Highlights
-
•
First report in literature of curative management with radiotherapy for histiocytic sarcoma.
-
•
Radiotherapy to localised base of tongue histiocytic sarcoma was well tolerated.
-
•
No evidence of relapse following 5 years follow up.
Keywords: Histiocytic sarcoma, Oropharynx, Radiotherapy
Abstract
Histiocytic sarcoma (HS) is an exceedingly rare and aggressive neoplasm of lymphoid and haematopoietic tissues and expresses histological and phenotypical characteristics of mature histiocytes. There have only been a few cases of documented HS in the head and neck region. Whilst patients with HS often have nodal or disseminated disease, patients can present with localised disease. There are currently no established treatment guidelines, and reported cases of localised disease have been managed with primary surgery and adjuvant radiotherapy and/or chemotherapy. Here we present, the case of a 49 year old man with a HS of the base of tongue treated with radical radiotherapy to a dose of 60 Gy in 30 fractions, achieving disease free survival of greater than 5 years with minimal toxicity. To our knowledge, this is the first reported case of HS treated with radical radiotherapy, and suggests that when the potential morbidity of surgery for localised disease is significant, radiotherapy may represent an alternative treatment.
1. Introduction
Histiocytic sarcoma (HS) is an extremely rare neoplasm originating from hematopoietic cells and displays morphologic and immunophenotype features of mature histiocytes [1], [2], [3], [4], [5]. Overlapping histologic appearance with various mimics make diagnosis challenging [4], [5], [6]. Most patients present with unifocal or multifocal extra-nodal disease, for example involving the skin, connective tissues, gastrointestinal tract, lung and central nervous system, although lymph node presentations are also recognised [1], [6], [7]. Co-existing malignancy, particularly haematological, are associated with HS [7]. The clinical course is commonly aggressive and there is no accepted standard treatment approach [1], [3], [8]. In a recent population based study median survival was only 6 months with 5 year disease specific survival of 42.3% for males and 33.6% for females [7]. The majority of patients have disseminated disease at presentation [1]. Patients with localised extra-nodal disease have generally been managed surgically with or without adjuvant treatment in the form of chemotherapy or radiotherapy [1], [8]. However, surgery to some anatomical sites can carry significant morbidity. Here we present a case of localised HS of the base of tongue treated successfully with radical radiotherapy. To our knowledge, this is the first reported case of HS treated with radical radiotherapy as a single modality.
2. Case report
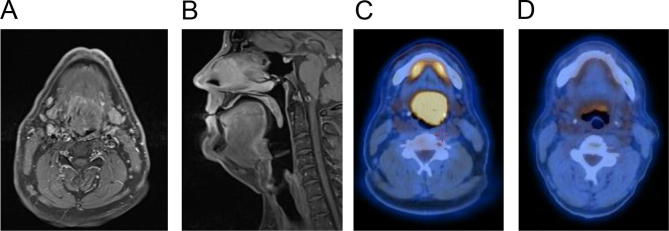
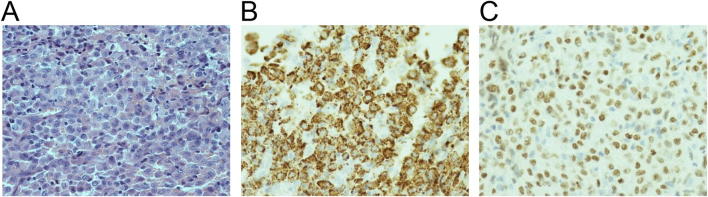
Our index case is that of a 49 year old man with no significant past medical history who presented initially in 2014 with mild odynophagia developing over a few months, followed by altered voice quality. There was no history of smoking. Direct visualisation by nasendoscopy demonstrated a base of the tongue mass. MRI revealed a large irregular mass measuring 44 mm by 40 mm extending from the tongue base to valleculae, involving the lingual surface of the epiglottis and no abnormal lymph nodes (Fig. 1A/B). 2-[Fluorine-18]-fluoro-2-deoxy-d-glucose (FDG) positron emission tomography–computed tomography (PET–CT) revealed an FDG avid base of tongue tumour with no evidence of nodal or distant disease (Fig. 1C). Histopathological analysis confirmed stratified squamous epithelium overlying fibro collagenous stroma and infiltrated by sheets of histiocytes with morphological atypia (Fig. 2A). Immunohistochemistry showed strong positivity for CD68 (Fig. 2B), PU-1 (Fig. 2C), lysozyme, CD45 and CD4. CD1a, CD123, CD34, CD117, CD20 and CD3 were negative. The features were consistent with a histiocytic neoplasm with main differentials being HS and monoblastic leukaemia. Bone marrow biopsy was normal and the diagnosis of HS was established. In view of localised disease and the morbidity of surgery (considered to require a total glossectomy), the patient was treated with radical full arc volumetric modulated arc therapy (VMAT) at a dose of 60 Gy in 30 fractions on an Elekta Agility™ linear accelerator. The clinical target volume encompassed the gross tumour volume with a 1 cm margin; bilateral nodal levels II–IVa in an elective dose level of 54 Gy in 30 fractions; a 4 mm margin was added to PTVs. He tolerated treatment well and did not require enteral feeding. A response assessment PET–CT 4 months post-treatment demonstrated a complete metabolic response (Fig. 1D). On long term follow up his only late treatment related morbidity is of Radiotherapy and Oncology Group (RTOG) grade 1 xerostomia. He remains relapse free at 5 years.
