Abstract
Despite innovations in surgical interventions, treatment of cartilage injury in osteoarthritic joints remains a challenge due to concomitant inflammation. Obstructing a single dominant inflammatory cytokine has shown remarkable clinical benefits in rheumatoid arthritis, and similar strategies are being suggested to target inflammatory pathways in osteoarthritis (OA). Here, we describe the utility of gelatin microspheres that are responsive to proteolytic enzymes typically expressed in arthritic flares, resulting in on-demand and spatiotemporally controlled release of anti-inflammatory cytokines for cartilage preservation and repair. These microspheres were designed with a net negative charge to sequester cationic anti-inflammatory cytokines, and the magnitude of the negative charge potential increased with an increase in crosslinking density. Collagenase-mediated degradation of the microspheres was dependent on the concentration of the enzyme. Release of anti-inflammatory cytokines from the loaded microspheres directly correlated with the degradation of the gelatin matrix. Exposure of the IL-4 and IL-13 loaded microspheres reduced the inflammation of chondrocytes up to 80%. Hence, the delivery of these microspheres in an OA joint can attenuate the stimulation of chondrocytes and the resulting secretion of catabolic factors such as proteinases and nitric oxide. The microsphere format also allows for minimally invasive delivery and is less susceptible to mechanically induced drug release. Consequently, bioresponsive microspheres can be an effective tool for cartilage preservation and arthritis treatment.
Keywords: chondrocytes, controlled drug release, IL-4, IL-10, IL-13, inflammation, osteoarthritis
1 |. INTRODUCTION
Osteoarthritis (OA) is the primary cause of disability among adults worldwide (Neogi, 2013). OA is a degenerative joint disease that affects one or more diarthrodial joints and is commonly associated with inflammation and tissue breakdown. OA is a whole-joint disease that includes changes in articular cartilage, subchondral bone, ligaments, and synovium and subsequent joint failure (Martel-Pelletier et al., 2016). OA starts with the “wear and tear” of the cartilage caused primarily due to trauma, obesity, and aging. Although the etiology of OA is still elusive (Boehme & Rolauffs, 2018), chondrocyte-mediated inflammatory events driven by the stimulation of innate immune receptors by damage-associated molecules are thought to be the primary cause. The damage-associated molecules activate the synovial macrophages (type A) and fibroblasts (FBs) (type B), causing synovitis. The activated synovial cells release catabolic and pro-inflammatory factors that lead to a positive-feedback loop of synovial cell activation, inflammation, and cartilage catabolism (Nefla, Holzinger, Berenbaum, & Jacques, 2016). When a significant amount of cartilage matrix is degraded, typical symptoms of OA are exhibited, including joint pain, tenderness, stiffness, loss of flexibility, grating sensation, and bone spurs.
Several therapeutic agents, including chondroprotective drugs and growth factors, anti-inflammatory cytokines, extracellular matrix constituents, and immunomodulatory stem cells, have been tested to attenuate the cartilage damage (Ying, Tyler, & Ming, 2014). However, the disease-modifying interventions that specifically suppress inflammation are thought to be most efficacious for the prevention and treatment of cartilage destruction (Joshi et al., 2018; Sokolove & Lepus, 2013). Among the many inflammatory cytokines, tumor necrosis factor-alpha (TNF-α) signaling and interleukin 1 beta (IL-1β) are the major mediators of cartilage destruction (Malemud, 2004). Although a low level of these factors may be required for normal homeostasis, inflammatory and oxidative conditions disrupt the balance and drive the pathogenesis of OA. Inhibitors of secretion/activity of TNF-α and IL-1β including receptor agonists and monoclonal antibodies are capable of mitigating cartilage breakdown (Kawaguchi et al., 2009; Martel-Pelletier, Mineau, Jolicoeur, Cloutier, & Pelletier, 1998; Scott & Kingsley, 2006). More importantly, the application of anti-inflammatory cytokines including IL-4, IL-10, and IL-13 are capable of reducing inflammation while stimulating protective chondrocyte metabolic response (Lubberts et al., 1999; Lubberts, Joosten, Helsen, & van den Berg, 1998; Ying et al., 2014). Studies have confirmed the inhibitory effects of anti-inflammatory cytokines on matrix metalloproteinases secretion, proteoglycans degradation, and chondrocyte apoptosis (Behrendt et al., 2016, 2018; Wojdasiewicz, Poniatowski, & Szukiewicz, 2014). Together, the anti-inflammatory cytokines are capable of exhibiting both anti-catabolic and anabolic effects for cartilage maintenance. Therefore, these anti-inflammatory cytokines have been proposed as therapeutic agents to modulate the inflammatory response and help prevent chondrocytes hypertrophy and subsequent osteophyte formation (Wojdasiewicz et al., 2014).
Other therapeutic agents include cartilage anabolic factors such as transforming growth factor-beta (TGFβ), FB growth factors, and insulin-like growth factor-I that are capable of partially recovering chondrocytes function (Andia & Maffulli, 2013; Ying et al., 2014). Similarly, chondroprotective drugs and matrix constituents such as glucosamine sulfate, chondroitin sulfate, hyaluronic acid, and diacerein are shown to decrease NF-kB activation mediated by IL-1β (Gouze et al., 2002). A key limitation of the anabolic factors and chondroprotective matrices is that they may mitigate the cartilage breakdown but cannot prevent the infiltration of immune cells. Nevertheless, their effects can be synergistically enhanced when co-delivered with anti-inflammatory cytokines (Ying et al., 2014). Alternatively, nonsteroidal anti-inflammatory drugs (NSAIDs) that reduce inflammation by inhibiting the cyclooxygenase enzymes (COX 1 & 2) are commonly prescribed to relieve the joint pain (Jiang et al., 2010; Noble, King, & Olutade, 2000). However, long-term usage of NSAIDs leads to gastro-intestinal complications and cardiovascular disease.
Together, delivering anti-inflammatory cytokines to the OA joints has the potential to suppress inflammation while stimulating or augmenting chondroprotective mechanisms. They are safe, allow quick cartilage recovery, and can be effective at almost all stages of the arthritis progression. However, they possess short articular half-lives, which reduce their therapeutic efficacy. For example, IL-10 has a half-life of 2–3 hr at body temperature (Suga, Keshavjee, & Liu, 2005). Therefore, an efficient drug delivery vehicle is essential to improve the drug residence time and hence enhance the therapeutic efficacy of anti-inflammatory cytokines.
