Abstract
Aim
Current major guidelines recommend risk stratification of the thyroid nodules, after each diagnostic evaluation, in order to focus attention on potentially risky nodules. The main aim of our study was to evaluate the performance of combined advanced ultrasound techniques in this process, compared with conventional stratification models, in order to reduce unnecessary fine-needle biopsies, respectively, surgery.
Material and Methods
We evaluated 261 cases (261 nodules) using conventional ultrasound (2B), real-time Doppler evaluation (4D) respectively, real-time elastography, using a linear multifrequency probe and a linear volumetric probe (Hitachi Prerius Machine, Hitachi Inc, Japan). All the nodules were classified using a risk stratification model comprising seven conventional US characteristics, two 4 D characteristics and a color map RTE aspect. The results were compared with the pathology results, considered the golden standard diagnosis.
Results
The prevalence of malignant nodules was 21.83% (57 cases). Conventional risk classification generated: 106 low-risk cases, 113 intermediate-risk and 42 high-risk cases. Our proposed risk classification changes the conventional risk classification with a risk upgrade in 27 cases and with a risk downgrade in 69 cases. The diagnostic quality of the combined risk stratification model was better, considering a low-risk category predictive for benignancy and a high category predictive for malignancy: Sensitivity: 80.88% versus 49.01%, respectively, Specificity: 91.22% versus 54.38. The diagnostic power differences were observed regardless of the nodule size.
Conclusion
Advanced ultrasound techniques did add diagnostic value in the presurgical risk assessment of the thyroid nodules.
Keywords: strain elastography, volumetric Doppler, complex ultrasound thyroid evaluation, risk stratification
Introduction
The prevalence of thyroid nodular pathology in the general population is high,1 increasing with age2 gender-dependent,3 lifestyle4,5 and iodine intake influenced.6–8 There is also a significant increase in thyroid cancer prevalence9 not only related to better diagnosis techniques,10 data in the literature describing a real increase per se.11 The increase initially explained by the diagnosis of a more subclinical form of the disease,12 is described to be similar in small (less than 1 cm) intermediate (2–4 cm) and even bigger than 4 cm nodules.13 This true increase was recently found in the United States population13 and is valid for papillary thyroid carcinoma variant.14 No significant change in the prevalence of the follicular variant, neither in medullar or anaplastic thyroid cancer was reported.9,13
Fine-needle aspiration cytology (FNAC) is considered to be the most cost-effective preoperative diagnostic tool15,16 but the indications for FNAC have changed comparatively with the previous guidelines,17,18 according to the ultrasound (US) characteristics suggestive for malignancy (taller than wider irregular margins, marked hypoechoic texture, micro and macro-calcifications, spiculated margins, extracapsular invasion). From the universal referral of all nodules bigger than 1 cm to FNAC,15,16 the current US risk stratification recommendations use a different size threshold in recommending FNAC: nodular diameter bigger than 1.5–2 cm if low risk versus diameter bigger than 1cm in high-risk cases.17,18 The problem is that there is not a complete overlap of the risk categories as identified by the American Thyroid Association (ATA) and the American Association of Clinical Endocrinology (AACE) guidelines. Also, the nodular size threshold required for FNAC recommendation is different in the two major guidelines: in the case of very low-risk nodules: 2.5 cm (AACE), respectively, of 2 cm (ATA), for low-risk nodules: 2 cm if proven growth (AACE) or just 1.5 cm (ATA); in the case of intermediate-risk nodules – size 2 cm (AACE) or 1 cm (ATA). The only category where the same threshold size is recommended is the high-risk category, in which all nodules bigger than 1 cm need further evaluation with FNAC. In addition, in order to simplify the risk stratification different Thyroid reporting and Data System (TI-RADS) models have emerged19–21 with good sensitivity and specificity, but with different recommendations regarding the moment of referral to FNAC according to risk and size.
Russ et al22 found that adding strain elastography findings in the TI-RADS model increases the sensitivity (96.7% versus 92.5%) but decreases the specificity (61% versus 44.7%). Our group previously demonstrated that adding quantitative strain elastography as the 6th parameter in the TI-RADS model, an excellent diagnostic quality (AUROC = 0.9567), could be obtained.23 High stiffness is currently considered a high-risk feature.17,18,24
It is known that FNAC is considered unclear in the Bethesda 3 and 4 diagnostic categories and in these situations the recommended management is divers: follow-up, lobectomy thyroidectomy or completion with molecular assays.17,18 In these cases, US pattern has to be taken into consideration.25
The aim of our study is to evaluate the efficacy of a multimodal ultrasound risk stratification model in the preoperative selection of the thyroid nodules. In order to avoid confounding factors due to the limited diagnostic availability and value of FNAC, we included in the analysis only nodules with proven pathology diagnostics after surgical resection.
Patients and Methods
This prospective study was performed in accordance with the Ethical Guidelines of the Helsinki Declaration and was approved by the Ethics Committee of our Center, No 78/15.01.2016. Written informed consent was obtained from all patients prior to inclusion.
