Abstract
Over the last two decades, the prevalence of obesity, and metabolic syndromes (MS) such as non-alcoholic fatty liver disease (NAFLD) and type 2 diabetes mellitus (T2DM), have dramatically increased. Bile acids play a major role in the digestion, absorption of nutrients, and the body’s redistribution of absorbed lipids as a function of their chemistry and signaling properties. As a result, a renewed interest has developed in the bile acid metabolic pathways with the challenge of gaining insight into novel treatment approaches for this rapidly growing healthcare problem. Of the two major pathways of bile acid synthesis in the liver, the foremost role of the acidic (alternative) pathway is to generate and control the levels of regulatory oxysterols that help control cellular cholesterol and lipid homeostasis. Cholesterol transport to mitochondrial sterol 27-hydroxylase (CYP27A1) by steroidogenic acute regulatory protein (StarD1), and the subsequent 7α-hydroxylation of oxysterols by oxysterol 7α-hydroxylase (CYP7B1) are the key regulatory steps of the pathway. Recent observations suggest CYP7B1 to be the ultimate controller of cellular oxysterol levels. This review discusses the acidic pathway and its contribution to lipid, cholesterol, carbohydrate, and energy homeostasis. Additionally, discussed is how the acidic pathway’s dysregulation not only leads to a loss in its ability to control cellular cholesterol and lipid homeostasis, but leads to inflammatory conditions.
Keywords: Bile acids, Metabolic syndromes (MS), Cardiovascular disease (CVD), Inflammation, Insulin resistance, Oxysterols
1. Introduction
Over the last two decades, prevalence of obesity and metabolic syndromes (MS) such as non-alcoholic fatty liver disease (NAFLD) and type 2 diabetes mellitus (T2DM) have dramatically increased. In most cases, NAFLD and T2DM share the same pathology with an estimated 70–80% of diabetic patients having NAFLD when closely examined.1 Today, NAFLD is the most common cause of chronic liver disease worldwide.2 Twenty-five percent of world’s adult population and up to 10% of children in developed countries are estimated to have NAFLD.2 The progression of non-alcoholic fatty liver (NAFL) to steatohepatitis (NASH) has already become a major contributor to the rising incidence in hepatocellular carcinoma (HCC), and the leading cause for liver transplantation in the United States.3 Intense efforts are being made to understand the mechanisms underlying this metabolic disease.
In NAFLD there is an excessive accumulation of triglycerides and cholesterol in the liver. Within the liver, cholesterol is metabolized to bile acids through two main biosynthetic pathways (Fig. 1). These pathways are tightly regulated by feedback mechanisms. In recent years, it has been reported that dysregulation of this feedback signaling network significantly contributes to pathologies of NAFLD.4–6 Dysregulation of the bile acid biosynthetic pathways and subsequent bile acid levels in the liver, gut, and peripheral tissues affect glucose and lipid homeostasis, as well as, energy expenditure through bile acid receptors such as farnesoid X receptor (FXR) and G protein-coupled bile acid receptor 1 (Gpbar-1 or TGR5).4,5 Albeit not considered a NAFLD model, there is evidence that excess accumulation of unusual bile acid metabolites produced by these pathways could initiate and potentiate hepatocyte toxicity and inflammation.7 As a result, a renewed interest has developed in the bile acid metabolic pathways.
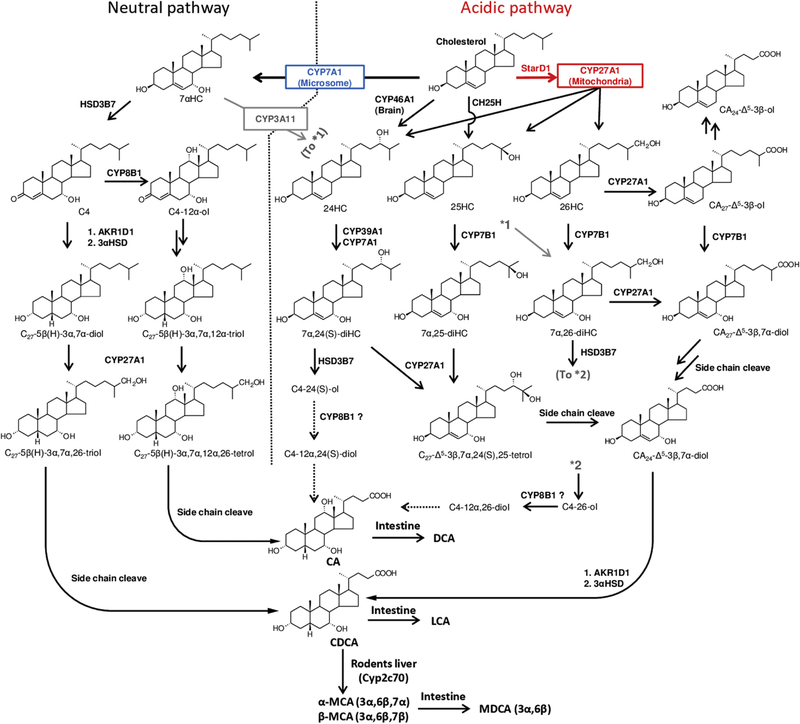
Fig. 1. Overview of the two major pathways of bile acid synthesis in mammalian liver.
The classic neutral pathway (left) is initiated by a highly regulated microsomal CYP7A1. The 7α-hydroxylation of cholesterol is the rate-determining step in this biochemical pathway. Produced 7αHC is then converted to C4 by HSD3B7. C4 is the branch point to CA and CDCA. The main stream of the acidic pathway (right) is initiated by mitochondrial CYP27A1. This enzyme has been shown to hydroxylate cholesterol to form 24HC, 25HC, 26HC and CA27-Δ5-3β-ol, which are then rapidly 7α-hydroxylated by microsomal CYP7B1 for reducing their regulatory abilities. In patients with CYP7B1 deficiency, CA27-Δ5-3β-ol is metabolized to CA24-Δ5-3β-ol without 7α-hydroxylation. In human and mice, CH25H in the endoplasmic reticulum also converts cholesterol to 25HC. In addition, 24HC produced in the brain is carried to the liver by ApoE and is also metabolized as same manner. CYP39A1 and CYP7A1 are reported to have 7α-hydroxylation activity toward 24HC in the liver. All the 7α-hydroxylated sterols and CA27-Δ5–3β,7α-diol are led to CA24-Δ5–3β,7α-diol by the multiple reactions of side chain cleaving, which is ultimately converted to CDCA. Most recently, Cyp3α11 is reported for (25S)-26-hydroxylation of 7αHC in mouse evidencing that mouse is capable of synthesis of down-stream sterols and bile acids bypassing CYP27A1 and CYP7B1. In human hepatocytes, 24HC is reported to be metabolized not only to CDCA but also to CA. Also, in rabbit, 26HC is shown to be metabolized to CA. Bile acids are conjugated with the amino acids, taurine or glycine, by the action of amino acid N-acyltransferase (BAAT). Conjugated bile acids are secreted into bile via bile salt export pump (BSEP). In the colon, conjugated bile acids are de-conjugated and 7α-dehydroxylated by bacterial bile salt hydrolase (BSH) and 7α-dehydroxylase, respectively. Abbreviations: AKR1D1, aldo-keto reductase 1D1; CA, cholic acid; CDCA, chenodeoxycholic acid; CH25H, cholesterol 25-hydroxylase; CYP7A1, cholesterol 7α-hydroxylase; CYP7B1, oxysterol 7α-hydroxylase; CYP8B1, sterol 12α-hydroxylase; CYP27A1, sterol 27-hydroxylase; CYP39A1, oxysterol 7α-hydroxylase 2; C4, 7α-hydroxy-4-cholesten-3-one; DCA, deoxycholic acid; HSD3B7, 3β-hydroxy-Δ5-C27-steroid oxidoreductase; LCA, lithocholic acid; MDCA, murideoxycholic acid; StarD1, steroidogenic acute regulatory protein; α-MCA, α-muricholic acid; β-MCA, β-muricholic acid; 3αHSD, 3α-hydroxysteroid dehydrogenase; 7αHC, 7α-Hydroxycholesterol; 24HC, 24(S)-hydroxycholesterol; 25HC, 25-hydroxycholesterol; 26HC, (25R)-26-hydroxycholesterol. Abbreviations of other sterol and bile acid intermediates are shown in Appendix.
Under physiological conditions in humans, the classical (or neutral) pathway accounts for most of the bile acid production, whereas the alternative (or acidic) pathway accounts for a much smaller portion (up to 10%) with more minor pathways contributing the remainder.8 However, with liver insult or in cirrhosis the acidic pathway can become predominant.9 From a developmental perspective, it is believed that the foremost role of the acidic pathway is to generate and control the levels of life sustaining regulatory oxysterols that help control cellular cholesterol and lipid homeostasis. Indeed, the acidic pathway is predominant in neonates.10–12 Oxysterols are known to regulate cholesterol synthesis and uptake through sterol responsive genes such as hydroxy-methylglutaryl-CoA reductase (HMGR) and low-density lipoprotein (LDR) receptor. They are also known to activate liver X receptor (LXR) to regulate fatty acid synthesis and cholesterol efflux; and, to activate peroxisome proliferator-activated receptor gamma (PPARγ) to increase fat movement out of cells.
This manuscript will review roles of the acidic pathway in regulation of cholesterol, carbohydrate, and energy homeostasis. It will discuss recent findings on the regulatory mechanism of this pathway and its contribution to metabolic syndrome development such as NAFLD and T2DM. Finally, it will review recent observations that have provided strong evidence for the acidic pathway’s novel role in cardiovascular disease (CVD), breast cancer, the gut microbiome, Alzheimer’s disease (AD), and Parkinson’s disease (PD).
2. Pathways of bile acid synthesis
The key steps of the two major pathways of bile acid synthesis are shown in Fig. 1. The neutral pathway is initiated by a highly regulated microsomal cytochrome P-450, cholesterol 7α-hydroxylase (CYP7A1). The 7α-hydroxylation of cholesterol is the rate-determining step in this biochemical pathway. 7α-Hydroxycholesterol (7αHC) is then converted to 7α-hydroxy-4-cholesten-3-one (C4) by 3β-hydroxy-Δ5-C27-steroid oxidoreductase (HSD3B7). C4 levels in serum generally reflect the rate of bile acid synthesis through the neutral pathway, and are often used as a biomarker for an estimation of the rate of bile acid synthesis in vivo.13 Subsequent, microsomal sterol 12α-hydroxylase (CYP8B1) determines the ratio of cholic acid (CA: 3α,7α,12α-trihydroxy-5β-cholanoic acid) to chenodeoxycholic acid (CDCA: 3α,7α-dihydroxy-5β-cholanoic acid).
