Abstract
Plaque psoriasis is a chronic debilitating condition, and biologic agents that inhibit tumor necrosis factor-α (TNF-α) are widely employed in management of the condition. Notwithstanding, several paradoxical adverse reactions have been reported with TNF-α inhibitors, including vasculitis, vitiligo, alopecia areata, sarcoidosis and other granulomatous diseases. Herein, we report the case of a 63-year-old man who developed vitiligo while on therapy with adalimumab following failure of conventional agents for plaque psoriasis. After discontinuation of adalimumab and initiation of secukinumab, vitiligo and other psoriatic symptoms gradually resolved. After 1 year of treatment, only small plaque areas were present in the flexor site with complete remission in the extensor area along with near complete resolution of depigmented areas. In this case of possible adalimumab-induced vitiligo in a patient with plaque psoriasis, secukinumab resolved both the symptoms of psoriasis and the likely adalimumab-related vitiligo.
INTRODUCTION
Plaque psoriasis is a chronic and debilitating condition that is related to increased proliferation of epidermal cells, impaired differentiation of keratinocytes and dilated dermal blood vessels as well as infiltrating inflammatory cells. In most cases, the disease is mild and can be controlled with topical therapy, including corticosteroids, vitamin D3 analogs, retinoids, calcineurin inhibitors and keratolytic agents. However, some patients develop moderate or severe psoriasis and require systemic therapy, such as phototherapy, acitretin, methotrexate or cyclosporine. The toxicity of these drugs and frequent lack of response has led to the development of biologic therapies. Among these, biologic drugs that inhibit tumor necrosis factor-α (TNF-α) (e.g. adalimumab) are widely employed in the management of plaque psoriasis and other dermatological inflammatory diseases. Notwithstanding their benefits, a wide range of paradoxical adverse reactions have been reported with TNF-α inhibitors [1]. These include, but are not limited to, dermatological (e.g. vasculitis, vitiligo and alopecia areata) and ophthalmic conditions (e.g. uveitis and scleritis) as well as sarcoidosis and other granulomatous diseases.
Herein, we report the case of a 63-year-old man who developed vitiligo while on therapy with adalimumab for plaque psoriasis. Following suspension of adalimumab, vitiligo and other psoriatic symptoms gradually resolved following initiation of secukinumab.
CASE REPORT
A 63-year-old male, overweight, with no comorbidities presented for plaque psoriasis primarily localized to the palm and dorsal surface of the hands in May 2015 (PASI 8.5). As topical therapy was ineffective, systemic therapy with cyclosporine was administered up to the end of 2016, but discontinued due to increased systolic blood pressure in the absence of noticeable clinical improvement. In Jan 2017, the patient was administered methotrexate (10 mg/week sc followed by folic acid 24 h later).
After 17 weeks on methotrexate, there was no significant clinical improvement with significant and severe involvement of the dorsal surface of the hands (Fig. 1). Given this, and following appropriate screening exams, the patient was given adalimumab in April 2017 (initial dose of 80 mg, followed by 40 mg every other week starting 1 week after the initial dose). After 6–7 months, gradual, clinically significant improvement in symptoms was observed, which lasted for ~1 year (parameters from routine blood analyses were always within normal ranges, although anti-adalimumab antibodies were not measured). After this time, the efficacy of adalimumab diminished. The plaques on the hands had improved, leaving areas of vitiligo on the patient’s palms, and progressive worsening of symptoms and appearance of plaques on the elbows and knees was seen (Fig. 2). Diagnosis of vitiligo was made based on objective examination, absence of family history and blood workup to exclude other conditions (anemia, hyperthyroidism and diabetes). All these exams were negative, reinforcing a diagnosis of vitiligo. In May 2018, adalimumab was discontinued and the patient was initiated on therapy with secukinumab (300 mg weekly for 4 weeks; 300 mg monthly thereafter). After 6 months of therapy with secukinumab, substantial clinical improvement was observed with a PASI score of 1.8. During this time, the areas of vitiligo underwent gradual repigmentation (Fig. 3). After 1 year of treatment with secukinumab, small plaque areas remained in the flexor site with complete remission in the extensor area along with near complete resolution of areas of depigmentation.
Figure 1.
