Abstract
Background
Improved efforts in screening and treating chronic hepatitis C virus (HCV) infection are expected to reduce its burden among adults on the liver transplantation (LT) waitlist (WL). We aim to evaluate birth cohort–specific liver disease etiology trends in US adults listed for and receiving LT.
Methods
We evaluated 2005–2016 United Network for Organ Sharing LT registry data to evaluate birth cohort–specific trends in LT WL registrants and recipients in the US. Annual trends in etiology of liver disease at listing were compared between the 1945–1965 birth cohort and the non–1945–1965 birth cohort, were stratified by presence of hepatocellular carcinoma (HCC vs. non-HCC), and were focused on the four leading indications for LT in the US, nonalcoholic steatohepatitis (NASH), HCV infection, alcoholic liver disease (ALD), and those with combined alcoholic cirrhosis with HCV (HCV/ALD).
Results
From 2005 to 2016, although HCV infection was a leading indication for LT WL registration among the 1945–1965 birth cohort patients until 2015, NASH overtook HCV infection as the leading indication in 2016. When stratified by HCC status, both ALD and NASH surpassed HCV infection as the leading indication among 1945–1965 birth cohort WL registrants without HCC, whereas HCV infection remained the leading indication among patients with HCC. When evaluating trends in patients who received LT, HCV infection remained the leading indication among the 1945–1965 birth cohort patients.
Conclusion
In 2016, NASH surpassed HCV infection as the leading indication for WL registration among the 1945–1965 birth cohort patients. Improved HCV screening, increased availability of effective HCV infection treatment, and rising prevalence of nonalcoholic fatty liver disease may explain changes in LT indication among this group.
Keywords: HCV, baby boomer, liver transplantation, HCC, direct-acting antiviral therapy
Abbreviations: ALD, alcoholic liver disease; DAA, direct acting antivirals; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; LT, liver transplantation; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; WL, waitlist
Hepatitis C virus (HCV) infection is the leading cause of cirrhosis and hepatocellular carcinoma (HCC) in the US. HCV infection–related liver disease is also associated with significant morbidity and mortality, accounting for more than 15,000 deaths annually and contributing to nearly 40% of all liver transplantation (LT) candidates.1, 2 Although efforts aimed at screening, linkage to care, and implementing effective antiviral therapies have significantly improved HCV infection–related outcomes, HCV infection burden in the US remains high, particularly among those in the 1945–1965 birth cohort. Recent studies report that approximately 70% of all patients with HCV infection in the US were born between 1945 and 1965.3 Contrary to the elevated incidence of HCV infection in the 1945–1965 birth cohort, incidence of HCV infection in the US population is decreasing overall, yet the rate of HCV infection–related deaths has risen owing to the propensity of the virus to cause chronic disease, including cirrhosis and HCC.
With the rising prevalence of obesity, coupled with the advent of direct acting antiviral (DAA) therapy, there is expected to be a shift away from HCV infection as the leading indication for waitlist (WL) registrants and LT recipients. A recent study using multiple data sets demonstrated dramatic trends in the burden of liver disease, particularly a decrease in WL registrants with HCV infection and LT recipients and an increase in WL registrants and LT recipients with nonalcoholic steatohepatitis (NASH) and alcoholic liver disease (ALD).4 In addition, a recent study using the United Network for Organ Sharing (UNOS) database found that in 2016, ALD became the leading indication for WL registration, surpassing HCV infection. Similarly, LT trends in 2016 demonstrated that ALD and NASH surpassed HCV infection as the first and second leading indicators for LT, respectively. Notably, in 2016, there was an 18% decline in HCV infection–related LT.5
Although HCV infection is currently the leading indication for HCC-related LT in the US, NASH-related HCC is also rising rapidly. One study evaluated patients from 2002 to 2012 who underwent LT and found that NASH was the second leading etiology of HCC-related LT, with a 4-fold increase since 2002.6 Although the incidence of HCC among NASH is significantly lower than HCV infection,7 with the advent of DAAs, HCV infection–related HCC is expected to decline, and NASH-related HCC is expected to become the leading indication of LT. In fact, in the next 10–20 years, NASH is predicted to become the leading indication of overall liver transplantation.8 Finally, in a study using the UNOS Organ Procurement and Transplantation Network database, researchers demonstrated a 170% increase in WL registrants for NASH, compared with a 45% increase for ALD and 14% increase for HCV infection (from 2004 to 2013).9
Application of DAA-based therapy is associated with decline in HCV infection–related WL and LT; however, there is little evidence currently available to assess these trends in the 1945–1965 birth cohort—a population with the highest predominance of HCV infection. The advances in the treatment and management from highly effective DAA therapy are rapidly changing the epidemiology of liver disease; thus, we aim to provide an updated birth cohort–specific analysis of WL and LT trends among US adults with end-stage liver disease.
