Abstract
Background
Spinal muscular atrophy (SMA) is caused by a homozygous deletion of the survival motor neuron 1 (SMN1) gene on chromosome 5, or a heterozygous deletion in combination with a (point) mutation in the second SMN1 allele. This results in degeneration of anterior horn cells, which leads to progressive muscle weakness. Children with SMA type II do not develop the ability to walk without support and have a shortened life expectancy, whereas children with SMA type III develop the ability to walk and have a normal life expectancy. This is an update of a review first published in 2009 and previously updated in 2011.
Objectives
To evaluate if drug treatment is able to slow or arrest the disease progression of SMA types II and III, and to assess if such therapy can be given safely.
Search methods
We searched the Cochrane Neuromuscular Specialised Register, CENTRAL, MEDLINE, Embase, and ISI Web of Science conference proceedings in October 2018. In October 2018, we also searched two trials registries to identify unpublished trials.
Selection criteria
We sought all randomised or quasi‐randomised trials that examined the efficacy of drug treatment for SMA types II and III. Participants had to fulfil the clinical criteria and have a homozygous deletion or hemizygous deletion in combination with a point mutation in the second allele of the SMN1 gene (5q11.2‐13.2) confirmed by genetic analysis.
The primary outcome measure was change in disability score within one year after the onset of treatment. Secondary outcome measures within one year after the onset of treatment were change in muscle strength, ability to stand or walk, change in quality of life, time from the start of treatment until death or full‐time ventilation and adverse events attributable to treatment during the trial period.
Treatment strategies involving SMN1‐replacement with viral vectors are out of the scope of this review, but a summary is given in Appendix 1. Drug treatment for SMA type I is the topic of a separate Cochrane Review.
Data collection and analysis
We followed standard Cochrane methodology.
Main results
The review authors found 10 randomised, placebo‐controlled trials of treatments for SMA types II and III for inclusion in this review, with 717 participants. We added four of the trials at this update. The trials investigated creatine (55 participants), gabapentin (84 participants), hydroxyurea (57 participants), nusinersen (126 participants), olesoxime (165 participants), phenylbutyrate (107 participants), somatotropin (20 participants), thyrotropin‐releasing hormone (TRH) (nine participants), valproic acid (33 participants), and combination therapy with valproic acid and acetyl‐L‐carnitine (ALC) (61 participants). Treatment duration was from three to 24 months. None of the studies investigated the same treatment and none was completely free of bias. All studies had adequate blinding, sequence generation and reporting of primary outcomes.
Based on moderate‐certainty evidence, intrathecal nusinersen improved motor function (disability) in children with SMA type II, with a 3.7‐point improvement in the nusinersen group on the Hammersmith Functional Motor Scale Expanded (HFMSE; range of possible scores 0 to 66), compared to a 1.9‐point decline on the HFMSE in the sham procedure group (P < 0.01; n = 126). On all motor function scales used, higher scores indicate better function.
Based on moderate‐certainty evidence from two studies, the following interventions had no clinically important effect on motor function scores in SMA types II or III (or both) in comparison to placebo: creatine (median change 1 higher, 95% confidence interval (CI) –1 to 2; on the Gross Motor Function Measure (GMFM), scale 0 to 264; n = 40); and combination therapy with valproic acid and carnitine (mean difference (MD) 0.64, 95% CI –1.1 to 2.38; on the Modified Hammersmith Functional Motor Scale (MHFMS), scale 0 to 40; n = 61).
Based on low‐certainty evidence from other single studies, the following interventions had no clinically important effect on motor function scores in SMA types II or III (or both) in comparison to placebo: gabapentin (median change 0 in the gabapentin group and –2 in the placebo group on the SMA Functional Rating Scale (SMAFRS), scale 0 to 50; n = 66); hydroxyurea (MD –1.88, 95% CI –3.89 to 0.13 on the GMFM, scale 0 to 264; n = 57), phenylbutyrate (MD –0.13, 95% CI –0.84 to 0.58 on the Hammersmith Functional Motor Scale (HFMS) scale 0 to 40; n = 90) and monotherapy of valproic acid (MD 0.06, 95% CI –1.32 to 1.44 on SMAFRS, scale 0 to 50; n = 31).
Very low‐certainty evidence suggested that the following interventions had little or no effect on motor function: olesoxime (MD 2, 95% –0.25 to 4.25 on the Motor Function Measure (MFM) D1 + D2, scale 0 to 75; n = 160) and somatotropin (median change at 3 months 0.25 higher, 95% CI –1 to 2.5 on the HFMSE, scale 0 to 66; n = 19). One small TRH trial did not report effects on motor function and the certainty of evidence for other outcomes from this trial were low or very low.
Results of nine completed trials investigating 4‐aminopyridine, acetyl‐L‐carnitine, CK‐2127107, hydroxyurea, pyridostigmine, riluzole, RO6885247/RG7800, salbutamol and valproic acid were awaited and not available for analysis at the time of writing.
Various trials and studies investigating treatment strategies other than nusinersen (e.g. SMN2‐augmentation by small molecules), are currently ongoing.
Authors' conclusions
Nusinersen improves motor function in SMA type II, based on moderate‐certainty evidence.
Creatine, gabapentin, hydroxyurea, phenylbutyrate, valproic acid and the combination of valproic acid and ALC probably have no clinically important effect on motor function in SMA types II or III (or both) based on low‐certainty evidence, and olesoxime and somatropin may also have little to no clinically important effect but evidence was of very low‐certainty. One trial of TRH did not measure motor function.
Plain language summary
Medicines for spinal muscular atrophy types II and III
What was the aim of this review?
This Cochrane Review aimed to look at the effects of medicines on spinal muscular atrophy (SMA) types II and III in terms of disability, muscle strength, ability to stand or walk, quality of life, and time to death or full‐time ventilation, within one year of beginning treatment. We also wanted to identify any harmful effects of the treatments during the trial period. Cochrane review authors collected relevant studies to answer this question and found 10 studies.
Key messages
Nusinersen given by intrathecal (into the spine) injection probably improves disability in SMA.
Creatine, phenylbutyrate, gabapentin, hydroxyurea, valproic acid and combination therapy with valproic acid and acetyl‐L‐carnitine probably have no clinically important effect on motor function (movements and actions of the muscles) in SMA types II and III, based on evidence from single completed, published trials.
Olesoxime and subcutaneous somatotropin may have little or no effect on motor function in SMA, but the reliability of the evidence was very low. One trial of intravenous (into a vein) thyrotropin‐releasing hormone (TRH) did not measure motor function and the reliability of the evidence was very low. All the studies had limitations in design or performance that could have affected the results.
What was studied in the review?
This review is of medicines for SMA types II and III. Symptoms of SMA first appear in childhood and adolescence. The main feature is increasing muscle weakness. Children with SMA type II will never be able to walk without support; they usually live into adolescence or longer, but with a shortened life expectancy. The age of onset of SMA type II is between six and 18 months. Children with SMA type III will walk independently but may lose the ability to walk at some time and they have a normal life expectancy. The age of onset of SMA type III is after 18 months.
What were the main results of the review?
We identified 10 trials, which included 717 participants. All trials compared a medicine with an inactive substance (placebo) or sham (pretend) procedure. The trials studied oral (by mouth) creatine (55 participants), oral gabapentin (84 participants), oral phenylbutyrate (107 participants), oral hydroxyurea (57 participants), intrathecal nusinersen (126 participants), oral olesoxime (165 participants), subcutaneous (under the skin) somatotropin (20 participants), intravenous TRH (nine participants), oral valproic acid (33 participants) or combination therapy with oral valproic acid and acetyl‐L‐carnitine (ALC) (61 participants). Treatment duration was from three to 24 months.
Nusinersen had a beneficial effect on motor function in people with SMA type II, when compared to a sham procedure. There were probably no beneficial effects on motor function in SMA types II/III for creatine, gabapentin, hydroxyurea, phenylbutyrate, valproic acid or combination therapy with valproic acid and ALC. Olesoxime and somatotropin may have no effect on motor function. The small TRH trial did not assess motor function and did not provide evidence of any reliability on other outcomes. We found all the studies to have limitations in design or performance that could have affected the results. Eight studies were (partially) funded by pharmaceutical companies, either by supplying the study drug or by giving financial support otherwise. In two studies investigating nusinersen and olesoxime, the pharmaceutical companies were involved in data analysis and reporting.
We are awaiting the results of nine completed trials investigating 4‐aminopyridine, ALC, CK‐2121707 hydroxyurea, pyridostigmine, riluzole, RO6885247/RG7800, salbutamol and valproic acid which were not available at the time of writing.
How up to date is this review?
The evidence is up to date to October 2018.
Summary of findings
Background
Description of the condition
Spinal muscular atrophy (SMA) is a neuromuscular disorder of childhood and adolescence with an annual incidence of 1 in 6000 to 1 in 12,000 people (Arkblad 2009; Cobben 2001; Nicole 2002). It is caused by degeneration of anterior horn cells in the spinal cord and characterised by progressive muscle weakness (Iannaccone 2001; Talbot 1999). Other parts of the peripheral nervous system such as the neuromuscular junction (NMJ), and possibly muscles and other organs, may also be affected (Braun 1995; Cifuentes‐Diaz 2002; Kariya 2008; Murray 2008).
SMA is an autosomal‐recessive disease caused by the homozygous deletion of the SMN1 gene, which has been mapped to chromosome 5q11.2‐13.3 (Brzustowicz 1990; Gilliam 1990; Lefebvre 1995; Melki 1990a; Melki 1990b). The deleted gene results in survival motor neuron (SMN) protein deficiency. Chromosome 5q11.2‐13.3 contains the duplicated SMN1 and SMN2 genes (Iannaccone 1998; Nicole 2002). The SMN1 and SMN2 genes are almost identical, but a crucial C to T nucleotide difference in exon 7 results in the exclusion of exon 7 from most SMN2 messenger ribonucleic acid (mRNA) copies (Lefebvre 1995; Lorson 1999). The functional SMN1 gene, which is transcribed into full‐length mRNA that produces the bulk of stable SMN protein, is lacking in people with SMA. The SMN2 gene, which is 80% to 90% transcribed into a truncated form lacking exon 7, only produces residual levels of full‐length SMN mRNA and protein (Cartegni 2006; Lorson 1999). The clinical severity of the disease is related to the number of copies of the SMN2 gene (Feldkotter 2002; Harada 2002; Piepers 2008; Swoboda 2005; Wadman 2017).
The cellular functions of the SMN protein are multiple (Sumner 2007), including ribonucleoprotein (RNP) assembly (Burghes 2009; Gendron 1999; Jablonka 2000; Lefebvre 1998; Pellizzoni 1998), motor axon outgrowth and axonal transport (McWhorter 2003; Rossoll 2003), protection against superoxide dismutase 1 (SOD1) toxicity (Zou 2007), endocytosis (Hosseinibarkooie 2016; Riessland 2017), and ubiquitin homeostasis (Wishart 2014).
Muscle weakness in SMA occurs predominantly in the axial and proximal muscle groups, with the lower limbs more affected than the upper limbs (Kroksmark 2001; Thomas 1994). In more severe cases of SMA, intercostal muscles are also weakened, usually with relative sparing of the diaphragm. Survival depends primarily on respiratory function and not necessarily on motor ability (Dubowitz 1995; Russman 1992; Talbot 1999). There is often a fine tremor in the fingers (Iannaccone 1998). Although the face is often spared, tongue fasciculations and facial weakness are not unusual findings (Iannaccone 1993). Cognitive function of people with SMA is normal (Iannaccone 1998; Thomas 1994). Electrophysiological examination shows denervation and reinnervation (Iannaccone 1998; Nicole 2002; Swoboda 2005).
Classification of SMA according to the International SMA Collaboration distinguishes five types (0 to IV), which are based on age of onset and maximal acquired motor function (Finkel 2015; Mercuri 2012; Munsat 1992). SMA types 0, I and IV represent the two ends of the spectrum of SMA, which are outside the scope of this review.
SMA type II is also known as intermediate SMA, juvenile SMA and chronic SMA. The age of onset is between six and 18 months. Children with SMA type II develop the ability to sit independently but are never able to walk without support. They often develop severe pulmonary and orthopaedic complications (Bertini 2005). The children generally survive beyond two years of age and usually live into adolescence or longer (Russman 1996; Zerres 1995; Zerres 1997).
SMA type III is known as Kugelberg‐Welander disease, Wohlfart‐Kugelberg‐Welander disease and mild SMA. The age of onset is after 18 months. Children with SMA type III develop the ability to walk independently at some time, although many lose this ability later in life. Most people with SMA type III have a normal life expectancy (Russman 1992; Zerres 1995; Zerres 1997). SMA type III is often further divided into SMA type IIIa (disease onset before 36 months of age) and SMA type IIIb (disease onset after 36 months of age) (Zerres 1995).
Description of the intervention
Drug treatment to modify the course of SMA types II and III is urgently needed. Management of SMA until recently has consisted of preventing or treating complications of the condition (Iannaccone 1998; Russman 2003; Finkel 2018; Mercuri 2018). Administration of agents capable of increasing the expression of SMN protein levels may improve the outcome in SMA (Feldkotter 2002; Gavrilina 2008; Lorson 1999). Transcriptional SMN2 activation, facilitation or correction of SMN2 splicing, translational activation and stabilisation of the full‐length SMN protein are possible therapeutic strategies for SMA. Other strategies are improvement of motor neuron viability by neuroprotective or neurotrophic agents (Lunn 2008; Thurmond 2008; Wirth 2006a). Recently, trials with splice‐site‐modulators (Chiriboga 2016; EMBRACE 2015; Finkel 2016; Finkel 2017 (ENDEAR); Mercuri 2018 (CHERISH); NCT01703988; NCT02052791; NCT02122952; NCT02268552; SHINE 2015), ribonucleic acid (RNA)‐degradation inhibitors (Butchbach 2010; Gogliotti 2013; van Meerbeke 2013), and compounds that replace the SMN1 gene have started (Appendix 1).
Various drugs that might slow down or cure SMA have been tested in open and (un)controlled studies of people with SMA types II and III, including thyrotropin‐releasing hormone (TRH) (Takeuchi 1994; Tzeng 2000), gabapentin (Merlini 2003; Miller 2001), phenylbutyrate (Mercuri 2004; Mercuri 2007; NPTUNE01 2007; STOPSMA 2007), creatine (Wong 2007), valproic acid (Conceicao 2010; JPRN‐JapicCTI‐163450 2016; Kissel 2014; Kissel 2011; NCT01671384; Saito 2014; SMART01; SMART02; SMART03; Swoboda 2009; Swoboda 2010), hydroxyurea (Chang 2002; Chen 2010; Liang 2008; NCT00568802), somatropin (Kirschner 2014), carnitine (Kissel 2014; Merlini 2007; Swoboda 2010), salbutamol (Giovannetti 2016; Khirani 2017; Kinali 2002; Morandi 2013; Pane 2008; Pasanisi 2014; Prufer de Queiroz Campos Araujo 2010; Tan 2011), riluzole (Abbara 2011; ASIRI 2008; Russman 2003), lamotrigine (Nascimento 2010), celecoxib (NCT02876094), olesoxime (Bertini 2017), SMN1 gene therapy (Mendell 2016; NCT02122952; Sproule 2016), SMN2 antisense oligonucleotides (ASO) (Chiriboga 2016; Mercuri 2018 (CHERISH); NCT01703988; NCT02052791; SHINE 2015), small molecules (JEWELFISH 2017; MOONFISH 2014; NCT02644668; SUNFISH 2016), and NMJ‐interactors (EMOTAS 2014; NCT01645787; SPACE).
Below we describe the working mechanisms, preclinical studies in SMA models, and results of studies and trials of the various drugs tested in people with SMA type II and III.
It was not clear on clinical grounds whether the patient populations in studies on coenzyme Q10, lithium carbonate and guanidine hydrochloride had a genetically confirmed diagnosis of SMA, partially because SMN gene analysis was not possible prior to 1991 (Angelini 1980; Folkers 1995; Il'ina 1980). Therefore, we have not discussed the therapeutic effects of these drugs.
In vitro and animal studies have found several other compounds to have an effect on SMN expression, but they are as yet untested in people with SMA. Therefore, they are outside the scoop of this review. See Appendix 2 for a brief description of these compounds.
SMN1 gene therapies are outside the scope of this review. We have added some information in Appendix 1 for overall completeness.
Antisense oligonucleotides
ASOs or 'morpholinos', are synthetic strands of nucleic acid that are able to interfere with (stimulate or inhibit) mRNA products of the target DNA sequence. In this way, ASOs can modify potential splice sites and interfere with splicing (Porensky 2013). Multiple ASOs for the SMN2 gene have been developed and investigated (Bogdanik 2015; Keil 2014; Nizzardo 2014; Osman 2014; Shababi 2012; Skordis 2003; Staropoli 2015; Zhou 2013; Zhou 2015). The intronic splice silencer in intron 7 of SMN2 is called nusinersen (formerly known as SMN Rx 39443, IONIS SMN Rx or ISIS‐SMN Rx). This compound specifically targets the splice silencer in intron 7 and ensures the inclusion of SMN2 exon 7, which results in increased SMN2 full‐length mRNA and protein production (Hua 2010). Nusinersen has subsequently demonstrated improved performance and survival in SMA animal models (Hua 2011; Passini 2011). Nusinersen is an intrathecally injected therapy.
Carnitine
L‐carnitine, an essential cofactor for the beta‐oxidation of long‐chain fatty acids, inhibits mitochondrial injury and apoptosis both in vitro and in vivo (Bigini 2002; Bresolin 1984). Acetyl‐L‐carnitine, the acetylated derivative of L‐carnitine, shows neuroprotective and neurotrophic activity in motor neuron cultures (Bigini 2002). L‐carnitine treatment restored the level of free carnitine in one animal model of SMA (Bresolin 1984).
Celecoxib
Treatment with celecoxib increased SMN RNA and protein levels in vitro and in models of severe SMA mice by activating the p38 pathway (Farooq 2013), and might have a neuroprotective effect by inhibition of glutamate release (Bezzi 1998). Glutamate is released after presynaptic depolarisation and if not efficiently cleared, leads to increased levels of free radicals, and potentially to degeneration of motor neurons (Bryson 1996).
Creatine
Creatine might have therapeutic benefit by increasing muscle mass and strength through its role as an energy shuttle between mitochondria and working musculature, and is thought to exert neuroprotective effects (Bessman 1981; Ellis 2004; Tarnopolsky 1999).
Gabapentin
Gabapentin has a neuroprotective role by diminishing the excitotoxicity of glutamate (Greensmith 1995; Merlini 2003; Taylor 1998).
Hydroxyurea
Hydroxyurea is a histone deacetylase inhibitor. Studies have suggested a therapeutic role for these agents in SMA, as they appear to activate SMN2 transcription (Darras 2007; Kernochan 2005; Wirth 2006b). In vitro, hydroxyurea increases SMN2 gene expression and production of SMN protein in cultured lymphocytes of people with SMA (Grzeschik 2005; Liang 2008).
Lamotrigine
Lamotrigine is a glutamate inhibitor and might prevent motor neuron death (Casanovas 1996).
Olesoxime
The experimental drug olesoxime (TRO19622) is thought to modulate the mitochondrial permeability transition pore (mPTP) opening, which might influence cell apoptosis of, for example, motor neurons (Bordet 2007; Bordet 2010).
Phenylbutyrate
Phenylbutyrate is a histone deacetylase inhibitor. In fibroblast cultures and leukocytes of people with SMA, phenylbutyrate increased SMN transcript expression (Also‐Rallo 2011; Andreassi 2004; Brahe 2005).
Valproate
Valproate is another histone deacetylase inhibitor that increases SMN protein in vitro by increasing transcription of SMN2 gene (Kernochan 2005; Weihl 2006). It also has an antiglutamatergic effect (Kim 2007). Valproate has been tested in various models of SMA and showed positive results on SMN expression in vitro (Brichta 2003; Brichta 2006; Sumner 2003) and in vivo (Piepers 2011).
Salbutamol
Some studies have documented positive effects of oral beta2‐adrenoceptor agonists on human skeletal muscle (Caruso 1995; Kindermann 2007; Mack 2014; Martineau 1992). Trials investigating effects of oral beta2‐adrenoceptor agonists in people with NMJ disorders have demonstrated improvement of motor function (Burke 2013; Liewluck 2011; Lorenzoni 2013; Rodríguez Cruz 2015). Since abnormal development of the NMJ and dysfunction of neuromuscular synaptic transmission occur in SMA (Braun 1995; Kariya 2008; Kong 2009; Murray 2008; Wadman 2012a), beta2‐adrenoceptor agonists might have an positive effect on muscles and NMJs in SMA. In fibroblasts of people with SMA, salbutamol increases the levels of SMN2 full‐length mRNA and the SMN protein (Angelozzi 2008). In 12 people with SMA types II and III who received six months of treatment with oral salbutamol, leukocytes showed a significant and constant increase in SMN2 full‐length transcript levels (Tiziano 2010).
Small molecules
RO6885247/RG7800
The small molecule RO6885247/RG7800 selectively modulates SMN2 splicing towards the inclusion of exon 7 and thereby stimulates production of full‐length SMN2 mRNA. Administration of RO6885247/RG7800 improves and almost rescues motor function and survival of SMA mice (Naryshkin 2014).
RO7034067/RG7916
The small molecule RO7034067/RG7916 modulates SMN2 splicing, but exact details of its structure and pharmacology are not available. One phase I trial with RO7034067/RG7916 combined with itraconazole in healthy volunteers showed a dose‐dependent increase of SMN2 mRNA transcripts, but results were only reported in a conference abstract, with further publication of data pending (NCT02633709; Sturm 2016).
CK‐2127107
CK‐2127107/CK‐107 (2‐aminoalkyl‐5‐N‐heteroarylpyrimidine) is a small‐molecule fast skeletal troponin activator candidate that has been tested in conditions of muscle weakness, fatigue and heart failure (Hwee 2015). It might have a beneficial effect in SMA because of muscle protection, increased muscle strength in skeletal muscle, and delay of onset and extent of muscle fatigue (Andrews 2018). One report of a phase I study in healthy men reported that the drug was well tolerated and there were no serious adverse events (Rudnicki 2016).
Somatropin
Somatropin, also called growth hormone or somatomedin C, is a small polypeptic hormone produced in the pituitary gland. It interacts with growth hormone receptors primarily in the muscles and liver, in which it induces insulin‐like growth factor‐1 (IGF‐1). Because of its primary role in liver and muscle metabolism, IGF‐1 seems to play an important role during muscle development and induces muscle regeneration after injury and denervation (Duan 2010). IGF‐1 stimulates myoblast and motor neuron proliferation, induces myogenic differentiation and generates myocyte hypertrophy in vitro and in vivo (Bosch‐Marcé 2011; Murdocca 2012). In vitro studies of motor neuron tissue cultures of rat spinal cord showed that IGF‐1 was one of the neuroprotective hormones that enhanced the survival of motor neurons and reduced their susceptibility to glutamate‐induced neurotoxicity (Corse 1999). One study showed that intracerebroventricular injections of IGF‐1 next to a SMN trans‐splicing RNA vector had a positive effect on disease severity and prolonged survival of severe SMA mice (Shababi 2011). One study showed that overexpression of IGF‐1 increased muscle mass, and that administration of a combination of IGF‐1 and trichostatin‐A improved survival and motor function in SMA mice (Bosch‐Marcé 2011). Biondi 2015 showed that underexpression of IGF‐1 receptors alone improved the motor function and the life span of SMA mice. Two studies investigated intracerebral injection of AVV‐IGF‐1 in SMA mice and showed variable results, with slightly improved motor function and survival due to prevention of muscle atrophy and preservation of NMJs (Tsai 2012; Tsai 2014).
Thyrotropin‐releasing hormone
The precise mechanism of action of TRH, a tripeptide produced by the hypothalamus, is unknown. It may have a neurotrophic effect on spinal motor neurons (Takeuchi 1994).
Riluzole
Riluzole is thought to have a neuroprotective effect on motor neurons by blocking the presynaptic release of glutamate. In a mouse model of SMA, riluzole attenuated disease progression (Haddad 2003).
Other neurotrophic factors
Other neurotrophic factors have been considered as potential therapies for motor neuron diseases (Apfel 2001). In a mouse model of SMA, cardiotrophin‐1 seemed effective in slowing down disease progression (Lesbordes 2003).
Neuromuscular junction interactors
Studies in SMN‐deficient mouse models of SMA have uncovered significant abnormalities in the morphology of the NMJ in SMA, in addition to the well‐known motor neuron degeneration (Braun 1995; Kariya 2008; Kong 2009; Murray 2008). Additionally, there was abnormal aggregation of acetylcholine receptors at the muscle endplates in people with SMA type I (Arnold 2004). Electrophysiological studies in people with SMA have shown neurophysiological alterations of the NMJ, which may correspond with the symptoms of fatigability (Dunaway 2014; Montes 2013; Wadman 2012a). Drugs such as pyridostigmine and neostigmine, which have an inhibitory effect on acetylcholinesterase, might directly interact with the NMJ and could improve its function. Other potential NMJ interactors are 3‐4 diaminopyridine (3‐4 DAP) and 4‐aminopyridine (4‐AP), which are potassium channel blockers that are presumed to prolong repolarisation and to facilitate the generation of the action potential at the NMJ.
Why it is important to do this review
There has been no treatment to slow progression or cure SMA types II or III (Bosboom 2009; Wadman 2012b).
Many studies have explored the effects of various drugs in SMA animal models or in people with SMA. Currently, several drugs and compounds tested in uncontrolled, unblinded and non‐randomised settings have shown possible positive effects on the course of SMA through neuroprotection (e.g. cardiotrophin‐1, creatine, gabapentin, lamotrigine and riluzole), induction of SMN2 activity (histone deacetylase inhibitors, e.g. valproic acid, phenylbutyrate and hydroxyurea), improvement of NMJ transmission (e.g. pyridostigmine), modification of SMN2 RNA (ASOs or small molecules, e.g. nusinersen), genetic restoration of the SMN1 gene using viral vectors, improvement of muscle metabolism and strength (e.g. creatine), and other (unknown) mechanisms (e.g. somatotropin, salbutamol, TRH). Overall, these studies provide conflicting evidence about the effects of these compounds on muscle strength, motor function and survival in SMA.
The number of studies and trials for drug treatment in SMA has expanded rapidly, which has created a need for a clear, thorough and systematic review of these trials and their results. We used Cochrane Systematic Review methods (Higgins 2011), and the GRADE approach (Atkins 2004), to review all randomised studies and trials on drug treatment in people with SMA types ii and III to analyse the effect of drug treatments on disability, muscle strength, ability to stand or walk, quality of life, time to death or full‐time ventilation and adverse events.
This is an update of a review first published in 2009 and first updated in 2012 (Bosboom 2009; Wadman 2012b). Drug treatment for SMA type I is the subject of a separate Cochrane Review (Wadman 2019).
Objectives
To evaluate if drug treatment is able to slow or arrest the disease progression of SMA types II and III, and to assess if such therapy can be given safely.
Methods
Criteria for considering studies for this review
Types of studies
All randomised or quasi‐randomised (alternate or other systematic treatment allocation) studies examining the effect of drug treatment designed to slow or arrest disease progression in children or adults with SMA types II and III. Placebo‐controlled cross‐over studies were also considered to be eligible for inclusion.
Types of participants
Children or adults with SMA types II and III fulfilling the criteria outlined in Table 11.
1. Diagnostic criteria for SMA types II and III.
| Primary criteria |
| SMA type II: age of onset between 6 and 18 months and have been able to sit independently, but never been able to walk without assistance. SMA type III: age of onset after 18 months and has/had the ability to walk without assistance. |
| Genetic analysis to confirm the diagnosis, with deletion or mutation of the SMN1 gene (5q11.2‐13.3) |
| Supporting criteria |
| Symmetrical muscle weakness of limb and trunk. |
| Proximal muscles more affected than distal muscles and lower limbs more than upper limbs. |
| No abnormality of sensory function. |
| Serum creatine kinase activity ≤ 5 times the upper limit of normal |
| Denervation on electrophysiological examination, and no nerve conduction velocities < 70% of the lower limit of normal. No abnormal sensory nerve action potentials. |
| Muscle biopsy showing atrophic fibres of both types, hypertrophic fibres of one type (usually type I), and in chronic cases type grouping. |
| No involvement of the central neurological systems, such as hearing or vision. |
Types of interventions
Any drug treatment, alone or in combination, designed to slow or arrest the progress of the disease compared to placebo (or sham) treatment, with no restrictions on the route of administration.
Types of outcome measures
We assessed outcome measures within or up to one year after the onset of treatment and compared to baseline. This is a list of the outcomes of interest within whichever studies are included in the review; we did not use outcomes as criteria for including studies.
Primary outcomes
Change in disability score (e.g. Gross Motor Function Measure (GMFM), Hammersmith Functional Motor Score (HFMS), Motor Function Measure (MFM) and SMA Functional Rating Scale (SMAFRS)) as determined by the original study authors.
Secondary outcomes
Change in muscle strength (e.g. dynamometry, isometric strength testing, manual muscle testing (MMT) or Medical Research Council (MRC) score).
Acquiring the ability to stand within one year after the onset of treatment.
Acquiring the ability to walk or improvement of walking within one year after the onset of treatment.
Change in quality of life as determined by quality of life scales.
Change in pulmonary function (forced vital capacity (FVC) as a percentage of FVC predicted for height). This was not stated in the original protocol, but many trials included a measure of pulmonary function or the strength of respiratory muscles.
Time from beginning of treatment until death or full‐time ventilation (a requirement for 16 hours of ventilation out of 24 hours regardless of whether this was with tracheostomy, a tube or mask).
Adverse events attributable to treatment during the whole study period, separated into severe (requiring or lengthening hospitalisation, life threatening or fatal) and others.
Search methods for identification of studies
Electronic searches
We searched the following databases on 22 October 2018.
Cochrane Neuromuscular Specialised Register (in the Cochrane Register of Studies (CRS); Appendix 3).
Cochrane Central Register of Studies (CENTRAL) (in the Cochrane Register of Studies Online (CRSO); Appendix 4).
MEDLINE (1991 to October week 43 2018; Appendix 5).
Embase (1991 to October week 43 2018; Appendix 6).
ISI Web of Science Conference Proceedings Citation Index (1991 to October 2018; Appendix 7).
We consulted the following registries on 22 October 2018 to identify additional trials that had not yet been published.
clinical trials registry of the US National Institute of Health (www.clinicaltrials.gov; Appendix 8).
the WHO international Clinical trials Registry (www.who.int/ictrp/en/; Appendix 9).
Searches were performed from 1991 onwards because at that time genetic analysis of the SMN1 gene became widely available and could be used to establish the diagnosis of SMA.
Searching other resources
We handsearched the reference lists of relevant cited studies, reviews, meta‐analyses, textbooks and conference proceedings to identify additional studies. We invite readers to suggest studies, particularly in other languages, that should be considered for inclusion.
Data collection and analysis
Selection of studies
For this updated review, two review authors (RW and AV) independently checked titles and abstracts obtained from literature searches to identify potentially relevant trials for full review.
We identified and excluded duplicates and collated multiple reports of the same study so that each study rather than each report was the unit of interest in the review.
From the full texts, two review authors (RW and AV) independently selected trials for inclusion that met the selection criteria. The review authors were not blinded to the trial author and source institution. The review authors resolved disagreement by reaching consensus. We presented an adapted PRISMA flowchart of study selection (Figure 1), and recorded details of excluded studies in the Characteristics of excluded studies table.
1.
Study flow diagram.
Data extraction and management
Two review authors (RW and AV) independently extracted data using a specially designed data extraction form. We extracted study characteristics from included studies on study design and setting, characteristics of participants (SMA type and age), eligibility criteria, intervention details, the outcomes assessed, source(s) of study funding and any conflicts of interest among investigators and recorded them in the Characteristics of included studies table.
We obtained missing data from the trial authors or pharmaceutical company whenever possible.
Disagreement did not occur, but we would have resolved differences by reaching consensus or with third party adjudication, if necessary.
Assessment of risk of bias in included studies
The 'Risk of bias' assessment took into account allocation concealment, security of randomisation, participant blinding (parent blinding), blinding of outcome assessors, incomplete outcome data (including use of intention‐to‐treat (ITT) analysis), selective reporting and 'other bias'. We scored each 'Risk of bias' item according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), as 'low', 'high' or 'unclear'.
Statistical considerations involved a trade‐off between bias and precision. We assessed the risk of bias as 'unclear' when too few details were available to make a judgement of 'high' or 'low' risk, when the risk of bias was genuinely unknown despite sufficient information about the conduct of the study, or when an entry was not relevant to a study. All studies were described by a precise risk of bias.
Two review authors (RW and AV) independently graded the risk of bias in included studies. In the event of disagreement, the review authors reassessed studies and reached agreement by consensus.
Measures of treatment effect
We initially intended to analyse continuous outcomes using mean differences (MDs) with 95% confidence intervals (CIs) in the outcome measures with standard deviation (SD) to quantify the effects of the drug treatment (such as change in disability scores, MRC muscle strength, quality of life) and dichotomous outcomes using a risk ratio (RR) with 95% CIs (such as ability to stand or walk and adverse events). We reported median
For survival or time to full‐time ventilation, we would have reported results from Kaplan‐Meier survival analyses if data been presented in this way.
Unit of analysis issues
We took into account the level at which randomisation occurred in cross‐over trials. We did not anticipate finding cluster‐randomised trials and did not anticipate that multiple observations for the same outcome would occur in the included studies.
Where multiple trial arms were reported in a single trial, we would have included only the treatment arms relevant to the review topic. If two comparisons (e.g. drug A versus placebo and drug B versus placebo) were combined in the same meta‐analysis, we would have followed the guidance in Section 16.5.4 of the Cochrane Handbook for Systematic Reviews of Interventions to avoid double‐counting (Higgins 2011). Our preferred approach would have been to perform a multiple‐treatments meta‐analysis using the indirect comparison method. In case of such analysis in the next update of this review, we will have expert statistical support, as well as subject expertise to analyse the data.
Cross‐over trials
If neither carry‐over nor period effects were present in cross‐over trials and individual participant data or the mean and SD (or standard error) of the participant‐specific differences between experimental intervention and control intervention measurements were available, we would have analysed continuous data using a paired t‐test in the two‐period, two‐intervention setting. The effect estimate would have been included in any meta‐analysis using the generic inverse variance function in Review Manager 5 (Review Manager 2014). In the absence of data for such an analysis, we would have analysed the treatment and placebo group as if they were parallel groups with the risk of a unit‐of‐analysis error. In the event of potential carry‐over or period effects, we would have analysed data from only the first period.
Dealing with missing data
We carefully evaluated important numerical data, such as the number of screened, randomised participants as well as ITT, as‐treated and per protocol populations. We investigated attrition rates (i.e. dropouts, losses to follow‐up and withdrawals), and critically appraised issues of missing data and imputation methods (e.g. last observation carried forward (LOCF)). In case of missing outcome data, we would have performed an ITT analysis. If SDs for outcomes were not reported, we would have imputed these values by assuming the SD of the missing outcome to be the mean of the SDs from studies where this information was reported (Higgins 2011).
Where there were missing data, we contacted the trial investigators, who provided additional data (Kirschner 2014; Kissel 2014; Miller 2001; Wong 2007).
Assessment of heterogeneity
In the event of substantial clinical, methodological or statistical heterogeneity, we would not have reported study results as the pooled effect estimate in a meta‐analysis. We would have identified heterogeneity by visual inspection of the forest plots and by using a standard Chi² test with a significance level of alpha = 0.1, in view of the low power of this test.
We would have examined heterogeneity using the I² statistic, which quantifies inconsistency across studies, to assess the impact of heterogeneity on the meta‐analysis.
We would have used the approximate guide to interpretation of the I² statistic as outlined in Chapter 11 of the Cochrane Handbook for Systematic Reviews of Interventions, as follows:
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity;
75% to 100%: considerable heterogeneity.
Assessment of reporting biases
We reviewed and included studies from trial registries to assess the magnitude of publication bias (Appendix 8; Appendix 9). If trials were completed but not yet published, we tried to retrieve results by contacting the principal investigators of the trials.
Data synthesis
We would only have pooled results of studies with the same class of drug treatment.
We would have calculated MDs or RRs with corresponding 95% CI for the pooled data if studies were sufficiently comparable. For continuous outcomes measured using different but comparable scales, we would have calculated standardised mean differences (SMD) and 95% CI, taking care to ensure a consistent direction of effect. If data were not sufficiently comparable between studies, we would have used the standard Review Manager 5 generic inverse variance (GIV) analysis using treatment effect differences with their standard errors.
The review authors estimated differences in medians and CI for the median from participant‐level data from Miller 2001 and Wong 2007 using a Hodges‐Lehmann estimator.
We would have pooled survival data using the GIV approach. If studies to be pooled had different follow‐up periods, we would have used appropriate adjustments, if necessary Poisson regression allowing for the aggregate person‐time‐at‐risk in the study groups.
When Chi² analysis showed the data to be heterogeneous, we would have used a random‐effects model with a maximum likelihood estimation, carrying out a sensitivity analysis with a fixed‐effect model (Mantel‐Haenszel RR method). Formal comparisons of intervention effects according to risk of bias would have been done using meta‐regression. The major approach to incorporating 'Risk of bias' assessments would have been to incorporate and restrict meta‐analyses to studies at low (or lower) risk of bias.
