Abstract
Objective
Researchers in the cognitive sciences have demonstrated the existence of processing capacity bottlenecks in the human brain. These capacity bottlenecks restrict our ability to process and act on environmental information. The purpose of the current study was to examine whether adults with attention-deficit/hyperactivity disorder (ADHD) show reduced capacity of working memory and response selection mechanisms.
Method
Thirty-eight young adults with ADHD and 33 comparison adults were assessed using two measures of processing capacity. A dual choice-response task (PRP task) measured response selection capacity, and an n-back task measured working memory capacity. These tasks measured capacity by assessing the degree to which increasing processing load disrupted performance.
Results
Results confirmed that performance declined as cognitive load was increased, and this was true for both groups on each task. On the PRP task, the performance decline resulting from increased cognitive load was more pronounced in the ADHD group than in the control group, indicative of reduced response selection capacity in the ADHD group. On the n-back task, however, there was no group difference in the degree to which increasing processing load disrupted performance.
Conclusion
Results indicate that adults with ADHD show a specific capacity reduction of response selection. This evidence suggests a dissociation between working memory and response selection capacities, and it may have implications for understanding cognitive dysfunction in adults with ADHD.
Keywords: ADHD, information processing capacity, working memory
Models of human cognition recognize the existence of capacity-limited stages of information processing (Kahneman, 1973). These capacity-limited stages are easily overloaded and limit our ability to attend to, retain, or respond to environmental information in certain contexts: if situational processing demands exceed an individual’s capacity, then his or her performance in that situation will be compromised as a result. Restrictions on human performance resulting from capacity-limited stages of processing are modeled by experimental tasks that overload these limited capacity channels. These tasks specifically measure capacity by manipulating the amount of information that must pass through the capacity-limited channel simultaneously while other stimulus qualities and demands of the behavioral response are held constant, and capacity limits are revealed by quantifying the degree to which increased processing load disrupts task performance. Using such methods, researchers have separated capacity demands from other task parameters, such as processing speed (Pashler, 1994) or attentional control (Goldberg, Patternson, Taqqu, & Wilder, 1998).
Tasks measuring processing capacity have been used to demonstrate capacity deficits in clinical groups, such as adults with Alzheimer’s dementia (Belleville, Rouleau, Van der Linden, & Collete, 2003). Another group characterized by chronic cognitive impairment is adults with ADHD (Barkley, Murphy, & Fischer, 2010; Nigg, 2001). This group shows wide-ranging deficits in behavioral and cognitive control mechanisms (e.g., Nigg, Butler, Huang-Pollock, & Henderson, 2002; Roberts, Fillmore, & Milich, 2011a). These deficits may result from, or exist in addition to, impairments in processing capacity. In the current study, we compared the performance of adults with and without ADHD on two behavioral tasks measuring the capacities of response selection and working memory processes. The purpose was to examine whether adults with ADHD show capacity reductions, and if so, to identify which cognitive processes show impaired capacity in this clinical group.
A large literature has documented that several cognitive processes are characterized by relatively limited capacities, and that these capacity bottlenecks tap into independent stages of information processing. For example, experimental evidence has suggested that the processing limitations underlying the restrictions on working memory capacity are functionally distinct from other capacity bottlenecks (Akyürek & Hommel, 2005). There is also evidence for dissociation among the brain regions underlying capacities of working memory and response selection (Marios & Ivanoff, 2005). Considering this evidence for the independence of limited-capacity channels, it may be that clinical groups characterized by cognitive deficits, such as individuals with ADHD, will show reduced capacity in some processes but not others.
One well-documented capacity-limited process becomes evident in multi-tasking situations: people have difficulty making two responses at once. Underlying this difficulty is the limited capacity of the response selection mechanism in the human brain. Initiating a controlled response occupies all or most of the processing available for response selection (Pashler, 1994). This limitation has been modeled in the lab with dual tasks that require participants to perform two tasks concurrently. One example of this method, the psychological refractory period (PRP) paradigm, requires participants to make a speeded choice response, and then perform another choice response task in rapid succession (Pashler, 1994). Neither the first (i.e., Task 1) nor the second (i.e., Task 2) task is challenging when performed alone; however, the allocation of cognitive resources to Task 1 results in a delay in processing Task 2, because people typically lack the processing resources needed to process both tasks simultaneously (Johnston & Heinz, 1978). This disruption in Task 2 performance is commonly known as an interference effect. The amount of dual-task interference can be changed by manipulating the temporal delay between tasks (i.e., stimulus onset asynchrony [SOA]), such that a shorter interval between Task 1 and Task 2 will result in more dual-task interference. Indeed, evidence that responding to Task 2 slows as the intertask SOA decreases has been reported in numerous studies (Pashler & Johnston, 1989; Van Selst & Jolicoeur, 1997; Van Selst, Ruthruff, & Johnston, 1999). Shorter SOAs produce the most dual-task interference, because under these conditions there is less time to complete the processes required by Task 1 before Task 2 is presented (Kahneman, 1973).
It is important to note that Task 1 does not fully engage the response selection mechanism, and additional capacity can be used to process Task 2 (Navu & Miller, 2002; but see Pashler [1994] for other theoretical accounts of dual-task interference). As an individual’s capacity increases, so does the ability to simultaneously process both tasks. Thus, less dual-task interference occurs in individuals with more capacity. The difference in reaction time on Task 2 between the shortest and longest intertask SOA is referred to as the PRP effect, and this score can be used as an index of response selection capacity. Individuals with less capacity show more pronounced increases in dual-task interference as the SOA between the tasks shortens.
Recognizing that the PRP task could be used to identify impairments of response selection capacity in clinical groups, Schachar and Logan (1990) examined PRP task performance in children with and without ADHD. These researchers found that the children with ADHD were slower to respond to Task 2 than were nonclinical children, despite their similar performance on a single choice-response task. Schachar and Logan interpreted this as evidence for impaired response selection capacity in the children with ADHD. The children with ADHD did not show more impairment as SOA shortened, however, so these results only partially support impaired response selection capacity in children with ADHD. It is also possible that impairments of cognitive processes that are more executive in nature (e.g., task switching) contributed to the slower responding in the ADHD group. Thus, it is currently unknown whether ADHD is associated with reduced response selection capacity.