Methods
Our retrospective cohort study included US adults (age ≥ 18) both with and without HCC listed for LT from 2005 to 2016 using the UNOS/OPTN LT registry. Etiology of liver disease was determined using disease diagnosis coding as provided by UNOS, and our analyses focused on the four leading indications for LT, including NASH, HCV infection, alcoholic cirrhosis (ALD), and combined alcohol cirrhosis with HCV (ALD/HCV). Trends in the etiology of liver disease at time of LT WL registration and at time of LT were stratified by the birth cohort (1945–1965 birth cohort and non–1945–1965 birth cohort) and presence of HCC.
Categorical variables were presented as frequencies (n) and proportions (%) and compared between groups using chi-square testing. Continuous variables were presented as means ± standard deviations and compared between groups using Student's t-test or analysis of variance testing. Statistical significance was met with two-tailed P-value < 0.05. All statistical analyses were performed using Stata statistical package, version 14 (StataCorp, College Station, TX). This study was reviewed and determined to qualify exempt status from the Alameda Health System Institutional Review Board.
Results
From 2005 to 2016, there were 72,740 patients in the 1945–1965 birth cohort listed for LT, compared with 15,802 patients listed for LT in the non–1945–1965 birth cohort. Among the 1945–1965 birth cohort patients, 16,369 (22.5%) were listed for ALD, 32,393 (44.5%) were listed for HCV infection, and 15,138 (20.8%) were listed for NASH. Comparatively, in the non–1945–1965 birth cohort, 6016 (38.1%) patients were listed for ALD, 4209 (26.6%) were listed for HCV infection, and 4636 (29.3%) were listed for NASH (Table 1).
Table 1.
Liver Disease Etiology Distribution of Patients Undergoing Liver Transplant Waitlist Registration and Receipt of Liver Transplantation by Birth Cohort, 2005–2016.
| Variables | 1945–1965 birth cohort | Non–1945–1965 birth cohort | ||
|---|---|---|---|---|
| Waitlist | Liver transplant | Waitlist | Liver transplant | |
| Overall total | 72,740 | 36,417 | 15,802 | 7637 |
| ALD | 16,369 | 7746 | 6016 | 2989 |
| HCV | 32,393 | 17,503 | 4209 | 2213 |
| NASH | 15,138 | 6569 | 4636 | 1979 |
| HCV/ALD | 8840 | 4599 | 941 | 456 |
| HCC total | 16,784 | 9841 | 2697 | 1477 |
| ALD | 1557 | 874 | 445 | 274 |
| HCV | 10,335 | 6563 | 1241 | 732 |
| NASH | 3390 | 1480 | 893 | 405 |
| HCV/ALD | 1502 | 924 | 118 | 66 |
| Non-HCC total | 55,956 | 26,576 | 13,195 | 6160 |
| ALD | 14,812 | 6872 | 5571 | 2715 |
| HCV | 22,058 | 10,940 | 3058 | 1481 |
| NASH | 11,748 | 5089 | 3743 | 1574 |
| HCV/ALD | 7338 | 3675 | 823 | 390 |
HCV, hepatitis C virus; ALD, alcoholic liver disease; NASH, nonalcoholic steatohepatitis; HCC, hepatocellular carcinoma.