The acidic pathway is initiated by sterol 27-hydroxylase (CYP27A1) located in the inner mitochondrial membrane. This enzyme has been shown to hydroxylate cholesterol to form (25R)-26-hydroxycholesterol (26HC) and 25-hydroxycholesterol (25HC), which are vital regulators of cholesterol/lipid homeostasis.14,15 Most recently, we have reported that CYP27A1 also metabolizes cholesterol to 24(S)-hydroxycholesterol (24HC).16 The enzymatic mechanism for generating three oxysterols, and what controls the ratios of these oxysterols are unclear. 26HC can be hydroxylated to form 3β-hydroxy-5-cholestenoic acid (CA27-Δ5-3β-ol) by additional CYP27A1. Of note, 26HC is often erroneously called as 27-hydroxycholesterol. However, the systematic name of this compound should be (25R)-26-hydroxycholesterol.17 Unlike liver specific CYP7A1, which is expressed in hepatocytes, CYP27A1 is widely expressed in all tissues in the body.11,18 The absence of CYP27A1 leads to a devastating clinical phenotype in humans, cere-brotendinous xanthomatosis (CTX).18,19 Although CYP27A1 catalyzes the first reaction of the acidic pathway, it is not the rate-limiting step of the entire pathway. Our laboratories have previously reported that cholesterol transport into the inner mitochondria membrane as mediated by the LXR induced steroidogenic acute regulatory protein (StarD1) is the essential rate-limiting step of this pathway.20,21 25HC, 26HC and CA27-Δ5–3β-ol are then rapidly 7α-hydroxylated by a microsomal oxysterol 7α-hydroxylase (CYP7B1), reducing their regulatory and cytotoxic properties. The resulting 7α-hydroxylated metabolites are then further converted to 3β,7α-dihydroxy-5-cholenoic acid (CA24-Δ5-3β,7α-diol) via multiple enzymatic reactions. This unsaturated bile acid is eventually converted to CDCA by Δ4-3-oxosteroid 5β-reductase (aldo-keto reductase 1D1, AKR1D1) and 3α-hydroxysteroid dehydrogenase (3αHSD).11 It should be noted that in the absence of CYP7B1, 26HC and CA27-Δ5-3β-ol cannot be metabolized to less toxic 7α-hydroxylated metabolites, expanding a developing toxic milieu. It is therefore not surprising that in the genetic CYP7B1 deficiency, infants rapidly show hepatic inflammation progresses to severe fibrosis.7,22–24
Although the main stream of this pathway is the conversion of cholesterol to CDCA via mitochondrial oxysterol generation, other pathways are also present in humans and mice. 25HC can be generated by cholesterol 25-hydroxylase (CH25H) in the endoplasmic reticulum, leading to the synthesis of CDCA. In addition, 24HC which is largely produced by cholesterol 24(S)-hydroxylase (CYP46A1) in the brain, is carried to the liver by apolipoprotein E (ApoE) and is also metabolized to bile acids.25,26 Oxysterol 7α-hydroxylase 2 (CYP39A1) and CYP7A1 hydroxylates 7α-position of 24HC,27,28 and the resulting 7α,24-diHC is converted to CDCA in a similar manner. Most recently, Griffith et al.29 reported that Cyp3a11 hydroxylated (25S)-26-position of 7αHC (and C4) in Cyp27a1 knockout mouse. This finding shows that cholesterol hydroxylation by Cyp27a1 is not the only point of entry to the acidic pathway of bile acid synthesis in mouse. The ability of synthesizing downstream sterols and bile acids without CYP27A1 and CYP7B1 maybe the reason why Cyp27a1 and Cyp7b1 knockout mice do not show clinical phenotypes such as autosomal recession,18,19 and liver failure as seen in human.7,22–24 Of note, most investigators believe that the acidic pathway generates only CDCA. However, in human hepatocytes, brain derived 24HC is reported to be metabolized not only to CDCA but also to CA.25 We have confirmed this bioconversion in the primary mouse hepatocytes as well.16 Also, Javitt’s group reported that 26HC and CA27-Δ5-3β-ol metabolized to CA in rabbit.30 For CA generation, 12α-hydroxylation on the steroid nucleus is essential. However, it is uncertain whether CYP8B1 can recognize these side chain oxidized sterols and cholestenoic acids as a substrate. To the best of our knowledge, CYP8B1 does not 12α-hydroxylate the side chain oxidized sterols, 25-hydroxy-4-cholesten-3-one (C4–25-ol) and (25R)-26-hydroxy-4-cholesten-3-one (C4–26-ol).31,32 Further investigation is needed to determine how CA is formed from these oxysterols and cholestenoic acids.
In mouse liver, most CDCA is rapidly metabolized to α-muricholic acid (α-MCA: 3α,6β,7α-trihydroxy-5β-cholanoic acid) and its 7β-epimer, β-muricholic acid (β-MCA: 3α,6β,7β-trihydroxy-5β-cholanoic acid).33 Bile acids are conjugated with the amino acids, taurine or glycine, by the action of amino acid N-acyltransferase (BAAT), and these conjugated bile acids are secreted into bile via bile salt export pump (BSEP). In the colon, conjugated bile acids are de-conjugated and 7α-dehydroxylated by bacterial bile salt hydrolase (BSH) and via the bile acid 7α-dehydroxylation pathway, respectively. As a result, CA and CDCA are converted to deoxycholic acid (DCA: 3α,12α-dihydroxy-5β-cholanoic acid) and lithocholic acid (LCA: 3α-hydroxy-5β-cholanoic acid), respectively. In the ileum, conjugated bile acids are secreted into portal blood via organic solute transporter α/β (OSTα/OSTβ) and circulated back to hepatocytes via Na-taurocholate co-transport peptide (NTCP). Approximately 95% bile acids are recovered and the 5% of bile acids that are lost in feces which can be compensated by de novo syn- thesis in the liver.
3. Bile acid pool and composition
Under physiological conditions, the total bile acid pool and its composition are strictly controlled by a coordinated regulation of expression of genes involved with synthesis, secretion, and transport of bile acids by the liver. The major components of human bile acid pool consist of CA, CDCA and DCA, and their ratio is roughly 4:4:2.34 However, the amount of DCA in the bile acid pool in humans can be quite variable. Bile acids in gallbladder bile are conjugated to either glycine or taurine, and the ratio is about 3 to 1.4,34 Increasing expression of CYP7A1 is correlated with increasing rates of bile acid synthesis, and increasing biliary bile acid and cholesterol secretion. Furthermore, increasing the rate of bile acid synthesis increases fecal excretion, and reduces serum cholesterol levels by stimulating LDL receptor-mediated uptake of LDL cholesterol; thus improving the hyperlipidemic manifestations of diet-induced obesity and diabetes.35
A shift of bile acid synthesis from the classic pathway to the acidic pathway alters the hydrophilicity of the bile acid pool which results in reduced intestinal cholesterol absorption in mice. Although small amounts of CA can be produced, CDCA (or MCAs in mice) is the main bile acid product synthesized from mitochondrial oxysterols. CA has a low critical micellar concentration (ca. 50 μM) and is highly efficient in mixed micelle formation with cholesterol and phosphatidylcholine in bile and intestine, which eases the absorption of dietary cholesterol by enterocytes. In this regard, Cyp8b1 deletion to reduce CA pool in ApoE knockout mice slows the development of atherosclerosis.36 Cyp8b1 knockout mice have improved glucose homeostasis by increased glucagon like peptide-1 (GLP-1) secretion.37 These mice are resistant to diet-induced obesity. It has been also shown that the increased ratio of serum 12α-hydroxylated bile acids (CA and DCA) to non-12α-hydroxylated bile acids (CDCA and LCA) is associated with insulin resistance in humans.38 The alteration in the hydrophilicity of the bile acid pool can also affect cellular bile acid signaling. CDCA is the most potent FXR agonist (EC50 = ca. 10 μM) followed by LCA and DCA.39–41 While, CA is the a much weaker FXR agonist (EC50 = ca. 0.59 mM), and UDCA and MCAs do not activate FXR.42 In Cyp7a1 knockout mice, the alternative pathway is stimulated to produce bile acids to maintain a smaller, but more hydrophilic bile acid pool with reduced TCA and increased TMCAs; which reduces cholesterol/lipid absorption, antagonizes intestinal FXR activation to reduce ceramide synthesis, and improves glucose tolerance and insulin sensitivity.43–46
4. Oxysterol signaling in lipid, cholesterol and glucose homeostasis
Oxysterols, 24HC, 25HC, and 26HC are key regulators of cellular cholesterol and lipid homeostasis. They have been reported to be endogenous LXR ligands.47 As illustrated in Fig. 2, activation of LXR can stimulate reverse cholesterol transport via high-density lipoprotein (HDL) and reduce the body’s cholesterol overload by inducing CYP7A1 to facilitate cholesterol metabolism through classical pathway, as well as, by inducing cholesterol transporters, including ATP-binding cassette transporters (ABCA1, ABCG5, ABCG8), and ApoE.48 In addition, LXR activation results in an increase in lipid synthesis in the liver through inducing the expression of sterol regulatory element binding protein-1c (SREBP-1c), fatty acid synthase (FAS), acetyl-CoA carboxylase 1 (ACC-1), and stearoyl-CoA desaturase 1 (SCD-1).49–51 LXR also controls the expression of genes involved in many other processes such as macrophage recruitment and activation, apoptosis and central nervous system myelination.52,53
Fig. 2. The acidic pathway of bile acid synthesis in metabolic regulations.