Patients
The study group included patients with a solid nodular goiter examined in our Ultrasound evaluation Unit between January 2016 and June 2018. In this period, we identified 564 patients with a nodular goiter; 261 of them were operated till the end of the enrolment period. For each case, a pathology report was obtained and was considered the golden standard for further analysis. The exclusion criterion was the absence of a pathology report. The ultrasound evaluation, comprising conventional grayscale, SE and tri-dimensional CD, was performed prior to surgery by one operator with more than 10 years’ experience in conventional US and 5 years in elastography, less than 2 months before surgery. In cases with a multinodular goiter, the nodule with a high risk on ultrasound evaluation according to our criteria was considered for statistical analysis. FNAC was not performed in all cases due to lack of acceptance, 51 cases, or no need in multinodularity associated with compression or autonomy, 34 cases. Rapid growth, uninodular lesion with autonomy, intermediate and high-risk ultrasound patterns were the indication FNAC.
Because FNAC was not performed in all cases, it was not included in the final analysis of the present study.
Table 1 is presenting the proposed risk classification in our study.
Table 1.
Proposed Risk Classification Model
| Technique | Low Risk | Intermediate Risk | High Risk |
|---|---|---|---|
| US | Oval Mild hypo echoic No risk sign |
Hypo echoic Sub capsular position Inhomogeneity |
Taller than wide Sub capsular Intense hypo echoic Calcification Suspect lymph nodes |
| SE | ES 1 +2 | ES 3 | ES 4 |
| V | Intact thyroid capsule No vascularization |
Altered thyroid capsule Intranodular increased vascularization |
Abbreviations: US, conventional ultrasound; SE, Real-Time Elastography; V, Volumetric Real-time three-dimensional ultrasound.
Ultrasound Evaluation
All ultrasound evaluations were performed using the Hitachi Preirus (Hitachi Medical Corporation, Tokyo, Japan) machine with a 5–18 MHz linear multifrequency probe and a 5–13MHz linear volumetric probe. SE was performed using mild external pressure.26 Images obtained with inadequate external pressure were not used in the final analysis. The color map types (ES) were interpreted following the Asteria criteria:26 ES 1 (complete green) and 2 color map (predominant green) – soft lesions, ES 3 (mixed green + blue images) intermediate lesion and ES 4 (complete blue lesion) – high stiffness lesion.
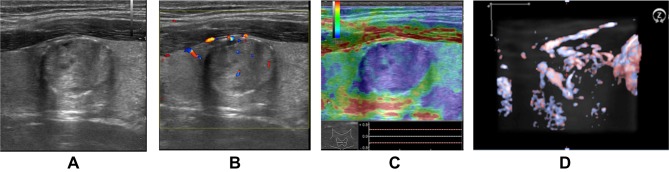
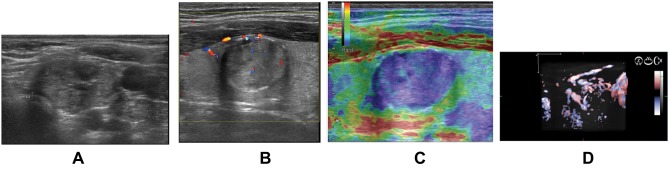
Volumetric Doppler evaluation offers a tri-dimensional image of the scanned region, with the nodule in the center of the image, the rotational scanning, in all three axis, if needed, in order to evaluate the degree of vascularization spreading from the surrounding thyroid parenchyma into the nodular lesion.27,28 A significant difference in 3D Doppler evaluation is seen in cases with similar 2D CD pattern images. Figures 1 and 2 present differences (2b versus 1b) observed in volumetric Doppler images, compared with the similar monoplane CD pattern (1a and 2a). For this reason, we used volumetric Doppler evaluation instead of monoplane Doppler evaluation.
Figure 1.

(A) Low intra- and peri-nodular vascularisation pattern observed on 2D examination. (B) Low intra- and peri-nodular vascularisation observed on 3D examination (same thyroid nodule).
Figure 2.
(A) Low intra- and peri-nodular vascularisation observed in 2D examination. (B) Increased intra- and peri-nodular vascularisation observed in 3D examination (same nodule).
Slices of 0.2 mm, in each X, Y and Z plane, represent the second type of obtained images. In cases with abundant vascularization, the volumetric view was completed, as previously described28 with the biplane combined views, observing all the planes from one border to the opposite border of the nodule.
The following findings of the nodules and thyroid gland were evaluated: conventional US patterns: shape, ecogenicity, homogeneity, margins, position, presence of calcification and of suspect lymph nodes;29 SE - color map code, ES 1–4; volumetric evaluation: capsule integrity and perinodular/intranodular vascularization.
As detailed in Table 1 we classified the nodules as low, intermediate and high-risk for malignancy.
Surgical Intervention
The surgeon of our team performed unilateral lobectomy or total thyroidectomy. The surgical treatment was recommended due to the presence of laterocervical level VI or III lymph nodes with noninflamatory observed on ultrasound associated to high-risk thyroidal nodules, in 34 cases, intermediate and positive FNAC result (Bethesda III, IV, V, and VI) in 109 cases, compression-only effects in 32 cases, functional autonomy (defined by suppressed TSH levels in the absence of thyroid hormone therapy) in 15 cases, rapid growth in 20 cases, cosmetic reasons in 17 cases, respectively, multinodularity associated with compression or autonomy in 34 cases.
