The predictive ability of metabolic profiling to detect obesity-induced perturbations in metabolism has not been clearly established. Complex aetiologies interacting with environmental factors highlight the need to understand how specific manipulations alter metabolite profiles in this state. The aim of this study was to determine if targeted metabolomic profiling could be employed as a reliable tool to detect dietary-induced insulin resistance in a small subset of experimental animals (n = 10/treatment). Following weaning, male C57BL/6J littermates were randomly divided into two dietary groups: chow and high fat. Following 12 weeks of dietary manipulation, mice were fasted for 5 h prior to serum collection. The resultant high fat-fed animals were obese and insulin resistant as shown by a euglycaemic-hyperinsulinaemic clamp. Sera were analysed by proton nuclear magnetic resonance spectroscopy, and 46 known compounds were identified and quantified. Multivariate analysis by orthogonal partial least squares discriminant analysis, a projection method for class separation, was then used to establish models of each treatment. Models were able to predict class separation between diets with 90% accuracy. Variable importance plots revealed the most important metabolites in this discrimination to include lysine, glycine, citrate, leucine, suberate and acetate. These metabolites are involved in energy metabolism and may be representative of the perturbations taking place with insulin resistance. Results show metabolomics to reliably describe the metabolic effects of insulin resistance in a small subset of samples and are an initial step in establishing metabolomics as a tool to understand the biochemical signature of insulin resistance.
Introduction
Metabolic profiling or ‘metabolomics’ describes the identification and quantification of numerous small molecular weight compounds in biological fluid samples. To date, the ability of metabolomics to predict nutritional responsiveness and chronic disease states is largely debatable because of the variability and complexity of data sets [1,2]. Diseases such as obesity, diabetes and cardiovascular disease not only involve multiple genes but also are strongly influenced by environmental factors including diet and exercise. In addition, factors such as age, sex and ethnicity are known to influence metabolomic profiles [3–5]. As such, there is an acute need to generate metabolomic data in highly controlled experimental models to determine the effects of specific manipulations on proton nuclear magnetic resonance spectroscopy profiles.
The aim of the present study was to determine whether metabolomics can be used as a tool to detect whole body insulin resistance from a single serum sample. As human samples are highly variable, the C57BL/6J laboratory mouse was chosen as an experimental model. When fed a high-fat diet, the C57BL/6J mouse became obese and insulin resistant compared with chow-fed mice of the same strain [6]. As insulin resistance is dietary induced over a prolonged period of time, this model shares many similarities with human insulin resistance and type 2 diabetes. Common features include obesity, hyper-insulinaemia, hyperlipidaemia and mild hypertension. These similarities combined with genetic homology and strict control over environmental conditions make this model ideal for exploring the influence of dietary- induced insulin resistance on metabolic profiling. In addition, a small subset of animals were examined and utilized for data generation as this most closely reflects the numbers used in laboratory experiments. This detail is important if metabolomics is to be used in a phenotyping capacity for the characterization of mutant and transgenic mouse models of human disease.
Employing 1H-NMR spectroscopy to analyse serum in control and insulin-resistant animals, we show targeted profiling of global spectra to be clearly distinguishable. In addition, results show that both body mass and diet class are important determinants of metabolite profiles in insulin resistance. Such data are the initial steps in establishing and validating metabolomics as a tool to characterize and understand the biochemical signature of insulin resistance.
Experimental Procedures
Mouse Maintenance
Procedures were approved by the University of Calgary Animal Care and Use Committee and abide by the Canadian Association for Laboratory Animal Science guidelines for animal experimentation. Insulin clamp experiments were approved by the Vanderbilt University Animal Care and Use Committee. Animals were maintained in a humidity-controlled room with a 12-h light: dark cycle. Following weaning (3 weeks), male C57BL/6J littermates were randomly segregated into two groups and maintained in microisolator cages for 1 week. Following this acclimatization period, animals received either chow or high-fat diet for 12 weeks (58R3 -Testdiet; Purina, Richmond, IN, USA). Energy density (%kcal/g) for chow and high-fat diets was 23% protein, 21% fat and 55% carbohydrate and 15% protein, 59% fat and 26% carbohydrate respectively.