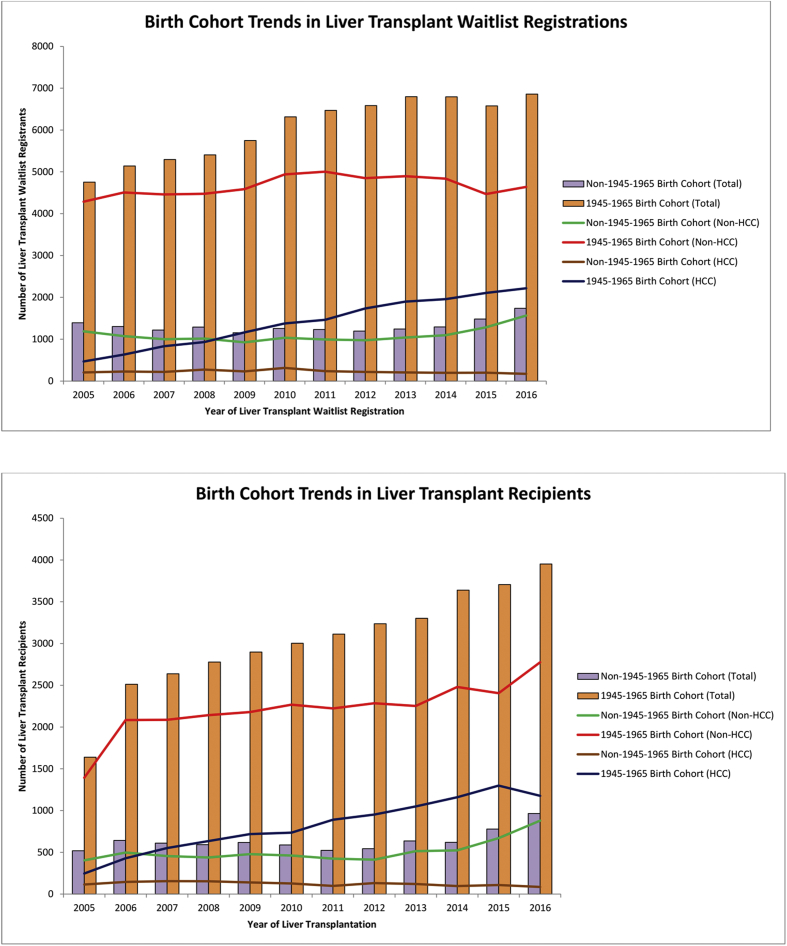
From 2005 to 2016, the number of patients listed for LT among those in the 1945–1965 birth cohort increased by 44.2% (4755 to 6859; Figure 1a). Among these 1945–1965 birth cohort patients, although the majority of patients were non-HCC (8.2% increase from 2005 to 2016 [4287–4641]), those with HCC demonstrated the greatest proportional increase (373% increase from 2005 to 2016 [468–2218]). Among the non–1945–1965 birth cohort patients, the number of patients listed for LT increased by 24.8% (1393–1739), including a 31.8% increase among patients without HCC and a 15.6% increase among patients with HCC (Figure 1a).
Figure 1.
Annual trends of birth cohort–specific prevalence of patients listed for liver transplantation and receiving liver transplantation in the United States.
Annual trends of those who received LT showed similar patterns to those listed for LT. From 2005 to 2016, the number of patients who received LT increased by 140% among those in the 1945–1965 birth cohort (from 1640 to 3952) (Figure 1b). Among these 1945–1965 birth cohort patients, non-HCC LT increased by 99% (from 1394 to 2776), whereas HCC LT increased 378% (from 246 to 1176). The number of patients receiving LT among those in the non–1945–1965 birth cohort increased by 85% (from 519 to 965) from 2005 to 2016, including a 117% increase (from 408 to 879) among patients without HCC, but a 25% decrease among patients with HCC.
