Abstract
Background
Baicalin, a natural product isolated from Scutellaria radix, has been reported to exert anti-oxidant and anti-apoptotic effects on skin, but the underlying mechanism remains poorly understood. This study aimed to investigate the possible mechanism of anti-UVB effect of baicalin in human skin fibroblasts.
Methods
Cell proliferation was estimated by CCK-8 Kit. Apoptotic incidence was detected by flow cytometry with Annexin V-PE/PI apoptosis detection kit. Autophagy was determined by the evaluation of fluorescent LC3 puncta and Western blotting. Cell signalling was analysed by Western blotting.
Results
Baicalin exerted cytoprotective effects in UVB-induced HSFs. Moreover, baicalin increased autophagy and suppressed UVB-induced apoptosis of HSFs. Pretreatment with 3-MA, an autophagy inhibitor, attenuated baicalin-induced HSFs autophagy and promoted apoptosis. Baicalin activated AMPK, which leads to suppression of basal mTOR activity in cultured HSFs. Administration of compound C, an AMPK inhibitor, abrogated AMPK phosphorylation and increased mTOR phosphorylation and apoptosis compared with baicalin alone.
Conclusion
Taken together, these results indicate the important role of mTOR inhibition in UVB protection by baicalin and provide a new target and strategy for better prevention of UV-induced skin disorders.
Keywords: autophagy, baicalin, ultraviolet B, apoptosis, AMPK
Introduction
Skin acts as the protection barrier of our body by defending against harmful environmental factors, such as ultraviolet light (UV) radiation, pathogens and toxic chemicals, but is usually firstly damaged and causes a variety of skin disorders. Exposure to UV (mostly UVA and UVB) is considered as one of the major hazards involving in human skin carcinogenesis, mainly associated with UV-induced DNA damage.1,2 The most important way to eliminate these damaged cells is through apoptosis, which acts as a protection mechanism to avoid malignant transformation.3 However, dysregulated apoptosis may destroy the integrity and function of the skin, causing skin disorders such as sunburn, psoriasis and skin cancer.4
Due to its wavelength (280–320nm), UVB is mostly absorbed in the epidermis which contains keratinocytes,5,6 but is also able to reach the underlying papillary dermis where fibroblasts are.7 Radiation of UVB can cause DNA damage and inducing apoptosis in both epidermis and dermis.3,8 In response to UVB radiation, p53 signaling pathway is activated, including up-regulation of genes coding for pro-apoptotic Bax and Bak proteins and trans-repression of anti-apoptotic Bcl-2, Bcl-xL,9–11 leading to cell cycle arrest and apoptosis.
UV radiation and DNA damage also induce autophagy,12 an evolutionarily conserved catabolic program by which cytoplasmic material and intracellular organelles are engulfed in autophagosomes, degraded by the lysosomes, ultimately recycling macromolecules to maintain homeostasis. Recent studies showed that, upon UV radiation, autophagy is driven by p53 through negative regulation of mTOR and activation of AMPK.13 It has also been reported that autophagy could counteract apoptosis at the level of Bcl-2-interacting protein-1 (Beclin-1; ATG6), which is pivotal both in initial steps in autophagosome formation and apoptosis. Although it is believed that keratinocytes are the major cell type impacted by UVB radiation,14–16 recent studies suggest more susceptibility of fibroblasts to UVB radiation than keratinocytes. Specifically, changes of fibroblast p21 have been shown to be higher than keratinocyte p21 after UVB radiation, leading to stronger changes in the level of p53 in fibroblasts than keratinocytes.17 Moreover, fibroblasts are known to take integral roles in the dermal-epidermal crosstalk by involving in several epidermal biological pathways such as keratinocyte proliferation, differentiation and migration.18
Baicalin, a flavonoid compound extracted from the dried roots of Scutellaria baicalensis Georgi, has multiple pharmacological activities including anti-oxidant, anti-bacterial, antiviral, and anti-inflammatory effects.19,20 Mounting evidence has revealed that baicalin has an inhibitory effect on UVB-induced photo-damage, reducing the increased apoptosis, ROS production, cyclobutene pyrimidine dimers (CPDs) formation and oxidative DNA adducts.21,22 A recent study showed that baicalin could reduce UVB-induced apoptosis in HaCaT in a dose-dependent manner.20 Given the fact that UVB could reach dermis containing fibroblast, together with the discussions earlier on the important roles of fibroblasts in mediating various cutaneous processes, it is of great interest to study the photoprotection effects and molecular mechanisms of baicalin on human skin fibroblasts (HSFs). The purpose of this study was to investigate whether baicalin can protect HSFs from UVB radiation-induced apoptosis and to determine the molecular mechanisms.
Materials and Methods
Cell Culture and UVB Radiation
Human dermal fibroblasts, which were obtained from four Chinese donors aged 8–12 years by means of a foreskin circumcision, were cultured in the Cell Resource Centre, IBMS, Chinese Academy of Medical Sciences and Peking Union Medical College. Purified Baicalin (BR, 90%) was purchased from the Yuanye Bio-Technology company (Shanghai, China). The cells were cultured in DMEM, which contained 25 mM glucose, 10% FBS, and 1% penicillin-streptomycin in an atmosphere of 5% CO2 at 37°C, and placed in a culture flask with a 25-cm2 surface area. The medium was replaced every 3 days. The cultured dermal fibroblasts were digested and passaged with 0.25% trypsin after the cell confluence reached approximately 80%. The fibroblasts from passages 3–6 were selected for the following experiments and seeded at a confluence of 70%-80% 24 h before experiments. The UVB dosage employed was established during pilot experiments. UVB irradiation was delivered with a Philips TL 20W/12 lamp (Eindhoven, The Netherlands), which emits UVB radiation at a wavelength of 280–320 nm and with a peak at 313 nm at a power of 2.3–2.4 W. Radiation output was monitored with a Waldmann UV-meter (Waldmann, Villigen-Schwenningen, Germany). Prior to UVB irradiation, the medium was removed and covered with phosphate-buffered saline (PBS). The cells were irradiated at a distance of ~15 cm from 1 lamp with an irradiation dosage of 600 J/m2 for UVB. Irradiation time was calculated according to the output power measured by the UV meter. After UV radiation, the cells were incubated in DMEM supplemented with 10% FBS and 1% penicillin–streptomycin in an atmosphere of 5% CO2 at 37°C till further treatment.