Polymeric biomaterials are generally attractive for controlled release of macromolecular substances such as peptides and large proteins owing to their chemical tunability (Fenton, Olafson, Pillai, Mitchell, & Langer, 2018). Several materials have been tested for articular drug delivery including chitosan (Zhou et al., 2015), poly(lactic-co-glycolic) acid, nanosilicates (Cross, Carrow, Ding, Singh, & Gaharwar, 2019), Poly(N-isopropylacrylamide) (McMasters, Poh, Lin, & Panitch, 2017), and avidin (Bajpayee, Quadir, Hammond, & Grodzinsky, 2016). However, clinical translation has, so far is minimal. Here, we describe a microsphere drug delivery system that is responsive to OA flares, resulting in on-demand and spatiotemporally controlled release of cytokines for immunomodulation and subsequent cartilage preservation (Figure 1). The microspheres studied were made out of gelatin crosslinked by genipin, which allows ionic complexation and sequestration of charged anti-inflammatory cytokines. Gelatin polypeptides were chosen due to their easy tunability (Gómez-Guillén, Giménez, López-Caballero, & Montero, 2011) and lower immunogenicity compared to their precursor collagen due to the removal of aromatic groups (Lynn, Yannas, & Bonfield, 2004; Rose et al., 2014). Furthermore, genipin is more biocompatible than chemical crosslinkers (Tsai, Huang, Sung, & Liang, 2000) and the degree of crosslinking can be controlled through the concentration and incubation time (Solorio, Dhami, Dang, Vieregge, & Alsberg, 2012; Solorio, Zwolinski, Lund, Farrell, & Stegemann, 2010). The microspheres are injectable and exhibit degradation and drug release controlled by the proteolytic enzymes characteristically expressed by the inflamed chondrocytes and macrophages in OA. These drug delivery vehicles can titrate the drug release to synchronize with the inflammatory response while reducing the washout of drugs during periods of low disease activity. Based on the endogenous mechanisms, the anti-inflammatory cytokines IL-4, IL-10, and IL-13 were chosen for this study. The charge potential of the microspheres to sequester the cationic cytokines was investigated. The degradation of the microspheres and the release kinetics of the cytokines were characterized using enzymatic treatment. We show that controlling the crosslinking density and hence the charge potential allows regulating both the cytokine loading and releasing capacity of the microspheres. Finally, we show that inflammatory cell-mediated release of cytokines from gelatin microspheres can alleviate the inflammation of IL-1β and lipopolysaccha-ride (LPS)-activated chondrocytes. Such biomaterial-based approaches can be used to synchronize drug release during inflammation, and thereby prolong the therapeutic effects and the residence time of anti-inflammatory cytokines.
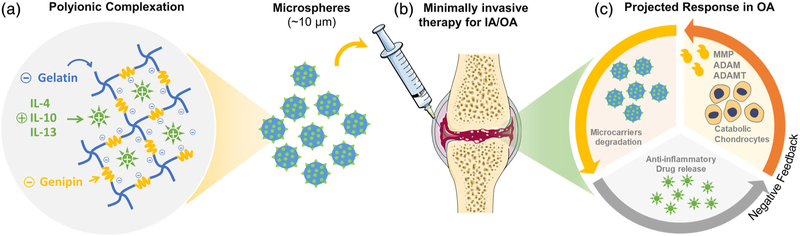
FIGURE 1.
Bioresponsive microspheres for delivery of the anti-inflammatory cytokines in osteoarthritis (OA). (a) The crosslinking of gelatin using amine-reactive genipin imparts a net negative charge on microspheres, which is used to sequester cationic cytokines electrostatically. (b) The microcarriers allow for minimally invasive delivery and are less susceptible to mechanically induced drug release and are conformant to the intra-articular space. (c) The microspheres exhibit degradation and drug release controlled by the proteolytic enzymes characteristically expressed by the inflamed chondrocytes and macrophages in OA. These drug delivery vehicles can titrate the drug release to synchronize with the inflammatory response while reducing the washout of drugs during periods of low disease activity
2 |. MATERIALS AND METHODS
2.1 |. Microsphere fabrication
Gelatin microspheres were made by emulsification of solubilized gelatin and subsequent crosslinking using genipin. Briefly, gelatin from porcine skin (type A, 175 bloom, Sigma) was dissolved in calcium and magnesium-free phosphate-buffered saline (1× PBS; Invitrogen, Carls-bad, CA) to make a 6 wt % stock solution. The 1–2 ml of stock solution was then dispensed dropwise to polydimethylsiloxane (PDMS) bath kept at 40°C. The mixture was stirred for 5 min using a dual radial-blade impeller at 2,000 rpm to emulsify the gelatin solution in PDMS. After the emulsification process, the emulsion was cooled in an ice bath and mixed for an additional 25 min to promote gelation. Then 1 wt % genipin (Wako Chemicals) in PBS was added to the emulsion (2 ml genipin solution per 90 ml emulsion) and mixed at 1,500 rpm for an additional 30 min in the ice bath to initiate crosslinking. The mixture was then collected in 50 ml centrifuge tubes and mixed with 0.1 vol/vol % of non-ionic surfactant solution and vigorously mixed to encourage phase separation and centrifugation at 200g for 5 min. After centrifugation, the PDMS supernatant was decanted without disturbing the pelleted microsphere. The microspheres were washed three times with the surfactant solution. The influence of surfactants on emulsification was also tested by adding 0.1 vol/vol % of non-ionic surfactant solution during the emulsification of gelatin in PDMS solution. Two surfactants Pluronic® L101 (denoted as L101, BASF, Milford, CT) and Tween® 20 (denoted as Tween, Sigma) with different hydrophilic–lipophilic balance (HLB) were tested. L101 has an HLB in the range of 1–7, due to a large hydrophobic end, whereas Tween has an HLB around 17 due to its large hydrophilic chain (Kim & Hsieh, 2001). These structural differences not only influence their properties as an emulsifier but also determine their cytotoxicity (Annamalai, Naik, Prout, Putnam, & Stegemann, 2018). The microspheres made from 1 ml of gelatin hydrogel were then suspended in 5 ml of 1 wt % genipin in PBS to promote complete crosslinking. The mixture was incubated at room temperature, and samples were collected at 3, 6, 12, and 24 hr and washed with 100% ethanol to remove genipin. The microspheres were then washed three times with deionized water to remove left-over salts and stored in −80°C. Then, the frozen microspheres were lyophilized to remove residual water and stored at −20°C for further characterization.
2.2 |. Cell culture
A mouse chondrogenic cell line (ATDC-5, derived from teratocarci-noma AT805, Sigma) and a primary human lung FB cell line (Lonza, Walkersville, MD) obtained from commercial sources were used to characterize gelatin microspheres. ATDC-5 is a commonly used chondrogenic cell line, which is shown to emulate the articular cartilage phenotype (Mayhew et al., 2014). Hence, ATDC-5 was deemed suitable for characterizing the bioresponsive therapeutic delivery system in this study. On the other hand, FBs are commonly recommended for cytotoxicity analysis of medical devices (Isama, Matsuoka, Haishima, & Tsuchiya, 2002). They are robust for vector-mediated transduction of fluorescent proteins allowing for easy cell tracking (Annamalai, Rioja, Putnam, & Stegemann, 2016). Furthermore, synovial FBs are vital players in arthritic flares (Huber et al., 2006) and are active in the synovium—the intended delivery site of the microspheres. Hence, a FB cell line was deemed suitable for the cytotoxicity analysis of microspheres. For morphological analysis and temporal visualization of cell proliferation using fluorescent micros-copy, the FB was transduced with a lentiviral vector expressing green fluorescent protein (GFP) as described previously (Annamalai et al., 2016). Stably transfected cells were selected using puromycin treatment. Finally, highly fluorescent FB was sorted out using cell sorter (MoFlo Astrios EQ, Beckman Coulter) and used for experiments. The chondrogenic cells were cultured in Dulbecco’s modified eagle medium (DMEM)/F12-Glutamax culture media (ThermoFisher, Waltham, MA) supplemented with 5% fetal bovine serum (FBS, ThermoFisher) and 1% penicillin–streptomycin (ThermoFisher). The FBs were cultured in DMEM (ThermoFisher) supplemented with 10% FBS and 1% penicillin–streptomycin. The cell response to degradation and cytokine release from the microspheres were characterized by metabolic performance, cell proliferation, and inflammation assays. The cell response was characterized in both 2D (tissue culture-treated surface) and 3D pellet cultures (chondrocytes only).
