Abstract
Hepatocellular carcinoma (HCC) is one of the major causes of morbidity, mortality, and healthcare expenditure in patients with chronic liver disease in India. The Indian National Association for Study of the Liver (INASL) had published its first guidelines on diagnosis and management of HCC (The Puri Recommendations) in 2014, and these guidelines were very well received by the healthcare community involved in diagnosis and management of HCC in India and neighboring countries. However, since 2014, many new developments have taken place in the field of HCC diagnosis and management, hence INASL endeavored to update its 2014 consensus guidelines. A new Task Force on HCC was constituted that reviewed the previous guidelines as well as the recent developments in various aspects of HCC that needed to be incorporated in the new guidelines. A 2-day round table discussion was held on 5th and 6th May 2018 at Puri, Odisha, to discuss, debate, and finalize the revised consensus statements. Each statement of the guideline was graded according to the Grading of Recommendations Assessment Development and Evaluation system with minor modifications. We present here the 2019 Update of INASL Consensus on Prevention, Diagnosis, and Management of Hepatocellular Carcinoma in India: The Puri-2 Recommendations.
Keywords: liver cancer, transplant, RFA, TACE, targeted therapy
Abbreviations: AFP, alpha-fetoprotein; AIH, autoimmune hepatitis; ALT, alanine aminotransferase; DAA, direct-acting antiviral; DALY, disability-adjusted life-year; DNA, deoxyribonucleic acid; Gd-BOPTA, gadolinium benzyloxypropionictetraacetate; Gd-EOB-DTPA, gadolinium ethoxybenzyl diethylenetriamine penta-acetic acid; GRADE, Grading of Recommendations Assessment Development and Evaluation; HBeAg, hepatitis B envelope antigen; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HIV, human immunodeficiency virus; IARC, International Agency for Research on Cancer; IFN, interferon; INASL, Indian National Association for Study of the Liver; MiRNA, micro-RNA; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; PIVKA, protein induced by vitamin K absence; RNA, ribonucleic acid; SVR, sustained virological response; TACE, trans-arterial chemoembolization; TARE, transarterial radioembolization; TNF, tumor necrosis factor; WHO, World Health Organization
Primary liver cancer or hepatocellular carcinoma (HCC) is one of the major causes of mortality among patients with chronic liver disease.1 The incidence and prevalence of HCC is rising in India, mainly because of the epidemic of non-alcoholic fatty liver disease and is poised to become the leading cause of cancer in India.2,3 Although there are many consensus guidelines on HCC management from USA, Europe and Asia, most of these fail to address India specific issues on HCC.4, 5, 6, 7, 8, 9, 10, 11 The Indian National Association for Study of the Liver (INASL) had published its first guidelines on diagnosis and management of HCC (The Puri Recommendations1) in 2014, and these guidelines were very well received by the healthcare community involved in diagnosis and management of HCC in India and neighboring countries. However, since 2014 many new developments have taken place in the field of HCC diagnosis and management, hence INASL endeavored to update its 2014 consensus guidelines. A new Task Force on HCC.
For the development of these revised guidelines, the task force reviewed the previous guidelines as well as the recent developments that have happened in various aspects of HCC that needed to be incorporated in the new guidelines. A 2-day round table discussion was held on 5th and 6th May 2018 at Puri, Odisha, to discuss, debate, and finalize the revised consensus statements. Each topic was discussed considering the most relevant data available in literature and the final consensus statements were formulated according to both scientific evidence and clinical expertise of the involved physicians. Only those statements were accepted which were unanimously approved by the majority of the members of the taskforce. Each statement of the guideline was graded according to the Grading of Recommendations Assessment Development and Evaluation (GRADE) system with minor modifications.12 The strength of recommendations (strong or weak) thus reflects the quality (grade) of underlying evidence (I, II-1, II-2, II-3, and III) (Table 1).
Table 1.
Modified Grading of Recommendations, Assessment, Development and Evaluation (GRADE).
| Quality of evidence | Criteria |
| I | Randomized controlled trials |
| II-1 | Controlled trials without randomization |
| II-2 | Cohort or case-control analytical studies |
| II-3 | Multiple time series, dramatic uncontrolled experiments |
| III | Opinions of respected authorities, descriptive epidemiology |
| Strength of recommendations | Criteria |
| Strong | Factors influencing the strength of the recommendation included the quality of the evidence, presumed patient-important outcomes, and cost |
| Weak | Variability in preferences and values, or more uncertainty. Recommendation is made with less certainty, higher cost, or resource consumption |
Epidemiology of HCC
Global epidemiology of HCC
HCC is among the leading causes of cancer death globally.11 According to the Global Burden of Disease Study 2015, there were 854,000 incident cases of and 810,000 deaths due to liver cancer globally in 2015, contributing to 20,578,000 disability-adjusted life-years. Number of cases with incident liver cancer increased by 75% between 1990 and 2015; of this, 47% can be explained by changing population age structures, 35% by population growth, and −8% to changing age-specific incidence rates. The male-to-female ratio for age-standardized liver cancer mortality was 2.8. Globally, hepatitis B virus (HBV) is estimated to account for 33% of liver cancer deaths, alcohol for 30%, hepatitis C virus (HCV) for 21%, and other causes for 16%; these relative figures show substantial variation between countries.13
Epidemiology of HCC in India
Data on the epidemiology of HCC from India are sparse and of variable and uncertain quality. Data on incidence of HCC as reported by various population-based registries across the country show a marked (>30-fold) variation in crude and age-adjusted incidence rates of HCC. However, it is uncertain whether the data from these different registries can be directly compared. Estimates based on these data suggest that crude incidence rate of HCC in India in the year 2015 was 2.8 cases per 100,000 population per year (males: 3.9, females: 1.6), and the crude mortality rate was 2.7 per 100,000 population per year.2 The registry data also suggest a slight increase in the incidence of HCC over time, but whether this reflects a true increase remains uncertain. In the only observational study, incidence of HCC in patients with cirrhosis was 1.6 per 100 person-years of follow-up, with fairly wide confidence intervals (CIs) (0.55–2.64).14
| Consensus statements | Level | Grade |
|---|---|---|
|
||
|
II-2 | |
|
II-2 |
Risk factors for HCC
HBV and HCV
Cirrhosis due to any cause may be complicated by development of HCC. The risk is higher in persons with cirrhosis due to chronic viral hepatitis than that due to other causes. Also, the risk of HCC is higher when hepatitis has progressed to cirrhosis. Available data clearly indicate that in HBV-related or HCV-related chronic liver disease, the risk of HCC is higher with concomitant HIV co-infection; HBV/HCV co-infection; chronic alcohol abuse; obesity; diabetes mellitus; or aflatoxin exposure.15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26
Among non-cirrhotic persons with chronic HBV infection, HBeAg positivity is associated with a higher risk of HCC than HBeAg negativity, though HBeAg-negative persons have a higher risk than persons who do not have HBV infection.27 In Indian HBV patients, HCC is more often associated with HBV genotype D28 infection/mixed genotype infection; high HBV DNA levels (>10,000 copies/mL); persistently elevated serum ALT levels; and high level of hepatitis B surface antigen.28,29 A recent meta-analysis showed that AA genotype in TNF-α G308A and TNF-α G238A and CT genotype in TNF-α C863T might increase HBV–HCC risk. Therefore, HBV infection seemed to be a more important factor for tumorigenesis of HCC than genetic predisposition in G308A of TNF-α, and interaction between TNF-α C863T polymorphisms and HBV infection might be associated with increased HCC risk.30
In non-cirrhotic patients with chronic HCV infection, HCC is most often associated with advanced fibrosis though up to 10% of HCV-related HCC cases have only mild degrees of fibrosis.31,32 Anti-HCV positivity33 and high serum HCV RNA levels34 are factors associated with higher risk of developing HCC.
The risk of HCC occurrence or recurrence following direct-acting antiviral (DAA) for HCV therapy remains unclear. An unexpectedly high rate of HCC recurrence after treatment with DAAs was recently reported.35 The aims of a meta-analysis by Cabibbo et al. were to estimate the recurrence and survival probabilities of HCV-related early HCC following complete response (CR) after potentially curative treatment and to identify predictors of recurrence and survival. This meta-analysis showed that recurrence risk and survival are extremely variable in patients with successfully treated HCV-related HCC.36 A subsequent meta-analysis showed that there is no evidence that HCC occurrence or recurrence is different between patients receiving DAA or interferon (IFN) therapy.37 In fact, sustained virological response (SVR) post DAA reduces the incidence of HCC. Cirrhosis, low albumin, low platelet, and alpha-fetoprotein (AFP) level posttreatment are indicators of high HCC occurrence even after SVR.38 Long-term follow-up studies are required to assess surveillance strategy in patients treated with DAA.
Alcohol
Chronic alcohol consumption is an important risk factor for HCC development. However, the incidence of HCC associated with alcoholic cirrhosis is lower than the incidence associated with cirrhosis of other etiologies, including chronic HCV, chronic HBV, and hereditary hemochromatosis. It appears that the linkage between compensated alcoholic liver disease-associated cirrhosis and HCC is medium to high risk, with the risk increasing with age and with quantity and duration of alcohol consumption and is more pronounced in females. Studies evaluating HCC risk in patients with cirrhosis associated exclusively with alcohol consumption (with no evidence of ongoing viral hepatitis infection) have suggested that the 10-year cumulative incidence may range from 6.8 to 28.7%.39
Diabetes mellitus
There is increasing evidence from observational studies suggests that DM is an important risk factor for HCC. In a systematic review of 26 case–control and cohort studies, diabetes was associated significantly with HCC in 16 studies with a pooled odds ratio of 2.5. The significant association between HCC and diabetes was independent of alcohol use or viral hepatitis.40 Presence of diabetes mellitus has also been shown to increase the risk of HCC in patients with chronic HCV and HBV infection.19,41
Not only diabetes, even patients with prediabetes are at higher risk for HCC. In a meta-analysis by Xu et al. of 8 cohort studies and 1 case–control study with 1384594 individuals, it was found that patients with prediabetes showed an increased HCC risk (HR [hazards ratio] = 1.21; 95% CI, 1.13–1.30; P < 0.00001).42
Obesity
Existing literature supports obesity to be an important risk factor for HCC. In a prospectively studied population of more than 900,000 US adults (404,576 men and 495,477 women) who were free of cancer at enrollment in 1982, there were 57,145 deaths from cancer during 16 years of follow-up. Men and women with body mass index ≥35 were at 4.5 times and 1.6 times higher risk for HCC in comparison to those with normal weight, respectively.43 In addition as mentioned earlier, obesity is also an important co-factor and has been shown to increase the risk of HCC by 4 times in patients with CHC.18,41
Non-alcoholic fatty liver disease
Literature suggests that the incidence of HCC developing in patients with cirrhosis due to non-alcoholic steatohepatitis (NASH) ranges from 2.4% over 7 years to 12.8% over 3 years.44 In addition, the Western and Indian data suggest that the commonly labeled “cryptogenic” cirrhosis leading to HCC, when investigated further turn out to have histological or clinical features of non-alcoholic fatty liver disease (NAFLD) in at least half of these patients.45, 46, 47 Additionally, strong evidence now exists showing that a proportion of NASH can progress to HCC with the absence of cirrhosis.48,49 In a recent meta-analyses, Younossi et al. reported that in patients with NAFLD, the annual incidence of HCC was 0.44 per 1000 person-years, whereas for those with NASH, the annual incidence of HCC was 5.29 per 1000 person-years.50
Autoimmune hepatitis
According to a recent meta-analysis, patients with autoimmune hepatitis (AIH)-related cirrhosis are at an increased risk for HCC while AIH patients without cirrhosis at index diagnosis, particularly those identified from general populations, are at an extremely low risk of HCC.51
Genetic risk factors
Micro-RNAs (miRNAs) are small, non-coding, single-stranded RNA molecules with a typical length of 22 nucleotides. They also play an important role in physiologic and pathologic processes including cell differentiation, proliferation, apoptosis, and carcinogenesis and have been implicated in the initiation and progression of various cancers. A meta-analysis by Dong et al. supports that the miR-146a rs2910164 was associated with a decreased risk of hepatocellular carcinoma.52 Another meta-analysis by Yu et al. supported the proposition that the polymorphisms of miR-146a rs2910164, miR-196a2 rs11614913, and miR-196a2 rs11614913 may contribute to the susceptibility of HCC.53
Among other genetic risk factors, HLA-DRB1 *1 and *11 allele polymorphisms were found to be protective factors, while *12 and *14 allele polymorphisms were risk factors for HCC development.54 Another meta-analysis demonstrated that DNA repair genes (XPD gene Asp312Asn and XRCC1 gene Arg399Gln) might be candidate susceptibility loci for HCC.55 Indian data suggest that the variants in low penetrance gene such as GSTM1 and GSTT1 are associated with an increased HCC risk. Further, an influence of GSTM1/T1 null genotypes may contribute in the etiology of HCC in patients with higher cigarette and alcohol consumption.56
| Consensus statements | Level | Grade |
|---|---|---|
|
II-2 | |
|
II-2 | |
|
II-2 | |
|
II-2 | |
|
II-2 | |
|
II-2 | |
|
II-2 | |
|
II-2 | |
|
II-2 | Strong |
Prevention of HCC
Universal precautions
Universal precautions refer to the practice, in medicine, of avoiding contact with patients' bodily fluids, by means of the wearing of non-porous articles such as medical gloves, goggles, and face shields. Universal precautions are intended to prevent parenteral, mucous membrane, and non-intact skin exposures of healthcare workers to blood-borne pathogens. INASL reiterates that universal precautions to avoid transmission of blood-borne viruses in healthcare settings should be adopted.57,58
Hepatitis B vaccination
Vaccination against hepatitis B reduces the risk of HCC, and the World Health Organization recommends hepatitis B vaccination for all children worldwide. Because perinatal or early postnatal transmission is the most important source of chronic HBV infection globally, all infants (including low birth weight and premature infants) should receive their first dose of hepatitis B vaccine as soon as possible after birth, ideally within 24 h.59 Unvaccinated children up to 5 years age should also be vaccinated to reduce the risk of HCC.
Antiviral therapy in patients with HBV and HCV
In patients with HBV, now accumulating evidence indicates that antiviral therapy with the current nucleotide analogs (NAs) entecavir or tenofovir, prescribed to control hepatic inflammation and prevent or reverse liver fibrosis can also reduce the risk of HCC, especially in Asian patients. The reduction of HCC risk may be by ∼30% in cirrhotic patients and by ∼80% in non-cirrhotic patients.60 However, the risk is not eliminated even in the vast majority of patients who remain in virological remission under entecavir/tenofovir. Therefore, patients at increased baseline HCC risk should continue to undergo HCC surveillance even if they have achieved complete long-term inhibition of viral replication and improvements in liver histology.61 Regarding patients with HCV, in a recent meta-analysis of 59 studies by Bang et al., it was shown that antiviral treatment was associated with reduced development of HCC, and this effect was intensified when SVR was achieved.62 Thus, effective antiviral therapy should be started in all eligible patients with chronic hepatitis B or C infection to prevent HCC.
Prevention of HCC due to metabolic conditions
Epidemiological evidence links obesity, type 2 diabetes, and NAFLD to the development of HCC, which is rising at an alarming rate in a number of countries including India. While there are no prospective randomized clinical trial data supporting the use of specific therapies to reduce HCC risk in individuals with the metabolic syndrome and NAFLD, evidence from murine models and cohort studies has accumulated in recent years. The simplest preventive measures include lifestyle modification, including prevention of obesity and control of metabolic diseases, such as diabetes and NAFLD.63,64
HCC prevention by reducing alcohol consumption
Many case–control studies have reported that chronic ethanol consumption is associated with an approximately 2-fold increased odds ratio for HCC.65 In 1988, the International Agency for Research on Cancer identified ethanol as a cancer-causing agent and listed a causal relationship between ethanol consumption and cancers of the digestive tract, liver, and breast.66,67 Further, according to the recent Liver Cancer Pooling Project, which is a consortium of 14 US-based prospective cohort studies that includes data from 1,518,741 individuals, compared with non-drinkers, heavy alcohol consumption (≥7 drinks/day) was associated with an 87% increased HCC risk while light-to-moderate alcohol consumption of <3 drinks/day appeared to be inversely associated with HCC risk.68
A meta-analysis suggested that the risk of HCC falls by 6–7% a year after cessation of alcohol, but there remains a large uncertainty around this estimate both statistically and in its interpretation. It is estimated that a time period of 23 years is required after drinking cessation, with a correspondingly large 95% CI of 14–70 years, for the risk of HCC to be equal to that of never drinkers.69,70 In view of these evidences, it is advisable that steps should be taken to reduce alcohol consumption.
Because patients with alcoholic cirrhosis are at an increased risk of developing HCC, all patients with alcoholic cirrhosis should be counseled and encouraged to achieve abstinence to reduce the risk of development of HCC.71 Further, patients with alcohol-related cirrhosis should be screened for cofactors, including obesity, diabetes, cigarette smoking, and hepatitis B and C virus infections, and if detected, they should be managed appropriately.71
Role of statins for HCC prevention
Recently, the use of statins has been reported to reduce the risk of HCC, especially in patients with HBV or HCV.72,73 The suppressive effects of statins on carcinogenesis could involve their pleiotropic effects through both HMG-CoA-dependent and HMG-CoA-independent pathways, such as effects on inflammation, immunomodulation, angiogenesis, apoptosis, and proliferation. Statins also reduce liver fibrosis progression and cirrhosis, as well as portal hypertension in HBV and HCV patients, thus indirectly reducing the risk of HCC. In a recent meta-analysis, 6 cohort studies involving 11,8961 participants with 9530 incident cases of HCC, statistically significant association was observed between increasing statins intake and HCC risk reduction (OR = 0.46, 95%CI: 0.24–0.68, P < 0.001).74 Prospective randomized clinical trials are currently underway to determine the role of statins in HCC chemoprevention. Pending the results of these trials, statins cannot be recommended for HCC chemoprevention outside of clinical trials.
Role of coffee and green tea in HCC prevention
An inverse association has been reported between coffee drinking and the risk of HCC. In a recent meta-analysis of 12 prospective cohort studies that investigated the association between coffee consumption and the risk of HCC, the summary relative risks (RRs) for HCC were 0.66 (95% CI: 0.55–0.78) for regular, 0.78 (95% CI: 0.66–0.91) for low, and 0.50 (95% CI: 0.43–0.58) for high coffee consumption, respectively. The summary RR for an increment of 1 cup per day was 0.85 (95% CI: 0.81–0.90).75 Similarly, according to another meta-analysis involving 18 cohort studies and 8 case–control studies (including >2.2 million participants), an extra 2 cups per day of coffee was associated with a 35% reduction in the risk of HCC.76 Another meta-analysis of 10 studies showed that the increasing green tea intake might have a preventive effect against HCC.77 However, these associations are weak and cannot be the basis for any recommendation for the general population.
Role of metformin in HCC prevention
Metformin has recently attracted great attention for antitumor effect in a wide range of malignancies including liver cancer, through both insulin-dependent and insulin-independent mechanisms.78 However, the evidence for a cancer preventive effect for metformin has not been consistently demonstrated. To better understand the effect of metformin use on HCC risk in diabetic patients, a meta-analysis of 19 studies involving 550,882 diabetic subjects was conducted, which suggested that metformin use reduced the ratio of liver cancer by 48% (OR = 0.52; 95% CI, 0.40–0.68) compared with non-users. After adjusting for hepatitis B/C virus infection, cirrhosis, obesity, behavioral factors, and time-related bias, the association was stable, pooled OR ranged from 0.42 to 0.75. However, more randomized trials are still needed to verify the results.79
| Consensus statements | Level | Grade |
|---|---|---|
|
III | Strong |
|
II-2 | Strong |
|
I | Strong |
|
II-2 | Strong |
|
I | Strong |
|
I | Strong |
|
I | Strong |
Surveillance for HCC
HCC surveillance can detect early tumors that are potentially amenable to treatment; hence, all patients at higher risk of developing HCC and who are eligible for HCC therapy are candidates for regular HCC surveillance. Even though the evidence is not of the highest quality, several prospective and retrospective studies, multiple modeling studies, and a randomized-controlled trial, all have concluded that surveillance is beneficial.80 Level of awareness of physicians managing patients of chronic liver disease is a major factor in surveillance of HCC; there is a need for greater healthcare provider awareness to improve HCC surveillance.81
Following patients should be subjected to surveillance for HCC: Child's A and B cirrhotic patients of any etiology; Child's C cirrhotic patients of any etiology who are listed for liver transplantation (LT); patients with chronic hepatitis B who have increased risk for HCC according to risk scores such as CU-HCC or PAGE-B; and chronic HCV with advanced fibrosis.
Tzartzeva et al. in a meta-analysis of 32 studies, comprising 13,367 patients, compared the performance of surveillance imaging, with or without AFP. They found that ultrasound alone has a low sensitivity to detect early stage HCC in patients with cirrhosis, and addition of AFP to ultrasound significantly increases sensitivity of early HCC detection in clinical practice.82 The pooled sensitivities of ultrasound with and without AFP for early-stage HCC were 63% (95% CI, 48%–75%) and 45% (95% CI, 30%–62%), respectively (P = 0.002). The benefit of AFP as an adjunct test to ultrasound was consistent across subgroups, including prospective studies, studies conducted in the United States, and studies conducted after the year 2000.82 Another smaller meta-analysis of 11 studies by Caviglia et al. suggested that the use of protein induced by vitamin K antagonist-II (PIVKA-II) + AFP in addition to US examination may improve the effectiveness of surveillance among patients at risk for HCC development.83 However, larger studies will be needed before PIVKA-II can be recommended for surveillance.
