2 | 341 (16.6) | 713 (26.9) | ||
| 3 | 687 (33.5) | 772 (29.1) | ||
| 4 | 510 (24.8) | 431 (16.2) | ||
| ≥ 5 | 399 (19.4) | 328 (12.4) | ||
Data are presented as number (%).
GnRHa = gonadotropin-releasing hormone agonist, COPD = chronic obstructive pulmonary disease, NHI = national health insurance, DM = diabetes mellitus.
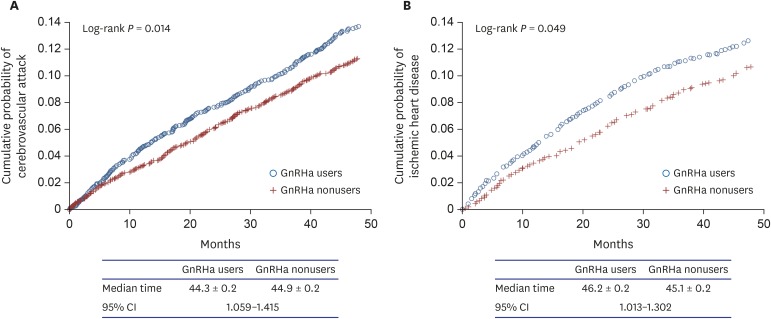
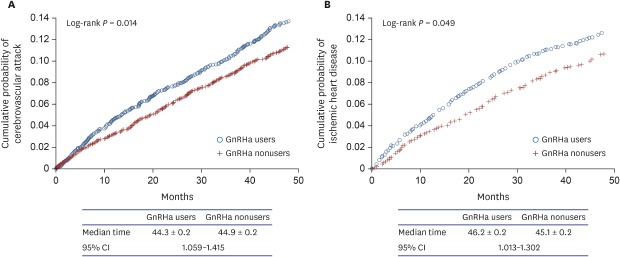
Kaplan-Meier curves for each of the outcomes—CVA and IHD—as univariate analyses are shown in Fig. 1. GnRHa users were more likely than nonusers to develop CVA and IHD (log rank, P = 0.013 and 0.048, respectively). The median time from the start of follow-up to the development of CVA was 44.3 ± 0.2 (standard error) months for GnRHa users and 44.9 ± 0.2 months for nonusers with 95% confidence interval of 1.059 to 1.415. However, in the multivariate analysis with Cox proportional hazards models, we found that GnRHa use was not independently associated with development of CVA and IHD (Table 2). Important significant predictors of CVA and IHD development were age as a continuous variable, prior hypertension, DM, chronic obstructive pulmonary disease, and CCI as a continuous variable (Table 2). Treatment modalities including radical prostatectomy and/or radiotherapy were not associated with the outcomes. Independent predictors of each disease outcome are shown in Table 2.
Fig. 1. Kaplan-Meier curves for the outcomes comparing GnRHa users to nonusers. (A) cerebrovascular attack, (B) ischemic heart disease.
GnRHa = gonadotropin-releasing hormone agonist, CI = confidence interval.
Table 2. Multivariate Cox proportional hazard model predicting risk of cerebrovascular attack, IHD, MI, and DM.
