Abstract
P4 medicine is an evolving approach to personalized medicine. The four Ps offer a means to: Predict who will develop disease and comorbidities; Prevent rather than react to disease; Personalize diagnosis and treatment; have patients Participate in their own care. P4 medicine is very applicable to obstructive sleep apnea (OSA) because each OSA patient has a different pathway to disease and its consequences. OSA has both structural and physiological mechanisms with different clinical subgroups, different molecular profiles, and different consequences. This may explain why there are different responses to alternative therapies, such as intraoral devices and hypoglossal nerve stimulation therapy. Currently, technology facilitates patients to participate in their own care from screening for OSA (snoring and apnea apps) to monitoring response to therapy (sleep monitoring, blood pressure, oxygen saturation, heart rate) as well as monitoring their own CPAP compliance. We present a conceptual framework that provides the basis for a new, P4 medicine approach to OSA and should be considered more in depth: predict and prevent those at high risk for OSA and consequences, personalize the diagnosis and treatment of OSA and build in patient participation to manage OSA.
Keywords: Sleep apnea, obstructive; Precision medicine; Continuous positive airway pressure; Obesity; Intraoral devices
1. INTRODUCTION
All areas of medicine are moving towards a more personalized approach. There is acceptance that important individual differences exist between patients with what seems like the same disease. These differences are related to genetic differences, epigenetic differences, and to environmental influences (Figure 1). These individual differences may manifest in the following: different underlying structural and physiological risk factors; different clinical presentations of disease, including symptom complexes; different molecular signatures of disease; different clinical consequences; and different response to therapy. Developing a personalized approach requires consideration of all of these domains (Figure 1).
Figure 1:
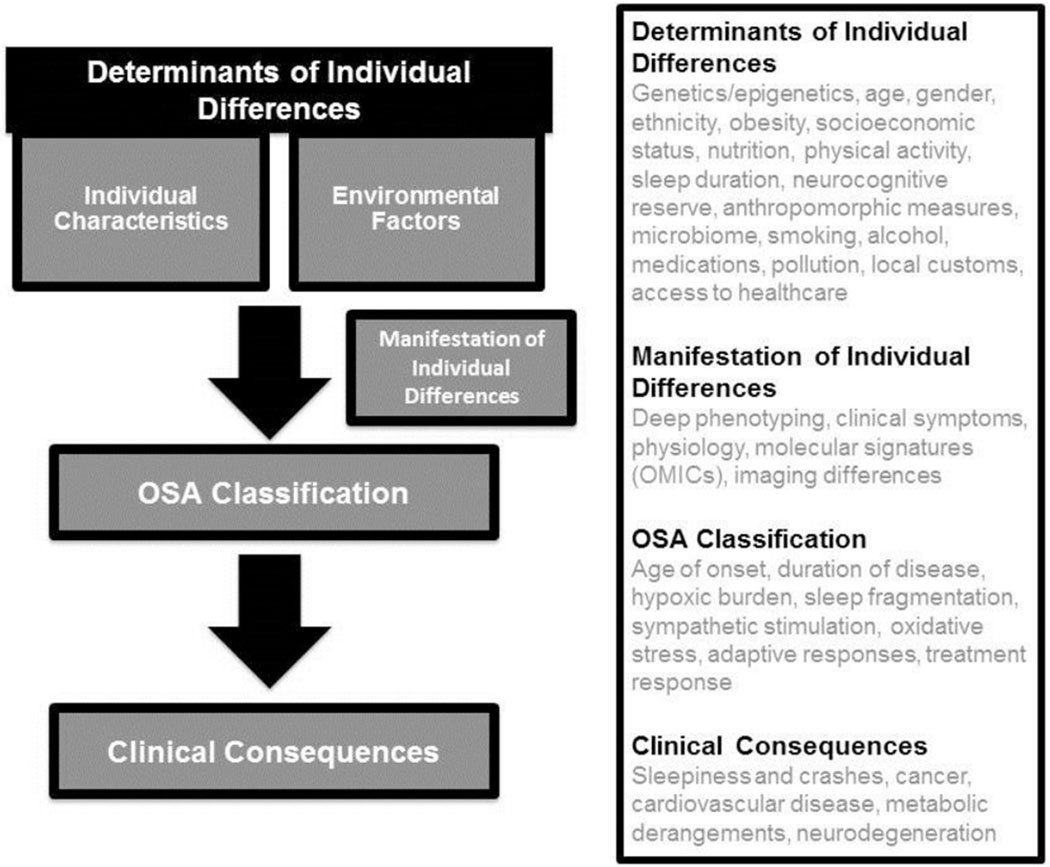
The Determinants of Individual Differences is composed of many Individual Characteristics and Environmental Factors. These many individual determinants can be analysed into many types of Manifestations of Individual Differences. Collectively, the determinants of individual differences and the manifestation of individual differences should determine an OSA classification schema that predicts clinical consequences. As we collect extensive raw data on individuals with OSA and perform predictive analytics on this raw data, a new OSA classification schema e.g. “how severe is my OSA?” would be able to predict an individual’s clinical consequences e.g. “what does this mean to me?”
In general, to personalize medicine for the individual, it is important to use unbiased approaches to first identify subgroups of patients within a disease by employing relevant mathematical techniques such as unsupervised machine learning and cluster analysis. In addition, use of ‘OMIC’ technologies allow unbiased characterization of cells including genes, RNA, proteins and metabolites (referred to as genomics, transcriptomics, proteomics, and metabolomics respectively). Collectively, OMIC strategies have the potential to convey a complete picture of cellular activity allowing for not only unbiased discovery of novel mechanisms, but also, comparisons between and within individuals. While techniques that evaluate genetic and epigenetic differences have evolved substantially over the last decade, new strategies to evaluate environmental influences are emerging.
This general concept of personalized medicine has led to substantial investments to make this new approach a reality. In the United States, the Precision Medicine Initiative was announced by President Obama in January 20151. This initiative will recruit 1.0 million individuals, obtain detailed genetic information (potentially whole genome sequencing), bank samples for biomarker assessment, procure clinical information from electronic medical records as well as phenotyping information using mobile technology. Given the recent explosion in mobile technology to assess sleep and circadian rhythms2, including apps to assess sleep-disordered breathing3, sleep medicine is well positioned to be an integral part of this Precision Medicine initiative.
One approach to personalized medicine is P4 medicine, a term introduced by Leroy Hood (for previous review on application of P4 medicine to obstructive sleep apnea, see 4). The four P’s are: prediction, prevention, personalization, and participation. Science will help clinicians predict who will develop disease, thus providing an opportunity to prevent disease rather than react to disease with the goal being to maintain health. If disease does develop, then a personalized approach to diagnose and treat individuals will optimize outcomes. Technology is constantly advancing, making patient participation in their own wellness an integral part of personalized medicine, from screening for disease, to monitoring and organizing their treatment online, as well as using social media, telemedicine, and “peer buddies” to improve care.
1.1. P4 Medicine in Other Areas of Health
An unbiased approach to identify subgroups of Parkinson’s patients using Big Data methodology was performed on data archived by the Parkinson’s Progression Markers Initiative (PPMI).5 Using imaging, genetics, clinical and demographic data, several model-based methods were compared to several model-free methods. It was acknowledged that the science has not yet developed to perform predictive analytics on raw data. However, when using derived biomarkers for each subject, model-free methods had a higher accuracy of diagnostic classifications and disease predictions compared to model-based techniques.5 As the authors have made their unbiased approach to Big Data publically available, validation and reproducibility can be performed not only with other Parkinson’s disease data sets but any other complex disease with Big Data.
The Hundred Person Wellness Project (HPWP) was launched in 2014 by the Institute for Systems Biology, directed by Dr. Leroy Hood, to test the P4 medicine paradigm. As wellness is difficult to define, 100 “well” individuals submitted to testing (whole-genome sequencing, assessment of the gut microbiome, clinical laboratory tests) and quantified self-measures (physical activity, sleep, weight, blood pressure, personality, lifestyle factors) to develop metrics of wellness and markers of early onset diseases. As test costs decrease, technology and computational approaches improve, and people increase their role in their own health and wellness, the shift from reactionary medicine to being proactive may become a reality.