Another process characterized by limited capacity is working memory storage. Working memory is a collection of cognitive mechanisms that act to store information to guide goal-directed behavior (Baddeley, 1992). Working memory capacity describes the amount of information that can be retained simultaneously for this purpose (Daneman & Carpenter, 1980). There are numerous ways to measure working memory capacity, and in the current study, we used the n-back task. This task requires participants to hold several pieces of information while concurrently performing a choice-response task based on the retained information. The amount of information that must be retained can be manipulated to increase or decrease cognitive load. Increasing the amount of information that participants must retain in working memory decreases their accuracy on the choice-response task, because fewer processing resources are available to retain the necessary information (e.g., Callicott et al., 1999). Processing capacity can be measured as the degree to which accuracy declines as memory load increases, because people with less capacity will have more difficulty retaining the necessary information as the processing demands increase (Casbon, Curtin, Lang, & Patrick, 2003).
Evidence for impaired working memory capacity in adults with ADHD has been mixed. Several studies have examined behavioral performance and neuroanatomical patterns of activation of adults with ADHD performing the n-back task (Ehlis, Bähne, Herrmann, & Fallgatter, 2007; Valera, Faraone, Biederman, Poldrack, & Seidman, 2005). In general, those with ADHD did not show more pronounced impairment as processing load increased. In fact, Valera and colleagues (2005) reported null group differences in behavioral performance on the task, despite having a sufficient sample size to detect behavioral differences (n = 40). However, both of these studies used a relatively limited number of working memory load conditions, such that the highest memory load condition required participants to retain two chunks of information, which may have been insufficient to overload capacity in either group. Further, both of these studies found that the ADHD group showed reduced activity in a brain region associated with working memory (i.e., lateral prefrontal cortex), which, in the study by Ehlis and colleagues (2008), became more pronounced as memory load increased. This may suggest that performance deficits would become apparent as memory load increases and capacity limits are reached. Indeed, other studies using tasks modeling larger memory loads have demonstrated reduced working memory capacity in adults with ADHD (Finke et al., 2011).
In the current study, we examined processing capacity of working memory and response selection in groups of young adults with and without ADHD. Response selection capacity was measured using a PRP task, and working memory capacity was measured using an n-back task. The PRP task required participants to process information to make two discrete responses, whereas the n-back task required participants to process large amounts of information to guide a single response. Thus, both tasks required similar choice-responses but differed in the type of processing capacity that was tasked.
We hypothesized that individuals with ADHD would show reduced response selection capacity. Specifically, we expected that that the ADHD group would show more pronounced increases in dual-task interference as SOA was shortened (i.e., larger PRP score) relative to the control group. This prediction was based on prior work suggesting reduced response selection capacity in children with ADHD (Schachar & Logan, 1990). Considering the mixed evidence on working memory capacity in adults with ADHD (Finke et al., 2011; Valera et al., 2005), we made no specific predictions regarding working memory capacity in the ADHD group. We employed a wider range of working memory load conditions (i.e., 3-back condition) to possibly detect capacity deficits that were not apparent in prior studies (Ehlis, 2008). However, considering evidence for the functional and anatomical independence of working memory and response selection capacities (Marios & Ivanoff, 2005), and the lack of deficits of working memory capacity reported in prior studies (e.g., Valera et al., 2005), it seemed possible that those in the ADHD group would show intact working memory capacity.
Method
Participants
All participants signed an informed consent form and the project was approved by the University of Kentucky Institutional Review Board. Thirty-eight individuals with ADHD and 33 individuals with no history of ADHD participated in the study. Volunteers were recruited through advertisements (i.e., newspaper ads and posters) seeking adults for a study of neurological and motor functioning. Participants were predominately Caucasian (n = 62), although several participants identified as African American (n = 4), Asian (n = 4), and biracial (n = 1). Participation was limited to individuals who were between the ages of 19 and 30 and had no uncorrected vision problems. Volunteers with recent psychiatric episodes related to severe psychiatric illness (i.e., bipolar disorder, schizophrenia), as determined through self-report, were not invited to participate. Demographic information is shown in Table 1.
Table 1:
Demographic and diagnostic information by group
| Group |
||||||
|---|---|---|---|---|---|---|
| Control (n = 33) |
ADHD (n = 38) |
|||||
| Mean | SD | Mean | SD | t | d | |
| Demographic | ||||||
| Age | 22.1 | 1.7 | 21.3 | 1.7 | 1.8 | 0.43 |
| Education | 14.9 | 1.6 | 15.1 | 1.1 | 0.6 | 0.14 |
| IQ: Verbal | 106.7 | 6.7 | 104.5 | 11.0 | 1.1 | 0.26 |
| IQ: Nonverbal | 110.4 | 8.4 | 107.3 | 8.4 | 1.8 | 0.43 |
| IQ: Composite | 109.5 | 6.7 | 106.6 | 9.2 | 1.7 | 0.41 |
| Diagnostic | ||||||
| CAARS | ||||||
| DSM-IA | 51.0 | 11.1 | 74.6 | 11.2 | 8.1*** | 2.18 |
| DSM-HI | 46.4 | 9.7 | 63.9 | 14.3 | 5.5*** | 1.48 |
| DSM-Tot | 49.0 | 11.4 | 72.6 | 11.1 | 8.0*** | 2.16 |
| DSM | 2.4 | 2.9 | 8.7 | 4.0 | 7.4*** | 1.78 |
| AASRS | 8.1 | 5.6 | 19.9 | 6.3 | 8.3*** | 2.00 |
Note. Group contrasts were tests by independent sample t tests. For comparisons involving CAARS variables, n = 57, df = 55. For all other comparisons, n = 71, df = 69. Age is reported in years. Education refers to years of education completed. IQ: Verbal, IQ: Nonverbal, and IQ: Composite refers to respective scaled scores of the Kaufman Brief Intelligence Test. CAARS scores are T-scores; DSM-IA is DSM-IV Inattentive Symptoms, DSM-HI is DSM-IV Hyperactive-Impulsive Symptoms; DSM-Tot is DSM-IV ADHD Symptoms Total; and Index is ADHD Index. DSM refers to symptom count on the ADHD symptoms checklist. AASRS refers to total score on the ADD/H Adolescent Self-Report Scale—Short Form.
p < .001.
To ensure that members of the ADHD group were actively experiencing ADHD symptoms, only volunteers who were currently prescribed medication for ADHD were invited to participate. Participants in the ADHD group reported several different prescriptions, including mixed amphetamine salts (n = 29), methylphenidate (n = 7), and dextroamphetamine (n = 2). Further, these participants were asked to provide informed consent for their medical records to be accessed for the purpose of confirming their diagnosis. The ADHD group included only individuals whose diagnosis could be confirmed through medical records. Participants were asked to discontinue the use of their medication for 24 hours prior to the study to ensure that they were unmedicated during the testing sessions, and the experimenter verbally confirmed compliance with this request at the beginning of each session.