| Variables | CVA | IHD | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| GnRHa use | 0.926 (0.791–1.111) | 0.359 | 0.931 (0.785–0.186) | 0.135 | |
| Age at diagnosis (continuous) | 1.069 (1.057–1.095) | < 0.001 | 1.039 (1.019–1.010) | < 0.001 | |
| Urban vs. suburban/rural | 1.221 (0.958–1.498) | 0.063 | 1.617 (1.305–2.028) | < 0.001 | |
| NHI vs. Medicaid | 1.704 (1.198–2.307) | 0.002 | 1.342 (0.931–2.012) | 0.098 | |
| Prior medication use | |||||
| Statin | 1.264 (0.953–1.676) | 0.104 | 0.395 (0.497–0.915) | 0.021 | |
| Antihypertensive | 1.064 (0.923–1.598) | 0.099 | 0.826 (0.628–0.986) | 0.037 | |
| Anticoagulant | 0.907 (0.696–1.182) | 0.470 | 0.986 (0.758–1.193) | 0.895 | |
| Antiplatelet therapy | 1.317 (0.917–1.891) | 0.135 | 0.710 (0.458–0.965) | 0.009 | |
| Prior antiandrogen use | 0.897 (0.548–1.015) | 0.088 | 0.812 (0.595–1.152) | 0.128 | |
| Medical history | |||||
| Hypertension | 1.200 (1.019–1.412) | 0.028 | 1.721 (1.398–1.998) | < 0.001 | |
| DM | 1.302 (1.023–1.521) | 0.017 | 1.652 (1.345–2.003) | < 0.001 | |
| Liver disease | 1.150 (0.744–1.778) | 0.530 | 1.329 (0.902–2.025) | 0.386 | |
| Other cancer | 1.206 (0.982–1.482) | 0.074 | 0.986 (0.605–1.152) | 0.098 | |
| CKD | 0.876 (0.483–1.592) | 0.665 | 1.425 (0.898–2.197) | 0.197 | |
| COPD | 1.427 (1.115–1.826) | 0.005 | 1.398 (1.108–1.912) | 0.003 | |
| Asthma | 1.232 (0.920–1.651) | 0.161 | 1.205 (0.831–1.612) | 0.295 | |
| Radical prostatectomy | 1.205 (0.865–2.057) | 0.395 | 1.207 (0.776–1.912) | 0.562 | |
| Radiotherapy | 1.198 (0.698–2.157) | 0.385 | 1.386 (0.837–2.514) | 0.473 | |
| CCI (continuous) | 1.051 (1.009–1.112) | 0.002 | 1.038 (1.021–1.103) | 0.002 | |
Values in bold type are statistically significant at P < 0.05.
IHD = ischemic heart disease, MI = myocardial infarction, DM = diabetes mellitus, CVA = cerebrovascular attack, HR = hazard ratio, CI = confidence interval, GnRHa = gonadotropin-releasing hormone agonist, NHI = national health insurance, CKD = chronic kidney disease, COPD = chronic obstructive pulmonary disease, CCI = Charlson comorbidity index.
To investigate the impact of duration of GnRHa use on the development of cardiovascular disease, we performed further analyses by categorizing the cohort into four groups according to duration of GnRHa use: non users, 12 months or less, 13 to 24 months, 25 to 36 months, and 37 months or more. In multivariate analysis, GnRHa use was still non associated with both outcomes, and increasing duration of use was not associated with increased risk of cardiovascular disease (Table 3). In contrast, age and previous diagnosis of hypertension remained as independent predictors of the development of CVA and IHD (data not shown).
Table 3. Association between duration of GnRHa use and cerebrovascular attack, IHD, MI, and DMa .
| Variables | CVA | IHD | |||
|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | ||
| Duration of GnRHa use, mon | |||||
| No use | Reference | Reference | |||
| ≤ 12 | 1.302 | 0.910–1.895 | 0.654 | 0.517–1.685 | |
| 13–24 | 0.982 | 0.598–1.180 | 1.194 | 0.752–1.594 | |
| 25–36 | 1.652 | 1.021–2.025 | 1.359 | 0.845–1.726 | |
| ≥ 37 | 1.215 | 0.877–1.524 | 1.217 | 0.612–1.783 | |
| P value | 0.090 | 0.139 | |||
Statistically significant values indicate bold.
GnRHa = gonadotropin-releasing hormone agonist, IHD = ischemic heart disease, MI = myocardial infarction, DM = diabetes mellitus, CVA = cerebrovascular attack, HR = hazard ratio, CI = confidence interval.
aMultivariate Cox regression models were used to estimate primary outcomes. All models were adjusted for age at diagnosis, residence (urban vs. suburban/rural), socioeconomic status (National Health Insurance vs. Medicaid), prior medication, prior antiandrogen use, past medical history, and comorbidity index.