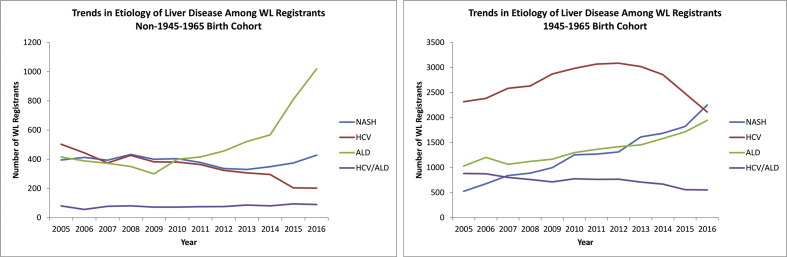
When stratified by liver disease etiology, HCV infection remained the leading indication for LT WL registration among the 1945–1965 birth cohort patients from 2005 to 2015. However, in 2016, NASH overtook HCV infection to become the leading indication for LT WL registration, accounting for 32.8% of patients in the 1945–1965 birth cohort listed for LT (Figure 2a). HCV infection as an indication for LT WL registration among the 1945–1965 birth cohort patients peaked in 2012 and has since then declined by a total of 31.5%, whereas NASH as an indication for WL registration during this same period increased by 71.4% (Figure 2a). ALD as an indication for LT WL registration among the 1945–1965 birth cohort patients increased by 88.7% (from 1030 to 1944) from 2005 to 2016 and was the third leading indication for LT WL registration among 1945–1965 birth cohort patients in 2016 (Figure 2a).
Figure 2.
Liver disease etiology–specific trends in patients listed for liver transplantation among the 1945–1965 birth cohort patients and non–1945–1965 birth cohort patients. WL, waitlist; HCV, hepatitis C virus; ALD, alcoholic liver disease; NASH, nonalcoholic steatohepatitis; HCC, hepatocellular carcinoma.
Among the non–1945–1965 birth cohort patients, HCV infection–related LT WL registration also decreased, whereas NASH-related LT WL registration remained stable (Figure 2b). However, beginning in 2011, ALD has become the leading indication for LT WL registration among the non–1945–1965 birth cohort patients. From 2005 to 2016, ALD WL registration increased by 145% (from 415 to 1019; Figure 2b). Interestingly, ALD as an indication for LT WL registration among the non–1945–1965 birth cohort patients increased about 4% per year from 2005 to 2014, followed by a steep increase of 39.5% per year from 2014 to 2016 (Figure 2b).
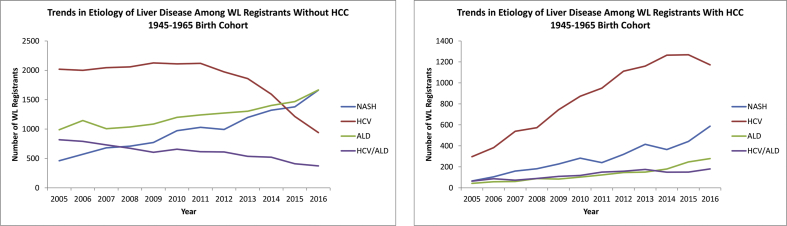
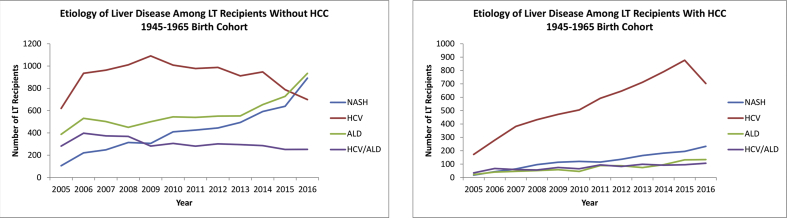
When stratified by HCC status, among the 1945–1965 birth cohort patients without HCC, those with HCV infection as an indication for LT WL registration demonstrated a steep decline beginning in 2011, whereas both ALD and NASH surpassed HCV infection as the leading indication for LT WL registration (Figure 3a). In addition, from 2005 to 2016, HCV infection decreased by 53%, ALD increased by 68%, and NASH increased by 260% (Figure 3a).