2.3 |. Cell proliferation and viability assay
The cell proliferation was assessed through the quantification of double-stranded DNA using Quant-iT™ Picogreen™ dsDNA assay kit (Invitrogen), and the metabolic performance was quantified using PrestoBlue™ Reagent (Invitrogen). Briefly, cells were seeded at a cell density of 5,000 cells/cm2 in a 24-well plate, and after 48 hr of incubation, microspheres were added. To avoid aggregation, 5 mg of microsphere was suspended in 1 ml of PBS and sonicated on ice for 2 min at 10% amplitude using a probe sonifier (Branson, Danbury, CT). Then, 100 μg of microspheres were added to each well on top of the cells and cell proliferation and metabolic activity were quantified at days 0, 1, 3, and 7 using corresponding assays. The values were then compared with untreated controls.
2.4 |. Inflammatory cytokine treatment and quantification of inflammation
The capability of drug-loaded microspheres to resurrect chondrocytes under osteoarthritic conditions was tested as follows. Microspheres loaded with recombinant murine interleukin 4 (IL-4, Peprotech, Rocky Hill, NJ) with a molecular weight of 13.5 kDa, recombinant murine interleukin 10 (IL-10, Peprotech) with a molecular weight of 18.7 kDa, and recombinant murine interleukin 13 (IL-13, Peprotech) with a molecular weight of 12.3 kDa, along with corresponding positive and negative control conditions were tested. First, the chondrocytes were seeded at a cell density of 50,000 cells/cm2 in a 24-well plate and stimulated with inflammatory cytokines and endotoxin to mimic the inflammatory environment of OA. Several inflammatory cytokines and endotoxin were tested, including recombinant murine IL-1β (Peprotech), LPS (Sigma), interferon-gamma (IFNγ, Peprotech), and TNF-α (Peprotech) to induce an inflammatory chondrocyte phenotype. Then, the inflammatory state of chondrocytes was quantified by measuring the nitric oxide (NO) production using Griess Reagent kit (Invitrogen), as described previously (Hayashi, Abe, Yamate, Taguchi, & Jasin, 1997). Briefly, 75 μl of culture supernatant was mixed with 75 μl of Griess reagent (1% sulfanilamide in 5% phosphoric acid and 1% N-(1-naphthyl) ethylenediamine dihydrochloride), incubated for 5 min at 37°C, and the optical density was measured at 540 nm using a plate reader (Synergy H1™, Biotek, Winooski, VT).
2.5 |. Microspheres characterization
The physical and biochemical properties of the microspheres were thoroughly characterized using confocal imaging and analysis, zeta potential analysis, enzymatic degradation analysis, cytokine loading and release studies, and cell culture studies. The crosslinking of gelatin using amine-reactive genipin imparted a net negative charge on microspheres and a unique fluorescence response at 590/620 Ex/Em wavelengths. The distinct fluorescence was used for the physical and morphological characterization of the microspheres. Confocal stacks of the microspheres were taken using Nikon A1 confocal microsphere for morphological analysis and quantification of size variability. The confocal stacks were processed using Image J software (National Institute of Health) to measure the diameter of the microspheres. A violin plot was created to show the size distribution and variability of the microspheres among different conditions.
The zeta potential of the microspheres was measured using a Zetasizer (Malvern, Westborough, MA). The zeta potential of the microspheres is the assessment of the charge affinity that can be used to drive the polyionic complexation with the cationic cytokines, including IL-4, IL-10, and IL-13. The microspheres were suspended in a neutral sucrose solution (13 wt %) as per the manufacturer’s recommendations to avoid aggregation and settling of the microspheres. The microspheres were sonicated for 2 min at 10% amplitude in ice using probe sonifier before each measurement.
For cytokine loading, the lyophilized microspheres were swelled in PBS and suspended in concentrated cytokine stock solution (20–40 μg/ml) overnight at 37°C. For loading 1 mg of dry microspheres, 10–20 μl of the cytokine stock solution was supplied and incubated under static conditions. The loading concentrations were determined empirically. Loaded microspheres were then washed in PBS containing 10 mg/ml albumin to remove unbound cytokines. The loading efficiency was determined by measuring the concentrations of cytokines in the wash buffer using the respective cytokine ELISA kit (R&D Systems). The supplier’s recommended stock concentrations of 40, 40, and 20 μg/ml, for IL-4, IL-10, and IL-13, respectively, showed maximum loading based on the release rates. However, no detectable unbound peptides were seen in the loading solution even at higher loading concentration. This is presumably due to the very low half-lives of unbound cytokines in aqueous solution at 37°C. Hence, the loading was reported based on the total protein released after degradation instead of the unbound protein detected in the loading solution, which can be misleading.
For enzymatic degradation and release studies, 100 μg of microspheres were sonicated, loaded with cytokines, and suspended in collagenase solution (Worthington, CLSPA: Activity 1,090 Units/mg) and kept at 37°C until complete degradation. The degradation of the crosslinked microspheres was measured fluorometrically by measuring the fluorescence of the supernatant every 5 min for 48 hr at 590/620 Ex/Em. The fluorescence of the crosslinked microspheres also allowed the visualization of their morphology. The cytokines released from the degrading microspheres were measured by ELISA (R&D Systems).
For cell-mediated degradation and release studies, microspheres loaded with recombinant murine IL-4, IL-10, IL-13, and corresponding positive and negative control conditions were tested. First, the chondrocytes were seeded at a cell density of 50,000 cells/cm2 in a 24-well plate and stimulated with inflammatory cytokines (IL-1β, Peprotech; IFNγ, Peprotech; TNF-α, Peprotech) and endotoxin (LPS, a toll-like receptor 4 [TLR4] activator, Sigma) to mimic the inflammatory environment of OA. After 24 hr, 1 μg of cytokine-loaded and control microspheres were added to each well of the 24-well plate. Culture media samples were collected at specific time points. The inflammatory state of chondrocytes was quantified by measuring the NO production in the culture media samples using the Griess Reagent kit, as described in Section 2.4.
2.6 |. Statistical analysis
All measurements were performed at least in triplicate. Data are presented as mean ± 1 SD. Analysis of variance (ANOVA) was used for multigroup comparisons, and Student’s t test with a 95% confidence limit (two-tailed and unequal variance) was used for paired comparisons, and the p-values adjusted with Bonferroni correction wherever applicable. Differences with p < .05 were considered statistically significant.
3 |. RESULTS AND DISCUSSION
3.1 |. Microsphere fabrication and influence of surfactant treatment
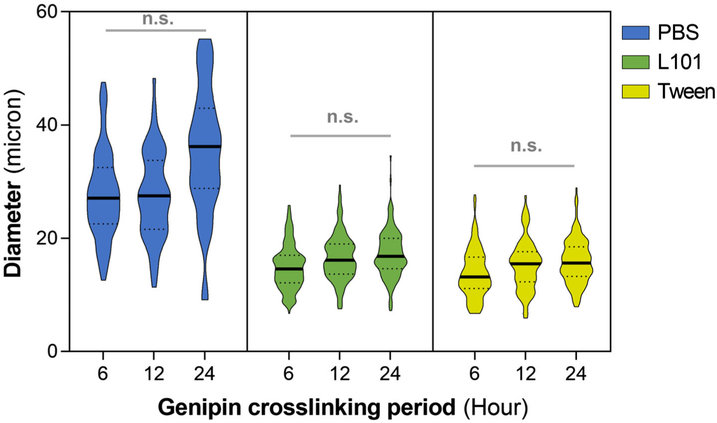
The bioresponsive microspheres were fabricated by water-in-oil emulsification of a gelatin solution, which produced spherical droplets on the same size scale as mammalian cells (10–30 μm). As the sol–gel transition of gelatin is around 30°C, it is imperative to crosslink the matrix to enhance stability under physiological conditions. Genipin, a relatively nontoxic natural crosslinking agent compared to other chemical crosslinkers (Tsai et al., 2000), was employed to stabilize the gelatin matrix, which yielded insoluble microspheres. The size of the microspheres can be modified through various factors, including the speed of emulsification and the viscosity of gelatin and PDMS solutions. The application of surfactants was investigated to attain a homogenous size distribution of microspheres, which enables a more predictable degradation and drug release rates. Tween and L101 are nonionic surfactants commonly used in biotechnology and pharmaceutical applications (Gates, Grad, Birek, & Lee, 1994; Tomlinson, Demeule, Lin, & Yadav, 2015). The amphiphilic surfactant molecules localize to the water–oil interface, which is thermodynamically favored (Lawrence & Rees, 2000). A one-way ANOVA of the size distribution showed that the addition of the surfactants (0.1% L101 or 0.1% Tween) during emulsification yielded microspheres with significantly smaller diameters compared to the non-surfactant PBS control (p < .01, Table 1). The surfactant treatment also reduced the size variability, providing a more homogenous population of microspheres compared to PBS control (Figure 2).
