Abstract
Highly oxygenated molecules (HOMs) are a class of compounds associated with secondary organic aerosols exhibiting high oxygen to carbon (O:C) ratios and often originating from the oxidation of biogenic compounds. Here, the photooxidation and ozonolysis of isoprene were examined under a range of conditions to identify HOM tracers for aged isoprene aerosol. The HOM tracers were identified as silylated derivatives by gas chromatography-mass spectrometry and by detecting their parent compounds by liquid chromatography-high resolution mass spectrometry. In addition to the previously observed methyltetrols and 2-methylglyceric acid, seven tracer compounds were identified, including 2-methyltartronic acid (MTtA), 2-methylerythronic acid (2MeTrA), 3-methylerythronic acid (3MeTrA), 2-methylthreonic acid (2MTrA), 3-methylthreonic acid (3MTrA), erythro-methyltartaric acid (e-MTA), and threo-methyltartaric acid (t-MTA). The molecular structures were confirmed with authentic standards synthesized in the laboratory. The presence of some of these HOMs in the gas and particle phases simultaneously provides evidence of their gas/particle partitioning. To determine the contributions of aged isoprene products to ambient aerosols, we analyzed ambient PM2.5 samples collected in the southeastern United States in summer 2003 and at two European monitoring stations located in Zielonka and Godów (Poland). Our findings show that methyltartaric acids (MTA) and 2- and 3-methylthreonic acids (and their stereoisomers) are representative of aged isoprene aerosol because they occur both in the laboratory chamber aerosol obtained and in ambient PM2.5. On the basis of gas chromatography-mass spectrometry (GC-MS) analysis, their concentrations were found to range from 0.04 ng for 3-methylthreonic acid to 6.3 ng m−3 for methyltartaric acid at the southeast site in Duke Forest, NC, USA.
Graphical Abstract
1. Introduction
Organic aerosols (OAs) make up a significant fraction of PM2.5: that is, particles having an aerodynamic diameter less than 2.5 μm.(1,2) The origin of OAs is from fossil fuel combustion, biomass burning, and other primary sources, as well as from the oxidation of volatile and semivolatile organic compounds in the atmosphere. Secondary organic aerosols (SOAs), which can contribute significantly to ambient PM2.5, are also formed from the heterogeneous oxidation of compounds in the particle phase.(3) Exposure to PM2.5 is known to be a detriment to human health, to degrade visibility, and to influence the energy budget of the earth.(4–6) The oxidation products of isoprene forming an organic aerosol represent a major input to the global SOA burden.(7,8)
Isoprene has been the subject of extensive studies in aerosol chemistry ever since 2-methylthreitol (2MTr) and 2-methylerythritol (2MeT; collectively methyltetrols, MTs) were discovered to arise from the oxidation of isoprene in the Amazon rainforest.(9,10) While Claeys et al.(9) identified the compounds, Edney et al.(10) provided direct evidence for their formation from laboratory irradiations of isoprene in the presence of oxides of nitrogen. Since that time, additional isoprene particle phase products, including 2-methylglyceric acid (2MGA), C5-alkene triols, C4–C5-organosulfates/organonitrates, and dihydroperoxydiols, have been detected in urban, rural, and remote environments.(11–18) Polar organic compounds upon silylation result in reduced polarity, enhanced volatility, and increased thermal stability and enable a GC-MS analysis for a number of involatile or too unstable compounds. However, appropriate caution should be taken, for example, with desulfation reactions associated with primary organosulfates, and corrections might be warranted when methyltetrols are analyzed.(19–23)
It is now thought that much, if not the majority, of SOAs in PM2.5 results from organics that have undergone several generations of atmospheric oxidation. Multiple insights(24–26) have illuminated the nature of this oxidized carbon, referred to as “aged carbon”, giving rise to highly oxygenated molecules (HOMs).(27) For example, Sato et al.(28) reported the presence of such species following ozonolysis reactions of β-pinene producing gas and particle products which in turn react with hydroxyl radicals (OH). In contrast, Ehn et al.,(29) Kristensen et al.,(30) and Jokinen et al.(31) have suggested that highly oxidized organic compounds are formed at early stages in the oxidation of monoterpenes. Rissanen et al.(32) have proposed a mechanistic description of such extremely low volatility organic compounds from the ozonolysis of cyclohexene. Several recent studies of isoprene oxidation have shown the prevalence of low-volatility highly oxidized molecules in SOAs.(33,34) Considerable effort is now underway to characterize organic species associated with biogenic SOA aging to incorporate it into atmospheric models.(25,26,35–37) However, there are few metrics which can be used to adequately assess the degree of oxidation of such HOMs in air quality model predictions.
Here, we report on the formation of such particulate carbon compounds (having oxygen to carbon mass ratios greater than unity; O:C ≥ 1) from the laboratory oxidation of isoprene (ISO) by O3 and OH radicals. These experiments have been conducted under a range of nitrogen oxide and acidic sulfate aerosol concentrations. Analytical methods coupling gas chromatography to mass spectrometry (GC-MS) and liquid chromatography to high-resolution Orbitrap mass spectrometry (LC-HR Orbitrap MS) have led to the identification of a novel class of tracer compounds from samples obtained from controlled chamber experiments. Syntheses of reference compounds in our laboratory allowed for definitive structural identifications of the markers. Moreover, PM2.5 samples from urban, rural, and remote areas from Europe and the United States have indicated that this novel class of compounds (hydroxy carboxylic acids) is found in ambient samples at levels near or exceeding that of methyltetrols.(9,10)
2. Experimental Methods
All chemicals were purchased at the highest purity available from Aldrich Chemical Co. (Milwaukee, WI) and were used without further purification. Solvents used in GC-MS (GC2 quality) were from Burdick and Jackson (Muskegon, MI). For LC-MS, water (resistivity 18.2 MΩ cm) was purified with a Milli-Q Advantage system from Merck (Poland) and methanol (ChromaSolv-Grade) was purchased from Sigma-Aldrich (Poland). The synthesis procedures of 2-methylthreonic acid, 3-methylthreonic acid, and methyltartaric acid involved generating trihydroxymethyl ester salts followed by a dihydroxylation reaction to afford reference products. Detailed procedures for the synthesis are provided in section S1 in the Supporting Information.
The experiments were conducted in a 14.5 m3 fixed-volume chamber with walls having a 40 μm Teflon coating. The standard photolytic system produced radiation in the actinic region of the spectrum (300–400 nm). For irradiations in the presence of nitrogen oxides (NOx), OH was produced by the conventional chain-propagating mechanism where NO oxidizes ozone and peroxy radicals formed in the system. In other experiments, OH was generated in the absence of NOx by photolyzing H2O2, where additional UV sunlamps increased the radiation by 280–320 nm. Additional details on the chamber operation, procedures, and instruments have been reported in our previous papers.(38,39) Chamber isoprene concentrations were measured by GC/FID (Hewlett-Packard, Palo Alto, CA; Model 5890). Ozone was measured with a Bendix ozone monitor (Model 8002, Lewisburg, WV). NO and NOx were monitored with a TECO oxides of nitrogen analyzer (Model 42C, Franklin, MA).
Particle samples from the chamber were collected onto 47 mm glass fiber filters at 16.7 L min–1 for 1 h just after adding all the reactants in the static (batch) mode and 24 h in the dynamic (flow) mode. They were sonicated for 1 h in 5 mL of methanol. Prior to extraction, known masses of cis-ketopinic acid (KPA) and d50-tetracosane (TCS) were spiked on each filter as internal/recovery standards. The extraction efficiency was determined for 3-methylthreonic acid (84.7 (±4.2)%), and methyltartaric acid (87.5 (±4.1)%) using HPLC-Q-ToF MS. Similar values were reported previously on the basis of similar organic compounds.(38,39) Each extract was evaporated to dryness under a gentle stream of N2 at room temperature and then derivatized with 200 μL of BSTFA (N,O-bis(trimethylsilyl)trifluoroacetamide: 1% trimethylchlorosilane) and 100 μL of pyridine at 70 °C for 1 h.(38,41) The silylated extracts were analyzed for organic compounds, and only targeted species are reported in this study. The organic compounds were analyzed by GC-MS, with details being given in section 3 in the Supporting Information.
