ABSTRACT
Background
Knowledge of the evolution of BMI and skeletal muscle index (SMI) measurements during advanced cancer and their relationships with disease progression (PD) is relevant to improve the timing of interventions that may improve cachexia-associated outcomes.
Objectives
We investigated BMI and SMI trajectories and their associations with PD in metastatic colorectal cancer (mCRC) patients during consecutive palliative systemic regimens.
Methods
In a secondary analysis of the primary CAIRO3 trial, we included 533 mCRC patients with BMI measurements repeated every 3 wk and 95 randomly selected patients with SMI measurements repeated every 9 wk. We studied 2 periods: p1, during first-line maintenance capecitabine + bevacizumab or observation until the first progression of disease (PD1); and p2, during capecitabine + oxaliplatin + bevacizumab or another reintroduction treatment from PD1 until the second progression of disease (PD2). BMI and SMI trajectories were modeled separately throughout both periods, and joint longitudinal-survival modeling was used to investigate the relationships between slopes in BMI and SMI with PD at 9 and 3 wk pre-PD. A multivariate longitudinal joint model was used to investigate the association between the BMI trajectory and PD at time of PD, independent of SMI.
Results
During p1, the slopes in BMI and SMI were associated with early PD1 [HRs for 9-wk BMI: 1.54 (95% CI: 1.33, 1.76); 9-wk SMI: 1.38 (95% CI: 0.87, 1.89), NS; 3-wk BMI: 1.74 (95% CI: 1.48, 1.99); 3-wk SMI: 2.65 (95% CI: 1.97, 3.32)]. During p2, only the slope in SMI was related to PD2 [9-wk BMI: 1.09 (95%: CI: 0.73, 1.45), NS; 9-wk SMI: 1.64 (95% CI: 1.25, 2.04); 3-wk BMI: 1.17 (95% CI: 0.77, 1.57); 3-wk SMI: 1.11 (95% CI: 0.70, 1.53)]. In models mutually adjusting for BMI and SMI, SMI was associated with PD in p1 [p1 ( n = 95), HR BMI: 1.32 (95% CI: 0.74, 2.39), NS; p1, HR SMI: 1.50 (95% CI: 1.04, 2.14); p2 ( n = 50), BMI: 0.98 (95% CI: 0.55, 1.75), NS; p2, HR SMI: 1.11 (95% CI: 0.61, 2.05), NS].
Conclusions
In mCRC patients during palliative systemic treatment, SMI losses, irrespective of BMI losses, may be a marker for the early initiation of cachexia interventions.
Keywords: cachexia, body composition, skeletal muscle index, body mass index, metastastic colorectal cancer, disease progression, palliative systemic treatment
Introduction
Cancer cachexia is a metabolic disorder that occurs in up to 50–80% of metastatic cancer patients (1–3). It is driven by a negative protein and energy balance, due to a variable combination of reduced food intake and abnormal metabolism, induced by the tumor and oncologic treatment (2). The progressive worsening of metabolic abnormalities will lead to involuntary, progressive weight loss; a reduced quality of life; poor treatment tolerance and response; and, eventually, death (4).
Despite the long recognition of the clinical consequences of cachexia, prevention, early identification, and treatment remain challenging (4, 5). Cachexia is obvious in its (very) advanced phase, due to the gross losses of muscle and fat, but at that point the opportunity for successful rehabilitation has long passed (4). Recent results have shown that advanced cancer patients may have an exploitable anabolic potential prior to reaching the refractory phase of cachexia, thereby creating a strong rationale for the early implementation of cachexia interventions (6, 7). However, due to the lack of diagnostic criteria for its early stages, clinicians have continued to focus on weight loss alone. Weight loss is a crude assessment of fat and muscle mass loss, and does not reflect detailed body composition changes (8, 9). It is now becoming increasingly clear that skeletal muscle index (SMI) losses may be a key element of weight loss that is associated with poor outcomes (3, 8–10). SMI losses can occur in the absence of weight losses, at least during early stages of advanced cancer and cachexia, and may therefore be a key element in the identification of early protein energy malnutrition disorders. The presence of protein energy malnutrition in early-stage cachexia could support the initiation of earlier cachexia interventions (3, 5, 6).
The need to investigate BMI and SMI losses or gains over the disease trajectory in more detail, in order to help diagnose the early and possible reversible cachexia phases, has been underlined in several recent publications (4–6). Previous studies found that oncologic treatment and cancer progression may have impacts on both BMI and SMI losses, but at what time and to what extent are currently unknown (6, 11, 12). Here, we investigated how the detailed trajectory of BMI and SMI changes were associated with disease progression in a cohort of metastatic colorectal cancer (mCRC) patients who were treated with different consecutive treatment strategies as part of a Phase III randomized study (13). We hypothesized that a SMI loss, and not a BMI loss, typically precedes progressive disease. We used serial BMI measures, acquired every 3 weeks during 2 consecutive palliative systemic treatment periods, and used computed tomography (CT) scans, repeated every 9 weeks, to measure SMIs. Finally, in patients with both BMI and SMI measures, we examined the relationship between the BMI trajectory and disease progression, independent of SMI.
