Abstract
Background:
Anterior cruciate ligament (ACL) tears result in significant quadriceps muscle atrophy that is resistant to recovery despite extensive rehabilitation. Recent work suggests an elevated fibrotic burden in the quadriceps muscle after the injury, which may limit recovery. Elucidating the mechanisms and cell types involved in the progression of fibrosis is critical for developing new treatment strategies.
Purpose:
To identify factors contributing to the elevated fibrotic burden found after the injury.
Study Design:
Descriptive laboratory study.
Methods:
After an ACL injury, muscle biopsy specimens were obtained from the injured and noninjured vastus lateralis of young adults (n = 14, mean ± SD: 23 ± 4 years). The expression of myostatin, transforming growth factor β, and other regulatory factors was measured, and immunohistochemical analyses were performed to assess turnover of extracellular matrix components.
Results:
Injured limb skeletal muscle demonstrated elevated myostatin gene (P < .005) and protein (P < .0005) expression, which correlated (R2 = 0.38, P < .05) with fibroblast cell abundance. Immunohistochemical analysis showed that human fibroblasts express the activin type IIB receptor and that isolated primary human muscle-derived fibroblasts increased proliferation after myostatin treatment in vitro (P < .05). Collagen 1 and fibronectin, primary components of the muscle extracellular matrix, were significantly higher in the injured limb (P < .05). The abundance of procollagen 1-expressing cells as well as a novel index of collagen remodeling was also elevated in the injured limb (P < .05).
Conclusion:
These findings support a role for myostatin in promoting fibrogenic alterations within skeletal muscle after an ACL injury.
Clinical Relevance:
The current work shows that the cause of muscle quality decline after ACL injury likely involves elevated myostatin expression, and future studies should explore therapeutic inhibition of myostatin to facilitate improvements in muscle recovery and return to sport.
Keywords: fibroblast, quadriceps, extracellular matrix, collagen
Quadriceps weakness can persist for 2 years after anterior cruciate ligament (ACL) injury,2,19,23 and this protracted weakness has been linked to an increased risk of osteoarthritis development and a reduced ability to return to full activity levels.3-5,21,29,39 Recent research showed that derangements in muscle quality and morphology significantly contribute to strength deficits.18,26,27 Specifically, we and others have shown fiber atrophy and fibrosis in the quadriceps,33,38 as well as alterations within its cellular architecture that may underlie observed reductions in muscle quality after ACL tear.15 Identification of the factors contributing to these deleterious changes is critical for the advancement of evidence-based therapy.
Myostatin—growth differentiation factor 8 (GDF8), a member of the transforming growth factor β (TGFβ) family—is a well-characterized negative regulator of muscle size.1,24,41 Myostatin overexpression has also been associated with fibrosis and fibroblast proliferation in skeletal muscle.11,28 Deposition of collagen and extracellular matrix (ECM) components disrupts muscle architecture and function, reducing muscle quality.16 Mechanistic determination of downstream fibrotic consequences of myostatin expression within muscle is needed to evaluate its therapeutic potential. Our previous work showed an increased fibrotic burden within the quadriceps muscle after an ACL injury,15,33 which may have functional consequences through reduced muscle quality. Of particular importance in the origin of muscle fibrosis is the TGFβ superfamily. In the current study, we sought to elucidate the contribution of various TGFβ superfamily members (TGFβ1, activin A, GDF11, and myostatin) to our observed increased muscle fibrotic burden. Heightened inflammatory signaling has also been associated with increased indices of muscle fibrosis,34,42 and cytokines can initiate a downstream signaling cascade promoting the expression of myostatin.43
Rodent models of ACL injury involving surgical transection of the ligament showed elevated myostatin expression in the injured limb quadriceps at 7 days after transection.10 The invasive nature of surgically transecting the ACL in a rodent model as well as ACL reconstruction surgery of humans raises the intriguing question of whether the ACL tear or the surgical procedure induces the observed elevation in circulating myostatin that has been reported.30 To determine whether ACL injury is sufficient to promote elevated myostatin expression within the quadriceps, we collected muscle biopsy specimens from patients who experienced an ACL tear. We hypothesized that acute ACL injury stimulates myostatin expression that contributes to the fibrotic burden and fibrogenic cell expansion after ACL injury.15,33 Abbreviations used in this article are defined in Table 1.
TABLE 1.
Abbreviations Used
| Abbreviation | Definition |
|---|---|
| ACVR2B | Activin type IIB receptor |
| BSA | 1% bovine serum albumin |
| C1orf43 | Chromosome 1 open reading frame 43 |
| CHP | Collagen hybridizing peptide |
| ECM | Extracellular matrix |
| EMC7 | ER membrane protein complex subunit 7 |
| FAP | Fibrogenic/adipogenic progenitor cells |
| GDF | Growth differentiation factor |
| HRP | Horseradish peroxidase |
| IL | Interleukin |
| MMP | Matrix metalloproteinase |
| MSTN | Myostatin |
| PBS | phosphate-buffered saline |
| PDGF | Platelet-derived growth factor |
| REEP5 | Receptor expression-enhancing protein 5 |
| Tcf 4 | Transcription factor 4 |
| TGFß | Transforming growth factor beta |
| TIMP1 | Issue inhibitor matrix metalloproteinase 1 |
| TNFα | Tumor necrosis factor alpha |
| VCP | Valosin-containing protein |
METHODS
Ethical Approval
The study was approved by the institutional review board at the University of Kentucky and performed in accordance with the ethical standards of the 1964 Declaration of Helsinki. Informed written consent was obtained from each patient before participation in this study.
Study Design
To qualify, potential participants could not have had a total knee dislocation or any previous ACL reconstructions or tears other than the current injury. The diagnosis of the ACL injury was made by 1 of 2 orthopaedic surgeons (D.L.J., M.L.I.). Of the 14 patients in the study, 10 had a meniscus tear, and all had a complete tear of the ACL. We previously used the same cohort to investigate the effect of an ACL injury and reconstruction on muscle fiber type, atrophy, and fibrosis.15,33 The current study includes additional patients (n = 4) for gene expression analysis. Given our findings showing fibrogenic cell expansion, we further investigated the molecular mechanisms involved in the promotion of muscle fibrosis among those same participants; however, the data presented here have not yet been reported.