P4 medicine principles are increasingly being applied to cancer. Studies in breast cancer,6 lung cancer and lymphoma 7, 8 have stratified patients into distinct subgroups to assess disease and molecular markers to decide treatment. Novel patient participation within cancer research include “citizen-driven trials” and “crowd sourcing” clinical trials. Citizen-driven trials are initiated by patients themselves i.e. the Multiple Myeloma Research Foundation9 and the Adenoid Cystic Carcinoma Research Foundation10 with varying degrees of physician involvement. Crowd-sourcing trials first identifies and enrolls patients then distributes them to various studies. More than 330,000 women have been recruited for the one million “Army of Women” through the partnership of the Dr. Susan Love Research Foundation and the Avon Foundation11 and distributed to 34 studies.12 In addition, the Army of Women is collaborating with the cancer Biomedicine Informatics Grid (caBIG) and the City of Hope Medical Center to process longitudinal data in breast cancer patients where enrollment and tracking of new data are done entirely online by patients and advocates.13
2. THE CASE FOR CHANGE – ONE SIZE DOES NOT FIT ALL FOR OBSTRUCTIVE SLEEP APNEA
OSA is a common disorder worldwide that is increasing in prevalence as obesity rates increase14 (For review of recent epidemiological data on worldwide studies of OSA prevalence in different populations, see 15). Substantial individual differences in multiple domains is the problem of the “one-size-fits-all” approach to OSA patients (Table 1). However, it is these individual differences that provide an opportunity to develop a personalized approach.
Table 1.
DOMAINS OF DIFFERENCES BETWEEN PATIENTS WITH OSA
| 1. Differences in structural and physiological risk factors | • Structural ○ Craniofacial dimensions ○ Size of soft tissue structures ■ Possible link between obesity and fat deposition • Physiological ○ Collapsibility of the upper airway (Pcrit) ○ Upper airway dilator muscle response to negative pharyngeal pressure ○ Overall loop gain of ventilator control system. ○ Arousal threshold to airway narrowing. ○ Heart rate response to arousals |
| 2. Differences in Clinical Characteristics | • Ethnic differences • Gender differences • Differences in clinical presentation: OSA subgroups based on symptoms: disturbed sleep, minimally symptomatic, excessively sleepy |
| 3. Differences in molecular signatures | • OMIC data correlated to physiological data (e.g. arousals and oxygen desaturations) • OMIC data correlated to consequences (e.g. genes of pathways associated with cancer) |
| 4. Differences in consequences of OSA | • Physiological differences (e.g. arousal index and heart rate response to arousals) may predict consequences (e.g. hypertension) • Individuals have variable increased risk of cardiovascular, metabolic and neurodegenerative diseases and cancer. |
2.1. Differences in Risk Factors Between Patients with OSA
There are both structural and physiological risk factors for OSA, the nature of which varies between subjects. Structural risk factors include craniofacial dimensions16 and size of soft tissue structures.17 There are also physiological mechanisms that increase the risk for OSA (Table 1),18–20 the magnitude of which also varies between subjects.
2.1.1. Differences in structural risk factors
Craniofacial risk factors for OSA are heritable16 as are soft tissue risk factors.21 The relevant gene variants have, however, not yet been identified. Craniofacial risk factors are discussed below. Soft tissue risk factors include an enlarged tongue, soft palate and lateral pharyngeal walls.17 We know that obesity is an important risk factor for OSA, but we do not yet know how obesity leads to OSA. There are likely several contributing mechanisms. One is central abdominal obesity, which can reduce both lung volume and caudal traction on the pharyngeal airway making it more collapsible. This may explain why males have an increase in OSA prevalence as they more frequently demonstrate a central abdominal obesity pattern.22 Another mechanism is that fat deposition in and around the pharynx may directly increase pharyngeal collapsibility23 and reflect increased neck circumference. Lastly, fat deposits in the tongue as a causative mechanism24 has been proposed to link obesity to OSA. Elegant imaging studies have shown increased tongue fat in individuals with OSA compared to controls after controlling for differences in BMI.24 Thus, tongue fat may be a particular distribution of fat that increases the risk for OSA. Fat distribution is genetically determined,25 for example, the gene variant (TRIB2) has been found to be associated with pericardial fat, but not with overall level of obesity.26 Thus it is conceivable that there are gene variants that control the amount of central abdominal obesity, pharyngeal fat and tongue fat.
2.1.2. Differences in physiological risk factors
Differences in physiological mechanisms within different individuals may lead to different pathways of disease and may also explain why some obese individuals do not develop OSA.19 For example, there are patients with OSA who do not have particularly collapsible airways but have high overall loop gain of their ventilatory control system.18 Could such individuals benefit from oxygen therapy to reduce loop gain? Patients may also have a low arousal threshold, thereby leading to shorter events and sleep fragmentation. Such patients may benefit from sedative medications to increase arousal threshold.27 A recent study shows that a combination of oxygen and a sedative can treat OSA in a subset of patients.28 Other patients may have reduced upper airway muscle responsiveness to negative intraluminal pressure. This can be modulated pharmacologically by desipramine.29 Enhanced muscle responsiveness contributes to protection of obese individuals who do not get OSA.19
2.2. Differences Clinical Characteristics of OSA
2.2.1. Ethnic Differences
Excellent epidemiological data from around the world has reported the prevalence of OSA in many different ethnicities.15 While there are some similar patterns, there are also key differences. To date, few studies have directly compared underlying biological mechanisms between ethnicities to explain these differences.
OSA in Asians occurs at a lower BMI compared to Caucasians, and data suggest this may be due to craniofacial differences.30 In a study comparing Caucasians living in Australia to Chinese living in Hong Kong (matched for OSA severity), Caucasians with OSA were more overweight and had larger tongues, while the Chinese had craniofacial bony restriction.30 Also, for a given BMI, OSA severity is higher in Chinese suggesting that Chinese are less tolerant to the effects of obesity.30 This finding is supported by studies from Singapore31 and the US.32
African Americans have the highest prevalence of excessive daytime sleepiness [self-report sleepiness score (ESS>10)] in two US studies – the Sleep Heart Health Study (SHHS)33 and the Multi-Ethnic Study of Atherosclerosis Sleep Cohort (MESA).32 More studies need to include African Americans to further understand why they report more excessive daytime sleepiness and the underlying biological mechanism that drives this. Knowing this may help us understand the basis of sleepiness in OSA patients.
Hispanics tend to have a high prevalence of OSA, although prevalence varies between countries. Mexico reported an overall prevalence of OSA syndrome (ESS≥11 and AHI ≥15 events/h) to be 10.1%34 while Brazil reports a 16.9% prevalence of moderate-severe OSA (AHI ≥15).35 In the US, Hispanics living in South Florida had a very high prevalence of moderate sleep apnea (63%), but Hispanics recruited in the Sleep Heart Health Study (Arizona, California, Massachusetts, Maryland, Minnesota, New York and Washington) had a more modest prevalence (17%) of moderate sleep apnea.33 Hispanics represent the most diverse ethnicity providing opportunities to asses not only the biology that drives OSA but other interesting environmental influences from geography to diet.
OSA prevalence studies in Caucasians are now available from Norway (2011),36 France (2011),37 the United States (2013),14 and Switzerland (2015).38 In general, OSA is correlated to obesity. From the United States,14 the Wisconsin Sleep Cohort Study reported an increase in the prevalence of sleep-disordered breathing, from 1988–1994 (14%) compared to 2007–2010 (55%) (depending on the age, sex and definition of OSA) and parallels increases in obesity.14
2.2.2. Gender Differences
Numerous population studies demonstrate OSA is more common in men than women. One explanation is that OSA is underdiagnosed in women, possibly up to 90% of women39 because women present differently compared to men.40 For example, when controlling for age, obesity and OSA severity, women report lower functional status, higher subjective complaints of excessive daytime sleepiness and mood disturbances as well as having a poorer reaction time on the psychomotor vigilance task.41 Another explanation for a higher prevalence of OSA in men is that they are more likely to have central abdominal obesity22 and increased neck circumference.42 The problem with underdiagnosing women with OSA is that this may put women at increased risk for co-morbidities.