Cell Proliferation Assay
We analyzed cell proliferation using a CCK-8 Kit (Beyotime Institute of Biotechnology, Nantong, Jiangsu, China) according to the manufacturer’s protocol. One hundred microliters of the HSF cells (2×103 cells/well) were inoculated in 96-well plates and five parallel wells were used for each treatment. The cells were subjected to different treatments and incubated at 37°C, 5% CO2 for 24 h. 10 μL of CCK-8 solution was added to each well and the cells were incubated for another 3 h. In this process, WST-8 [2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium] in the solution is reduced by the dehydrogenase activates in the cells to form a yellow-colored formazan dye. Cell density was analyzed by measuring the absorbance at 450 nm using a Varioskan Flash (Thermo Scientific, USA).
Flow Cytometry
Apoptotic incidence was detected by flow cytometry with Annexin V-PE/PI apoptosis detection kit (BD Biosciences, USA). Briefly, after treatment, cells were collected and suspended in 1× binding buffer, and 1 × 105 cells were counted and incubated with 5 μL of Annexin V-PE and 5 μL of PI for 30 min at room temperature in the dark. Then, 1× ending buffer was added and flow cytometry analysis was performed within 1 h. Apoptotic cells were stained as Annexin V (+)/PI (−) (early apoptotic cells) and Annexin V (+)/PI (+) (late apoptotic cells).
Western Blotting Assay
We lysed the cells in 62.5 mM Tris-HCl (pH 6.8) containing 2% w/v SDS, then measured the protein concentration using the Pierce BCA assay (Thermo Fisher Scientific, Rockford, IL, USA). We added mercaptoethanol and bromophenol blue to render the final composition equivalent to the Laemmli sample buffer, then the samples were fractionated using SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and blotted onto Immobilon-P membrane (Millipore, Billerica, MA, USA). Primary antibodies for PARP, cleaved caspase-3 and LC3 (Cell Signaling Technology, Danvers, MA, USA), antibodies for AMPK, p-AMPK, mTOR, and p-mTOR (Abcam, Cambridge, UK), antibodies for Bcl-2 and Bax (Proteintech, Chicago, IL, USA), and antibodies for beclin-1 and GAPDH (Santa Cruz Biotechnology, Santa Cruz, CA, USA) were used. Secondary antibodies of rabbit anti-mouse HRP (1:1000 dilution) or goat anti-rabbit HRP (1:1000 dilution) were used (Biotime, Haimen, China). Visualization of antibody binding was done using Pierce ECL reagents (Thermo Fisher Scientific, CA, USA). The quantification of protein bands was established by Band-Scan software (PROZYME, San Leandro®, California).
Evaluation of Autophagy by Measuring the Fluorescent LC3 Puncta
The tandem fluorescent GFP-LC3 adenoviral vectors (Ad-tf-LC3) were purchased from HanbioCo (HanbioCo. Ltd, Shanghai, China). Fibroblasts cultured on coverslips were transduced with Ad-tf-LC3 at 15 MOI for 24 h. After adenovirus transduction, the cells were washed with PBS, fixed with 4% paraformaldehyde for 20 min, mounted with a reagent, and viewed with confocal microscopy. Autophagy was determined by analyzing the formation of LC3 puncta of autophagosomes in cells. Specifically, the number of GFP dots was determined by manually counting fluorescent puncta in five fields from three different sample preparations. The number of dots per cell was obtained by dividing the total number of dots by the number of nuclei in each microscopic field. At least 150 GFP-positive cells per condition were counted and the graphs were plotted as percentage of GFP-LC3 dot-positive cells over the total number of transfected cells.
Statistical Analysis
All experiments were repeated at least three times and representative experiments are shown. Data are presented as means±SEM. Differences were evaluated by one-way analysis of variance post hoc Dunnet’s, using computer program GraphPad Prism (GraphPad Software, San Diego, CA). A p-value of less than 0.05 was considered statistically significant.
Results
Baicalin Protects Against UVB-Induced Phototoxicity in HSFs
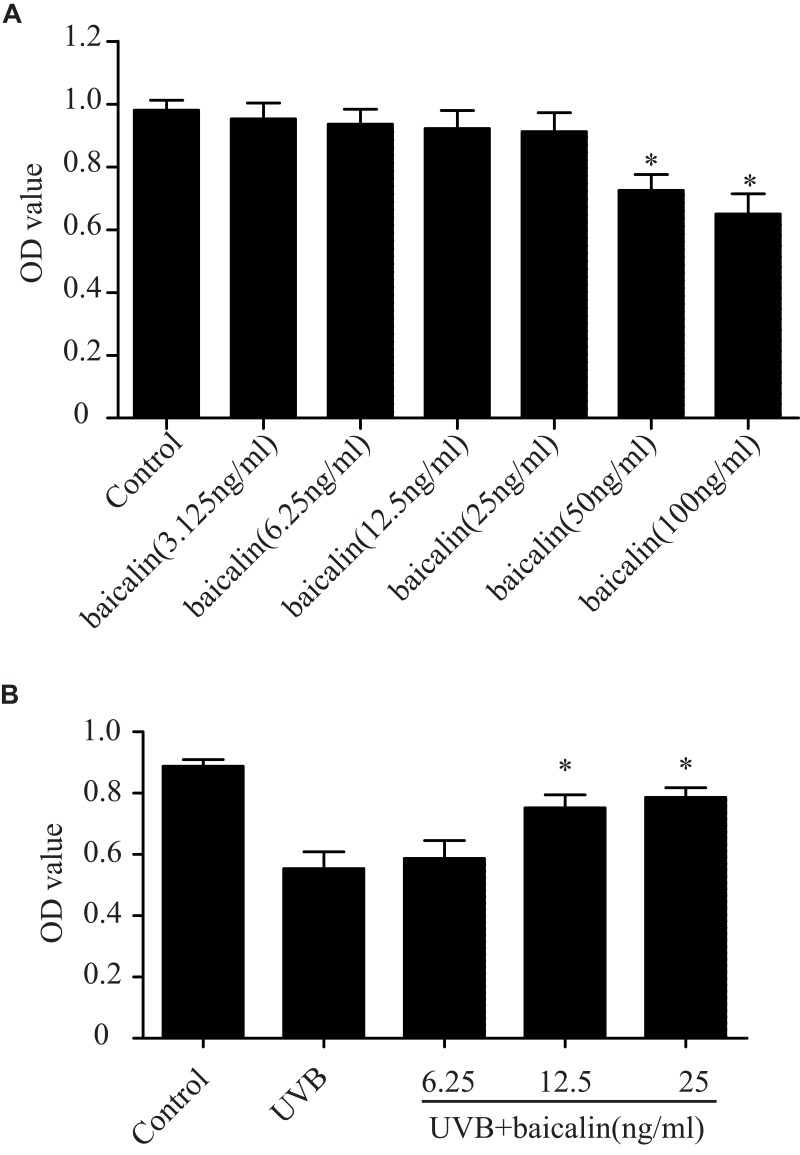
Before detecting the cytoprotective effect of baicalin in UVB-induced HSFs, we first confirmed the toxic effect of baicalin on HSFs. HSFs were treated with different concentrations of baicalin (3.125, 6.25, 12.5, 25, 50, 100 ng/mL) and incubated for 24 hrs. As Figure 1A shows, the cell viability was significantly reduced when pretreated with 50 ng/mL and 100 ng/mL baicalin (p < 0.05), while other concentrations of baicalin were considered safe to HSFs. In another set of experiment, after being irradiated with 600 J/m2 of UVB, HSFs were cultured with varied concentrations of baicalin (6.25, 12.5, and 25 ng/mL) for 24 hrs. As the results in Figure 1B show, the 12.5 ng/mL and 25 ng/mL baicalin treatment remarkably increased cell viability compared to UVB treatment alone. These data indicate that baicalin could protect HSFs from phototoxicity induced by UVB, and the concentration of 25 ng/mL was chosen to be used in the following studies.