'Summary of findings' tables
We created 'Summary of findings' tables using the following outcomes depending on the outcomes used in the included studies:
change in disability score (e.g. GMFM, HFMS and MFM);
change in muscle strength (e.g. dynamometry, isometric strength testing, MMT or MRC;
acquiring the ability to stand or walk within one year after the onset of treatment;
change in quality of life as determined by quality of life scales;
change in pulmonary function (FVC; reported preferably as a percentage of FVC predicted for height or age (or both) or alternatively as total volume in litres);
time from beginning of treatment until death or full‐time ventilation;
adverse events (reported preferably as number of adverse events or alternatively as number of people with adverse events).
We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the certainty of a body of evidence (studies that contributed data for the prespecified outcomes). We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) using GRADEpro GDT software (gradepro.org). We justified all decisions to down‐ or upgrade the certainty of studies using footnotes and made comments to aid reader's understanding of the review where necessary. Although studies might be graded as high risk in any of the GRADE domains, we would not have excluded the particular study.
Subgroup analysis and investigation of heterogeneity
We would have attempted to determine potential reasons for heterogeneity by examining individual study and subgroup characteristics.
We would have performed subgroup analyses as follows to explore the influence of the following factors (if applicable) on effect sizes:
SMA type (II versus III);
SMN2 copy number.
Subgroup analysis based on SMA type and SMN2 copy number is needed, since the subgroups contain a different disease course with potential, significant different effects from or interaction with the intervention.
We would have compared subgroups using the formal tests for subgroup differences in Review Manager 5 (Review Manager 2014).
Sensitivity analysis
We would have performed sensitivity analyses as follows to explore the influence of the following factors (if applicable) on effect sizes. We would have restricted the analysis:
by taking into account risk of bias;
to outlier studies (very long, very large, very short or very small) to establish the extent to which they dominated the results.
We would also have tested the robustness of the results by repeating the analysis using different measures of effect size (RR, odds ratios, etc.) and different statistical models (fixed‐effect and random‐effects models).
Non‐randomised evidence
We did not include non‐randomised studies in our review. In the Discussion section, we reviewed the results from open and uncontrolled studies.
Results
Description of studies
Results of the search
For this updated review, the numbers of new references found by the searches were: Cochrane Neuromuscular Specialised Register 67 (37 new), CENTRAL 173 (90 new), MEDLINE 676 (351 new), Embase 196 (123 new) and ISI Web of Knowledge 402 (277 new).
Studies with no published data yet, were named by their acronym, or after their trial register code (www.clinicaltrial.gov).
See Figure 1 for a flow diagram of the study selection process.
Included studies
Ten trials fulfilled the selection criteria and remained for inclusion (see Included studies). There were two studies with the same class of drug treatment (valproic acid) (Kissel 2014; Swoboda 2010), but one of these trials used L‐carnitine as add‐on medication (Swoboda 2010). Two studies only included people with SMA type II (Mercuri 2007; Mercuri 2018 (CHERISH)), one study only included ambulatory people with SMA type III (Kissel 2014), and one study included only non‐ambulatory children and adolescents with SMA types II and IIIa (Bertini 2017). Six studies did not make a distinction between the SMA subtypes for inclusion (Chen 2010; Kirschner 2014; Miller 2001; Swoboda 2010; Tzeng 2000; Wong 2007).
Oral creatine versus placebo
Wong 2007 was a double‐blind randomised placebo‐controlled trial that compared oral creatine with placebo in 55 participants divided into two age groups. Of the 22 participants aged two to five years, 10 received creatine 2 g once a day and 12 received placebo. Of the 33 participants aged five to 18 years, 17 received creatine 5 g once a day and 16 received placebo. Duration of treatment was six months with follow‐up at nine months.
Muscle strength for knee extension, knee flexion and elbow flexion were measured bilaterally with the Richmond Quantitative Measurement System. Hand grip strength was measured bilaterally with handheld dynamometry. The best scores were added to obtain a total, upper body and lower body quantitative muscle testing (QMT) score.
Treatment efficacy for each age group was evaluated by ITT analysis of continuous endpoints using analysis of covariance (ANCOVA), which included the qualifying screening measure as the baseline covariate, treatment group as between‐subject effect, time as within‐subject effect and a subject by time interaction.
The primary endpoint was the change in GMFM from baseline. Secondary endpoints were the changes in muscle strength and pulmonary function tests (e.g. FVC) from baseline in children five to 18 years of age, and change in quality of life (assessed by a neuromuscular module of the parent questionnaire for the Pediatric Quality of Life Inventory (PedsQL)) from baseline. Adverse events were routinely assessed at each visit.
Oral gabapentin versus placebo
Miller 2001 was a double‐blind, randomised, placebo‐controlled trial that compared oral gabapentin 1200 mg three times a day with placebo in 84 participants at least 21 years old. Duration of treatment was 12 months with follow‐up at quarterly intervals while on the treatment.
Muscle strength was measured bilaterally by maximum voluntary isometric contraction (MVIC) of elbow flexion and hand grip. Linear regression analysis was used to determine the change in muscle strength, FVC, SMAFRS and a combined measure of the functional capacity of the lower limbs and quality of life (mini‐Sickness Impact Profile: mini‐SIP) over time.
Treatment efficacy was determined by comparing the mean percentage change for the treatment and placebo groups in the ITT population (defined as participants with at least two study visits: 37 participants in the treatment group and 39 participants in the placebo group) using the Mann‐Whitney test.
The primary endpoint was mean percentage change in muscle strength from baseline. Secondary endpoints were the mean percentage change of FVC, SMAFRS and mini‐SIP from baseline, and the occurrence of adverse events. Adverse events were systematically assessed at each visit.
Oral hydroxyurea versus placebo
A phase II/III double‐blind randomised placebo‐controlled trial compared oral hydroxyurea with placebo in 57 participants with SMA types II and III aged above five years (Chen 2010). Participants received an escalated daily dose over four weeks to a final daily dose of 20 mg/kg/day hydroxyurea or placebo. For the first four weeks, participants received 10 mg/kg/day and for the second four weeks the dose was escalated to 15 mg/kg/day. Duration of treatment was 18 months. Follow‐up of post‐treatment effects was at six months.
The safety and tolerability of hydroxyurea were measured through serum level measurement. Muscle strength and motor function were measured with the MMT and the GMFM. The GMFM and MMT were performed in all 57 participants. The Modified Hammersmith Functional Motor Scale (MHFMS) was performed in 28 participants with SMA type II and 10 participants with SMA type III who were already non‐ambulatory at the beginning of the trial. Lung function was evaluated by FVC measurements. In all participants, quantitative full‐length SMN mRNA was measured. Adverse events and serious adverse events were monitored at each assessment by a full blood count, chemistry profiles of liver and renal function, and completion of a questionnaire.
Treatment efficacy was evaluated by ITT analysis with a LOCF approach. Changes in GMFM, MHFMS, MMT, FVC and serum full‐length SMN mRNA were analysed by ANCOVA. Measures at time points of the treatment period and the post‐treatment period for primary and secondary endpoints were compared by mixed models with adjusted covariates. A two‐tailed t‐test was used to compare the incidence of adverse events and serious adverse events during the treatment phase.
The primary endpoints were GMFM, MMT and serum full‐length SMN mRNA level. Secondary endpoints were the MHFMS and FVC. Adverse events were systematically assessed by a questionnaire at each visit.
Intrathecal nusinersen versus sham procedure
Mercuri 2018 (CHERISH) was a phase III double‐blind randomised, sham‐procedure controlled study that compared intrathecally injected nusinersen with a sham procedure in 126 participants with SMA type II, aged two to 12 years. Inclusion criteria included a minimal score of 10 and maximum score of 54 on the HMFSE. Participants were randomised 2:1 (nusinersen: sham‐procedure) to receive intrathecally injected nusinersen or the sham‐procedure. Participants received their treatment or sham‐procedure at days one, 29, 85 and 274.
Participants in the treatment group received nusinersen 12 mg intrathecally. The sham‐procedure consisted of a small needle prick on the lower back at the location where the lumbar puncture (LP) injection is normally made. The needle would break the skin but no LP injection or needle insertion occurred. The needle prick was covered with the same bandage that was used to cover the LP injection in the treatment group. Treatment period was planned to be 15 months, but was stopped after interim analysis showing beneficiary effects of nusinersen compared to the sham procedure.
Motor abilities were assessed by HFMSE and Upper Limb Module Test (ULMT). Motor milestone development was monitored, including standing and walking with or without support. Assessment of vital signs, weight changes and neurological examination were included. Adverse events and serious adverse events were monitored using laboratory parameters, urine analysis and electrocardiogram.
The interim analysis on treatment efficacy was done by ITT analysis with a LOCF approach and multiple‐imputation method to account for missing data. Interim analysis was performed when all the children had been enrolled for at least six months and at least 39 children had completed their 15‐month assessment. In the final analysis, the least squares mean changes in the total HFMSE score, the number of World Health Organization motor milestones achieved per child, and the Revised Upper Limb Module (RULM) score and least‐squares MDs in change between groups were based on an ANCOVA, with group assignment as a fixed effect and with adjustment for each child's age at screening and the value at baseline. The primary endpoint was change from baseline between treatment groups in the HFMSE. Secondary outcome measures were dichotomised analysis of the HFMSE scores (responder analysis of a 3‐point change in HFMSE), change from baseline in ULMT, milestone development and adverse events. Adverse events were systematically assessed at each visit.
Oral olesoxime versus placebo
Bertini and colleagues performed a double‐blind, randomised, placebo‐controlled trial compared oral olesoxime with placebo in 165 non‐ambulatory participants with SMA types II and IIIa aged three to 25 years (Bertini 2017). Participants were randomised 2:1 (olesoxime:placebo) to receive oral liquid suspension of olesoxime 10 mg/kg once daily or oral liquid suspension of placebo. Treatment period was 24 months.
Participants started with a screening and baseline visit. Follow‐up visits were scheduled four and 13 weeks after baseline, with follow‐up every 13 weeks during the treatment period of 24 months.
Motor abilities were assessed by MFM at weeks 26, 52, 78 and 104, and the HMFS at weeks 13, 39, 65, 91 and 104. Children younger aged younger than six years (n = 48) were assessed with the adapted version of the MFM‐32, the MFM‐20, where participants older than six years (n = 112) were tested with the MFM‐32. Electromyography, including compound muscle action potential (CMAP) and motor unit number estimation (MUNE) assessments of ulnar and hypothenar nerves, were performed at weeks 26, 52, 78 and 104. FVC was tested at weeks 13, 26, 39, 52, 78 and 104. The PedsQL was tested every visit. Clinical examination and electrocardiogram were performed every visit as well. Safety laboratory studies were performed at baseline and every consecutive visit. Serum levels of olesoxime were reviewed at weeks four, 13 and 52.
Data analysis was done with ITT analyses using mixed‐effects repeated measure model. An interim analysis was performed at 12 months with predefined criteria to assess whether the trial should be continued or terminated.
The primary endpoint was the change from baseline between treatment groups in MFM, parts D1+D2. Secondary outcome measures were responder analyses of change from baseline in total MFM scores and individual MFM domains and change from baseline in HFMS, CMAP amplitude, MUNE, Clinical Global Impression, FVC and PedsQL. Adverse events were systematically assessed at each visit.
Oral phenylbutyrate versus placebo
Mercuri 2007 was a phase II, double‐blind, randomised, placebo‐controlled trial that compared oral phenylbutyrate 500 mg/kg/day, divided into five doses and using an intermittent schedule (seven days on treatment, seven days off treatment), with placebo in 107 participants with SMA type II. Duration of treatment was 13 weeks with follow‐up at the end of the study period (also at 13 weeks).
Motor function was assessed in all participants. In addition, muscle strength and FVC were assessed in children older than five years. Muscle strength was measured by handheld dynamometry of elbow flexion, hand grip, 3‐point pinch, knee flexion and knee extension; the best scores were added to obtain an arm megascore and a leg megascore.
Treatment efficacy was evaluated by ITT analysis in 90 participants (45 in the treatment group and 45 in the placebo group) with continuous endpoints at five and 13 weeks' follow‐up using ANCOVA which included the baseline outcome values as covariates, treatment group and age as between‐patient factors, time as a within‐patient factor, and possible interaction between treatment group, time and age.
The primary endpoint was the change in HFMS from baseline. Secondary endpoints were the change in muscle strength and FVC from baseline and the occurrence of adverse events. Adverse events were systematically assessed at each visit by means of a questionnaire.
Subcutaneous somatotropin versus placebo
A double‐blind, randomised, placebo‐controlled, cross‐over pilot trial compared subcutaneous injections of somatropin with subcutaneous injections of placebo in 20 participants with SMA types II and III aged between six and 36 years old (Kirschner 2014). Participants were randomised to two cohorts, in which one started with treatment for 12 weeks and crossed over to placebo for 12 weeks after a washout period of eight weeks, while the other cohort started with placebo for 12 weeks and crossed over to treatment for 12 weeks after a washout period of eight weeks. During the 12‐week period, participants received either somatotropin 0.015 mg/kg/day subcutaneously in the first week which was increased after one week up to 0.03 mg/kg/day for weeks two to 12 or placebo at the same dose regimen. Duration of the trial was 40 weeks. Follow‐up after the last treatment was eight weeks.
Muscle strength was measured with MVIC using hand‐held myometry and MRC scale in elbow flexion, handgrip, knee flexion and knee extension at baseline and weeks four, 12, 20, 24 and 32. Motor function was evaluated with the HFMSE, 10‐metre walking time and Gowers' time at baseline and weeks four, 12, 20, 24 and 32. Pulmonary functioning test were assessed with FVC and peak cough flow at baseline and weeks 12, 20 and 32. Laboratory studies included IGF‐1 serum concentrations and endocrinological measurements at baseline and weeks four, 12, 20, 24, 32 and 40.
Data analysis was done with a modified ITT concept using t‐test and Wilcoxon test.
The primary endpoint was change in quantitative muscle strength of upper limb using hand‐held myometry in elbow flexion and handgrip. Secondary outcomes measures were change in quantitative muscle strength of lower limb, muscle strength with MMT in seven muscles, change in HFMSE, change in Gowers' time, change in qualitative Gowers' manoeuvre, change in FVC and peak cough flow, and adverse events. Adverse events were systematically assessed at each visit.
Intravenous thyrotropin‐releasing hormone versus placebo
Tzeng 2000 was a double‐blind, randomised, placebo‐controlled trial that compared intravenous TRH 0.1 mg/kg once a day with placebo. Six participants were treated with TRH and three received placebo. The duration of treatment was 29 days over a 34‐day period with follow‐up and conclusion of the study at five weeks.
Muscle strength was evaluated by dynamometry of the deltoids, biceps, triceps, wrist extensors, hand grip, hip flexors, quadriceps and hamstrings.
Comparisons of total mean muscle strength and electrodiagnostic measures at baseline and at the end of the five‐week study period were made using paired t‐tests.
The primary endpoint was the change in total mean muscle strength from baseline. Secondary endpoints were change in electrodiagnostic measures and the occurrence of adverse events related to the treatment. Adverse events were collected when spontaneous reported by the participants.
Oral valproic acid (valproate) plus acetyl‐L‐carnitine versus placebo
One double‐blind, placebo‐controlled trial compared combination therapy with oral valproic acid and acetyl‐L‐carnitine to placebo in 61 non‐ambulatory children aged between two and eight years (Swoboda 2010). Thirty‐one children received treatment with valproic acid 125 mg, given in divided doses two to three times a day and sufficient to maintain overnight trough levels of 100 mg/dL, and acetyl‐L‐carnitine doses at 50 mg/kg/day divided into two daily doses. Thirty children received a double placebo. The duration of treatment was 12 months in the active treatment arm and six months in the placebo. After six months the placebo group switched over to active treatment per protocol.
In all participants, the MHFMS and GMFM were used to measure functional motor ability at baseline, three, six and 12 months after the start of treatment. The degree of innervation by the ulnar nerve was estimated using maximum ulnar CMAP amplitude. Myometry measurements were performed in children aged five years and older (24 children) with no significant contractures: three times for right and left elbow flexion and for right and left knee extension. Also in the children aged five years and older, pulmonary function testing was performed, which included FVC, forced expiratory volume (FEV), and maximum inspiratory and expiratory pressures (MIP and MEP). Quality of life was assessed using the PedsQL, filled in by parents at each visit. Children aged five years or older completed the age‐appropriate PedsQL. Bone mineral density and bone mineral content were measured with dual‐energy X‐ray absorptiometry (DEXA).
All analyses were performed on an ITT population of 61 people that was defined as all participants randomised to receive study medication. The analysis of variance (ANOVA) test was used to compare treatment groups for change in MHFMS from the baseline data. Non‐normally distributed data were tested with the Wilcoxon rank sum test.
The primary endpoints were laboratory safety data, adverse event data and change in MHFMS from baseline after six months. Secondary endpoints included measurement from baseline at six and 12 months in MHFMS, estimates of CMAPs, DEXA, body composition and bone density, quantitative SMN mRNA and quality of life. Adverse events were systematically assessed at each visit.
Oral valproic acid (valproate) versus placebo
Kissel 2014, a double‐blind, placebo‐controlled, cross‐over trial, compared oral valproic acid with placebo in 33 ambulatory participants with SMA type III aged above 18 years old. Participants were divided over two cohorts. Cohort one (16 participants) was first treated with oral valproic acid 10 mg/kg/day to 20 mg/kg/day divided over two or three doses (doses depending on serum levels of valproic acid with preferred levels of 50 mg/dL) for six months, after this period this cohort switched to equal dosage of oral placebo for six months. Cohort two (17 participants) started with six months' treatment with placebo and afterwards crossed over to oral valproic acid 10 mg/kg/day to 20 mg/kg/day divided over two to three doses (doses depending on serum levels of valproic acid with preferred levels of 50 mg/dL) for six months. Total duration of the trial was 12 months.
Participants started with two baseline visits within a six‐week period to assure that the methodologies were reliable and to assure test–retest stability. Clinical assessments were done at three, six and 12 months. Motor abilities were assessed by maximum voluntary isometric contraction testing (MVICT) in bilateral elbow flexors, elbow extensors, knee flexors, knee extensors and grip. Functional motor abilities were tested with modified SMAFRS, the ability to climb four standard stairs and endurance during the six‐minute walk test. Muscle mass was measured by DEXA scanning. The degree of innervation by the ulnar nerve was estimated using maximum ulnar CMAP. Pulmonary function testing was performed, which included FVC, FEV and MIP. Quality of life was assessed using the mini‐SIP. Safety laboratory studies (chemistry profile, blood and platelet count, transaminases, carnitine profile, amylase, lipase, valproic acid levels) were performed at baseline, two to three weeks after initiation, at thee, six and 12 months and one additional time between six and 12 months. Serum levels of SMN protein and mRNA were performed.
The two baseline visits and the visit closest to the start of the treatment were used as baseline evaluation. Changes from baseline between treatment and placebo at six months were analysed with t‐tests and at 12 months with mixed‐effects models.
The primary endpoint was the change in MVICT at six months. Secondary outcomes included laboratory safety data, adverse event and change in muscle scores of upper and lower extremities, SMAFRS, CMAPs of the ulnar nerve, DEXA, muscle mass, pulmonary functioning tests, SMN protein levels and mRNA levels from baseline after six and 12 months. Adverse events were systematically assessed at each visit.
Funding
In three trials, pharmaceutical companies were involved in funding, analysis, reporting of results, or a combination of these (Bertini 2017; Kirschner 2014; Mercuri 2018 (CHERISH)). In six trials, pharmaceutical companies provided the study drug without cost and they had no involvement in study design, or analysis and reporting of results (Kissel 2014; Mercuri 2007; Miller 2001; Swoboda 2010; Tzeng 2000; Wong 2007). Authors of one trial were reported to have a patent on the study drug (Chen 2010).
Excluded studies
We identified and assessed 59 studies (36 new) for possible inclusion in the review. We excluded 36 studies (see Characteristics of excluded studies table) because they were not randomised or were uncontrolled (Abbara 2011; Brahe 2005; Brichta 2006; Chang 2002; Chiriboga 2016; Darbar 2011; EMOTAS 2014; Folkers 1995; Giovannetti 2016; JEWELFISH 2017; JPRN‐JapicCTI‐163450 2016; Kato 2009; Khirani 2017; Kinali 2002; Kissel 2011; Liang 2008; NCT02876094; Mercuri 2004; Merlini 2003; Nascimento 2010; NCT01703988; NCT02052791; NCT03709784; NPTUNE01 2007; OLEOS; Pane 2008; Piepers 2011; Prufer de Queiroz Campos Araujo 2010; Saito 2014; SHINE 2015; SMART01; SMART03; Swoboda 2009; Tan 2011; Tsai 2007; Weihl 2006). We could exclude 13 unpublished studies because they were not randomised or were uncontrolled, with eight of these studies still being ongoing (EMOTAS 2014; JEWELFISH 2017; JPRN‐JapicCTI‐163450 2016; NCT02876094; NCT03709784; OLEOS; SHINE 2015; SMART03), and five studies being completed but not yet published at time of the search (NCT01703988; NCT02052791; NPTUNE01 2007; Prufer de Queiroz Campos Araujo 2010; SMART01).
Studies awaiting classification
Nine trials were completed but no data were available for analysis (ASIRI 2008; CHICTR‐TRC‐10001093; Merlini 2007; MOONFISH 2014; Morandi 2013; NCT00568802; NCT01645787; NCT02644668; SPACE) (see Characteristics of studies awaiting classification table). Results of two trials, the EUROsmart trial with acetyl‐L‐carnitine (Merlini 2007), and a trial with salbutamol (Morandi 2013), were only published in conference abstracts which did not include enough data for analysis (Merlini 2010; Morandi 2013). We also could not obtain the results of three completed randomised, placebo‐controlled trials with hydroxyurea (NCT00568802), with riluzole in SMA types II and III (ASIRI 2008), and with 4AP in adults with SMA type III (NCT01645787). One trial was terminated for safety reasons and results are not yet published (MOONFISH 2014). We tried to obtain data and preliminary results for all of these completed but unpublished trials, but data were not available upon request at time of writing.
We could not obtain information about the trial methods or results of the completed two‐armed trial on rat nerve growth factor and, therefore, this study is awaiting classification (CHICTR‐TRC‐10001093).
Ongoing studies
Four trials were ongoing at the time of this search (EMBRACE 2015; NCT01671384; SMART02; SUNFISH 2016) (see Characteristics of ongoing studies table).
Risk of bias in included studies
The 'Risk of bias' assessments for the 10 included trials are shown in the Characteristics of included studies table and summarised in Figure 2 and Figure 3.
2.
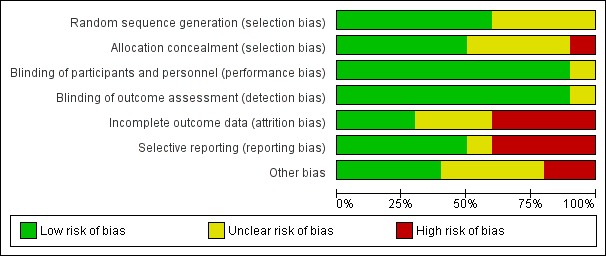
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all 10 included studies.
3.
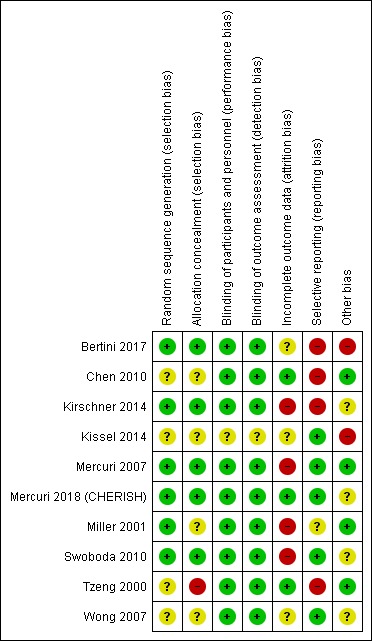
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The randomisation method was not clear in four trials (Chen 2010; Kissel 2014; Tzeng 2000; Wong 2007), but was at low risk of bias in the remaining six trials. Allocation concealment was not clear in four trials (Chen 2010; Kissel 2014; Miller 2001; Wong 2007), but adequately reported in five trials, which we judged at low risk of bias (Bertini 2017; Kirschner 2014; Mercuri 2007; Mercuri 2018 (CHERISH); Swoboda 2010). Allocation concealment was at high risk of bias in one trial (Tzeng 2000), which used a coin toss method. In one trial, there were baseline differences probably due to inadequate randomisation, since muscle strength in the cohort of children aged five to 18 years in the creatine treatment group was slightly weaker than in the placebo group (Wong 2007).
Blinding
Blinding of parents, participants and observers were adequate and at low risk of bias in all trials except Kissel 2014, for which the risk of bias related to blinding was unclear.
Incomplete outcome data
Four trials were at high risk of bias from attrition and three at unclear risk of bias. The method for modified ITT analysis was unknown in Kirschner 2014 and we assessed this trial at high risk of bias. In one trial, the risk of attrition bias was high because fewer than expected numbers of participants provided data for some outcome measures (Swoboda 2010). A difference in the number of children over five years of age providing data on myometry and FVC was unexplained, with data on adverse events limited in Mercuri 2007. This resulted in a high‐risk assessment. Follow‐up was below 80% in two trials (Miller 2001; Wong 2007). Miller 2001 performed an ITT analysis but participants withdrew for unknown reasons and the number analysed was not the number initially included. We judged the risk of bias in Miller 2001 to be high and in Wong 2007 to be unclear. Participants withdrew for unknown reasons in two other studies; however, ITT analysis possibly minimised the risk of bias, which we judged unclear (Bertini 2017: n = 17; Kissel 2014: n = 4).
Risk of attrition bias in the other trials was low (Chen 2010; Mercuri 2018 (CHERISH); Tzeng 2000).
Selective reporting
Primary outcome measures were adequately stated in all trials. Trial authors provided data to complete analysis of primary and secondary outcomes, including muscle strength (Kirschner 2014; Kissel 2014; Miller 2001; Wong 2007), disability scores (Kirschner 2014; Kissel 2014; Miller 2001; Wong 2007), pulmonary function (Kirschner 2014; Kissel 2014; Wong 2007), quality of life (Kissel 2014; Miller 2001; Wong 2007), and adverse events (Kissel 2014; Miller 2001; Wong 2007).
We assessed four trials at high risk of reporting bias. Two studies dichotomised data post hoc for analysis (Bertini 2017; Chen 2010). A third study measured quality of life but the reporting was incomplete (Kirschner 2014). We also judged Tzeng 2000 at high risk of reporting bias. Although all outcomes were reported, the statistical plan was limited and unclear. Miller 2001 was at unclear risk of bias, as adverse events were not reported. We judged the other five trials at low risk of selective reporting (Kissel 2014; Mercuri 2007; Mercuri 2018 (CHERISH); Swoboda 2010; Wong 2007),
Other potential sources of bias
Two studies were at high risk from other potential sources of bias (Bertini 2017; Kissel 2014) and four at unclear risk (Kirschner 2014; Mercuri 2018 (CHERISH); Swoboda 2010; Wong 2007).
The cross‐over design with potential carry‐over effects placed two studies at unclear risk of bias (Kirschner 2014; Swoboda 2010) and one study at high risk of bias (Kissel 2014).
In four trials, there were baseline differences, with other potential bias graded as unclear (Mercuri 2018 (CHERISH); Swoboda 2010; Wong 2007) or high (Bertini 2017). In Swoboda 2010, there were baseline differences in gender as the valproic acid plus acetyl‐L‐carnitine treatment group consisted of 36.6% females compared to 56% females in the placebo group, and there were differences in body mass index. In Mercuri 2018 (CHERISH), we judged the risk as unclear, since the baseline differences resulted in more severely affected children in the nusinersen‐treated group. However, there was a beneficial significant effect on motor function in the nusinersen group, the effects of nusinersen even being underestimated in more severely affected children. No definite conclusions on this subject could be drawn. In Bertini 2017, we judged the risk of bias as high because there were differences in mean and median ages between the olesoxime and placebo group, with a higher mean and median age in the placebo group, and the proportion of males and females was uneven between the two treatment groups.
Four trials were at low risk of other potential sources of bias (Chen 2010; Mercuri 2007; Miller 2001; Tzeng 2000).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9; Table 10
Summary of findings for the main comparison. Oral creatine compared to placebo for children with SMA types II and III.
| Oral creatine compared to placebo for children with SMA types II and III | ||||||
| Patient or population: children with SMA types II and III Setting: outpatient clinic Intervention: oral creatine Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with oral creatine | |||||
| Change in disability score assessed with: GMFM Scale: 0–264 Follow‐up: 9 months | The median change in disability score was –1 | Median change 1 higher (1 lower to 2 higher) | — | 40 (1 RCT) | ⊕⊕⊕⊝ Moderatea | — |
| Change in total muscle strength (total muscle strength) assessed with: quantitative muscle testing (in pounds) Follow‐up: 9 months | The mean change in total muscle strength was 2.42 pounds | MD 1.25 pounds lower (10.1 lower to 7.6 higher) | — | 22 (1 RCT) | ⊕⊕⊝⊝ Lowb,c | Only participants aged ≥ 5 years. |
| Acquiring the ability to stand or walk | Not measured | |||||
| Change in quality of life assessed with: Parent Questionnaire for the PedsQL Neuromuscular Module Scale: 0–100 Follow‐up: 9 months | The median change in quality of life was 2 | Median change 7 lower (11 lower to 3 higher) | — | 38 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | Higher scores on the PedsQL indicate better quality of life. |
| Change in pulmonary function assessed with: FVC (in % predicted) Follow‐up: 9 months | The mean change in pulmonary function was –0.83 % predicted | MD 0.56 % predicted higher (10.8 lower to 11.9 higher) | — | 23 (1 RCT) | ⊕⊕⊝⊝ Lowb,c | Only participants aged ≥ 5 years. |
| Time from beginning of treatment until death or full‐time ventilation | 1 death occurred in the placebo group in 28 participants (36 per 1000) | 0 deaths occurred in the treatment group among 27 participants (0 per 1000) | — | 40 (1 RCT) | ⊕⊕⊕⊝ Moderatea | — |
| Adverse events related to treatment | 571 per 1000 | 480 per 1000 (291 to 800) | 0.84 (0.51 to 1.4) | 40 (1 RCT) | ⊕⊕⊝⊝ Lowd,e | There were 43 events in 16/28 participants in placebo group and 55 events in 13/27 participants treated with creatine. Adverse events were systematically, prospectively collected at every study visit. Adverse events included mainly respiratory infections. |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; FVC: forced vital capacity; GMFM: Gross Motor Function Measure; MD: mean difference; MHFMS: Modified Hammersmith Functional Motor Scale; MMT: Manual Muscle Testing; PedsQL: Pediatric Quality of Life Inventory; RCT: randomised controlled trial; RR: risk ratio; SMA: spinal muscular atrophy. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
a Downgraded one level for imprecision because of the small sample size. b Downgraded one level due to inconsistency. Unknown cohort representation (outcome reported for 22 of the randomised participants). c Downgraded one level because of imprecision. Small sample size, inadequately for optimal information size (OIS). Cut off for OIS was the calculated sample size of the trial. d Downgraded one level for risk of bias. No information on type of adverse events included. e Downgraded one level for imprecision because the small sample size is unlikely to have captured uncommon adverse events.
Summary of findings 2. Oral gabapentin compared to placebo for adults with SMA types II and III.
| Oral gabapentin compared to placebo for adults with SMA types II and III | ||||||
| Patient or population: adults with SMA types II and III Setting: outpatient clinic Intervention: oral gabapentin Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with oral gabapentin | |||||
| Change in disability score assessed with: SMAFRS Scale: 0–50 Follow‐up: 12 months | The median change in the SMAFRS score was 0 in the gabapentin group (37 participants) and –2 in the placebo group (34 participants) | — | 66 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | Higher scores on the SMAFRS indicate better function. | |
| Change in muscle strength assessed as: % change in total muscle strength from baseline Follow‐up: 12 months | The mean change in muscle strength was –2.2% | MD 3.3% higher (6.9 lower to 14 higher) | — | 50 (1 RCT) | ⊕⊕⊝⊝ Lowb,c | — |
| Acquiring ability to walk Follow‐up: 12 months | 0/35 participants in the placebo group developed the ability to walk at 9 or 12 months' follow‐up | 0/38 participants treated with oral gabapentin developed the ability to walk at 9 or 12 months' follow‐up | — | 73 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | — |
| Change in quality of life assessed with: change (%) from baseline in mini‐SIP Scale: 0–19 Follow‐up: 12 months | The mean change in quality of life was –0.26% | MD 0.36% higher (0.29 lower to 1 higher) | — | 67 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | Higher scores on the mini‐SIP indicate poorer health status. |
| Change in pulmonary function assessed with: FVC (in % predicted) Follow‐up: 12 months | The mean change in pulmonary function was –2.9 % predicted | MD 1.1 % predicted lower (4.1 lower to 1.9 higher) | — | 65 (1 RCT) | ⊕⊕⊝⊝ Lowb,c | Data from analysis of participants who completed ≥ 2 visits. |
| Time from beginning of treatment to death or full‐time ventilation Follow‐up: 12 months | 0 reported deaths and 0 participants required full‐time ventilation | — | 84 (1 RCT) | ⊕⊕⊕⊝ Moderatea | — | |
| Adverse events related to treatment Follow‐up: median 12 months | Adverse events were reported to be infrequent and not statistically different between treatment groups. Numerical data on adverse events were not available. | — | 65 (1 RCT) | ⊕⊕⊝⊝ Lowd,e | Adverse events were systematically, prospectively collected at every study visit. | |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; FVC: forced vital capacity; MD: mean difference; mini‐SIP: mini‐Sickness Impact Profile; PedsQL: Pediatric Quality of Life Inventory; RCT: randomised controlled trial; RR: risk ratio; SMA: spinal muscular atrophy; SMAFRS: Spinal Muscular Atrophy Functional Rating Scale. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
a Downgraded one level for imprecision because the small sample size. b Downgraded one level for risk of bias. Incomplete data at 12‐month follow‐up and it was unclear why cases dropped out. Three cases (two treated, one placebo) were excluded from analysis because of extreme outcomes (greater than three standard deviations). c Downgraded one level because of imprecision; small sample size, inadequate for optimal information size (OIS). d Downgraded one level because no data on adverse events were available. e Downgraded one level for imprecision because the small sample size is unlikely to have captured uncommon adverse events.
Summary of findings 3. Oral hydroxyurea compared to placebo for children and adults with SMA types II and III.
| Oral hydroxyurea compared to placebo for children and adults with SMA types II and III | ||||||
| Patient or population: children and adults with SMA types II and III Setting: outpatient clinic Intervention: oral hydroxyurea Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with oral hydroxyurea | |||||
| Change in disability score assessed with: GMFM Scale: 0–264 Follow‐up: 18 months | The mean change in disability score was 2.02 | MD 1.88 lower (3.89 lower to 0.13 higher) | — | 57 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | Higher scores on the GMFM indicate better function. |
| Change in disability score assessed with: MHFMS Scale: 0–40 Follow‐up: 18 months | The mean change in disability score was 0.04 | MD 0.02 lower (0.12 lower to 0.07 higher) | — | 38 (1 RCT) | ⊕⊕⊕⊝ Moderateb | Only performed in non‐ambulatory participants. |
| Change in muscle strength assessed with: MMT Scale: 16–80 Follow‐up: 18 months | The mean change in muscle strength was –0.03 | MD 0.55 lower (2.65 lower to 1.55 higher) | — | 57 (1 RCT) | ⊕⊕⊕⊝ Moderateb | — |
| Acquiring the ability to stand or walk | Not measured | |||||
| Change in quality of life | Not measured | |||||
| Change in pulmonary function assessed with: FVC (in litres) Follow‐up: 18 months | The mean change in pulmonary function was –0.22 L | MD 0.01 L higher (0.25 lower to 0.26 higher) | — | 57 (1 RCT) | ⊕⊕⊝⊝ Lowb,c | — |
| Time from beginning of treatment until death or full‐time ventilation | 1 participant died in the treatment group after 5 visits (after 8 months of treatment), due to respiratory complications. | — | 57 (1 RCT) | ⊕⊕⊕⊝ Moderateb | Also reported as the 1 serious adverse event. | |
| Adverse events related to treatment Follow‐up: 18 months | All participants experienced adverse events. 129 events occurred in the 20 participants in the placebo group. 224 events occurred in the 37 participants in the hydroxyurea group. | — | 57 (1 RCT) | ⊕⊕⊕⊝ Moderated | Adverse events were systematically, prospectively collected using a questionnaire at every study visit. Adverse events: laboratory disturbances (e.g. neutropenia, thrombocytopenia, high transaminases), respiratory complaint, gastrointestinal complaints, rash, neurological symptoms, unspecified. 1 participant died in the treatment group due to respiratory complications.d | |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; FVC: forced vital capacity; GMFM: Gross Motor Function Measure; MD: mean difference; RCT: randomised controlled trial; SMA: spinal muscular atrophy. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
a Downgraded one level for imprecision. CIs were very wide. b Downgraded one level for imprecision because of small sample size (inadequate for optimal information size (OIS)). Cut‐off for OIS was the calculated sample size of the trial. c Downgraded one level for indirectness, because of discrepancy in results of respiratory failure (results in text and figures appeared different). d Downgraded one level for imprecision because the small sample size is unlikely to have captured uncommon adverse events.