UHPLC-ESI-MS/MS analysis was performed for organic acids using a Dionex UltiMate 3000 RSLC system (Thermo Fisher Scientific) coupled to an Orbitrap Fusion Tribrid mass spectrometer (Thermo Fisher Scientific) equipped with a heated electrospray ionization source (HESI). UHPLC-ESI-MS/MS was used as an independent method to confirm the identity of the targeted isoprene HOM compounds measured by GC-MS. Compounds were separated on an Acquity UPLC HSS T3 column, 2.1 mm i.d. × 100 mm, particle size 1.8 μm, pore size 100 Å (Waters), at 40 °C. Water with 10 mM ammonium formate and methanol with 10 mM ammonium formate were used as solvents A and B, respectively. Samples were loaded directly onto an analytical column at a flow rate of 0.3 mL min–1 of 100% solvent A. The injection volumes were 1 and 20 μL for the survey scans and the MS/MS spectra scans, respectively. Compounds were eluted from the column at a flow rate of 0.3 mL min–1 with the following linear gradient: 0 min, 100% A; 3 min, 100% A; 6 min, 100% B; 9 min, 100% B; 12 min, 100% A. The eluted compounds were ionized in the negative ion mode with a capillary voltage of 2.5 kV in an HESI source. The ion source working parameters optimized on the total ion current (TIC) values were as follows: sheath gas flow 35 L min–1, auxiliary gas flow 10 L min–1, ion transfer tube temperature 325 °C, and vaporizer temperature 275 °C. Nitrogen was used as the sheath and auxiliary gas. Survey scans were recorded in the Orbitrap mass analyzer at a resolving power of 60000 in the m/z range of 50–300. From each survey scan the most abundant singly charged ions were fragmented by higher energy collision-induced dissociation (HCD). The cycle time was 3 s. The product ions were analyzed in the Orbitrap analyzer at a resolving power of 15000. After fragmentation, the masses were excluded for 30 s from further fragmentation. Collision energy values were optimized to obtain good fragmentation of the compounds studied. Survey scans and MS/MS spectra were evaluated manually using Xcalibur 3.0 (Thermo Fisher Scientific). Blanks and background samples were extracted and analyzed identically as the field/chamber samples and gave negligible signals of the targeted SOA compounds.
Ambient PM2.5 samples were collected from several sites in Europe and the United States: two regional background monitoring stations located in Zielonka and Godów (Poland) and one site at the Duke University Research Forest (DF; Blackwood Division). Extended analysis of field samples is reported in the paper associated with the HOM formation mechanism, time series, and implication to ambient PM2.5.(42) Detailed descriptions of the field sites and sampling procedures have been reported previously(43,44) and are described in section S2 in the Supporting Information.
Initial conditions for a subset of experiments, representative of a large group conducted during the past 15 years in our laboratory, are given in Table 1. Two sets of experiments were conducted. In the first set, four experiments (1–3 and 7) were carried out with the chamber operated in a batch mode. In the second set, four experiments (4–6 and 8) were conducted with the chamber operated in a flow mode with a residence time of 4 h. A further description of each experiment is reported in section S3 in the Supporting Information.
Table 1.
Initial and Input Conditions for Isoprene Experiments
| experiment | ISO (ppm) | NO (ppm) | O3 (ppm) | H2O2 (ppm) | acidic seed (mg L−1) | hexane (ppm) | RH (%) | T (°C) | residence time (h) |
|---|---|---|---|---|---|---|---|---|---|
| 1b (SE) | 0.68 | 0.990 | <3 | 19 | |||||
| 2b (SE) | 0.68 | 1.030 | 350 | <3 | 20 | ||||
| 3e (SE) | 3.65 | 0.427 | 30 | 20 | |||||
| 4c (DE) | 3.08 | 0.300 | 30 | 29 | 4.08 | ||||
| 5c (DE) | 3.14 | 0.300 | 30 | 29 | 4.08 | ||||
| 6 (DE) | 2.20 | 4.7 | <3 | 25 | 4.60 | ||||
| 7 (SE) | 2.78 | 5.8 | <5 | 21 | |||||
| 8 (DE)d,g | 1.40 | 0.296 | 60 | 44 | 27 | 3.80 | |||
| 8 (DE)d,f | 1.64 | 0.347 | 49 | 28 | 3.86 |
In experiments without NOx, H2O2 served as the OH precursor. Abbreviations: DE, dynamic mode; SE, static mode (initial concentrations are given).
Detailed information about some experiments used in this study can be found in Kleindienst et al.(45)
Detailed information about some experiments used in this study can be found in Surratt et al.(46)
Detailed information about some experiments used in this study can be found in Lewandowski et al.(47) and Nestorowicz et al.(43)
Detailed information about some experiments used in this study can be found in Jaoui et al.(42) Experiment 8 was conducted in two stages.(47)
Atomizing solution: (NH4)2SO4 at 0.5 mg L−1.
atomizing solution: (NH4)2SO4 at 41 mg L−1 and H2SO4 at 60 mg L−1. Chambers often exhibit a NOx background (<1 ppb) that is present even when NOx is not deliberately added.
3. Results and Discussion
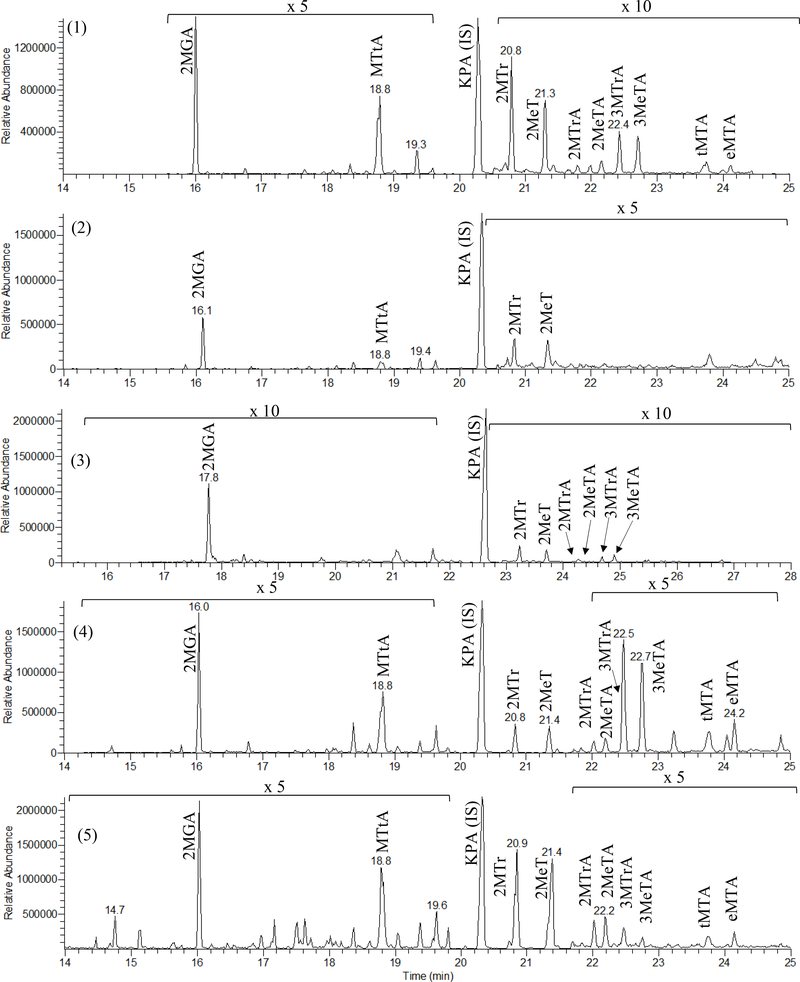
While the putative isoprene HOM tracers can be found in field samples, they are most readily isolated and identified by laboratory experiments. Here, the conditions of individual experiments represent the types of atmospheric reactions likely to occur. The tracers have been identified using the method of Jaoui et al.(38,41) The GC-MS (methane CI) extracted-ion chromatograms in Figure 1 use the selected ions (m/z 165, 321, 335, 409, 423, and 437) to best illustrate the presence of these tracers. The chromatograms for the series of five experiment systems, representative of a larger group of experiments, have used the initial reactant conditions given in Table 1. The experiments include: (1) ISO/ozone (O3), (2) ISO/O3/n-hexane, (3) irradiated ISO/NOx (gas-phase products), (4) irradiated ISO/NOx (SOA), and (5) ISO/NOx in the presence of acidic sulfate aerosol.
