Abstract
The intracellular transport system in neurons is specialized to an extraordinary degree, enabling the delivery of critical cargo to sites in axons or dendrites that are far removed from the cell center. Vesicles formed in the cell body are actively transported by kinesin motors along axonal microtubules to presynaptic sites that can be located over a meter away. Both growth factors and degradative vesicles carrying aged organelles or aggregated proteins take the opposite route, driven by dynein motors. Distance is not the only challenge; precise delivery of cargos to sites of need must also be accomplished. For example, localized delivery of presynaptic components to hundreds of thousands of ‘en passant’ synapses distributed along the length of a single axon in some neuronal subtypes provides a layer of complexity that must be successfully navigated to maintain synaptic transmission. Here, we review recent advances in the field of axonal transport with a focus on conceptual developments, and highlight our growing quantitative understanding of neuronal trafficking and its role in maintaining the synaptic function that underlies higher cognitive processes such as learning and memory.
Print page summary
Background:
Neurons are polarized cells with extreme geometries. Multiple dendrites and one axon generally emerge from a single cell body and establish synaptic contacts with their partners. Synaptic maintenance and plasticity rely on the active delivery of newly synthesized components to these sites, which may be localized up to a meter from the cell body. Cargos that are actively trafficked along the axon include synaptic vesicle precursors, mitochondria, signaling endosomes, autophagosomes, lysosomes, and mRNA granules. In axons, these cargos use cytoplasmic dynein and kinesin motors to navigate a uniformly polarized microtubule network to reach their destinations. In dendrites, microtubules are organized in a more complex, bipolar pattern that is effectively navigated by a distinct subset of neuronal motor proteins and is less understood overall.
Advances:
Traditionally, the 1 meter-long axon of human lower motor neurons has been considered as a striking example of the long distances cargo must be conveyed from soma to synaptic terminal. Advances in connectomics and axonal tracing techniques are providing us with an increasingly accurate depiction of the morphology and size of axonal arbors in the central nervous system (CNS) and the many synaptic connections that mediate neuronal function. In humans, it is estimated that the axonal arbor of some neuronal populations in the CNS can range up to hundreds of meters in total length and contain thousands of ‘en passant’ synapses along its extent. Improved and complementary in vitro and in vivo imaging approaches are now allowing the elucidation of increasingly intricate mechanisms by which the activity of dynein and kinesin motors regulate organelle transport along axons. Recently, efforts have focused on identifying the adaptor proteins that specify motor-cargo selectivity and the regulatory mechanisms that govern the directed transport of cargo carrying opposing motor proteins. In parallel, significant advances are being made in our understanding of how the axonal microtubule network is organized and how changes at the microtubule level can affect motor activity to finely regulate axonal transport. A picture is now emerging whereby several regulatory layers exquisitely interplay to direct multiple steps of axonal transport including how motors initiate transport and how they respond to local cues to deliver cargo with high precision along the axon. This specific delivery is required to maintain synaptic function.
Outlook:
The intracellular transport system in neurons is specialized to an extraordinary degree, enabling the delivery of critical cargo to sites in axons or dendrites that are far removed from the cell center. Distance is not the only challenge. Localized delivery of presynaptic components provides another layer of complexity that must be successfully navigated to maintain synaptic transmission. Innovative approaches to determine the mechanisms regulating axonal transport and cargo delivery, the number and lifetime of presynaptic components, and the metabolic requirements to maintain synaptic activity are required. These advances will be key to assembling a more comprehensive and quantitative framework of axonal transport and its central role in presynaptic operation. A growing number of mutations across the molecular machinery involved in axonal transport are being identified. These mutations cause a range of neurodevelopmental and neurodegenerative diseases, underlining the importance of axonal transport in maintaining proper neuronal function. Both nerve injury and chemotherapy can also disrupt trafficking pathways in neurons. Hopefully, the mechanistic insights being developed now will provide a framework for the design of successful therapeutic interventions for both genetic and trauma-induced disruptions in axonal transport and synaptic function in the future.
Presynaptic maintenance and plasticity require both the delivery of newly synthesized cargo originating from the neuronal cell body, and the clearance of aged synaptic components. Both supply and clearance require that organelles effectively navigate long distances and the extreme geometries that axons can display in vivo. With our improved understanding of the dimension, complexity, and organization of the axonal arbor of some neuronal populations in the central nervous system (CNS), there is a concurrent evolution of the concept of axonal transport as a central mechanism for axonal function and presynaptic operation. While progress has begun to disentangle the “distance to travel” problem, new questions emerge regarding how the transport of multiple cargoes is specifically regulated to maintain the numerous presynaptic sites along the axon, each properly supplied with all the necessary components critical to ensure reliable neurotransmission. Thus, the effective trafficking of cargos within the neuron is the baseline requirement for the function of neuronal circuits, the ability to learn and create memories.
Microtubules and motors
Microtubules are the tracks used by the molecular motors cytoplasmic dynein and kinesin for long distance transport in neurons. Microtubules are formed from the head-to-tail polymerization of α- and β-tubulin heterodimers into protofilaments that assemble laterally to form a hollow tube. Neuronal microtubules are generally composed of 13 protofilaments, although differences exist among species and neuron types (1). Since αβ-tubulin heterodimers are assembled with the α-tubulin facing one end (minus-end) and β-tubulin the other (plus-end) of the microtubule, the overall polymer structure is polar. The microtubule minus-end region is relatively stable, whereas the plus-end can be more dynamic, actively growing and shrinking by addition and loss of tubulin heterodimers (2).
Microtubule-based motors are essential for the major long-range transport of cargos along the axon, moving over distances of up to a meter and speeds of 1–3 μm/s. There is a superfamily of more than 45 kinesins expressed in mammals, grouped into distinct families based on sequence homology and structural similarities. Motors from four of these families, kinesin-1, kinesin-2, kinesin-3 and kinesin-4 family motors, traffic cargos along the axon in the anterograde direction, moving outward from the soma. Other kinesins have been implicated in microtubule organization and remodeling (3). In contrast to this diversity, the retrograde transport of cargos toward the soma is driven by a single motor, cytoplasmic dynein. Dynein’s specificity for cargo is regulated by a broad group of activating adaptors (4). Myosin motors moving along actin filaments contribute to short-range cargo movement in neurons, particularly at synapses (3); it is becoming clear that microtubule- and actin-based dynamics tightly interact and that this interplay has important roles, especially in neural development and regeneration.
Axonal transport is typically divided into “fast” and “slow” components. The “fast” component includes the transport of vesicular cargo and organelles and generally occurs at relatively high velocity (>0.5 μm/sec), while the “slow” component includes the transport of soluble cargo such as synapsin or filamentous cargo such as neurofilaments and is significantly slower (<0.1 μm/sec) (5). Reliable transport and accurate delivery of fast-moving cargo to specialized regions in the axon relies on the integration of multiple internal and external signals that ultimately affect the motor-microtubule interaction. The multiple mechanisms mediating the axonal transport of cargo functionally converge to ensure the maintenance of presynaptic homeostasis, by replenishing presynapses with fresh or additional proteins or organelles such as synaptic vesicles and mitochondria, and removing old and defective synaptic components (6).
Axonal microtubule polarity
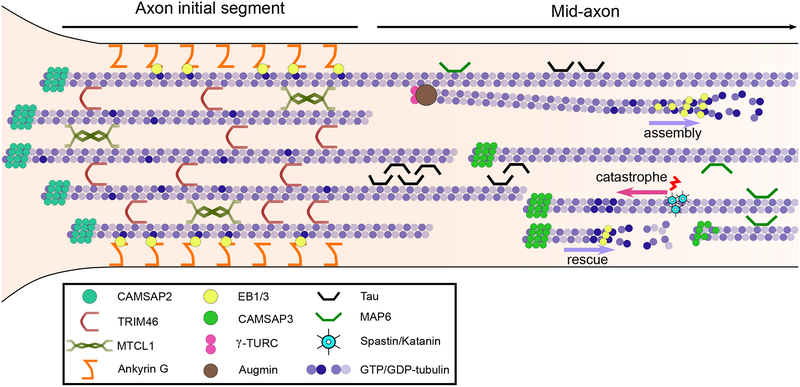
A characteristic feature of the axonal microtubule network is its “plus-end out” arrangement (7–9). It is not yet fully clear how axonal microtubule polarity is specified and maintained, but the formation and integrity of the axon initial segment (AIS) is emerging as an important determinant of this process. The AIS acts as a barrier separating the somatodendritic and axonal compartments (10). Dynein activity in the AIS is critical for maintaining axonal identity and microtubule polarity (11–13). The critical AIS component Ankyrin G interacts indirectly with peripheral AIS microtubules through end-binding (EB) proteins (14), which in the AIS are bound all along the microtubule lattice (14), rather than binding specifically to dynamic microtubule plus ends as is more commonly observed throughout the neuron. The microtubule cross-linkers MTCL1 (15) and TRIM46 (16, 17) also localize to the AIS. MTCL1 maintains Ankyrin G localization (15) and TRIM46 is a microtubule cross-linker that bundles parallel microtubules (16, 17). TRIM46 knock-down induces a dramatic increase in mixed microtubule polarity within the axon (16), suggesting that maintaining uniform microtubule polarity locally in the AIS is critical to maintaining the uniform microtubule polarity found throughout the axon.
The selective sorting of parallel microtubule cross-linkers to the axon can potentially facilitate the maintenance of the uniformly polarized microtubule network in this compartment. Early work has shown that prior to axon specification, the microtubule network is uniformly plus-end out in all minor neuritic processes (18). Selective microtubule stabilization in one minor process determines axon specification (19) and only after this event, the dendritic microtubule network polarity becomes progressively mixed (18). CAMSAP2, a microtubule minus-end-binding protein that stabilizes microtubules (20, 21) plays an important role in specifying neuronal polarity (22). Interestingly, CAMSAP2 accumulates proximally to the AIS in developing neurons (22). It is conceivable that in the early stages of axonal differentiation, axonal microtubule polarity is specified by the selective bundling of plus-end out microtubules in the AIS by TRIM46 and the minus-end stabilization of the bundled microtubules by CAMSAP2. This initial microtubule array could thus provide the template from which a uniformly polarized microtubule network can extend along the axon (Fig. 1).
Fig. 1. Axonal microtubule organization and polarity.
Stabilization of the microtubule “plus-end out” arrangement in the axon initial segment (AIS) is critical for axonal identity and likely acts as a template to establish the uniform polarity of the axonal microtubule network along the mid-axon.
Extension of the axonal microtubule network
Axonal elongation requires the persistent generation of properly oriented microtubules. In neurons, the centrosome gradually shuts down during development and neurite extension is sustained through acentrosomal microtubule nucleation (23) via the γ-tubulin ring complex (γTuRC) (22, 24). Augmin has recently been identified as an important factor for steering the polarity of axonal microtubule nucleation (24). Augmin recruits γTuRC to microtubule lattices to nucleate microtubule branches and together with NEDD1, likely controls the geometry of microtubule network assembly (24). Recently, SSNA1 was reported to induce branched microtubule polymerization (25). SSNA1 localization at axonal branch points (25) suggests that it may play a role in specifying microtubule plus-end out polarity to newly formed axonal branches. Microtubule plus-end guidance into axonal branches is assisted by the actin cytoskeleton via interactions with septin 7, which is located at the base of filopodia (26), and drebrin, which is located along the proximal region of filopodia (27).
Stabilization of newly formed microtubules is required to maintain axonal polarity. Mid- and distal axonal microtubules can be stabilized by the minus-end binding protein CAMSAP3 (28). The microtubule network along the axon can be further stabilized by interacting with endoplasmic reticulum (ER) tubules, likely through direct binding of the ER-shaping protein P180 to the microtubule lattice (29). Microtubule-associated proteins (MAPs), including MAP6 and Tau also stabilize axonal microtubules and assist the formation of axonal microtubule bundles (30, 31). In contrast to TRIM46 in the AIS (16), Tau does not have intrinsic parallel microtubule cross-linking properties (32). Nevertheless, evidence suggests that Tau-mediated bundling of distal microtubules is important to steer microtubule polarity and growth during axonal development (33).
The microtubule severing activity of katanin and spastin has been shown to amplify microtubule mass in vitro (34, 35). Microtubule severing followed by growth of the two resulting polymers can potentially assist in maintaining axonal microtubule polarity (36, 37). However, this mechanism has not yet been directly demonstrated in neurons. In fact, microtubule-severing function in neurons in vivo remains ambiguous. While spastin knock-out in Drosophila lead to a decreased microtubule density at terminal neuromuscular junction (NMJ) synapses (38), spastin knock-out mice show the opposite phenotype with increased axonal microtubule mass in NMJs (39). Future studies will be required to elucidate the roles of microtubule severing enzymes on axonal and presynaptic microtubule organization.