TABLE 1.
The influence of surfactant treatments on microspheres size distribution
| PBS | L101 | Tween | Statistical analysis# | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Hours of crosslinking | Mean ± SD (μm) | Median (μm) | Mean ± SD (μm) | Median (μm) | Mean ± SD (μm) | Median (μm) | PBS vs. L101 | PBS vs. Tween | L101 vs. Tween |
| 6 | 27.9 ± 7.7 | 27.1 | 14.2 ± 3.8 | 14.6 | 13.9 ± 4.3 | 13.2 | p < .001 | p < .001 | p = .092 |
| 12 | 27.7 ± 7.7 | 27.5 | 16.6 ± 4.1 | 16.2 | 15.3 ± 4.4 | 15.5 | p < .001 | p < .001 | p = .080 |
| 24 | 35.4 ± 11.1 | 36.2 | 17.3 ± 4.2 | 16.8 | 16.0 ± 3.9 | 15.6 | p < .001 | p < .001 | p = .018 |
FIGURE 2.
The influence of surfactant treatments on microspheres size distribution. The addition of nonionic surfactants Tween and L101 (0.1%) during gelatin emulsification yielded homogenous microspheres with a narrower size distribution compared to control PBS condition. The degree of crosslinking of the gelatin matrix, varied by the incubation period in the genipin crosslinking solution, had no significant effect on the microsphere size and its distribution. The solid line within the plot indicates the median and the dotted lines on the lower and upper ends represent the 25th and 75th quartile, respectively. n.s. indicates no statistical significance
This effect is due to the stabilization of the droplets by the surfactant molecules that prevent droplet growth (coalescence) (Rowe, 1965). In addition, smaller droplets can be formed more easily in the surfactant-treated conditions because of lower dynamic interfacial tensions. The size of the microspheres is also crucial in determining the loading capacity of the vehicles because the internal loading volume is proportional to the square of the radius. Although smaller microspheres can limit the drug loading capacity, they can minimize mechanically induced drug release and facilitate smoother articular movements of the knee. The degree of crosslinking of the gelatin matrix, varied by the incubation period in the genipin crosslinking solution, had no significant effect on the microsphere size and its distribution (Figure 2). Overall, the surfactant treatment not only reduced the mean size of the microspheres but also narrowed the size distribution of microspheres compared to that of PBS control condition.
3.2 |. Cytotoxicity of surfactant-treated microspheres
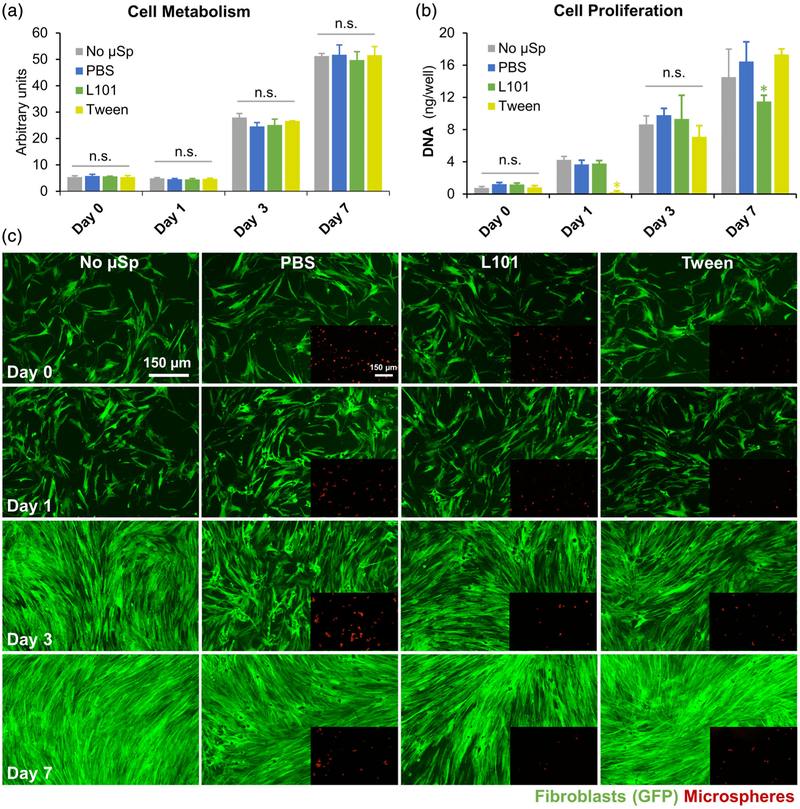
The cytotoxicity of the microspheres fabricated through surfactant treatment and genipin crosslinking was investigated through the quantification of cell metabolism and proliferation. Human FBs were chosen as a test cell type and were co-cultured with microspheres fabricated with different treatments. No change in cell metabolism was noticed when the cells were co-cultured with microspheres (Figure 3a). However, the rate of cell proliferation quantified through a DNA assay showed slightly altered growth rates in surfactant-treated conditions. The L101 treatment, although it did not exhibit any short-term cytotoxicity, affected the cell proliferation in the long-term cultures, as evident by a ~20% reduction in cell proliferation on day 7 compared to the control (Figure 3b). On the other hand, Tween treatment showed short-term cytotoxicity as seen from the reduced cell proliferation rate at 24 hr, but the cells recovered within 7 days of culture (Figure 3b). The fluorescent micrographs of the GFP-FBs taken at various time points showed no differences in cell morphology and proliferation among the different conditions (Figure 3c). The differences in the cytotoxicity of the surfactants are likely due to the differences in HLB of the surfactants and their subsequent ability to penetrate the cell membrane. Although the effects are minor, Tween was chosen as the standard surfactant for microsphere fabrication due to its improved ability to maintain cell viability over the long term. For further characterization and microsphere use, multiple washes were incorporated for the thorough removal of any residual surfactants in the microspheres.
FIGURE 3.