Methods
Patients
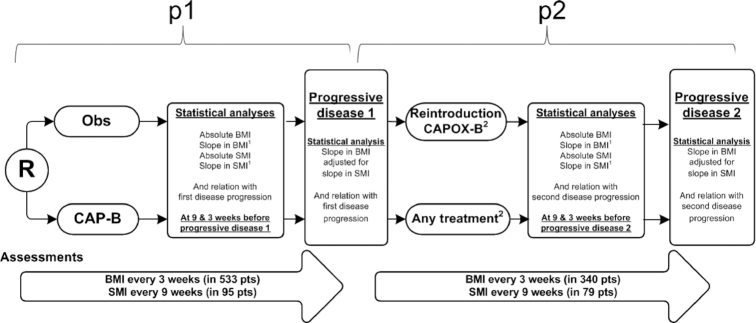
This is a secondary analysis of the already finished randomized Phase III CAIRO3 trial (13). In CAIRO3, 557 previously untreated mCRC patients with stable disease or better after 6 cycles of capecitabine + oxaliplatin + bevacizumab (CAPOX-B) were randomized to observation or to maintenance treatment with capecitabine + bevacizumab (CAP-B; Figure 1). Upon their first disease progression (PD1), patients received a reintroduction treatment with CAPOX-B or, if not feasible (e.g., due to persisting neuropathy), any other treatment until the second disease progression (PD2). Other eligibility criteria for CAIRO3 participation included an age ≥18 years, histological proof of colorectal cancer, and a WHO performance status ≤1 (on a scale of 0 to 4). For this analysis, we included patients for whom BMI or SMI measures were available during 2 consecutive treatment periods: that is, during CAP-B or observation (p1) or during CAPOX-B or another reintroduction treatment (p2).
FIGURE 1.
Study design and statistical analyses. 1Slopes in BMI and SMI were determined by using joint longitudinal survival models and, throughout the manuscript, are presented as hazard ratios for disease progression per each unit of BMI or SMI loss per month. Mixed models contained a random intercept and slope per patient, and fixed effects were sex and treatment (i.e., CAP-B vs observation during p1 or CAPOX-B vs other reintroduction treatment during p2). The Cox models were adjusted for sex, age, lactate dehydrogenase level, treatment (CAP-B vs observation in both p1 and p2), and WHO performance status. 2During CAIRO3 and after first progression of disease, patients were to receive the same treatment as the initial treatment given before CAIRO3 randomization (i.e., CAPOX-B). In cases of persisting neuropathy at grade 2 or higher, or any other reason preventing the reintroduction of CAPOX, the reintroduction treatment was chosen at the discretion of the treating physician. CAP-B, capecitabine + bevacizumab; CAPOX-B; capecitabine + oxaliplatin + bevacizumab; Obs, observation; p1, period 1; p2, period 2; PD, disease progression; R, randomization; SMI, skeletal muscle index.
Determinant: BMI measurements
Patients’ heights and body weights (at the start of every cycle of systemic treatment) were measured as part of the daily clinical practice to determine the appropriate dose of systemic treatment (i.e., by calculating the body surface area). Height was measured at the time of CAIRO3 randomization and body weight was measured every 3 weeks at the start of every cycle of systemic treatment during the maintenance and observation treatments; during CAPOX-B reintroduction treatment, BMI was calculated as body weight divided by height in meters squared.
Determinant: muscle mass measurements
Due to feasibility, we collected the 9-weekly repeated CT scans of patients from the 7 hospitals that had the highest accruals in CAIRO3. SMI measurements were performed by 2 trained analysts (SAK and MJO) using a software tool (Slice-o-matic, version 5.0; Tomovision). A single slice evaluation method at the third vertebral level (L3) and thresholds for Hounsfield units (−29 to 150) were used to identify and quantify the skeletal muscle area at the L3 level (14, 15). The skeletal muscle area at the L3 level has been proven to highly correlate with the total body skeletal muscle mass (r2 = 0.86) (14) and with poor clinical outcomes, including survival, treatment-related toxicities, and a reduced quality of life (16–18). The SMI was calculated by the skeletal muscle area at L3 in cm2, divided by the patient's height in m2 (19).
Outcome measures
Survival times were calculated from the start of each time interval. The primary outcome measures were PD1 within 1 year and PD2 within 9 months.
Statistical analysis
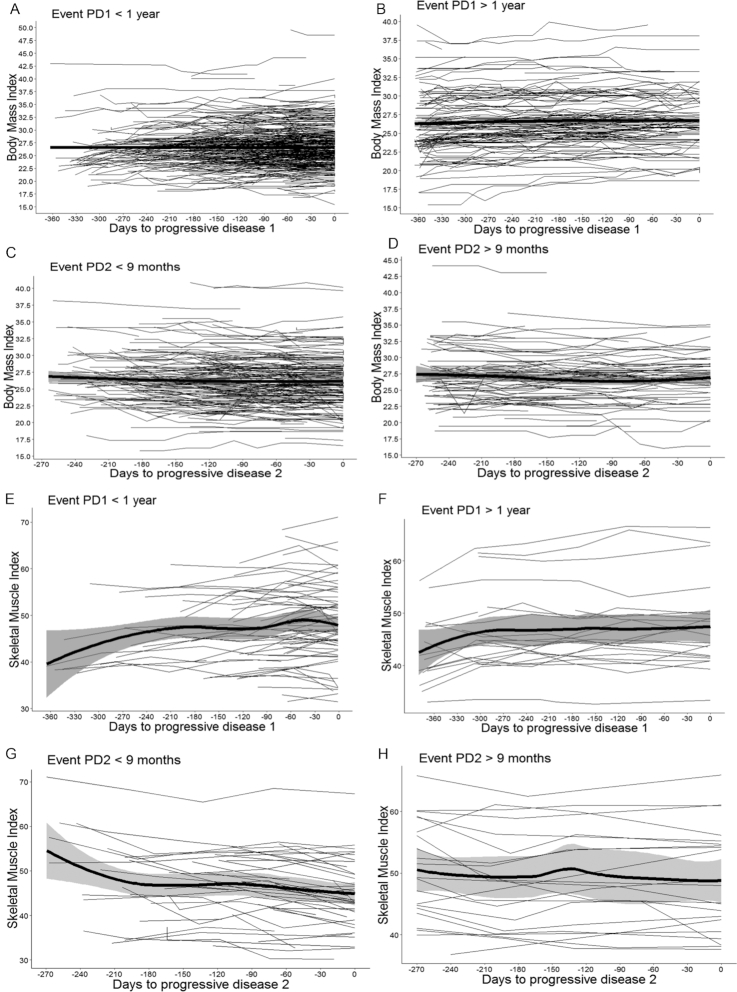
All analyses were carried out using R, version 3.5.1. To gain visual insight into the BMI and SMI trajectories over time during p1 and p2, the individual patient trajectories and the group means with 95% CI bands were plotted [ggplot2 package (20); Figure 2]. The trajectories are displayed separately for patients with available BMI measures, or with available SMI measures, and for those who reached the endpoint or who were censored. The time scales were adjusted to each patient's time to disease progression by setting the time of disease progression to 0 and calculating the number of days between the CT scans and disease progression.