DISCUSSION
There has been great interest in the adverse cardiovascular effects of ADT, given the suggestions that low serum testosterone level is associated with coronary artery disease.14,15 Several studies have reported an association between ADT and increased risk of developing cardiovascular disease. A claims-based analysis of more than 70,000 Pca patients reported that GnRHa use increased the risk of incident DM, coronary heart disease, MI, and sudden cardiac death.1 A report based on the Surveillance, Epidemiology, and End Results (SEER)-Medicaid database found that patients who received ADT for at least 1 year had a 20% higher risk of cardiovascular morbidity than control patients.16 Another study using linked administrative databases showed that men aged 66 years or older with Pca who were given at least 6 months of ADT had an increased risk of DM,17,18 but not of MI or sudden cardiac death.19 Thus, the findings of studies of the association between ADT and cardiovascular disease that have suggested a positive correlation remain somewhat mixed, making the issue quite confusing.
The above-mentioned studies have a few weaknesses. First, most studies did not randomly assign the patients either to ADT or to s control group. This of course makes it possible that factors related to ADT might also be related to cardiovascular disease. Second, these studies might have overlooked the possibility that patients receiving regular prescriptions of ADT visit the hospital more frequently, which would make them more likely to be diagnosed with diseases of interest.1 Third, different studies focused on different outcomes. For example, some studies analyzed the incidence rates, while others investigated disease-related morbidity or mortality.1,16,19
Although our study may have similar limitations, our results are based on the entire Korean population, which included all Pca patients from the whole nation during the study period, rather than sampling a certain range of the population (e.g., database from Medicaid or from different insurers), as was done in the previous studies.1,19 Analyzing the patient cohort from the whole nation may have reduced possible bias that might have resulted from patient cohort sampling. In this nation-wide, population-based study of men with Pca, we found that ADT with GnRHa was not associated with increased risks of cardiovascular disease. The result did not change when the duration of GnRHa use was taken into consideration. These results were confirmed after accounting for oral antiandrogen use and additional clinical information, such as age at diagnosis, medications and previous diagnoses that might have been related to the outcomes of interest, and SES. Our analysis found that the important factors related to increased risk of developing cardiovascular disease were the patient’s age and previous diagnosis of hypertension and/or DM.
Several studies reported results supporting our findings. Studies investigating morbidity related to cardiovascular events had demonstrated similar results,16,19 and as for mortality, a few prior randomized trials had found that there was no association between ADT and cardiovascular mortality.11,12
We hypothesized that the main reason that ADT use seemed to be related to cardiovascular disease in previous studies was discrepancies in patient characteristics between the ADT and control groups. This might have caused a few defects in controlling for confounding factors in the analysis. As seen in our data, GnRHa nonusers tended to be younger, reside more in urban areas, and have higher SES (fewer included in Medicaid). Notably, although there were no differences in past medical history between GnRHa users and nonusers, a significant difference was observed in prior use of relevant medications (Table 1). This suggests that nonusers may visit medical facilities more often and receive more appropriate treatment for their health issues, which can greatly affect the development of cardiovascular disease. Furthermore, nonusers had significantly less comorbidity than users as calculated by the CCI. This indicates that nonusers were healthier than users from the beginning, which could lead to the misleading conclusion that GnRH use is associated with major health problems. Our hypothesis is further supported by the current finding that most of the variables that were shown to predict the development of either CVA or IHD independently in the multivariate analysis, such as age at diagnosis, SES, and CCI (Table 2), also differed significantly between GnRHa users and nonusers (Table 1). Therefore, our findings can be interpreted as indicating that factors such as age, SES, and comorbidities, and not ADT, may have affected the development of the diseases of interest.