Pathology Examination
Thyroid pathology specialists, in the Pathology Department, made the pathology diagnosis. Imunohistochemical evaluation, HBME, CH-19, K067 and TTF reactions were performed, if needed.
Statistical Analysis
Data were collected and analyzed using SPSS v.17 statistical software package (SPSS Inc, Chicago, IL, USA). Clinical and laboratory characteristics of the patients were expressed as a mean, standard deviation (SD), median, and range. Prior to analysis, variables were tested for normality using the Shapiro–Wilk test for homogeneity of variances with Levene’s test. Sensitivity, specificity, positive and negative predictive values, and likelihood ration were calculated for each risk category, using pathology reports as the golden standard. In cases with multinodular goiter, we used, in the statistical analysis, only the characteristics of the diagnostic nodule, the nodule considered as conclusive for the diagnosis in the pathology report.
Results
From the total of 261 analyzed patients, we analyzed 261 nodules. The final pathology diagnostic was: thyroid cancer in 57 cases (21.83%): papillary carcinoma 45 cases, follicular carcinoma 5 cases, Hurthle cell carcinoma 3 cases, 2 borderline malignancy cases and 2 medullary carcinoma patients.
The risk stratifications of the 261 evaluated thyroid nodules, according to different ultrasound characteristics, are presented in Table 2.
Table 2.
Low Intermediate and High-Risk Categories, According to the Conventional, Conventional + Elastography Respectively Conventional + Elastography + Volumetric Characteristics
| Risk US Technique |
Low Risk | Intermediate Risk | High Risk | Total Cases | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BN | CA | T | BN | CA | T | BN | CA | T | ||
| US (no of cases) | 100 | 6 | 106 | 93 | 20 | 113 | 11 | 31 | 42 | 261 |
| US (%of cases) |
100% | |||||||||
| US +SE (No of cases) |
165 | 3 | 168 | 33 | 9 | 42 | 6 | 45 | 51 | 261 |
| US +SE (%of cases) |
100% | |||||||||
| US+SE+V (No of cases) |
165 | 2 | 167 | 33 | 3 | 36 | 6 | 52 | 58 | 261 |
| US+SE+V (%of cases) |
100% | |||||||||
Abbreviations: BN, confirmed benignity (pathology report); CA, confirmed malignancy (pathology report); T, Total number of cases in the category, US, conventional ultrasound; SE, Real-Time Elastography; V, Volumetric Real-time three-dimensional ultrasound.
We used a stepwise evaluation of the nodules. After initial grayscale evaluations, when considering the elastographic and volumetric characteristics, we reassess the risk category. Risk upgrade was made in 9.19% of cases, from low to intermediate risk 4 cases and from intermediate to high risk, another 20 cases. Risk downgrade was made in 26.81% of cases, from intermediate to low risk in 65 cases, respectively, 5 cases where downgraded from the high-risk category.
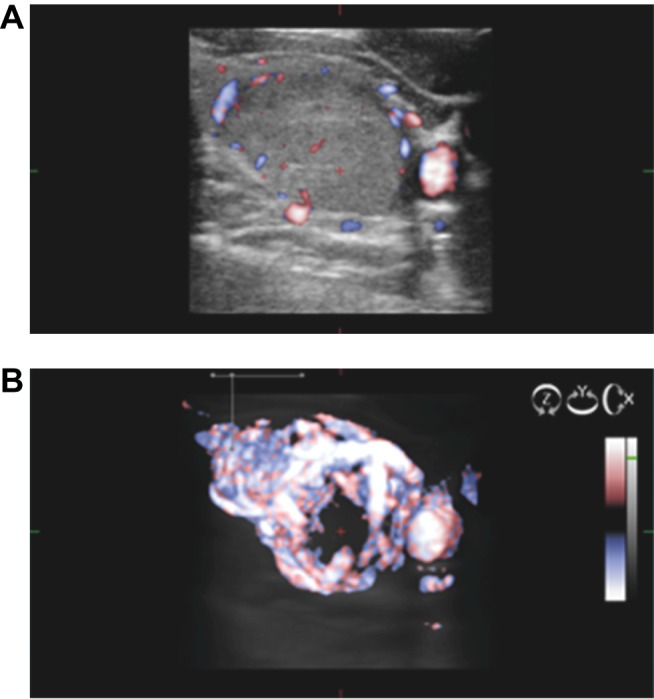
Figure 3 presents a case of risk downgrade because of low stiffness and low vascularization in volumetric Doppler.
Figure 3.
2B Intermediate risk nodule with normal volumetric aspect (B) and normal stiffness (A) (ES color map 1 and 2 code) is reconsidered as a low-risk lesion.
Figure 4 presents a case with a risk upgrade, from a low-grade lesion, because of an increased US stiffness and increased vascularization (volumetric evaluation). The same upgrade is described in Figure 5, but from an intermediate grade lesion, because of an increased US stiffness and increased vascularization (volumetric evaluation). The risk upgrade was considered even in the presence of only one high-risk characteristic, increased volumetric vascularization, despite the normal stiffness and the intermediate-risk category, according to the conventional US characteristics, as seen in Figure 6.
Figure 4.