FIGURE 2.
BMI and SMI trajectories over time. Patient-specific longitudinal trajectories of BMI and SMI over time for patients who reached the endpoint or who were censored. Trajectories in black represent the individual patient BMI and SMI trajectories. Solid, bold lines in black denote the mean populations of BMI and SMI trajectories over time, with 95% CI bars. The time scales were adjusted to each patient's time to disease progression (setting the time of disease progression to 0). (A, B, E, and F) The longitudinal BMI and SMI trajectories across patients that experienced progression/were censored during p1 (i.e., during CAP-B and observation). BMI trajectories, irrespective of reaching the endpoint within 1 year or not, did not show great fluctuations over time. Also, SMI levels in patients who were censored and did not reach the endpoint remained relatively stable, with a slight increasing tendency over time. By contrast, in patients who experienced an event, SMI levels showed a decrease as they got closer to the event. (A) The 95% CI bar is smaller than the broad black solid line and, therefore, not clearly visible in the Figure. (C, D, G, and H) The longitudinal SMI and BMI trajectories across patients that experienced progression/were censored during p2 (i.e., during CAPOX-B or other reintroduction treatment). Again, BMI levels, irrespective of reaching the endpoint within 9 months or not, remained relatively stable over time. Also, SMI levels in patients who were censored and did not reach the endpoint remained relatively stable, with a slight decreasing tendency over time, whereas in patients who experienced an event, SMI levels showed a decrease as they got closer to the event. CAP-B, capecitabine + bevacizumab; CAPOX-B; capecitabine + oxaliplatin + bevacizumab; PD1, first disease progression; PD2, second disease progression; p1, period 1; p2, period 2; SMI, skeletal muscle index.
To investigate the relationships of BMI and SMI trajectories over time with disease progression during p1 and p2, we used joint modeling. In this approach, longitudinal and survival data are combined, taking into account the dependence of longitudinal data and time-to-event data (21). Thereby, it becomes possible to study how the longitudinal trajectory of a biomarker relates to the time-to-event outcome, while taking into account the unbalanced follow-up times and any biological variation in the longitudinal biomarker trajectory of interest (21). In addition, a joint model reliably estimates the risk of the event, given a specific biomarker trajectory independent of prognostic factors that have an effect on both the biomarker and the event of interest (21).
Separate models for BMI and SMI trajectories were performed. For the longitudinal analysis (i.e., the individual BMI and SMI trajectories over time), we used a mixed-effects model [LME4 package (22)]. We included the baseline values of BMIs and SMIs in the outcome vector (23). To account for the between-patient heterogeneity in trajectories, a random intercept and slope was added for each patient. The follow-up time was calculated from the start of the 2 treatment periods (i.e., randomization or PD1). Since only a small number of patients had follow-up times that exceeded 1 year during p1 and 9 months during p2, we defined these 2 time points as the end of follow-up for the respective periods. To take into account the non-linear changes in BMIs and SMIs over time, follow-up times were modeled using natural cubic splines with 3 knots, which—compared to the model with linear time—increased the model fit, as indicated by the Akaike Information Criterion. Sex and treatment (CAP-B vs observation during p1 or CAPOX-B vs other reintroduction treatment during p2) were added to the model as fixed effects. To test whether BMI and SMI changes over time varied between the different treatments, we tested the treatment x time interaction. This increased the model fit, but led to convergence issues in the joint models and, therefore, was not included in the final models.
The relationships between BMI and SMI trajectories and disease progression were investigated by combining the mixed-effect models with Cox proportional hazard models (JM package) (24). In the Cox models, the event of interest for p1 was PD1 within 1 year and the event of interest for p2 was PD2 within 9 months. The Cox models were adjusted for the following potential confounders: sex (male/female), age (continuous), lactate dehydrogenase level (normal/elevated), treatment (CAP-B vs observation), and WHO performance status (0/1). The BMI and SMI trajectories were modeled throughout each period. We investigated the relationships between absolute levels and slopes of BMIs and SMIs at 9 and 3 weeks before progressive disease, using lag functions (Figure 1). These time points were chosen because they represent the time points in which the CT scans or BMI assessments preceding progressive disease were acquired. Survival estimates for absolute BMIs and SMIs are presented as HRs per each unit lower in BMI or SMI, and slopes in BMIs and SMIs are presented as HRs per each unit BMI or SMI loss per month, all with 95% CIs.
Finally, to investigate the relationships between both BMI and SMI trajectories and disease progression, we repeated the analysis with models mutually adjusting the BMI trajectory and SMI trajectory for each other (multivariate mixed models and joint model Bayes; JMbayes package) (25). Since the lag function was not available for the JMbayes package, the relationships between SMI and BMI trajectories and disease progression were only investigated at the time of disease progression.
Results
Of the 557 CAIRO3 patients, 533 (96%) were included, with a total of 6549 BMI measures during the follow-up period. Of 104 CAIRO3 patients from the hospitals with the highest CAIRO3 recruitments, 95 patients had a total of 854 CT scans evaluable for SMI measures (Figure 1). The patient and treatment characteristics of the included patients are described in Table 1. No clinically meaningful differences in characteristics were found between the 4 patient groups for whom BMI data were available [at start p1 (n = 533) or at start p2 (n = 340)] or for whom SMI data were available [at start p1 (n = 95) and at start p2 (n = 79)]. In addition no clinically meaningful differences were found between patients included in this analysis and the total group of CAIRO3 patients (data not shown).
