Abstract
Background
Up to one in ten patients undergoing cystectomy with urinary diversion develop a ureteroenteric stricture (UES). Despite unrecognized ureteral obstruction contributing to infection, nephrolithiasis, and/or progression of kidney disease, the long-term natural history and risk factors associated with UES remains understudied. Herein, we report our single institutional experience with the long-term incidence, clinical presentation, and risk factors associated with UES formation following urinary diversion.
Methods
We reviewed 2,285 patients who underwent RC with urinary diversion between 1980–2008. UES was defined as radiographic evidence of ureteral obstruction at the level of the ureteroenteric anastomosis. The diagnosis of benign UES was confirmed by pathology. UES-free survival was estimated using the Kaplan-Meier method. The association between clinicopathologic features and the development of a UES were assessed using multivariable models.
Results
A total of 192 (8%) patients developed a benign UES, at a median of 7 months (IQR 4–24) following RC, with 5% occurring after 10 years. Seventy seven percent of patients exhibited signs and/or symptoms of ureteral obstruction. Patients who developed a UES had a greater body mass index (BMI) (28 vs. 27), operative time (330 vs. 301 minutes) and were more likely to experience a <30-day Clavien ≥3 complication (all P<0.05). Receipt of abdominal radiation and smoking history were not significantly associated with UES stricture risk. On multivariable analysis, only greater BMI (per 1-unit increase) (OR 1.06, 95% CI: 1.02–1.09; P=0.0009) and <30-day Clavien ≥3 complication (OR 2.85, 95% CI: 1.90–4.28; P<0.0001) were associated with the development of a UES. Development of UES was associated with renal function deterioration.
Conclusions
UES was identified in 8% of patients following RC with urinary diversion, with the majority presenting with symptoms. While the majority of these occur in the first 2 years after surgery, a patients’ risk for the development of this complication persists beyond 10 years. Due to the adverse sequelae of UES, long-term functional and imaging surveillance following urinary diversion is warranted, and early reconstruction should be considered.
Keywords: Ureteroenteric stricture (UES), ureteral stricture, radical cystectomy, urinary diversion
Introduction
In 2019, bladder cancer remains the fifth most common non-skin malignancy with an estimated 17,670 deaths annually (1). While radical cystectomy is the gold standard surgical treatment option for high-risk non-invasive and muscle invasive bladder cancer, it remains associated with significant postoperative morbidity (2-6). In fact, despite advancements in surgical technique and postoperative enhanced care pathways, it is estimated that up to 60% of patients experience early (<30 day) complications (5,6); and approximately 40% of patients experience long-term urinary reconstruction related adverse events requiring costly intervention including ureteroenteric anastomotic stricture (UES) (7).
Often patients with UES present with recurrent urinary tract infections, nephrolithiasis, or progressive impairment of renal function. Accordingly, the formation of a UES is one of the few long-term complications that may require aggressive surgical reconstruction (8). Of particular importance is the strong association between urinary diversion and progressive renal dysfunction, which is associated with an increased risk of all-cause mortality, cardiovascular events, and hospitalizations (9-13). Although renal deterioration within this population of patients may be multifactorial and related to advanced age, associated comorbidities, and nephrotoxic chemotherapy; urinary tract obstruction secondary to UES consistently remains the leading cause of end stage renal disease following radical cystectomy (11,12).
It is hypothesized that UES formation is the result of tissue ischemia and/or inflammation with subsequent scar formation. Nevertheless, despite a reported incidence of up to 13%, the etiology of UES remains unclear, as few studies have consistently demonstrated that any clinical or surgical feature is associated with stricture diagnosis (8,14-16). Furthermore, although the majority of UES are diagnosed early within 18 months of radical cystectomy, the attendant risk beyond this timeframe is poorly understood as the majority of current data lacks long-term follow-up (5,8,14). Therefore, given the potential for significant morbidity related to UES formation we sought to characterize the long-term natural history, clinical presentation, and associated risk factors among patients undergoing radical cystectomy with urinary diversion for bladder cancer.
Methods
After Institutional Review Board approval we evaluated the Mayo Clinic Cystectomy Registry to identify patients undergoing radical cystectomy and urinary diversion for bladder cancer between 1980 and 2008. Patients subsequently diagnosed with benign UES were identified while those with a history of extrinsic compression or malignant ureteral obstruction were excluded.