Induction of the acidic pathway increases oxysterols which primarily activates LXR. It results in an increase in lipogenesis through inducing the expression of SREBP-1c, ChREBP, FAS, ACC-1, and SCD-1. Upon fed state, glucose enters hepatocyte through the glucose transporter GLUT2 and is catalyzed into Acetyl-CoA via glycolysis and citrate cycle. LPK, a key enzyme in glycolysis, is under the transcriptional control of ChREBP. Therefore, chronic LXR activation strongly contribute to steatosis development. Whereas, LXR also plays a beneficial role in the degradation and the excretion of cholesterol whose excess can cause liver inflammation. Activation of LXR can stimulate reverse cholesterol transport and reduce the body’s cholesterol overload by inducing CYP7A1 to facilitate cholesterol metabolism through classical pathway, as well as, by inducing cholesterol transporters, including ABCA1, ABCG5/8, and ApoE. However, as seen in NASH, chronic oxysterol excess may also increase the inflammation due to their lipotoxicity. Furthermore, oxysterols are able to bind to INSIG, thereby inducing a close interaction between INSIG and SCAP, thus preventing SREBP export to the Golgi and subsequent activation. This interaction is central in the control exerted by some oxysterols on cholesterol metabolism. Abbreviations: ABCA1, ATP-binding cassette transporter A1; ABCG5/8, ATP-binding cassette transporter G5/G8; ACC-1, acetyl-CoA carboxylase 1; ChREBP, carbohydrate response element (ChRE)-binding protein; CYP7A1, cholesterol 7α-hydroxylase; CYP7B1, oxysterol 7α-hydroxylase; FAS, fatty acid synthase; HDL, high-density lipoprotein; INSIG, insulin induced gene protein; LPK, liver pyruvate kinase; LXR, liver X receptor; SCAP, sterol regulatory element binding protein (SREBP) cleavage-activating protein; SCD-1, stearoyl-CoA desaturase 1; SREBP-1c, sterol regulatory element binding protein-1c.
Oxysterols are able to bind and regulate the function of several other proteins which play a direct or indirect role in the regulation of cholesterol and lipids homeostasis.47 Insulin induced gene protein (INSIG) is a regulatory protein that controls the maturation of sterol regulatory element binding protein (SREBP), a transcription factor which upregulates the expression of enzymes involved in cholesterol and fatty acid biosynthesis.54 It has been shown that 25HC is able to bind INSIG, inducing an interaction between INSIG and SREBP cleavage-activating protein (SCAP) preventing SREBP-1c exporting and increased lipid biosynthesis. A similar binding to INSIG was observed for 24HC, 22(R)HC and 26HC.54 This interaction is central to control of oxysterols on cholesterol and lipid synthesis.
Niemann-Pick type C protein 1 (NPC1) is a membrane glycol-protein which resides primarily in the late endosomes, and functions to mobilize LDL-derived cholesterol out of the endosomes.55 This protein shares the sequence homology with other regulatory protein involved in the cholesterol homeostasis such as SREBP and SCAP.56 Mutation or absence of NPC1 leads to a failure to transport cholesterol out of the late endosomes to the mitochondria and, thereby, decreasing the synthesis of 26HC through mitochondrial CYP27A1, and of 25HC through the endoplasmic reticulum/Golgi CH25H, respectively. Decreased cholesterol transport leads to reduced oxysterol synthesis and activation of LXR. Interestingly, Ma et al.57 have also provided evidence that oxysterol LXR activation is able to act in a feed-forward manner and up-regulate expression of NPC1 protein; driving the clearance of endosomal cholesterol.
5. The regulatory mechanism of the acidic pathway of bile acid synthesis
In the neutral pathway, two mechanisms have been proposed for the feed-back regulation of CYP7A1 gene transcription. In the liver, primary bile acids activate FXR to induce small heterodimer partner (SHP), which represses trans-activation of the CYP7A1 and CYP8B1 genes. In the intestine, primary bile acids activate FXR to induce fibroblast growth factor 15 (FGF15, or human orthologue FGF19), which is circulated to the liver to activate hepatic FGF receptor 4/Klotho signaling to inhibit CYP7A1 gene transcription via extracellular regulated protein kinases 1/2 (ERK1/2)/c-Jun of the mitogen-activated protein kinase (MAPK) pathway.58,59 In the liver, conjugated bile acids-activated sphingosine-1-phosphate receptor 2 (S1PR2) signaling activates the ERK1/2 and serine/threonine-protein kinase (AKT) signaling pathways to possibly modulate CYP7A1/CYP8B1 activity.60
The acidic pathway appears to lack the striking response seen with CYP7A1 to feeding of bile acids or cholesterol, at least in mice.61 However, this pathway is highly regulated in several different ways. As illustrated in Fig. 3, the pathway creates a feed-forward loop driven by LXR activating oxysterols. The cellular oxysterol levels are controlled by cholesterol delivery to mitochondria by StarD1 as well as by oxysterol metabolism by microsomal CYP7B1. Indeed, in primary rodents’ hepatocytes, an increased expression of StarD1 significantly elevated oxysterol synthesis and subsequent bile acid synthesis.20,21 Additionally, Cyp7b1 knockout mice have significantly higher levels of hepatic oxysterols, 24HC, 25HC and 26HC.62 Of importance, CYP27A1 appears not to be a rate-determining enzyme of oxysterol synthesis. In this regard, overexpression of Cyp27a1 in the primary rodent hepatocyte cultures only slightly elevated oxysterol and bile acid synthesis, whose rate was much lower than compared to StarD1 overexpression.20 This is due to low cholesterol content in the inner mitochondrial membrane. Interestingly, little is known about regulation of StarD1 in the liver. StarD1 has been reported to be regulated by cytokines, PPARγ, RXR agonists, and by modified LDL.63–65
Fig. 3. The regulatory factors of the acidic pathway of bile acid synthesis.
The pathway is regulated by a feed-forward manner by oxysterols. Cholesterol transport into mitochondria, where CYP27A1 generates oxysterols, is mediated by StarD1 which is induced by the LXR activation. The intercellular oxysterol levels are also controlled by CYP7B1. Insulin signaling pathway is primarily involved in the regulation of CYP7B1. Insulin resistance significantly suppressed CYP7B1 expression. In brain, GH-Stat5 signaling may activate TGR5 to up-regulate expression of CYP7B1. The cold exposure significantly increases Cyp7b1 mRNA and protein expression in mice. In the intestine, conjugated bile acid can be deconjugated by BSH. CDCA (TCDCA) is further bio-transformed (7α-dehydroxylated) to secondary LCA (TLCA). In the cold-mediated Cyp7b1 induction, increased fecal taurine excretion was also observed. Taurine can provide pathobionts in the gut with its’ terminal electron acceptor (SO3 group), allowing for growth and expansion of deltaproteobacteria, Biophila wadsworthia in the gut. In BAT, secondary bile acids activate TGR5 to increase thermogenesis. In the intestinal L cells, secondary bile acids also activate TGR5 to increase cAMP and stimulate GLP-1 secretion. GLP-1 stimulates insulin secretion from β cells to improve insulin sensitivity which in turn enhance the Cyp7b1 induction. In NASH fibrosis, increased CYP7B1 expression has been reported,85–88 suggesting that this mechanism is working for protecting against hepatotoxicity. Although Cyp7b1 is not a direct FXR target gene, induction of FXR-MAFG pathway may account for the repression of Cyp7b1 expression indirectly. Abbreviations: BAT, brown adipose tissue; BSH, bile salt hydrolase; CYP27A1, sterol 27-hydroxylase; CYP7B1, oxysterol 7α-hydroxylase; FXR, farnesoid X receptor; GH, growth hormone; GLP-1, glucagon like peptide-1; LCA, lithocholic acid; LXR, liver X receptor; MAFG, MAF bZIP transcription factor G; Stat5, signal transducer and activator of transcription 5; StarD1, steroidogenic acute regulatory protein; TCDCA, taurochenodeoxycholic acid; TGR5, G protein-coupled bile acid receptor; TLCA, taurolithocholic acid.
Several regulators have been reported for the expression of CYP7B1. Peripheral observations have provided evidence for the insulin signaling pathway as involved in the regulation of this enzyme. Reduced Cyp7b1 mRNA expression was observed in the mouse models of insulin resistance, i.e. selective liver-specific insulin receptor knockout mice;66 and, lower expression of Cyp7b1 mRNA was observed in T2DM mice.67,68 Recently, induction of Cyp7b1 was reported to associate with the mechanisms involved in adaptive thermogenesis.69 Mice bred in a colder room showed significantly higher expressions of hepatic Cyp7b1 mRNA and protein compared to those bred at room temperature. These cold exposed mice had elevated fecal bile acid excretion and an altered gut microbiome. Taurine-conjugated lithocholic acid (TLCA), a secondary bile acid generated from CDCA by gut bacteria, is a potent TGR5 agonist which possibly activate brown adipose tissue (BAT) thermogenesis.70 Also, TGR5 activation leads to increased cAMP and stimulation of glucagon-like peptide1 (GLP-1) secretion from intestinal endocrine L cells. TGR5 activation leads to augmentation of insulin secretion from pancreatic β cells and stimulation of GLP-1 synthesis from α cells. Thus, enhanced glucose tolerance and insulin sensitivity is correlated with Cyp7b1 induction, making a feed-forward loop. This mechanism occurs independent of FXR regulation.
Chiang et al.71 indicated that the induction of Cyp7b1 expression by TGR5 may also go through pulsatile growth hormone (GH) signaling. This study showed significantly lower expression of hepatic Cyp7b1 mRNA and protein activity in fasted TGR5 knockout mice. This lower expression of Cyp7b1 was considered due to high level of non-pulsatile GH secretion (GH resistance) of these mice. Since Cyp7b1 was shown to be down-regulated in the hepatocyte signal transducer and activator of transcription 5 A/B (Stat5A/B) deficient mice,72,73 GH-Stat5 signaling is a possible inducer of Cyp7b1. However, there is also a report that transcriptional activity of Cyp7b1 was not induced by GH.74 Therefore, further investigation is needed to resolve this issue.
It has been reported that CYP7B1 has no FXR responsive element.75 However, our lab and others have observed modest down-regulation of murine Cyp7b1 by bile acid feeding at both the specific activity and mRNA levels.61,76 This may be explained by an indirect feed-back regulation by the FXR-MAF bZIP transcription factor G (MAFG) pathway.77 In this pathway, bile acid activation of FXR induces the transcriptional repressor, MAFG, which then binds directly to a responsive element in the murine Cyp7b1 gene promoter. Although Cyp7b1 is not a direct FXR target gene, induction of an FXR-dependent pathway may account for the repression of Cyp7b1 when hepatic bile acid levels are elevated.