Cytotoxicity of the microspheres. (a) Human fibroblasts (FB) co-cultured with microspheres fabricated with different treatments exhibited no change in cell metabolism. (b) However, the rate of cell proliferation (DNA assay) showed slightly altered growth rates in microspheres produced with surfactants. The L101 treatment, although it did not exhibit any short-term cytotoxicity, showed a ~20% reduction in cell proliferation on day 7 compared to the control. Tween treatment, on the other hand, showed short-term cytotoxicity, but the cells recovered within 7 days of culture. (c) The fluorescent micrographs of the GFP-fibroblasts taken at various time points demonstrated no differences in cell morphology and proliferation among the different conditions. Scale bars indicate 150 μm in length. n.s. indicates no statistical significance and * indicates p < .05
3.3 |. Influence of crosslinking density on the charge potential of microspheres
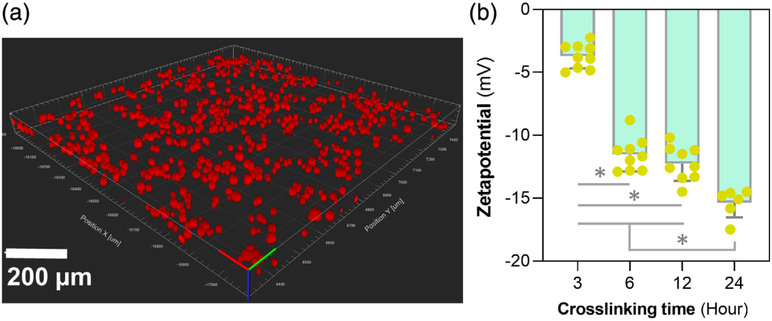
The crosslinking density of the microspheres can be controlled by varying the genipin incubation period (Annamalai et al., 2018; Solorio et al., 2010). The genipin crosslinking reaction occurs in two steps: First, the primary amines in gelatin form an intermediate through Michael addition, followed by a secondary amide link formation with the genipin ester group through nucleophilic substitution (Annamalai, Turner, et al., 2018). The influence of the crosslinking density on the charge potential of the microspheres was characterized by measuring zeta potential. Based on the cytotoxicity analyses discussed above, Tween was used as the standard surfactant for microsphere fabrication (Figure 4a, confocal images of microspheres fabricated with Tween). The increase in crosslinking density correlated with a decrease in surface charge of the microspheres as measured through the zeta potential (Figure 4b). A slight but not significant reduction in zeta potential was seen between the microspheres that were crosslinked with genipin for 6 and 12 hr. However, microspheres crosslinked for 24 hr showed a drastic decrease in the zeta potential (p < .05). The charge potential of the microsphere is a crucial driving force in ionically complexing the cationic cytokines, including IL-4 (pI = 9.17), IL-10 (pI = 8.19), and IL-13 (pI = 8.69) to the gelatin matrix. The control over the crosslinking density and hence, the charge potential helps to optimize the cytokines loading and releasing capacity of the microspheres.
FIGURE 4.
Charge potential of the microspheres. (a) Confocal images of microspheres fabricated with Tween treatment showing spherical morphology. (b) The zeta potential measurements of the microspheres showed a correlation between the increase in crosslinking density of the gelatin matrix and a decrease in surface charge potential. * indicates p < .05
3.4 |. Enzymatic degradation and cytokine release from microspheres
The bioresponsivity of the microspheres is the result of their selective degradability to the enzymes characteristically expressed in OA. During inflammation, many contributing cells secrete matrix-degrading catabolic enzymes at levels not usually seen under physiological conditions. These catabolic enzymes can degrade the crosslinked-gelatin matrix in a concentration-dependent manner. The resulting bioresponsivity can be optimized through the method and the extent of crosslinking. In this study, the microspheres were treated with genipin to ensure a crosslinking density of ~90% of the primary amines such that the degradation happens only during the onset of an inflammatory flare.
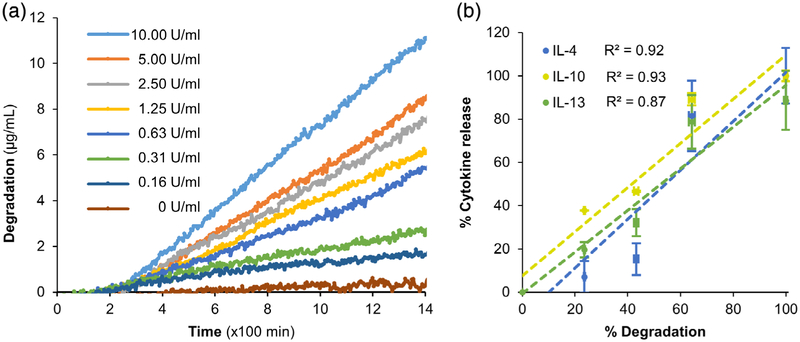
To demonstrate the bioresponsivity of the microspheres, the microspheres were subjected to enzymatic degradation at increasing collagenase concentrations. Treatment of gelatin microspheres with different concentrations of collagenase at 37°C exhibited a dose-dependent degradation rate (Figure 5a). The degradation rate was insignificant under control conditions (complete culture media, 0 U/ml of collagenase), while at high collagenase concentrations (>5 U/ml), there was a rapid degradation of the microspheres. At 5 U/ml collagenase treatment, a steady rate of degradation of microspheres was seen that lasted for more than 48 hr. Hence, 5 U/ml collagenase treatment was used to characterize the cytokine release from the microspheres. The anti-inflammatory cytokines were loaded into the crosslinked microspheres through electrostatic sequestration by the negatively charged microspheres. The microspheres were loaded with 200–400 ng of IL-4, IL-10, or IL-13 per mg of the microspheres. Upon collagenase degradation, the cytokines were released from the microspheres at rates that linearly correlated with the rate of degradation of the microspheres (Figure 5b).
FIGURE 5.
Enzymatic degradation of microspheres and release of cytokines. (a) Collagenase treatment of gelatin microspheres at 37°C exhibited a dose-dependent degradation. The degradation rate was insignificant in culture media (0 U/ml of collagenase), while rapid degradation of the microspheres was seen at high collagenase concentrations (>5 U/ml). (b) Upon collagenase degradation, the cytokines IL-4, IL-10, and IL-13 were released from the respective microspheres at rates that linearly correlated with the rate of degradation
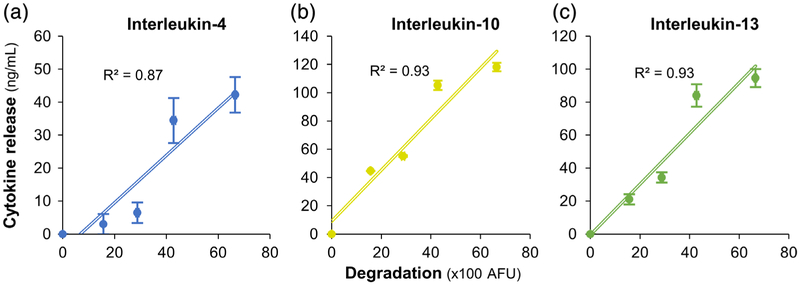
We were able to achieve a maximum loading and subsequent release of 40 ng/mg of IL-4 (Figure 6a), 120 ng/mg of IL-10 (Figure 6b), and 100 ng/mg of IL-13 (Figure 6c). In all formulations, the release rate of the cytokines linearly correlated with the rate of microsphere degradation (Figure 5, R2 ≥ 0.87, p < .05). These results demonstrate the on-demand delivery of cytokines from the microspheres in a catabolic microenvironment.
FIGURE 6.