2.2.3. Differences in clinical presentation
Clinical symptoms of OSA vary between patients. Using cluster analysis in the Icelandic Sleep Apnea Cohort, three distinct subgroups of severe OSA patients were identified.43 The three subgroups were as follows: disturbed sleep, a group where the main symptoms related to difficulty sleeping—an insomnia group; minimally symptomatic, a group that were relatively asymptomatic but did report snoring and witnessed apnoeas; and excessively sleepy, a group where the average Epworth Sleepiness Score (ESS) was higher than the other two groups and one-third reported falling asleep while driving.
Other groups have subsequently published cluster analyses, one from Greece44 and one from France.45 These studies used, however, a different strategy since they included OSA severity (from the polysomnogram) as an input variable. Although, the question being addressed in these studies is different than the Iceland study, there is general agreement that there are excessively sleepy and relatively asymptomatic subgroups.
It is important to determine whether these clinical subgroups identified in Iceland are generalizable as was discussed in a recent commentary on the study.46 The results may reflect identification bias and who is referred to the University Hospital in Iceland for initiation of CPAP therapy. Generalizability of these clinical subgroups is currently being assessed by the Sleep Apnea Global Interdisciplinary Cohort (SAGIC), of which the authors of this article are members.
2.3. Differences in Molecular Signatures in OSA
Currently there has been two applications of OMIC approaches to the study of OSA. First, OMICs was applied as part of a strategy to screen for OSA, using urinary proteomics 47 to detect protein modifications in response to obstructive events with arousals and oxygen desaturations. Overnight urine was collected in children with OSA, children who snore but do not have OSA, and controls. Results showed that children with OSA had levels of 16 proteins that were different in urine compared to the other two groups. Four of the 16 proteins were able to positively predict OSA in children and when combinatorial analyses were used for these proteins, predictive ability to detect OSA compared to the other groups reached a sensitivity of 100% and a specificity of 96.5%. While apps are most likely the future for OSA screening, these proteomic data sheds light on the effects of arousals and oxygen desaturation on an individual’s clinical consequence of OSA.
A second application of OMICs in OSA is assessing response to CPAP treatment. Gene expression in peripheral circulating cells before and after CPAP therapy was assessed in a small sample of patients using microarrays.48 While there was no significant change in gene expression in any single gene, there was a significant change in the expression of 964 genes associated with cancer pathways. The data show substantial variability in CPAP response between subjects, wherein some subjects showed a large change in expression and others showed none. This genomic data raise the idea that individual differences may have prognostic information and support recent evidence that OSA is an independent risk factor for cancer incidence and increased cancer mortality.49, 50
2.4. Differences in Consequences of OSA
Differences in physiology may also be responsible for different consequences of OSA. For example a patient’s arousal index and heart rate response to arousals differs between patients with OSA51 and this difference is heritable, as revealed by studies in twins.52 Variations in heart rate response may reflect differences in sympathetic responses and may be predictive of who develops hypertension.
OSA is a systemic disorder that is a risk factor for many consequences including hypertension,53 stroke,54 heart attack,54 atrial fibrillation,55 insulin resistance,56 diabetes,57 neurodegeneration,58 cancer49, 50 and mortality.59, 60 The basis for why some but not all patients with identical OSA severity develop these consequences is unknown, and is an area of inquiry. If we can identify which OSA patients are at increased risk for specific consequences, this may lead to prevention of disease by assertively targeting therapy to these individuals.
3. PREDICTION
3.1. Predicting Who Has OSA
Combining information about obesity (BMI), increased age and gender can be used to predict the likelihood of OSA.61 In addition, symptoms such as witnessed apnoeas and loud habitual snoring also help predict the likelihood of OSA. Multiple instruments have been developed to utilize this information. The Berlin questionnaire dichotomizes individuals into low and high risk for OSA62 and has relatively high sensitivity but low specificity. The STOP-BANG questionnaire63 gives points for gender, age above a threshold (50 years of age), and BMI above a threshold (e.g., 35 kg/m2), to assess likelihood of OSA.
However, these instruments cannot accommodate the continuous nature of these variables e.g. as obesity and age increase, so does the likelihood of OSA. Strategies to utilize more data in OSA have been developed including: multi-variable apnoea prediction based on a logistic regression model (input variables: age, BMI, gender and a symptom score64); a likelihood ratio approach (input variables: neck circumference, hypertension, habitual snoring and bed partner reports of nocturnal gasping/choking65); and an artificial neural network approach (input variables: age, history of hypertension, history of diabetes mellitus, BMI, neck circumference and response to 4 questions that included snoring and witnessed apnoeas 66). There have been limited comparisons of which approach is superior. It is conceivable that discrimination to predict OSA could be improved if multiple prediction algorithms were applied to the same data.
There may be other sources of relevant information. For example, a logistic regression model using craniofacial variables from digital photography67—face width, eye width, cervicomental angle and mandibular length—demonstrated an 86.0% sensitivity, 59.1% specificity and area under the receiver operating curve (AUC) of 0.82 to predict OSA.68 Combining this with clinical information improved the AUC to 0.87.68 However, as science progresses, we will be able to analyze raw data more comprehensively as well as connections between the raw data. Raw data could include every data point on a polysomnogram, every pixel on a digital photograph, all OMIC data, and so on. In addition, using connections between raw data may add discriminatory power. For example, while the arousal index is an important input variable, whether an arousal increases sympathetic activity,69 increases heart rate response 70 and causes blood pressure fluctuations71 may add discriminatory power to identify OSA subjects compared to controls. As model-free, unbiased approaches are being developed for raw data, concurrently, we need to compile and centralize derived data we already have (clinical symptoms, age, gender, obesity measure, presence of hypertension, heart rate variability and craniofacial measures) and evaluate different unbiased approaches and structured approaches of predictive analytics.
3.2. Predicting OSA using Genetics
Another potential source of information to predict who has OSA is genetic information. This is an area where there has been limited progress and will be considered elsewhere in this series (i.e. Respirology, Invited Review Series, Respiratory sleep disorders).
3.3. Predicting Who Will Benefit From Therapy
Another important goal of P4 medicine is to predict who will benefit from a particular form of OSA therapy. For example, some patients do not benefit from use of an intraoral device and may get worse. A number of different approaches have been studied to predict who will benefit, but results are inconsistent72, 73 (for helpful discussion, see 74). Recently a new approach that relates various physiological risk factors for OSA to responses to intraoral devices has been proposed.75 This approach is based on passive upper airway collapsibility and loop gain being predictors of reducing OSA severity, with subjects with high loop gain having a high residual AHI when using an intraoral device.75 While interesting, this small sample requires future, larger prospective studies to see if these physiological risk factors can be used clinically to predict who would benefit from intraoral devices.74
3.4. Predicting Outcome Response To Therapy
Another important goal of P4 medicine is to predict response to therapy for specific consequences of OSA. Recently a new approach measured 84 microRNAs in circulating cells of OSA subjects with resistant hypertension on CPAP therapy. They report a three microRNA signature that identified patients with OSA who showed a beneficial blood pressure response to CPAP therapy compared to those with no changes in blood pressure response.76 Moving forward, studies should consider using OMIC signatures to predict response to therapy.
4. PREVENTION
The ultimate goal for healthcare is to move from treating disease to maintaining wellness. This can be achieved by prevention at the primary, secondary, and tertiary levels. In simple terms, primary prevention is concerned with preventing the onset of disease, secondary prevention deals with latent diseases and attempts to prevent an asymptomatic disease from progressing to symptomatic disease, and tertiary prevention attempts to reduce the complications caused by symptomatic disease. Phenotypic characterization of OSA using clinical and molecular data is central to understanding the heterogeneity of disease trajectory as well as to develop rational preventative approaches to inform public health policy.
4.1. Primary prevention
Primary prevention recognizes developmental biology as the origins of health and disease. The “Barker hypothesis” proposes that the period of gestation (and perhaps preconception) has significant impact on the developmental health and wellbeing of an individual from infancy to adulthood, and highlights the importance of epigenetics.77 Recent population based data found an association between prematurity and subsequent development of OSA in childhood78 and may be due to dentofacial developmental abnormalities that leads to upper airway narrowing. Another study found an association between breastfeeding (for longer than one month) and a decreased risk of habitual snoring and witnessed apnoeas in children with a family history of asthma.79 This may be due to better oropharyngeal development.
