Abstract
Resistant and generalized fear are hallmark symptoms of Post-Traumatic Stress Disorder (PTSD). Given PTSD is highly comorbid with addiction disorders indicates a maladaptive interaction between fear and reward circuits. To investigate learning processes underlying fear, reward and safety, we trained male rats to discriminate among a fear cue paired with footshock, a reward cue paired with sucrose and an explicit safety cue co-occurring with the fear cue in which no footshocks were delivered. In an attempt to emulate aspects of PTSD, we pre-exposed male rats to a stressor (15 unsignaled footshocks) before training them to fear, reward and safety cues, and subsequent fear and reward extinction. Prior stress did not produce any significant impairments on conditioned inhibition to a safety cue compared to non-stressed controls. However, in subsequent fear extinction, prior stress profoundly impaired fear reduction to an extinguished fear cue. Prior stress also significantly reduced reward seeking to a reward-associated cue throughout training. Together, our data show that prior stress did not affect conditioned inhibition of fear to the same extent as impairing fear extinction. These results have interesting implications on how safety circuits are organized and impacted by stress, leading to possibly new avenues of research on mechanisms of stress disorders, such as PTSD.
Keywords: Stress, Conditioned inhibition, Extinction, Safety
1. Introduction
Persistent alterations to defensive and approach behaviors can occur after a traumatic event, interfering with daily activities and well-being. Post-traumatic stress disorder (PTSD) develops in up to 20 % of individuals exposed to a trauma [1]. Individuals with PTSD display elevated defensive behaviors resistant to extinction/exposure therapy (reviewed in [2]), and to the fear-reducing effects of safety cues [3]. Many of these individuals also show comorbidity with a substance-use disorder [4]; these individuals also seem particularly resistant to treatment [5]. Thus, the ability to adaptively regulate fear, safety and reward behaviors can be strongly influenced by stress.
Behavioral therapy for anxiety and addiction disorders often involves repeated unreinforced exposures to the fear- or reward-related cue, a procedure known as extinction. Extinction of a fear-related cue is related to but distinct from safety conditioning, a form of conditioned inhibition (reviewed in [6,7]). During conditioned inhibition training, a safety cue in conjunction with a fear cue signifies no aversive outcome whereas the fear cue on its own does result in an aversive outcome. If a safety cue can inhibit the excitatory fear response elicited by a fear cue and displays retarded acquisition of aversive valence when later paired with the aversive outcome, it has passed both the summation and retardation of acquisition tests, respectively, and can be classified as a conditioned inhibitor [8].
Our previous studies [9-13] have shown male Long Evans rats readily learned to suppress fear responding to a learned fear cue if in the presence of a safety cue. They also did not develop a fear response to the safety cue when it was later paired with shock [9], thus classifying the safety cue as a conditioned inhibitor. Using this paradigm, we have shown this conditioned inhibition of fear requires the infralimbic region of the prefrontal cortex (IL) [10], is disrupted when D1-receptor mediated dopamine signaling in the basolateral amygdala is altered [12], and that individual neurons in the basal amygdala display safety-specific responding [9]. We have also shown that a subset of safety-specific amygdala neurons became responsive to the fear cue upon fear extinction as fear behavior decreased [14]. However, we have yet to explore the effects of a prior stressor on this adaptive behavior. In an attempt to emulate aspects of PTSD, we exposed male rats to a stressor before training them to fear, reward and safety cues, and subsequent fear and reward extinction.
One model, stress-enhanced fear learning (SEFL), has been shown to accelerate fear learning that is also resistant to extinction [15-17], modeling key symptoms seen in PTSD. SEFL has also been shown to produce long-lasting nonassociative sensitization resulting in fear learning to a conditioning procedure that normally does not produce a fear memory [18]. Moreover, SEFL has been shown to increase voluntary alcohol intake [19], making it a potentially useful tool to interrogate mechanisms of anxiety and addiction comorbidity. Due to the demonstrated effects this paradigm has on fear and reward-seeking expression, we utilized this procedure in assessing how prior stress can affect conditioned inhibition of fear and fear extinction within the same animal. Our data show that prior stress did not affect conditioned inhibition of fear but did severely impair fear extinction, while also reducing reward seeking.
2. Materials and methods
2.1. Subjects
A total of 36 adult male Long Evans rats (Blue Spruce; Envigo, Indianapolis), were single-housed and handled for 1 week prior to testing. Female rats were not used in the present study because we have previously shown that females, in this paradigm, did not show conditioned inhibition of fear or fear extinction without any prior stress [13]. All procedures were performed during the light cycle and approved by the Purdue Animal Care and Use Committee. Rats had ad libitum access to food and water throughout.
2.2. Behavioral apparatus
Training chambers were Plexiglas boxes (32 cm length x25 cm width x30 cm height) encased in sound-attenuating chambers (Med Associates, ST Albans, VT). 10 % liquid sucrose was delivered through a recessed port in the center of one wall. Port entries and exits were monitored through an infrared beam. Two lights (28v, 100 mA) located on either side of the port served as the 20 s continuous light cue. A house light (28v, 100 mA) at the top of the wall opposite the port provided constant illumination in context B. Auditory cues were delivered via a “tweeter” speaker (ENV-224BM) located at the top of the same wall as the port. A grid floor delivered footshocks via a constant current aversive stimulator (ENV-414S). A side-view video camera located on the door of the sound-attenuating cubicle recorded behavior for offline video analyses.