6. The acidic pathway in NAFLD and T2DM
Dysregulated hepatic lipid metabolism contributes to NAFLD, and a condition which ranges from simple steatosis (NAFL) to steatosis combined with inflammation and necrosis (NASH) which can further progress to hepatic fibrosis and cirrhosis. In most cases, NAFLD and T2DM shares the same pathology. An estimated 70–80% of diabetic and obese patients have NAFLD condition.1 In NAFL and T2DM,66–68,78 reduced hepatic CYP7B1 expression has been reported. With down-regulation of CYP7B1, oxysterols (i.e. 25HC and 26HC) are expected to be elevated in livers of these patients. Indeed, Ikegami et al.79 reported a significant increase in the serum levels of 4bHC, 25HC and 26HC in NAFLD patients. Together with cholesterol, chronically increased oxysterol levels in hepatocytes likely account for liver inflammation.80–83 Unpublished observations from our laboratory show a clear correlation with suppression of Cyp7b1 mRNA, increased oxysterol levels, onset of inflammation with Western diet fed mice.84 In support of these findings, a genetic deficiency of CYP7B1 in children leads to aggressive inflammation and fibrosis in association with a marked increase in these oxysterols and their 7α-dehydroxylated metabolites, CA27-Δ5-3β-ol and 3β-hydroxy-5-cholenoic acid (CA24-Δ5-3β-ol).7 (See Fig. 1) Dai et al.22 reported that a patient with CYP7B1 deficiency developed significant steatosis along with hepatitis. This observation suggested oxysterols can stimulate lipogenesis through activation of LXR/SREBP-1c pathway.49–51 In NAFL, lipogenesis is markedly up-regulated in concert with insulin resistance. This observation may provide an explanation for the increasing de novo fat synthesis in the face of already existing lipid excess. In this regard, elevated oxysterols, due to reduced CYP7B1 expression, chronically activates the LXR/SREBP-1c signaling to stimulate fatty acid synthesis. Therefore, increased oxysterols secondary to reduced CYP7B1 appear to initially contribute to the development of steatosis as they continuously accumulate along with 7α-dehydroxylated metabolites as previously described by Setchell et al.7 in a manner similar to CYP7B1 deficient children. This dysregulation of oxysterol metabolism may drive NAFL to NASH (See Fig. 2).
Unlike in simple steatosis, elevated CYP7B1 expression has been reported in NASH fibrotic liver.85–88 In these patients, serum total bile acid level is elevated with decreased CA composition suggesting that the acidic pathway is predominant.38,89–92 Also, taurine conjugated bile acids are increased, whereas, unconjugated and glycine conjugated bile acids are decreased.93 An increased BAAT and decreased CYP8B1 expressions may help explain bile acid pool size/composition changes in patients with NASH.85 The altered expression of bile acid metabolism in NASH livers is believed to be a defense mechanism by altering the overall bile acid profile to protect against hepatotoxicity. In the rodent model of LCA-induced cholestasis,94 the alternative pathway is used for maintaining a smaller but more hydrophilic bile acid pool with reduced TCA and increased TMCAs and TDCA, which antagonizes intestinal FXR activation to reduce ceramide synthesis, and improve glucose tolerance and insulin sensitivity. It should be noted that this mechanism seems to work closely with gut microbiome, which will be discussed in the next section. Despite these observations, the mechanism of up-regulation of CYP7B1 in NASH liver remains unclear. Since the existing insulin resistance should down-regulate CYP7B1, another mechanism of induction of CYP7B1 must be activated. In summary, in NAFL and T2DM, elevated hepatic oxysterol levels appear able to drive lipogenesis through the LXR mediated upregulation of SREBP-1c, ChREBP, FAS, ACC-1, and SCD-1 in the presence of hepatic lipid excess.
7. Contribution of the acidic pathway to gut microbiome and metabolic syndromes
Bile acids appear to be a major regulator of the gut microbiota. Previously, we reported linkage of the liver health to fecal bile acid concentrations and gut microbiota composition.95–97 As cirrhosis progressed, bacterial dysbiosis was observed which was linked to lower bile acid levels entering the intestine. In the ileum and large bowel, conjugated primary bile acids (i.e. TCA and TCDCA) are hydrolyzed to free bile acids (i.e. CA and CDCA) by BSH expressed in bacteria genera Bacteroides, Clostridium, Bifidobacterium, and Lactobacillus. Primary free bile acids, CA and CDCA, are bio-transformed (7α-dehydroxylated) to toxic secondary bile acids DCA and LCA, respectively, that can change the bile acid pool/composition and alter host physiology. A small population of intestinal species in the genus Clostridium, including C. scindens, hiranonis, C. hylemonae (Clostridium cluster XVIa), and C. sordellii (Clostridium cluster XI) are capable of this biotransformation.98
The cold-mediated Cyp7b1 induced mice showed increased fecal bile acid excretion as well as an altered microbiome.69 They were shown to have a lower composition of Lachnospiraceae and Deferribacteraceae family members, whereas elevated composition of Clostridiales and Porphyromonadaceae family members. Interestingly, increased fecal taurine excretion was also observed in mice exposed to cold temperature. Taurine is known to provide pathobionts in the gut with its’ terminal electron acceptor (SO3 group), allowing for growth and expansion of deltaproteobacteria, Biophila wadsworthia in the gut.99 Indeed, high-fat diet is associated with increased taurine-conjugation in humans.100 The mechanism of cold-mediated Cyp7b1 induction and that of the beneficial effects to the organism from the microbiome profile alteration remains unclear. It could be hypothesized that the alteration of bile acid profile affects the relative activation of two bile acid receptors, FXR and TGR5, which possibly changes the lipid and glucose metabolisms. Further investigation is needed.
In some studies, CYP7B1 expression in NASH livers has shown to be elevated,85–88 and the bile acid pool is increased with an increased ratio of secondary bile acids.89,92,101 However, NASH represents a continuum of disease from mild inflammation to fibrotic and necrotic findings; and most reports do not clearly separate this continuum. Therefore, the presentation of mild disease vs. more extensive findings are likely markedly different. With that understanding, there are reports that several differing bacterial species are associated with NAFLD. For instance, the abundance of Proteobacteria, Enterobacteria, and Escherichia,102 or Bacteroides appears to be higher in patients with NASH as compared to matched healthy individuals.103 It is also reported that the fecal microbiome of children with NASH showed abundant Gammapro-teobacteria and Prevotella compared to that of non-NASH obese children.104 Of note, an increase in Proteobacteria and decrease in Firmicutes were observed during progression of NAFLD, suggesting that the gut microbiome is in a state of flux during disease progression.105 Furthermore, elevated taurine metabolizing bacteria, Biophila and Escherichia, are reported in patients with NAFLD.89 As already mentioned, high-fat diet is known to associate with increased taurine-conjugation in humans.100 The increased bile acid pool and secondary bile acid composition (higher conjugated LCA) could suggest reduced intestinal and hepatic FXR signaling, and predict upregulation of TGR5 signaling; a milieu which has anti-inflammatory effects, promotes energy expenditure, and improves insulin resistance.
8. NAFLD and breast cancer in association with increased circulating 26HC
26HC has been shown to bind to estrogen receptor (ER)-α and is described as selective estrogen receptor modulator (SERM).106,107 Wu et al.108 demonstrated increased 26HC content in normal tissue of ER+ breast cancer patients as compared to cancer free controls; and, actual tumor 26HC content to be even higher. Furthermore, diminished CYP7B1 expression in tumor tissue correlated with increased 26HC levels, suggesting CYP7B1 to be the rate-controlling step. These findings were corroborated by Nelson et al.109 Increased circulating 26HC levels are known to correlate with hypercholesterolemia, and be a risk factor for ER+ breast cancers and decreased response to endocrine therapies.110 Hence, higher circulating 26HC in NAFLD,66–68,79 due to reduced hepatic CYP7B1 would seem to be a risk factor for tumor stimulation in ER+ breast cancer. However, circulating 26HC have not yet been found to correlate; suggestive that in breast tumor, 26HC may be locally modulated.108 Continued study is needed to determine the level of regulation.
9. AD and PD
Approximately 25% of the cholesterol in the body is found in the brain, which accounts for 2% of the brain’s weight.111 As cholesterol is not able to pass the blood-brain membrane barrier (BBB), all brain cholesterol is synthesized de novo. Cholesterol is converted to the more hydrophilic 24HC by CYP46A1 which can now cross the BBB and be excreted out of the brain.112 CYP46A1 is preferentially expressed in neurons and some astrocytes.113 The reported export rate of cholesterol from brain by 24HC is about 4–7 mg/day.112,114,115 Additionally, cholesterol can be converted to 26HC by CYP27A1 expressed in neurons, astrocytes and oligodendrocytes. However, most brain 26HC originates from the circulation,114,116 and, its’ tissue concentration is much lower than that of 24HC.117 The import rate of 26HC from circulation to brain is about 4–5 mg/day.112,118 Thus, 24HC plays as an “exported oxysterol” out of brain, while 26HC plays as an “imported oxysterol” to brain. The reason and mechanism of this completely opposite direction of two oxysterol is unknown. Major pathways of brain cholesterol metabolism and transport are summarized in the Fig. 4.
Fig. 4. Major cholesterol metabolic pathways in brain.
Cholesterol is synthesized de novo in brain. Cholesterol is not able to pass blood-brain barrier (BBB). The most predominant pathway of cholesterol excretion is by 24(S)-hydroxylation which is catalyzed by CYP46A1 expressed exclusively in the brain, particularly in neurons and some astrocyte. Cholesterol can also be converted to 26HC in the brain by CYP27A1 expressed in neurons, astrocytes and oligodendrocytes. However, most brain 26HC originates from the circulation. The import rate of 26HC from circulation to brain is reported to be 4–5 mg/24 h. 25-Hydroxylation can also be occurred in brain. 25HC and 26HC are 7α-hydroxylated by CYP7B1. Resulting 7α,25-diHC and 7α,26-diHC are converted to 3-oxo-Δ4 forms (C4–25-ol and C4–26-ol) and excreted out of brain. In AD, higher brain 26HC have been reported. A decreased brain 24HC and increased its’ level in plasma as well as cerebrospinal fluid are reported. Significance of oxysterols to the pathogenesis of AD and PD are not yet defined. However, increased 26HC in MS can be transported to the brain which possibly alters the levels and ratio of 26HC and 24HC. Abbreviations: BAs, bile acids; CH25H, cholesterol 25-hydroxylase; CYP7B1, oxysterol 7α-hydroxylase; CYP27A1, sterol 27-hydroxylase; CYP46A1, cholesterol 24(S)-hydroxylase; HSD3B7, 3β-hydroxy-Δ5-C27-steroid oxidoreductase; 24HC, 24(S)-hydroxycholesterol; 25HC, 25-hydroxycholesterol; 26HC, (25R)-26-hydroxycholesterol. Abbreviations of other sterol and bile acid intermediates are shown in Appendix.