Axonal microtubule organization
Multiple electron microscopy (EM) studies agree that axonal microtubules are discontinuous structures. Estimates of microtubule length range from 0.05 to 40 μm in developing axons of cultured hippocampal neurons (40), and up to several hundred microns in some neuronal subtypes (41–43). These individual microtubules are organized to form a tiled array along the axon, allowing for continuous transport of cargos from soma to axon tip or tip to soma by kinesin and dynein motors. In myelinated axons, EM work has shown that microtubules are mostly continuous through nodes of Ranvier (42). In these specialized regions of shortened axonal diameter, the microtubule number remained virtually unchanged but the microtubule bundle was found to be more compacted (42) and to contain fasciculated microtubules, a feature that was previously thought to be unique to the AIS (44).
Technical challenges have thus far curtailed a thorough characterization of the axonal microtubule network, particularly at presynaptic sites. However, live fluorescence imaging approaches have been applied recently to investigate axonal microtubule organization. A novel approach based on the quantitative analysis of fluorescently-tagged patronin/CAMSAP and tubulin in was successfully applied to quantify microtubule number, length, and spacing along the axon in C. elegans neurons (45). This strategy confirmed the seminal findings of Chalfie and Thomson who investigated microtubule organization using EM (46), with the additional advantage that Yogev et al. (45) were able to spatially localize microtubule plus- and minus-ends within the tiled microtubule array of the axon while simultaneously monitoring the dynamicity of individual microtubules. This approach, collectively with other studies using fluorescence-based imaging techniques, including spinning disk confocal microscopy (47) and super-resolution microscopy (48), also confirmed that microtubules are more densely packed in the axon shaft, and become sparser and more splayed at growth cones and axonal termini. Distal microtubules are more dynamic and enriched in tyrosinated α-tubulin (48), a marker of newly polymerized microtubules (discussed below and see Box 1). Both the lower microtubule density and more dynamic nature of these microtubules have significant implications for the initiation of retrograde transport, as detailed below.
Box 1. Challenges in tackling the nanoscale distribution of tubulin modifications and isoforms.
Neuronal microtubules are highly modified and are likely an ensemble of multiple tubulin isoforms however, the spatial distribution of tubulin modifications and isoforms along the neuronal cytoskeleton at the nanoscale level is still unclear. Most of what is known about microtubule modifications in neurons stems from employing a handful of antibodies that target acetylated, tyrosinated/detyrosinated, and glutamylated (≥1 glutamate-long side-chain) and polyglutamylated tubulin (≥3 glutamate-long side-chain). Generally, these antibodies have a size of 15 nm and their optical detection requires binding with a secondary antibody of a similar size, resulting in a ~30 nm complex. Given that tubulin subunits are 4 nm apart, this approach is not suitable to resolve small-scale modifications. The problem is further compounded by the packed arrangement of the axonal microtubule array. Nano-antibodies against tubulin have been developed (194). Further increasing the repertoire to target different tubulin modifications and isoforms, and combining it with improved super-resolution microscopy techniques, such as expansion microscopy or the recently developed motor-PAINT (195), will reveal a more detailed picture of tubulin diversity along the axon and help understand how it affects the activity of motors proteins.
Analysis of microtubule dynamics in primary hippocampal neurons revealed that EB3 puncta initiate and terminate preferentially near or at presynaptic sites, indicating that synaptic regions show higher microtubule dynamicity than non-synaptic regions along the axon (49). These findings indicate that en passant synapses along the axon are enriched in highly dynamic microtubule plus-ends. An analogous microtubule organization was described in peripheral motor neuron axons of adult mice, where EB3 dynamics were found to be higher in the presynaptic areas of the NMJ compared to the non-synaptic intercostal sections of the axon (50). Together with previous EM work describing that microtubules in synaptic areas of olfactory axons are shorter compared to microtubules in the nerve proper (43), a picture of how the axonal microtubule network is organized is emerging. These studies suggest that long microtubules support efficient fast transport of vesicles along the axon, and in synaptic areas, shorter, more dynamic microtubules promote cargo pausing and local delivery to presynaptic sites.
Axonal microtubule diversity
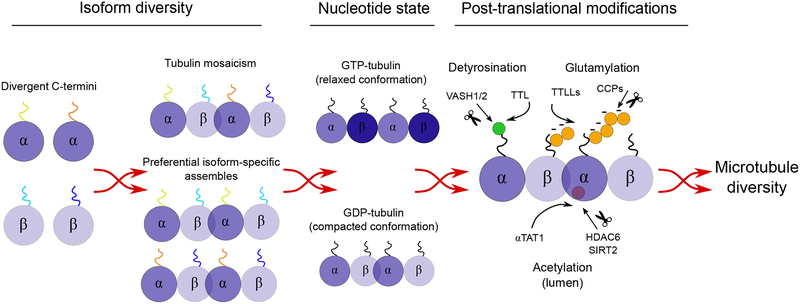
Microtubules provide the guiding tracks for motor movement along the axon, influencing motor activity through multiple levels of regulation. The tubulin nucleotide state, microtubule post-translational modifications (PTMs), and tubulin isoform composition act in a concerted way to create cytoskeleton specializations and locally regulate transport (Fig. 2).
Fig. 2. Axonal microtubule diversity.
From numerous tubulin isoforms, to distinct nucleotide states and several PTMs, microtubule lattices can in theory display a large number of combinatorial arrangements. This diversity can presumably be shown either at the single microtubule level (multitude of tubulin isoforms, nucleotide states, and PTMs distributed across the same microtubule lattice), or at the microtubule population level (uniformity of tubulin isoforms, nucleotide state and PTM distribution across microtubule populations, possibly conferring these a specific function).
Nucleotide state of tubulin
GTP-bound tubulin heterodimers incorporate into the growing tip of a microtubule. Tubulin polymerization and incorporation into the microtubule lattice catalyzes hydrolysis of the GTP bound to the β-subunit (51). This hydrolysis changes the conformation of the mammalian microtubule lattice from an expanded to a compacted state (52), influencing the affinity of several microtubule binding proteins. For example, EB proteins have a higher affinity for tubulin in a GTP-bound state and this underpins the dynamic recruitment of EBs to the growing microtubule tip where they regulate microtubule dynamics (53). In contrast, the lower affinity of the kinesin-3 motor KIF1A for GTP-tubulin facilitates release of this motor and its cargo from the microtubule upon encountering a dynamic microtubule plus-end (49).
For decades, the prevalent view was that GTP-tubulin incorporation was confined to the microtubule tip. However, this idea has been recently challenged by a series of elegant in vitro studies (34, 54, 55). This work shows that lattice defects originating from either mechanical stress or enzymatic activity can be rescued by the local incorporation of free tubulin, presumably in the GTP state. The controlled generation of GTP-tubulin islands along the microtubule lattice may locally regulate the function of specific microtubule effectors. Indeed, GTP-rich microtubules were detected in the AIS (56), where EB proteins are known to bind the length of microtubules (14), and at en passant synapses, where GTP-rich microtubules specify synaptic cargo delivery (49). It is unclear from these in vitro studies (34, 54, 55) whether and at what point the incorporation of GTP tubulin into the microtubule lattice is followed by GTP hydrolysis. Nevertheless, a system whereby microtubules undergo self-renewal through the incorporation of free tubulin heterodimers into a preexisting polymer could potentially extend the lifetime of a single microtubule indefinitely, akin to a “ship of Theseus” paradox. Notably, by bypassing the need to depolymerize the long axonal microtubules, this model would allow the continuous renewal of the microtubule network without interrupting active transport. Moreover, GTP-islands can create points of microtubule rescue and regrowth (54, 57, 58), and may be the mechanism underlying the high local microtubule dynamicity at presynaptic sites. Nevertheless, although incorporation of photo-converted tubulin into a preexisting microtubule lattice has been reported in cells (54), the extent with which cells and in particular neurons, rely on this mechanism to maintain their microtubule network remains unclear and needs to be further investigated.
Additional mechanisms may also affect the lattice structure of the microtubule, and thus the relative binding affinity of molecular motors and other microtubule-associated proteins. For example, recent work indicates that the binding of the motor protein kinesin-1 to GDP-microtubules is sufficient to induce the expansion of the microtubule lattice to a pseudo GTP-state (59, 60). This change in conformation is cooperative, with a ~10% binding saturation of a kinesin-1 motor domain predicted to be sufficient to expand the lattice across the whole microtubule (60). However, in vivo, the binding of kinesin-1 to microtubules is tightly regulated by auto-inhibition, so it will be important to assess the possible impact of this mechanism on the binding of other motors or effectors that are sensitive to the nucleotide- and conformation-states of the microtubule lattice.
Post-translational modifications of tubulin
Tubulin can be subjected to a wide range of PTMs. Acetylation, detyrosination, and polyglutamylation are particularly prevalent in axonal microtubules (61).
α-tubulin is acetylated in K40 by α-tubulin acetyltransferase (αTAT1) and deacetylated by histone deacetylase 6 (HDAC6) and sirtuin 2 (SIRT2) (62). It has long been proposed that α-tubulin acetylation regulates axonal transport however, the exact mechanism whereby the acetylation of a luminal tubulin residue can modify motor activity remains elusive (62). Recently, it was shown that acetylation increases microtubule resistance to mechanical breakage (63). Tubulin acetylation might be especially important for increasing the longevity of axonal microtubules given their long length and shape, which may be bent at axonal turns or by the sudden migration of microglia through the neuropil (64). Studies suggest that tubulin acetylation may also play a role in specifying proper axonal pathfinding. Loss of α-tubulin acetylation increased axonal microtubule dynamics (65), leading to excessive axonal branching in the cortex (65, 66), and distortion of the dentate gyrus in mice (67). Although spatial learning and memory were not impaired, mice in which α-tubulin acetylation was disrupted displayed increased anxiety-like behavior (65).
Detyrosinated microtubules are thought to be longer-lived than microtubules composed of tyrosinated α-tubulin, as the removal of the terminal tyrosine residue preferentially occurs once α-tubulin subunits are incorporated into polymer (68, 69), while retyrosination occurs on free tubulin subunits (70). There is a microtubule detyrosination gradient running down the axon of developing sensory neurons, with newer tyrosinated microtubules enriched at the distal axon (48). Tyrosination is enriched at the tips of growing microtubules (70) and in regions with high microtubule dynamicity (48), but a thorough picture of the distribution of microtubule tyrosination state at the nanoscale level along synapsing axons is still lacking (Box 1). The regulation of the α-tubulin tyrosination cycle is critical for proper neuronal differentiation and outgrowth, and influences axonal transport. Mice lacking tubulin tyrosine ligase (TTL) display disorganization of the cortical layers and die soon after birth (71). The recent identification of vasohibins (VASH1 and VASH2) as enzymes catalyzing the removal of the tyrosine residue from α-tubulin (68, 69) opens the door to the interrogation of how the complete tyrosination cycle affects neuronal function and in particular, axonal transport.
Negatively charged glutamate residues can be sequentially added or removed from the C-terminal chain of α- and β-tubulin by the tubulin tyrosine ligase-like (TTLL) and cytosolic carboxypeptidase (CCP) enzyme families, respectively (72, 73). The graded nature of this modification allows the fine control of several microtubule effectors, including spastin (74), and likely kinesin and dynein motors (see Initiation of transport section). Although axonal microtubules are generally highly glutamylated, it is unclear how the graded control of this modification is maintained along the axon and at presynaptic sites (Box 1). Importantly, loss-of-function mutations in the deglutamylase CCP1 affect mitochondrial transport in neurons (75) and cause infantile-onset neurodegeneration (76).