Animal Experimentation
At the end of the dietary period, animals (n = 10/treatment) were fasted for 5 h prior to being anaesthetized with pentobarbital and weighed. Whole blood (~1 ml) was obtained by a cardiac puncture and placed on ice and allowed to clot for 30 min. Samples were then centrifuged for 10 min at 1000 g and sera collected prior to storage at −80 °C. Blood glucose was assessed in anaesthetized mice (One Touch; LifeScan, British Columbia, Canada). Non-esterified fatty acids (NEFA) were measured spectrophotometrically (Wako NEFA C kit; Wako Chemicals, Richmond, VA, USA). Immunoreactive insulin was assayed with a double antibody method [7]. Abundant data on chow and high fat-fed C57BL/6J mouse have been previously published [6,8–10]. Insulin sensitivity as assessed by in vivo, euglycaemic-hyper- insulinaemic clamp (n = 16/treatment, chow and high fat) are previously described [9]. Briefly, clamps were conducted following a postoperative recovery period of ~5 days. The recovery period was a sufficient time for body weight to be restored within 10% of presurgery body weight. On the day of the study, conscious and unrestrained mice were placed in a 1-l plastic container lined with bedding and fasted for 5 h before an experiment; Micro-Renathane (0.033 OD) tubing was connected to the catheter leads and infusion syringes. Following this, a baseline (t = −90 min) arterial blood sample (150 μl) was drawn for the measurement of arterial blood glucose, haematocrit, plasma insulin and NEFA. The remaining erythrocytes were washed with 0.9% heparinized saline and reinfused. Mice were then infused with insulin (4 mU/kg/min). To maintain glycae- mia during insulin experiments, arterial blood glucose (5 μl; HemoCue, Lake Forest, CA, USA) was measured at ~10-min intervals and glucose (50%) administered into the venous catheter. Mice also received saline-washed erythrocytes from a donor mouse as needed in order to maintain haematocrit within 5% of incoming haematocrit.
Metabolite Sample Preparation
Serum samples were thawed and filtered twice using 3- kDa Nanosep microcentrifuge filters, prewashed to reduce contamination. The filtrate was transferred to clean microfuge tubes; the final sample volume ranged from 100 to 400 μl. Samples were brought to 650 ml by addition of D20, 140 μl of phosphate buffer containing dimethyl-silapentane-sulphonate (DSS, final concentration 0.5 mM) and 40 μl of sodium azide. Final sample pH was adjusted to 7 ± 0.2.
Spectrum Acquisition
One-dimensional nuclear overhauser effect spectroscopy (NOESY) spectra were acquired using an automated NMR Case sample changer on a 600-MHz Bruker Ultrashield spectrometer. The NOESY pulse sequence had a mixing time of 100 min and a water presaturation pulse. Initial samples for each batch were shimmed to ensure half-height linewidth of ~1.1 Hz for the DSS peak at 0.0 ppm. Spectra were acquired with 256 scans (batch 1) or 768 scans (batch 2), then zero filled and Fourier transformed to 32 k or 64 k points. Baseline correction was performed manually using a spline function.
Sample Fitting
Processed spectra were imported into Chenomx software (version 4.6) for quantification. For some samples, additional preprocessing was required in Chenomx Processor, in the form of a reference deconvolution, water region deletion and/or additional baseline correction. In total, 46 compounds were quantified from each spectra, for a total of 1000 individual measurements. Three test spectra were randomly chosen and concentrations for each compound were averaged over the three test profiles. These averages were used as the starting concentration vector for fitting or refitting all the acquired spectra (including the three used for the previous spectra). All 20 spectra were randomly ordered (within acquisition batches) for fitting in Chenomx Profiler. For each spectrum, the 46 compounds were sorted by decreasing concentration and then fit for concentration and translation in that order. The three test profiles were compared with the corresponding profiles to test for consistency. Each compound concentration was then normalized by dividing the measured concentration into the total concentration of all metabolites in that sample (excluding glucose and lactate because of excessively large volumes which otherwise dominate the normalization) using a PERL script.
Statistical Analysis
For measures of body mass, blood glucose, NEFA and insulin sensitivity, a two-way ANOVA was performed to detect statistical differences (p < 0.05). Differences within the ANOVA were determined using Tukey’s post hoc test. All data are reported as means ± s.e. Urea and propylene glycol were excluded from the data set because of difficulty in quantification and contamination by experimental methods respectively.
In addition to univariate tests, multivariate analysis was conducted using SIMCA-P software (Umetrics, Sweden) to better assess the concentration changes. A supervised orthogonal partial least squares discriminant analysis (OPLS-DA) approach was chosen (model 1 - diet). This allows for a direct comparison of the variance between diet type (y variable) and metabolite concentrations (x variable) [11]. Additional modelling was performed by OPLS to examine variation of body mass (y variable) in relation to metabolite concentrations (x variable) (model 2 - mass).
Validation
The accuracy of each model was tested with three established methods. Leave-some-out testing is a process whereby seven ‘partial models’ are built, each based on a different subset of samples. The (average) ability of the submodels to predict the diet (or mass) of the excluded animals gives a measure of the original model’s strength. Secondly, the models were tested by randomly shuffling the y variables and building alternate models based on this partially incorrect data.
The strongest form of model validation is external validation (EV) using entirely separate samples. Ten additional samples were acquired using the same diet and acquisition protocol. The spectra were profiled in random order without knowledge of diet or mass and then imported into SIMCA as a secondary data set. The y values of the EV samples were then predicted using model 1 - diet and model 2 - mass, which is indicative of each experiment’s reproducibility.