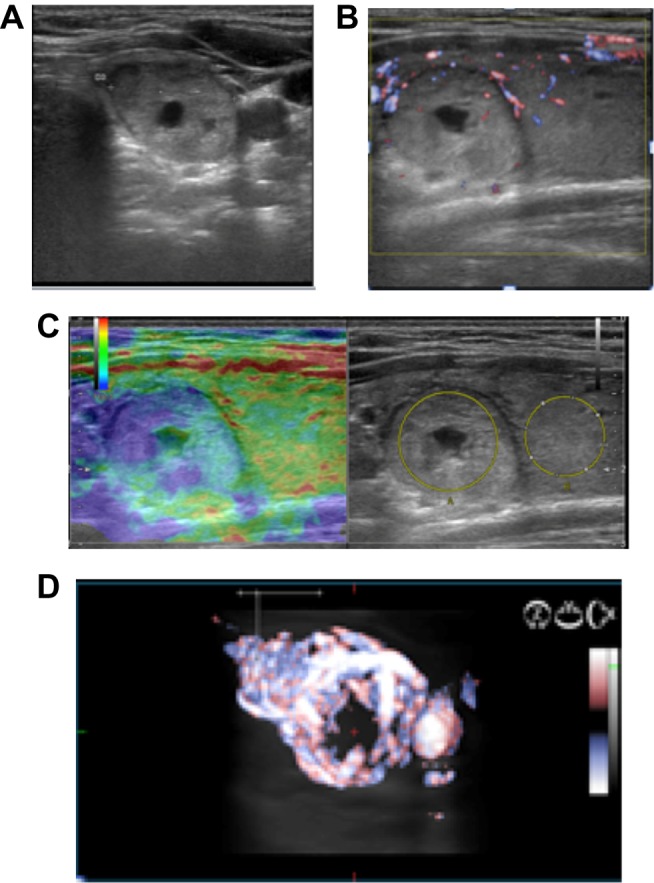
Low-risk nodules (A) with increased stiffness (B) and increased vascularization in volumetric evaluation (C) versus color Doppler (D) were reconsidered as intermediate risk lesions.
Figure 5.
Intermediate risk case (A) with increased stiffness (C) was reconsidered as high risk, regardless of color Doppler (B) or volumetric aspect (D).
Figure 6.
Intermediate risk case (A) was reconsidered as high risk, in the presence of high-risk volumetric characteristics (B) despite intermediate stiffness (C).
We analyzed the sensibility, specificity and accuracy, in differentiating benign versus malignant lesions, of the conventional only US risk stratification, respectively, conventional + ES and volumetric Doppler characteristics. The results are presented in Table 3. We did consider low-risk nodules as a predictor for benign lesions. Since intermediate-risk category is a gray zone of predictive diagnostic, when evaluating the prediction of malignancy, we performed 2 separate analyses: considering just the high-risk nodules as suggestive for malignancy and considering intermediate and high-risk nodules suggestive for malignancy.
Table 3.
Comparison of Diagnostic Quality (Sensitivity, Specificity, Accuracy) for Risk Category Assessment by Means of US Only, US+SE and US+SE+V Models
| Risk Category by Ultrasound Evaluation | Correct Diagnostic | Se (%) | Sp (%) | Acc (%) |
|---|---|---|---|---|
| Low Risk | ||||
| US | 100/204 | 49.01 | 89.47 | 57.85 |
| US+SE | 165/204 | 80.88 | 94.70 | 83.90 |
| US+SE+V | 165/204 | 80.88 | 96.49 | 84.29 |
| High Risk | ||||
| US | 31/57 | 54.38 | 94.60 | 90.03 |
| US+SE | 45/57 | 78.94 | 97.05 | 93.10 |
| US+SE+V | 52/57 | 91.22 | 97.05 | 98.08 |
| Intermediate + High Risk | ||||
| US | 51/57 | 91.71 | 49.01 | 57.85 |
| US+SE | 54/57 | 94.73 | 80.88 | 83.90 |
| US+SE+V | 55/57 | 96.43 | 80.88 | 91.18 |
Note: Final pathology report 204 benign cases, 57 malignant cases.
Abbreviations: Se, sensitivity; Sp, specificity; Acc, accuracy; SE, Real-Time Elastography; V, Volumetric Real-time three-dimensional ultrasound.
The diagnostic power of this modified risk stratification was also studied in respect to the size of the nodule. The sensitivity and specificity of low risk (suggestive for benign lesions) and intermediate + high-risk cases (suggestive for malignant lesions) for all types of ultrasound evaluations are listed according to the size of the nodules in Table 4. Sensibility, specificity and accuracy were considered in nodules lower than 2 cm, between 2 and 4 cm, respectively, higher than 4 cm in the highest diameter.
Table 4.