Quadriceps Muscle Biopsy Specimens
Muscle biopsy specimens from the injured and noninjured limbs were collected during the same visit for each patient. Patients were completely weightbearing at the time of data collection in the current study and were ambulating without an assistive device. In addition, patients were given a home exercise program to follow and were encouraged to perform range of motion and strengthening exercises. Percutaneous muscle biopsy specimens (250 mg) from the vastus lateralis muscle were performed with a Bergström 5-mm muscle biopsy needle with suction.35 Approximately 50 mg was mounted in tragacanth gum on cork and flash frozen in liquid nitrogen–cooled 2-methylbutane, with the remainder of the muscle tissue snap frozen in liquid nitrogen for RNA/ protein analyses. Samples were stored at −80°C until processing. Human skeletal muscle–derived fibroblasts were obtained from 3 age-matched muscle biopsy donors, which were donated by the Center for Muscle Biology at the University of Kentucky.
Total RNA Isolation and Quantitative Reverse Transcription Polymerase Chain Reaction
Total RNA from whole tissue was isolated with the miR-Neasy Mini Kit (Qiagen) according to the manufacturer’s instruction. RNA content, integrity, and purity were assessed with the NanoDrop 2000 (Thermo Fisher) and the 2100 Bioanalyzer (Agilent). First-strand cDNA synthesis from total RNA (1 μg) was performed with Superscript IV VILO Master Mix with ezDNase enzyme (Thermo Fisher). The resultant cDNA was amplified with Radiant SYBR Green Master Mix (Alkali Scientific) plus 0.3 mmol/L of gene-specific upstream and downstream primers (myostatin/ GDF8, TGFβ, activin a, GDF11, IL-6, TNFα, C1orf43, EMC7, VCP, REEP5, MMP2, MMP9, MMP13, MMP1, TIMP1) during 40 cycles on a QuantStudio 3 Fast Realtime Cycler (Thermo Fisher). The expression of the specific genes of interest was generated with the ΔΔCt method and reported as relative arbitrary units. Expression cutoffs were set at a cycle threshold of 35. Genes were normalized to the geometric mean of VCP, REEP5, EMC7, and C1orf43.14 Primer sequences are available upon request.
Western Blot Analysis
Muscle samples were homogenized by bead milling with 0.5-mm zirconium oxide beads (Bullet Blender; Next Advance) in ice-cold RIPA buffer plus phosphatase and protease inhibitor mini tablets, EDTA free (Thermo Fisher). Protein concentration was determined by the Bradford method (Thermo Fisher). Thirty or fifty micrograms of protein were loaded onto 4% to 20% SDS polyacrylamide gels (Bio-Rad) and transferred onto PVDF membranes at 50V for 1 hour at 4°C. Membranes were blocked for 1 hour at room temperature (RT) with 2% bovine serum albumin before the addition of primary antibodies overnight at 4°C with gentle agitation: anti-GDF8/myostatin (ab71808; Abcam), anti-phospho-SMAD3 (Ser423/425, 9520; Cell Signaling Technologies), anti-SMAD3 (9523; Cell Signaling Technologies), anti-TGFβ (3709; Cell Signaling Technologies), anti-TNFα (18-kDA soluble form, 8184; Cell Signaling Technologies), anti-β-tubulin (2128; Cell Signaling Technologies), and anti-β-actin (4970; Cell Signaling Technologies). After washing, blots were incubated for 1 hour at RT with IRDye 680LT goat anti-rabbit IgG (LI-COR) or IRDye 800CW goat anti-mouse IgG (LI-COR), or blots were incubated in a horseradish peroxidase (HRP)-conjugated secondary antibody for 1 hour at RT. Chemiluminescent solution (ECL Plus; Amersham BioSciences) was applied to each blot, and after a 5-minute incubation, optical density measurements were obtained with a phosphoimager (Bio-Rad), and densitometric analysis was performed with Quantity One software (v 4.5.2; Bio-Rad) or the Odyssey Infrared Imaging System (v 3.0.21; LI-COR). Results are expressed as arbitrary densitometric units.
Immunohistochemistry
Frozen tissue was sectioned (7 μm), and slides were air-dried for 1 hour. Transcription factor 4 (Tcf4)/transcription factor 7–like 2 (TCF7L2) and platelet-derived growth factor receptor α (PDGFRα) immunohistochemical methods were published previously.15 Briefly, sections were fixed in 4% paraformaldehyde, followed by epitope retrieval with sodium citrate (10 mM, pH 6.5) at 92°C for 10 minutes. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide in phosphate-buffered saline (PBS) for 7 minutes, followed by a blocking step in 2.5% normal horse serum (Vector Laboratories). Overnight incubations with rabbit anti-Tcf4 (2569, 1:100; Cell Signal Technologies) or goat anti-PDGFRα (AF-307-NA, 1:100; R&D Systems) were carried out at 4°C. After washing, donkey anti-rabbit biotin secondary (1:1000; Jackson ImmunoResearch) or chicken anti-goat biotin secondary (1:1000; Jackson ImmunoResearch) was incubated for 1 hour at RT. Signal amplification of Tcf4 was achieved with streptavidin-HRP, followed by tyramide signal amplification Alexa Fluor 488 (Thermo Fisher). Sections were then costained with 4′,6-diamidino-2-phenylindole (DAPI; D35471, Life Technologies).
For Tcf4-activin type IIB receptor (ACVR2B) staining, slides were fixed in 4% paraformaldehyde, followed by epitope retrieval with sodium citrate (10 mM, pH 6.5) at 92°C for 10 minutes. Endogenous peroxidase activity was blocked, followed by a blocking step in 2.5% normal horse serum. Slides were then incubated with primary antibody: Tcf4 (2553, mouse monoclonal, 1:100; Cell Signal Technologies) and ACVR2B (AP7105a, rabbit, 1:100; Abgent) were incubated overnight at 4°C. The next day, slides were incubated in goat anti-mouse biotin secondary, AF647 wheat germ agglutinin (Thermo Fisher; to label the ECM), and goat anti-rabbit AF555 (Thermo Fisher), followed by streptavidin-HRP and then tyramide signal amplification with Alexa Fluor 488 (Thermo Fisher). Sections were then costained with DAPI.