RC was performed by various surgeons at our institution, using standard techniques, during the study timeframe. All RC cases were done through an open surgical approach. Management of the ureteroenteric anastomosis was per individual surgeon discretion and not standardized; that is, some surgeons routinely performed a running anastomosis, whereas others utilized an interrupted suture technique. Similarly, the choice to anastomose each ureter individually (Bricker), or in a combined fashion (Wallace), was at the discretion of the treating surgeon. For all cases, the ureteroenteric anastomosis was formed using a freely refluxing technique with a temporary ureteral stent for a period of 2–4 weeks.
Clinicopathologic features evaluated included age, race, gender, body mass index (BMI), smoking history, Eastern Cooperative Oncology Group (ECOG) performance status, and pathologic tumor stage. Operative features assessed included type of urinary diversion, estimated blood loss (EBL), and operative time. We also evaluated postoperative complications associated with urinary diversion including <30-day Clavien-Dindo classification, urine leak, urinary tract infection, and intraabdominal abscess.
The retrospective nature of our study precluded standardized postoperative surveillance in all patients. However, follow-up after RC at our institution is recommended quarterly for the first 2 years after surgery, semiannually for the next 2 years and annually thereafter in patients without evidence of recurrence. Functional surveillance includes history, physical examination, urinalysis, serum electrolyte panel, and anatomical imaging of the upper urinary tract. For patients followed elsewhere the cystectomy registry monitors outcomes annually by correspondence with the patient and treating physician.
UES was defined as the presence of ipsilateral upper urinary tract dilation as identified on abdominal imaging, combined with evidence of anatomical obstruction via fluoroscopic, endoscopic, or nuclear imaging techniques. Patients were defined as presenting with asymptomatic obstruction if there were no identifiable sequelae as a result of UES and this was identified incidentally on surveillance imaging. Acute kidney injury was defined by an increase in creatinine of 0.3 mg/dL or greater (17). The diagnosis of benign UES was confirmed by pathology and/or cytology. The management of UES was at the discretion of the treating surgeon, but most commonly managed with temporizing stent exchange.
Time to UES presentation was defined as the time to diagnosis of UES from the date of urinary diversion. Manner of presentation (symptomatology) and management after diagnosis was additionally assessed. Continuous variables were summarized with medians/inter-quartile ranges (IQR) and compared using t-test. Categorical variables were summarized using frequencies/percentages and were compared using Fisher’s exact test and chi-square tests. UES-free survival was estimated using the Kaplan-Meier method. Cox proportional hazard regression models were used to evaluate the association of clinicopathologic factors with the diagnosis of benign UES. All tests were 2-sided with P<0.05 considered statistically significant. Statistical analyses were done with SAS Version 9.4 (Cary, NC).
Results
Of the 2,285 eligible patients who underwent radical cystectomy with urinary diversion at our institution between 1980 and 2008, 192 (8%) developed a benign UES. Clinicopathological characteristics are presented in Table 1. Patients who developed a UES had significantly greater BMI (median 28.3 vs. 26.9; P=0.0001) and operative times (330 vs. 301 minutes; P=0.011) relative to those without UES diagnosis. There was no difference in gender, age, smoking history, receipt of radiation, chemotherapy, or BCG treatment between cohorts. While the majority of patients underwent ileal conduit urinary diversion (77%), there was no difference in the incidence of UES based upon diversion type (P=0.08). Postoperatively, those with a UES were more likely to experience a UTI within 30 days of surgery (15% vs. 7%; P=0.02) and a maximum 30-day Clavien-Dindo complication grade of ≥3 (24% vs. 11%; P<0.0001).