Results
Animal Characteristics
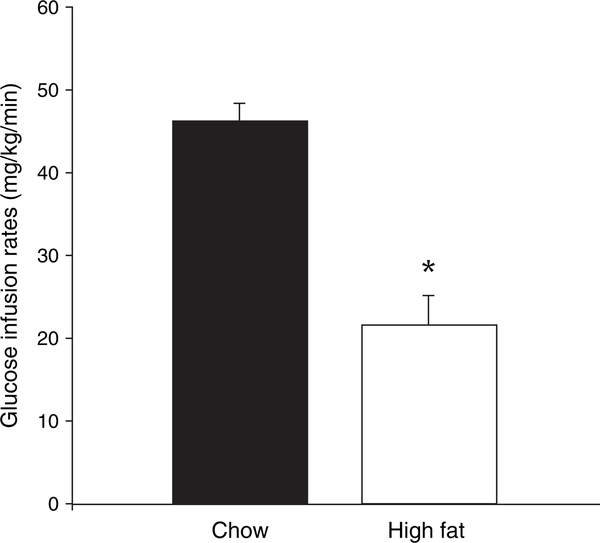
Following dietary manipulation, high fat-fed animals became obese with average weights of 30 ± 0.9 g and 41 ± 1.5 g for control and high fat-fed animals respectively (p < 0.05). Fasting blood glucose levels in anaesthetized animals were 11.0 ± 0.5 mM for chow and 14.8 ± 0.8 mM for high fat-fed animals (p < 0.05). Fasting NEFA levels were not different between groups with values of 0.90 ± 0.04 and 1.02 ± 0.05 mM for chow and high fat-fed groups respectively. A separate subset of animals was used for the in vivo, euglycaemic and hyperinsulinaemic clamps. During the clamps, blood glucose did not differ between chow and high fat-fed groups at any time. Baseline insulin levels were 21 ± 10 μU/ml for chow and 68 ± 10 μU/ml for high fat-fed animals (p > 0.05). Average blood glucose during the clamps was 7.4 ± 0.3 and 7.6 ± 0.2 mM for chow and high fat-fed animals respectively (p < 0.05). Glucose infusion rates required to maintain euglycaemia are shown in figure 1. This study and other studies [6,8,10,12,13] showed high-fat feeding in the C57BL/6J mouse to cause significant obesity and insulin resistance, disposing of ~50% less glucose compared with chow-fed animals.
Fig. 1.
Mean glucose infusion rates during a euglycaemic- hyperinsulinaemic clamp (4 mU/mg/min insulin and 7.0 mM glucose clamp with variable infusion) for chow and high fat-fed C57BL/6J mice. *p < 0.05 for chow vs. high fat-fed mice. Data are reported as means ± s.e., n = 16 mice/dietary treatment.
Metabolites
The univariate mean and standard deviation of each compound’s original serum concentration along with the ANOVA p value for significant separation between those compounds in a univariate model (model 1) are shown in table 1. According to the multivariate model, 22 metabolites were higher in the high fat-fed animals (table 1). Of these, only absolute concentrations of citrate were significantly different (univariate p < 0.05). Another 24 metabolites were lower with high-fat feeding (table 2). In this group, glycerine, lysine, suberate, acetate, leucine, valine, trimethylamine N-oxide, hippurate and arginine were significantly lower.
Table 1.
Positive contributors to multivariate analysis
| Metabolite | Control | High fat | p Value |
|---|---|---|---|
| Citrate | 92 ± 12 | 154 ± 30 | 0.011 |
| Citrulline | 19 ± 3 | 24 ± 3 | 0.097 |
| Asparagine | 11 ± 2 | 14 ± 2 | 0.108 |
| Glycerol | 157 ± 35 | 225 ± 49 | 0.115 |
| 3-Hydroxybutyrate | 86 ± 25 | 125 ± 33 | 0.190 |
| Carnitine | 13 ± 1 | 11 ± 1 | 0.234 |
| 2-Hydroxybutyrate | 12 ± 2 | 19 ± 8 | 0.267 |
| O-Acetylcarnitine | 6 ± 1 | 9 ± 3 | 0.271 |
| Threonine | 60 ± 10 | 69 ± 9 | 0.390 |
| 3-Methyl-2-oxovalerate | 6 ± 2 | 5 ± 1 | 0.549 |
| Aspartate | 9 ± 1 | 8 ± 0 | 0.564 |
| O-Phosphocholine | 4 ± 2 | 6 ± 4 | 0.645 |
| Alanine | 165 ± 21 | 156 ± 19 | 0.667 |
| Serine | 49 ± 8 | 51 ± 8 | 0.750 |
| Glutamine | 233 ± 34 | 242 ± 20 | 0.763 |
| 2-Oxoglutarate | 10 ± 1 | 11 ± 2 | 0.806 |
| Urea | 259 ± 67 | 273 ± 66 | 0.826 |
| Taurine | 361 ± 70 | 374 ± 102 | 0.879 |
| Nicotinate | 4 ± 1 | 4 ± 1 | 0.883 |
| Methionine | 13 ± 1 | 13 ± 1 | 0.893 |
| Creatine | 71 ± 9 | 70 ± 11 | 0.916 |
| Ornithine | 30 ± 5 | 31 ± 2 | 0.970 |
These metabolites increase with high-fat feeding compared with controls (chow fed). Values are stated as mean (μM) ± s.e.,n = 10/dietary treatment, p values for each individual metabolite are listed.