Summary of findings 4. Intrathecal injected nusinersen compared to sham procedure for children with SMA type II.
| Intrathecal injected nusinersen compared to sham procedure for children with SMA type II | |||||||
| Patient or population: children with SMA type II Setting: hospital visits (24 hours' observation at trial site after first procedure, 6 hours' observation after subsequent injections) Intervention: intrathecal injected nusinersen Comparison: sham procedure | |||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | ||
| Risk with sham procedure | Risk with intrathecal injected nusinersen | ||||||
| Change in disability score assessed with: HFMSE Score: 0–66 Follow‐up: mean 15 months | The mean change in HFMSE in the control group was –1.9 points | The mean change in HFMSE in the nusinersen‐treated group was 5.9 points higher than in the sham procedure group (3.7 higher to 8.1 higher) | MD 5.9 (3.7 to 8.1) | 126 (1 RCT) | ⊕⊕⊕⊝ Moderatea | ||
| Change in disability score (3 point‐change) assessed with: HFMSE Follow‐up: mean 15 months | 262 per 1000 | 471 per 1000 (259 to 812) |
RR 1.8 (0.99 to 3.1) |
126 (1 RCT) | ⊕⊕⊕⊝ Moderatea | 11/42 participants in the sham‐controlled group showed a 3‐point change on the HFMSE. 48/84 participants in the nusinersen group showed a 3‐point change on the HFMSE. | |
| Change in muscle strength | Not measured | ||||||
| Acquiring the ability to stand or walk assessed with: WHO Motor Milestone criteria Follow‐up: 15 months | Acquiring the ability to stand | 1/42 children in the sham‐controlled group acquired the ability to stand alone. | 1/84 children treated with nusinersen acquired the ability to stand alone. | RR 0.5 (0.03 to 7.80) | 126 (1 RCT) | ⊕⊕⊝⊝ Lowb | |
| Acquiring the ability to walk | 0/42 children in the sham‐controlled group acquired the ability to walk with assistance. | 1/84 children treated with nusinersen acquired the ability to walk with assistance. | RR 1.5 (0.06 to 36.1) | 126 (1 RCT) | ⊕⊕⊝⊝ Lowb | ||
| Change in quality of life | Not measured | ||||||
| Change in pulmonary function | Not measured | ||||||
| Time from beginning of treatment until death or full‐time ventilation | Not measured | ||||||
| Adverse events related to treatment Follow‐up: mean 15 months | 1000 per 1000 | 900 per 1000 | RR 0.9 (0.9 to 1.0) | 126 (1 RCT) | ⊕⊕⊕⊝ Moderatec | 78/84 (93%) participants treated with nusinersen experienced an adverse event, while 42/42 (100%) participants treated in the sham‐controlled group had any adverse event. Adverse events were systematically, prospectively collected at every study visit. Adverse events included proteinuria, hyponatraemia, transient low platelet counts, vasculitis, pyrexia, headache, vomiting, back pain and epistaxis. |
|
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HFMSE: Hammersmith Functional Motor Measure Expanded; MD: mean difference; MHFMS: Modified Hammersmith Functional Motor Scale; MMT: manual muscle testing; RCT: randomised controlled trial; RR: risk ratio; SMA: spinal muscular atrophy; WHO: World Health Organization. | |||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||||
a Downgraded one level for imprecision because of the small sample size. b Downgraded two levels for imprecision because of small sample size, low event rate and wide CI. c Downgraded one level for imprecision because the small sample size is unlikely to have captured uncommon adverse events.
Summary of findings 5. Oral olesoxime compared to placebo for non‐ambulatory children and adolescents with SMA types II and III.
| Oral olesoxime compared to placebo for non‐ambulatory children and adolescents with SMA types II and III | ||||||
| Patient or population: non‐ambulatory children and adolescents with SMA types II and III Setting: outpatient clinic Intervention: oral olesoxime Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with oral olesoxime | |||||
| Change in disability score assessed with: MFM (D1+D2) Scale: 0–75 Follow‐up: 24 months | The mean change in disability score was –1.82 | MD 2 higher (0.25 lower to 4.25 higher) | — | 160 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c | Higher scores on the MFM indicate better function. Combined analysis of participants assessed with MFM‐32 or MFM‐20. |
| Change in disability score assessed with: MFM total score Scale: 0–96 Follow‐up: 24 months | The mean change in disability score was –1.45 | MD 2.04 higher (0.21 lower to 4.28 higher) | — | 160 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c | Higher scores on the MFM indicate better function. Combined analysis of participants assessed with MFM‐32 or MFM‐20. |
| Change in disability score assessed with: MFM responder analysis Follow‐up: 24 months | Study population | RR 1.43 (–0.98 to 2.08) | 160 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c | Higher scores on the MFM indicate better function. Participants were classified as 'responders' in case MFM‐32 or MFM‐20 showed no change or better scores compared to baseline, and 'non‐responders'. | |
| 39 per 100 | 55 per 100 (–38 to 80) | |||||
| Change in disability score assessed with: HFMS Scale: 0–40 Follow‐up: 24 months | The mean change in disability score was –1.72 | MD 0.94 higher (0.28 lower to 2.17 higher) | — | 160 (1 RCT) | ⊕⊕⊝⊝ Lowb,c | Higher scores on the HFMS indicate better function.a |
| Change in muscle strength | Not measured | |||||
| Acquiring the ability to stand or walk | Not measured | |||||
| Change in quality of life assessed with: PedsQL Neuromuscular Module Score: 0–100 Follow‐up: 24 months | — | MD 0.25 (4.58 lower to 5.08 higher) | — | 108 (1 RCT) | ⊕⊕⊝⊝ Lowb,c | Higher scores on the PedsQL indicate a better quality of life. Scores on participants aged > 5 years. |
| Change in pulmonary function assessed with: FVC (in % predicted) Follow‐up: 24 months | The mean change in pulmonary function was +6.16 % predicted | MD 1.88 % predicted lower (3.14 lower to 6.91 higher ) | — | 102 (1 RCT) | ⊕⊕⊝⊝ Lowb,c | — |
| Time from beginning of treatment until death or full‐time ventilation Follow‐up: 24 months | 2 participants died; 1 with cardiac arrest (olesoxime group) and 1 with increased bronchial secretions (placebo group). Deaths were not deemed to be related to treatment. | — | 160 (1 RCT) | ⊕⊕⊕⊝ Moderatec | — | |
| Adverse events related to treatment Follow‐up: 24 months | 1000 per 1000 | 950 per 1000 | RR 0.95 (0.91 to 0.99) | 165 (1 RCT) | ⊕⊕⊕⊝ Moderated | 612 events occurred in 57 participants in the placebo group. 1104 events occurred in 108 participants in the olesoxime group. Adverse events were systematically, prospectively collected at every study visit. Adverse events: (upper) respiratory tract infection, gastroenteritis, influenza, vomiting, abdominal pain, diarrhoea, cough, pyrexia, pain in extremity, scoliosis, arthralgia, fall, headache. |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; FVC: forced vital capacity; HFMS: Hammersmith Functional Motor Score; MD: mean difference; MFM: Motor Function Measure; MMT: manual muscle testing; PedsQL: Pediatric Quality of Life Inventory; RCT: randomised controlled trial; RR: risk ratio; SMA: spinal muscular atrophy. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
a Downgraded one level for indirectness because trial authors combined two different outcome measures (MFM‐32 and MFM20) to assess the primary outcome with no correction in analysis. b Downgraded one level for risk of bias because of differences between baseline groups. c Downgraded one level for imprecision because of the small sample size. d Downgraded one level for imprecision because the small sample size was unlikely to have captured uncommon adverse events.
Summary of findings 6. Oral phenylbutyrate compared to placebo for children with SMA type II.
| Oral phenylbutyrate compared to placebo for children with SMA type II | |||||||
| Patient or population: children with SMA type II Setting: outpatient clinic Intervention: oral phenylbutyrate Comparison: placebo | |||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | ||
| Risk with placebo | Risk with oral phenylbutyrate | ||||||
| Change in disability score assessed with: Hammersmith Functional Motor Scale (HFMS) Scale: 0–40 Follow‐up: 13 weeks | The mean change in disability score was 0.73 | MD 0.13 lower (0.84 lower to 0.58 higher) | ‐ | 90 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | Higher scores on the HFMS indicate better function. | |
| Change in muscle strength assessed with: handheld dynamometer (in Newtons) Follow‐up: 13 weeks | Leg megascore | The mean change in muscle strength (leg megascore) was 3.22 N | MD 1.04 N higher (2.46 lower to 4.54 higher) | — | 70 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | Children aged > 5 years had additional assessment of muscle strength by myometry. |
| Arm megascore | The mean change in muscle strength (arm megascore) was –0.42 N | MD 1.98 N higher (1.67 lower to 5.63 higher) | — | 72 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | Children aged > 5 years had additional assessment of muscle strength by myometry. | |
| Acquiring the ability to stand or walk | Not measured | ||||||
| Change in quality of life | Not measured | ||||||
| Change in pulmonary function assessed with: FVC (% predicted) Follow‐up: 13 weeks | The mean change in pulmonary function was –0.01 % predicted | MD 0.04 % predicted higher (0.07 lower to 0.15 higher) | — | 67 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | Children aged > 5 years had additional assessment of FVC. | |
| Time from beginning of treatment until death or full‐time ventilation Follow‐up: mean 13 weeks | No deaths were reported. No data available on ventilation. | — | 107 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | — | ||
| Adverse events related to treatment Follow‐up: 13 weeks | 994 per 1000 | 292 per 1000 (118 to 740) | RR 3.1 (1.25 to 7.84) | 107 (1 RCT) | ⊕⊕⊕⊝ Moderatec | 5/53 participants had ≥ 1 adverse events in the phenylbutyrate and placebo group. 19/54 participants had ≥ 1 adverse events in the phenylbutyrate group. Adverse events were systematically and prospectively collected at every study visit. Adverse events included rash, drowsiness with hallucinations, nausea and constipation. No full report on types of adverse events was available. 3 participants discontinued the trial because of severe drowsiness, rash or constipation.b |
|
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; FVC: forced vital capacity; HFMS: Hammersmith Functional Motor Score; MD: mean difference; MFM: Motor Function Measure; RCT: randomised controlled trial; RR: risk ratio; SMA: spinal muscular atrophy. | |||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||||
a Downgraded one level because of risk of bias. b Downgraded one level for imprecision because the small sample size. c Downgraded one level because of imprecision on small sample size. Small sample size is unlikely to have captured uncommon adverse events.
Summary of findings 7. Subcutaneous somatotropin compared to placebo for children and adults with SMA types II and III.
| Subcutaneous somatotropin compared to placebo for children and adults with SMA types II and III | |||||||
| Patient or population: children and adults with SMA types II and III Setting: outpatient clinic Intervention: subcutaneous somatotropin Comparison: placebo | |||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | ||
| Risk with placebo | Risk with subcutaneous somatotropin | ||||||
| Change in disability score assessed with: HFMSE Scale: 0–66 Follow‐up: 3 months | The median change in disability score was –1.05 | Median change 0.25 higher (1 lower to 2.5 higher) | — | 19 (1 cross‐over RCT) | ⊕⊝⊝⊝ Very lowa,b | Higher scores on the HFMSE indicate better function. | |
| Change in muscle strength assessed with: MMT with hand‐held myometry from Citec (in Newtons) Follow‐up: 3 months | Upper limbs | The mean change in muscle strength (upper limbs) was 0.30 N | MD 0.08 N lower (3.79 lower to 3.95 higher) | — | 19 (1 cross‐over RCT) | ⊕⊕⊝⊝ Lowb,c | — |
| Lower limbs | The mean change in muscle strength (lower limbs) was 0.95 N | MD 2.23 N higher (2.19 lower to 6.63 higher) | — | 19 (1 cross‐over RCT) | ⊕⊕⊝⊝ Lowb,c | — | |
| Acquiring the ability to stand or walk | Not measured | ||||||
|
Change in quality of life Follow‐up: 40 weeks |
The trial report states that the trial found no significant differences in quality of life between the somatotropin‐treated group and the placebo group. | — | 19 (1 cross‐over RCT) | ⊕⊝⊝⊝ Very lowb,d | |||
| Change in pulmonary function assessed with: FVC (in litres) Follow‐up: 3 months | The mean change in pulmonary function was –0.11 L | MD 0.22 L higher (0.02 lower to 0.4 higher) | — | 19 (1 cross‐over RCT) | ⊕⊕⊝⊝ Lowb,c | — | |
| Time from beginning of treatment until death or full‐time ventilation Follow‐up: mean 40 weeks | No participant died or required full‐time ventilation in either group | — | 19 (1 cross‐over RCT) | ⊕⊕⊝⊝ Lowb,c | — | ||
| Adverse events related to treatment Follow‐up: 40 weeks | 368 per 1000 | 578 per 1000 (278 to 1000) | RR 1.57 (0.78 to 3.17) | 19 (1 cross‐over RCT) | ⊕⊕⊝⊝ Lowc,e | 23 adverse events occurred, 14 during somatotropin treatment and 9 during placebo treatment. Adverse events were systematically, prospectively collected at every study visit. Adverse events included headache, arthralgia, myalgia, oedema, elevated serum thyroid‐stimulating hormone and myalgia. | |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; FVC: forced vital capacity; HFMSE: Hammersmith Functional Motor Score Expanded; MD: mean difference; MFM: Motor Function Measure; MMT: Manual Muscle Testing; RCT: randomised controlled trial; RR: risk ratio; SMA: spinal muscular atrophy. | |||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||||
a Downgraded two levels due to risk of bias. HFMSE ranges were not available and because of the potential carry‐over effect due to the cross‐over design. b Downgraded one level for imprecision because of very small study size. c Downgraded one level because of potential bias from carry‐over effects due to the cross‐over design. d Downgraded two levels due to risk of bias. The report provided no information about how quality of life was measured and did not provide numerical data. There was a potential carry‐over effect due to the cross‐over design. e Downgraded for imprecision because the small sample size is unlikely to have captured uncommon adverse events.
Summary of findings 8. Intravenous thyrotropin releasing hormone compared to placebo for children with SMA types II and III.
| Intravenous thyrotropin releasing hormone compared to placebo for children with SMA types II and III | ||||||
| Patient or population: children with SMA types II and III Setting: in hospital treatment Intervention: intravenous TRH Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with intravenous TRH | |||||
| Change in disability score | Not measured | |||||
| Change in muscle strength assessed with: hand‐held dynamometry (CSD‐500, Amitec; in pounds) Scale: 0–6 Follow‐up: 5 weeks | The mean change in muscle strength was 0.48 pounds | MD 0.34 pounds higher (0.54 lower to 1.22 higher) | — | 9 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | — |
| Acquiring the ability to stand or walk | Not measured | |||||
| Change in quality of life | Not measured | |||||
| Change in pulmonary function | Not measured | |||||
| Time to death or full‐time ventilation | Not measured but no deaths reported | — | 9 (1 RCT) | ⊕⊕⊝⊝ Lowa,b |
— | |
| Adverse events related to treatment | No events in 3 participants treated with placebo | 12 events in 6 participants treated with TRH | — | 9 (1 RCT) | ⊕⊝⊝⊝ Very lowb,c,d | Adverse events included abdominal discomfort, flushing, nausea and vomiting. |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; SMA: spinal muscular atrophy; TRH: thyrotropin‐releasing hormone. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
a Downgraded one level for sample size. b Downgraded two levels for baseline imbalance and lack of allocation concealment. c Downgraded one level for imprecision because the small sample size is unlikely to have captured uncommon adverse events. d Downgraded one level for indirectness, because data on adverse events was not collected systematically.
Summary of findings 9. Oral valproic acid plus acetyl‐L‐carnitine compared to placebo for non‐ambulatory children with SMA types II and III.
| Oral valproic acid + acetyl‐L‐carnitine compared to placebo for non‐ambulatory children with SMA types II and III | ||||||
| Patient or population: non‐ambulatory children with SMA types II and III Setting: outpatient clinic Intervention: oral valproic acid + acetyl‐L‐carnitine Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with oral valproic acid + acetyl‐L‐carnitine | |||||
| Change in disability score assessed with: MHFMS Scale: 0–40 Follow‐up: 6 months | The mean change in disability score was 0.18 | MD 0.64 higher (1.1 lower to 2.38 higher) | — | 61 (1 RCT) | ⊕⊕⊕⊝ Moderatea | Higher scores on the MHFMS indicate better function. |
| Change in muscle strength assessed with: myometry with myometer (in kg) Follow‐up: 6 months | The mean change in muscle strength was –0.25 kg | MD 1.43 kg higher (0.69 lower to 3.56 higher) | — | 16 (1 RCT) | ⊕⊕⊝⊝ Lowb | Only performed in participants aged > 5 years. |
| Acquiring the ability to stand or walk | Not measured | |||||
| Change in quality of life assessed with: PedsQL Scale: 0–100 Follow‐up: 6 months | The mean change in quality of life was 0.3 | MD 2.2 lower (9.27 lower to 4.87 higher) | — | 54 (1 RCT) | ⊕⊝⊝⊝ Very lowa,c,d | Higher scores on the PedsQL indicate better quality of life. Only 54 participants completed PedsQL at follow‐up. Characteristics of this subset are unknown. |
| Change in pulmonary function assessed with: FVC (in % predicted) Follow‐up: 6 months | No numerical data available for analysis | — | 24 (1 RCT) | ⊕⊕⊝⊝ Lowb,e | Only performed in participants aged > 5 years. | |
| Time from beginning of treatment until death or full‐time ventilation Follow‐up: 6 months | 0 deaths or no need for full‐time ventilation | — | 61 (1 RCT) | ⊕⊕⊕⊝ Moderatea | — | |
| Adverse events related to treatment Follow‐up: 12 months | 581 per 1000 | 755 per 1000 (534 to 1000) | RR 1.32 (0.92 to 1.89) | 61 (1 RCT) | ⊕⊕⊕⊝ Moderatef | 18/31 participants in the placebo group had ≥ 1 adverse events. 23/30 participants in the valproic acid + acetyl‐L‐carnitine group had ≥ 1 adverse events. Adverse events were systematically, prospectively collected at every study visit. |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; FVC: forced vital capacity; MD: mean difference; MHFMS: Modified Hammersmith Functional Motor Scale; PedsQL: Pediatric Quality of Life Inventory; RCT: randomised controlled trial; SMA: spinal muscular atrophy. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
a Downgraded for imprecision because the small sample size. b Downgraded two levels because of very small sample size, inadequately for optimal information size (OIS). Cut off for OIS was the calculated sample size of the trial. c Downgraded one level due to risk of bias. Only a subset of participants completed PedsQL at follow‐up. d Downgraded one level due to inconsistency. Only a subset of participants completed follow‐up. e Downgraded one level due to risk of bias. Data on pulmonary function was not available. f Downgraded for imprecision because the small sample size is unlikely to have captured uncommon adverse events.
Summary of findings 10. Oral valproic acid compared to placebo for ambulatory adults with SMA type III.
| Oral valproic acid compared to placebo for ambulatory adults with SMA type III | |||||||
| Patient or population: ambulatory adults with SMA type III Setting: outpatient clinic Intervention: oral valproic acid Comparison: placebo | |||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | ||
| Risk with placebo | Risk with oral valproic acid | ||||||
| Change in disability score assessed with: SMAFRS Scale: 0–50 Follow‐up: 6 months | The mean change in disability score was –0.35 | MD 0.06 higher (1.32 lower to 1.44 higher) | — | 31 (1 cross‐over RCT) | ⊕⊕⊝⊝ Lowa,b | Higher scores on the SMAFRS indicate better function. | |
| Change in muscle strength assessed with: MVICT (in Newtons) Follow‐up: 6 months | Arms | The mean change in muscle strength of arms was –0.01 N | MD 0.23 N lower (1.03 lower to 0.57 higher) | — | 30 (1 cross‐over RCT) | ⊕⊕⊝⊝ Lowa,b | — |
| Legs | The mean change in muscle strength of legs was 0.35 N | MD 0.37 N lower (1.09 lower to 0.35 higher) | — | 30 (1 cross‐over RCT) | ⊕⊕⊝⊝ Lowa,b | — | |
| Acquiring the ability to stand or walk | Not measured | ||||||
| Change in quality of life assessed with: mini‐SIP Scale: 0–19 Follow‐up: 6 months | The mean change in quality of life was 0.91 | MD 1.1 lower (3.8 lower to 1.6 higher) | — | 28 (1 cross‐over RCT) | ⊕⊕⊝⊝ Moderatea | Higher score on the mini‐SIP indicates a poorer health status. | |
| Change in pulmonary function assessed with: FVC (in % predicted) Follow‐up: 6 months | The mean change in pulmonary function was 0.53% predicted | MD 1.24% predicted lower (4.71 lower to 2.23 higher) | — | 24 (1 cross‐over RCT) | ⊕⊕⊕⊝ Lowa,b | — | |
| Time from beginning of treatment until death or full‐time ventilation Follow‐up: 6 months | No deaths or full‐time ventilation | — | 33 (1 cross‐over RCT) | ⊕⊕⊕⊝ Lowa,b | — | ||
| Adverse events related to treatment Follow‐up: 12 months | 455 per 1000 | 364 per 1000 (200 to 655) | RR 0.80 (0.44 to 1.44) | 33 (1 cross‐over RCT) | ⊕⊕⊝⊝ Lowa,c | 96 adverse events occurred, 66 in the placebo group and 30 in the valproic acid group. Adverse events were systematically, prospectively collected at every study visit and included upper airway tract infection or symptoms, dizziness, headache, peripheral neuropathy, tremor, fatigue, pain, abdominal pain, nausea, vomiting, decreased platelet count, weight gain and alopecia.a,b | |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; FVC: forced vital capacity; MD: mean difference; mini‐SIP: mini‐Sickness Impact Profile; MVICT: maximum voluntary isometric contraction testing; RCT: randomised controlled trial; SMA: spinal muscular atrophy; SMAFRS: Spinal Muscular Atrophy Rating Scale; TRH: thyrotropin‐releasing hormone. | |||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||||
a Downgraded one level because of potential carry‐over effect due to the cross‐over design. b Downgraded one level for imprecision because of very small study size. c Downgraded for imprecision because the small sample size is unlikely to have captured uncommon adverse events.
Meta‐analysis was not possible due to the extensive variation in the drug treatments, outcomes and outcome measures, analyses, follow‐ups, study designs and the reporting of results in the 10 studies included in the review. We present the detailed results of each trial in tables (Table 12; Table 13; Table 14; Table 15; Table 16; Table 17; Table 18; Table 19; Table 20; Table 21).
2. Oral creatine versus placebo outcomes (Wong 2007).
| Creatine | Placebo | Difference (95% CI) | P value | |
| All ages | ||||
| Number of participants randomised | 27 | 28 | — | — |
| Number (%) of participants evaluable for analysis disability | 18 (67%) | 22 (79%) | — | — |
| Median change in disability score (GMFM) | 0 | ‐1 | 1 (‐1 to 2) | 0.19 |
| Number (%) of participants evaluable for analysis QoL | 17 (63%) | 21 (75%) | — | — |
| Median change in quality of life (PedsQL, neuromuscular module) | ‐5 | 2 | ‐7 (‐11 to 3) | 0.31 |
| Age 2 to 5 years | ||||
| Number of participants randomised | 8 | 12 | — | — |
| Number (%) of participants evaluable for analysis disability | 7 (88%) | 10 (83%) | — | — |
| Median change in disability score (GMFM) | 1 | ‐2 | 1.5 (‐4 to 9) | 0.18 |
| Number (%) of participants evaluable for analysis QoL | 6 (75%) | 9 (75%) | — | — |
| Median change in QoL (PedsQL, neuromuscular module) | 4.5 | 3 | 2 (‐8 to 13) | 0.71 |
| Age 5 to 18 years | ||||
| Number of participants randomised | 19 | 16 | — | — |
| Number (%) of participants evaluable for analysis disability and QoL | 11 (58%) | 12 (75%) | — | — |
| Median change in disability score (GMFM) | ‐1 | ‐0.5 | 0 (‐2 to 2) | 0.77 |
| Number (%) of participants evaluable for analysis QoL | 11 (58%) | 12 (75%) | — | — |
| Median change in quality of life (PedsQL, neuromuscular module) | ‐6 | 0 | ‐6 (‐15 to 2) | 0.11 |
| Number (%) of participants evaluable for analysis of muscle strength | 11 (58%) | 11 (69%) | — | — |
| Mean (SD) change in arms muscle strength (QMT) | ‐0.34 (6.98) | 1.49 (8.50) | ‐1.83 (‐8.75 to 5.09) | 0.59 |
| Mean (SD) change in legs muscle strength (QMT) | 1.51 (4.21) | 0.93 (3.06) | 0.58 (‐2.70 to 3.85) | 0.72 |
| Mean (SD) change in total muscle strength (QMT) | 1.17 (9.67) | 2.42 (10.3) | ‐1.25 (‐10.1 to 7.6) | 0.77 |
| Number (%) of participants evaluable for analysis pulmonary function | 11 (58%) | 12 (75%) | — | — |
| Mean (SD) change in pulmonary function (FVC % of predicted value in litres) | ‐0.27 (14.5) | ‐0.83 (11.5) | 0.56 (‐10.75 to 11.87) | 0.92 |
| Creatine | Placebo | Risk ratio (95% CI) | P value | |
| All ages | ||||
| Number of adverse events | 55 | 43 | — | — |
| Number of participants with adverse events | 13/27 | 16/28 | 0.84 (0.51 to 1.40) | 0.59 |
| Number of severe adverse events | NA | NA | — | — |
| Number of participants with severe adverse events | NA | 1 (death by respiratory failure) | — | — |
CI: confidence interval; FVC: forced vital capacity; GMFM: Gross Motor Function Measure; NA: not available; PedsQL: Pediatric Quality of Life Inventory; QMT: quantitative muscle test; QoL: quality of life; SD: standard deviation.
3. Oral gabapentin versus placebo outcomes (Miller 2001a).
| Gabapentin | Placebo | Difference (95% CI) | P value | |
| Follow‐up at 12 months | ||||
| Number (%) of participants evaluable for analysis disability | 32 (80%) | 34 (77%) | — | — |
| Median change in disability score (SMAFRS) | 0 | ‐2 | 1 (‐1 to 4) | 0.30 |
| Number (%) of participants evaluable for analysis quality of life | 33 (83%) | 34 (77%) | — | — |
| Mean change in quality of life (mini‐SIP) | 0.09 | ‐0.26 | 0.36 (‐0.29 to 1) | 0.28 |
| Number (%) of participants evaluable for analysis grip muscle strength | 30 (75%) | 31 (70%) | — | — |
| Mean change in grip muscle strength (MVC) in percentage from baseline | 0.08 | ‐4.5 | 4.6 (‐2.5 to 11.6) | 0.57 |
| Number (%) of participants evaluable for analysis arms muscle strength | 28 (70%) | 29 (66%) | — | — |
| Mean change in arms muscle strength (MVC) in percentage from baseline | 0.05 | ‐1.8 | 1.9 (‐2.5 to 6.2) | 0.39 |
| Number (%) of participants evaluable for analysis feet muscle strength | 28 (70%) | 29 (66%) | — | — |
| Mean change in feet muscle strength (MVC) in percentage from baseline | 0.36 | 0.70 | 0.29 (‐8.3 to 8.9) | 0.95 |
| Number (%) of participants evaluable for analysis total muscle strength | 25 (63%) | 25 (57%) | — | — |
| Mean change in total muscle strength (MVC) in percentage from baseline | 1.2 | ‐2.2 | 3.3 (‐6.9 to 14) | 0.52 |
| Number (%) of participants evaluable for analysis development of walking | 38 (95%) | 35 (80%) | — | — |
| Number of participants with development of walking | 0 | 0 | — | — |
| Number (%) of participants evaluable for analysis pulmonary function | 31 (78%) | 34 (77%) | — | — |
| Mean change in pulmonary function (FVC % of predicted value) | ‐4.0 | ‐2.9 | ‐1.1 (‐4.1 to 1.9) | 0.48 |
| Gabapentin | Placebo | Risk ratio (95% CI) | P value | |
| Number of adverse events | NA | NA | — | — |
| Number of participants with adverse events | NA | NA | — | — |
| Number of severe adverse events | 0 | 0 | — | — |
| Number of participants with severe adverse events | 0 | 0 | — | — |
CI: confidence interval; FVC: forced vital capacity; mini‐SIP: mini Sickness Impact Profile; MVC: maximum voluntary contraction; NA: not available; SMAFRS: Spinal Muscular Atrophy‐Functional Rating Scale.
4. Oral hydroxyurea versus placebo outcomes (Chen 2010).
| Hydroxyurea | Placebo | Difference (95% CI) | P value | |
| All ages | ||||
| Number of participants randomised | 37 | 20 | — | — |
| Number (%) of participants evaluable for analysis disability (GMFM) | 37 (100%) | 20 (100%) | — | — |
| Mean change (SE) in disability score (GMFM) | 0.14 (0.57) | 2.02 (0.88) | ‐1.88 (‐3.89 to 0.13) | 0.07 |
| Number (%) of participants evaluable for analysis muscle strength (MMT) | 37 (100%) | 20 (100%) | — | — |
| Mean change (SE) in muscle strength (MMT) | ‐0.58 (0.56) | ‐0.03 (0.98) | ‐0.55 (‐2.65 to 1.55) | 0.60 |
| Number (%) of participants evaluable for pulmonary function (FVC in litres) | 37 (100%) | 20 (100%) | — | — |
| Mean (SE) change in pulmonary function (FVC in litres) | ‐0.21 (0.06) | ‐0.22 (0.12) | ‐0.01 (‐0.25 to 0.26) | 0.93 |
| Number (%) of participants evaluable for analysis disability (MHFMS) | 26 (100%) | 12 (100%) | — | — |
| Mean change (SE) in disability score (MHFMS) | 0.02 (0.03) | 0.04 (0.04) | ‐0.02 (‐0.12 to 0.07) | 0.70 |
| Hydroxyurea | Placebo | Risk ratio (95% CI) | P value | |
| Number of adverse event episodes | 224 | 129 | — | 0.65 |
| Mean number (SD) of adverse event episodes per participant | 6.05 (3.43) | 6.45 (2.91) | ‐0.4 (‐2.21 to 1.41) | 0.66 |
| Number of severe adverse event episodes | 19 | 10 | — | 0.96 |
| Mean number (SD) of severe adverse event episodes per participant | 0.51 (1.04) | 0.50 (0.83) | 0.01 (‐0.77 to 0.79) | 0.96 |
| Number (%) of participants discontinued intervention | 2 (5%) | 0 (0%) | — | — |
FVC: forced vital capacity; GMFM: Gross Motor Function Measure; MHFMS: Modified Hammersmith Functional Motor Scale; MMT: manual muscle testing; SD: standard deviation; SE: standard error.
5. Intrathecal injected nusinersen versus placebo outcomes (Mercuri 2018).
| Nusinersen | Placebo | Difference (95% CI) | P value | |
| Number of participants randomised | 84 | 42 | — | — |
| Interim analysisa | 84b | 42b | — | — |
| Mean change in HFMSE | 4.0 | ‐1.9 | 5.9 (3.7 to 8.1) | < 0.001 |
| Final analysis | 84c | 42c | — | — |
| Mean change in HFMSE | 3.9 | ‐1.0 | 4.9 (3.1 to 6.7) | — |
| Nusinersen | Placebo | Difference (95% CI) | P value | |
| Percentage of participants with 3‐point‐change on HFMSE (%) | 57 | 26 | 30.5 (12.7 to 48.3) | < 0.001 |
| Percentage (number) of children who achieved ≥ 1 new WHO motor milestone (% (number)) | 20 (13) | 6 (2) | 14 (‐7 to 34) | 0.08 |
| Nusinersen | Placebo | Risk ratio (95% CI) | P value | |
| Percentage (number) of children who achieved ability to stand alone | 2 (1) | 3 (1) | 0.5 (0.03 to 7.80) | — |
| Percentage (number) of children who achieved ability to walk with assistance | 2 (1) | 0 (0) | 1.5 (0.06 to 36.1) | — |
| Number of participants with adverse events | 78 | 42 | 0.9 (0.9 to 1.0) | 0.01 |
CI: confidence interval; HFMSE: Hammersmith Functional Motor Scale Expanded; WHO: World Health Organization.
a Conducted in children who completed at least six months of the trial. Data of children who had not yet completed the 15‐month period were imputed. b Included 35 participants in the nusinersen group and 19 participants in the control group who completed the 15‐month assessment. Data for 49 participants in the nusinersen group and 23 participants in the control group were imputed. c The number of children with observed data for the 15‐month assessment was 66 in the nusinersen group and 34 in the control group, and the number of children with imputed data was 18 in the nusinersen group and eight in the control group.
6. Oral olesoxime versus placebo outcomes (Bertini 2017).
| Olesoxime | Placebo | Estimated difference (95% CI)a | P value | |
| Number of participants randomised | 108 | 57 | — | — |
| Number (%) of participants evaluable for analysis MFM‐32 D1+D2 | 103 (95%) | 57 (100%) | — | — |
| Meanab change (95% CI) in disability score (MFM D1+D2) at 24 months | ‐0.18 (‐1.30 to 1.66) | ‐1.82 (‐3.68 to 0.04) | 2.00 (‐0.25 to 4.25) | 0.07 |
| Number (%) of participants evaluable for analysis MFM total score | 103 (95%) | 57 (100%) | — | — |
| Meanab change (95% CI) in disability score (MFM total score) at 24 months | 0.59 (‐0.9 to 2.070 | ‐1.45 (‐3.31 to 0.41) | 2.04 (‐0.21 to 4.28) | 0.08 |
| Number (%) of participants evaluable for analysis HFMS | 103 (95%) | 57 (100%) | — | — |
| Meanb change (95% CI) in disability score (HFMS) at 24 months | ‐0.78 (‐1.60 to 0.04) | ‐1.72 (‐2.74 to ‐0.70) | 0.94 (‐0.28 to 2.17) | 0.13 |
| Number (%) of participants evaluable for analysis of nerve innervation value (CMAP) (in mV) | 70 (65%) | 34 (60%) | — | — |
| Meanb (95% CI) change in total amplitude CMAP from baseline to 24 months | ‐0.07 (‐0.49 to 0.36) | ‐0.16 (‐0.74 to 0.43) | NA | 0.79 |
| Number (%) of participants evaluable for analysis of MUNE | 58 (54%) | 30 (53%) | — | — |
| Meanb (95% CI) change in total MUNE from baseline to 24 months | ‐4.51 (‐12.21 to 3.18) | ‐6.69 (‐16.86 to 3.48) | NA | 0.71 |
| Number (%) of participants evaluable for FVC | 64 (59%) | 38 (67%) | — | — |
| Meanb (95% CI) change in FVC from baseline to 24 months | 4.28 (‐0.32 to 8.88) | 6.16 (1.00 to 11.33) | ‐1.88 (‐3.14 to 6.91) | 0.57 |
| Olesoxime | Placebo | Difference (95% CI) | P value | |
| Number (%) of participants evaluable for analysis of quality of life (PedsQL) from baseline to 24 months | 71 (66%) | 37 (65%) | — | — |
| Mean (SD) change in quality of life (PedsQL) from baseline to 24 months | NA | NA | 0.25 (‐4.58 to 5.08) | 0.92 |
| Olesoxime | Placebo | Risk ratio (95% CI) | P value | |
| Number (%) of participants evaluable for responder analysis MFM‐32 score D1+D2 (24 months) | 103 (95%) | 57 (100%) | — | — |
| Proportion of participants with good response according to MFM‐32 score D1+D2c | 56 (54) | 22 (39) | 1.43 (‐0.98 to 2.08) | 0.06 |
| Number of adverse events | 1104 (64) | 612 (36) | — | — |
| Number (%) of participants with adverse events | 103 (95%) | 57 (100%) | 0.95 (0.91 to 0.99) | 0.02 |
| Number (%) of participants with severe adverse events | 18 (17%) | 14 (25%) | 0.67 (0.37 to 1.26) | — |
| Number of deaths | 1 | 1 | — | — |
| Number (%) of participants discontinued intervention | 10 (9%) | 7 (12%) | — | — |
CI: confidence interval; CMAP: compound muscle action potential; FVC: forced vital capacity; HFMS: Hammersmith Functional Motor Scale; MFM: Motor Function Measure; MFM D1+D2: functional domains 1 and 2 of the Motor Function Measure; MUNE: motor unit number estimation; NA: not available; PedsQL: Pediatric Quality of Life Inventory; SD: standard deviation.
a Least squared mean of MFM, including MFM‐32 and MFM‐20 assessments. b Calculated with available data. c Participants with no change or improvement of scores were considered 'responders', participants with a decline in score were considered 'non‐responders'.
7. Oral phenylbutyrate versus placebo outcomes (Mercuri 2007).
| Phenylbutyrate | Placebo | Difference (95% CI) | P value | |
| All ages | ||||
| Number of participants randomised | 54 | 53 | — | — |
| Number (%) of participants evaluable for analysis of HFMS | 45 (83%) | 45 (85%) | — | — |
| Mean (SD) HFMS | 12.1 (9.60) | 12.8 (9.86) | ‐0.7 (‐4.78 to 3.78) | 0.70 |
| Mean (SD) change in HFMSa | 0.60 (0.22) | 0.73 (0.29) | ‐0.13 (‐0.84 to 0.58) | 0.70 |
| Age > 5 years | ||||
| Mean (SD) change in muscle strength arm megascore (dynamometry)a | 1.56 (6.94) | ‐0.42 (8.61) | 1.98 (‐1.67 to 5.63) | 0.74 |
| Mean (SD) change muscle strength leg megascore (dynamometry)a | 4.26 (8.64) | 3.22 (6.26) | 1.04 (‐2.46 to 4.54) | 0.78 |
| Number (%) of participants evaluable for analysis of HFMS | 26 (48%) | 23 (43%) | — | — |
| Mean (SD) change in pulmonary function (FVC % of predicted value in litres)a | 0.03 (0.17) | ‐0.01 (0.27) | 0.04 (‐0.07 to 0.15) | 0.39 |
| Phenylbutyrate | Placebo | Risk ratio (95% CI) | P value | |
| All ages | ||||
| Number of participants with adverse events | 19 | 5 | 3.1 (1.25 to 7.84) | 0.01 |
| Number of withdrawals because of adverse events | 6 | 3 | 1.86 (0.49 to 7.11) | 0.9 |
| Number of severe adverse events | 1 | 2 | — | — |
| Number of participants with severe adverse events | 1 | 2 | 1.96 (0.18 to 21.0) | 1.0 |
CI: confidence interval; FVC: forced vital capacity; HFMS: Hammersmith Functional Motor Scale; SD: standard deviation;
aMean change from baseline.