The current study has a few drawbacks. Because of the limitations of the HIRA database, which does not include data on tumor characteristics, such as tumor stage and Gleason score, the effects of the tumor itself on cardiovascular diseases and DM could not be identified. All patients in our study had relatively short follow-up period of around 4 years, and further analysis of long-term results is required. We also did not include mortality data, and therefore care should be taken when applying these results to the clinical setting. Furthermore, although radical prostatectomy was included in the analysis and found to be not associated with the outcome, there may still be a remaining risk of bias masking the effect of GnRHa on the outcome. The effect of major surgery should be refined in the future study. The most important advantage of the current study is that it included the entire Korean population of Pca patients during the study period, because the entire Korean population is covered by either National Health Insurance or Medicaid (approximately 97% and 3%, respectively). All patients in this study were diagnosed during the same 6-month period, started follow-up at a similar time (from July 1, 2012 until December 31, 2012), and completed follow-up at the same time (December 31, 2016) to minimize confounding factors as it is in the prospective study.
In conclusion, ADT using GnRHa did not seem to increase the risk of developing cardiovascular disease. The increased incidence of such diseases seen in men with ADT is believed to be due to inequalities in patient characteristics, including age, SES, and comorbidities. However, our result does not exclude the possibility that ADT increases mortality related to cardiovascular adverse effects, because we could not analyze overall survival. Further randomized, long-term studies are warranted to establish strategies for ADT use in the clinical setting, especially in patients at high risk for developing cardiovascular disease.
Footnotes
Funding: This research was supported by Korean Urologic Oncology Society Research Fund 2017 (KUOS17-02).
Disclosure: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Shim M, Cho JS.
- Data curation: Shim M, Bang WJ, Oh CY, Lee YS.
- Formal analysis: Shim M.
- Funding acquisition: Shim M, Cho JS.
- Investigation: Shim M.
- Methodology: Shim M.
- Project administration:
- Resources: Shim M, Ju YS.
- Software: Ju YS.
- Supervision: Jeon SS, Ahn H, Ju YS, Cho JS.
- Validation: Shim M, Ahn H.
- Writing - original draft: Shim M.
- Writing - review & editing: Shim M.
SUPPLEMENTARY MATERIALS
Details of the HIRA database
Inclusion and exclusion criteria
Codes used to identify medications
Codes used to identify diagnosis
References
- 1.Keating NL, O'Malley AJ, Freedland SJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy: observational study of veterans with prostate cancer. J Natl Cancer Inst. 2010;102(1):39–46. doi: 10.1093/jnci/djp404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown BW, Brauner C, Minnotte MC. Noncancer deaths in white adult cancer patients. J Natl Cancer Inst. 1993;85(12):979–987. doi: 10.1093/jnci/85.12.979. [DOI] [PubMed] [Google Scholar]
- 3.Mason MD, Parulekar WR, Sydes MR, Brundage M, Kirkbride P, Gospodarowicz M, et al. Final report of the intergroup randomized study of combined androgen-deprivation therapy plus radiotherapy versus androgen-deprivation therapy alone in locally advanced prostate cancer. J Clin Oncol. 2015;33(19):2143–2150. doi: 10.1200/JCO.2014.57.7510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Warde P, Mason M, Ding K, Kirkbride P, Brundage M, Cowan R, et al. Combined androgen deprivation therapy and radiation therapy for locally advanced prostate cancer: a randomised, phase 3 trial. Lancet. 2011;378(9809):2104–2111. doi: 10.1016/S0140-6736(11)61095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones CU, Hunt D, McGowan DG, Amin MB, Chetner MP, Bruner DW, et al. Radiotherapy and short-term androgen deprivation for localized prostate cancer. N Engl J Med. 2011;365(2):107–118. doi: 10.1056/NEJMoa1012348. [DOI] [PubMed] [Google Scholar]