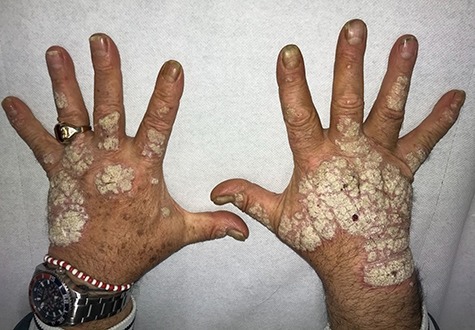
Dorsal surface of the hands prior to initiation of therapy with adalimumab.
Figure 2.
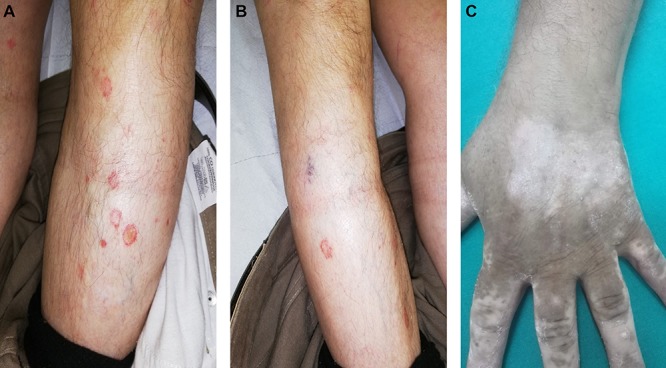
Plaques on the knee (A) and elbow (B) and appearance of vitiligo (C) following the loss of efficacy of adalimumab.
Figure 3.
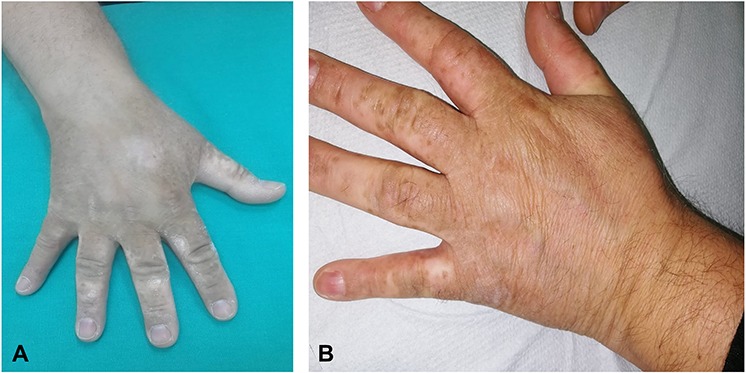
Images of the hands showing gradual repigmentation (A) and near complete resolution of vitiligo at 1 year after starting therapy with secukinumab (B).
DISCUSSION
Secukinumab is a human monoclonal antibody that targets interleukin-17A (IL-17A) and approved for moderate-to-severe plaque psoriasis, psoriatic arthritis and ankylosing spondylitis. Multiple randomized controlled trials evaluating secukinumab versus placebo, etanercept (ERASURE and FIXTURE), and ustekinumab (CLEAR and CLARITY) have established the efficacy of this drug in patients with moderate-to-severe psoriasis (reviewed in Yang et al. [2]). In general, secukinumab has been shown to be effective and well tolerated in treatment of plaque psoriasis [3]. Moreover, secukinumab has demonstrated a favorable safety profile for ~5 years of treatment [4]. In the present case, following the loss of efficacy of adalimumab and development of vitiligo, the initiation of secukinumab was associated with near complete resolution of symptoms within 1 year of treatment. During that time, no adverse effects of secukinumab were observed; and of note, areas of vitiligo also resolved with ongoing maintenance treatment. The safety profile of secukinumab has been, reported to be comparable to that of other IL-17 inhibitors, well tolerated for at least 5 years of treatment and is mostly associated with only mild side effects that rarely lead to discontinuation of therapy [2].
It is known that there is an association between vitiligo and psoriasis [1], and it cannot be entirely excluded that the psoriasis erupted on an area of vitiligo in the present case. However, the patient asked for referral to a dermatologist for psoriasis, and not vitiligo, and was repeatedly queried about previous vitiligo, which he denied. Vitiligo has been reported as a paradoxical adverse event following the use of several anti-TNF-α agents including adalimumab [1]. The adverse event is considered paradoxical since TNF-α is considered to be a potential cytokine involved in the pathogenesis of vitiligo. In fact, TNF-α inhibits differentiation of melanocytes from stem cells and also inhibits the functioning of melanocytes by inducing apoptosis. In treatment of vitiligo, however, anti-TNF-α agents have provided uncertain results, and several reports have documented development of vitiligo during anti-TNF-α therapy [5, 6]. Herein, a case of possible adalimumab-induced vitiligo in a patient with plaque psoriasis is reported.