Isoform diversity of tubulin
The differential expression and incorporation of tubulin isoforms remains one of the least understood aspects contributing to microtubule diversity in neurons. Yeast express two α- and one β-tubulin, Drosophila four α- and 3–4 β-tubulins, C. elegans nine α- and six β-tubulins, while mice express seven α- and β-tubulin genes and humans express eight α- and nine β-tubulin genes. Members within each tubulin sub-family typically show >90% homology and identical structural properties. Functional specialization of tubulin isoforms has been demonstrated, but only in a few systems such as Drosophila axonemes (1). Mutations in tubulin genes can lead to severe neurological disorders, so both the expression pattern of these genes across neuronal populations and the localization of the resulting tubulin isoforms across neuronal compartments is an area of active research (77). The degree of isoform mosaicism in neuronal microtubules is still unknown, and whether specific tubulin isoforms are preferentially modified post-translationally, or whether tubulin isoform composition directly affects motor activity and/or MAP recruitment to microtubules also remains unclear. Only recently, improved systems for recombinant tubulin expression and isolation have allowed the examination of the impact of specific tubulin isoforms on microtubule dynamics and structure (78). Given the different levels of microtubule dynamicity (9, 49) and length (43) in synaptic and non-synaptic regions, it is tempting to speculate that microtubules assembled from particular isoforms may be more prevalent in certain neuronal regions, but further studies will be required to investigate these open questions (Box 2).
Box 2. Traveling cost of replenishing synaptic vesicles at the presynaptic compartment.
In the human cortex, the total ATP expenditure of a single pyramidal neuron on processes involving ion channel operation during activity and mediating axonal neurotransmission is ~4.8×109 ATP/sec (196, 197), 0.92×109 ATP/sec is consumed to maintain the resting membrane potential (196). Evidence indicates that the energy budget of a single cortical neuron is conserved among humans and rodents at a total of 4.95×1014 ATP/day (196), which are generated from glycolysis and mitochondrial oxidative phosphorylation (198). Using the mouse basal forebrain cholinergic neuron (see Axonal cargo distribution and delivery section) as a model and considering that SVPs are mostly delivered in the anterograde direction (49), the total distance traveled by SVPs throughout the whole axonal arbor each day is between 2.8 to 5.6 km. Because kinesin hydrolyzes one ATP molecule per step, a total of 3.5×1011to 7×1011 ATP molecules would be spent in this process (for simplicity we are assuming one single kinesin-3 dimer mediating the transport of one SVP). This indicates that only 0.07–0.14% of the total ATP budget is dedicated to transporting SVPs throughout the axonal arbor of mouse basal forebrain cholinergic neurons. This proportion will likely be higher in rat dopaminergic neurons, which have even larger axonal arbors with ~500,000 presynapses, or in human serotonergic neurons, which are estimated to extend axons for 350 meters (124). Nevertheless, even considering the energy expenditure of axonal SVP transport together with an estimate of ATP spent in the transport of other axonal and dendritic cargoes, the ATP directly devoted to microtubule-based transport is likely <1% of the total ATP budget of a single neuron. This highlights how efficient axonal transport and presynaptic replenishment are from an energetic point-of-view.
Regulation of the motors driving axonal transport
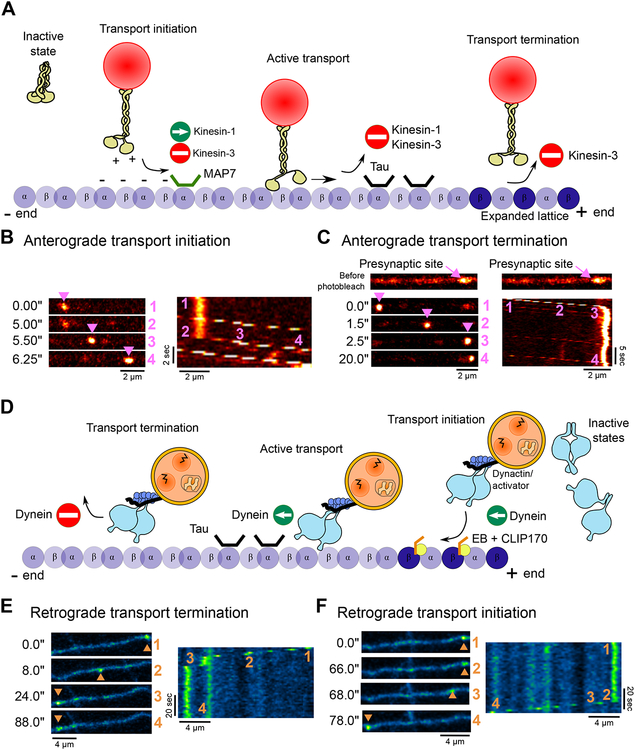
The axonal transport of organelles and other cargos is regulated by diverse mechanisms, including modification of the microtubule track through PTMs and/or MAP binding, the biophysical properties of the motor proteins involved, localized activation of motors by adaptors or other binding partners, and the integration of opposing motors bound to the same cargo by associated scaffolding proteins. These mechanisms lead to cargo-specific differences in transport along the axon, so that mitochondria are trafficked differently than autophagosomes for example, but also compartment-specific regulation, so that regulation of transport may be differentially tuned in the proximal axon or distal tip. Distinct phases of transport can be identified, including transport initiation, active transport, and localized delivery (Fig. 3).
Fig. 3. Axonal transport is regulated at multiple levels.
Despite having distinct biophysical properties, (A) kinesin and (D) dynein undergo similar transport phases. From an inactive unbound state, the motors bind to cargo and are recruited to the microtubule where they initiate active transport. MAPs, such as Tau or MAP7 can differentially affect motors and induce transport termination in a motor-specific manner. Kinesin-3 can sense the nucleotide state of the microtubule lattice and rapidly detaches upon encountering a dynamic GTP microtubule plus-end. Initiation (B) and termination (C) of synaptic vesicle precursor (SVP) transport along an axon of a live hippocampal neuron in culture expressing synaptophysin-mScarlet. SVPs are transported mostly in the anterograde direction by kinesin-3 and pause preferentially at presynaptic sites, which are enriched in dynamic GTP-rich microtubule plus-ends. Initiation (E) and termination (F) of autophagosome transport along the axon of a live cultured hippocampal neuron expressing LC3-GFP. Autophagosomes are mostly generated distally in the axon and driven by dynein in the retrograde direction towards the soma.
In the next sections, we focus on the mechanisms regulating the activity of kinesin-1 and −3 motors (kinesin-1: KIF5A/B/C; kinesin-3: KIF1A) because these are the best-studied motors mediating anterograde cargo transport in the axon. We also discuss the mechanisms regulating cytoplasmic dynein, the sole retrograde motor for axonal transport.
Initiation of transport
Mechanisms regulating transport initiation are critical in allowing kinesin-driven cargos entry into the axon to sustain cargo supply to presynapses. Mechanisms regulating transport initiation are also critical to govern the motility of dynein-dependent cargo, allowing cargo such as autophagosomes to escape the distal axon and thus clear aging or defective axonal components. Transport initiation mechanisms may also contribute to restarting motility following transient pausing and/or cargo detachment from microtubules, as motors must then bind to an adjacent microtubule to resume transport along the mid-axon.
Free cytosolic kinesin-1 and −3 are locked in an autoinhibited state. Upon binding to cargo, there is a conformation change that unlocks the kinesin motor and allows it to bind strongly to the microtubule. Track engagement initially occurs through an electrostatic interaction between positive charges in the kinesin motor domain and negative charges at the tubulin surface (79), and is stabilized upon ADP/ATP nucleotide exchange in the kinesin motor domain (80). The mode of kinesin/microtubule binding is mostly conserved between kinesin-1 and −3 however, kinesin-3 displays approximately 250x higher affinity for microtubules compared to kinesin-1 (80). The interaction between the positively charged loop-12 (K-loop) of kinesin-3 and the negatively charged C-terminal tails of α- and β-tubulin significantly contributes to this difference (81–83). In fact, it is likely that the regulation of the surface charge of the microtubule through the modification of the glutamate side-chain length of the tubulin C-terminal tail (74) can act as a mechanism to modulate kinesin-3 recruitment to the microtubule track along the axon. The nucleotide state of the microtubule can also directly influence motor binding. Kinesin-3 shows lower binding affinity to GTP- than GDP-like lattices, a feature that is specified by the motor loop-11 region (49). Kinesin-1 was reported to have higher affinity for GTP-like microtubules (56), but others observed this motor binding equally well to GTP- and GDP-like lattices (49, 84).
Motor subunits, accessory proteins, and MAPs can provide another regulatory layer modulating motor-microtubule interaction and transport initiation. For example, kinesin-1 activity is regulated in many contexts by associated kinesin light chains, which contribute to auto-inhibition and cargo binding (85). Kinesin-3 is negatively regulated by kinesin-binding protein (KBP), which binds directly to the kinesin-3 motor domain to inhibit motor-microtubule binding (86). KBP does not bind to kinesin-1 motors and thus can act as a selective motor regulator along the axon, although the mechanistic basis for this selectivity remains unclear.
Tau is an axonal MAP that inhibits the binding and motility of kinesin-1 (87, 88) and −3 (88) along microtubules by partially blocking the tubulin surface region with which the kinesin microtubule binding domain interacts (89). MAP7, on the other hand, promotes microtubule binding of kinesin-1 but inhibits binding of kinesin-3 (88). Detailed studies have shown that MAP7 interacts with the stalk domain of kinesin-1 (88, 90); this is thought to stabilize a conformation that facilitates binding to the microtubule (90). This effect is important for proper axonal cargo distribution, as the MAP7D2 isoform was recently shown to preferentially localize to microtubules in the proximal axon and regulate kinesin-1 recruitment and axonal sorting of kinesin-1 cargo (91).
Dynein activity is also tightly regulated in the neuron. The dynein complex can exist in two inactive forms, an auto-inhibited “phi (Φ)-particle” (92, 93) and an “open conformation” that does not undergo productive motility (93). To initiate active transport, dynein in the open conformation must bind to dynactin, a multi-subunit complex required to activate dynein-driven transport along the axon (94). The binding of an activating adaptor, such as BICD2 or Hook1 (4, 95–97) to form a tripartite dynein-dynactin-activator complex is also required to promote processive motility to the microtubule minus end. Dynein’s activating adaptors are cargo-specific, acting both to enhance the stability of the dynein-dynactin interaction and to mediate cargo interactions (4). For example, Hook1 activates the dynein-dependent retrograde motility of signaling endosomes, but is not required for the motility of mitochondria or autophagosomes (98).
Binding to dynactin promotes the binding of the dynein motor to microtubules by reorienting the dynein dimer to a more optimal binding conformation (96) and by mediating a direct interaction with microtubules through its glycine-rich (CAP-Gly) domain (99). The affinity of dynactin for microtubules is directly regulated by tubulin tyrosination (48, 100), a factor that spatially specifies the initiation of dynein-driven transport along the microtubule lattice. Dynein is robustly recruited to the dynamic plus-end of microtubules as part of a molecular programme that involves the sequential recruitment of EB and CLIP-170 proteins (48, 101). This mechanism is critical for dynein-based transport initiation at distal regions of the axon (48, 101), but might also play a role at presynaptic sites given the high dynamicity of the local microtubule network (49).
Active transport
Once transport is initiated, sustained motor activity conveys cargo along the axon, mediating cargo distribution to appropriate destinations such as presynapses or the axon terminal. The intrinsic biophysical properties of the motor are a major determinant on how far a cargo is transported prior to detachment from the microtubule. Kinesin-1 motors take 8 nm steps, which corresponds to the repeating αβ-tubulin dimer unit in the microtubule lattice. The two heads of kinesin-1 step alternately in a hand-over-hand fashion to produce processive motility. In vitro, kinesin-1 can take over 100 steps prior to detachment, totaling a distance of around 1 μm, while dimeric kinesin-3 can take over 500 steps and cover a distance of ~8 μm in a single run (49). In contrast to kinesin, dynein step size can be variable (8–32 nm) (102). The binding of dynein to dynactin and an activating adaptor such as BICD2 or Hook1 (4, 95, 103) modulates motor processivity and velocity, resulting in run lengths of 5–10 μm along a single microtubule, and velocities of 0.8 – 1.3 μm/s (104). Mechanistically, longer run lengths and faster velocities can be explained by the capacity of certain activating adaptors, including BICD2 and Hook3 to recruit two dynein motors to a single dynactin complex (104, 105).
In vivo, the biophysical properties of motor proteins are tightly regulated by the formation of high-order motor assemblies, or by interaction with accessory proteins. Motors can team up in a homo- (106) or heteromeric (107) assemblies to drive organelle transport. Non-invasive force measurements have determined that synaptic vesicle precursors in C. elegans neurons can be transported by 1 to 4 active kinesin-3 dimers (106). Estimates of motor number on axonal cargos such as late endosomes/lysosomes range from 1–4 kinesin motors and 1–5 dynein motors (50). Measurements of motor number using quantitative super-resolution microscopy confirm these estimates (108). Cooperation affects dynein and kinesin function differentially. While dynein teams optimize force production (109), multiple kinesin-1 motors bound to a single cargo mostly affect microtubule binding rates (110, 111).