TABLE 1.
Patient, tumor, and treatment characteristics1
| Patients with BMI measures | Patients with SMI measures | |||
|---|---|---|---|---|
| At randomization, start p1 (n = 533) | At PD1, start p2 (n = 340) | At randomization, start p1 (n = 95) | At PD1, start p2 (n = 79) | |
| Age, mean in years (SD) | 65.8 (8.1) | 63.3 (8.8) | 65.7 (8.2) | 64.4 (8.1) |
| ≤70 | 405 (76%) | 250 (74%) | 60 (63%) | 51 (65%) |
| >70 | 128 (24%) | 90 (27%) | 35 (37%) | 28 (35%) |
| Sex | ||||
| Female | 189 (36%) | 103 (32%) | 36 (38%) | 29 (37%) |
| Male | 344 (65%) | 231 (68%) | 59 (62%) | 50 (63%) |
| Primary site | ||||
| Colon only | 266 (50%) | 170 (50%) | 49 (52%) | 40 (51%) |
| Rectum only | 153 (29%) | 96 (28%) | 25 (26%) | 20 (25%) |
| Rectosigmoid | 114 (21%) | 74 (22%) | 21 (22%) | 19 (24%) |
| Resection primary tumor | ||||
| Yes | 314 (59%) | 210 (62%) | 55 (58%) | 42 (53%) |
| No | 219 (41%) | 130 (38%) | 40 (42%) | 37 (47%) |
| WHO performance status | ||||
| 0 | 334 (63%) | Only available at randomization | 60 (63%) | Only available at randomization |
| 1 | 199 (37%) | — | 35 (37%) | — |
| Number of metastatic sites | ||||
| 1 | 218 (43%) | Only available at randomization | 34 (36%) | Only available at randomization |
| >1 | 291 (57%) | — | 55 (58%) | — |
| Missing | 24 | — | 10 | — |
| Lactate dehydrogenase, IU/L | ||||
| Elevated | 298 (56%) | Only available at randomization | 57 (60%) | Only available at randomization |
| Normal | 235 (44%) | — | 38 (40%) | — |
| BMI, mean (SD), kg/m2 | 27.0 (4.3) | 26.6 (4.0) | 26.5 (4.9) | 26.9 (4.4) |
| Underweight (<18.5) | 3 (1%) | 4 (1%) | 1 (1%) | 1 (2%) |
| Normal weight (18.5–25) | 165 (31%) | 109 (32%) | 43 (46%) | 14 (30%) |
| Overweight (25–30) | 253 (48%) | 172 (51%) | 32 (34%) | 23 (49%) |
| Obese (30+) | 112 (21%) | 55 (16%) | 17 (18%) | 9 (19%) |
| Unknown | 0 | 0 | 2 | 32 |
| SMI, mean (SD) | ||||
| Males | NA2 | NA2 | 48.2 (7.2) | 48.9 (8.3) |
| Females | NA2 | NA2 | 41.0 (6.0) | 40.9 (5.0) |
| Sarcopenia3 | ||||
| Males | NA2 | NA2 | 25 (42%) | 13 (34%) |
| Females | NA2 | NA2 | 16 (44%) | 9 (31%) |
| Best response to initial CAPOX-B treatment | ||||
| Complete or partial response | 350 (66%) | 222 (65%) | 71 (75%) | 59 (75%) |
| Stable disease | 183 (34%) | 118 (35%) | 24 (25%) | 20 (25%) |
| Treatment arm | ||||
| Maintenance CAP-B | 273 (51%) | 129 (38%) | 50 (53%) | 39 (49%) |
| Observation | 260 (49%) | 211 (62%) | 45 (47%) | 40 (51%) |
| Reintroduction treatment | ||||
| CAPOX-B | 293 (55%) | 289 (85%) | 43 (45%) | 41 (52%) |
| Other | 240 (45%) | 51 (15%) | 52 (55%) | 38 (48%) |
Data are shown at start of 2 treatment periods for the BMI and the SMI groups. Data are shown as n(%) unless otherwise noted. CAP-B, capecitabine + bevacizumab; CAPOX-B; capecitabine + oxaliplatin + bevacizumab; NA, not applicable; PD1, first disease progression; PD2, second disease progression; p1, period 1; p2, period 2; SMI, skeletal muscle index.
Was only determined in the group of patients with available computed tomography scans.
Sarcopenia was defined as SMIs of <43 cm2/m2 for males with a BMI <25 cm2/m2, <53 cm2/m2 for males with a BMI ≥25 cm2/m2, and <41 cm2/m2 for females (9).
BMI and SMI trajectories over time
Figure 2 shows the BMI and SMI trajectories during p1 and p2. During both periods, large variations in BMIs and SMIs across patients were observed. Fluctuations in BMIs and SMIs over time were detected in all patients, but appeared more pronounced in the SMI trajectories, compared to the BMI trajectories. Furthermore, the BMI trajectories remained relatively stable during both periods, while for the SMI trajectories, there was a general increase over time during p1 and a general decrease during p2.
The BMI trajectories over time were comparable between patients who did and did not reach the endpoint of progressive disease. In contrast, the SMI trajectories during p1 displayed a decrease preceding the endpoint in patients who experienced PD1 within 1 year, and were relatively stable, on average, in patients who did not reach the endpoint within 1 year. During p2, the SMI levels of patients who reached the endpoint of PD2 within 9 months decreased more steeply preceding the endpoint, and remained relatively stable in patients who did not experience PD2 within 9 months.