Table 1. Comparisons of clinicopathologic features by ureteroenteric stricture status.
| Variable | No UES (N=2,093) | UES (N=192) | Total (N=2,285) | P value |
|---|---|---|---|---|
| Male gender, no. [%] | 1,701 [81] | 158 [82] | 1,859 [81] | 0.73 |
| Age, yr. [median; IQR] | 68.0 [61.0–75.0] | 69.0 [63.0–75.0] | 68.0 [62.0–75.0] | 0.21 |
| BMI, median [IQR] | 26.9 [24.3–30.0] | 28.3 [25.6–31.6] | 27.0 [24.4–30.1] | 0.0001 |
| ECOG, no. [%] | 0.24 | |||
| 0 | 1,652 [80] | 158 [83] | 1,810 [80] | |
| 1 | 309 [15] | 28 [15] | 337 [15] | |
| 2+ | 112 [5] | 5 [3] | 117 [5] | |
| Smoking history, no. [%] | 1,672 [81] | 150 [79] | 1,821 [81] | 0.43 |
| Preop GFR, median [IQR] | 61.3 [48.0-72.6] | 60.7 [49.0-71.3] | 61.2 [48.1-72.5] | 0.80 |
| Type of Diversion, no. [%] | 0.08 | |||
| Ileal Conduit | 1,522 [77] | 150 [82] | 1,672 [77] | |
| Neobladder | 461 [23] | 32 [18] | 493 [23] | |
| Operative time, min; median [IQR] | 301 [255–355] | 330 [262–365] | 304 [256–356] | 0.011 |
| EBL, cc, median [IQR] | 700 [450–1,000] | 750 [500–1,100] | 700 [450–1,000] | 0.09 |
| Perioperative chemotherapy, no. [%] | 556 [27] | 57 [30] | 613 [27] | 0.35 |
| Preop BCG treatment, no. [%] | 418 [20] | 39 [20] | 457 [20] | 0.91 |
| Pelvic radiation, no. [%] | 256 [12] | 28 [15] | 284 [12] | 0.34 |
| Pathologic T-stage, no. [%] | 0.12 | |||
| <pT2 | 955 [46] | 99 [52] | 1,054 [46] | |
| pT2 | 361 [17] | 36 [19] | 397 [18] | |
| pT3/4 | 766 [37] | 56 [29] | 822 [36] | |
| Pathologic N-stage, no. [%] | 0.11 | |||
| pN0/X | 1,738 [84] | 168 [88] | 1,906 [84] | |
| pN+ | 331 [16] | 22 [12] | 353 [16] | |
| <30-day urine leak, no. [%] | 78 [4] | 11 [6] | 89 [4] | 0.18 |
| <30-day UTI, no. [%; N=896] | 61 [7] | 11 [15] | 72 [8] | 0.02 |
| <30-day abdominal abscess, no. [%] | 52 [3] | 1 [0] | 53 [2] | 0.08 |
| Max Clavien ≥3, no. [%] | 239 [11] | 46 [24] | 285 [13] | <0.0001 |
IQR, interquartile range; UES, ureteroenteric stricture; BMI, body mass index; BCG, Bacillus Calmette-Guerin; ECOG, Eastern Cooperative Oncology Group; EBL, estimated blood loss; GFR, glomerular filtration rate; UTI, urinary tract infection.
Natural history
At a median postoperative follow up of 10.7 years (IQR 7.3–15.5), the cumulative UES-free survival at 1, 3, 5, and 10 years was 94%, 93%, 91%, and 90% (Figure 1). The diagnosis of a UES tended to occur early (median 7 months) with 75% diagnosed within 2 years following surgery. After 2 years, there remained a 0.4%/year UES incidence with 5% (N=10) developing UES beyond 10 years. The most common site of UES was left (N=102; 53%) followed by right (N=54; 28%) and lastly bilateral (N=36; 18%).
Figure 1.
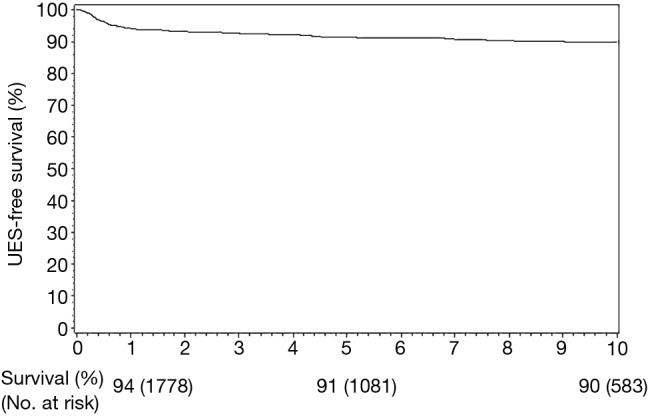
Ureteroenteric stricture free survival following radical cystectomy and urinary diversion.