Enzymatic degradation and release of cytokines. A maximum loading and subsequent release of (a) 40 ng/mg of IL-4, (b) 120 ng/mg of IL-10, and (c) 100 ng/mg of IL-13 were achieved using the microspheres. In all conditions, the release rate of the cytokines linearly correlated with the rate of microsphere degradation (R2 ≥ 0.87, p < .05)
3.5 |. Chondrocyte stimulated with inflammatory agents create an osteoarthritic phenotype
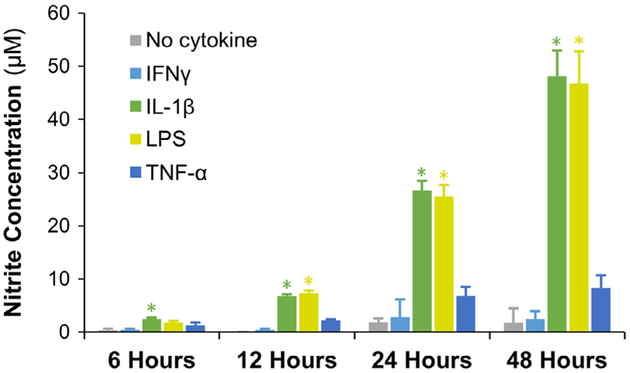
During OA, the anabolic pathways of chondrocytes including collagen type II and aggrecan production are turned off while metalloproteinases and NO production are upregulated (Rahmati, Mobasheri, & Mozafari, 2016). Such an osteoarthritic chondrocyte phenotype would be the ideal culture model to validate the efficacy of drug-loaded microspheres. To create such a phenotype, inflammatory mediators that are commonly implicated in chondrocyte activation were studied. Specifically, LPS (a TLR4 activator), IL-1β, IFNγ, and TNF-α were tested. The resulting osteoarthritic chondrocyte phenotype was evaluated and compared through the quantification of NO, the key mediator in the progression of OA (Studer, Jaffurs, Stefanovic-Racic, Robbins, & Evans, 1999). Murine IL-1β (2 ng/ml), LPS (200 ng/ml), IFNγ (10 ng/ml), or TNF-α (10 ng/ml) were individually supplemented in the growth media and supplied to the mouse chondrogenic cells (ATDC-5). The cells were then cultured for 48 hr, and cellular NO production was measured at various time points using the Griess assay (Figure 7). The assay indicated that TNF-α and IFNγ treatment did not induce any increase in the NO concentration of the cells. However, IL-1β and LPS showed a significant increase in NO production within 12 hr. The chondrocyte activation by IL-1β is mainly induced by the activation of nuclear factor κB, which is ubiquitously expressed transcription factor that mediates inflammatory and catabolic event in OA (Marcu et al., 2010). TLR4, on the other hand, is a type of pattern-recognition receptor that binds not only LPS but also host-derived debris known as damage-associated damage patterns generated during catabolic events (Gómez, Villalvilla, Largo, Gualillo, & Herrero-Beaumont, 2014). Therefore, IL-1β and LPS can faithfully replicate the chondrocyte phenotype exhibited in OA. Hence, they were chosen for the activation of chondrocytes and subsequent testing of drug-loaded microspheres.
FIGURE 7.
Chondrocyte activation using anti-inflammatory agents. Chondrocytes subjected to TNF-α and IFNγ treatment did not show any increase in the NO production. However, IL-1β and LPS stimulated chondrocytes showed a significant increase in NO production within 12 hr compared to control and other treatment conditions. Therefore, IL-1β and LPS treatment were chosen to reproduce the OA chondrocyte phenotype. * indicates p < .05
3.6 |. Microspheres modulated the inflammatory phenotype of activated chondrocytes
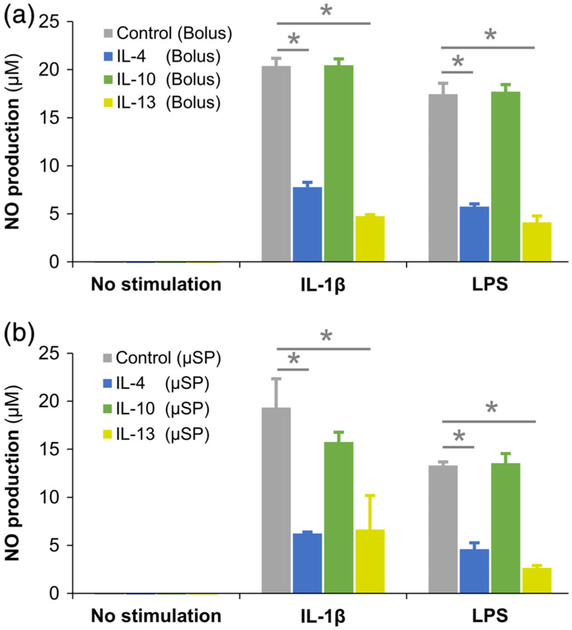
Chondrocytes were stimulated with murine IL-1β (2 ng/ml) or LPS (200 ng/ml) to create an OA phenotype that was validated by their upregulated NO production (Figure 7). The activated chondrocytes were then treated with IL-4, IL-10, or IL-13 loaded microspheres (maximum load 40, 120, and 100 ng of respective cytokine/mg of microspheres) and the NO production was quantified at day 3. The NO production by the microspheres treated cultures was compared to that of the corresponding bolus treatment cultures in which 200 mg of respective cytokines were directly supplemented in the growth media and replenished every 24 hr (Figure 8a). The NO production of the chondrocytes showed that the IL-4 and IL-13 released from the microspheres successfully reduced the chondrocyte inflammation by 65–80% within 3 days (Figure 8b, Table 2) while IL-10 showed only a marginal effect with no significant decrease in NO production compared to untreated controls. IL-10, although a potent anti-inflammatory cytokine, is known to primarily elicit anti-inflammatory activity only in synergy with other anti-inflammatory cytokines (van Roon, Lafeber, & Bijlsma, 2001). Hence, only a marginal suppression of inflammation is seen in IL-10 treated conditions.
FIGURE 8.
Modulation of inflammation by cytokine-loaded microspheres. NO production by the chondrocytes treated with (a) bolus supplementation of 200 mg of cytokines in the culture media and (b) microsphere treatment condition were quantified and compared. The results showed that the IL-4 and IL-13 released from the microspheres successfully reduced the chondrocyte inflammation by 65–80% within 3 days while IL-10 showed only a marginal effect with no significant decrease in NO production compared to untreated controls. Compared to bolus treatment, the microspheres mediated delivery of the anti-inflammatory cytokines exhibited comparable or better performance. * indicates p < 0.05
TABLE 2.
Percent reduction in nitric oxide production by activated chondrocytes after treatment with anti-inflammatory cytokines (compared to untreated control)
| Reduction in NO production compared to untreated control | ||||||
|---|---|---|---|---|---|---|
| Bolus treatment | Microsphere treatment | Significance of the change in NO production between bolus and microsphere treatment | ||||
| IL-1 β (%) | LPS (%) | IL-1β (%) | LPS (%) | IL-1β | LPS | |
| IL-4 | 61.83 ± 2.4 | 67.07 ± 1.6 | 67.73 ± 0.7 | 65.36 ± 4.9 | p < .05 | n.s. |
| IL-10 | −0.4 ± 3.2 | −1.4 ± 4.3 | 21.01 ± 5.3 | −1.83 ± 7.4 | p < .05 | n.s. |
| IL-13 | 76.59 ± 0.7 | 76.39 ± 3.7 | 65.7 ± 18.3 | 80.02 ± 1.8 | n.s. | n.s. |
The last two columns show the statistical significance in nitric oxide production by activated chondrocytes between microsphere treatment and bolus treatment. n.s. indicates no statistical significance.