Table 2.
Negative contributors to multivariate analysis
| Metabolite | Control | High fat | p Value |
|---|---|---|---|
| Glycine | 97 ± 16 | 42 ± 16 | 0.002 |
| Lysine | 88 ± 12 | 60 ± 5 | 0.005 |
| Suberate | 8 ± 2 | 4 ± 1 | 0.005 |
| Acetate | 148 ± 29 | 88 ± 14 | 0.014 |
| Leucine | 58 ± 8 | 41 ± 5 | 0.019 |
| Valine | 73 ± 11 | 51 ± 6 | 0.023 |
| Trimethylamine N-oxide | 13 ± 3 | 7 ± 3 | 0.038 |
| Hippurate | 79 ± 21 | 48 ± 11 | 0.042 |
| Arginine | 44 ± 15 | 22 ± 3 | 0.049 |
| Phenylalanine | 30 ± 4 | 23 ± 2 | 0.064 |
| Lactate | 3390 ± 483 | 2546 ± 532 | 0.103 |
| Isobutyrate | 9 ± 1 | 7 ± 1 | 0.114 |
| Succinate | 33 ± 22 | 8 ± 3 | 0.117 |
| Tyrosine | 29 ± 4 | 24 ± 3 | 0.149 |
| Isoleucine | 38 ± 10 | 26 ± 7 | 0.166 |
| Uridine | 6 ± 2 | 5 ± 1 | 0.202 |
| Allantoin | 39 ± 7 | 31 ± 7 | 0.250 |
| Tryptophan | 8 ± 2 | 6 ± 1 | 0.290 |
| Glutamate | 43 ± 24 | 29 ± 5 | 0.334 |
| Fumarate | 4 ± 1 | 3 ± 1 | 0.373 |
| Formate | 19 ± 6 | 15 ± 3 | 0.374 |
| Pyruvate | 72 ± 21 | 60 ± 12 | 0.499 |
| Benzoate | 139 ± 49 | 115 ± 34 | 0.570 |
| Choline | 13 ± 3 | 11 ± 3 | 0.625 |
| Proline | 65 ± 7 | 64 ± 5 | 0.906 |
These metabolites decrease with high-fat feeding compared with controls (chow fed). Values are stated as mean (μM) ± s.e.,n = 10/dietary treatment, p values for each individual metabolite are listed.
Model Generation
The importance of the multivariate model is the ability to identify the set of metabolites most directly responsive to the high-fat diet. In OPLS models, variation in the x component represents changes in metabolite concentration, while y is correlated to variation in either diet (model 1) or body mass (model 2). Other sources of variation can be isolated in subsequent orthogonal components so that their impact is not interpreted as part of the diet response but rather alternate sources of variation (batch, exercise, instrument instability, etc.).
Model 1 - Diet
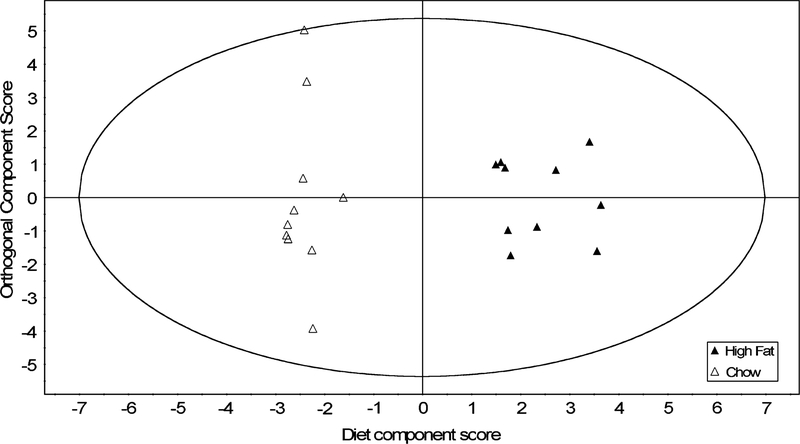
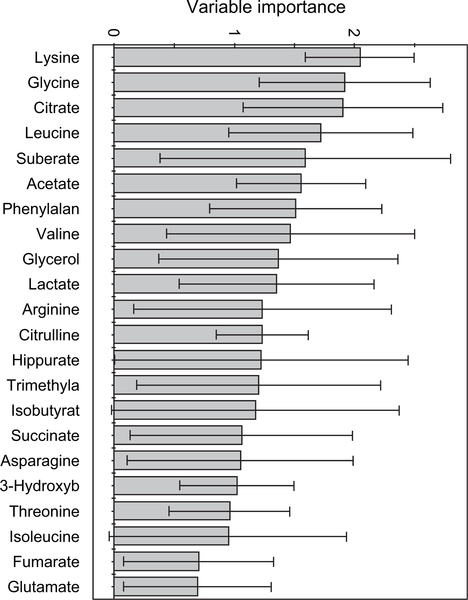
Chow and high-fat diets resulted in clearly distinguishable spectra. Acetate, formate and pyruvate were elevated, while uridine, creatine, tyrosine and the ketone body 3-hydroxybutyrate were simultaneously depressed. The variable importance of each metabolite in model 1 (figure 2) is shown in figure 3. The most significant metabolites included amino acids (Lys, Gly, Leu, Phe and Val) and energy metabolites (citrate, acetate, glycerol, suberate and lactate). Model 1 (figure 2) had one sample (of 20) outside the 95% confidence interval, which is within acceptable tolerances.