BMI trajectory and disease progression
Table 2 shows the regression estimates of the joint model analysis. During p1, 79% of patients experienced disease progression within 1 year, with a median time to PD1 of 174 days (range 12–365 days). The absolute BMI was not related to a higher risk of early PD1 [HR per each unit BMI lower at 9 weeks pre-PD1 1.00 (95% CI: 0.98–1.03), NS; HR per each unit BMI lower at 3 weeks pre-PD1 1.00 (0.98–1.03), NS]. The slope in BMIs preceding PD1 showed a statistically significantly higher risk of early PD1 [HR per unit BMI loss/month at 9 weeks pre-PD1 1.54 (95% CI: 1.33–1.76); HR per unit BMI loss/month at 3 weeks pre-PD1 1.74 (95% CI: 1.48–1.99)].
TABLE 2.
SMI and BMI trajectories and disease progression joint model estimates1
| Pts at risk, n | HR (95% CI) | P value | |
|---|---|---|---|
| During CAP-B treatment or observation, p1 | |||
| BMI | |||
| Absolute BMI at PD1 | 533 | 1.00 (0.98, 1.03) | 0.50 |
| Slope in BMI at PD1 | 533 | 1.76 (1.46, 2.05) | <0.01 |
| Absolute BMI 9 weeks pre-PD1 | 533 | 1.00 (0.98, 1.03) | 0.88 |
| Slope in BMI 9 weeks pre-PD1 | 533 | 1.54 (1.33, 1.76) | <0.01 |
| Absolute BMI 3 weeks pre-PD1 | 533 | 1.00 (0.98, 1.03) | 0.73 |
| Slope in BMI 3 weeks pre-PD1 | 533 | 1.74 (1.48, 1.99) | <0.01 |
| SMI | |||
| Absolute SMI at PD1 | 95 | 0.99 (0.96, 1.02) | 0.53 |
| Slope in SMI at PD1 | 95 | 3.05 (2.33, 3.78) | <0.01 |
| Absolute SMI 9 weeks pre-PD1 | 95 | 0.99 (0.96, 1.02) | 0.47 |
| Slope in SMI 9 weeks pre-PD1 | 95 | 1.38 (0.87, 1.89) | 0.22 |
| Absolute SMI 3 weeks pre-PD1 | 95 | 0.99 (0.96, 1.02) | 0.45 |
| Slope in SMI 3 weeks pre-PD1 | 95 | 2.65 (1.97, 3.32) | <0.01 |
| BMI and SMI, mutually adjusted | |||
| BMI change at PD1 | 95 | 1.32 (0.74, 2.39) | 0.35 |
| SMI change at PD1 | 95 | 1.50 (1.04, 2.14) | 0.03 |
| During CAPOX-B or other reintroduction treatment, p2 | |||
| BMI | |||
| Absolute BMI at PD2 | 340 | 1.03 (1.00, 1.06) | 0.07 |
| Slope in BMI at PD2 | 340 | 1.11 (0.61, 1.60) | 0.69 |
| Absolute BMI 9 weeks pre-PD2 | 340 | 1.03 (1.00, 1.06) | 0.10 |
| Slope in BMI 9 weeks pre-PD2 | 340 | 1.09 (0.73, 1.45) | 0.64 |
| Absolute BMI 3 weeks pre-PD1 | 340 | 1.03 (1.00, 1.06) | 0.10 |
| Slope in BMI 3 weeks pre-PD1 | 340 | 1.17 (0.77, 1.57) | 0.64 |
| SMI | |||
| Absolute SMI at PD2 | 79 | 1.00 (1.00, 1.02) | 0.05 |
| Slope in SMI at PD2 | 79 | 1.13 (0.77, 1.50) | 0.08 |
| Absolute SMI 9 weeks pre-PD2 | 79 | 1.01 (1.01, 1.03) | 0.05 |
| Slope in SMI 9 weeks pre-PD2 | 79 | 1.64 (1.25, 2.04) | 0.01 |
| Absolute SMI 3weeks pre-PD2 | 79 | 1.00 (1.00, 1.02) | 0.04 |
| Slope in SMI 3 weeks pre-PD2 | 79 | 1.11 (0.70, 1.53) | 0.61 |
| BMI and SMI, mutually adjusted | |||
| BMI change at PD2 | 50 | 0.98 (0.55, 1.75) | 0.96 |
| SMI change at PD2 | 50 | 1.11 (0.61, 2.05) | 0.72 |
This table shows the associations between BMI and SMI trajectories and times to disease progression, analyzed by a joint longitudinal survival model analysis. Mixed models contained a random intercept and slope per patient, and fixed effects were sex and treatment (i.e., CAP-B vs observation during p1 or CAPOX-B vs other reintroduction treatment during p2). The Cox models were adjusted for sex, age, lactate dehydrogenase level, treatment (CAP-B vs observation in both p1 and p2), and WHO performance status. Bold text indicate statistically significant results. CAP-B, capecitabine + bevacizumab; CAPOX-B; capecitabine + oxaliplatin + bevacizumab; PD1, first disease progression; PD2, second disease progression; p1, period 1; p2, period 2; pts, patients; SMI, skeletal muscle index.
During p2, 340 patients with available BMI measures were included, of which 73% experienced disease progression within 9 months and the median time to PD2 was 192 days (range 20–270). Neither the absolute BMI nor the slope in BMI was related to PD2 [HR per each unit BMI lower at 9 weeks pre-PD2 1.03 (95% CI: 1.00–1.06); HR per each unit BMI lower at 3 weeks pre-PD2 1.03 (95% CI: 1.00–1.06); HR per unit BMI loss at 9 weeks pre-PD2 1.09 (95% CI: 0.73–1.45), NS; HR per unit BMI loss at 3 weeks pre-PD2 1.17 (95% CI: 0.77–1.57), NS].