Fig. 1.
Histiocytic sarcoma of the base of tongue in a 49 year old man, treated with radical radiotherapy 60 Gy in 30 fractions. Prior to treatment a T1-weighted MRI with gadolinium enhancement demonstrated base of tongue mass in A) axial and B) sagittal views, and a FDG-PET-CT showed highly avid base of tongue (SUVmax 16) mass in C). FDG-PET-CT 4 months following completion of radiotherapy showed a complete metabolic response in D).
Fig. 2.
Histopathological findings from base of tongue biopsy demonstrating histiocytic sarcoma: A) Haematoxylin and Eosin, B) CD68, C) PU-1 staining, demonstrating strong positivity for CD68 showing monocyte lineage and PU-1 showing myeloid differentiation.
3. Discussion
HS is defined by the WHO classification as malignant proliferation of cells which resemble mature histiocytes [4], [5]. It originates from the microcytic-macrophage system cells [3]. There are only a few hundred cases of true HS documented in the literature [7], [9] and current epidemiological data estimates HS accounts for <1% of tumours in lymph nodes or soft tissue [10].
HS is more common in adults with a median age of 63 in a population based study [7], exhibiting a bimodal age distribution with a small peak around 0–29 years and larger peak at 50–69 years [4]. There is a slight male preponderance (approximately 1.5:1) [4], [7], [9]. HS has not been linked to any predisposing hereditary or environmental factors [9]. Pathogenesis has been uncertain, although in a recent study of 28 cases of HS, genomic profiling revealed possible driver mutations involving the RAS-MAPK signaling pathway (MAP2K1, KRAS, NRAS, BRAF, PTPN11, NF1, CBL) in 57% and mutations resulting in activation of the PI3K signaling pathway (PTEN, MTOR, PIK3R1, PIK3CA) in 21% [10].
Presentation is usually an asymptomatic lump or symptoms related to involved surrounding structures [4], [9]. Systemic symptoms such as asthenia, fevers/night sweats, fatigue and weakness are not uncommon [4]. Diagnosis is made on immunohistochemical characterisation, however, this can be challenging due to overlapping immunohistochemical findings noted in tumours of dendritic macrophage lineage [4], [5]. Immunohistochemistry is positive for CD68, CD163 (CD163 is a haemoglobin scavenger receptor exclusively correlated with cells of histiocytic lineage) and lysozyme [4], [5], [11].
Due to its extremely low incidence and varied presentation there is no documented standard of care in the current literature [1], [12]. Localised disease is routinely managed with primary surgery [1], with the option of adjuvant radiotherapy [2], [8]; in addition chemotherapy has occasionally been used adjuvantly [1]. Chemotherapy has been more widely reported for metastatic disease, with a variety of schedules based around those used for non-Hodgkin lymphoma or sarcomas [1]. To our knowledge this is the first case of the use of radical radiotherapy as a single treatment modality for localised HS. The decision to treat with radiotherapy was made based on the likely morbidity associated with a glossectomy and patient choice. There is an almost complete lack of evidence to guide the optimal dose and fractionation regimen for localised HS and the dose-fractionation regime we used was based on trying to achieve a likely curative dose with acceptable toxicity. Higher radiation doses are generally used for head and neck squamous cell carcinomas (e.g., 70 Gy in 35 fractions) but considerably lower doses are usually employed for haematological malignancies. In view of the myeloid progenitor cell of origin of HS [1] a dose lower than conventional head and neck squamous cell cancer doses was chosen with the aim of achieving disease control whilst minimising toxicity. Few radiotherapy doses for HS have been reported in the literature. In a case report of HS of the thyroid gland and left neck lymph nodes, a dose of 40 Gy in 20 fractions was employed in a sequential chemo-radiotherapy schedule with a complete response achieved [12]. In a reported case of brain HS an adjuvant dose of 54 Gy to tumour bed following subtotal resection was used with gradual resolution of residual disease, although the patient subsequently developed distant disease [2]. Based on these data it is not possible to define an optimal dose for treatment of HS. We electively irradiated bilateral lymph nodes levels II–IVa, although the risk of regional lymph node dissemination and consequently whether elective nodal treatment is required remains unclear. These varied treatment approaches and dose fractionation schedules reflect the limited available data and uncertainty regarding the radiosensitivity of HS in addition to the overall role of radiotherapy for localised disease.