For collagen 1, procollagen 1, fibronectin, and tenascin C staining, slides were fixed in ice-cold acetone (−20°C) for 10 minutes, rinsed with PBS, and blocked in 1% bovine serum albumin (BSA) in PBS for 1 hour at RT. Slides were then incubated with primary antibody: fibronectin (ab2413, rabbit, 1:100; Abcam), tenascin C (AB19013, rabbit, 1:100; Millipore), collagen 1 (ab34710, rabbit, 1:200), and procollagen (SP1.D8, mouse monoclonal, supernatant; Developmental Studies Hybridoma Bank) were incubated overnight at 4°C. The SP1.D8 procollagen 1 antibody was obtained from the Developmental Studies Hybridoma Bank, created by the National Institute of Child Health and Human Development of the National Institutes of Health, and maintained at the Department of Biology of The University of Iowa. The next day, slides were incubated in goat anti-mouse IgG1 AF555 and goat anti-rabbit AF488 (Thermo Fisher) for 1 hour at RT. Slides were costained with DAPI.
For collagen 4–collagen hybridizing peptide, immunohistochemical methods were modified according to the manufacturer’s instruction (3Helix). In brief, sections were fixed in ice-cold acetone (−20°C) for 10 minutes and blocked in 2.5% normal horse serum for 1 hour at RT. 3Helix-5-FAM conjugate (3Helix) was diluted to a working solution (20 μM), placed on a heating block at 80°C for 5 minutes to denature 3Helix trimers, and then quickly cooled on wet ice for 2 minutes. Immediately after cooling, anti-collagen 4 (ab6586, rabbit, 1:200; Abcam) was added to the 3Helix-PBS solution, and slides were incubated overnight at 4°C. The next day, slides were washed in PBS, incubated with goat anti-rabbit AF555, and then costained with DAPI.
For CD11b/CD206 staining, immunohistochemical methods were modified as previously published.25 Sections were fixed in ice-cold acetone (−20°C) for 10 minutes, briefly washed in PBS, blocked for endogenous peroxidase activity with 3% hydrogen peroxide in PBS for 15 minutes, and placed in a streptavidin/biotin blocking kit (Vector Laboratories) for 15 minutes each and then overnight at 4°C in 2.5% normal horse serum. On day 2, slides were incubated with mouse IgG1 anti-CD11b (1:100; Cell Sciences) in 2.5% normal horse serum overnight at 4°C. On the third day, the sections were rinsed in PBS, followed by incubation with goat anti-mouse biotin secondary for 90 minutes at RT and then by streptavidin-HRP and tyramide signal amplification with Alexa Fluor 488 (Thermo Fisher) for 15 minutes at RT. Sections were placed back into the streptavidin/biotin blocking kit and 2.5% normal horse serum for 1 hour and incubated overnight with goat anti-CD206 (1:200; R&D Systems) in 2.5% normal horse serum at 4°C. On the fourth day, sections were incubated in rabbit anti-goat biotinylated secondary antibody (1:1000; Jackson ImmunoResearch) for 90 minutes at RT. Streptavidin AF594 was then added to the section for 1 hour, followed by nuclei visualization with DAPI.
Fibroblast Isolation
Primary fibroblasts were isolated from skeletal muscle samples by collagenase type II (2 mg/mL; Thermo Fisher) and dispase II (1 mg/mL; Sigma-Aldrich) digestion for 60 minutes with agitation at 37°C, filtered (40 mm; Miltenyi Biotec), and plated on uncoated 100-mm tissue culture plastic for 1 hour at 37°C in growth media, 20% fetal bovine serum (Atlanta Biologicals), and DMEM (Dulbec-co’s modified Eagle medium; Sigma-Aldrich). To remove contaminating myoblasts, adherent cells were subjected to magnetic antibody cell sorting with human anti-fibroblast microbeads via a fibroblast-specific antigen (130-050-601; Miltenyi Biotec). Cell labeling was carried out in 0.5% BSA (Thermo Fisher) and Hank’s Balanced Salt Solution (Thermo Fisher) for 15 minutes at 4°C. Cells were washed once, resuspended in 0.5% BSA/Hank’s Balanced Salt Solution, and sorted with AutoMacs Pro (Miltenyi Biotec). The positive cell fraction was then resuspended in growth media and plated on 6-well dishes (VWR). Immunocytochemistry was performed after plating to assess fibroblast purity with a known fibroblast marker (Tcf4), and cells used in experiments were >98% Tcf4+.
Myostatin Treatment and EdU Labeling
Myostatin dosage and duration were adapted as previously described.28 The fibroblasts isolated from 3 human patients were run in duplicate, with each patient serving as a genetic control to compare treatment effects. Before myostatin treatment, fibroblasts were serum starved in DMEM for 12 hours, followed by 300 ng/mL of human-recombinant GDF8/myostatin (Abcam) or vehicle (200μM HCl) for 12 hours. Four hours before collection, 10μM 5-ethynyl-2’-deoxyuridine (EdU; Thermo Fisher) was directly added to the media. Cells were washed twice with PBS to remove residual EdU. Cells were then detached and spun onto glass slides with a Shandon Cyto-spin 4 Cytocentrifuge (Thermo Fisher) at 1000 rpm for 3 minutes. Slides were air-dried for 15 minutes and fixed with 4% paraformaldehyde for 20 minutes at RT. Click-It EdU fluorescent labeling with Alexa Fluor 647 (Thermo Fisher) was conducted according to the manufacturer’s instructions, followed by costaining with DAPI.
Image Acquisition and Analysis
Images were captured at 200× total magnification with an upright microscope (Carl Zeiss), and analysis was carried out with Zen 2 core software (Carl Zeiss). Fibroblasts (Tcf4+/DAPI+), fibrogenic/adipogenic progenitor cells (FAPs; PDGFRα+/DAPI+), and macrophages (CD11b+/CD206– and CD11b+/CD206+) were identified as previously described.15,25 Cell counts were then normalized to the number of myofibers present within each cross section or the total area of the biopsy specimen. EdU labeling quantification was performed with ZEN 2 image processing software. Thresholds were set to first identify all DAPI+ events, followed by all EdU+ events, and the relative ratio of EdU+ cells to total DAPI+ cells was tallied. The total number of cells per slide ranged from 15,000 to 23,000. The data are presented as percentage Edu+/DAPI+. Collagen 1, collagen 4, and fibronectin were quantified to measure expansion of key components of the ECM with the thresholding feature of Zeiss AxioVision software (v 4.9), and the area occupied by collagen 1, collagen 4, and fibronectin was independently expressed relative to the total muscle area (mm2). Collagen hybridizing peptide analysis was conducted in a similar manner to determine areas of active collagen remodeling. The binding area of the collagen hybridizing peptide was determined with the thresholding feature of AxioVision and expressed relative to the total muscle area (mm2). All immunohistochemical images were analyzed by a single assessor (B.D.P., C.R.B.) in a blinded manner to the limb injury status.