Figure 1.
Baicalin protects against the UVB-induced phototoxicity in HSFs. (A) Effect of baicalin at different dosages on HSFs viability. Baicalin was applied to the HSFs at 3.125, 6.25, 12.5, 25, 50, 100 ng/mL. After 24 hrs, the cultured cell viability was assayed by using a CCK-8 assay kit. Values are given as means±SEM (n=5). *p < 0.05 versus Control. (B) Baicalin protects cultured HSFs against UVB-induced cell viability. HSFs were irradiated with 600 J/m2 of UVB, and then cultured with 6.25, 12.5, and 25 ng/mL baicalin. Twenty-four hours after UVB irradiation, the cell viability was assayed by using a CCK-8 assay kit. Values are given as means±SEM (n=6). *p < 0.05 versus UVB.
Baicalin Suppresses UVB-Induced Apoptosis in HSFs
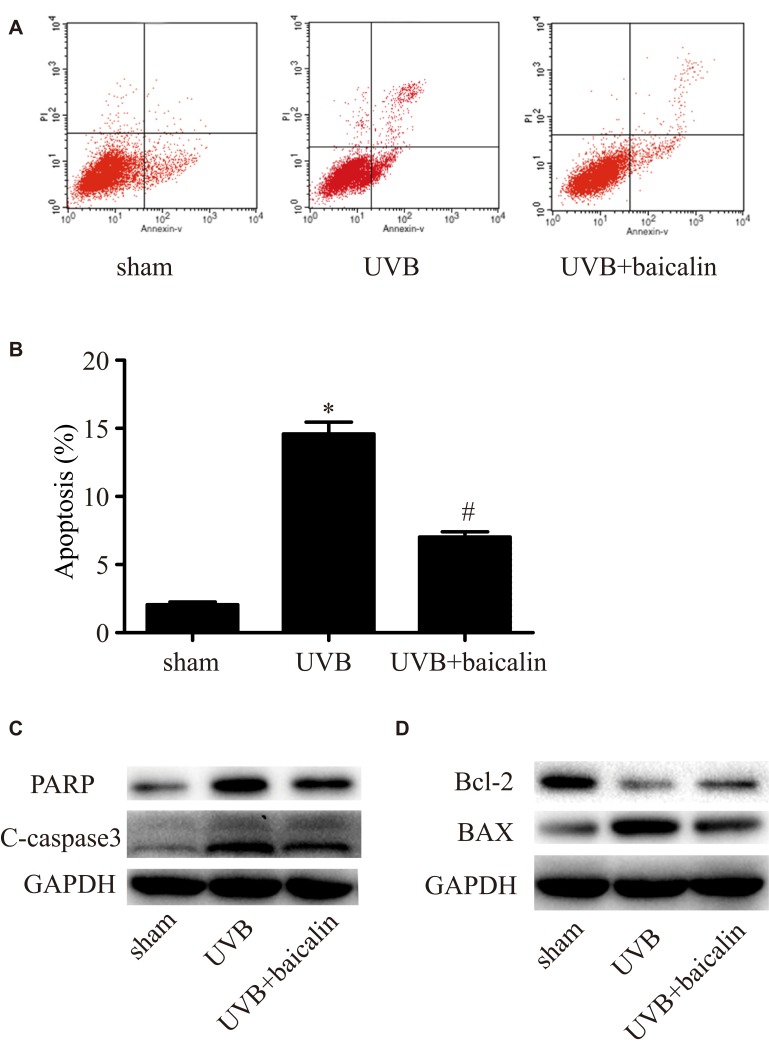
To investigate whether baicalin affects apoptosis induced by UVB in HSFs, Annexin V/PI staining and flow cytometry were applied. As Figure 2A and B shows, compared to the sham group, UVB irradiation (600 J/m2) significantly induced apoptosis of HSFs (p < 0.05), which is in accordance with previous studies,23,24 whilst baicalin treatment (25 ng/mL) inhibited apoptosis remarkably (p < 0.05). We further analyzed the possible pathway involved by exploring the expression of caspase 3, the key mediator in apoptosis, and PARP, the typical substrate for caspase 3. Firstly, Figure 2C shows that caspase3 and PARP in HSFs were elevated after UVB irradiation, but UVB+baicalin treatment could decrease the elevation of both caspase3 and PARP. Secondly, as Figure 2D shows, there were decreased Bcl-2 (an anti-apoptotic protein) and increased BAX (a pro-apoptotic protein) after UVB irradiation, which were inhibited by the baicalin treatment. These results suggest that baicalin could suppress the UVB-induced apoptosis through decreasing caspase3, PARP and BAX, and increasing Bcl-2 levels.
Figure 2.
Baicalin suppresses UVB-induced apoptosis in HSFs. (A, B) Cellular apoptosis was assayed by annexin V-FITC and PI counterstaining, and analyzed with flow cytometry. The original flow cytometry figures are shown in (A) and the apoptosis rates are shown in (B). Values are given as means±SEM (n=5). *p < 0.05 versus sham, #p < 0.05 versus UVB. (C, D) HSFs were treated with indicated treatments. Then, PARP, cleaved caspase-3, Bcl-2 expression, and BAX expression were detected by Western blotting analysis. (C) Representative image of immunoblots for PARP and cleaved caspase-3. GAPDH was used as a loading control. (D) Representative image of immunoblots for Bcl-2 and BAX. GAPDH was used as a loading control. The concentration of baicalin in these studies was 25 ng/mL.