Figure 1.
GC-MS extracted ion chromatograms of products associated with the oxidation of isoprene. Ions extracted are m/z 321 (2-methylglyceric acid, 2MGA), m/z 335 (2-methyltartronic acid, MTtA), m/z 409 (2-methylthreitol, 2MTr; 2-methylerythritol, 2MeT), m/z 423 (2-methylthreonic acid, 2MTrA; 2-methylerythronic acid, 2MeTA; 3-methylthreonic acid, 3MTrA; 3-methylerythronic acid, 3MeTA), and m/z 437 (erythro-methyltartaric acid, eMTA; threo-methyltartaric acid, tMTA). The m/z 165 (ketopinic acid, KPA) represents the internal standard used. The different experiment types (1)–(5) are noted in the panels. Retention times may differ due to differing chromatographic conditions.
Two additional experiments (6, 7) with irradiated ISO/OH in the absence of NOx were carried out to determine to what extent the HOM tracers appear at low NOx conditions. For experiment (2) the n-hexane added scavenged OH formed during ozonolysis. Gas-phase products detected in experiment (3) provide a comparison with tracer compounds found in the particle phase.
Two groups of products were found in each of the four particle-phase systems, albeit at various concentrations. Figure 1 displays GS-MS chromatograms of the two groups of products detected in the five different experiments. Group 1 consists of seven HOM tracers: 2-methyltartronic acid (MTtA), 2-methylthreonic acid (2MTrA), 2-methylerythronic acid (2MeTA), 3-methylthreonic acid (3MTrA), 3-methylerythronic acid (3MeTA), erythro-methyltartaric acid (eMTA), and threo-methyltartaric acid (tMTA). These are easily seen in panel 4 of Figure 1 from the isoprene/NOx irradiation. These compounds have been given in Table 2 in the order of GC retention times. Table 2 gives the chemical formulas, O:C mass ratio, the five most abundant ions associated with each TMS derivative in methane-CI mode, the molecular weights of the underivatized (MW) and TMS-derivatized compounds (MWBSTFA), exact masses from LC-HRMS analysis for the corresponding [M – H]− pseudomolecular ions, and the proposed chemical structures of the compounds. Group 2 compounds are the previously reported isoprene particle phase tracer compounds, 2MGA, 2MTrT, and 2MeT, and are also detected in the experimental systems reported in this study.(9,10,13,35,40,45)Figure 1 shows that in the isoprene-O3 experiment (panel 1) all seven group 1 tracer compounds are at levels equal to or substantially lower than those of traditional isoprene group 2 tracers. Moreover, once the OH scavenger is added to the same system (panel 2), the group 1 tracers all but disappear. The group 1 tracers are substantially present in the particle phase from the isoprene oxidation in the presence of NOx, (panel 4), although they again are generally below the level of detection in the gas phase (panel 3).
Table 2.
Isoprene HOMs Identified in Isoprene Experiments
| IUPAC/common nomenclature | Formula | O/C Mass ratio (by wt) | m/z BSTFA Derivative (methane-CI) | MW (MWBSTFA) Exact mass [M − H]− (error) (g mol−1) | Proposed Structure c |
|---|---|---|---|---|---|
| 2-hydroxy-2-methyl-1,3-propanedicarboxylic acid/2-methyltartronic acid (MTtA)a | C4H6O5 | 1.67 | 335, 261, 217, 379, 73 | 134 (350) 133.0139 (+ 0.0009) |
 |
| threo-2,3,4-trihydroxy-2-methyl-1-butanecarboxylic acid/2-methylthreonic acid (2MTrA)b | C5H10O5 | 1.33 | 423, 117, 73, 393, 349 | 150 (438) 149.0454 (+ 0.0009) |
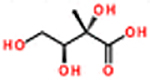 |
| erythro-2,3,4-trihydroxy-2-methyl-1-butanecarboxylic acid/2-methylerythronic acid (2MeTA)b | C5H10O5 | 1.33 | 423, 117, 73, 393, 349 | 150 (438) 149.0454 (+ 0.0009) |
 |
| threo-2,3,4-trihydroxy-3-methyl-1-butanecarboxylic acid/3-methylthreonic acid (3MTrA)c | C5H10O5 | 1.33 | 423, 349, 73, 147, 393 | 150 (438) 149.0454 (+ 0.0009) |
 |
| erythro-2,3,4-trihydroxy-3-methyl-1-butanecarboxylic acid/3-methylerythronic acid (3MeTA)c | C5H10O5 | 1.33 | 423, 349, 73, 147, 393 | 150 (438) 149.0454 (+ 0.0009) |
 |
| threo-2,3-dihydroxy-3-methyl-1,4-butanedicarboxylic acid/threo-methyltartaric acid (tMTA)d | C5H8O6 | 1.60 | 437, 319, 73, 481, 453 | 164 (452) 163.0246 (+ 0.0008) |
 |
| erythro-2,3-dihydroxy-3-methyl-1,4-butanedicarboxylic acid/erythro-methyltartaric acid (eMTA)d | C5H8O6 | 1.60 | 437, 319, 73, 481, 453 | 164 (452) 163.0246 (+ 0.0008) |
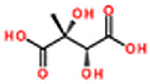 |
Not detected by LC-HRMS.
2MeTA and 2MTrA coelute by LC-HRMS given as 2MTrA.
3MeTA and 3MTrA coelute by LC-HRMS given as 3MTrA.
eMTA and tMTA coelute by LC-HRMS given as MTA.
For all compounds except MTtA only one diastareoisomeric formula is shown for simplicity.
The m/z values are ordered by increasing retention times. Exact masses for the parent compounds are from the Orbitrap LC-HRMS analyses.
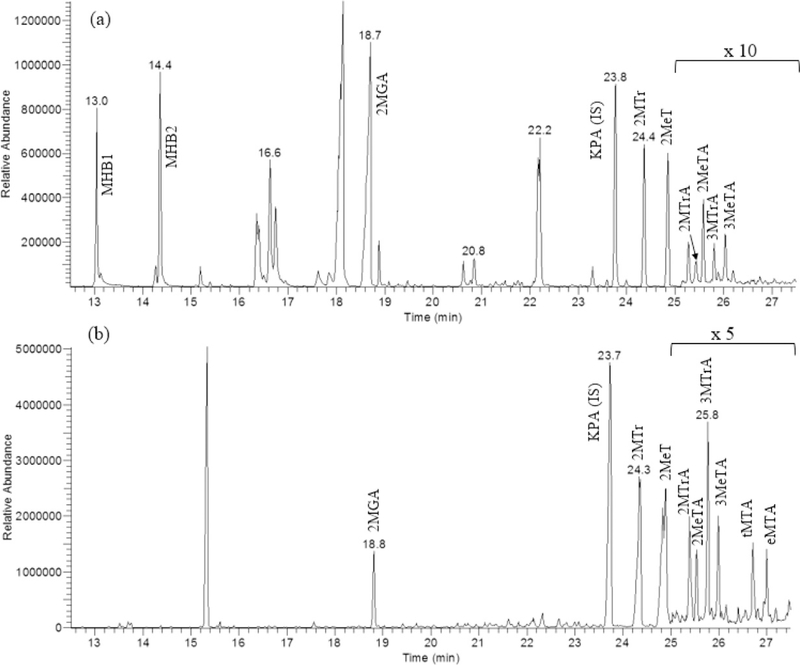
As indicated from the comparison of experiments (1) and (2), OH radicals play the dominant role in the formation of the Group 1 tracers. Samples collected from experiments (6) and (7) (Table 1) using an XAD-denuder are characteristic of the gas phase. The gas- and particle-phase products from the NOx-free irradiation are shown in Figure 2. The gas-phase products are identified as 2-methyl-4-hydroxybut-2-enal and 3-methyl-4-hydroxybut-2-enal (collectively MHB) and are labeled in the Master Chemical Mechanism as HC4CCHO and HC4ACHO, respectively.(48) They elute at respective retention times of 13.05 and 14.39 min and are completely absent from the particle phase, given they are volatile and have an O:C ratio of 0.53. In contrast, among group 1 compounds in Figure 2a, tMTA and eMTA (collectively MTA) are the compounds detected in the particle phase only, as shown in Figure 2b. Their relatively high O:C ratio of 1.60 contributes to their condensation into the particle phase.
Figure 2.