2.3. Behavioral paradigm
2.3.1. Prior stress (context A)
During stress exposure, rats were placed in training chambers for a total of 90 min with the house light off and a vanilla odor present. A Plexiglas wall insert was placed in front of the wall containing the reward port so the rats did not have access to the reward port. Sucrose was not present in the pump lines leading to the reward port so rats could not smell sucrose during this session. Rats assigned to the stress group (n = 18) received 15 unsignaled footshocks (1.0 mA, 0.5 s, ITI 2–2.5 min.) throughout the session. Rats assigned to the control group (n = 18) received no footshocks. Videos were not collected in context A. All subsequent training sessions (reward, habituation, DC and extinction) took place in context B, described below.
2.3.2. Reward and habituation training (context B)
Stress (or associated control) was followed 1d later by a total of 5 reward sessions (R1-R5) administered on separate days in context B. Context B consisted of no specific odor with the house light turned on for the entire session. Access to the reward port was unrestricted and sucrose was present in the pump lines. Each session consisted of 25 paired presentations of a 20 s reward cue with a 3 s delivery of a 10 % sucrose solution (100 μL) into the port accessible to the animals. Sucrose delivery initiated pseudorandomly 10–20 s after reward cue onset. The intertrial interval (ITI) was 90–130 s. After the last reward session, rats received an additional reward session with included an additional 5 trials of the future fear cue, unreinforced, and 5 trials of the future safety cue (ITI, 90–130 s). This session, habituation (HAB), allowed the rats to habituate to the novel cues and reduce any baseline freezing to these cues without producing latent inhibition [9].
2.3.3. Discriminative conditioning (DC) sessions (context B)
One day after habituation, rats received four sessions of discriminative conditioning (DC). Within the DC sessions, rats were exposed to four different trial types: reward cue + sucrose, fear cue + footshock, safety cue, and fear + safety cues. Each session consisted of 15 trials of the reward cue-sucrose pairing (20 s 3 kHz continuous auditory cue + 3 s sucrose delivery initiated 18 s after cue onset), 4 trials of fear cue-footshock pairings (20 s 11 kHz pulsing auditory cue +.45 mA footshock, 0.5 s at cue offset), 15 trials of simultaneous presentation of the 20 s fear and safety cues (11 kHz pulsing auditory cue + 2 lights on the sides of the port) without footshock, and 10 trials of the 20 s safety cue presented alone (2 lights on the sides of the port) without footshock. Trials were presented in a pseudorandom order (ITI 100–140 s). These cues were not counter-balanced for this study; our previous data have showed no differences in learning acquisition among these cues [9,13].
2.3.4. Extinction training & test (context B)
Extinction training was administered one day after the final DC session. This session exposed animals to a total of 40 trials: 20 trials of the 20 s reward cue and 20 trials of the 20 s fear cue without sucrose or footshock delivery (ITI, 90–140 s), presented in a pseudorandom order. The extinction test was administered one day after extinction training and involved a total of 30 presentations of the 4 trial types presented in the DC sessions, each lasting 20 s. Similar to extinction training, the reward and fear cues were unreinforced during the extinction test and consisted of 10 trials of the reward cue, 10 trials of the fear cue, 5 trials of the fear + safety cue, and 5 trials of the safety cue, delivered in a pseudorandom order. The purpose of this test was to a) assess for reward and fear extinction memory and b) assess how extinction training affected responding to the safety cue.
2.4. Data analyses
Fear behavior was assessed offline from videos, by measuring freezing behavior of the rat, defined as complete immobility with the exception of respiratory movements, which is an innate defensive behavior [20,21]. Contextual freezing was calculated by quantifying the total time spent freezing during the first 5 min of reward session 1 (R1) before any cue presentations; data were expressed as percentage of time freezing (+/− SEM). Differences in contextual freezing were assessed with an unpaired t-test between control and stress groups. For cued freezing, the total time spent freezing during the presentation of each 20 s cue was quantified and expressed as percentage of time freezing (+/− SEM). Reward behavior was assessed by measuring the time spent inside the reward port, as well as when the nose of the animal was positioned towards the entrance of the port in response to each 20 s cue. Behavioral scoring was conducted by hand and completed by multiple scorers and Pearson’s correlations of behavioral values between scorers were greater than r = 0.80. Behavioral data were analyzed with two-way repeated measures ANOVAs followed by post hoc Dunnett’s or Sidak’s multiple comparisons tests with GraphPad Prism 7.