Baicalin Increases UVB-Induced Autophagy in HSFs
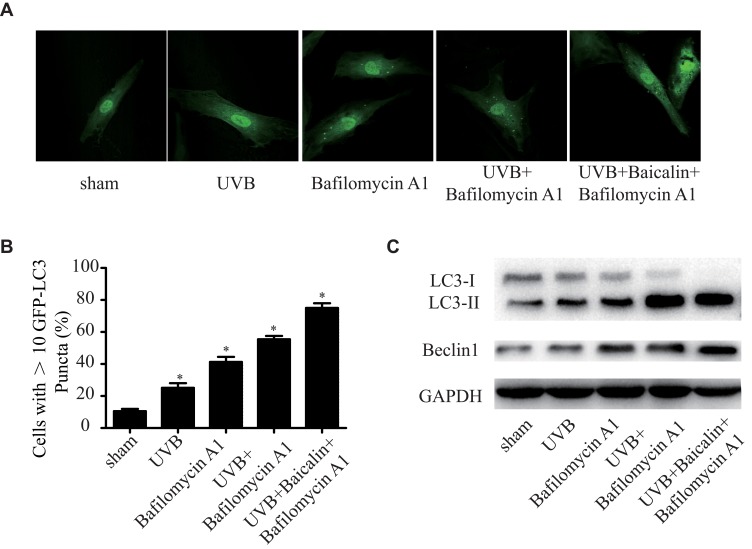
It has been proven that UVB exposure promotes autophagy in dermis,25 whilst baicalin also induces autophagy in varied human cancer cells.26,27 As we have found that baicalin could protect HSFs against UVB-induced cell death and apoptosis, we further investigated the effect of baicalin on UVB-induced autophagy. As Figure 3A and B shows, UVB exposure could significantly increase the number of GFP-LC3-containing puncta and baicalin treatment increased the elevation. Bafilomycin A1, an autophagy inhibitor that prevents maturation of autophagic vacuoles by inhibiting fusion between autophagosomes and lysosomes,28 was applied to detect how baicalin affected the autophagy flux. As the data shown in Figure 3A and B, compared to UVB+ Bafilomycin A1, the UVB+Baicalin+Bafilomycin A1 treatment significantly increased the number of GFP-LC3-containing puncta, suggesting that baicalin prompted the UVB-induced autophagic activity by inducing autophagosome generation, not through inhibiting autophagosome lysosomal fusion. Next, we studied the generation of autophagosomes by detecting the LC3 conversion (LC3-I to LC3-II), for the expression of LC3-II is the indicator of the number of autophagosomes.29 The Western blot results (Figure 3C) illustrated that UVB exposure could increase the ratio of LC3-II/LC3-I and the expression of beclin-1, the critical player in the regulation of autophagy. Combined with GFP-LC3-containing puncta data, the Bafilomycin application failed at eliminating the elevation, which further confirmed that baicalin could increase the UVB-induced autophagy flux.
Figure 3.
Baicalin increases UVB-induced autophagy in HSFs. HSFs were pretreated with Bafilomycin A1 (100 nM) for 2 h, then treated with 25 ng/mL baicalin. (A) Representative confocal images of GFP-LC3 in fibroblasts. Scale bars = 50μm. (B) The percentage of cells with induced autophagy was determined as the percentage of GFP-LC3 cells with greater than 10 GFP-LC3 puncta per cell (means ±SEM of the independent experiments, *p < 0.05). (C) LC3-II and Beclin1 expressions were detected by Western blotting analysis. GAPDH was used as a loading control. The concentration of baicalin in these studies was 25 ng/mL.
3-MA Inhibits the Effects of Baicalin on UVB-Induced Autophagy and Apoptosis in HSFs
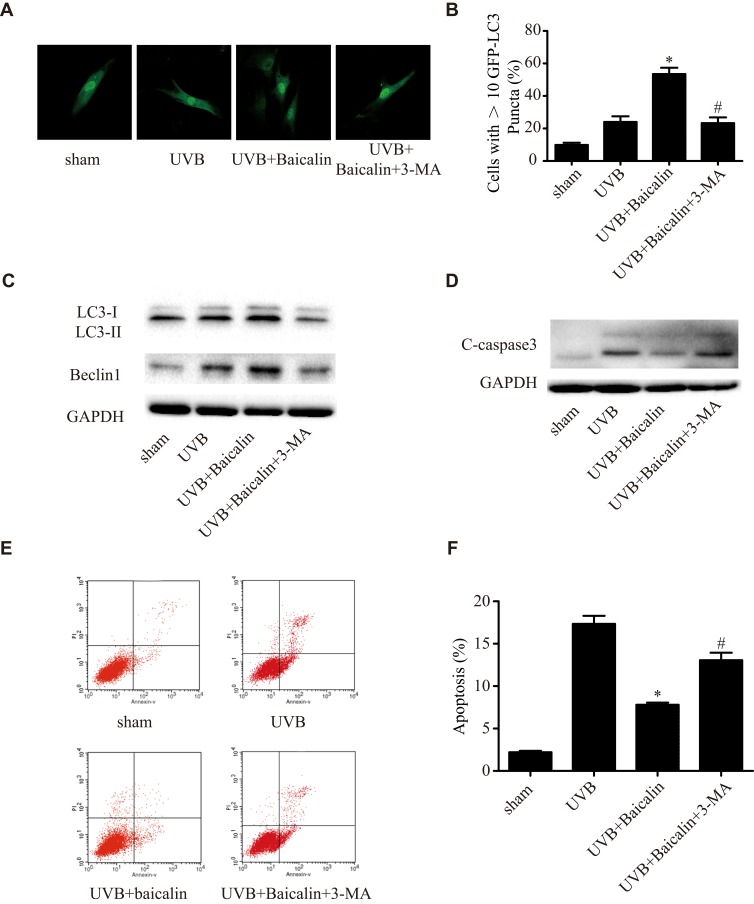
To further confirm the effect of baicalin on UVB-induced autophagy, we adopted another inhibitor of autophagy, 3-methyladenine (3-MA) in our experiment before the Baicalin treatment. Figure 4A and B shows, 3-MA could significantly decrease the number of GFP-LC3-containing puncta compared with Baicalin+UVB treatment. The ratio of LC3-II/LC3-I and the expression of beclin-1 (Figure 4C), which were induced by Baicalin+UVB treatment, were both remarkably suppressed by 3-MA. We also detected that 3-MA could inhibit the suppressive effect of Baicalin on UVB-induced apoptosis. As Figure 4E and F shows, compared with Baicalin+UVB treatment, 3-MA increased apoptosis level of HSFs significantly, and in accordance with it, the expression of caspase3 was also elevated (Figure 4D). These results suggest that the inhibition of autophagy could eliminate the effects of baicalin on UVB-induced autophagy and apoptosis, in other words, baicalin suppresses UVB-induced apoptosis through inducing autophagy flux.