Most organelle cargos examined to date, including late endosomes/lysosomes, mitochondria and autophagosomes, generally have oppositely directed dynein and kinesin motors bound simultaneously (112). The coordination between opposing motors can be governed by scaffolding proteins that mediate organelle-motor interaction (112). JIP1 is a scaffolding protein that regulates the trafficking of both autophagosomes and APP along the axon by binding opposing dynein and kinesin motors, and selectively regulating their activity (113, 114). Similarly, TRAKs1&2 are scaffolding proteins associated with mitochondria that are also thought to selectively regulate bound dynein and kinesin motors to control mitochondrial motility along the axon (115). Together, both adaptors and scaffolding proteins provide cargo-specific regulation of organelle trafficking along the axon (4, 112). Motor activity can be further tuned by modulations of the phosphorylation state of the motors or their associated adaptors and scaffolding proteins (116).
Recent evidence also implicates MAPs in motor regulation. The inhibitory effect of Tau on kinesin motility (87) was shown to preferentially induce dynein-mediated minus-end-directed transport of vesicles (117). Contrastingly, by promoting kinesin-1 recruitment and motility (88, 90, 118), decoration of microtubules with MAP7 biased vesicle transport to the plus-end (118). Given that MAP7 also exerts an inhibitory effect on kinesin-3 (88) these studies raise the interesting possibility that certain microtubules may be selectively decorated with MAPs to act as highways for specific motor or organelle populations, or alternatively to favor transport in a specific direction.
Transport, distribution and delivery of axonal cargo
Synaptic vesicle precursors.
Synaptic vesicle precursors (SVPs) are vesicles enriched in synaptic components such as synaptophysin, synaptotagmin, and vesicular amino acid transporters (e.g. VGLUT1) that replenish synaptic vesicle and active zone components at presynaptic sites. SVP transport is mainly driven by kinesin-3 (119), but kinesin-1-mediated transport of vesicles carrying active zone components has been reported (120). SVP movement along the axon is highly processive and efficient, averaging >3 μm/sec with few pauses (49); these pauses occur preferentially at microtubule termini (45). Presynaptic delivery of SVPs is specified by the high local density of GTP-rich dynamic microtubule plus-ends and the intrinsic low affinity kinesin-3 displays for GTP-rich microtubule lattices (49). DENN/MADD (121) and Arl-8 (122, 123) have also been implicated in the regulation of SVP presynaptic delivery. By binding the small GTPase Rab3 and the kinesin-3 stalk domain, DENN/MADD links the SVP to the microtubule (121). Because DENN/MADD binds GDP-Rab3 less efficiently, the nucleotide state of Rab3 can regulate SVP association with kinesin-3. Similarly, the small GTPase Arl-8 has been shown to bind and activate kinesin-3 when GTP-bound, but in the GDP state the binding is minimal (123). This GTPase nucleotide switch may be an important step to prevent SVP from escaping the presynapse by disengaging the SVP from the motor or preventing the SVP/motor complex from resuming transport after detaching from the microtubule.
The scale of successfully maintaining presynaptic site homeostasis and replenishing SVPs in a timely- and spatially-precise manner is becoming increasingly clear with advances in axonal tracing techniques, transport studies, and more accurate determination of protein lifetimes in neurons. For instance, the axonal arbor length of a cholinergic neuron projecting from the mouse basal forebrain is on average 30 cm and can branch >1000 times (124). Assuming that a presynapse is present at each branch point and an average intersynaptic distance of 4 μm along the entire axon, a simple model can be built in which a 4000 μm-long primary axon branches 296 μm-long secondary axons 1000 times. This results in 75 presynapses per branch and 75,000 total distributed throughout the entire axonal arbor. Presynaptic boutons contain ~200 synaptic vesicles (125). Studies in cultured neurons estimate that the average presynaptic protein lifetime is around 3.5 days (126) and that half of the synaptic vesicles residing at presynapses are turned over in <30 hours (127). Presynaptic protein lifetimes are 2–4 times longer in vivo (128). Using this range to scale-up synaptic vesicle lifetime, we can estimate that presynaptic sites are replenished with 20–40 new SVPs per day. This totals 1.5 – 3 million SVPs travelling a total distance of 2.8 to 5.6 km along the axon to replenish presynapses every day and a staggering 17–35 SVPs being generated each second in the cell body (Box 2). Early studies have determined that the exit flux from the Golgi in cell lines can reach 4,000 proteins per second (129). Given that a synaptic vesicle is composed of ~250 proteins (130), 16 synaptic vesicles could be produced at that rate. Considering that numerous other vesicles of the secretory pathway (e.g. lysosomes, postsynaptic cargoes) need to be simultaneously generated, one can contemplate the astonishingly high metabolic and secretory capacity of the ER and Golgi complex in neurons. Because synaptic vesicle lifetime is inversely proportional to the number of exo-endocytosis cycles it undergoes (127), it is likely that the replenishment rate of synaptic vesicles is modulated across neuronal populations with distinct firing patterns.
Mitochondria.
A significant portion of the neuronal metabolic capacity derives from mitochondria. The proper positioning of these organelles along the axon is critical for synaptic function. Mitochondrial movement is mainly regulated by kinesin-1 and dynein (115). In peripheral axons with terminal synapses, mitochondria tend to move processively in a single direction with an anterograde bias (131). In CNS axons, mitochondrial motility decreases in parallel with age and synaptic connectivity (132, 133). About half of mitochondria in the axon are stationed at presynaptic sites (133, 134) where they are an important ATP source (135) and regulate Ca2+ levels (134, 136). Delivery of mitochondria to presynaptic sites is controlled by local Ca2+ influx, which causes the accessory protein Miro on the mitochondrial outer membrane to change conformation and inhibit kinesin-mediated transport (137, 138). The acetylation state of Miro, which is in part controlled by the deacetylase HDAC6, inversely affects the sensitivity of Miro to Ca2+ (139). Because both kinesin and dynein interact with Miro via TRAK proteins (115), it is likely that this mechanism affects dynein-mediated transport as well. Despite the transient nature of Ca2+ influx at presynapses, mitochondria can remain stationed at these sites for days (133). The mechanisms controlling the long-term anchoring of mitochondria to the presynapse are still unclear, but there is evidence suggesting that syntaphilin docks mitochondria to presynaptic microtubules (140). Local myosin-actin interactions (141) and stable mitochondria-ER or mitochondria-plasma membrane contacts might also play a role.
Dense-core vesicles.
Dense-core vesicles (DCVs) convey a wide variety of neuropeptides along axons in a neuron type-dependent manner (142). DCV transport is mainly mediated by kinesin-3 (143) and dynein (144), although kinesin-1 might also be involved (145). Anterograde-moving DCVs frequently pause at presynaptic sites (49), where they are preferentially retained in an activity-dependent manner (49, 146, 147).
Late endosomes/lysosomes.
The anterograde transport of LAMP-1-positive vesicles (late endosomes/lysosomes) (148, 149) in axons is mainly mediated by kinesin-1 through an association with the BORC-Arl-8-SKIP complex (150), although kinesin-2 might also play a role (50). Dynein drives the retrograde motility of these organelles, regulated by JIP3 (151) and RILP in conjunction with Rab7 (152, 153). LAMP-1-positive vesicles move as rapidly as SVPs but pause more frequently (49). These pauses occur randomly along the axon, rather than the preferential pausing at synapses observed for SVPs (49) and likely underpin the even distribution of LAMP-1 vesicles observed throughout the axonal compartment (150). Late endosomes and lysosomes are involved in many processes. They are critical players in neuronal degradative pathways (154), Ca2+ regulation (155), and recently, late endosomes (Rab7- and LAMP-1-positive vesicles) were observed in association with RNA granules and to serve as platforms for local translation along the axon (156). Lysosomal dysfunction particularly affects presynaptic maintenance and homeostasis (157). An even distribution of these vesicles maintained by a random pausing pattern might be optimal to ensure efficient fusion with autophagosomes and sustain protein synthesis along the whole axonal compartment. Nevertheless, given the heterogeneous nature of these organelles (50, 148), a more thorough analysis of their transport and distribution using multiple markers simultaneously might reveal a more biased distribution of certain subpopulations to specific axonal sites and uncover their specialized roles.
Autophagosomes.
Autophagosomes and signaling endosomes are two vesicle populations characterized by robust retrograde transport throughout the axon. Both vesicle populations are generated distally in the axon (154, 158, 159) where dynein (160) and dynactin (101) are enriched. Autophagosomes initially show bidirectional oscillatory movement before engaging in processive retrograde transport (154). The accessory protein JIP1 was shown to be critical in resolving the tug-of-war between opposing motors by inhibiting kinesin-1 and allowing unopposed dynein-based movement (114). Huntingtin and HAP1 are also implicated in the regulation of autophagosome-bound motors (161). Robust retrograde transport of autophagosomes is intermittently interrupted along the axon; these pauses may correspond to fusion events with late-endosomes/lysosomes. Ultimately, axonal autophagosomes/autophagolysosomes are conveyed to the soma for the final steps in the degradation of engulfed proteins and organelles and the recycling of their components.
Signaling endosomes.
Dynein activity is also crucial for signaling endosome formation and processive transport to the cell body (98); both in transit and upon arrival in the soma these organelles regulate signaling pathways affecting neuronal survival, neuronal development, and synapse formation (159). The motility of signaling endosomes is regulated by the dynein adaptors Hook1 (98) and RILP (153). Given the diversity of signaling endosomes (162), future research will be required to elucidate whether distinct populations use different adaptors/activators depending on their maturation state or whether specific dynein adaptors/activators bind to particular receptor-ligand duos.
Cytosolic cargos.
Soluble protein supply to the axon can be sustained by slow axonal transport of proteins generated in the soma (5). Slow axonal transport (0.2–10 mm/day) has been directly shown for cytoskeleton elements, including tubulin, actin, and dynein (5, 160, 163), and also some presynaptic components, such as synapsin (164). Except for actin (163), slow axonal transport is largely dependent on microtubule-based fast transport and in particular, kinesin-1 movement through the transient association of the soluble cargo with the motor (160, 164). It is unknown to what extent neurons rely on slow axonal transport to supply soluble proteins to presynaptic sites. However, it is clear that the sole reliance on this mechanism would severely limit the capacity of a human central cholinergic neuron to rapidly adjust the supply of soluble protein throughout its entire >100 meters-long axonal arbor (124) upon sudden surges in local protein demand. Moreover, a soluble protein synthesized in the soma would take over 100 days to reach the tip of a 1 meter-long human lower motor neuron axon, 450 days to the tip of the 4.5 meter-long recurrent laryngeal nerve axon in the giraffe, and 8 years to the tip of a 30 meter-long spinal axon in blue whales. These numbers are incompatible with the lifetime of most cytosolic proteins in neurons (half-life tubulin: ~30 days; dynein heavy-chain: ~6 days) (128).
RNA transport.
Axons are populated by a wide range of mRNA transcripts (165), which are actively translated in vivo (166). Local translation of mRNAs in the axon circumvents the limitations related with short protein lifetimes and in principle, can rapidly respond to changes in protein demand and tune local protein supply. However, this mechanism is likely limited to expression of cytosolic proteins because there is limited Golgi in the axon, at least in rodents (158) – it is possible that in larger mammals there is a higher prevalence of Golgi in the axon to locally synthesize membrane-associated proteins rather than waiting days for export from the soma. In neurons, RNA-binding proteins bind to mRNA forming specialized granules with liquid-like properties (167) and evidence suggests that their transport can be directly mediated by kinesin-1 and dynein (168, 169). Recently, it was shown that axonal mRNA distribution can also be achieved by hitchhiking on late endosomes (156). Critically, the mechanisms controlling mRNA stability or the distribution of ribosomes necessary for local translation along the axon remain unclear. Future studies are needed to address these questions and reveal the key roles of local translation in axonal and presynaptic function (Box 3).
Box 3. Outstanding questions.