Fig. 2.
An orthogonal partial least squares discriminant analysis scores plot shows the separation between samples along each of the two model components in model 1. The first (horizontal) component is the variation associated by the model with metabolite-based interclass differences. The second, vertical component represents subject variation unrelated to diet. n = 10 mice/dietary treatment.
Fig. 3.
The variable importance plot (VIP) generated from model 2 shows the relative contribution that each metabolite makes to the difference between classes. Metabolites with VIP scores above 1 are considered to be strong contributors; anything below 0.5 is considered non-significant. Only compounds with an error smaller than their magnitude (95% confidence in their significance) are shown.
Model 2 - Mass
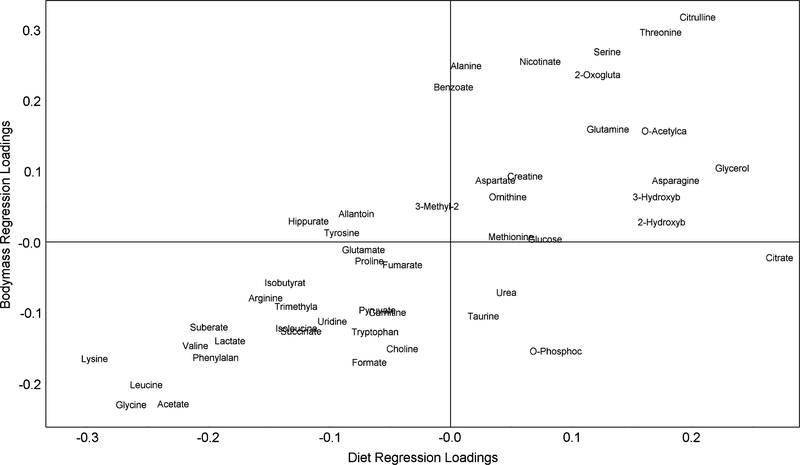
A second OPLS model, created from the same metabolite data, used the mass (y variable) for regression (figure 4, r2 = 0.89, q2 = 0.52). The purpose of this second model was to compare the metabolic changes associated with the cause (diet) with those associated with the effect (change in mass). Validation was performed on this model as well as scores, loadings and cross-validation statistics. The mass measurements of the chow-fed animals were clustered in a small range (35.4 ± 4.3 g). However, the high fat-fed animals were more disperse (42.8 ± 9.1 g), showing some overlap with the chow-fed mass range. Upon inspection, the high-fat animals showed a bimodal distribution and as a result, the body mass model (model 2) could not separate animals into diet classes. All animals with higher body mass were among those fed a high-fat diet (80%); however, not all animals on a high-fat diet exhibited a significant increase in body mass (20%). It was, however, a good cross-validation predictor of body mass based on metabolic profile. Model 2 showed substantially different compounds of importance compared with model 1. Of possible interest was the substantial drop in the significance of citrate and suberate. In contrast, the significance of alanine, 2-oxoglutarate, benzoate and formic acid all increased in this model.
Fig. 4.
A comparison of metabolite loading co-efficients in model 2 - mass vs. model 1 - diet. Co-efficients on the diagonal react similarly in both models, whereas off-axis metabolites show a differential change. Those in the top-right corner are elevated in high-fat animals and high-mass animals and vice versa. Citrate, which falls to the right of the x axis, shows elevated concentrations in fat-fed animals, but does not change in concert with body mass. Conversely, Alanine and benzoate concentrations are higher in animals with elevated body mass, but do not respond directly to diet.
Internal Validation
Model 1 cross-validation accuracy (YPredCV) was 18/20 or 90%. If Hotelling’s 95% confidence interval was used to exclude outliers from this prediction, the accuracy improved but the sample size shrinks. Overall, the sample size is too small for the model to serve as a classifier (predictor for third-party samples), but is sufficiently robust for biological interpretation.
External Validation
Because cross validation cannot detect sampling bias, particularly in small sample sets, additional EV on samples was performed. Seven of 10 EV samples fell within the model’s expected parameter range. The body mass of the EV samples predicted using model 2 correlated reasonably well with the experimental mass of the animals, with a correlation co-efficient of 0.28. It is important to restate that these predictions are not intended to describe the power of the model as a clinical or externally predictive tool. Rather, they are intended to verify that the model is not overfit to the small number of samples used. Given the limited sample size to build the models (n = 10/dietary treatment), a 70% membership is reasonable.
