Abstract
Bile acids facilitate nutrient absorption and are endogenous ligands for nuclear receptors that regulate lipid and energy metabolism. The brain–gut–liver axis plays an essential role in maintaining overall glucose, bile acid, and immune homeostasis. Fasting and feeding transitions alter nutrient content in the gut, which influences bile acid composition and pool size. In turn, bile acid signaling controls lipid and glucose use and protection against inflammation. Altered bile acid metabolism resulting from gene mutations, high-fat diets, alcohol, or circadian disruption can contribute to cholestatic and inflammatory diseases, diabetes, and obesity. Bile acids and their derivatives are valuable therapeutic agents for treating these inflammatory metabolic diseases.
Keywords: bile acid signaling, bile acid therapy, nonalcoholic fatty liver disease, FXR, TGR5
1. INTRODUCTION
Bile acids are physiological agents that are required for nutrient absorption, distribution, metabolism, and excretion. Bile acids also are nutrient sensors and metabolic regulators that activate nuclear farnesoid X receptor (FXR) and Takeda G protein–coupled receptor 5 (TGR5; also known as G protein–coupled bile acid receptor-1, or GPBAR-1) signaling to integrate lipid, glucose, and energy metabolism and maintain metabolic homeostasis. Bile acids are synthesized in the liver and are secreted into the gastrointestinal tract to absorb nutrients postprandially and to control gut microbial overgrowth; meanwhile, gut microbes metabolize bile acids, which determines the circulating bile acid composition and pool size and regulates host metabolism. Nutrient availability, as determined by fasting and refeeding, modulates bile acid synthesis, the gut microbiome, and circulating hormones to regulate whole-body metabolic homeostasis and prevent metabolic diseases. Activation of bile acid receptor signaling protects the gastrointestinal tract from inflammation and injury. Dysregulation of bile acid homeostasis by mutations in genes of bile acid synthesis and transport, high-fat diets, drugs, and circadian disruption have been linked to cholestatic liver diseases, inflammatory bowel diseases, type 2 diabetes, obesity, and alcoholic and nonalcoholic fatty liver diseases. Drugs targeting bile acid receptors have been developed as potential therapeutic treatments for metabolic syndrome. This review focuses on recent advances in understanding bile acid homeostasis in human health and diseases, nutrient and gut microbiota regulation of bile acid metabolism, and the use of bile acids as therapeutic agents for metabolic diseases.
2. BILE ACID SYNTHESIS AND BIOTRANSFORMATION
2.1. Bile Acid Synthesis
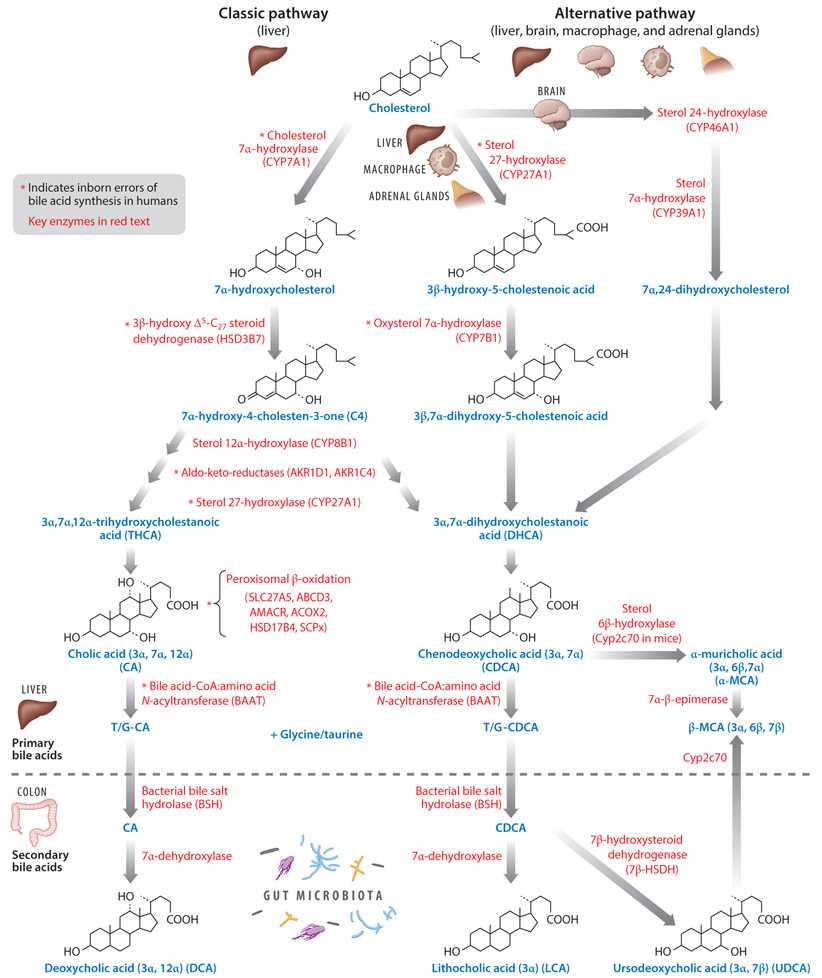
Bile acids are the end products of cholesterol catabolism in the liver. There are two major bile acid biosynthesis pathways: the classic pathway (neutral) and the alternative (acidic) pathway (16). The classic bile acid synthesis pathway is initiated by the microsomal rate-limiting enzyme cholesterol 7α-hydroxylase (CYP7A1), which converts cholesterol to 7α-hydroxycholesterol. 7α-hydroxycholesterol is then converted to 7α-hydroxy-4-cholesten-3-one (C4) by 3β-hydroxyΔ5-C27-steroid dehydrogenase (HSD3B7) (Figure 1). C4 is a common serum biomarker used for indicating bile acid synthesis and is the precursor for the two primary bile acids synthesized in the human liver, cholic acid (CA) and chenodeoxycholic acid (CDCA). Microsomal sterol 12α-hydroxylase (CYP8B1) hydroxylates C4 to 7α,12α-dihydroxy-4-cholesten-3-one, which is followed by the enzymatic activity of aldo-keto reductases (AKR1D1 and AKR1C4) and the mitochondrial sterol 27-hydroxylase (CYP27A1) to form 3α,7α,12α-trihydroxycholestanoic acid (THCA), the precursor of CA. Without 12α-hydroxylation, C4 is converted to 3α,7α-dihydroxycholestanoic acid (DHCA), the precursor of CDCA. THCA and DHCA are transported into the peroxisome for steroid side chain cleavage, similar to fatty acid β-oxidation. Bile acid-coenzyme A (CoA) synthase (BACS, SLC27A5) first activates THCA and DHCA to form acyl-CoA thioesters, which are then transported into the peroxisomes via the peroxisomal bile acid-acyl transporter ABCD3, where α-methylacyl-CoA racemase (AMACR), acyl-CoA oxidase (ACOX2), and D-bifunctional enzyme (HSD17B4) complete racemization, hydration, and dehydration steps, and, finally, sterol carrier protein x (SCPx) cleaves a propionyl-CoA from the steroid side chain of THCA and DHCA to form cholyl-CoA and chenodeoxycholyl-CoA, respectively. Cholyl-CoA and chenodeoxycholyl-CoA are conjugated to taurine or glycine by bile acid-CoA:amino acid N-acyltransferase (BAAT) to form T/G-CA and T/G-CDCA, respectively (Figure 1) (134).
Figure 1.
Bile acid synthesis pathways. In the liver, cholesterol is catabolized to bile acids by two pathways involving ~17 enzymes. The classic bile acid synthesis pathway is initiated by CYP7A1 in the liver, and the alternative bile acid synthesis pathway is initiated by CYP27A1 in the liver, macrophages, and adrenal glands, and CYP46A1 in the brain. Key enzymes involved in steroid ring modification, peroxisomal β-oxidation, cleavage of the steroid side chain, and bile acid conjugation are shown. In humans, CA and CDCA are the primary bile acids synthesized in the liver. In mice, Cyp2c70 converts CDCA to α- and β-MCA. In the intestine, primary bile acids undergo biotransformation. Conjugated bile acids are deconjugated by gut bacteria BSH, then bacteria 7α-dehydroxylase converts CA and CDCA to DCA and LCA, respectively. In human intestine, a small amount of CDCA can be converted to UDCA by 7β-HSDH. In mice, Cyp2c70 can also convert UDCA to β-MCA. The location of the hydroxyl group in each of the primary and secondary bile acids are indicated. Abbreviations: 7β-HSDH, 7β-hydroxysteroid dehydrogenase; ABCD3, bile acid-acyl transporter 3; ACOX2, acyl-CoA oxidase 2; AKR1D1 and AKR1C4, Δ4–3-oxosteroid-5β-reductase (aldo-keto reductases); AMACR, α-methylacyl-CoA racemase; BAAT: bile acid–CoA:amino acid N-acyltransferase; BSH: bile salt hydrolase; C4, 7α-hydroxy-4-cholesten-3-one; CA, cholic acid; CDCA, chenodeoxycholic acid; Cyp2c70, sterol 6β-hydroxylase; CYP7A1, cholesterol 7α-hydroxylase; CYP7B1, oxysterol 7α-hydroxylase; CYP8B1, sterol 12α-hydroxylase; CYP27A1, sterol 27-hydroxylase; CYP39A1, sterol 7α-hydroxylase; CYP46A1, sterol 24-hydroxylase; DCA, deoxycholic acid; HSD3B7, 3β-hydroxy-Δ5-C27-steroid dehydrogenase; HSD17B4, D-bifunctional protein; LCA, lithocholic acid; MCA, muricholic acid; SCPx, sterol carrier protein x; SLC27A5, long-chain acyl-coenzyme A synthase [also known as bile acid-coenzyme A synthase (BACS)]; UDCA: ursodeoxycholic acid.
In the alternative bile acid synthesis pathway, CYP27A1 initiates the conversion of cholesterol to both 27-hydroxycholesterol and 3β-hydroxy-5-cholestenoic acid in the liver, macrophages, and adrenal glands. Oxysterol 7α-hydroxylase (CYP7B1) then hydroxylates the C7 position, forming 7α,27-dihydroxycholesterol and 3β,7α-dihydroxy-5-cholestenoic acid. In the brain, sterol 24-hydroxylase (CYP46A1) converts cholesterol to 24-hydroxycholesterol, which is then hydroxylated at the 7α position by a specific sterol 7α-hydroxylase (CYP39A1) in the liver. These oxysterols generated in extrahepatic tissues can then act as substrates for the synthesis of CDCA and CA (details in 17). The classic bile acid synthesis pathway is tightly regulated by a negative feedback mechanism (discussed in Section 3), while the alternative pathway may be constitutively active. In humans, the classic pathway dominates bile acid synthesis, while in rodents the alternative pathway may account for as much as 50% of bile acid synthesis. Since CYP7A1 is not expressed in the fetal liver, bile acids are produced via the alternative pathway in neonates. CA (3α, 7α, 12α) and CDCA (3α, 7α) are the two primary bile acids synthesized in the human liver. CDCA is a hydrophobic bile acid. In mice, the majority of CDCA is further converted to α-muricholic acid (α-MCA; 3α, 6β, 7α) by the rodent-specific enzyme sterol-6β-hydroxylase (Cyp2c70), which can be epimerized to its 7β epimer, β-MCA (3α, 6β, 7β), by 7α-β-epimerase. Cyp2c70 may also hydroxylate the gut bacteria–produced secondary bile acid, ursodeoxycholic acid (UDCA; 3α, 7β), to β-MCA (3α, 6β, 7β) (129). α-MCA and β-MCA are considered as primary bile acids produced in rodent liver and are highly soluble and nontoxic bile acids. In humans, small amounts of CDCA (~2%) are converted to its 7β epimer, UDCA, by bacterial 7β-hydroxysteroid dehydrogenase (7β-HSDH) as a secondary bile acid, which is a highly soluble and nontoxic bile acid.
Bile acid synthesis pathways in humans and mice are remarkably similar; however, bile acid composition in the bile acid pools is very different. In mice, CA (~50%) and α- plus β-MCAs (~50%) are the predominant primary bile acids synthesized, whereas in the human liver, CA and CDCA make up the majority of primary bile acids. Bile acids form sodium salts (bile salts) under physiological pH. To increase solubility and reduce cytotoxicity, bile acids are immediately conjugated to taurine and glycine. In humans, bile acids are conjugated to glycine and taurine in a ratio of 3:1, while most bile acids (~95%) are taurine conjugated in mice.