Statistical Analysis
Data are presented as mean ± SD. Wilcoxon signed rank tests were performed between injured and noninjured limbs to compare each dependent variable, with significance set at P ≤ .05. Pearson correlations were tested by assessing the linear fit between the number of Tcf4+ fibroblasts and PDGFRα+ FAPs and myostatin protein expression in the injured limb. All analyses were performed with GraphPad Prism 7.0.
RESULTS
Twelve men and 2 women with ACL tears completed the study (mean ± SD: age, 23 ± 4 years; weight, 80 ± 15 kg; height, 1.78 ± 0.07 m). The mean time from injury to biopsy completion was 80 ± 30 days. There was no significant relationship between the time from injury to biopsy and any of the outcome variables (data not shown). Expression of genes encoding TGFβ family members, as well as downstream effectors, was analyzed by quantitative reverse transcription polymerase chain reaction. Myostatin displayed a nearly 2-fold elevation in mRNA abundance (P < .005) (Figure 1A), whereas TGFβ1, activin A, and GDF11 were unaffected by ACL tear in skeletal muscle (Figure 1, B-D). TGFβ protein expression showed no difference between limbs, supportive of TGFβ mRNA expression (Figure 2A). Myostatin protein levels mirrored the observed elevation in gene expression (P < .005) (Figure 2B), confirming that the transcription and translation of myostatin in muscle are elevated after an ACL tear. SMAD3, a downstream target of the myostatin signaling cascade, was also investigated. Higher phospho-SMAD3 was observed in the injured limb when compared with the noninjured limb (P < .0005) (Figure 2C). Previous immunohistochemical analysis revealed that interstitial cells outside the dystrophin border displayed elevated p-SMAD3 staining in the injured limb,15 supportive of our current findings. Elevated myostatin expression was also correlated with the abundance of fibrogenic cells within the injured limb vastus lateralis. A significant linear correlation was found between Tcf4+ fibroblast abundance and myostatin protein expression (P < .05) (Figure 3A). While it did not achieve statistical significance, PDGFRα+ FAPs demonstrated a similar positive trend when plotted against myostatin protein expression (P = .08) (Figure 3B).
Figure 1.
Increased myostatin (MSTN) mRNA expression is observed following anterior cruciate ligament tear in quadriceps skeletal muscle. Gene expression, measured by quantitative polymerase chain reaction, from injured (I) and noninjured (NI) vastus lateralis muscle. Target genes (A) MSTN, (B) TGFβ1, (C) activin A, and (D) GDF11 were normalized to the geomean of 4 stable housekeeping genes and further normalized to the relative expression in NI control muscle. Values are presented as mean ± SD relative quotient (RQ), N = 14. **P < .005 vs NI limb.
Figure 2.
Elevated myostatin (MSTN) protein expression and phosphorylation of SMAD3 in anterior cruciate ligament–injured quadriceps muscle. Whole muscle protein lysates were probed with (A) anti-TGFβ1, (B) anti-GDF8/MSTN, and (C) anti-p-SMAD3 and anti-SMAD3 antibodies, in addition to stable housekeeping proteins β-actin and β-tubulin. Band intensities were collected from the noninjured (NI) and injured (I) muscle samples, and differences were represented as a fold change in arbitrary units (AUs) across limbs. Values are presented as mean ± SD, n = 11. **P < .005 vs NI limb. ***P < .0005 vs NI limb. SMAD3, Small mothers against decapentaplegic 3.
Figure 3.
Myostatin (MSTN) protein expression correlates with fibrogenic cell abundance in quadriceps muscle following anterior cruciate ligament injury. Correlation of (A) Tcf4+ fibroblasts and (B) PDGFRα+ FAPs relative to myofiber number with myostatin protein expression in the injured limb. Values are presented as mean ± SD, n = 10. AU, arbitrary unit.
In evaluating other potential skeletal muscle perturbations after ACL injury, we investigated inflammatory cytokine gene expression of interleukin 6 (IL-6) and tumor necrosis factor α (TNFα). We report that despite significant time from injury, IL-6 (P < .0005) (Figure 4A) and TNFα (P < .05) (Figure 4B) were significantly higher in the injured limb than the noninjured limb. TNFα protein expression was also elevated in the injured limb, supportive of its higher mRNA expression (P < .05) (Figure 4C). Despite elevated expression of proinflammatory cytokines within the muscle, we observed no differences in macrophage abundance between limbs (Figure 5). Macrophages are often denoted by their polarity as M1, classically activated proinflammatory macrophages, or M2, alternatively activated anti-inflammatory macrophages. We observed no differences in M1 (CD11b+/CD206−) or M2 (CD11b+/CD206+)25 macrophage abundance within the injured limb muscle.
Figure 4.
Elevated inflammatory marker mRNA and protein expression is observed following anterior cruciate ligament tear in quadriceps skeletal muscle. Gene expression is measured by quantitative polymerase chain reaction from injured (I) and noninjured (NI) vastus lateralis muscle. Target genes (A) IL-6 and (B) TNFα were normalized to the geomean of 4 stable housekeeping genes and then further normalized to the relative expression in NI control muscle. Values are presented as mean ± SD relative quotient (RQ). Whole muscle protein lysates were probed with (C) anti-TNFα in addition to a stable housekeeping protein β-tubulin. Band intensities were represented as a fold change in arbitrary units (AUs) across limbs, N = 14. *P < .05 vs noninjured limb. ***P < .0005 vs NI limb.
Figure 5.
Macrophage abundance is not different in the quadriceps muscle following anterior cruciate ligament tear. Representative images of (A) DAPI, (B) CD11b, (C) CD206, and (D) merged immunohistochemistry demonstrating an M1 macrophage (CD11b+/CD206−, yellow arrowhead) and an M2 macrophage (CD11b+/CD206+, white arrowhead). Abundance of (E) M1 and (F) M2 macrophages in the injured (I) and noninjured (NI) limbs. Values are represented as mean macrophages per fiber ± SD, n = 10. Scale bar = 50 μm.