In the present patient, secukinumab resolved both the symptoms of psoriasis after failure of adalimumab and the likely treatment-related vitiligo. In this regard, it is interesting that secukinumab targets IL-17A, which has been implicated in the pathogenesis of vitiligo. IL-17 has been firmly implicated in the development of several inflammatory disorders, including psoriasis, in which elevated levels of IL-17A have been found in sera from patients [7]. Moreover, levels of IL-17 have been found to be elevated in patients with vitiligo [8], and expression levels of IL-17A messenger RNA are also increased in vitiligo lesions [9]. Indeed, the efficacy of narrowband ultraviolet B (NB-UVB) therapy in vitiligo has been suggested to be related to its ability to modulate the expression of IL-17A [10]. Thus, the possibility of significant correlation between expression of IL-17 and autoimmune vitiligo is reinforced by the present case in which vitiligo resolved following administration of the anti-IL-17A agent secukinumab.
In conclusion, the present case of plaque psoriasis is of interest as the patient was refractory to both topical agents and methotrexate. Following an initial period of improvement of symptoms with adalimumab, loss of efficacy was observed along with the appearance of vitiligo. Following discontinuation of adalimumab and administration of secukinumab, symptoms related to psoriasis gradually resolved almost completely and vitiligo lesions progressively repigmented within 1 year. No adverse effects of secukinumab were observed, and the patient is continuing on maintenance therapy with the drug. Lastly, the mechanism of action of secukinumab, namely targeting IL-17A, is consistent with its positive effects on vitiligo.
ACKNOWLEDGEMENTS
Editorial assistance for the manuscript was provided by Health Publishing & Services srl and supported by an unrestricted grant from Novartis Farma Italy SpA.
Conflict of interest statement
None declared.
Funding
None.
Consent
Written informed consent was obtained from the patient.
Guarantor
GP is the guarantor of this article.
References
- 1. Toussirot E, Aubin F. Paradoxical reactions under TNF-alpha blocking agents and other biological agents given for chronic immune-mediated diseases: an analytical and comprehensive overview. RMD Open 2016;2:e000239. doi: 10.1136/rmdopen-2015-000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yang EJ, Beck KM, Liao W. Secukinumab in the treatment of psoriasis: patient selection and perspectives. Psoriasis (Auckl) 2018;875–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Langley RG, Elewski BE, Lebwohl M, et al. . Secukinumab in plaque psoriasis--results of two phase 3 trials. N Engl J Med 2014;371:326–38. [DOI] [PubMed] [Google Scholar]
- 4. Bissonnette R, Luger T, Thaci D, et al. . Secukinumab demonstrates high sustained efficacy and a favourable safety profile in patients with moderate-to-severe psoriasis through 5 years of treatment (SCULPTURE Extension Study). J Eur Acad Dermatol Venereol 2018;32:1507–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Posada C, Florez A, Batalla A, Alcazar JJ, Carpio D. Vitiligo during treatment of Crohn's disease with adalimumab: adverse effect or co-occurrence? Case Rep Dermatol 2011;3:28–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ramirez-Hernandez M, Marras C, Martinez-Escribano JA. Infliximab-induced vitiligo. Dermatology 2005;210:79–80. [DOI] [PubMed] [Google Scholar]
- 7. Martin DA, Towne JE, Kricorian G, et al. . The emerging role of IL-17 in the pathogenesis of psoriasis: preclinical and clinical findings. J Invest Dermatol 2013;133:17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Khan R, Gupta S, Sharma A. Circulatory levels of T-cell cytokines (interleukin [IL]-2, IL-4, IL-17, and transforming growth factor-beta) in patients with vitiligo. J Am Acad Dermatol 2012;66:510–1. [DOI] [PubMed] [Google Scholar]
- 9. Bassiouny DA, Shaker O. Role of interleukin-17 in the pathogenesis of vitiligo. Clin Exp Dermatol 2011;36:292–7. [DOI] [PubMed] [Google Scholar]
- 10. Malerba M, Damiani G, Radaeli A, et al. . Narrowband ultraviolet B phototherapy in psoriasis reduces proinflammatory cytokine levels and improves vitiligo and neutrophilic asthma. Br J Dermatol 2015;173:1544–5. [DOI] [PubMed] [Google Scholar]