Diagnostic Values of Different Risk Stratification Models According to the Nodule Diameter
| Diameter | < 2 cm | 2–4 cm | > 4 cm | T | ||||
|---|---|---|---|---|---|---|---|---|
| Cases (No) | 158 | 69 | 34 | 261 | ||||
| CA (No) | 26 | 24 | 7 | 57 | ||||
| CA (%) | 16.45% | 34.7% | 20.56 | 21.83 | ||||
| Technique | Se (%) | Sp (%) | Se (%) | Sp (%) | Se (%) | Sp (%) | ||
| US | L | 59.09 | 96.15 | 26.66 | 79.16 | 37.03 | 100 | |
| I+H | 57.69 | 99.24 | 50.00 | 84.44 | 57.14 | 74.01 | ||
| US+SE | L | 94.96 | 96.5 | 51.11 | 91.66 | 45.94 | 100 | |
| I+H | 80.76 | 99.24 | 79.16 | 95.55 | 71.40 | 88.8 | ||
| US+SE+V | L | 94.6 | 96.5 | 51.11 | 95.88 | 45.94 | 100 | |
| I+H | 92.30 | 99.24 | 91.66 | 95.55 | 85.71 | 88.80 | ||
Abbreviations: CA, Cancer, Se, sensitivity; Sp, specificity; SE, Real-Time Elastography; V, Volumetric Real-time three-dimensional ultrasound; T, Total number of cases, L – Low, I, Intermediate, H, High.
Discussions
In our study, we evaluated the combined integrated ultrasound model in the stratification risk thyroid nodules. We combined conventional ultrasound recommended parameters17–20,30 with high stiffness18,22,23 on elastography and alteration in the thyroid capsule integrity or increased intranodular vascularization observed on the real-time tri-dimensional technique.27,28
Our study is emerging because of the general recommendation of diagnostic risk stratification, in order to reduce the number of unnecessary FNAC and surgeries.17–19 An accurate presurgical diagnostic is mandatory for the surgeon, in order to choose the best surgical technique and to control de invasiveness of the surgery.31,32
The results are convincing: better sensitivity, specificity and accuracy in combined models US+SE+V and US+SE better than US evaluation alone: increasing sensitivity in case of soft nodules, and soft nodules with no alteration in volumetric evaluation in detection of benign lesions: 49.01%-80.88%-80.88%, with an important increase also of the specificity when integrating information: 89.47%-94.7%-66.49%. Our results were similar to other integrated thyroid nodule models,22 describing a 98.8 positive predictive value for the combined ultrasound risk stratification model, meaning the rate of mistake equals 0.02, in the presence of benign characteristics in all used ultrasound techniques (grayscale, elastography and volumetric evaluation).
The calculated risk for malignancy was different for the 3 used models, with a lower malignancy risk in the low-risk category in the 2B+RTE+volumetric model (1.19%) versus 5.6% in the conventional 2 B model, borderline risk in the intermediate group (8.33% versus 17.69%), with a suggestive risk in the high-risk group (89.65% versus 73.80%). The results are explained by downgrading the risk category, using mainly the elastography details. The same combined approach was used by other authors, with better stratification results33,34 due to the decrease of false-positive results. Higher stiffness increased the risk.17,18,34 The increase in sensitivity is consistent, independent of the nodule size. Even if combined diagnostic quality seems better in small nodules, the improvement of the multimodal ultrasound is observed regardless of the nodular size. In the literature, the results regarding nodular size impact on ultrasound diagnostic qualities are still controversial: some report the TIRADS is better in small size nodules (<1 cm) and ATA criteria better in nodules bigger than 2 cm.35 Others do state than there is no difference in US risk patterns in nodules bigger or smaller than 3 cm,36 although there is a slightly higher malignancy risk in nodules smaller than 2 cm.36 Also, in nodules with a high suspicion US pattern, there was no significant association between the malignancy risk and nodule size.36 Other studies do show an increase in the malignancy risk as the size of the nodule increases37–40 with the threshold of 2 cm37 or of 3–4 cm39,40 predictive for malignancy, but others described the opposite, with differences regarding the size.41,42
The impact of the size might be associated with the histological type of cancer, since PTC do show high-risk ultrasound characteristics and non-PTC cancer show intermediate to low risk characteristics.36 In our study, the 2 B model classified 27/45 PTC (58.69%) as high risk and 16/45 (28.07%) as intermediate risk, compared with the complex combined model that considered 44/45 PTC (97.77%) as high-risk lesions.
All 3 Hurthle cell carcinomas were considered as high risk, both in the simple and complex ultrasound model. In the group of FTC cases, the 2 B model considered only 3 cases low risk (60%) and 2 cases intermediate risk (40%), compared with the complex model that assigned 1 case as a high risk (20%), with the others as a low and intermediate risk. Even this complex model is nonperforming in the imagistic diagnostic of FTCs because of low stiffness observed in FTC.43
Because of the great discrepancy of cases in the intermediary risk category, between 5% and 80%22,44–46 and because of the decrease in specificity, as seen in Table 3, we did consider only low risk as a clear indicator for benignancy and high risk as a consistent indicator for malignancy.
Volumetric evaluation did not increase the number of the correct classified benign nodules (164/204) benign; in high-risk nodules, volumetric evaluation did add further identified cases.
Using this stratification, the overall sensitivity of the combined method was higher using RTE added information (80.88% versus 49.01%) or RTE + 4D information (80.88% versus 49.01%) with an increase also in specificity, better for added V+SE versus SE only extra information (91.22–78.44 compared with 54.38%).
The retrospective analysis of the study group suggests that in the presence of complex low-risk characteristics, the cancer probability is very low. Nonperforming FNAC in these cases would have overlooked 2 cancer cases (3.5%). The intermediate-risk category remains the gray zone of thyroid ultrasound evaluation – no general recommendations can be made for this special category.