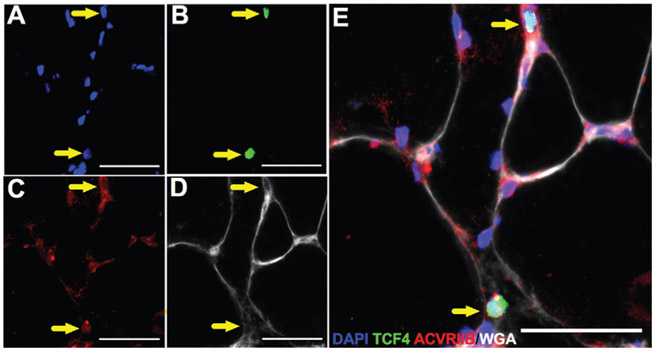
Myostatin initiates its downstream cellular signaling cascade after its binding to ACVR2B.6 Previous data from rodent models demonstrated ACVR2B localization to various cell lineages within skeletal muscle milieu, including myonuclei, satellite cells, and interstitial cells.6 Figure 6 shows localized expression of the Tcf4 fibroblast marker with ACVR2B in human skeletal muscle. To more definitively ascertain the effect of myostatin on fibrogenic cell proliferation, primary skeletal muscle–derived fibroblasts were treated with recombinant human myostatin. Treatment with myostatin induced a significantly greater proliferative response in fibroblasts as assessed by EdU incorporation (P < .05) (Figure 7).
Figure 6.
Activin type IIB receptor (ACVR2B) is expressed by human skeletal muscle fibroblasts. Representative images of (A) DAPI, (B) Tcf4, (C) ACVR2B, and (D) wheat germ agglutinin staining in human skeletal muscle. (E) Merged immunohistochemical image demonstrating ACVR2B+/Tcf4+ cells (yellow arrows). Scale bar = 50 μm.
Figure 7.
Myostatin treatment induces proliferation of human skeletal muscle–derived fibroblasts. (A-D) Representative images of EdU-labeled fibroblasts following 12-hour VEH or MSTN (300 ng/mL) treatment. (E) Myostatin treatment results in a 70% increase in EdU+ fibroblast frequency. Values are presented as percentage EdU+/DAPI+, mean ± SD, n = 3, with isolates assessed in duplicate. *P < .05 vs VEH-treated group. Scale bar = 200 μm. DAPI, 4#,6-diamidino-2-phenylindole; EdU, 5-ethynyl-2′-deoxyuridine; VEH, vehicle.
We also sought to identify specific ECM protein abundance and estimate ECM turnover with a novel collagen hybridizing peptide. Our findings demonstrate significantly higher collagen 1 deposition in the injured limb as compared with the noninjured limb (P < .005) (Figure 8, A and B). The elevated collagen 1 abundance was supported by a significantly greater number of cells expressing procollagen 1 within the injured limb versus the noninjured limb (P < .05) (Figure 8C). There was, however, no change in collagen 4 staining between injury conditions (Figure 8, D and E). The collagen hybridizing peptide binds to unfolded collagen chains and was found to be significantly greater in the injured limb quadriceps muscle as compared with the noninjured limb (P < .005) (Figure 8F). Fibronectin, another major interstitial ECM component, was significantly higher in the injured limb (P < .05) (Figure 9, A-C). In support of greater ECM turnover, we also show elevated abundance of tenascin C (P < .05) (Figure 9, D-F), a hexameric glycoprotein that is upregulated within injured skeletal muscle and thought to decrease cellular adhesion, promote migration, and inhibit premature fusion.22
Figure 8.
Elevated abundance of collagen 1, procollagen 1–positive cells, and CHP in the injured limb quadriceps muscle. (A) Representative images of collagen 1 (green) and procollagen 1 (red) staining in injured (I) and noninjured (NI) limbs. (B) Percentage area of collagen 1 staining in the I and NI limbs. (C) Number of procollagen 1+ cells relative to muscle area in the I and NI limbs. (D) Representative images of collagen 4 (red) and CHP (green) in I and NI limbs. Percentage area of (E) collagen 4 staining and (F) CHP staining in the I and NI limbs. Values are represented as mean ± SD, n = 10. *P < .05 vs from NI limb. **P < .005 vs noninjured limb. Scale bar = 100 μm.
Figure 9.
Fibronectin and tenascin C accumulation in the injured limb quadriceps muscle following an anterior cruciate ligament tear. Representative images of fibronectin staining (red) in the (A) noninjured and (B) injured limbs. (C) Percentage area of fibronectin staining in the noninjured and injured limbs. Representative images of tenascin C staining (red), collagen 4 (green), and DAPI (blue) in the (D) noninjured and (E) injured limbs. (F) Percentage area of tenascin C staining in the noninjured and injured limbs. Values are presented as mean ± SD, n = 10. *P < .05 vs noninjured limb. Scale bar = 100 μm. I, injured; NI, noninjured. DAPI, 4#,6-diamidino-2-phenylindole.
Additional factors involved in ECM turnover revealed no significant alterations in gene expression between the noninjured and injured limbs (all results reported as mean ± SD relative quotient, N = 14, P > .05): MMP2 (noninjured, 0.7905 ± 0.292; injured, 0.8311 ± 0.3077), MMP9 (1.559 ± 1.191, 2.233 ± 2.047), MMP13 (0.9479 ± 1.171, 1.926 ± 1.914), and TIMP1 (0.61636 ± 0.1838; 0.7623 ± 0.3407). MMP1 was also investigated but failed to reach cycle threshold cutoff levels.
DISCUSSION
An ACL tear produces a myriad of detrimental effects within the injured limb quadriceps muscle. Our findings expand on what is known by showing an upregulation of myostatin expression within the quadriceps muscle after an ACL tear that is associated with increased fibrogenic cell density. Additionally, isolated muscle fibroblasts exhibit increased proliferative rates after exposure to myostatin. As we previously showed an accumulation of ECM components within the quadriceps after an ACL tear, the results from our observational study provide support for the contribution of myostatin in the origin of fibrotic tissue deposition.
Pathological fibrosis has been traditionally linked to signaling cascades initiated by the TGFβ superfamily.31 Intriguingly, we found that myostatin and not other TGFβ familial members (TGFβ1, GDF11, activin A) is significantly elevated in the injured limb muscle before reconstruction. Recent work showed increases in systemic TGFβ serum levels after ACL reconstructive surgery,30 but our muscle expression data do not show any effect of ACL tear on TGFβ mRNA or protein expression. Our findings do support previous work in rodent ACL injury models as well as findings demonstrating increased human myostatin serum levels after ACL reconstruction.10,30 We show for the first time elevated myostatin gene and protein expression within the injured limb muscle, which suggests that the source of systemic myostatin may be from the injured limb skeletal muscle.17 An important finding from our study was that the ACL tear alone is sufficient to drive myostatin expression in human patients. Controversy exists regarding whether the elevations in myostatin are due to the ACL tear itself or the invasive nature of surgical reconstruction.10,29 In our study, the collection of muscle biopsy specimens before reconstruction demonstrates that the ACL tear is a sufficient stimulus to induce myostatin expression, likely promoting rapid maladaptation within the muscle shortly after the ligament tear. Paired with subsequent induction of myostatin after reconstructive surgery, there would appear to exist a window of myostatin induction that we hypothesize is crucial in the observed reduction in muscle quality. Future studies will establish a time course of myostatin induction to delineate this effect.