Clinical presentation and risk factors
At the time of diagnosis 77% (N=147) exhibited signs and/or symptoms of ureteral obstruction (Table 2) including acute kidney injury 62% (N=115), recurrent symptomatic UTI 36% (N=69), pain 26% (N=50), and hematuria 6% (N=12). The incidence of postoperative stage 3 chronic kidney disease or greater among patients with UES and those without UES was 77.2% and 57.9% respectively (P<0.0001, Figure 2).
Table 2. Presenting symptoms at diagnosis of ureteroenteric stricture.
| Variable | No. [%] |
|---|---|
| Asymptomatic at presentation | 45 [23] |
| Symptomatic at presentation | 147 [77] |
| Pain | 50 [26] |
| Hematuria | 12 [6] |
| Urinary tract infection | 69 [36] |
| Sepsis | 11 [6] |
| Acute kidney injury | 115 [62] |
| Other | 41 [22] |
Figure 2.
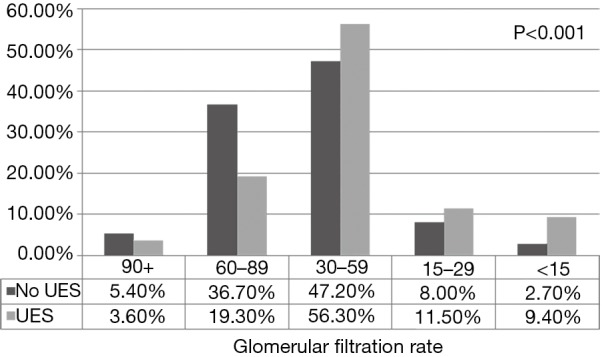
Renal function at last follow up stratified by UES status. UES, ureteroenteric stricture.
We next assessed for clinical characteristics associated with UES diagnosis (Table 3). On multivariable analysis after adjusting for preoperative hydronephrosis, operative time, and pathologic stage, only greater BMI (per 1-unit increase) (OR 1.06, 95% CI: 1.02–1.09; P=0.0009) and <30 day Clavien ≥3 complication (OR 2.85, 95% CI: 1.90–4.28; P<0.0001) were independently associated with UES diagnosis. Smoking (OR 0.87; P=0.62), receipt of chemotherapy (OR 1.17; P=0.35), radiation treatment (OR 1.25; P=0.35), and postoperative ureteroenteric urine leak (OR 1.56; P=0.18) were not associated with risk of UES.
Table 3. Risk factors for ureteroenteric stricture development.
| Variable | Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | ||
| Age at surgery (continuous) | 1.01 | 0.99–1.02 | 0.35 | ||||
| Gender (ref. female) | 1.07 | 0.73–1.58 | 0.73 | ||||
| BMI (continuous) | 1.06 | 1.03–1.09 | <0.0001 | 1.05 | 1.02–1.09 | 0.038 | |
| Preoperative GFR (continuous) | 1.00 | 0.99–1.01 | 0.95 | ||||
| ECOG (ref. ≤1) | 0.47 | 0.19–1.16 | 0.12 | ||||
| Chemotherapy | 1.17 | 0.84–1.62 | 0.35 | ||||
| Radiation therapy | 1.25 | 0.80–1.87 | 0.35 | ||||
| Smoking | 0.87 | 0.60–1.24 | 0.62 | ||||
| BCG treatment | 1.02 | 0.71–1.48 | 0.91 | ||||
| Preoperative hydronephrosis | 0.67 | 0.47–0.97 | 0.03 | 0.79 | 0.45–1.39 | 0.41 | |
| Operative time, min. (continuous) | 1.01 | 1.00–1.01 | 0.01 | 1.01 | 0.96–1.05 | 0.83 | |
| EBL, cc (continuous) | 1.00 | 1.00–1.00 | 0.21 | ||||
| Pathologic stage (pT3/4 vs. ≤T2) | 0.71 | 0.50–0.99 | 0.06 | 0.95 | 0.54–1.66 | 0.83 | |
| ≤30–day urine leak | 1.56 | 0.82–2.97 | 0.18 | ||||
| ≤30–day UTI | 2.18 | 1.09–4.35 | 0.03 | 1.64 | 0.78–3.46 | 0.19 | |
| ≤30–day abscess | 0.21 | 0.03–1.49 | 0.12 | ||||
| Max Clavien complication (grade ≥3 vs. <3) | 2.44 | 1.71–3.50 | <0.0001 | 2.07 | 1.18–3.63 | 0.01 | |
OR, odds ratio; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; BMI, body mass index; BCG, Bacillus Calmette-Guerin; EBL, estimated blood loss; GFR, glomerular filtration rate; UTI, urinary tract infection.