In some cases, primary prevention of OSA in children can halt or attenuate the trajectory to adult OSA if there is early intervention to modify risk factors. For example, adenotonsillary hypertrophy is a major risk factor of childhood OSA with surgical intervention being the main therapy that is often successful. Dentofacial morphology is another risk factor of OSA that may be caused by upper airway obstruction during childhood (e.g., nasal obstruction, adenotonsillar hypertrophy), leading to incremental craniofacial abnormalities that exacerbate airway narrowing. A range of dentofacial orthopaedics procedures, including rapid maxillary expansion, maxillary advancement and mandibular advancement have been demonstrated to improve OSA in children.80, 81 Hence, it would be important to identify and treat these abnormalities in children. Developing predictive tools of who should receive early intervention offer a real prospect at primary prevention of OSA.
4.2. Secondary prevention
The prevalence of OSA syndrome (i.e., symptomatic OSA) is much lower than the prevalence of sleep-disordered breathing in the population.82 Hence the majority of individuals are asymptomatic. OSA increases with age, weight gain and alcohol consumption.83 Furthermore, chronic snoring is thought to be a key factor in the progression toward OSA through the vibratory trauma it induces in the upper airway, leading to local inflammation and impairment of neuromuscular reflexes.84 Secondary prevention would involve identifying OSA patients that are asymptomatic as early as possible to halt or slow disease progression. Early interventions could include improving sleep hygiene (goal of attaining 6-8 hours of sleep), weight loss, alcohol reduction, and management of snoring (e.g., oral appliance therapy, management of nasal obstruction).
4.3. Tertiary prevention
As discussed above, OSA is associated with many consequences and tertiary prevention would attempt to reduce these complications in symptomatic OSA patients. As susceptibility to consequences varies considerably between individuals we first need to understand which OSA subgroup is associated with which complications. For example, in the Iceland study discussed above, the minimally symptomatic group had a greater association with cardiometabolic complications43 such as hypertension, ischaemic heart disease and diabetes. Thus, by knowing what complications are likely to occur by OSA subgroup and how OMIC signatures predict these subgroups will enable clinicians to better target therapy to reduce long-term complications.
5. PERSONALIZATION
5.1. Personalizing the diagnosis of OSA
Personalized medicine should consider individual differences when making a diagnosis, from genetics to environmental exposures to clinical presentation and its effects on pathways to disease. OSA has long been defined and treated according to a single metric – the apnea-hypopnea index (AHI). Although in-lab polysomnogram studies provide a massive amount of information, only a few derived indices are used. As discussed above, OSA research is starting to identify different clinically relevant OSA subgroups using symptom profiles, pathophysiological disturbances, and molecular signatures. Ongoing endeavours to incorporate deep extreme phenotyping of large OSA samples across multiple dimensions and using unbiased mathematical approaches will undoubtedly identify novel and clinically relevant subgroups, further personalizing the diagnosis of OSA.
5.2. Personalizing the management of OSA
Fundamental to the concept of personalized approaches is the idea that individuals with different characteristics will be treated in different ways. While CPAP is the main modality of treatment, alternative treatment options include intraoral mandibular advancement devices, surgical approaches, and hypoglossal nerve stimulation.85 While each treatment option is different in the mode of action, common to all of these alternative approaches is that efficacy tends to vary between individuals. Using overnight physiological phenotyping studies 18 that assess how four key physiological traits contribute to upper airway collapse in individuals with OSA (Table 1) may be a systematic way of personalizing treatment.
5.2.1. Treating obesity
While weight loss is important in reducing OSA severity, there is variability to the amount of improvement.86 It may be that specific patient phenotypes are the most amenable to weight loss therapy for examples, those with a more restricted craniofacial skeleton may have a better response to weight loss therapy.87 Therefore, craniofacial phenotype could be used to predict which patients would benefit the most from weight loss interventions.
5.2.2. Continuous Positive Airway Pressure (CPAP)
CPAP has long held the position of first line treatment for OSA and is an excellent treatment for most individuals with demonstrated improvement of symptoms and other health outcomes. However individual differences with respect to CPAP therapy are known to exist. While >4 hours of use/night is considered to be good usage, some health outcome measures require greater usage than this arbitrary threshold.88 Furthermore, >50% of those started on CPAP abandon it all together within 6 months of treatment initiation, even with intensive cognitive behavioural therapy interventions.89 Suboptimal usage therefore reduces the real world effectiveness of this treatment. Despite these issues with adequate implementation, CPAP remains the standard of care as the traditional ‘one-size-fits-all’ approach to treatment.
5.2.3. Oral appliances
Oral appliances (mandibular advancement splints) are the leading alternative treatment option to CPAP. Oral appliances hold the lower jaw in a more forward position to enlarge and stabilize the airway. In trials where patients experience both oral appliances and CPAP, and when effective, patients generally preferred oral appliance over CPAP.72 More recently, technology has become available to objectively measure oral appliance usage through embedded temperature sensing chips. Initial studies confirm high rates of adherence and also good concordance between subjective and objective estimates of usage time.90, 91
Oral appliances have demonstrated variable efficacy in the treatment of OSA, with one-fourth of severe OSA patients experiencing complete resolution of OSA with an oral appliance alone and approximately one-third of the patients not experiencing any clinical benefit.92 Thus the uncertainty surrounding the efficacy of oral appliances has resulted in its use largely as a secondary treatment option if CPAP is not tolerated. Currently there are limited data and techniques to identify in advance who will or will not benefit from intraoral devices.
5.2.4. Hypoglossal nerve stimulation and surgical options
Likewise, studies for hypoglossal nerve stimulation show that even though very careful selection criteria were employed, approximately one-third of patients do not have clinically relevant responses.85 The basis for this lack of response is unknown. In addition, while surgical treatments for OSA may not have the same efficacy in the reduction in AHI compared to CPAP, compliance is essentially 100%. Moving forward, comparative effectiveness research based on personalized responses to therapy must be considered.
5.2.5. Other therapies
Combination treatment for OSA can be personalized for an additive, or synergistic, effect. For example targeting sedatives specifically to OSA patients with a low arousal threshold may allow stable sleep and breathing to occur in these particular patients.27, 93 Oxygen supplementation to patients with ventilatory control system hypersensitivity (i.e., high loop gain) may be of value.94 Combining therapies such as oral appliances and positional therapy,95 genioglossus stimulation and mandibular advancement,96 oxygen and sedatives28 are effective in certain OSA subgroups.
6. PARTICIPATION
The participatory aspect of P4 medicine takes the patient from a passive receiver of care to actively managing his or her own wellness. The P4 strategy flips the model of physicians as the gatekeepers of expert advice and sole manager of medical care, to one where patients are engaged to use social networking and digital monitoring in addition to consultation with physicians. P4 medicine requires large amounts of input data to inform prediction analytics. As technology drives down the price of mobile devices, monitoring health is no longer restricted to clinical settings thereby allowing healthcare consumers to collect their own data (e.g., sleep monitoring, blood pressure, oxygen saturation and heart rate), manage their response to treatment97 and contribute to the other three Ps of this approach to medicine.
6.1. Patient-centred outcomes
A central concept in participatory medicine is recognition of patient centred outcomes, defined as outcomes that are the most meaningful to patients and health care providers. In 2010, the USA established the Patient-Centred Outcomes Research Institute (PCORI) to incorporate patient-centred outcomes into comparative effectiveness research.98 The tenant of PCORI is to produce faster implementation by starting with the end users (patients and health care stakeholders) and tailoring clinical research to what is most meaningful to them.
The Sleep Apnea Patient-Centred Outcomes Network (SAPCON) is a collaborative group of patients, researchers, and clinicians that generates patient-centred comparative and implementation research.99 Another similar initiative is Sustainable Methods, Algorithms, and Research Tools for Delivering Optimal Care Study (SMART DOCS) that assesses the effects of a patient-centred outcome and coordinated-care management (PCCM), participatory environment.100 This study randomized two groups, one group receiving the current care model of OSA, and the other group receiving a novel, more participatory approach to OSA. The more participatory model facilitates communication between patients and health care providers to identify and obtain outcomes important to the patient.