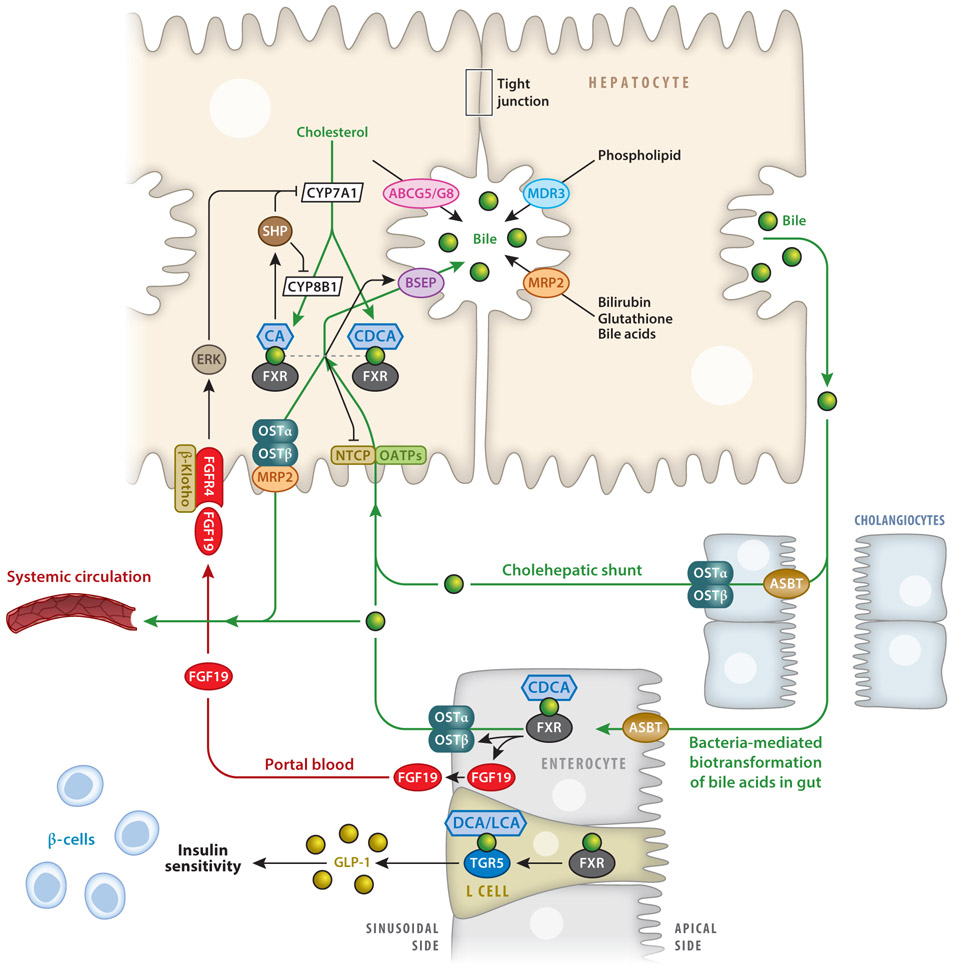
Conjugated bile acids are secreted across the canalicular membrane through active transport via the bile salt export pump (BSEP) (Figure 2). Here, bile acids rapidly form mixed micelles with cholesterol and phospholipids. Cholesterol is transported into bile via the adenosine triphosphate (ATP)-binding cassette subfamily G member 5 (ABCG5)–ABCG8 transporter protein, while phospholipids are transported by multidrug resistance protein 3 (MDR3). Bilirubin and glutathione are transported into bile via multidrug resistance-related protein 2 (MRP2), and typically bilirubin must be conjugated to glucuronic acid prior to excretion into bile. Bile continues to form as bile acids, electrolytes, and bicarbonate move into the canalicular space and are eventually secreted into the biliary tree formed by cholangiocytes. Finally, bile is secreted via the common bile duct into the gallbladder for storage.
Figure 2.
FXR and TGR5 regulation of bile acid synthesis and enterohepatic circulation of bile acids. In hepatocytes, FXR induces SHP to inhibit CYP7A1 and CYP8B1 gene transcription. FXR induces BSEP and MDR3, leading to the secretion into bile of, respectively, bile acids and phospholipids. FXR inhibits bile acid uptake via NTCP. In cholangiocytes, small amounts of bile acids are reabsorbed by ASBT and excreted via OSTα and -β, and these are then recycled to hepatocytes via cholehepatic shunting. In enterocytes, 95% of bile acids are reabsorbed via ASBT and may activate FXR, which induces FGF19. FGF19 in portal blood is circulated back to hepatocytes to activate FGFR4 and ERK signaling, inhibiting CYP7A1 and CYP8B1 transcription. Bile acids inhibit ASBT, but FXR induces OSTα and -β. In enteroendocrine L cells, FXR induces TGR5 to stimulate GLP-1 secretion. Abbreviations: ABCG5/G8, ATP-binding cassette subfamily G member 5 and 8; ASBT, apical sodium-dependent bile salt transporter; BSEP, bile salt export pump; CDCA, chenodeoxycholic acid; DCA, deoxycholic acid; ERK, extracellular signal-regulated protein kinase; FGF19, fibroblast growth factor 19; FGFR4, fibroblast growth factor receptor 4; FXR, farnesoid X receptor; GLP-1, glucagon-like peptide 1; LCA, lithocholic acid; MDR3, multidrug resistance protein 3; MRP2, MDR-related protein 2; NTCP, Na+-taurocholate cotransporting polypeptide; OATP, organic anion transport polypeptide; OST, organic solute transporter; SHP, small heterodimer partner; TGR5, Takeda G protein–coupled receptor 5.
2.2. Bile Acid Transformation in the Intestine
Secreted bile acids are reabsorbed in the intestine, mostly in the ileum. In the ileum and colon, gut bacterial bile salt hydrolase (BSH) deconjugates taurine- and glycine-conjugated bile acids, forming free bile acids. BSH activity is high in the Gram-positive bacteria genera Clostridium, Enterococcus, Bifidobacterium, and Lactobacillus, and in the Gram-negative genus Bacteroides. In the colon, bacterial 7α-dehydroxylase, mostly originating from the Clostridium clusters Eubacterium and Clostridium XIVa, removes a 7α-HO group from CA and CDCA to form, respectively, deoxycholic acid (DCA; 3α, 12α) and lithocholic acid (LCA; 3α) (Figure 1) (116). DCA and LCA are highly insoluble and toxic. DCA concentration is high in the colon (millimolar range) and has the strongest bactericidal activity. DCA is a promoter of colon cancer. LCA is the most hydrophobic bile acid, and its toxicity is reduced via sulfonation in the liver and intestine by bile salt sulfotransferases, leading to its excretion in urine and feces. The remaining bile acids are then reconjugated to glycine and taurine and enter portal circulation. In humans, CA, CDCA, and DCA are present in a ratio of approximately 4:4:2, and the glycine to taurine bile acids ratio is 3:1 in the human bile acid pool, while TCA and tauro-α-MCA plus tauro-β-MCA are present in a ratio of approximately 1:1; ~95% of bile acids are taurine conjugated in the mouse bile acid pool.
3. BILE ACID HOMEOSTASIS
3.1. Enterohepatic Circulation of Bile Acids
Meal ingestion triggers the release of cholecystokinin from the pancreas, which stimulates gallbladder contractions and releases bile acids into the gastrointestinal tract. In the ileum, bile acids facilitate nutrient absorption and are efficiently reabsorbed by enterocytes via the apical sodium-dependent bile salt transporter (ASBT). Bile acids are transported across the enterocyte to the sinusoidal membrane where organic solute transporter-α and -β (OSTα and -β) efflux bile acids into portal blood (Figure 2); here, they are taken up by hepatocytes via Na+-taurocholate cotransporting polypeptide (NTCP) and organic anion transporting polypeptides (OATPs). Bile acids lost through fecal excretion are replaced by de novo synthesis in the liver. This recycling of the bile acids occurs six to eight times per day in humans and efficiently reabsorbs about 95% of bile acids. A small subset of unconjugated bile acids secreted into the canaliculi may be absorbed directly by cholangiocytes and is transported back to the liver via the cholehepatic shunt (Figure 2).
3.2. Bile Acid–Activated Receptors in the Regulation of Bile Acid Homeostasis
Bile acid homeostasis is maintained through tight regulation of the synthesis, absorption, and excretion of bile acids by specific receptors and transporters located in the liver and intestine. Bile acids are endogenous ligands of nuclear receptors, including FXR (84), the pregnane X receptor (PXR) (44), and the vitamin D receptor (VDR) (83). Bile acids also activate TGR5 (87), sphingosine-1-phosphate receptor 2 (S1PR2) (128), and the muscarinic receptor (113).
3.2.1. Farnesoid X receptor.
FXR was the first bile acid–activated nuclear receptor identified (84). Ligand-activated FXR and retinoid X receptor heterodimers bind to an inverse repeat of the AGGTCA sequence, with one nucleotide spacing (IR1) on the target gene promoter. It has been proposed that FXR induces the nuclear receptor small heterodimer partner (SHP), which inhibits hepatic nuclear factor 4 and liver-related homolog 1 to inhibit transactivation of the CYP7A1 and CYP8B1 genes (Figure 2). Taurochenodeoxycholic acid (TCDCA) is the most potent endogenous FXR agonist [half of the maximum effective concentration (EC50) = 17 μM]. TCA is a major bile acid, but it is a weak FXR agonist (EC50 = ~0.6 mM). Therefore, it is unlikely that the physiological concentrations of TCA in hepatocytes can activate the FXR/SHP pathway to inhibit bile acid synthesis. However, in a cholestatic disease state, bile acids accumulate in hepatocytes and may activate the FXR/SHP pathway to inhibit CYP7A1 gene transcription. An early study of bile fistula in rats showed that intraduodenal infusion, but not intravenous infusion, of TCA inhibited Cyp7a1 messenger RNA expression levels, suggesting that intestinal factors induced by TCA are required for bile acid feedback inhibition of CYP7A1 gene transcription (100). A more recent study identified fibroblast growth factor 15 (FGF15, or human FGF19) as an intestinal FXR-induced hormone, which activates the hepatic FGF receptor 4 (FGFR4)–β-Klotho complex to inhibit CYP7A1 gene transcription (Figure 2) (57). FGFR4 may activate extracellular signal-regulated protein kinase-1 and -2 (ERK1 and -2) to inhibit CYP7A1 gene transcription (126). Bile acid concentrations are much higher in the gut compared with the liver, and this intestinal FXR/FGF19 (or FGF15) to liver FGFR4 /ERK1/2 signaling pathway is more likely the physiological mechanism regulating bile acid feedback.
In the ileum, FXR induces OSTα and -β to efflux bile acids into the portal circulation. Bile acids inhibit NTCP by a mechanism involving FXR/SHP-mediated repression of the retinoid X receptor and the retinoic acid receptor.
3.2.2. Pregnane X receptor and vitamin D receptor.
PXR and VDR are activated by LCA in the intestine. PXR induces CYP3A4 (mouse ortholog, Cyp3a11), the major drug-metabolizing enzyme in the intestine and liver. In mice, PXR can be activated by 5β-cholestane-3α,7α,12α-triol (triol) to induce Cyp3a11, which metabolizes triol to bile acids (28). PXR also induces sulfotransferases, which conjugate a sulfur group to LCA for detoxification and secretion. VDR is expressed in hepatic stellate cells and in the intestine. Activation of intestinal VDR induced CYP3A4 and reduced inflammation to protect against colonic cancer (83), while activation of VDR inhibited hepatic stellate cell activation and protected against liver fibrosis (23). Lastly, both PXR and VDR inhibit CYP7A1 gene transcription (49, 74).