3. Results
3.1. Prior stress increased contextual freezing to the novel context
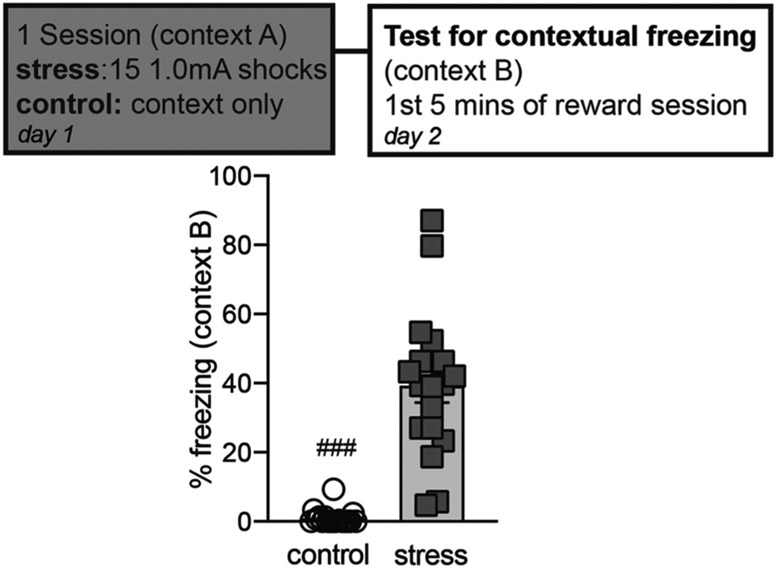
We first tested whether or not this particular stress exposure increased contextual freezing to the novel context. Male rats were either exposed to 15 1.0 mA footshocks in context A (stress, n = 18), or to just the context (control, n = 18) (Fig. 1). One day later rats were placed in a novel context (context B) for the first reward session (R1), and contextual freezing was measured during the first 5 min prior to any cue presentation. Averaged percent time freezing during the first 5 min of R1 was significantly higher in the stress group compared to the control group (unpaired t-test, p < 0.0001). Looking at the individual data points revealed the majority of the stress rats showed 20–60 % freezing to context B. Interestingly some rats showed very high (~80 %) fear generalization to context B and some showed very little (< 10 %).
Fig. 1.
Prior stress elevated contextual freezing levels in a novel context. Rats that were previously exposed to 15 1.0 mA shocks in context A showed significantly elevated contextual freezing levels 1 day later in context B. Control rats were placed in context A without shocks. Averaged percent time freezing for the first 5 min is shown of the first reward session (R1) before any cue presentations. Rats that were previously stressed showed significantly higher contextual freezing than control rats. Means +/− SEM. ###p < 0.0001.
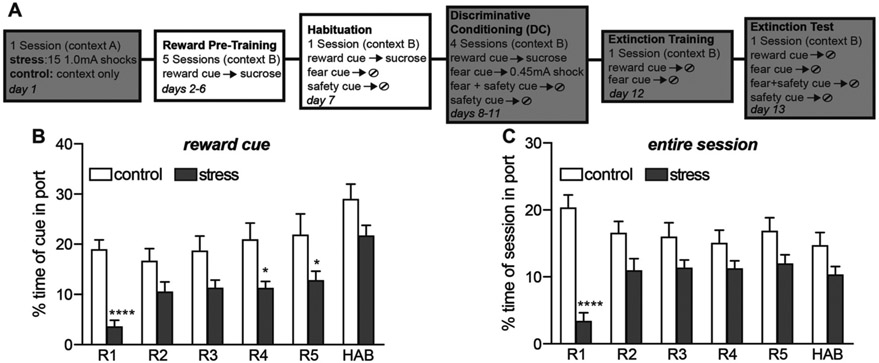
3.2. Prior stress reduced reward seeking during early reward training
One day after the stress, or control, procedure all rats underwent 5 reward training sessions in which the reward cue was paired with sucrose delivery. This was followed by 1 habituation session that included reward cue-sucrose pairings as well as 5 presentations of the future fear and safety cues, one auditory cue and one light cue
The percent time spent at or in the reward port during each reward cue across each reward and habituation session was quantified (Fig. 2B). A two-way repeated measures ANOVA showed a significant stress by session interaction (F(5, 170) = 2.99, p = 0.01) and significant main effects of stress (F(1, 34) = 10.15, p = 0.003) and session (F(5, 170) = 25.61, p < 0.0001). Rats that were previously stressed showed significantly lower reward seeking than controls during the reward cue during sessions R1, R4 and R5 (post hoc Sidak’s: p < 0.0001, p = 0.03, p = 0.04, respectively). The averaged percent time spent in/at the reward port was also calculated for the entire session regardless of cue presentation (Fig. 2C), to assess if reward seeking was also reduced outside of the cue presentation. A two-way repeated measures ANOVA again showed a significant stress by session interaction (F(5, 170) = 20.03, p < 0.0001) and significant main effects of stress (F(1, 34) = 11.22, p = 0.002) and session (F(5, 170) = 2.64, p = 0.03). The stress group showed significantly lower reward seeking during R1 (p < 0.0001), with no significant differences from controls in subsequent sessions. Thus, with the exception of the first reward session (R1, Fig. 2C), the stress group did not show any differences in reward seeking outside of the cue presentation. Taken together, these data indicate prior stress reduced reward seeking during the reward cue, particularly in the first session.
Fig. 2.