(a) Gas-phase and (b) particle-phase tracer compounds in experiments with the irradiation of an isoprene/H2O2 mixture, which produces OH in the absence of NOx. The gas-phase extracts from denuder samples were analyzed by GC-MS. Methyltetrols and isomers of methylthreonic acid appear to partition between the gas and particle phases, while the methyltartaric acids are absent from the gas phase presumably because of their low volatility (high O:C ratio).
Figure 2a also shows a significant gas-phase presence of the group 2 tracers (2MGA and MTs). The origin of these gas-phase components is of considerable interest due to previous assumptions that they are formed exclusively in the particle phase.(9,10) Their presence in the gas phase might result from decomposition of particle-bound oligomers(49) or by conventional particle–gas partitioning. However, the substantial amounts in the gas phase, particularly for the methyltetrols, suggests a potential gas-phase mechanism.(49,50) These observations serve as the basis for a revised mechanism presented by Jaoui et al.(42)
3.1. Structural Characterization of 2MeTA, 3MeTA, 2MTrA, 3MTrA, eMTA, tMTA, and MTtA
Representative EI and CI mass spectra for the BSTFA derivatives of group 1 tracers are presented in Figures S1 and S2 (section S4) in the Supporting Information. For the GC-MS interpretation, the CI mass spectra are generally the best starting point for establishing empirical formulas and structures of the ISO-HOMs. Generally, for TMS derivatives of acids, the CI mass spectra show a strong base peak of the M+ + 1 or M+ – 15 ion which allows inference of their molecular weights. In contrast, the peak for M+ + 1 is very weak or missing in the EI spectra, whereas the M+ – 117 peak is found at high abundance in both EI and CI spectra, indicative of the presence of one or more organic acid groups.(41) Additional distinctive ions allowed differentiation between derivatized acid and alcohol groups, and the expected molecular ions were observed.(41)
As a descriptive example, two sets of two peaks are found in the Figure 1 (panel 4) chromatogram. The retention times are 22.03 and 22.20 min for the first set and 22.49 and 22.76 min for the second set. The four peaks display similar fragment and adduct ions across the range m/z 50–500 Da. The compounds corresponding to these four peaks are 2MTrA, 2MeTA, 3MTrA, and 3MeTA, respectively. For example, the CI spectrum in Figure S2b, which corresponds to the robust peak eluting at 22.49 min (3MTrA in Figures 1–4), shows characteristic product ions at m/z 73, 423 [M+ – 15], 349 [M+ – 89], 321 [M+ – 117], 333 [M+ – 105], and 305 [M+ – 133], and adduct ions at m/z 439 [M+ + 1], 467 [M+ + 29], and 479 [M+ + 41]. The same set of product ions is also present in the later-eluting compound (3MeTA) at 22.76 min. This ionic pattern is consistent with the presence of four OH groups, the molecular weight (MW) of 438 Da for the derivatized compound and 150 Da molecular weight for the parent compound with the two peaks representing two diastereomers. Mass spectral peaks at m/z 321 [M•+ – 117] and 333 [M•+ – 105] are consistent with a compound having both carboxylic acid (COOH) and alcoholic (OH) functional groups.(38,41) In contrast, the EI spectrum has far greater fragmentation and only a subset of these peaks is present (e.g., the EI base peak of m/z 73 representing the TMS group).
Figure 4.
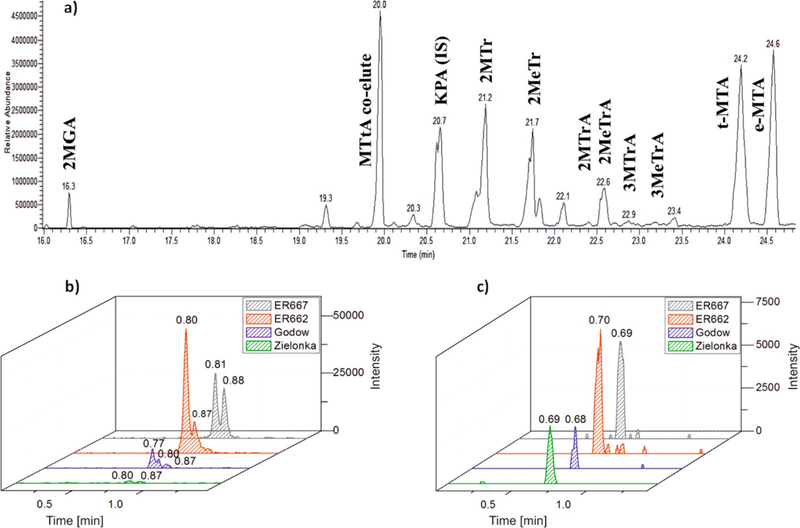
(a) GC-MS extracted ion chromatogram in CI (CH4) mode from the trimethylsilylated PM2.5 sample collected in summer 2003 at Duke Forest, NC, USA, showing isoprene aging SOA compounds in addition to methyltetrols and 2-methylglyceric acid. Extracted ions are m/z 165, 331, 409, 423, and 437. (b) LC-Orbitrap MS extracted ion chromatograms (m/z 149) and (c) (m/z 163) recorded for ambient fine aerosols collected in Godów and Zielonka (Poland) and for comparison smog chamber ISO SOA generated in the USA. The EPA smog chamber was operated under acidic (ER662) and nonacidic (ER667) conditions.
Figure 1 (panel 4) shows two peaks eluting approximately 1.0 and 1.5 min after 3MeTA (RT = 22.72 min) having some of the same identifying fragments and adducts as the 2MTrA, 3MTrA, 2MTrA, and 3MeTA compounds. These compounds are detected in all SOA samples from Figure 1 except for the isoprene/ozone/hexane system (Figure 1, panel 2) where they are below the limit of detection. This observation suggests the importance of photochemical systems where OH is present. The EI and CI mass spectra associated with the first peak (RT = 23.72) are shown in Figure S2c,d, respectively. The CI mass spectrum shows TMS-characteristic ions at m/z 73 and 147. Identifying fragments were found at m/z 437 [M•+ – 15], 363 [M•+ – 89], 319 [M•+ – 133], and adducts at 453 [M•+ + 1], 481 [M•+ + 29], and 493 [M•+ + 41]. These fragments and adducts are consistent with the presence of four OH groups and a MW of 452 Da for the derivatized compound and 164 Da for the parent compound. The EI fragmentation of m/z 437 [M•+ – 15], 363 [M•+ – 89], and other ions are consistent with the CI analysis. The two peaks have been identified as threo-methyltartaric acid (tMTA) and erythro-methyltartaric acid (eMTA), respectively.
Figure 2 shows two extracted ion chromatograms from the isoprene/H2O2 experiment associated with the gas (a) and particle phase (b), respectively. Ions extracted are m/z 165 (KPA), m/z 321 (2MGA), m/z 335 (MTtA), m/z 409 (2MTr, 2MeT), m/z 423 (2MTrA, 2MeTA, 3MTrA, 3MeTA), m/z 437 (eMTA, tMTA), and m/z 83 (MHB). The mass spectra for the two early peaks in the gas-phase spectrum (RT = 13.05, 14.39 min) are shown in Figure S3 and correspond to the isomeric hydroxy-methyl-butenals (MHB1, MHB2). Both mass spectra follow the well-established pattern of fragments and adducts described above. From the analysis, each compound has a derivatized mass of 172 Da and a nominal parent mass of 100 Da. A detailed interpretation of the GC-MS data reveals the presence of single OH and carbonyl groups and an empirical formula of C5H8O2. The compounds have also been detected in the NOx systems, but at far lower intensities. These compounds are believed to be the first-generation oxidation products of isoprene that may possibly oxidize further to produce the ISO-HOM tracers as discussed in a detailed mechanism presented by Jaoui et al.(42)
For the LC-HR Orbitrap MS/MS analysis, mass spectra of selected laboratory-synthesized compounds 2MTrA, 3-MTrA, and MTA (section S1 in the Supporting Information) have been used to verify the isoprene product identifications in the laboratory and field extracts. For example, the proposed structures of 3MTrA, 2MTrA, and 2MTA were confirmed with LC-Orbitrap MS analysis (Figure 3a,b) by a comparison of the retention time and mass spectral signature of the standard with those from the peak in Figure 1 (panel 4). The registered MS2 spectra of standards and analytes are nearly identical (Figure 3c–h). The slightly distorted relative abundances of diagnostic ions observed in the LC-ESI Orbitrap MS/MS spectra of the synthesized standard and the MW 164 unknown from isoprene SOA are explained by the coelution of erythro and threo isomers of MTA during the reversed-phase chromatography. The proposed patterns for 3MTrA, 2MTrA, and MTA fragmentation are given in Figure S4.