Among the many open questions in neuronal cell biology, how is the sub-compartmentalization of the neuron specified is one of the most fundamental. An increasing number of molecular players involved in the stabilization of the microtubule network at the AIS have been described, but the exact mechanisms that sort these into the neurite that is to become the axon, and/or exclude them from pre-dendritic processes, are still unclear. There are at least 20 kinesin motor isoforms expressed in hippocampal neurons (199). The function of most of these motors in neurons and their relevance for axonal transport is still unclear. Furthermore, the roles of dynein activating adaptors in defining dynein cargo recognition and transport regulation in neurons will also be an active area of research. In addition, a detailed depiction of the microtubule network organization along whole axons is still absent. Improved cryo-EM techniques and computational approaches to reconstruct serial sections will play an important role in unveiling this. Correlative light and electron microscopy approaches will assist in deepening our understanding of microtubule dynamics and organization. A particular area of interest will be to unambiguously demonstrate that incorporation of native GTP-tubulin into a preexisting microtubule lattice occurs in vivo. Local translation in the axon potentially has important implications for the replenishment of soluble proteins and maintaining a healthy pool of mitochondria at presynaptic sites. The mechanisms that mediate the distribution and stability of mRNA, and the transport and/or assembly of ribosomes throughout the axonal arbor are not well understood and is an area of interest for future studies. Finally, the growing list of known mutations affecting components of the axonal transport machinery and causative of a broad range of neurological disorders highlights gene-editing approaches as a promising research avenue to uncover suitable therapeutic strategies for these disorders.
Axonal transport and disease
From motor proteins to adaptors to tubulin subunits, mutations affecting components of the axonal transport machinery have been implicated in a broad range of neurological disorders. Advances in DNA sequencing techniques have identified mutations in kinesin-1 and kinesin-3 as causative of a broad range of neurological disorders, including hereditary spastic paraplegia (HSP), amyotrophic lateral sclerosis (ALS), epilepsy, and severe intellectual deficits (170–173). Similarly, mutations in dynein, primarily in the DYNC1H1 gene encoding the cytoplasmic dynein heavy chain, have been implicated in a range of neurodevelopmental and neurodegenerative diseases (reviewed in 174), including Charcot-Marie Tooth disease type 2O (CMT-2O) and spinal muscular atrophy-lower extremity predominant (SMA-LED). Mutations in kinesin- and dynein-binding proteins have also been implicated in disease. For example, missense mutations on the kinesin-3 inhibitor KBP were found to be causative of Goldberg-Shprintzen syndrome (GOSHS) (175), a severe neurological disorder characterized by microcephaly and mental retardation. Mutations in the dynein-binding protein Lis1 cause severe lissencephaly, primarily due to defects in neuronal migration, but recent work has also established a critical role for Lis1 in maintaining axonal transport in the adult brain (176). Mutations in the DCTN1 gene encoding the p150Glued subunit of dynactin affect the initiation of retrograde transport (101); these mutations are directly implicated in Perry syndrome, an aggressive form of Parkinsonism (177). A distinct mutation within the same domain causes a rare form of motor neuron disease, HMN7B (178). Similarly, mutations in BICD2, an activating adaptor for dynein, lead to spinal muscular atrophy (179–181), characterized by lower motor neuron degeneration.
Mutations in associated effectors or scaffolding proteins can also lead to disease. For example, Rab7 is anchored to the membrane of late-endosomes/lysosomes and is involved in dynein/dynactin motor complex recruitment (152). Mutations in Rab7 have been implicated in Charcot-Marie-Tooth disease (182) and shown to disrupt endosome axonal transport in sensory neurons (183). Huntingtin can act as a motor scaffold coordinating the activity of kinesin/dynein motor complexes (112). Pathogenic expansion of the polyQ repeat region of huntingtin deleteriously affects the scaffolding functions of the protein, disrupting the axonal transport of multiple cargoes, including autophagosomes and mitochondria (161, 184, 185). Finally, mutations that alter microtubule organization or regulation can cause disease. For example, mutations in various α- and β-tubulin isoforms lead to brain malformations and neurodevelopment disorders (186). Two have been located at or near the GTP nucleotide-binding pocket of β-tubulin (187, 188) and likely affect the nucleotide-state transition directly, while one in α-tubulin was recently shown to directly impair dynein motor activity (189).
Axonal dynamics are a tightly orchestrated mechanism, required to maintain neuronal health and function through a lifespan that may reach 90–100 years, so it is not surprising that mutations that disrupt these dynamics are tightly associated with both neurodevelopmental and neurodegenerative disease. However, it remains unclear why mutations in genes required for axonal transport result in such a wide variety of diseases, affecting very different stages of development or distinct neuronal subtypes. For example, mutations within the DYNC1H1 gene encoding the cytoplasmic dynein heavy chain can induce either intellectual disability or distal limb weakness, or both (174). As we learn more about the specific interactions among motors, adaptors, and cargo being transported, the pathobiology induced by mutations in the axonal transport machinery may become clearer.
Directly targeting the transport machinery and the microtubule network to restore rates of axonal transport has long been considered a promising therapeutic strategy to slow the progression of neurodegenerative diseases. More recently, the pharmacological modulation of microtubule dynamics with microtubule-stabilizing drugs has been shown to promote axonal regeneration after axonal injury (190–192). It is known that axonal transport plays a critical role in sustaining axonal regeneration (193); whether the pro-regeneration effects of microtubule-stabilizing drugs are directly associated with a boosting of anterograde and retrograde trafficking to and from the injury site remains to be elucidated.
Concluding remarks
Axonal transport sustains synaptic and neuronal function by delivering the necessary components to support neurotransmission at presynaptic sites and carrying aged organelles or signaling vesicles to the soma. From the functional diversity provided by tubulin isoforms and PTMs to the different ways motors engage with microtubules or interact with accessory proteins to bind cargo, the distinct layers that regulate axonal transport are many but coalesce to efficiently direct the distribution of a wide range of cargo along the axonal arbor. While significant advances in single-molecule and in vivo approaches are enabling a deeper understanding of how these regulatory layers interplay, they are also uncovering the true scale of the task that maintains hundreds of thousands of synapses operating effectively within the same neuron. Further application of innovative and complementary approaches will continue to build a coherent view of the mechanisms that maintain axonal and synaptic homeostasis through life (Fig. 4).
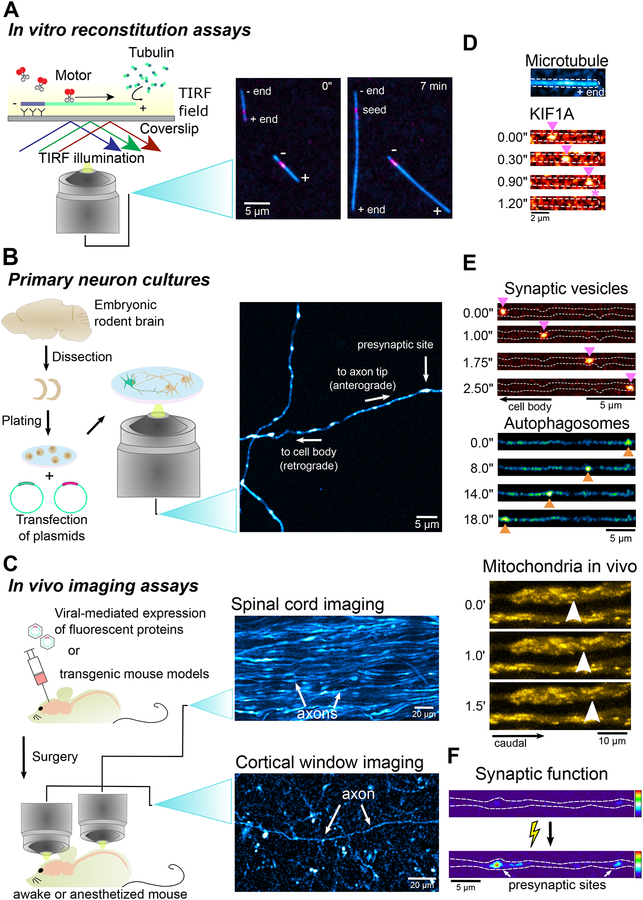
Fig. 4. Range of imaging approaches to address questions in the field of axonal transport.
A wide range of imaging assays can be employed to investigate the dynamics of biological processes in systems with different levels of biological complexity. For example: A) Combining in vitro reconstitution assays with total internal reflection fluorescence (TIRF) microscopy allows the assessment of the dynamic behavior of single motors on microtubules. The ability to easily manipulate the system by adding or removing components enables the elucidation of how single factors (e.g. MAPs) affect motor activity or what are the minimum components required in the system to observe a specific dynamic behavior. Panels show the basic setup of an in vitro reconstitution assay and TIRF microscopy imaging of polymerization of fluorescently-labelled tubulin. B) Primary rodent CNS neurons in culture undergo a well-described polarized developmental process akin to what is observed in vivo. Neurons develop an axon and multiple dendrites, establish synaptic contacts with their neighbors, and fire action potentials spontaneously. The combined ability to easily manipulate the neurons genetically and pharmacologically, express fluorescent reporters and use imaging techniques with high spatial and temporal resolution such as spinning disk confocal microscopy, make this system ideal to investigate the mechanisms underlying organelle dynamics in a physiologically-relevant context. Panels show a basic protocol to image primary neuron cultures and a confocal microscopy image of hippocampal axons in culture expressing fluorescently-tagged synaptophysin. C) The development of Cre mouse lines, improved adeno-associated viral vectors, and the advent of CRISPR/Cas9 gene-editing techniques have enabled more straightforward strategies of genetically manipulating mice, generating transgenic mouse lines, and expressing fluorescent reporters even in a neuron sub-type-specific manner. Innovative surgical techniques, including spinal cord exposure and cortical window implementation allow long-term optical access to the spinal cord and superficial layers of the cortex. By employing two-photon microscopy techniques, it is thus possible to interrogate organelle dynamics and synaptic function in neurons in their native environment. Panels show a simple strategy to perform in vivo imaging assays, an image of axonal bundles in the spinal cord of a Thy1-YFP mouse, and a cholinergic axon in cortical layer I/II of a ChAT-Cre ROSA26-EGFPf mouse. D) TIRF imaging of a single dimeric KIF1A motor labeled with TMR actively moving on a fluorescently-labelled microtubule in an in vitro reconstitution assay. E) Synaptic vesicle precursors (labeled with synaptophyin-mScarlet) move mostly in the anterograde direction, while autophagosomes (labeled with LC3-GFP) show robust retrograde movement along the axons of live neurons in culture. Two-photon microscopy imaging of mitochondrial dynamics in spinal cord axons of a Thy1-MitoCFP mouse. F) VGLUT1-pHluorin signal in a primary hippocampal neuron before and after electrical-field stimulation. An increased repertoire of fluorescent pH and Ca2+ indicators are allowing the study of how a variety of processes and mechanisms regulating axonal transport affects synaptic fuhnction and neuronal activity.
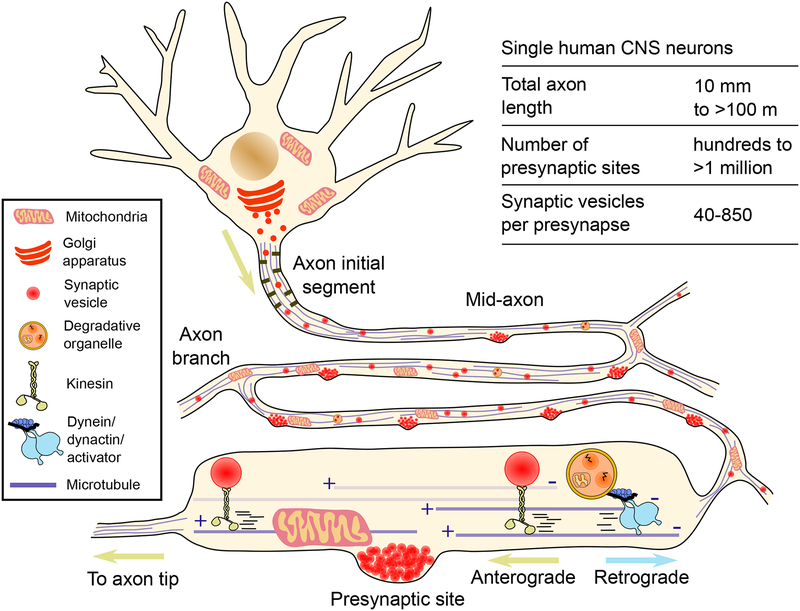
Fig. 0. Axonal transport drives cargo through extreme geometries.
Neurons in the human central nervous system (CNS) display highly complex axonal arbors that can branch thousands of times, reach hundreds of meters in total length, and contain hundreds of thousands of presynaptic sites distributed ‘en passant’. The axonal transport machinery is critical for synaptic function by delivering new synaptic vesicles to and removing aged organelles from presynaptic sites.