Prior stress reduced reward seeking during reward pre-training. (A) Schematic depicting experimental outline. During reward pre-training and habituation sessions, rats received 25 cue-sucrose pairings per session. (B) Averaged percent time spent in the reward port during the reward cue for the five reward pre-training sessions (R1-5) and habituation session (HAB). Rats that were previously stressed showed lower reward seeking during the reward cue. (C) Averaged percent time spent in the reward port for each entire session, regardless of reward cue presentation. Rats that were previously stressed showed significantly lower reward seeking during R1, but then were not significantly different from controls afterwards. Means + SEM. *p < 0.05, ****p < 0.0001, between group comparisons.
In our previous studies [9-13], we included a habituation session where the future fear and safety cues were presented 5 times each to reduce any baseline freezing that might occur to the novel cues before discriminative conditioning. To be consistent with our prior work, we included a habituation session in the current study. During habituation, the percent time spent at or in the reward port, and the percent time freezing, were analyzed during the presentation of the future fear and safety cues. Two-way repeated measures ANOVAs on port behavior and freezing showed significant stress by cue interactions for both behaviors (F(1, 30) = 10.27, p = 0.003, F(1, 30) = 6.82, p = 0.01, respectively) and main effects of stress (F(1, 30) = 6.13, p = 0.02) and cue (F(1, 30) = 41.01, p < 0.0001) for port behavior. Both groups showed higher port behavior to the auditory cue than the light cue (post hoc Sidak’s: controls, p < 0.0001; stress, p = 0.04), likely reflecting that the reward cue was also an auditory cue. There were no significant differences in freezing levels to the auditory cue versus light cue within the control (p = 0.22) or stress (p = 0.08) group.
3.3. Prior stress reduced reward seeking during discriminative conditioning
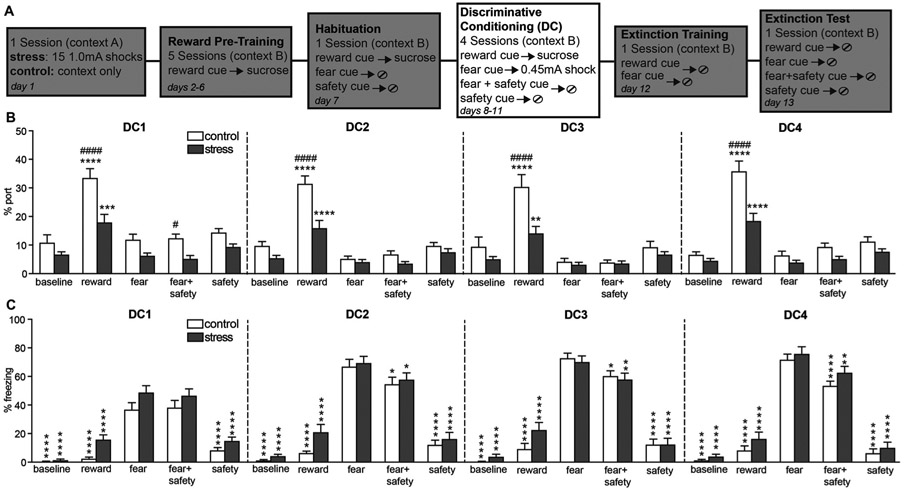
During each of the 4 discriminative conditioning sessions, rats were presented with four types of cued trials: reward cue-sucrose, fear cue-shock, fear + safety cue with no footshock, and the safety cue presented alone without footshock. Again, time spent in/at the port was quantified to assess reward seeking (Fig. 3B).
Fig. 3.
Prior stress reduced reward seeking during discriminative conditioning but did not affect conditioned inhibition of freezing. (A) Schematic depicting experimental outline. During the 4 DC sessions, rats were presented with four types of cued trials: reward cue-sucrose, fear cue-shock, fear + sfety cue with no footshock, and the safety cue presented alone without footshock. (B) Averaged percent time spent in the port during each cue across the 4 DC sessions, as well as a 5 min baseline (BL) period at the beginning of each session. Both groups showed significantly higher reward seeking to the reward cue compared to all other cues. However, rats that were previously stressed showed significantly lower reward seeking during the reward cue compared to controls. Means + SEM. #p < 0.05, ####p < 0.0001 within cue, between group comparison. **p < 0.01, ***p < 0.001, ****p < 0.0001 within group, between cue comparison. (C) Averaged percent time spent freezing during each cue across the 4 DC sessions, as well as a 5 min baseline (BL) period at the beginning of each session. During DC2-4 both groups showed significantly higher freezing to the fear cue over all other cues. *p < 0.05, **p < 0.01, ****p < 0.0001 within group, compared to fear cue. Means + SEM.
Two-way repeated measures ANOVAs on percent time in/at the port during each cue and 5 min cue-free baseline (BL) period for each of the 4 discriminative conditioning (DC) sessions, showed significant stress by cue interactions for each session, and significant main effects of stress for each session and cue for each session (Table 1). Both groups showed significantly higher reward seeking to the reward cue compared to all other cues in every DC session (post hoc Dunnett’s multiple comparisons test, DC1-4 p < 0.0001). However, the stress group showed significantly lower reward seeking during the reward cue compared to the control group in every DC session (post hoc Sidak’s multiple comparisons test: DC1 p < 0.001, DC2 p < 0.0001, DC3 p < 0.01, DC4 p < 0.0001).