Figure 3.
Liver disease etiology–specific trends in 1945–1965 birth cohort patients listed for liver transplantation with and without hepatocellular carcinoma. WL, waitlist; HCV, hepatitis C virus; ALD, alcoholic liver disease; NASH, nonalcoholic steatohepatitis; HCC, hepatocellular carcinoma.
Among the 1945–1965 birth cohort patients with HCC, HCV infection remained the leading indication for LT WL registration throughout the study period, and those with HCV infection more than doubled the number of patients with NASH—the second leading indication among those in this cohort (Figure 3b).
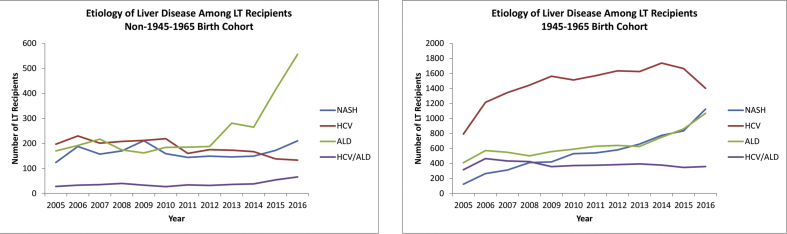
When evaluating 1945–1965 birth cohort patients who actually received a LT during the study period, while HCV infection remained the leading etiology among LT recipients, HCV infection peaked in 2014 and has since then been on the decline (Figure 4a). In 2016, HCV infection accounted for 35.4% of all 1945–1965 birth cohort LT recipients, followed by NASH at 28.4% and ALD at 27.0% (Figure 4a). Among the non–1945–1965 birth cohort patients, ALD has become and remains the leading etiology among LT recipients since 2012 (Figure 4b). In 2016, ALD accounted for 57.6% of LT recipients among the non–1945–1965 birth cohort patients.
Figure 4.
Liver disease etiology–specific trends in patients receiving liver transplantation among the 1945–1965 birth cohort patients and non–1945–1965 birth cohort patients. LT, liver transplantation; HCV, hepatitis C virus; ALD, alcoholic liver disease; NASH, nonalcoholic steatohepatitis; HCC, hepatocellular carcinoma.
When stratified by HCC status, among the 1945–1965 birth cohort patients without HCC, while HCV infection remained the leading indication for LT during the majority of the study period, NASH and ALD overtook HCV infection, and in 2016, ALD was the leading indication for LT accounting for 33.6%, followed by NASH at 32.1% and HCV infection at 25.2% (Figure 5a). HCV infection as an indication for LT among 1945–1965 birth cohort patients with HCC far surpassed all other etiologies, and despite peaking in 2015, HCV infection still accounted for 59.8% of HCC-related LT among those in this group in 2016 (Figure 5b).
Figure 5.
Liver disease etiology–specific trends in 1945–1965 birth cohort patients receiving liver transplantation with and without hepatocellular carcinoma. LT, liver transplantation; HCV, hepatitis C virus; ALD, alcoholic liver disease; NASH, nonalcoholic steatohepatitis; HCC, hepatocellular carcinoma.
Discussion
Historically, chronic HCV infection has been the primary indication for liver transplant registration for the 1945–1965 birth cohort in the US. The present study indicates that the number of 1945–1965 birth cohort patients listed for LT has steadily risen since 2005, and in 2016, NASH surpassed HCV infection as the leading indication for 1945–1965 birth cohort LT registrants. These trends are especially evident in the 1945–1965 birth cohort patients listed for transplantation who do not have HCC. We have identified factors that may be responsible for this emerging pattern, including oral DAAs, implementation of one-time HCV screening, and aging of the 1945–1965 birth cohort population.