Compared to bolus treatment, microsphere-mediated delivery of the anti-inflammatory cytokines exhibited a comparable or better performance (Table 2). It should be noted that 2–5 times more cytokines were added to the bolus treatments compared to the microsphere treatment and were replenished every 24 hr to account for the short half-lives of the cytokines. These results show that the microspheres are efficient in titrating the drug release depending on the inflammatory response while reducing drug washout. Furthermore, the microspheres are biodegradable and can be quickly removed from the synovium once digested by the catabolic factors. Their breakdown products are nontoxic and did not show any agonistic influence on inflammatory pathways. This is noteworthy because the extended residence of nondigestible substances is known to cause inflammation and contribute to OA. Furthermore, the microcarrier format allows for minimally invasive delivery, is less susceptible to mechanically induced drug release, and is conformant to the intra-articular space. Taken together, bioresponsive microspheres can be an effective tool for cartilage preservation and arthritis treatment. Moreover, localized delivery of therapeutics can avoid undesirable systemic effects commonly seen with the use of other anti-inflammatory substances such as cyclooxygenase-2 inhibitors (Zarghi & Arfaei, 2011).
4 |. CONCLUSIONS
We have created bioresponsive gelatin microspheres that can prolong the half-life of anti-inflammatory cytokines while reducing their washout during periods of low disease activity. The gelatin microspheres are made through a facile emulsification procedure followed by appropriate post-processing that allowed tailoring the crosslinking density and the charge potential of the matrix. The negative charge potential of the microspheres allowed ionic complexation and sequestration of cationic anti-inflammatory cytokines. We previously showed that the genipin crosslinking density could be optimized such that the microspheres can be preferentially degraded by inflammatory cells and not by other noninflammatory cells (Annamalai, Turner, et al., 2018). Applying the same principle, the microspheres were made preferentially degradable by the catabolic factors symptomatically secreted by the inflamed chondrocytes and macrophages in OA. We have shown that the therapeutics sequestered in the microspheres stay intact for a prolonged period hence extending their half-lives. The therapeutics maintain their potency upon release and fully exhibit biological function. The enzymatic degradation of the microspheres was concentration-dependent, and the release of the cytokines linearly correlated with the degradation rates. The IL-4 and IL-13 loaded microspheres co-cultured with osteoarthritic chondrocytes reduced their inflammation by up to 80%. Such on-demand delivery systems that are synchronized with the catabolic responses may find general utility in wound healing, particularly in preventing inflammation-mediated cartilage damage in OA.
ACKNOWLEDGMENTS
The research reported in this publication was supported in part by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01AR062636, to J.P.S.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding information
National Institute of Arthritis and Musculoskeletal and Skin Diseases, Grant/Award Number: R01AR062636
REFERENCES
- Andia I, & Maffulli N (2013). Platelet-rich plasma for managing pain and inflammation in osteoarthritis. Nature Reviews Rheumatology, 9, 721–730. [DOI] [PubMed] [Google Scholar]
- Annamalai RT, Naik T, Prout H, Putnam AJ, & Stegemann JP (2018). Biofabrication of injectable fibrin microtissues for minimally-invasive therapies: Application of surfactants. Biomedical Materials, 13(4), 045005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annamalai RT, Rioja AY, Putnam AJ, & Stegemann JP (2016). Vascular network formation by human microvascular endothelial cells in modular fibrin microtissues. ACS Biomaterials Science & Engineering, 2 (11), 1914–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annamalai RT, Turner PA, Carson WF IV, Levi B, Kunkel S, & Stegemann JP (2018). Harnessing macrophage-mediated degradation of gelatin microspheres for spatiotemporal control of BMP2 release. Biomaterials, 161, 216–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajpayee AG, Quadir MA, Hammond PT, & Grodzinsky AJ (2016). Charge based intra-cartilage delivery of single dose dexamethasone using Avidin nano-carriers suppresses cytokine-induced catabolism long term. Osteoarthritis and Cartilage, 24(1), 71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrendt P, Feldheim M, Preusse-Prange A, Weitkamp JT, Haake M, Eglin D, … Kurz B (2018). Chondrogenic potential of IL-10 in mechanically injured cartilage and cellularized collagen ACI grafts. Osteoarthritis and Cartilage, 26(2), 264–275. [DOI] [PubMed] [Google Scholar]
- Behrendt P, Preusse-Prange A, Klüter T, Haake M, Rolauffs B, Grodzinsky AJ, … Kurz B (2016). IL-10 reduces apoptosis and extracellular matrix degradation after injurious compression of mature articular cartilage. Osteoarthritis and Cartilage, 24(11), 1981–1988. [DOI] [PubMed] [Google Scholar]
- Boehme KA, & Rolauffs B (2018). Onset and progression of human osteoarthritis—Can growth factors, inflammatory cytokines, or differential miRNA expression concomitantly induce proliferation, ECM degradation, and inflammation in articular cartilage? International Journal of Molecular Sciences, 19(8), 2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross LM, Carrow JK, Ding X, Singh KA, & Gaharwar AK (2019). Sustained and prolonged delivery of protein therapeutics from two-dimensional nanosilicates. ACS Applied Materials & Interfaces, 11(7), 6741–6750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton OS, Olafson KN, Pillai PS, Mitchell MJ, & Langer R (2018). Advances in biomaterials for drug delivery. Advanced Materials, 30(29), 1705328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates KA, Grad H, Birek P, & Lee PI (1994). A new bioerodible polymer insert for the controlled release of metronidazole. Pharmaceutical Research, 11(11), 1605–1609. [DOI] [PubMed] [Google Scholar]
- Gómez R, Villalvilla A, Largo R, Gualillo O, & Herrero-Beaumont G (2014). TLR4 signalling in osteoarthritis—Finding targets for candidate DMOADs. Nature Reviews Rheumatology, 11, 159. [DOI] [PubMed] [Google Scholar]
- Gómez-Guillén MC, Giménez B, López-Caballero ME, & Montero MP (2011). Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocolloids, 25 (8), 1813–1827. [Google Scholar]
- Gouze JN, Bianchi A, Bécuwe P, Dauça M, Netter P, Magdalou J, … Bordji K (2002). Glucosamine modulates IL-1-induced activation of rat chondrocytes at a receptor level, and by inhibiting the NF-κB pathway. FEBS Letters, 510(3), 166–170. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Abe E, Yamate T, Taguchi Y, & Jasin HE (1997). Nitric oxide production by superficial and deep articular chondrocytes. Arthritis and Rheumatism, 40(2), 261–269. [DOI] [PubMed] [Google Scholar]
- Huber LC, Distler O, Tarner I, Gay RE, Gay S, & Pap T (2006). Synovial fibroblasts: Key players in rheumatoid arthritis. Rheumatology, 45(6), 669–675. [DOI] [PubMed] [Google Scholar]
- Isama K, Matsuoka A, Haishima Y, & Tsuchiya T (2002). Proliferation and differentiation of normal human osteoblasts on dental Au-Ag-Pd casting alloy: Comparison with cytotoxicity to fibroblast L929 and V79 cells. Materials Transactions, 43(12), 3155–3159. [Google Scholar]