These data suggest that, among currently available tests, ultrasound in combination with AFP may be the most effective strategy for HCC surveillance in patients with cirrhosis. Hence, it is recommended that 6-monthly ultrasound abdomen by experienced personnel plus AFP level is the recommended surveillance test. To increase the effectiveness of surveillance, outreach strategies could be used to double the percentage of patients with cirrhosis who underwent ultrasound screening for HCC.84
| Consensus statements | Level | Grade |
|---|---|---|
|
I | Strong |
|
III | Strong |
|
I | Strong |
|
I | Strong |
Diagnosis of HCC
If a nodule of size >1 cm is detected in the cirrhotic liver during surveillance or random ultrasonogram, a dynamic (tri-phasic or four-phasic) computed tomography (CT) or magnetic resonance imaging (MRI) scan should be done, preferably at centers equipped with appropriate equipment and expertise. These imaging modalities are recommended as first-line diagnostic tools for HCC when the nodule is detected in cirrhotic liver. The typical vascular pattern depicted by HCC on dynamic CT or MRI consists of arterial enhancement, stronger than the surrounding liver (wash-in), and hypodensity or hyposignal intensity compared with the surrounding liver (wash-out) in the venous phase. This happens because in neoplastic lesions, active neoangiogenesis results in stronger arterial vascularization, compared with the surrounding liver parenchyma. Simultaneously, a progressive decrease of the portal supply takes place, which leads to a decrease of portal blood within the lesion compared with the surrounding liver parenchyma in venous phases. This typical pattern has a sensitivity of around 60% with a 96–100% specificity for HCC,74 and in presence of this pattern, no tissue diagnosis is required. However, to achieve standardization in reporting of dynamic CT and MRI, LI-RADS lexicon should be followed.85
However, these classical features of HCC (hypervascularity of the nodule in arterial phase and washout in porto-venous phase) can only be applied to cirrhotic patients having nodule(s) ≥1 cm, because of the high pretest probability. If a nodule of size <1 cm is detected in the liver, a 3-monthly follow-up is recommended for 1 year using ultrasound for any enlargement in size. If there is any change in pattern or growth, dynamic CT/dynamic MRI should be done. Nodules <1 cm may also be evaluated for HCC with gadoxetic acid-enhanced MRI (Gd-EOB-DTPA or Gd-BOPTA). In a meta-analysis, it was found that gadoxetic acid-enhanced MRI has superior diagnostic ability to dynamic CT in patients with small lesions. In patients with any-sized lesions, there is no evidence that gadoxetic acid-enhanced MRI is superior to either dynamic CT or dynamic MRI.86
Nodular lesions that show an imaging pattern atypical for HCC on one of the dynamic scans (CT or MRI) should undergo the other dynamic scan (CT or MRI). MR scans should preferably be gadoxetic acid-enhanced (Gd-EOB-DTPA or Gd-BOPTA). If on second scan, the features are typical of HCC in the setting of chronic liver disease, then biopsy is not necessary for confirmation of diagnosis. If on both the scans, the features are atypical of HCC, then histological confirmation for diagnosis of HCC is required.
In patients with renal failure (eGFR < 30 ml/min), contrast enhanced ultrasound with SonoVue is recommended for a lesion detected on ultrasonography (US). According to a meta-analysis by Huang et al., contrast enhanced ultra sonography (CEUS) shows a diagnostic ability comparable to that of contrast enhanced co-axial tomography (CECT) in detecting small hepatocellular carcinoma.87 In another meta-analysis of 16 studies, CEUS was found to be useful for diagnosis of small HCC with relatively high sensitivity and specificity.88
| Consensus statements | Level | Grade |
|---|---|---|
|
I | Strong |
|
I | Strong |
|
I | Strong |
|
I | Strong |
|
I | Strong |
|
I | Strong |
|
II-3 | Weak |
|
II-3 | Weak |
Role of histology in HCC diagnosis
The imaging modalities (dynamic CT and MRI) are recommended as first-line diagnostic tools for HCC in cirrhotic liver. Tissue diagnosis of HCC is not required in majority of cases. However, in certain exceptional situations, tissue diagnosis may be required. Tissue diagnosis is indicated when imaging and other findings are equivocal or not typical or suspected HCC, which are smaller than 2 cm. Tissue diagnosis may also be indicated in larger lesions in non-cirrhotic livers.
The histological diagnosis of HCC is based on the resemblance of tumor cells to hepatocytes. The diagnosis is straightforward when HCC is well differentiated, and its neoplastic hepatocytes produce bile and are virtually identical to normal ones, showing also the same immunohistochemical profile. It is challenging when lesions are less differentiated, and the neoplastic cells lose their hepatocyte trait or develop changes seen in other tumors (e.g. clear cell change).89 Histological distinction of some small HCCs from benign/dysplastic nodules may be difficult. Immunohistochemistry would be necessary for confirming the diagnosis and prognostic subclassification in most cases. Wherever feasible, CK19 immunostaining may be done to exclude a combined HCC-cholangicarcinoma since this has a poorer prognosis when compared with pure HCC. Several histopathological parameters that had been shown to be significant predictors of prognosis are tumor number, size, cell differentiation and grade, presence of satellite nodules, and pTNM stage.90 These should be the minimum requirements for reporting of HCC. For grading of HCC, either standard 4 scale Edmonson Steiner System91 (Grade I-IV) or 4 Grade system (Well Differentiated/Moderately Differentiated/Poorly Differentiated/Undifferentiated) should be followed.92 As far as possible, histological variants must be indicated, e.g. trabecular, macrotrabecular, acinar, pseudoglandular, solid, clear, fibrolamellar HCC, steatohepatitic HCC, scirrhous HCC, and mixed HCC-cholangio carcinoma (CCA). Presence of microemboli must be indicated. All resected specimens should be submitted for histopathologic evaluation, and in these cases, the state of adjacent/rest of liver must be highlighted including cirrhosis, chronic hepatitis, NAFLD, metabolic liver disease, etc. Infiltrated or clear margins of a resected specimen must also be indicated.
Fine Needdle Aspiration Cytology (FNACs) may not yield sufficient material for immunohistochemistry unless cell-blocks are prepared. Hence, needle core biopsies, at least 2 are recommended. Needle-tract seeding refers to implantation of tumor cells by contamination, when instruments like biopsy needles are used to examine, excise, or ablate a tumor. Needle-tract seeding of HCC may deter liver biopsy. The incidence of needle-tract seeding varies in the literature between 0% and 7.69%, with a median of 2.7%.93,94 Apparently, the larger the needle diameter and the number of passes, or the lower the degree of tumor differentiation, the higher the risk of seeding.
| Consensus statements | Level | Grade |
|---|---|---|
|
II-2 | Strong |
|
II-2 | Strong |
|
II-2 | Strong |
|
II-2 | Strong |
|
II-3 | Weak |
|
II-1 | Strong |
|
II-2 | Strong |
Role of positron emission tomography scan in management of HCC
Positron emission tomography (PET) using 18F-fluorodeoxyglucose (18F-FDG) is widely used for assessing a variety of malignancies and, however, has poor sensitivity in the evaluation of HCC.95 Therefore, there is no significant role of 18F-FDG PET/CT in diagnosis of HCC except for detection of distant metastases. A recent meta-analysis suggested that 18F-FDG PET with or without CT can diagnose extrahepatic metastases or local residual/recurrent HCC with high specificity (95%) but low sensitivity (64%).9618F-FDG PET/CT may also play an important role in prognostication of HCC. Higher the standardised uptake value (SUV) (uptake) poor is prognosis.97 Nowadays, 18F-FDG PET is also advised in the pretransplant evaluation of HCC patients because of its ability to predict HCC recurrence after LT.98
| Consensus statements | Level | Grade |
|---|---|---|
|
II-2 | Strong |
|
II-3 | Weak |
Diagnostic and prognostic biomarkers
Tumor markers
AFP is the only tumor marker that has undergone extensive evaluation. Estimation of serum AFP remains a useful test for management of patients with HCC. The test, when used with the conventional cut-off point of 500 ng/mL, has a sensitivity of about 50% and a specificity of more than 90% in detecting the presence of HCC in a patient with coexisting liver disease.99 However, AFP alone is not recommended either in surveillance or diagnosis of HCC.
Serum AFP estimation may also be useful in monitoring response to therapy, particularly as more effective loco-regional and systemic treatments are becoming available. Indeed, there is preliminary evidence that changes in serum AFP may be a more accurate and sensitive way of determining the degree of response to treatment than conventional imaging procedures that rely on physical determination of tumor size.99 It may, perhaps, be time to add changes in serum AFP to the conventional imaging criteria for assessing response and tumor recurrence hence recommended to be done before loco-regional therapy.
High levels of tumor markers are associated with worse prognosis in HCC patients.100,101 A recent systematic review of 13 studies had suggested that AFP >1000 ng/mL is associated with poorer outcomes from LT for HCC.101
PIVKA-II is another biomarker for HCC used for diagnosis as well as prognosis. Combined with AFP, the sensitivity and specificity of HCC diagnosis can be improved to 94% and 98%, respectively. PIVKA-II alone or in combination with AFP and/or AFP-L3 has been shown to be effective in predicting the treatment response and clinical outcome of hepatectomy, LT, loco-regional therapy, systemic therapy, and radiotherapy. Japanese clinical guidelines recommend the combined use of PIVKA-II and AFP for the diagnosis of HCC, management of high-risk population, and prognosis of anticancer treatment.102,103
Neutrophil to lymphocyte ratio and platelet to lymphocyte ratio
A recent meta-analysis including 24 articles comprising 6318 patients revealed that a high neutrophil to lymphocyte ratio (NLR) before treatment predicted a poor over all survival (OS) and poor recurrence free survival (RFS). Moreover, an increased platelet to lymphocyte ratio (PLR) predicted a poor OS and earlier HCC recurrence. In addition, both the NLR and PLR were identified as independent risk factors for predicting OS and RFS in HCC patients in a subgroup analysis of different treatment types, including curative or palliative therapy.104 Another meta-analysis by Zhao et al.105 and Song et al.106 also have suggested that PLR could be used as prognostic marker in HCC.
Long non-coding RNAs
Increasing evidences have shown that long non-coding RNAs (lncRNAs) are involved in cancer diagnosis and prognosis. A recent meta-analysis by Hao et al.107 of 19 studies including 1454 patients with HCC, suggested that lncRNAs show a moderate diagnostic accuracy for HCC. The pooled sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, diagnostic odds ratio, and Are Under Curve (AUC) for lncRNAs in the diagnosis of HCC were 0.83, 0.80, 4.2, 0.21, 20, and 0.88, respectively.107 Another meta-analysis of 10 studies showed that lncRNAs were a high diagnostic value for HCC, and its expression could potentially be used as auxiliary biomarker in confirming HCC.108
Molecular classification of HCC
Cancer is a disease of the genome, and a large number of genetic and epigenetic alterations are accumulated during the process of hepatocarcinogenesis.109 Recent developments using comprehensive genomic tools have enabled the identification of the molecular heterogeneity in human HCC. Consequently, several molecular classifications of HCC have been described using different approaches particularly with the genetic, chromosomal, transcriptomic, miRNA, and methylation profiling. An analysis of the biological features of HCC is necessary for personalized therapy. However, the full understanding of the HCC molecular classification requires additional comprehensive studies using both genomic and pathway analyses. A refinement of the molecular classification of HCC, taking into account the geographical and genetic diversity of the patients, will be essential for an efficient design of the forthcoming personalized clinical treatments. Hence, molecular classification of HCC is not ready for clinical application.
| Consensus statements | Level | Grade |
|---|---|---|
|
I | |
|
II-2 | Strong |
|
I | |
|
II-2 | Strong |
|
II-2 | Weak |
Staging of HCC
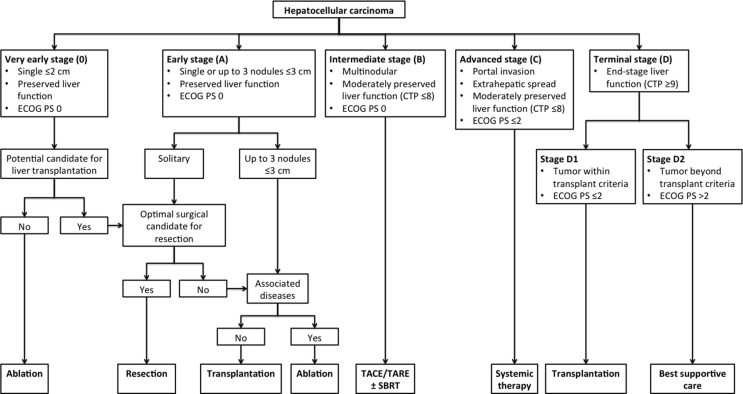
Staging of HCC is necessary for guiding management decision, prognostication, and uniformity of research protocols. However, there is no globally accepted staging system that allows for comparison of current management protocols among heterogeneous populations. Despite the limitations of the Barcelona-Clinic Liver Cancer (BCLC) staging system, it remains the most validated and reliable system for prognostication. However, modification of BCLC staging system was needed to include recent data on loco-regional and systemic therapies for HCC and expanded criteria for transplantation. The INASL modified BCLC staging system is given in Figure 1. One of the most important modifications of the BCLC system proposed was with regard to end-stage cirrhosis. Patients with end-stage liver cirrhosis with heavily impaired liver functions (Child-Pugh class C) but with tumor size within Milan criteria and PS ≤ 2 should be considered for LT.
Figure 1.
INASL-modified BCLC staging. INASL, Indian National Association for Study of the Liver; BCLC, Barcelona-Clinic Liver Cancer; TACE, transarterial chemoembolization; TARE, transarterial radioembolization; SBRT, stereotactic body radiotherapy.
For appropriate staging of HCC a thorough assessment of extra-hepatic spread is essential. For this a PET CT is recommended. However, a CT scan of abdomen plus chest and a bone scan may also be used.
The treatment stage migration concept refers to patients who at first glance would be treated with the option that corresponds to their BCLC stage, but because of any coexisting comorbidity, technical issue, or even treatment failure/progression but still within the original stage cannot be treated by the initial suggested treatment. These patients then move to the treatment that would correspond to the next stage.110 It is usually applied with a left to right direction in the scheme. For example, BCLC stage A patients failing local ablation: offer TACE. BCLC stage B patients not responding to at least 2 cycles of TACE: offer sorafenib.
| Consensus statements | Level | Grade |
|---|---|---|
|
III | Strong |
|
III | Weak |
|
III | Weak |
|
II-2 | Strong |
Treatment of very early and early HCC (BCLC 0 and A)
Treatment options for very early and early HCC
Liver resection
The indications of liver resection (LR) are as follows: (i) non-cirrhotic HCC, provided an R0 resection can be carried out leaving an adequate liver remnant; (ii) resectable solitary HCC in cirrhotic liver in patients with no clinically relevant portal hypertension (Hepatic Venous pressure Gradient [HVPG] ≤ 10 mmHg and platelet count, ≥100,000), good liver function, and adequate liver remnant, when ablation is not possible for tumors ≤2 cm (BCLC-0) or as an alternative to LT for tumors >2 cm in size.
When opting for resection, anatomical resection is the preferred approach, especially in patients with a small (<5 cm) solitary tumor and good liver function. In a recent meta-analysis of 25 studies, including 10,216 patients, Tan et al. demonstrated better outcomes following anatomical resection compared with non-anatomical resection.111 Anterior approach is superior to conventional approach and has been shown by Hao et al.112,113 Recent meta-analysis has suggested that laparoscopic minor LR in cirrhotic patients with HCC is safe, with improved short-term outcomes and comparable long-term survival and should be considered as standard care.114 Li et al. suggested that laparoscopic surgery minimized the release of circulating tumor cells compared with open surgery for HCC.115 Therefore, INASL recommends that in experienced centers, LR may be considered via laparoscopic/minimally invasive approaches, especially for solitary tumors (≤5 cm) in favorable locations. Wide surgical margin (≥1 cm) should be aimed for because it has been shown that it can significantly improve prognosis.116 Neoadjuvant or adjuvant therapies (including sorafenib) have not proven to improve outcome of patients successfully treated with curative resection.
Ablative therapies
Image-guided percutaneous ablation is regarded as one of the most common and effective treatment modalities for very early and early HCC. It includes percutaneous ethanol injection (PEI), microwave ablation (MWA), radiofrequency ablation (RFA), irreversible electroporation (IRE), and cryoablation. Ablation is minimally invasive and easily repeatable for recurrence. PEI used to be the standard in ablation. However, of all ablative modalities, RFA is the preferred modality for HCC and has now replaced PEI as the most frequently used ablative therapy. There is evidence that RFA is superior to ethanol injection, in terms of better survival, local control of the disease, fewer treatment sessions needed to complete treatment, and reduced local tumor recurrence rate.117,118 Also there have been studies where RFA was combined with PEI for better results.119 New-generation MWA can create a larger ablation volume in a shorter time period. One meta-analysis indicated a similar efficacy between RFA and MWA with an apparent superiority of MWA in larger neoplasms.120 The initial Indian experience with IRE, which is a non-thermal ablation method that delivers short electric pulses to induce cell death due to apoptosis, was promising; however, it requires further studies, especially in terms of long-term outcomes.121 Neoadjuvant or adjuvant therapies (including sorafenib) have not proven to improve outcome of patients successfully treated with ablation.122
Liver transplantation
LT has been accepted worldwide as the most effective treatment modality for patients with HCC. Majority of the HCC arise from a cirrhotic liver, and because of the presence of impaired liver function, curative therapies such as partial hepatectomy and tumor ablation are not suitable options. LT is thought to be an ideal treatment for cirrhotic patients with HCC because it removes the tumor with the largest possible margin and replaces it with a non-cirrhotic liver.123 The most common indication for LT is HCC within Milan criteria (single tumor ≤5 cm or multiple tumors ≤3 nodules ≤3 cm in size, without vascular invasion). UCSF criteria have been the most validated expanded criteria (single nodule ≤6.5 cm or 2–3 nodules ≤4.5 cm and total tumor diameter ≤8 cm) for selection without compromising results.
Post-LT immunosuppression needs to be tailored in patients with HCC because calcineurin inhibitors (CNIs) promote tumor growth. High blood CNI level predisposes to early tumor recurrence. Hence, CNI minimization should be attempted in all patients transplanted for HCC taking care of risk of rejection. mTOR inhibitors may delay the recurrence of HCC and improve overall recurrence-free survival. mTOR inhibitors can be used in patients transplanted for HCC.
Choice of treatment for very early and early HCC
In non-cirrhotic liver
HCC in non-cirrhotic liver is generally more advanced at the time of diagnosis as compared with cirrhotic HCC, as non-cirrhotic HCC is usually detected late because of the occurrence of cancer-related symptoms, outside any scheduled surveillance program.124 However, in spite of large size, these tumors have a much higher amenability to hepatic resection,125 because of the low risk of liver failure even after extended parenchymal mutilation.124 According to a recent systematic review, hepatectomy for non-cirrhotic HCC carries low perioperative morbidity and mortality and offers favorable long-term outcomes.126 Hence, in patients with a non-cirrhotic HCC, resection is the treatment of choice provided, and an R0 resection can be carried out leaving an adequate liver remnant.
Solitary tumor size ≤2 cm in cirrhotic liver
In a cirrhotic patient, with resectable solitary HCC of size ≤2 cm (BCLC-0), the clinical outcome of RFA is comparable to LR.127 A meta-analysis by Jia et al. that included 15 studies found that the OS and DFS were equivalent for patients receiving RFA versus resection in patients with small solitary tumors (<3 cm) and good liver status based on Child-Pugh score.128 Cochrane network meta-analysis129 also found no evidence of a difference in all-cause mortality at maximal follow-up between surgery and RFA in people eligible for surgery. All-cause mortality at maximal follow-up was higher with percutaneous acetic acid injection and PEI than with RFA in people not eligible for surgery.129 Hence INASL recommends that in cirrhotic patients, with resectable solitary HCC of size ≤2 cm (BCLC-0), RFA should be offered as the first line treatment option, provided the tumor is in favorable location (i.e. neither subcapsular nor centrally located or adjoining gall bladder).
Solitary tumor size >2 cm in cirrhotic liver
For resectable solitary tumor of size >2 cm, LT should be considered as the preferred option if within transplant criteria because of better outcome of LT than LR. A meta-analysis of 9 studies by Menahem et al.130 compared the effects of LT and LR on OS and DFS in patients within Milan criteria. The meta-analysis found no between-group difference in 1-, 3-, and 5-year OS rates. However, 10-year OS rates were better in patients who underwent LT. A comparison of DFS rates showed similar results at 1 year, but better 3- and 5-year DFS rates in patients who underwent LT than LR.130
Resection can be a treatment option for resectable solitary tumor >2 cm only when the patient has good liver function, there is no clinically significant portal hypertension (HVPG ≤10 mmHg and platelet count ≥100,000), and adequate liver remnant can be ensured. The relevance platelet count ≥100,000 was documented by Zhang et al. in a meta-analysis which found that preoperative platelet count could act as a significant biomarker in the prognosis of HCC, especially a platelet count of ≥100 × 103/cumm.131
If LT and resection cannot be done, RFA should be offered provided the tumor is <3 cm and in favorable location; and RFA + TACE should be offered if tumor size is between 3 and 5 cm. However, the results of RFA + TACE are slightly inferior to LR. According to a recent meta-analysis,132 although TACE + RFA combined therapy and LR had a similar 1-year OS, 3-year OS, 1-year RFS, and 3-year RFS rates for early HCC, the 5-year OS rate and 5-year RFS rate were lower in patients with TACE + RFA than in those with LR. Thus, the authors concluded that LR is associated with better long-term survival outcomes and a lower recurrence rate than TACE + RFA for patients with early HCC and is the optimal choice for patients with early HCC.132
Multiple tumors in cirrhotic liver within transplant criteria
LT is the best treatment option for adult patients with cirrhosis and HCC within Milan criteria (single tumor ≤5 cm or multiple tumors ≤3 nodules ≤3 cm in size, without vascular invasion). The Milan criteria remain the gold standard criteria for selection of patients with HCC for LT in the DDLT setting. In this setting, the UCSF criteria have been also validated in several studies and have yielded similar outcomes. Major vascular invasion and extrahepatic metastases are an absolute contraindication for LT for HCC.
In countries where LDLT is predominant, Milan criteria may be too restrictive. Hence, currently most LDLT centers follow expanded criteria and have acceptable results. UCSF criteria have been the most validated expanded criteria (single nodule ≤6.5 cm or 2–3 nodules ≤4.5 cm and total tumor diameter ≤8 cm) for selection without compromising results. In a well-matched cohort, there is no difference in overall survival and disease-free survival of LT for HCC, with respect to the type of graft (living vs. deceased donor). In LDLT predominant centers, primary LT is a better treatment strategy in a Child A cirrhotic with initially resectable and transplantable HCC (early HCC as per BCLC staging) compared with upfront resection ± salvage transplantation for recurrence.
If LT is not an option for patients meeting Milan criteria combination of TACE plus RFA should be offered. Minor LR may also be considered in these patients with mild portal hypertension when complete resection is possible with adequate tumor free margin.