Treatment of HCV infection has rapidly evolved over the past decade, notably the emergence of oral DAA agents, which target various stages of the HCV life cycle.10, 11, 12 It is clear that more widespread availability of DAA therapy has contributed to the decline of HCV infection in the 1945–1965 birth cohort overall, as well as prevention of disease progression that has lead to declines in HCV infection–related end-stage liver disease requiring WL registration and LT after 2011.13, 14, 15, 16 In recent years, interferon-free all-oral DAA combination therapy has shown favorable safety profiles and sustained virologic response (SVR) rates of more than 95%.17 Importantly, DAAs have also shown to be effective in patients with advanced liver disease.18 In fact, DAA-based HCV therapy may improve hepatic dysfunction to the point where patients no longer require LT and are removed from the WL.19 More and more evidence support that DAAs contribute to declining HCV infection–related liver WL and LT. Moreover, we are beginning to observe these effects specifically in the 1945–1965 birth cohort, which traditionally has had the highest prevalence of HCV-infected individuals.
Another important factor that is likely responsible for the decline in HCV infection rates in the 1945–1965 birth cohort is the increasing implementation of one-time HCV screening for all persons born between 1945 and 1965. Most patients with chronic HCV infection are asymptomatic and remain undiagnosed until the disease progresses to decompensated liver disease or HCC. One-time screening offers early diagnosis and treatment, therefore preventing progression to cirrhosis. The recent availability of oral DAAs along with the implementation of one-time screening may have increased the number of patients successfully treated and substantially reduced the burden of HCV infection in the US. A final potential contribution to the decline in 1945–1965 birth cohort WL registrants with HCV infection is the advanced age and comorbidities of the population that unfortunately may have disqualified patients from LT.3
HCV infection remains the leading etiology among adults with HCC listed for and undergoing LT in the US. As those in the 1945–1965 birth cohort with HCV infection, there is an accompanying increased likelihood for HCC, especially for those who have developed cirrhosis.20, 21 Our data show a decline in HCV-associated HCC in the 1945–1965 birth cohort, which is likely attributed to highly effective DAA therapy resulting in high cure rates that delay or even prevent the progression to HCC. A recent retrospective study of 3271 US veterans (mean age, 55.8 years) using the Veterans Affairs Health Care System found that DAA-induced SVR was associated with a 71% reduction in the risk of HCC.21 These data suggest that eradication of HCV infection with DAA therapy reduces the risk of HCC. However, it is generally recognized that HCC risk in patients with HCV infection increases dramatically once they develop cirrhosis, and the need for LT in this population is independent—regardless of whether HCV infection is cured. The 1945–1965 birth cohort patients who have already developed cirrhosis from HCV infection will need continued HCC surveillance; therefore, emphasis on treatment should be placed on early diagnosis before the onset of cirrhosis.
NASH has become the leading indication of liver transplant WL registrants in the 1945–1965 birth cohort owing to concurrent advancements in HCV infection treatment and one-time HCV screening, along with the rising incidence of NASH in the 1945–1965 birth cohort. Nonalcoholic fatty liver disease (NAFLD) is recognized as the most common chronic liver disease in the US. NAFLD affects between 80 and 100 million people; among whom, nearly 25% have NASH.22 It is well established that the hepatic manifestations of metabolic syndrome (MS) are associated with increased risk of NAFLD. MS has been shown to affect approximately one-third of the US population23 and has been reported in nearly 50% of those aged 60 years or older.24 Thus, factors including increased screening, effective treatment of HCV infection, and an aging population with increasing metabolic comorbidities have contributed to the relative importance of NASH and specifically its ascent to become the leading indication for LT among the 1945–1965 birth cohort patients. Although NASH has become the leading indication for LT in the 1945–1965 birth cohort, it is also interesting to observe that ALD has risen to become the leading indication among the non–1945–1965 birth cohort patients. This observation is consistent with prior reports of ALD rising to become the leading indication overall,5 and this observation is further supported by increasing data showing the rising burden of ALD and alcoholic cirrhosis, particularly among younger cohorts in the US.25, 26
Despite these important observations, an important limitation of our study to consider is the focus on patients who have made it to LT WL registration and not on patients who have not successfully been referred to and listed for LT. Thus, potential delays in referral to LT evaluation and potential comorbidities that may have precluded LT WL registration are not accurately captured in this data set and study. As is typical of observational registry-based studies, our study is also subject to potential misclassification bias as our diagnosis of liver disease etiology relied primarily on disease diagnosis coding provided by the UNOS data registry. Furthermore, specific to patients with concurrent HCC, the timely receipt of lack of receiving appropriate HCC surveillance impacts the tumor stage at diagnosis and thus eligibility for LT WL registration and receipt of LT. However, data regarding HCC screening and surveillance practices were not available for inclusion in this study. In addition, data specifically regarding HCV infection therapies were not available, and thus, receipt of therapy before or during the LT WL period or the type of DAA therapies received could not be incorporated into our analyses. Despite these limitations, our data provide important epidemiological data regarding liver disease etiology trends particularly for the 1945–1965 birth cohort, which is not only the cohort with the major predominance of HCV infections in the US but also the cohort that represents most patients listed for and undergoing LT in the US.