Figure 3.
LC-Orbitrap MS extracted ion chromatograms (m/z 149 and 163) acquired for (a, b) ER667 smog chamber SOA and authentic standards. Negative ion electrospray product ion mass spectra (MS2) of (c) the MW 150 unknown from smog chamber ISO SOA (ER667) eluting at RT 0.88 min, (d) the MW 150 unknown from smog chamber ISO SOA (ER667) eluting at RT 0.81 min, (e) the MW 164 unknown from smog chamber ISO SOA (ER667) eluting at RT 0.69 min, (f) 3-methylthreonic acid, (g) 2-methylthreonic acid, and and (h) 2-methyltartaric acid. The asterisk (*) indicates the contamination of the MS system.
The LC-HR Orbitrap MS/MS analysis provides spectra having highly accurate mass data that remove possible ambiguities for molecules having the same nominal mass but different empirical formulas. Figure 3a,b displays LC-Orbitrap MS extracted ion chromatograms (m/z 149 and 163) for authentic standards of 2MTrA, 3MTrA, and MTA together with SOA from the ISO/NOx chamber experiment (experiment (8)). Figure 3c–h displays the product ion mass spectra (MS2) of a MW 150 unknown from chamber isoprene products eluting at RT 0.88 min (Figure 3c), a MW 150 unknown from the isoprene product eluting at RT 0.81 min (Figure 3d), a MW 164 unknown from the isoprene product eluting at RT 0.69 min Figure 3e), the standard for 3-methylthreonic acid (Figure 3f), the standard for 2-methylthreonic acid (Figure 3g), and the standard for 2-methyltartaric acid (Figure 3h). The elemental composition of the deprotonated compound shown in Figure 3a,c has an exact mass of 149.0454 Da, thereby giving an empirical formula of C5H10O5, which is entirely consistent with the GC-MS characterization. This empirical formula has been observed in other studies of isoprene oxidation and is believed to be a major HOM component of isoprene-based PM.(33) Another compound consistently shown by the GC and LC analyses is methyltartronic acid. The peak is the earliest one to elute of the seven compounds. Figure S5 shows the LC-HR Orbitrap MS data of the compound which elutes from an HSS T3 reversed-phase column at 0.73 min (Figure S5a,b) and the TMS derivative from GC-MS analysis (Figure S5d,e). A plausible fragmentation pathway for methyltartronic acid is shown in Figure S5c. Additional mass spectral discussion of MTtA is reported in the section S5 in the Supporting Information.
While the LC-HR Orbitrap MS/MS provides highly reliable empirical formulas for the tracers of the putative ISO-HOMs, the relatively poor resolution power of the liquid chromatography rendered a single peak for the 2MTrA and 2MeTA, similarly to 3MTrA and 3MeTA and the two isomers of MTA (Figure 3a,b and Figure S5a). In contrast, GC-MS showed a good separation for trimethylsilylated ISO-HOMS isomers (Figures 1 and 2). The presence of two asymmetric carbon atoms (two chiral centers) in isoprene HOM acids (Table 2), except for MTtA, makes each of them exist as a pair of diastereoisomers. Therefore, their formation under atmospheric and/or laboratory conditions may result in a racemic mixture of two statistically possible diastereoisomers. For example, the peak at RT 22.4 min assigned to 3-methylerythronic acid in GC/MS chromatograms (Figure 1, panel 1) corresponds to the equimolar mixture of the stereoisomers (2R,3S)-2,3,4-trihydroxy-3-methyl-1-butanecarboxylic acid and (2S,3R)-2,3,4-trihydroxy-3-methyl-1-butanecarboxylic acid. However, the consistency of the GC-MS and LC-Orbitrap MS/MS identifications, together with the use of authentic compound standards, proves that the derivatization process does not compromise the integrity of the parent compounds. At the present time, we find no evidence that MTtA, 2MTrA, 2MeTA, 3MTrA, 3MeTA, tMTA, and eMTA have been previously reported as structurally elucidated particle-phase isoprene oxidation products.
3.2. Detection in Ambient Samples
We established the presence of the compounds from field samples from two campaigns: (1) at Zielonka and Godów, Poland, in summer 2017(43) and (2) at Duke Forest, NC, USA, in summer 2003.(44) Duke Forest samples were extracted, derivatized, and GC-MS analyzed in 2003 at the time of collection, and the resulting chromatograms were reanalyzed for the targeted compounds reported in this study. Figure 4a shows a GC-MS extracted ion chromatogram of a PM2.5 DF sample. A comparison of mass spectra from the laboratory and field samples is shown in Figure S6 as the TMS derivatives of MTA. Minor differences in the abundances between the laboratory and field CI adducts and fragments can occur when (1) the mass spectra are collected under slightly different reagent gas pressures in the ion source and (2) coelution takes the place of other compounds in the field samples (Figure 4a).
Figure 4a shows a subset of the group 1 tracers which appeared at the DF site. The concentrations were estimated in three ambient samples from DF with a single BSTFA derivatization.(38,41) The method for quantification given in section S6 in the Supporting Information is consistent with our analysis of the group 2 samples. Using the calibration factors from that method, average concentrations for individual compounds were determined. MTtA was not quantified because it coeluted with other constituents. Moreover, 2-MTrA, 2-MeTA, 3-MTrA, and 3-MeTA were found to be minor constituents occurring at low levels in comparison to the MTA. The concentrations for MTA ranged from 1.5 to 6.3 ng m–3. By comparison, levels of the group 2 compounds, 2MGA and 2MTr + 2MeT, ranged from 1.2 to 4.4 ng m–3 and from 5.3 to 11.9 ng m–3, respectively. Our findings suggest that further atmospheric processing of primary isoprene oxygenates might be occurring via the oxidation of terminal hydroxyl groups over a wide range of conditions. Henceforth, using the GC-MS approach, we focus our attention on methyltartaric acid as the most viable atmospheric tracer for aged isoprene aerosol.
These tracer compounds were also detected at Zielonka and Godów using LC-Orbitrap MS (Figure 4b,c). Findings from chamber samples are also presented in Figure 4 for comparison. The production of the group 1 compounds is apparent in the experiments with NOx (Figure 4b,c). These compounds were confirmed in the field samples by a comparison of the retention times and mass spectral signatures of the standards (Figures 3 and 4). The registered MS2 spectra of these HOMs in the field samples are nearly identical with those of the synthesized standards. The ambient PM2.5 samples in Figure 4b show modest levels of 2MTrA and 3MTrA, suggesting that they form at low yields rapidly, convert to other compounds, or are removed by another mechanism. From Figure 4c, the peaks eluting at 0.68 and 0.69 min for the PM2.5 samples show that MTA is detected in the PM2.5 samples in Zielonka and Godów. Note that these HOM compounds were not detected in SOA generated in chamber experiments conducted during the past 15 years from the oxidation of several hydrocarbons, including but not limited to α-pinene, β-pinene; d-limonene, Δ3-carene, toluene, benzene, p-, m-, and o-xylenes, naphthalene, 1,3-butadiene, a series of C4, C5, and C6 alkenea, dienes, aldehydes, and alcohols, β-caryophyllene, α-humulene, α-cedrene, α-farnesene, β-farnesene, etc.
In summary, we showed that the oxidation of isoprene under a range of conditions produced particle-phase tracers indicative of aged isoprene aerosol. Seven tracer compounds, 2-methyltartronic acid, 2-methylthreonic acid, 2-methylerythronic acid, 3-methylthreonic acid, 3-methylerythronic acid, erythro-methyltartaric acid, and threo-methyltartaric acid, were identified using molecular structure analysis and confirmed using synthesized standards. Their detection in ambient aerosol samples from selected locations in Europe and the USA provided the evidence that isoprene HOMs are present in ambient aerosol and give molecular evidence for isoprene aging. The results have potential implications for monitoring and modeling the formation and growth of aerosol in areas affected by isoprene oxidation, for prediction of the properties of aerosol particles, for determination of the uptake of liquid water under humid conditions influencing scattering more incident light, and the formation of cloud droplets.