Discussion
The C57BL/6J mouse is a commonly employed experimental model of metabolic disease as it readily develops obesity and insulin resistance when fed a high-fat diet [14–17]. The model is advantageous over genetically induced mouse models (e.g. db/db and ob/ob) as obesity occurs gradually and is environmentally induced. Specifically, high-fat feeding results in a decline in whole body glucose disposal, increased fatty acid utilization, impaired glucose tolerance and cardiac dysfunction [6,8–10,12,13]. Here, we present a first step in validating 1H-NMR-based metabolic profiling techniques to detect dietary-induced insulin resistance in this model.
Results showed NMR-based spectroscopy to be a predictor of dietary-induced insulin resistance in an experimental model of limited sample size. EV showed that both diet and body mass could be predicted with reasonable success. The variable importance plot, a measure of which metabolites significantly discriminate chow and high fat-fed animals, shows lysine, glycine, citrate, leucine, suberate and acetate to be altered. These metabolites are all involved in energy metabolism and may be representative of the perturbations taking place with insulin resistance.
Of interest, results show the amino acids leucine, glycine and lysine to be depressed in high fat-fed animals compared with chow-fed animals. There has been renewed interest in the role of leucine in dietary-induced insulin resistance [18,19]. This amino acid interacts with insulin signalling through the rapamycin (mTOR) pathway and is important in protein synthesis and substrate selection. Analogous to the present findings, plasma leucine has been shown to be depressed with high-fat feeding in the Sprague-Dawley rat with a 22% reduction in levels following 4 weeks of dietary manipulation [20]. In humans, plasma leucine levels are depressed with type 2 diabetes compared with healthy volunteers but restored with 6 weeks of rosiglitazone treatment [21]. Of note, these results are for plasma and not for urine that typically shows elevated branched-chain amino acid levels with the disease [21].
Other altered metabolites include glycine and citrate that were elevated with high-fat feeding compared with controls. Glycine is a key metabolite in nucleic acid synthesis and is known to fluctuate with changes in energy status. Starvation results in large increases in glycine, signalling energy transition and energy conservation [22]. Likewise, citrate is produced from the Trichloroacetic Acid (TCA) cycle and has been previously shown to be increased in alloxan diabetic rats as well as ketoacidotic humans with type 1 diabetes [23]. Endogenously produced from fatty acid and glucose metabolism, concentrations are known to be sensitive to insulin and glucose levels [24]. Citrate becomes elevated with high-fat feeding as a consequence of hyperglycaemia, insulin resistance, a heavy reliance on fatty acid utilization and a decreased liver clearance [25]. Previous reports show this metabolite to be increased with hyperglycaemia and lowered upon insulin administration in both experimental animal models and humans [26–29]. Despite these findings, the use and applicability of citrate as a disease marker in metabolomic studies are controversial and should be interpreted with caution [30].
Correlations between the degree of insulin resistance induced by high-fat feeding and metabolomic profile could not be performed in the present study as insulin resistance and metabolite profiling were performed on separate subsets of animals because of limited serum volume. However, results show metabolite profiling to be a predictor of body mass. This correlation may be weaker than expected as only 80% of the animals on high-fat diet became obese, the other 20% maintained body masses in the range of chow-fed animals (>38 g). Such variation in dietary-induced obesity in inbred rodent strains has been previously reported and is thought to arise from subtle polygenic differences between animals [31–34]. Additionally, the present study examined metabolites from a single serum sample. Given this, we could not determine which tissues were primarily responsible for the observed metabolomic alterations with high-fat feeding.
Examination of the literature shows the use of 1H-NMR spectroscopy to detect complex disease states in humans to be highly speculative at best [1,2]. Mixed results are, in part, because of the complex aetiology of these conditions and the heterogeneous nature of the populations sampled. To date, the majority of studies have examined freezer samples of sera/plasma collected on a large number of patients and show poor assessment of disease in metabolomic profiles. A more appropriate strategy is to examine samples collected under more defined conditions. An excellent example of this can be found in the work of Salek et al. [35] who compared spectral profiles in urine from humans, rats and mice with type 2 diabetes. In this study, metabolomic profiles of unmedicated human subjects, the db/db mouse and fa/fa rat (genetically induced models of diabetes caused by leptin receptor mutation) were analysed. Changes in metabolites involved in nucleotide, methylamine metabolism and TCA cycle intermediates were common between the animal and the human models. There were a greater number of metabolites altered in the animal vs. human models, an expected finding considering that these models exhibit extreme type 2 diabetic phenotypes. Likewise, these models had greater changes in the number, severity and type of metabolites with diabetes compared with the present study that employs a milder, dietary-induced model of the disease. Overall, the study showed that control and disease samples from each model could be clearly distinguished, data that may eventually provide novel biomarkers for tracking type 2 diabetes in urine.
Metabolomic technologies encompassing 1H-NMR allow the examination of a large number of metabolites from small volumes of serum. To the authors’ knowledge, this is the first examination of mouse serum from chow and high fat-fed C57BL/6J mice. Of note, this predictive ability was generated on a small subset of experimental animals as they most closely reflect numbers used in common laboratory experiments. Animals in the present study were fasted, negating any direct effect of the diets on metabolomic profiles. Limitations of this study include the measurement of whole serum and the presence of anaesthesia. The specific effects of anaesthesia on metabolite profiles are not known. However, given the volume of blood collected from each animal (1 ml), this was unavoidable.