8. Subcutaneous somatotropin versus placebo outcomes (Kirschner 2014).
| Total number of participants randomised (cross‐over setting) | 20 | — | — | |
| Somatropin | Placebo | Difference (95% CI) | P value | |
| Number of participants completing phase 1 of study protocol | 9/10 | 10/10 | — | — |
| Number of participants completing phase 2 of study protocol | 8/10 | 9/10 | — | — |
| Number (%) of participants evaluable for intention‐to‐treat analysis | 17 (90%) | 17 (80%) | — | — |
| Mean (SD) change in muscle strength of upper limb (hand‐held myometry in megascore of biceps and handgrip) (Newton) | ‐1.05 (6.42) | 0.30 (10.6) | ‐1.35 (‐7.12 to 4.42) | 0.97 |
| Number (%) of participants evaluable for analysis disability score (HFMSE) | 19 (95%) | 19 (95%) | — | — |
| Median (SD) change in disability score (HFMSE) | 0.05 (3.19) | ‐1.05 (5.28) | 0.25 (‐1 to 2.5) | 0.58 |
| Number (%) of participants evaluable for analysis arm megascore | 19 (95%) | 19 (95%) | — | — |
| Mean (SD; range) change in arm megascore (Newton) | ‐1.05 (6.42; ‐11 to 19) | 0.30 (10.60; ‐18 to 34.3) | 0.08 (‐3.79 to 3.95) | 0.97 |
| Number (%) of participants evaluable for analysis leg megascore | 19 (95%) | 19 (95%) | — | — |
| Mean (SD; range) change in leg megascore (Newton) | 2.96 (7.64; ‐10 to 24.5) | 0.95 (9.93; ‐18 to 22) | 2.23 (‐2.19 to 6.63) | 0.30 |
| Number (%) of participants evaluable for analysis muscle strength (MRC) | 19 (95%) | 19 (95%) | — | — |
| Mean (SD) change MRC (% of maximum score) | ‐2.31 (5.1) | 0.43 (7.0) | ‐2.74 (‐6.7 to 1.29) | 0.19 |
| Number (%) of participants evaluable for analysis pulmonary function (FVC) | 19 (95%) | 19 (95%) | — | — |
| Mean (SD; range) change in pulmonary function (FVC improvement in litres) | 0.12 (0.25; ‐0.4 to 0.5) | ‐0.11 (0.40; ‐1.4 to 0.36) | 0.23 (‐0.01 to 0.45) | 0.08 |
| Somatropin | Placebo | Risk Ratio (95% CI) | P value | |
| Number of adverse events | 14 | 9 | — | — |
| Number of participants with adverse events | 11 | 7 | 1.57 (0.78 to 3.17) | 0.34 |
| Number (%) of participants discontinued intervention | 3 (15%) | 0 (0%) | — | — |
CI: confidence interval; FVC: forced vital capacity; HFMSE: Hammersmith Functional Motor Scale Expanded; MRC: Medical Research Council; SD: standard deviation.
9. Intravenous TRH versus placebo outcomes (Tzeng 2000).
| TRH | Placebo | Difference (95% CI) | |
| Number of participants randomised | 6 | 3 | — |
| Number (%) of participants evaluable for analysis | 6 (100%) | 3 (100%) | — |
| Mean (SD) change in muscle strength (dynamometry in pounds) | 0.82 (0.59) | 0.48 (0.29) | 0.34 (‐0.54 to 1.22) |
| TRH | Placebo | Risk ratio (95% CI) | |
| Number of adverse events | 12 | 0 | — |
| Number of participants with adverse events | NA | NA | — |
| Number of severe adverse events | 0 | 0 | — |
| Number of participants with severe adverse events | 0 | 0 | — |
CI: confidence interval; NA: not available; SD: standard deviation; TRH: thyrotropin‐releasing hormone.
10. Oral valproic acid plus acetylcarnitine versus placebo outcomes (Swoboda 2010).
| Valproic acid + acetyl‐L‐carnitine | Placebo | Difference (95% CI) | P value | |
| All ages | ||||
| Number of participants randomised | 31 | 30 | — | — |
| Number (%) of participants evaluable for analysis disability (MHFMS) | 28 (90%) | 28 (93%) | — | — |
| Mean change (SD) in disability score (MHFSM) at 6 months | 0.82 (2.88) | 0.18 (3.98) | 0.64 (‐1.1 to 2.38) | 0.50 |
| Number (%) of participants evaluable for analysis of nerve innervation value (CMAP) | 19 (61%) | 19 (63%) | — | — |
| Mean (SD) change in total amplitude CMAP from baseline to 6 months | 0.02 (0.70) | ‐0.10 (0.66) | 0.12 (‐0.33 to 0.57) | 0.59 |
| Number (%) of participants evaluable for analysis of quality of life (PedsQL) from baseline to 12 months | 27 (87%) | 27 (90%) | — | — |
| Mean (SD) change in quality of life (PedsQL) from baseline to 12 months | ‐1.9 (13.6) | 0.3 (12.9) | ‐2.2 (‐9.27 to 4.87) | 0.54 |
| Age < 3 years old | ||||
| Number (%) of participants evaluable for analysis disability (MHFMS) | 12 (52%) | 11 (48%) | — | — |
| Mean change (SD) in disability score (MHFSM) from baseline to 6 months | 1.33 (2.27) | 1.09 (5.37) | 0.24 (‐3.28 to 3.76) | 0.89 |
| Aged 3–8 years | ||||
| Number (%) of participants evaluable for analysis disability (MHFSM) | 18 (47%) | 17 (45%) | — | — |
| Mean change (SD) in disability score (MHFSM) from baseline to 6 months | 0.44 (3.29) | ‐0.41 (2.79) | 0.85 (‐1.25 to 2.95) | 0.42 |
| Aged ≥ 5 years | ||||
| Number of participants evaluable for analysis muscle strength in arms (myometry) | 7 | 7 | — | — |
| Mean (SD) change in arm muscle strength (myometry) from baseline to 6 months (kg) | 0.64 (0.6) | 0.07 (1.04) | 0.57 (‐0.45 to 1.58) | 0.23a |
| Number of participants evaluable for analysis muscle strength in legs (myometry) | 6 | 4 | — | — |
| Mean (SD) change in leg muscle strength (myometry) from baseline to 6 months (kg) | 0.55 (0.83) | ‐0.85 (2.22) | 1.40 (‐1.98 to 4.79) | 0.19a |
| Number of participants evaluable for analysis muscle strength in both arms and legs (myometry) | 7 | 8 | — | — |
| Mean (SD) change in total muscle strength (myometry) from baseline to 6 months (kg) | 1.18 (0.91) | ‐0.25 (2.47) | 1.43 (0.69 to 3.56) | 0.21 |
| Number of participants evaluable for analysis pulmonary function (FVC in % of predicted) | NA | NA | NAa | NAa |
| Mean (SD) change in pulmonary function (FVC in % of predicted) from baseline to 6 months | NA | NA | — | — |
| Valproic acid + acetyl‐L‐carnitine | Placebo | Risk ratio (95% CI) | P value | |
| All ages | ||||
| Number (%) of participants with adverse events (6 months) | 23 (77%) | 18 (58%) | 1.32 (0.92 to 1.89) | — |
| Number (%) of participants with severe adverse events (6 months) | 6 (20%) | 2 (6%) | 3.1 (0.60 to 12.1) | — |
CI: confidence interval; CMAP: compound maximum action potential; FVC: forced vital capacity; MHFMS: Modified Hammersmith Functional Motor Scale; PedsQL: Pediatric Quality of Life Inventory; SD: standard deviation.
a Underpowered.
11. Oral valproic acid versus placebo outcomes (Kissel 2014).
| Valproic acid | Placebo | Difference (95% CI)a | P value | |
| Number (%) of participants randomised first 6 months (cross‐over setting) | 16 (100%) | 17 (100%) | — | — |
| Number (%) of participants evaluable for analysis after 12 months | 30 (91%) | 30 (91%) | — | — |
| Analysis after 6 months before cross‐over | ||||
| Number (%) of participants evaluable for analysis SMAFRS | 14 (88%) | 17 (100%) | — | — |
| Mean (SD) change in SMAFRS | ‐0.29 (1.59) | ‐0.35 (2.32) | 0.06 (‐1.32 to 1.44) | 0.93 |
| Number (%) of participants evaluable for analysis upper extremity | 14 | 16 | — | — |
| Mean (SD) change in MVICT upper extremity (Newton) | ‐0.24 (1.17) | ‐0.01 (1.05) | ‐0.23 (‐1.03 to 0.57) | 0.54 |
| Number (%) of participants evaluable for analysis lower extremity | 13 | 16 | — | — |
| Mean (SD) change in lower extremity MVICT (Newton) | ‐0.02 (0.65) | 0.35 (1.30) | ‐0.37 (‐1.09 to 0.35) | 0.36 |
| Number (%) of participants evaluable for analysis pulmonary function (FVC) | 14 (88%) | 17 (100%) | — | — |
| Mean (SD; range) change in pulmonary function (FVC improvement in litres) | ‐0.04 (0.35) | 0.30 (1.20) | ‐0.34 (‐0.94 to 0.26) | 0.31 |
| Number of participants evaluable for QoL analysis | 14 | 17 | — | — |
| Mean (SD) change in mini‐SIP for QoL | ‐0.19 (2.80) | 0.91 (4.78) | ‐1.1 (‐3.8 to 1.6) | 0.53 |
| Valproic acid | Placebo | Risk ratio (95% CI)a | P value | |
| Number of adverse events | 30 | 66 | — | — |
| Number of participants with adverse events | 12 | 15 | 0.8 (0.44 to 1.44) | |
| Number of severe adverse events | 2 | 3 | — | — |
| Number of participants with severe adverse events | 2 | 2 | 1.0 (0.15 to 6.69) | — |
| Number (%) of participants discontinued intervention | NA | NA | — | — |
CI: confidence interval; FVC: forced vital capacity; mini‐SIP: mini‐Sickness Illness Profile; MVICT: maximum voluntary isometric contraction; NA: not available; QoL: quality of life; SD: standard deviation; SMAFRS: Spinal Muscular Atrophy Functional Rating Scale.
aCalculated with available data.
We re‐analysed the data from four trials according to our predefined primary and secondary outcome measures (Kissel 2014; Miller 2001; Tzeng 2000; Wong 2007). To enable this analysis, we obtained the raw study data from the principal investigators of two studies (Miller 2001; Wong 2007). We performed additional analysis on the available data of one study to retrieve MDs and CIs of data (Kissel 2014). We obtained additional data for one study to complete information on effect sizes and CIs (Kirschner 2014). The results of this re‐analysis are shown separately for each included trial in Table 12, Table 13, Table 18, Table 19, and Table 21.
Oral creatine versus placebo
Wong 2007 compared creatine versus placebo and reported outcomes at nine months. See Table 12 for numerical results and Table 1.
Primary outcome
Change in disability score
Change in disability, assessed by the GMFM was a primary outcome in Wong 2007. Trial authors supplied additional data on GMFM for re‐analysis. The change in disability scores showed little or no difference between the treatment and placebo groups (n = 40; moderate‐certainty evidence, downgraded one level for imprecision owing to a small sample size).
Secondary outcomes
Change in muscle strength
The study measured muscle strength via quantitative myometry, but (re‐)analysis of data showed no evidence of a difference for change in hand, arm, feet, leg or total muscle strength between the treatment and placebo groups (n = 22; low‐certainty evidence, downgraded one level for imprecision owing to a small sample size and one level for inconsistency due to unknown cohort representation).
Acquiring the ability to stand within one year after the onset of treatment
The study did not measure ability to stand.
Acquiring the ability to walk or improvement of walking within one year after the onset of treatment
The study did not measure ability to walk.
Change in quality of life
The study measured quality of life using the mini‐SIP and PedsQL. There was no evidence of a difference in quality of life measures between the treatment and placebo groups (n = 38; low‐certainty evidence, downgraded one level for imprecision owing to a small sample size and one level for inconsistency).
Change in pulmonary function
The study measured change in FVC in participants more than five years old. There was no evidence of a difference in pulmonary function between the treatment and placebo groups (n = 23; low‐certainty evidence, downgraded one level for imprecision owing to a small sample size and one level for inconsistency due to unknown cohort representation).
Time from beginning of treatment until death or full‐time ventilation
One participant in Wong 2007 died. The death was reported not to be related to the study treatment and occurred in the placebo group. None of the participants reached the state of more than 16 hours' ventilation a day (n = 40; moderate‐certainty evidence, downgraded one level for imprecision owing to a small sample size).
Adverse events, separated into severe and others
In Wong 2007, adverse events rates were similar in the creatine and placebo groups: 13/27 participants who received creatine and 16/28 participants who received placebo had an adverse event (n = 40; low‐certainty evidence, downgraded two levels for study limitations (risk of bias) and because it is unlikely the trial captured uncommon adverse events (risk of imprecision). The report did not include information on type of adverse events. Data on the number of adverse events were available for analysis, but the trial authors were unable to provide other data on (severe) adverse events on request.
Oral gabapentin versus placebo
Miller 2001 compared gabapentin versus placebo and reported outcomes at 12 months. See Table 13 for numerical results and Table 2.
Primary outcome
Change in disability score
Change in disability, measured with the SMAFRS, was a primary outcome in Miller 2001.
There was no evidence of a difference for change in disability scores between the treatment and placebo groups (n = 66; low‐certainty evidence, downgraded two levels for study limitations (risk of bias and imprecision)).
Secondary outcomes
Change in muscle strength
The trial measured muscle strength using quantitative myometry. There was no evidence of a difference for change in hand, arm, feet, leg or total muscle strength between the treatment and placebo groups.
In Miller 2001, some participants were unable to perform all of the muscle strength tests and, therefore, these were not included in the analyses. Moreover, the raw data from this trial showed several extreme values of muscle strength in one particular participating centre. Therefore, we re‐analysed the data with and without these outliers, but this did not result in a different statistical outcome. For a limited number of participants in this trial, data were available at 12 months' follow‐up. Re‐analysis of these limited data also showed no clinically or statistically significant difference for change in muscle strength between the treatment and placebo groups (for total muscle strength, n = 50; low‐certainty evidence, downgraded one level for study limitations (risk of bias) and one level for imprecision; Table 13).
Acquiring the ability to stand within one year after the onset of treatment
The study did not measure ability to stand.
Acquiring the ability to walk or improvement of walking within one year after the onset of treatment
In Miller 2001, none of the participants who were unable to walk before treatment acquired this ability after treatment, and none of the participants who could walk lost this ability in either the treatment or placebo group (n = 73; low‐certainty evidence, downgraded one level for study limitations (risk of bias) and one level for imprecision).
Change in quality of life
Quality of life was measured with the use of mini‐SIP. There was no evidence of a difference in quality of life between the treatment and placebo groups (n = 73; low‐certainty evidence, downgraded one level for study limitations (risk of bias) and one level for imprecision).
Change in pulmonary function
Miller 2001 included only adults and measured the change in FVC. There was no evidence of a difference in pulmonary function between the treatment and placebo groups (n = 65; low‐certainty evidence, downgraded one level for study limitations (risk of bias) and one level for imprecision).
Time from beginning of treatment until death or full‐time ventilation
No deaths were reported and none of the participants reached the state of more than 16 hours' ventilation a day (n = 84; moderate‐certainty evidence, downgraded one level for imprecision).
Adverse events, separated into severe and others
Miller 2001 did not provide specific information on adverse events (n = 65; low‐certainty evidence, downgraded one level for risk of bias and one level for imprecision).
Oral hydroxyurea versus placebo
Chen 2010 compared oral hydroxyurea versus placebo, with a follow‐up of 18 months. See Table 14 for numerical results. and Table 3.
Primary outcome
Change in disability score
Chen 2010 measured change in disability as a primary outcome using the GMFM. There was no evidence of a difference in change from baseline between the treatment and placebo groups (n = 57; low‐certainty evidence, downgraded two levels for imprecision (wide CIs and small sample size)). The trial also measured the MHFMS in non‐ambulatory participants as a secondary outcome. There were no clinically or statistically significant difference between the hydroxyurea and placebo groups (n = 38; moderate‐certainty evidence, downgraded one level for imprecision).
Secondary outcomes
Change in muscle strength
Muscle strength was measured via quantitative myometry. There was no evidence of a difference for change in hand, arm, feet, leg or total muscle strength between the treatment and placebo groups (n = 57; moderate‐certainty evidence, downgraded one level for imprecision).
Acquiring the ability to stand within one year after the onset of treatment
The study did not measure ability to stand.
Acquiring the ability to walk or improvement of walking within one year after the onset of treatment
The study did not measure ability to walk.
Change in quality of life
The study did not measure quality of life.
Change in pulmonary function
Chen 2010 measured change in FVC in participants more than five years old. There was no evidence of a difference in pulmonary function between the treatment and placebo groups (n = 57; low‐certainty evidence, downgraded one level for indirectness and one level for imprecision).
Time from beginning of treatment until death or full‐time ventilation
One participant in the treatment group died. The death was reported not to be related to the study treatment. No other deaths were reported and none of the participants reached the state of more than 16 hours' ventilation a day (n = 57; moderate‐certainty evidence, downgraded one level for imprecision).
Adverse events, separated into severe and others
In Chen 2010, all participants had at least one adverse event (n = 57; moderate‐certainty evidence, downgraded one level for imprecision) and 19/61 participants had a severe adverse event.
Intrathecal nusinersen versus sham procedure
Mercuri 2018 (CHERISH) compared intrathecal nusinersen versus placebo. Results were reported after 15 months of treatment. See Table 15 for numerical results and Table 4.
Primary outcome
Change in disability score
Change in disability measured using the HFMSE was a primary outcome in Mercuri 2018 (CHERISH). There were significant differences in HFMSE score in favour of the nusinersen‐treated participants compared to the sham procedure‐treated participants (n = 126; moderate‐certainty evidence, downgraded one level for imprecision). More participants in the nusinersen‐treated group than the sham procedure‐treated group had a 3‐point change in disability score (n = 126; moderate‐certainty evidence, downgraded one level for imprecision).
Secondary outcomes
Change in muscle strength
Mercuri 2018 (CHERISH) did not report change in muscle strength.
Acquiring the ability to stand within one year after the onset of treatment
One child treated with nusinersen and one child treated with the sham procedure acquired the ability to stand alone (n = 126; low‐certainty evidence, downgraded two levels for imprecision).
Acquiring the ability to walk or improvement of walking within one year after the onset of treatment
One child treated with nusinersen acquired the ability to walk with assistance compared to no children in the sham‐controlled group (n = 126; low‐certainty evidence, downgraded two levels for imprecision).
Change in quality of life
The study did not measure quality of life.
Change in pulmonary function
The study did not measure pulmonary function.
Time from beginning of treatment until death or full‐time ventilation
No deaths were reported and none of the participants reached the state of more than 16 hours' ventilation a day (n = 126; moderate‐certainty evidence).
Adverse events, separated into severe and others
In Mercuri 2018 (CHERISH), 78/84 (93%) participants treated with nusinersen experienced an adverse event, while 42/42 (100%) participants treated with the sham procedure had any adverse event (n = 126; moderate‐certainty evidence, downgraded one level for imprecision). Serious adverse events were reported in 14/84 (17%) participants in the nusinersen group and in 12/42 (29%) participants in the sham procedure group.
Oral olesoxime versus placebo
Bertini 2017 compared oral olesoxime versus placebo and reported outcomes at 24 months. See Table 16 for numerical results and Table 5.
Primary outcome
Change in disability score
Change in disability, measured using the MFM was a primary outcome in Bertini 2017. There was no evidence of a difference in change in disability scores between the treatment and placebo groups (n = 160; very low‐certainty evidence, downgraded one level for study limitations (risk of bias), one level for imprecision and one level for indirectness). The trial also measured motor function using the HFMS, with little or no difference in the change from baseline between the treatment and placebo groups (n = 160; low‐certainty evidence, downgraded one level for study limitations (risk of bias) and one level for imprecision).
Secondary outcomes
Change in muscle strength
The study did not measure muscle strength.
Acquiring the ability to stand within one year after the onset of treatment
The study did not measure ability to stand.
Acquiring the ability to walk or improvement of walking within one year after the onset of treatment
The study did not measure ability to walk.
Change in quality of life
Bertini 2017 measured quality of life using the PedsQL Neuromuscular Module. There was no evidence of a difference in quality of life between the olesoxime‐treated group and the placebo group (n = 108; low‐certainty evidence, downgraded one level for study limitations and one level for imprecision).
Change in pulmonary function
Bertini 2017 measured the change in FVC in participants more than five years old. There was no evidence of a difference in pulmonary function between the treatment and placebo groups (n = 102; low‐certainty evidence, downgraded one level for study limitations and one level for imprecision).
Time from beginning of treatment until death or full‐time ventilation
Two participants died, one in the olesoxime group and one in the placebo group. Deaths were reported not to be related to the study treatment. There were no other deaths and none of the participants reached the state of more than 16 hours' ventilation a day (n = 160; moderate‐certainty evidence, downgraded one level for imprecision).
Adverse events, separated into severe and others
Bertini 2017 reported that 103 (95%) participants receiving olesoxime and 57 (100%) participants receiving placebo had at least one adverse event, with a total of 1104 adverse events in the olesoxime and 612 in the placebo group (n = 165; moderate‐certainty evidence, downgraded one level for imprecision). Severe adverse events occurred in 18 (17%) participants in the olesoxime group and 14 (25%) participants in the placebo group (Bertini 2017).
Oral phenylbutyrate versus placebo
Mercuri 2007 compared oral phenylbutyrate versus placebo. See Table 17 for numerical results and Table 6.
Primary outcome
Change in disability score
Change in disability, measured on the HFMSE was a primary outcome in Mercuri 2007. There was no evidence of a difference for change in disability scores between the treatment and placebo groups (n = 90; low‐certainty evidence, downgraded one level because of risk of bias and one level for imprecision).
Secondary outcomes
Change in muscle strength
Muscle strength was measured via quantitative myometry. There was no evidence of a difference for change in arm or leg megascore between the treatment and placebo groups (both measurements n = 70; low‐certainty evidence, downgraded one level for study limitations and one level for imprecision).
Acquiring the ability to stand within one year after the onset of treatment
The study did not measure ability to stand.
Acquiring the ability to walk or improvement of walking within one year after the onset of treatment
The study did not measure ability to walk.
Change in quality of life
The study did not measure quality of life.
Change in pulmonary function
Mercuri 2007 measured the change in FVC in participants aged more than five years. There was no evidence of a difference in pulmonary function between the treatment and placebo groups (n = 67; low‐certainty evidence, downgraded one level for study limitations and one level for imprecision).
Time from beginning of treatment until death or full‐time ventilation
No deaths were reported (n = 107; low‐certainty evidence, downgraded one level for imprecision and one level for risk of bias).
Adverse events, separated into severe and others
In Mercuri 2007, 2/54 participants treated with phenylbutyrate, compared with 1/53 participants treated with placebo had adverse events. Only one person in each group had a severe adverse event (n = 107; moderate‐certainty evidence, downgraded one level for imprecision).
Subcutaneous somatotropin versus placebo
Kirschner 2014, which was a cross‐over study, compared subcutaneous somatotropin with placebo. See Table 18 for numerical results and Table 7.
Primary outcome
Change in disability score
Kirschner 2014 measured disability as a secondary outcome using the HFMSE. Trial authors supplied additional data on the HFMSE for re‐analysis.
There was no evidence of a difference between the treatment and placebo periods in the change in disability scores (n = 19; very low‐certainty evidence, downgraded two levels for risk of bias and one level for imprecision).
Secondary outcomes
Change in muscle strength
Muscle strength was measured via quantitative myometry (MMT). Trial authors supplied additional data upon request. There was no evidence of a difference between the treatment and placebo periods for change in muscle strength in the upper limbs (n = 19; low‐certainty evidence) or in the lower limbs (n = 19; low‐certainty evidence, downgraded one level for risk of bias and one level for imprecision).
Acquiring the ability to stand within one year after the onset of treatment
The study did not measure ability to stand.
Acquiring the ability to walk or improvement of walking within one year after the onset of treatment
The study did not measure ability to walk.
Change in quality of life
The trial report states that significant differences were not detected between somatotropin and placebo in quality of life measures, but the report does not specify the measures used or provide numerical data.
Change in pulmonary function
Kirschner 2014 measured the change in FVC in participants aged more than five years. Trial authors supplied additional data on pulmonary function for re‐analysis. There was no evidence of a difference in pulmonary function between the treatment and placebo periods (n = 19; low‐certainty evidence, downgraded one level for risk of bias and one level for imprecision).
Time from beginning of treatment until death or full‐time ventilation
No deaths were reported and none of the participants reached more than 16 hours' ventilation a day (n = 19; low‐certainty evidence, downgraded one level for risk of bias and one level for imprecision).
Adverse events, separated into severe and others
In Kirschner 2014, 11 participants (55%) experienced 14 adverse events attributed to treatment while receiving somatotropin. Five events were classified as 'moderate' and two as 'severe', which resulted in termination of trial participation. In the placebo phase, a slightly smaller proportion of participants experienced adverse events, with seven participants reporting nine adverse events (n = 19; low‐certainty evidence, downgraded one level for risk of bias and one level for imprecision).
Intravenous thyrotropin‐releasing hormone versus placebo
Tzeng 2000 compared intravenous TRH versus placebo. See Table 19 for numerical results and Table 8.
Primary outcome
Change in disability score
The study did not measure change in disability score.
Secondary outcomes
Change in muscle strength
Tzeng 2000 measured muscle strength via quantitative myometry. There was no evidence of a difference for change in hand, arm, feet, leg or total muscle strength except in one participant treated with TRH (n = 9; very low‐certainty evidence, downgraded one level for imprecision and two levels for study limitations).
No comparison was made by the study investigators between the treatment and placebo groups because the study size was considered too small.
Acquiring the ability to stand within one year after the onset of treatment
The study did not measure ability to stand.
Acquiring the ability to walk or improvement of walking within one year after the onset of treatment
The study did not measure ability to walk.
Change in quality of life
The study did not measure quality of life.
Change in pulmonary function
The study did not measure pulmonary function.
Time from beginning of treatment until death or full‐time ventilation
No deaths were reported and none of the participants reached the state of more than 16 hours' ventilation a day (n = 9; low‐certainty evidence, downgraded one level for imprecision and one level for study limitations).
Adverse events, separated into severe and others
In Tzeng 2000, the six participants treated with TRH had 12 adverse events, compared to no adverse events in the three participants in the placebo group (n = 9; very low‐certainty evidence, downgraded two levels for imprecision and one level for study limitations and indirectness).
Oral valproic acid (valproate) plus acetyl‐L‐carnitine versus placebo
Swoboda 2010 compared oral valproate plus ACL versus placebo. See Table 20 for numerical results and Table 9.
Primary outcome
Change in disability score
Change in disability was a primary outcome in Swoboda 2010. The scale used was the GMFM. There was no evidence of a difference for change in disability scores between the treatment and placebo groups (n = 61; moderate‐certainty evidence, downgraded one level for imprecision).
Secondary outcomes
Change in muscle strength
Swoboda 2010 measured change in muscle strength via quantitative myometry. There was no evidence of a difference for change in hand, arm, feet, leg or total muscle strength between the treatment and placebo groups (n = 16; low‐certainty evidence, downgraded two levels for imprecision because of very small sample size).
Acquiring the ability to stand within one year after the onset of treatment
The study did not measure ability to stand.
Acquiring the ability to walk or improvement of walking within one year after the onset of treatment
The study did not measure ability to walk.
Change in quality of life
Quality of life was measured in Swoboda 2010 with the Profile Pediatric Quality of Life Inventory. There was no evidence of a difference in quality of life between the treatment and placebo groups (n = 16; very low‐certainty evidence, downgraded one level for risk of bias, one level for indirectness and one level for imprecision).
In Swoboda 2010 there was no statistically significant association between quality of life and change in MHFMS, but there was a non‐significant trend towards deterioration of quality of life as MHFMS declined.
Change in pulmonary function
Swoboda 2010 measured the change in FVC in participants aged more than five years. There was no evidence of a difference in pulmonary function between the treatment and placebo groups. The trial was noted to have insufficient power to observe a statistically significant association (n = 24; low‐certainty evidence, downgraded one level for risk of bias and one level for imprecision).
Time from beginning of treatment until death or full‐time ventilation
No deaths were reported and none of the participants reached the state of more than 16 hours' ventilation a day (n = 61; moderate‐certainty evidence, downgraded one level for imprecision).
Adverse events, separated into severe and others
In Swoboda 2010, 23/30 (77%) participants receiving treatment with valproic acid plus ALC had one or more adverse event compared to 18/31 (58%) participants in the placebo group. Severe adverse events occurred in 20% of the participants treated with valproic acid plus ALC and in 6% of the placebo group (n = 61; moderate‐certainty evidence, downgraded one level for imprecision).
Oral valproic acid (valproate) versus placebo
Kissel 2014 compared oral valproic acid (valproate) versus placebo. See Table 21 for numerical results and Table 10.
Primary outcome
Change in disability score
Change in disability was a secondary outcome in Kissel 2014. The scale used was the SMAFRS. The trial authors made additional data available. There was no evidence of a difference for change in disability scores between treatment and placebo periods (n = 31; low‐certainty evidence, downgraded one level for study limitations and one level for imprecision).
Secondary outcomes
Change in muscle strength
Muscle strength was measured via quantitative myometry. There was no evidence of a difference for change in hand, arm, feet, leg or total muscle strength between treatment and placebo periods(n = 30; low‐certainty evidence, downgraded one level for study limitations and one level for imprecision).
Acquiring the ability to stand within one year after the onset of treatment
The study did not measure ability to stand.
Acquiring the ability to walk or improvement of walking within one year after the onset of treatment
The study did not measure ability to walk.
Change in quality of life
Change in quality of life was assessed with the mini‐SIP. Additional data were made available by the trial authors for re‐analysis. There was no evidence of a difference between treatment and placebo periods (n = 28; moderate‐certainty evidence, downgraded one level for study limitations).
Change in pulmonary function
Kissel 2014 included only adults and measured the change in FVC. Additional data were made available by the trial authors for re‐analysis. There was no evidence of a difference in pulmonary function between treatment and placebo periods (n = 24; low‐certainty evidence, downgraded one level for study limitations and one level for imprecision).
Time from beginning of treatment until death or full‐time ventilation
No deaths were reported and none of the participants reached the state of more than 16 hours' ventilation a day (n = 33; low‐certainty evidence, downgraded one level for imprecision and one level for study limitations)
Adverse events, separated into severe and others
The trial reported 96 adverse events. Trial authors supplied additional information on the types and number of adverse events. Thirty of the adverse events occurred in the valproic acid treatment period and 60 occurred in the placebo treatment period. Two of the events led to early termination of trial participation. Additionally, there were five serious adverse events; two in the valproic acid treatment period and three in the placebo treatment period, but all five were classified as unrelated to treatment (Kissel 2014) (n = 33; low‐certainty evidence, downgraded one level for imprecision and one level for study limitations).
Discussion
Summary of main results
We found 10 randomised controlled trials (including 717 participants) with data available to evaluate the efficacy of drug treatment in people with SMA types II and III (Bertini 2017; Chen 2010; Mercuri 2007; Mercuri 2018 (CHERISH); Miller 2001; Kirschner 2014; Kissel 2014; Swoboda 2010; Tzeng 2000; Wong 2007). Two of these trials included only people with SMA type II (Mercuri 2007; Mercuri 2018 (CHERISH)), and one trial only included ambulatory participants with SMA type III (Kissel 2014). Two RCTs included solely non‐ambulatory participants with SMA types II and III (Swoboda 2010), and types II and IIIa (Bertini 2017). The treatments investigated were oral creatine, oral gabapentin, oral hydroxyurea, intrathecally injected nusinersen, oral olesoxime, oral phenylbutyrate, subcutaneous injections of somatropin, intravenous TRH, and oral therapy with valproic acid with or without oral acetyl‐L‐carnitine.
Intrathecally injected nusinersen was an effective treatment for the improvement of motor function in SMA type II (Mercuri 2018 (CHERISH)), with moderate‐certainty evidence of improvement on the primary outcome (HFMSE) in the treatment group compared to a mean decline of motor scores in the sham‐procedure group.
Although open and uncontrolled trials with other drugs had seemed promising, none of the other included nine trials showed any efficacy on any of the primary outcome measures (Bertini 2017; Chen 2010; Mercuri 2007; Miller 2001; Kirschner 2014; Kissel 2014; Swoboda 2010; Tzeng 2000; Wong 2007).
One RCT did not demonstrate efficacy of oral gabapentin in adults aged 21 years and older with SMA types II and III (Miller 2001). Efficacy of oral hydroxyurea was not established in one trial in 57 participants (Chen 2010).
One RCT of olesoxime in 165 participants with SMA types II and III suggested beneficial effects from olesoxime compared to placebo with stabilisation or slight improvement of motor function in post hoc responder analysis (dichotomous analysis), but there was no effect on the original primary or secondary outcomes (Bertini 2017). The RCT in 107 participants with SMA type II showed no efficacy after three months of treatment with phenylbutyrate (Mercuri 2007). The cross‐over RCT of subcutaneous somatotropin in 20 participants with SMA types II and III showed no effect on muscle strength, motor function or pulmonary function (Kirschner 2014). Two trials investigated the effects of valproic acid in SMA, but both the trial of the combined therapy of valproic acid plus ALC in non‐ambulatory and the trial of monotherapy with valproic acid showed no significant improvement of motor function and muscle strength compared to placebo treatment (Kissel 2014; Swoboda 2010).
Our confidence in these findings of little or no effect was very low for TRH; low to very low for olesoxime and somatotropin; low for valproic acid, phenylbutyrate, gabapentin and hydroxyurea; and moderate for nusinersen, creatine and valproic acid plus ALC.
Nine additional RCTs investigating 4‐aminopyridine, ALC, CK‐2121707 hydroxyurea, pyridostigmine, riluzole, RO6885247/RG7800, salbutamol and valproic acid were completed but no data for analysis were available at the time of writing and they could not be included in the final assessment (ASIRI 2008; CHICTR‐TRC‐10001093; Merlini 2007; MOONFISH 2014; Morandi 2013; NCT00568802; NCT01645787; NCT02644668; SPACE). We consider it unlikely that the results of Merlini 2007 (last update received 2007), NCT00568802 (last update received 2008) and ASIRI 2008 (last update received 2011) will be published in the future, because publication of the results has already been delayed many years.
Evidence from other studies in spinal muscular atrophy
We discuss the results of treatment with each of these drugs from unreported and non‐randomised trials in SMA types II and III. Result of treatment in SMA type I is the topic of another Cochrane Review (Wadman 2019).
Carnitine
One RCT of treatment with ALC in 110 people with SMA types II and III is completed, but results are not available (Merlini 2007).
Celecoxib
One open‐label trial in children and adults with SMA types II and III is planned to investigate the effect of different dosages of celecoxib on SMN protein levels in peripheral leukocytes (NCT02876094).
CK‐2127107
One phase II RCT in 72 participants with SMA types II, III and IV to investigate safety and efficacy of CK‐2127107 150 mg or 450 mg daily compared to placebo is completed, but results are not yet available (see Characteristics of studies awaiting classification; NCT02644668).
Creatine
There are no known trials or studies on creatine in SMA, apart from the trial included in this review (Wong 2007).
Gabapentin
In one large randomised, unblinded and uncontrolled trial with gabapentin in 120 participants with SMA types II and III, there was a trend for improvement in the strength in favour of gabapentin treatment observed after 12 months, with no effect on FVC or other functional tests (Merlini 2003).
Hydroxyurea
In one uncontrolled pilot trial in two people with SMA type I, five people with SMA type II and two people with SMA type III, hydroxyurea showed an improvement in muscle strength without adverse effects (Chang 2002). One larger randomised uncontrolled trial from the same investigators, included 33 people with SMA types II and III and treated them with three different doses of hydroxyurea for eight weeks (Liang 2008). This trial showed increased SMN gene expression and a trend towards improvement in clinical outcome measures.
Lamotrigine
One case series of two people with SMA types II (aged 28 years) and III (aged 37 years) described the use of lamotrigine 50 mg/day for 10 years and reported no deterioration in motor function over five years of treatment (Nascimento 2010).
Neuromuscular junction interactors
Two out of four participants with SMA type II and III reported improved endurance in daily activities after taking pyridostigmine 4 mg/day divided over multiple daily doses (Wadman 2012a). One placebo‐controlled, cross‐over trial in people with SMA types II to IV on the effect of the acetylcholine esterase inhibitor pyridostigmine versus placebo is completed, but results are not yet available (SPACE). One double‐blind RCT in 12 participants with SMA type III aged 18 to 50 years has investigated the effect of 4‐aminopyridine 10 mg twice daily versus placebo. This trial is completed and results are pending (NCT01645787).