6.2. Participatory treatment management
OSA management is particularly suited to a participatory approach due to the enabling role of monitoring technology readily available in both diagnosis and treatment of disease. Portable monitoring devices can be used in the home for diagnosis and wearable devices can be used to track sleep-wake patterns through activity. In terms of treatment, CPAP machines have long had the capability to record objective compliance data in hours of usage, and this uniquely positions the sleep field for patient engagement. Oral appliances now have similar capabilities of measuring hours of treatment usage using temperature sensors embedded in the device.91
6.2.1. CPAP management
Modern CPAP machines now have wireless connectivity that can transmit data to cloud storage. This information is accessible to healthcare providers in order to monitor adherence and efficacy of therapy and detect any issues of therapy (such as mask leaks). There are now apps available for patients (e.g., Philips Respironics SleepMapper, Monroeville, PA, USA and ResMed Airview, San Diego, CA, USA) through which they can directly monitor and enhance their treatment. Features include goal setting, reports on mask fit, hours of usage, and AHI. Having access to such information should lead to patients participating more in their own healthcare as well as facilitate better communication with clinicians.
The long-term benefits on patient health of participatory treatment still need to be ascertained. The application of participatory care using a web-based technology to enhance OSA treatment has recently been demonstrated in a trial assessing different strategies to improve adherence to CPAP treatment.101 The parallel group study randomized newly diagnosed OSA patient before commencement of CPAP therapy to one of three conditions: 1) usual care (no access to their own treatment usage data), 2) free access to their own treatment usage data through a web-based platform displaying their treatment usage, or 3) access to the web-based platform plus a financial incentive ($30/night in the first week for adequate treatment usage). The participants randomized to conditions 2 and 3 had ad libitum access to their recorded treatment usage (mask on time) sent through a wireless modem to a web-based portal. This study showed that feedback to participants through the website improved daily hours of CPAP usage compared to usual care. Interestingly the financial incentive did not provide any further benefit in increase treatment adherence beyond that of data access.
7. SUMMARY AND CONCLUSIONS
P4 medicine is very relevant to OSA. In this review we have laid out the case to use this approach and presented initial data supporting its application. Wide availability of OMIC strategies, new unbiased data analytical approaches and new mobile technologies that allow patients to participate in their own care, will support the P4 approach to OSA.
It is, however, a journey that is just beginning. We need more studies to assess whether the clinical subgroups of OSA exist in other ethnicities and whether subgroups have different clinical consequences, and response to different therapies. New approaches to in-depth physiological phenotyping remain investigational until more studies correlate these indices to OSA classification and clinical consequences.102 Novel OMIC approaches needs to be performed in a systematic way to predict, prevent and personalize disease in high-risk patients, then undergo extensive validation before biomarkers can be routinely used in clinical practice.
The future of OSA research (Table 2) and the clinical implementation of these strategies is exciting. Today’s technology not only makes the P4 approach to OSA feasible but collectively we can transform the way we approach the common disorder of OSA.
Table 2.
RESEARCH AND CLINICAL DIRECTIONS FOR P4 MEDICINE IN OSA
| • Confirming clinical subgroups and relevance to outcomes. |
| • Developing personalized approaches to therapy based on clinical subgroups. |
| • Application of all OMIC approaches to identify molecular signatures that will provide prognostic information. |
| • Elucidating the genetic variants that confer risk or protection for OSA. |
| • Enhancing clinical phenotypes based on new physiological approaches and applying these in routine care. |
| • Understanding the reasons why some patients with OSA develop hypertension, CV disease, diabetes, etc. why others do not. |
| • Evaluating whether treatment of OSA can affect the rate of progression of neurodegenerative disease and in which types of patients. |
| • Development of mobile approaches for diagnosis and follow-up management of OSA. |
| • Enhanced prediction tools based on information from several domains—obesity measure, symptoms, facial photography, etc. |
| • Developing approaches to prevent OSA in individuals with upper airway compromise before they develop clinical disease. |
| • Developing personalized approaches to therapy. |
| • Understanding basis of different response to intra-oral devices and hypoglossal nerve stimulation. |
| • Widespread participatory care for patients with OSA based on new technologies. |
ACKNOWLEDGEMENTS:
NIH Program Project Grant HL094307.
Biographies
Dr. Diane Lim is an Assistant Professor at the University of Pennsylvania. Her research focus is studying the effects of cyclical intermittent hypoxia (CIH) on cancer in mouse models. Additional research interests include correlating OMIC data and physiological PSG signals to mechanisms of CIH injury in mice and clinical outcomes of OSA in humans.
Dr. Kate Sutherland is a research fellow at the Charles Perkins Centre, University of Sydney and Royal North Shore Hospital, Sydney Australia. Her current research areas include phenotypic characterisation of obstructive sleep apnea, particularly of anatomic risk factors (craniofacial structure and obesity) and improving treatment outcomes of non-PAP therapies (e.g. oral appliances and weight loss).
Dr. Peter Cistulli is ResMed Chair in Sleep Medicine at the University of Sydney, where co-leads the Sleep Research Theme at the Charles Perkins Centre. He is also Director of the Sleep Medicine program at Royal North Shore Hospital. His research interests are in the areas of OSA pathophysiology and novel treatments for OSA.
Dr. Allan Pack is the Director of the Center for Sleep and Circadian Neurobiology and Chief of the Division of Sleep Medicine at the University of Pennsylvania. His active areas of research include a functional genomics/genetics approach to sleep and its disorders, in particular obstructive sleep apnea. Dr. Pack is developing a personalized approach to this common disorder.
REFERENCES:
- 1.Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med. 2015; 372: 793–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ko PR, Kientz JA, Choe EK, Kay M, Landis CA, Watson NF. Consumer Sleep Technologies: A Review of the Landscape. J Clin Sleep Med. 2015; 11: 1455–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nandakumar R, Gollakota S, Watson N. Contactless sleep apnea detection on smartphones. MobiSys’15 ACM, Florence, Italy, 2015; 1–13. [Google Scholar]
- 4.Pack AI. Application of Personalized, Predictive, Preventative, and Participatory (P4) Medicine to Obstructive Sleep Apnea. A Roadmap for Improving Care? Ann Am Thorac Soc. 2016; 13: 1456–67. [DOI] [PubMed] [Google Scholar]
- 5.Dinov ID, Heavner B, Tang M, Glusman G, Chard K, Darcy M, Madduri R, Pa J, Spino C, Kesselman C, Foster I, Deutsch EW, Price ND, Van Horn JD, Ames J, Clark K, Hood L, Hampstead BM, Dauer W, Toga AW. Predictive Big Data Analytics: A Study of Parkinson’s Disease Using Large, Complex, Heterogeneous, Incongruent, Multi-Source and Incomplete Observations. PLoS ONE. 2016; 11: e0157077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C, Cameron D, Dowsett M, Barrios CH, Steger G, Huang CS, Andersson M, Inbar M, Lichinitser M, Lang I, Nitz U, Iwata H, Thomssen C, Lohrisch C, Suter TM, Ruschoff J, Suto T, Greatorex V, Ward C, Straehle C, McFadden E, Dolci MS, Gelber RD. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005; 353: 1659–72. [DOI] [PubMed] [Google Scholar]
- 7.Schadt EE, Lamb J, Yang X, Zhu J, Edwards S, Guhathakurta D, Sieberts SK, Monks S, Reitman M, Zhang C, Lum PY, Leonardson A, Thieringer R, Metzger JM, Yang L, Castle J, Zhu H, Kash SF, Drake TA, Sachs A, Lusis AJ. An integrative genomics approach to infer causal associations between gene expression and disease. Nat Genet. 2005; 37: 710–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monti S, Savage KJ, Kutok JL, Feuerhake F, Kurtin P, Mihm M, Wu B, Pasqualucci L, Neuberg D, Aguiar RC, Dal Cin P, Ladd C, Pinkus GS, Salles G, Harris NL, Dalla-Favera R, Habermann TM, Aster JC, Golub TR, Shipp MA. Molecular profiling of diffuse large B-cell lymphoma identifies robust subtypes including one characterized by host inflammatory response. Blood. 2005; 105: 1851–61. [DOI] [PubMed] [Google Scholar]
- 9.Multiple Myeloma Research Foundation. 2009. Multiple Myeloma Research Consortium (MMRC) activates clinical trials 30–40 percent faster than industry standard. MMRFSM; http://www.themmrf.org/archive/outdated-content/press-releases-061809.html 2017. [Google Scholar]
- 10.Adenoid Cystic Carcinoma Research Foundation. 2010. http://www.accrf.org/ Accessed: January 17, 2017.