ADHD diagnosis was also confirmed with self-report measures of ADHD symptomatology These measures were based on the Diagnostic and Statistical Manual of Mental Disorders criteria for ADHD (4th ed. [DSM-IV]; American Psychiatric Association, 1994). All participants completed the ADD/H Adolescent Self-Report Scale—Short Form (AASRS; Robin & Vandermay, 1996) and an ADHD Symptom Checklist of 18 DSM-IV-based items adapted to assess symptoms of ADHD as they occur in adults. The AASRS—Short Form assessed symptoms experienced within the last month; this provided confirmation that participants were currently experiencing ADHD symptoms. The diagnostic cutoff score for this was 10, as recommended by the authors. The DSM-IV questionnaire was composed from items that were conceptually related to DSM-IV criteria and loaded highly onto the ADHD symptomatology factor on the Young ADHD Questionnaire—Self Report (Young, 2004). Most of the participants in the ADHD (n = 29) and the control groups (n = 28) also completed the Conners Adult ADHD Rating Scale—Self Report: Long Version (CAARS--S:L; Conners, Erhardt, & Sparrow, 1999). This measure was added to the assessment battery after data from thirteen participants had been collected. Our reason for adding this measure was to improve the validity of the diagnostic confirmation method. Similar to the other diagnostic measures, the CAARS—S: L is adapted from the DSM-IV criteria for ADHD. In addition, this measure benefits from a large normative sample of adults (Conners et al., 1999). The diagnostic cutoff on this measure was a T score of 66 on either the DSM-IV Inattentive Symptoms subscale or the DSM-IV Hyperactive/Impulsive Symptoms subscale. Volunteers with ADHD who completed the entire assessment battery were invited to participate if they scored at or above the cutoff on at least two of three questionnaires. Volunteers with ADHD who completed the AASRS and the DSM-IV- based questionnaire were invited to participate if they scored at or above the cutoff on at least one questionnaire. A licensed clinical psychologist with over 30 years of experience in diagnosing ADHD reviewed all available information pertaining to diagnostic status (i.e., symptom ratings scales, available clinical records).
Participants completed the 30-item Barratt Impulsiveness Scale (Patton, Stanford, & Barratt, 1995) as an additional measure of impulsivity. The BIS served to further validate group classification. Impulsivity is a core feature of ADHD; the scale was administered to confirm that the groups differed in trait impulsivity. This questionnaire measures impulsivity through items such as “I act on impulse” and “I consider myself always careful”. Participants indicate how frequently each statement applies to them on a 4-point Likert scale (never, occasionally, often, almost always). Possible score totals range from 30 to 120, with higher scores indicating greater total levels of impulsiveness. A t test confirmed that the ADHD group was more impulsive than the control group, t (68) = 5.9, p < .001, d = 1.56.
All participants were screened using health questionnaires and a medical history interview. These measures assessed participants’ current or past medical disorders, including serious physical disease, impaired cardiovascular functioning, chronic obstructive pulmonary disease, seizure, head trauma, CNS tumors, or current severe psychiatric illness. Participants in the control group reported past or current diagnoses of depression (n = 3), anxiety (n = 1), substance abuse (n = 1), and bipolar disorder (n = 1). Those in the ADHD group reported past or current diagnoses of depression (n = 5), anxiety (n = 1), substance abuse (n = 1), and bipolar disorder (n = 1).
Tasks
PRP task
This was a dual task that required participants to respond to two choice-responses in close succession (i.e., Task 1 and Task 2). Task 1 was a visual choice response task in which participants were presented with a “1” or “2” and required to press the corresponding key on the number pad of a standard keyboard. The numbers were presented in black against a white background. The numbers remained visible for 2,000 ms or terminated when a response occurred. Task 2 was an auditory choice response task during which participants distinguished between a high (1,000 Hz) and low tone (125 Hz). The tone was presented for 500 ms. Participants pressed the “a” key when the high tone was presented and the “z” key when the low tone was presented. Responses that occurred more than 2,000 ms after the presentation of Task 2 were not recorded.
Trials began with the presentation of a fixation point (*) for 250 ms followed by a random variable delay of 120, 180, or 240 ms. The delay was followed by the presentation of Task 1 (i.e., “1” or “2”), and this was followed by the presentation of Task 2 (i.e., high or low tone). The tasks were separated by a variable SOA (i.e., 50 ms, 200 ms, 600 ms, or 800 ms). The intertrial interval was 2,200 ms. A feedback message (e.g., the word “INCORRECT”) was displayed on the screen during the intertrial interval following any incorrect response to encourage accurate responding. Participants were instructed to emphasize speeded responding by responding as quickly as possible to each task as soon as each stimulus was presented. They were specifically instructed not to link responses (i.e., not to wait until the Task 2 stimulus was presented before responding to Task 1). They completed a two minute training block of the PRP task prior to completing the full task, and the experimenter provided corrective feedback if response linking was observed.
The task consisted of 192 trials. Task 1 stimuli (i.e., “1” and “2”) were presented during an equal number of trials (i.e., 96 trials). The same was true for Task 2. The four SOAs were used during an equal number of trials (i.e., 48 trials). There were 16 possible combinations of these variables for a trial (e.g., a possible combination: Task 1 = “1”, SOA = 50 ms, Task 2 = high tone). Each combination was presented 12 times during the task in a random order. The task required approximately 10 minutes to complete.
The main criterion variable of interest from the PRP task was RT on Task 2 at each SOA. Both a PRP score and a PRP slope were calculated for each participant. The PRP score was the difference in RT on Task 2 between the longest and shortest SOAs. PRP slope was calculated by entering each participant’s data into a bivariate regression model with SOA as the independent variable and RT as the dependent variable. The resulting slope of this model was the participant’s PRP slope.
N-back task
The n-back task was used to measure working memory capacity. During this task, participants were required to store a series of letters in memory until making a choice response based on that letter during a later trial. The task consisted of 270 trials. During each trial, participants were presented with a letter. They were instructed to remember this letter for a set number of trials and determine whether this letter matched one presented during a later trial. The number of trials that participants were required to hold each letter in memory was manipulated to change cognitive load. Participants completed this task under three cognitive load conditions (i.e., 1-back, 2-back, and 3-back). In the 1-back condition, participants pressed the “1” key on a standard keyboard if the letter from the current trial matched the letter presented in the previous trial (target) and the “2” key if the two letters did not match (nontarget). In the 2- and 3- back conditions, participants indicated whether the current stimulus matched the stimulus from 2 and 3 trials prior, respectively. Thus, under the higher cognitive load conditions, participants were required to hold more information in memory. Participants were instructed to emphasize accurate responding.