Nusinersen
Two phase I/II studies (one open‐label phase I study and its long‐term extension) in 28 participants with SMA types II or III aged two to 14 years showed no safety or tolerability concerns with intrathecal nusinersen treatment (Chiriboga 2016; Darras 2013; Haché 2016). One RCT investigating one dose of nusinersen compared to a sham‐procedure is ongoing and includes participants with atypical SMA type I, while excluding infants with a typical type I presentation (age at onset less than six months and having two SMN2 copies) (EMBRACE 2015). One trial is including genetically confirmed, presymptomatic infants with probable SMA type I (NURTURE 2015). One trial in 34 children with SMA types II and III, aged two to 14 years that investigated the effects of three doses of nusinersen at four different doses has been completed, and results are pending (NCT01703988). One open‐label trial in 52 children with SMA types II and III testing one dose of nusinersen is completed, but results are pending (NCT02052791). One open‐label extension study (SHINE 2015) evaluated the effects of continuous treatment in patients previously participating in Mercuri 2018 (CHERISH) and Finkel 2017 (ENDEAR). The US Food and Drug Administration (FDA) and European Medicines Agency (EMA) approved the use of nusinersen for SMA types I to IV.
Olesoxime
One open‐label extension study with olesoxime was started (see OLEOS) to analyse long‐term effects in non‐ambulatory participants with SMA types II and III who participated in the previous study (Bertini 2017), but the pharmaceutical company announced that the trial and further development of olesoxime would be cancelled due to unsatisfactory results (Roche 2018).
Phenylbutyrate
One pilot study with phenylbutyrate 500 mg/kg/day using an intermittent schedule (seven days on and seven days off) in participants with SMA type II suggested positive effects on motor function after nine weeks of treatment with oral phenylbutyrate (Mercuri 2004). One multicentre phase I/II open‐label trial intentionally evaluating multiple dosage levels of sodium phenylbutyrate to determine the maximum tolerated dose or the highest dose that can be safely given to children with SMA types II or III was terminated after the inclusion of nine participants due to poor compliance to the study drug administration of 500 mg/kg/day (NPTUNE01 2007). Analysis of data is pending but is probably underpowered. One phase I/II open‐label study on sodium phenylbutyrate 450 mg/kg/day to 600 mg/kg/day in 14 presymptomatic infants genetically confirmed to have SMA with suspected SMA type I or II according to family history and SMN2 copy number, has been completed, but results are pending (see STOPSMA 2007).
Riluzole
One study on pharmacokinetics of oral riluzole in 14 participants aged six to 20 years with SMA types II and III indicated that a dose of 50 mg/day of riluzole showed the same daily exposure of riluzole as in the indicated levels in previous trials with people with amyotrophic lateral sclerosis (Abbara 2011). One RCT with riluzole in 141 participants with SMA types II and III is completed, but has not been published and data were not available (ASIRI 2008).
Salbutamol
One case series of nine people with SMA type II treated with salbutamol showed positive effects on pulmonary function and patient perception of motor function (Pasanisi 2014; Tan 2011). In one pilot trial with 13 participants with SMA types II and III, there was a significant increase in muscle strength and pulmonary function after six months of salbutamol treatment (Kinali 2002). The next open‐label trial involving 23 participants with SMA type II presented a significant improvement in functional scores after six and 12 months of treatment with salbutamol without any major adverse effects (Pane 2008). One open‐label trial with 28 participants with SMA types I to III, aged one to 20 years, showed an increase in motor function (HFMS) and pulmonary function in 25% of participants and stability of functional scores in the rest of participants. The results of this study have not been published and are only available through conference reports (Prufer de Queiroz Campos Araujo 2010). One pilot study in 10 participants with SMA types II to IV reported an improvement on perceived motor function, disability and fatigue after salbutamol treatment (Giovannetti 2016). Pulmonary function, including maximal static inspiratory pressure, sniff nasal inspiratory pressure and slow vital capacity, showed effects of one‐year treatment with daily oral salbutamol in seven children with SMA type II and III compared to a natural history cohort of children with SMA type II (Khirani 2017). Only one RCT with salbutamol is completed and suggested that salbutamol induced improvement of motor performance in the majority of the 45 adults with SMA type III. However, this trial has not been published and limited information is only available through conference abstracts (Morandi 2013).
Small molecules
RO6885247/RG7800
One phase I randomised, double‐blind, placebo‐controlled, multiple‐dose study to investigate the safety, tolerability, pharmacokinetics and pharmacodynamics of RO6885247/RG7800 in people with SMA types I, II and III was started in November 2014, but later terminated due to potential safety reasons in December 2016 (for details see Studies awaiting classification; MOONFISH 2014).
RO7034067 or RG7916 (risdiplam)
One open‐label trial including children and adults aged 12 to 60 years with SMA types II and III, previously treated with an SMN2 anti‐sense oligonucleotide, is recruiting participants (JEWELFISH 2017). One RCT with RO7034067/RG7916 has started recruiting children and adults with SMA types II and III (SUNFISH 2016). One trial will include genetically confirmed, presymptomatic infants with SMA (RAINBOWFISH), but has not started at time of writing this review.
Somatropin
There are no known trials or studies on somatotropin in SMA, apart from the trial included in this review (Kirschner 2014).
Thyrotropin‐releasing hormone
A positive effect of TRH in SMA was considered since one uncontrolled study found improvement in motor function and electromyographic findings in participants with SMA types II and III after TRH therapy (Takeuchi 1994).
Valproic acid
Two case series and four open‐label studies showed an increase of SMN transcripts levels in almost all participants with oral valproic acid, but only possibly a beneficial effect on pulmonary function and variable results on motor abilities and muscle strength (Darbar 2011; Kissel 2011; Saito 2014; Swoboda 2009; Tsai 2007; Weihl 2006). One retrospective uncontrolled case series reported improvement of motor function in seven adults with SMA types III and IV during treatment with valproic acid (Weihl 2006). In another retrospective case series, global muscle strength improved in two children and one adolescent with SMA types II and III, but there was no effect in three other participants (Tsai 2007). One prospective case series of seven people with SMA types II and III showed increased SMN transcripts, overall improvement of pulmonary function and improved motor function (Saito 2014). One open‐label trial with 12‐month treatment of valproic acid and L‐carnitine in 33 children with SMA type III found no effects on motor function (Kissel 2011). One open‐label trial with valproic acid in 42 children and adults with SMA types I, II and III showed only slight improvement in gross motor function in younger non‐ambulatory type II children, variable responses of SMN transcripts in blood and carnitine depletion during treatment (Swoboda 2009). Results from the open‐label study in 13 participants aged one to seven years with SMA types I to III are pending (SMART01).
There are four ongoing studies with valproic acid in SMA, including one RCT investigating monotherapy with valproic acid in children with SMA types I and II aged one to seven years (SMART02), one RCT investigating combined therapy of valproic acid plus L‐carnitine in children aged two to 15 years with SMA types II and III (NCT01671384), and two open‐label trials including people with SMA types I, II and III (JPRN‐JapicCTI‐163450 2016; SMART03).
Overall completeness and applicability of evidence
All trials included in this review investigated the effects of drug treatment in children or adults (or both) with SMA types II and III, in terms of disability, muscle strength, ability to stand or walk, quality of life, time to death or full‐time ventilation and adverse events. The trials investigated 10 different treatments, additionally the inclusion criteria and outcome measures varied between trials, which makes it impossible to perform meta‐analyses.
A major issue in SMA, irrespective of the investigated therapy, is the timing of the treatment in relation to its potential effect. Previous experimental studies suggested that there is a limited window of opportunity to rescue or stabilise motor neuron function in the early or presymptomatic stages of the disease. None of the 10 trials identified for inclusion in this review primarily included people who had just been diagnosed. One phase I/II study with phenylbutyrate in presymptomatic infants genetically confirmed to have SMA, and suspected to have SMA type I or II according to family history and SMN2 copy number, has been completed and results are pending (STOPSMA 2007). One trial was started with nusinersen treatment in presymptomatic infants with genetically confirmed SMA (NURTURE 2015).
The practice of supportive care, e.g. pulmonary, nutritional and orthopaedic supportive therapy, in children and adults with SMA types II and III probably differs between centres and countries (Bladen 2014). Practice guidelines for the clinical care of children and adults with SMA are given in the consensus statement for standard care in SMA (Finkel 2018; Mercuri 2018). For future trials it is important that the level of supportive care is explicitly mentioned to avoid baseline differences in the treatment arms and between participating centres.
Certainty of the evidence
None of the included trials were completely free of bias according to the Cochrane 'Risk of bias' tool (Higgins 2011).
The randomisation method was not clear in four trials (Chen 2010; Kissel 2014; Tzeng 2000; Wong 2007). Allocation concealment was not clear in four trials (Chen 2010; Kissel 2014; Miller 2001; Wong 2007) and at high risk of bias in Tzeng 2000. Blinding of parents, participants and observers were adequate in all trials except Kissel 2014, for which the risk of bias related to blinding of participants and personnel and outcome assessors was unclear. We graded the risk of attrition bias high in four trials (Kirschner 2014; Mercuri 2007; Miller 2001; Swoboda 2010), and unclear in three trials (Bertini 2017; Kissel 2014; Wong 2007). Reporting bias was suspected in four trials (Bertini 2017; Chen 2010; Kirschner 2014; Tzeng 2000), and unclear in one trial (Miller 2001).
The cross‐over design with potential carry‐over effects in three studies placed two studies at unclear risk of bias (Kirschner 2014; Swoboda 2010), and one study at high risk of bias (Kissel 2014). Baseline differences resulted in potential bias in four trials, which we graded at either an unclear risk of bias (Mercuri 2018 (CHERISH); Swoboda 2010), or a high risk of bias (Bertini 2017; Wong 2007).
Grading unexplained heterogeneity or inconsistency of results was not possible, since we could not pool data in a meta‐analysis for any drug treatment. We downgraded the evidence one level for imprecision when studies were small or included an insufficient number of participants according to the power analysis of the study.
One issue that is noteworthy and unique for SMA is that the phenotype of patients varies significantly among and within SMA types II and III. Additionally, SMN2 copy number correlates with disease severity. Therefore, studies should consider SMA type and SMN2 copy number as a stratification criterion. None of the included studies incorporated SMN2 copy number in the inclusion criteria or subgroup analyses, which might have influenced results.
Motor assessments were all done with methods validated in SMA or other neuromuscular disorders. However, the disability scores and techniques to measure muscle strength currently used are possibly not sensitive enough to detect subtle changes in muscle strength and motor function, and may therefore underestimate or fail to detect a potential beneficial effect of treatment (type II error).
We are confident that we have minimised the risk of publication bias by evaluating all trial registries and screening trials and studies awaiting publication.
Potential biases in the review process
There may be some potential for bias in this review process as there were changes to the protocol. These included additions and deletions to the outcomes and alterations to the reporting of adverse events, as reported in Differences between protocol and review. None of these changes were made as a result of the findings of the included studies but rather to improve the structure of the review.
All the included trials were relatively small and had a short‐term follow‐up period. We did not extensively report on adverse events. We could not exclude the possibility of missing uncommon adverse events in our review.
We are confident that we have identified all clinically relevant trials, as we conducted a comprehensive search of all published literature and clinical trial registries, and three of the review authors regularly attend international conferences on SMA.
The results of our review might be biased since, at the time of writing, the results had not been published from nine completed trials, investigating 4‐AP (NCT01645787), ALC (Merlini 2007), CK‐2127107 (NCT02644668), hydroxyurea (NCT00568802), pyridostigmine (SPACE), riluzole (ASIRI 2008), rat nerve growth factor (CHICTR‐TRC‐10001093), RO06885247/RG7800 (MOONFISH 2014), and salbutamol (Morandi 2013).
The review authors are investigators in trials of different drug treatments in SMA. The search and selection of trials were not, however, biased by the review authors' involvement in these trials. Data analysis of the creatine trial (Wong 2007, with Dr Iannaccone as investigator and author) was performed by Drs Wadman and Vrancken. Data analysis for the olesoxime trial was checked by Dr Iannaccone, as Drs Wadman, van der Pol and Vrancken were site investigators (Bertini 2017).
Agreements and disagreements with other studies or reviews
To the best of our knowledge, there are no other systematic reviews considering the whole spectrum of drug treatments in SMA. Several reviews have also identified and discussed various drug treatments in SMA (Anderton 2015; Arnold 2013; Darras 2007; Lewelt 2012; Nurputra 2013; Stavarachi 2010; Swoboda 2007; Tisdale 2015), with some focusing specifically on preclinical studies (Seo 2013), genetic therapies (Donnelly 2012; Zanetta 2014), solely histone deacetylase inhibitor therapies (Mohseni 2013), SMN‐inducing therapies (Kaczmarek 2015), or small molecule and molecular therapies (Zanetta 2014). Our conclusions are in line with these reviews.
Although we have tried to give an overview of the efficacy of drug treatment with gabapentin, creatine, nusinersen, valproic acid with and without acetylcarnitine, somatotropin, TRH, phenylbutyrate, olesoxime and hydroxyurea in preclinical studies, studies with animal models of SMA or studies in participants with SMA (Discussion), the overview on non‐randomised and preclinical trials and studies was not based on a systematic review and potential studies might have been missed.
Authors' conclusions
Implications for practice.
Nusinersen probably improves motor function in spinal muscular atrophy (SMA) type II (moderate‐certainty evidence).
Creatine, gabapentin, hydroxyurea, phenylbutyrate, thyrotrophin releasing hormone, valproic acid and the combination of valproic acid and acetyl‐L‐carnitine probably have no clinically important effect on motor function in SMA II/III (low‐ to moderate‐certainty evidence). Olesoxime and somatotropin may have no effect on motor function (low‐ to very low‐certainty evidence). We are uncertain about the effect of thyrotropin‐releasing hormone as the evidence is very low certainty.
Implications for research.
Nusinersen is the only drug therapy for SMA for which there is moderate‐certainty evidence of benefit. New therapies or treatment strategies should preferably either be compared to nusinersen or be evaluated as an add‐on therapy to nusinersen.
Most trials investigating new therapies are focused on the early phases of the disease, since motor improvement or lack of decline is the easiest way to establish drug efficacy. However, therapies for those who already have a prolonged disease duration should also be sought to prevent disease progression, conserve motor function and improve quality of life.
What's new
| Date | Event | Description |
|---|---|---|
| 22 October 2018 | New search has been performed | Protocol modification in primary and secondary outcomes. Protocol modification in SMA type. Transformation of disability scores into MRC scores has been removed, since it is not possible for the included study population. We revised the methods to conform to current Cochrane standards. |
| 20 June 2017 | New citation required and conclusions have changed | Updated search October 2018. Four new trials included. Inclusion of 'Summary of findings' tables. Re‐evaluation of 'Risk of bias' assessments. One author (J Wokke) retired from authorship. Fay‐Lynn Asselmann joined the author team. |
History
Protocol first published: Issue 4, 2006 Review first published: Issue 1, 2009
| Date | Event | Description |
|---|---|---|
| 15 February 2012 | Amended | 'Declaration of interest' and 'Contributions of authors' updated; no other changes to text. |
| 15 February 2012 | New citation required but conclusions have not changed | Change in listing of authors to include Dr WL van der Pol. This corrects an error in the authorship of this update. |
| 31 March 2011 | New citation required but conclusions have not changed | RI Wadman included as new lead author. |
| 8 March 2011 | New search has been performed | Databases were searched and review was updated. Two new trials were found. |
| 30 July 2008 | Amended | Converted to new review format. |
Acknowledgements
Editorial support from the Cochrane Neuromuscular for earlier versions of this review was funded for the original review by the TREAT NMD European Union Grant 036825.
This project was supported by the National Institute for Health Research (NIHR) via Cochrane Infrastructure funding to Cochrane Neuromuscular. The views and opinions expressed herein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service or the Department of Health. Cochrane Neuromuscular is also supported by the MRC Centre for Neuromuscular Diseases.
We used a standard template provided by Cochrane Neuromuscular to complete some of the mandatory sections of the methods.
Appendices
Appendix 1. SMN1 gene therapies
SMN1 gene therapy
Studies that aim to study the repair of the SMN1 deletion by the introduction of the SMN1 gene have started. Viral vectors, such as the self‐complementary adeno‐associated virus (scAAV9) are used to incorporate the SMN1 gene. In vitro studies in fibroblasts of people with SMA (Azzouz 2004; Dominguez 2011), and in vivo studies in mice (Benkhelifa‐Ziyyat 2013; Dominguez 2011), primates, and pigs with SMA phenotype have shown promising results on SMN1‐expression with effect on motor function and survival (Duque 2015; Foust 2010; Glascock 2012a; Glascock 2012b; Passini 2011; Robbins 2014; Valori 2010). These studies also indicate that intramuscular or intravenous injection of the adeno‐associated virus (AAV) results in widespread dissemination of the gene, including penetration of the central nervous system (Benkhelifa‐Ziyyat 2013; Foust 2010; Glascock 2012a; Meyer 2015).
One phase I study with intravenous AVXS‐101 (scAAV9.CB.SMN) in infants with SMA type I has been completed and showed all 15 participants to be alive and event‐free at 20 months of age, as compared with a rate of survival of 8% in a historical cohort. Participants included in the study also showed improvement on the CHOP‐INTEND (Children's Hospital of Philadelphia Infant Test of Neuromuscular Disorders; 0 to 66 scale) (increase of 9.8 points at one month and 15.4 points at three months, as compared with a decline in this score in a historical cohort) (Mendell 2016; Mendell 2017).
One phase I study with intrathecal AVXS‐101 has started in 27 children with SMA type II aged six to 60 months testing three different doses (6.0 × 1013 vg or AVXS‐101, 1.2 × 1013 vg of AVXS‐101 or 2.4 × 1013 vg of AVXS‐101) (STRONG).
Appendix 2. Other experimental factors studied in vivo and vitro
Several compounds have an effect on SMN expression in vivo and in vitro. These include 2,4‐diaminoquinazolines (RG3039 and D156844) (Butchbach 2010; Gogliotti 2013; Jarecki 2005; Singh 2008; Thurmond 2008, van Meerbeke 2013), 2,4‐diaminoquinazoline inhibitors of the decapping scavenger enzyme DcpS (DAQ‐DcpSi) (Cherry 2017), 2‐(4,6‐dimethylpyrazolo[1,5‐a]pyrazin‐2‐yl)‐7‐(4‐methylpiperazin‐1‐yl)‐4H‐pyrido[1,2‐a]pyrimidin‐4‐one (Woll 2016), 3‐(6,8‐Dimethylimidazo[1,2‐a]pyrazin‐2‐yl)‐7‐(4‐methylpiperazin‐ 1‐yl)‐1H‐isochromen‐1‐one (Woll 2016), 4PBA (Butchbach 2016), 5‐(N‐ethyl‐N‐isopropyl)‐amiloride (Yuo 2008), AAV8.LSP.dnMstn (Liu 2016), AAV8.LSP.sActRIIB (Liu 2016), aclarbicin (Andreassi 2004; Ting 2007), amikacin (Wolstencroft 2005), BAY 55‐9837 (Hadwen 2014), bortezomib (Kwon 2011), butyrate prodrug pivaloyloxymethyl butyrate (AN9) (Edwards 2016), dacinostat (Mohseni 2016), E1mov11 (Osman 2016), edavarone (Ando 2017); fasudil (Bowerman 2012), Genetics/G418 (Heier 2009; Heier 2015), Indoprofen (Lunn 2004), loganin (Tseng 2016), M344 (Riessland 2006), ML372 (Abera 2016), panobinostat/LBH589 (Garbes 2009), Pip6a‐PMO (Hammond 2016), PTK‐SMA1 (Hastings 2009), quercetin (Uzunalli 2015; Wishart 2014), quisinostat/JNJ‐26481585 (Schreml 2013), romidepsin (Hauke 2009), scAAV9‐siPTEN (Ning 2010; Little 2015), securinine (Chen 2017), SMN‐AS1 (d'Ydewalle 2017; Woo 2017), sodium vanadate (Liu 2014; Ting 2007; Zhang 2001), suberoylanilide hydroxamic acid (Hahnen 2006; Mohseni 2016; Riessland 2010), TC007 (Mattis 2009a; Mattis 2009b; Mattis 2012), (S)‐3‐(6,8‐dimethylimidazo[1,2‐a]pyrazin‐2‐yl)‐7‐(4‐ethyl‐3‐ methylpiperazin‐1‐yl)‐2H‐chromen‐2‐one (Woll 2016), tobramycin (Wolstencroft 2005), trichostatin A (Avila 2007; Liu 2014; Ting 2007), triptolode (Hsu 2012), VK563 (Butchbach 2016), and Y‐27632 (Bowerman 2010). Every compound has its own potential and way of interacting with the SMN complex. Description of the working mechanism of each compound goes behind the scope of this review, since none of the compounds have yet been investigated in human studies.
At the time of writing, it is unclear whether treatment with any of these drugs has a beneficial clinical effect on the disease course of SMA types II and III.
Appendix 3. Cochrane Neuromuscular Specialised Register (CRS) search strategy
#1 MeSH DESCRIPTOR Muscular Atrophy, Spinal Explore All [REFERENCE] [STANDARD] #2 MeSH DESCRIPTOR Muscular Disorders, Atrophic [REFERENCE] [STANDARD] #3 "spinal muscular" NEXT atroph* [REFERENCE] [STANDARD] #4 Werdnig NEXT Hoffman* [REFERENCE] [STANDARD] #5 Kugelberg next Welander [REFERENCE] [STANDARD] #6 #1 or #2 or #3 or #4 or #5 [REFERENCE] [STANDARD] #7 (#1 or #2 or #3 or #4 or #5) AND (INREGISTER) [REFERENCE] [STANDARD]
Appendix 4. Cochrane Central Register of Controlled Trials (CENTRAL) (CRSO) search strategy
Search run on 22 October 2018
#1 MESH DESCRIPTOR Muscular Atrophy, Spinal EXPLODE ALL TREES 32 #2 Werdnig near Hoffman* 2 #3 Kugelberg near Welander 1 #4 spinal near muscul* near atroph* 66 #5 #1 or #2 or #3 or #4 72
Appendix 5. MEDLINE (OvidSP) search strategy
Database: Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) <1991 to Present> Ovid MEDLINE(R) 1946 to October week 43 2018 Search strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 randomized controlled trial.pt. (469833) 2 controlled clinical trial.pt. (95075) 3 randomized.ab. (405868) 4 placebo.ab. (192439) 5 drug therapy.fs. (2035863) 6 randomly.ab. (286433) 7 trial.ab. (428139) 8 groups.ab. (1764357) 9 or/1‐8 (4191699) 10 exp animals/ not humans.sh. (4669484) 11 9 not 10 (3613302) 12 exp Muscular Atrophy, Spinal/ (4602) 13 muscular disorders, atrophic/ (426) 14 spinal muscular atroph$.mp. (4867) 15 (Werdnig adj Hoffman$).mp. (393) 16 (Kugelberg adj Welander).mp. (189) 17 or/12‐16 (6876) 18 11 and 17 (709) 19 remove duplicates from 18 (604) 20 limit 19 to yr="1991 ‐Current" (539)
Appendix 6. Embase (OvidSP) search strategy
Database: Embase <1991 to 2018 week 43> Search strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 crossover‐procedure.sh. (54096) 2 double‐blind procedure.sh. (137638) 3 single‐blind procedure.sh. (27791) 4 randomized controlled trial.sh. (465768) 5 (random$ or crossover$ or cross over$ or placebo$ or (doubl$ adj blind$) or allocat$).tw,ot. (1349705) 6 trial.ti. (215268) 7 or/1‐6 (1506890) 8 (animal/ or nonhuman/ or animal experiment/) and human/ (1735631) 9 animal/ or nonanimal/ or animal experiment/ (3746312) 10 9 not 8 (3037555) 11 7 not 10 (1393170) 12 spinal muscular atrophy/ or hereditary spinal muscular atrophy/ (6331) 13 (Werdnig adj Hoffman$).mp. (627) 14 (Kugelberg adj Welander).mp. (333) 15 spinal muscul$ atroph$.mp. (7028) 16 or/12‐15 (7410) 17 11 and 16 (148) 18 limit 17 to yr="1991 ‐Current" (141) 19 remove duplicates from 18 (132)
Appendix 7. ISI Web of Science Conference Proceedings search strategy
WOS 22 October 2018 Indexes=CPCI‐S, CPCI‐SSH Timespan=1991‐2018 #6 8 #5 AND #4 #5 211,588 TS=(random* or placebo or "single blind" or "double blind*" or crossover or "cross‐over" ) #4 543 #1 or #2 or #3 #3 3 TS=(Kugelberg AND Welander) #2 14 TS=(Werdnig AND Hoffman*) #1 536 TS=("muscular atrophy" NEAR spinal)
Appendix 8. ClinicalTrials.gov search strategy
1 spinal muscular atrophy
2 drug therapy OR treatment OR trial
Appendix 9. WHO International Clinical Trials Registry Platform search strategy
1 spinal muscular atrophy
2 SMA
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bertini 2017.
| Methods | Randomised, placebo‐controlled, double‐blind trial | |
| Participants | 165 non‐ambulatory participants with SMA types II or IIIa aged 3–25 years Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Oral liquid olesoxime (TRO19622: cholest‐4‐en‐3‐one, oxime) 10 mg/kg once a day or placebo Treatment duration: 24 months |
|
| Outcomes | Primary outcome: change over 24 months in functional outcome score D1+D2 of MFM Secondary outcomes: change in total MFM score, HFMS, electrophysiological measures (CMAP and MUNE), pulmonary function (FVC), quality of life (PedsQL), and Global Clinical Impression from baseline, responder analysis of MFM, laboratory assessments, ECG and adverse events |
|
| Funding | AFM‐Téléthon and Trophos SA (a wholly owned member of the Hoffmann La Roche Group since 2015) | |
| Conflicts of interest | Several investigators declared grants and consultancy fees from Hoffmann La Roche, Trophos and other commercial entities. 8 investigators were current or former employees of Trophos or Hoffmann La Roche, 3 were stockholders and 2 authors were named on a patent pending for olesoxime. Roche also funded medical writing support. | |
| Notes | ClinicalTrials.gov id: NCT01302600 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned, centrally generated with validated randomisation software (SAS version 9.2). |
| Allocation concealment (selection bias) | Low risk | Computer‐generated allocation by independent statistician. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and all investigators, site personal and sponsor study personal was ensured. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of participants and all investigators, site personal and sponsor study personal was ensured. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 17 participants withdrew for unknown reasons. |
| Selective reporting (reporting bias) | High risk | Data were dichotomised post hoc. Investigators were employees of the pharmaceutical company and they were involved in data collection and analysis. |
| Other bias | High risk | Primary outcome measure was used in 2 different forms (MFM‐32 and MFM‐20) and, therefore, not truly comparable. Treatment groups have differences in included age ranges. |
Chen 2010.
| Methods | Randomised, placebo‐controlled, double‐blind trial | |
| Participants | 57 participants aged ≥ 5 years who fulfilled international classification criteria for SMA types II or III and with a homozygous deletion of the SMN1 gene Inclusion criteria:
Disease severity was categorised according to the International Classification for SMA, which is based on age at disease onset and maximum function. Exclusion criteria:
|
|
| Interventions | Oral hydroxyurea in escalating dose from 10 mg/kg to 20 mg/kg over 8 weeks (5 mg/kg increase per 4 weeks) or placebo in increasing dose over 8 weeks Duration of treatment: 18 months Follow‐up: 6 months post‐treatment |
|
| Outcomes | Change in functional score (GMFM), change in functional score in non‐ambulatory patients (HFMS), change in muscle strength (MMT), change in pulmonary function (FVC), adverse events | |
| Funding | Quote: "Supported by Department of Health, Executive Yuan, Taiwan (DOH96‐TD‐I‐111‐TM013) and in part by Sun's KMU‐SMA fund." | |
| Conflicts of interest | Most authors reported research support from Department of Health, Executive Yuan, Taiwan and Sun's KMU‐SMA fund. 2 authors reported patents regarding hydroxyurea treatment for SMA and method for diagnosis of SMA. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned. No methods of randomisation described. |
| Allocation concealment (selection bias) | Unclear risk | No details of randomisation given. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants, families, investigators, study co‐ordinators, evaluators and statisticians were blinded. Randomisation unit and study pharmacist were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants, families, investigators, study co‐ordinators, evaluators and statisticians were blinded. Randomisation unit and study pharmacist were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | High risk | Data on muscle strength dichotomised post hoc. |
| Other bias | Low risk | None identified. |
Kirschner 2014.
| Methods | Randomised, double‐blind, placebo‐controlled cross‐over trial | |
| Participants | 20 participants with SMA types II and III, genetically confirmed with SMN1‐ deletion or mutation, aged 6–36 years Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Subcutaneous somatotropin (first week dose 0.015 mg/kg/day; week 2–12 dose 0.03 mg/kg/day) or placebo subcutaneous (first week dose 0.015 mg/kg/day; week 2–12 dose 0.03 mg/kg/day) Treatment of 12 weeks with 1 treatment (somatropin or placebo) followed by 8 weeks' washout, afterwards second cross‐over treatment period (somatropin or placebo) of 12 weeks is started |
|
| Outcomes | Change in quantitative muscle strength of upper limb using hand‐held myometry in elbow flexion and handgrip, change in quantitative muscle strength of lower limb, muscle strength with MMT in 7 muscles, change in motor function (HFMSE), change in Gowers' time, change in qualitative Gowers' manoeuvre, change in pulmonary function (FVC and peak cough flow), adverse events | |
| Funding | Quote: "NovoNordisk Pharma GmbH provided the trial drug and some financial support for conducting this trial. NovoNordisk had no influence on the trial design, how the trial was conducted or the data analysis." | |
| Conflicts of interest | Author conflicts of interest not stated | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned. Computer‐generated allocation by central pharmacy. |
| Allocation concealment (selection bias) | Low risk | Computer‐generated allocation by central pharmacy. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants, physicians and physiotherapists were blinded. Statistical analysis of primary outcome was blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants, physicians and physiotherapists were blinded. Statistical analysis of primary outcome was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 3 participants withdrew during somatotropin treatment. They were included in the modified ITT (unknown method) analysis. |
| Selective reporting (reporting bias) | High risk | Quality of life is mentioned as an outcome, but no scale is mentioned. However, the report states that the trial found no difference between somatotropin and placebo groups in quality of life. |
| Other bias | Unclear risk | Potential bias from cross‐over study design; cross‐over design implies risk of carryover effect. |
Kissel 2014.
| Methods | Randomised, double‐blind, placebo‐controlled cross‐over trial | |
| Participants | 33 ambulatory adults with SMA type III Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Oral valproic acid 10–20 mg/kg/day (doses adequate to reach serum levels 50–100 mg/dL) divided over 2–3 doses or placebo orally Cross‐over of treatment after 6 months for a consecutive period of 6 months |
|
| Outcomes | Change in maximum voluntary isometric contraction testing for separate muscles (bilateral elbow flexors, elbow extensors, knee flexors, knee extensors and grip) and total muscle score, change in muscle strength measured by hand‐held dynamometer of elbow flexors/extensors and knee flexors/extensor, change in SMAFRS, change in CMAP of ulnar nerve, change in mRNA levels, change in SMN protein levels, change in pulmonary function (FVC, FEV1, MIP), change in muscle mass measured by DEXA, change in endurance assessed through 6‐minute walk test, change in function assessed in time to climb 4 standard stairs, change in mini‐SIP, adverse events | |
| Funding | Quote: "Funded by Families of Spinal Muscular Atrophy and also by grants from the Center for Clinical and Translational Sciences, University of Utah (UL1RR025764), and the Center for Clinical and Translational Sciences, Ohio State University (UL1RR025755)." Abbott Pharmaceuticals provided valproic acid and placebo |
|
| Conflicts of interest | Authors reported receipt of grants and funding from Families of SMA and other non‐governmental, charitable, governmental, academic and pharmaceutical company sources. 2 authors report receipt of drugs from Abbott Pharmaceuticals for clinical trials in SMA. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned. Unknown method. Since the trial was part of the previous Carni‐VAL‐I trial and most procedures were the same, one could suppose there was central randomisation by telephone; however, this was not exactly stated in the article or supplementary material. |
| Allocation concealment (selection bias) | Unclear risk | Randomly assigned. Unknown method. Since the trial was part of the previous Carni‐VAL‐I trial and most procedures were the same, one could suppose there was central randomisation by telephone; however, this was not exactly stated in the article or supplementary material. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants, physicians and investigators were blinded. 1 investigator was not blinded. Study compliance (tablet counts and valproic acid levels) were checked by unblinded investigator. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Participants, physicians and investigators were blinded. 1 investigator was not blinded. Study compliance (tablet counts and valproic acid levels) were checked by unblinded investigator. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 4 participants withdrew, but were included in ITT analysis. |
| Selective reporting (reporting bias) | Low risk | Incomplete data reported in article, but data available on request. |
| Other bias | High risk | Potential bias by design: cross‐over design implies risk of carryover effect. No report on a washout period between the 2 treatment periods. |
Mercuri 2007.
| Methods | Randomised, placebo‐controlled, double‐blind trial | |
| Participants | 107 participants who fulfilled international classification criteria for SMA type II Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Phenylbutyrate 500 mg/kg/day 7 days orally, divided in 5 doses using an intermittent schedule (7 days on and 7 days off) or placebo Duration of treatment: 3 months Follow‐up: 3 months |
|
| Outcomes | Functional score (HFMS), change in functional score. Subgroup aged > 5 years: change in muscle strength arm and leg (myometry), change in pulmonary function (FVC), adverse events | |
| Funding | Financial support from Famiglie SMA, Italy and Associazione per lo Studio delle Atrofie Spinali Musculari Infantili (ASAMSI) Fyrklövern Scandinavia AB, Sweden provided triButyrate Medication provided by pharmaceutical company, but no details about the involvement of the company in study procedures. |
|
| Conflicts of interest | Quote: "The authors report no conflicts of interest." | |
| Notes | Muscle strength was measured bilaterally for elbow flexion, hand grip and 3‐point pinch. Muscle strength was measured bilaterally for knee flexion and knee extension. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned. Central allocation. |
| Allocation concealment (selection bias) | Low risk | Central allocation. Only randomisation unit and pharmacy had access to assignment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Only randomisation unit and pharmacy had access to assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Only randomisation unit and pharmacy had access to assignment. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Myometry and FVC were measured in children aged > 5 years, but a different number of children were reported in the 2 groups. No report on explaining this difference. Unclear reports on adverse events. Quote: "The efficacy analyses were conducted according to the original randomization assignment (intention to treat), using the last observation carried forward approach for missing follow‐up data" |
| Selective reporting (reporting bias) | Low risk | There was clear evidence that reported results corresponded to all intended outcome measurements. |
| Other bias | Low risk | No other bias identified |
Mercuri 2018 (CHERISH).
| Methods | Randomised sham‐procedure controlled, double‐blind trial. Randomisation 2:1 (nusinersen:sham procedure) | |
| Participants | 126 participants with SMA types II, aged 2–12 years Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Intrathecal injection with nusinersen 12 mg or sham procedure | |
| Outcomes | Change in HFMSE, achievement of new motor milestones, change in Upper Limb Module Test, vital signs, weight changes, laboratory changes, ECG | |
| Funding | Ionis Pharmaceuticals and Biogen Inc | |
| Conflicts of interest | Investigators collected data, which were held and analysed by Biogen. The first manuscript was written by the authors and the senior industry author of Biogen, medical‐writing assistance was paid for by Biogen. | |
| Notes | As of December 2016 the study was stopped and participants were transitioned to the open‐label SHINE study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned by central and electronic procedure. |
| Allocation concealment (selection bias) | Low risk | Computer‐generated allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study personnel who deliver the treatment were not involved in the assessments of participants. Key personnel for assessments and parents of participants were not present during procedure. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study personnel who deliver the treatment were not involved in the assessments of participants. Key personnel for assessments and parents of participants were not present during procedure. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Trial stopped early for benefit on the basis of interim analyses. ITT and efficacy outcomes reported. Data imputed for ITT analyses. Supplementary data report sensitivity analyses. Quote: "Sensitivity analyses of the primary end point using final data were consistent with the results of the final analysis." |
| Selective reporting (reporting bias) | Low risk | Protocol available. There was clear evidence that reported results corresponded to all intended outcome measurements. Extensive data reporting in article and supplementary material. |
| Other bias | Unclear risk | Baseline differences with more severely affected children in the nusinersen‐treated group. |
Miller 2001.
| Methods | Randomised, placebo‐controlled, double‐blind trial (2 × 2 block design) | |
| Participants | 84 participants who fulfilled international classification criteria for SMA types II or III and have an homozygous deletion of the SMN1 gene Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Gabapentin 1200 mg 3 times a day or placebo Duration of treatment: 12 months Follow‐up: at quarterly time intervals while on treatment |
|
| Outcomes | Change in disability score (SMAFRS), change in muscle strength, development of walking, change in pulmonary function (FVC), change in quality of life (SIP), adverse events | |
| Funding | Andrew's Buddies, MDA, Warner‐Lambert, and Families of SMA | |
| Conflicts of interest | Not stated | |
| Notes | Muscle strength of elbow flexion and hand grip was measured bilaterally. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned. Randomisation in blocks of 4 participants (2 placebo, 2 gabapentin) with equalised randomisation per centre. No methods of randomisation described. |
| Allocation concealment (selection bias) | Unclear risk | Randomisation performed by research pharmacist. Precise method of allocation concealment not known. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Only research pharmacist was not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Only research pharmacist was not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Reasons for dropout of 19 participants not mentioned. ITT analysis performed with a different number of participants than initially included. |
| Selective reporting (reporting bias) | Unclear risk | Adverse events not reported. |
| Other bias | Low risk | None identified |
Swoboda 2010.