In the DDLT setting, where waiting time for LT is more than 6 months, bridging therapy may be recommended for those within listing criteria to reduce the chances of tumor progression during waiting period. A recent meta-analysis by Kulik et al. of 63 comparative and non-comparative studies showed that in patients with HCC listed for LT, the use of LRT is associated with a non-significant trend toward improved waitlist and posttransplant outcomes, though there was a high risk of selection bias in the available evidence.133 However, another meta-analysis by Huang and Lu of 12 studies including 1504 patients suggested that preoperative LRT had no impact on survival following LT for HCC. The RFS rate also had no association with preoperative LRT.134 The choice LRT for bridging therapy to be offered will depend on local availability of expertise and experience as well as patients ability to afford (in privately funded centers). However, caution needs to be applied when choosing TACE because a recent meta-analysis by Si et al.,135 which evaluated the influence of preoperative TACE on LT showed that preoperative TACE had no obvious effect on improving overall survival but resulted in a higher rate of vascular complications and a reduction of disease-free survival.135
Multiple tumors in cirrhotic liver within transplant criteria: downstaging therapy
In patients beyond, the Milan criteria LT may be considered if patient can be successfully downstaged into the Milan criteria using locoregional therapy. There is no standard, agreed-upon waiting period following downstaging to determine efficacy of downstaging and subsequent optimal timing for LT. In patients beyond Milan criteria, if LT is not an option using expanded criteria or downstaging, feasibility of LR should be assessed, preferably in a multidisciplinary setting.
Role of sorafenib as adjuvant treatment in combination with other modalities in early HCC
Sorafenib is not indicated in early HCC (BCLC 0 or A) or intermediate stage HCC (BCLC B), either alone or in combination with other modalities. According to a meta-analysis published in 2017, there was no convincing evidence of sorafenib as an effective adjuvant therapy in patients with HCC after resection.136 Bruix et al. conducted a large, multicentric, phase 3, double-blind, placebo-controlled study on patients with HCC who achieved a complete radiological response after surgical resection or local ablation to test the efficacy and safety of sorafenib versus placebo as adjuvant therapy (STORM trial). However, the study noted no difference in median recurrence-free survival between the two groups.122 A subsequent meta-analysis also showed no advantage of combining sorafenib with RFA.137 There was also no apparent benefit of preemptive sorafenib therapy in liver transplant recipients with HCC on explant.138 In patients on transplant waiting list, there was no advantage of adding sorafenib to TACE as bridging therapy.139 Thus, the current literature does not support Sorafenib use as neo-adjuvant or adjuvant therapy with resection, ablation, or LT in early HCC.
| Consensus statements | Level | Grade |
|---|---|---|
|
II-2 | Strong |
|
I | Strong |
|
I | Strong |
|
II-2 | Strong |
|
II-1 | Strong |
|
II-1 | Strong |
|
II-1 | Strong |
|
II-3 | Weak |
|
II-3 | Strong |
|
II-2 | Strong |
|
II-2 | Strong |
|
II-3 | Weak |
|
II-3 | Weak |
|
II-3 | Weak |
|
II-2 | Weak |
|
II-2 | Strong |
|
II-2 | Weak |
|
II-2 | Strong |
|
II-2 | Weak |
Treatment of intermediate stage HCC (BCLC B)
TACE and TAE
Transcatheter arterial embolization (TAE) was first reported by Doyon et al. in 1974 as a novel treatment for malignant liver tumors.140 Later, Nakakuma showed that utilization of lipiodol allowed inclusion of chemotherapy with TAE, and thus the practice of transarterial chemoembolization (TACE) became widespread.141 TACE combines conventional TAE with regional chemotherapy to selectively induce ischemia and chemotherapy effects within the tumor while minimizing damage to the untreated liver.142 For the treatment of HCC classified as intermediate stage according to the BCLC staging system (BCLC-B) lipiodol TACE (also known as conventional TACE, cTACE) is recommended as the standard of care.
The primary indication of TACE is inoperable, large, or multinodular, non-invasive tumors isolated to the liver in patients who are asymptomatic and have less than 50% tumor volume and do not have any hepatic decompensation. TACE is also indicated in patients of BCLC stage A, in whom local ablation has technical limitations and for downstaging patients for transplantation. TACE in combination with RFA is also recommended in patients with tumor size of 3–5 cm. According to a meta-analysis by Yang et al., the use of TACE plus RFA for intermediate stage hepatocellular carcinoma can attain higher tumor response rates and improve survival rates than TACE alone.143 TACE is contraindicated in patients with poorly compensated advanced liver disease (Child class C); patients with encephalopathy, refractory to medical management; poor performance status (Eastern Co-operative Oncology Group [ECOG] status > 2); uncorrectable coagulopathy; hepatofugal blood flow; main portal vein thrombosis; patients with contraindications to contrast media; and pregnancy.
A systematic review of 7 RCTs, involving 545 patients showed that arterial embolization improved survival compared with control.144 According to another recent systematic review,145 the objective response rate with lipiodol TACE is 52.5% (95% CI: 43.6–61.5). The OS is 70.3% at 1 year, 51.8% at 2 years, 40.4% at 3 years, and 32.4% at 5 years. The median OS is 19.4 months (95% CI: 16.2–22.6). Liver enzyme abnormalities are the most commonly observed adverse events, followed by the symptoms associated with postembolization syndrome. The overall procedural mortality rate is 0.6%, and the most common cause of death is related to acute liver insufficiency.145
Two TACE techniques have been used since 2004, conventional TACE (cTACE) and TACE with drug-eluting beads (DEB-TACE). Drug-eluting beads (DEBs) were developed to slowly release chemotherapeutic agents and to increase ischemia intensity and duration.146 DEB-TACE has comparable local response and comparable overall survival to cTACE but has less systemic side-effects. Hence, DEB-TACE may be preferred in select patients. According to a meta-analysis by Chen et al., compared with cTACE, DEB-TACE therapy significantly improved 1-, 2-, and 3-year OS rates and the 1- and 2-year RFS rates.147 A meta-analysis suggested that cone-beam CT could significantly increase detection of tumors and tumor feeding arteries during TACE148 (Level of evidence II-2). Thus cone-beam CT should be considered as an adjunct tool to digital substraction angiography (DSA) during TACE.
Bland trans-arterial embolization (TAE) is also efficacious for HCC. A network meta-analysis of RCTs by Katsanos et al. showed that chemoembolization and radioembolization for unresectable HCC may improve tumor objective response and patient survival but are not more effective than bland particle embolization.149 However, the studies comparing TAE and TACE are few and of low to moderate quality, hence at present, bland TAE is not recommended.
TACE is indicated in patients with unresectable HCC. For patients with resectable HCC, LR is superior to TACE. A meta-analysis 4 cohort studies including 861 patients looking at optimal treatment for solitary HCC ≥5 cm compared LR and TACE to an absence of viable tumor. The results suggested that LR resulted in greater survivability and time to disease progression than TACE for solitary HCC ≥5 cm. The authors concluded that where a patient is fit for surgery, has adequate liver function, and a favorable tumor, resection should be considered.150
TACE combined with other modalities
TACE can also be combined with other loco-regional therapies in select patients. A network meta-analysis of RCTs by Katsanos et al. suggested that chemoembolization combined with external radiotherapy or local liver ablation may significantly improve tumor response and patient survival rates over embolization monotherapies.149 Another network meta-analysis suggests that TACE + external beam radiotherapy (EBRT) was more effective than the other seven minimally invasive procedures (TACE, DEB-TACE, transarterial radioembolization [TARE], TACE + high intensity focussed ultrasonography [HIFU], TACE + PEI, TACE + sorafenib, and trans arterial Ethanol Ablation [TEA]), and therefore, it is considered as the optimal treatment for unresectable HCC.151 There is emerging evidence to suggest that TACE in combination with sorafenib is more effective than TACE alone.152
TARE
TARE, also known as selective internal radiation therapy (SIRT), is a form of radiation therapy that involves embolization in conjunction with a radiotherapy agent into the arteries that supply the HCC. It is indicated in select group of patients with advanced HCC, such as patients with portal vein thrombosis with good liver function (Child A). There are two main categories of radioembolic agents approved for clinical use. First category is based on micron-range particulates that encapsulate or adsorb therapeutic radionuclides, like yttrium-90-bearing glass spheres (Therasphere®) or polymeric selective internal radiation spheres (SIR-spheres®). Second category is lipiodol or related embolic substances tagged with therapeutic radionuclides (e.g. rhenium-188 or iodine-131). The therapeutic effect of all these types is based on local deposition of radiation dose by high-energy beta radiation. Worldwide yttrium-90–based microspheres are more commonly used form of TARE.
Although, TARE can be successfully used in all patients in whom TACE is indicated, however, literature has not documented any superiority of TARE over TACE. In patients who are suitable for both TACE and TARE, TACE should be preferred, because in these patients, the median survival of TACE has been found to be comparable to TARE. In a meta-analysis that compared clinical outcomes of TACE versus TARE in unresectable HCC, there was no statistically significant difference in survival for up to 4 years between the two groups (HR = 1.06; 95% CI 0.81–1.46, P = 0.567). Also, there was no difference in partial or CR rates between the two groups.153
TARE is probably more suitable in situations where TACE cannot be done or is relatively contraindicated. Thus, TARE is indicated in select group of patients with advanced HCC, such as patients with portal vein thrombosis. A recent systematic review demonstrated Y-90 TARE achieved a median disease control rate of 74.3% and median survival of 9.7 months in patients of HCC with portal vein tumor thrombosis.154 TARE is also suitable in patients whom TACE had failed. Vilgrain et al. in an open-label randomized controlled phase 3 trial (SARAH Trial), compared the efficacy and safety of sorafenib to that of SIRT with 90Y resin microspheres in patients with locally advanced or intermediate-stage HCC after unsuccessful TACE. They found that overall survival did not significantly differ between the groups receiving sorafenib and SIRT. However, tumor response and quality of life (QOL) were significantly better in the SIRT group than in the sorafenib group, and safety was better in the SIRT group than in the sorafenib group. The authors suggested that quality of life and tolerance might help when choosing between the two treatments.155 In an another meta-analysis, Ludwig et al.156 showed greater 1-year survival benefit of DEB-TACE over 90Y-radioembolization. DEB-TACE also had a favorable 2- and 3-year survival benefit trend over 90Y-radioembolization. There was no significant difference for tumor response detected. However, in the meta-analysis adjusted indirect comparison method was applied because insufficient direct evidence between 90Y-radioembolization and DEB-TACE were available. The authors recommended that direct comparison of these methods for a more robust evaluation is warranted.156 In a direct comparison of cTACE and Y90 radioembolization in patients of BCLC stages A or B, Salem et al. found Y90 radioembolization to provide significantly longer time to progression (TTP) than cTACE. Also, Y90 radioembolization provided better tumor control and could reduce dropout from transplant waitlists.157
TARE is contraindicated in BCLC-D patients, Child C status, patients who have contraindications to angiography, patients with prior external beam radiotherapy, patients with significant hepatopulmonary shunt (>20%), and patients with extra hepatic metastases.
Role of sorafenib as adjuvant treatment in intermediate HCC
The START trial published in 2015 reported that a combination of TACE and sorafenib on an interrupted dosing schedule is well tolerated in patients with intermediate stage unresectable HCC.158 Subsequently, a post hoc analysis of the START trial suggested that with a high patient tolerance to an interrupted sorafenib dosing schedule, the combination of TACE with sorafenib was associated with improved OS in early-intermediate stage HCC when compared with treatment with TACE alone.159 According to a 2016 meta-analysis that included 4 RCTs, including a total of 887 patients with early or intermediate stage HCC, tested the efficacy and safety of TACE plus sorafenib. The pooled results showed that TACE plus sorafenib significantly improved TTP. Nevertheless, the OS, ORR, and DCR were not improved. Moreover, the incidence of treatment-related adverse events (AEs) was higher in the TACE plus sorafenib group.160 Hence, addition of sorafenib to TACE is not recommended at present, till more data emerges. According to a Cochrane network meta-analysis published in 2017, that included 3 RCTs, including 430 participants, there was no evidence that people with intermediate-stage HCC would benefit from sorafenib either alone or when TACE was used as a co-intervention.161 However, it may still be given to those patients of intermediate stage HCC (BCLC-B) who are not suitable for or progressing despite locoregional therapy.
| Consensus statements | Level | Grade |
|---|---|---|
|
I | Strong |
|
I | Strong |
|
I | Strong |
|
I | Strong |
|
I | Strong |
|
II-2 | Weak |
|
I | Strong |
|
II-2 | Weak |
Treatment of advanced HCC (BCLC C)
The stage BCLC C includes a heterogeneous population, which can be subclassified according to clinical features, performance status, macrovascular invasion, and extrahepatic spread. Depending upon the reason for their allocation to this stage the OS can vary widely from 38.6 months to 3.1 months.162 Sorafenib is the standard treatment in patients with stage BCLC C.
Since the approval of sorafenib for patients with advanced HCC in 2007, many drugs have failed in the first- and second-line setting. Fortunately, between 2017 and 2018, four drugs (regorafenib, lenvatinib, cabozantinib, and ramucirumab) were found to be effective and tolerable for patients with HCC as the first- or second-line therapy.163
Recently, data are now emerging that TARE can also be offered to patients of stage BCLC C. Vilgrain et al. in an open-label randomized controlled phase 3 trial (SARAH Trial), compared the efficacy and safety of sorafenib to that of SIRT with 90Y resin microspheres in patients with advanced HCC. They found that OS did not significantly differ between the groups receiving sorafenib and SIRT. The authors suggested that quality of life and tolerance might help when choosing between the two treatments.155
Molecular targeted therapy
Sorafenib
Sorafenib is an oral multikinase inhibitor, which works by inhibiting the activity of several tyrosine kinases involved in tumor angiogenesis and progression, including vascular endothelial growth factor receptor (VEGFR-2/3), platelet-derived growth factor receptor (PDGF-R), Flt3 and c-Kit, and also targets Raf kinases involved in the MAPK/ERK pathway. Two large RCTs have documented improved survival with sorafenib compared with placebo.164,165 According to a recent meta-analysis by Finn et al., in patients with advanced HCC and CP A liver function, sorafenib is the only treatment that has been shown to improve OS in randomized studies.166 High-quality data supporting the use of other treatment modalities in this setting, or in the setting of patients with less compensated (CP B) liver disease, are lacking.166 Hence, sorafenib is recommended as a first-line treatment for advanced HCC (BCLC C) with preserved liver function (child A; selected child B).
The optimal dose of sorafenib is 400 mg twice daily with optimal management of adverse effects to improve survival. However, sorafenib should be initiated at a reduced dose to minimize the adverse events and increase tolerability. Sorafenib should be continued till radiologic progression. A meta-analysis demonstrated that the occurrence of sorafenib-related side-effects (such as diarrhea, hypertension, and skin toxicities) is associated with a better OS in sorafenib-treated HCC patients.167 Another similar meta-analysis suggested that presence of dermatologic adverse events was associated with a lower mortality when compared with those patients without them with a pooled HR of 0.45 (95% CI: 0.38–0.53).168 Presence of microvascular invasion, high AFP, and high NLR were prognostic factors of poorer OS in patients being treated with Sorafenib.169
Lenvatinib
Lenvatinib is a tyrosine kinase inhibitor (TKI) of VEGF receptors 1–3, FGF receptors 1–4, PDGF receptor α, RET, and KIT and in a phase 2 trial showed activity in advanced HCC.170 Subsequently an open-label, phase 3, multicenter, non-inferiority trial, which included 954 patients with HCC not suitable for surgery, ablative therapy, or TACE, compared overall survival in patients treated with lenvatinib versus sorafenib as a first-line treatment. The median survival time for lenvatinib of 13.6 months (95% CI 12.1–14.9) was non-inferior to sorafenib (12.3 months, 10.4–13.9; HR 0.92, 95% CI 0.79–1.06), meeting criteria for non-inferiority. The progression-free survival (PFS; 7.4 versus 3.7 months) and TTP (8.9 versus 3.7 months) were favorable for those treated with lenvatinib; however, these benefits did not translate into better OS. The most common any-grade adverse events were hypertension (42%), diarrhea (39%), decreased appetite (34%), and decreased weight (31%) for lenvatinib, and palmar-plantar erythrodysesthesia (52%), diarrhea (46%), hypertension (30%), and decreased appetite (27%) for sorafenib. The investigators concluded that lenvatinib was non-inferior to sorafenib in overall survival in untreated advanced HCC.171 In a cost-effectiveness analysis of lenvatinib treatment for patients with unresectable HCC compared with sorafenib in Japan, lenvatinib was found to be more cost-effective than sorafenib.172
Regorafenib
Till 2016, there was no systemic treatments for patients with HCC whose disease progressed during sorafenib treatment. Regorafenib is a multikinase inhibitor and has a similar structure to sorafenib and became the first systemic treatment approved as a second-line therapy for HCC based on the RESORCE trial.173 Apart from inhibiting different kinases such as RAF-1; B-RAF; VEGFR 1, 2, and 3; and PDGFRβ, it also inhibits FGFR1 oncogenic mutants of KIT, RET, and BRAF. The RESORCE trial173 was a double-blind, parallel-group, phase 3 trial done at 152 sites in 21 countries, adults with HCC who tolerated sorafenib (≥400 mg/day for ≥20 of last 28 days of treatment), progressed on sorafenib, and had Child-Pugh A liver function. Regorafenib was found to improve OS with a HR of 0·63 (95% CI 0·50–0·79; one-sided P < 0·0001).173 With the development of regorafenib, the first sequential treatment option (sorafenib-regorafenib) has become available in the therapeutic management of HCC. Those patients with advanced HCC who start sorafenib treatment, tolerate it according to the RESORCE trial definition, and develop radiologic tumor progression are candidates for regorafenib treatment.174 A later subanalysis of the RESORCE trial175 showed that the median OS was 26.0 months in the regorafenib group and 19.2 months in the placebo group when survival was assessed from the first dose of sorafenib treatment.
Cabozantinib
Cabozantinib is a multi-TKI of MET, VEGF receptors, and AXL and was shown to prolong the overall survival in patients who progressed after sorafenib compared with the placebo. According to the results of a phase 2 placebo-controlled randomized discontinuation study, cabozantinib was found to have clinical activity in HCC patients, including objective tumor responses, disease stabilization, and reductions in AFP.176 Subsequently, a randomized phase 3 trial of cabozantinib versus placebo (CELESTIAL trial)177 as a second-line treatment for patients with HCC previously treated with sorafenib, documented a median OS of 10.2 months with cabozantinib and 8.0 months with placebo with a HR of 0.76 (95% CI, 0.63–0.92). The most frequent AEs were hand foot skin reaction, arterial hypertension, and transaminase elevation. Grade 3–4 AEs were 68% in the cabozantinib arm and 36% in the placebo arm. The rate of discontinuation and death because of treatment-related AEs were 16% and 1.3% in the cabozantinib arm and 3% and 0.4% in the placebo arm, respectively.177
Ramucirumab
Ramucirumab is a fully human recombinant immunoglobulin G (IgG) 1 monoclonal antibody to inhibit a single target of VEGFR2. In a randomized, double-blind, multicenter, phase 3 trial, ramucirumab was compared with placebo as second-line treatment in patients with advanced HCC following first-line therapy with sorafenib (the REACH trial), and ramucirumab was not found to significantly improve survival over placebo.178 However, in a subgroup analysis of the REACH trial of the Japanese patients, ramucirumab treatment did improve OS, including in patients with a baseline AFP level of 400 ng/mL or greater. Moreover, improvements in PFS and objective response rate were also demonstrated. The safety profile of ramucirumab was acceptable and well tolerated in Japanese patients.179 Another study that looked at patient-focused outcome results from the REACH study ramucirumab was associated with no worsening of QoL. In patients with baseline AFP ≥400 ng/mL, the significant survival benefit observed in patients treated with ramucirumab was coupled with a trend in patient-focused outcome benefits.180 In a subsequent clinical trial (REACH-2 trial)181 that enrolled only patients with AFP ≥400 ng/mL, ramucirumab was found to improve the overall survival compared with placebo. Thus, ramucirumab became the first biomarker-driven systemic treatment.
Immune checkpoint inhibitors
In contrast with classical chemotherapy or molecular therapies that target cancer cells directly, immunotherapies aim to block immune-escape mechanisms of tumors and, consecutively, induce a strong and predominantly T-cell mediated immune response against cancer cells. The most successful form of immunotherapy to date has been the blockade of the immune checkpoints CTLA-4 (cytotoxic T-lymphocyte-associated protein 4) and PD-1/PD-L1 receptors (programmed cell death protein 1/programmed death-ligand 1).182 To date, several clinical trials have evaluated the efficacy of antibodies against PD-1, PD-L1, and CTLA-4, both as monotherapy as well as in combinatorial therapy approaches in patients with advanced HCC.182 Nivolumab, pembrolizumab, tislelizumab, and camrelizumab are fully humanized monoclonal antibodies against PD-1, whereas durvalumab and atezolizumab target PD-L1. Tremelimumab is a monoclonal antibody that binds to CTLA-4. The efficacy of these antibodies is currently being investigated in various clinical trials as mono- or combination therapy in advanced HCC.182
Nivolumab
Nivolumab is a PD-1 immune checkpoint inhibitor. In a phase 1/2, open-label, non-comparative, dose escalation, and expansion trial (CheckMate 040) with histologically confirmed advanced HCC with or without HCV or HBV infection, nivolumab showed a manageable safety profile, including acceptable tolerability. The objective response rate was 20% (95% CI 15–26) in patients treated with nivolumab 3 mg/kg in the dose-expansion phase and 15% (95% CI 6–28) in the dose-escalation phase.183 This trial led to the approval of nivolumab by Federal Drug Administration (FDA) for the treatment of HCC in patients who have been previously treated with sorafenib. It will be of great interest to learn about the results of ongoing trial CheckMate-459, where nivolumab is being compared with sorafenib (NCT02576509) as the first-line agent.