Table 1.
Summary of two-way repeated-measures ANOVA analyses for reward seeking and freezing behaviors during the four discriminative conditioning (DC) sessions.
| Reward seeking | |||
|---|---|---|---|
| Session | Cue x group effects | Main effect of cue | Main effect of group |
| DC1 | F(4,136) = 4.91, p = 0.001 | F(4,136) = 49.03,p < 0.0001 | F(1,34) = 14.70, p = 0.0005 |
| DC2 | F(4,136) = 10.81, p < 0.0001 | F(4,136) = 78.34, p < 0.0001 | F(1,34) = 13.08,p = 0.001 |
| DC3* | F(4,116) = 7.62, p < 0.0001 | F(4,116) = 42.01, p < 0.0001 | F(1,29) = 8.24, p = 0.008 |
| DC4** | F(4,128) = 12.08, p < 0.0001 | F(4,128) = 99.30, p < 0.0001 | F(1,32) = 11.86, p = 0.002 |
| Freezing | |||
| Session | Cue x group effects | Main effect of cue | Main effect of group |
| DC1 | F(4,136) = 1.82, p = 0.13 | F(4,136) = 120.00, p < 0.0001 | F(1,34) = 5.65, p = 0.02 |
| DC2 | F(4,136) = 1.47, p = 0.21 | F(4,136) = 189.10, p < 0.0001 | F(1,34) = 1.88, p = 0.18 |
| DC3* | F(4,116) = 2.32, p = 0.06 | F(4,116) = 204.00, p < 0.0001 | F(1,29) = 0.34 p = 0.57 |
| DC4** | F(4,128) = 0.56, p = 0.69 | F(4,128) = 283.30, p < 0.0001 | F(1,32) = 2.17, p = 0.15 |
video files for 5 control animals were corrupted for this session (n = 13 controls, n = 18 trauma).
video files for 2 control animals were corrupted for this session (n = 16 controls, n = 18 trauma).
These data show that the reduced levels of reward seeking in the stress group that was seen in reward pre-training not only persisted into discriminative conditioning sessions but became even more pronounced, likely to the presence of fear cue-shock trials. Despite the lower levels of reward seeking, the stress group still showed significantly higher reward seeking during the reward cue compared to the other cues, indicating intact reward discrimination.
3.4. Prior stress did not affect conditioned inhibition of freezing
During each of the discriminative conditioning sessions, freezing levels were also assessed during each cue (Fig. 3C). Two-way repeated measures ANOVAs on percent freezing during each cue and 5 min cue-free baseline (BL) period for each of the 4 discriminative conditioning (DC) sessions, did not show significant stress by cue interactions for any session but did show main effects of cue for every session (Table 1). There was also a main effect of stress during DC1 (Table 1); the stress group showed higher freezing levels compared to the control group. During DC2-4 both groups showed higher freezing to the fear cue over all other cues (post hoc Dunnett’s multiple comparisons tests, p < 0.05), indicating the prior stress did not affect conditioned inhibition of freezing to the fear cue in the presence of a safety cue.
These data suggest that prior stress did not significantly impair the ability to suppress fear in response to a safety cue or reward discrimination. Because conditioned inhibition of fear was not significantly impaired, we also examined how fear reduction via fear extinction would be affected.
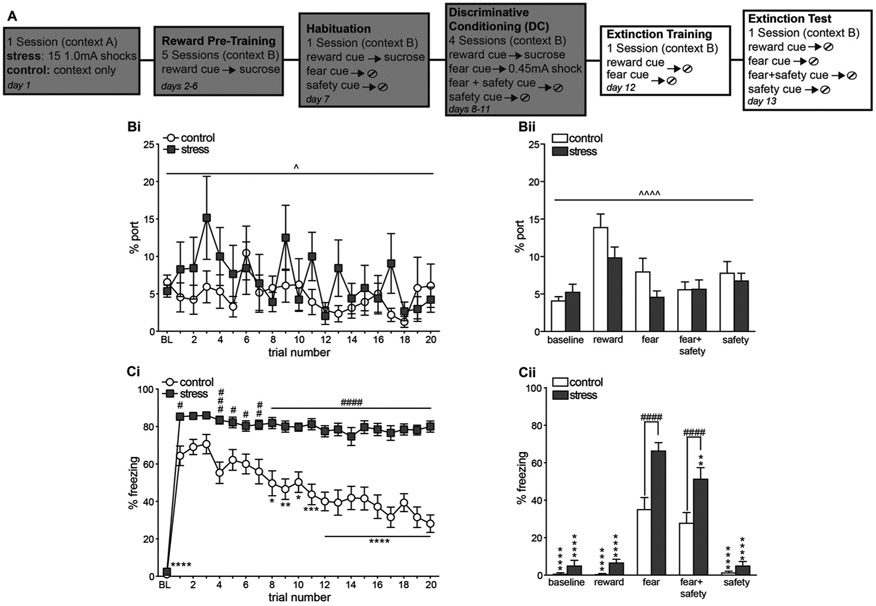
3.5. Prior stress significantly impaired subsequent fear extinction
One day after DC4 all rats received 1 session of extinction training in which both the fear and reward cues were extinguished within the same session (Fig. 4Bi, Ci). One day later all rats received an extinction test where all cues were presented (Fig. 4Bii, Cii).