3.2.3. Takeda G protein–coupled receptor 5.
TGR5, a Gαs protein–coupled receptor, is activated by several bile acids: LCA (EC50 = 0.3 μM) > DCA (EC50 = 1 μM) > CDCA > UDCA. TGR5 is widely expressed in the epithelial cells of the gastrointestinal system, including in the intestine, spleen, cholangiocytes, gallbladder, hepatic sinusoidal endothelial cells, and hepatic macrophages (Kupffer cells). TGR5 activates cyclic adenosine monophosphate/protein kinase A (cAMP/PKA) signaling to stimulate energy metabolism in brown adipose tissue and is involved in gallbladder refilling, secretion of glucagon-like peptide-1 (GLP-1) from L cells, and control of gastrointestinal motility (5, 75, 131, 132). Activation of TGR5 protects the liver from bile acid overload during liver regeneration, markedly decreases lipopolysaccharide (LPS)-induced cytokine production in primary macrophages, and protects adipose tissue from inflammation and associated insulin resistance. TGR5 is highly expressed in cholangiocytes, and selective agonists protect against LPS-induced inflammation. TGR5 expression levels are further increased in cholangiocarcinoma cells, and activation of TGR5 by LCA increases reactive oxygen species and ERK1 and -2 phosphorylation, causing cholangiocyte proliferation. A recent study showed that TGR5 plays a key role in fasting-induced hepatic steatosis via FGF21/growth hormone/Stat5 signaling, which regulates CYP7B1, a sexually dimorphic and male-predominant gene in the alternative bile acid synthesis pathway (25). A recent study reports that FXR and TGR5 are colocalized in enteroendocrine cells, and FXR induces TGR5 to stimulate GLP-1 secretion (103), which stimulates insulin secretion from pancreatic β-cells, tying bile acids to glucose regulation. This suggests that FXR binds to the TGR5 gene promoter to induce TGR5 expression and provides evidence for direct FXR and TGR5 cross talk in regulating insulin and glucose sensitivity.
3.2.4. Sphingosine-1-phosphate receptor 2.
Tauro-conjugated bile acids are activators of S1PR2, a Gαi protein–coupled receptor. It has been reported that S1PR2 activates the protein kinase B (AKT)/insulin and ERK1 and ERK2 signaling pathways and regulates hepatic lipid metabolism (95, 128). S1PR2 is highly expressed in cholangiocytes, and its expression is increased in cholangiocarcinoma (80). TCA activates S1PR2 and stimulates cholangiocarcinoma proliferation, migration, and invasion.
3.3. The Brain–Gut–Liver Axis in Bile Acid Homeostasis
The brain–gut–liver axis refers to the bidirectional system of communication arising from the central nervous system (CNS), which sends efferent signals to the gut lumen, and afferent signals in the gut are transmitted through the autonomic nervous system to the CNS. Central control of the gut is exerted via direct neuronal signaling as well as hormonal release, and it mediates food intake, gut motility, mucosal production, glucose use in the liver, and inflammatory and immune responses. In turn, the gut microbiome can affect neural physiology via the production of LPSs. Chronic liver diseases, including cholestasis and cirrhosis, are often associated with hepatic encephalopathy (HE) (46, 88, 118), and research indicates that gut-derived toxins and hyperammonemia, coupled with compromised intestinal barrier function, contribute to the pathology of HE.
Bile acids can modulate hormone release from the hypothalamus. In human patients with cholestasis and rodent models of bile duct ligation, TCA and glycochenodeoxycholic acid cross the blood–brain barrier and are taken up by neurons expressing ASBT, where they act to suppress the hypothalamic–pituitary–adrenal (HPA) axis (90). Interestingly, TCA-activated S1PR2 signaling has been shown to cause inflammation in HE (89), while other bile acids (in particular, UDCA) have been implicated as having protective effects against neurological disorders such as Parkinson’s, Huntington’s, and Alzheimer’s diseases. TGR5 may act as a neurosteroid receptor, and its expression was reduced in the brains of patients with HE (66), although the exact function of TGR5 in the brain is still unclear.
Circadian rhythms also modulate bile acid, lipid, and glucose homeostasis and originate from so-called core clock genes whose rhythmic transcription and translation result in protein accumulation and degradation that oscillates with a period of ~24 h. These neural signals then synchronize the cellular rhythms in all peripheral tissues and coordinate cellular processes, such as nutrient usage and the prevention of metabolically futile cycles, as well as control of whole-body physiology, such as sleep–wake cycles, blood pressure, and body temperature. Homeostatic maintenance of physiological timing is critical to ensuring appropriate metabolic responses to a changing environment. Desynchronization of circadian timing, due to chronic jet lag, long-term shift work, or sleep disorders, contributes to the development of metabolic syndrome, cardiovascular events, inflammatory gastrointestinal diseases, and cancer. It has been reported that ~10% of the mouse liver transcriptome (2) and ~20% of the proteome (114) exhibit circadian rhythmicity, indicating that many aspects of liver metabolism are likely under circadian control. In humans, the serum C4 level, an indicator of bile acid synthesis, peaks during the day (between 1:00 p.m. and 9:00 p.m.), which is opposite to the circadian rhythm of the lathosterol level that indicates cholesterol synthesis (38). A small study of human participants reported that circulating conjugated and unconjugated bile acids are asynchronous, and transintestinal flux of conjugated bile acids increased serum FGF19 levels and suppressed bile acid synthesis. Interestingly, unconjugated bile acids peaked at night, possibly related to intestinal bacterial deconjugation (3). Serum FGF19 levels also exhibit a diurnal rhythm, peaking 90–120 min after the postprandial rise in serum bile acids (82), coinciding with a role for FGF19 in the physiological control of bile acid synthesis. Short-term sleep disruption in mice resulted in widespread suppression of core clock and clock-controlled genes, including reduced Cyp7a1 gene expression and altered bile acid homeostasis (33). Chronic jet lag was shown to induce hepatocellular carcinoma in wild-type mice, and this effect was worsened in Fxr knockout mice, indicating that bile acid and cholesterol homeostasis are critical in preventing nonalcoholic fatty liver disease (NAFLD) and hepatocellular carcinoma (HCC) (67). Additionally, microbial BSH altered bile acid composition, host metabolic genes involved in lipid metabolism, and circadian rhythms in the liver and intestine (63).
Delineation of chronic metabolic and inflammatory liver disease pathways is critical to understanding the neural changes that occur in these conditions and will open new avenues of treatment and help to reduce side effects. Presently, drug development contrasts two approaches: broad-spectrum drugs, such as bile acid receptor agonists, versus manipulation of the microbiome, which is highly individualized. Large-scale and interdisciplinary studies will be required to advance our knowledge of how bile acids and the brain–gut–liver axis function to maintain homeostasis and how this axis can be utilized to prevent disease progression.
4. NUTRIENT REGULATION OF BILE ACID HOMEOSTASIS AND METABOLISM
Nutrient homeostasis is maintained by the liver during fasting and feeding. Bile acids act as nutrient sensors and metabolic regulators of hepatic homeostatic pathways, including glucose and lipid metabolism and the detoxification of drugs and xenobiotics. Bile acids aid in the absorption of fats, drugs, nutrients, and vitamins, and, in turn, bile acid synthesis and homeostasis are regulated by the fasting-to-feeding transition, nutrient content and diet, and the gut microbiome. Overnutrition and high-fat diets lead to metabolic syndrome, diabetes, and NAFLD. NAFLD is a hepatic manifestation of metabolic syndrome, with a prevalence of ~24% in the global population (145).
4.1. Fasting and Feeding in Bile Acid, Lipid, and Glucose Metabolism
During the immediate postprandial state, blood glucose and insulin levels are increased, and glucose is transported to tissues to be utilized for energy metabolism and for glycogen synthesis in the liver and muscle. Feeding induces CYP7A1 gene expression but suppresses CYP8B1 gene expression, while fasting induces CYP8B1 and suppresses CYP7A1 gene expression (102). The opposing effects of nutrient regulation on these two important genes may alter the rate of bile acid synthesis and bile acid composition, which affect intestinal nutrient absorption and hepatic lipid and glucose metabolism during fasting and refeeding cycles (Table 1). The postprandial increase in serum insulin and glucose levels is correlated with the increase of bile acid synthesis and serum GLP-1 and triglyceride levels. FGF19 is induced in the late postprandial states. Serum insulin and glucose levels are decreased in postabsorptive states and during starvation, and the levels are correlated with the decrease of bile acids and serum GLP-1 and triglycerides. In starvation states, serum free fatty acids, FGF21, and glucagon are increased to stimulate energy metabolism, and this induces gluconeogenesis and glucose release from glycogens to prevent hypoglycemia.
Table 1.
Nutrient regulation of bile acid, lipid, and glucose metabolism
| Hormones and metabolites |
Time after feeding | ||
|---|---|---|---|
| Postprandial (1–5 h) | Postabsorptive (6–12 h) |
Fasting (12–24 h) | |
| Insulin, glucose | ↑ | ↓ | ↓ |
| Bile acids | ↑ | ↓ | ↓ |
| GLP-1 | ↑ | ↓ | ↓ |
| FGF19 | ↑ | ↓ | ↓ |
| Triglycerides, VLDL | ↑ | ↓ | ↓ |
| Free fatty acids, FGF21 | ↓ | ↓ | ↑ |
| Glucagon, glycogen | ↓ | ↓ | ↑ |
↑ indicates increase; ↓ indicates decrease.
Abbreviations: FGF, fibroblast growth factor; GLP-1, glucagon-like peptide 1; VLDL, very low-density lipoprotein.
4.1.1. Metabolic hormones and nutrient sensors.
Bile acids modulate glucose metabolism via both FXR and TGR5: Bile acids activate intestinal FXR to produce and release FGF19, while activation of TGR5 by bile acids in intestinal L cells stimulates rapid secretion of GLP-1. GLP-1 is an incretin that stimulates insulin secretion from pancreatic β-cells, and bile acid activation of FXR also stimulates insulin secretion. Feeding-induced FGF19 and fasting-induced FGF21 are metabolic regulators with different physiological functions, but they function similarly in stimulating energy metabolism and insulin sensitivity, and in reducing weight via direct actions within the CNS (71).
4.1.2. Fibroblast growth factor 19.
FXR induces FGF19, which activates the ERK pathway and improves glucose tolerance and insulin sensitivity (69). FGF19 increased energy metabolism and metabolic rate, and reduced weight in diet-induced obese (DIO) mice (36). When delivered centrally, FGF19 improved glucose tolerance and insulin sensitivity independently of weight loss and insulin signaling (85), and it may involve suppression of the hypothalamic–pituitary–adrenal axis (107). Patients with metabolic syndrome and obesity have reduced circulating FGF19 levels. Meanwhile, patients with type 2 diabetes are known to have increased CYP7A1 gene expression and bile acid pool size, due to hyperglycemia, indicating a dysregulation of the FXR/FGF19 negative feedback pathway (40).
4.1.3. Glucagon-like peptide-1.
GLP-1 circulates to pancreatic β-cells and binds to the GLP-1 receptor to stimulate the release of insulin. GLP-1 also increases hepatic glycogen production and improves whole-body glucose tolerance. GLP-1 has a short half-life of ~2 min before its degradation by dipeptidyl peptidase 4 (DPP4). DPP4 is altered in islets of patients with type 2 diabetes, and DDP4 inhibitor–based therapies have had moderate success in reducing glucose intolerance and insulin resistance (137). GLP-1 receptor agonists have shown promise for the management of type 2 diabetes and may also result in weight loss (125).
4.1.4. Fibroblast growth factor 21.
FGF21 is a nutrient-sensing hormone produced in hepatocytes and adipose tissue in response to prolonged fasting. In fasting and starvation, glucagon stimulates cAMP production to induce hormone-sensitive phospholipase A in adipose tissue, which hydrolyzes triglycerides to release free fatty acids that circulate to the liver and muscle. In liver and adipose tissue, free fatty acids activate peroxisome proliferator–activated receptor α (PPARα) to induce FGF21. FGF21 induces PPARγ coactivator 1α (PGC-1α) and stimulates gluconeogenesis and fatty acid oxidation to improve glucose tolerance (68, 108). FGF21 also activates the FGFR–β-Klotho complex in hepatocytes and adipose tissue to mimic insulin action. FGF21 inhibits growth hormone/Stat5 signaling as an adaptive response to fasting (58). FGF21 has been shown to stimulate insulin sensitivity via several mechanisms. In the liver, FGF21 inhibits mechanistic target of rapamycin complex 1 (or mTORC1) to stimulate hepatic insulin sensitivity. In adipose tissue, FGF21 activates PPARγ to stimulate fatty acid oxidation and improve insulin sensitivity. FGF21 stimulates adiponectin secretion from adipose tissue to reduce ceramide and blood glucose and to enhance insulin sensitivity in ob/ob mice and DIO mice (53). FGF21 also directly regulates hepatic metabolism independently of PGC-1α (35). In obese human patients, circulating FGF21 is paradoxically induced and may indicate that obesity is an FGF21-resistant state (41). However, FGF21 may potentially be utilized as a therapeutic to treat obesity and metabolic disease (21, 37, 130).