Tremelimumab
Tremelimumab is a fully human monoclonal antibody that binds to CTLA-4 on the surface of activated T lymphocytes. Sangro et al. first showed positive results with tremelimumab in a pilot trial on 27 patients with advanced HCC and HCV infection.184 The disease control rate was 76.4%, and 3 patients had a partial response (PR). Subsequently, in an open-label study on 32 patients with HCC, tremelimumab was given at 2 dose levels (3.5 and 10 mg/kg i.v.) every 4 weeks for 6 doses, followed by 3-monthly infusions until off-treatment criteria were met. On day 36, patients underwent subtotal RFA or chemoablation. No dose-limiting toxicities were encountered. The authors concluded that tremelimumab in combination with tumor ablation is a potential new treatment for patients with advanced HCC and leads to the accumulation of intratumoral CD8+ T cells. Positive clinical activity was seen, with a possible surrogate reduction in HCV viral load.185
Pembrolizumab
Pembrolizumab is another fully humanized anti-PD-1 monoclonal antibody that has shown significant anti-tumor activity in a variety of cancers. The KEYNOTE-224 study was a non-randomized, multicenter, open-label, phase 2 trial of pembrolizumab conducted in 47 centers across 10 countries. The study showed a median OS similar to nivolumab in the second-line at 12.9 months with a disease control rate of 61% and ORR of 18%.186 Consequently, the FDA granted provisionary approval for pembrolizumab as second-line treatment for advanced HCC, pending results from the randomized phase III trial.
Treatment of HCC with portal vein tumor thrombosis
Portal vein tumor thrombosis (PVTT) is one of the most common complications in HCC and is the dominant form of macrovascular invasion of HCC.187 PVTT usually indicates poor prognosis due to a rapidly progressive disease (PD) course, worsening liver function, complications connected with portal hypertension, and limited options and poorer tolerance to treatment. Although patients with PVTT as well those with metastatic HCC, both are clubbed together in stage BCLC C; however, ideally HCC with PVTT should not be grouped with metastatic HCC because newer therapeutic options have shown promising results for PVTT in case control studies.
Sorafenib is the treatment of choice for patients with PVTT. Sorafenib therapy marginally improves survival in patients with HCC and PVTT. In a subgroup analysis188 of SHARP trial164 patients with HCC having PVTT, the median survival time was slightly longer in the sorafenib group (8.1 months) than in the placebo group (4.9 months). TARE with Y-90 is another alternative for select patients of with PVTT and good liver function (Child-Pugh A). A recent systematic review aimed to assess the safety and effectiveness of Y-90 radioembolization for HCC and PVTT.154 The radiological response achieved was: CR in 3.2%, PR in 16.5%, stable disease (SD) in 31.3%, and PD in 28%. The median survival was 9.7 months for all patients, including the median OS were 12.1, 6.1 months of Child-Pugh class A and B patients, and the median OS were 6.1, 13.4 months of main and branch PVTT patients, respectively.154 TACE with or without radiotherapy or systemic therapy may also be considered an option in segmental PVTT. A meta-analysis of 13 studies by Silva et al. sought to examine the role of TACE in the treatment of HCC with PVT in either the main portal vein (MPV) or portal vein branches (PVB). The median OS was 8 (95% CI 5–15) months. Survival rates after 1, 3, and 5 years were 29%, 4%, and 1%, respectively. Patients with MPV thrombosis had worse survival than PVB patients but similar mRECIST response rates. The authors concluded that TACE was a safe treatment for a highly selected population of HCC patients with PVT. Despite worse survival rates compared with PVB thrombosis, PVT in the MPV should not be considered an absolute contraindication to TACE.189 Zhang et al. conducted a meta-analysis of 5 retrospective studies with 973 patients to compare the effectiveness and safety of TACE plus sorafenib versus TACE alone for HCC with PVTT. The authors found that TACE plus sorafenib improved OS, ORR, TTP, and DCR for HCC patients with PVTT compared with TACE alone.190 A small RCT found that combined use of RFA of both HCC nodule and MPVTT plus sorafenib is much better than the use of sorafenib alone in cirrhotics with invasion of main portal trunk. Results of the RCT clearly indicated that 3-year survival of such kind of patients can be increased adding the alleged percutaneous thrombectomy to sorafenib alone in a safe and effective manner.191
| Consensus statements | Level | Grade |
|---|---|---|
|
I | Strong |
|
II-1 | Strong |
|
I | Weak |
|
II-2 | Weak |
|
I | Strong |
|
I | Strong |
|
I | Strong |
|
II-2 | Weak |
|
II-2 | Strong |
|
I | Strong |
|
II-2 | Weak |
|
II-3 | Weak |
Treatment of HCC stage BCLC-D
The BCLC staging system incorporates 3 major parameters including tumor burden, severity of cirrhosis and performance status to predict the prognosis of HCC. However, patients are classified as BCLC stage D only on the basis of Child-Turcotte-Pugh (CTP) class C or PS 3–4, because of very limited survival time after diagnosis with or without anti-cancer treatments.192 The median survival time of untreated patients of BCLC stage D is 6 months.193 Tumor burden, including size and number of tumor nodule(s), vascular invasion, and extra-hepatic involvement, which profoundly influences the outcome of HCC patients, is not considered a criterion for BCLC stage D. This makes the BCLC stage D very heterogeneous; for example, patients with CTP class C, extra-hepatic metastases, and multiple co-morbidities are considered the same BCLC stage as patients with PS 3, minimal cirrhosis, and a small resectable HCC nodule. The treatment allocation to BCLC stage C patients according to standard guidelines (best supportive care110) may actually decrease their survival compared with patients who do not adhere to standard guidelines.194 To resolve this limitation of BCLC stage D, Hsu et al. constructed an easy-to-use nomogram taking into account the tumor burden as a pivotal prognostic factor.192 They divided the BCLC stage C patients into 3 categories: within the Milan criteria, with distant involvement and vascular invasion, and the rest. They suggested that BCLC stage D patients with mild cirrhosis and small tumor burden might potentially benefit from surgical resection or TACE. Similarly, selected patients with CTP class C and small tumor burden could choose LT or ablation to effectively prolong their survival.192 A multicenter, cohort study suggested that patients judged as potentially eligible for LT according to the following criteria: absence of macroscopic vascular invasion or metastases, age 70 years or younger, and absence of relevant extrahepatic comorbidities, even if in BCLC stage D, results in survival benefit.195 Survival of BCLC-D patients receiving LT was significantly higher than that of patients receiving systemic therapies or supportive care (137 versus 5 months).196 Thus, INASL recommends that LT if feasible should be considered in BCLC-D HCC if PS and extent of tumor permits. If LT not feasible, then, after improvement of ascites, jaundice, and/or hepatic encephalopathy with conservative care, ablative therapies, or transarterial tumor therapies may be performed, when possible.
Supportive treatment including management of pain, ascites, variceal bleeding, nutrition, and psychological support should be part of management of BCLC-D HCC patients. Antiviral treatment in BCLC-D might stabilize the liver function, thus increasing the therapeutic window for tumor-specific therapies in these patients.
For pain management of mild intensity acetaminophen (paracetamol) up to 3 g/day can be utilized and is safe. Opioids should be used for the management of pain not controlled by paracetamol.197 Routine artificial nutrition is not justified in patients in the terminal stage HCC, however, in individual cases, dietary counseling, and artificial nutrition can slow down nutritional deprivation, avoid dehydration, and improve the quality of life. Management of psychosocial and spiritual issues should be a part of the care of terminal HCC patients.1,197
| Consensus statements | Level | Grade |
|---|---|---|
|
III | Weak |
|
III | Weak |
|
III | Strong |
|
III | Weak |
|
III | Strong |
|
III | Weak |
|
III | Strong |
Emerging therapy for HCC
Stereotactic body radiotherapy for HCC
Although HCC is a radiosensitive tumor, utilization of EBRT has historically been limited by the relative sensitivity of adjacent normal liver parenchyma.198 However, with the advent of stereotactic body radiotherapy (SBRT), this paradigm is gradually changing. SBRT is a special form of EBRT that accurately delivers a high dose of radiation in fewer treatment fractions to an extracranial target.199 SBRT represents a significant advance and a promising tool in HCC management, improving the therapeutic ratio by limiting the dose to adjacent normal hepatic parenchyma, and escalating dose to the tumor. SBRT now represents an effective locally ablative therapy for HCC with a favorable toxicity profile and this modality should be explored further.198
Seo et al. compared RFA with SBRT for HCC smaller than 3 cm using a Markov model. The investigators showed that the expected overall survival of SBRT was nearly identical to RFA in HCCs smaller than 3 cm, but SBRT might have an advantage for tumors 2 cm and larger.200 A Cochrane meta-analysis by Abdel-Rahman and Elsayed,201 that included 9 RCTs with 879 participants, found very low- and low-quality evidence suggesting that combined EBRT and TACE may be associated with lower mortality and increased complete and overall response rates, despite an increased toxicity as expressed by a higher rise of bilirubin and ALT. Although high-quality RCTs are needed to assess further the role of EBRT for unresectable HCC, this modality appears promising.201 Another network meta-analysis suggests that TACE + EBRT was more effective than the other seven minimally invasive procedures (TACE, DEB-TACE, TARE, TACE + HIFU, TACE + PEI, TACE + sorafenib, and TEA), and therefore, it may be considered as the optimal treatment for unresectable HCC.151 Thus in BCLC stage B patients, SBRT can be an option for residual or recurrent lesions after TACE as part of combination therapy.
In patients with BCLC stage C with thrombus involving the main branch of portal vein, SBRT followed by sorafenib is a treatment option. A large series reported the outcome of 985 patients who were treated with radiotherapy for HCC with PVTT. More than 50% responded to treatment, and the median OS was 10.2 months. Predictors of improved survival included higher radiation dose and combination treatment with other liver-directed therapies.202 In a study that compared response to sorafenib versus radiotherapy in unresectable HCC with major PVTT using propensity-score analysis,203 radiotherapy was associated with higher OS than sorafenib (4.8 vs. 10.9 months). 5% to 10% of patients treated with radiotherapy were alive 5 years after treatment, whereas no patient in the sorafenib group survived more than 2 years.203
| Consensus statements | Level | Grade |
|---|---|---|
|
II-2 | Strong |
|
II-2 | Strong |
|
II-2 | Weak |
Cytotoxic, hormonal, and other agents for HCC
Hepatic arterial infusion chemotherapy
In the last decade, cisplatin-, epirubicin-, interferon-, or 5-fluorouracil–based transcatheter hepatic arterial infusion chemotherapy (HAIC) has been increasingly used for patients with HCC stage BCLC C in Asian countries. HAIC was suggested as a promising treatment as it can inhibit tumor growth through antiangiogenic mechanisms with less toxicity and fewer systemic side-effects than the maximum tolerated dose therapy. A meta-analysis204 of 10 studies including 1264 patients showed that HAIC was associated with significantly higher 1-, 2-, and 3-year OS than sorafenib. Compared with sorafenib, HAIC was also associated with superior CR, PR, and objective response rate (ORR). The authors concluded that HAIC can be considered as an alternative treatment option for patients with HCCs of BCLC stage C.204 A multicenter open-labeled randomized phase II trial in chemo-naïve patients with advanced HCC with Child-Pugh scores of 5–7 evaluated the effect of addition of HAIC with cisplatin (SorCDDP) to Sorafenib for the treatment of advanced HCC. The median survival in the Sorafenib and SorCDDP arms were 8.7 and 10.6 months, respectively. The median time to progression and the response rate were, respectively, 2.8 months and 7.3% in the Sorafenib arm and 3.1 months and 21.7% in the SorCDDP arm. The adverse events were more frequent in the SorCDDP arm than in the Sorafenib arm, but well-tolerated. Thus SorCDDP yielded favorable overall survival when compared with Sorafenib in patients with advanced HCC.205 However, use of HAIC is still investigational, and its use in clinical practice cannot be recommended outside of clinical trial.
Cytotoxic and hormonal agents
Prior to the arrival of sorafenib in 2008, doxorubicin was routinely used as a single drug for advanced HCC, but had shown inefficacy, with a response rate of about 15–20%. Other cytotoxic agents, such as epirubicin, cisplatin, 5-fluorouracil, etoposide and their combinations, demonstrate even lower efficacy.206 Combination chemotherapy with cytotoxic agents yielded higher response rates, however, some randomized controlled studies comparing promising combination therapies with no treatment or single agents failed to show any advantage in terms of the overall survival. Similarly, numerous RCTs and meta-analyses of hormonal therapies, such as anti-estrogen, anti-androgen, tamoxifen, octreotide, and interferon therapies have shown no significant survival advantage.207 Thus, these treatments are discouraged in advanced HCC.
Other agents
Thalidomide is not only capable of inhibiting angiogenesis, but also modulating immunity. A meta-analysis of 23 RCTs involving 1836 patients showed that thalidomide plus TACE was significantly superior in increasing 6-month, 1-year, 1.5-year, and 2-year survival rates. It also improved ORR, DCR, cellular immunity and reduced VEGF, when compared with TACE group.208 Another recent meta-analysis of 12 RCTs also showed that showed that TACE plus thalidomide was significantly superior than TACE alone in terms of 1-year, 2-year, 3-year survival rates, PFS, objective response rate, and disease control rate. However, the authors added that this finding was not definitive due to the poor quality of included studies.209 Thus till more evidence emerges from carefully designed and conducted RCTs thalidomide cannot be recommended for HCC either alone or in combination with other modalities.
Another agent arsenic trioxide (As2O3) was evaluated in a meta-analysis of 18 RCTs involving 1412 participants to determine whether As2O3 and TACE therapy achieved better therapeutic effects compared with TACE alone for HCC. It was found that adjuvant As2O3 therapy combined with TACE achieved better therapeutic effects compared with TACE alone. Both the intravenous administration of As2O3 and the arterial administration of As2O3 were found to be good options for clinical practice.210 A RCT of patients of HCC with pulmonary metastasis TACE plus an intravenous infusion of As2O3 was found to effectively controlled pulmonary metastasis and prolonged OS compared with TACE alone.211 However, large RCTs are needed before it can be recommended for use.
| Consensus statements | Level | Grade |
|---|---|---|
|
II-2 | Strong |
|
II-2 | Strong |
Adoptive immunotherapy
Immunotherapy has a potential to offer systemic, nontoxic, and durable antitumor effects, and therefore is highly attractive as a treatment option for HCC. Adoptive immunotherapy (AIT) or adoptive cell therapy (ACT) is a highly personalized cancer therapy that involves administration to the cancer-bearing host of immune cells with direct anticancer activity. ACT using naturally occurring tumor-reactive lymphocytes has mediated durable, complete regressions in cancer patients, probably by targeting somatic mutations exclusive to each cancer. In addition, the ability to genetically engineer lymphocytes to express conventional T cell receptors or chimeric antigen receptors has further extended the successful application of ACT for cancer treatment.212
There is good data to suggest that adjuvant AIT with cytokine-induced killer cells improves survival in patients undergoing resection or loco-regional therapy. Multiple meta-analyses have suggested that AIT is safe and effective in reducing mortality and tumor recurrence for patients with HCC after curative therapies213,214 or palliative therapies.215, 216, 217, 218, 219 However, studies from India on AIT are lacking. Hence, it cannot be recommended for clinical use outside of clinical trials, till more data emerges from India.
| Consensus statements | Level | Grade |
|---|---|---|
|
I | Weak |
Treatment response evaluation and follow-up schedule
Response evaluation in patients treated with loco-regional therapies and targeted therapies
The performance of the treatment response criteria is usually evaluated on their ability to reflect the biological effects induced by anti-cancer treatments and, more importantly, in predicting survival. When assessing the effects of the loco-regional treatments for HCC, evidence unequivocally suggests that the criteria based on viable tumor measurement (e.g. mRECIST and EASL criteria) are superior to criteria based on total tumor measurement. This superiority is due to the inability of the latter in discriminating the areas of treatment-induced necrosis from the viable tumor. According to a meta-analysis, both mRECIST and EASL criteria showed a very good concordance in HCC patients undergoing loco-regional treatments. Objective response according to both the criteria confirmed a strong prognostic value in terms of overall survival. This prognostic value appeared to be very similar between the two methods.220
For treatment response evaluation following systemic therapies, the choice of criteria is still not clear, and at least five different criteria have been proposed: RECIST, EASL, mRECIST, RECICL and Choi criteria.221 However, it is now generally suggested that criteria based on viable tumor assessment (EASL, mRECIST, RECICL) may be better compared with the RECIST. Lencioni et al. investigated whether objective response by mRECIST criteria accurately predicted OS in patients with advanced HCC treated with systemic targeted therapies.222 Individual patient data from the BRISK-PS randomized phase III trial223 comparing brivanib vs. placebo were used to analyze objective response by mRECIST criteria as a predictor of OS in a time-dependent covariate analysis. The study demonstrated that objective response is an independent predictor of survival and qualifies as a potential surrogate end-point for overall survival in this patient population who were treated by systemic therapy.222
Therefore, INASL recommends that the treatment response evaluation should be done by dynamic CT or MRI studies using the mRECIST criteria, both for loco-regional therapy and for systemic therapy. It is preferable to use same investigation modality (dynamic CT or MRI) in follow up as used at diagnosis for assessing clinical response.
The response should be categorized as: CR, PR, SD, or PD (Table 2 CR and PR patients should be considered as responders and SD and PD patients as non-responders).
Table 2.
Modified Response Evaluation Criteria in Solid Tumors (mRECIST) for the Assessment of HCC Response to Locoregional and Systemic Therapy.
| Assessment category | mRECIST criteria |
|---|---|
| CR (complete response) | Disappearance of any intratumoral arterial enhancement in all target lesions |
| PR (partial response) | At least 30% decrease in the sum of diameters of viable target lesions |
| SD (stable disease) | Any cases that do not qualify for either PR or PD |
| PD (progressive disease) | At least 20% increase in the sum of the diameters of viable target lesions |
HCC, hepatocellular carcinoma.
The timing of initial treatment response evaluation and subsequent follow-up
The timing of initial treatment response evaluation and subsequent follow-up should depend on treatment modality used.1,224 For patients who underwent resection, dynamic CT or MRI studies should be done every 3 months for the first year and then every 6 months for another year. After 2 years, if there has been no recurrence, the patients should be subjected to routine surveillance. For patients who have undergone percutaneous ablation, the initial response evaluation should be done at 4 weeks. Subsequently, dynamic CT or MRI studies every 3 months for the first year and then every 6 months for another year and then routine surveillance. For patients who have undergone LT, ultrasonography plus AFP should be done every 3 months for the first year and then every 6 months as routine surveillance. For patients who have undergone TACE the initial response evaluation should be at 4 weeks. Subsequently, dynamic CT or MRI studies every 3 months for the first year and then every 6 months for another year and then routine surveillance. For patients undergoing systemic therapy, dynamic CT or MRI for tumor progression should be done every 3 months to guide therapy decisions.
Decision about repeat RFA/TACE based on treatment response
Radiological progression in spite of two sessions of RFA or TACE indicates futility of these procedures. In practice, RFA/TACE should not be repeated when substantial necrosis is not achieved after two RFA/TACE treatments or when there is progression or liver function impairment, worsening of performance status (PS), or the appearance of portal vein tumor thrombosis or extrahepatic metastases. Tumor burden, BCLC stage at baseline, and Child-Pugh score are other factors for RFA/TACE retreatment decision-making and for consideration of alternative therapy after failure of these treatments.
| Consensus statements | Level | Grade |
|---|---|---|
|
I | Strong |
|
III | Weak |
|
II-2 | Strong |
Treatment of HCC with extrahepatic spread and HCC with rupture
Extrahepatic spread
According to the BCLC staging, patients with extrahepatic spread (EHS) of HCC, still remain in BCLC stage C. The median OS of a patients with EHS is 11.2 months.162 Although the standard recommended treatment for patients with BCLC stage C is sorafenib; however, it may be not effective when there is EHS. Two RCTs164,165 in their subgroup analysis compared sorafenib versus placebo in patients with EHS, and sorafenib showed no statistically significant improvement of OS over placebo.166 Hence, while the intrahepatic primary tumor will require sorafenib, the EHS needs to be tackled by other modalities like radiotherapy.
It has been observed that radiotherapy can be used to alleviate pain in patients with bone metastasis and relieve of symptoms from localized pulmonary225 or lymph node metastases.226 In a retrospective study of 91 patients who received median radiation dose of 40 Gy (range, 20–66 Gy) for bone metastasis, the pain response rate was 81.4%.227
HCC with rupture
Ruptured HCC is a rare, but life-threatening presentation of HCC that often requires acute intervention. According to a recently published systematic review of 67 studies on ruptured HCC, the leading causes of death in the short-term was bleeding complications in 34% and hepatic failure in 30%.228 A wide range of therapeutic strategies including TACE, TAE, hepatectomy, or their combination has been employed for management of ruptured HCC. TAE/TACE seems to be the most effective modality in controlling bleeding from ruptured HCC.229
| Consensus statements | Level | Grade |
|---|---|---|
|
II-2 | Strong |
|
II-2 | Strong |
Recurrence of HCC after successful curative treatment
Recurrence of HCC after resection
It is generally recognized that intrahepatic recurrence of HCC may have a monoclonal (or monocentric) origin when it develops from an intrahepatic metastasis or have a multiclonal (or multicentric) origin when it arises from de novo carcinogenesis because of long-term chronic inflammation and cirrhosis. Recurrence occurring within 1-2 year of surgery is typically defined as intrahepatic metastasis, while recurrence occurring later than 2 year after resection is late recurrence of HCC because of de novo carcinogenesis.
The long-term survival after LR remains unsatisfactory because of the high incidence of intrahepatic recurrence (up to 68%–98% of patients).230 Hence, HCC is increasingly being managed by LR first then salvage LT in case of recurrence within accepted criteria. Many reports compared the safety of the salvage against the primary surgery in the setting of deceased donation, but the difference in LDLT setting is not sufficiently defined. Salvage LDLT is believed to be a more challenging surgery than primary LDLT because of operative field adhesions, in addition to the inherent difficulties particularly short vasculo-biliary stumps.231 A recent systematic review by Murali et al. compared primary liver transplant with loco-regional therapy with curative intent (CLRT) followed by salvage liver transplantation (SLT).232 The authors concluded that CLRT-SLT may be offered as first-line therapy to patients with HCC and well-compensated cirrhosis instead of primary LT because it may lead to better utilization of donor liver. However, a large proportion of patients with HCC recurrence after CLRT may not be candidates for SLT. Only 32.5% patients with HCC recurrence after CLRT actually received SLT, as the rest were not medically eligible. Thus, the disease free survival (DFS) was worse with CLRT-SLT compared with LT.232 According to another meta-analysis by Xiong et al., the 3-year and 5-year overall survival rates were inferior in SLT, which shows that PLT is a better treatment strategy for transplantable HCC. However, considering the severe organ limitation and the feasibility and safety of SLT, it provides a better option for patients with HCC recurrence after curative resection.233 According to another recent systematic review of 7 retrospective studies, the efficacy of SLT might be superior to that of CLRT in the treatment of recurrent HCC. However, considering the similar overall survival rate and current situation of donor shortage, repeat hepatectomy is still an important option for recurrence HCC.230 According to a meta-analysis by Erridge et al., there does not appear to be a significant difference in survival between patients undergoing repeat hepatectomy or ablation for recurrent HCC.234 Another meta-analysis also could not demonstrate any superiority of repeat hepatectomy or RFA over the other in terms of DFS and OS.235 Hence, INASL recommends that for treatment of recurrence after resection, a repeat hepatectomy, ablation, or a SLT could be performed in selected patients. Other therapies such as chemoembolization or sorafenib could be proposed to those patients who are unable to undergo resection, ablation or SLT.