The letters presented during the n-back task were randomly presented consonants other than “X”. Each trial began with the presentation of a stimulus letter for 500 ms. The letters were black print and presented in the center of a white computer screen. Participants were given 3,000 ms to make a response, and stimulus presentation was response terminated. Participants completed three blocks of each memory load condition. Blocks were completed in a fixed order of ascending difficulty (i.e., 1-back, 2-back, and 3-back). Each block consisted of 30 trials, and breaks were provided between blocks. The target and critical characters did not match on the majority (i.e., two-thirds) of the trials. Participants performed a practice version of each load condition before starting the first block of that condition.
The criterion variables of interest on the n-back task were response accuracy and response time. Response accuracy was defined as the proportion of trials where the participant responded yes to a target or no to a nontarget (i.e., hits and correct rejections). Working memory capacity was measured as the change in response accuracy as memory load increased. We also calculated measures of discriminability (A’) and response bias (B″) to test for group differences in response strategy (Macmillan & Creelman, 1990).
Procedure
This study took place in a laboratory setting in the university’s Department of Psychology. These tasks were administered as part of a larger testing battery that included other measures of cognitive functioning. Participants first attended an individual familiarization session in which they became acquainted with the tasks and provided background information. After providing informed consent, participants were interviewed and completed questionnaires concerning their health status, drug and alcohol use, impulsivity, and demographic characteristics. The experimenter then administered the Kaufman Brief Intelligence Scale (K-BIT) to assess IQ. All participants completed the ADHD scales. The testing familiarization sessions were separated by a minimum of 24 hours.
The testing session began with the participant completing preliminary questionnaires (e.g., verification that participants had not taken any medication). Following the completion of these questionnaires, participants performed the n-back task and the PRP task. They were allowed breaks as needed between tasks to minimize fatigue effects. After the testing session concluded, the participants were debriefed and compensated approximately $50.
Results
Demographics and Diagnostic Criteria
A chi-square analysis found that gender make-up was independent of group, χ² (1, n = 71) = 0.02, p = .899. Moreover, no significant gender differences in task performance were observed, ps > .166. As seen in Table 1, the age difference between groups approached significance, p = .065, but age was not correlated with task performance. Although IQ was correlated with slope on the n-back task, the main effect of group on n-back accuracy remained significant when IQ was included in this analysis as a covariate, p = .037. Further, the group difference in IQ was not significant, so results are presented without the inclusion of IQ as a covariate.
PRP Task Performance
Task 1
A 2 (group) x 4 (SOA) mixed-design analysis of variance (ANOVA) on Task 1 RT found a main effect of SOA, F (3, 207) = 5.3, p = .002, resulting from slower RT as SOA increased. There was no significant main effect of group (p = .057) or group x SOA interaction (p = .186). Collapsed across groups, RTs in the 50, 200, 600, and 800 ms SOA conditions were 497.2 ms (SD = 108.2 ms), 508.6 ms (SD = 115.1 ms), 522.5 ms (SD = 162.2 ms), and 539.6 ms (SD = 185.5 ms), respectively. Performance on Task 1 was almost error free in both groups (M = 0.99, SD = 0.01).
Task 2
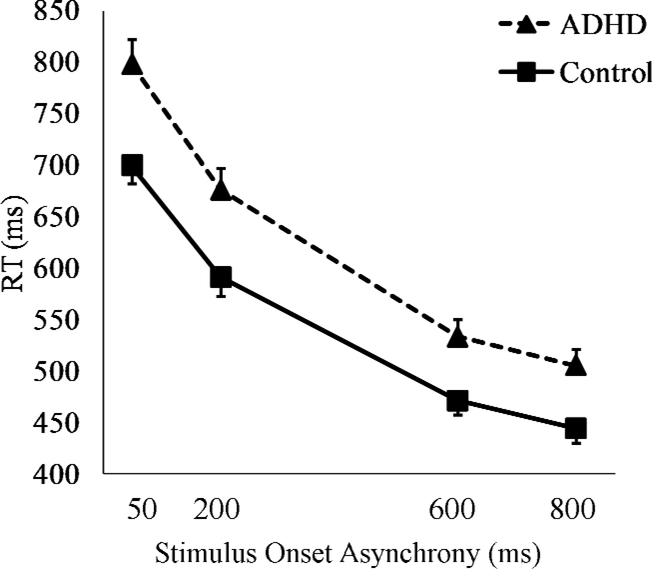
RTs on Task 2 for each group are presented in Figure 1. A 2 (group) x 4 (SOA) mixed-design analysis of variance (ANOVA) was used to examine group differences in RT. The ADHD group was significantly slower to respond than the control group across SOA conditions, F (1, 69) = 9.8, p = .003. In both groups, RT slowed as SOA decreased, and this slowing was significant, F (3, 207) = 626.6, p < .001. Table 2 presents group differences in RT at each SOA, and shows that the group difference became larger as SOA shortened. This pattern indicates that shortening the interval between the two tasks was more detrimental to the performance of the ADHD group. Indeed, a significant group x SOA interaction, F (3, 204) = 3.2, p = .026, confirmed that the ADHD group showed more dual-task interference than did the control group. Table 2 also presents PRP slope and PRP score of each group. Both PRP slope and PRP score were larger in the ADHD group, ps < .05, owing to greater increase in RT as SOA decreased. Accuracy was similar between the ADHD group (M = 0.95, SD = 0.03) and the control group (M = 0.95, SD = 0.04), t (69) = 1.5, p = .141, d = 0.36.
Figure 1.
Mean response time (+SE) on Task 2 separated by group and SOA.
Table 2:
Task 2 descriptive statistics and group differences
| SOA |
||||||
|---|---|---|---|---|---|---|
| 50 | 200 | 600 | 800 | PRP effect | PRP Slope | |
| ADHD | 798.0 (147.2) | 676.1 (132.8) | 532.9 (104.0) | 505.4 (95.1) | 292.7 (79.0) | 0.44 (0.14) |
| Control | 699.9 (102.5) | 591.0 (106.1) | 470.8 (79.4) | 443.8 (80.8) | 256.0 (65.3) | 0.39 (0.09) |
| Difference | 98.1 | 85.1 | 62.1 | 61.6 | 36.7 | 0.05 |
| t value | 3.3** | 3.0** | 2.8** | 2.9** | 2.1* | 1.7* |
Note. Reported values under the SOA heading are mean (SD) RT on Task 2 of the PRP task.
Group differences are reported in the difference row. Differences were analyzed using one tailed t-tests.
p < .05
p < .01.