Acknowledgements
The authors would like to thank Nicolas Snaidero, Marina Herwerth, and Thomas Misgeld for assistance in the acquisition of the in vivo imaging data shown in Fig. 4. We acknowledge support from NIH grant R35 GM126950 to E.L.F.H. P.G-D. was supported by the Elite Network Bavaria via the ‘Biomedical Neuroscience’ program and by the Deutsche Forschungsgemeinschaft (German Research Foundation) within the framework of the Munich Cluster for Systems Neurology (EXC 2145 SyNergy). P.G-D. was additionally supported by an Alexander von Humboldt Research Fellowship.
References and Notes
- 1.Chaaban S, Brouhard GJ, A microtubule bestiary: structural diversity in tubulin polymers. Molecular biology of the cell 28, 2924–2931 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akhmanova A, Steinmetz MO, Control of microtubule organization and dynamics: two ends in the limelight. Nature reviews. Molecular cell biology 16, 711–726 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Nirschl JJ, Ghiretti AE, Holzbaur ELF, The impact of cytoskeletal organization on the local regulation of neuronal transport. Nature reviews. Neuroscience 18, 585–597 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olenick MA, Holzbaur ELF, Dynein activators and adaptors at a glance. Journal of cell science 132, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roy S, Seeing the unseen: the hidden world of slow axonal transport. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry 20, 71–81 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vijayan V, Verstreken P, Autophagy in the presynaptic compartment in health and disease. The Journal of cell biology 216, 1895–1906 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baas PW, Deitch JS, Black MM, Banker GA, Polarity orientation of microtubules in hippocampal neurons: uniformity in the axon and nonuniformity in the dendrite. Proceedings of the National Academy of Sciences of the United States of America 85, 8335–8339 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stepanova T et al. , Visualization of microtubule growth in cultured neurons via the use of EB3-GFP (end-binding protein 3-green fluorescent protein). The Journal of neuroscience : the official journal of the Society for Neuroscience 23, 2655–2664 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kleele T et al. , An assay to image neuronal microtubule dynamics in mice. Nature communications 5, 4827 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leterrier C, The Axon Initial Segment: An Updated Viewpoint. The Journal of neuroscience : the official journal of the Society for Neuroscience 38, 2135–2145 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuijpers M et al. , Dynein Regulator NDEL1 Controls Polarized Cargo Transport at the Axon Initial Segment. Neuron 89, 461–471 (2016). [DOI] [PubMed] [Google Scholar]
- 12.Zheng Y et al. , Dynein is required for polarized dendritic transport and uniform microtubule orientation in axons. Nature cell biology 10, 1172–1180 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klinman E, Tokito M, Holzbaur ELF, CDK5-dependent activation of dynein in the axon initial segment regulates polarized cargo transport in neurons. Traffic 18, 808–824 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leterrier C et al. , End-binding proteins EB3 and EB1 link microtubules to ankyrin G in the axon initial segment. Proceedings of the National Academy of Sciences of the United States of America 108, 8826–8831 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Satake T et al. , MTCL1 plays an essential role in maintaining Purkinje neuron axon initial segment. The EMBO journal 36, 1227–1242 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Beuningen SFB et al. , TRIM46 Controls Neuronal Polarity and Axon Specification by Driving the Formation of Parallel Microtubule Arrays. Neuron 88, 1208–1226 (2015). [DOI] [PubMed] [Google Scholar]
- 17.Harterink M et al. , TRIM46 Organizes Microtubule Fasciculation in the Axon Initial Segment. The Journal of neuroscience : the official journal of the Society for Neuroscience 39, 4864–4873 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baas PW, Black MM, Banker GA, Changes in microtubule polarity orientation during the development of hippocampal neurons in culture. The Journal of cell biology 109, 3085–3094 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Witte H, Neukirchen D, Bradke F, Microtubule stabilization specifies initial neuronal polarization. The Journal of cell biology 180, 619–632 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hendershott MC, Vale RD, Regulation of microtubule minus-end dynamics by CAMSAPs and Patronin. Proceedings of the National Academy of Sciences of the United States of America 111, 5860–5865 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang K et al. , Microtubule minus-end stabilization by polymerization-driven CAMSAP deposition. Developmental cell 28, 295–309 (2014). [DOI] [PubMed] [Google Scholar]
- 22.Yau KW et al. , Microtubule minus-end binding protein CAMSAP2 controls axon specification and dendrite development. Neuron 82, 1058–1073 (2014). [DOI] [PubMed] [Google Scholar]
- 23.Stiess M et al. , Axon extension occurs independently of centrosomal microtubule nucleation. Science 327, 704–707 (2010). [DOI] [PubMed] [Google Scholar]
- 24.Sanchez-Huertas C et al. , Non-centrosomal nucleation mediated by augmin organizes microtubules in post-mitotic neurons and controls axonal microtubule polarity. Nature communications 7, 12187 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Basnet N et al. , Direct induction of microtubule branching by microtubule nucleation factor SSNA1. Nature cell biology 20, 1172–1180 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu J et al. , Septin-driven coordination of actin and microtubule remodeling regulates the collateral branching of axons. Current biology : CB 22, 1109–1115 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ketschek A et al. , Drebrin coordinates the actin and microtubule cytoskeleton during the initiation of axon collateral branches. Developmental neurobiology 76, 1092–1110 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pongrakhananon V et al. , CAMSAP3 maintains neuronal polarity through regulation of microtubule stability. Proceedings of the National Academy of Sciences of the United States of America 115, 9750–9755 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farias GG et al. , Feedback-Driven Mechanisms between Microtubules and the Endoplasmic Reticulum Instruct Neuronal Polarity. Neuron 102, 184–201 e188 (2019). [DOI] [PubMed] [Google Scholar]
- 30.Tortosa E et al. , Dynamic Palmitoylation Targets MAP6 to the Axon to Promote Microtubule Stabilization during Neuronal Polarization. Neuron 94, 809–825 e807 (2017). [DOI] [PubMed] [Google Scholar]
- 31.Chung PJ et al. , Tau mediates microtubule bundle architectures mimicking fascicles of microtubules found in the axon initial segment. Nature communications 7, 12278 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prezel E et al. , Tau can switch microtubule network organizations: from random networks to dynamic and stable bundles. Molecular biology of the cell 29, 154–165 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Biswas S, Kalil K, The Microtubule-Associated Protein Tau Mediates the Organization of Microtubules and Their Dynamic Exploration of Actin-Rich Lamellipodia and Filopodia of Cortical Growth Cones. The Journal of neuroscience : the official journal of the Society for Neuroscience 38, 291–307 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vemu A et al. , Severing enzymes amplify microtubule arrays through lattice GTP-tubulin incorporation. Science 361, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuo YW, Trottier O, Mahamdeh M, Howard J, Spastin is a dual-function enzyme that severs microtubules and promotes their regrowth to increase the number and mass of microtubules. Proceedings of the National Academy of Sciences of the United States of America 116, 5533–5541 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kapitein LC, Hoogenraad CC, Building the Neuronal Microtubule Cytoskeleton. Neuron 87, 492–506 (2015). [DOI] [PubMed] [Google Scholar]
- 37.Leterrier C, Dubey P, Roy S, The nano-architecture of the axonal cytoskeleton. Nature reviews. Neuroscience 18, 713–726 (2017). [DOI] [PubMed] [Google Scholar]
- 38.Sherwood NT, Sun Q, Xue M, Zhang B, Zinn K, Drosophila spastin regulates synaptic microtubule networks and is required for normal motor function. PLoS biology 2, e429 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brill MS et al. , Branch-Specific Microtubule Destabilization Mediates Axon Branch Loss during Neuromuscular Synapse Elimination. Neuron 92, 845–856 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu W, Baas PW, Changes in microtubule number and length during axon differentiation. The Journal of neuroscience : the official journal of the Society for Neuroscience 14, 2818–2829 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bray D, Bunge MB, Serial analysis of microtubules in cultured rat sensory axons. Journal of neurocytology 10, 589–605 (1981). [DOI] [PubMed] [Google Scholar]
- 42.Tsukita S, Ishikawa H, The cytoskeleton in myelinated axons: serial section study. Biomedical Research 2, 424–237 (1981). [Google Scholar]
- 43.Burton PR, Microtubules of frog olfactory axons: their length and number/axon. Brain research 409, 71–78 (1987). [DOI] [PubMed] [Google Scholar]
- 44.Nakazawa E, Ishikawa H, Occurrence of fasciculated microtubules at nodes of Ranvier in rat spinal roots. Journal of neurocytology 24, 399–407 (1995). [DOI] [PubMed] [Google Scholar]
- 45.Yogev S, Cooper R, Fetter R, Horowitz M, Shen K, Microtubule Organization Determines Axonal Transport Dynamics. Neuron 92, 449–460 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chalfie M, Thomson JN, Organization of neuronal microtubules in the nematode Caenorhabditis elegans. The Journal of cell biology 82, 278–289 (1979). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burnette DT et al. , Myosin II activity facilitates microtubule bundling in the neuronal growth cone neck. Developmental cell 15, 163–169 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nirschl JJ, Magiera MM, Lazarus JE, Janke C, Holzbaur EL, alpha-Tubulin Tyrosination and CLIP-170 Phosphorylation Regulate the Initiation of Dynein-Driven Transport in Neurons. Cell reports 14, 2637–2652 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guedes-Dias P et al. , Kinesin-3 Responds to Local Microtubule Dynamics to Target Synaptic Cargo Delivery to the Presynapse. Current biology : CB 29, 268–282 e268 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hendricks AG et al. , Motor coordination via a tug-of-war mechanism drives bidirectional vesicle transport. Current biology : CB 20, 697–702 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brouhard GJ, Rice LM, Microtubule dynamics: an interplay of biochemistry and mechanics. Nature reviews. Molecular cell biology 19, 451–463 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang R, LaFrance B, Nogales E, Separating the effects of nucleotide and EB binding on microtubule structure. Proceedings of the National Academy of Sciences of the United States of America 115, E6191–E6200 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van de Willige D, Hoogenraad CC, Akhmanova A, Microtubule plus-end tracking proteins in neuronal development. Cellular and molecular life sciences : CMLS 73, 2053–2077 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aumeier C et al. , Self-repair promotes microtubule rescue. Nature cell biology 18, 1054–1064 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schaedel L et al. , Microtubules self-repair in response to mechanical stress. Nature materials 14, 1156–1163 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nakata T, Niwa S, Okada Y, Perez F, Hirokawa N, Preferential binding of a kinesin-1 motor to GTP-tubulin-rich microtubules underlies polarized vesicle transport. The Journal of cell biology 194, 245–255 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dimitrov A et al. , Detection of GTP-tubulin conformation in vivo reveals a role for GTP remnants in microtubule rescues. Science 322, 1353–1356 (2008). [DOI] [PubMed] [Google Scholar]
- 58.Tropini C, Roth EA, Zanic M, Gardner MK, Howard J, Islands containing slowly hydrolyzable GTP analogs promote microtubule rescues. PloS one 7, e30103 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Peet DR, Burroughs NJ, Cross RA, Kinesin expands and stabilizes the GDP-microtubule lattice. Nature nanotechnology 13, 386–391 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shima T et al. , Kinesin-binding-triggered conformation switching of microtubules contributes to polarized transport. The Journal of cell biology 217, 4164–4183 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Park JH, Roll-Mecak A, The tubulin code in neuronal polarity. Current opinion in neurobiology 51, 95–102 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Janke C, Montagnac G, Causes and Consequences of Microtubule Acetylation. Current biology : CB 27, R1287–R1292 (2017). [DOI] [PubMed] [Google Scholar]
- 63.Portran D, Schaedel L, Xu Z, Thery M, Nachury MV, Tubulin acetylation protects long-lived microtubules against mechanical ageing. Nature cell biology 19, 391–398 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tonnesen J, Inavalli V, Nagerl UV, Super-Resolution Imaging of the Extracellular Space in Living Brain Tissue. Cell 172, 1108–1121 e1115 (2018). [DOI] [PubMed] [Google Scholar]
- 65.Dan W et al. , alpha-Tubulin Acetylation Restricts Axon Overbranching by Dampening Microtubule Plus-End Dynamics in Neurons. Cerebral cortex 28, 3332–3346 (2018). [DOI] [PubMed] [Google Scholar]