In our study, we observed that using elastography and the volumetric information increased stiffness or thyroid capsule rupture, did upgrade the risk category in the high-risk class. Observation of low stiffness can decrease the risk category of the nodule. This risk reassignment did decrease the intermediary risk category by 68.14%. Similar approaches are described for RTE29,30,33 or volumetric27 alone. Accordingly, publications using combined conventional, strain elastography and volumetric Doppler evaluation are few in the literature.
There are several limitations to our study. The number of cases smaller than 2 cm, and higher than 2 cm or 4 cm is not equal. The size distribution in our study group does not necessarily overlap the distribution in the general population, so a clear conclusion of the cancer prevalence according to size cannot be made in our cohort. Populational studies are needed in order to answer the question about nodule size prevalence. The costs of volumetric evaluations can be limited if using pre-existing compatible ultrasound platforms. We could not evaluate the economic ratio of cost benefits, of the method, considering the ultrasound machine cost versus the hospitalization cost for each identified and treated thyroid cancer case. To our knowledge, we are the first to incorporate volumetric evaluation besides elastography and conventional ultrasonography, in a risk stratification model for thyroid evaluation: increased intranodular vascular branching, alteration of the capsule integrity and high stiffness in elastography do upgrade the risk category.
Conclusion
Our proposed risk stratification model, not described before, combines elastographic results and volumetric Doppler characteristics with conventional grayscale characteristics. The model adds diagnostic value in the preoperative evaluation of thyroid nodules. Revaluating the risk category does reduce the intermediate-risk case. In cases with a low risk, in all US used techniques, FNAC can be avoided, regardless of the nodule size. The proposed stepwise US+SE+V evaluation, with a reconsideration of the risk category, increases the diagnostic confidence of solid thyroid nodules.
Funding Statement
“This research was funded by a research grant: grant number: SMIS 45997/21.01.2014- “Cresterea calitatii actului medical prin valorificarea potentialului IT” funder: Guvernul Romaniei, Ministerul Comunicatiilor si societatii informationale, axa POS CCE.
Abbreviations
2B, conventional ultrasound; RTE, real-time elastography; V, volumetric Doppler; Se, Sensitivity; Sp, Specificity; Acc, Accuracy; FNAC, Fine-needle aspiration cytology; PTC, papillary thyroid carcinoma; FTC, follicular thyroid carcinoma.
Author Contributions
DS, IS and VI performed the process of patients’ diagnostic and evaluation. IM conceived the study and performed the statistical analysis, while DNa and DNe participated in the design of the study and helped to draft the manuscript. VF performed the surgeries. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Vanderpump MPJ. The epidemiology of thyroid diseases In: Braverman LE, Utiger RE, editors. Werner and Ingbar’s the THYRIOD: A Fundamental and Clinical Text. 9th ed. Philadelphia: JB Lippincott-Raven; 2005:398–496. [Google Scholar]
- 2.Hegedus L. Clinical practice. The thyroid nodule. N Engl J Med. 2004;351(17):1764–1771. [DOI] [PubMed] [Google Scholar]
- 3.Vanderpump MPJ. The epidemiology of thyroid disease. Br Med Bull. 2011;99:39–51. doi: 10.1093/bmb/ldr030 [DOI] [PubMed] [Google Scholar]
- 4.Barrere X, Valeix P, Preziosi P, et al. Determinants of thyroid volume in healthy French adults participating in the SU.VI.MAX cohort. Clin Endocrinol. 2000;52:273–278. doi: 10.1046/j.1365-2265.2000.00939.x [DOI] [PubMed] [Google Scholar]
- 5.Knudsen N, Bullow I, Laurberg P, Perrild H, Ovesen P, Jorensen T. High occurence of thyroid multinodularity and low occcurence of subclinical hypothyroidism among tobacco smokers in large population study. Journal of Endocrinology. 2002;175:571–576. [DOI] [PubMed] [Google Scholar]
- 6.Hashemipour M, Amini M, Aminorroaya A, et al. High prevalence of goiter in an iodine replete area: do thyroid auto-antibodies play a role? Asia Pac J Clin Nutr. 2007;16(3):403–410. [PubMed] [Google Scholar]
- 7.Teng W, Shan Z, Teng X, Guan H, TEng D, Jin Y, Yu X, FAc C, Chong W, Yang F, Dai H, Yu Y, Li J, Chen Y, Zhao D, SHi X, Xu F, MAo J, Gu X, Yang R, Tong Y, Wang W, Gao T, Li C. Effect of iodine intakeon thyroid disease in China. N Eng J MEd. 2006; 29;354:2783–2793. [DOI] [PubMed] [Google Scholar]