Discussion
In a large cohort of patients undergoing radical cystectomy and urinary diversion with extended follow-up, we found that only greater BMI and <30-day Clavien complication ≥3 were independently associated with UES diagnosis. Accordingly, there was no association with receipt of radiation therapy, type of urinary diversion, or postoperative urine leak. While the 75% of UES where diagnosed within 2 years following radical cystectomy, subsequent risk extended beyond 10 years.
The natural history of UES has historically developed within 18 months following radical cystectomy (5,8,14). While the majority of UES developed within 2 years in our cohort, we saw continued stricture formation up to 10 years post-operatively. Importantly, 77% presented with symptoms (acute kidney injury, UTI, pain, and hematuria) suggesting upper tract urinary obstruction. In this setting, the treating urologist should have a low suspicion for obtaining functional imaging to rule out upper tract obstruction in patients with urinary diversion. Our findings also demonstrate that one in four patients may develop silent obstruction which can only be diagnosed radiographically. These findings highlight the necessity of lifelong follow up with serum electrolyte assessment and anatomical imaging (CT urogram, MR urogram, renal ultrasound and/or loopogram). We recommend following the guidelines put forth by the NCCN and EAU for bladder cancer surveillance, which stress the need for early frequent (every 3–6 months) imaging for the first 2 years and then spacing the follow up to yearly (3,4). However, as the guidelines describe, follow up should be lifelong as we have shown obstruction can occur in a delayed fashion.
Furthermore, while preoperative renal function was comparable between patients with and without UES, at last follow up, UES was associated with higher stages of chronic kidney disease. These findings are consistent with Eisenberg et al., who demonstrated that UES was the strongest risk factor for developing worsened renal function after urinary diversion (12). Similarly, Makino and colleagues found UES to be the sole risk factor for early renal function deterioration (18). While there is limited data regarding renal function following UES reconstruction, Wenske et al. reviewed 100 patients undergoing ureteral reconstruction for ureteral obstruction/injury (19). They found that patients with CKD stage 3 or worse, were more likely to have continued renal deterioration after repair whereas those with eGFR >60 mL/min/1.73 m2 were more likely to have stable or improved renal function following surgical correction. Our study and these reports stress the potential benefit of early reconstruction prior to development of permanent renal deterioration and the need for future research into outcomes following reconstruction.
The etiology of benign UES formation following urinary diversion remains unclear. While several studies have evaluated multiple potential contributing factors (i.e., radiation, BMI, surgical technique), there remains a large discrepancy between author conclusions (8,14,20-22). Despite this uncertainty, the underlying mechanism predisposing to benign UES disease has remained consistent—tissue ischemia and/or chronic inflammation with resultant scar formation and upper tract obstruction. With this hypothesis in mind, contributing factors to the development of UES are multifactorial and may be divided into preoperative, intraoperative, and postoperative risk factors.