Figure 4.
3-MA inhibits the effects of Baicalin on UVB-induced autophagy and apoptosis in HSFs. (A) Representative confocal images of GFP-LC3 in fibroblasts. Scale bars = 50μm. (B) The percentage of cells with induced autophagy was determined as the percentage of GFP-LC3 cells with greater than 10 GFP-LC3 puncta per cell (means ±SEM of the independent experiments, *p < 0.05 versus UVB, #p < 0.05 versus UVB+Baicalin). (C) LC3-II and Beclin1 expressions were detected by Western blotting analysis. GAPDH was used as a loading control. (D) C-caspase3 expression was detected by Western blotting analysis. GAPDH was used as a loading control. (E, F) Cellular apoptosis was assayed by annexin V-FITC and PI counterstaining, and analyzed with flow cytometry. The original flow cytometry figures are shown in (E) and the apoptosis rates are shown in (F). Values are given as means±SEM (n=5). *p < 0.05 versus UVB, #p < 0.05 versus UVB+Baicalin. The concentration of baicalin in these studies was 25 ng/mL.
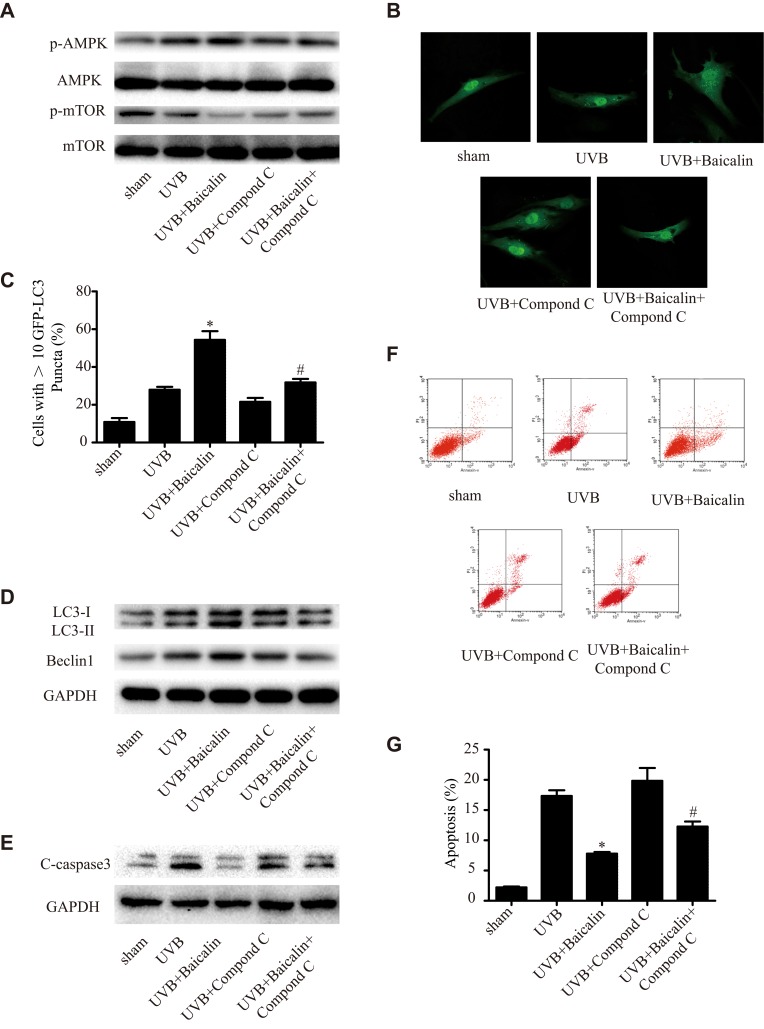
Baicalin Increases UVB-Induced Autophagy in HSFs via the AMPK-mTOR Pathway
In order to detect the underlying mechanism of Baicalin promoting UVB-induced autophagy in HSFs, we studied the AMPK-mTOR pathway. As Figure 5A shows, Baicalin could significantly increase the phosphorylation of AMPK and decrease the phosphorylation of mTOR, while the application of compound C, an inhibitor of AMPK, could remarkably abrogate the effect of baicalin on AMPK and mTOR. Compound C also decreased the number of GFP-LC3-containing puncta, the ratio of LC3-II/LC3-I, and the expression of beclin-1 (Figure 5B and C), suggesting the inhibition of AMPK could suppress the induction of Baicalin on autophagy induced by UVB in HSFs. As Figure 5E shows, compared with the UVB+Baicalin group, the addition of compound C could elevate the expression of caspase 3, and Figure 5F and G shows that compound C also increased the apoptosis level. All these results suggest that the inhibition of AMPK could attenuate the effect of Baicalin on autophagy and apoptosis in HSFs.
Figure 5.
(A) Western blot analysis of AMPK, mTOR, and their phosphorylated forms. (B) Representative confocal images of GFP-LC3 in fibroblasts. Scale bars = 50μm. (C) The percentage of cells with induced autophagy was determined as the percentage of GFP-LC3 cells with greater than 10 GFP-LC3 puncta per cell. *p < 0.05 versus UVB, #p < 0.05 versus UVB+Baicalin. (D) LC3-II and Beclin1 expression were detected by Western blotting analysis. GAPDH was used as a loading control. (E) C-caspase3 expression was detected by Western blotting analysis. GAPDH was used as a loading control. (F, G) Cellular apoptosis was assayed by annexin V-FITC and PI counterstaining, and analyzed with flow cytometry. The original flow cytometry figures are shown in (F), and the apoptosis rates are shown in (G). Values are given as means±SEM (n=5). *p < 0.05 versus UVB, #p < 0.05 versus UVB+Baicalin. The concentration of baicalin in these studies was 25 ng/mL.