Fig. 4.
Prior stress impaired subsequent fear extinction. (A) Schematic depicting experimental outline. One day after DC4 all rats received 1 session of extinction training in which both the fear and reward cues were extinguished within the same session. One day later all rats received an extinction test where all cues were presented. (Bi) Averaged percent time spent in the port during each reward cue presentation during extinction training as well as a 5 min baseline (BL) period at the beginning of the session. Main effect of trial ^p < 0.05. (Bii) Averaged percent time spent in the port during each cue 1 day after extinction training. Main effect of cue ^^^^p < 0.0001. (Ci) Averaged percent time spent freezing during each fear cue presentation during extinction training as well as a 5 min baseline (BL) period at the beginning of the session. Compared to trial 1, control animals showed significantly reduced freezing during trials 8–20 (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001). Freezing levels in the stress group did not significantly decrease at any time point. The stress group also showed significantly higher freezing levels than the control group for every trial except trials 2 and 3 (#p < 0.05, ##p < 0.01, ###p < 0.001, ####p < 0.0001). (Cii) Averaged percent time spent freezing during each cue 1 day after extinction training. Freezing in the stress group was significantly higher than controls during the fear and fear + safety cues (####p < 0.0001). The stress group also showed significantly lower freezing to all cues compared to the fear cue (**p < 0.01, ****p < 0.0001), indicating intact conditioned inhibition. Means +/− SEM.
A two-way repeated measures ANOVA on percent time spent in/at the port during each reward cue trial during extinction training, as well as a 5 min baseline (BL) period at the beginning of the session, did not show a significant stress by trial interaction (F(20,600) = 1.36, p = 0.14) or main effect of stress (F(1,30) = 0.80, p = 0.38), but did show a main effect of trial (F(20,600) = 1.87, p = 0.01). Overall there were no differences in reward seeking between the stress and control groups during extinction training (Fig. 4). For the extinction test one day later a two-way repeated measures ANOVA on percent time spent in/at the port during each cue and BL period did not show a significant stress by trial interaction (F(4,120) = 2.30, p = 0.06) or main effect of stress (F(1,30) = 1.66, p = 0.21). However, there was a main effect of cue (F(4,120) = 14.69, p < 0.0001); there was more reward seeking during the reward cue compared to the other cues and BL.
During extinction training, a significant stress by trial interaction (F(20,600) = 7.21, p < 0.0001), and main effects of stress (F(1,30) = 52.13, p < 0.0001) and trial (F(20,600) = 46.71, p < 0.0001), were found on percent time freezing during each fear cue trial and 5 min baseline (BL) period. Compared to trial 1, control animals showed significantly reduced freezing during trials 8–20 (post hoc Dunnett’s: trials 8 and 10, p < 0.05; trial 9, p < 0.01; trial 11, p < 0.001; trials 12–20, p < 0.0001), as well as compared to BL (p < 0.0001). Compared to trial 1, the BL of the stress and control groups were significantly lower (p < 0.0001). However, freezing levels in the stress group did not significantly decrease at any time point after trial 1, demonstrating a lack of fear extinction. The stress group also showed significantly higher freezing levels than the control group for every trial except trials 2 and 3 (post hoc Bonferroni’s: trials 1, 5 and 6, p < 0.05; trial 7, p < 0.01; trial 4, p < 0.001; trials 8–20, p < 0.0001). For the extinction test one day later, a significant stress by cue interaction (F(4, 120) = 9.27, p < 0.0001), and main effects of cue (F(4, 120) = 124.4, p < 0.0001) and stress (F(1,30) = 15.33, p = 0.0005) were found on the percent time freezing during each cue and BL period. Freezing was significantly higher in the stress group compared to the control group during the fear (p < 0.0001) and fear + safety (p < 0.0001) cues. The control group showed comparable freezing levels to the fear and fear + safety cues, and freezing to the fear cue was significantly higher than BL, and during the reward and safety cues (post hoc Dunnett’s, p < 0.0001). In the stress group, freezing was significantly higher to the fear cue than BL (p < 0.0001), reward cue (p < 0.0001, fear + safety cue (p < 0.01), and safety cue (p < 0.0001). Thus, even though extinction of freezing to the fear cue was not apparent in the stress group, there was significant inhibition of freezing during the fear + safety cue, indicating intact conditioned inhibition.