One could hypothesize, it is only with conditions of excess such as occurs with dysregulation of cholesterol metabolism in NAFLD (i.e. acidic pathway of cholesterol metabolism) that increased 26HC transport to the brain occurs. Or, as with what has been found to date in breast cancer, 26HC synthesized within the brain may be more locally modulated by CYP7B1 under yet to be understood conditions of metabolic dysregulation; allowing for a slowers more gradual accumulation and onset of oxysterol induced toxicity. Or, whether oxysterols might initiate inflammation in the brain as found in the aortas of Cyp7b1 knockout mice, is also unclear (See Section 10). However, as in breast tissue and in endothelial tissue, it appears the oxysterols and their metabolism could play an important role.
Described below are several possible pathologic manifestations of excess brain oxysterol levels. The roles of oxysterols in the pathogenesis of neurodegenerative diseases such as AD and PD are not yet completely defined. However, oxysterol homeostasis is tightly regulated, and their specific levels and ratio are strictly maintained depending on each part of brain. For instance, the ratio of 26HC/24HC is maintained as about 1/8 in the frontal cortex, 1/5 in the occipital cortex, and 1/10 in the basal ganglia.119 Alterations in these oxysterol levels and ratios may have widespread pathological ramifications. AD is characterized histopathologically by the deposition of amyloid-β (Aβ) plaques and neurofibrillary tangles-containing hyperphosphorylated tau protein in the brain. It has been reported that in human neuroblastoma SH-SY5Y cells,120,121 and in rodents organotypic slices,122,123 26HC increases amyloid-β protein precursor (AβPP), Aβ and phosphorylated tau levels; while, 24HC has been shown to facilitate the cleavage of AβPP to the non-amyloidogenic pathway.121 Indeed, higher 26HC with decreased 24HC has been reported in AD brain as well as in plasma and cerebrospinal fluid.119,124–126 While, PD is characterized by the aggregation of α-synuclein protein in Lewy body inclusions and the death of dopaminergic neurons in the substantia nigra. The treating human neuroblastoma SH-SY5Y cells with 26HC found a marked increase both in soluble and insoluble α-synuclein.127 Conversely, treatment with 24HC reduces levels of α-synuclein levels SH-SY5Y cells. These findings suggested that increased 26HC levels with reduced 24HC may have significant contribution to these pathologies. The regulation of AD- and PD-related proteins by oxysterol are worthy of further investigation in vivo.
In NAFL and insulin resistance, plasma 26HC and 25HC are markedly increased.79 These elevated oxysterols are likely transported to the brain; altering their specific levels and ratios. Our preliminary data (unpublished) show significant elevation in 26HC and 25HC levels in the brains of Cyp7b1 knockout mice (Table 1). Adenovirus (Ad)-StarD1 infection to these mice led to further elevation of these oxysterol levels in their brain suggesting these oxysterols are likely peripherally delivered rather than locally synthesized. Thus, MS associated oxysterols are possibly associated with neurodegeneration by induction of these disease characteristic proteins at least in AD and PD. Further animal and human studies are necessary to confirm the hypothesis of vascular oxysterol driven neurodegeneration.
Table 1.
Brain oxysterol levels of Cyp7b1 knockout mice.
| Group | 24HC | 25HC | 26HC |
|---|---|---|---|
| WT Control (N = 3) | 41.3 ± 0.15 | 0.04 ± 0.03 | 0.75 ± 0.14 |
| Cyp7b1−/− (N = 8) | 40.4 ± 0.62 | 7.17 ± 0.59* | 6.78 ± 0.21* |
| Cyp7b1−/− (Ad-StarD1 overexpression, N = 4) | 37.7 ± 1.92 | 12.5 ± 1.27* | 9.77 ± 0.35* |
Ad-β-Gal (Control) or Ad-StarD1 recombinant virus was injected to from the tail vain of 13-Week old B6/129 mice (WT) or Cyp7b1 knockout mice to overexpress the selected gene as described.16 One week after adenovirus injection, mice were briefly anesthetized, and brain was harvested. Oxysterol levels were quantified by high performance liquid chromatography (HPLC) as described in the literature.16 By two tails unpaired t-test (homoscedastic),
P ≤ 0.001 vs. WT Control. Abbreviations: 24HC, 24(S)-hydroxycholesterol; 25HC, 25-hydroxycholesterol; 26HC, (25R)-26-hydroxycholesterol; Ad, adenovirus; StarD1, steroidogenic acute regulatory protein; WT, wild type.
10. CVD
It is well appreciated that CVD is the leading cause of morbidity and mortality in NAFLD patients. Abundant evidence exists that associates NAFLD with endothelial dysfunction, increased coronary arterial calcifications and increased carotid intima media thickness.128,129 Endovascular atherosclerotic plaques are known to contain high 26HC levels. As in breast cancer, existing evidence supports its source as being locally modulated.108 In response to subintimal cholesterol accumulation, signaling occurs, drawing in monocytes/macrophages to metabolize/remove the cholesterol/lipid, respectively. With inability to clear excess cholesterol/lipid, macrophages become lipid laden foam cells with well-described metabolic consequences.130 Interestingly, during their differentiation from monocytes to macrophages, increasing expression of StarD1 follows.65 And, although macrophages are unable to metabolize cholesterol to bile acids, there is rapid CYP27A1 hydroxylation of the mitochondrial StarD1 delivered cholesterol with active regulable CYP7B1 to reduce the oxysterols cytotoxic properties. The most abundant oxysterol synthesized is 26HC, accounting for the high endovascular plaque levels. Interestingly, although oxysterols can act as LXR ligands, attenuate cholesterol synthesis, and are precursors to bile acids, within macrophages oxysterol synthesis has been proposed as a physiologic means to deliver sterols from the peripheral tissues to the liver; however, this remains controversial.62,131–133
Therefore, factors controlling CYP7B1’s activity likely play a major role in the levels of tissue oxysterols. Umetani et al.134 utilizing Cyp7b1 knockout and ApoE knockout mice models without altering dietary lipid intake, demonstrated that elevated tissue 26HC promoted atherogenesis; with strong supportive findings linking initiation to 26HC-ERα interaction. However, despite evidence that hormone replacement may not be beneficial to post-menopausal women, the fact remains that the risk of coronary heart disease increases dramatically after menopause. The marked inflammation and rapid progression to fibrosis within the liver in CYP7B1 deficient children in their first year of life is strong support that oxysterols with their hydrophobic bile acid metabolites such a CA27-Δ5-3β-ol, an additional hydroxylation metabolite of 26HC, through toxicity induce subsequent inflammation.7
As in the liver, accumulating oxysterol levels within cardiovascular tissue are inflammatory. Whether oxysterols are carried to or formed within the tissue, or whether they directly initiate cytotoxicity or stimulate inflammation through receptor interaction such as described by Umetani et al.134 growing evidence suggests that with accumulating oxysterols the stage is set for the initiation and continued propagation of inflammation.
11. Conclusion remarks
Our recent observations have demonstrated that CYP27A1 makes three key regulatory oxysterols and renewed the importance of CYP7B1 as the key regulator of these oxysterol levels.16 Furthermore, preliminary observations have shown that StarD1 inhibition improves hepatocyte mitochondrial free cholesterol overload and injury.135 Finally, currently unpublished observations from our laboratory have shown a direct toxicity due to increased oxysterol levels as a function of CYP7B1’s pathophysiologic downregulation.84
Interestingly, there is a report that a feeding of Chardonnay grapeseed flower successfully induced hepatic Cyp7b1 expression in mice and improved fatty liver.136 Another report on a diet induced NAFL model mouse showed that UDCA supplementation induced Cyp7b1 mRNA, and the mouse improved fasting glucose level and hepatic steatosis.137 To our knowledge, these are the only reports for successful induction of CYP7B1 in vivo by exogeneous agents. However, recent findings show insulin signaling to be a prime up-regulator of CYP7B1. Therefore, it is reasonable to think incretin mimetics such as GLP-1 agonists and dipeptidyl peptidase-4 (DPP-4) inhibitors play a role as inducers of CYP7B1. Thus, recent findings are now being able to mechanistically connect insulin resistance to the transition of NAFL to NASH. More specifically in preliminary observations, our laboratory has been able to show a direct correlation amongst a suppressed CYP7B1, elevated oxysterol levels, and hepatocellular injury. The recent observations with adaptive thermogenesis provide for a potential physiological role for this pathway. The understanding of regulation of CYP7B1 and modulation of intercellular oxysterols would be a key to development of the MS intervention. In addition, measurement of serum oxysterols and their specific metabolites is potentially useful for specific non-invasive biomarkers for these pathogenic conditions.
Therefore, although the acidic pathway of cholesterol metabolism has been relegated one of lesser importance as it plays only a minor role in the liver in the rates of bile acid biosynthesis, the pathway has an important role in producing regulatory oxysterols and for making a specific bile acid pool; both of which are required for controlling lipid, cholesterol, carbohydrate metabolisms, and energy expenditure. Under physiological conditions, oxysterols have a finite half-life as they do not accumulate in cells. The half-life of 26HC in plasma is ~1 h.138 This short half-life of oxysterols help explains their roles as acute regulators of cellular cholesterol and lipid homeostasis. However, the chronic excess of oxysterols and the subsequently generated metabolites can be injurious to hepatocytes as well as have inflammatory ramifications in extrahepatic tissues. Targeting oxysterol metabolism represents a new treatment strategy for all inflammatory metabolic diseases.
Supplementary Material
Acknowledgements
Authors thank Dr. Phillip B Hylemon, Virginia Commonwealth University, for his thoughtful comments and critical reading of the manuscript. This work was supported by Gilead Sciences Liver Research Award 2016 and Virginia Commonwealth University DOIM Pilot Project Grant (4111393) to G. Kakiyama; and Veterans Administration Merit Award I01 BX000197–07 to W. M. Pandak.