In conclusion, results show NMR-based spectroscopy to be a predictor of dietary-induced insulin resistance and body mass in mice. Such results are a first step in validating the technique for further experimental use. It is important to note that we are not suggesting that metabolomic profiling be used as a surrogate for the detection of insulin resistance or insulin clamp studies. Indeed, future work will need to establish the relationships between the severity of insulin resistance and metabolomic profile.
Acknowledgements
We gratefully acknowledge the technical assistance of Ria Driessen, Lin Su and Olivia T. Brusslers. The encouragement and guidance of Dr David Wishart and Dr Christoph Sensen were greatly appreciated. Supported by the Alberta Heritage Foundation for Medical Research (J. S. and H. J. V.), the Heart and Stroke Foundation of Canada (J. S.), the Canadian Institutes for Health Research (J. S. and H. J. V.), the National Science and Engineering Council of Canada (H. J. V.) and Genome Canada (J. S.). D. H. W. is supported by National Institutes of Health Grants U24 DK-59637 and RO1 DK-54902. No other known disclosures or potential conflicts to declare.
References
- 1.Roussel R, Mentre F, Bouchemal N et al. NMR-based prediction of cardiovascular risk in diabetes. Nat Med 2007; 13: 399–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kirschenlohr HL, Griffin JL, Clarke SC et al. Proton NMR analysis of plasma is a weak predictor of coronary artery disease. Nat Med 2006; 12: 705–710. [DOI] [PubMed] [Google Scholar]
- 3.Berger A, Milgram E, Mitchell M et al. The metabolomics of aging. FASEB J 2007; 823.4 [Google Scholar]
- 4.Lutz U, Lutz RW, Lutz WK. Metabolic profiling of glucuronides in human urine by LC-MS/MS and partial least-squares discriminant analysis for classification and prediction of gender. Anal Chem 2006; 78: 4564–4571. [DOI] [PubMed] [Google Scholar]
- 5.Kardia SR, Chu J, Sowers MR. Characterizing variation in sex steroid hormone pathway genes in women of 4 races/ethnicities: the Study of Women’s Health Across the Nation (SWAN). Am J Med 2006; 119: S3–S15. [DOI] [PubMed] [Google Scholar]
- 6.Fueger PT, Bracy DP, Malabanan CM, Pencek RR, Granner DK, Wasserman DH. Hexokinase II overexpression improves exercise-stimulated but not insulin-stimulated muscle glucose uptake in high-fat-fed C57BL/6J mice. Diabetes 2004; 53: 306–314. [DOI] [PubMed] [Google Scholar]
- 7.Morgan CR, Lazarow AL. Immunoassay of insulin: two antibody system. Plasma insulin of normal, subdiabetic, and diabetic rats. Am J Med Sci 1963; 257: 415–419. [Google Scholar]
- 8.Ayala JE, Bracy DP, Julien BM, Rottman JN, Fueger PT, Wasserman DH. Chronic treatment with sildenafil improves energy balance and insulin action in high fat-fed conscious mice. Diabetes 2007; 56: 1025–1033. [DOI] [PubMed] [Google Scholar]
- 9.Shearer J, Fueger PT, Bracy DP, Wasserman DH, Rottman JN. Partial gene deletion of heart-type fatty acid-binding protein limits the severity of dietary-induced insulin resistance. Diabetes 2005; 54: 3133–3139. [DOI] [PubMed] [Google Scholar]
- 10.Park SY, Cho YR, Kim HJ et al. Unraveling the temporal pattern of diet-induced insulin resistance in individual organs and cardiac dysfunction in C57BL/6 mice. Diabetes 2005; 54: 3530–3540. [DOI] [PubMed] [Google Scholar]
- 11.Beckwith-Hall BM, Brindle JT, Barton RH et al. Application of orthogonal signal correction to minimise the effects of physical and biological variation in high resolution 1H NMR spectra of biofluids. Analyst 2002; 127: 1283–1288. [DOI] [PubMed] [Google Scholar]
- 12.Rao R, Hao CM, Redha R, Wasserman DH, McGuinness OP, Breyer MD. Glycogen synthase kinase 3 inhibition improves insulin-stimulated glucose metabolism but not hypertension in high-fat-fed C57BL/6J mice. Diabetologia 2007; 50: 452–460. [DOI] [PubMed] [Google Scholar]
- 13.Wu JJ, Roth RJ, Anderson EJ et al. Mice lacking MAP kinase phosphatase-1 have enhanced MAP kinase activity and resistance to diet-induced obesity. Cell Metab 2006; 4: 61–73. [DOI] [PubMed] [Google Scholar]
- 14.Clee SM, Attie AD. The genetic landscape of type 2 diabetes in mice. Endocr Rev 2007; 28: 48–83. [DOI] [PubMed] [Google Scholar]