4.2. Bile Acids and Nonalcoholic Fatty Liver Disease
NAFLD is the most common chronic liver disease, with a prevalence of ~30% in the US population, and it is a risk factor for type 2 diabetes, obesity, cardiovascular disease, and HCC (18, 31). Bile acid composition and pool size are altered in insulin-resistant patients, characterized by increased hydrophobic plasma bile acids and 12α-hydroxylated bile acids (47). CYP8B1 determines the ratio of 12α-hydroxylated bile acids to non-12α-hydroxylated bile acids and, thus, the hydrophobicity of the bile acid pool. Mice deficient in Cyp8b1 have an increased bile acid pool with increased T-α-MCA and T-β-MCA and reduced dietary cholesterol absorption as well as diet-induced obesity (9, 78). The loss of Cyp8b1 prevents hypercholesterolemia and gallstone formation in diabetic mice, and it prevents atherosclerosis in Apoe knockout mice (124); also, it improves glucose homeostasis by increasing GLP-1 secretion (65). Mice overexpressing Cyp7a1 have an increased bile acid pool consisting of increased TCDCA and an absence of TCA. These mice are resistant to high-fat diet-induced obesity and glucose intolerance; this may be due to activation of FXR signaling by TCDCA (76, 77). Hence, the bile acid pool size and bile acid composition affect glucose homeostasis, and modulation of bile acid synthesis and composition could provide new avenues for the treatment of metabolic disorders.
5. THE GUT MICROBIOTA AND BILE ACID METABOLISM IN NONALCOHOLIC FATTY LIVER DISEASE
High-fat diets, circadian disruption, time of feeding and fasting, drugs, and hormones can shape the gut microbiota to alter bile acid homeostasis and host metabolism (136). Bile acids, especially DCA, are potent antimicrobial agents that control bacterial overgrowth and maintain intestinal barrier function. The biotransformation of primary bile acids into secondary bile acids occurs in the colon (Section 2.2). Gut bacteria determine bile acid composition and pool size to modulate intestinal FXR and TGR5 signaling, which regulate host metabolism. In human feces, 90% of the bacteria belong to two phyla: Gram-positive Firmicutes and Gram-negative Bacteroidetes. The relative abundance of Firmicutes and Bacteroidetes is associated with obesity in mice and humans. A higher ratio of Firmicutes to Bacteroidetes enables the gut microbiota to extract energy more efficiently from high-fat diets, thus increasing adiposity and obesity.
An animal-based saturated-fat diet promotes dysbiosis by increasing the abundance of bile-tolerant bacteria, decreasing the ratio of Firmicutes to Bacteroidetes (20), and significantly inducing TCA and DCA (22). Interestingly, a recent study demonstrated the transmission of obese- and lean-associated metabolic phenotypes to germ-free mice via gut microbe–host interactions, although the underlying mechanism of gut microbial control of host metabolism is not clear (115).
Gut bacteria utilize bile acids, polysaccharides, cellulose, and starch to produce short-chain fatty acids (such as acetate, butyrate, and propionate) and amino acids for energy metabolism and growth. BSH and 7α- and 7β-HSDH activities are high in the genera Clostridium, Enterococcus, Bifidobacterium, Listeria, and Lactobacillus of the phylum Firmicutes, and in the genus Bacteroides of the phylum Bacteroidetes. The abundance of these bacteria in the gut microbiome determines the extent of secondary bile acid synthesis. In these anaerobic bacteria, 7α- and 7β-HSDH activities catalyze multiple steps of secondary bile acid synthesis. Microbial metabolites, bile acid species, and bacterial BSH activity play important roles in regulating host glucose, lipid, and energy metabolism (63). A recent study reports that bacteria of the phylum Bacteroidetes exhibit selective BSH deconjugation of bile acids based on the hydroxyl group pattern on the steroid ring (143). This study demonstrated that altering BSH selectivity may alter gut bile acid composition to affect host metabolism and gene expression.
5.1. Bile Acids and Nonalcoholic Steatohepatitis
Nonalcoholic steatohepatitis (NASH) is a progressive form of NAFLD that may lead to cirrhosis and HCC (12, 18). NASH patients have increased circulating bile acids, especially conjugated primary bile acids, decreased secondary bile acids, and an increased ratio of conjugated CA to CDCA (112). The cause of this alteration in circulating bile acids in NASH patients is not clear, although the gut microbiota may play a role. CA has the lowest critical micellar concentration for the solubilization of cholesterol in the gallbladder, and it efficiently absorbs dietary cholesterol in the intestine (93). Diets that are high in fat, high in cholesterol, and high in fructose cause hepatic inflammation and NASH (60, 99, 138). The molecular mechanism of progression from simple steatosis to fibrosis is not completely understood. It is likely that dysbiosis affects bile acid biotransformation and bile acid pool size, contributing to NASH progression and type 2 diabetes. A recent detailed analysis demonstrated a significant increase in the minor Gram-negative phylum Proteobacteria, while Gram-positive Firmicutes decreased in advanced fibrosis (81). It appears that dysbiosis in the form of increased Gram-negative bacteria may precede fibrosis, and microbial biomarkers could eventually be used as a reliable and noninvasive tool for diagnosing moderate-to-advanced fibrosis.
5.2. Intestinal Farnesoid X Receptor in Bile Acid Metabolism
The gut microbiota and intestinal FXR interact to control metabolic homeostasis (43). Mice fed a high-fat diet and treated with tempol, an antioxidant, had reduced Lactobacillus and BSH activity, which increased concentrations of T-β-MCA. T-β-MCA antagonizes intestinal FXR/FGF19 signaling to increase bile acid synthesis and improve DIO and diabetes (73, 120). Further studies of intestine-specific Fxr−/− mice and antagonism of intestinal FXR by glycine-MCA demonstrated that intestinal FXR played a critical role in protecting against DIO and diabetes (61, 62). These studies also indicated that intestinal FXR induced the synthesis of ceramides and stimulated hepatic gluconeogenesis (140). Ceramides are known to cause endoplasmic reticulum stress, cause mitochondrial oxidative stress, and contribute to NAFLD (43). Paradoxically, the intestine-selective FXR agonist fexaramine altered serum bile acid composition, promoted adipose tissue browning, and reduced diet-induced obesity in DIO mice (30). It was later discovered that fexaramine treatment altered the gut microbiota by increasing the abundance of the anaerobic Gram-positive Acetatifactor (phylum Firmicutes) and Gram-negative Bacteroides (phylum Bacteroidetes). These bacteria have high 7α- and 7β-HSDH activities, which convert CDCA and UDCA to LCA. LCA and DCA are potent TGR5 agonists in the colon and stimulate postprandial glucose-induced GLP-1 secretion from L cells and improve insulin sensitivity in obese diabetic mice (101). The activation of TGR5 in adipose tissue promoted white adipose tissue browning and energy metabolism and prevented obesity (101). A recent study reported that aged Atg7ΔCD11c mice had reduced weight compared with control Atg7f/f mice, but their weight became similar when they were cohoused or received fecal transplants. Fecal RNA sequencing identified increased Bacteroides acidifaciens in Atg7ΔCD11c mice compared with Atg7f/f mice. Interestingly, C57BL/6J mice fed B. acidifaciens gained less weight and fat mass, and they had increased serum insulin and GLP-1, suggesting that B. acidifaciens may prevent obesity and improve insulin sensitivity (141). B. acidifaciens has been isolated from human feces. It is likely that B. acidifaciens treatment stimulates intestinal FXR/TGR5/GLP-1 signaling to stimulate energy metabolism and increase insulin sensitivity.
5.3. Alcohol and Bile Acid Metabolism
Chronic alcohol consumption may lead to steatosis and progression to inflammation, fibrosis, or HCC. Women have an increased risk of alcoholic fatty liver disease due to lower levels of alcohol dehydrogenase and higher levels of estrogen (39). Alcoholic patients exhibit increased serum bile acids and cholestatic liver injury, and alcohol increases bile acid pool size and reduces biliary bile flow and fecal excretion. A recent study showed that chronic-plus-binge alcohol feeding reduced Cyp7a1 expression but increased the bile acid pool by increasing intestinal bile acid absorption, and the deficiency in Cyp7a1 expression exacerbated alcohol-induced liver injury (26). The microbiome is involved in mediating the effects of alcohol in the liver and gut, and dysbiosis is a common result of chronic alcohol consumption in humans. Another study reported that chronic ethanol feeding in mice induced bacterial expression of cholylglycine hydrolase and, subsequently, increased levels of unconjugated bile acids. These effects and the symptoms of alcoholic liver disease were reduced with antibiotics or the administration of an FXR-specific agonist (51). Germ-free mice that were humanized with stool from patients with cirrhosis plus alcoholism had increased secondary bile acids in the intestine compared with mice with stool from cirrhosis-only patients or healthy controls (64). In humans, chronic alcohol consumption was associated with long-term changes in colonic bacterial composition (94) and increased secondary bile acids and expression of intestinal bile acid transporter (8).
5.4. Bariatric Surgery
Bariatric surgery is one of the most effective treatments for obesity, and evidence suggests that bile acid physiology may play a role. Bile acid pool size and FGF19 levels are increased following bariatric surgery, and this quickly improves insulin sensitivity and glycemic control in obese patients (96). Interestingly, serum FGF19 increased after bariatric surgery, but not after conventional diet-induced weight loss (41). Additionally, serum bile acids and GLP-1 levels also increased following bariatric surgery (4). The mechanisms of rapid improvement of glycemic control and diabetes remission after gastric bypass remain unknown. A recent study of serum bile acid profiles during the 5 years following gastric bypass and duodenal switch surgeries showed substantial increases in total serum bile acid concentrations (117). The greater increases in unconjugated and glycine-conjugated primary bile acids after duodenal switch compared with gastric bypass suggest that a constitutively increased bile acid pool may be due to shorter enterohepatic circulation of bile acids, which alters the gut microbiome and metabolites to improve metabolism. It is likely that increased bile acids after bariatric bypass and duodenal switch surgeries reduce weight and increase insulin sensitivity by activating both FXR and TGR5 signaling to improve metabolic function.
6. BILE ACID–RELATED LIVER DISEASES
Inborn errors of bile acid metabolism have been identified in neonates. Bile acid synthesis intermediates accumulate in the liver and cause injury and cholestasis. Cholestatic liver diseases include biliary atresia, a developmental defect of the biliary tree in neonates (11), congenital cholestasis, and acquired cholestasis. Patients often have end-stage liver disease and require liver transplantation.
6.1. Inborn Errors of Bile Acid Metabolism
Deficiencies of bile acid synthesis, conjugation, and secretion cause the accumulation of cholesterol, bile acids, and toxic intermediates in hepatocytes. Of the 17 enzymes involved, inborn errors of bile acid synthesis have been identified in 13 enzymes in the classic and alternative bile acid synthesis pathways (Figure 1) (134). Deficiency of the rate-limiting enzyme CYP7A1 due to a frameshift nonsense mutation has been reported in only one family (111). Patients with this homozygote CYP7A1 mutation have dyslipidemia and premature gallstone and cardiovascular disease, consistent with the critical role of bile acid synthesis in maintaining whole-body cholesterol homeostasis. Analysis of liver and stool samples indicated increased hepatic cholesterol content, reduced biliary bile acid secretion, and induction of the alternative bile acid synthesis pathway. Genetic linkage analysis of CYP7A1 polymorphisms in the promoter region (A-204C, A-278C) and single nucleotide polymorphisms (rs7833904, rs8192870, rs2081687, rs3808607) have associated CYP7A1 gene mutations with increased risks of gallstones, hyperlipidemia, and cardiovascular disease (1, 13, 59).
In contrast to mild liver and cardiovascular diseases in CYP7A1 deficiency, mutation of the CYP7B1 gene in infants causes severe neonatal cholestasis and fibrosis (122) and progressive spastic paraplegia (133). In these infants, toxic 3β-monohydroxy-Δ5 bile acids accumulate and cause cholestatic liver injury, giant cell hepatitis, and severe fibrosis and cirrhosis. Mutation of the CYP8B1 gene has not been reported in humans, although deletion of the Cyp8b1 gene in mice reduces the absorption of dietary cholesterol and fat, prevents atherosclerosis, and improves glucose homeostasis and NAFLD (9, 65). More than 200 mutations in the human CYP27A1 gene have been identified in the rare lipid storage disorder cerebrotendinous xanthomatosis, which is characterized by the accumulation of cholesterol and cholestenol (bile alcohol) in xanthomas (xanthomatosis) and in the brain, and these occur with premature atherosclerosis and progressive neurological disorders (119). Deficiency of CYP27A1 reduces CDCA and causes the accumulation of cholesterol and 5β-cholestane-3α,7α,12α-triol, which is converted to bile alcohols that form xanthomas in tendons. Patients with cerebrotendinous xanthomatosis can be effectively treated with CDCA, which inhibits CYP7A1 and bile acid synthesis to alleviate the accumulation of cholesterol metabolites and, if administered early, reverses the pathophysiological process (6).