While the chemical characteristics of SOA products formed from isoprene oxidation have been reported in several studies, this is the first study to find specific isoprene SOA products due to aerosol aging. These hydroxy carboxylic acids are found to be an important class of compounds in isoprene SOA, in that their concentrations in ambient PM2.5 samples are near or exceed the levels of the methyltetrols in the same samples. Additional analysis of a wide range of field studies and proposed formation mechanisms are forthcoming.(42)
Supplementary Material
Acknowledgments
The views expressed in this article are those of the author(s) and do not necessarily represent the views or policies of the U.S. Environmental Protection Agency. The research at the Institute of Physical Chemistry, Polish Academy of Sciences, was partially supported by funding from the Polish National Science Centre grant (No. OPUS8-2014/15/B/ST10/04276). The work of K.S. was supported by funds from the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement No. 711859 and by financial resources for science in the years 2017–2021 awarded by the Polish Ministry of Science and Higher Education for the implementation of an international cofinanced project. The authors also thank Dr. Julio Torres Elguera for valuable discussions and technical assistance in LC-HRMS/MS experiments. The LC-HRMS/MS experiments were carried out at the Biological and Chemical Research Centre, University of Warsaw, established within the project cofinanced by European Union from the European Regional Development Fund under the Operational Programme Innovative Economy 2007–2013.
Footnotes
Supporting Information
• Detailed procedures for the preparation of the reference compounds and some of their TMS derivatives, description of chamber experiments, schemes containing the fragmentation pathways, field study information, and discussion of additional derivatization experiments.
References
- 1.Nozière B; Kalberer M; Claeys M; Allan J; D’Anna B; Decesari S; Finessi E; Glasius M; Grgić I; Hamilton JF; Hoffmann T; Iinuma Y; Jaoui M; Kahnt A; Kampf CJ; Kourtchev I; Maenhaut W; Marsden N; Saarikoski S; Schnelle-Kreis J; Surratt JD; Szidat S; Szmigielski R; Wisthaler A The molecular identification of organic compounds in the atmosphere: State of the art and challenges. Chem. Rev 2015, 115 (10), 3919–3983, DOI: 10.1021/cr5003485 [DOI] [PubMed] [Google Scholar]
- 2.Hallquist M; Wenger JC; Baltensperger U; Rudich Y; Simpson D; Claeys M; Dommen J; Donahue NM; George C; Goldstein AH; Hamilton JF; Herrmann H; Hoffmann T; Iinuma Y; Jang M; Jenkin ME; Jimenez JL; Kiendler-Scharr A; Maenhaut W; McFiggans G; Mentel Th. F.; Monod A; Prévôt ASH; Seinfeld JH; Surratt JD; Szmigielski R; Wildt J The formation, properties, and impact of secondary organic aerosol: current and emerging issues. Atmos. Chem. Phys 2009, 9, 5155–5236, DOI: 10.5194/acp-9-5155-2009 [DOI] [Google Scholar]
- 3.Zheng GJ; Duan FK; Su H; Ma YL; Cheng Y; Zheng B; Zhang Q; Huang T; Kimoto T; Chang D; Pöschl U; Cheng YF; He KB Exploring the severe winter haze in Beijing: the impact of synoptic weather, regional transport and heterogeneous reactions. Atmos. Chem. Phys 2015, 15 (6), 2969–2983, DOI: 10.5194/acp-15-2969-2015 [DOI] [Google Scholar]
- 4.Burnett R; Chen H; Szyszkowicz M; Fann N; Hubbell B; Arden Pope C III; Apte JS; Brauer M; Cohen A; Weichenthal S; Coggins J; Di Q; Brunekreef B; Frostad J; Lim SS; Kan H; Walker KD; Thurston GD; Hayes RB; Lim CC; Turner MC; Jerrett M; Krewski D; Gapstur SM; Diver WR; Ostro B; Goldberg D; Crouse DL; Martin RV; Peters P; Pinault L; Tjepkema M; van Donkelaar A; Villeneuve PJ; Miller AB; Yin P; Zhou M; Wang L; Janssen NAH; Marra M; Atkinson RW; Tsang H; Quoc Thach T; Cannon JB; Allen RT; Hart JE; Laden F; Cesaroni G; Forastiere F; Weinmayr G; Jaensch A; Nagel G; Concin H; Spadaro JV Global estimates of mortality associated with long-term exposure to outdoor fine particulate matter. Proc. Natl. Acad. Sci. U. S. A 2018, 115 (38), 9592–9597, DOI: 10.1073/pnas.1803222115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim KH; Kabir E; Kabir S A review on the human health impact of airborne particulate matter. Environ. Int 2015, 74, 136–143, DOI: 10.1016/j.envint.2014.10.005 [DOI] [PubMed] [Google Scholar]
- 6.Cohen AJ; Brauer M; Burnett R; Anderson HR; Frostad J; Estep K; Balakrishnan K; Brunekreef B; Dandona L; Dandona R; Feigin V; Freedman G; Hubbell B; Jobling A; Kan H; Knibbs L; Liu Y; Martin R; Morawska L; Pope CA 3rd; Shin H; Straif K; Shaddick G; Thomas M; van Dingenen R; van Donkelaar A; Vos T; Murray CJL; Forouzanfar MH Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: An analysis of data from the global burden of diseases study 2015. Lancet 2017, 389 (10082), 1907–1918, DOI: 10.1016/S0140-6736(17)30505-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carlton AG; Wiedinmyer C; Kroll JH A review of Secondary Organic Aerosol (SOA) formation from isoprene. Atmos. Chem. Phys 2009, 9 (14), 4987–5005, DOI: 10.5194/acp-9-4987-2009 [DOI] [Google Scholar]
- 8.Henze DK; Seinfeld JH Global secondary organic aerosol from isoprene oxidation. Geophys. Res. Lett 2006, 33, L09812, DOI: 10.1029/2006GL025976 [DOI] [Google Scholar]
- 9.Claeys M; Graham B; Vas G; Wang W; Vermeylen R; Pashynska V; Cafmeyer J; Guyon P; Andreae MO; Artaxo P; Maenhaut W Formation of secondary organic aerosols through photooxidation of isoprene. Science 2004, 303, 1173–1176, DOI: 10.1126/science.1092805 [DOI] [PubMed] [Google Scholar]
- 10.Edney EO; Kleindienst TE; Jaoui M; Lewandowski M; Offenberg JH; Wang W; Claeys M Formation of 2-methyltetrols and 2-methylglyceric acid in secondary organic aerosol from laboratory irradiated isoprene/NOx/SO2/air mixtures and their detection in ambient PM2.5 samples collected in the eastern United States. Atmos. Environ 2005, 39, 5281–5289, DOI: 10.1016/j.atmosenv.2005.05.031 [DOI] [Google Scholar]
- 11.Spolnik G; Wach P; Rudzinski KJ; Skotak K; Danikiewicz W; Szmigielski R Improved UHPLC-MS/MS methods for analysis of isoprene-derived organosulfates. Anal. Chem 2018, 90 (5), 3416–3423, DOI: 10.1021/acs.analchem.7b05060 [DOI] [PubMed] [Google Scholar]
- 12.Szmigielski R Evidence for C5 organosulfur secondary organic aerosol components from in-cloud processing of isoprene: Role of reactive SO4 and SO3 radicals. Atmos. Environ 2016, 130, 14–22, DOI: 10.1016/j.atmosenv.2015.10.072 [DOI] [Google Scholar]