- 15.Leiter EH. Obesity genes and diabetes induction in the mouse. Crit Rev Food Sci Nutr 1993; 33: 333–338. [DOI] [PubMed] [Google Scholar]
- 16.Raab RM, Bullen J, Kelleher J, Mantzoros C, Stephanopoulos G. Regulation of mouse hepatic genes in response to diet induced obesity, insulin resistance and fasting induced weight reduction. Nutr Metab (Lond) 2005; 2: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alevizos I, Misra J, Bullen J et al. Linking hepatic transcriptional changes to high-fat diet induced physiology for diabetes-prone and obese-resistant mice. Cell Cycle 2007; 6: 1631–1638. [DOI] [PubMed] [Google Scholar]
- 18.Layman DK, Walker DA. Potential importance of leucine in treatment of obesity and the metabolic syndrome. J Nutr 2006; 136: 319S–323S. [DOI] [PubMed] [Google Scholar]
- 19.Newgard CB. Metabolomics applied to understanding of overlaps between metabolic syndrome and cardiovascular disease. FASEB J 2007. (Session #415). [Google Scholar]
- 20.Calles-Escandon J, Cunningham J, Felig P. The plasma amino acid response to cafeteria feeding in the rat: influence of hyperphagia, sucrose intake, and exercise. Metabolism 1984; 33: 364–368. [DOI] [PubMed] [Google Scholar]
- 21.van Doorn M, Vogels J, Tas A et al. Evaluation of metabolite profiles as biomarkers for the pharmacological effects of thiazolidinediones in type 2 diabetes mellitus patients and healthy volunteers. Br J Clin Pharmacol 2007; 63: 562–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adibi SA. Influence of dietary deprivations on plasma concentration of free amino acids of man. J Appl Physiol 1968; 25: 52–57. [DOI] [PubMed] [Google Scholar]
- 23.DeVilliers DC Jr, Dixit PK, Lazarow A. Citrate metabolism in diabetes. I. Plasma citrate in alloxan-diabetic rats and in clinical diabetes. Metabolism 1966; 15: 458–465. [DOI] [PubMed] [Google Scholar]
- 24.Piloquet H, Ferchaud-Roucher V, Duengler F, Zair Y, Maugere P, Krempf M. Insulin effects on acetate metabolism. Am J Physiol Endocrinol Metab 2003; 285: E561–E565. [DOI] [PubMed] [Google Scholar]
- 25.Sjostrom P Der Citratgehalt in Blutserum als Diagnosticum bie Krandheiten der Lever und der Gallenwege. Acta Chir Scand 1937; (Suppl.): 49. [Google Scholar]
- 26.Natelson S, Pincus JB, Lugovoy JK. Response of citric acid levels to oral administration of glucose. I. Normal adults and children. J Clin Invest 1948; 27: 446–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Penttila IM, Pollanen LO. Effect of insulin acid tolbutamide on blood citric acid in rabbits. Scand J Clin Lab Invest 1959; 11: 322–325. [DOI] [PubMed] [Google Scholar]
- 28.Pincus JB, Natelson S, Lugovoy JK. Response of citric acid levels to oral administration of glucose. II. Abnormalities observed in the diabetic and convulsive state. J Clin Invest 1948; 27: 450–453. [PubMed] [Google Scholar]
- 29.Tudorow J, Dikow A. Das verhalten des Citronensaures-piegels in blut wahrend der Glucosebelastung beigesunden. Clin Chim Acta 1960; 5: 762.13777171 [Google Scholar]
- 30.Robertson DG. Metabonomics in toxicology: a review. Toxicol Sci 2005; 85: 809–822. [DOI] [PubMed] [Google Scholar]
- 31.West DB, Boozer CN, Moody DL, Atkinson RL. Dietary obesity in nine inbred mouse strains. Am J Physiol 1992; 262: R1025–R1032. [DOI] [PubMed] [Google Scholar]
- 32.Schemmel R, Mickelsen O, Gill JL. Dietary obesity in rats: body weight and body fat accretion in seven strains of rats. J Nutr 1970; 100: 1041–1048. [DOI] [PubMed] [Google Scholar]
- 33.Levin BE, Dunn-Meynell AA, Balkan B, Keesey RE. Selective breeding for diet-induced obesity and resistance in Sprague-Dawley rats. Am J Physiol 1997; 273: R725–R730. [DOI] [PubMed] [Google Scholar]
- 34.Levin BE, Triscari J, Hogan S, Sullivan AC. Resistance to diet-induced obesity: food intake, pancreatic sympathetic tone, and insulin. Am J Physiol 1987; 252: R471–R478. [DOI] [PubMed] [Google Scholar]
- 35.Salek RM, Maguire ML, Bentley E et al. A metabolomic comparison of urinary changes in type 2 diabetes in mouse, rat, and human. Physiol Genomics 2007; 29: 99–108. [DOI] [PubMed] [Google Scholar]
- 36.Weljie AM, Dowlatabadi R, Miller J, Vogel HJ, Jirik FR. An inflammatory arthritis-associated metabolite biomarker pattern revealed by 1H NMR spectroscopy. J Proteome Res 2007; 6(9): 3456–64. [DOI] [PubMed] [Google Scholar]