Recurrence of HCC after ablation
HCC often recurs after RFA and recurrences are roughly divided in early recurrences (<2 years after ablation) due to tumor metastasis and late recurrences (>2 years after ablation) due to de novo carcinogenesis in cirrhotic parenchyma.236 Local recurrence can result to either from incomplete local treatment or from tumor aggressiveness, while distant recurrence is related to tumor metastasis or de novo carcinogenesis. Incomplete ablation accounts for most tumor recurrences, and there is compelling evidence that complete ablation of HCC is required to prevent recurrence.237 Tumor features (size and number of nodules, serum biomarkers, histological, and immunohistochemical features) as well as severity of liver disease and virological features influence tumor recurrence rate after percutaneous ablation. Any recurrence after successful curative ablative therapies should be evaluated and treated as new tumor.
Recurrence of HCC after liver transplantation
According to a meta-analysis of 61 studies, the mean HCC recurrence rate was 16% of all LTs for HCC and the median time from LT to HCC recurrence was 13 months (range 2–132 months). The majority of patients (67%) presented with HCC extra-hepatic recurrences, involving lung, bone, adrenal gland, peritoneal lymph nodes, and rarely the brain. The OS after HCC recurrence was 13 months.238 The prognosis of recurrence is poor despite numerous proposals of the therapeutic option. Lower levels of immunosuppressive therapy, and the use of mammalian targets of rapamycin (mTORs) is a potential preventive strategy to reduce HCC recurrence post-LT.239
Treatment for intrahepatic recurrence after transplantation is challenging. The treatment of choice for HCC recurrence is surgical resection,240 because it is effective in prolonging patient survival despite the technical difficulty in resecting graft livers. Besides surgical resection, different kinds of treatment are also in use, including TACE, ablation, and SBRT. Systemic treatment based on the combination of an mTOR inhibitor with sorafenib can also be used.241
| Consensus statements | Level | Grade |
|---|---|---|
|
II-2 | Strong |
|
II-2 | Strong |
|
II-2 | Strong |
|
III-3 | Weak |
Adjuvant antivirals for HCC
HCV-related HCC
IFN has been shown to improve outcomes following ablation or resection of HCC. A recent meta-analysis sought to determine the prognostic impact of SVR achieved through IFN-based regimens in HCV-related HCC. The meta-analysis concluded that SVR was associated with improved OS and RFS in patients with HCV who had undergone resection or locoregional therapy for HCC.242 Whether the high rates of SVR achieved with IFN-free regimens have an effect on the risk of recurrence following resection or ablation of HCC is currently debated. The EASL recommends that since these HCC patients frequently have cirrhosis or advanced fibrosis, they should receive appropriate antiviral therapy for their liver disease, while careful HCC surveillance is required in these patients. INASL also recommends that therapy with DAAs should be considered simultaneously along with curative therapies in HCV-related HCC.
In patients with HCV-related HCC, who have an indication for LT, the ideal timing for antiviral therapy (before or after LT) remains debated. The EASL243 recommends that HCV-related HCC patients on the transplant waiting list, LT must be considered as the main therapeutic goal, and the antiviral treatment decision must be made on a case-by-case basis through a multidisciplinary discussion. Antiviral treatment with DAAs can be initiated before LT to prevent recurrence of infection, provided that it does not interfere with the management of the patient on the waiting list. Antiviral treatment can also be delayed until after transplantation, when there likelihood of a higher SVR than pretransplant period.243
HBV-related HCC
There is now enough evidence that HBV-related HCC should be given adjuvant antiviral therapy along with curative or palliative therapy for HCC. In a meta-analysis that included 21 studies containing 8072 patients found that adjuvant nucleoside/nucleotide analog (NA) significantly improved RFS and OS after curative treatment.244 Another meta-analysis found significant improvements for the OS and PFS in the NA-treated group compared with the control group for patients with HBV-related HCC after unresectable treatment.245 Antiviral agents with high genetic barrier to resistance (entecavir and TDF) should be used as adjuvant therapy because only they reduced the risk of HCC recurrence compared with other antivirals, especially in patients with high baseline viral load.246
HBsAg negative, anti-HBcAb positive patients are at risk of HBV reactivation post TACE.247 Hence anti-HBc should be tested prior to TACE in all patients who are HBsAg negative. If anti-HBc is positive, serum HBV DNA should be tested, and if found detectable, the patient should receive pre-emptive antiviral prophylaxis with entecavir or tenofovir. If anti-HBc is positive, but serum HBV DNA is negative, and these patients should be monitored with HBsAg, alanine transaminase (ALT), and HBV DNA testing every 3 months during TACE therapy and up to 6 months after. Pre-emptive antiviral therapy with entecavir or tenofovir should be started immediately on detection of HBsAg or HBV DNA positivity.248
| Consensus statements | Level | Grade |
|---|---|---|
|
II-2 | Weak |
|
I | Strong |
|
II-2 | Weak |
Pediatric aspects of HCC
HCC is a rare malignancy in childhood. However, it is the second most common primary liver cancer after hepatoblastoma in older children and adolescents.249 It accounts for about 27% of primary liver cancers in children.250 The tumor may be seen in the setting of chronic liver disease or de novo without any underlying liver cirrhosis. In contrast to adults, most pediatric HCC arise de-novo, without underlying liver cirrhosis.
In a study from Delhi on 35 liver explants,251 2 patients (5.7%) had evidence of HCC when the preop ultrasonography (USG) and AFP did not suggest the presence of HCC. Thorough examination of the explant was recommended as presence of HCC would imply postop screening for recurrence. In another study from Chennai, 8 of 12 HCCs studied were discovered on the explant (66.7%) with majority being secondary to metabolic liver disease and none secondary to hepatitis B.252 A case report of a 12-year-old child with Budd–Chiari syndrome who had undergone transjugular intrahepatic portosystemic shunt (TIPS) and presenting with a ruptured HCC has been described from Mumbai.253
Etiology
The liver disease could be of viral, cholestatic, or metabolic etiology. HBV has been reported to be the causative agent of HCC in 64% children with HCC in Hong Kong and is a major cause of HCC in countries with high prevalence of HBV.254 However, with HBV vaccination of the neonates, the incidence of HCC has declined over the years.255 HCV does not cause HCC in children or young adults. HCCs are described in the setting of Budd–Chiari syndrome in adults but also reported in children.253 There is a high risk of developing HCCs in childhood or adolescence in patients with tyrosinemia type 1, a condition associated with liver failure in infancy or cirrhosis at a later stage in childhood. A tyrosine, phenylalanine restricted diet and use of nitisinone significantly reduces the risk of development of HCC.256 Biliary atresia may be predisposed to development of a malignancy albeit rare, even after the child has had a successful Kasai surgery as these children often develop cirrhosis.257 Patients with progressive familial intrahepatic cholestasis (PFIC) type 2 present with pruritus and jaundice secondary to bile salt export pump deficiency and are at up to 10% risk for development of HCC by 2 years of age.258 In cholestatic syndromes that may not be associated with cirrhosis like Alagille's, PFIC 1, and PFIC 3, there is low risk of development of a liver malignancy.259 Patients with glycogen storage disorders (GSDs) type I develop adenomas usually at puberty which may transform in to malignancy.260 GSD type IV patients rapidly progress to cirrhosis and are therefore at high risk of HCC.261 Untreated Gaucher disease has a higher incidence of development hepatocellular carcinoma.262
Diagnosis and differential diagnosis
Investigations for diagnosis do not differ from those in adults. Liver biopsy is recommended in those without underlying cirrhosis and features suspicious of malignancy. Liver space occupying lesions in the children include hemangiomas, mesenchymal hamartomas, focal nodular hyperplasia, and adenomas, as well as malignant tumors, most commonly hepatoblastoma (HB) or metastases from distant primaries.
Hepatoblastomas are the commonest primary liver tumors in children. Hepatoblastoma can be a part of multisystem syndromes such as Beckwith-Wiedemann overgrowth disorder, which may be associated with several childhood malignancies. HB can also occur in very low birth weight children.263 HB typically occurs in children less than 5 years and usually less than 2 years of age in the absence of underlying cirrhosis. If there is cirrhosis, HCC needs to be considered. The presence of fetal type of epithelial cells in a HB makes differentiation from a HCC extremely difficult on histopathology. On the other hand, in the presence of embryonal type of epithelial or mesenchymal cells, histopathological differentiation from an HCC is easily possible.264 HCCs may histologically have features transitioning between a hepatoblastoma and a HCC, and these were earlier referred to as “transitional liver cell tumors”.265 The recent pediatric liver tumor consensus classification mentions “hepatocellular neoplasm not otherwise specified” to highlight this group of tumors.266
Prognosis
A pretreatment extent of disease, a staging, and risk stratification system for liver tumors has been designed by the International Childhood Liver Tumor Strategy Group to describe the radiographic appearance of tumors before treatment and can prognosticate the survival.267
Prevention
Regular 6-monthly surveillance with ultrasonography and AFP in diseases known to be associated with HCC is warranted.
Therapy
Surgical resection
Surgical resection is the best option as in adults. Unlike adults where systemic chemotherapy has limited benefit, childhood HCCs have shown response rates of 50% with neoadjuvant combination chemotherapy of cisplatin and doxorubicin (PLADO).268 The better response may be related to “de novo” tumors and normal liver function. Also, the tumor may have elements of a hepatoblastoma, thereby improving response to chemotherapy. Pediatric liver tumor specialists currently recommend that children with HCC should receive PLADO with or without sorafenib, as more intensive regimens have not yielded better results. But the role of postoperative chemotherapy in pediatric patients is unknown. The suggestion is that this therapy may be used as neoadjuvant before resection or in those with unresectable or metastatic disease.269,270
TACE
In a recent retrospective study of 65 children with HCC from China,271 the authors concluded that HCCs in children presented in a more advanced stage compared with adults with a 5 year survival of 15.8% Multiple tumors were seen in 61.5%, 30.8% had portal vein tumor thrombus and 16.9% had distant metastasis. In advanced-stage HCC, the overall survival of patients who underwent TACE was longer than that of patients who underwent supportive therapy. Very few case series for TACE exist.
Liver transplantation
There is limited experience in LT for pediatric HCCs with survival ranging from 25 to 60% at 5 years in unresectable tumors.272,273 The American Association for Transplantation and the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition recommend that LT in childhood HCC could be considered in children with no extrahepatic tumor or gross vascular invasion on imaging, irrespective of the number, or size of the lesions. Therefore, Milan criteria may not hold, and each patient needs to be considered individually.274,275
Long-term survival
McAteer et al.276 reported unadjusted 5-year survival for the younger children (0–4 year) of 53% was better than 32%for older children. It was also better in males (40%) vs. females (26%). Asian children fared worse (13%), than white (33%) and black (46%) children. The United Network for Organ Sharing transplant survival data revealed in 152 transplants for liver tumors, and there were 43 transplants for HCC. The respective 1-year, 5-year, and 10-year patient survivals after LT were 86%, 63%, and 58%, respectively.277
| Consensus statements | Level | Grade |
|---|---|---|
|
I | |
|
I | |
|
I | |
|
II-2 | |
|
I | Strong |
|
I | Strong |
|
II-2 | Weak |
|
II-3 | |
|
II-3 | Weak |
|
II-3 | |
|
II-2 | Weak |
TACE, transarterial chemoembolization; HCC, hepatocellular carcinoma; RFA, radiofrequency ablation; PFIC, progressive familial intrahepatic cholestasis.
These updated guidelines provide a data-supported approach to the diagnosis, staging, and management of patients of HCC in India as of 2019. They are aimed at providing the best possible care to the patients of HCC in India according to the current evidence. They are also aimed to ensure a uniformity of diagnostic and treatment approaches of HCC in the entire country and to serve as framework for future research on HCC in India. As more evidence is generated, especially from India, in next 3–4 years, these guidelines will need to be further updated and revised.
Conflicts of interest
The authors have none to declare.
References
- 1.Kumar A., Acharya S.K., Singh S.P. The Indian national association for study of the liver (INASL) consensus on prevention, diagnosis and management of hepatocellular carcinoma in India: the Puri recommendations. J Clin Exp Hepatol. 2014;4(suppl 3):S3–S26. doi: 10.1016/j.jceh.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Three year report of PBCR 2012-2014. http://ncdirindia.org/NCRP/ALL_NCRP_REPORTS/PBCR_REPORT_2012_2014/ALL_CONTENT/Printed_Version.htm. Accessed August 12, 2018.
- 3.Younossi Z., Stepanova M., Ong J.P. Nonalcoholic steatohepatitis is the fastest growing cause of hepatocellular carcinoma in liver transplant candidates. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2019;17:748–755. doi: 10.1016/j.cgh.2018.05.057. e3. [DOI] [PubMed] [Google Scholar]
- 4.Marrero J.A., Kulik L.M., Sirlin C.B. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American association for the study of liver diseases. Hepatol Baltim Md. 2018;68:723–750. doi: 10.1002/hep.29913. [DOI] [PubMed] [Google Scholar]
- 5.Heimbach J.K., Kulik L.M., Finn R.S. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatol Baltim Md. 2018;67:358–380. doi: 10.1002/hep.29086. [DOI] [PubMed] [Google Scholar]
- 6.Forner A., Reig M., Varela M. Diagnosis and treatment of hepatocellular carcinoma. Update consensus document from the AEEH, SEOM, SERAM, SERVEI and SETH. Med Clin (Barc). 2016;146:511.e1–511.e22. doi: 10.1016/j.medcli.2016.01.028. [DOI] [PubMed] [Google Scholar]
- 7.Carrilho F.J., Mattos A.A. de, Vianey A.F. Brazilian society of hepatology recommendations for the diagnosis and treatment of hepatocellular carcinoma. Arq Gastroenterol. 2015;52(suppl 1):2–14. doi: 10.1590/S0004-28032015000500001. [DOI] [PubMed] [Google Scholar]
- 8.Korean Liver Cancer Study Group (KLCSG), National Cancer Center, Korea (NCC) 2014 Korean liver cancer study group-national cancer center Korea practice guideline for the management of hepatocellular carcinoma. Korean J Radiol. 2015;16:465–522. doi: 10.3348/kjr.2015.16.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Korean Liver Cancer Study Group (KLCSG), National Cancer Center, Korea (NCC) 2014 KLCSG-NCC Korea practice guideline for the management of hepatocellular carcinoma. Gut Liver. 2015;9:267–317. doi: 10.5009/gnl14460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kudo M., Matsui O., Izumi N. Surveillance and diagnostic algorithm for hepatocellular carcinoma proposed by the Liver Cancer Study Group of Japan: 2014 update. Oncology. 2014;87(suppl 1):7–21. doi: 10.1159/000368141. [DOI] [PubMed] [Google Scholar]
- 11.European Association for the Study of the Liver Electronic address: easloffice@easloffice.eu, European association for the study of the liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 12.Atkins D., Best D., Briss P.A. Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Global Burden of Disease Liver Cancer Collaboration. Akinyemiju T., Abera S. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: results from the global burden of disease study 2015. JAMA Oncol. 2017;3:1683–1691. doi: 10.1001/jamaoncol.2017.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paul S.B., Sreenivas V., Gulati M.S. Incidence of hepatocellular carcinoma among Indian patients with cirrhosis of liver: an experience from a tertiary care center in northern India. Indian J Gastroenterol Off J Indian Soc Gastroenterol. 2007;26:274–278. [PubMed] [Google Scholar]
- 15.Dika I.E., Harding J.J., Abou-Alfa G.K. Hepatocellular carcinoma in patients with HIV. Curr Opin HIV AIDS. 2017;12:20–25. doi: 10.1097/COH.0000000000000335. [DOI] [PubMed] [Google Scholar]
- 16.Li L., Lan X. Association between hepatitis B virus/hepatitis C virus infection and primary hepatocellular carcinoma risk: a meta-analysis based on Chinese population. J Cancer Res Ther. 2016;12(suppl ment):C284–C287. doi: 10.4103/0973-1482.200763. [DOI] [PubMed] [Google Scholar]
- 17.Donato F., Tagger A., Chiesa R. Hepatitis B and C virus infection, alcohol drinking, and hepatocellular carcinoma: a case-control study in Italy. Brescia HCC Study. Hepatol Baltim Md. 1997;26:579–584. doi: 10.1002/hep.510260308. [DOI] [PubMed] [Google Scholar]
- 18.Ohki T., Tateishi R., Sato T. Obesity is an independent risk factor for hepatocellular carcinoma development in chronic hepatitis C patients. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2008;6:459–464. doi: 10.1016/j.cgh.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 19.Dyal H.K., Aguilar M., Bartos G. Diabetes mellitus increases risk of hepatocellular carcinoma in chronic hepatitis C virus patients: a systematic review. Dig Dis Sci. 2016;61:636–645. doi: 10.1007/s10620-015-3983-3. [DOI] [PubMed] [Google Scholar]
- 20.Moudgil V., Redhu D., Dhanda S., Singh J. A review of molecular mechanisms in the development of hepatocellular carcinoma by aflatoxin and hepatitis B and C viruses. J Environ Pathol Toxicol Oncol Off Organ Int Soc Environ Toxicol Cancer. 2013;32:165–175. doi: 10.1615/jenvironpatholtoxicoloncol.2013007166. [DOI] [PubMed] [Google Scholar]
- 21.Asim M., Sarma M.P., Thayumanavan L., Kar P. Role of aflatoxin B1 as a risk for primary liver cancer in north Indian population. Clin Biochem. 2011;44:1235–1240. doi: 10.1016/j.clinbiochem.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 22.Kumar M., Kumar R., Hissar S.S. Risk factors analysis for hepatocellular carcinoma in patients with and without cirrhosis: a case-control study of 213 hepatocellular carcinoma patients from India. J Gastroenterol Hepatol. 2007;22:1104–1111. doi: 10.1111/j.1440-1746.2007.04908.x. [DOI] [PubMed] [Google Scholar]
- 23.Paul S.B., Chalamalasetty S.B., Vishnubhatla S. Clinical profile, etiology and therapeutic outcome in 324 hepatocellular carcinoma patients at a tertiary care center in India. Oncology. 2009;77:162–171. doi: 10.1159/000231886. [DOI] [PubMed] [Google Scholar]
- 24.Pal S., Ramachandran J., Kurien R.T. Hepatocellular carcinoma continues to be diagnosed in the advanced stage: profile of hepatocellular carcinoma in a tertiary care hospital in South India. Trop Dr. 2013;43:25–26. doi: 10.1177/0049475512473600. [DOI] [PubMed] [Google Scholar]
- 25.Sarma M.P., Asim M., Medhi S., Bharathi T., Diwan R., Kar P. Viral genotypes and associated risk factors of hepatocellular carcinoma in India. Cancer Biol Med. 2012;9:172–181. doi: 10.7497/j.issn.2095-3941.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arora A., Sharma P., Tyagi P. Hepatitis B virus infection can cause hepatocellular carcinoma in less advanced liver cirrhosis: a comparative study of 142 patients from north India. J Clin Exp Hepatol. 2013;3:288–295. doi: 10.1016/j.jceh.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang H.-I., Lu S.-N., Liaw Y.-F. Hepatitis B e antigen and the risk of hepatocellular carcinoma. N Engl J Med. 2002;347:168–174. doi: 10.1056/NEJMoa013215. [DOI] [PubMed] [Google Scholar]
- 28.Vivekanandan P., Torbenson M., Ramakrishna B. Hepatitis B virus-associated hepatocellular carcinoma from India: role of viral genotype and mutations in CTNNB1 (beta-catenin) and TP53 genes. J Gastrointest Cancer. 2011;42:20–25. doi: 10.1007/s12029-010-9222-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Asim M., Malik A., Sarma M.P. Hepatitis B virus BCP, Precore/core, X gene mutations/genotypes and the risk of hepatocellular carcinoma in India. J Med Virol. 2010;82:1115–1125. doi: 10.1002/jmv.21774. [DOI] [PubMed] [Google Scholar]