Progressive intrahepatic cholestasis and neonatal cholestasis can be caused by several mutations in the HSD3B7 gene (54). Bile acid therapy is effective in treating HSD3B7-deficient patients. AKR1D1 is required for the synthesis of 5β-reduced steroids, and AKR1D1 gene mutations have been identified in infants with hepatitis and liver failure. The oxidation reaction that cleaves the three-carbon steroid side chain from steroid intermediates to form C24 bile acids occurs in peroxisomes. Thus, it is unsurprising that defects in peroxisome biogenesis affect seven enzymatic and transport reactions (SLC27A5, ABCD3, AMACR, ACOX2, HSD17B4, BAAT, and SCPx) involved in the β-oxidation of DHCA and THCA to, respectively, CDCA and CA (Figure 1). Deficiencies in peroxisomal bile acid synthesis enzymes cause the accumulation of the cytotoxic C27 intermediates DHCA and THCA (32).
6.2. Cholestatic Liver Diseases
Cholestasis is a chronic liver condition resulting from obstructed hepatic bile flow, leading to the accumulation of cytotoxic bile acids in the liver and increased bile acids in the systemic circulation. Chronic cholestasis leads to fibrosis, cirrhosis, liver failure, and a higher risk of HCC or cholangiocarcinomas. Intrahepatic cholestasis arises from genetic mutations of bile acid transporter genes and autoimmune destruction of small bile ductules, while extrahepatic cholestasis results from obstruction by stones or tumors of the bile duct or pancreas (127). Biliary atresia causes extrahepatic cholestasis and vitamin deficiency in infants (135), and patients with cholestasis usually exhibit malnutrition due to a lack of nutrient absorption.
6.2.1. Congenital cholestasis.
Congenital cholestasis is usually early onset and is associated with jaundice, pruritus, and growth failure. Progressive familial intrahepatic cholestasis (PFIC) and benign recurrent intrahepatic cholestasis are autosomal recessive diseases associated with genetic mutations in ATP8B1 (PFIC1), BSEP (PFIC2), and ABCB4 (MDR3) (PFIC3) (27, 127). Mutation of the tight junction protein 2 gene (TJP2), localized to hepatic tight junctions, causes progressive cholestasis in humans and has been referred to as PFIC4. More recently, children with neonatal cholestasis associated with an FXR mutation have also been reported (42). These patients had undetectable hepatic expression of BSEP, normal or near-normal serum γ-glutamyl transferase (GGT), a marker of ductular damage, and rapid progression to end-stage liver disease.
In PFIC1, the ATP8B1 gene encodes a P-type cation transporter. ATP8B1 is a phospholipid flippase, which transports phosphatidylserine from the outer to the inner leaflet of the plasma membrane to maintain phospholipid asymmetry in the membrane, with higher phosphatidylcholine in the outer layer. The current hypothesis is that the loss of function of ATP8B1 alters membrane structure, resulting in impaired bile acid transport. In PFIC2, a lack of functional BSEP at the apical hepatocyte membrane causes hepatic bile acid accumulation, giant cell hepatitis, hepatocellular necrosis, and gallstone disease. In PFIC3, mutations of the ABCB4 (MDR3) gene, which encodes the apical phospholipid transporter (a floppase, P-glycoprotein), cause the accumulation of bile acids in hepatocytes, and biliary injury results from chronic exposure to high concentrations of nonmicellar bile acids. Decreased phospholipid secretion results in unstable mixed micelles that favor cholesterol crystallization and small bile duct obstruction. High serum levels of GGT are a characteristic feature of PFIC3 and distinguish it from PFIC1 and PFIC2.
6.2.2. Acquired cholestasis.
Intrahepatic cholestasis of pregnancy (ICP) affects ~1% of pregnant women. ICP is primarily diagnosed during the third trimester, and pruritus is the usual initial clinical presentation (24), although it is reversible and rapidly resolves after delivery. Environmental, hormonal, and genetic factors are thought to be involved in the pathogenesis, although ATP8B1 mutations have also been identified (92). ABCB4 and ABCB11 mutations occur in severe and recurrent ICP (144), and genetic variations of the BSEP gene may also be associated with ICP (104).
Primary biliary cholangitis (PBC), also known as primary biliary cirrhosis, is an acquired chronic cholestasis associated with autoimmune destruction of the small bile ducts, portal infiltration, and fibrosis. The majority of PBC patients are middle-aged females, while primary sclerosing cholangitis (PSC) patients are mostly male. PSC is associated with injury to and fibrosis of both intrahepatic and extrahepatic bile ducts, resulting in biliary strictures and obstructed bile flow.
Infants and children receiving total parenteral nutrition often have bile acid malabsorption, cholestasis, portal inflammation, and fibrosis (45). Total parenteral nutrition is used to treat infants with gastrointestinal abnormalities, short bowel syndrome, malnutrition, inflammatory bowel disease, and idiopathic diarrhea. Parenteral nutrition–associated cholestasis is associated with mutations in ABCB4 (MDR3) in preterm infants (142). UDCA can be used as bile acid replacement therapy to improve lipid absorption in patients with parenteral nutrition–associated cholestasis.
7. BILE ACID THERAPY
7.1. Bile Acid Replacement Therapy
Bile acid replacement therapy has been used to increase bile acids and reduce the concentrations of toxic bile acid intermediates that accumulate in patients with inborn errors of bile acid metabolism (Table 2). UDCA is a highly hydrophilic bile acid that is present in low amounts in humans. UDCA increases the hydrophilicity of the bile acid pool to decrease toxicity, and it has been used to dissolve cholesterol gallstones (79) and to prevent cholelithiasis in pregnancy. Currently, UDCA is the approved first-line therapy for PBC (29) because it significantly improves liver function and delays the need for liver transplantation. UDCA can also provide beneficial effects for ICP and PFIC3 patients. UDCA stimulates bile flow and promotes biliary HCO3− secretion (10), and it also exhibits anti-inflammatory and antiapoptotic effects (10, 110). However, ~40% of PBC patients do not respond to UDCA therapy. Fibrates, which activate PPARα to inhibit bile acid synthesis (86), are used alone or in combination with UDCA to treat PBC. Norursodeoxycholic acid (norUDCA) is a side chain–shortened C23 homolog of UDCA, which undergoes cholehepatic shunting to promote HCO3− secretion, and it exhibits potent anticholestatic and anti-inflammatory effects in experimental models (48). A recent randomized controlled Phase 2 trial showed that norUDCA significantly reduced alkaline phosphatase in a dose-dependent manner in PSC patients after 12 weeks of treatment (34).
Table 2.
Bile acid-based therapies
| Type of therapy | Effects |
|---|---|
| Bile acid replacement | |
| CDCA | Treats inborn errors of bile acid synthesis; reduces hypertriglyceridemia in gallstone patients |
| UDCA | Dissolves gallstones; reduces bile acid cytotoxicity in circulating pool |
| Bile acid sequestrants (bile acid–binding resins) | |
| Cholestyramine, colesevelam | Dissolves gallstones; treats diabetes but increases triglycerides; stimulates bile acid synthesis (CYP7A1); decreases FGF19; activates TGR5/GLP-1 signaling and improves glycemic control; stimulates thermogenesis in brown adipose tissue |
| FXR agonists | |
| Obeticholic acid, INT-747 (6-ethyl-chenodeoxycholic acid), Ocaliva (Intercept Pharmaceuticals) | Anti-inflammatory; used to treat cholestasis, NASH, diabetes, obesity, and PBC |
| NorUDCA | Used to treat PBC and PSC |
| Fexaramine (intestine-restricted FXR agonist) | Used to treat obesity and diabetes |
| FXR antagonists | |
| UDCA, glycine-MCA | Used to treat obesity and diabetes |
| TGR5 agonists | |
| INT-777 [6α-etdyl-23(S)-metdyl-cholic acid] | Anti-inflammatory; used to treat obesity and NASH |
| Dual FXR and TGR5 agonists | |
| INT-767 (6α-etdyl-3α, 7α, 23-trihydroxy-24-nor-5β-cholan-23-sulfate sodium salt) | Used to treat NASH |
Abbreviations: CDCA, chenodeoxycholic acid; CYP7A1, cholesterol 7α-hydroxylase; FGF, fibroblast growth factor; FXR, farnesoid X receptor; GLP-1, glucagon-like peptide 1; MCA, muricholic acid; NASH, nonalcoholic steatohepatitis; PBC, primary biliary cirrhosis; PSC, primary sclerosing cholangitis; TGR5, Takeda G protein–coupled receptor 5; UDCA, ursodeoxycholic acid.
7.2. Bile Acid Sequestrants
Bile acid sequestrants are a class of drugs that bind negatively charged bile acids in the intestine to prevent reabsorption and reduce the size of the bile acid pool. This results in reduced FGF19, which increases CYP7A1 gene transcription and hepatic bile acid synthesis. Bile acid sequestrants may increase DCA in the colon to stimulate TGR5-mediated secretion of GLP-1, which increases insulin secretion, improves glycemic control, and stimulates thermogenesis in brown adipose tissue (Table 2) (109, 123). By increasing bile acid synthesis, bile acid sequestrants increase hepatic uptake of low-density lipoprotein (LDL) cholesterol, thus reducing hypercholesterolemia. However, bile acid sequestrants may increase serum triglycerides and cause dyslipidemia. Cholestyramine and colestipol are classic bile acid sequestrants used to treat cholesterol gallstone disease and hypercholesterolemia in human patients. Colesevelam, a second-generation bile acid sequestrant, improves glycemic control in patients with type 2 diabetes (50).
7.3. Bile Acid Therapy for Nonalcoholic Steatohepatitis
Bile acid–based therapies targeting FXR and TGR5 have been developed to treat cholestasis and NASH. These drug therapies are based on the anti-inflammatory effect of activation of FXR and TGR5 signaling in the liver, adipose tissue, and intestine in mouse models of cholestasis, diet-induced obesity, insulin resistance, and NASH. Studies of the pharmacological activation of FXR and TGR5 signaling by bile acid–based compounds in mouse models have now been successfully applied to human cholestasis and NASH patients.
7.3.1. Farnesoid X receptor agonists.
Obeticholic acid (OCA; Ocaliva, Intercept Pharmaceuticals), a 6α-ethyl CDCA derivative, is a selective FXR agonist (EC50 = 0.099 μM) (105). OCA decreases bile acid synthesis and alleviates inflammation in experimental cholestasis (105); however, increased serum LDL cholesterol and decreased high-density lipoprotein cholesterol may result. OCA therapy improved NASH scores in PBC patients who had inadequate responses to UDCA and in noncirrhotic NASH patients (52, 97, 98). A multinational, randomized, doubleblind, Phase 2 study of OCA monotherapy reported improvement of inflammation in PBC patients treated with OCA for 6 years (70). Pruritus is a common side effect of bile acid therapy and was the main reason for discontinuing use of OCA at a higher dosage (50 mg). The US Food and Drug Administration recently approved OCA for treating PBC patients who do not respond to UDCA, and a Phase 3 clinical trial of OCA for NASH is underway.
7.3.2. Farnesoid X receptor antagonists.
UDCA is a weak FXR antagonist proven to be of some therapeutic value in treating obese NAFLD patients (91). Antagonism of intestinal FXR signaling by glycine-MCA altered the gut microbiota, reduced ceramide synthesis to reduce hepatic inflammation and steatosis, stimulated adipose tissue beigeing, and reduced diet-induced obesity (62). Caffeic acid phenethyl ester inhibits intestinal FXR-induced ceramide synthesis and decreases mitochondrial acetyl-CoA levels, pyruvate carboxylase activity, and gluconeogenesis (140). Intestinal FXR antagonists may have therapeutic potential for the treatment of NASH.
7.3.3. Takeda G protein–coupled receptor 5 agonists.
The TGR5-selective agonist INT-777 (6α-ethyl-23(S)-methyl-cholic acid; EC50 = 0.82 μM) stimulates adipose tissue thermogenesis and energy metabolism and reduces inflammation (106). INT-777 stimulates GLP-1 secretion and increases insulin sensitivity, but it does not affect serum lipids (103). INT-777 may have anti-inflammasome effects in pancreatic acinar cells and may also reduce neuroinflammation (72, 139). UDCA analogs have been shown to activate TGR5 (55, 56). BA501 (6β-3α,7β-dihydroxy-5β-cholan-24-ol), a non-bile-acid steroid derivative of UDCA, is a selective TGR5 agonist and reduces lipid deposition and vascular injury in DIO mice (121).