- 11.Love SM, Mills D, Eraklis E, Hurlbert M. The Love/Avon Army of Women: A “just in time” resource to encourage research in women. J Clin Oncol. 2009; 27: 1535. [Google Scholar]
- 12.Army of Women. 2010. Army of Women; https://www.armyofwomen.org/ Accessed: December 21 2016. [Google Scholar]
- 13.Bouchie A Coming soon: a global grid for cancer research. Nat Biotechnol. 2004; 22: 1071–3. [DOI] [PubMed] [Google Scholar]
- 14.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013; 177: 1006–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lim DC, Pack AI. Obstructive Sleep Apnea: Update and Future. Annu Rev Med. 2016. [DOI] [PubMed] [Google Scholar]
- 16.Chi L, Comyn FL, Keenan BT, Cater J, Maislin G, Pack AI, Schwab RJ. Heritability of craniofacial structures in normal subjects and patients with sleep apnea. Sleep. 2014; 37: 1689–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwab RJ, Gupta KB, Gefter WB, Metzger LJ, Hoffman EA, Pack AI. Upper airway and soft tissue anatomy in normal subjects and patients with sleep-disordered breathing. Significance of the lateral pharyngeal walls. Am J Respir Crit Care Med. 1995; 152: 1673–89. [DOI] [PubMed] [Google Scholar]
- 18.Eckert DJ, White DP, Jordan AS, Malhotra A, Wellman A. Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am J Respir Crit Care Med. 2013; 188: 996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sands SA, Eckert DJ, Jordan AS, Edwards BA, Owens RL, Butler JP, Schwab RJ, Loring SH, Malhotra A, White DP, Wellman A. Enhanced upper-airway muscle responsiveness is a distinct feature of overweight/obese individuals without sleep apnea. Am J Respir Crit Care Med. 2014; 190: 930–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Owens RL, Edwards BA, Eckert DJ, Jordan AS, Sands SA, Malhotra A, White DP, Loring SH, Butler JP, Wellman A. An Integrative Model of Physiological Traits Can be Used to Predict Obstructive Sleep Apnea and Response to Non Positive Airway Pressure Therapy. Sleep. 2015; 38: 961–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwab RJ. Genetic determinants of upper airway structures that predispose to obstructive sleep apnea. Respir Physiol Neurobiol. 2005; 147: 289–98. [DOI] [PubMed] [Google Scholar]
- 22.Martinez-Rivera C, Abad J, Fiz JA, Rios J, Morera J. Usefulness of truncal obesity indices as predictive factors for obstructive sleep apnea syndrome. Obesity (Silver Spring). 2008; 16: 113–8. [DOI] [PubMed] [Google Scholar]
- 23.Isono S. Obesity and obstructive sleep apnoea: mechanisms for increased collapsibility of the passive pharyngeal airway. Respirology. 2012; 17: 32–42. [DOI] [PubMed] [Google Scholar]
- 24.Kim AM, Keenan BT, Jackson N, Chan EL, Staley B, Poptani H, Torigian DA, Pack AI, Schwab RJ. Tongue fat and its relationship to obstructive sleep apnea. Sleep. 2014; 37: 1639–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malis C, Rasmussen EL, Poulsen P, Petersen I, Christensen K, Beck-Nielsen H, Astrup A, Vaag AA. Total and regional fat distribution is strongly influenced by genetic factors in young and elderly twins. Obes Res. 2005; 13: 2139–45. [DOI] [PubMed] [Google Scholar]
- 26.Fox CS, White CC, Lohman K, Heard-Costa N, Cohen P, Zhang Y, Johnson AD, Emilsson V, Liu CT, Chen YD, Taylor KD, Allison M, Budoff M, Rotter JI, Carr JJ, Hoffmann U, Ding J, Cupples LA, Liu Y. Genome-wide association of pericardial fat identifies a unique locus for ectopic fat. PLoS Genet. 2012; 8: e1002705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eckert DJ, Malhotra A, Wellman A, White DP. Trazodone increases the respiratory arousal threshold in patients with obstructive sleep apnea and a low arousal threshold. Sleep. 2014; 37: 811–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Edwards BA, Sands SA, Owens RL, Eckert DJ, Landry S, White DP, Malhotra A, Wellman A. The Combination of Supplemental Oxygen and a Hypnotic Markedly Improves Obstructive Sleep Apnea in Patients with a Mild to Moderate Upper Airway Collapsibility. Sleep. 2016; 39: 1973–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taranto-Montemurro L, Edwards BA, Sands SA, Marques M, Eckert DJ, White DP, Wellman A. Desipramine Increases Genioglossus Activity and Reduces Upper Airway Collapsibility during Non-REM Sleep in Healthy Subjects. Am J Respir Crit Care Med. 2016; 194: 878–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee RW, Vasudavan S, Hui DS, Prvan T, Petocz P, Darendeliler MA, Cistulli PA. Differences in craniofacial structures and obesity in Caucasian and Chinese patients with obstructive sleep apnea. Sleep. 2010; 33: 1075–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tan A, Cheung YY, Yin J, Lim WY, Tan LW, Lee CH. Prevalence of sleep-disordered breathing in a multiethnic Asian population in Singapore: A community-based study. Respirology. 2016; 21: 943–50. [DOI] [PubMed] [Google Scholar]
- 32.Chen X, Wang R, Zee P, Lutsey PL, Javaheri S, Alcantara C, Jackson CL, Williams MA, Redline S. Racial/Ethnic Differences in Sleep Disturbances: The Multi-Ethnic Study of Atherosclerosis (MESA). Sleep. 2015; 38: 877–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baldwin CM, Ervin AM, Mays MZ, Robbins J, Shafazand S, Walsleben J, Weaver T. Sleep disturbances, quality of life, and ethnicity: the Sleep Heart Health Study. J Clin Sleep Med. 2010; 6: 176–83. [PMC free article] [PubMed] [Google Scholar]
- 34.Bouscoulet LT, Vazquez-Garcia JC, Muino A, Marquez M, Lopez MV, de Oca MM, Talamo C, Valdivia G, Pertuze J, Menezes AM, Perez-Padilla R. Prevalence of sleep related symptoms in four Latin American cities. J Clin Sleep Med. 2008; 4: 579–85. [PMC free article] [PubMed] [Google Scholar]
- 35.Tufik S, Santos-Silva R, Taddei JA, Bittencourt LR. Obstructive sleep apnea syndrome in the Sao Paulo Epidemiologic Sleep Study. Sleep Med. 2010; 11: 441–6. [DOI] [PubMed] [Google Scholar]
- 36.Hrubos-Strom H, Randby A, Namtvedt SK, Kristiansen HA, Einvik G, Benth J, Somers VK, Nordhus IH, Russell MB, Dammen T, Omland T, Kvaerner KJ. A Norwegian population-based study on the risk and prevalence of obstructive sleep apnea. The Akershus Sleep Apnea Project (ASAP). J Sleep Res. 2011; 20: 162–70. [DOI] [PubMed] [Google Scholar]
- 37.Sforza E, Chouchou F, Collet P, Pichot V, Barthelemy JC, Roche F. Sex differences in obstructive sleep apnoea in an elderly French population. Eur Respir J. 2011; 37: 1137–43. [DOI] [PubMed] [Google Scholar]
- 38.Heinzer R, Vat S, Marques-Vidal P, Marti-Soler H, Andries D, Tobback N, Mooser V, Preisig M, Malhotra A, Waeber G, Vollenweider P, Tafti M, Haba-Rubio J. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. The Lancet Respiratory medicine. 2015; 3: 310–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jefferson FA, Bliwise D, Paul KN, Clark K. Sex differences in the REM sleep response to OSA. Gordon Research Conference on Sleep Regulation and Function, 2013; Abstr #2-22. [Google Scholar]
- 40.Runyon JJ, Maislin G, Schwab RJ. Gender differences in symptoms associated with sleep apnea. Sleep Rev. 2001; Winter. [Google Scholar]
- 41.Ye L, Pien GW, Ratcliffe SJ, Weaver TE. Gender differences in obstructive sleep apnea and treatment response to continuous positive airway pressure. J Clin Sleep Med. 2009; 5: 512–8. [PMC free article] [PubMed] [Google Scholar]