- 30.Xiao Q., Fu B., Chen P., Liu Z.Z., Wang W., Ye Q. Three polymorphisms of tumor necrosis factor-alpha and hepatitis B virus related hepatocellular carcinoma: a meta-analysis. Medicine (Baltim) 2016;95 doi: 10.1097/MD.0000000000005609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bruden D.J.T., McMahon B.J., Townshend-Bulson L. Risk of end-stage liver disease, hepatocellular carcinoma, and liver-related death by fibrosis stage in the hepatitis C Alaska Cohort. Hepatol Baltim Md. 2017;66:37–45. doi: 10.1002/hep.29115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lewis S., Roayaie S., Ward S.C., Shyknevsky I., Jibara G., Taouli B. Hepatocellular carcinoma in chronic hepatitis C in the absence of advanced fibrosis or cirrhosis. AJR Am J Roentgenol. 2013;200:W610–W616. doi: 10.2214/AJR.12.9151. [DOI] [PubMed] [Google Scholar]
- 33.Caselmann W.H., Alt M. Hepatitis C virus infection as a major risk factor for hepatocellular carcinoma. J Hepatol. 1996;24(2 suppl l):61–66. [PubMed] [Google Scholar]
- 34.Sarma M.P., Asim M., Medhi S., Bharathi T., Kar P. Hepatitis C virus related hepatocellular carcinoma: a case control study from India. J Med Virol. 2012;84:1009–1017. doi: 10.1002/jmv.23290. [DOI] [PubMed] [Google Scholar]
- 35.Kozbial K., Moser S., Schwarzer R. Unexpected high incidence of hepatocellular carcinoma in cirrhotic patients with sustained virologic response following interferon-free direct-acting antiviral treatment. J Hepatol. 2016;65:856–858. doi: 10.1016/j.jhep.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 36.Cabibbo G., Petta S., Barbàra M. A meta-analysis of single HCV-untreated arm of studies evaluating outcomes after curative treatments of HCV-related hepatocellular carcinoma. Liver Int Off J Int Assoc Study Liver. 2017;37:1157–1166. doi: 10.1111/liv.13357. [DOI] [PubMed] [Google Scholar]
- 37.Waziry R., Hajarizadeh B., Grebely J. Hepatocellular carcinoma risk following direct-acting antiviral HCV therapy: a systematic review, meta-analyses, and meta-regression. J Hepatol. 2017;67:1204–1212. doi: 10.1016/j.jhep.2017.07.025. [DOI] [PubMed] [Google Scholar]
- 38.Guarino M., Sessa A., Cossiga V. Direct-acting antivirals and hepatocellular carcinoma in chronic hepatitis C: a few lights and many shadows. World J Gastroenterol. 2018;24:2582–2595. doi: 10.3748/wjg.v24.i24.2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Joshi K., Kohli A., Manch R., Gish R. Alcoholic liver disease: high risk or low risk for developing hepatocellular carcinoma? Clin Liver Dis. 2016;20:563–580. doi: 10.1016/j.cld.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 40.El-Serag H.B., Hampel H., Javadi F. The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2006;4:369–380. doi: 10.1016/j.cgh.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 41.Chen C.-L., Yang H.-I., Yang W.-S. Metabolic factors and risk of hepatocellular carcinoma by chronic hepatitis B/C infection: a follow-up study in Taiwan. Gastroenterology. 2008;135:111–121. doi: 10.1053/j.gastro.2008.03.073. [DOI] [PubMed] [Google Scholar]
- 42.Xu W.-G., Qian Y.-F., Wu J. The effect of prediabetes on hepatocellular carcinoma risk: a systematic review and meta-analysis. Minerva Med. 2017;108:185–190. doi: 10.23736/S0026-4806.16.04601-2. [DOI] [PubMed] [Google Scholar]
- 43.Calle E.E., Rodriguez C., Walker-Thurmond K., Thun M.J. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 44.White D.L., Kanwal F., El-Serag H.B. Association between nonalcoholic fatty liver disease and risk for hepatocellular cancer, based on systematic review. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2012;10:1342–1359. doi: 10.1016/j.cgh.2012.10.001. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marrero J.A., Fontana R.J., Su G.L., Conjeevaram H.S., Emick D.M., Lok A.S. NAFLD may be a common underlying liver disease in patients with hepatocellular carcinoma in the United States. Hepatol Baltim Md. 2002;36:1349–1354. doi: 10.1053/jhep.2002.36939. [DOI] [PubMed] [Google Scholar]
- 46.Duseja A., Sharma B., Kumar A. Nonalcoholic fatty liver in a developing country is responsible for significant liver disease. Hepatol Baltim Md. 2010;52:2248–2249. doi: 10.1002/hep.23838. [DOI] [PubMed] [Google Scholar]
- 47.David D., Raghavendran A., Goel A. Risk factors for non-alcoholic fatty liver disease are common in patients with non-B non-C hepatocellular carcinoma in India. Indian J Gastroenterol Off J Indian Soc Gastroenterol. 2017;36:373–379. doi: 10.1007/s12664-017-0785-x. [DOI] [PubMed] [Google Scholar]
- 48.Kawada N., Imanaka K., Kawaguchi T. Hepatocellular carcinoma arising from non-cirrhotic nonalcoholic steatohepatitis. J Gastroenterol. 2009;44:1190–1194. doi: 10.1007/s00535-009-0112-0. [DOI] [PubMed] [Google Scholar]
- 49.Issa D., Alkhouri N. Nonalcoholic fatty liver disease and hepatocellular carcinoma: new insights on presentation and natural history. Hepatobiliary Surg Nutr. 2017;6:401–403. doi: 10.21037/hbsn.2017.07.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Younossi Z.M., Koenig A.B., Abdelatif D., Fazel Y., Henry L., Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatol Baltim Md. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 51.Tansel A., Katz L.H., El-Serag H.B. Incidence and determinants of hepatocellular carcinoma in autoimmune hepatitis: a systematic review and meta-analysis. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2017;15:1207–1217. doi: 10.1016/j.cgh.2017.02.006. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dong S., Miao A.-Y., Lei W., Chen Q.-W. miR-146a rs2910164 and hepatocellular carcinoma: a meta-analysis. Minerva Med. 2017;108:287–292. doi: 10.23736/S0026-4806.16.04764-9. [DOI] [PubMed] [Google Scholar]
- 53.Yu J.-Y., Hu F., Du W., Ma X.-L., Yuan K. Study of the association between five polymorphisms and risk of hepatocellular carcinoma: a meta-analysis. J Chin Med Assoc JCMA. 2017;80:191–203. doi: 10.1016/j.jcma.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 54.Liu L., Guo W., Zhang J. Association of HLA-DRB1 gene polymorphisms with hepatocellular carcinoma risk: a meta-analysis. Minerva Med. 2017;108:176–184. doi: 10.23736/S0026-4806.16.04571-7. [DOI] [PubMed] [Google Scholar]
- 55.Shi Y.-H., Wang B., Xu B.-P. The association of six non-synonymous variants in three DNA repair genes with hepatocellular carcinoma risk: a meta-analysis. J Cell Mol Med. 2016;20:2056–2063. doi: 10.1111/jcmm.12896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Asim M., Khan L.A., Husain S.A. Genetic polymorphism of glutathione S transferases M1 and T1 in Indian patients with hepatocellular carcinoma. Dis Markers. 2010;28:369–376. doi: 10.3233/DMA-2010-0717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bierer B.E. Universal precautions: necessary safety procedures when handling human blood, body fluids, and specimens. Curr Protoc Im. 2017;118:A.3P.1–A.3P.3. doi: 10.1002/cpim.29. [DOI] [PubMed] [Google Scholar]
- 58.Ahmed R., Bhattacharya S. Universal screening versus universal precautions in the context of preoperative screening for HIV, HBV, HCV in India. Indian J Med Microbiol. 2013;31:219–225. doi: 10.4103/0255-0857.115623. [DOI] [PubMed] [Google Scholar]
- 59.Hepatitis B vaccines: WHO position paper—July 2017. Releve Epidemiol Hebd. 2017;92:369–392. [PubMed] [Google Scholar]
- 60.Papatheodoridis G.V., Chan H.L.-Y., Hansen B.E., Janssen H.L.A., Lampertico P. Risk of hepatocellular carcinoma in chronic hepatitis B: assessment and modification with current antiviral therapy. J Hepatol. 2015;62:956–967. doi: 10.1016/j.jhep.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 61.Vlachogiannakos J., Papatheodoridis G. Hepatocellular carcinoma in chronic hepatitis B patients under antiviral therapy. World J Gastroenterol. 2013;19:8822–8830. doi: 10.3748/wjg.v19.i47.8822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bang C.S., Song I.H. Impact of antiviral therapy on hepatocellular carcinoma and mortality in patients with chronic hepatitis C: systematic review and meta-analysis. BMC Gastroenterol. 2017;17:46. doi: 10.1186/s12876-017-0606-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Saran U., Humar B., Kolly P., Dufour J.-F. Hepatocellular carcinoma and lifestyles. J Hepatol. 2016;64:203–214. doi: 10.1016/j.jhep.2015.08.028. [DOI] [PubMed] [Google Scholar]
- 64.Reeves H.L., Zaki M.Y.W., Day C.P. Hepatocellular carcinoma in obesity, type 2 diabetes, and NAFLD. Dig Dis Sci. 2016;61:1234–1245. doi: 10.1007/s10620-016-4085-6. [DOI] [PubMed] [Google Scholar]
- 65.Ramadori P., Cubero F.J., Liedtke C., Trautwein C., Nevzorova Y.A. Alcohol and hepatocellular carcinoma: adding fuel to the flame. Cancers. 2017;9 doi: 10.3390/cancers9100130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McKillop I.H., Schrum L.W., Thompson K.J. Role of alcohol in the development and progression of hepatocellular carcinoma. Hepatic Oncol. 2016;3:29–43. doi: 10.2217/hep.15.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Alcohol drinking. IARC Working Group Lyon, 13-20 October 1987. IARC Monogr Eval Carcinog Risks Hum. 1988;44:1–378. [PMC free article] [PubMed] [Google Scholar]
- 68.Petrick J.L., Campbell P.T., Koshiol J. Tobacco, alcohol use and risk of hepatocellular carcinoma and intrahepatic cholangiocarcinoma: the Liver Cancer Pooling Project. Br J Canc. 2018;118:1005–1012. doi: 10.1038/s41416-018-0007-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Testino G., Leone S., Borro P. Alcohol and hepatocellular carcinoma: a review and a point of view. World J Gastroenterol. 2014;20:15943–15954. doi: 10.3748/wjg.v20.i43.15943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Heckley G.A., Jarl J., Asamoah B.O., G-Gerdtham U How the risk of liver cancer changes after alcohol cessation: a review and meta-analysis of the current literature. BMC Canc. 2011;11:446. doi: 10.1186/1471-2407-11-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.European Association for the Study of the Liver Electronic address: easloffice@easloffice.eu, European association for the study of the liver. EASL clinical practice guidelines: management of alcohol-related liver disease. J Hepatol. 2018;69:154–181. doi: 10.1016/j.jhep.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 72.Singh S., Singh P.P., Singh A.G., Murad M.H., Sanchez W. Statins are associated with a reduced risk of hepatocellular cancer: a systematic review and meta-analysis. Gastroenterology. 2013;144:323–332. doi: 10.1053/j.gastro.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 73.Nishio T., Taura K., Nakamura N. Impact of statin use on the prognosis of patients with hepatocellular carcinoma undergoing liver resection: a subgroup analysis of patients without chronic hepatitis viral infection. Surgery. 2018;163:264–269. doi: 10.1016/j.surg.2017.09.026. [DOI] [PubMed] [Google Scholar]
- 74.Yi C., Song Z., Wan M., Chen Y., Cheng X. Statins intake and risk of liver cancer: a dose-response meta analysis of prospective cohort studies. Medicine (Baltim) 2017;96 doi: 10.1097/MD.0000000000007435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bravi F., Tavani A., Bosetti C., Boffetta P., La Vecchia C. Coffee and the risk of hepatocellular carcinoma and chronic liver disease: a systematic review and meta-analysis of prospective studies. Eur J Cancer Prev Off J Eur Cancer Prev Organ ECP. 2017;26:368–377. doi: 10.1097/CEJ.0000000000000252. [DOI] [PubMed] [Google Scholar]
- 76.Kennedy O.J., Roderick P., Buchanan R., Fallowfield J.A., Hayes P.C., Parkes J. Coffee, including caffeinated and decaffeinated coffee, and the risk of hepatocellular carcinoma: a systematic review and dose-response meta-analysis. BMJ Open. 2017;7 doi: 10.1136/bmjopen-2016-013739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ni C.-X., Gong H., Liu Y., Qi Y., Jiang C.-L., Zhang J.-P. Green tea consumption and the risk of liver cancer: a meta-analysis. Nutr Cancer. 2017;69:211–220. doi: 10.1080/01635581.2017.1263754. [DOI] [PubMed] [Google Scholar]
- 78.Morales D.R., Morris A.D. Metformin in cancer treatment and prevention. Annu Rev Med. 2015;66:17–29. doi: 10.1146/annurev-med-062613-093128. [DOI] [PubMed] [Google Scholar]
- 79.Ma S., Zheng Y., Xiao Y., Zhou P., Tan H. Meta-analysis of studies using metformin as a reducer for liver cancer risk in diabetic patients. Medicine (Baltim) 2017;96 doi: 10.1097/MD.0000000000006888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Díaz-González Á., Forner A. Surveillance for hepatocellular carcinoma. Best Pract Res Clin Gastroenterol. 2016;30:1001–1010. doi: 10.1016/j.bpg.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 81.Panda D. Role of surveillance in prevention of hepatocellular carcinoma. J Clin Exp Hepatol. 2014;4(suppl 3):S43–S49. doi: 10.1016/j.jceh.2014.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tzartzeva K., Obi J., Rich N.E. Surveillance imaging and alpha fetoprotein for early detection of hepatocellular carcinoma in patients with cirrhosis: a meta-analysis. Gastroenterology. 2018;154:1706–1718. doi: 10.1053/j.gastro.2018.01.064. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Caviglia G.P., Ribaldone D.G., Abate M.L. Performance of protein induced by vitamin K absence or antagonist-II assessed by chemiluminescence enzyme immunoassay for hepatocellular carcinoma detection: a meta-analysis. Scand J Gastroenterol. April 2018:1–7. doi: 10.1080/00365521.2018.1459824. [DOI] [PubMed] [Google Scholar]
- 84.Singal A.G., Tiro J.A., Marrero J.A. Mailed outreach program increases ultrasound screening of patients with cirrhosis for hepatocellular carcinoma. Gastroenterology. 2017;152:608–615. doi: 10.1053/j.gastro.2016.10.042. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ayuso C., Rimola J., Vilana R. Diagnosis and staging of hepatocellular carcinoma (HCC): current guidelines. Eur J Radiol. 2018;101:72–81. doi: 10.1016/j.ejrad.2018.01.025. [DOI] [PubMed] [Google Scholar]
- 86.Duncan J.K., Ma N., Vreugdenburg T.D., Cameron A.L., Maddern G. Gadoxetic acid-enhanced MRI for the characterization of hepatocellular carcinoma: a systematic review and meta-analysis. J Magn Reson Imag JMRI. 2017;45:281–290. doi: 10.1002/jmri.25345. [DOI] [PubMed] [Google Scholar]
- 87.Huang J., Chen W., Yao S. Assessing diagnostic value of contrast-enhanced ultrasound and contrast-enhanced computed tomography in detecting small hepatocellular carcinoma: a meta-analysis. Medicine (Baltim) 2017;96 doi: 10.1097/MD.0000000000007555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Deng H., Shi H., Lei J., Hu Y., Li G., Wang C. A meta-analysis of contrast-enhanced ultrasound for small hepatocellular carcinoma diagnosis. J Cancer Res Ther. 2016;12(suppl ment):C274–C276. doi: 10.4103/0973-1482.200756. [DOI] [PubMed] [Google Scholar]
- 89.Quaglia A. Hepatocellular carcinoma: a review of diagnostic challenges for the pathologist. J Hepatocell Carcinoma. 2018;5:99–108. doi: 10.2147/JHC.S159808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rastogi A. Changing role of histopathology in the diagnosis and management of hepatocellular carcinoma. World J Gastroenterol. 2018;24:4000–4013. doi: 10.3748/wjg.v24.i35.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Edmondson H.A., Steiner P.E. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer. 1954;7:462–503. doi: 10.1002/1097-0142(195405)7:3<462::aid-cncr2820070308>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 92.Martins-Filho S.N., Paiva C., Azevedo R.S., Alves V.A.F. Histological grading of hepatocellular carcinoma-A systematic review of literature. Front Med. 2017;4:193. doi: 10.3389/fmed.2017.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sparchez Z., Mocan T. Contemporary role of liver biopsy in hepatocellular carcinoma. World J Hepatol. 2018;10:452–461. doi: 10.4254/wjh.v10.i7.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Silva M.A., Hegab B., Hyde C., Guo B., Buckels J.a.C., Mirza D.F. Needle track seeding following biopsy of liver lesions in the diagnosis of hepatocellular cancer: a systematic review and meta-analysis. Gut. 2008;57:1592–1596. doi: 10.1136/gut.2008.149062. [DOI] [PubMed] [Google Scholar]
- 95.Dubash S.R., Idowu O.A., Sharma R. The emerging role of positron emission tomography in hepatocellular carcinoma. Hepatic Oncol. 2015;2:191–200. doi: 10.2217/hep.15.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liao X., Wei J., Li Y. 18F-FDG PET with or without CT in the diagnosis of extrahepatic metastases or local residual/recurrent hepatocellular carcinoma. Medicine (Baltim) 2018;97 doi: 10.1097/MD.0000000000011970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Na S.J., Oh J.K., Hyun S.H. 18F-FDG PET/CT can predict survival of advanced hepatocellular carcinoma patients: a multicenter retrospective cohort study. J Nucl Med Off Publ Soc Nucl Med. 2017;58:730–736. doi: 10.2967/jnumed.116.182022. [DOI] [PubMed] [Google Scholar]
- 98.Yaprak O., Acar S., Ertugrul G., Dayangac M. Role of pre-transplant 18F-FDG PET/CT in predicting hepatocellular carcinoma recurrence after liver transplantation. World J Gastrointest Oncol. 2018;10:336–343. doi: 10.4251/wjgo.v10.i10.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Johnson P.J. The role of serum alpha-fetoprotein estimation in the diagnosis and management of hepatocellular carcinoma. Clin Liver Dis. 2001;5:145–159. doi: 10.1016/s1089-3261(05)70158-6. [DOI] [PubMed] [Google Scholar]
- 100.Nomura F., Ohnishi K., Tanabe Y. Clinical features and prognosis of hepatocellular carcinoma with reference to serum alpha-fetoprotein levels. Analysis of 606 patients. Cancer. 1989;64:1700–1707. doi: 10.1002/1097-0142(19891015)64:8<1700::aid-cncr2820640824>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 101.Hakeem A.R., Young R.S., Marangoni G., Lodge J.P.A., Prasad K.R. Systematic review: the prognostic role of alpha-fetoprotein following liver transplantation for hepatocellular carcinoma. Aliment Pharmacol Ther. 2012;35:987–999. doi: 10.1111/j.1365-2036.2012.05060.x. [DOI] [PubMed] [Google Scholar]
- 102.Kudo M., Izumi N., Kokudo N. Management of hepatocellular carcinoma in Japan: consensus-based clinical practice guidelines proposed by the Japan society of hepatology (JSH) 2010 updated version. Dig Dis Basel Switz. 2011;29:339–364. doi: 10.1159/000327577. [DOI] [PubMed] [Google Scholar]
- 103.Xing H., Yan C., Cheng L. Clinical application of protein induced by vitamin K antagonist-II as a biomarker in hepatocellular carcinoma. Tumour Biol J Int Soc Oncodevelopmental Biol Med. October 2016 doi: 10.1007/s13277-016-5443-x. [DOI] [PubMed] [Google Scholar]
- 104.Zheng J., Cai J., Li H. Neutrophil to lymphocyte ratio and platelet to lymphocyte ratio as prognostic predictors for hepatocellular carcinoma patients with various treatments: a meta-analysis and systematic review. Cell Physiol Biochem Int J Exp Cell Physiol Biochem Pharmacol. 2017;44:967–981. doi: 10.1159/000485396. [DOI] [PubMed] [Google Scholar]
- 105.Zhao Y., Si G., Zhu F. Prognostic role of platelet to lymphocyte ratio in hepatocellular carcinoma: a systematic review and meta-analysis. Oncotarget. 2017;8:22854–22862. doi: 10.18632/oncotarget.15281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Song W., Wang K., Zhong F.-P., Fan Y.-W., Peng L., Zou S.-B. Clinicopathological and prognostic significance of platelet-to-lymphocyte ratio in patients with hepatocellular carcinoma. Oncotarget. 2016;7:81830–81838. doi: 10.18632/oncotarget.13244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hao Q.-Q., Chen G.-Y., Zhang J.-H., Sheng J.-H., Gao Y. Diagnostic value of long noncoding RNAs for hepatocellular carcinoma: a PRISMA-compliant meta-analysis. Medicine (Baltim) 2017;96 doi: 10.1097/MD.0000000000007496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zheng C., Hao H., Chen L., Shao J. Long noncoding RNAs as novel serum biomarkers for the diagnosis of hepatocellular carcinoma: a systematic review and meta-analysis. Clin Transl Oncol Off Publ Fed Span Oncol Soc Natl Cancer Inst Mex. 2017;19:961–968. doi: 10.1007/s12094-017-1626-1. [DOI] [PubMed] [Google Scholar]
- 109.Zucman-Rossi J. Molecular classification of hepatocellular carcinoma. Dig Liver Dis Off J Ital Soc Gastroenterol Ital Assoc Study Liver. 2010;42(suppl 3):S235–S241. doi: 10.1016/S1590-8658(10)60511-7. [DOI] [PubMed] [Google Scholar]
- 110.Díaz-González Á., Reig M., Bruix J. Treatment of hepatocellular carcinoma. Dig Dis Basel Switz. 2016;34:597–602. doi: 10.1159/000445275. [DOI] [PubMed] [Google Scholar]
- 111.Tan Y., Zhang W., Jiang L., Yang J., Yan L. Efficacy and safety of anatomic resection versus nonanatomic resection in patients with hepatocellular carcinoma: a systemic review and meta-analysis. PLoS One. 2017;12 doi: 10.1371/journal.pone.0186930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hao S., Chen S., Tu C., Huang T. Anterior approach to improve the prognosis in HCC patients via decreasing dissemination of EpCAM+ circulating tumor cells. J Gastrointest Surg Off J Soc Surg Aliment Tract. 2017;21:1112–1120. doi: 10.1007/s11605-017-3410-5. [DOI] [PubMed] [Google Scholar]
- 113.Hao S., Fan P., Chen S., Tu C., Wan C. Anterior approach to improve the long-term outcome in patients with large-size hepatocellular carcinoma having liver resection. J Surg Oncol. 2016;114:872–878. doi: 10.1002/jso.24433. [DOI] [PubMed] [Google Scholar]