7.3.4. Dual farnesoid X receptor and Takeda G protein–coupled receptor 5 agonists.
INT-767 (6α-ethyl-3α,7α,23-trihydroxy-24-nor-5β-cholan-23-sulfate sodium salt) is an FXR (EC50 = 0.03 μM) and TGR5 (EC50 = 0.63 μM) dual agonist. INT-767 is a more potent FXR agonist than OCA. INT-767 inhibits bile acid synthesis, reduces liver injury, stimulates adipose tissue browning and TGR5-mediated GLP-1 secretion, and improves insulin sensitivity in DIO mice (7, 19, 103). INT-767 also prevents HCC in Abcb4 (Mdr3)-deficient mice (14). INT-767 is in a Phase 1 clinical trial for NASH. BA502, another dual FXR and TGR5 agonist, promotes adipose tissue browning, reduces hepatic steatosis in DIO mice, and reduces liver fibrosis induced by CCl4 (15).
8. CONCLUSIONS AND FUTURE PERSPECTIVES
Bile acids play a critical role in nutrient absorption and metabolism in the intestine and liver, and they regulate lipid, glucose, and energy homeostasis. Emerging trends in bile acid research must elucidate the complex interactions between bile acids and gut microbes in homeostatic and disease conditions, and the specific targeting of hepatic FXR or intestinal FXR and TGR5 signaling should be further developed into drug therapies for metabolic diseases.
ACKNOWLEDGMENTS
This research was supported by grants DK44442 and DK58379 from the National Institute of Diabetes and Digestive and Kidney Disease, National Institutes of Health.
Abbreviations:
- FXR
farnesoid X receptor
- TGR5
Takeda G protein–coupled receptor 5; also known as G protein–coupled bile acid receptor-1
- CYP7A1
cholesterol 7α-hydroxylase
- CA
cholic acid
- CDCA
chenodeoxycholic acid
- CYP8B1
sterol 12α-hydroxylase
- CYP27A1
sterol 27-hydroxylase
- CYP7B1
oxysterol 7α-hydroxylase
- MCA
muricholic acid
- UDCA
ursodeoxycholic acid
- DCA
deoxycholic acid
- LCA
lithocholic acid
- FGF15
fibroblast growth factor 15
- FGF19
fibroblast growth factor 19
- GLP-1
glucagon-like peptide-1
- NAFLD
nonalcoholic fatty liver disease
- HCC
hepatocellular carcinoma
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Abdullah MM, Eck PK, Couture P, Lamarche B, Jones PJH. 2018. The combination of single nucleotide polymorphisms rs6720173 (ABCG5), rs3808607 (CYP7A1), and rs760241 (DHCR7) is associated with differing serum cholesterol responses to dairy consumption. Appl. Physiol. Nutr. Metab 43:1090–93 [DOI] [PubMed] [Google Scholar]
- 2.Akhtar RA, Reddy AB, Maywood ES, Clayton JD, King VM, et al. 2002. Circadian cycling of the mouse liver transcriptome, as revealed by cDNA microarray, is driven by the suprachiasmatic nucleus. Curr. Biol 12:540–50 [DOI] [PubMed] [Google Scholar]
- 3.Al-Khaifi A, Straniero S, Voronova V, Chernikova D, Sokolov V, et al. 2018. Asynchronous rhythms of circulating conjugated and unconjugated bile acids in the modulation of human metabolism. J. Intern. Med 284:546–59 [DOI] [PubMed] [Google Scholar]
- 4.Albaugh VL, Flynn CR, Cai S, Xiao Y, Tamboli RA, Abumrad NN. 2015. Early increases in bile acids post Roux-en-Y gastric bypass are driven by insulin-sensitizing, secondary bile acids. J. Clin. Endocrinol. Metab 100:E1225–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alemi F, Poole DP, Chiu J, Schoonjans K, Cattaruzza F, et al. 2013. The receptor TGR5 mediates the prokinetic actions of intestinal bile acids and is required for normal defecation in mice. Gastroenterology 144:145–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amador MDM, Masingue M, Debs R, Lamari F, Perlbarg V, et al. 2018. Treatment with chenodeoxycholic acid in cerebrotendinous xanthomatosis: clinical, neurophysiological, and quantitative brain structural outcomes. J. Inherit. Metab. Dis 41:799–807 [DOI] [PubMed] [Google Scholar]
- 7.Baghdasaryan A, Claudel T, Gumhold J, Silbert D, Adorini L, et al. 2011. Dual farnesoid X receptor/TGR5 agonist INT-767 reduces liver injury in the Mdr2−/− (Abcb4−/−) mouse cholangiopathy model by promoting biliary HCO−3 output. Hepatology 54:1303–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bajaj JS, Kakiyama G, Zhao D, Takei H, Fagan A, et al. 2017. Continued alcohol misuse in human cirrhosis is associated with an impaired gut–liver axis. Alcohol. Clin. Exp. Res 41:1857–65 [DOI] [PubMed] [Google Scholar]
- 9.Bertaggia E, Jensen KK, Castro-Perez J, Xu Y, Di Paolo G, et al. 2017. Cyp8b1 ablation prevents Western diet–induced weight gain and hepatic steatosis because of impaired fat absorption. Am. J. Physiol. Endocrinol. Metab 313:E121–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beuers U 2006. Drug insight: Mechanisms and sites of action of ursodeoxycholic acid in cholestasis. Nat. Clin. Pract. Gastroenterol. Hepatol 3:318–28 [DOI] [PubMed] [Google Scholar]
- 11.Bezerra JA, Wells RG, Mack CL, Karpen SJ, Hoofnagle JH, et al. 2018. Biliary atresia: clinical and research challenges for the twenty-first century. Hepatology 68:1163–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brunt EM. 2010. Pathology of nonalcoholic fatty liver disease. Nat. Rev. Gastroenterol. Hepatol 7:195–203 [DOI] [PubMed] [Google Scholar]
- 13.Cai Q, Wang ZQ, Cai Q, Li C, Chen EZ, Jiang ZY. 2014. Relationship between CYP7A1-204A>C polymorphism with gallbladder stone disease and serum lipid levels: a meta-analysis. Lipids Health Dis. 13:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cariello M, Peres C, Zerlotin R, Porru E, Sabba C, et al. 2017. Long-term administration of nuclear bile acid receptor FXR agonist prevents spontaneous hepatocarcinogenesis in Abcb4−/− mice. Sci. Rep 7:11203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carino A, Cipriani S, Marchiano S, Biagioli M, Santorelli C, et al. 2017. BAR502, a dual FXR and GPBAR1 agonist, promotes browning of white adipose tissue and reverses liver steatosis and fibrosis. Sci. Rep 7:42801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chiang JYL. 2009. Bile acids: regulation of synthesis. J. Lipid Res 50:1955–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiang JYL, Ferrell JM. 2018. Bile acid metabolism in liver pathobiology. Gene Expr. 18:71–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen JC, Horton JD, Hobbs HH. 2011. Human fatty liver disease: old questions and new insights. Science 332:1519–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Comeglio P, Cellai I, Mello T, Filippi S, Maneschi E, et al. 2018. INT-767 prevents NASH and promotes visceral fat brown adipogenesis and mitochondrial function. J. Endocrinol 238:107–27 [DOI] [PubMed] [Google Scholar]
- 20.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, et al. 2014. Diet rapidly and reproducibly alters the human gut microbiome. Nature 505:559–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Degirolamo C, Sabba C, Moschetta A. 2015. Therapeutic potential of the endocrine fibroblast growth factors FGF19, FGF21 and FGF23. Nat. Rev. Drug Discov 15:51–69 [DOI] [PubMed] [Google Scholar]
- 22.Devkota S, Wang Y, Musch MW, Leone V, Fehlner-Peach H, et al. 2012. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10−/− mice. Nature 487:104–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ding N, Yu RT, Subramaniam N, Sherman MH, Wilson C, et al. 2013. A vitamin D receptor/SMAD genomic circuit gates hepatic fibrotic response. Cell 153:601–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dixon PH, Williamson C. 2016. The pathophysiology of intrahepatic cholestasis of pregnancy. Clin. Res. Hepatol. Gastroenterol 40:141–53 [DOI] [PubMed] [Google Scholar]
- 25.Donepudi AC, Boehme S, Li F, Chiang JY. 2017. G-protein-coupled bile acid receptor plays a key role in bile acid metabolism and fasting-induced hepatic steatosis in mice. Hepatology 65:813–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Donepudi AC, Ferrell JM, Boehme S, Choi HS, Chiang JYL. 2018. Deficiency of cholesterol 7α-hydroxylase in bile acid synthesis exacerbates alcohol-induced liver injury in mice. Hepatol. Commun 2:99–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Droge C, Bonus M, Baumann U, Klindt C, Lainka E, et al. 2017. Sequencing of FIC1, BSEP and MDR3 in a large cohort of patients with cholestasis revealed a high number of different genetic variants. J. Hepatol 67:1253–64 [DOI] [PubMed] [Google Scholar]
- 28.Dussault I, Yoo HD, Lin M, Wang E, Fan M, et al. 2003. Identification of an endogenous ligand that activates pregnane X receptor–mediated sterol clearance. PNAS 100:833–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dyson JK, Hirschfield GM, Adams DH, Beuers U, Mann DA, et al. 2015. Novel therapeutic targets in primary biliary cirrhosis. Nat. Rev. Gastroenterol. Hepatol 12:147–58 [DOI] [PubMed] [Google Scholar]
- 30.Fang S, Suh JM, Reilly SM, Yu E, Osborn O, et al. 2015. Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance. Nat. Med 21:159–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farrell GC, Larter CZ. 2006. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology 43(Suppl. 1):S99–112 [DOI] [PubMed] [Google Scholar]
- 32.Ferdinandusse S, Denis S, Dacremont G, Wanders RJ. 2009. Toxicity of peroxisomal C27-bile acid intermediates. Mol. Genet. Metab 96:121–28 [DOI] [PubMed] [Google Scholar]
- 33.Ferrell JM, Chiang JY. 2015. Short-term circadian disruption impairs bile acid and lipid homeostasis in mice. Cell. Mol. Gastroenterol. Hepatol 1:664–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fickert P, Hirschfield GM, Denk G, Marschall HU, Altorjay I, et al. 2017. norUrsodeoxycholic acid improves cholestasis in primary sclerosing cholangitis. J. Hepatol 67:549–58 [DOI] [PubMed] [Google Scholar]
- 35.Fisher FM, Estall JL, Adams AC, Antonellis PJ, Bina HA, et al. 2011. Integrated regulation of hepatic metabolism by fibroblast growth factor 21 (FGF21) in vivo. Endocrinology 152:2996–3004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fu L, John LM, Adams SH, Yu XX, Tomlinson E, et al. 2004. Fibroblast growth factor 19 increases metabolic rate and reverses dietary and leptin-deficient diabetes. Endocrinology 145:2594–603 [DOI] [PubMed] [Google Scholar]
- 37.Gaich G, Chien JY, Fu H, Glass LC, Deeg MA, et al. 2013. The effects of LY2405319, an FGF21 analog, in obese human subjects with type 2 diabetes. Cell Metab. 18:333–40 [DOI] [PubMed] [Google Scholar]
- 38.Galman C, Angelin B, Rudling M. 2005. Bile acid synthesis in humans has a rapid diurnal variation that is asynchronous with cholesterol synthesis. Gastroenterology 129:1445–53 [DOI] [PubMed] [Google Scholar]
- 39.Gao B, Bataller R. 2011. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology 141:1572–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gerhard GS, Styer AM, Wood GC, Roesch SL, Petrick AT, et al. 2013. A role for fibroblast growth factor 19 and bile acids in diabetes remission after Roux-en-Y gastric bypass. Diabetes Care 36:1859–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gomez-Ambrosi J, Gallego-Escuredo JM, Catalan V, Rodriguez A, Domingo P, et al. 2017. FGF19 and FGF21 serum concentrations in human obesity and type 2 diabetes behave differently after diet- or surgically-induced weight loss. Clin. Nutr 36:861–68 [DOI] [PubMed] [Google Scholar]
- 42.Gomez-Ospina N, Potter CJ, Xiao R, Manickam K, Kim MS, et al. 2016. Mutations in the nuclear bile acid receptor FXR cause progressive familial intrahepatic cholestasis. Nat. Commun 7:10713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gonzalez FJ, Jiang C, Patterson AD. 2016. An intestinal microbiota–farnesoid X receptor axis modulates metabolic disease. Gastroenterology 151:845–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goodwin B, Gauthier KC, Umetani M, Watson MA, Lochansky MI, et al. 2003. Identification of bile acid precursors as endogenous ligands for the nuclear xenobiotic pregnane X receptor. PNAS 100:223–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guglielmi FW, Regano N, Mazzuoli S, Fregnan S, Leogrande G, et al. 2008. Cholestasis induced by total parenteral nutrition. Clin. Liver Dis 12:97–110 [DOI] [PubMed] [Google Scholar]