Local expression of myostatin within the damaged ligament could serve a protective function through increased collagen synthesis. Indeed, fibroblasts isolated from the ACL are responsive to myostatin and react with increased ECM biosynthesis and proliferation in an attempt to repair the damaged ligament.17 However, the diffusion of myostatin to the surrounding quadriceps muscle would have negative consequences: mitigation of skeletal muscle growth and increased muscle ECM biosynthesis. Myostatin is able to positively regulate its own expression through activation of downstream effectors in the SMAD family of transcription factors,1 allowing for sustained overexpression in the muscle after an ACL injury and surgical reconstruction, which can impede proper rehabilitation and recovery. The ACL-injured evolutionary perspective, induction of myostatin after an ACL injury would be protective of the ligament through increased collagen synthesis within the damaged ligament and via suppression of muscle growth. Suppression of muscle growth would prevent overload and reinjury of the healing ligament as well as knee instability from excessive anterior translation of the tibia. However, modern ACL reconstruction utilizes an autograft or allograft to replace the damaged ligament, minimizing any beneficial effect of myostatin-induced ligament collagen synthesis. In fact, performing ACL reconstruction soon after the initial injury is associated with arthrofibrosis of the knee joint capsule,36 leading to excessive stiffness, and is potentially due to the elevated level of myostatin within the tibiofemoral joint.
The ACL-injured limb muscle also demonstrated elevated expression of proinflammatory cytokines IL-6 and TNFα. IL-6 initiates a downstream signaling cascade through activation of Stat3 (signal transducer and activator of transcription 3), which can promote the expression of myostatin.43 Heightened inflammatory signaling has also been associated with increased indices of muscle fibrosis during various chronic conditions34,42 as well as acute orthopaedic injuries.40 The current cross-sectional study design does not allow for us to elucidate the temporal relationship between these factors, but it does provide intriguing evidence for an inflammation-myostatin-ECM accumulation pathology. While we report altered expression of proinflammatory cytokines in the injured limb muscle, we show no differences in macrophage abundance or polarity between limbs. We did observe heterogeneity in the density of macrophages within the injured limb muscle, and future studies will seek to better define the role of macrophages in a larger subset of patients. The time after ACL injury to muscle sample collection demonstrated some variability among patients; however, we did not observe significant correlations between days after injury and any of our outcome measures. It should be noted as well that patients were completely weightbearing during the time of biopsy specimen collection, minimizing the contribution of unloading to our observed increases in myostatin expression. Unloading signals that could promote myostatin expression would likely decrease over time; however, as our patients were walking without any assistive device and were encouraged to perform range of motion and strengthening exercises, we believe that the contribution of unloading-induced myostatin expression was minimal in our sample.
The cell type–dependent effects of myostatin are most apparent in the analysis of cyclin D1 protein expression, which is elevated in fibroblasts after myostatin treatment.28 The higher phospho-SMAD3 in the muscle homogenate is indicative of a profibrotic state within the skeletal muscle milieu. We report that myostatin protein expression from whole muscle homogenates correlated significantly with the elevated frequency of Tcf4+ fibroblasts, supporting the concept that elevated myostatin in skeletal muscle after an ACL tear has the potential to promote fibroblast cell proliferation. We confirmed our clinical observations through in vitro experiments with primary human skeletal muscle fibroblasts exposed to human recombinant myostatin. We showed that myostatin exposure produced similar increases in fibroblast proliferation (as assessed by EdU incorporation) as previously observed in mouse primary fibroblasts.28 Note that fibroblasts also express myostatin,37 and the increased abundance of fibroblasts within muscle may be contributing to the elevated myostatin expression that we observed. The current cross-sectional study design does not, however, allow us to infer a temporal relationship between myostatin and fibroblast density changes. Immunohistochemical colocalization analysis also revealed that human fibroblasts express ACR2B, the primary receptor for myostatin, underpinning the regulatory potential of myostatin in the origin of muscle fibrotic burden.
Myostatin expression also showed a trend for a positive correlation with the abundance of PDGFRα+ FAPs within the injured limb muscle. Aberrant ECM deposition within muscle has largely been attributed to fibroblasts and FAPs, which are the profibrotic cells in skeletal muscle.13,44 Systematic increases in muscle fibroblasts and FAPs after an ACL injury are likely responsible for elevated ECM deposition in the injured vastus lateralis, and our results from the current cross-sectional study suggest that this may be due to elevations in myostatin expression. Work in mouse skeletal muscle after injury shows that FAPs and Tcf4+ fibroblasts express PDGFRa, suggesting that these populations might be overlapping.9,32 We demonstrated in human muscle that cells expressing PDGFRα concurrently express Tcf4, but a larger subset of Tcf4+ cells do not express PDGFRα.15 Intriguingly, Tcf4+/PDGFRα-cell abundance was elevated in the injured limb, but more work is needed to ascertain direct contributions of these cell populations.15
We showed that overall collagen abundance is up after ACL injury, and in the current study, we report elevated abundance of collagen 1 and procollagen 1–expressing cells but no difference in collagen 4. Collagen 4, which is associated with the basal lamina, is likely differentially regulated as compared with collagen 1 after an ACL injury. Fibronectin, a downstream target of SMAD3 in fibroblasts and another major ECM component, was higher in the injured limb.12,20 Results from our collagen hybridizing peptide provide intriguing data supporting elevated collagen remodeling in muscle after an ACL injury. The collagen hybridizing peptide adheres to areas of dynamic collagen remodeling, where it can access and bind loose collagen strands. In static conditions, the triple helix of collagen is inaccessible to the hybridizing peptide. Results gleaned from our collagen hybridizing peptide experiments cannot ascertain if increased collagen remodeling is due to elevated synthesis or degradation (or both); however, in combination with greater levels of cells expressing procollagen 1, it would suggest that increased collagen 1 deposition is occurring. These data are supported by elevated expression of tenascin C within the injured limb muscle. Tenascin C expression is highest in areas undergoing greater mechanical stress, such as tendons and myotendinous junctions, and is thought to add mechanical stability.22 Tenascin C was recently shown to be upregulated after a muscle injury and during increased loading, where it is thought to promote remodeling of the ECM and decrease cellular adhesion.7,8 Combined with our collagen hybridizing peptide results, we show likely elevated turnover of muscle collagens and other ECM components after an ACL injury. Previous literature in rodent models reports significant perturbations in ECM-related genes; however, our findings display no substantial changes in MMP1, MMP2, MMP9, MMP13, or TIMP1.41 Clear perturbations in ECM remodeling after an ACL tear highlight the need for novel therapies to address these deleterious adaptations.15,33
CONCLUSION
In conclusion, the expansion of fibrogenic cells reduces muscle quality through altered ECM remodeling after an ACL tear. Our data show a rapid induction of myostatin before surgical reconstruction that appears to be key in the cause of muscle maladaptation after ACL tear.