- 6.Shahinian VB, Kuo YF, Freeman JL, Orihuela E, Goodwin JS. Increasing use of gonadotropin-releasing hormone agonists for the treatment of localized prostate carcinoma. Cancer. 2005;103(8):1615–1624. doi: 10.1002/cncr.20955. [DOI] [PubMed] [Google Scholar]
- 7.Sharifi N, Gulley JL, Dahut WL. Androgen deprivation therapy for prostate cancer. JAMA. 2005;294(2):238–244. doi: 10.1001/jama.294.2.238. [DOI] [PubMed] [Google Scholar]
- 8.Hellerstedt BA, Pienta KJ. The current state of hormonal therapy for prostate cancer. CA Cancer J Clin. 2002;52(3):154–179. doi: 10.3322/canjclin.52.3.154. [DOI] [PubMed] [Google Scholar]
- 9.Jespersen CG, Nørgaard M, Borre M. Androgen-deprivation therapy in treatment of prostate cancer and risk of myocardial infarction and stroke: a nationwide Danish population-based cohort study. Eur Urol. 2014;65(4):704–709. doi: 10.1016/j.eururo.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 10.D'Amico AV, Denham JW, Crook J, Chen MH, Goldhaber SZ, Lamb DS, et al. Influence of androgen suppression therapy for prostate cancer on the frequency and timing of fatal myocardial infarctions. J Clin Oncol. 2007;25(17):2420–2425. doi: 10.1200/JCO.2006.09.3369. [DOI] [PubMed] [Google Scholar]
- 11.Roach M, 3rd, Bae K, Speight J, Wolkov HB, Rubin P, Lee RJ, et al. Short-term neoadjuvant androgen deprivation therapy and external-beam radiotherapy for locally advanced prostate cancer: long-term results of RTOG 8610. J Clin Oncol. 2008;26(4):585–591. doi: 10.1200/JCO.2007.13.9881. [DOI] [PubMed] [Google Scholar]
- 12.Efstathiou JA, Bae K, Shipley WU, Hanks GE, Pilepich MV, Sandler HM, et al. Cardiovascular mortality after androgen deprivation therapy for locally advanced prostate cancer: RTOG 85-31. J Clin Oncol. 2009;27(1):92–99. doi: 10.1200/JCO.2007.12.3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53(12):1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 14.Alexandersen P, Haarbo J, Christiansen C. The relationship of natural androgens to coronary heart disease in males: a review. Atherosclerosis. 1996;125(1):1–13. doi: 10.1016/0021-9150(96)05864-9. [DOI] [PubMed] [Google Scholar]
- 15.Smith MR, Lee H, Nathan DM. Insulin sensitivity during combined androgen blockade for prostate cancer. J Clin Endocrinol Metab. 2006;91(4):1305–1308. doi: 10.1210/jc.2005-2507. [DOI] [PubMed] [Google Scholar]
- 16.Saigal CS, Gore JL, Krupski TL, Hanley J, Schonlau M, Litwin MS, et al. Androgen deprivation therapy increases cardiovascular morbidity in men with prostate cancer. Cancer. 2007;110(7):1493–1500. doi: 10.1002/cncr.22933. [DOI] [PubMed] [Google Scholar]
- 17.Basaria S, Muller DC, Carducci MA, Egan J, Dobs AS. Hyperglycemia and insulin resistance in men with prostate carcinoma who receive androgen-deprivation therapy. Cancer. 2006;106(3):581–588. doi: 10.1002/cncr.21642. [DOI] [PubMed] [Google Scholar]
- 18.Berruti A, Dogliotti L, Terrone C, Cerutti S, Isaia G, Tarabuzzi R, et al. Changes in bone mineral density, lean body mass and fat content as measured by dual energy x-ray absorptiometry in patients with prostate cancer without apparent bone metastases given androgen deprivation therapy. J Urol. 2002;167(6):2361–2367. [PubMed] [Google Scholar]
- 19.Alibhai SM, Duong-Hua M, Sutradhar R, Fleshner NE, Warde P, Cheung AM, et al. Impact of androgen deprivation therapy on cardiovascular disease and diabetes. J Clin Oncol. 2009;27(21):3452–3458. doi: 10.1200/JCO.2008.20.0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Details of the HIRA database
Inclusion and exclusion criteria
Codes used to identify medications
Codes used to identify diagnosis