- 46.Hadjihambi A, Arias N, Sheikh M, Jalan R. 2018. Hepatic encephalopathy: a critical current review. Hepatol. Int 12:135–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haeusler RA, Astiarraga B, Camastra S, Accili D, Ferrannini E. 2013. Human insulin resistance is associated with increased plasma levels of 12α-hydroxylated bile acids. Diabetes 62:4184–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Halilbasic E, Fiorotto R, Fickert P, Marschall HU, Moustafa T, et al. 2009. Side chain structure determines unique physiologic and therapeutic properties of norursodeoxycholic acid in Mdr2−/− mice. Hepatology 49:1972–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Han S, Chiang JY. 2009. Mechanism of vitamin D receptor inhibition of cholesterol 7α-hydroxylase gene transcription in human hepatocytes. Drug Metab. Dispos 37:469–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hansen M, Sonne DP, Mikkelsen KH, Gluud LL, Vilsboll T, Knop FK. 2017. Bile acid sequestrants for glycemic control in patients with type 2 diabetes: a systematic review with meta-analysis of randomized controlled trials. J. Diabetes Complicat 31:918–27 [DOI] [PubMed] [Google Scholar]
- 51.Hartmann P, Hochrath K, Horvath A, Chen P, Seebauer CT, et al. 2018. Modulation of the intestinal bile acid/farnesoid X receptor/fibroblast growth factor 15 axis improves alcoholic liver disease in mice. Hepatology 67:2150–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hirschfield GM, Mason A, Luketic V, Lindor K, Gordon SC, et al. 2015. Efficacy of obeticholic acid in patients with primary biliary cirrhosis and inadequate response to ursodeoxycholic acid. Gastroenterology 148:751–61.e8 [DOI] [PubMed] [Google Scholar]
- 53.Holland WL, Adams AC, Brozinick JT, Bui HH, Miyauchi Y, et al. 2013. An FGF21–adiponectin–ceramide axis controls energy expenditure and insulin action in mice. Cell Metab. 17:790–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang HY, Zhou H, Wang H, Chen YX, Fang F. 2016. Novel mutations in the 3β-hydroxy-Δ5-C27-steroid dehydrogenase gene (HSD3B7) in a patient with neonatal cholestasis. Chin. Med.J 129:98–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ibrahim E, Diakonov I, Arunthavarajah D, Swift T, Goodwin M, et al. 2018. Bile acids and their respective conjugates elicit different responses in neonatal cardiomyocytes: role of Gi protein, muscarinic receptors and TGR5. Sci. Rep 8:7110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Iguchi Y, Nishimaki-Mogami T, Yamaguchi M, Teraoka F, Kaneko T, Une M. 2011. Effects of chemical modification of ursodeoxycholic acid on TGR5 activation. Biol. Pharm. Bull 34:1–7 [DOI] [PubMed] [Google Scholar]
- 57.Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, et al. 2005. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2:217–25 [DOI] [PubMed] [Google Scholar]
- 58.Inagaki T, Lin VY, Goetz R, Mohammadi M, Mangelsdorf DJ, Kliewer SA. 2008. Inhibition of growth hormone signaling by the fasting-induced hormone FGF21. Cell Metab. 8:77–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Iwanicki T, Balcerzyk A, Niemiec P, Nowak T, Ochalska-Tyka A, et al. 2015. CYP7A1 gene polymorphism located in the 5′ upstream region modifies the risk of coronary artery disease. Dis. Markers 2015:185969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jensen T, Abdelmalek MF, Sullivan S, Nadeau KJ, Green M, et al. 2018. Fructose and sugar: a major mediator of non-alcoholic fatty liver disease. J. Hepatol 68:1063–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jiang C, Xie C, Li F, Zhang L, Nichols RG, et al. 2015. Intestinal farnesoid X receptor signaling promotes nonalcoholic fatty liver disease. J. Clin.Investig 125:386–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jiang C, Xie C, Lv Y, Li J, Krausz KW, et al. 2015. Intestine-selective farnesoid X receptor inhibition improves obesity-related metabolic dysfunction. Nat. Commun 6:10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Joyce SA, MacSharry J, Casey PG, Kinsella M, Murphy EF, et al. 2014. Regulation of host weight gain and lipid metabolism by bacterial bile acid modification in the gut. PNAS 111:7421–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kang DJ, Hylemon PB, Gillevet PM, Sartor RB, Betrapally NS, et al. 2017. Gut microbial composition can differentially regulate bile acid synthesis in humanized mice. Hepatol. Commun 1:61–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kaur A, Patankar JV, de Haan W, Ruddle P, Wijesekara N, et al. 2015. Loss of Cyp8b1 improves glucose homeostasis by increasing GLP-1. Diabetes 64:1168–79 [DOI] [PubMed] [Google Scholar]
- 66.Keitel V, Gorg B, Bidmon HJ, Zemtsova I, Spomer L, et al. 2010. The bile acid receptor TGR5 (Gpbar-1) acts as a neurosteroid receptor in brain. Glia 58:1794–805 [DOI] [PubMed] [Google Scholar]
- 67.Kettner NM, Voicu H, Finegold MJ, Coarfa C, Sreekumar A, et al. 2016. Circadian homeostasis of liver metabolism suppresses hepatocarcinogenesis. Cancer Cell 30:909–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kharitonenkov A, Wroblewski VJ, Koester A, Chen YF, Clutinger CK, et al. 2007. The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21. Endocrinology 148:774–81 [DOI] [PubMed] [Google Scholar]
- 69.Kir S, Beddow SA, Samuel VT, Miller P, Previs SF, et al. 2011. FGF19 as a postprandial, insulin-independent activator of hepatic protein and glycogen synthesis. Science 331:1621–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kowdley KV, Luketic V, Chapman R, Hirschfield GM, Poupon R, et al. 2018. A randomized trial of obeticholic acid monotherapy in patients with primary biliary cholangitis. Hepatology 67:1890–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lan T, Morgan DA, Rahmouni K, Sonoda J, Fu X, et al. 2017. FGF19, FGF21, and an FGFR1/β-klotho-activating antibody act on the nervous system to regulate body weight and glycemia. Cell Metab. 26:709–18.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li B, Yang N, Li C, Li C, Gao K, et al. 2018. INT-777, a bile acid receptor agonist, extenuates pancreatic acinar cells necrosis in a mouse model of acute pancreatitis. Biochem. Biophys. Res. Commun 503:38–44 [DOI] [PubMed] [Google Scholar]
- 73.Li F, Jiang C, Krausz KW, Li Y, Albert I, et al. 2013. Microbiome remodelling leads to inhibition of intestinal farnesoid X receptor signalling and decreased obesity. Nat. Commun 4:2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li T, Chiang JY. 2005. Mechanism of rifampicin and pregnane x receptor (PXR) inhibition of human cholesterol 7α-hydroxylase gene (CYP7A1) transcription. Am. J. Physiol. Gastrointest. Liver Physiol 288:G74–84 [DOI] [PubMed] [Google Scholar]
- 75.Li T, Holmstrom SR, Kir S, Umetani M, Schmidt DR, et al. 2011. The G protein–coupled bile acid receptor, TGR5, stimulates gallbladder filling. Mol. Endocrinol 25:1066–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li T, Matozel M, Boehme S, Kong B, Nilsson LM, et al. 2011. Overexpression of cholesterol 7α-hydroxylase promotes hepatic bile acid synthesis and secretion and maintains cholesterol homeostasis. Hepatology 53:996–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li T, Owsley E, Matozel M, Hsu P, Novak CM, Chiang JY. 2010. Transgenic expression of cholesterol 7α-hydroxylase in the liver prevents high-fat diet-induced obesity and insulin resistance in mice. Hepatology 52:678–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li-Hawkins J, Gafvels M, Olin M, Lund EG, Andersson U, et al. 2002. Cholic acid mediates negative feedback regulation of bile acid synthesis in mice. J. Clin. Investig 110:1191–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lioudaki E, Ganotakis ES, Mikhailidis DP. 2011. Lipid lowering drugs and gallstones: a therapeutic option? Curr. Pharm. Des 17:3622–31 [DOI] [PubMed] [Google Scholar]
- 80.Liu R, Zhao R, Zhou X, Liang X, Campbell DJ, et al. 2014. Conjugated bile acids promote cholangiocarcinoma cell invasive growth via activation of sphingosine 1-phosphate receptor 2. Hepatology 60:908–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Loomba R, Seguritan V, Li W, Long T, Klitgord N, et al. 2017. Gut microbiome–based metagenomic signature for non-invasive detection of advanced fibrosis in human nonalcoholic fatty liver disease. Cell Metab. 25:1054–62.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lundasen T, Galman C, Angelin B, Rudling M. 2006. Circulating intestinal fibroblast growth factor 19 has a pronounced diurnal variation and modulates hepatic bile acid synthesis in man. J. Intern. Med 260:530–36 [DOI] [PubMed] [Google Scholar]
- 83.Makishima M, Lu TT, Xie W, Whitfield GK, Domoto H, et al. 2002. Vitamin D receptor as an intestinal bile acid sensor. Science 296:1313–16 [DOI] [PubMed] [Google Scholar]
- 84.Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, et al. 1999. Identification of a nuclear receptor for bile acids. Science 284:1362–65 [DOI] [PubMed] [Google Scholar]
- 85.Marcelin G, Jo YH, Li X, Schwartz GJ, Zhang Y, et al. 2014. Central action of FGF19 reduces hypothalamic AGRP/NPY neuron activity and improves glucose metabolism. Mol. Metab 3:19–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Marrapodi M, Chiang JY. 2000. Peroxisome proliferator–activated receptor α (PPARα) and agonist inhibit cholesterol 7α-hydroxylase gene (CYP7A1) transcription. J. Lipid Res 41:514–20 [PubMed] [Google Scholar]
- 87.Maruyama T, Miyamoto Y, Nakamura T, Tamai Y, Okada H, et al. 2002. Identification of membrane-type receptor for bile acids (M-BAR). Biochem. Biophys. Res. Commun 298:714–19 [DOI] [PubMed] [Google Scholar]
- 88.McMillin M, DeMorrow S. 2016. Effects of bile acids on neurological function and disease. FASEB J. 30:3658–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.McMillin M, Frampton G, Grant S, Khan S, Diocares J, et al. 2017. Bile acid-mediated sphingosine-1-phosphate receptor 2 signaling promotes neuroinflammation during hepatic encephalopathy in mice. Front. Cell. Neurosci 11:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McNeilly AD, Macfarlane DP, O’Flaherty E, Livingstone DE, Mitic T, et al. 2010. Bile acids modulate glucocorticoid metabolism and the hypothalamic–pituitary–adrenal axis in obstructive jaundice. J. Hepatol 52:705–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mueller M, Thorell A, Claudel T, Jha P, Koefeler H, et al. 2015. Ursodeoxycholic acid exerts farnesoid X receptor–antagonistic effects on bile acid and lipid metabolism in morbid obesity. J. Hepatol 62:1398–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mullenbach R, Bennett A, Tetlow N, Patel N, Hamilton G, et al. 2005. ATP8B1 mutations in British cases with intrahepatic cholestasis of pregnancy. Gut 54:829–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Murphy C, Parini P, Wang J, Bjorkhem I, Eggertsen G, Gafvels M. 2005. Cholic acid as key regulator of cholesterol synthesis, intestinal absorption and hepatic storage in mice. Biochim. Biophys. Acta 1735:167–75 [DOI] [PubMed] [Google Scholar]