SMI trajectory and disease progression
In the patients with available SMI measures during p1 (n = 95), 77% experienced disease progression within 1 year, with a median time to PD1 of 175 days (range 32–270). The absolute SMI was not related to early PD1 [HR per each unit SMI lower at 9 weeks pre-PD1 0.99 (95% CI: 0.96–1.02), NS; HR per each unit SMI lower at 3 weeks pre-PD1 0.99 (95% CI: 0.96–1.02), NS]. The slope in SMI at 9 weeks was non-significantly associated with a higher risk of early PD1 and the slope in SMI at 3 weeks preceding PD1 was significantly associated with a higher risk of early PD1 (HR per unit SMI loss/month at 9 weeks pre-PD1 1.38 (95% CI: 0.87–1.89), NS; HR per unit SMI loss/month at 3 weeks pre-PD1 2.65 (95% CI: 1.97–3.32)].
During p2, in patients with available SMI measures, 73% of patients experienced disease progression within 9 months and the median time to PD2 was 197 days (range 19–270). The absolute SMI and the slope in SMI were statistically significantly related to PD2 at 9 weeks, and at 3 weeks pre-PD2 only the absolute SMI was related to PD2 [HR per each unit SMI lower at 9 weeks pre-PD2 1.01 (95% CI: 1.01–1.03); HR per each unit SMI lower at 3 weeks pre-PD2 1.00 (95% CI: 1.00–1.02); HR per unit SMI loss/month at 9 weeks pre-PD2 1.64 (95% CI: 1.25–2.04); HR per unit SMI loss/month at 3 weeks pre-PD2 1.11 (95% CI: 0.70–1.53), NS].
BMI and SMI trajectories
In the sample of patients with BMI and SMI measures (p1 n = 95 patients; p2 n = 50 patients), the BMI trajectory was not associated with PD, independently of the SMI trajectory, during either treatment period [HR slope in BMI during p1, 1.32 (95% CI: 0.74–2.39), NS; HR slope in BMI during p2, 0.98 (95% CI: 0.55–1.75), NS]. In contrast, the slope in SMI, independent of the BMI trajectory, was significantly associated with PD1 during p1 [HR slope in SMI 1.50 (95% CI: 1.04–2.14)] and non-significantly associated with PD2 during p2 [HR slope in SMI 1.11 (95% CI: 0.61–2.05), NS].
Discussion
In this study, we examined the trajectories of BMIs and SMIs over time, and their relationships with PD in mCRC patients receiving consecutive palliative systemic treatments of different intensities. During less intensive treatment with CAP-B or observation, both the slopes of BMIs and SMIs were significantly related to early PD. However, after mutual adjustment, only SMI remained significant. During more intensive CAPOX-B or other reintroduction treatments, the absolute SMI and slope in SMI (at 9 weeks pre-PD), but not the absolute BMI or slope in BMI, were significantly associated with shorter times to PD2. After mutual adjustment, the association between the SMI slope and PD2 was attenuated, although non-significantly, and the association disappeared for the BMI slope. This indicates that an SMI loss, irrespective of a body weight loss, is important in the relationship with PD.
In previous studies, it was observed that BMI or SMI losses at the start of or during treatment were associated with early PD (6, 9, 11, 16, 17). In contrast to our study, most of these studies did not use >2 repeated measures over time, which may have decreased their predictive values. Measuring BMI or SMI changes between 2 time points implies a linear change over time, whereas these changes may follow a more complex pattern. This pattern might be of specific interest when determining the relationships between BMI and SMI changes and PD (26). Indeed, our data indicate that neither BMI nor SMI losses are linear processes, and that accelerations—especially of SMI losses (although non-significantly on some time points)—during both treatment periods typically precede PD. This was observed during 2 periods in which palliative, systemic treatments with different intensities were administered.
Our findings provide important information for use in defining cachexia stages and cachexia treatment strategies. Cachexia can develop progressively through various stages: from pre-cachexia to cachexia to refractory cachexia. The identification of the different stages is relevant because of the potential reversibility of cachexia during the early stages, while during later stages the consequences of extensive tumor burdens and catabolism may outweigh potential benefits from cachexia treatments (2). The cachexia and refractory cachexia stages can be classified according to the degree of absolute BMI depletion, combined with the degree of ongoing weight loss and muscularity (2, 4). The pre-cachexia stage is defined by metabolic derangements (e.g., anorexia and impaired glucose tolerance), which precede substantial, involuntary weight loss (i.e., ≤5%) and are difficult to identify (4, 5). It is becoming increasingly clear that the early metabolic changes in cachexia may specifically affect protein metabolism (and not fat metabolism), thereby affecting muscle mass, but not fat mass (5, 6). Muscle mass loss, irrespective of weight loss, may therefore serve as a potential marker for the early detection of the pre-cachexia stage. Indeed, our data show that an SMI loss is the main aspect of weight loss that is associated with PD during first-line maintenance with CAP-B or observation. Also, during consecutive, more intensive CAPOX-B treatment, an SMI loss at 9 weeks pre-PD, but not a BMI loss, was associated with PD. Our data thereby supports the development or implementation of early interventions that aim to decrease or reverse SMI losses, with the aim of delaying or preventing cachexia progression and the associated poor clinical outcomes. This recommendation is in line with several previous studies, which have described that patients during the early course of metastatic disease may still have exploitable anabolic potential (6, 15, 27). Furthermore, since eventually all patients will develop PD, our data also suggest that cachexia interventions may already be of value for patients who are responding to treatment, even if not necessarily losing SMI at that time, because it can be expected that they will (start to) lose SMI at the time of PD. This recommendation is further supported by the observation that SMI losses during treatment follow a non-linear process, which sometimes only occurs shortly preceding PD (e.g., during p1), emphasizing the difficulty of implementing cachexia interventions at the time of actual SMI losses.
Cachexia interventions include nutritional and exercise interventions. Meeting energy and protein requirements are key in obtaining relevant clinical effects (28–30), but likely need to be combined with exercise, due to its essential role in restoring muscle depletion (31).