N-back Task Performance
Accuracy
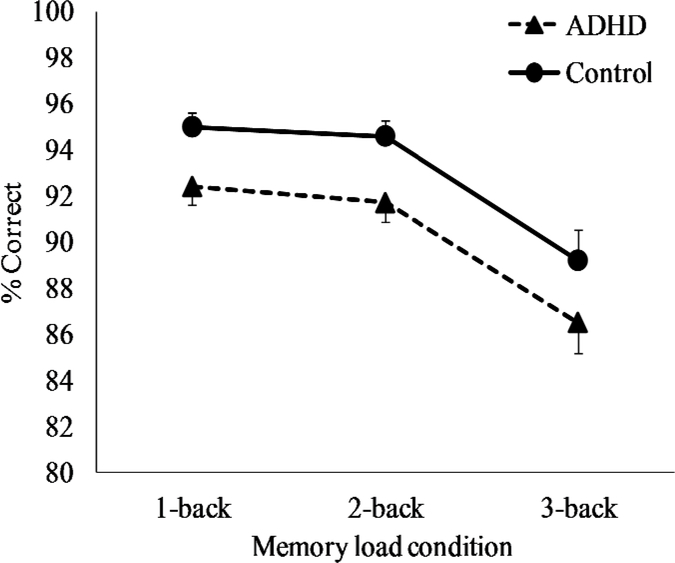
Accuracy on the n-back task was analyzed using a 2 (group) X 3 (cognitive load) mixed-design ANOVA. Figure 2 presents accuracy scores separated by group and load condition. As seen in the figure, the ADHD group (M = 0.90, SD = 0.05) was less accurate than the control group (M = 0.93, SD = 0.04) across all load conditions, and this was confirmed by the significant main effect of group, F (1, 69) = 6.1, p = .016. The entire sample became less accurate as memory load increased, as indicated by a significant main effect of memory load condition, F (2, 138) = 39.8, p < .001. There was no significant group x memory load interaction, F (2, 138) < 0.1, p = .971, and Figure 2 shows that the groups displayed comparable declines in accuracy as memory load increased.
Figure 2.
Response accuracy (+SE) on the n-back task separated by group and memory load condition.
RT
Table 3 presents RTs on the n-back task. The table shows that the ADHD group was slower to respond than the control group in the 1-back and 2-back conditions, but the group difference was reduced in the 3-back condition. A 2 (group) x 3 (cognitive load) mixed-design ANOVA examined these group differences. The main effect of group was marginally significant, F (1, 69) = 3.4, p = .068, owing to slower responding in the ADHD group. Both groups were slower to respond as memory load increased, F (2, 138) = 111.8, p < .001. There was a significant group x memory load interaction, F (2, 138) = 3.5, p = .031. As seen in Table 3, the group difference in RT was significant in the 1- and 2-back conditions but not in the 3-back condition.
Table 3:
N-back task RT, A', and B″, separated by group and load condition
| Load Condition |
|||
|---|---|---|---|
| 1-back | 2-back | 3-back | |
| ADHD | |||
| RT | 612.1 (122.8) | 842.1 (240.4) | 908.9 (236.1) |
| A' | 0.95 (0.03) | 0.94 (0.04) | 0.90 (0.07) |
| B″ | 0.32 (0.55) | 0.48 (0.44) | 0.67 (0.38) |
| Control | |||
| RT | 540.1 (82.1) | 714.4 (152.1) | 898.9 (241.2) |
| A' | 0.97 (0.02) | 0.97 (0.03) | 0.92 (0.06) |
| B″ | 0.23 (0.56) | 0.37 (0.58) | 0.60 (0.45) |
| Difference | |||
| RT | 72.0 (2.9)** | 127.7 (2.7)** | 10.0 (1.6) |
| A' | 0.02 (2.7)** | 0.02 (2.7)** | 0.02 (1.6)t |
| B″ | 0.10 (0.7) | 0.12 (0.9) | 0.07 (0.7) |
Note. Parenthetical values in ADHD and control rows are standard deviations. Parenthetical values in the difference rows are t values for the between-group comparisons.
p < .01
p = .06
Signal Detection
A’ (i.e., discriminability) and B″ (i.e., response bias) values are reported in Table 3. A pair of 2 (group) X 3 (cognitive load) mixed-design ANOVA examined group differences in these measures. The control group showed better discriminability than the ADHD group, F (1, 69) = 6.4, p = .014. The group difference in response bias was not significant, F (1, 69) = 1.9, p = .173. There was a main effect of cognitive load on discriminability, F (2, 138) = 44.5, p < .001, and response bias, F (2, 138) = 10.2, p < .001. Participants showed poorer discriminability and responded more conservatively as cognitive load increased. The group X cognitive load interaction was not significant for either measure (ps > .782).
Discussion
The current experiment examined whether adults with ADHD show reduced processing capacity using two behavioral tasks that measured capacities of two cognitive mechanisms. The PRP task measured response selection capacity by requiring participants to quickly process information to make two response selections. The n-back task measured working memory capacity by requiring participants to retain an increasingly large amount of information in working memory. Both groups showed declines in performance on both tasks as cognitive load increased. This is consistent with prior literature and supports the notion that these tasks can be used to measure processing capacity (Carter et al., 1998; Schachar & Logan, 1990). Two group differences in PRP task performance were noted. First, the ADHD group was slower to respond overall on Task 2. This supports a general difficulty with task switching in adults with ADHD and extends the findings of Schachar and Logan (1990) to an adult sample. Second, participants in the ADHD group showed more pronounced slowing on Task 2 as the intertask SOA shortened. This finding supports our first hypothesis that adults with ADHD would show reduced response selection capacity. On the n-back task, the ADHD group showed poorer discriminability and appeared to respond slower than did the control group, although the latter difference was not significant. The group difference in accuracy was similar across memory load conditions, however, which does not support a group difference in working memory capacity. Further, both groups showed a more conservative response style memory load increased, as indicated by load-dependent increases in B″ and RT. Taken together, these findings support a specific deficit in response selection capacity in adults with ADHD and may suggest a dissociation between working memory and response selection capacity in this clinical group.