- 94.Mutlu EA, Gillevet PM, Rangwala H, Sikaroodi M, Naqvi A, et al. 2012. Colonic microbiome is altered in alcoholism. Am. J. Physiol. Gastrointest. Liver Physiol 302:G966–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nagahashi M, Takabe K, Liu R, Peng K, Wang X, et al. 2015. Conjugated bile acid–activated S1P receptor 2 is a key regulator of sphingosine kinase 2 and hepatic gene expression. Hepatology 61:1216–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nemati R, Lu J, Dokpuang D, Booth M, Plank LD, Murphy R. 2018. Increased bile acids and FGF19 after sleeve gastrectomy and Roux-en-Y gastric bypass correlate with improvement in type 2 diabetes in a randomized trial. Obes. Surg 28:2672–86 [DOI] [PubMed] [Google Scholar]
- 97.Neuschwander-Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, et al. 2015. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet 385:956–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nevens F, Andreone P, Mazzella G, Strasser SI, Bowlus C, et al. 2016. A placebo-controlled trial of obeticholic acid in primary biliary cholangitis. N. Engl. J. Med 375:631–43 [DOI] [PubMed] [Google Scholar]
- 99.Ouyang X, Cirillo P, Sautin Y, McCall S, Bruchette JL, et al. 2008. Fructose consumption as a risk factor for non-alcoholic fatty liver disease. J. Hepatol 48:993–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pandak WM, Heuman DM, Hylemon PB, Chiang JY, Vlahcevic ZR. 1995. Failure of intravenous infusion of taurocholate to down-regulate cholesterol 7α-hydroxylase in rats with biliary fistulas. Gastroenterology 108:533–44 [DOI] [PubMed] [Google Scholar]
- 101.Pathak P, Cen X, Nichols RG, Ferrell JM, Boehme S, et al. 2018. Intestine farnesoid X receptor agonist and the gut microbiota activate G-protein bile acid receptor-1 signaling to improve metabolism. Hepatology 68:1574–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pathak P, Li T, Chiang JY. 2013. Retinoic acid-related orphan receptor α regulates diurnal rhythm and fasting induction of sterol 12α-hydroxylase in bile acid synthesis. J. Biol. Chem 288:37154–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pathak P, Liu H, Boehme S, Xie C, Krausz KW, et al. 2017. Farnesoid X receptor induces Takeda G-protein receptor 5 cross-talk to regulate bile acid synthesis and hepatic metabolism. J. Biol. Chem 292:11055–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pauli-Magnus C, Lang T, Meier Y, Zodan-Marin T, Jung D, et al. 2004. Sequence analysis of bile salt export pump (ABCB11) and multidrug resistance p-glycoprotein 3 (ABCB4, MDR3) in patients with intrahepatic cholestasis of pregnancy. Pharmacogenetics 14:91–102 [DOI] [PubMed] [Google Scholar]
- 105.Pellicciari R, Fiorucci S, Camaioni E, Clerici C, Costantino G, et al. 2002. 6α-ethyl-chenodeoxycholic acid (6-ECDCA), a potent and selective FXR agonist endowed with anticholestatic activity. J. Med. Chem 45:3569–72 [DOI] [PubMed] [Google Scholar]
- 106.Pellicciari R, Gioiello A, Macchiarulo A, Thomas C, Rosatelli E, et al. 2009. Discovery of 6α-ethyl-23(S)-methylcholic acid (S-EMCA, INT-777) as a potent and selective agonist for the TGR5 receptor, a novel target for diabesity. J. Med. Chem 52:7958–61 [DOI] [PubMed] [Google Scholar]
- 107.Perry RJ, Lee S, Ma L, Zhang D, Schlessinger J, Shulman GI. 2015. FGF1 and FGF19 reverse diabetes by suppression of the hypothalamic–pituitary–adrenal axis. Nat. Commun 6:6980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Potthoff MJ, Inagaki T, Satapati S, Ding X, He T, et al. 2009. FGF21 induces PGC-1α and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. PNAS 106:10853–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Potthoff MJ, Potts A, He T, Duarte JA, Taussig R, et al. 2013. Colesevelam suppresses hepatic glycogenolysis by TGR5-mediated induction of GLP-1 action in DIO mice. Am. J. Physiol. Gastrointest. Liver Physiol 304:G371–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Poupon R 2012. Ursodeoxycholic acid and bile-acid mimetics as therapeutic agents for cholestatic liver diseases: an overview of their mechanisms of action. Clin. Res. Hepatol. Gastroenterol 36(Suppl. 1):S3–12 [DOI] [PubMed] [Google Scholar]
- 111.Pullinger CR, Eng C, Salen G, Shefer S, Batta AK, et al. 2002. Human cholesterol 7α-hydroxylase (CYP7A1) deficiency has a hypercholesterolemic phenotype. J. Clin. Investig 110:109–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Puri P, Daita K, Joyce A, Mirshahi F, Santhekadur PK, et al. 2018. The presence and severity of nonalcoholic steatohepatitis is associated with specific changes in circulating bile acids. Hepatology 67:534–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Raufman JP, Cheng K, Zimniak P. 2003. Activation of muscarinic receptor signaling by bile acids: physiological and medical implications. Dig. Dis. Sci 48:1431–44 [DOI] [PubMed] [Google Scholar]
- 114.Reddy AB, Karp NA, Maywood ES, Sage EA, Deery M, et al. 2006. Circadian orchestration of the hepatic proteome. Curr. Biol 16:1107–15 [DOI] [PubMed] [Google Scholar]
- 115.Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, et al. 2013. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 341:1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ridlon JM, Harris SC, Bhowmik S, Kang DJ, Hylemon PB. 2016. Consequences of bile salt biotransformations by intestinal bacteria. Gut Microbes 7:22–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Risstad H, Kristinsson JA, Fagerland MW, le Roux CW, Birkeland KI, et al. 2017. Bile acid profiles over 5 years after gastric bypass and duodenal switch: results from a randomized clinical trial. Surg. Obes. Relat. Dis 13:1544–53 [DOI] [PubMed] [Google Scholar]
- 118.Romero-Gomez M, Montagnese S, Jalan R. 2015. Hepatic encephalopathy in patients with acute decompensation of cirrhosis and acute-on-chronic liver failure. J. Hepatol 62:437–47 [DOI] [PubMed] [Google Scholar]
- 119.Salen G, Steiner RD. 2017. Epidemiology, diagnosis, and treatment of cerebrotendinous xanthomatosis (CTX). J. Inherit. Metab. Dis 40:771–81 [DOI] [PubMed] [Google Scholar]
- 120.Sayin SI, Wahlstrom A, Felin J, Jantti S, Marschall HU, et al. 2013. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 17:225–35 [DOI] [PubMed] [Google Scholar]
- 121.Sepe V, Renga B, Festa C, D’Amore C, Masullo D, et al. 2014. Modification on ursodeoxycholic acid (UDCA) scaffold: discovery of bile acid derivatives as selective agonists of cell-surface G-protein coupled bile acid receptor 1 (GP-BAR1). J. Med. Chem 57:7687–701 [DOI] [PubMed] [Google Scholar]
- 122.Setchell KDR, Schwarz M, O’Connell NC, Lund EG, Davis DL, et al. 1998. Identification of a new inborn error in bile acid synthesis: mutation of the oxysterol 7alpha-hydroxylase gene causes severe neonatal liver disease. J. Clin. Investig 102:1690–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shang Q, Saumoy M, Holst JJ, Salen G, Xu G. 2010. Colesevelam improves insulin resistance in a diet-induced obesity (F-DIO) rat model by increasing the release of GLP-1. Am. J. Physiol. Gastrointest. Liver Physiol 298:G419–24 [DOI] [PubMed] [Google Scholar]
- 124.Slatis K, Gafvels M, Kannisto K, Ovchinnikova O, Paulsson-Berne G, et al. 2010. Abolished synthesis of cholic acid reduces atherosclerotic development in apolipoprotein E knockout mice. J. Lipid Res 51:3289–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Smits MM, van Raalte DH, Tonneijck L, Muskiet MH, Kramer MH, Cahen DL. 2016. GLP-1 based therapies: clinical implications for gastroenterologists. Gut 65:702–11 [DOI] [PubMed] [Google Scholar]
- 126.Song KH, Li T, Owsley E, Strom S, Chiang JY. 2009. Bile acids activate fibroblast growth factor 19 signaling in human hepatocytes to inhibit cholesterol 7alpha-hydroxylase gene expression. Hepatology 49:297–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Srivastava A. 2014. Progressive familial intrahepatic cholestasis. J. Clin. Exp. Hepatol 4:25–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Studer E, Zhou X, Zhao R, Wang Y, Takabe K, et al. 2012. Conjugated bile acids activate the sphingosine-1-phosphate receptor 2 in primary rodent hepatocytes. Hepatology 55:267–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Takahashi S, Fukami T, Masuo Y, Brocker CN, Xie C, et al. 2016. Cyp2c70 is responsible for the species difference in bile acid metabolism between mice and humans. J. Lipid Res 57:2130–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Talukdar S, Zhou Y, Li D, Rossulek M, Dong J, et al. 2016. A long-acting FGF21 molecule, PF-05231023, decreases body weight and improves lipid profile in non-human primates and type 2 diabetic subjects. Cell Metab. 23:427–40 [DOI] [PubMed] [Google Scholar]
- 131.Thomas C, Auwerx J, Schoonjans K. 2008. Bile acids and the membrane bile acid receptor TGR5—connecting nutrition and metabolism. Thyroid 18:167–74 [DOI] [PubMed] [Google Scholar]
- 132.Thomas C, Gioiello A, Noriega L, Strehle A, Oury J, et al. 2009. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 10:167–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tsaousidou MK, Ouahchi K, Warner TT, Yang Y, Simpson MA, et al. 2008. Sequence alterations within CYP7B1 implicate defective cholesterol homeostasis in motor-neuron degeneration. Am. J. Hum. Genet 82:510–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Vaz FM, Ferdinandusse S. 2017. Bile acid analysis in human disorders of bile acid biosynthesis. Mol. Asp. Med 56:10–24 [DOI] [PubMed] [Google Scholar]
- 135.Venkat VL, Shneider BL, Magee JC, Turmelle Y, Arnon R, et al. 2014. Total serum bilirubin predicts fat-soluble vitamin deficiency better than serum bile acids in infants with biliary atresia. J. Pediatr. Gastroenterol. Nutr 59:702–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wahlstrom A, Sayin SI, Marschall HU, Backhed F. 2016. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab. 24:41–50 [DOI] [PubMed] [Google Scholar]
- 137.Widlansky ME, Puppala VK, Suboc TM, Malik M, Branum A, et al. 2017. Impact of DPP-4 inhibition on acute and chronic endothelial function in humans with type 2 diabetes on background metformin therapy. Vasc. Med 22:189–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wouters K, van Gorp PJ, Bieghs V, Gijbels MJ, Duimel H, et al. 2008. Dietary cholesterol, rather than liver steatosis, leads to hepatic inflammation in hyperlipidemic mouse models of nonalcoholic steatohepatitis. Hepatology 48:474–86 [DOI] [PubMed] [Google Scholar]
- 139.Wu X, Lv YG, Du YF, Hu M, Reed MN, et al. 2018. Inhibitory effect of INT-777 on lipopolysaccharide-induced cognitive impairment, neuroinflammation, apoptosis, and synaptic dysfunction in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 88:360–74 [DOI] [PubMed] [Google Scholar]
- 140.Xie C, Jiang C, Shi J, Gao X, Sun D, et al. 2017. An intestinal farnesoid X receptor–ceramide signaling axis modulates hepatic gluconeogenesis in mice. Diabetes 66:613–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yang JY, Lee YS, Kim Y, Lee SH, Ryu S, et al. 2017. Gut commensal Bacteroides acidifaciens prevents obesity and improves insulin sensitivity in mice. Mucosal Immunol. 10:104–16 [DOI] [PubMed] [Google Scholar]
- 142.Yang XF, Liu GS, Li MX. 2015. Analysis of mutations of MDR3 exons 9 and 23 in infants with parenteral nutrition–associated cholestasis. Exp. Ther. Med 10:2361–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yao L, Seaton SC, Ndousse-Fetter S, Adhikari AA, DiBenedetto N, et al. 2018. A selective gut bacterial bile salt hydrolase alters host metabolism. eLife 7:e37182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yeap SP, Harley H, Thompson R, Williamson KD, Bate J, et al. 2018. Biliary transporter gene mutations in severe intrahepatic cholestasis of pregnancy: diagnostic and management implications. J. Gastroenterol. Hepatol 34:425–35 [DOI] [PubMed] [Google Scholar]
- 145.Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, et al. 2018. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol 15:11–20 [DOI] [PubMed] [Google Scholar]