- 66.Xu Z et al. , Microtubules acquire resistance from mechanical breakage through intralumenal acetylation. Science 356, 328–332 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim GW, Li L, Ghorbani M, You L, Yang XJ, Mice lacking alpha-tubulin acetyltransferase 1 are viable but display alpha-tubulin acetylation deficiency and dentate gyrus distortion. The Journal of biological chemistry 288, 20334–20350 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Aillaud C et al. , Vasohibins/SVBP are tubulin carboxypeptidases (TCPs) that regulate neuron differentiation. Science 358, 1448–1453 (2017). [DOI] [PubMed] [Google Scholar]
- 69.Nieuwenhuis J et al. , Vasohibins encode tubulin detyrosinating activity. Science 358, 1453–1456 (2017). [DOI] [PubMed] [Google Scholar]
- 70.Szyk A, Deaconescu AM, Piszczek G, Roll-Mecak A, Tubulin tyrosine ligase structure reveals adaptation of an ancient fold to bind and modify tubulin. Nature structural & molecular biology 18, 1250–1258 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Erck C et al. , A vital role of tubulin-tyrosine-ligase for neuronal organization. Proceedings of the National Academy of Sciences of the United States of America 102, 7853–7858 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.van Dijk J et al. , A targeted multienzyme mechanism for selective microtubule polyglutamylation. Molecular cell 26, 437–448 (2007). [DOI] [PubMed] [Google Scholar]
- 73.Rogowski K et al. , A family of protein-deglutamylating enzymes associated with neurodegeneration. Cell 143, 564–578 (2010). [DOI] [PubMed] [Google Scholar]
- 74.Valenstein ML, Roll-Mecak A, Graded Control of Microtubule Severing by Tubulin Glutamylation. Cell 164, 911–921 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Magiera MM et al. , Excessive tubulin polyglutamylation causes neurodegeneration and perturbs neuronal transport. The EMBO journal 37, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shashi V et al. , Loss of tubulin deglutamylase CCP1 causes infantile-onset neurodegeneration. The EMBO journal 37, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Breuss MW, Leca I, Gstrein T, Hansen AH, Keays DA, Tubulins and brain development - The origins of functional specification. Molecular and cellular neurosciences 84, 58–67 (2017). [DOI] [PubMed] [Google Scholar]
- 78.Pamula MC, Ti SC, Kapoor TM, The structured core of human beta tubulin confers isotype-specific polymerization properties. The Journal of cell biology 213, 425–433 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Woehlke G et al. , Microtubule interaction site of the kinesin motor. Cell 90, 207–216 (1997). [DOI] [PubMed] [Google Scholar]
- 80.Atherton J et al. , Conserved mechanisms of microtubule-stimulated ADP release, ATP binding, and force generation in transport kinesins. eLife 3, e03680 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Okada Y, Hirokawa N, Mechanism of the single-headed processivity: diffusional anchoring between the K-loop of kinesin and the C terminus of tubulin. Proceedings of the National Academy of Sciences of the United States of America 97, 640–645 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Soppina V, Verhey KJ, The family-specific K-loop influences the microtubule on-rate but not the superprocessivity of kinesin-3 motors. Molecular biology of the cell 25, 2161–2170 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lessard DV et al. , Polyglutamylation of tubulin’s C-terminal tail controls pausing and motility of kinesin-3 family member KIF1A. The Journal of biological chemistry 294, 6353–6363 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li Q, King SJ, Xu J, Native kinesin-1 does not bind preferentially to GTP-tubulin-rich microtubules in vitro. Cytoskeleton 74, 356–366 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pernigo S, Lamprecht A, Steiner RA, Dodding MP, Structural basis for kinesin-1:cargo recognition. Science 340, 356–359 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kevenaar JT et al. , Kinesin-Binding Protein Controls Microtubule Dynamics and Cargo Trafficking by Regulating Kinesin Motor Activity. Current biology : CB 26, 849–861 (2016). [DOI] [PubMed] [Google Scholar]
- 87.Dixit R, Ross JL, Goldman YE, Holzbaur EL, Differential regulation of dynein and kinesin motor proteins by tau. Science 319, 1086–1089 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Monroy BY et al. , Competition between microtubule-associated proteins directs motor transport. Nature communications 9, 1487 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kellogg EH et al. , Near-atomic model of microtubule-tau interactions. Science 360, 1242–1246 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hooikaas PJ et al. , MAP7 family proteins regulate kinesin-1 recruitment and activation. The Journal of cell biology 218, 1298–1318 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pan X et al. , MAP7D2 Localizes to the Proximal Axon and Locally Promotes Kinesin-1-Mediated Cargo Transport into the Axon. Cell reports 26, 1988–1999 e1986 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Torisawa T et al. , Autoinhibition and cooperative activation mechanisms of cytoplasmic dynein. Nature cell biology 16, 1118–1124 (2014). [DOI] [PubMed] [Google Scholar]
- 93.Zhang K et al. , Cryo-EM Reveals How Human Cytoplasmic Dynein Is Auto-inhibited and Activated. Cell 169, 1303–1314 e1318 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Waterman-Storer CM et al. , The interaction between cytoplasmic dynein and dynactin is required for fast axonal transport. Proceedings of the National Academy of Sciences of the United States of America 94, 12180–12185 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.McKenney RJ, Huynh W, Tanenbaum ME, Bhabha G, Vale RD, Activation of cytoplasmic dynein motility by dynactin-cargo adapter complexes. Science 345, 337–341 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Urnavicius L et al. , The structure of the dynactin complex and its interaction with dynein. Science 347, 1441–1446 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Reck-Peterson SL, Redwine WB, Vale RD, Carter AP, The cytoplasmic dynein transport machinery and its many cargoes. Nature reviews. Molecular cell biology 19, 382–398 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Olenick MA, Dominguez R, Holzbaur ELF, Dynein activator Hook1 is required for trafficking of BDNF-signaling endosomes in neurons. The Journal of cell biology 218, 220–233 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Waterman-Storer CM, Karki S, Holzbaur EL, The p150Glued component of the dynactin complex binds to both microtubules and the actin-related protein centractin (Arp-1). Proceedings of the National Academy of Sciences of the United States of America 92, 1634–1638 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.McKenney RJ, Huynh W, Vale RD, Sirajuddin M, Tyrosination of alpha-tubulin controls the initiation of processive dynein-dynactin motility. The EMBO journal 35, 1175–1185 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Moughamian AJ, Holzbaur EL, Dynactin is required for transport initiation from the distal axon. Neuron 74, 331–343 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mallik R, Carter BC, Lex SA, King SJ, Gross SP, Cytoplasmic dynein functions as a gear in response to load. Nature 427, 649–652 (2004). [DOI] [PubMed] [Google Scholar]
- 103.Schlager MA, Hoang HT, Urnavicius L, Bullock SL, Carter AP, In vitro reconstitution of a highly processive recombinant human dynein complex. The EMBO journal 33, 1855–1868 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Urnavicius L et al. , Cryo-EM shows how dynactin recruits two dyneins for faster movement. Nature 554, 202–206 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Grotjahn DA et al. , Cryo-electron tomography reveals that dynactin recruits a team of dyneins for processive motility. Nature structural & molecular biology 25, 203–207 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hayashi K, Hasegawa S, Sagawa T, Tasaki S, Niwa S, Non-invasive force measurement reveals the number of active kinesins on a synaptic vesicle precursor in axonal transport regulated by ARL-8. Physical chemistry chemical physics : PCCP 20, 3403–3410 (2018). [DOI] [PubMed] [Google Scholar]
- 107.Encalada SE, Szpankowski L, Xia CH, Goldstein LS, Stable kinesin and dynein assemblies drive the axonal transport of mammalian prion protein vesicles. Cell 144, 551–565 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cella Zanacchi F, Manzo C, Magrassi R, Derr ND, Lakadamyali M, Quantifying Protein Copy Number in Super Resolution Using an Imaging-Invariant Calibration. Biophysical journal 116, 2195–2203 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rai AK, Rai A, Ramaiya AJ, Jha R, Mallik R, Molecular adaptations allow dynein to generate large collective forces inside cells. Cell 152, 172–182 (2013). [DOI] [PubMed] [Google Scholar]
- 110.Gutierrez-Medina B, Buendia Padilla M, Gutierrez-Esparza AJ, Oaxaca Camacho AR, Differential effect of multiple kinesin motors on run length, force and microtubule binding rate. Biophysical chemistry 242, 28–33 (2018). [DOI] [PubMed] [Google Scholar]
- 111.Feng Q, Mickolajczyk KJ, Chen GY, Hancock WO, Motor Reattachment Kinetics Play a Dominant Role in Multimotor-Driven Cargo Transport. Biophysical journal 114, 400–409 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fu MM, Holzbaur EL, Integrated regulation of motor-driven organelle transport by scaffolding proteins. Trends in cell biology 24, 564–574 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fu MM, Holzbaur EL, JIP1 regulates the directionality of APP axonal transport by coordinating kinesin and dynein motors. The Journal of cell biology 202, 495–508 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fu MM, Nirschl JJ, Holzbaur ELF, LC3 binding to the scaffolding protein JIP1 regulates processive dynein-driven transport of autophagosomes. Developmental cell 29, 577–590 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.van Spronsen M et al. , TRAK/Milton motor-adaptor proteins steer mitochondrial trafficking to axons and dendrites. Neuron 77, 485–502 (2013). [DOI] [PubMed] [Google Scholar]
- 116.Gibbs KL, Greensmith L, Schiavo G, Regulation of Axonal Transport by Protein Kinases. Trends in biochemical sciences 40, 597–610 (2015). [DOI] [PubMed] [Google Scholar]
- 117.Chaudhary AR, Berger F, Berger CL, Hendricks AG, Tau directs intracellular trafficking by regulating the forces exerted by kinesin and dynein teams. Traffic 19, 111–121 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chaudhary AR et al. , MAP7 regulates organelle transport by recruiting kinesin-1 to microtubules. The Journal of biological chemistry 294, 10160–10171 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Okada Y, Yamazaki H, Sekine-Aizawa Y, Hirokawa N, The neuron-specific kinesin superfamily protein KIF1A is a unique monomeric motor for anterograde axonal transport of synaptic vesicle precursors. Cell 81, 769–780 (1995). [DOI] [PubMed] [Google Scholar]
- 120.Cai Q, Pan PY, Sheng ZH, Syntabulin-kinesin-1 family member 5B-mediated axonal transport contributes to activity-dependent presynaptic assembly. The Journal of neuroscience : the official journal of the Society for Neuroscience 27, 7284–7296 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Niwa S, Tanaka Y, Hirokawa N, KIF1Bbeta- and KIF1A-mediated axonal transport of presynaptic regulator Rab3 occurs in a GTP-dependent manner through DENN/MADD. Nature cell biology 10, 1269–1279 (2008). [DOI] [PubMed] [Google Scholar]
- 122.Wu YE, Huo L, Maeder CI, Feng W, Shen K, The balance between capture and dissociation of presynaptic proteins controls the spatial distribution of synapses. Neuron 78, 994–1011 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Niwa S et al. , Autoinhibition of a Neuronal Kinesin UNC-104/KIF1A Regulates the Size and Density of Synapses. Cell reports 16, 2129–2141 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wu H, Williams J, Nathans J, Complete morphologies of basal forebrain cholinergic neurons in the mouse. eLife 3, e02444 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Schikorski T, Stevens CF, Quantitative ultrastructural analysis of hippocampal excitatory synapses. The Journal of neuroscience : the official journal of the Society for Neuroscience 17, 5858–5867 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cohen LD et al. , Metabolic turnover of synaptic proteins: kinetics, interdependencies and implications for synaptic maintenance. PloS one 8, e63191 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Truckenbrodt S et al. , Newly produced synaptic vesicle proteins are preferentially used in synaptic transmission. The EMBO journal 37, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fornasiero EF et al. , Precisely measured protein lifetimes in the mouse brain reveal differences across tissues and subcellular fractions. Nature communications 9, 4230 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hirschberg K et al. , Kinetic analysis of secretory protein traffic and characterization of golgi to plasma membrane transport intermediates in living cells. The Journal of cell biology 143, 1485–1503 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Takamori S et al. , Molecular anatomy of a trafficking organelle. Cell 127, 831–846 (2006). [DOI] [PubMed] [Google Scholar]
- 131.Misgeld T, Schwarz TL, Mitostasis in Neurons: Maintaining Mitochondria in an Extended Cellular Architecture. Neuron 96, 651–666 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lewis TL Jr., Turi GF, Kwon SK, Losonczy A, Polleux F, Progressive Decrease of Mitochondrial Motility during Maturation of Cortical Axons In Vitro and In Vivo. Current biology : CB 26, 2602–2608 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Smit-Rigter L et al. , Mitochondrial Dynamics in Visual Cortex Are Limited In Vivo and Not Affected by Axonal Structural Plasticity. Current biology : CB 26, 2609–2616 (2016). [DOI] [PubMed] [Google Scholar]