Acknowledgments
One or more of the authors has declared the following potential conflict of interest or source of funding: This work was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health (NIH; grant K23 AR062069 to B.N. and grant R01 AR072061 to C.S.F.) and the John Sealy Memorial Endowment Fund to C.S.F. C.S.F. is a former KL2 scholar supported by the Claude D. Pepper Older Americans Independence Center, University of Texas Medical Branch (National Institute on Aging, NIH; grant P30 AG024832). Additional support came from a pilot grant from the Center for Muscle Biology, University of Kentucky. D.L.J. and M.L.I. have received hospitality payments from Smith & Nephew. The procollagen 1 (SP1.D8) antibody was developed by H. Furthmayr and obtained from the Developmental Studies Hybridoma Bank, created by the National Institute of Child Health and Human Development of the NIH and maintained at the Department of Biology, The University of Iowa. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Footnotes
This content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Allen DL, Unterman TG. Regulation of myostatin expression and myoblast differentiation by FoxO and SMAD transcription factors. Am J Physiol Cell Physiol. 2007;292(1):C188–C199. [DOI] [PubMed] [Google Scholar]
- 2.Angelozzi M, Madama M, Corsica C, et al. Rate of force development as an adjunctive outcome measure for return-to-sport decisions after anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther. 2012;42(9):772–780. [DOI] [PubMed] [Google Scholar]
- 3.Ardern CL, Taylor NF, Feller JA, Webster KE. Fifty-five per cent return to competitive sport following anterior cruciate ligament reconstruction surgery: an updated systematic review and meta-analysis including aspects of physical functioning and contextual factors. Br J Sports Med. 2014;48(21):1543–1552. [DOI] [PubMed] [Google Scholar]
- 4.Ardern CL, Taylor NF, Feller JA, Webster KE. Return-to-sport outcomes at 2 to 7 years after anterior cruciate ligament reconstruction surgery. Am J Sports Med. 2012;40(1):41–48. [DOI] [PubMed] [Google Scholar]
- 5.Ardern CL, Webster KE, Taylor NF, Feller JA. Return to sport following anterior cruciate ligament reconstruction surgery: a systematic review and meta-analysis of the state of play. Br J Sports Med. 2011;45(7):596–606. [DOI] [PubMed] [Google Scholar]
- 6.Babcock LW, Knoblauch M, Clarke MS. The role of myostatin and activin receptor IIB in the regulation of unloading-induced myofiber type-specific skeletal muscle atrophy. J Appl Physiol (1985). 2015; 119(6):633–642. [DOI] [PubMed] [Google Scholar]
- 7.Calve S, Isaac J, Gumucio JP, Mendias CL. Hyaluronic acid, HAS1, and HAS2 are significantly upregulated during muscle hypertrophy. Am J Physiol Cell Physiol. 2012;303(5):C577–C588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calve S, Odelberg SJ, Simon HG. A transitional extracellular matrix instructs cell behavior during muscle regeneration. Dev Biol. 2010;344(1):259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Contreras O, Rebolledo DL, Oyarzun JE, Olguin HC, Brandan E. Connective tissue cells expressing fibro/adipogenic progenitor markers increase under chronic damage: relevance in fibroblast-myofibroblast differentiation and skeletal muscle fibrosis. Cell Tissue Res. 2016; 364(3):647–660. [DOI] [PubMed] [Google Scholar]
- 10.Delfino GB, Peviani SM, Durigan JL, et al. Quadriceps muscle atrophy after anterior cruciate ligament transection involves increased mRNA levels of atrogin-1, muscle ring finger 1, and myostatin. Am J Phys Med Rehabil. 2013;92(5):411–419. [DOI] [PubMed] [Google Scholar]
- 11.Dong J, Dong Y, Chen Z, Mitch WE, Zhang L. The pathway to muscle fibrosis depends on myostatin stimulating the differentiation of fibro/adipogenic progenitor cells in chronic kidney disease. Kidney Int. 2017;91(1):119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong Y, Lakhia R, Thomas SS, et al. Interactions between p-Akt and Smad3 in injured muscles initiate myogenesis or fibrogenesis. Am J Physiol Endocrinol Metab. 2013;305(3):e367–e375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dulauroy S, Di Carlo SE, Langa F, Eberl G, Peduto L. Lineage tracing and genetic ablation of ADAM12(+) perivascular cells identify a major source of profibrotic cells during acute tissue injury. Nat Med. 2012; 18(8):1262–1270. [DOI] [PubMed] [Google Scholar]
- 14.Eisenberg E, Levanon EY. Human housekeeping genes, revisited. Trends Genet. 2013;29(10):569–574. [DOI] [PubMed] [Google Scholar]
- 15.Fry CS, Johnson DL, Ireland ML, Noehren B. ACL injury reduces satellite cell abundance and promotes fibrogenic cell expansion within skeletal muscle. J Orthop Res. 2017;35(9):1876–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fry CS, Lee JD, Jackson JR, et al. Regulation of the muscle fiber microenvironment by activated satellite cells during hypertrophy. FASEB J. 2014;28(4):1654–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fulzele S, Arounleut P, Cain M, et al. Role of myostatin (GDF-8) signaling in the human anterior cruciate ligament. J Orthop Res. 2010; 28(8):1113–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grapar Zargi T, Drobnic M, Vauhnik R, Koder J, Kacin A. Factors predicting quadriceps femoris muscle atrophy during the first 12 weeks following anterior cruciate ligament reconstruction. Knee. 2017;24(2): 319–328. [DOI] [PubMed] [Google Scholar]
- 19.Hiemstra LA, Webber S, MacDonald PB, Kriellaars DJ. Knee strength deficits after hamstring tendon and patellar tendon anterior cruciate ligament reconstruction. Med Sci Sports Exerc. 2000;32(8):1472–1479. [DOI] [PubMed] [Google Scholar]
- 20.Isono M, Chen S, Hong SW, Iglesias-de la Cruz MC, Ziyadeh FN. Smad pathway is activated in the diabetic mouse kidney and Smad3 mediates TGF-beta-induced fibronectin in mesangial cells. Biochem Biophys Res Commun. 2002;296(5):1356–1365. [DOI] [PubMed] [Google Scholar]