The finding that adults with ADHD showed reduced response selection capacity is important because it supports a previously unreported component of the ADHD behavioral phenotype, and it has a number of implications. It provides a potential explanation for their poorer performance on tasks with high response demands (e.g., manual inhibition tasks; Roberts, Fillmore, & Milich, 2011b). These deficits may result from reduced response selection capacity. For example, the notion that individuals with ADHD show inhibitory control deficits is heavily based on studies that have used the stop-signal paradigm (Logan, Cowan, & Davis, 1984) to measure this cognitive function (Barkley, 1997; Schachar, Tannock, & Logan, 1993). The stop-signal task requires respondents to make a speeded choice response, but to inhibit that response when they are presented with a stop-signal tone. Children and adults with ADHD often take longer to inhibit a response than do individuals without the disorder (Lijffijt et al., 2005), and this is cited as evidence for impaired inhibitory control in this group. However, it is also possible that individuals with ADHD lack the cognitive resources to effectively process the stop signal while concurrently processing the choice response task, so the stop signal simply takes longer for them to process (Pashler, 1994; Schachar & Logan, 1990). Indeed, research has suggested that selecting a go or no go response engages the response selection mechanism in much the same manner as a two-choice response (De Jong, 1993). Thus, inhibitory deficits as measured by the stop-signal task could result from reduced ability to simultaneously process two response demands rather than a specific inhibitory deficit. This notion is also supported by evidence suggesting overlap in the neuroanatomical regions engaged during the PRP task (Jiang, 2004) and the stop-signal task (Aron, Fletcher, Bullmore, Sahakian, & Robbins, 2003) (i.e., right inferior frontal gyrus).
Second, reduced processing capacity may contribute to adaptive impairments experienced by adults with ADHD, such as employment difficulties (Murphy & Barkley, 1996) and poor driving habits (Barkley, Murphy, & Kwasnik, 1996), because multitasking ability has been associated with these activities (Levy, Pashler, & Boer, 2006; Melamed, Stern, Rahmani, Groswasser, & Najenson, 1985). Indeed, rarely in everyday activities are people required to focus on a single stream of information for an extended time. Instead, people are routinely required to quickly shift attention between several stimuli and make responses based on this information. Accordingly, dual-task models that task response selection capacity may more accurately model the cognitive demands of human-environment interactions outside of the lab setting than do tasks that place low processing demands on capacity-limited channels.
Analyses of PRP performance also found that RT on Task 1 was faster at shorter SOAs. This may indicate that participants disregarded the instructions and shared processing resources between Task 1 and Task 2. This seems unlikely, however. If participants were indeed processing Task 2 before Task 1 was complete, then responses to Task 1 would have slowed at shorter SOAs. Instead, this slower responding to Task 1 at longer SOAs may indicate that some participants were waiting for both tasks to be presented before responding to either task. Ideally, RT on Task 1 would be unaffected by SOA; however, few participants showed this slowing on Task 1, and the number was comparable between groups. Further, the degree to which increasing SOA slowed responding on Task 1 was similar between groups, as indicated by the nonsignificant interaction, so it is unlikely that this unexpected slowing on Task 1 affected RT on Task 2 differently between groups.
Consistent with prior literature, the ADHD group was less accurate on the n-back task, which is indicative of poorer working memory functioning in this group (Marchetta, Hurks, Krabbendarn, & Jolles, 2008). Considering the effects of cognitive load on performance, however, we found no evidence that adults with ADHD show reduced working memory capacity. Further, these findings cannot be attributed to group differences in response style, because response bias (i.e., B″) was similar between groups. If adults with ADHD do not show working memory capacity deficits, then questions arise as to why affected adults reliably show working memory impairment (Boonstra, Oosterlaan, Sergeant, & Buitelaar, 2005). Working memory is comprised of numerous supporting (e.g., interference control; Barkley, 1997) and central (e.g., central executive; Baddeley, 1992) mechanisms, and impairments of working memory can result from dysfunction in any of these mechanisms. Our findings suggest that working memory dysfunction in adults with ADHD may result from dysfunction of another mechanism, such as stimulus encoding or consolidation in working memory, or, at the other end, difficulties with response selection or execution, rather than problems with storage capacity. Indeed, this may explain why the group difference was similar at each processing load, because the processing demands at the stage of encoding/consolidation and response selection/execution are identical between memory load conditions.
It should be noted, however, that our results conflict with a recent study by Finke and colleagues (2011), who reported evidence for deficits in working memory capacity, but not perceptual encoding or attentional inhibition, in a group of adults with ADHD. Our methods differed from those of Finke and colleagues in several respects, the most important being that these researchers used a different psychophysical task to assess working memory capacity. Although the n-back task has been used in many studies to measure working memory capacity, it may be sensitive to other cognitive functions beyond working memory capacity that are more executive in nature (e.g., updating, inhibiting distracters; Miyake, Friedman, Emerson, Witzki, Howerter & Wager, 2000). For example, the ADHD group may have overcome working memory capacity deficits by using rehearsal strategies—this may explain their slower responding in the 1- and 2-back conditions. Despite these potential shortcomings of the n-back task, it is compelling that previous studies utilizing the n-back task in similar designs have demonstrated load-dependent performance deficits in groups characterized by impaired working memory capacity, such as alcohol-intoxicated individuals and individuals with schizophrenia (Goldberg et al., 1998; Carter et al., 1998; Casbon et al., 2003; Gjerde, 1983; Steele & Josephs, 1990).
Despite the similar effects of load condition on accuracy between the groups, it is possible that group differences would have become more pronounced at higher memory-load conditions (i.e., 4-back, 5-back). Our data appear to suggest otherwise, however, because the group difference in RT was reduced at higher processing loads, suggesting that the ADHD group’s performance became more similar to that of the control group as processing load increased. It is possible that group differences would continue to diminish under higher memory load conditions. However, this interpretation assumes that the n-back task is subject to a speed-accuracy trade-off (Wickelgren, 1977), and it is unclear whether using a slower and more cautious response style improves accuracy on this task.
Thus far, we have discussed results and implications of each task separately; however, when taken together, these results may indicate a potential dissociation between working memory and response selection capacity in adults with ADHD. There are several possible reasons for this dissociation. First, there are a number of differences between the n-back task and the PRP task in addition to the differences in the types of capacity being assessed. The n-back task presented visual stimuli in a fixed order while emphasizing accurate responding, whereas the PRP task presented visual and auditory stimuli in a random order while emphasizing response speed. It is possible that the observed effects were driven by any of these methodological task differences. However, the results may also suggest dissociation between working memory capacity and response selection capacity in adults with ADHD. Dual-task interference on the PRP task appears to reflect capacity limitations of a central mechanism of response selection (Marois & Ivanoff, 2005; Tombu & Jolicoeur, 2003), and this cognitive mechanism appears to be independent of other capacity-limited channels (but see Tombu et al. [2011] for evidence that a unified attentional bottleneck is underlying diverse capacity limitations). Indeed, our results suggest that clinical groups, such as adults with ADHD, may show specific impairment of response selection capacity while other limited capacity mechanisms remain intact.