- 13.Kleindienst TE; Jaoui M; Lewandowski M; Offenberg JH; Lewis CW; Bhave PV; Edney EO Estimates of the contributions of biogenic and anthropogenic hydrocarbons to secondary organic aerosol at a southeastern U.S. location. Atmos. Environ 2007, 41 (37), 8288–8300, DOI: 10.1016/j.atmosenv.2007.06.045 [DOI] [Google Scholar]
- 14.Hatch LE; Creamean JM; Ault AP; Surratt JD; Chan MN; Seinfeld JH; Edgerton ES; Su Y; Prather KA Measurements of isoprene-derived organosulfates in ambient aerosols by aerosol time-of-flight mass spectrometry - Part 1: Single particle atmospheric observations in Atlanta. Environ. Sci. Technol 2011, 45 (12), 5105–5111, DOI: 10.1021/es103944a [DOI] [PubMed] [Google Scholar]
- 15.Liu J; D’Ambro EL; Lee BH; Lopez-Hilfiker FD; Zaveri RA; Rivera-Rios JC; Keutsch FN; Iyer S; Kurten T; Zhang Z; Gold A; Surratt JD; Shilling JE; Thornton JA Efficient isoprene secondary organic aerosol formation from a Non-IEPOX pathway. Environ. Sci. Technol 2016, 50 (18), 9872–9880, DOI: 10.1021/acs.est.6b01872 [DOI] [PubMed] [Google Scholar]
- 16.Piletic IR; Edney EO; Bartolotti LJ Barrier-less reactions with loose transition states govern the yields and lifetimes of organic nitrates derived from isoprene. J. Phys. Chem. A 2017, 121 (43), 8306–8321, DOI: 10.1021/acs.jpca.7b08229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Surratt JD; Chan AWH; Eddingsaas NC; Chan M; Loza CL; Kwan AJ; Hersey SP; Flagan RC; Wennberg PO; Seinfeld JH Reactive intermediates revealed in secondary organic aerosol formation from isoprene. Proc. Natl. Acad. Sci. U. S. A 2010, 107 (15), 6640–6645, DOI: 10.1073/pnas.0911114107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fang W; Gong L; Zhang O; Cao M; Li Y; Sheng L Measurements of secondary organic aerosol formed from OH-initiated photo-oxidation of isoprene using online photoionization aerosol mass spectrometry. Environ. Sci. Technol 2012, 46, 3898–3904, DOI: 10.1021/es204669d [DOI] [PubMed] [Google Scholar]
- 19.Takano R; Matsuo M; Kamei-Hayashi K; Hara S; Hirase SA Novel regioselective desulfation method specific to carbohydrate 6-sulfate using silylation reagents. Biosci., Biotechnol., Biochem 1992, 56 (10), 1577–1580, DOI: 10.1271/bbb.56.1577 [DOI] [Google Scholar]
- 20.Kolender AA; Matulewicz MC Desulfation of sulfated galactans with chlorotrimethylsilane. Characterization of b-carrageenan by 1H NMR spectroscopy. Carbohydr. Res 2004, 339, 1619–1629, DOI: 10.1016/j.carres.2004.03.029 [DOI] [PubMed] [Google Scholar]
- 21.Bedini E; Laezza A; Ladonisi A Chemical derivatization of sulfated glycosaminoglycans. Eur. J. Org. Chem 2016, 2016, 3018–3042, DOI: 10.1002/ejoc.201600108 [DOI] [Google Scholar]
- 22.Bedini E; Laezza A; Parrilli M A review of chemical methods for the selective sulfation and desulfation of polysaccharides. Carbohydr. Polym 2017, 174 (15), 1224–1239, DOI: 10.1016/j.carbpol.2017.07.017 [DOI] [PubMed] [Google Scholar]
- 23.Cui T; Zeng Z; dos Santos EO; Zhang Z; Chen Y; Zhang Y; Rose CA; Budisulistiorini SH; Collins LB; Bodnar WM; de Souza RAF; Martin ST; Machado CMD; Turpin BT; Gold A; Ault AP; Surratt JD Development of a hydrophilic interaction liquid chromatography (HILIC) method for the chemical characterization of water-soluble isoprene epoxydiol (IEPOX)-derived secondary organic aerosol. Environ. Sci.: Processes Impacts 2018, 20, 1524–1536, DOI: 10.1039/C8EM00308D [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paulot F; Crounse JD; Kjaergaard HG; Kürten A; St. Clair JM; Seinfeld JH; Wennberg PO Unexpected epoxide formation in the gas-phase photooxidation of isoprene. Science 2009, 325, 730–733, DOI: 10.1126/science.1172910 [DOI] [PubMed] [Google Scholar]
- 25.Jimenez JL; Canagaratna MR; Donahue NM; Prevot ASH; Zhang Q; Kroll JH; DeCarlo PF; Allan JD; Coe H; Ng NL; Aiken AC; Docherty KS; Ulbrich IM; Grieshop AP; Robinson AL; Duplissy J; Smith JD; Wilson KR; Lanz VA; Hueglin C; Sun YL; Tian J; Laaksonen A; Raatikainen T; Rautiainen J; Vaattovaara P; Ehn M; Kulmala M; Tomlinson JM; Collins DR; Cubison MJ; Dunlea EJ; Huffman JA; Onasch TB; Alfarra MR; Williams PI; Bower K; Kondo Y; Schneider J; Drewnick F; Borrmann S; Weimer S; Demerjian K; Salcedo D; Cottrell L; Griffin R; Takami A; Miyoshi T; Hatakeyama S; Shimono A; Sun JY; Zhang YM; Dzepina K; Kimmel JR; Sueper D; Jayne JT; Herndon SC; Trimborn AM; Williams LR; Wood EC; Middlebrook AM; Kolb CE; Baltensperger U; Worsnop DR Evolution of organic aerosols in the atmosphere. Science 2009, 326 (5959), 1525–1529, DOI: 10.1126/science.1180353 [DOI] [PubMed] [Google Scholar]
- 26.Stolzenburg D; Fischer L; Vogel AL; Heinritzi M; Schervish M; Simon M; Wagner AC; Dada L; Ahonen LR; Amorim A; Baccarini A; Bauer PS; Baumgartner B; Bergen A; Bianchi F; Breitenlechner M; Brilke S; Buenrostro Mazon S; Chen D; Dias A; Draper DC; Duplissy J; El Haddad I; Finkenzeller H; Frege C; Fuchs C; Garmash O; Gordon H; He X; Helm J; Hofbauer V; Hoyle CR; Kim C; Kirkby J; Kontkanen J; Kurten A; Lampilahti J; Lawler M; Lehtipalo K; Leiminger M; Mai H; Mathot S; Mentler B; Molteni U; Nie W; Nieminen T; Nowak JB; Ojdanic A; Onnela A; Passananti M; Peta T; Quelever LLJ; Rissanen MP; Sarnela N; Schallhart S; Tauber C; Tome A; Wagner R; Wang M; Weitz L; Wimmer D; Xiao M; Yan C; Ye P; Zha Q; Baltensperger U; Curtius J; Dommen J; Flagan RC; Kulmala M; Smith JN; Worsnop DR; Hansel A; Donahue NM; Winkler PM Rapid growth of organic aerosol nanoparticles over a wide tropospheric temperature range. Proc. Natl. Acad. Sci. U. S. A 2018, 115 (37), 9122–9127, DOI: 10.1073/pnas.1807604115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berndt T; Herrmann H; Sipila M; Kulmala M Highly oxidized second-generation products from the gas-phase reaction of OH radicals with isoprene. J. Phys. Chem. A 2016, 120 (51), 10150–10159, DOI: 10.1021/acs.jpca.6b10987 [DOI] [PubMed] [Google Scholar]
- 28.Sato K; Jia T; Tanabe K; Morino Y; Kajii Y; Imamura T Terpenylic acid and nine-carbon multifunctional compounds formed during the aging of beta-pinene ozonolysis secondary organic aerosol. Atmos. Environ 2016, 130, 127–135, DOI: 10.1016/j.atmosenv.2015.08.047 [DOI] [Google Scholar]
- 29.Ehn M; Thornton JA; Kleist E; Sipilä M; Junninen H; Pullinen I; Springer M; Rubach F; Tillmann R; Lee B; Lopez-Hilfiker F; Andres S; Acir I-H; Rissanen M; Jokinen T; Schobesberger S; Kangasluoma J; Kontkanen J; Nieminen T; Kurtén T; Nielsen LB; Jørgensen S; Kjaergaard HG; Canagaratna M; Dal Maso M; Berndt T; Petäjä T; Wahner A; Kerminen V-M; Kulmala M; Worsnop DR; Wildt J; Mentel TF A large source of low-volatility secondary organic aerosol. Nature 2014, 506, 476–479, DOI: 10.1038/nature13032 [DOI] [PubMed] [Google Scholar]