- 21.Jansson KA, Linko E, Sandelin J, Harilainen A. A prospective randomized study of patellar versus hamstring tendon autografts for anterior cruciate ligament reconstruction. Am J Sports Med. 2003;31(1):12–18. [DOI] [PubMed] [Google Scholar]
- 22.Jarvinen TA, Kannus P, Jarvinen TL, Jozsa L, Kalimo H, Jarvinen M. Tenascin-C in the pathobiology and healing process of musculoskeletal tissue injury. Scand J Med Sci Sports. 2000;10(6):376–382. [DOI] [PubMed] [Google Scholar]
- 23.Kline PW, Morgan KD, Johnson DL, Ireland ML, Noehren B. Impaired quadriceps rate of torque development and knee mechanics after anterior cruciate ligament reconstruction with patellar tendon autograft. Am J Sports Med. 2015;43(10):2553–2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kollias HD, McDermott JC. Transforming growth factor-beta and myostatin signaling in skeletal muscle. J Appl Physiol (1985). 2008;104(3):579–587. [DOI] [PubMed] [Google Scholar]
- 25.Kosmac K, Peck BD, Walton RG, et al. Immunohistochemical identification of human skeletal muscle macrophages. Bio Protoc. 2018; 8(12):e2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krishnan C, Williams GN. Factors explaining chronic knee extensor strength deficits after ACL reconstruction. 2011;29(5):633–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuenze CM, Blemker SS, Hart JM. Quadriceps function relates to muscle size following ACL reconstruction. J Orthop Res. 2016; 34(9):1656–1662. [DOI] [PubMed] [Google Scholar]
- 28.Li ZB, Kollias HD, Wagner KR. Myostatin directly regulates skeletal muscle fibrosis. J Biol Chem. 2008;283(28):19371–19378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lindstrom M, Strandberg S, Wredmark T, Fellander-Tsai L, Henriksson M. Functional and muscle morphometric effects of ACL reconstruction: a prospective CT study with 1 year follow-up. Scand J Med Sci Sports. 2013;23(4):431–442. [DOI] [PubMed] [Google Scholar]
- 30.Mendias CL, Lynch EB, Davis ME, et al. Changes in circulating biomarkers of muscle atrophy, inflammation, and cartilage turnover in patients undergoing anterior cruciate ligament reconstruction and rehabilitation. Am J Sports Med. 2013;41(8):1819–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Montesano R, Orci L. Transforming growth factor-beta stimulates collagen-matrix contraction by fibroblasts–implications for wound-healing. Proc Natl Acad Sci U S A. 1988;85(13):4894–4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murphy MM, Lawson JA, Mathew SJ, Hutcheson DA, Kardon G. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development. 2011;138(17):3625–3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noehren B, Andersen A, Hardy P, et al. Cellular and morphological alterations in the vastus lateralis muscle as the result of ACL injury and reconstruction. J Bone Joint Surg Am. 2016;98(18):1541–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pincu Y, Linden MA, Zou K, Baynard T, Boppart MD. The effects of high fat diet and moderate exercise on TGFbeta1 and collagen deposition in mouse skeletal muscle. Cytokine. 2015;73(1):23–29. [DOI] [PubMed] [Google Scholar]
- 35.Shanely RA, Zwetsloot KA, Triplett NT, Meaney MP, Farris GE, Nieman DC. Human skeletal muscle biopsy procedures using the modified Bergstrom technique. J Vis Exp. 2014;91:51812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shelbourne KD, Wilckens JH, Mollabashy A, DeCarlo M. Arthrofibrosis in acute anterior cruciate ligament reconstruction: the effect of timing of reconstruction and rehabilitation. Am J Sports Med. 1991;19(4):332–336. [DOI] [PubMed] [Google Scholar]
- 37.Shenoy PS, Bose B, Sharma M, McFarlane C, Kambadur R. Lack of myostatin reduces MyoD induced myogenic potential of primary muscle fibroblasts. J Cell Biochem. 2014;115(11):1908–1917. [DOI] [PubMed] [Google Scholar]
- 38.Thomas AC, Villwock M, Wojtys EM, Palmieri-Smith RM. Lower extremity muscle strength after anterior cruciate ligament injury and reconstruction. J Athl Train. 2013;48(5):610–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tourville TW, Jarrell KM, Naud S, Slauterbeck JR, Johnson RJ, Beynnon BD. Relationship between isokinetic strength and tibiofemoral joint space width changes after anterior cruciate ligament reconstruction. Am J Sports Med. 2014;42(2):302–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilde JM, Gumucio JP, Grekin JA, et al. Inhibition of p38 mitogen-activated protein kinase signaling reduces fibrosis and lipid accumulation after rotator cuff repair. J Shoulder Elbow Surg. 2016;25(9): 1501–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wurtzel CN, Gumucio JP, Grekin JA, et al. Pharmacological inhibition of myostatin protects against skeletal muscle atrophy and weakness after anterior cruciate ligament tear. J Orthop Res. 2017;35(11):2499–2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yarar-Fisher C, Bickel CS, Kelly NA, et al. Heightened TWEAK-NF-kappaB signaling and inflammation-associated fibrosis in paralyzed muscles of men with chronic spinal cord injury. Am J Physiol Endocrinol Metab. 2016;310(9):e754–e761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang L, Pan J, Dong Y, Tweardy DJ, Garibotto G, Mitch WE. Stat3 activation links a C/EBPdelta to myostatin pathway to stimulate loss of muscle mass. Cell Metab. 2013;18(3):368–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zou Y, Zhang RZ, Sabatelli P, Chu ML, Bonnemann CG. Muscle interstitial fibroblasts are the main source of collagen VI synthesis in skeletal muscle: implications for congenital muscular dystrophy types Ullrich and Bethlem. J Neuropathol Exp Neurol. 2008;67(2): 144–154. [DOI] [PubMed] [Google Scholar]