- Jiang D, Zou J, Huang L, Shi Q, Zhu X, Wang G, & Yang H (2010). Efficacy of intra-articular injection of celecoxib in a rabbit model of osteoarthritis. International Journal of Molecular Sciences, 11(10), 4106–4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi N, Yan J, Levy S, Bhagchandani S, Slaughter KV, Sherman NE, … Karp JM (2018). Towards an arthritis flare-responsive drug delivery system. Nature Communications, 9(1), 1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi A, Nakaya H, Okabe T, Tensho K, Nawata M, Eguchi Y, … Wakitani S (2009). Blocking of tumor necrosis factor activity promotes natural repair of osteochondral defects in rabbit knee. Acta Orthopaedica, 80(5), 606–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, & Hsieh Y-L (2001). Wetting and absorbency of nonionic surfactant solutions on cotton fabrics. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 187–188, 385–397. [Google Scholar]
- Lawrence MJ, & Rees GD (2000). Microemulsion-based media as novel drug delivery systems. Advanced Drug Delivery Reviews, 45(1), 89–121. [DOI] [PubMed] [Google Scholar]
- Lubberts E, Joosten LA, van Den Bersselaar L, Helsen MM, Bakker AC, van Meurs JB, … van Den Berg WB (1999). Adenoviral vector-mediated overexpression of IL-4 in the knee joint of mice with collagen-induced arthritis prevents cartilage destruction. Journal of Immunology, 163(8), 4546–4556. [PubMed] [Google Scholar]
- Lubberts E, Joosten LAB, Helsen MMA, & van den Berg WB (1998). Regulatory role of interleukin 10 in joint inflammation and cartilage destruction in murine streptococcal cell wall (SCW) arthritis. More therapeutic benefit with IL-4/IL-10 combination therapy than with IL-10 treatment alone. Cytokine, 10(5), 361–369. [DOI] [PubMed] [Google Scholar]
- Lynn AK, Yannas IV, & Bonfield W (2004). Antigenicity and immunogenicity of collagen. Journal of Biomedical Materials Research Part B: Applied Biomaterials, 71B(2), 343–354. [DOI] [PubMed] [Google Scholar]
- Malemud CJ (2004). Cytokines as therapeutic targets for osteoarthritis. BioDrugs, 18(1), 23–35. [DOI] [PubMed] [Google Scholar]
- Marcu KB, Otero M, Olivotto E, Borzi RM, & Goldring MB (2010). NF-κB signaling: Multiple angles to target OA. Current Drug Targets, 11(5), 599–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel-Pelletier J, Barr AJ, Cicuttini FM, Conaghan PG, Cooper C, Goldring MB, … Pelletier JP (2016). Osteoarthritis. Nature Reviews Disease Primers, 2, 16072. [DOI] [PubMed] [Google Scholar]
- Martel-Pelletier J, Mineau F, Jolicoeur FC, Cloutier JM, & Pelletier JP (1998). In vitro effects of diacerhein and rhein on interleukin 1 and tumor necrosis factor-alpha systems in human osteoarthritic synovium and chondrocytes. The Journal of Rheumatology, 25(4), 753–762. [PubMed] [Google Scholar]
- Mayhew M, Kevorkian L, Swingler T, Bevan D, Stubberfield C, Moore A, & Gavrilović J (2014). A modified and enhanced atdc5 chondrogenesis model produces an articular-like phenotype. Osteoarthritis and Cartilage, 22, S169. [Google Scholar]
- McMasters J, Poh S, Lin JB, & Panitch A (2017). Delivery of anti-inflammatory peptides from hollow PEGylated poly(NIPAM) nanoparticles reduces inflammation in an ex vivo osteoarthritis model. Journal of Controlled Release, 258, 161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nefla M, Holzinger D, Berenbaum F, & Jacques C (2016). The danger from within: Alarmins in arthritis. Nature Reviews Rheumatology, 12(11), 669–683. [DOI] [PubMed] [Google Scholar]
- Neogi T (2013). The epidemiology and impact of pain in osteoarthritis. Osteoarthritis and Cartilage, 21(9), 1145–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble SL, King DS, & Olutade JI (2000). Cyclooxygenase-2 enzyme inhibitors: Place in therapy. American Family Physician, 61(12), 3669–3676. [PubMed] [Google Scholar]
- Rahmati M, Mobasheri A, & Mozafari M (2016). Inflammatory mediators in osteoarthritis: A critical review of the state-of-the-art, current prospects, and future challenges. Bone, 85, 81–90. [DOI] [PubMed] [Google Scholar]
- Rose JB, Pacelli S, El Haj AJ, Dua HS, Hopkinson A, White LJ, & Rose FRAJ (2014). Gelatin-based materials in ocular tissue engineering. Materials, 7(4), 3106–3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe EL (1965). Effect of emulsifier concentration and type on the particle size distribution of emulsions. Journal of Pharmaceutical Sciences, 54, 260–264. [DOI] [PubMed] [Google Scholar]
- Scott DL, & Kingsley GH (2006). Tumor necrosis factor inhibitors for rheumatoid arthritis. The New England Journal of Medicine, 355(7), 704–712. [DOI] [PubMed] [Google Scholar]
- Sokolove J, & Lepus CM (2013). Role of inflammation in the pathogenesis of osteoarthritis: Latest findings and interpretations. Therapeutic Advances in Musculoskeletal Disease, 5(2), 77–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solorio L, Zwolinski C, Lund AW, Farrell MJ, & Stegemann JP (2010). Gelatin microspheres crosslinked with genipin for local delivery of growth factors. Journal of Tissue Engineering and Regenerative Medicine, 4(7), 514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solorio LD, Dhami CD, Dang PN, Vieregge EL, & Alsberg E (2012). Spatiotemporal regulation of chondrogenic differentiation with controlled delivery of transforming growth factor-β1 from gelatin microspheres in mesenchymal stem cell aggregates. Stem Cells Translational Medicine, 1(8), 632–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studer R, Jaffurs D, Stefanovic-Racic M, Robbins PD, & Evans CH (1999). Nitric oxide in osteoarthritis. Osteoarthritis and Cartilage, 7(4), 377–379. [DOI] [PubMed] [Google Scholar]
- Suga M, Keshavjee S, & Liu M (2005). Instability of cytokines at body temperature. The Journal of Heart and Lung Transplantation, 24(4), 504–505. [DOI] [PubMed] [Google Scholar]
- Tomlinson A, Demeule B, Lin B, & Yadav S (2015). Polysorbate 20 degradation in biopharmaceutical formulations: Quantification of free fatty acids, characterization of particulates, and insights into the degradation mechanism. Molecular Pharmaceutics, 12(11), 3805–3815. [DOI] [PubMed] [Google Scholar]
- Tsai CC, Huang RN, Sung HW, & Liang HC (2000). In vitro evaluation of the genotoxicity of a naturally occurring crosslinking agent (genipin) for biologic tissue fixation. Journal of Biomedical Materials Research, 52(1), 58–65. [DOI] [PubMed] [Google Scholar]
- van Roon JAG, Lafeber FPJG, & Bijlsma JWJ (2001). Synergistic activity of interleukin-4 and interleukin-10 in suppression of inflammation and joint destruction in rheumatoid arthritis. Arthritis and Rheumatism, 44(1), 3–12. [DOI] [PubMed] [Google Scholar]
- Wojdasiewicz P, Poniatowski ŁA, & Szukiewicz D (2014). The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators of Inflammation, 2014, 561459–561459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying Z, Tyler P, & Ming P (2014). Anti-inflammatory strategies in cartilage repair. Tissue Engineering Part B: Reviews, 20(6), 655–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarghi A, & Arfaei S (2011). Selective COX-2 inhibitors: A review of their structure-activity relationships. Iranian Journal of Pharmaceutical Research, 10(4), 655–683. [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Liu SQ, Peng H, Yu L, He B, & Zhao Q (2015). In vivo anti-apoptosis activity of novel berberine-loaded chitosan nanoparticles effectively ameliorates osteoarthritis. International Immunopharmacology, 28(1), 34–43. [DOI] [PubMed] [Google Scholar]