Discussion
UVB is one of the major etiological factors that induce DNA damage and trigger apoptosis as well as autophagy,30 which results in sunburn and skin cancers including non-melanoma carcinoma and malignant melanoma formation.8,31 Traditional photoprotection methods are usually wearing of tropical sunscreen or oral administration of antioxidants such as vitamins, minerals, polyphenols and carotenoids.32 Recently, natural compounds have been widely explored in their photoprotection effects. Baicalin, a famous natural product of traditional Chinese medicine with antioxidant, anti-inflammatory, anti-apoptosis and pro-autophagy effects, is brought into focus for the protective function to photodamage and carcinogenesis.20,33 Baicalin is proved to be photoprotective to fibroblasts after UVB irradiation by the anti-photoaging effect.34 It is also effective in inhibiting UVB-induced photodamage in keratinocytes by harvesting reactive oxygen species,20 reducing the increased apoptosis rate and suppressing oxidative DNA adducts, in which process the relevant cytokine secretion and the expression of several genes like p53 and p21 were reduced.35 Meanwhile, baicalin has been shown to induce autophagy in a variety of cell types including hepatocellular carcinoma cells,26,36 breast cancer cells,37 and even mice brain.38 In this context, we examined whether baicalin could protect fibroblast cells against UVB radiation via reducing apoptosis and inducing autophagy, and explored if the underlying mechanism of autophagy induction is via activation of AMPK and downregulation of mTOR.13
In the present study, we found that baicalin increased cell viability (Figure 1A) and suppressed apoptosis (Figure 2A and B) of human fibroblast cells induced by UVB at 600 J/m2, the dose level high enough to caused fibroblast cells to undergo apoptosis.39,40 UVB radiation is capable of activating several apoptotic pathways by inducing DNA damage and activating tumor suppressor gene p53.24 As UVB radiation activates p53 signaling pathway, the downstream pro-apoptotic Bax is upregulated and anti-apoptotic Bcl-2 is suppressed, leading cell arrest and apoptosis. Indeed, the addition of baicalin downregulated Bax and increased Bcl-2 as shown in Figure 2D, suggesting that baicalin may suppress UVB-induced apoptosis via downregulating p53 signaling pathway. Additionally, the caspase signaling pathway is known to regulate the apoptotic responses.41 By Western blot analysis, we found that caspase-3 and PARP, both of which are activated during apoptosis, were inhibited by baicalin treatment compared to UVB radiation alone (Figure 2C). The result agrees with one of our previous studies showing that exposure of HSFs to UVA activates caspase-3, and the treatment with baicalin decrease caspase-3 significantly. These promising results indicated that multiple pathways participating in reducing UVB-induced apoptosis in fibroblasts by baicalin treatment.
Besides apoptosis, UVB radiation also induces the autophagy of HSF. Beclin-1, a biomarker of autophagy, is known to be involved in the formation of autophagosomes.42 The conversion of the LC3-I protein to LC3-II is another hallmark of autophagy induction during autophagosome formation.43 As shown in Figure 3, with the addition of Bafilomycin A1, which inhibits the autophagosome-lysosome fusion, baicalin treatment could still increase the number of GFP-LC3-containing puncta, upregulate the expression of beclin-1 and the ratio of LC3-II/LC3-I. These results suggest that baicalin prompted the UVB-induced autophagic activity by inducing autophagosome generation, not through inhibiting autophagosome-lysosome fusion.
Apoptosis and autophagy are complex and interconnected cellular processes that play a major role in determining cellular fate. Autophagy has been demonstrated to induce apoptosis under different stresses in some in vitro studies, but it has also been found to maintain cellular homeostasis under different stresses by inhibiting apoptosis. To explore the roles of baicalin between the interaction of autophagy and apoptosis within UVB-induced fibroblast cells, we applied 3-MA to HSF to inhibit autophagy and studied the alternation of biomarkers after that. Specifically, 3-MA blocks autophagosome formation by inhibition of phosphatidylinositol 3-kinases (PI3K), which controls the activation of mTOR, a key regulator of autophagy. As Figure 4A–C shows, 3-MA significantly decreased the number of GFP-LC3-containing puncta, suppressed the expression of beclin-1 and ratio of LC3-II/LC3-I induced by UVB+Baicalin. These results indicated that 3-MA could inhibit autophagy induced by UVB+Baicalin. Then, we further examined the apoptosis level shown in Figure 4E and F. In consistency with the autophagy level, the apoptosis induced by UVB+Baicalin was upregulated by 3-MA, and the elevated caspase-3 level (Figure 4D) further confirmed these results. These data suggested that baicalin may suppress UVB-induced apoptosis through inducing autophagy flux.
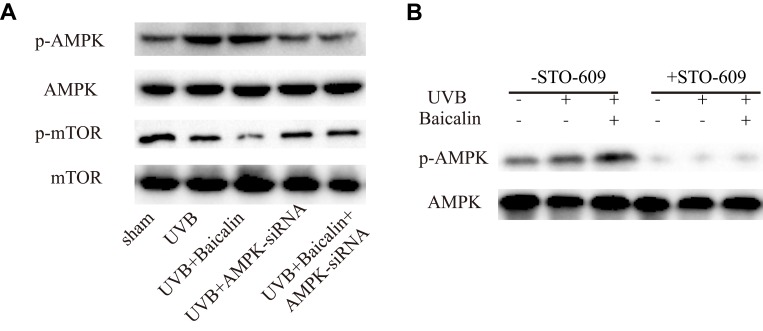
We finally move to investigating the signaling pathways of baicalin-induced autophagy after UVB irradiation. Upon UV radiation, it has been revealed that autophagy is driven by p53 through negative regulation of mTOR and activation of AMPK, and autophagy could counteract apoptosis at the level of Beclin-1, which both involves in autophagy and apoptosis.21 AMPK is a serine/threonine protein kinase that consists of three subunits: a catalytic α-subunit and regulatory β- and γ-subunits.44 Activated AMPK (p-AMPK) leads to phosphorylation and activation of the TSC1/2 complex, which inhibits mTOR activity.45 mTOR is a serine/threonine protein kinase that regulates cell metabolism, proliferation, death, and survival.22 In this study, firstly, we confirmed that baicalin could increase the UVB-induced phosphorylation of AMPK and decrease the phosphorylation of mTOR (Figure 5A). Then, we applied compound C to inhibit AMPK and found that the effect of baicalin on UVB-induced AMPK and mTOR status was abrogated. At the same time, the number of GFP-LC3-containing puncta, the ratio of LC3-II/LC3-I, and the expression of beclin-1 (Figure 5B, C, and D) were all downregulated, suggesting the inhibition of AMPK could suppress the effect of baicalin on UVB-induced autophagy in HSFs. Additionally, as Figure 5E–G shows, the addition of compound C could elevate the expression of caspase 3 and increase the apoptosis level compared to UVB+Baicalin treatment. Obviously, the inhibition of AMPK could attenuate the effect of Baicalin on UVB-induced autophagy and apoptosis in HSFs. With siRNA to inhibit the AMPK expression (Figure 6), we confirmed its effect on the phosphorylation of mTOR and the involvement of AMPK after the treatment of baicalin in HSFs.
Figure 6.