In conclusion, while the number of 1945–1965 birth cohort patients listed for LT continues to increase, in 2016, NASH surpassed HCV infection as the leading indication for WL registration in this group. This phenomenon is multifactorial and reflects not only the success of HCV screening programs and increasing availability and implementation of DAAs but also the aging population with comorbid metabolic factors that increase the risk of NAFLD.
Author contributions
Farah Shirazi contributed to study concept and design, analysis and interpretation of data, and critical revision of the manuscript for important intellectual content and has approved the final draft submitted. Jennifer Wang contributed to analysis and interpretation of data and critical revision of the manuscript for important intellectual content and has approved the final draft submitted. Robert J. Wong contributed to study concept and design, acquisition of data, analysis and interpretation of data, critical revision of the manuscript for important intellectual content, statistical analysis, and study supervision and has approved the final draft submitted.
Conflicts of interest
Dr. Wong serves as a consultant or on the advisory board/speakers' bureau of Gilead Sciences and on the speakers bureau of Salix, and he reports grants from Gilead Sciences and AbbVie, outside the submitted work.
Financial support
No financial support was provided for this study. Dr. Wong is supported by an AASLD Foundation Clinical and Translational Research Award in Liver Diseases (2017–2019).
References
- 1.Davis G., Alter M., El-Serag Aging of the hepatitis C virus (HCV)- infected persons in the US: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology. 2010;138:513–521. doi: 10.1053/j.gastro.2009.09.067. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong G, Wasley A., Simard E. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705–714. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 3.Smith B., Morgan R., Beckett G. Hepatitis C virus testing of persons born during 1945-1965: recommendations from the Centers for Disease Control and Prevention. Ann Intern Med. 2012;157:817–822. doi: 10.7326/0003-4819-157-9-201211060-00529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldberg D., Ditah I., Saeian K. Changes in the prevalence of hepatitis C virus infection, nonalcoholic steatohepatitis, and alcoholic liver disease among patients with cirrhosis or liver failure on the waitlist for liver transplantation. Gastroenterology. 2017;152:1090–1099. doi: 10.1053/j.gastro.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cholankeril G., Ahmed A. Alcoholic liver disease replaces hepatitis C virus infection as the leading indication for liver transplantation in the United States. Clin Gastroenterol Hepatol. 2018;16:1356–1358. doi: 10.1016/j.cgh.2017.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong R., Cheung R., Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology. 2014;59:2188–2195. doi: 10.1002/hep.26986. [DOI] [PubMed] [Google Scholar]
- 7.Ascha M., Hanouneh I., Lopez R. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology. 2010;51:1972–1978. doi: 10.1002/hep.23527. [DOI] [PubMed] [Google Scholar]
- 8.Charlton M., Burns J., Pedersen R. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology. 2011;141:1249–1253. doi: 10.1053/j.gastro.2011.06.061. [DOI] [PubMed] [Google Scholar]
- 9.Wong R., Aguilar M., Cheung R. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148:547–555. doi: 10.1053/j.gastro.2014.11.039. [DOI] [PubMed] [Google Scholar]