- 30.Kristensen K; Cui T; Zhang H; Gold A; Glasius M; Surratt JD Dimers in α-pinene secondary organic aerosol: Effect of hydroxyl radical, ozone, relative humidity, and aerosol acidity. Atmos. Chem. Phys 2014, 14, 4201–4218, DOI: 10.5194/acp-14-4201-2014 [DOI] [Google Scholar]
- 31.Jokinen T; Berndt T; Makkonen R; Kerminen V-M; Junninen H; Paasonen P; Stratmann F; Herrmann H; Guenther AB; Worsnop DR; Kulmal M; Ehn M; Sipilä M Production of extremely low volatile organic compounds from biogenic emissions: Measured yields and atmospheric implications. Proc. Natl. Acad. Sci. U. S. A 2015, 112 (23), 7123–7128, DOI: 10.1073/pnas.1423977112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rissanen MP; Kurtén T; Sipilä M; Thornton JA; Kangasluoma J; Sarnela N; Junninen H; Jørgensen S; Schallhart S; Kajos MK; Taipale R; Springer M; Mentel TF; Ruuskanen T; Petäjä T; Worsnop DR; Kjaergaard HG; Ehn M The formation of highly oxidized multifunctional products in the ozonolysis of cyclohexene. J. Am. Chem. Soc 2014, 136 (44), 15596–15606, DOI: 10.1021/ja507146s [DOI] [PubMed] [Google Scholar]
- 33.Krechmer J; Coggon MM; Massoli P; Nguyen TB; Crounse JD; Hu W; Day DA; Tyndall GS; Henze DK; Rivera-Rios JC; Nowak JB; Kimmel JR; Mauldin RL III; Stark H; Jayne JT; Sipila M; Junninen H; St. Clair JM; Zhang X; Feiner PA; Zhang L; Miller DO; Brune WH; Keutsch FN; Wennberg PO; Seinfeld JH; Worsnop DR; Jimenez JL; Canagaratna MR Formation of low volatility organic compounds and secondary organic aerosol from isoprene hydroxyhydroperoxide low-NO oxidation. Environ. Sci. Technol 2015, 49 (17), 10330–10339, DOI: 10.1021/acs.est.5b02031 [DOI] [PubMed] [Google Scholar]
- 34.D’Ambro EL; Møller KH; Lopez-Hilfiker FD; Schobesberger S; Liu J; Shilling JE; Lee BH; Kjaergaard HG; Thornton JA Isomerization of second-generation isoprene peroxy radicals: epoxide formation and implications for secondary organic aerosol yields. Environ. Sci. Technol 2017, 51 (9), 4978–4987, DOI: 10.1021/acs.est.7b00460 [DOI] [PubMed] [Google Scholar]
- 35.Lewandowski M; Piletic IR; Kleindienst TE; Offenberg JH; Beaver MR; Jaoui M; Docherty KS; Edney EO Secondary organic aerosol characterization at field sites across the United States during the spring-summer period. Int. J. Environ. Anal. Chem 2013, 93, 1084–1103, DOI: 10.1080/03067319.2013.803545 [DOI] [Google Scholar]
- 36.Zhang X; Lambe AT; Upshur MA; Brooks WA; Be AG; Thomson RJ; Geiger FM; Surratt JD; Zhang Z; Gold A; Graf S; Cubiso MJ Highly oxygenated multifunctional compounds in α-pinene secondary organic aerosol. Environ. Sci. Technol 2017, 51, 5932–5940, DOI: 10.1021/acs.est.6b06588 [DOI] [PubMed] [Google Scholar]
- 37.Szmigielski R; Surratt JD; Gomez-Gonzalez Y; Van der Veken P; Kourtchev I; Vermeylen R; Blockhuys F; Jaoui M; Kleindienst TE; Lewandowski M; Offenberg JH; Edney EO; Seinfeld JH; Maenhaut W; Claeys M 3-methyl-1,2,3-butanetricarboxylic acid: an atmospheric tracer for terpene secondary organic aerosol. Geophys. Res. Lett 2007, 34 (6), L24811, DOI: 10.1029/2007GL031338 [DOI] [Google Scholar]
- 38.Jaoui M; Corse E; Kleindienst TE; Offenberg JH; Lewandowski M; Edney EO Analysis of secondary organic aerosol compounds from the photooxidation of d-limonene in the presence of NOX and their detection in ambient PM2.5. Environ. Sci. Technol 2006, 40 (12), 3819–3828, DOI: 10.1021/es052566z [DOI] [PubMed] [Google Scholar]
- 39.Jaoui M; Kamens R Mass balance of gaseous and particulate products analysis from α-pinene/NOx/air in the presence of natural sunlight. J. Geophys. Res 2001, 106, 12541–12558, DOI: 10.1029/2001JD900005 [DOI] [Google Scholar]
- 40.Kleindienst TE; Edney EO; Lewandowski M; Offenberg JH; Jaoui M Secondary organic carbon and aerosol yields from the irradiations of isoprene and alpha-pinene in the presence of NOx and SO2. Environ. Sci. Technol 2006, 40, 3807–3812, DOI: 10.1021/es052446r [DOI] [PubMed] [Google Scholar]
- 41.Jaoui M; Kleindienst TE; Lewandowski M; Edney EO Identification and quantification of aerosol polar oxygenated compounds bearing carboxylic and/or hydroxyl groups. 1. Method development. Anal. Chem 2004, 76, 4765–4778, DOI: 10.1021/ac049919h [DOI] [PubMed] [Google Scholar]
- 42.Jaoui M; Szmigielski R; Rudzinski KJ; Piletic IR; Lewandowski M; Riedel TP; Kleindienst TE Formation mechanisms of organic hydroxy acid tracers for aged isoprene aerosol: Implication to ambient PM2.5. Manuscript in preparation, 2019. [Google Scholar]
- 43.Nestorowicz K; Jaoui M; Rudziński KJ; Lewandowski M; Kleindienst T; Danikiewicz W; Szmigielski R Atmospheric oxidation of isoprene: influence of aerosol acidity and relative humidity on chemical composition of secondary organic aerosol. Atmos. Chem. Phys 2018, 18, 18101–18121, DOI: 10.5194/acp-18-18101-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Geron C Carbonaceaous aerosol characteristics over a Pinus Taeda Plantation: Results from the CELTIC experiment. Atmos. Environ 2011, 45, 794–801, DOI: 10.1016/j.atmosenv.2010.07.015 [DOI] [Google Scholar]
- 45.Kleindienst TE; Lewandowski M; Offenberg JH; Jaoui M; Edney EO Ozone-isoprene reaction: Re-examination of the formation of secondary organic aerosol. Geophys. Res. Lett 2007, 34, L01805, DOI: 10.1029/2006GL027485 [DOI] [Google Scholar]
- 46.Surratt JD; Lewandowski M; Offenberg JH; Jaoui M; Kleindienst TE; Edney EO; Seinfeld JH Effect of acidity on secondary organic aerosol formation from isoprene. Environ. Sci. Technol 2007, 41, 5363–5369, DOI: 10.1021/es0704176 [DOI] [PubMed] [Google Scholar]
- 47.Lewandowski M; Jaoui M; Offenberg JH; Krug JD; Kleindienst TE Atmospheric oxidation of isoprene and 1,3-butadiene: influence of aerosol acidity and relative humidity on secondary organic aerosol. Atmos. Chem. Phys 2015, 15 (7), 3773–3783, DOI: 10.5194/acp-15-3773-2015 [DOI] [Google Scholar]
- 48.Jenkin ME; Young JV; Rickard AR The MCM v3.3.1 degradation scheme for isoprene. Atmos. Chem. Phys 2015, 15, 11433–11459, DOI: 10.5194/acp-15-11433-2015 [DOI] [Google Scholar]
- 49.Lopez-Hilfiker FD; Mohr C; D’Ambro EL; Lutz A; Riedel TP; Gaston CJ; Iyer S; Zhang Z; Gold A; Surratt JD; Lee BH; Kurten T; Hu WW; Jimenez J; Hallquist M; Thornton JA Molecular composition and volatility of organic aerosol in the Southeastern U.S.: Implications for IEPOX derived SOA. Environ. Sci. Technol 2016, 50 (5), 2200–2209, DOI: 10.1021/acs.est.5b04769 [DOI] [PubMed] [Google Scholar]
- 50.Kleindienst TE; Lewandowski M; Offenberg JH; Jaoui M; Edney EO The formation of secondary organic aerosol from the isoprene + OH reaction in the absence of NOx. Atmos. Chem. Phys 2009, 9, 6541–6558, DOI: 10.5194/acp-9-6541-2009 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.