- 8.Xin Sun, Zhongyan Shan, Weiping Teng. Effects of increased iodine intake on thyroid disease. Endocronol MEtab (Seoul) 2014;29(3):240–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pellegriti G, Frasca F, Regalbuto C, Squatrito S, Vigneri R. Worldwide increasing incidence of thyroid cancer: update on epidemiology and risk factors. J Cancer Epidemiol. 2013;2013:965212. doi: 10.1155/2013/965212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grodski S, Brown T, Sidhu S, et al. Increasing incidence of thyroid cancer is due to increased pathologic detection. Surgery. 2008;144(6):1038–1043. doi: 10.1016/j.surg.2008.08.023 [DOI] [PubMed] [Google Scholar]
- 11.Howlender H, Noone AM, Krapcho M, Garshell J, Miller D, Alterkruse SF et al. Bethesd, MD:2016 Apr, SEER Cancer Statistics Review, 1975-2013, national Cancer Institute. Available from: http://seer.cancer.gov/csr/1975_2013/ based on November 2015 SEER data submission, posted to the SEER web site. [Google Scholar]
- 12.Kent WD, Hall SF, Houlded RL, et al. Incresed incidence of differentiated thyroid carcinoma and detection of subclinical disease. CMAJ. 2007;177(11):135701361. doi: 10.1503/cmaj.061730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kent WD, Hall SF, Isotalo PA, Houlden RL, George RL, Groome PA. Increased incidence of differentiated thyroid cancer carcinoma and detection of subclinical disease. CMAJ 2007;177(11):1357–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aschebrook-Kilfoy B, Grogan R, Ward M, Kaplan E, Devesa S. Follicular thyroid cancer incidence patterns in the United States, 1980–2009. Thyroid. 2013;23(8):1015–1021. doi: 10.1089/thy.2012.0356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gharib H, Papini E, Paschke R, et al. American Association of Clinical Endocrinologist, Associazione Medici Endocrinologi, and European Thyroid Association medical guidelines for clinical practice for the diagnosis and management of thyroid nodules. Endocrine Pract. 2010;16(suppl 1):1–4. doi: 10.4158/10024.GL [DOI] [PubMed] [Google Scholar]
- 16.American Thyroid Association (ATA) Guidelines Task Force on Thyroid Nodulas and Differentiated Thyroid Cancer, Cooper DS, Doherty DM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzafferi EL, McIver B, Pacini B, Schlumberger M, Stewart DL, Tuttle RM. Revised ATA management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009;19(11):1167–1214. [DOI] [PubMed] [Google Scholar]
- 17.Haugen BR, Alexander EK, Bible KC, et al. 2015 ATA management guidelines for adults with thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;1(26):1–133. doi: 10.1089/thy.2015.0020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gharib H, PApini E, Garber J, et al. AACE guidelines for clinical practice for the diagnostic and management of thyroid nodules. Endocrine Pract. 2016;22(1):1–60. doi: 10.4158/EP161208.GL [DOI] [Google Scholar]
- 19.Moifo B, Takoeta EO, Tambe J, et al. Reliability of Thyroid Imaging Reporting and Data System (TIRADS) classification in differentiating Benign from malignant thyroid nodules. Open J Radiol. 2013;03:103–110. doi: 10.4236/ojrad.2013.33016 [DOI] [Google Scholar]
- 20.Wei X, Li Y, Zhang S, et al. Thyroid imaging reporting and data system (Ti-RADS) in the diagnostic value of thyroid nodules: a systematic review. Tumor Biol. 2014;35(7):6769–6776. doi: 10.1007/s13277-014-1837-9 [DOI] [PubMed] [Google Scholar]
- 21.Rust FM, Meyer G, Dauth N, et al. Interobserver agreement of Thyroid Reporting and Data System (TIRADS) and strain elastography for assessment of thyroid nodules. PLoS ONE. 2014. doi: 10.1371/journal.pone.0077927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Russ G, Royer B, Bigorgne C, et al. Prospective evaluation of thyroid imaging system reporting and data system on 4550 nodules with and without elastography. Eur J Endocrinol. 2013;168:649–655. doi: 10.1530/EJE-12-0936 [DOI] [PubMed] [Google Scholar]
- 23.Stoian D, Timar B, Derban M, et al. TI-RADS: the impact of qualitative strain elastography for better stratification of the cancer risk. Med Utrason. 2015;17(3):327–333. doi: 10.11152/mu.2013.2066.173.dst [DOI] [PubMed] [Google Scholar]
- 24.Dudea SM, Botar-Jid C. Ultrasound elastography in thyroid disease. Med Ultrason. 2015;17:74–96. doi: 10.11152/mu.2013.2066.171.smd [DOI] [PubMed] [Google Scholar]
- 25.So-hyeon H, Hyejin L, Min-Sun C, et al. Malignancy risk and related factors of atypia of undetermined significance/follicular lesion of undetermined significance thyroid fine needle aspiration. Internatiol J Endocrinol. 2018:ID 4521984. doi: 10.1155/2018/4521984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Asteria C, Giovanardi A, Pizzocarlo L, et al. US- Elastography in the diagnostic of benign and malignant thyroid nodules. Thyroid. 2008;18(5):5239531. doi: 10.1089/thy.2007.0323 [DOI] [PubMed] [Google Scholar]