- 114.Twaij A., Pucher P.H., Sodergren M.H., Gall T., Darzi A., Jiao L.R. Laparoscopic vs open approach to resection of hepatocellular carcinoma in patients with known cirrhosis: systematic review and meta-analysis. World J Gastroenterol. 2014;20:8274–8281. doi: 10.3748/wjg.v20.i25.8274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li W., Zhou X., Huang Z. Laparoscopic surgery minimizes the release of circulating tumor cells compared to open surgery for hepatocellular carcinoma. Surg Endosc. 2015;29:3146–3153. doi: 10.1007/s00464-014-4041-5. [DOI] [PubMed] [Google Scholar]
- 116.Zhong F.-P., Zhang Y.-J., Liu Y., Zou S.-B. Prognostic impact of surgical margin in patients with hepatocellular carcinoma: a meta-analysis. Medicine (Baltim) 2017;96 doi: 10.1097/MD.0000000000008043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bouza C., López-Cuadrado T., Alcázar R., Saz-Parkinson Z., Amate J.M. Meta-analysis of percutaneous radiofrequency ablation versus ethanol injection in hepatocellular carcinoma. BMC Gastroenterol. 2009;9:31. doi: 10.1186/1471-230X-9-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yang B., Zan R., Wang S. Radiofrequency ablation versus percutaneous ethanol injection for hepatocellular carcinoma: a meta-analysis of randomized controlled trials. World J Surg Oncol. 2015;13:96. doi: 10.1186/s12957-015-0516-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhu Z.X., Liao M.H., Wang X.X., Huang J.W. Radiofrequency ablation with or without ethanol injection for hepatocellular carcinoma: a systematic review and meta-analysis. Minerva Med. 2016;107:381–391. [PubMed] [Google Scholar]
- 120.Facciorusso A., Di Maso M., Muscatiello N. Microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma: a systematic review and meta-analysis. Int J Hyperth Off J Eur Soc Hyperthermic Oncol North Am Hyperth Group. 2016;32:339–344. doi: 10.3109/02656736.2015.1127434. [DOI] [PubMed] [Google Scholar]
- 121.Kalra N., Gupta P., Gorsi U. Irreversible electroporation for unresectable hepatocellular carcinoma: initial experience. Cardiovasc Intervent Radiol. 2019;42:584–590. doi: 10.1007/s00270-019-02164-2. [DOI] [PubMed] [Google Scholar]
- 122.Bruix J., Takayama T., Mazzaferro V. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2015;16:1344–1354. doi: 10.1016/S1470-2045(15)00198-9. [DOI] [PubMed] [Google Scholar]
- 123.Ma K.W., Cheung T.T. When to consider liver transplantation in hepatocellular carcinoma patients? Hepatic Oncol. 2017;4:15–24. doi: 10.2217/hep-2016-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Trevisani F., Frigerio M., Santi V., Grignaschi A., Bernardi M. Hepatocellular carcinoma in non-cirrhotic liver: a reappraisal. Dig Liver Dis Off J Ital Soc Gastroenterol Ital Assoc Study Liver. 2010;42:341–347. doi: 10.1016/j.dld.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 125.Sotiropoulos G.C., Lang H., Frilling A. Resectability of hepatocellular carcinoma: evaluation of 333 consecutive cases at a single hepatobiliary specialty center and systematic review of the literature. Hepato-Gastroenterology. 2006;53:322–329. [PubMed] [Google Scholar]
- 126.Zhou Y., Lei X., Wu L., Wu X., Xu D., Li B. Outcomes of hepatectomy for noncirrhotic hepatocellular carcinoma: a systematic review. Surg Oncol. 2014;23:236–242. doi: 10.1016/j.suronc.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 127.Ng K.K.C., Chok K.S.H., Chan A.C.Y. Randomized clinical trial of hepatic resection versus radiofrequency ablation for early-stage hepatocellular carcinoma. Br J Surg. 2017;104:1775–1784. doi: 10.1002/bjs.10677. [DOI] [PubMed] [Google Scholar]
- 128.Jia J.B., Zhang D., Ludwig J.M., Kim H.S. Radiofrequency ablation versus resection for hepatocellular carcinoma in patients with Child-Pugh A liver cirrhosis: a meta-analysis. Clin Radiol. 2017;72:1066–1075. doi: 10.1016/j.crad.2017.07.024. [DOI] [PubMed] [Google Scholar]
- 129.Majumdar A., Roccarina D., Thorburn D., Davidson B.R., Tsochatzis E., Gurusamy K.S. Management of people with early- or very early-stage hepatocellular carcinoma: an attempted network meta-analysis. Cochrane Database Syst Rev. 2017;3:CD011650. doi: 10.1002/14651858.CD011650.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Menahem B., Lubrano J., Duvoux C. Liver transplantation versus liver resection for hepatocellular carcinoma in intention to treat: an attempt to perform an ideal meta-analysis. Liver Transplant Off Publ Am Assoc Study Liver Dis Int Liver Transplant Soc. 2017;23:836–844. doi: 10.1002/lt.24758. [DOI] [PubMed] [Google Scholar]
- 131.Zhang Z., Zhang Y., Wang W. Thrombocytopenia and the outcomes of hepatectomy for hepatocellular carcinoma: a meta-analysis. J Surg Res. 2017;210:99–107. doi: 10.1016/j.jss.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 132.Yi P.-S., Huang M., Zhang M., Xu L., Xu M.-Q. Comparison of transarterial chemoembolization combined with radiofrequency ablation therapy versus surgical resection for early hepatocellular carcinoma. Am Surg. 2018;84:282–288. [PubMed] [Google Scholar]
- 133.Kulik L., Heimbach J.K., Zaiem F. Therapies for patients with hepatocellular carcinoma awaiting liver transplantation: a systematic review and meta-analysis. Hepatol Baltim Md. 2018;67:381–400. doi: 10.1002/hep.29485. [DOI] [PubMed] [Google Scholar]
- 134.Huang X., Lu S. Impact of preoperative locoregional therapy on recurrence and patient survival following liver transplantation for hepatocellular carcinoma: a meta-analysis. Scand J Gastroenterol. 2017;52:143–149. doi: 10.1080/00365521.2016.1236396. [DOI] [PubMed] [Google Scholar]
- 135.Si T., Chen Y., Ma D. Transarterial chemoembolization prior to liver transplantation for patients with hepatocellular carcinoma: a meta-analysis. J Gastroenterol Hepatol. 2017;32:1286–1294. doi: 10.1111/jgh.13727. [DOI] [PubMed] [Google Scholar]
- 136.Shang J., Xu S., Zhang J., Ran X., Bai L., Tang H. Efficacy of sorafenib in patients with hepatocellular carcinoma after resection: a meta-analysis. Oncotarget. 2017;8:109723–109731. doi: 10.18632/oncotarget.21299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chen L., Ma X., Liu X., Cui X. Sorafenib combined with radiofrequency ablation as treatment for patients with hepatocellular carcinoma: a systematic review and meta-analysis. J BUON Off J Balk Union Oncol. 2017;22:1525–1532. [PubMed] [Google Scholar]
- 138.Satapathy S.K., Das K., Kocak M. No apparent benefit of preemptive sorafenib therapy in liver transplant recipients with advanced hepatocellular carcinoma on explant. Clin Transplant. 2018;32 doi: 10.1111/ctr.13246. [DOI] [PubMed] [Google Scholar]
- 139.Hoffmann K., Ganten T., Gotthardtp D. Impact of neo-adjuvant Sorafenib treatment on liver transplantation in HCC patients - a prospective, randomized, double-blind, phase III trial. BMC Canc. 2015;15:392. doi: 10.1186/s12885-015-1373-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Doyon D., Mouzon A., Jourde A.M., Regensberg C., Frileux C. [Hepatic, arterial embolization in patients with malignant liver tumours (author's transl)] Ann Radiol (Paris) 1974;17:593–603. [PubMed] [Google Scholar]
- 141.Nakakuma K., Tashiro S., Hiraoka T. Studies on anticancer treatment with an oily anticancer drug injected into the ligated feeding hepatic artery for liver cancer. Cancer. 1983;52:2193–2200. doi: 10.1002/1097-0142(19831215)52:12<2193::aid-cncr2820521203>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 142.Poliektov N., Johnson D.T. Treatment of liver tumors with transarterial chemoembolization. Semin Interv Radiol. 2018;35:350–355. doi: 10.1055/s-0038-1673423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yang D.-J., Luo K.-L., Liu H. Meta-analysis of transcatheter arterial chemoembolization plus radiofrequency ablation versus transcatheter arterial chemoembolization alone for hepatocellular carcinoma. Oncotarget. 2017;8:2960–2970. doi: 10.18632/oncotarget.13813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Llovet J.M., Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatol Baltim Md. 2003;37:429–442. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 145.Lencioni R., de Baere T., Soulen M.C., Rilling W.S., Geschwind J.-F.H. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: a systematic review of efficacy and safety data. Hepatol Baltim Md. 2016;64:106–116. doi: 10.1002/hep.28453. [DOI] [PubMed] [Google Scholar]
- 146.Raoul J.-L., Forner A., Bolondi L., Cheung T.T., Kloeckner R., de Baere T. Updated use of TACE for hepatocellular carcinoma treatment: how and when to use it based on clinical evidence. Cancer Treat Rev. 2019;72:28–36. doi: 10.1016/j.ctrv.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 147.Chen P., Yuan P., Chen B., Sun J., Shen H., Qian Y. Evaluation of drug-eluting beads versus conventional transcatheter arterial chemoembolization in patients with unresectable hepatocellular carcinoma: a systematic review and meta-analysis. Clin Res Hepatol Gastroenterol. 2017;41:75–85. doi: 10.1016/j.clinre.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 148.Pung L., Ahmad M., Mueller K. The role of cone-beam CT in transcatheter arterial chemoembolization for hepatocellular carcinoma: a systematic review and meta-analysis. J Vasc Interv Radiol JVIR. 2017;28:334–341. doi: 10.1016/j.jvir.2016.11.037. [DOI] [PubMed] [Google Scholar]
- 149.Katsanos K., Kitrou P., Spiliopoulos S., Maroulis I., Petsas T., Karnabatidis D. Comparative effectiveness of different transarterial embolization therapies alone or in combination with local ablative or adjuvant systemic treatments for unresectable hepatocellular carcinoma: a network meta-analysis of randomized controlled trials. PLoS One. 2017;12 doi: 10.1371/journal.pone.0184597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Stevens C.L., Awad A., Abbas S.M., Watters D.A.K. Systematic review and meta-analysis of hepatic resection versus transarterial chemoembolization for solitary large hepatocellular carcinoma. HPB. 2017;19:653–658. doi: 10.1016/j.hpb.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 151.Tao R., Li X., Ran R. A mixed analysis comparing nine minimally invasive surgeries for unresectable hepatocellular carcinoma patients. Oncotarget. 2017;8:5460–5473. doi: 10.18632/oncotarget.12348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Jin P.-P., Shao S.-Y., Wu W.-T. Combination of transarterial chemoembolization and sorafenib improves outcomes of unresectable hepatocellular carcinoma: an updated systematic review and meta-analysis. Jpn J Clin Oncol. 2018;48:1058–1069. doi: 10.1093/jjco/hyy138. [DOI] [PubMed] [Google Scholar]
- 153.Lobo L., Yakoub D., Picado O. Unresectable hepatocellular carcinoma: radioembolization versus chemoembolization: a systematic review and meta-analysis. Cardiovasc Intervent Radiol. 2016;39:1580–1588. doi: 10.1007/s00270-016-1426-y. [DOI] [PubMed] [Google Scholar]
- 154.Jia Z., Jiang G., Tian F., Zhu C., Qin X. A systematic review on the safety and effectiveness of yttrium-90 radioembolization for hepatocellular carcinoma with portal vein tumor thrombosis. Saudi J Gastroenterol Off J Saudi Gastroenterol Assoc. 2016;22:353–359. doi: 10.4103/1319-3767.191139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Vilgrain V., Pereira H., Assenat E. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncol. 2017;18:1624–1636. doi: 10.1016/S1470-2045(17)30683-6. [DOI] [PubMed] [Google Scholar]
- 156.Ludwig J.M., Zhang D., Xing M., Kim H.S. Meta-analysis: adjusted indirect comparison of drug-eluting bead transarterial chemoembolization versus 90Y-radioembolization for hepatocellular carcinoma. Eur Radiol. 2017;27:2031–2041. doi: 10.1007/s00330-016-4548-3. [DOI] [PubMed] [Google Scholar]
- 157.Salem R., Gordon A.C., Mouli S. Y90 radioembolization significantly prolongs time to progression compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology. 2016;151:1155–1163. doi: 10.1053/j.gastro.2016.08.029. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chao Y., Chung Y.-H., Han G. The combination of transcatheter arterial chemoembolization and sorafenib is well tolerated and effective in Asian patients with hepatocellular carcinoma: final results of the START trial. Int J Cancer. 2015;136:1458–1467. doi: 10.1002/ijc.29126. [DOI] [PubMed] [Google Scholar]
- 159.Lee T.-Y., Lin C.-C., Chen C.-Y. Combination of transcatheter arterial chemoembolization and interrupted dosing sorafenib improves patient survival in early-intermediate stage hepatocellular carcinoma: a post hoc analysis of the START trial. Medicine (Baltim) 2017;96 doi: 10.1097/MD.0000000000007655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zeng J., Lv L., Mei Z.-C. Efficacy and safety of transarterial chemoembolization plus sorafenib for early or intermediate stage hepatocellular carcinoma: a systematic review and meta-analysis of randomized controlled trials. Clin Res Hepatol Gastroenterol. 2016;40:688–697. doi: 10.1016/j.clinre.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 161.Roccarina D., Majumdar A., Thorburn D., Davidson B.R., Tsochatzis E., Gurusamy K.S. Management of people with intermediate-stage hepatocellular carcinoma: an attempted network meta-analysis. Cochrane Database Syst Rev. 2017;3:CD011649. doi: 10.1002/14651858.CD011649.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Giannini E.G., Bucci L., Garuti F. Patients with advanced hepatocellular carcinoma need a personalized management: a lesson from clinical practice. Hepatol Baltim Md. 2018;67:1784–1796. doi: 10.1002/hep.29668. [DOI] [PubMed] [Google Scholar]
- 163.Kim D.Y. New systemic therapies for advanced hepatocellular carcinoma. Korean J Gastroenterol Taehan Sohwagi Hakhoe Chi. 2019;73:10–15. doi: 10.4166/kjg.2019.73.1.10. [DOI] [PubMed] [Google Scholar]
- 164.Llovet J.M., Ricci S., Mazzaferro V. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 165.Cheng A.-L., Kang Y.-K., Chen Z. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 166.Finn R.S., Zhu A.X., Farah W. Therapies for advanced stage hepatocellular carcinoma with macrovascular invasion or metastatic disease: a systematic review and meta-analysis. Hepatol Baltim Md. 2018;67:422–435. doi: 10.1002/hep.29486. [DOI] [PubMed] [Google Scholar]
- 167.Abdel-Rahman O., Lamarca A. Development of sorafenib-related side effects in patients diagnosed with advanced hepatocellular carcinoma treated with sorafenib: a systematic-review and meta-analysis of the impact on survival. Expert Rev Gastroenterol Hepatol. 2017;11:75–83. doi: 10.1080/17474124.2017.1264874. [DOI] [PubMed] [Google Scholar]
- 168.Díaz-González Á., Sanduzzi-Zamparelli M., Sapena V. Systematic review with meta-analysis: the critical role of dermatological events in patients with hepatocellular carcinoma treated with sorafenib. Aliment Pharmacol Ther. 2019;49:482–491. doi: 10.1111/apt.15088. [DOI] [PubMed] [Google Scholar]
- 169.Bruix J., Cheng A.-L., Meinhardt G., Nakajima K., De Sanctis Y., Llovet J. Prognostic factors and predictors of sorafenib benefit in patients with hepatocellular carcinoma: analysis of two phase III studies. J Hepatol. 2017;67:999–1008. doi: 10.1016/j.jhep.2017.06.026. [DOI] [PubMed] [Google Scholar]
- 170.Ikeda K., Kudo M., Kawazoe S. Phase 2 study of lenvatinib in patients with advanced hepatocellular carcinoma. J Gastroenterol. 2017;52:512–519. doi: 10.1007/s00535-016-1263-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kudo M., Finn R.S., Qin S. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet Lond Engl. 2018;391:1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 172.Kobayashi M., Kudo M., Izumi N. Cost-effectiveness analysis of lenvatinib treatment for patients with unresectable hepatocellular carcinoma (uHCC) compared with sorafenib in Japan. J Gastroenterol. February 2019 doi: 10.1007/s00535-019-01554-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Bruix J., Qin S., Merle P. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Lond Engl. 2017;389:56–66. doi: 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- 174.Sanduzzi-Zamparelli M., Díaz-Gonzalez Á, Reig M. New systemic treatments in advanced hepatocellular carcinoma. Liver Transplant Off Publ Am Assoc Study Liver Dis Int Liver Transplant Soc. 2019;25:311–322. doi: 10.1002/lt.25354. [DOI] [PubMed] [Google Scholar]
- 175.Finn R.S., Merle P., Granito A. Outcomes of sequential treatment with sorafenib followed by regorafenib for HCC: additional analyses from the phase III RESORCE trial. J Hepatol. 2018;69:353–358. doi: 10.1016/j.jhep.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 176.Kelley R.K., Verslype C., Cohn A.L. Cabozantinib in hepatocellular carcinoma: results of a phase 2 placebo-controlled randomized discontinuation study. Ann Oncol Off J Eur Soc Med Oncol. 2017;28:528–534. doi: 10.1093/annonc/mdw651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Abou-Alfa G.K., Meyer T., Cheng A.-L. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379:54–63. doi: 10.1056/NEJMoa1717002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zhu A.X., Park J.O., Ryoo B.-Y. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2015;16:859–870. doi: 10.1016/S1470-2045(15)00050-9. [DOI] [PubMed] [Google Scholar]
- 179.Kudo M., Hatano E., Ohkawa S. Ramucirumab as second-line treatment in patients with advanced hepatocellular carcinoma: Japanese subgroup analysis of the REACH trial. J Gastroenterol. 2017;52:494–503. doi: 10.1007/s00535-016-1247-4. [DOI] [PubMed] [Google Scholar]
- 180.Chau I., Peck-Radosavljevic M., Borg C. Ramucirumab as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib: patient-focused outcome results from the randomised phase III REACH study. Eur J Cancer Oxf Engl 1990. 2017;81:17–25. doi: 10.1016/j.ejca.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 181.Zhu A.X., Kang Y.-K., Yen C.-J. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:282–296. doi: 10.1016/S1470-2045(18)30937-9. [DOI] [PubMed] [Google Scholar]
- 182.Marquardt J.U., Saborowski A., Czauderna C., Vogel A. The changing landscape of systemic treatment of advanced hepatocellular carcinoma: new targeted agents and immunotherapies. Target Oncol. February 2019 doi: 10.1007/s11523-019-00624-w. [DOI] [PubMed] [Google Scholar]
- 183.El-Khoueiry A.B., Sangro B., Yau T. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet Lond Engl. 2017;389:2492–2502. doi: 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Sangro B., Gomez-Martin C., de la Mata M. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol. 2013;59:81–88. doi: 10.1016/j.jhep.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 185.Duffy A.G., Ulahannan S.V., Makorova-Rusher O. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J Hepatol. 2017;66:545–551. doi: 10.1016/j.jhep.2016.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zhu A.X., Finn R.S., Edeline J. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19:940–952. doi: 10.1016/S1470-2045(18)30351-6. [DOI] [PubMed] [Google Scholar]
- 187.Wang J.-C., Xia A.-L., Xu Y., Lu X.-J. Comprehensive treatments for hepatocellular carcinoma with portal vein tumor thrombosis. J Cell Physiol. 2019;234:1062–1070. doi: 10.1002/jcp.27324. [DOI] [PubMed] [Google Scholar]
- 188.Bruix J., Raoul J.-L., Sherman M. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a phase III trial. J Hepatol. 2012;57:821–829. doi: 10.1016/j.jhep.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Silva J.P., Berger N.G., Tsai S. Transarterial chemoembolization in hepatocellular carcinoma with portal vein tumor thrombosis: a systematic review and meta-analysis. HPB. 2017;19:659–666. doi: 10.1016/j.hpb.2017.04.016. [DOI] [PubMed] [Google Scholar]
- 190.Zhang X., Wang K., Wang M. Transarterial chemoembolization (TACE) combined with sorafenib versus TACE for hepatocellular carcinoma with portal vein tumor thrombus: a systematic review and meta-analysis. Oncotarget. 2017;8:29416–29427. doi: 10.18632/oncotarget.15075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Giorgio A., Merola M.G., Montesarchio L. Sorafenib combined with radio-frequency ablation compared with sorafenib alone in treatment of hepatocellular carcinoma invading portal vein: a western randomized controlled trial. Anticancer Res. 2016;36:6179–6183. doi: 10.21873/anticanres.11211. [DOI] [PubMed] [Google Scholar]
- 192.Hsu C.-Y., Liu P.-H., Ho S.-Y. Impact of tumor burden on prognostic prediction for patients with terminal stage hepatocellular carcinoma: a nomogram study. PLoS One. 2017;12 doi: 10.1371/journal.pone.0188031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Giannini E.G., Farinati F., Ciccarese F. Prognosis of untreated hepatocellular carcinoma. Hepatol Baltim Md. 2015;61:184–190. doi: 10.1002/hep.27443. [DOI] [PubMed] [Google Scholar]
- 194.Kikuchi L., Chagas A.L., Alencar R.S.S.M. Adherence to BCLC recommendations for the treatment of hepatocellular carcinoma: impact on survival according to stage. Clin Sao Paulo Braz. 2017;72:454–460. doi: 10.6061/clinics/2017(08)01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Vitale A., Morales R.R., Zanus G. Barcelona Clinic Liver Cancer staging and transplant survival benefit for patients with hepatocellular carcinoma: a multicentre, cohort study. Lancet Oncol. 2011;12:654–662. doi: 10.1016/S1470-2045(11)70144-9. [DOI] [PubMed] [Google Scholar]
- 196.D'Avola D., Iñarrairaegui M., Pardo F. Prognosis of hepatocellular carcinoma in relation to treatment across BCLC stages. Ann Surg Oncol. 2011;18:1964–1971. doi: 10.1245/s10434-011-1551-4. [DOI] [PubMed] [Google Scholar]