4. Discussion
In this study, we investigated the effect of prior stress on reward seeking, conditioned inhibition of fear and fear extinction in a paradigm that includes cued reward, fear and safety trials. Overall, we found prior stress greatly reduced reward seeking early in reward pre-training (R1) and throughout the discriminative conditioning (DC) sessions. Interestingly, we found no effect of prior stress on conditioned inhibition of fear in response to a safety cue but a clear impairment on extinction of cued fear. During a test for fear extinction, animals with prior stress still showed high levels to the fear cue and, interestingly, still showed significant suppression of fear to the fear cue when in the presence of the safety cue. Together, our data show that prior stress did not affect conditioned inhibition of fear but did severely impair fear extinction.
Based on prior literature using this particular stress exposure, termed ‘stress-enhanced fear learning (SEFL)’ [15,16,18,22], we expected to observe a failure to downregulate freezing in response to the fear cue when in the presence of the safety cue; i.e. a lack of conditioned inhibition. Most of the prior research using SEFL to investigate alterations in fear regulation have done so by assessing its impact on contextual fear conditioning (reviewed in [17], whereas our study assessed cue-induced fear during conditioned inhibition and extinction. Most striking in our results was the reduction in freezing to the fear cue when the safety cue was also present, but the absolute lack of reduced freezing to the fear cue across extinction in the prior stress group. We hypothesize that animals that received the prior stress can effectively learn about explicit safety cues that are always associated with safety, as was the case in our study. However, they are not as adept at learning to modify their fear response to an already learned fear cue, resulting in the lack of fear extinction.
This dissociation between conditioned inhibition of fear and fear extinction after prior stress is potentially very interesting. To our knowledge, no other study has compared these two types of inhibitory learning against each other. In our paradigm, one situation ties an explicit cue (safety cue) with an expected outcome (no footshock), but in the other, the expectancy needs to be updated in response to extinction training, from fear cue predicting shock to fear cue predicting no shock. These data do seem to be at odds with findings in individuals with PTSD that do not show conditioned inhibition when a fear and safety cue are co-presented [3]. One obvious difference between the clinical data and ours is the age of the trauma/stress memory. Individuals diagnosed with PTSD often do not receive treatment until many weeks after the trauma, sometimes years [23]. Perhaps our conditioned inhibition results would differ if we increased the time between the stress exposure and the beginning of reward training. Regardless, it is very striking that on one day the prior stress group showed reduced freezing to the safety cue but on the very next day they did not show any reduction of freezing during extinction of the fear cue. Then one day after this extinction training, they once again showed reduced freezing to the fear + safety cue but an unextinguished fear response to the fear cue. This demonstrates that age of the trauma memory alone cannot explain the differences seen in our study.
Not only did prior stress not affect freezing to the fear + safety cue, it also had no observable effect on fear versus safety cue discrimination. Several groups study safety learning by assessing how well an animal discriminates between a fear cue (CS+) and safety cue (CS−) (reviewed in [7]). It is important to note that a safety cue that is well discriminated from a fear cue does not necessarily guarantee that the safety cue will act as a conditioned inhibitor. This can only be tested by directly pitting it against a fear cue to determine if the fear elicited by the fear cue is inhibited by the safety cue, like we tested here. To reinforce this point, individuals with PTSD have been shown to discriminate between fear and safety cues presented separately, but fail to inhibit their fear potentiated startle response when they are presented in compound [24].
Prior stress also had interesting implications for reward seeking behavior, in which it was significantly reduced during the first reward session (R1) and then increased to almost match controls by the fifth reward session (R5). During the stress session, the reward port was physically blocked off with no sucrose present, preventing rats from associating reward-relevant contextual cues with the stress context. However, there was clear generalization of fear to context B in most of the animals, as seen during the first 5 min of the first reward session (Fig. 1), which may have conflicted with approach behavior to the reward port, impeding reward acquisition. Alternatively, or in parallel, the generalized fear in the stress group may be itself serving as an aversive unconditioned stimulus that then becomes associated with the appetitive sucrose reward being presented during the reward sessions; this second-order type of conditioning effect would then reduce, or conflict with, the rewarding properties of the sucrose and affect approach behavior. As the 5 reward sessions progressed, animals with prior stress may have learned to increase their reward seeking behavior as they concurrently learned these reward sessions did not contain footshocks. This in of itself may suggest that extinction of generalized fear that was induced by the novel context was occurring in these animals. Then, in response to DC1, with the introduction of cued footshocks, reward seeking once again decreased for all DC sessions in animals that received prior stress. So, despite showing unaffected conditioned inhibition, animals with prior stress did show reduced reward seeking during the same sessions, indicating their behavior was negatively affected.
Patients suffering from PTSD are impaired in both fear extinction and safety conditioning (reviewed in [2]). In an attempt to emulate aspects of PTSD in humans, the present study utilized the SEFL procedure to expose male rats to unsignaled footshocks prior to training them to discriminate among fear, reward and safety cues followed by fear and reward extinction. Our data showed that prior stress impaired fear extinction but not conditioned inhibition. Since these are two types of inhibitory learning, these results have interesting implications on how safety circuits are organized and impacted by stress, leading to possible new avenues of research on mechanisms and treatment options for stress disorders, such as PTSD.
Acknowledgements
We thank Yolanda Jonker and Signe Hobaugh for excellent animal care. This work was supported by NIMHR01MH110425 to SS.
Footnotes
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.bbr.2019.112414.