Although the current study contributes to our understanding of information processing deficits in adults with ADHD, there are some limitations. First, all of the participants in the ADHD group were prescribed stimulant medication and were unmedicated at the time of testing. This was done to ensure that the sample was actively experiencing symptoms; however, it is unclear to what extent medication carryover effects influenced the results. Second, the use of a single ADHD group may have obscured differences between subtypes. Third, participation was limited to individuals between 18 and 29 years old. It is unclear to what extent these findings would generalize to older adults with ADHD.
In sum, the current research provides new information on information processing capacity in adults with ADHD. Specifically, adults with ADHD show deficits in response selection capacity, but they do not show deficits in working memory capacity, at least as measured in an n-back model. This limited processing capacity may have implications for understanding cognitive dysfunction in adults with ADHD. Instead of understanding cognitive dysfunction in this population in terms of isolated deficits in specific cognitive mechanisms (e.g., selective attention, inhibitory control), these deficits may relate to limitations of processing capacity. Further, these results may support a dissociation between working memory and response selection capacity in a clinical group.
Acknowledgments
This research was supported by the National Institute on Drug Abuse grants DA021027 and DA005312 and National Institute on Alcohol Abuse and Alcoholism grants AA012895 and AA018274.
References
- Akyürek EG, & Hommel B (2005). Short-term memory and the attentional blink: Capacity versus content. Memory & Cognition, 33, 654–663. [DOI] [PubMed] [Google Scholar]
- Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, & Robbins TW (2003) Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nature Neuroscience, 6, 115–116. doi: 10.1038/nn1003 [DOI] [PubMed] [Google Scholar]
- Baddeley A (1992). Working memory. Science, 255, 556–559. doi: 10.1126/science.1736359 [DOI] [PubMed] [Google Scholar]
- Barkley RA (1997). Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychological Bulletin, 121, 65–94. doi: 10.1037/0033-2909.121.1.65 [DOI] [PubMed] [Google Scholar]
- Barkley RA, Murphy KR, & Bush T (2001). Executive functioning and olfactory identification in young adults with attention deficit-hyperactivity disorder. Neuropsychology, 15, 211–220. doi: 10.1037//0894-4105.15.2.211 [DOI] [PubMed] [Google Scholar]
- Barkley RA, Murphy KR, & Fischer M (2010). ADHD in adults: What the science says. New York, NY: Guilford Press. [Google Scholar]
- Barkley RA, Murphy KR, & Kwasnik D (1996). Motor vehicle driving competencies and risks in teens and young adults with attention deficit hyperactivity disorder. Pediatrics, 98, 1089–1095. [PubMed] [Google Scholar]
- Belleville S, Rouleau N, Van der Linden M, & College F (2003). Effect of manipulation and irrelevant noise on working memory capacity of patients with Alzheimer’s dementia. Neuropsychology, 17, 69–81. doi: 10.1037/0894-4105.17.1.69 [DOI] [PubMed] [Google Scholar]
- Boonstra AM, Oosterlaan J, Sergeant JA, & Buitelaar JK (2005). Executive functioning in adult ADHD: A meta-analytic review. Psychological Medicine, 35, 1097–1108. doi: 10.1017/S003329170500499X [DOI] [PubMed] [Google Scholar]
- Callicott JH, Mattay VS, Bertolino A, Finn K, Coppola R, Frank JA, … Weinberger DR (1999). Physiological characteristics of capacity constraints in working memory as revealed by functional MRI. Cerebral Cortex, 9, 20–26. doi: 10.1093/cercor/9.1.20 [DOI] [PubMed] [Google Scholar]
- Carter CS, Perlstein W, Ganguli R, Brar J, Mintun M, & Cohen JD (1998). Functional hypofrontality and working memory dysfunction in schizophrenia. American Journal of Psychiatry, 155, 1285–1287. [DOI] [PubMed] [Google Scholar]
- Casbon TS, Curtin JJ, Lang AR, & Patrick CJ (2003). Deleterious effects of alcohol intoxication: Diminished cognitive control and its behavioral consequences. Journal of Abnormal Psychology, 112, 476–487. doi: 10.1037/0021-843X.112.3.476 [DOI] [PubMed] [Google Scholar]
- Conners CK, Erhardt D, & Sparrow EP (1999). Conners’ Adult ADHD Rating Scales. Toronto, Canada: Multi-Health Systems. [Google Scholar]
- Daneman M, & Carpenter PA (1980). Individual differences in working memory and reading. Journal of Verbal Learning and Verbal Behavior, 19, 450–466. doi: 10.1016/S0022-5371(80)90312-6 [DOI] [Google Scholar]
- De Jong R (1993). Multiple bottlenecks in overlapping task performance. Journal of Experimental Psychology: Human Perception and Performance, 19, 965–980. doi: 10.1037/0096-1523.19.5.965 [DOI] [PubMed] [Google Scholar]
- Finke K, Schwarzkopf W, Müller U, Frodl T, Müller HJ, Schneider WX,…Hennig-Fast K (2011). Disentangling the adult attention-deficit hyperactivity disorder endophenotype: Parametric measurement of attention. Journal of Abnormal Psychology, 120, 890–901. doi: 10.1037/a0024944 [DOI] [PubMed] [Google Scholar]
- Gjerde PF (1983). Attentional capacity dysfunction and arousal in schizophrenia. Psychological Bulletin, 93, 57–72. doi: 10.1037/0033-2909.93.1.57 [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Patterson KJ, Taqqu Y, & Wilder K (1998). Capacity limitations in short-term memory in schizophrenia: Tests of competing hypotheses. Psychological Medicine, 28, 665–673. [DOI] [PubMed] [Google Scholar]
- Johnston WA, & Heinz SP (1978). Flexibility and capacity demands of attention. Journal of Experimental Psychology: General, 107, 420–435. [Google Scholar]
- Kahneman D (1973). Attention and effort. Englewood Cliffs, NJ: Prentice-Hall. [Google Scholar]
- Levy J, Pashler H, & Boer E (2006). Central interference in driving: Is there any stopping the psychological refractory period? Psychological Science, 17, 228–235. doi: 10.1111/j.1467-9280.2006.01690.x [DOI] [PubMed] [Google Scholar]