Several studies have examined patient related characteristics and their association with subsequent UES formation (8,14). Multiple studies, including our own, have not been able to reliably demonstrate that radiation is a poor prognosticator towards stricture formation. Katkoori and colleagues retrospectively evaluated 526 patients undergoing radical cystectomy and urinary diversion with a median follow up of 28 months (14). In their study, they found no difference in patients with and without prior radiation treatment (1.5% vs. 1.3%). Likewise, in the largest retrospective analysis to date of 1,964 patients treated from 1971 to 2008, Shah and colleagues found no significant association with pretreatment clinical characteristics including receipt of preoperative radiation or chemotherapy and subsequent UES formation (23). Radiation causes progressive and obliterative microvascular tissue damage within, and surrounding, the treatment field resulting in compromised vascularity of the tenuous pelvic ureter. Once transected and mobilized, terminal perfusion of the distal ureter is dependent upon proximal blood supply from accessory branches arising from the aorta, gonadal, and renal arteries. As a result, ureter arising below the iliac vasculature is often compromised in patients with pelvic irradiation. To minimize UES risk in this population, we recommend the judicious use of the healthy well vascularized non-pelvic ureter for ureteral reconstruction. To facilitate the objective assessment of ureteral viability, promising new adjuvant techniques have been described to evaluate tissue perfusion intraoperatively through the use of fluorescent chromophores (24).
In our study, increasing BMI was associated with UES. That is, for every 1-unit increase in BMI, we noted an independent 9% increased risk of UES formation. This has been previously reported in the robotic cystectomy literature by Ahmed and colleagues (20). This finding highlights the complexity of urinary diversion and its susceptibility to both systemic factors and anatomic challenges. Obesity is associated with increasing operative complexity, blood loss, and complications (25,26). Increasing intraabdominal adiposity may predispose to insufficient ureteral mobilization and external compression with ischemia, especially when passing the ureter under the bulky sigmoid mesentery root. To further complicate urinary reconstruction in obese patients, the subcutaneous distance that a conduit must traverse to achieve satisfactory stoma formation is often substantial and may place undue tension on the conduit mesentery and ureteroenteric anastomosis, both risk factors for ischemia. Lastly, increasing BMI is a surrogate for metabolic syndrome and insulin resistance, both associated with impaired wound healing and microvascular disease (27).
The impact of operative technique on functional outcomes following radical cystectomy cannot be understated. This includes an emphasis on meticulous and judicious ureteral dissection and a no-touch tension free anastomosis. Nevertheless, several nuances exist including type of anastomosis (Bricker vs. Wallace), suture technique (running vs. interrupted), and operative approach (open vs. robotic) (20-22). While previous studies have demonstrated conflicting results regarding the most optimal reconstructive technique, consistently the left ureter has been more likely to develop a UES than the right (23). It is hypothesized to be the result of additional ureteral length and dissection necessary and the need to tunnel the ureter under the sigmoid mesentery (15). While we were unable to determine any difference in UES outcomes based upon operative technique, at our institution we heavily emphasize minimal ureteral manipulation during dissection, preservation of existing periureteral vasculature, excision of all pelvic ureter when feasible, particularly in the setting of prior radiation, and complete dissection of the sigmoid mesentery to the level of the inferior mesenteric artery to allow for a tension-free, compression-free course of the left ureter.
In the post-operative setting, tissue inflammation may predispose to impaired healing and risk of ureteral scar formation. We observed that severe complications (Clavien classification ≥3) were independently associated with UES. Consistent with the reported literature (16,20,28), post-operative UTI was associated with UES formation. This process may result as a consequence of the profound tissue reaction, edema, and inflammation associated with symptomatic urinary tract infection. Alternatively, early urinary tract infection following diversion may be the sequelae of rapid and early stricture formation with acute urinary obstruction. A complicated post-operative course including anastomotic urine leak, intraabdominal abscess, and complicated urinary tract infection should raise suspicion for evolving UES formation and warrant close postoperative functional surveillance due to the elevated risk of ureteral scar formation.
The current study has several notable limitations. This is a retrospective study at a tertiary referral center, and therefore the distribution of clinicopathologic factors may not be applicable to all settings. In addition, multiple urologists at our institution perform cystectomy with urinary diversion, and therefore we cannot reliably discern differences in operative technique, such as the course of the left ureter under the sigmoid mesentery, and its influence on UES formation. Given the referral nature of our practice, follow up visits and imaging often occurs locally, and therefore, our database is partially reliant on correspondence from local providers and patients. Without long-term follow up, we cannot comment on stricture rates with robotic techniques as robotic cystectomy and intracorporeal diversion has only become increasingly common in our department over the last few years. Furthermore, we have recently moved towards reconstructing UES with robotic-assistance which we hope to report on soon. Anecdotally, we have found several advantages including precise stricture identification with the utilization of combined antegrade ureteroscopy and in console imaging overlay (TilePro®), and the ability to assess tissue perfusion using near infrared fluorescence and indocyanine green. In spite of these limitations, we believe our findings further the understanding of benign UES following radical cystectomy and highlight the need for continued contemporary studies assessing the effect of robotic assistance and the utility of adjuvant maneuvers to assess intraoperative ureteral perfusion and viability.