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.livres.2019.05.001.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Cusi K Role of obesity and lipotoxicity in the development of nonalcoholic steatohepatitis: pathophysiology and clinical implications. Gastroenterology. 2012;142:711–725 (e6). [DOI] [PubMed] [Google Scholar]
- 2.Younossi Z, Tacke F, Arrese M, et al. Global perspectives on non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. Hepatology. 2018. 10.1002/hep.30251 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 3.Younossi Z, Anstee QM, Marietti M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11–20. [DOI] [PubMed] [Google Scholar]
- 4.Chiang JYL. Bile acid metabolism and signaling in liver disease and therapy. Liver Res. 2017;1:3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Molinaro A, Wahlström A, Marschall HU. Role of bile acids in metabolic control. Trends Endocrinol Metab. 2018;29:31–41. [DOI] [PubMed] [Google Scholar]
- 6.Arab JP, Karpen SJ, Dawson PA, Arrese M, Trauner M. Bile acids and nonalcoholic fatty liver disease: molecular insights and therapeutic perspectives. Hepatology. 2017;65:350–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Setchell KD, Schwarz M, O’Connell NC, et al. Identification of a new inborn error in bile acid synthesis: mutation of the oxysterol 7alpha-hydroxylase gene causes severe neonatal liver disease. J Clin Invest. 1998;102: 1690–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Russell DW. The enzymes, regulation, and genetics of bile acid synthesis. Annu Rev Biochem. 2003;72:137–174. [DOI] [PubMed] [Google Scholar]
- 9.Vaz FM, Ferdinandusse S. Bile acid analysis in human disorders of bile acid biosynthesis. Mol Aspects Med. 2017;56:10–24. [DOI] [PubMed] [Google Scholar]
- 10.Arnon R, Yoshimura T, Reiss A, Budai K, Lefkowitch JH, Javitt NB. Cholesterol 7-hydroxylase knockout mouse: a model for monohydroxy bile acid-related neonatal cholestasis. Gastroenterology. 1998;115:1223–1228. [DOI] [PubMed] [Google Scholar]
- 11.Javitt NB. 25R,26-Hydroxycholesterol revisited: synthesis, metabolism, and biologic roles. J Lipid Res. 2002;43:665–670. [PubMed] [Google Scholar]
- 12.Nakagawa M, Setchell KD. Bile acid metabolism in early life: studies of amniotic fluid. J Lipid Res. 1990;31:1089–1098. [PubMed] [Google Scholar]
- 13.Honda A, Yamashita K, Numazawa M, et al. Highly sensitive quantification of 7alpha-hydroxy-4-cholesten-3-one in human serum by LC-ESI-MS/MS. J Lipid Res. 2007;48:458–464. [DOI] [PubMed] [Google Scholar]
- 14.Li X, Pandak WM, Erickson SK, et al. Biosynthesis of the regulatory oxysterol, 5-cholesten-3beta,25-diol 3-sulfate, in hepatocytes. J Lipid Res. 2007;48: 2587–2596. [DOI] [PubMed] [Google Scholar]
- 15.Cali JJ, Russell DW. Characterization of human sterol 27-hydroxylase. A mitochondrial cytochrome P-450 that catalyzes multiple oxidation reaction in bile acid biosynthesis. J Biol Chem. 1991;266:7774–7778. [PubMed] [Google Scholar]
- 16.Kakiyama G, Marques D, Takei H, et al. Mitochondrial oxysterol biosynthetic pathway gives evidence for CYP7B1 as controller of regulatory oxysterols. J Steroid Biochem Mol Biol. 2019;189:36–47. [DOI] [PubMed] [Google Scholar]
- 17.Fakheri RJ, Javitt NB. 27-Hydroxycholesterol, does it exist? On the nomenclature and stereochemistry of 26-hydroxylated sterols. Steroids. 2012;77, 575–557. [DOI] [PubMed] [Google Scholar]
- 18.Björkhem I Cerebrotendinous xanthomatosis. Curr Opin Lipidol. 2013;24: 283–287. [DOI] [PubMed] [Google Scholar]
- 19.Javitt NB, Kok E, Cohen B, Burstein S. Cerebrotendinous xanthomatosis: reduced serum 26-hydroxycholesterol. J Lipid Res. 1982;23:627–630. [PubMed] [Google Scholar]
- 20.Pandak WM, Ren S, Marques D, et al. Transport of cholesterol into mitochondria is rate-limiting for bile acid synthesis via the alternative pathway in primary rat hepatocytes. J Biol Chem. 2002;277:48158–48164. [DOI] [PubMed] [Google Scholar]
- 21.Ren S, Hylemon PB, Marques D, et al. Overexpression of cholesterol transporter StAR increases in vivo rates of bile acid synthesis in the rat and mouse. Hepatology. 2004;40:910–917. [DOI] [PubMed] [Google Scholar]
- 22.Dai D, Mills PB, Footitt E, et al. Liver disease in infancy caused by oxysterol 7 alpha-hydroxylase deficiency: successful treatment with chenodeoxycholic acid. J Inherit Metab Dis. 2014;37:851–861. [DOI] [PubMed] [Google Scholar]
- 23.Ueki I, Kimura A, Nishiyori A, et al. Neonatal cholestatic liver disease in an Asian patient with a homozygous mutation in the oxysterol 7alpha-hydroxylase gene. J Pediatr Gastroenterol Nutr. 2008;46:465–469. [DOI] [PubMed] [Google Scholar]
- 24.Mizuochi T, Kimura A, Suzuki M, et al. Successful heterozygous living donor liver transplantation for an oxysterol 7alpha-hydroxylase deficiency in a Japanese patient. Liver Transpl. 2011;17:1059–1065. [DOI] [PubMed] [Google Scholar]
- 25.Bjorkhem I, Andersson U, Ellis E, et al. From brain to bile. Evidence that conjugation and omega-hydroxylation are important for elimination of 24S-hydroxycholesterol (cerebrosterol) in humans. J Biol Chem. 2001;276: 37004–37010. [DOI] [PubMed] [Google Scholar]
- 26.Björkhem I Five decades with oxysterols. Biochimie. 2013;95:448–454. [DOI] [PubMed] [Google Scholar]
- 27.Li-Hawkins J, Lund EG, Bronson AD, Russell DW. Expression cloning of an oxysterol 7alpha-hydroxylase selective for 24-hydroxycholesterol. J Biol Chem. 2000;275:16543–16549. [DOI] [PubMed] [Google Scholar]
- 28.Norlin M, Toll A, Björkhem I, Wikvall K. 24-hydroxycholesterol is a substrate for hepatic cholesterol 7alpha-hydroxylase (CYP7A). J Lipid Res. 2000;41: 1629–1639. [PubMed] [Google Scholar]
- 29.Griffiths WJ, Crick PJ, Meljon A, et al. Additional pathways of sterol metabolism: evidence from analysis of CYP27A1−/− mouse brain and plasma. Biochim Biophys Acta Mol Cell Biol Lipids. 2019;1864:191–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ayaki Y, Kok E, Javitt NB. Cholic acid synthesis from 26-hydroxycholesterol and 3-hydroxy-5-cholestenoic acid in the rabbit. J Biol Chem. 1989;264: 3818–3821. [PubMed] [Google Scholar]
- 31.Griffiths WJ, Abdel-Khalik J, Crick PJ, Yutuc E, Wang Y. New methods for analysis of oxysterols and related compounds by LC-MS. J Steroid Biochem Mol Biol. 2016;162:4–26. [DOI] [PubMed] [Google Scholar]
- 32.Honda A, Yoshida T, Xu G, et al. Significance of plasma 7alpha-hydroxy-4-cholesten-3-one and 27-hydroxycholesterol concentrations as markers for hepatic bile acid synthesis in cholesterol-fed rabbits. Metabolism. 2004;53: 42–48. [DOI] [PubMed] [Google Scholar]
- 33.Takahashi S, Fukami T, Masuo Y, et al. Cyp2c70 is responsible for the species difference in bile acid metabolism between mice and humans. J Lipid Res. 2016;57:2130–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li J, Dawson PA. Animal models to study bile acid metabolism. Biochim Biophys Acta (BBA) - Mol Basis Dis. 2018. 10.1016/j.bbadis.2018.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li T, Matozel M, Boehme S, et al. Overexpression of cholesterol 7alpha-hydroxylase promotes hepatic bile acid synthesis and secretion and maintains cholesterol homeostasis. Hepatology. 2011;53:996–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Slätis K, Gåfvels M, Kannisto K, et al. Abolished synthesis of cholic acid reduces atherosclerotic development in apolipoprotein E knockout mice. J Lipid Res. 2010;51:3289–3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaur A, Patankar JV, de Haan W, et al. Loss of Cyp8b1 improves glucose homeostasis by increasing GLP-1. Diabetes. 2015;64:1168–1179. [DOI] [PubMed] [Google Scholar]
- 38.Haeusler RA, Astiarraga B, Camastra S, Accili D, Ferrannini E. Human insulin resistance is associated with increased plasma levels of 12alpha-hydroxylated bile acids. Diabetes. 2013;62:4184–4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pellicciari R, Costantino G, Fiorucci S. Farnesoid X receptor: from structure to potential clinical applications. J Med Chem. 2005;48:5383–5403. [DOI] [PubMed] [Google Scholar]
- 40.Fiorucci S, Rizzo G, Donini A, Distrutti E, Santucci L. Targeting farnesoid X receptor for liver and metabolic disorders. Trends Mol Med. 2007;13:298–309. [DOI] [PubMed] [Google Scholar]
- 41.Otte K, Kranz H, Kober I, et al. Identification of farnesoid X receptor beta as a novel mammalian nuclear receptor sensing lanosterol. Mol Cell Biol. 2003;23: 864–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li Y, Jadhav K, Zhang Y. Bile acid receptors in non-alcoholic fatty liver disease. Biochem Pharmacol. 2013;86:1517–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ishibashi S, Schwarz M, Frykman PK, Herz J, Russell DW. Disruption of cholesterol 7alpha-hydroxylase gene in mice. I. Postnatal lethality reversed by bile acid and vitamin supplementation. J Biol Chem. 1996;271:18017–18023. [DOI] [PubMed] [Google Scholar]
- 44.Schwarz M, Lund EG, Setchell KD, et al. Disruption of cholesterol 7alpha-hydroxylase gene in mice. II. Bile acid deficiency is overcome by induction of oxysterol 7alpha-hydroxylase. J Biol Chem. 1996;271:18024–18031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Erickson SK, Lear SR, Deane S, et al. Hypercholesterolemia and changes in lipid and bile acid metabolism in male and female CYP7A1-deficient mice. J Lipid Res. 2003;44:1001–1009. [DOI] [PubMed] [Google Scholar]
- 46.Ferrell JM, Boehme S, Li F, Chiang JY. Cholesterol 7alpha-hydroxylase-deficient mice are protected from high-fat/high-cholesterol diet-induced metabolic disorders. J Lipid Res. 2016;57:1144–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mutemberezi V, Guillemot-Legris O, Muccioli GG. Oxysterols: from cholesterol metabolites to key mediators. Prog Lipid Res. 2016;64:152–169. [DOI] [PubMed] [Google Scholar]