- Logan GD, Cowan WB, & Davis KA (1984). On the ability to inhibit simple and choice reaction time responses: A model and a method. Journal of Experimental Psychology: Human Perception and Performance, 10, 276–291. doi: 10.1037/0096-1523.10.2.276 [DOI] [PubMed] [Google Scholar]
- Macmillan NA, & Creelman CD (1990). Response bias: Characteristics of detection theory, threshold theory, and “nonparametric” indexes. Psychological Bulletin, 107, 401–413. doi: 10.1037/0033-2909.107.3.401 [DOI] [Google Scholar]
- Marchetta NDJ, Hurks PPM, Krabbendarn L, & Jolles J (2008). Interference control, working memory, concept shifting, and verbal fluency in adults with attention-deficit/hyperactivity disorder (ADHD). Neuropsychology, 22, 74–84. doi: 10.1037/0894-4105.22.1.74 [DOI] [PubMed] [Google Scholar]
- Marois R, & Ivanoff J (2005). Capacity limits of information processing in the brain. Trends in Cognitive Sciences, 9, 296–305. doi: 10.1016/j.tics.2005.04.010 [DOI] [PubMed] [Google Scholar]
- Melamed S, Stern M, Rahmani L, Groswasser Z, & Najenson T (1985). Attention capacity limitation, psychiatric parameters and their impact on work involvement following brain injury. Scandinavian Journal of Rehabilitation Medicine, 12, 21–26. [PubMed] [Google Scholar]
- Miyake A Friedman NP, Emerson MJ, Witzki AH, Howerter A, & Wager TD (2000). The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive Psychology, 41, 49–100. doi: 10.1006/cogp.1999.0734 [DOI] [PubMed] [Google Scholar]
- Murphy K, & Barkley RA (1996). Attention deficit hyperactivity disorder adults: Comorbidities and adaptive impairments. Comprehensive Psychiatry, 37, 393–401. doi: 10.1016/S0010-440X(96)90022-X [DOI] [PubMed] [Google Scholar]
- Navu D, & Miller J (2002). Queuing or sharing? A critical evaluation of the single-bottleneck notion. Cognitive Psychology, 44, 193–251. doi: 10.1006/cogp.2001.0767 [DOI] [PubMed] [Google Scholar]
- Nigg JT (2001). Is ADHD a disinhibitory disorder? Psychological Bulletin, 127, 571–598. doi: 10.1037//0033-2909.127.5.571 [DOI] [PubMed] [Google Scholar]
- Nigg JT, Butler KM, Huang-Pollock CL, & Henderson JM (2002). Inhibitory processes in adults with persistent childhood onset ADHD. Journal of Consulting and Clinical Psychology, 70, 153–157. doi: 10.1037//0022-006x.70.1.153 [DOI] [PubMed] [Google Scholar]
- Pashler H (1994). Dual-task interference in simple tasks: Data and theory. Psychological Bulletin, 116, 220–244. doi: 10.1037/0033-2909.116.2.220 [DOI] [PubMed] [Google Scholar]
- Pashler H, & Johnston JC (1989). Chronometric evidence for central postponement in temporally overlapping tasks. Quarterly Journal of Experimental Psychology, 41A, 19–45. doi: 10.1080/14640748908402351 [DOI] [Google Scholar]
- Patton JH, Stanford MS, & Barratt ES (1995). Factor structure of the Barratt impulsiveness scale. Journal of Clinical Psychology, 51, 768–774. [DOI] [PubMed] [Google Scholar]
- Roberts W, Fillmore MT, & Milich R (2011a). Separating automatic and intentional inhibitory mechanisms of attention in adults with attention-deficit/hyperactivity disorder. Journal of Abnormal Psychology, 120, 223–233. doi: 10.1037/a0021408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts W, Fillmore MT, & Milich R (2011b). Linking impulsivity and inhibitory control using manual and oculomotor response inhibition tasks. Acta Psychologica, 138, 419–428. doi: 10.1016/j.actpsy.2011.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robin AL, & Vandermay SJ (1996). Validation of a measure for adolescent self-report of attention deficit disorder symptoms. Journal of Developmental & Behavioral Pediatrics, 17, 211–215. doi: 10.1097/00004703-199608000-00001 [DOI] [PubMed] [Google Scholar]
- Schachar R, & Logan G (1990). Are hyperactive children deficient in attentional capacity. Journal of Abnormal Child Psychology, 18, 493–513. [DOI] [PubMed] [Google Scholar]
- Schachar RJ, Tannock R, & Logan G (1993). Inhibitory control, impulsiveness, and attention deficit hyperactivity disorder. Clinical Psychology Review, 13, 721–739. doi: 10.1016/S0272-7358(05)80003-0 [DOI] [Google Scholar]
- Steele CM, & Josephs RA (1990). Alcohol myopia: Its prized and dangerous effects. The American Psychologist, 45, 921–933. doi: 10.1037/0003-066X.45.8.921 [DOI] [PubMed] [Google Scholar]
- Tombu MN, Asplund CL, Dux PE, Godwin D, Martin JW, & Marois R (2011). A unified attentional bottleneck in the human brain. PNAS, 108, 13426–13431. doi: 10.1073/pnas.1103583108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombu M, & Jolicoeur P (2003). A central capacity sharing model of dual-task performance. Journal of Experimental Psychology: Human Perception and Performance, 29, 3–18. doi: 10.1037/0096-1523.29.1.3 [DOI] [PubMed] [Google Scholar]
- Valera EM, Faraone SV, Biederman J, Poldrack RA, Seidman LJ (2005). Functional neuroanatomy of working memory in adults with attention-deficit/hyperactivity disorder. Biological Psychiatry, 57, 439–447. doi: 10.1016/j.biopsych.2004.11.034 [DOI] [PubMed] [Google Scholar]
- Van Selst M, & Jolicoeur P (1997). Decision and response in dual-task interference. Cognitive Psychology, 33, 266–307. doi: 10.1006/cogp.1997.0662 [DOI] [PubMed] [Google Scholar]
- Van Selst M, Ruthruff E, & Johnston JC (1999). Can practice eliminate the psychological refractory period effect? Journal of Experimental Psychology: Human Perception and Performance, 25, 1268–1283. doi: 10.1037/0096-1523.25.5.1268 [DOI] [PubMed] [Google Scholar]
- Wickelgren WA (1977). Speed-accuracy tradeoff and information processing dynamics. Acta Psychologica, 41, 67–85. doi: 10.1016/0001-6918(77)90012-9 [DOI] [Google Scholar]
- Young S (2004). The YAQ-S and YAQ-I: The development of self and informant questionnaires reporting on current adult ADHD symptomatology, comorbid and associated problems. Personality and Individual Differences, 36, 1211–1223. doi: 10.1016/S0191-8869(03)00212-5 [DOI] [Google Scholar]