Abstract
Hidradenitis suppurativa (HS) is a chronic and recurrent inflammatory skin disorder affecting mainly the areas rich in apocrine sweat glands, such as the axillae, groins and buttocks. The role of mechanical pressure and friction due to clothing in the pathogenesis of HS lesions has been previously stressed. Here, we report 2 middle-aged men who presented with HS lesions/HS-like lesions on their amputation stump and review 2 additional cases from the literature. Management was challenging as 2 patients needed tumor necrosis αinhibitor while deroofing/surgery was the option for the 2 others. These cases highlight that mechanical pressure and friction are environmental factors that can play a role in the pathogenesis of HS lesions.
Keywords: Amputation, Friction, Hidradenitis suppurativa, Prosthesis, Stump
Established Facts
Hidradenitis suppurativa may locate specifically in areas of chronic friction and pressure as an equivalent of a Koebner phenomenon.
Novel Insights
Two cases of difficult-to-treat hidradenitis suppurativa or hidradenitis suppurativa-like lesions located to lower limb amputation stumps are reported.
Introduction
Hidradenitis suppurativa (HS) is a chronic and recurrent inflammatory skin disorder affecting mainly the areas rich in apocrine sweat glands [1, 2]. HS patients have been reported to have a reduced life time expectancy, highlighting the need for a holistic treatment approach in this patient group [3]. Patients present painful inflammatory nodules, abscesses, comedones, scarring and tunneling sinus tracts mainly in the axillary, inguinal and anogenital regions [1, 2]. However, ectopic locations of HS in other body parts have been reported, and the role of chronic mechanical trauma and frictions has been questioned as a potential factor [4, 5]. Friction avoidance is among the list of lifestyle modifications suggested to HS patients [1, 2]. Amputation stumps are peculiar areas subjected to pressure, shear and friction. Acne mechanica [6] and epidermoid cysts [7] have been reported on amputation stumps as well as HS [8, 9]. We report here 2 additional cases of HS localized on amputation stumps.
Case Reports
Case 1
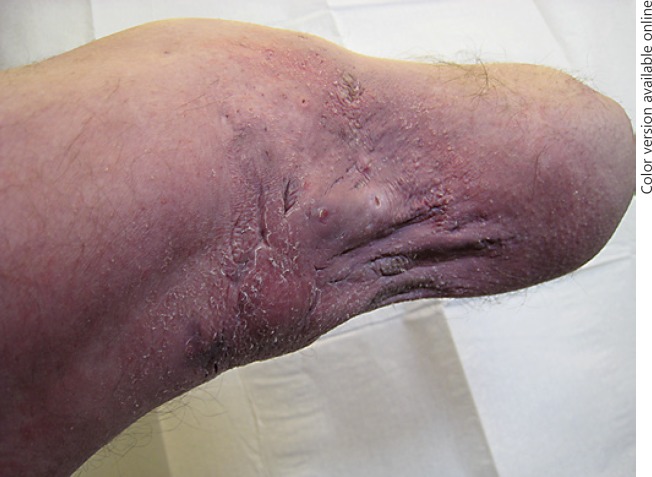
The patient was a 55-year-old man with a history of metabolic syndrome (diabetes type 2, hypertension, hypercholesterolemia), overweight (BMI 28.9) and Charcot-Marie-Tooth disease. He was a former smoker, still using snuff tobacco. He was first referred to us for the management of recurrent boils on his amputation stump in 2015. Amputation had been performed over a decade previously, at the end of 2001, because of his neurological condition. Within the next year recurrent boils appeared strictly on the stump. Upon examination, on the stump fistulas, abscesses and scars evocative of localized HS were found (Fig. 1). Physical examination revealed also folliculitis of the back, with open comedos and folliculitis of thighs. However, the patient did not have any other typical features of HS. Local antibiotics (clindamycin), oral rifampicin-clindamycin and acitretin were inefficient. The patient used multiple treatments combined such as local corticosteroids, acitretin and oral cephalexin. Tumor necrosis factor α inhibitor (adalimumab) was initiated in summer 2017 at the recommended regimen for HS (160 at week 0, 80 at week 2 and 40 mg weekly starting at week 4) [2]. Efficacy was notable after 3 months with relapses when treatment was withdrawn. At 1 year of follow-up, the patient still presents 1 or 2 flares monthly which is way better than before, and the patient is satisfied with such a result. Of note, he had developed an erysipelas under adalimumab.