- 27.Slapa RZ, Slowinska-Srzednicka J, Szopinski K, Jakubowski W. Gray-scale three-dimensional sonography of thyroid nodules: feasibility of the method and preliminary studies. Eur Radiol. 2006;16:428–436. doi: 10.1007/s00330-005-2903-x [DOI] [PubMed] [Google Scholar]
- 28.Slapa RZ, Jakubowski WS, Slowinska- SRzednicka J, et al. Advantages and disadvantages of 3D ultrasound of thyroid nodules including thin slice volume rendering. Thyroid Res. 2010;4:1–12. doi: 10.1186/1756-6614-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stoian D, Cornianu M, Dobrescu A, Lazar F. Nodular thyroid cancer. Diagnostic value of real time elastography. Chirurgia. 2012;107(1):39–46. [PubMed] [Google Scholar]
- 30.Xue J, Cao XL, Shi L, Lin CH, Wang J, Wang L. The diagnostic value of combination of TI-RADS and ultrasound elastography in the differentiation of benign and malignant thyroid nodules. Clin Imaging. 2016;40:913–916. doi: 10.1016/j.clinimag.2016.04.014 [DOI] [PubMed] [Google Scholar]
- 31.Conzo G, Calo PG, Tartaglia E, et al. Contriversies in the surgical management of follicular neoplasms. Retrospective analysis od 721 patients. Int J Surg. 2014;12(Suppl 1):29–34. [DOI] [PubMed] [Google Scholar]
- 32.Conzo G, Docimo G, Mauriello C, et al. The current status of lymph node dissection in the treatment of papillary thyroid cancer. A literature review. Clin Ter. 2013;164(4):e343–346. [DOI] [PubMed] [Google Scholar]
- 33.Russ G, Leboulleux S, Leenhardt L, et al. Thyroid incidentalomas: epidemiology, risk stratification with ultrasound and workup. Eur Thyroid J. 2014;3:154–163. doi: 10.1159/000365289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ha EJ, Baek JH, Na DJ. Risk stratification of thyroid nodules on ultrasonography: current status and perspectives. Thyroid. 2017;12. doi: 10.1089/thy.2016.0654 [DOI] [PubMed] [Google Scholar]
- 35.Xu T, Gu JY, Xu SH, et al. Thyroid nodule sizes influenced the diagnosticperformance of TIRADS and ultrasound patterns of 2015 ATA guidelines. Sci Rep. 2017;24(7):43183. doi: 10.1038/srep43183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hong MJ, Na DG, Baek JH, et al. Impact if nodule size in malignancy risk differs according to the ultrasonography pattern of thyroid nodules. Korean J Radiol. 2018;19(3):534–541. doi: 10.3348/kjr.2018.19.3.534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kamran SC, Marqusee E, Kim MI, et al. Thyroid nodule size and prediction of cancer. J Clin Endocrinol Metab. 2013;98:564–570. doi: 10.1210/jc.2012-2968 [DOI] [PubMed] [Google Scholar]
- 38.Kuru B, Gulcelik NE, Gulcelik MA, Dincer H. Predictive index for carcinoma of thyroid nodules and its integration with fine-needle aspiration cytology. Head Neck. 2009;31:856–866. doi: 10.1002/hed.v31:7 [DOI] [PubMed] [Google Scholar]
- 39.Hammad AY, Noureldine SI, Hu T, Ibrahim Y, Masoodi HM, Kandil E. A meta-analysis examining the independent association between thyroid nodule size and malignancy. Gland Surg. 2016;5:312–317. doi: 10.21037/gs [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shin JJ, Caragacianu D, Randolph GW. Impact of thyroid nodule size on prevalence and post-test probability of malignancy: a systematic review. Laryngoscope. 2015;125:263–272. doi: 10.1002/lary.24784 [DOI] [PubMed] [Google Scholar]
- 41.Frates MC, Benson CB, Doubilet PM, et al. Prevalence and distribution of carcinoma in patients with solitary and multiple thyroid nodules on sonography. J Clin Endocrinol Metab. 2006;91:3411–3417. doi: 10.1210/jc.2006-0690 [DOI] [PubMed] [Google Scholar]
- 42.Deveci MS, Deveci G, LiVolsi VA, Gupta PK, Baloch ZW. Concordance between thyroid nodule sizes measured by ultrasound and gross pathology examination: effect on patient management. Diagn Cytopathol. 2007;35:579–583. doi: 10.1002/(ISSN)1097-0339 [DOI] [PubMed] [Google Scholar]
- 43.Yurong H, Xueming L, Zhiyu L, Xiufang Z, Meifeng C, Zhiyahn L. Real-time ultrasound elastography in te differential diagnosis of benign and malignant thyroid nodules. J Ultrasound Med. 2009;28:861–867. doi: 10.7863/jum.2009.28.7.861 [DOI] [PubMed] [Google Scholar]
- 44.Horvath E, Majlis S, Rossi R, et al. An ultrasonogram reporting system for thyroid nodules stratifying cancer risk for clinical management. JCEM. 2009;94(5):1748–1751. doi: 10.1210/jc.2008-1724 [DOI] [PubMed] [Google Scholar]
- 45.Kwak JY, Han KH, Yoon JH, et al. Thyroid imaging reporting and data system for US feartures of nodules. Radiology. 2011;260(11):892–899. doi: 10.1148/radiol.11110206 [DOI] [PubMed] [Google Scholar]
- 46.Tessle FN, Middleton WM, Grant GE. ACR thyroid imaging, reporting and data system: white paper of the ACR TI-RADS Committee. J Coll Am Radiol. 2017;14(5):587–595. doi: 10.1016/j.jacr.2017.01.046 [DOI] [PubMed] [Google Scholar]