- 134.Lewis TL Jr., Kwon SK, Lee A, Shaw R, Polleux F, MFF-dependent mitochondrial fission regulates presynaptic release and axon branching by limiting axonal mitochondria size. Nature communications 9, 5008 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rangaraju V, Calloway N, Ryan TA, Activity-driven local ATP synthesis is required for synaptic function. Cell 156, 825–835 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Vaccaro V, Devine MJ, Higgs NF, Kittler JT, Miro1-dependent mitochondrial positioning drives the rescaling of presynaptic Ca2+ signals during homeostatic plasticity. EMBO reports 18, 231–240 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Macaskill AF et al. , Miro1 is a calcium sensor for glutamate receptor-dependent localization of mitochondria at synapses. Neuron 61, 541–555 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang X, Schwarz TL, The mechanism of Ca2+ -dependent regulation of kinesin-mediated mitochondrial motility. Cell 136, 163–174 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kalinski AL et al. , Deacetylation of Miro1 by HDAC6 blocks mitochondrial transport and mediates axon growth inhibition. The Journal of cell biology 218, 1871–1890 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chen Y, Sheng ZH, Kinesin-1-syntaphilin coupling mediates activity-dependent regulation of axonal mitochondrial transport. The Journal of cell biology 202, 351–364 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pathak D, Sepp KJ, Hollenbeck PJ, Evidence that myosin activity opposes microtubule-based axonal transport of mitochondria. The Journal of neuroscience : the official journal of the Society for Neuroscience 30, 8984–8992 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.van den Pol AN, Neuropeptide transmission in brain circuits. Neuron 76, 98–115 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lo KY, Kuzmin A, Unger SM, Petersen JD, Silverman MA, KIF1A is the primary anterograde motor protein required for the axonal transport of dense-core vesicles in cultured hippocampal neurons. Neuroscience letters 491, 168–173 (2011). [DOI] [PubMed] [Google Scholar]
- 144.Kwinter DM, Lo K, Mafi P, Silverman MA, Dynactin regulates bidirectional transport of dense-core vesicles in the axon and dendrites of cultured hippocampal neurons. Neuroscience 162, 1001–1010 (2009). [DOI] [PubMed] [Google Scholar]
- 145.Lim A, Rechtsteiner A, Saxton WM, Two kinesins drive anterograde neuropeptide transport. Molecular biology of the cell 28, 3542–3553 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wong MY et al. , Neuropeptide delivery to synapses by long-range vesicle circulation and sporadic capture. Cell 148, 1029–1038 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cavolo SL, Bulgari D, Deitcher DL, Levitan ES, Activity Induces Fmr1-Sensitive Synaptic Capture of Anterograde Circulating Neuropeptide Vesicles. The Journal of neuroscience : the official journal of the Society for Neuroscience 36, 11781–11787 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Cheng XT et al. , Characterization of LAMP1-labeled nondegradative lysosomal and endocytic compartments in neurons. The Journal of cell biology 217, 3127–3139 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yap CC, Digilio L, McMahon LP, Garcia ADR, Winckler B, Degradation of dendritic cargos requires Rab7-dependent transport to somatic lysosomes. The Journal of cell biology 217, 3141–3159 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Farias GG, Guardia CM, De Pace R, Britt DJ, Bonifacino JS, BORC/kinesin-1 ensemble drives polarized transport of lysosomes into the axon. Proceedings of the National Academy of Sciences of the United States of America 114, E2955–E2964 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gowrishankar S, Wu Y, Ferguson SM, Impaired JIP3-dependent axonal lysosome transport promotes amyloid plaque pathology. The Journal of cell biology 216, 3291–3305 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Johansson M et al. , Activation of endosomal dynein motors by stepwise assembly of Rab7-RILP-p150Glued, ORP1L, and the receptor betalll spectrin. The Journal of cell biology 176, 459–471 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ye M, Lehigh KM, Ginty DD, Multivesicular bodies mediate long-range retrograde NGF-TrkA signaling. eLife 7, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Maday S, Wallace KE, Holzbaur EL, Autophagosomes initiate distally and mature during transport toward the cell soma in primary neurons. The Journal of cell biology 196, 407–417 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Padamsey Z, Foster WJ, Emptage NJ, Intracellular Ca(2+) Release and Synaptic Plasticity: A Tale of Many Stores. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry, 1073858418785334 (2018). [DOI] [PubMed] [Google Scholar]
- 156.Cioni JM et al. , Late Endosomes Act as mRNA Translation Platforms and Sustain Mitochondria in Axons. Cell 176, 56–72 e15 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sambri I et al. , Lysosomal dysfunction disrupts presynaptic maintenance and restoration of presynaptic function prevents neurodegeneration in lysosomal storage diseases. EMBO molecular medicine 9, 112–132 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Maday S, Holzbaur EL, Autophagosome biogenesis in primary neurons follows an ordered and spatially regulated pathway. Developmental cell 30, 71–85 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Harrington AW, Ginty DD, Long-distance retrograde neurotrophic factor signalling in neurons. Nature reviews. Neuroscience 14, 177–187 (2013). [DOI] [PubMed] [Google Scholar]
- 160.Twelvetrees AE et al. , The Dynamic Localization of Cytoplasmic Dynein in Neurons Is Driven by Kinesin-1. Neuron 90, 1000–1015 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wong YC, Holzbaur EL, The regulation of autophagosome dynamics by huntingtin and HAP1 is disrupted by expression of mutant huntingtin, leading to defective cargo degradation. The Journal of neuroscience : the official journal of the Society for Neuroscience 34, 1293–1305 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Villarroel-Campos D, Schiavo G, Lazo OM, The many disguises of the signalling endosome. FEBS letters 592, 3615–3632 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ganguly A et al. , A dynamic formin-dependent deep F-actin network in axons. The Journal of cell biology 210, 401–417 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Scott DA, Das U, Tang Y, Roy S, Mechanistic logic underlying the axonal transport of cytosolic proteins. Neuron 70, 441–454 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Jung H, Yoon BC, Holt CE, Axonal mRNA localization and local protein synthesis in nervous system assembly, maintenance and repair. Nature reviews. Neuroscience 13, 308–324 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Shigeoka T et al. , Dynamic Axonal Translation in Developing and Mature Visual Circuits. Cell 166, 181–192 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Gopal PP, Nirschl JJ, Klinman E, Holzbaur EL, Amyotrophic lateral sclerosis-linked mutations increase the viscosity of liquid-like TDP-43 RNP granules in neurons. Proceedings of the National Academy of Sciences of the United States of America 114, E2466–E2475 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kanai Y, Dohmae N, Hirokawa N, Kinesin transports RNA: isolation and characterization of an RNA-transporting granule. Neuron 43, 513–525 (2004). [DOI] [PubMed] [Google Scholar]
- 169.Czaplinski K, Understanding mRNA trafficking: are we there yet? Seminars in cell & developmental biology 32, 63–70 (2014). [DOI] [PubMed] [Google Scholar]
- 170.Nicolas A et al. , Genome-wide Analyses Identify KIF5A as a Novel ALS Gene. Neuron 97, 1268–1283 e1266 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Poirier K et al. , Mutations in TUBG1, DYNC1H1, KIF5C and KIF2A cause malformations of cortical development and microcephaly. Nature genetics 45, 639–647 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lee JR et al. , De novo mutations in the motor domain of KIF1A cause cognitive impairment, spastic paraparesis, axonal neuropathy, and cerebellar atrophy. Human mutation 36, 69–78 (2015). [DOI] [PubMed] [Google Scholar]
- 173.Esmaeeli Nieh S et al. , De novo mutations in KIF1A cause progressive encephalopathy and brain atrophy. Annals of clinical and translational neurology 2, 623–635 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Moughamian AJ, Holzbaur ELF, [Cytoplasmic dynein dysfunction and neurodegenerative disease] in King SM (Ed.), Dyneins: Structure, Biology and Disease: Dynein Mechanics, Dysfunction, and Disease, vol. 2, Academic Press, Elsevier, 2018, pp. 286–315. [Google Scholar]
- 175.Dafsari HS et al. , Goldberg-Shprintzen megacolon syndrome with associated sensory motor axonal neuropathy. American journal of medical genetics. Part A 167, 1300–1304 (2015). [DOI] [PubMed] [Google Scholar]
- 176.Hines TJ et al. , An Essential Postdevelopmental Role for Lis1 in Mice. eNeuro 5, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Farrer MJ et al. , DCTN1 mutations in Perry syndrome. Nature genetics 41, 163–165 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Puls I et al. , Mutant dynactin in motor neuron disease. Nature genetics 33, 455–456 (2003). [DOI] [PubMed] [Google Scholar]
- 179.Neveling K et al. , Mutations in BICD2, which encodes a golgin and important motor adaptor, cause congenital autosomal-dominant spinal muscular atrophy. American journal of human genetics 92, 946–954 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Oates EC et al. , Mutations in BICD2 cause dominant congenital spinal muscular atrophy and hereditary spastic paraplegia. American journal of human genetics 92, 965–973 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Peeters K et al. , Molecular defects in the motor adaptor BICD2 cause proximal spinal muscular atrophy with autosomal-dominant inheritance. American journal of human genetics 92, 955–964 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Cogli L, Piro F, Bucci C, Rab7 and the CMT2B disease. Biochemical Society transactions 37, 1027–1031 (2009). [DOI] [PubMed] [Google Scholar]
- 183.Zhang K et al. , Defective axonal transport of Rab7 GTPase results in dysregulated trophic signaling. The Journal of neuroscience : the official journal of the Society for Neuroscience 33, 7451–7462 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Guedes-Dias P et al. , Mitochondrial dynamics and quality control in Huntington’s disease. Neurobiology of disease 90, 51–57 (2016). [DOI] [PubMed] [Google Scholar]
- 185.Saudou F, Humbert S, The Biology of Huntingtin. Neuron 89, 910–926 (2016). [DOI] [PubMed] [Google Scholar]
- 186.Romaniello R et al. , Tubulin genes and malformations of cortical development. European journal of medical genetics 61, 744–754 (2018). [DOI] [PubMed] [Google Scholar]
- 187.Poirier K et al. , Mutations in the neuronal ss-tubulin subunit TUBB3 result in malformation of cortical development and neuronal migration defects. Human molecular genetics 19, 4462–4473 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Romaniello R et al. , A novel mutation in the beta-tubulin gene TUBB2B associated with complex malformation of cortical development and deficits in axonal guidance. Developmental medicine and child neurology 54, 765–769 (2012). [DOI] [PubMed] [Google Scholar]
- 189.Aiken J, Moore JK, Bates EA, TUBA1A mutations identified in lissencephaly patients dominantly disrupt neuronal migration and impair dynein activity. Human molecular genetics 28, 1227–1243 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Hellal F et al. , Microtubule stabilization reduces scarring and causes axon regeneration after spinal cord injury. Science 331, 928–931 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Sengottuvel V, Leibinger M, Pfreimer M, Andreadaki A, Fischer D, Taxol facilitates axon regeneration in the mature CNS. The Journal of neuroscience : the official journal of the Society for Neuroscience 31, 2688–2699 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ruschel J et al. , Axonal regeneration. Systemic administration of epothilone B promotes axon regeneration after spinal cord injury. Science 348, 347–352 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Blanquie O, Bradke F, Cytoskeleton dynamics in axon regeneration. Current opinion in neurobiology 51, 60–69 (2018). [DOI] [PubMed] [Google Scholar]
- 194.Mikhaylova M et al. , Resolving bundled microtubules using anti-tubulin nanobodies. Nature communications 6, 7933 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Tas RP et al. , Differentiation between Oppositely Oriented Microtubules Controls Polarized Neuronal Transport. Neuron 96, 1264–1271 e1265 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Hyder F, Rothman DL, Bennett MR, Cortical energy demands of signaling and nonsignaling components in brain are conserved across mammalian species and activity levels. Proceedings of the National Academy of Sciences of the United States of America 110, 3549–3554 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Zhu XH et al. , Quantitative imaging of energy expenditure in human brain. NeuroImage 60, 2107–2117 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Yellen G, Fueling thought: Management of glycolysis and oxidative phosphorylation in neuronal metabolism. The Journal of cell biology 217, 2235–2246 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Silverman MA et al. , Expression of kinesin superfamily genes in cultured hippocampal neurons. Cytoskeleton 67, 784–795 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]