Fig. 1.
HS-like lesions on the left amputation stump.
Case 2
The patient was a 41-year-old man referred for the management of HS. He was an active smoker (10 pack-years) and obese (BMI 31.6). He had a first-degree relative with HS symptoms. HS began when he was 26 on the inner thighs. Other affected sites were the chest and abdominal folds. HS had previously been treated with local excisions and a 6-month course of doxycycline (with significant improvement during the course). A pilonidal sinus disease was excised in 2007 with local recurrence in 2017 treated with repeated excisions. Lower limb amputation was performed in 2013 after a traffic accident. Hurley II boils, abscesses and fistulas appeared in 2017 on the stump, preventing the patient from wearing a permanent prosthesis. A 6-month course of doxycycline again induced a significant improvement of HS, including stump lesions, followed by relapse under treatment (Fig. 2). Combination by ofloxacin and clindamycin as previously reported [10] was inefficient. Lack of social care reimbursement of adalimumab in France prevented us from initiating this treatment. Surgery on active lesions on the pubis and axillary fold is planned before stump surgery.
Fig. 2.
a Boil on the upper part of a surgery scar on the inner side of the thigh. b Small boils on the posterior side of the thigh.
Discussion/Conclusion
We report about 2 new cases of HS features localized on amputation stumps. The stump is exposed to constant friction, pressures and shear force, increased humidity and prolonged and moist contact with the prosthesis, resulting in exposure to its constituent chemical compounds [11]. Skin problems on amputation stumps affect from 16 to 70% of the patients according to several series [12, 13, 14]. Mechanically induced lesions such as verrucous hyperplasia, follicular hyperkeratosis or epidermoid cysts are broadly observed in 1.5–4% of the amputees [13, 14]. To our knowledge, no case of HS lesions has been specifically reported in those series.
Our first patient did not meet the criteria for HS as he did not have any personal or familial history of HS, nor lesions in any typical areas of HS (namely axillae, groins or buttocks), so that the diagnosis remains HS-like lesions. Some authors have put forward mechanical stress as a trigger for the outburst of new lesions of HS [4, 5, 15, 16]. For instance, Boer [4] reported 14 patients with HS who had abdominal lesions corresponding to the waistband. In his series, patients were all with a BMI >30 and most of all smokers [4]. Such a phenomenon was also observed in the bra strap area in a woman [5]. To the best of our knowledge, a total of 4 cases (3 men [mean age 45] and a 24-year-old woman) of HS lesions/HS-like lesions on a leg stump have been reported (Table 1) [8, 9]. Clinical presentation was highly variable: age at amputation ranged from 4 to 39 years and delay for onset from 1 to 8 years after amputation. Two patients had HS symptoms elsewhere, while 2 others had not. Treatment outcome varied with efficacy of biologics in 2 cases, initial efficacy of antibiotics in 1 and deroofing in 1. In men, overweight/obesity, diabetes and smoking were found as cofactors.
Table 1.