- 42.Gaines J, Vgontzas AN, Fernandez-Mendoza J, Kritikou I, Basta M, Bixler EO. Gender differences in the association of sleep apnea and inflammation. Brain Behav Immun. 2015; 47: 211–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ye L, Pien GW, Ratcliffe SJ, Bjornsdottir E, Arnardottir ES, Pack AI, Benediktsdottir B, Gislason T. The different clinical faces of obstructive sleep apnoea: a cluster analysis. Eur Respir J. 2014; 44: 1600–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vavougios GD, George DG, Pastaka C, Zarogiannis SG, Gourgoulianis KI. Phenotypes of comorbidity in OSAS patients: combining categorical principal component analysis with cluster analysis. J Sleep Res. 2016; 25: 31–8. [DOI] [PubMed] [Google Scholar]
- 45.Bailly S, Destors M, Grillet Y, Richard P, Stach B, Vivodtzev I, Timsit JF, Levy P, Tamisier R, Pepin JL. Obstructive Sleep Apnea: A Cluster Analysis at Time of Diagnosis. PLoS ONE. 2016; 11: e0157318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ryan CM, Kendzerska T, Wilton K, Lyons OD. The different clinical faces of obstructive sleep apnea (OSA), OSA in older adults as a distinctly different physiological phenotype, and the impact of OSA on cardiovascular events after coronary artery bypass surgery. Am J Respir Crit Care Med. 2015; 192: 1127–9. [DOI] [PubMed] [Google Scholar]
- 47.Gozal D, Jortani S, Snow AB, Kheirandish-Gozal L, Bhattacharjee R, Kim J, Capdevila OS. Two-dimensional differential in-gel electrophoresis proteomic approaches reveal urine candidate biomarkers in pediatric obstructive sleep apnea. Am J Respir Crit Care Med. 2009; 180: 1253–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gharib SA, Seiger AN, Hayes AL, Mehra R, Patel SR. Treatment of obstructive sleep apnea alters cancer-associated transcriptional signatures in circulating leukocytes. Sleep. 2014; 37: 709–14, 14A–14T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nieto FJ, Peppard PE, Young T, Finn L, Hla KM, Farre R. Sleep-disordered breathing and cancer mortality: results from the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med. 2012; 186: 190–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Campos-Rodriguez F, Martinez-Garcia MA, Martinez M, Duran-Cantolla J, Pena Mde L, Masdeu MJ, Gonzalez M, Campo F, Gallego I, Marin JM, Barbe F, Montserrat JM, Farre R. Association between obstructive sleep apnea and cancer incidence in a large multicenter Spanish cohort. Am J Respir Crit Care Med. 2013; 187: 99–105. [DOI] [PubMed] [Google Scholar]
- 51.Azarbarzin A, Ostrowski M, Younes M, Keenan BT, Pack AI, Staley B, Kuna ST. Arousal responses during overnight polysomnography and their reproducibility in healthy young adults. Sleep. 2015; 38: 1313–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gao X, Azarbarzin A, Keenan BT, Ostrowski M, Pack FM, Staley B, Maislin G, Pack AI, Younes M, Kuna ST. Heritability of heart rate response to arousals in twins. Sleep. 2017. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Konecny T, Kara T, Somers VK. Obstructive sleep apnea and hypertension: an update. Hypertension. 2014; 63: 203–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gottlieb DJ, Yenokyan G, Newman AB, O’Connor GT, Punjabi NM, Quan SF, Redline S, Resnick HE, Tong EK, Diener-West M, Shahar E. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation. 2010; 122: 352–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Matassini MV, Brambatti M, Guerra F, Scappini L, Capucci A. Sleep-disordered breathing and atrial fibrillation: review of the evidence. Cardiol Rev. 2015; 23: 79–86. [DOI] [PubMed] [Google Scholar]
- 56.Iftikhar IH, Hoyos CM, Phillips CL, Magalang UJ. Meta-analyses of the Association of Sleep Apnea with Insulin Resistance, and the Effects of CPAP on HOMA-IR, Adiponectin, and Visceral Adipose Fat. J Clin Sleep Med. 2015; 11: 475–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Anothaisintawee T, Reutrakul S, Van Cauter E, Thakkinstian A. Sleep disturbances compared to traditional risk factors for diabetes development: Systematic review and meta-analysis. Sleep Med Rev. 2016; 30: 11–24. [DOI] [PubMed] [Google Scholar]
- 58.Yaffe K, Laffan AM, Harrison SL, Redline S, Spira AP, Ensrud KE, Ancoli-Israel S, Stone KL. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011; 306: 613–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marshall NS, Wong KK, Cullen SR, Knuiman MW, Grunstein RR. Sleep apnea and 20-year follow-up for all-cause mortality, stroke, and cancer incidence and mortality in the busselton health study cohort. J Clin Sleep Med. 2014; 10: 355–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Young T, Finn L, Peppard PE, Szklo-Coxe M, Austin D, Nieto FJ, Stubbs R, Hla KM. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008; 31: 1071–8. [PMC free article] [PubMed] [Google Scholar]
- 61.Lyons MM, Keenan BT, Li J, Khan T, Elkasabanny N, Walsh CM, Williams NN, Pack AI, Gurubhagavatula I. Symptomless multi-variable apnea prediction index assesses OSA risk and adverse outcomes in elective surgery. Sleep. 2017. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999; 131: 485–91. [DOI] [PubMed] [Google Scholar]
- 63.Chung F, Abdullah HR, Liao P. STOP-Bang Questionnaire: A Practical Approach to Screen for Obstructive Sleep Apnea. Chest. 2016; 149: 631–8. [DOI] [PubMed] [Google Scholar]
- 64.Maislin G, Pack AI, Kribbs NB, Smith PL, Schwartz AR, Kline LR, Schwab RJ, Dinges DF. A survey screen for prediction of apnea. Sleep. 1995; 18: 158–66. [DOI] [PubMed] [Google Scholar]
- 65.Flemons WW, Whitelaw WA, Brant R, Remmers JE. Likelihood ratios for a sleep apnea clinical prediction rule. Am J Respir Crit Care Med. 1994; 150: 1279–85. [DOI] [PubMed] [Google Scholar]
- 66.Teferra RA, Grant BJ, Mindel JW, Siddiqi TA, Iftikhar IH, Ajaz F, Aliling JP, Khan MS, Hoffmann SP, Magalang UJ. Cost minimization using an artificial neural network sleep apnea prediction tool for sleep studies. Ann Am Thorac Soc. 2014; 11: 1064–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee RW, Chan AS, Grunstein RR, Cistulli PA. Craniofacial phenotyping in obstructive sleep apnea--a novel quantitative photographic approach. Sleep. 2009; 32: 37–45. [PMC free article] [PubMed] [Google Scholar]
- 68.Lee RW, Petocz P, Prvan T, Chan AS, Grunstein RR, Cistulli PA. Prediction of obstructive sleep apnea with craniofacial photographic analysis. Sleep. 2009; 32: 46–52. [PMC free article] [PubMed] [Google Scholar]
- 69.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995; 96: 1897–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Penzel T, Kantelhardt JW, Grote L, Peter JH, Bunde A. Comparison of detrended fluctuation analysis and spectral analysis for heart rate variability in sleep and sleep apnea. IEEE Trans Biomed Eng. 2003; 50: 1143–51. [DOI] [PubMed] [Google Scholar]
- 71.Camargo S, Riedl M, Anteneodo C, Kurths J, Penzel T, Wessel N. Sleep apnea-hypopnea quantification by cardiovascular data analysis. PLoS ONE. 2014; 9: e107581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sutherland K, Vanderveken OM, Tsuda H, Marklund M, Gagnadoux F, Kushida CA, Cistulli PA. Oral appliance treatment for obstructive sleep apnea: an update. J Clin Sleep Med. 2014; 10: 215–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Okuno K, Pliska BT, Hamoda M, Lowe AA, Almeida FR. Prediction of oral appliance treatment outcomes in obstructive sleep apnea: A systematic review. Sleep Med Rev. 2016; 30: 25–33. [DOI] [PubMed] [Google Scholar]
- 74.Cistulli PA, Sutherland K. Deep Phenotyping in Obstructive Sleep Apnea. A Step Closer to Personalized Therapy. Am J Respir Crit Care Med. 2016; 194: 1317–8. [DOI] [PubMed] [Google Scholar]