- 197.Kumar M., Panda D. Role of supportive care for terminal stage hepatocellular carcinoma. J Clin Exp Hepatol. 2014;4(suppl 3):S130–S139. doi: 10.1016/j.jceh.2014.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Yu Y., Feng M. Radiotherapy for hepatocellular carcinoma. Semin Radiat Oncol. 2018;28:277–287. doi: 10.1016/j.semradonc.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 199.Guckenberger M., Andratschke N., Alheit H. Definition of stereotactic body radiotherapy: principles and practice for the treatment of stage I non-small cell lung cancer. Strahlenther Onkol Organ Dtsch Rontgengesellschaft Al. 2014;190:26–33. doi: 10.1007/s00066-013-0450-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Seo Y.-S., Kim M.-S., Yoo H.-J. Radiofrequency ablation versus stereotactic body radiotherapy for small hepatocellular carcinoma: a Markov model-based analysis. Cancer Med. 2016;5:3094–3101. doi: 10.1002/cam4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Abdel-Rahman O., Elsayed Z. External beam radiotherapy for unresectable hepatocellular carcinoma. Cochrane Database Syst Rev. 2017;3:CD011314. doi: 10.1002/14651858.CD011314.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Im J.H., Yoon S.M., Park H.C. Radiotherapeutic strategies for hepatocellular carcinoma with portal vein tumour thrombosis in a hepatitis B endemic area. Liver Int Off J Int Assoc Study Liver. 2017;37:90–100. doi: 10.1111/liv.13191. [DOI] [PubMed] [Google Scholar]
- 203.Nakazawa T., Hidaka H., Shibuya A. Overall survival in response to sorafenib versus radiotherapy in unresectable hepatocellular carcinoma with major portal vein tumor thrombosis: propensity score analysis. BMC Gastroenterol. 2014;14:84. doi: 10.1186/1471-230X-14-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Ni J.-Y., Liu S.-S., Sun H.-L. Transcatheter hepatic arterial infusion chemotherapy vs sorafenib in the treatment of patients with hepatocellular carcinoma of Barcelona Clinic Liver Cancer stage C: a meta-analysis of Asian population. OncoTargets Ther. 2018;11:7883–7894. doi: 10.2147/OTT.S156844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ikeda M., Shimizu S., Sato T. Sorafenib plus hepatic arterial infusion chemotherapy with cisplatin versus sorafenib for advanced hepatocellular carcinoma: randomized phase II trial. Ann Oncol Off J Eur Soc Med Oncol. 2016;27:2090–2096. doi: 10.1093/annonc/mdw323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Cao H., Phan H., Yang L.-X. Improved chemotherapy for hepatocellular carcinoma. Anticancer Res. 2012;32:1379–1386. [PubMed] [Google Scholar]
- 207.Ikeda M., Mitsunaga S., Ohno I. Systemic chemotherapy for advanced hepatocellular carcinoma: past, present, and future. Dis Basel Switz. 2015;3:360–381. doi: 10.3390/diseases3040360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Cao D.-D., Xu H.-L., Liu L. Thalidomide combined with transcatheter artierial chemoembolzation for primary hepatocellular carcinoma: a systematic review and meta-analysis. Oncotarget. 2017;8:44976–44993. doi: 10.18632/oncotarget.16689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Yang W., Wang D., Huang L. Thalidomide combined with transcatheter arterial chemoembolization (TACE) for intermediate or advanced hepatocellular carcinoma: a systematic review and grade Approach. Asian Pac J Cancer Prev APJCP. 2018;19:2043–2055. doi: 10.22034/APJCP.2018.19.8.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Lv X.-H., Wang C.-H., Xie Y. Arsenic trioxide combined with transarterial chemoembolization for primary liver cancer: a meta-analysis. J Gastroenterol Hepatol. 2017;32:1540–1547. doi: 10.1111/jgh.13789. [DOI] [PubMed] [Google Scholar]
- 211.Hu H.T., Yao Q.J., Meng Y.L. Arsenic trioxide intravenous infusion combined with transcatheter arterial chemoembolization for the treatment of hepatocellular carcinoma with pulmonary metastasis: long-term outcome analysis. J Gastroenterol Hepatol. 2017;32:295–300. doi: 10.1111/jgh.13529. [DOI] [PubMed] [Google Scholar]
- 212.Rosenberg S.A., Restifo N.P. Adoptive cell transfer as personalized immunotherapy for human cancer. Science. 2015;348:62–68. doi: 10.1126/science.aaa4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Mo H.-Y., Liao Y.-Y., You X.-M. Timely meta-analysis on the efficacy of adoptive immunotherapy for hepatocellular carcinoma patients after curative therapy. PLoS One. 2017;12 doi: 10.1371/journal.pone.0174222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Wang H., Liu A., Bo W. Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma patients after curative resection, a systematic review and meta-analysis. Dig Liver Dis Off J Ital Soc Gastroenterol Ital Assoc Study Liver. 2016;48:1275–1282. doi: 10.1016/j.dld.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 215.Ding M., Wang Y., Chi J. Is adjuvant cellular immunotherapy essential after TACE-predominant minimally-invasive treatment for hepatocellular carcinoma? A systematic meta-analysis of studies including 1774 patients. PLoS One. 2016;11 doi: 10.1371/journal.pone.0168798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Li Y.-C., Zhao L., Wu J.-P., Qu C.-X., Song Q.-K., Wang R.-B. Cytokine-induced killer cell infusion combined with conventional treatments produced better prognosis for hepatocellular carcinoma patients with barcelona clinic liver cancer B or earlier stage: a systematic review and meta-analysis. Cytotherapy. 2016;18:1525–1531. doi: 10.1016/j.jcyt.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 217.Cai X.-R., Li X., Lin J.-X. Autologous transplantation of cytokine-induced killer cells as an adjuvant therapy for hepatocellular carcinoma in Asia: an update meta-analysis and systematic review. Oncotarget. 2017;8:31318–31328. doi: 10.18632/oncotarget.15454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.He G., Zheng C., Huo H. TACE combined with dendritic cells and cytokine-induced killer cells in the treatment of hepatocellular carcinoma: a meta-analysis. Int Immunopharmacol. 2016;40:436–442. doi: 10.1016/j.intimp.2016.09.015. [DOI] [PubMed] [Google Scholar]
- 219.Yu R., Yang B., Chi X. Efficacy of cytokine-induced killer cell infusion as an adjuvant immunotherapy for hepatocellular carcinoma: a systematic review and meta-analysis. Drug Des Devel Ther. 2017;11:851–864. doi: 10.2147/DDDT.S124399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Vincenzi B., Di Maio M., Silletta M. Prognostic relevance of objective response according to EASL criteria and mRECIST criteria in hepatocellular carcinoma patients treated with loco-regional therapies: a literature-based meta-analysis. PLoS One. 2015;10 doi: 10.1371/journal.pone.0133488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Tovoli F., Renzulli M., Granito A., Golfieri R., Bolondi L. Radiologic criteria of response to systemic treatments for hepatocellular carcinoma. Hepatic Oncol. 2017;4:129–137. doi: 10.2217/hep-2017-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Lencioni R., Montal R., Torres F. Objective response by mRECIST as a predictor and potential surrogate end-point of overall survival in advanced HCC. J Hepatol. 2017;66:1166–1172. doi: 10.1016/j.jhep.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 223.Llovet J.M., Decaens T., Raoul J.-L. Brivanib in patients with advanced hepatocellular carcinoma who were intolerant to sorafenib or for whom sorafenib failed: results from the randomized phase III BRISK-PS study. J Clin Oncol Off J Am Soc Clin Oncol. 2013;31:3509–3516. doi: 10.1200/JCO.2012.47.3009. [DOI] [PubMed] [Google Scholar]
- 224.Arora A., Kumar A. Treatment response evaluation and follow-up in hepatocellular carcinoma. J Clin Exp Hepatol. 2014;4(suppl 3):S126–S129. doi: 10.1016/j.jceh.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Sun T., He J., Zhang S., Sun J., Zeng M., Zeng Z. Simultaneous multitarget radiotherapy using helical tomotherapy and its combination with sorafenib for pulmonary metastases from hepatocellular carcinoma. Oncotarget. 2016;7:48586–48599. doi: 10.18632/oncotarget.9374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Chen Y.-X., Zeng Z.-C., Fan J. Defining prognostic factors of survival after external beam radiotherapy treatment of hepatocellular carcinoma with lymph node metastases. Clin Transl Oncol Off Publ Fed Span Oncol Soc Natl Cancer Inst Mex. 2013;15:732–740. doi: 10.1007/s12094-012-0997-6. [DOI] [PubMed] [Google Scholar]
- 227.Jung I.-H., Yoon S.M., Kwak J. High-dose radiotherapy is associated with better local control of bone metastasis from hepatocellular carcinoma. Oncotarget. 2017;8:15182–15192. doi: 10.18632/oncotarget.14858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Moris D., Chakedis J., Sun S.H. Management, outcomes, and prognostic factors of ruptured hepatocellular carcinoma: a systematic review. J Surg Oncol. 2018;117:341–353. doi: 10.1002/jso.24869. [DOI] [PubMed] [Google Scholar]
- 229.Sahu S.K., Taneja S., Kalra N. Rupture of hepatocellular carcinoma: a tale of 20 cases from a tertiary care center in northern India. J Gastrointest Cancer. February 2018 doi: 10.1007/s12029-018-0063-x. [DOI] [PubMed] [Google Scholar]
- 230.Wang H.-L., Mo D.-C., Zhong J.-H. Systematic review of treatment strategy for recurrent hepatocellular carcinoma: salvage liver transplantation or curative locoregional therapy. Medicine (Baltim) 2019;98 doi: 10.1097/MD.0000000000014498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Yong C.-C., Elsarawy A.M., Wang S.-H. The surgical challenges of salvage living donor liver transplantation for Hepatocellular carcinoma; the cumulative experience of 100 cases - a retrospective cohort study and a propensity score analysis. Int J Surg Lond Engl. 2018;54(Pt A):187–192. doi: 10.1016/j.ijsu.2018.04.041. [DOI] [PubMed] [Google Scholar]
- 232.Murali A.R., Patil S., Phillips K.T., Voigt M.D. Locoregional therapy with curative intent versus primary liver transplant for hepatocellular carcinoma: systematic review and meta-analysis. Transplantation. 2017;101:e249–e257. doi: 10.1097/TP.0000000000001730. [DOI] [PubMed] [Google Scholar]
- 233.Xiong Q., Geng T.-T., He L., Gao H. Harm and benefits of salvage transplantation for hepatocellular carcinoma: an updated meta-analysis. Transplant Proc. 2016;48:3336–3347. doi: 10.1016/j.transproceed.2016.09.047. [DOI] [PubMed] [Google Scholar]
- 234.Erridge S., Pucher P.H., Markar S.R. Meta-analysis of determinants of survival following treatment of recurrent hepatocellular carcinoma. Br J Surg. 2017;104:1433–1442. doi: 10.1002/bjs.10597. [DOI] [PubMed] [Google Scholar]
- 235.Gavriilidis P., Askari A., Azoulay D. Survival following redo hepatectomy vs radiofrequency ablation for recurrent hepatocellular carcinoma: a systematic review and meta-analysis. HPB. 2017;19:3–9. doi: 10.1016/j.hpb.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 236.Ganne-Carrié N., Nault J.-C., Ziol M. Predicting recurrence following radiofrequency percutaneous ablation for hepatocellular carcinoma. Hepatic Oncol. 2014;1:395–408. doi: 10.2217/hep.14.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Ikemoto T., Shimada M., Yamada S. Pathophysiology of recurrent hepatocellular carcinoma after radiofrequency ablation. Hepatol Res Off J Jpn Soc Hepatol. 2017;47:23–30. doi: 10.1111/hepr.12705. [DOI] [PubMed] [Google Scholar]
- 238.de'Angelis N., Landi F., Carra M.C., Azoulay D. Managements of recurrent hepatocellular carcinoma after liver transplantation: a systematic review. World J Gastroenterol. 2015;21:11185–11198. doi: 10.3748/wjg.v21.i39.11185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Guerrini G.P., Berretta M., Tarantino G. Multimodal oncological approach in patients affected by recurrent hepatocellular carcinoma after liver transplantation. Eur Rev Med Pharmacol Sci. 2017;21:3421–3435. [PubMed] [Google Scholar]
- 240.Mazzola A., Costantino A., Petta S. Recurrence of hepatocellular carcinoma after liver transplantation: an update. Future Oncol Lond Engl. 2015;11:2923–2936. doi: 10.2217/fon.15.239. [DOI] [PubMed] [Google Scholar]
- 241.Chok K.S. Management of recurrent hepatocellular carcinoma after liver transplant. World J Hepatol. 2015;7:1142–1148. doi: 10.4254/wjh.v7.i8.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Manthravadi S., Paleti S., Pandya P. Impact of sustained viral response postcurative therapy of hepatitis C-related hepatocellular carcinoma: a systematic review and meta-analysis. Int J Cancer. 2017;140:1042–1049. doi: 10.1002/ijc.30521. [DOI] [PubMed] [Google Scholar]
- 243.European Association for the Study of the Liver Electronic address: easloffice@easloffice.eu, European association for the study of the liver. EASL recommendations on treatment of hepatitis C 2018. J Hepatol. 2018;69:461–511. doi: 10.1016/j.jhep.2018.03.026. [DOI] [PubMed] [Google Scholar]
- 244.Liu G.-M., Huang X.-Y., Shen S.-L., Hu W.-J., Peng B.-G. Adjuvant antiviral therapy for hepatitis B virus-related hepatocellular carcinoma after curative treatment: a systematic review and meta-analysis. Hepatol Res Off J Jpn Soc Hepatol. 2016;46:100–110. doi: 10.1111/hepr.12584. [DOI] [PubMed] [Google Scholar]
- 245.He L., Liu X., Zhao Y. Efficacy of nucleot(s)ide analogs therapy in patients with unresectable HBV-related hepatocellular carcinoma: a systematic review and meta-analysis. Dis Markers. 2017;2017:7075935. doi: 10.1155/2017/7075935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Cho H., Ahn H., Lee D.H. Entecavir and tenofovir reduce hepatitis B virus-related hepatocellular carcinoma recurrence more effectively than other antivirals. J Viral Hepat. 2018;25:707–717. doi: 10.1111/jvh.12855. [DOI] [PubMed] [Google Scholar]
- 247.Jang J.W., Kim Y.W., Lee S.W. Reactivation of hepatitis B virus in HBsAg-negative patients with hepatocellular carcinoma. PLoS One. 2015;10 doi: 10.1371/journal.pone.0122041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Arora A., Anand A.C., Kumar A. INASL guidelines on management of hepatitis B virus infection in patients receiving chemotherapy, biologicals, immunosupressants, or corticosteroids. J Clin Exp Hepatol. 2018;8:403–431. doi: 10.1016/j.jceh.2018.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Khanna R., Verma S.K. Pediatric hepatocellular carcinoma. World J Gastroenterol. 2018;24:3980–3999. doi: 10.3748/wjg.v24.i35.3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Moore S.W., Davidson A., Hadley G.P. Malignant liver tumors in South African children: a national audit. World J Surg. 2008;32:1389–1395. doi: 10.1007/s00268-008-9526-8. [DOI] [PubMed] [Google Scholar]
- 251.Bhatia V., Seth S., Kapoor A., Sibal A. Incidentally detected hepatocellular carcinoma in cirrhotic children. Indian J Pediatr. 2014;81:826. doi: 10.1007/s12098-013-1188-3. [DOI] [PubMed] [Google Scholar]
- 252.Palaniappan K., Borkar V.V., Safwan M. Pediatric hepatocellular carcinoma in a developing country: is the etiology changing? Pediatr Transplant. 2016;20:898–903. doi: 10.1111/petr.12754. [DOI] [PubMed] [Google Scholar]
- 253.Suri A., Sharma V.K., Ranade P.R., Marar S., Nagral A. Ruptured hepatocellular carcinoma in a child with Budd-Chiari syndrome. Indian Pediatr. 2016;53:833–834. doi: 10.1007/s13312-016-0941-x. [DOI] [PubMed] [Google Scholar]
- 254.Chan K.L., Fan S.T., Tam P.K.H., Chiang A.K.S., Chan G.C.F., Ha S.Y. Paediatric hepatoblastoma and hepatocellular carcinoma: retrospective study. Hong Kong Med J Xianggang Yi Xue Za Zhi. 2002;8:13–17. [PubMed] [Google Scholar]
- 255.Chang M.-H. Hepatitis B virus and cancer prevention. Recent results Cancer Res Fortschritte Krebsforsch Progres. Dans Rech Sur Cancer. 2011;188:75–84. doi: 10.1007/978-3-642-10858-7_6. [DOI] [PubMed] [Google Scholar]
- 256.McKiernan P.J. Nitisinone in the treatment of hereditary tyrosinaemia type 1. Drugs. 2006;66:743–750. doi: 10.2165/00003495-200666060-00002. [DOI] [PubMed] [Google Scholar]
- 257.Hirzel A.C., Madrazo B., Rojas C.P. Two rare cases of hepatocellular carcinoma after Kasai procedure for biliary atresia: a recommendation for close follow-up. Case Rep Pathol. 2015;2015:982679. doi: 10.1155/2015/982679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Knisely A.S., Strautnieks S.S., Meier Y. Hepatocellular carcinoma in ten children under five years of age with bile salt export pump deficiency. Hepatol Baltim Md. 2006;44:478–486. doi: 10.1002/hep.21287. [DOI] [PubMed] [Google Scholar]
- 259.Bhadri V.A., Stormon M.O., Arbuckle S., Lam A.H., Gaskin K.J., Shun A. Hepatocellular carcinoma in children with Alagille syndrome. J Pediatr Gastroenterol Nutr. 2005;41:676–678. doi: 10.1097/01.mpg.0000179759.60048.c4. [DOI] [PubMed] [Google Scholar]
- 260.Franco L.M., Krishnamurthy V., Bali D. Hepatocellular carcinoma in glycogen storage disease type Ia: a case series. J Inherit Metab Dis. 2005;28:153–162. doi: 10.1007/s10545-005-7500-2. [DOI] [PubMed] [Google Scholar]
- 261.de Moor R.A., Schweizer J.J., van Hoek B., Wasser M., Vink R., Maaswinkel-Mooy P.D. Hepatocellular carcinoma in glycogen storage disease type IV. Arch Dis Child. 2000;82:479–480. doi: 10.1136/adc.82.6.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Weinreb N.J., Lee R.E. Causes of death due to hematological and non-hematological cancers in 57 US patients with type 1 Gaucher Disease who were never treated with enzyme replacement therapy. Crit Rev Oncog. 2013;18:177–195. doi: 10.1615/critrevoncog.2013005921. [DOI] [PubMed] [Google Scholar]
- 263.Tanaka Y., Inoue T., Horie H. International pediatric liver cancer pathological classification: current trend. Int J Clin Oncol. 2013;18:946–954. doi: 10.1007/s10147-013-0624-8. [DOI] [PubMed] [Google Scholar]
- 264.Luo J.-H., Ren B., Keryanov S. Transcriptomic and genomic analysis of human hepatocellular carcinomas and hepatoblastomas. Hepatol Baltim Md. 2006;44:1012–1024. doi: 10.1002/hep.21328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Prokurat A., Kluge P., Kościesza A., Perek D., Kappeler A., Zimmermann A. Transitional liver cell tumors (TLCT) in older children and adolescents: a novel group of aggressive hepatic tumors expressing beta-catenin. Med Pediatr Oncol. 2002;39:510–518. doi: 10.1002/mpo.10177. [DOI] [PubMed] [Google Scholar]
- 266.López-Terrada D., Alaggio R., de Dávila M.T. Towards an international pediatric liver tumor consensus classification: proceedings of the Los Angeles COG liver tumors symposium. Mod Pathol Off J U S Can Acad Pathol Inc. 2014;27:472–491. doi: 10.1038/modpathol.2013.80. [DOI] [PubMed] [Google Scholar]
- 267.Roebuck D.J., Aronson D., Clapuyt P. 2005 PRETEXT: a revised staging system for primary malignant liver tumours of childhood developed by the SIOPEL group. Pediatr Radiol. 2007;37:123–132. doi: 10.1007/s00247-006-0361-5. quiz 249-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Otte J.-B. Progress in the surgical treatment of malignant liver tumors in children. Cancer Treat Rev. 2010;36:360–371. doi: 10.1016/j.ctrv.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 269.Schmid I., Häberle B., Albert M.H. Sorafenib and cisplatin/doxorubicin (PLADO) in pediatric hepatocellular carcinoma. Pediatr Blood Cancer. 2012;58:539–544. doi: 10.1002/pbc.23295. [DOI] [PubMed] [Google Scholar]
- 270.Schmid I., von Schweinitz D. Pediatric hepatocellular carcinoma: challenges and solutions. J Hepatocell Carcinoma. 2017;4:15–21. doi: 10.2147/JHC.S94008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Wang J., Mao Y., Liu Y. Hepatocellular carcinoma in children and adolescents: clinical characteristics and treatment. J Gastrointest Surg Off J Soc Surg Aliment Tract. 2017;21:1128–1135. doi: 10.1007/s11605-017-3420-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Tagge E.P., Tagge D.U., Reyes J. Resection, including transplantation, for hepatoblastoma and hepatocellular carcinoma: impact on survival. J Pediatr Surg. 1992;27:292–296. doi: 10.1016/0022-3468(92)90849-3. discussion 297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Reyes J.D., Carr B., Dvorchik I. Liver transplantation and chemotherapy for hepatoblastoma and hepatocellular cancer in childhood and adolescence. J Pediatr. 2000;136:795–804. [PubMed] [Google Scholar]
- 274.Squires R.H., Ng V., Romero R. Evaluation of the pediatric patient for liver transplantation: 2014 practice guideline by the American association for the study of liver diseases, American society of transplantation and the North American society for pediatric Gastroenterology, hepatology, and nutrition. J Pediatr Gastroenterol Nutr. 2014;59:112–131. doi: 10.1097/MPG.0000000000000431. [DOI] [PubMed] [Google Scholar]
- 275.Squires R.H., Ng V., Romero R. Evaluation of the pediatric patient for liver transplantation: 2014 practice guideline by the American association for the study of liver diseases, American society of transplantation and the North American society for pediatric Gastroenterology, hepatology and nutrition. Hepatol Baltim Md. 2014;60:362–398. doi: 10.1002/hep.27191. [DOI] [PubMed] [Google Scholar]
- 276.McAteer J.P., Goldin A.B., Healey P.J., Gow K.W. Hepatocellular carcinoma in children: epidemiology and the impact of regional lymphadenectomy on surgical outcomes. J Pediatr Surg. 2013;48:2194–2201. doi: 10.1016/j.jpedsurg.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 277.Austin M.T., Leys C.M., Feurer I.D. Liver transplantation for childhood hepatic malignancy: a review of the United Network for Organ Sharing (UNOS) database. J Pediatr Surg. 2006;41:182–186. doi: 10.1016/j.jpedsurg.2005.10.091. [DOI] [PubMed] [Google Scholar]