Characteristics of all cases of HS on amputation stumps
| Reference | Gender, age, years | Family history of HS | Habitus, comorbidities | Other sites affected by HS | Age at leg amputation, years | Delay for HS occurrence on leg stump, years | Administered treatments | Efficiency |
|---|---|---|---|---|---|---|---|---|
| Henderson [7], 2006 | Female, 24 | No? | Not available | Axilla Groin |
4 | 8 | Minocycline Topical clindamycin Intralesional corticosteroids Etanercept |
Minimal Minimal Minimal Significant |
| De Winter [8], 2012 | Male, 44 | No | Smoker BMI = 31.3 Diabetes type II Hypopituitarism |
None | 39 | 5 | Several courses of antibiotics Deroofing | Minimal Significant |
| Present case 1 | Male, 51 | No | Ex-smoker, snuff tobacco BMI = 28.9 Diabetes type II Hypertension Hypercholesterolemia Charcot-Marie-Tooth disease |
None | 38 | 1 | Topical clindamycin Rifampicin-clindamycin Acitretin Adalimumab |
None None None Significant |
| Present case 2 | Male, 41 | Yes (first-degree relative) | Smoker BMI = 31.6 Pilonidal sinus disease |
Inner thighs Abdominal fold |
36 | 1 | Doxycycline Ofloxacin-clindamycin |
Initially significant, relapse None |
At first glance, it is rather surprising that a stump can be the elective localization of HS lesions as it is not an area considered to be rich in apocrine sweat glands. Apocrine glands have a role in thermoregulation. They lower the surface tension of the sweat so that it forms a sheet rather than drops on the skin [17, 18]. The stump is exposed to constant friction, pressures and shear force. Besides, the stump is fitted with a prosthesis which creates a warm environment and possibly increases sweating. Friction and skin confinement within a prosthesis would be both important in the physiopathogeny of the lesions in predisposed patients. Skin confinement may favor apocrine gland development and thereby apocrine gland disease. Overweight, obesity and tobacco are additional factors that could take part here in the phenomenon, even though they are not the sole triggers of the disease [19]. In the aforementioned studies of skin lesions in amputees [13, 14], neither smoking nor BMI were analyzed. Boer [4] reported a patient with abdominal waistband-induced HS lesions that improved with weight loss. In the case of amputation, the situation is more challenging as mechanical factors on the stump are unavoidable. In case of severe flare, it can be suggested to the patient to temporarily use a wheelchair or crutches, at the cost of an impact on the patient's quality of life. Even though the role of overweight/obesity and smoking on the stump lesions remains open, lifestyle modifications should be emphasized due to additional benefits for the patients' health.
In conclusion, HS or HS-like lesions may develop locally on leg stumps because of mechanical stress in patients with additional HS risk factors. Treatment is challenging as pressure and shear forces applied on the prosthesis are almost unavoidable and may expose to treatment failure. To date, no treatment appeared specifically beneficial. Biologics were necessary in half of the cases to provide relief.
Statement of Ethics
Subjects have given their consent to publish details and photos of the case.
Disclosure Statement
Dr. Nicolas Kluger has served as a consultant to AbbVie and has received research funding from AbbVie and speaker fees from AbbVie (2015–2016) and travel grants (2018) from AbbVie Finland. P. Guillem received honoraria from AbbVie and Novartis as a consultant and provided lectures for AbbVie, Brothier, Cicaplus, Coloplast and Novartis.
Author Contributions
M.K. and K.I.: acquisition, critical revision of the work. N.K. and P.G.: design of the work, acquisition, analysis and interpretation of the data, drafting of the work.
References