- 10.Liang T., Ghany M. Current and future therapies for hepatitis C virus infection. N Engl J Med. 2013;368:1907–1917. doi: 10.1056/NEJMra1213651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chhatwal J., Wang X., Ayer T. Hepatitis C Disease Burden in the United States in the era of oral direct-acting antivirals. Hepatology. 2016;64:1442–1450. doi: 10.1002/hep.28571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poordad F., Mccone J., Bacon B. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1195–1206. doi: 10.1056/NEJMoa1010494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobson I., Gordon S., Kowdley K. POSITRON Study Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med. 2013;368:1867–1877. doi: 10.1056/NEJMoa1214854. [DOI] [PubMed] [Google Scholar]
- 14.Pungpapong S., Aqel B., Leise M. Multicenter experience using simeprevir and sofosbuvir with or without ribavirin to treat hepatitis C genotype 1 after liver transplant. Hepatology. 2015;61:1880–1886. doi: 10.1002/hep.27770. [DOI] [PubMed] [Google Scholar]
- 15.Lawitz E., Sulkowski M., Ghalib R. Simeprevir plus sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non-responders to pegylated interferon and ribavirin and treatment-naive patients: the COSMOS randomised study. Lancet. 2014;384:1756–1765. doi: 10.1016/S0140-6736(14)61036-9. [DOI] [PubMed] [Google Scholar]
- 16.Manns M., McHutchison J., Gordon S. Peginterferon alfa-2b plus riba-virin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–965. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 17.Kohli A., Shaffer A., Sherman A., Kottilil S. Treatment of hepatitis C: a systematic review. J Am Med Assoc. 2014;312:631–640. doi: 10.1001/jama.2014.7085. [DOI] [PubMed] [Google Scholar]
- 18.Charlton M., Everson G., Flamm S., SOLAR-1 Investigators Ledipasvir and sofosbuvir plus ribavirin for treatment of HCV infection in patients with advanced liver disease. Gastroenterology. 2015;149:649–659. doi: 10.1053/j.gastro.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 19.Saab S., Park S., Mizokami M. Safety and efficacy of ledipasvir/sofosbuvir for the treatment of genotype 1 hepatitis C in subjects aged 65 years or older. Hepatology. 2016;63:1112–1119. doi: 10.1002/hep.28425. [DOI] [PubMed] [Google Scholar]
- 20.Lemon S., McGivern Is hepatitis C virus carcinogenic? Gastroenterology. 2012;142:1274–1278. doi: 10.1053/j.gastro.2012.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morgan R., Baack B., Smith B. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158:329–337. doi: 10.7326/0003-4819-158-5-201303050-00005. [DOI] [PubMed] [Google Scholar]
- 22.Loomba R., Sanyal A. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013;10:686–690. doi: 10.1038/nrgastro.2013.171. [DOI] [PubMed] [Google Scholar]
- 23.Mozumdar A., Liguori G. Persistent increase of prevalence of metabolic syndrome among US adults: NHANES III to NHANES 1999-2006. Diabetes Care. 2011;34:216–219. doi: 10.2337/dc10-0879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aguilar M., Bhuket T., Torres S. Prevalence of the metabolic syndrome in the United States, 2003–2012. J Am Med Assoc. 2015;313:1973–1974. doi: 10.1001/jama.2015.4260. [DOI] [PubMed] [Google Scholar]
- 25.Tapper E.B., Parikh N.D. Mortality due to cirrhosis and liver cancer in the United States, 1999-2016: observational study. BMJ. 2018;362:k2817. doi: 10.1136/bmj.k2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong T., Dang K., Ladhani S. Prevalence of alcoholic fatty liver disease among adults in the United States, 2001–2016. J Am Med Assoc. 2019;321:1723–1725. doi: 10.1001/jama.2019.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]