| Methods | Randomised, placebo‐controlled, double‐blind trial | |
| Participants | 61 non‐ambulatory participants with SMA types II or III Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Oral liquid carnitine 50 mg/kg/day in 2 doses in combination with oral valproic acid capsules in 2–3 doses to maintain overnight serum level trough 50–100 mg/dL or liquid placebo twice daily in combination with placebo capsule 2–3 times a day Duration of treatment: 12 months in active treatment and 6 months in placebo group. The placebo group switched to active treatment after 6 months per protocol Total follow‐up: 12 months |
|
| Outcomes | Primary: safety, motor function assessments, change in functional score (MHFMS) Secondary: include change in quality of life (PedsQL), change in innervation via maximum ulnar CMAP, adverse events. Change in muscle strength and change in pulmonary function were measured in participants aged ≥ 5 years |
|
| Funding | Quote: "Abbott Pharmaceutical provided VPA [valproic acid] and placebo, and Sigma‐Tau Pharmaceutical provided L‐carnitine, at no cost." | |
| Conflicts of interest | Quote: "The authors have declared that no competing interests exist." | |
| Notes | Baseline differences in body mass index and gender between the different treatment groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned using a permuted block design balancing for institution. |
| Allocation concealment (selection bias) | Low risk | Randomisation was performed using a permuted block design balancing for institution. Randomisation was performed centrally by telephone. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and key study personnel. Medical monitor was unblinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of participants and key study personnel. Medical monitor was unblinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not all outcome measures are available for analysis. Missing data were not fully explained. |
| Selective reporting (reporting bias) | Low risk | Protocol available. There was clear evidence that reported results correspond to all intended outcome measurements. |
| Other bias | Unclear risk | Partial cross‐over design after 6 months. |
Tzeng 2000.
| Methods | Randomised (ratio 2:1), placebo‐controlled, double‐blind trial | |
| Participants | 9 participants who fulfilled international classification criteria for SMA types II or III Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Thyrotropin‐releasing hormone 0.1 mg/kg intravenous once a day or placebo Duration of treatment: 29 days of treatment over a 34‐day period Follow‐up: 35 days |
|
| Outcomes | Change in muscle strength (dynamometry), adverse events | |
| Funding | Quote: "Supported, in part, by Ferring Laboratories, Inc., Suffern, New York, the manufacturer of Thyrel™ who made the study medication, Thyrel™, available without cost to the subjects, and, in part, by grant H133 P 70011, Advanced Multidisciplinary Fellowship in Rehabilitation Outcomes Research to the Department of Physical Medicine and Rehabilitation, UMDNJ‐New Jersey Medical School, from the National Institute on Disability and Rehabilitation Research." | |
| Conflicts of interest | Not reported | |
| Notes | Groups were not equal at baseline, with only women, only SMA II and older participants in the placebo group. Muscle strength was measured bilaterally of deltoid, biceps, triceps, wrist extension, hand grip, hip flexion, knee extension and knee flexion. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned (2:1). Patients randomised by double coin flip for each patient. Any heads‐tails combination was a participant; a tails–tails combination was a control; and a heads–heads combination was rejected and the flip repeated. Potential bias by inclusion of first arrival method. Quote: "Once either the control or subject group was full, all subsequent arriving patients were placed into the remaining group." |
| Allocation concealment (selection bias) | High risk | Randomisation done on a first arrival basis. Quote: "Once either the control or subject group was full, all subsequent arriving patients were placed into the remaining group." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All investigators and participants were blinded. Only the pharmacist was unblinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All investigators and participants were blinded. Only the pharmacist was unblinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts. |
| Selective reporting (reporting bias) | High risk | All outcomes were reported. The statistical analysis plan is limited and unclear. |
| Other bias | Low risk | None identified. |
Wong 2007.
| Methods | Randomised, placebo‐controlled, double‐blind trial | |
| Participants | 55 participants who fulfilled international classification criteria for SMA types II or III Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Creatine: aged 2–5 years, 2 g once a day or placebo; age 5–18 years, 5 g once a day or placebo Duration of treatment: 6 months Follow‐up: 9 months |
|
| Outcomes | 2–5 years and 5–18 years: change in disability score (GMFM), change in quality of life, adverse events 5–18 years: change in quantitative muscle strength (QMT), change in pulmonary function |
|
| Funding | National Institutes of Health, the Muscular Dystrophy Association, and Andrew's Buddies Experimental and Applied Sciences; Golden, Colorado supplied creatine |
|
| Conflicts of interest | Not reported | |
| Notes | Creatine group at baseline slightly weaker; follow‐up inadequate (> 20% dropout rate and < 9 months' follow‐up). Muscle strength was measured bilaterally for hand grip, elbow flexion, knee extension and knee flexion according to the Richmond Quantitative Measurement System | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned, method of randomisation unknown. |
| Allocation concealment (selection bias) | Unclear risk | Randomisation at central site. Method not known. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants, families, investigators, evaluators and study co‐ordinators blinded; the study statistician blinded to group membership. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants, families, investigators, evaluators and study co‐ordinators blinded; the study statistician blinded to group membership. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | High dropout rate partially described. |
| Selective reporting (reporting bias) | Low risk | Outcome measurements were limited and inconsistent in the published report, but the trialists provided additional data for analysis. |
| Other bias | Unclear risk | None identified. |
CMAP: compound muscle action potential; CSF: cerebrospinal fluid; DEXA: dual energy X‐ray; ECG: electrocardiogram; FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; GMFM: Gross Motor Function Measure; HFMS(E): Hammersmith Functional Motor Scale (Expanded); ITT: intention‐to‐treat; MFM: Motor Function Measure; MHFMS: Modified Hammersmith Functional Motor Scale; mini‐SIP: mini‐Sickness Impact Profile; MIP: maximum inspiratory pressure; MMT: manual muscle testing; mRNA: messenger ribonucleic acid; MUNE: motor unit number estimation; PedsQL: Pediatric Quality of Life Inventory; QMT: quantitative muscle testing; SMA: spinal muscular atrophy; SMAFRS: Spinal Muscular Atrophy Functional Rating Scale; SMN: survival motor neuron.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abbara 2011 | Riluzole. Non‐randomised, open‐label study to assess pharmacokinetics of riluzole in people with SMA types II and III. |
| Brahe 2005 | Phenylbutyrate. Not randomised, not controlled. Pilot trial. Study on the effect of phenylbutyrate on human SMN expression in blood. |
| Brichta 2006 | Valproic acid. Not randomised, not controlled. Pilot trial. Study on the effect of valproic acid on human SMN expression in blood. |
| Chang 2002 | Hydroxyurea. Not randomised, not controlled. Pilot trial. Study on the effect of hydroxyurea on clinical manifestations and human SMN expression in blood. |
| Chiriboga 2016 | Nusinersen. Phase 1, 4 groups, dose‐escalating trial. Not randomised, not controlled. |
| Darbar 2011 | Valproic acid. Open‐label trial, not controlled. No placebo given. |
| EMOTAS 2014 | Pyridostigmine. Open‐label trial. Not randomised, not controlled. Study ongoing, but can be excluded. |
| Folkers 1995 | Coenzyme Q10. Not randomised, not controlled. Observational study that included 1 participant with SMA type III/IV. |
| Giovannetti 2016 | Salbutamol. No placebo given. |
| JEWELFISH 2017 | RG7916 or RO7034067. Open‐label trial, no placebo given. Study ongoing, but can be excluded. |
| JPRN‐JapicCTI‐163450 2016 | Sodium valproate. Open‐label trial, no placebo given. Study ongoing, but can be excluded. |
| Kato 2009 | Thyrotropin. Case report. |
| Khirani 2017 | Salbutamol. Not randomised, not controlled. Pilot trial. |
| Kinali 2002 | Albuterol. Not randomised, not controlled. Pilot trial. |
| Kissel 2011 | Valproic acid. Open‐label trial, not controlled. |
| Liang 2008 | Hydroxyurea. Not controlled. |
| Mercuri 2004 | Phenylbutyrate. Not randomised, not controlled. Pilot trial. |
| Merlini 2003 | Gabapentin. Not controlled (no placebo given, compared treatment with no treatment, not blinded). |
| Nascimento 2010 | Lamotrigine. Case series. Not controlled, not randomised. |
| NCT01703988 | Nusinersen. Open‐label study. Study completed; results pending. |
| NCT02052791 | Nusinersen. open‐label. Study completed; results pending. |
| NCT02876094 | Celecoxib. Open‐label, non‐randomised study. Primary outcome SMN protein level, no clinical scores or evaluation. Study ongoing, but can be excluded. |
| NCT03709784 | Nusinersen. Observational study in adults treated with nusinersen. Study ongoing, but can be excluded. |
| NPTUNE01 2007 | Sodium phenylbutyrate. Dose‐escalating study. Non‐randomised, not controlled. No placebo given. Trial terminated due to poor compliance with study drug administration. |
| OLEOS | Olesoxime. Not randomised, not controlled. No placebo given. Only people who participated in previous trials (TRO19622CLEQ11150‐1 or TRO19622CLEQ1275‐1) to be included. Study ongoing, but can be excluded. |
| Pane 2008 | Salbutamol. Not randomised, not controlled. Pilot trial. |
| Piepers 2011 | Valproic acid. Not controlled, not randomised. Case series. |
| Prufer de Queiroz Campos Araujo 2010 | Salbutamol. Pilot trial. Not controlled (no placebo given), not randomised. |
| Saito 2014 | Valproic acid. Case series. Not controlled (no placebo given), not randomised. |
| SHINE 2015 | Nusinersen. Open‐label, not randomised. No placebo or sham‐procedure given. Study ongoing, but can be excluded. |
| SMART01 | Valproic acid. Open‐label trial. Not randomised, not controlled (no placebo given). Study completed, no published data yet. |
| SMART03 | Valproic acid. Open‐label trial. Not randomised, not controlled (no placebo given). Study recruiting participants, but can be excluded. |
| Swoboda 2009 | Valproic acid and carnitine. Not controlled (no placebo given). Open‐label trial. |
| Tan 2011 | Salbutamol. Not randomised, not controlled. Case series. |
| Tsai 2007 | Valproic acid. Not randomised, not controlled. |
| Weihl 2006 | Valproic acid. Not randomised, not controlled. Retrospective study on people with SMA types III and IV. |
SMA: spinal muscular atrophy; SMN: survival motor neuron.
Characteristics of studies awaiting assessment [ordered by study ID]
ASIRI 2008.
| Methods | Randomised, placebo‐controlled, double‐blind trial |
| Participants | People with SMA types II or III, aged 6–20 years. |
| Interventions | Riluzole 50 mg/day orally or placebo Duration of treatment: 24 months Follow‐up: 24 months |
| Outcomes | Motor function (MFM scale), spirometry (FVC), Measure of Functional Independence, adverse events and tolerance evaluation. |
| Notes | Study completed (December 2011), but no results available. |
CHICTR‐TRC‐10001093.
| Methods | Randomised, parallel, controlled trial. Placebo was mentioned, but unknown if the trial was placebo‐controlled. |
| Participants | 40 participants with SMA type II, aged 3–8 years |
| Interventions | Rat nerve growth factor injection for 6 months |
| Outcomes | Change in Pediatric Evaluation of Disability Inventory, change in electromyogram. |
| Notes | Study completed, but no results available. Unknown date of completion; last update received April 2017 on WHO International Clinical Trials Registry Platform. |
Merlini 2007.
| Methods | Randomised, placebo‐controlled, double‐blind trial |
| Participants | 110 participants with SMA types II or III, aged ≥ 4 years |
| Interventions | Acetyl‐L‐carnitine 50 mg/kg/day (maximum 3 g/day) (route not mentioned) or placebo Duration of treatment: 9 months Follow‐up: 12 months |
| Outcomes | Change in muscle strength arm and leg (myometry), change in functional score (time to walk 10 m and to arise from floor), change in pulmonary function (FVC), change in quality of life (Short Form‐36 Health Survey questionnaire (SF‐36) or Childhood Health Assessment Questionnaire. |
| Notes | Study status unknown (last report on trial notified in 2007). No results available |
MOONFISH 2014.
| Methods | Phase I, randomised, double‐blind, placebo‐controlled trial |
| Participants | 64 participants with SMA types I, II or III, aged 2–55 years or < 7 months |
| Interventions | RO6885247 orally once daily for 12 weeks or placebo orally once daily for 12 weeks |
| Outcomes | Safety (incidence of adverse events), pharmacokinetics (plasma concentrations of RO6885247 and RO6885247 exposure), pharmacodynamics (SMN protein levels in blood and in vivo splicing of SMN2 mRNA in blood), effect on CMAP, effect on electrical impedance myography. |
| Notes | Recruitment of participants suspended since April 2015 for safety reasons. In parallel to the MOONFISH trial, Hoffmann‐La Roche have been investigating the effects of the long‐term use of RG7800 in animals. These animal studies are a standard requirement in the development of new medicines. In this study, they observed an unexpected safety finding in the eye of animals and subsequently immediately suspended dosing in the MOONFISH trial as a precautionary measure. Trial terminated in July 2015. No results available at time of writing. |
Morandi 2013.
| Methods | Randomised, placebo‐controlled, double‐blind trial |
| Participants | 45 adults with SMA type III |
| Interventions | Salbutamol or identical placebo tablets at the same dosing schedule |
| Outcomes | Change in manual muscle testing, North Star Ambulatory Assessment scale, 6MWT and FVC. Molecular analyses included SMN2 gene copy number, SMN2 transcript and SMN protein levels. |
| Notes | Study completed, but results are awaiting. Unknown date of completion; last update received March 2012 on WHO International Clinical trials Registry Platform. |
NCT00568802.
| Methods | Randomised, placebo‐controlled, double‐blind trial |
| Participants | Participants with SMA types II and III, aged 1–10 years |
| Interventions | Hydroxyurea (dose and route) or placebo Duration of treatment: not mentioned |
| Outcomes | Motor function (Gross Motor Function Measure and timed motor tests), adverse events, pulmonary function, motor unit number estimation, SMN protein and SMN mRNA |
| Notes | Study completed, not enough data or results available for analysis or inclusion (or both). Unknown date of completion, last date received 2008. |
NCT01645787.
| Methods | Randomised, double‐blind, placebo‐controlled cross‐over trial |
| Participants | 12 participants with SMA type III, aged 18–50 years |
| Interventions | 4‐AP 10 mg twice daily or placebo twice daily Short‐term treatment trial in which participants are treated for 2 weeks with 4‐AP and placebo in random sequence followed by a long treatment trial of 6 weeks in which participants are also treated with placebo and 4‐AP. |
| Outcomes | 6MWT, Hammersmith Functional Motor Scale Expanded, manual muscle testing, change in motor unit estimation and nerve conduction studies |
| Notes | Study completed (September 2015), but no results available yet. |
NCT02644668.
| Methods | Phase II, randomised, double‐blind, placebo‐controlled, 2 dose cohorts |
| Participants | 72 participants, aged ≥ 12 years with genetically confirmed diagnosis of SMA and clinically SMA types II, III or IV |
| Interventions | Cohort 1: 36 participants with SMA type II, III or IV randomised 2:1 to CK‐2127107 150 mg or placebo. Cohort 2: 36 participants with SMA type II, III or IV randomised 2:1 to CK‐2127107 450 mg (or lower) or placebo. Both cohorts were also divided into 18 ambulatory versus 18 non‐ambulatory participants. |
| Outcomes | Change from baseline and slope of change from baseline in FVC, MIP, MEP, HHD, HFMSE, RULM, TUG, 6MWT, and safety and tolerability measurements |
| Notes | Study completed (May 2018), but no results are available yet. |
SPACE.
| Methods | Phase II, randomised, double‐blind, placebo‐controlled cross‐over trial |
| Participants | 45 participants with SMA types II, III and IV, aged ≥ 12 years |
| Interventions | Oral pyridostigmine (days 1–3: 2 mg/kg/day over 4 doses per day; days 4–7: 4 mg/kg/day over 4 doses per day; weeks 2–8: 6 mg/kg/day over 5 doses per day) or oral placebo (days 1–3: 2 mg/kg/day over 4 doses per day; days 4–7: 4 mg/kg/day over 4 doses per day; weeks 2–8: 6 mg/kg/day over 5 doses per day). Cross‐over took place after 8 weeks with 1 week of washout. |
| Outcomes | Change in time to complete repeated 9 hole peg test, change in MFM scores, change in time to complete shuttle walk test, time to complete shuttle box and block test, change in pulmonary function (FVC), change in SMAFRS, change in PedsQL, fatigue and fatigability questionnaires, VAS scores, change in CMAP, and change in decremental response during repetitive nerve stimulation. |
| Notes | Study completed (January 2018), but no results are available yet. |
4‐AP: 4‐aminopyridine; 6MWT: 6‐minute walk test; CMAP: compound muscle action potential; FVC: forced vital capacity; HFMS(E): Hammersmith Functional Motor Scale (Expanded); HHD: hand‐held dynamometry; MFM: Motor Function Measure; MEP: maximum expiratory pressure; MIP: maximum inspiratory pressure; PedsQL: Pediatric Quality of Life Inventory; RULM: Revised Upper Limb Module; TUG: Time Up and Go; RULM; Revised Upper Limb Module; SMA: spinal muscular atrophy; SMN: survival motor neuron; SMAFRS: Spinal Muscular Atrophy Functional Rating Scale; TUG: Timed Up and Go; VAS: visual analogue scale; WHO: World Health Organization.
Characteristics of ongoing studies [ordered by study ID]
EMBRACE 2015.
| Trial name or title | A phase II, randomised, double‐blind, sham‐procedure controlled study to assess the safety and tolerability and explore the efficacy of IONIS 396443 (BIIB058) administered intrathecally in subjects with spinal muscular atrophy who are not eligible to participate in the clinical studies IONIS 396443‐CS3B or IONIS 396443‐CS4 |
| Methods | Phase II, randomised, double‐blind, sham‐procedure‐controlled study |
| Participants | 21 participants with genetically confirmed SMA with onset of clinical signs and symptoms consistent with SMA at ≤ 6 months of age and have documentation of 3 SMN2 copies OR onset of clinical signs and symptoms consistent with SMA at ≤ 6 months of age, > 7 months of age (211 days) at screening, and have documentation of 2 SMN2 copies OR onset of clinical signs and symptoms consistent with SMA at > 6 months of age, are ≤ 18 months of age at screening, and have documentation of 2 or 3 SMN2 copies |
| Interventions | Multiple intrathecal injection of nusinersen (also called IONIS‐SMN Rx or IONIS 396443) or multiple sham procedure with placebo |
| Outcomes | Number of adverse events and serious adverse events, change from baseline in clinical laboratory parameters, change from baseline in electrocardiogram, change from baseline in vital signs, change from baseline in neurological examination including motor function, change in plasma concentration of nusinersen and change in cerebrospinal fluid concentration of nusinersen |
| Starting date | June 2015 |
| Contact information | Biogen |
| Notes | Study ongoing, but not recruiting participants. |
NCT01671384.
| Trial name or title | Randomised placebo‐controlled trial of valproic acid and levocarnitine in children with spinal muscular atrophy aged 2–15 years |
| Methods | Randomised, double‐blind, placebo‐controlled trial |
| Participants | 60 participants with SMA types II or III, aged 2–15 years at time of inclusion Diagnosis of SMA has been made by the presence of exon7 deletion of SMNT gene OR by normal/mildly elevated creatine phosphokinase with electrodiagnostic characteristics suggestive of neurogenic weakness, normal motor and sensory nerve conduction velocities and muscle biopsy showing neurogenic atrophy or evidence of reinnervation (or both) |
| Interventions | Valproic acid (dose unknown) and levocarnitine 50 mg/kg/day (maximum 1000 mg) divided over 2 doses combined with physiotherapy or placebo combined with physiotherapy |
| Outcomes | Change in muscle strength (MMT on a 5‐point scale), change in modified HFMS, change in FVC, adverse effects, changes in haematology, liver function and valproic acid levels |
| Starting date | August 2013 Estimated completion date December 2016 |
| Contact information | G Sheffali, MD, Additional Professor Department of Pediatrics, All India Institute of Medical Sciences, New Delhi, India |
| Notes | Study ongoing and recruiting participants |
SMART02.
| Trial name or title | Multicenter cooperative and investigator initiated clinical trial using valproic acid in childhood onset spinal muscular atrophy: confirmatory trial (SMART02) |
| Methods | Phase IIB, randomised, double‐blind, placebo‐controlled trial |
| Participants | 28 participants with SMA types I and II, aged 1–7 years |
| Interventions | Oral valproic acid or placebo, 12.5 mg/kg or 25 mg/kg once a day after supper. Treatment period: 40 weeks |
| Outcomes | HFMSE, HFMS, motor function, WHO motor milestones |
| Starting date | January 2016 |
| Contact information | Kayoko Saito, Institute of Medical Genetics, Tokyo Women's Medical University, Japan |
| Notes | Study ongoing and recruiting participants |
SUNFISH 2016.
| Trial name or title | A study to Investigate the safety, tolerability, pharmacokinetics, pharmacodynamics and efficacy of RO7034067 in type 2 and 3 spinal muscular atrophy (SMA) participants (SUNFISH) |
| Methods | Randomised, placebo‐controlled, double‐blind trial |
| Participants | 186 participants with SMA types II and III, aged 2–25 years old with a confirmed diagnosis of 5q‐autosomal‐recessive SMA |
| Interventions | Participants start with treatment in part 1 of the study: Part 1A includes adolescents and adults (aged 12–25 years) with SMA type II or III, ambulatory or non‐ambulatory, receiving 12 weeks of placebo or RO7034067 Part 1B: children with SMA type II or III (aged 2–11 years), ambulatory or non‐ambulatory, receiving 12 weeks of placebo or RO7034067 After 12 weeks of treatment, participants can participate in part 2 of the trial if they meet inclusion criteria. Part 2: participants aged 2–25 years with SMA type II or III, non‐ambulatory with RULM entry item 1A ≥ 2 who have the ability to sit independently as assessed by item 9 of the MFM. 1 group will start with 12 months of placebo and will switch to treatment with RO7034067. 1 group will be treated for 24 months with RO7034067. All participants in part 2 will be offered the opportunity to enter an open‐label extended phase after completing part 2. |
| Outcomes | Safety (incidence of adverse events), pharmacokinetics (plasma concentrations of RO6885247 and RO6885247 exposure), pharmacodynamics (SMN protein levels in blood and in vivo splicing of SMN2 mRNA in blood), change from baseline in motor scores (MFM‐32, HFMSE, RULM), change from baseline in pulmonary function (FVC, SNIP, FEV1, peak cough flow), change from baseline in quality of life (PedsQL) and (severe) adverse events |
| Starting date | October 2016 |
| Contact information | Hoffmann‐La Roche |
| Notes | Study ongoing and recruiting participants |
FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; HFMS(E): Hammersmith Functional Motor Scale (Expanded); MFM: Motor Function Measure; MMT: manual muscle testing; mRNA: messenger ribonucleic acid; PedsQL: Pediatric Quality of Life Inventory; RULUM: Revised Upper Limb Module; SNIP: sniff nasal inspiratory pressure; RULM; Revised Upper Limb Module; SMA: spinal muscular atrophy; WHO: World Health Organization.
Differences between protocol and review
The 'Risk of bias' methodology was revised in the first update according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We expanded the methods according to current standards in the 2017 update, as meta‐analysis may be possible in future updates. We included 'Summary of findings' tables. We did not consider use of explicit inclusion and exclusion criteria or explicit outcome criteria as part of our 'Risk of bias' analysis as these considerations relate to indirectness of evidence, which is one of the GRADE criteria. We considered baseline imbalance in relation to allocation bias.
The search strategy was adjusted. Searches were performed from 1991 onwards because at that time genetic analysis of the SMN1 gene became widely available and could be used to establish the diagnosis of SMA.
We adjusted the definition on SMA types II and III in Table 11 and added the highest achieved motor milestones (sitting, walking) as discriminators of the SMA types II and III. This was not stated in table 1 of original protocol and the update of 2011, although it was mentioned in the main text.
Change in forced vital capacity (FVC), as a percentage of FVC predicted for height, was added as a secondary outcome measure. This was not stated in the original protocol but many trials used this as a measure of pulmonary function or the strength of respiratory muscles. Transformation of disability scores into Medical Research Council scores has been removed in the update of 2019, since the transformation is not possible for the included study population and its clinical scores.
The 'Adverse events' section was revised and we did not discuss the adverse events from the included trials in relation to the adverse effects of drug treatment in non‐randomised literature. We have outlined the most common adverse events of treatments in the 'Summary of findings' tables (see Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 10; Table 9).
RW joined the review for the 2011 update and FA joined this update. At the 2019 update, J Wokke retired from authorship.
We added some additional information to the methods to update them to current reporting standards and specify methods for any future meta‐analysis We:
clarified that we did not use outcomes for study selection;
searched clinical trials registries to identify unpublished and ongoing studies;
added a PRISMA flow chart to illustrate the study selection process;
specified methods for use if studies with multiple eligible intervention arms are identified;
specified rules of thumb levels for assessment of heterogeneity from Higgins 2011;
specified use of random‐effects model with a sensitivity analysis using a fixed‐effect analysis, should meta‐analysis be possible;
stated that we would use standardised mean difference if different scales were used to measure the same construct;
included 'Summary of findings' tables and the GRADE analysis according to Cochrane requirements.
Contributions of authors
All authors contributed substantially to the concept and design of the review.
WB and AV performed data extraction and analyses for the original review in 2009.
WB wrote the first draft of the original review and the other co‐authors contributed to subsequent revisions for important intellectual content.
RW, AV and WP updated the review in 2011 and 2019 and the other authors approved the revisions.
Sources of support
Internal sources
University Medical Center Utrecht, Department of Neurology and Neuromuscular diseases, Utrecht, Netherlands.
University Medical Center Utrecht, Department of Neurology, Utrecht, Netherlands.
University Medical Center Utrecht, Department of Biostatics and Clinical Epidemiology, Utrecht, Netherlands.
Cochrane Neuromuscular Disease Group, King's College London School of Medicine, London, UK.
University of Texas Southwestern Medical Center, Department of Pediatrics, Dallas, Texas, USA.
Onze Lieve Vrouwe Gasthuis West, Department of Neurology, Amsterdam, Netherlands.
External sources
No sources of support supplied
Declarations of interest
RW: was involved as investigator at a participating centre in the trials on the safety and efficacy of cholest‐4‐en‐3‐one, oxime for children with SMA types II and IIIa (Bertini 2017; OLEOS), and is involved as an investigator in the monocentre placebo‐controlled trial of pyridostigmine in children and adults with SMA types II to IV (SPACE). She does not receive any funding from the pharmaceutical industry.
WP: receives research support from the Prinses Beatrix Spierfonds, Stichting Spieren voor Spieren, Netherlands ALS foundation. His employer receives fees for consultancy services to Biogen, Avexis and Novartis. WP was an investigator at a participating centre in the trials on the safety and efficacy of cholest‐4‐en‐3‐one, oxime for children with SMA types II and IIIa (Bertini 2017; OLEOS), and is an investigator in the monocentre placebo‐controlled trial of pyridostigmine in children and adults with SMA types II to IV (SPACE).
WB: none.
FA: none.
LB: serves on scientific advisory boards for the Prinses Beatrix Spierfonds, Thierry Latran Foundation, Biogen Idec and Cytokinetics; received an educational grant from Baxter International Inc; serves on the editorial board of Amyotrophic Lateral Sclerosis and the Journal of Neurology, Neurosurgery and Psychiatry; and receives research support from the Prinses Beatrix Fonds, Netherlands ALS Foundation, The European Community's Health Seventh Framework Programme (grant agreement number 259867), and The Netherlands Organization for Health Research and Development (Vici Scheme, JPND (SOPHIA, STRENGTH)). LB was an investigator at a participating centre in the trials on the safety and efficacy of cholest‐4‐en‐3‐one, oxime for children with SMA types II and IIIa (Bertini 2017; OLEOS) and is an investigator of the monocentre placebo‐controlled trial on pyridostigmine in children and adults with SMA types II to IV (SPACE).
SI: Dr Iannaccone was involved in the trial of riluzole as one of the investigators and authors (Russman 2003). She was involved in a trial of the efficacy of creatine for children with spinal muscular atrophy types II and III as investigator and author (Wong 2007) and she was involved in a trial of the efficacy of riluzole (not published). She has received support for research from AveXis, Biogen and Scholar Rock for clinical trials in SMA patients and from Sarepta, Reveragen, Mallinckrodt, Fibrogen, and PTC Therapeutics for clinical trials in muscular dystrophy. She has been a consultant for AveXis, Biogen, Sarepta, Audentes, Catabasis and Genentech/Roche.
AV: none known. He was involved as investigators at a participating centre in the trials on the safety and efficacy of cholest‐4‐en‐3‐one, oxime for children with SMA types II and IIIa (Bertini 2017; OLEOS), and is an investigator in the monocentre placebo‐controlled trial of pyridostigmine in children and adults with SMA types II to IV (SPACE).
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Bertini 2017 {published data only}
- Bertini E, Dessaud E, Mercuri E, Muntoni F, Kirschner J, Reid C, et al. Safety and efficacy of olesoxime in patients with type 2 or non‐ambulatory type 3 spinal muscular atrophy: a randomised, double‐blind, placebo‐controlled phase 2 trial. Lancet Neurology 2017;16(7):513‐22. Corrections in Lancet Neurology 2017; 16(8):584. [DOI: 10.1016/S1474-4422(17)30085-6; 3134415] [DOI] [PubMed] [Google Scholar]
- NCT01302600. Safety and efficacy of olesoxime (TRO19622) in 3‐25 years SMA patients. clinicaltrials.gov/ct2/show/NCT01302600 (first received 24 February 2011).
Chen 2010 {published data only}
- Chen TH, Chang JG, Yang YH, Mai HH, Liang WC, Wu YC, et al. Randomized, double‐blind, placebo‐controlled trial of hydroxyurea in spinal muscular atrophy. Neurology 2010;75(24):2190‐7. [PUBMED: 21172842] [DOI] [PubMed] [Google Scholar]
- NCT00485511. A trial of hydroxyurea in spinal muscular atrophy. clinicaltrials.gov/ct2/show/NCT00485511 (first received 13 June 2007).
Kirschner 2014 {published data only}
- Kirschner J, Schorling D, Hauschke D, Rensing‐Zimmermann C, Wein U, Grieben U, et al. Somatropin treatment of spinal muscular atrophy: a placebo‐controlled, double‐blind crossover pilot study. Neuromuscular Disorders 2014;24(2):134‐42. [PUBMED: 24300782] [DOI] [PubMed] [Google Scholar]
- NCT00533221. Pilot study of growth hormone to treat SMA type II and III. clinicaltrials.gov/ct2/show/NCT00533221 (first received 21 September 2007).
Kissel 2014 {published data only}
- Kissel JT, Elsheikh B, King WM, Freimer M, Scott CB, Kolb SJ, et al. SMA valiant trial: a prospective, double‐blind, placebo‐controlled trial of valproic acid in ambulatory adults with spinal muscular atrophy. Muscle & Nerve 2014;49(2):187‐92. [PUBMED: 23681940] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00481013. Valproic acid in ambulant adults with spinal muscular atrophy (VALIANTSMA). clinicaltrials.gov/ct2/show/NCT00481013.
Mercuri 2007 {published data only}
- Mercuri E, Bertini E, Messina S, Solari A, D'Amico A, Angelozzi C, et al. Randomized, double‐blind, placebo‐controlled trial of phenylbutyrate in spinal muscular atrophy. Neurology 2007;68(1):51‐5. [PUBMED: 17082463] [DOI] [PubMed] [Google Scholar]
Mercuri 2018 (CHERISH) {published data only}
- Mercuri E, Darras BT, Chiriboga CA, Day JW, Campbell C, Connolly AM, et al. Nusinersen versus sham control in later‐onset spinal muscular atrophy. New England Journal of Medicine 2018;378(7):625‐35. [IONIS 396443‐CS4] [DOI] [PubMed] [Google Scholar]
- NCT02292537. A study to assess the efficacy and safety of nusinersen (ISIS 396443) in participants with later‐onset spinal muscular atrophy (SMA) (CHERISH). clinicaltrials.gov/ct2/show/NCT02292537 (first received 17 November 2014).
Miller 2001 {published data only}
- Miller RG, Moore DH, Dronsky V, Bradley W, Barohn R, Bryan W, et al. A placebo‐controlled trial of gabapentin in spinal muscular atrophy. Journal of the Neurological Sciences 2001;191(1‐2):127‐31. [PUBMED: 11677003] [DOI] [PubMed] [Google Scholar]
Swoboda 2010 {published data only}
- NCT00227266. Valproic acid and carnitine in patients with spinal muscular atrophy. clinicaltrials.gov/ct2/show/study/NCT00227266 (first received 27 September 2005).
- Swoboda KJ, Scott CB, Crawford TO, Simard LR, Reyna SP, Krosschell KJ, et al. SMA CARNI‐VAL trial part I: double‐blind, randomized, placebo‐controlled trial of L‐carnitine and valproic acid in spinal muscular atrophy. PloS One 2010;5(8):e12140. [PUBMED: 20808854] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tzeng 2000 {published data only}
- Tzeng AC, Cheng J, Fryczynski H, Niranjan V, Stitik T, Sial A, et al. A study of thyrotropin‐releasing hormone for the treatment of spinal muscular atrophy: a preliminary report. American Journal of Physical Medicine & Rehabilitation 2000;79(5):435‐40. [PUBMED: 10994885] [DOI] [PubMed] [Google Scholar]
Wong 2007 {published data only}
- Wong BL, Hynan LS, Iannaccone ST, AmSMART Group. A randomized, placebo‐controlled trial of creatine in children with spinal muscular atrophy. Journal of Clinical Neuromuscular Disease 2007;8(3):101‐10. [EMBASE: 46673514] [Google Scholar]
References to studies excluded from this review
Abbara 2011 {published data only}
- Abbara C, Estournet B, Lacomblez L, Lelièvre B, Ouslimani A, Lehmann B, et al. Riluzole pharmacokinetics in young patients with spinal muscular atrophy. British Journal of Clinical Pharmacology 2011;71(3):403‐10. [PUBMED: 21284699] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brahe 2005 {published data only}
- Brahe C, Vitali T, Tiziano FD, Angelozzi C, Pinto AM, Borgo F, et al. Phenylbutyrate increases SMN gene expression in spinal muscular atrophy patients. European Journal of Human Genetics 2005;13(2):256‐9. [PUBMED: 15523494] [DOI] [PubMed] [Google Scholar]
Brichta 2006 {published data only}
- Brichta L, Holker I, Haug K, Klockgether T, Wirth B. In vivo activation of SMN in spinal muscular atrophy carriers and patients treated with valproate. Annals of Neurology 2006;59(6):970‐5. [PUBMED: 16607616] [DOI] [PubMed] [Google Scholar]
Chang 2002 {published data only}
- Chang JG, Tsai FJ, Wang WY, Jong YJ. Treatment of spinal muscular atrophy by hydroxyurea. American Journal of Human Genetics 2002; Vol. 71 Suppl 4:2402.
Chiriboga 2016 {published data only}
- Chiriboga CA, Swoboda KJ, Darras BT, Iannaccone ST, Montes J, Vivo DC, et al. Results from a phase 1 study of nusinersen (ISIS‐SMNRx) in children with spinal muscular atrophy. Neurology 2016;86(10):890‐7. [ISIS 396443 ‐ CS1; ISIS 396443 ‐ CS10; NCT01494701; NCT01780246] [DOI] [PMC free article] [PubMed] [Google Scholar]
Darbar 2011 {published data only}
- Darbar IA, Plaggert PG, Resende MBD, Zanotelli E, Reed UC. Evaluation of muscle strength and motor abilities in children with type II and III spinal muscle atrophy treated with valproic acid. BMC Neurology 2011;11:36. [PUBMED: 21435220] [DOI] [PMC free article] [PubMed] [Google Scholar]
EMOTAS 2014 {unpublished data only}
- NCT02227823. Safety and efficacy study of pyridostigmine on patients with spinal muscular atrophy type 3. www.clinicaltrials.gov/show/NCT02227823 (first received 20 August 2014). [1376 ; NCT02227823]
Folkers 1995 {published data only}
- Folkers K, Simonsen R. Two successful double‐blind trials with coenzyme Q10 (vitamin Q10) on muscular dystrophies and neurogenic atrophies. Biochimica et Biophysica Acta 1995;1271(1):281‐6. [PUBMED: 7599221] [DOI] [PubMed] [Google Scholar]
Giovannetti 2016 {published data only}
- Giovannetti AM, Pasanisi MB, Černiauskaitė M, Bussolino C, Leonardi M, Morandi L. Perceived efficacy of salbutamol by persons with spinal muscular atrophy: a mixed methods study. Muscle & Nerve 2016;54(5):843‐9. [DOI] [PubMed] [Google Scholar]
- Pasanisi MB, Giovannetti AM, Bussolino C, Campanella A, Leonardi M, Morandi L. Perception of efficacy in adult patients affected by spinal muscular atrophy (SMA) treated with salbutamol. Neuromuscular Disorders 2014;24(9‐10):914. [Google Scholar]
JEWELFISH 2017 {unpublished data only}
- NCT03032172. A study of RO7034067 in adult and pediatric participants with spinal muscular atrophy (Jewelfish). clinicaltrials.gov/show/NCT03032172 (first received 24 January 2017).
JPRN‐JapicCTI‐163450 2016 {unpublished data only}
- JPRN‐JapicCTI‐163450. Phase 3 study of K‐828‐SP. apps.who.int/trialsearch/Trial2.aspx?TrialID=JPRN‐JapicCTI‐163450 (first received November 2016).