- 48.Hong C, Tontonoz P. Liver X receptors in lipid metabolism: opportunities for drug discovery. Nat Rev Drug Discov. 2014;13:433–444. [DOI] [PubMed] [Google Scholar]
- 49.Repa JJ, Liang G, Ou J, et al. Regulation of mouse sterol regulatory element- binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXRalpha and LXRbeta. Genes Dev. 2000;14:2819–2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Joseph SB, Laffitte BA, Patel PH, et al. Direct and indirect mechanisms for regulation of fatty acid synthase gene expression by liver X receptors. J Biol Chem. 2002;277:11019–11025. [DOI] [PubMed] [Google Scholar]
- 51.Talukdar S, Hillgartner FB. The mechanism mediating the activation of acetyl-coenzyme A carboxylase-alpha gene transcription by the liver X receptor agonist T0–901317. J Lipid Res. 2006;47:2451–2461. [DOI] [PubMed] [Google Scholar]
- 52.Lee SD, Tontonoz P. Liver X receptors at the intersection of lipid metabolism and atherogenesis. Atherosclerosis. 2015;242:29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meffre D, Shackleford G, Hichor M, et al. Liver X receptors alpha and beta promote myelination and remyelination in the cerebellum. Proc Natl Acad Sci USA. 2015;112:7587–7592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Radhakrishnan A, Ikeda Y, Kwon HJ, Brown MS, Goldstein JL. Sterol-regulated transport of SREBPs from endoplasmic reticulum to Golgi: oxysterols block transport by binding to Insig. Proc Natl Acad Sci U S A. 2007;104:6511–6518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Higgins ME, Davies JP, Chen FW, Ioannou YA. Niemann-Pick C1 is a late endosome-resident protein that transiently associates with lysosomes and the trans-Golgi network. Mol Genet Metab. 1999;68:1–13. [DOI] [PubMed] [Google Scholar]
- 56.Ioannou YA. The structure and function of the Niemann-Pick C1 protein. Mol Genet Metab. 2000;71:175–1781. [DOI] [PubMed] [Google Scholar]
- 57.Ma X, Hu YW, Mo ZC, et al. NO-1886 up-regulates Niemann-Pick C1 protein (NPC1) expression through liver X receptor alpha signaling pathway in THP-1 macrophage-derived foam cells. Cardiovasc Drugs Ther. 2009;23:199–206. [DOI] [PubMed] [Google Scholar]
- 58.Jahn D, Rau M, Hermanns HM, Geier A. Mechanisms of enterohepatic fibro- blast growth factor 15/19 signaling in health and disease. Cytokine Growth Factor Rev. 2015;26:625–635. [DOI] [PubMed] [Google Scholar]
- 59.Ferrebee CB, Dawson PA. Metabolic effects of intestinal absorption and enterohepatic cycling of bile acids. Acta Pharm Sin B. 2015;5:129–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Studer E, Zhou X, Zhao R, et al. Conjugated bile acids activate the sphingosine-1-phosphate receptor 2 in primary rodent hepatocytes. Hepatology. 2012;55: 267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schwarz M, Lund EG, Lathe R, Björkhem I, Russell DW. Identification and characterization of a mouse oxysterol 7alpha-hydroxylase cDNA. J Biol Chem. 1997;272:23995–24001. [DOI] [PubMed] [Google Scholar]
- 62.Li-Hawkins J, Lund EG, Turley SD, Russell DW. Disruption of the oxysterol 7alpha-hydroxylase gene in mice. J Biol Chem. 2000;275:16536–16542. [DOI] [PubMed] [Google Scholar]
- 63.Ning Y, Chen S, Li X, Ma Y, Zhao F, Yin L. Cholesterol, LDL, and 25- hydroxycholesterol regulate expression of the steroidogenic acute regulatory protein in microvascular endothelial cell line (bEnd.3). Biochem Biophys Res Commun. 2006;342:1249–1256. [DOI] [PubMed] [Google Scholar]
- 64.Ma Y, Ren S, Pandak WM, et al. The effects of inflammatory cytokines on steroidogenic acute regulatory protein expression in macrophages. Inflamm Res. 2007;56:495–501. [DOI] [PubMed] [Google Scholar]
- 65.Borthwick F, Taylor JM, Bartholomew C, Graham A. Differential regulation of the STARD1 subfamily of START lipid trafficking proteins in human macrophages. FEBS Lett. 2009;583:1147–1153. [DOI] [PubMed] [Google Scholar]
- 66.Biddinger SB, Haas JT, Yu BB, et al. Hepatic insulin resistance directly promotes formation of cholesterol gallstones. Nat Med. 2008;14:778–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen C, Hu B, Wu T, et al. Bile acid profiles in diabetic (db/db) mice and their wild type littermates. J Pharm Biomed Anal. 2016;131:473–481. [DOI] [PubMed] [Google Scholar]
- 68.Nojima K, Sugimoto K, Ueda H, Babaya N, Ikegami H, Rakugi H. Analysis of hepatic gene expression profile in a spontaneous mouse model of type 2 diabetes under a high sucrose diet. Endocr J. 2013;60:261–274. [DOI] [PubMed] [Google Scholar]
- 69.Worthmann A, John C, Rühlemann MC, et al. Cold-induced conversion of cholesterol to bile acids in mice shapes the gut microbiome and promotes adaptive thermogenesis. Nat Med. 2017;23:839–849. [DOI] [PubMed] [Google Scholar]
- 70.Kawamata Y, Fujii R, Hosoya M, et al. A G protein-coupled receptor responsive to bile acids. J Biol Chem. 2003;278:9435–9440. [DOI] [PubMed] [Google Scholar]
- 71.Donepudi AC, Boehme S, Li F, Chiang JY. G-protein-coupled bile acid receptor plays a key role in bile acid metabolism and fasting-induced hepatic steatosis in mice. Hepatology. 2017;65:813–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Holloway MG, Cui Y, Laz EV, Hosui A, Hennighausen L, Waxman DJ. Loss of sexually dimorphic liver gene expression upon hepatocyte-specific deletion of Stat5a-Stat5b locus. Endocrinology. 2007;148:1977–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schwarz M, Lund EG, Russell DW. Two 7 alpha-hydroxylase enzymes in bile acid biosynthesis. Curr Opin Lipidol. 1998;9:113–118. [DOI] [PubMed] [Google Scholar]
- 74.Connerney J, Lau-Corona D, Rampersaud A, Waxman DJ. Activation of male liver chromatin accessibility and STAT5-dependent gene transcription by plasma growth hormone pulses. Endocrinology. 2017;158:1386–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wu Z, Martin KO, Javitt NB, Chiang JY. Structure and functions of human oxysterol 7alpha-hydroxylase cDNAs and gene CYP7B1. J Lipid Res. 1999;40: 2195–2203. [PubMed] [Google Scholar]
- 76.Pandak WM, Hylemon PB, Ren S, et al. Regulation of oxysterol 7alpha-hydroxylase (CYP7B1) in primary cultures of rat hepatocytes. Hepatology. 2002;35:1400–1408. [DOI] [PubMed] [Google Scholar]
- 77.de Aguiar Vallim TQ, Tarling EJ, Ahn H, et al. MAFG is a transcriptional repressor of bile acid synthesis and metabolism. Cell Metab. 2015;21: 298–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhou C, Pridgen B, King N, Xu J, Breslow JL. Hyperglycemic Ins2AkitaLdlr−/− mice show severely elevated lipid levels and increased atherosclerosis: a model of type 1 diabetic macrovascular disease. J Lipid Res. 2011;52: 1483–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ikegami T, Hyogo H, Honda A, et al. Increased serum liver X receptor ligand oxysterols in patients with non-alcoholic fatty liver disease. J Gastroenterol. 2012;47:1257–1266. [DOI] [PubMed] [Google Scholar]
- 80.Guillemot-Legris O, Mutemberezi V, Muccioli GG. Oxysterols in metabolic syndrome: from bystander molecules to bioactive lipids. Trends Mol Med. 2016;22:594–614. [DOI] [PubMed] [Google Scholar]
- 81.Clare K, Hardwick SJ, Carpenter KL, Weeratunge N, Mitchinson MJ. Toxicity of oxysterols to human monocyte-macrophages. Atherosclerosis. 1995;118: 67–75. [DOI] [PubMed] [Google Scholar]
- 82.Lemaire-Ewing S, Prunet C, Montange T, et al. Comparison of the cytotoxic, pro-oxidant and pro-inflammatory characteristics of different oxysterols. Cell Biol Toxicol. 2005;21:97–114. [DOI] [PubMed] [Google Scholar]
- 83.Kim BY, Son Y, Eo SK, Park YC, Kim K. Diclofenac inhibits 27-hydroxycholesterol-induced inflammation. Biochem Biophys Res Commun. 2016;478:1456–1461. [DOI] [PubMed] [Google Scholar]
- 84.Kakiyama G, Marques D, Takei H, et al. Inflammasome activation by chronic down regulation of CYP7B1 and its causative increased oxysterol accumulation, represents the key initial step in fatty liver’s progression toward inflammation. Gastroenterology. 2017;152:S1069. [Google Scholar]
- 85.Lake AD, Novak P, Shipkova P, et al. Decreased hepatotoxic bile acid composition and altered synthesis in progressive human nonalcoholic fatty liver disease. Toxicol Appl Pharmacol. 2013;268:132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Naito H, Jia X, Yetti H, et al. Importance of detoxifying enzymes in differentiating fibrotic development between SHRSP5/Dmcr and SHRSP rats. Environ Health Prev Med. 2016;21:368–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yetti H, Naito H, Yuan Y, et al. Bile acid detoxifying enzymes limit susceptibility to liver fibrosis in female SHRSP5/Dmcr rats fed with a high-fat- cholesterol diet. PLoS One. 2018;13:e0192863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pircher PC, Kitto JL, Petrowski ML, et al. Farnesoid X receptor regulates bile acid-amino acid conjugation. J Biol Chem. 2003;278:27703–27711. [DOI] [PubMed] [Google Scholar]
- 89.Jiao N, Baker SS, Chapa-Rodriguez A, et al. Suppressed hepatic bile acid signalling despite elevated production of primary and secondary bile acids in NAFLD. Gut. 2018;67:1881–1891. [DOI] [PubMed] [Google Scholar]
- 90.Ferslew BC, Xie G, Johnston CK, et al. Altered bile acid metabolome in patients with nonalcoholic Steatohepatitis. Dig Dis Sci. 2015;60:3318–3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Min HK, Kapoor A, Fuchs M, et al. Increased hepatic synthesis and dysregulation of cholesterol metabolism is associated with the severity of nonalcoholic fatty liver disease. Cell Metab. 2012;15:665–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mouzaki M, Wang AY, Bandsma R, et al. Bile acids and dysbiosis in non-alcoholic fatty liver disease. PLoS One. 2016;11:e0151829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li H, Wang L, Yan X, et al. A proton nuclear magnetic resonance metabo-nomics approach for biomarker discovery in nonalcoholic fatty liver disease. J Proteome Res. 2011;10:2797–2806. [DOI] [PubMed] [Google Scholar]