- 75.Edwards BA, Andara C, Landry S, Sands SA, Joosten SA, Owens RL, White DP, Hamilton GS, Wellman A. Upper-Airway Collapsibility and Loop Gain Predict the Response to Oral Appliance Therapy in Patients with Obstructive Sleep Apnea. Am J Respir Crit Care Med. 2016; 194: 1413–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sanchez-de-la-Torre M, Khalyfa A, Sanchez-de-la-Torre A, Martinez-Alonso M, Martinez-Garcia MA, Barcelo A, Lloberes P, Campos-Rodriguez F, Capote F, Diaz-de-Atauri MJ, Somoza M, Gonzalez M, Masa JF, Gozal D, Barbe F. Precision Medicine in Patients With Resistant Hypertension and Obstructive Sleep Apnea: Blood Pressure Response to Continuous Positive Airway Pressure Treatment. J Am Coll Cardiol. 2015; 66: 1023–32. [DOI] [PubMed] [Google Scholar]
- 77.Wadhwa PD, Buss C, Entringer S, Swanson JM. Developmental origins of health and disease: brief history of the approach and current focus on epigenetic mechanisms. Seminars in reproductive medicine. 2009; 27: 358–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Raynes-Greenow CH, Hadfield RM, Cistulli PA, Bowen J, Allen H, Roberts CL. Sleep apnea in early childhood associated with preterm birth but not small for gestational age: a population-based record linkage study. Sleep. 2012; 35: 1475–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brew BK, Marks GB, Almqvist C, Cistulli PA, Webb K, Marshall NS. Breastfeeding and snoring: a birth cohort study. PLoS ONE. 2014; 9: e84956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Camacho M, Chang ET, Song SA, Abdullatif J, Zaghi S, Pirelli P, Certal V, Guilleminault C. Rapid maxillary expansion for pediatric obstructive sleep apnea: A systematic review and meta-analysis. Laryngoscope. 2016. [DOI] [PubMed] [Google Scholar]
- 81.Ngiam J, Cistulli PA. Dental treatment for paediatric obstructive sleep apnea. Paediatric respiratory reviews. 2015; 16: 174–81. [DOI] [PubMed] [Google Scholar]
- 82.Arnardottir ES, Bjornsdottir E, Olafsdottir KA, Benediktsdottir B, Gislason T. Obstructive sleep apnoea in the general population: highly prevalent but minimal symptoms. Eur Respir J. 2016; 47: 194–202. [DOI] [PubMed] [Google Scholar]
- 83.Peppard PE, Austin D, Brown RL. Association of alcohol consumption and sleep disordered breathing in men and women. J Clin Sleep Med. 2007; 3: 265–70. [PMC free article] [PubMed] [Google Scholar]
- 84.Boyd JH, Petrof BJ, Hamid Q, Fraser R, Kimoff RJ. Upper airway muscle inflammation and denervation changes in obstructive sleep apnea. Am J Respir Crit Care Med. 2004; 170: 541–6. [DOI] [PubMed] [Google Scholar]
- 85.Strollo PJ Jr., Soose RJ, Maurer JT, de Vries N, Cornelius J, Froymovich O, Hanson RD, Padhya TA, Steward DL, Gillespie MB, Woodson BT, Van de Heyning PH, Goetting MG, Vanderveken OM, Feldman N, Knaack L, Strohl KP. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med. 2014; 370: 139–49. [DOI] [PubMed] [Google Scholar]
- 86.Dixon JB, Schachter LM, O’Brien PE, Jones K, Grima M, Lambert G, Brown W, Bailey M, Naughton MT. Surgical vs conventional therapy for weight loss treatment of obstructive sleep apnea: a randomized controlled trial. JAMA. 2012; 308: 1142–9. [DOI] [PubMed] [Google Scholar]
- 87.Sutherland K, Phillips CL, Yee BJ, Grunstein RR, Cistulli PA. Maxillomandibular Volume Influences the Relationship between Weight Loss and Improvement in Obstructive Sleep Apnea. Sleep. 2016; 39: 43–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Weaver TE, Maislin G, Dinges DF, Bloxham T, George CF, Greenberg H, Kader G, Mahowald M, Younger J, Pack AI. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep. 2007; 30: 711–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bartlett D, Wong K, Richards D, Moy E, Espie CA, Cistulli PA, Grunstein R. Increasing adherence to obstructive sleep apnea treatment with a group social cognitive therapy treatment intervention: a randomized trial. Sleep. 2013; 36: 1647–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dieltjens M, Braem MJ, Vroegop AV, Wouters K, Verbraecken JA, De Backer WA, Van de Heyning PH, Vanderveken OM. Objectively measured vs self-reported compliance during oral appliance therapy for sleep-disordered breathing. Chest. 2013; 144: 1495–502. [DOI] [PubMed] [Google Scholar]
- 91.Vanderveken OM, Dieltjens M, Wouters K, De Backer WA, Van de Heyning PH, Braem MJ. Objective measurement of compliance during oral appliance therapy for sleep-disordered breathing. Thorax. 2013; 68: 91–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sutherland K, Takaya H, Qian J, Petocz P, Ng AT, Cistulli PA. Oral Appliance Treatment Response and Polysomnographic Phenotypes of Obstructive Sleep Apnea. J Clin Sleep Med. 2015; 11: 861–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Carter SG, Berger MS, Carberry JC, Bilston LE, Butler JE, Tong BK, Martins RT, Fisher LP, McKenzie DK, Grunstein RR, Eckert DJ. Zopiclone Increases the Arousal Threshold without Impairing Genioglossus Activity in Obstructive Sleep Apnea. Sleep. 2016; 39: 757–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wellman A, Malhotra A, Jordan AS, Stevenson KE, Gautam S, White DP. Effect of oxygen in obstructive sleep apnea: role of loop gain. Respir Physiol Neurobiol. 2008; 162: 144–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dieltjens M, Vroegop AV, Verbruggen AE, Wouters K, Willemen M, De Backer WA, Verbraecken JA, Van de Heyning PH, Braem MJ, de Vries N, Vanderveken OM. A promising concept of combination therapy for positional obstructive sleep apnea. Sleep Breath. 2015; 19: 637–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Oliven R, Tov N, Odeh M, Gaitini L, Steinfeld U, Schwartz AR, Oliven A. Interacting effects of genioglossus stimulation and mandibular advancement in sleep apnea. J Appl Physiol (1985). 2009; 106: 1668–73. [DOI] [PubMed] [Google Scholar]
- 97.Hood L, Flores M. A personal view on systems medicine and the emergence of proactive P4 medicine: predictive, preventive, personalized and participatory. New biotechnology. 2012; 29: 613–24. [DOI] [PubMed] [Google Scholar]
- 98.Frank L, Basch E, Selby JV. The PCORI perspective on patient-centered outcomes research. JAMA. 2014; 312: 1513–4. [DOI] [PubMed] [Google Scholar]
- 99.Redline S, Baker-Goodwin S, Bakker JP, Epstein M, Hanes S, Hanson M, Harrington Z, Johnston JC 2nd, Kapur VK, Keepnews D, Kontos E, Lowe A, Owens J, Page K, Rothstein N. Patient Partnerships Transforming Sleep Medicine Research and Clinical Care: Perspectives from the Sleep Apnea Patient-Centered Outcomes Network. J Clin Sleep Med. 2016; 12: 1053–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kushida CA, Nichols DA, Holmes TH, Miller R, Griffin K, Cardell CY, Hyde PR, Cohen E, Manber R, Walsh JK. SMART DOCS: a new patient-centered outcomes and coordinated-care management approach for the future practice of sleep medicine. Sleep. 2015; 38: 315–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kuna ST, Shuttleworth D, Chi LQ, Schutte-Rodin S, Friedman E, Guo HY, Dhand S, Yang L, Zhu JS, Bellamy SL, Volpp KG, Asch DA. Web-based access to positive airway pressure usage with or without an initial financial incentive improves treatment use in patients with obstructive sleep apnea. Sleep. 2015; 38: 1229–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Terrill PI, Edwards BA, Nemati S, Butler JP, Owens RL, Eckert DJ, White DP, Malhotra A, Wellman A, Sands SA. Quantifying the ventilatory control contribution to sleep apnoea using polysomnography. Eur Respir J. 2015; 45: 408–18. [DOI] [PMC free article] [PubMed] [Google Scholar]