Kato 2009 {published data only}
- Kato Z, Okuda M, Okumura Y, Arai T, Teramoto T, Nishimura M, et al. Oral administration of the thyrotropin‐releasing hormone (TRH) analogue, taltireline hydrate, in spinal muscular atrophy. Journal of Child Neurology 2009;24(8):1010‐2. [PUBMED: 19666885] [DOI] [PubMed] [Google Scholar]
Khirani 2017 {published data only}
- Khirani S, Dabaj I, Amaddeo A, Olmo Arroyo J, Ropers J, Tirolien S, et al. Effect of salbutamol on respiratory muscle strength in spinal muscular atrophy. Pediatric Neurology 2017;73:78‐87. [DOI] [PubMed] [Google Scholar]
Kinali 2002 {published data only}
- Kinali M, Mercuri E, Main M, Biasia F, Karatza A, Higgins R, et al. Pilot trial of albuterol in spinal muscular atrophy. Neurology 2002;59(4):609‐10. [PUBMED: 12196659] [DOI] [PubMed] [Google Scholar]
Kissel 2011 {published data only}
- Kissel JT, Scott CB, Reyna SP, Crawford TO, Simard LR, Krosschell KJ, et al. SMA CARNIVAL trial part II: a prospective, single‐armed trial of L‐carnitine and valproic acid in ambulatory children with spinal muscular atrophy. PloS One 2011;6(7):e21296. [PUBMED: 21754985] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liang 2008 {published data only}
- Liang WC, Yuo CY, Chang JG, Chen YC, Chang YF, Wang HY, et al. The effect of hydroxyurea in spinal muscular atrophy cells and patients. Journal of the Neurological Sciences 2008;268(1‐2):87‐94. [PUBMED: 18166199] [DOI] [PubMed] [Google Scholar]
Mercuri 2004 {published data only}
- Mercuri E, Bertini E, Messina S, Pelliccioni M, D'Amico A, Colitto F, et al. Pilot trial of phenylbutyrate in spinal muscular atrophy. Neuromuscular Disorders 2004;14(2):130‐5. [PUBMED: 14733959] [DOI] [PubMed] [Google Scholar]
Merlini 2003 {published data only}
- Merlini L, Solari A, Vita G, Bertini E, Minetti C, Mongini T, et al. Role of gabapentin in spinal muscular atrophy: results of a multicenter, randomized Italian study. Journal of Child Neurology 2003;18(8):537‐41. [PUBMED: 13677579] [DOI] [PubMed] [Google Scholar]
Nascimento 2010 {published data only}
- Nascimento OJ, Orsini M, Quintanilha G. Lamotrigine on motor symptoms of spinal muscular atrophies. Revista de Neurologia 2010;50(2):127‐8. [PUBMED: 20112222] [PubMed] [Google Scholar]
NCT01703988 {unpublished data only}
- Darras BT, Chiriboga C, Swoboda K, Iannaccone S, Montes J, Rausch N, et al. Results of a first‐in‐human phase I study to assess the safety, tolerability, and dose range finding of a single intrathecal dose of ISIS‐SMNRx in patients with spinal muscular atrophy. Annals of Neurology 2013;74 Suppl 17:S128. [Google Scholar]
- NCT01703988. An open‐label safety, tolerability and dose‐range finding study of multiple doses of ISIS SMNRx in patients with spinal muscular atrophy (SMNRx ‐ CS2). clinicaltrials.gov/show/NCT01703988 (first received 8 October 2012). [IONIS SMNRx‐CS2 ; NCT01703988]
NCT02052791 {unpublished data only}
- NCT02052791. An open‐label safety and tolerability study of IONIS SMNRx in patients with spinal muscular atrophy who previously participated in IONIS SMNRx‐CS2 or IONIS SMNRx‐CS10. www.clinicaltrials.gov/show/NCT02052791 (first received 30 January 2014). [IONIS SMNRx‐CS12 ; ISIS 396443‐CS12 ; NCT02052791]
NCT02876094 {unpublished data only}
- NCT02876094. Effect of low‐dose celecoxib on SMN2 in patients with spinal muscular atrophy (SMA). www.clinicaltrials.gov/show/NCT02876094 (first received 9 August 2016). [NCT02876094]
NCT03709784 {unpublished data only}
- NCT03709784. Spinraza in adult spinal muscular atrophy (SAS). clinicaltrials.gov/ct2/show/NCT03709784 (first received 17 October 2018).
NPTUNE01 2007 {unpublished data only}
- NCT00439569. Clinical trial of sodium phenylbutyrate in children with spinal muscular atrophy types II or III. clinicaltrials.gov/show/NCT00439569 (first received 21 February 2007). [HHSN265200423611C; N01NS42361_NPTUNE01; NCT00439569; NPTUNE01]
OLEOS {unpublished data only}
- NCT02628743. A study to evaluate long term safety, tolerability, and effectiveness of olesoxime in patients with spinal muscular atrophy. clinicaltrials.gov/show/NCT02628743 (first received 1 December 2015). [2015‐001589‐25; BN29854 ; EUCTR2015‐001589‐25‐GB; NCT02628743]
Pane 2008 {published data only}
- Pane M, Staccioli S, Messina S, D'Amico A, Pelliccioni M, Mazzone ES, et al. Daily salbutamol in young patients with SMA type II. Neuromuscular Disorders 2008;18(7):536‐40. [PUBMED: 18579379] [DOI] [PubMed] [Google Scholar]
Piepers 2011 {published data only}
- Piepers S, Cobben JM, Sodaar P, Jansen MD, Wadman RI, Meester‐Delver A, et al. Quantification of SMN protein in leucocytes from spinal muscular atrophy patients: effects of treatment with valproic acid. Journal of Neurology, Neurosurgery, and Psychiatry 2011;82(8):850‐2. [PUBMED: 20551479] [DOI] [PubMed] [Google Scholar]
Prufer de Queiroz Campos Araujo 2010 {published data only}
- Prufer de Queiroz Campos Araujo A. Long‐term open salbutamol trial in spinal muscular atrophy. Journal of Neurology 2010;257 (Suppl 1):S101. [PUBMED: 20495927]20495927 [Google Scholar]
Saito 2014 {published data only}
- Saito T, Nurputra DK, Harahap NI, Harahap IS, Yamamoto H, Muneshige E, et al. A study of valproic acid for patients with spinal muscular atrophy. Neurology and Clinical Neuroscience 2014;3:49‐57. [Google Scholar]
SHINE 2015 {unpublished data only}
- NCT02594124. An open‐label study (SHINE) for patients with spinal muscular atrophy (SMA) who participated in studies with IONIS‐SMNRx. clinicaltrials.gov/show/NCT02594124 (first received 30 October 2015). [EUCTR2015‐001870‐16‐DE; IONIS SMNRx‐CS11; ISIS 396443‐CS11 ; NCT02594124]
SMART01 {unpublished data only}
- SMART01. Multicenter cooperative and investigator initiated clinical trial using valproic acid in childhood onset spinal muscular atrophy. dbcentre3.jmacct.med.or.jp/jmactr/App/JMACTRE02_04/JMACTRE02_04.aspx?kbn=3&seqno=5544 (first received August 2014). [JPRN‐SMA‐IIA00190]
SMART03 {unpublished data only}
- SMART03. Multicenter cooperative and investigator initiated clinical trial using valproic acid in childhood onset spinal muscular atrophy: continuous administration trial. dbcentre3.jmacct.med.or.jp/jmactr/App/JMACTRE02_04/JMACTRE02_04.aspx?kbn=3&seqno=4602 (first received August 2016).
Swoboda 2009 {published data only}
- Swoboda KJ, Scott CB, Reyna SP, Prior TW, LaSalle B, Sorenson SL, et al. Phase II open label study of valproic acid in spinal muscular atrophy. PloS One 2009;4(5):e5268. [PUBMED: 19440247] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tan 2011 {published data only}
- Tan C, Williams AN. Oral salbutamol in 2 wheelchair bound cases of SMA type II. Archives of Disease in Childhood 2011;96 Suppl 1:G77(P). [Google Scholar]
Tsai 2007 {published data only}
- Tsai LK, Yang CC, Hwu WL, Li H. Valproic acid treatment in six patients with spinal muscular atrophy. European Journal of Neurology 2007;14(12):e8‐9. [PUBMED: 18028187] [DOI] [PubMed] [Google Scholar]
Weihl 2006 {published data only}
- Weihl CC, Connolly AM, Pestronk A. Valproate may improve strength and function in patients with type III/IV spinal muscle atrophy. Neurology 2006;67(3):500‐1. [PUBMED: 16775228] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
ASIRI 2008 {unpublished data only}
- NCT00774423. Study to evaluate the efficacy of riluzole in children and young adults with spinal muscular atrophy (SMA) (ASIRI). clinicaltrials.gov/show/NCT00774423 (first received 16 October 2008). [NCT00774423; P040904]
CHICTR‐TRC‐10001093 {unpublished data only}
- CHICTR‐TRC‐10001093. Rat nerve growth factor injection in the treatment of children with spinal muscular atrophy: a randomized controlled trial. www.chictr.org.cn/showprojen.aspx?proj=8445 (first received 11 February 2010). [ChiCTR‐TRC‐10001093]
Merlini 2007 {published data only}
- Merlini L, Basoglu B, Dohna‐Schwake C, Febrer A, Hausmanova‐Petrusewicz I, Jedrzejowska M, et al. European spinal muscular atrophy RCT of acetyl‐L‐carnitine in SMA. Neuromuscular Disorders 2007;17:780‐1. [Google Scholar]
MOONFISH 2014 {unpublished data only}
- NCT02240355. A study of RO6885247 in adult and pediatric patients with spinal muscular atrophy (MOONFISH). clinicaltrials.gov/show/NCT02240355 (first received 11 September 2014). [2014‐002246‐41; BP29420 ; NCT02240355]
Morandi 2013 {published data only}
- Morandi L, Abiusi E, Pasanisi MB, Lomastro R, Fiori S, Pietro L, et al. Salbutamol tolerability and efficacy in adult type III SMA patients: results of a multicentric, molecular and clinical, double‐blind, placebo‐controlled study. Neuromuscular Disorders 2013;9(10):771. [Google Scholar]
- Tiziano FD, Lomastro R, Pietro L, Pasanisi MB, Fiori S, Angelozzi C, et al. Phase‐ll multicenter double‐blind, placebo‐controlled study of tolerability and efficacy of salbutamol in adult type III SMA patients. Acta Myologica 2012;31:97. [Google Scholar]
NCT00568802 {unpublished data only}
- NCT00568802. A pilot therapeutic trial using hydroxyurea in type II and type III spinal muscular atrophy patients. clinicaltrials.gov/show/NCT00568802 (first received 4 December 2007). [NCT00084006; NCT00568802; SU‐11012007‐781 79233]
NCT01645787 {unpublished data only}
- NCT01645787. Short and long term treatment with 4‐AP in ambulatory SMA patients. clinicaltrials.gov/show/NCT01645787 (first received 5 July 2012). [AAAI7400 ; NCT01645787]
NCT02644668 {unpublished data only}
- NCT02644668. A study of CK‐2127107 in patients with spinal muscular atrophy. clinicaltrials.gov/show/NCT02644668 (first received 23 December 2015). [CY 5021; NCT02644668]
- Rudnicki S, Andrews J, Malik F, Wolff A, Day J. CK‐2127107 a selective activator of the fast skeletal muscle troponin complex, for the potential treatment of spinal muscular atrophy. Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration 2016;17(Suppl 1):P261. [Google Scholar]
SPACE {unpublished data only}
- SPACE. SPACE trial SMA and pyridostigmine in adults and children; experimental trial to assess effect of pyridostigmine compared to placebo in patients with spinal muscular atrophy types 2, 3 and 4. apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2011‐004369‐34‐NL (first received October 2016). [EUCTR2011‐004369‐34‐NL]
References to ongoing studies
EMBRACE 2015 {published data only}
- NCT02462759. A study to assess the safety and tolerability of ISIS 396443 (IONIS SMNRx) in participants with spinal muscular atrophy (SMA) (EMBRACE). clinicaltrials.gov/show/NCT02462759 (first received 14 May 2015). [232SM202 ; EUCTR2014‐003657‐33‐DE; NCT02462759]
NCT01671384 {published data only}
- NCT01671384. Valproate and levocarnitine in children with spinal muscular atrophy. clinicaltrials.gov/show/NCT01671384 (first received 13 August 2012). [NCT01671384]
SMART02 {unpublished data only}
- SMART02. Multicenter cooperative and investigator initiated clinical trial using valproic acid in childhood onset spinal muscular atrophy: confirmatory trial. dbcentre3.jmacct.med.or.jp/JMACTR/App/JMACTRS06/JMACTRS06.aspx?seqno=5544 (first received 12 January 2016). [27‐3594; JPRN‐JMA‐IIA00231]
SUNFISH 2016 {unpublished data only}
- SUNFISH. A study to investigate the safety, tolerability, pharmacokinetics, pharmacodynamics and efficacy of RO7034067 in type 2 and 3 spinal muscular atrophy participants (Sunfish). clinicaltrials.gov/show/NCT02908685 (first received 19 September 2016).
Additional references
Abera 2016
- Abera MB, Xiao J, Nofziger J, Titus S, Southall N, Zheng W, et al. ML372 blocks SMN ubiquitination and improves spinal muscular atrophy pathology in mice. JCI Insight 2016;1(19):e88427. [DOI] [PMC free article] [PubMed] [Google Scholar]
Also‐Rallo 2011
- Also‐Rallo E, Alias L, Martinez‐Hernandez R, Caselles L, Barcelo MJ, Baiget M, et al. Treatment of spinal muscular atrophy cells with drugs that upregulate SMN expression reveals inter‐ and intra‐patient variability. European Journal of Human Genetics 2011;19:1059‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Anderton 2015
- Anderton RS, Mastaglia FL. Advances and challenges in developing a therapy for spinal muscular atrophy. Expert Review of Neurotherapeutics 2015;15(8):895‐908. [DOI] [PubMed] [Google Scholar]
Ando 2017
- Ando S, Funato M, Ohuchi K, Kameyama T, Inagaki S, Seki J, et al. Edaravone is a candidate agent for spinal muscular atrophy: in vitro analysis using a human induced pluripotent stem cells‐derived disease model. European Journal of Pharmacology 2017;814:161‐8. [DOI] [PubMed] [Google Scholar]
Andreassi 2004
- Andreassi C, Angelozzi C, Tiziano FD, Vitali T, Vincenzi E, Boninsegna A, et al. Phenylbutyrate increases SMN expression in vitro: relevance for treatment of spinal muscular atrophy. European Journal of Human Genetics 2004;12(1):59‐65. [DOI] [PubMed] [Google Scholar]
Andrews 2018
- Andrews JA, Miller TM, Vijayakumar V, Stoltz R, James JK, Meng L, et al. CK‐2127107 amplifies skeletal muscle response to nerve activation in humans. Muscle & Nerve 2018;57(5):729‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Angelini 1980
- Angelini C, Micaglio GF, Trevisan C. Guanidine hydrochloride in infantile and juvenile spinal muscular atrophy. A double blind controlled study. Acta Neurologica 1980;2(6):460‐5. [PUBMED: 7027754] [PubMed] [Google Scholar]
Angelozzi 2008
- Angelozzi C, Borgo F, Tiziano FD, Martella A, Neri G, Brahe C. Salbutamol increases SMN mRNA and protein levels in spinal muscular atrophy cells. Journal of Medical Genetics 2008;45(1):29‐31. [DOI] [PubMed] [Google Scholar]
Apfel 2001
- Apfel SC. Neurotrophic factor therapy – prospects and problems. Clinical Chemistry and Laboratory Medicine 2001;39(4):351‐5. [DOI] [PubMed] [Google Scholar]
Arkblad 2009
- Arkblad E, Tulinius M, Kroksmark AK, Henricsson M, Darin N. A population‐based study of genotypic and phenotypic variability in children with spinal muscular atrophy. Acta Paediatrica 2009;98(5):865‐72. [DOI] [PubMed] [Google Scholar]
Arnold 2004
- Arnold AS, Gueye M, Guettier‐Sigrist S, Courdier‐Fruh I, Coupin G, Poindron P, et al. Reduced expression of nicotinic AChRs in myotubes from spinal muscular atrophy I patients. Laboratory Investigation 2014;84(10):1271‐8. [DOI] [PubMed] [Google Scholar]
Arnold 2013
- Arnold WD, Burghes AH. Spinal muscular atrophy: the development and implementation of potential treatments. Annals of Neurology 2013;74(3):348‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Avila 2007
- Avila AM, Burnett BG, Taye AA, Gabanella F, Knight MA, Hartenstein P, et al. Trichostatin A increases SMN expression and survival in a mouse model of spinal muscular atrophy. Journal of Clinical Investigation 2007;117(3):659‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Azzouz 2004
- Azzouz M, Le T, Ralph GS, Walmsley L, Monani UR, Lee DC, et al. Lentivector‐mediated SMN replacement in a mouse model of spinal muscular atrophy. Journal of Clinical Investigation 2004;114(12):1726‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Benkhelifa‐Ziyyat 2013
- Benkhelifa‐Ziyyat S, Besse A, Roda M, Duque S, Astord S, Carcenac R, et al. Intramuscular scAAV9‐SMN injection mediates widespread gene delivery to the spinal cord and decreases disease severity in SMA mice. Molecular Therapy 2013;21(2):282‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bertini 2005
- Bertini E, Burghes A, Bushby K, Estournet‐Mathiaud B, Finkel RS, Hughes RA, et al. 134th ENMC International Workshop: outcome measures and treatment of spinal muscular atrophy, 11‐13 February 2005, Naarden, The Netherlands. Neuromuscular Disorders 2005;15(11):802‐16. [DOI] [PubMed] [Google Scholar]
Bessman 1981
- Bessman SP, Geiger PJ. Transport of energy in muscle: the phosphorylcreatine shuttle. Science 1981;211(4481):448‐52. [DOI] [PubMed] [Google Scholar]
Bezzi 1998
- Bezzi P, Carmignoto G, Pasti L, Vesce S, Rossi D, Rizzini BL, et al. Prostaglandins stimulate calcium‐dependent glutamate release in astrocytes. Nature 1998;391(6664):281‐5. [DOI] [PubMed] [Google Scholar]
Bigini 2002
- Bigini P, Larini S, Pasquali C, Muzio V, Mennini T. Acetyl‐L‐carnitine shows neuroprotective and neurotrophic activity in primary culture of rat embryo motoneurons. Neuroscience Letters 2002;329(3):334‐8. [DOI] [PubMed] [Google Scholar]
Biondi 2015
- Biondi O, Branchu J, Salah AB, Houdebine L, Bertin L, Chali F, et al. IGF‐1R Reduction triggers neuroprotective signaling pathways in spinal muscular atrophy mice. Journal of Neuroscience 2015;35(34):12063‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bladen 2014
- Bladen CL, Thompson R, Jackson JM, Garland C, Wegel C, Ambrosini A, et al. Mapping the differences in care for 5,000 spinal muscular atrophy patients, a survey of 24 national registries in North America, Australasia and Europe. Journal of Neurology 2014;261(1):152‐63. [PUBMED: 24162038] [DOI] [PubMed] [Google Scholar]
Bogdanik 2015
- Bogdanik LP, Osborne MA, Davis C, Martin WP, Austin A, Rigo F, et al. Systemic, postsymptomatic antisense oligonucleotide rescues motor unit maturation delay in a new mouse model for type II/III spinal muscular atrophy. Proceedings of the National Academy of Sciences of United States of America 2015;112(43):E5863‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bordet 2007
- Bordet T, Buisson B, Michaud M, Drouot C, Galéa P, Delaage P, et al. Identification and characterization of cholest‐4‐en‐3‐one, oxime (TRO19622), a novel drug candidate for amyotrophic lateral sclerosis. Journal of Pharmacology and Experimental Therapeutics 2007;322(2):709‐20. [DOI] [PubMed] [Google Scholar]
Bordet 2010
- Bordet T, Berna P, Abitbol J‐L, Pruss RM. Olesoxime (TRO19622): a novel mitochondrial‐targeted neuroprotective compound. Pharmaceuticals (Basel) 2010;3(2):345‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bosch‐Marcé 2011
- Bosch‐Marcé M, Wee CD, Martinez TL, Lipkes CE, Choe DW, Kong L, et al. Increased IGF‐1 in muscle modulates the phenotype of severe SMA mice. Human Molecular Genetics 2011;20(9):1844‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bowerman 2010
- Bowerman M, Beauvais A, Anderson CL, Kothary R. Rho‐kinase inactivation prolongs survival of an intermediate SMA mouse model. Human Molecular Genetics 2010;19(8):1468‐78. [DOI] [PubMed] [Google Scholar]
Bowerman 2012
- Bowerman M, Murray LM, Boyer JG, Anderson CL, Kothary R. Fasudil improves survival and promotes skeletal muscle development in a mouse model of spinal muscular atrophy. BMC Medicine 2012;7(10):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Braun 1995
- Braun S, Croizat B, Lagrange MC, Warter JM, Poindron P. Constitutive muscular abnormalities in culture in spinal muscular atrophy. Lancet 1995;345(8951):694‐5. [PUBMED: 7741893] [DOI] [PubMed] [Google Scholar]
Bresolin 1984
- Bresolin N, Freddo L, Tegazzin V, Bet L, Armani M, Angelini C. Carnitine and acyltransferase in experimental neurogenic atrophies: changes with treatment. Journal of Neurology 1984;231(4):170‐5. [DOI] [PubMed] [Google Scholar]
Brichta 2003
- Brichta L, Hofmann Y, Hahnen E, Siebzehnrubl FA, Raschke H, Blumcke I, et al. Valproic acid increases the SMN2 protein level: a well‐known drug as a potential therapy for spinal muscular atrophy. Human Molecular Genetics 2003;12(19):2481‐9. [DOI] [PubMed] [Google Scholar]
Bryson 1996
- Bryson HM, Fulton B, Benfield P. Riluzole. A review of its pharmacodynamic and pharmacokinetic properties and therapeutic potential in amyotrophic lateral sclerosis. Drugs 1996;52(4):549‐63. [DOI] [PubMed] [Google Scholar]
Brzustowicz 1990
- Brzustowicz LM, Lehner T, Castilla LH, Penchaszadeh GK, Wilhelmsen KC, Daniels R, et al. Genetic mapping of chronic childhood‐onset spinal muscular atrophy to chromosome 5q11.2‐13.3. Nature 1990;344(6266):540‐1. [DOI] [PubMed] [Google Scholar]
Burghes 2009
- Burghes AH, Beattie CE. Spinal muscular atrophy: why do low levels of survival motor neuron protein make motor neurons sick?. Nature Reviews Neuroscience 2009;10(8):597‐609. [DOI] [PMC free article] [PubMed] [Google Scholar]
Burke 2013
- Burke G, Hiscock A, Klein A, Niks EH, Main M, Manzur AY, et al. Salbutamol benefits children with congenital myasthenic syndrome due to DOK7 mutations. Neuromuscular Disorders 2013;23(2):170‐5. [DOI] [PubMed] [Google Scholar]
Butchbach 2010
- Butchbach ME, Singh J, Thorsteinsdottir M, Saieva L, Slominski E, Thurmond J, et al. Effects of 2,4‐diaminoquinazoline derivatives on SMN expression and phenotype in a mouse model for spinal muscular atrophy. Human Molecular Genetics 2010;19(3):454‐67. [PUBMED: 19897588] [DOI] [PMC free article] [PubMed] [Google Scholar]
Butchbach 2016
- Butchbach ME, Lumpkin CJ, Harris AW, Saieva L, Edwards JD, Workman E, et al. Protective effects of butyrate‐based compounds on a mouse model for spinal muscular atrophy. Experimental Neurology 2016;279:13‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cartegni 2006
- Cartegni L, Hastings ML, Calarco JA, Stanchina E, Krainer AR. Determinants of exon 7 splicing in the spinal muscular atrophy genes, SMN1 and SMN2. American Journal of Human Genetics 2006;78(1):63‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Caruso 1995
- Caruso JF, Signorile JF, Perry AC, Leblanc B, Williams R, Clark M, et al. The effects of albuterol and isokinetic exercise on the quadriceps muscle group. Medicine and Science in Sports and Exercise 1995;27(11):1471‐6. [PubMed] [Google Scholar]
Casanovas 1996
- Casanovas A, Ribera J, Hukkanen M, Riveros‐Moreno V, Esquerda JE. Prevention by lamotrigine, MK‐801 and N omega‐nitro‐l‐arginine methyl ester of motoneuron cell death after neonatal axotomy. Neuroscience 1996;71:313‐25. [DOI] [PubMed] [Google Scholar]
Chen 2017
- Chen YC, Chang JG, Liu TY, Jong YJ, Cheng WL, Yuo CY. Securinine enhances SMN2 exon 7 inclusion in spinal muscular atrophy cells. Biomedicine & Pharmacotherapy 2017;88:708‐14. [DOI] [PubMed] [Google Scholar]
Cherry 2017
- Cherry JJ, DiDonato CJ, Androphy EJ, Calo A, Potter K, Custer SK, et al. n vitro and in vivo effects of 2,4 diaminoquinazoline inhibitors of the decapping scavenger enzyme DcpS: context‐specific modulation of SMN transcript levels. PloS One 2017;12(9):eCollection 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cifuentes‐Diaz 2002
- Cifuentes‐Diaz C, Nicole S, Velasco ME, Borra‐Cebrian C, Panozzo C, Frugier T, et al. Neurofilament accumulation at the motor endplate and lack of axonal sprouting in a spinal muscular atrophy mouse model. Human Molecular Genetics 2002;11(12):1439‐47. [PUBMED: 12023986] [DOI] [PubMed] [Google Scholar]
Cobben 2001
- Cobben JM, Visser M, Scheffer H. From gene to disease; 'survival' motor neuron protein and hereditary proximal spinal muscle atrophy. Nederlands Tijdschrift voor Geneeskunde 2001;145(52):2525‐7. [PubMed] [Google Scholar]
Conceicao 2010
- Conceicao E, Silva T, Umbertine R, Maria Joaquina D. Analysis of motor skill acquisition among children with type I spinal muscular atrophy submitted to medication with valproic acid. Amyotrophic Lateral Sclerosis 2010;11(Suppl 1):63‐71. [Google Scholar]
Corse 1999
- Corse AM, Bilak MM, Bilak SR, Lehar M, Rothstein JD, Kuncl RW. Preclinical testing of neuroprotective neurotrophic factors in a model of chronic motor neuron degeneration. Neurobiology of Disease 1999;6:335‐46. [DOI] [PubMed] [Google Scholar]
d'Ydewalle 2017
- d'Ydewalle C, Ramos DM, Pyles NJ, Ng SY, Gorz M, Pilato CM, et al. The antisense transcript SMN‐AS1 regulates SMN expression and Is a novel therapeutic target for spinal muscular atrophy. Neuron 2017;93(1):66‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Darras 2007
- Darras BT, Kang PB. Clinical trials in spinal muscular atrophy. Current Opinion in Pediatrics 2007;19(6):675‐9. [DOI] [PubMed] [Google Scholar]
Darras 2013
- Darras BT, Chiriboga C, Swoboda K, Iannaccone S, Montes J, Rausch N, et al. Results of a first‐in‐human phase I study to assess the safety, tolerability, and dose range finding of a single intrathecal dose of ISIS‐SMNRx in patients with spinal muscular atrophy. Annals of Neurology 2013;74(Suppl 17):S128. [Google Scholar]
Dominguez 2011
- Dominguez E, Marais T, Chatauret N, Benkhelifa‐Ziyyat S, Duque S, Ravassard P, et al. Intravenous scAAV9 delivery of a codon‐optimized SMN1 sequence rescues SMA mice. Human Molecular Genetics 2011;20(4):681‐93. [DOI] [PubMed] [Google Scholar]
Donnelly 2012
- Donnelly EM, Boulis NM. Update on gene and stem cell therapy approaches for spinal muscular atrophy. Expert Opinion on Biological Therapy 2012;11:1463‐71. [DOI] [PubMed] [Google Scholar]
Duan 2010
- Duan C, Ren H, Gao S. Insulin‐like growth factors (IGFs), IGF receptors, and IGF‐binding proteins: roles in skeletal muscle growth and differentiation. General and Comparative Endocrinology 2010;167:344‐51. [DOI] [PubMed] [Google Scholar]
Dubowitz 1995
- Dubowitz V. Chaos in the classification of SMA: a possible resolution. Neuromuscular Disorders 1995;5(1):3‐5. [DOI] [PubMed] [Google Scholar]
Dunaway 2014
- Dunaway S, Montes J, Garber CE, Carr B, Kramer SS, Kamil‐Rosenberg S, et al. Performance of the timed "up & go" test in spinal muscular atrophy. Muscle & Nerve 2014;50(2):273‐7. [DOI] [PubMed] [Google Scholar]
Duque 2015
- Duque SI, Arnold WD, Odermatt P, Li X, Porensky PN, Schmelzer L, et al. A large animal model of spinal muscular atrophy and correction of phenotype. Annals of Neurology 2015;77(3):399‐414. [DOI] [PMC free article] [PubMed] [Google Scholar]
Edwards 2016
- Edwards JD, Butchbach ME. Effect of the butyrate prodrug pivaloyloxymethyl butyrate (AN9) on a mouse model for spinal muscular atrophy. Journal of Neuromuscular Disorders 2016;3(4):511‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ellis 2004
- Ellis AC, Rosenfeld J. The role of creatine in the management of amyotrophic lateral sclerosis and other neurodegenerative disorders. CNS Drugs 2004;18(14):967‐80. [DOI] [PubMed] [Google Scholar]
Farooq 2013
- Farooq F, Abadía‐Molina F, MacKenzie D, Hadwen J, Shamim F, O'Reilly S, et al. Celecoxib increases SMN and survival in a severe spinal muscular atrophy mouse model via p38 pathway activation. Human Molecular Genetics 2013;22(17):3415‐24. [DOI] [PubMed] [Google Scholar]
Feldkotter 2002
- Feldkotter M, Schwarzer V, Wirth R, Wienker TF, Wirth B. Quantitative analyses of SMN1 and SMN2 based on real‐time lightCycler PCR: fast and highly reliable carrier testing and prediction of severity of spinal muscular atrophy. American Journal of Human Genetics 2002;70(2):358‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Finkel 2015
- Finkel R, Bertini E, Muntoni F, Mercuri E, ENMC SMA Workshop Study Group. 209th ENMC International Workshop: outcome measures and clinical trial readiness in spinal muscular atrophy 7‐9 November 2014, Heemskerk, The Netherlands. Neuromuscular Disorders 2015;25(7):593‐602. [DOI] [PubMed] [Google Scholar]
Finkel 2016
- Finkel RS, Chiriboga CA, Vajsar J, Day JW, Montes J, Vivo DC, et al. Treatment of infantile‐onset spinal muscular atrophy with nusinersen: a phase 2, open‐label, dose‐escalation study. Lancet 2016;388(10063):3017‐26. [NCT01839656] [DOI] [PubMed] [Google Scholar]
Finkel 2017 (ENDEAR)
- Finkel RS, Mercuri E, Darras BT, Connolly AM, Kuntz NL, Kirschner J. Nusinersen versus sham control in infantile‐onset spinal muscular atrophy. New England Journal of Medicine 2017;377(18):1723‐32. [ENDEAR; IONIS SMNRx‐CS3B ; ISIS 396442‐CS3B ; NCT02193074] [DOI] [PubMed] [Google Scholar]
Finkel 2018
- Finkel RS, Mercuri E, Meyer OH, Simonds AK, Schroth MK, Graham RJ, et al. Diagnosis and management of spinal muscular atrophy: part 2: pulmonary and acute care; medications, supplements and immunizations; other organ systems; and ethics. Neuromuscular Disorders 2018;28(3):197‐207. [DOI] [PubMed] [Google Scholar]
Foust 2010
- Foust KD, Wang X, McGovern VL, Braun L, Bevan AK, Haidet AM, et al. Rescue of the spinal muscular atrophy phenotype in a mouse model by early postnatal delivery of SMN. Nature Biotechnology 2010;28(3):271‐4. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Garbes 2009
- Garbes L, Riessland M, Hölker I, Heller R, Hauke J, Tränkle C, et al. LBH589 induces up to 10‐fold SMN protein levels by several independent mechanisms and is effective even in cells from SMA patients non‐responsive to valproate. Human Molecular Genetics 2009;18(19):3645‐58. [DOI] [PubMed] [Google Scholar]
Gavrilina 2008
- Gavrilina TO, McGovern VL, Workman E, Crawford TO, Gogliotti RG, DiDonato CJ, et al. Neuronal SMN expression corrects spinal muscular atrophy in severe SMA mice while muscle‐specific SMN expression has no phenotypic effect. Human Molecular Genetics 2008;17(8):1063‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gendron 1999
- Gendron NH, MacKenzie AE. Spinal muscular atrophy: molecular pathophysiology. Current Opinion in Neurology 1999;12(2):137‐42. [DOI] [PubMed] [Google Scholar]
Gilliam 1990
- Gilliam TC, Brzustowicz LM, Castilla LH, Lehner T, Penchaszadeh GK, Daniels RJ, et al. Genetic homogeneity between acute and chronic forms of spinal muscular atrophy. Nature 1990;345(6278):823‐5. [DOI] [PubMed] [Google Scholar]
Glascock 2012a
- Glascock JJ, Shababi M, Wetz MJ, Krogman MM, Lorson CL. Direct central nervous system delivery provides enhanced protection following vector mediated gene replacement in a severe model of spinal muscular atrophy. Biochemical and Biophysical Research Communications 2012;417:376‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Glascock 2012b
- Glascock JJ, Osman EY, Wetz MJ, Krogman MM, Shababi M, Lorson CL. Decreasing disease severity in symptomatic, SMN(–/–);SMN2(+/+), spinal muscular atrophy mice following scAAV9‐SMN delivery. Human Gene Therapy 2012;23(3):330‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gogliotti 2013
- Gogliotti RG, Cardona H, Singh J, Bail S, Emery C, Kuntz N, et al. The DcpS inhibitor RG3039 improves survival, function and motor unit pathologies in two SMA mouse models. Human Molecular Genetics 2013;22(20):4048‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Greensmith 1995
- Greensmith L, Vrbova G. Possible strategies for treatment of SMA patients: a neurobiologist's view. Neuromuscular Disorders 1995;5(5):359‐69. [DOI] [PubMed] [Google Scholar]
Grzeschik 2005
- Grzeschik SM, Ganta M, Prior TW, Heavlin WD, Wang CH. Hydroxyurea enhances SMN2 gene expression in spinal muscular atrophy cells. Annals of Neurology 2005;58(2):194‐202. [DOI] [PubMed] [Google Scholar]
Haché 2016
- Haché M, Swoboda KJ, Sethna N, Farrow‐Gillespie A, Khandji A, Xia S, et al. Intrathecal injections in children with spinal muscular atrophy: nusinersen clinical trial experience. Journal of Child Neurology 2016;31(7):899‐906. [DOI] [PMC free article] [PubMed] [Google Scholar]
Haddad 2003
- Haddad H, Cifuentes‐Diaz C, Miroglio A, Roblot N, Joshi V, Melki J. Riluzole attenuates spinal muscular atrophy disease progression in a mouse model. Muscle & Nerve 2003;28(4):432‐7. [DOI] [PubMed] [Google Scholar]
Hadwen 2014
- Hadwen J, MacKenzie D, Shamim F, Mongeon K, Holcik M, MacKenzie A, et al. VPAC2 receptor agonist BAY 55‐9837 increases SMN protein levels and moderates disease phenotype in severe spinal muscular atrophy mouse models. Orphanet Journal of Rare Diseases 2014;9:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hahnen 2006
- Hahnen E, Eyüpoglu IY, Brichta L, Haastert K, Tränkle C, Siebzehnrübl FA, et al. In vitro and ex vivo evaluation of second‐generation histone deacetylase inhibitors for the treatment of spinal muscular atrophy. Journal Neurochemistry 2006;98(1):193‐202. [DOI] [PubMed] [Google Scholar]
Hammond 2016
- Hammond SM, Hazell G, Shabanpoor F, Saleh AF, Bowerman M, Sleigh JN, et al. Systemic peptide‐mediated oligonucleotide therapy improves long‐term survival in spinal muscular atrophy. Proceedings of the National Academy of Sciences in the United States of America 2016;113(39):10962‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harada 2002
- Harada Y, Sutomo R, Sadewa AH, Akutsu T, Takeshima Y, Wada H, et al. Correlation between SMN2 copy number and clinical phenotype of spinal muscular atrophy: three SMN2 copies fail to rescue some patients from the disease severity. Journal of Neurology 2002;249(9):1211‐9. [DOI] [PubMed] [Google Scholar]
Hastings 2009
- Hastings ML, Berniac J, Liu YH, Abato P, Jodelka FM, Barthel L, et al. Tetracyclines that promote SMN2 exon 7 splicing as therapeutics for spinal muscular atrophy. Science Translational Medicine 2009;1(5):5ra12. [PUBMED: 20161659] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hauke 2009
- Hauke J, Riessland M, Lunke S, Eyüpoglu IY, Blümcke I, El‐Osta A, et al. Survival motor neuron gene 2 silencing by DNA methylation correlates with spinal muscular atrophy disease severity and can be bypassed by histone deacetylase inhibition. Human Molecular Genetics 2009;18(2):304‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Heier 2009
- Heier CR, DiDonato CJ. Translational readthrough by the aminoglycoside geneticin (G418) modulates SMN stability in vitro and improves motor function in SMA mice in vivo. Human Molecular Genetics 2009;18(7):1310‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Heier 2015
- Heier CR, DiDonato CJ. ECG in neonate mice with spinal muscular atrophy allows assessment of drug efficacy. Frontiers in Bioscience (Elite Edition) 2015;1(7):107‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hosseinibarkooie 2016