(A) Western blot analysis of AMPK, mTOR, and their phosphorylated forms. (B) Representative Western blots showing the effect of the CaMKKb inhibitor STO-609 (10 mg/mL) on baicalin-induced AMPK and phosphorylation of AMPK protein expression.
In conclusion, the present study demonstrated that baicalin has the ability to protect UVB-irradiated HSFs from apoptosis by inducing autophagy, via the upregulation of AMPK phosphorylation and the downregulation of mTOR phosphorylation. Though more detail mechanisms need to be explored, baicalin is becoming considered as a potential therapeutic strategy to prevent the photodamage.
Acknowledgments
This study was supported by CAMS Innovation Fund for Medical Sciences (CIFMS-2017-I2M-1-017), the National Natural Science Foundation of China (grant nos. 81703152 and 81872545), the PUMC Youth Fund and Fundamental Research Funds for the Central Universities (2017310032) and the Jiangsu Province Natural Science Foundation (No. BK20170161).
Disclosure
The authors have no conflicts of interest to declare.
References
- 1.Pustisek N, Situm M. UV-radiationapoptosis and skin. Coll Antropol. 2011;35(Suppl 2):339–341. [PubMed] [Google Scholar]
- 2.P. National Toxicology. NTP 12th Report on Carcinogens, Report on carcinogens: carcinogen profiles, 12. 2011; iii–499. [PubMed]
- 3.Lee CH, Wu SB, Hong CH, Yu HS, Wei YH. Molecular mechanisms of UV-induced apoptosis and its effects on skin residential cells: the implication in UV-based phototherapy. Int J Mol Sci. 2013;14:6414–6435. doi: 10.3390/ijms14036414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raj D, Brash DE, Grossman D. Keratinocyte apoptosis in epidermal development and disease. J Invest Dermatol. 2006;126:243–257. doi: 10.1038/sj.jid.5700008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barrandon Y. The epidermal stem cell: an overview. Semin Dev Biol. 1993;4:209–215. doi: 10.1006/sedb.1993.1025 [DOI] [Google Scholar]
- 6.Maß P, Hoffmann K, Gambichler T, Altmeyer P, Mannherz HG. Premature keratinocyte death and expression of marker proteins of apoptosis in human skin after UVB exposure. Arch Dermatol Res. 2003;295:71–79. doi: 10.1007/s00403-003-0403-x [DOI] [PubMed] [Google Scholar]
- 7.Lee KS, Kim SJ, Ryoo YW, Kim BC. All-trans-retinoic acid down-regulates elastin promoter activity elevated by ultraviolet B irradiation in cultured skin fibroblasts. J Dermatol Sci. 1998;17:182–189. doi: 10.1016/S0923-1811(98)00004-8 [DOI] [PubMed] [Google Scholar]
- 8.Budden T, Bowden NA. The role of altered nucleotide excision repair and UVB-induced DNA damage in melanomagenesis. Int J Mol Sci. 2013;14:1132–1151. doi: 10.3390/ijms14011132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnstone RW, Ruefli AA, Lowe SW. Apoptosis: a link between cancer genetics and chemotherapy. Cell. 2002;108:153–164. doi: 10.1016/S0092-8674(02)00625-6 [DOI] [PubMed] [Google Scholar]
- 10.Pietsch EC, Sykes SM, McMahon SB, Murphy ME. The p53 family and programmed cell death. Oncogene. 2008;27:6507–6521. doi: 10.1038/onc.2008.315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yin X, Cao L, Kang R, et al. UV irradiation resistance-associated gene suppresses apoptosis by interfering with BAX activation. EMBO Rep. 2011;12:727–734. doi: 10.1038/embor.2011.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen LH, Chu PM, Lee YJ, et al. Targeting protective autophagy exacerbates UV-triggered apoptotic cell death. Int J Mol Sci. 2012;13:1209–1224. doi: 10.3390/ijms13011209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maiuri MC, Galluzzi L, Morselli E, Kepp O, Malik SA, Kroemer G. Autophagy regulation by p53. Curr Opin Cell Biol. 2010;22:181–185. doi: 10.1016/j.ceb.2009.12.001 [DOI] [PubMed] [Google Scholar]
- 14.Black AT, Gray JP, Shakarjian MP, Laskin DL, Heck DE, Laskin JD. Distinct effects of ultraviolet B light on antioxidant expression in undifferentiated and differentiated mouse keratinocytes. Carcinogenesis. 2008;29:219–225. doi: 10.1093/carcin/bgm242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jans J, Garinis GA, Schul W, et al. Differential role of basal keratinocytes in UV-induced immunosuppression and skin cancer. Mol Cell Biol. 2006;26:8515–8526. doi: 10.1128/MCB.00807-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reich A, Mędrek K. Effects of narrow band UVB (311 nm) irradiation on epidermal cells. Int J Mol Sci. 2013;14:8456–8466. doi: 10.3390/ijms14048456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gęgotek A, Biernacki M, Ambrożewicz E, Surażyński A, Wroński A, Skrzydlewska E. The cross-talk between electrophiles, antioxidant defence and the endocannabinoid system in fibroblasts and keratinocytes after UVA and UVB irradiation. J Dermatol Sci. 2016;81:107–117. [DOI] [PubMed] [Google Scholar]
- 18.Fernandez TL, Van Lonkhuyzen DR, Dawson RA, Kimlin MG, Upton Z. In vitro investigations on the effect of dermal fibroblasts on keratinocyte responses to ultraviolet B radiation. Photochem Photobiol. 2014;90:1332–1339. doi: 10.1111/php.2014.90.issue-6 [DOI] [PubMed] [Google Scholar]
- 19.Grzegorczyk-Karolak I, Wysokinska H, Olas B. Studies on the antioxidant properties of extracts from the roots and shoots of two Scutellaria species in human blood plasma. Acta Biochimica Polonica. 2015;62:253–258. doi: 10.18388/abp.2014_944 [DOI] [PubMed] [Google Scholar]
- 20.Chang WS, Lin EY, Hsu SW, et al. Baicalin scavenged reactive oxygen species and protected human keratinocytes against UVB-induced cytotoxicity. In Vivo. 2016;30:605–610. [PubMed] [Google Scholar]
- 21.Chao JI, Su WC, Liu HF. Baicalein induces cancer cell death and proliferation retardation by the inhibition of CDC2 kinase and survivin associated with opposite role of p38 mitogen-activated protein kinase and AKT. Mol Cancer Ther. 2007;6:3039–3048. doi: 10.1158/1535-7163.MCT-07-0281 [DOI] [PubMed] [Google Scholar]