Conclusions
UES was identified in 8% of patients following RC with urinary diversion, with the majority presenting with symptoms. While 75% of these occur in the first 2 years after surgery, a patients’ risk for the development of this complication persists beyond 10 years following RC. Development of UES was associated with renal deterioration and only greater BMI and <30-day Clavien ≥3 complication were associated with stricture formation. Due to the adverse sequelae of UES, long-term functional and imaging surveillance following urinary diversion is warranted, and early reconstruction should be considered.
Acknowledgments
None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by Mayo Clinic’s IRB (13-009715) and informed consent was taken from all the patients.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- 2.Chang SS, Bochner BH, Chou R, et al. Treatment of Non-Metastatic Muscle-Invasive Bladder Cancer: AUA/ASCO/ASTRO/SUO Guideline. J Urol 2017;198:552-9. 10.1016/j.juro.2017.04.086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spiess PE, Agarwal N, Bangs R, et al. Bladder Cancer, Version 5.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2017;15:1240-67. 10.6004/jnccn.2017.0156 [DOI] [PubMed] [Google Scholar]
- 4.Witjes JA, Compérat E, Cowan NC, et al. EAU guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2013 guidelines. Eur Urol 2014;65:778-92. 10.1016/j.eururo.2013.11.046 [DOI] [PubMed] [Google Scholar]
- 5.Shimko MS, Tollefson MK, Umbreit EC, et al. Long-term complications of conduit urinary diversion. J Urol 2011;185:562-7. 10.1016/j.juro.2010.09.096 [DOI] [PubMed] [Google Scholar]
- 6.Shabsigh A, Korets R, Vora KC, et al. Defining early morbidity of radical cystectomy for patients with bladder cancer using a standardized reporting methodology. Eur Urol 2009;55:164-74. 10.1016/j.eururo.2008.07.031 [DOI] [PubMed] [Google Scholar]
- 7.Hautmann RE, Hautmann SH, Hautmann O. Complications associated with urinary diversion. Nat Rev Urol 2011;8:667-77. 10.1038/nrurol.2011.147 [DOI] [PubMed] [Google Scholar]
- 8.Hautmann RE, de Petriconi R, Kahlmeyer A, et al. Preoperatively Dilated Ureters are a Specific Risk Factor for the Development of Ureteroenteric Strictures after Open Radical Cystectomy and Ileal Neobladder. J Urol 2017;198:1098-106. 10.1016/j.juro.2017.05.069 [DOI] [PubMed] [Google Scholar]
- 9.Madersbacher S, Schmidt J, Eberle JM, et al. Long-term outcome of ileal conduit diversion. J Urol 2003;169:985-90. 10.1097/01.ju.0000051462.45388.14 [DOI] [PubMed] [Google Scholar]
- 10.Samuel JD, Bhatt RI, Montague RJ, et al. The natural history of postoperative renal function in patients undergoing ileal conduit diversion for cancer measured using serial isotopic glomerular filtration rate and 99m technetium-mercaptoacetyltriglycine renography. J Urol 2006;176:2518-22; discussion 2522. 10.1016/j.juro.2006.07.146 [DOI] [PubMed] [Google Scholar]
- 11.Gilbert SM, Lai J, Saigal CS, et al. Downstream complications following urinary diversion. J Urol 2013;190:916-22. 10.1016/j.juro.2013.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eisenberg MS, Thompson RH, Frank I, et al. Long-term renal function outcomes after radical cystectomy. J Urol 2014;191:619-25. 10.1016/j.juro.2013.09.011 [DOI] [PubMed] [Google Scholar]
- 13.Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004;351:1296-305. 10.1056/NEJMoa041031 [DOI] [PubMed] [Google Scholar]
- 14.Katkoori D, Samavedi S, Adiyat KT, et al. Is the incidence of uretero-intestinal anastomotic stricture increased in patients undergoing radical cystectomy with previous pelvic radiation? BJU Int 2010;105:795-8. 10.1111/j.1464-410X.2009.08835.x [DOI] [PubMed] [Google Scholar]