4. Conclusion
HS is a rare disease without standardised therapeutic guidelines. Localised disease is routinely managed with surgery with consideration of adjuvant radiotherapy. However, for disease at some anatomical sites the morbidity of a surgical approach can be high. This case suggests that in these situations definitive radiotherapy can be an effective alternative treatment modality for localised disease.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Gounder M., Desai V., Kuk D., Agaram N., Arcila M., Durham B. Impact of surgery, radiation and systemic therapy on the outcomes of patients with dendritic cell and histiocytic sarcomas. Eur J Cancer. 2015;51:2413–2422. doi: 10.1016/j.ejca.2015.06.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cao M., Eshoa C., Schultz C., Black J., Zu Y., Chang C.C. Primary central nervous system histiocytic sarcoma with relapse to mediastinum: a case report and review of the literature. Arch Pathol Lab Med. 2007;131:301–305. doi: 10.5858/2007-131-301-PCNSHS. [DOI] [PubMed] [Google Scholar]
- 3.Hung Y.P., Qian X. Histiocytic sarcoma. Arch Pathol Lab Med. 2019 doi: 10.5858/arpa.2018-0349-RS. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi E., Nakamura S. Histiocytic sarcoma: an updated literature review based on the 2008 WHO classification. J Clin Exp Hematop. 2013;53:1–8. doi: 10.3960/jslrt.53.1. [DOI] [PubMed] [Google Scholar]
- 5.Swerdlow S.H., Campo E., Pileri S.A., Harris N.L., Stein H., Siebert R. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375–2390. doi: 10.1182/blood-2016-01-643569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou J., Liu Y., Wang W., Xu F.L. Histiocytic sarcoma of the neck: a case report. Mol Clin Oncol. 2018;9:54–57. doi: 10.3892/mco.2018.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kommalapati A., Tella S., Durkin M., Go R.S., Goyal G. Histiocytic sarcoma: a population-based analysis of incidence, demographic disparities, and long term outcomes. Blood. 2018;131:265–268. doi: 10.1182/blood-2017-10-812495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hornick J.L., Jaffe E.S., Fletcher C.D. Extranodal histiocytic sarcoma: clinicopathologic analysis of 14 cases of a rare epithelioid malignancy. Am J Surg Pathol. 2004;28:1133–1144. doi: 10.1097/01.pas.0000131541.95394.23. [DOI] [PubMed] [Google Scholar]
- 9.Barbato G., Tarantini A., Serra F., Cabry F., Farinetti A., Sorrentino L. A novel surgical approach with peritonectomy to extranodal multisystemic histiocytic sarcoma: a case report and literature review. Int J Surg Case Rep. 2019;59:213–216. doi: 10.1016/j.ijscr.2019.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shanmugam V., Griffin G.K., Jacobsen E.D., Fletcher C.D.M., Sholl L.M., Hornick J.L. Identification of diverse activating mutations of the RAS-MAPK pathway in histiocytic sarcoma. Mod Pathol. 2019;32:830–843. doi: 10.1038/s41379-018-0200-x. [DOI] [PubMed] [Google Scholar]
- 11.Wu W., Tanrivermis Sayit A., Vinters H.V., Pope W., Mirsadraei L., Said J. Primary central nervous system histiocytic sarcoma presenting as a postradiation sarcoma: case report and literature review. Hum Pathol. 2013;44:1177–1183. doi: 10.1016/j.humpath.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 12.De Vos F.Y., Gerding M.N., Arends J.W., Wegman J.J. Histiocytic sarcoma localised in the thyroid: a case report. Ann Hematol. 2008;87:681–682. doi: 10.1007/s00277-008-0473-0. [DOI] [PMC free article] [PubMed] [Google Scholar]