- Hosseinibarkooie S, Peters M, Torres‐Benito L, Rastetter RH, Hupperich K, Hoffmann A, et al. The power of human protective modifiers: PLS3 and CORO1C unravel impaired endocytosis in spinal muscular atrophy and rescue SMA phenotype. American Journal of Human Genetics 2016;99(3):647‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hsu 2012
- Hsu YY, Jong YJ, Tsai HH, Tseng YT, An LM, Lo YC. Triptolide increases transcript and protein levels of survival motor neurons in human SMA fibroblasts and improves survival in SMA‐like mice. British Journal of Pharmacology 2012;166(3):1114‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hua 2010
- Hua Y, Sahashi K, Hung G, Rigo F, Passini MA, Bennett CF, et al. Antisense correction of SMN2 splicing in the CNS rescues necrosis in a type III SMA mouse model. Genes & Development 2010;24(15):1634‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hua 2011
- Hua Y, Sahashi K, Rigo F, Hung G, Horev G, Bennett CF, et al. Peripheral SMN restoration is essential for long‐term rescue of a severe SMA mouse model. Nature 2011;478(7367):123‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hwee 2015
- Hwee DT, Kennedy AR, Hartman JJ, Ryans J, Durham N, Malik FI, et al. The small‐molecule fast skeletal troponin activator, CK‐2127107, improves exercise tolerance in a rat model of heart failure. Journal of Pharmacology and Experimental Therapeutics 2015;353:159‐68. [DOI] [PubMed] [Google Scholar]
Iannaccone 1993
- Iannaccone ST, Browne RH, Samaha FJ, Buncher CR. Prospective study of spinal muscular atrophy before age 6 years. DCN/SMA Group. Pediatric Neurology 1993;9(3):187‐93. [DOI] [PubMed] [Google Scholar]
Iannaccone 1998
- Iannaccone ST. Spinal muscular atrophy. Seminars in Neurology 1998;18(1):19‐26. [DOI] [PubMed] [Google Scholar]
Iannaccone 2001
- Iannaccone ST, Burghes AH. Spinal muscular atrophies. In: Pourmand R, Harati Y editor(s). Neuromuscular Disorders. Philadelphia (PA): Lippincott Williams and Wilkins, 2001:83‐98. [Google Scholar]
Il'ina 1980
- Il'ina NA, Antipova RI, Khokhlov AP. Use of lithium carbonate to treat Kugelberg‐Welander spinal amyotrophy [Primenenie uglekislogo litiia dlia lecheniia spinal'noi amiotrofii Kugel'berga‐Velandera]. Zhurnal Nevropatologii i Psikhiatrii Imeni S.S. Korsakova (Moscow, Russia) 1980;80(11):1657‐60. [PUBMED: 7456914] [PubMed] [Google Scholar]
Jablonka 2000
- Jablonka S, Rossoll W, Schrank B, Sendtner M. The role of SMN in spinal muscular atrophy. Journal of Neurology 2000;247 Suppl 1:I37‐42. [DOI] [PubMed] [Google Scholar]
Jarecki 2005
- Jarecki J, Chen X, Bernardino A, Coovert DD, Whitney M, Burghes A, et al. Diverse small‐molecule modulators of SMN expression found by high‐throughput compound screening: early leads towards a therapeutic for spinal muscular atrophy. Human Molecular Genetics 2005;14(14):2003‐18. [DOI] [PubMed] [Google Scholar]
Kaczmarek 2015
- Kaczmarek A, Schneider S, Wirth B, Markus Riessland M. Investigational therapies for the treatment of spinal muscular atrophy. Expert Opinion on Investigational Drugs 2015;24(7):967‐81. [DOI] [PubMed] [Google Scholar]
Kariya 2008
- Kariya S, Park GH, Maeno‐Hikichi Y, Leykekhman O, Lutz C, Arkovitz MS, et al. Reduced SMN protein impairs maturation of the neuromuscular junctions in mouse models of spinal muscular atrophy. Human Molecular Genetics 2008;17(16):2552‐69. [PUBMED: 18492800] [DOI] [PMC free article] [PubMed] [Google Scholar]
Keil 2014
- Keil JM, Seo J, Howell MD, Hsu WH, Singh RN, DiDonato CJ. A short antisense oligonucleotide ameliorates symptoms of severe mouse models of spinal muscular atrophy. Molecular Therapy – Nucleic Acids 2014;3:e174. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kernochan 2005
- Kernochan LE, Russo ML, Woodling NS, Huynh TN, Avila AM, Fischbeck KH, et al. The role of histone acetylation in SMN gene expression. Human Molecular Genetics 2005;14(9):1171‐82. [DOI] [PubMed] [Google Scholar]
Kim 2007
- Kim JE, Kim DS, Kwak SE, Choi HC, Song HK, Choi SY, et al. Anti‐glutamatergic effect of riluzole: comparison with valproic acid. Neuroscience 2007;147(1):136‐45. [DOI] [PubMed] [Google Scholar]
Kindermann 2007
- Kindermann W. Do inhaled beta(2)‐agonists have an ergogenic potential in non‐asthmatic competitive athletes?. Sports Medicine 2007;37(2):95‐102. [DOI] [PubMed] [Google Scholar]
Kong 2009
- Kong L, Wang X, Choe DW, Polley M, Burnett BG, Bosch‐Marcé M, et al. Impaired synaptic vesicle release and immaturity of neuromuscular junctions in spinal muscular atrophy mice. Journal of Neuroscience 2009;29(3):842‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kroksmark 2001
- Kroksmark AK, Beckung E, Tulinius M. Muscle strength and motor function in children and adolescents with spinal muscular atrophy II and III. European Journal of Paediatric Neurology 2001;5(5):191‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kwon 2011
- Kwon DY, Motley WW, Fischbeck KH, Burnett BG. Increasing expression and decreasing degradation of SMN ameliorate the spinal muscular atrophy phenotype in mice. Human Molecular Genetics 2011;20(18):3667‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 1995
- Lefebvre S, Burglen L, Reboullet S, Clermont O, Burlet P, Viollet L, et al. Identification and characterization of a spinal muscular atrophy‐determining gene. Cell 1995;80(1):155‐65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lefebvre 1998
- Lefebvre S, Burglen L, Frezal J, Munnich A, Melki J. The role of the SMN gene in proximal spinal muscular atrophy. Human Molecular Genetics 1998;7(10):1531‐6. [DOI] [PubMed] [Google Scholar]
Lesbordes 2003
- Lesbordes JC, Cifuentes‐Diaz C, Miroglio A, Joshi V, Bordet T, Kahn A, et al. Therapeutic benefits of cardiotrophin‐1 gene transfer in a mouse model of spinal muscular atrophy. Human Molecular Genetics 2003;12(11):1233‐9. [DOI] [PubMed] [Google Scholar]
Lewelt 2012
- Lewelt A, Newcomb TM, Swoboda KJ. New therapeutic approaches to spinal muscular atrophy. Current Neurology and Neuroscience Reports 2012;12(1):42‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liewluck 2011
- Liewluck T, Selcen D, Engel AG. Beneficial effects of albuterol in congenital endplate acetylcholinesterase deficiency and Dok‐7 myasthenia. Muscle & Nerve 2011;44(5):789‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Little 2015
- Little D, Valori CF, Mutsaers CA, Bennett EJ, Wyles M, Sharrack B, et al. PTEN depletion decreases disease severity and modestly prolongs survival in a mouse model of spinal muscular atrophy. Molecular Therapy 2015;23(2):270‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2014
- Liu H, Yazdani A, Murray LM, Beauvais A, Kothary R. The SMN‐independent beneficial effects of trichostatin A on an intermediate mouse model of spinal muscular atrophy. PloS One 2014;9(7):e101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2016
- Liu M, Hammers DW, Barton ER, Sweeney HL. Activin receptor type IIB inhibition improves muscle phenotype and function in a mouse model of spinal muscular atrophy. PloS One 2016;11(11):e0166803. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lorenzoni 2013
- Lorenzoni PJ, Scola RH, Kay CS, Filla L, Miranda AP, Pinheiro JM, et al. Salbutamol therapy in congenital myasthenic syndrome due to DOK7 mutation. Journal of the Neurological Sciences 2013;331:155‐7. [DOI] [PubMed] [Google Scholar]
Lorson 1999
- Lorson CL, Hahnen E, Androphy EJ, Wirth B. A single nucleotide in the SMN gene regulates splicing and is responsible for spinal muscular atrophy. Proceedings of the National Academy of Sciences of the United States of America 1999;96(11):6307‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lunn 2004
- Lunn MR, Root DE, Martino AM, Flaherty SP, Kelley BP, Coovert DD, et al. Indoprofen upregulates the survival motor neuron protein through a cyclooxygenase‐independent mechanism. Chemistry & Biology 2004;11(11):1489‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lunn 2008
- Lunn MR, Wang CH. Spinal muscular atrophy. Lancet 2008;371(9630):2120‐33. [DOI] [PubMed] [Google Scholar]
Mack 2014
- Mack SG, Cook DJ, Dhurjati P, Butchbach ME. Systems biology investigation of cAMP modulation to increase SMN levels for the treatment of spinal muscular atrophy. PloS One 2014;16(9):e115473. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martineau 1992
- Martineau L, Horan MA, Rothwell NJ, Little RA. Salbutamol, a beta 2‐adrenoceptor agonist, increases skeletal muscle strength in young men. Clinical Science (London) 1992;83(5):615‐21. [DOI] [PubMed] [Google Scholar]
Mattis 2009a
- Mattis VB, Ebert AD, Fosso MY, Chang CW, Lorson CL. Delivery of a read‐through inducing compound, TC007, lessens the severity of a spinal muscular atrophy animal model. Human Molecular Genetics 2009;18(20):3906‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mattis 2009b
- Mattis VB, Fosso MY, Chang CW, Lorson CL. Subcutaneous administration of TC007 reduces disease severity in an animal model of SMA. BMC Neuroscience 2009;30(10):142. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mattis 2012
- Mattis VB, Tom Chang CW, Lorson CL. Analysis of a read‐through promoting compound in a severe mouse model of spinal muscular atrophy. Neuroscience Letters 2012;525(1):72‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
McWhorter 2003
- McWhorter ML, Monani UR, Burghes AH, Beattie CE. Knockdown of the survival motor neuron (SMN) protein in zebrafish causes defects in motor axon outgrowth and pathfinding. Journal of Cell Biology 2003;162(5):919‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Melki 1990a
- Melki J, Abdelhak S, Sheth P, Bachelot MF, Burlet P, Marcadet A, et al. Gene for chronic proximal spinal muscular atrophies maps to chromosome 5q. Nature 1990;344(6268):767‐8. [DOI] [PubMed] [Google Scholar]
Melki 1990b
- Melki J, Sheth P, Abdelhak S, Burlet P, Bachelot MF, Lathrop MG, et al. Mapping of acute (type I) spinal muscular atrophy to chromosome 5q12‐q14. The French Spinal Muscular Atrophy Investigators. Lancet 1990;336(8710):271‐3. [DOI] [PubMed] [Google Scholar]
Mendell 2016
- Mendell JR, Al‐Zaidy S, Shell R, Arnold WD, Rodino‐Klapac L, Kissel JT, et al. Gene therapy for spinal muscular atrophy type 1 shows potential to improve survival and motor functional outcomes. Molecular Therapy 2016;24(Suppl 1):S190. [WOS:000375264200475] [Google Scholar]
Mendell 2017
- Mendell JR, Al‐Zaidy S, Shell R, Arnold WD, Rodino‐Klapac LR, Prior TW, et al. Gene‐replacement therapy for spinal muscular atrophy. New England Journal of Medicine 2017;377(18):1713‐22. [DOI] [PubMed] [Google Scholar]
Mercuri 2012
- Mercuri E, Bertini E, Iannaccone ST. Childhood spinal muscular atrophy: controversies and challenges. Lancet Neurology 2012;11(5):443‐52. [DOI] [PubMed] [Google Scholar]
Mercuri 2018
- Mercuri E, Finkel RS, Muntoni F, Wirth B, Montes J, Main M, et al. Diagnosis and management of spinal muscular atrophy: part 1: recommendations for diagnosis, rehabilitation, orthopedic and nutritional care. Neuromuscular Disorders 2018;28(2):103‐15. [DOI] [PubMed] [Google Scholar]
Merlini 2010
- Merlini L, Basoglu B, Dahna‐Schwake C, Febrer A, Hausmanova‐Petrusewicz I, Jedrzejonska M, et al. Eurosmart: European spinal muscular atrophy randomised trial of acetyl‐L‐carnitine in spinal muscular atrophy. Revue Neurologique 2010;166(3):359. [Google Scholar]
Meyer 2015
- Meyer K, Ferraiuolo L, Schmelzer L, Braun L, McGovern V, Likhite S, et al. Improving single injection CSF delivery of AAV9‐mediated gene therapy for SMA: a dose–response study in mice and nonhuman primates. Molecular Therapy 2015;23(3):477‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mohseni 2013
- Mohseni J, Zabidi‐Hussin ZA, Sasongko TH. Histone deacetylase inhibitors as potential treatment for spinal muscular atrophy. Genetics and Molecular Biology 2013;36(3):299‐307. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mohseni 2016
- Mohseni J, Al‐Najjar BO, Wahab HA, Zabidi‐Hussin ZA, Sasongko TH. Transcript, methylation and molecular docking analyses of the effects of HDAC inhibitors, SAHA and Dacinostat, on SMN2 expression in fibroblasts of SMA patients. Journal of Human Genetics 2016;61:823‐30. [DOI] [PubMed] [Google Scholar]
Montes 2013
- Montes J, Blumenschine M, Dunaway S, Alter AS, Engelstad K, Rao AK, et al. Weakness and fatigue in diverse neuromuscular diseases. Journal of Child Neurology 2013;28(10):1277‐83. [DOI] [PubMed] [Google Scholar]
Munsat 1992
- Munsat TL, Davies KE. International SMA consortium meeting (26‐28 June 1992, Bonn, Germany). Neuromuscular Disorders 1992;2(5‐6):423‐8. [DOI] [PubMed] [Google Scholar]
Murdocca 2012
- Murdocca M, Malgieri A, Luchetti A, Saieva L, Dobrowolny G, Leonibus E, et al. IPLEX administration improves motor neuron survival and ameliorates motor functions in a severe mouse model of spinal muscular atrophy. Molecular Medicine 2012;18:1076‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Murray 2008
- Murray LM, Comley LH, Thomson D, Parkinson N, Talbot K, Gillingwater TH. Selective vulnerability of motor neurons and dissociation of pre‐ and post‐synaptic pathology at the neuromuscular junction in mouse models of spinal muscular atrophy. Human Molecular Genetics 2008;17(7):949‐62. [PUBMED: 18065780] [DOI] [PubMed] [Google Scholar]
Naryshkin 2014
- Naryshkin NA, Weetall M, Dakka A, Narasimhan J, Zhao X, Feng Z, et al. SMN2 splicing modifiers improve motor function and longevity in mice with spinal muscular atrophy. Science 2014;345(6197):688‐93. [DOI] [PubMed] [Google Scholar]
NCT02122952
- NCT02122952. Gene transfer clinical trial for spinal muscular atrophy type 1. clinicaltrial.gov/show/NCT02122952 (first received 23 April 2014). [AVXS‐101‐CL‐101; NCT02122952]
NCT02268552
- NCT02268552. An open label study of LMI070 in type 1 spinal muscular atrophy (SMA). clinicaltrials.gov/show/NCT02268552 (first received 1 October 2014). [CLMI070X2201; NCT02268552]
NCT02633709
- NCT02633709. A study to investigate the safety, tolerability, pharmacokinetics and pharmacodynamics of RO7034067 (RG7916) given by mouth in healthy volunteers. clinicaltrials.gov/show/NCT02633709 (first received 15 December 2015). [2015‐004605‐16; BP29840; NCT02633709]
Nicole 2002
- Nicole S, Diaz CC, Frugier T, Melki J. Spinal muscular atrophy: recent advances and future prospects. Muscle & Nerve 2002;26(1):4‐13. [DOI] [PubMed] [Google Scholar]
Ning 2010
- Ning K, Drepper C, Valori CF, Ahsan M, Wyles M, Higginbottom A, et al. PTEN depletion rescues axonal growth defect and improves survival in SMN‐deficient motor neurons. Human Molecular Genetics 2010;19(16):3159‐68. [DOI] [PubMed] [Google Scholar]
Nizzardo 2014
- Nizzardo M, Simone C, Salani S, Ruepp MD, Rizzo F, Ruggieri M, et al. Effect of combined systemic and local morpholino treatment on the spinal muscular atrophy Δ7 mouse model phenotype. Clinical Therapeutics 2014;36(3):340‐56. [DOI] [PubMed] [Google Scholar]
Nurputra 2013
- Nurputra DK, Lai PS, Harahap NI, Morikawa S, Yamamoto T, Nishimura N, et al. Spinal muscular atrophy: from gene discovery to clinical trials. Annals of Human Genetics 2013;77(5):435‐63. [DOI] [PubMed] [Google Scholar]
NURTURE 2015
- NCT02386553. A study of multiple doses of IONIS SMNRx (ISIS 396443) delivered to infants with genetically diagnosed and presymptomatic spinal muscular atrophy (NURTURE). www.clinicaltrials.gov/show/NCT02386553 (first received 27 February 2015). [2014‐002098‐12; 232SM201; NCT02386553]
Osman 2014
- Osman EY, Miller MR, Robbins KL, Lombardi AM, Atkinson AK, Brehm AJ, et al. Morpholino antisense oligonucleotides targeting intronic repressor Element1 improve phenotype in SMA mouse models. Human Molecular Genetics 2014;23(18):4832‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Osman 2016
- Osman EY, Washington CW, Kaifer KA, Mazzasette C, Patitucci TN, Florea KM, et al. Optimization of morpholino antisense oligonucleotides targeting the intronic repressor element1 in spinal muscular atrophy. Molecular Therapy 2016;24(9):1592‐601. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pasanisi 2014
- Pasanisi MB, Giovannetti AM, Bussolino C, Campanella A, Leonardi M, Morandi L. Perception of efficacy in adult patients affected by spinal muscular atrophy (SMA) treated with salbutamol. Neuromuscular Disorders 2014;24:9‐10. [Google Scholar]
Passini 2011
- Passini MA, Bu J, Richards AM, Kinnecom C, Sardi SP, Stanek LM, et al. Antisense oligonucleotides delivered to the mouse CNS ameliorate symptoms of severe spinal muscular atrophy. Science Translational Medicine 2011;3(72):72ra18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pellizzoni 1998
- Pellizzoni L, Kataoka N, Charroux B, Dreyfuss G. A novel function for SMN, the spinal muscular atrophy disease gene product, in pre‐mRNA splicing. Cell 1998;95(5):615‐24. [DOI] [PubMed] [Google Scholar]
Piepers 2008
- Piepers S, Berg LH, Brugman F, Scheffer H, Ruiterkamp‐Versteeg M, Engelen BG, et al. A natural history study of late onset spinal muscular atrophy types 3b and 4. Journal of Neurology 2008;255(9):1400‐4. [DOI] [PubMed] [Google Scholar]
Porensky 2013
- Porensky PN, Burghes AH. Antisense oligonucleotides for the treatment of spinal muscular atrophy. Human Gene Therapy 2013;24(5):489‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
RAINBOWFISH
- RAINBOWFISH. A study of risdiplam in infants with genetically diagnosed and presymptomatic spinal muscular atrophy (Rainbowfish). clinicaltrials.gov/ct2/show/NCT03779334 (first received 18 December 2018). [BN40703 ; NCT03779334]
Review Manager 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Riessland 2006
- Riessland M, Brichta L, Hahnen E, Wirth B. The benzamide M344, a novel histone deacetylase inhibitor, significantly increases SMN2 RNA/protein levels in spinal muscular atrophy cells. Human Genetics 2006;120(1):101‐10. [DOI] [PubMed] [Google Scholar]
Riessland 2010
- Riessland M, Ackermann B, Förster A, Jakubik M, Hauke J, Garbes L, et al. SAHA ameliorates the SMA phenotype in two mouse models for spinal muscular atrophy. Human Molecular Genetics 2010;19(8):1492‐506. [DOI] [PubMed] [Google Scholar]
Riessland 2017
- Riessland M, Kaczmarek A, Schneider S, Swoboda KJ, Löhr H, Bradler C, et al. Neurocalcin delta suppression protects against spinal muscular atrophy in humans and across species by restoring impaired endocytosis. American Journal of Human Genetics 2017;100(2):297‐315. [DOI] [PMC free article] [PubMed] [Google Scholar]
Robbins 2014
- Robbins KL, Glascock JJ, Osman EY, Miller MR, Lorson CL. Defining the therapeutic window in a severe animal model of spinal muscular atrophy. Human Molecular Genetics 2014;23(17):4559‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Roche 2018
- Jethwa S, on behalf of the Roche SMA Team. www.treatsma.uk/wp‐content/uploads/2018/05/2018‐05‐30‐Olesoxime‐PG‐update‐May‐2018.pdf 30 May 2018.
Rodríguez Cruz 2015
- Rodríguez Cruz PM, Palace J, Ramjattan H, Jayawant S, Robb SA, Beeson D. Salbutamol and ephedrine in the treatment of severe AChR deficiency syndromes. Neurology 2015;85:1043‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rossoll 2003
- Rossoll W, Jablonka S, Andreassi C, Kröning AK, Karle K, Monani UR, et al. Smn, the spinal muscular atrophy‐determining gene product, modulates axon growth and localization of beta‐actin mRNA in growth cones of motoneurons. Journal of Cell Biology 2003;163(4):801‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rudnicki 2016
- Rudnicki S, Andrews J, Malik F, Wolff A, Day J. CK‐2127107 a selective activator of the fast skeletal muscle troponin complex, for the potential treatment of spinal muscular atrophy. Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration 2016;17:214. [Google Scholar]
Russman 1992
- Russman BS, Iannacone ST, Buncher CR, Samaha FJ, White M, Perkins B, et al. Spinal muscular atrophy: new thoughts on the pathogenesis and classification schema. Journal of Child Neurology 1992;7(4):347‐53. [DOI] [PubMed] [Google Scholar]
Russman 1996
- Russman BS, Buncher CR, White M, Samaha FJ, Iannaccone ST. Function changes in spinal muscular atrophy II and III. The DCN/SMA Group. Neurology 1996;47(4):973‐6. [DOI] [PubMed] [Google Scholar]
Russman 2003
- Russman BS, Iannaccone ST, Samaha FJ. A phase 1 trial of riluzole in spinal muscular atrophy. Archives of Neurology 2003;60(11):1601‐3. [DOI] [PubMed] [Google Scholar]
Schreml 2013
- Schreml J, Riessland M, Paterno M, Garbes L, Roßbach K, Ackermann B, et al. Severe SMA mice show organ impairment that cannot be rescued by therapy with the HDACi JNJ‐26481585. European Journal of Human Genetics 2013;21(6):643‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Seo 2013
- Seo J, Howell MD, Singh NN, Singh RN. Spinal muscular atrophy: an update on therapeutic progress. Biochimica Biophysica Acta 2013;1832(12):2180‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shababi 2011
- Shababi M, Glascock J, Lorson CL. Combination of SMN trans‐splicing and a neurotrophic factor increases the life span and body mass in a severe model of spinal muscular atrophy. Human Gene Therapy 2011;22(2):135‐44. [DOI] [PubMed] [Google Scholar]
Shababi 2012
- Shababi M, Lorson CL. Optimization of SMN trans‐splicing through the analysis of SMN introns. Journal Molecular Neuroscience 2012;46(3):459‐69. [DOI] [PubMed] [Google Scholar]
Singh 2008
- Singh J, Salcius M, Liu SW, Staker BL, Mishra R, Thurmond J, et al. DcpS as a therapeutic target for spinal muscular atrophy. ACS Chemical Biology 2008;3(11):711‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Skordis 2003
- Skordis LA, Dunckley MG, Yue B, Eperon IC, Muntoni F. Bifunctional antisense oligonucleotides provide a trans‐acting splicing enhancer that stimulates SMN2 gene expression in patient fibroblasts. Proceedings of National Academy of Sciences of the United States of America 2003;100(7):4114‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sproule 2016
- Sproule D, Al‐Zaidy S, Shell R, Arnold D, Rodino‐Klapac L, Kissel J, et al. Interim safety, efficacy and achievement of developmental milestones in this phase 1, first‐in‐human study of the systemic delivery of AVXS‐101, an AAV9‐mediated gene therapy for children with spinal muscular atrophy (SMA) type 1. Annals of Neurology 2016;80(Suppl 20):S368. [Google Scholar]
Staropoli 2015
- Staropoli JF, Li H, Chun SJ, Allaire N, Cullen P, Thai A, et al. Rescue of gene‐expression changes in an induced mouse model of spinal muscular atrophy by an antisense oligonucleotide that promotes inclusion of SMN2 exon 7. Genomics 2015;105(4):220‐8. [DOI] [PubMed] [Google Scholar]
Stavarachi 2010
- Stavarachi M, Apostol P, Toma M, Cimponeriu D, Gavrila L. Spinal muscular atrophy disease: a literature review for therapeutic strategies. Journal of Medicine and Life 2010;3(1):3‐9. [PMC free article] [PubMed] [Google Scholar]
STOPSMA 2007
- STOPSMA. Study to evaluate sodium phenylbutyrate in pre‐symptomatic infants with spinal muscular atrophy (STOPSMA). clinicaltrials.gov/show/NCT00528268 (first received 10 September 2007). [1R01HD054599‐01; 22183; NCT00528268]
STRONG
- STRONG. Study of intrathecal administration of AVXS‐101 for spinal muscular atrophy (STRONG). clinicaltrials.gov/ct2/show/NCT03381729 (first received 22 December 2017). [AVXS‐101‐CL‐102; NCT03381729]
Sturm 2016
- Sturm S, Gunther A, Nave S, Jordan P, Al Kotbi N, Parkar N, et al. The SMN2 splicing modifier RG7916 induces a dose‐dependent increase of full length SMN2 mRNA. Annals of Neurology 2016;80(Suppl 20):S293‐4. [Google Scholar]
Sumner 2003
- Sumner CJ, Huynh TN, Markowitz JA, Perhac JS, Hill B, Coovert DD, et al. Valproic acid increases SMN levels in spinal muscular atrophy patient cells. Annals of Neurology 2003;54(5):647‐54. [DOI] [PubMed] [Google Scholar]
Sumner 2007
- Sumner CJ. Molecular mechanisms of spinal muscular atrophy. Journal of Child Neurology 2007;22(8):979‐89. [DOI] [PubMed] [Google Scholar]
Swoboda 2005
- Swoboda KJ, Prior TW, Scott CB, McNaught TP, Wride MC, Reyna SP, et al. Natural history of denervation in SMA: relation to age, SMN2 copy number, and function. Annals of Neurology 2005;57(5):704‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Swoboda 2007
- Swoboda KJ, Kissel JT, Crawford TO, Bromberg MB, Acsadi G, D'Anjou G, et al. Perspectives on clinical trials in spinal muscular atrophy. Journal of Child Neurology 2007;22(8):957‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Takeuchi 1994
- Takeuchi Y, Miyanomae Y, Komatsu H, Oomizono Y, Nishimura A, Okano S, et al. Efficacy of thyrotropin‐releasing hormone in the treatment of spinal muscular atrophy. Journal of Child Neurology 1994;9(3):287‐9. [DOI] [PubMed] [Google Scholar]
Talbot 1999
- Talbot K. Spinal muscular atrophy. Journal of Inherited Metabolic Disease 1999;22(4):545‐54. [DOI] [PubMed] [Google Scholar]
Tarnopolsky 1999
- Tarnopolsky M, Martin J. Creatine monohydrate increases strength in patients with neuromuscular disease. Neurology 1999;52(4):854‐7. [DOI] [PubMed] [Google Scholar]
Taylor 1998
- Taylor CP, Gee NS, Su TZ, Kocsis JD, Welty DF, Brown JP, et al. A summary of mechanistic hypotheses of gabapentin pharmacology. Epilepsy Research 1998;29(3):233‐49. [DOI] [PubMed] [Google Scholar]
Thomas 1994
- Thomas NH, Dubowitz V. The natural history of type I (severe) spinal muscular atrophy. Neuromuscular Disorders 1994;4(5‐6):497‐502. [DOI] [PubMed] [Google Scholar]
Thurmond 2008
- Thurmond J, Butchbach ME, Palomo M, Pease B, Rao M, Bedell L, et al. Synthesis and biological evaluation of novel 2,4‐diaminoquinazoline derivatives as SMN2 promoter activators for the potential treatment of spinal muscular atrophy. Journal of Medicinal Chemistry 2008;51(3):449‐69. [DOI] [PubMed] [Google Scholar]
Ting 2007
- Ting CH, Lin CW, Wen SL, Hsieh‐Li HM, Li H. Stat5 constitutive activation rescues defects in spinal muscular atrophy. Human Molecular Genetics 2007;16(5):499‐514. [DOI] [PubMed] [Google Scholar]
Tisdale 2015
- Tisdale S, Pellizzoni L. Disease mechanisms and therapeutic approaches in spinal muscular atrophy. Journal of Neuroscience 2015;35(23):8691‐700. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tiziano 2010
- Tiziano FD, Lomastro R, Pinto AM, Messina S, D'Amico A, Fiori S, et al. Salbutamol increases survival motor neuron (SMN) transcript levels in leucocytes of spinal muscular atrophy (SMA) patients: relevance for clinical trial design. Journal of Medical Genetics 2010;47(12):856‐8. [DOI] [PubMed] [Google Scholar]
Tsai 2012
- Tsai LK, Chen YC, Cheng WC, Ting CH, Dodge JC, Hwu W‐L, et al. IGF‐1 delivery to CNS attenuates motor neuron cell death but does not improve motor function in type III SMA mice. Neurobiology of Disease 2012;45:272‐9. [DOI] [PubMed] [Google Scholar]
Tsai 2014
- Tsai LK, Chen CL, Ting CH, Lin‐Chao S, Hwu WL, Dodge JC, et al. Systemic administration of a recombinant AAV1 vector encoding IGF‐1 improves disease manifestations in SMA mice. Molecular Therapy 2014;22(8):1450‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tseng 2016
- Tseng YT, Chen CS, Jong YJ, Chang FR, Lo YC. Loganin possesses neuroprotective properties, restores SMN protein and activates protein synthesis positive regulator Akt/mTOR in experimental models of spinal muscular atrophy. Pharmacological Research 2016;111:58‐75. [DOI] [PubMed] [Google Scholar]
Uzunalli 2015
- Uzunalli G, Bora‐Tatar G, Dayangaç‐Erden D, Erdem‐Yurter H. Effects of flavonoid quercetin on survival of motor neuron gene expression. Cell Biology International 2015;39(3):350‐4. [DOI] [PubMed] [Google Scholar]
Valori 2010
- Valori CF, Ning K, Wyles M, Mead RJ, Grierson AJ, Shaw PJ, et al. Systemic delivery of scAAV9 expressing SMN prolongs survival in a model of spinal muscular atrophy. Science Translational Medicine 2010;2(35):35ra42. [DOI] [PubMed] [Google Scholar]
van Meerbeke 2013
- Meerbeke JP, Gibbs RM, Plasterer HL, Miao W, Feng Z, Lin MY, et al. The DcpS inhibitor RG3039 improves motor function in SMA mice. Human Molecular Genetics 2013;22(20):4074‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wadman 2012a
- Wadman RI, Vrancken AF, Berg LH, Pol WL. Dysfunction of the neuromuscular junction in spinal muscular atrophy types 2 and 3. Neurology 2012;79(20):2050‐5. [DOI] [PubMed] [Google Scholar]
Wadman 2017
- Wadman RI, Stam M, Gijzen M, Lemmink HH, Snoeck IN, Wijngaarde CA, et al. Association of motor milestones, SMN2 copy and outcome in spinal muscular atrophy types 0‐4. Journal of Neurology, Neurosurgery, and Psychiatry 2017;88(4):365‐7. [DOI] [PubMed] [Google Scholar]
Wadman 2019
- Wadman RI, Pol WL, Bosboom WMJ, Asselman F‐L, Berg LH, Iannaccone ST, Vrancken AFJE. Drug treatment for spinal muscular atrophy type I. Cochrane Database of Systematic Reviews 2019, Issue 12. [DOI: 10.1002/14651858.CD006281.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wirth 2006a
- Wirth B, Brichta L, Hahnen E. Spinal muscular atrophy: from gene to therapy. Seminars in Pediatric Neurology 2006;13(2):121‐31. [DOI] [PubMed] [Google Scholar]
Wirth 2006b
- Wirth B, Brichta L, Hahnen E. Spinal muscular atrophy and therapeutic prospects. Progress in Molecular and Subcellular Biology 2006;44:109‐32. [DOI] [PubMed] [Google Scholar]
Wishart 2014
- Wishart TM, Mutsaers CA, Riessland M, Reimer MM, Hunter G, Hannam ML, et al. Dysregulation of ubiquitin homeostasis and β‐catenin signaling promote spinal muscular atrophy. Journal of Clinical Investigation 2014;124(4):1821‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Woll 2016
- Woll MG, Qi H, Turpoff A, Zhang N, Zhang X, Chen G, et al. Discovery and optimization of small molecule splicing modifiers of survival motor neuron 2 as a treatment for spinal muscular atrophy. Journal of Medicinal Chemistry 2016;59(13):6070‐85. [DOI] [PubMed] [Google Scholar]
Wolstencroft 2005
- Wolstencroft EC, Mattis V, Bajer AA, Young PJ, Lorson CL. A non‐sequence‐specific requirement for SMN protein activity: the role of aminoglycosides in inducing elevated SMN protein levels. Human Molecular Genetics 2005;14(9):1199‐210. [DOI] [PubMed] [Google Scholar]
Woo 2017
- Woo CJ, Maier VK, Davey R, Brennan J, Li G, Brothers J, et al. Gene activation of SMN by selective disruption of lncRNA‐mediated recruitment of PRC2 for the treatment of spinal muscular atrophy. Proceedings of the National Academy of Sciences of the United States of America 2017;114(8):E1509‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yuo 2008
- Yuo CY, Lin HH, Chang YS, Yang WK, Chang JG. 5‐(N‐ethyl‐N‐isopropyl)‐amiloride enhances SMN2 exon 7 inclusion and protein expression in spinal muscular atrophy cells. Annals of Neurology 2008;63(1):26‐34. [DOI] [PubMed] [Google Scholar]
Zanetta 2014
- Zanetta C, Nizzardo M, Simone C, Monguzzi E, Bresolin N, Comi GP, et al. Molecular therapeutic strategies for spinal muscular atrophies: current and future clinical trials. Clinical Therapeutics 2014;36(1):128‐40. [DOI] [PubMed] [Google Scholar]
Zerres 1995
- Zerres K, Rudnik‐Schoneborn S. Natural history in proximal spinal muscular atrophy. Clinical analysis of 445 patients and suggestions for a modification of existing classifications. Archives of Neurology 1995;52(5):518‐23. [DOI] [PubMed] [Google Scholar]
Zerres 1997
- Zerres K, Rudnik‐Schoneborn S, Forrest E, Lusakowska A, Borkowska J, Hausmanowa‐Petrusewicz I. A collaborative study on the natural history of childhood and juvenile onset proximal spinal muscular atrophy (type II and III SMA): 569 patients. Journal of the Neurological Sciences 1997;146(1):67‐72. [DOI] [PubMed] [Google Scholar]
Zerres 1999
- Zerres K, Davies KE. 59th ENMC International Workshop: Spinal Muscular Atrophies: recent progress and revised diagnostic criteria 17‐19 April 1998, Soestduinen, The Netherlands. Neuromuscular Disorders 1999;9(4):272‐8. [DOI] [PubMed] [Google Scholar]
Zhang 2001
- Zhang ML, Lorson CL, Androphy EJ, Zhou J. An in vivo reporter system for measuring increased inclusion of exon 7 in SMN2 mRNA: potential therapy of SMA. Gene Therapy 2001;8(20):1532‐8. [DOI] [PubMed] [Google Scholar]
Zhou 2013
- Zhou H, Janghra N, Mitrpant C, Dickinson RL, Anthony K, Price L, et al. A novel morpholino oligomer targeting ISS‐N1 improves rescue of severe spinal muscular atrophy transgenic mice. Human Gene Therapies 2013;24(3):331‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhou 2015
- Zhou H, Meng J, Marrosu E, Janghra N, Morgan J, Muntoni F. Repeated low doses of morpholino antisense oligomer: an intermediate mouse model of spinal muscular atrophy to explore the window of therapeutic response. Human Molecular Genetics 2015;24(22):6265‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zou 2007
- Zou T, Ilangovan R, Yu F, Xu Z, Zhou J. SMN protects cells against mutant SOD1 toxicity by increasing chaperone activity. Biochemical and Biophysical Research Communication 2007;364(4):850‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Bosboom 2009
- Bosboom WM, Vrancken AF, Berg LH, Wokke JH, Iannaccone ST. Drug treatment for spinal muscular atrophy types II and III. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD006282.pub2] [DOI] [PubMed] [Google Scholar]
Wadman 2012b
- Wadman RI, Pol WL, Bosboom WM, Berg LH, Iannaccone ST, Vrancken AF. Drug treatment for spinal muscular atrophy types II and III. Cochrane Database of Systematic Reviews 2012, Issue 4. [DOI: 10.1002/14651858.CD006282.pub4] [DOI] [PubMed] [Google Scholar]