- 15.Tal R, Sivan B, Kedar D, et al. Management of benign ureteral strictures following radical cystectomy and urinary diversion for bladder cancer. J Urol 2007;178:538-42. 10.1016/j.juro.2007.03.142 [DOI] [PubMed] [Google Scholar]
- 16.Richards KA, Cohn JA, Large MC, et al. The effect of length of ureteral resection on benign ureterointestinal stricture rate in ileal conduit or ileal neobladder urinary diversion following radical cystectomy. Urol Oncol 2015;33:65.e1-8. 10.1016/j.urolonc.2014.05.015 [DOI] [PubMed] [Google Scholar]
- 17.Kellum JA, Lameire N, KDIGO AKI Guideline Work Group Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit Care 2013;17:204. 10.1186/cc11454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Makino K, Nakagawa T, Kanatani A, et al. Biphasic decline in renal function after radical cystectomy with urinary diversion. Int J Clin Oncol 2017;22:359-65. 10.1007/s10147-016-1053-2 [DOI] [PubMed] [Google Scholar]
- 19.Wenske S, Olsson CA, Benson MC. Outcomes of distal ureteral reconstruction through reimplantation with psoas hitch, Boari flap, or ureteroneocystostomy for benign or malignant ureteral obstruction or injury. Urology 2013;82:231-6. 10.1016/j.urology.2013.02.046 [DOI] [PubMed] [Google Scholar]
- 20.Ahmed YE, Hussein AA, May PR, et al. Natural History, Predictors and Management of Ureteroenteric Strictures after Robot Assisted Radical Cystectomy. J Urol 2017;198:567-74. 10.1016/j.juro.2017.02.3339 [DOI] [PubMed] [Google Scholar]
- 21.Kouba E, Sands M, Lentz A, et al. A Comparison of the Bricker Versus Wallace Ureteroileal Anastomosis in Patients Undergoing Urinary Diversion for Bladder Cancer. J Urol 2007;178:945-8; discussion 948-9. 10.1016/j.juro.2007.05.030 [DOI] [PubMed] [Google Scholar]
- 22.Evangelidis A, Lee EK, Karellas ME, et al. Evaluation of ureterointestinal anastomosis: Wallace vs Bricker. J Urol 2006;175:1755-8; discussion 1758. [DOI] [PubMed]
- 23.Shah SH, Movassaghi K, Skinner D, et al. Ureteroenteric Strictures After Open Radical Cystectomy and Urinary Diversion: The University of Southern California Experience. Urology 2015;86:87-91. 10.1016/j.urology.2015.03.014 [DOI] [PubMed] [Google Scholar]
- 24.Shen JK, Jamnagerwalla J, Yuh BE, et al. Real-time indocyanine green angiography with the SPY fluorescence imaging platform decreases benign ureteroenteric strictures in urinary diversions performed during radical cystectomy. Ther Adv Urol 2019;11:1756287219839631. 10.1177/1756287219839631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Svatek RS, Fisher MB, Matin SF, et al. Risk factor analysis in a contemporary cystectomy cohort using standardized reporting methodology and adverse event criteria. J Urol 2010;183:929-34. 10.1016/j.juro.2009.11.038 [DOI] [PubMed] [Google Scholar]
- 26.Roghmann F, Trinh QD, Braun K, et al. Standardized assessment of complications in a contemporary series of European patients undergoing radical cystectomy. Int J Urol 2014;21:143-9. 10.1111/iju.12232 [DOI] [PubMed] [Google Scholar]
- 27.Kota SK, Meher LK, Jammula S, et al. Aberrant angiogenesis: The gateway to diabetic complications. Indian J Endocrinol Metab 2012;16:918-30. 10.4103/2230-8210.102992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Large MC, Cohn JA, Kiriluk KJ, et al. The impact of running versus interrupted anastomosis on ureterointestinal stricture rate after radical cystectomy. J Urol 2013;190:923-7. 10.1016/j.juro.2013.02.091 [DOI] [PubMed] [Google Scholar]