- 1.Alikhan A, Sayed C, Alavi A, et al. North American Clinical Management Guidelines for Hidradenitis Suppurativa: a Publication from the United States and Canadian Hidradenitis Suppurativa Foundations Part I: Diagnosis, Evaluation, and the use of Complementary and Procedural Management. J Am Acad Dermatol. 2019 Mar 11; doi: 10.1016/j.jaad.2019.02.067. pii: S0190-9622(19)30367-6. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zouboulis CC, Desai N, Emtestam L, Hunger RE, Ioannides D, Juhász I, et al. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatol Venereol. 2015 Apr;29((4)):619–44. doi: 10.1111/jdv.12966. [DOI] [PubMed] [Google Scholar]
- 3.Tiri H, Jokelainen J, Timonen M, Tasanen K, Huilaja L. Substantially reduced life expectancy in patients with hidradenitis suppurativa: a Finnish nationwide registry study. Br J Dermatol. 2018 Dec;••• doi: 10.1111/bjd.17578. [DOI] [PubMed] [Google Scholar]
- 4.Boer J. Should Hidradenitis Suppurativa Be Included in Dermatoses Showing Koebnerization? Is It Friction or Fiction? Dermatology. 2017;233((1)):47–52. doi: 10.1159/000472252. [DOI] [PubMed] [Google Scholar]
- 5.Boer J, Mihajlovic D. Boils at Frictional Locations in a Patient with Hidradenitis Suppurativa. Acta Dermatovenerol Croat. 2016 Dec;24((4)):303–4. [PubMed] [Google Scholar]
- 6.Strauss RM, Harrington CI. Stump acne: a new variant of acne mechanica and a cause of immobility. Br J Dermatol. 2001 Mar;144((3)):647–8. doi: 10.1046/j.1365-2133.2001.04116.x. [DOI] [PubMed] [Google Scholar]
- 7.Allende MF, Levy SW, Barnes GH. Epidermoid cysts in amputees. Acta Derm Venereol. 1963;43:56–67. [PubMed] [Google Scholar]
- 8.Henderson RL., Jr Case reports: treatment of atypical hidradenitis suppurativa with the tumor necrosis factor receptor-Fc fusion protein etanercept. J Drugs Dermatol. 2006 Nov-Dec;5((10)):1010–1. [PubMed] [Google Scholar]
- 9.de Winter K, van der Zee HH, Prens EP. Is mechanical stress an important pathogenic factor in hidradenitis suppurativa? Exp Dermatol. 2012 Mar;21((3)):176–7. doi: 10.1111/j.1600-0625.2012.01443.x. [DOI] [PubMed] [Google Scholar]
- 10.Delaunay J, Villani AP, Guillem P, Tristan A, Boibieux A, Jullien D. Oral ofloxacin and clindamycin as an alternative to the classic rifampicin-clindamycin in hidradenitis suppurativa: retrospective analysis of 65 patients. Br J Dermatol. 2018 Jan;178((1)):e15–6. doi: 10.1111/bjd.15739. [DOI] [PubMed] [Google Scholar]
- 11.Meulenbelt HE, Geertzen JH, Dijkstra PU, Jonkman MF. Skin problems in lower limb amputees: an overview by case reports. J Eur Acad Dermatol Venereol. 2007 Feb;21((2)):147–55. doi: 10.1111/j.1468-3083.2006.01936.x. [DOI] [PubMed] [Google Scholar]
- 12.Chan KM, Tan ES. Use of lower limb prosthesis among elderly amputees. Ann Acad Med Singapore. 1990 Nov;19((6)):811–6. [PubMed] [Google Scholar]
- 13.Lyon CC, Kulkarni J, Zimerson E, Van Ross E, Beck MH. Skin disorders in amputees. J Am Acad Dermatol. 2000 Mar;42((3)):501–7. doi: 10.1016/s0190-9622(00)90227-5. [DOI] [PubMed] [Google Scholar]
- 14.Colgecen E, Korkmaz M, Ozyurt K, Mermerkaya U, Kader C. A clinical evaluation of skin disorders of lower limb amputation sites. Int J Dermatol. 2016 Apr;55((4)):468–72. doi: 10.1111/ijd.13089. [DOI] [PubMed] [Google Scholar]
- 15.Boer J, Nazary M, Riis PT. The Role of Mechanical Stress in Hidradenitis Suppurativa. Dermatol Clin. 2016 Jan;34((1)):37–43. doi: 10.1016/j.det.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 16.De Vita V, Fabbrocini G. Mechanical Stress as a Cause of Hidradenitis Suppurativa: A Lesson from a Patient with a Monster Hernia. Acta Dermatovenerol Croat. 2018 Oct;26((3)):260–1. [PubMed] [Google Scholar]
- 17.Porter AM. Why do we have apocrine and sebaceous glands? J R Soc Med. 2001 May;94((5)):236–7. doi: 10.1177/014107680109400509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lupi O. Ancient adaptations of human skin: why do we retain sebaceous and apocrine glands? Int J Dermatol. 2008 Jul;47((7)):651–4. doi: 10.1111/j.1365-4632.2008.03765.x. [DOI] [PubMed] [Google Scholar]
- 19.Wollina U, Langner D, Heinig B, Nowak A. Comorbidities, treatment, and outcome in severe anogenital inverse acne (hidradenitis suppurativa): a 15-year single center report. Int J Dermatol. 2017 Jan;56((1)):109–15. doi: 10.1111/ijd.13393. [DOI] [PubMed] [Google Scholar]