The strengths of this study include the collection of extensive, repeated BMI and CT scan data throughout systemic treatments; the standardized assessment of PD; and the clinical trial context, which helped reduce confounding by treatment. Furthermore, we made use of the joint model approach. This statistical approach allowed us to include all longitudinal measurements of the variable of interest over time and explore heterogeneous patterns between individuals that would not have been identified by a Cox regression model with time-varying covariates. The trajectories in BMI and SMI were more accurately reflected than they would have been in methods that would have analyzed only the first and last measurements (21), which contributed to more accurate estimates of their relationships with disease progression.
There are some limitations to our study. Firstly, the observational design of this study enabled the detection of associations, but did not allow for conclusions on causality. Residual confounding may have contributed to the observed results in our study. Secondly, we were only able to analyze SMI changes in a smaller sample of patients. This may have led to the absence of significant associations during p2. Thirdly, we were not able to investigate the relationships between the BMI and SMI trajectories and PD at 9 or 3 weeks before PD, but only at time of PD. This was because lag functions were not available in the JMbayes package. Finally, we did not have data on hospitalizations during CAIRO3 participation, which may have led to an underestimation of our results (e.g., when patients with long hospitalizations experienced more SMI losses and had a higher risk of early PD).
In conclusion, decreases in the BMI trajectories at 9 and 3 weeks pre-PD and in the SMI trajectory at 3 weeks pre-PD were associated with shorter times to PD in mCRC patients during maintenance CAP-B treatment or observation. SMIs and their trajectory (at 9 weeks pre-PD), but not BMIs or their trajectory, were associated with shorter times to PD during more intensive CAPOX-B or other reintroduction treatments. Independent of SMI losses, BMI losses were not associated with PD during either treatment period, while SMI losses were (although non-significantly during CAPOX-B treatment), indicating that the loss of SMI, irrespective of body weight loss, is important in the relationship with PD. These results indicate that serial SMI measurements may have the potential to identify those patients who are in early cachexia phases. These patients may benefit from the initiation of cachexia interventions, including nutritional interventions and physical exercise.
Acknowledgments
The authors’ responsibilities were as follows – SAK, MJO: acquired the data; SAK, MJO, RKS, BD MJ, CJAP, PHMP, AMM, MK: analyzed the data; SAK, BD, MJ, CJAP, PHMP, AMM, MK: interpreted the data; SAK PHMP, AMM, MK: wrote the manuscript and had full access to all of the data in the study; MJO, BD, MJ, CJAP: revised the manuscript; RKS: conducted the statistical analysis and revised the manuscript, including critical revision for statistical content; PHMP, AMM, MK: had final responsibility for the decision to submit the manuscript for publication. Author disclosures: BD and MJ work at Nutricia Research, which provided intellectual input to this study. SAK, RKS, PHMP, MJO, CJAP, MK, and AMM, no conflicts of interest.
Notes
This study was funded by the Dutch Colorectal Cancer Group (designed the CAIRO3 study) and the province of Utrecht, Netherlands.
Abbreviations used: CAP-B, capecitabine + bevacizumab; CAPOX-B, capecitabine + oxaliplatin + bevacizumab; CT, computed tomography; L3, third vertebral level; mCRC, metastatic colorectal cancer; PD, disease progression; SMI, skeletal muscle index.
References
- 1. Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, Bozzetti F, Fearon K, Hütterer E, Isenring E, Kaasa S et al.. ESPEN guidelines on nutrition in cancer patients. Clin Nutr. 2017;36:11–48. [DOI] [PubMed] [Google Scholar]
- 2. Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, Macdonald N, Mantovani G et al.. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011;12:489–95. [DOI] [PubMed] [Google Scholar]
- 3. Baracos VE, Martin L, Korc M, Guttridge DC, Fearon KCH. Cancer-associated cachexia. Nat Rev Dis Primers. 2018;4:1–18. [DOI] [PubMed] [Google Scholar]
- 4. Fearon K, Arends J, Baracos V. Understanding the mechanisms and treatment options in cancer cachexia. Nat Rev Clin Oncol. 2012;10:80–9. [DOI] [PubMed] [Google Scholar]
- 5. Antoun S, Rossoni C, Lanoy E. What’s next in using CT scans to better understand cachexia?. Curr Opin Support Palliat Care. 2018;12(4):427–33. [DOI] [PubMed] [Google Scholar]
- 6. Prado CM, Sawyer MB, Ghosh S, Lieffers JR, Esfandiari N, Antoun S, Baracos VE. Central tenet of cancer cachexia therapy: Do patients with advanced cancer have exploitable anabolic potential?. Am J Clin Nutr. 2013;98(4):1012–19. [DOI] [PubMed] [Google Scholar]
- 7. Kurk S, Peeters P, Dorresteijn B, de Jong P, Jourdan M, Creemers GJ, Erdkamp F, de Jongh F, Kint P, Poppema B et al.. Impact of skeletal muscle index (SMI) loss during palliative systemic treatment (Tx) on time to progression and overall survival (OS) in metastatic colorectal cancer (mCRC) patients. J Clin Oncol. 2017;35(Suppl 15):10087. [Google Scholar]
- 8. Prado CMM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, Baracos VE. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population-based study. Lancet Oncol. 2008;9:629–35. [DOI] [PubMed] [Google Scholar]