- 94.Beilke LD, Aleksunes LM, Holland RD, et al. Constitutive androstane receptor- mediated changes in bile acid composition contributes to hepatoprotection from lithocholic acid-induced liver injury in mice. Drug Metab Dispos. 2009;37:1035–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kakiyama G, Pandak WM, Gillevet PM, et al. Modulation of the fecal bile acid profile by gut microbiota in cirrhosis. J Hepatol. 2013;58:949–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bajaj JS, Ridlon JM, Hylemon PB, et al. Linkage of gut microbiome with cognition in hepatic encephalopathy. Am J Physiol Gastrointest Liver Physiol. 2012;302:G168–G175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bajaj JS, Hylemon PB, Ridlon JM, et al. Colonic mucosal microbiome differs from stool microbiome in cirrhosis and hepatic encephalopathy and is linked to cognition and inflammation. Am J Physiol Gastrointest Liver Physiol. 2012;303:G675–G685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ridlon JM, Kang DJ, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. J Lipid Res. 2006;47:241–259. [DOI] [PubMed] [Google Scholar]
- 99.Devkota S, Wang Y, Musch MW, et al. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10−/− mice. Nature. 2012;487: 104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hardison WG. Hepatic taurine concentration and dietary taurine as regulators of bile acid conjugation with taurine. Gastroenterolgy. 1978;75:71–75. [PubMed] [Google Scholar]
- 101.Mouzaki M, Comelli EM, Arendt BM, et al. Intestinal microbiota in patients with nonalcoholic fatty liver disease. Hepatology. 2013;58:120–127. [DOI] [PubMed] [Google Scholar]
- 102.Zhu L, Baker SS, Gill C, et al. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology. 2013;57:601–609. [DOI] [PubMed] [Google Scholar]
- 103.Boursier J, Mueller O, Barret M, et al. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology. 2016;63:764–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Michail S, Lin M, Frey MR, et al. Altered gut microbial energy and metabolism in children with non-alcoholic fatty liver disease. FEMS Microbiol Ecol. 2015;91:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Loomba R, Seguritan V, Li W, et al. Gut microbiome-based metagenomic signature for non-invasive detection of advanced fibrosis in human nonalcoholic fatty liver disease. Cell Metab. 2017;25:1054–1062 (e5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lappano R, Recchia AG, De Francesco EM, et al. The cholesterol metabolite 25-hydroxycholesterol activates estrogen receptor a-mediated signaling in cancer cells and in cardiomyocytes. PLoS One. 2011;6:e16631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.DuSell CD, Umetani M, Shaul PW, Mangelsdorf DJ, McDonnell DP. 27-hydroxycholesterol is an endogenous selective estrogen receptor modulator. Mol Endocrinol. 2008;22:65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wu Q, Ishikawa T, Sirianni R, et al. 27-Hydroxycholesterol promotes cell-autonomous, ER-positive breast cancer growth. Cell Rep. 2013;5:637–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nelson ER, Wardell SE, Jasper JS, et al. 27-Hydroxycholesterol links hyper-cholesterolemia and breast cancer pathophysiology. Science. 2013;342: 1094–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Karuna R, Holleboom AG, Motazacker MM, et al. Plasma levels of 27-hydroxycholesterol in humans and mice with monogenic disturbances of high density lipoprotein metabolism. Atherosclerosis. 2011;214:448–455. [DOI] [PubMed] [Google Scholar]
- 111.Dietschy JM, Turley SD. Thematic review series: brain Lipids. Cholesterol metabolism in the central nervous system during early development and in the mature animal. J Lipid Res. 2004;45:1375–1397. [DOI] [PubMed] [Google Scholar]
- 112.Björkhem I Crossing the barrier: oxysterols as cholesterol transporters and metabolic modulators in the brain. J Intern Med. 2006;260:493–508. [DOI] [PubMed] [Google Scholar]
- 113.Lavrnja I, Smiljanic K, Savic D, et al. Expression profiles of cholesterol metabolism-related genes are altered during development of experimental autoimmune encephalomyelitis in the rat spinal cord. Sci Rep. 2017;7:2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lütjohann D, Breuer O, Ahlborg G, et al. Cholesterol homeostasis in human brain: evidence for an age-dependent flux of 24S-hydroxycholesterol from the brain into the circulation. Proc Natl Acad Sci U S A. 1996;93:9799–9804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Björkhem I, Lütjohann D, Diczfalusy U, Ståhle L, Ahlborg G, Wahren J. Cholesterol homeostasis in human brain: turnover of 24S-hydroxycholesterol and evidence for a cerebral origin of most of this oxysterol in the circulation. J Lipid Res. 1998;39:1594–1600. [PubMed] [Google Scholar]
- 116.Björkhem I, Meaney S, Diczfalusy U. Oxysterols in human circulation: which role do they have? Curr Opin Lipidol. 2002;13:247–253. [DOI] [PubMed] [Google Scholar]
- 117.Brown J 3rd, Theisler C, Silberman S, et al. Differential expression of cholesterol hydroxylases in Alzheimer’s disease. J Biol Chem. 2004;279: 34674−34681. [DOI] [PubMed] [Google Scholar]
- 118.Heverin M, Meaney S, Lütjohann D, Diczfalusy U, Wahren J, Björkhem I. Crossing the barrier: net flux of 27-hydroxycholesterol into the human brain. J Lipid Res. 2005;46:1047–1052. [DOI] [PubMed] [Google Scholar]
- 119.Heverin M, Bogdanovic N, Lütjohann D, et al. Changes in the levels of cerebral and extracerebral sterols in the brain of patients with Alzheimer’s disease. J Lipid Res. 2004;45:186–193. [DOI] [PubMed] [Google Scholar]
- 120.Marwarha G, Dasari B, Prasanthi JR, Schommer J, Ghribi O. Leptin reduces the accumulation of Abeta and phosphorylated tau induced by 27-hydroxycholesterol in rabbit organotypic slices. J Alzheimer’s Dis. 2010;19: 1007–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Prasanthi JR, Huls A, Thomasson S, Thompson A, Schommer E, Ghribi O. Differential effects of 24-hydroxycholesterol and 27-hydroxycholesterol on beta-amyloid precursor protein levels and processing in human neuroblastoma SH-SY5Y cells. Mol Neurodegener. 2009;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Schrag M, Sharma S, Brown-Borg H, Ghribi O. Hippocampus of Ames dwarf mice is resistant to beta-amyloid-induced tau hyperphosphorylation and changes in apoptosis-regulatory protein levels. Hippocampus. 2008;18: 239–244. [DOI] [PubMed] [Google Scholar]
- 123.Sharma S, Prasanthi RPJ, Schommer E, Feist G, Ghribi O. Hypercholesterolemia-induced Abeta accumulation in rabbit brain is associated with alteration in IGF-1 signaling. Neurobiol Dis. 2008;32:426–432. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 124.Testa G, Staurenghi E, Zerbinati C, et al. Changes in brain oxysterols at different stages of Alzheimer’s disease: their involvement in neuro-inflammation. Redox Biol. 2016;10:24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Shafaati M, Marutle A, Pettersson H, et al. Marked accumulation of 27- hydroxycholesterol in the brains of Alzheimer’s patients with the Swedish APP 670/671 mutation. J Lipid Res. 2011;52:1004–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lütjohann D, Papassotiropoulos A, Björkhem I, et al. Plasma 24S-hydroxycholesterol (cerebrosterol) is increased in Alzheimer and vascular demented patients. J Lipid Res. 2000;41:195–198. [PubMed] [Google Scholar]
- 127.Rantham Prabhakara JP, Feist G, Thomasson S, Thompson A, Schommer E, Ghribi O. Differential effects of 24-hydroxycholesterol and 27-hydroxycholesterol on tyrosine hydroxylase and alpha-synuclein in human neuroblastoma SH-SY5Y cells. J Neurochem. 2008;107:1722–1729. [DOI] [PubMed] [Google Scholar]
- 128.Day CP, Anstee QM. Foreword. Nonalcoholic fatty liver disease. Semin Liver Dis. 2015;35:203–206. [DOI] [PubMed] [Google Scholar]
- 129.Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol. 2013;10: 330–344. [DOI] [PubMed] [Google Scholar]
- 130.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Brown AJ, Jessup W. Oxysterols and atherosclerosis. Atherosclerosis. 1999;142: 1–28. [DOI] [PubMed] [Google Scholar]
- 132.Tontonoz P, Mangelsdorf DJ. Liver X receptor signaling pathways in cardiovascular disease. Mol Endocrinol. 2003;17:985–993. [DOI] [PubMed] [Google Scholar]
- 133.Meir K, Kitsberg D, Alkalay I, et al. Human sterol 27-hydroxylase (CYP27) overexpressor transgenic mouse model. Evidence against 27-hydroxycholesterol as a critical regulator of cholesterol homeostasis. J Biol Chem. 2002;277:34036–34041. [DOI] [PubMed] [Google Scholar]
- 134.Umetani M, Ghosh P, Ishikawa T, et al. The cholesterol metabolite 27-hydroxycholesterol promotes atherosclerosis via proinflammatory processes mediated by estrogen receptor alpha. Cell Metab. 2014;20:172–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ribas V, Nunez S, Garcia-Ruiz C, et al. Hepatocyte-specific StARD1 deletion attenuates alcoholic steatohepatisis. Hepatology. 2015;62:859A. [Google Scholar]
- 136.Seo KH, Bartley GE, Tam C, et al. Chardonnay grape seed flour ameliorates hepatic steatosis and insulin resistance via altered hepatic gene expression for oxidative stress, inflammation, and lipid and ceramide synthesis in diet- induced obese mice. PLoS One. 2016;11:e0167680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ma H, Sales VM, Wolf AR, et al. Attenuated effects of bile acids on glucose metabolism and insulin sensitivity in a male mouse model of prenatal undernutrition. Endocrinology. 2017;158:2441–2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bodin K, Andersson U, Rystedt E, et al. Metabolism of 4 beta-hydroxycholesterol in humans. J Biol Chem. 2002;277:31534–31540. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.