References
- [1].Kilpatrick DG, Resnick HS, Milanak ME, Miller MW, Keyes KM, Friedman MJ, National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria, J. Trauma Stress 26 (2013) 537–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Zuj DV, Norrholm SD, The clinical applications and practical relevance of human conditioning paradigms for posttraumatic stress disorder, Prog. Neuropsychopharmacol. Biol. Psychiatry 88 (2019) 339–351. [DOI] [PubMed] [Google Scholar]
- [3].Jovanovic T, Kazama A, Bachevalier J, Davis M, Impaired safety signal learning may be a biomarker of PTSD, Neuropharmacology 62 (2012) 695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE, Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication, Arch. Gen. Psychiatry 62 (2005) 617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Blanco C, Xu Y, Brady K, Pérez-Fuentes G, Okuda M, Wang S, Comorbidity of posttraumatic stress disorder with alcohol dependence among US adults: results from National Epidemiological Survey on alcohol and related conditions, Drug Alcohol Depend. 132 (2013) 630–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Christianson JP, Fernando ABP, Kazama AM, Jovanovic T, Ostroff LE, Sangha S, Inhibition of fear by learned safety signals: a mini-symposium review, J. Neurosci 32 (2012) 14118–14124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sangha S, Diehl MM, Bergstrom HC, Drew MR, Know safety, no fear, Neurosci. Biobehav. Rev (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Rescorla RA, Pavlovian conditioned inhibition, Psychol. Bull 72 (1969) 77–94. [Google Scholar]
- [9].Sangha S, Chadick JZ, Janak PH, Safety encoding in the basal amygdala, J. Neurosci 33 (2013) 3744–3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Sangha S, Robinson PD, Greba Q, Davies DA, Howland JG, Alterations in reward, fear and safety cue discrimination after inactivation of the rat prelimbic and infralimbic cortices, Neuropsychopharmacology 39 (2014) 2405–2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sangha S, Greba Q, Robinson PD, Ballendine SA, Howland JG, Heightened fear in response to a safety cue and extinguished fear cue in a rat model of maternal immune activation, Front. Behav. Neurosci 8 (2014) 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ng KH, Pollock MW, Urbanczyk PJ, Sangha S, Altering D1 receptor activity in the basolateral amygdala impairs fear suppression during a safety cue, Neurobiol. Learn. Mem 147 (2018) 26–34. [DOI] [PubMed] [Google Scholar]
- [13].Greiner EM, Müller I, Norris MR, Ng KH, Sangha S, Sex differences in fear regulation and reward-seeking behaviors in a fear-safety-reward discrimination task, Behav. Brain Res 368 (2019) 111903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sangha S, Plasticity of fear and safety neurons of the amygdala in response to fear extinction, Front. Behav. Neurosci 9 (2015) 354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Rau V, Fanselow MS, Exposure to a stressor produces a long lasting enhancement of fear learning in rats, Stress 12 (2009) 125–133. [DOI] [PubMed] [Google Scholar]
- [16].Long VA, Fanselow MS, Stress-enhanced fear learning in rats is resistant to the effects of immediate massed extinction, Stress 15 (2012) 627–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Conoscenti MA, Fanselow MS, Dissociation in effective treatment and behavioral phenotype between stress-enhanced fear learning and learned helplessness, Front. Behav. Neurosci 13 (2019) 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Poulos AM, Zhuravka I, Long V, Gannam C, Fanselow M, Sensitization of fear learning to mild unconditional stimuli in male and female rats, Behav. Neurosci 129 (2015) 62–67. [DOI] [PubMed] [Google Scholar]
- [19].Meyer EM, Long V, Fanselow MS, Spigelman I, Stress increases voluntary alcohol intake, but does not alter established drinking habits in a rat model of posttraumatic stress disorder, Alcohol. Clin. Exp. Res 37 (2013) 566–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Blanchard RJ, Blanchard DC, Crouching as an index of fear, J. Comp. Physiol. Psychol 67 (1969) 370–375. [DOI] [PubMed] [Google Scholar]
- [21].Fendt M, Fanselow MS, The neuroanatomical and neurochemical basis of conditioned fear, Neurosci. Biobehav. Rev 23 (1999) 743–760. [DOI] [PubMed] [Google Scholar]
- [22].Perusini JN, Meyer EM, Long VA, Rau V, Nocera N, Avershal J, Maksymetz J, Spigelman I, Fanselow MS, Induction and expression of fear sensitization caused by acute traumatic stress, Neuropsychopharmacology 41 (2016) 45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Goldberg SB, Simpson TL, Lehavot K, Katon JG, Chen JA, Glass JE, Schnurr PP, Sayer NA, Fortney JC, Mental health treatment delay: a comparison among civilians and veterans of different service eras, Psychiatr. Serv 70 (2019) 358–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Jovanovic T, Sakoman AJ, Kozarić-Kovačić D, Meštrović AH, Duncan EJ, Davis M, Norrholm SD, Acute stress disorder versus chronic posttraumatic stress disorder: inhibition of fear as a function of time since trauma, Depress. Anxiety 30 (2013) 217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]