- 22.Min W, Ahmad I, Chang ME, Burns EM, Qian Q, Yusuf N. Baicalin protects keratinocytes from toll-like receptor-4 mediated DNA damage and inflammation following ultraviolet irradiation. Photochem Photobiol. 2015;91:1435–1443. doi: 10.1111/php.2015.91.issue-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baek JY, Park S, Park J, et al. Protective role of mitochondrial peroxiredoxin III against UVB-induced apoptosis of epidermal keratinocytes. J Invest Dermatol. 2017;137(6):1333–1342. [DOI] [PubMed] [Google Scholar]
- 24.Kong L, Wang S, Wu X, Zuo F, Qin H, Wu J. Paeoniflorin attenuates ultraviolet B-induced apoptosis in human keratinocytes by inhibiting the ROS-p38-p53 pathway. Mol Med Rep. 2016;13:3553–3558. doi: 10.3892/mmr.2016.4953 [DOI] [PubMed] [Google Scholar]
- 25.Cavinato M, Jansen-Dürr P. Molecular mechanisms of UVB-induced senescence of dermal fibroblasts and its relevance for photoaging of the human skin. Exp Gerontol. 2017;94:78–82. doi: 10.1016/j.exger.2017.01.009 [DOI] [PubMed] [Google Scholar]
- 26.Wang Z, Jiang C, Chen W, et al. Baicalein induces apoptosis and autophagy via endoplasmic reticulum stress in hepatocellular carcinoma cells. J BioMed Res Int. 2014;2014:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y-F, Li T, Tang Z-H, et al. Baicalein triggers autophagy and inhibits the protein kinase B/mammalian target of rapamycin pathway in hepatocellular carcinoma HepG2 cells. Phytother Res. 2015;29:674–679. doi: 10.1002/ptr.v29.5 [DOI] [PubMed] [Google Scholar]
- 28.Yamamoto A, Tagawa Y, Yoshimori T, Moriyama Y, Masaki R, Tashiro Y. Bafilomycin A1 prevents maturation of autophagic vacuoles by inhibiting fusion between autophagosomes and lysosomes in rat hepatoma cell line, H-4-II-E cells. Cell Struct Funct. 1998;23:33–42. doi: 10.1247/csf.23.33 [DOI] [PubMed] [Google Scholar]
- 29.Mizushima N, Yoshimori T. How to interpret LC3 immunoblotting. Autophagy. 2007;3:542–545. doi: 10.4161/auto.4600 [DOI] [PubMed] [Google Scholar]
- 30.Deshmukh J, Pofahl R, Haase I. Epidermal Rac1 regulates the DNA damage response and protects from UV-light-induced keratinocyte apoptosis and skin carcinogenesis. Cell Death Dis. 2017;8:e2664. doi: 10.1038/cddis.2017.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wangari-Talbot J, Chen S. Genetics of melanoma. Front Genet. 2012;3:330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parrado C, Philips N, Gilaberte Y, Juarranz A, González S. Oral Photoprotection: effective Agents and Potential Candidates. Front Med. 2018;5:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li D, Lin B, Yusuf N, et al. Proteomic analysis and functional studies of baicalin on proteins associated with skin cancer. Am J Chin Med (Gard City N Y). 2017;45:599–614. doi: 10.1142/S0192415X17500355 [DOI] [PubMed] [Google Scholar]
- 34.Zhang J-A, Yin Z, Ma L-W, et al. The protective effect of baicalin against UVB irradiation induced photoaging: an in vitro and in vivo study. PLoS ONE. 2014;9:e99703. doi: 10.1371/journal.pone.0099703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hall JR, Messenger ZJ, Tam HW, Phillips SL, Recio L, Smart RC. Long noncoding RNA lincRNA-p21 is the major mediator of UVB-induced and p53-dependent apoptosis in keratinocytes. Cell Death Dis. 2015;6:e1700. doi: 10.1038/cddis.2015.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang X, Tang X, Liu H, Li L, Hou Q, Gao J. Autophagy induced by baicalin involves downregulation of CD147 in SMMC-7721 cells in vitro. Oncol Rep. 2012;27:1128–1134. doi: 10.3892/or.2011.1599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yan W, Ma X, Zhao X, Zhang S. Baicalein induces apoptosis and autophagy of breast cancer cells via inhibiting PI3K/AKT pathway in vivo and vitro. Drug Des Devel Ther. 2018;12:3961–3972. doi: 10.2147/DDDT.S181939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fang J, Zhu Y, Wang H, et al. Baicalin protects mice brain from apoptosis in traumatic brain injury model through activation of autophagy. Front Neurosci;2019;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ishihara Y, Tsuno S, Ping B, et al. Hsa-miR-520d-5p promotes survival in human dermal fibroblasts exposed to a lethal dose of UV irradiation. NPJ Aging Mech Dis. 2016;2:16029. doi: 10.1038/npjamd.2016.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Britto SM, Shanthakumari D, Agilan B, Radhiga T, Kanimozhi G, Prasad NR. Apigenin prevents ultraviolet-B radiation induced cyclobutane pyrimidine dimers formation in human dermal fibroblasts. Mutat Res. 2017;821:28–35. doi: 10.1016/j.mrgentox.2017.06.002 [DOI] [PubMed] [Google Scholar]
- 41.Li X-Q, Cai L-M, Liu J, et al. Liquiritin suppresses UVB‑induced skin injury through prevention of inflammation, oxidative stress and apoptosis through the TLR4/MyD88/NF‑κB and MAPK/caspase signaling pathways. Int J Mol Med. 2018;42:1445–1459. doi: 10.3892/ijmm.2018.3720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wirawan E, Vande Walle L, Kersse K, et al. Caspase-mediated cleavage of Beclin-1 inactivates Beclin-1-induced autophagy and enhances apoptosis by promoting the release of proapoptotic factors from mitochondria. Cell Death Dis. 2010;1:e18. doi: 10.1038/cddis.2009.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aryal B, Denison TA, Gonzalez Y, Rao VA. Chapter 19 - Autophagy-based protein biomarkers for in vivo detection of cardiotoxicity in the context of cancer therapy In: Hayat MA, editor. Autophagy: Cancer, Other Pathologies, Inflammation, Immunity, Infection, and Aging. Amsterdam: Academic Press; 2014:289–307. [Google Scholar]
- 44.Bai L, Mei X, Shen Z, et al. Netrin-1 improves functional recovery through autophagy regulation by activating the AMPK/mTOR signaling pathway in rats with spinal cord injury. Sci Rep. 2017;7:42288. doi: 10.1038/srep42288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/S0092-8674(03)00929-2 [DOI] [PubMed] [Google Scholar]