- 9. Martin L, Birdsell L, MacDonald N, Reiman T, Clandinin MT, McCargar LJ, Murphy R, Ghosh S, Sawyer MB, Baracos VE. Cancer cachexia in the age of obesity: Skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31:1539–47. [DOI] [PubMed] [Google Scholar]
- 10. Caan BJ, Cespedes Feliciano EM, Prado CM, Alexeeff S, Kroenke CH, Bradshaw P, Quesenberry CP, Weltzien EK, Castillo AL, Olobatuyi TA et al.. Association of muscle and adiposity measured by computed tomography with survival in patients with nonmetastatic breast cancer. JAMA Oncol. 2018;4(6):798–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lieffers JR, Mourtzakis M, Hall KD, Mccargar LJ, Prado CMM, Baracos VE. A viscerally driven cachexia syndrome in patients with advanced colorectal cancer: Contributions of organ and tumor mass to whole-body energy demands 1–3. Am J Clin Nutr. 2009;89:1173–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Antoun S, Birdsell L, Sawyer MB, Venner P, Escudier B, Baracos VE. Association of skeletal muscle wasting with treatment with sorafenib in patients with advanced renal cell carcinoma: Results from a placebo-controlled study. J Clin Oncol. 2010;28:1054–60. [DOI] [PubMed] [Google Scholar]
- 13. Simkens LHJ, Van Tinteren H, May A, Ten Tije AJ, Creemers GJM, Loosveld OJL, De Jongh FE, Erdkamp FLG, Erjavec Z, Van Der Torren AME et al.. Maintenance treatment with capecitabine and bevacizumab in metastatic colorectal cancer (CAIRO3): A phase 3 randomised controlled trial of the Dutch Colorectal Cancer Group. Lancet. 2015;385:1843–52. [DOI] [PubMed] [Google Scholar]
- 14. Mourtzakis M, Prado CMM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008;33:997–1006. [DOI] [PubMed] [Google Scholar]
- 15. Kurk S, Peeters P, Dorresteijn B, de Jong P, Jourdan M, Kuijf H, Punt C, Koopman M, May A. Impact of different palliative systemic treatments on skeletal muscle mass in metastatic colorectal cancer patients. J Cachexia Sarcopenia Muscle. 2018;9:909–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Blauwhoff-Buskermolen S, Versteeg KS, de van der Schueren MA, den Braver NR, Berkhof J, Langius JA, Verheul HM. Loss of muscle mass during chemotherapy is predictive for poor survival of patients with metastatic colorectal cancer. J Clin Oncol. 2016;34:1339–44. [DOI] [PubMed] [Google Scholar]
- 17. Miyamoto Y, Baba Y, Sakamoto Y, Ohuchi M, Tokunaga R, Kurashige J, Hiyoshi Y, Iwagami S, Yoshida N, Watanabe M et al.. Negative impact of skeletal muscle loss after systemic chemotherapy in patients with unresectable colorectal cancer. PLOS One. 2015;10:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kurk S, Peeters P, Stellato R, Dorresteijn B, de Jong P, Jourdan M, Creemers GJ, Erdkamp F, de Jongh F, Kint P et al.. Skeletal muscle mass loss and dose limiting toxicities in metastatic colorectal cancer patients. J Cachexia Sarcopenia Muscle. 2019;10(4):808−13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shen W, Punyanitya M,Wang Z, Gallagher D, St-Onge M-P, Albu J, Heymsfield SB, Heshka S . Total body skeletal muscle and adipose tissue volumes: Estimation from a single abdominal cross-sectional image. J Appl Physiol. 2004;97:2333–8. [DOI] [PubMed] [Google Scholar]
- 20. Wickham H. Ggplot2: Elegant Graphics for Data Analysis. New York, NY: Springer-Verlag; 2009. [Google Scholar]
- 21. Ibrahim JG, Chu H, Chen LM. Basic concepts and methods for joint models of longitudinal and survival data. J Clin Oncol. 2010;28:2796–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bates D, Achler M, Bolker B, Walker S. Fitting linear mixed-effects models using LME4. J Stat Softw. 2015;67:1–48. [Google Scholar]
- 23. Glymour MM, Weuve J, Berkman LF, Kawachi I, Robins JM. When is baseline adjustment useful in analyses of change? An example with education and cognitive change. Am J Epidemiol. 2005;162:267–78. [DOI] [PubMed] [Google Scholar]
- 24. Rizopoulos D. JM: An R package for the joint modelling of longitudinal and time-to-event data. J Stat Softw. 2010;35:1–33.21603108 [Google Scholar]
- 25. Rizopoulos D. JMbayes: Joint modeling of longitudinal and time-to-event data under a Bayesian approach. Available from: https://CRAN.R-project.org/package=JMbayes. [DOI] [PubMed] [Google Scholar]
- 26. Bayar MA, Antoun S, Lanoy E. Statistical approaches for evaluating body composition markers in clinical cancer research. Expert Rev Anticancer Ther. 2017;17:311–18. [DOI] [PubMed] [Google Scholar]
- 27. Winter A, MacAdams J, Chevalier S. Normal protein anabolic response to hyperaminoacidemia in insulin-resistant patients with lung cancer cachexia. Clin Nutr. 2012;31:765–73. [DOI] [PubMed] [Google Scholar]
- 28. Prado CMM, Bekaii-Saab T, Doyle LA, Shrestha S, Ghosh S, Baracos VE, Sawyer MB. Skeletal muscle anabolism is a side effect of therapy with the MEK inhibitor: Selumetinib in patients with cholangiocarcinoma. Br J Cancer. 2012;106:1583–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Deutz NE, Safarb A, Schutzlera S, Memelinkc R, Ferrandoa A, Spencerd H, van Helvoort A, Wolfe RR.. Muscle protein synthesis in cancer patients can be stimulated with a specially formulated medical food. Clin Nutr. 2011;30(6):759–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sánchez-Lara K, Turcott JG, Juárez-Hernández E, Nuñez-Valencia C, Villanueva G, Guevara P, De la Torre-Vallejo M, Mohar A, Arrieta O. Effects of an oral nutritional supplement containing eicosapentaenoic acid on nutritional and clinical outcomes in patients with advanced non-small cell lung cancer: randomised trial. Clin Nutr. 2014;33:1017–23. [DOI] [PubMed] [Google Scholar]
- 31. Bozzetti F. Forcing the vicious circle: Sarcopenia increases toxicity, decreases response to chemotherapy and worsens with chemotherapy. Ann Oncol. 2017;28:2107–18. [DOI] [PubMed] [Google Scholar]