Abstract
Despite their clinical and biological importance, the cell biology of obligate intracellular bacteria is less well understood than that of many free-living model organisms. One reason for this is that they are mostly genetically intractable. As a consequence, it is not possible to engineer strains expressing fluorescent proteins and therefore fluorescence light microscopy – a key tool in host-pathogen cell biology studies – is difficult. Strain diversity also limits the universality of antibody-based immunofluorescence approaches. Here, we have developed a universal labelling protocol for intracellular bacteria based on a clickable methionine analog. Whilst we have applied this to obligate intracellular bacteria, we expect it to be useful for labelling free living bacteria as well as other intracellular pathogens.
1. Introduction
Obligate intracellular bacteria cause a range of human and veterinary diseases around the world. The two main orders of obligate intracellular bacteria are the Rickettsiales and Chlamydiales. Chlamydiales cause sexual- and aerosol-transmitted diseases in humans and are the leading cause of non-congenital blindness worldwide. The Rickettsiales are spread by arthropod vectors and most have animal reservoirs. Rickettsial species cause a wide range of human diseases including typhus (Rickettsia prowazekii), Rocky Mountain Spotted Fever (Rickettsia rickettsii) scrub typhus (Orientia tsutsugamushi), Anaplasmosis (Anaplasma spp.) and Ehrlichiosis (Ehrlichia spp.) (Luce-Fedrow et al., 2018; Fang et al., 2017; Battilani et al., 2017; Saito and Walker, 2016), whilst A. marginale causes disease in cattle (Kocan et al., 2003). The Rickettsiales Wolbachia is not known to cause disease but is a widely distributed endosymbiont of arthropods and nematodes (Miller, 2013).
Fluorescence light microscopy is an important tool for understanding host-pathogen cell biology, especially in the case of obligate intracellular bacteria where a visualization of the interactions between bacteria and host is indispensable for an understanding of their interactions. Most obligate intracellular bacteria remain genetically intractable (McClure et al., 2017; Salje, 2017) and therefore fluorescent protein-based approaches to labeling bacteria are not possible. Immunofluorescence based approaches have been extremely powerful and are currently the main tool for labelling obligate intracellular bacteria. However, the Rickettsiales are a very diverse order and antibodies generally need to be generated specifically for each organism. Even where genetic tools are available, this needs to be repeated for any new environmental and clinical isolates limiting workflow and throughput. For this reason, we have been developing universal tools to label obligate intracellular bacteria.
We recently reported the use of a panel of fluorescent reporters that could be used to label bacteria for live cell imaging (Atwal et al., 2016). In the current work we have built on this by developing protocols for a methionine-based probe. In addition to being used to delineate intracellular bacteria, this probe reports on the metabolic activity of bacteria under study.
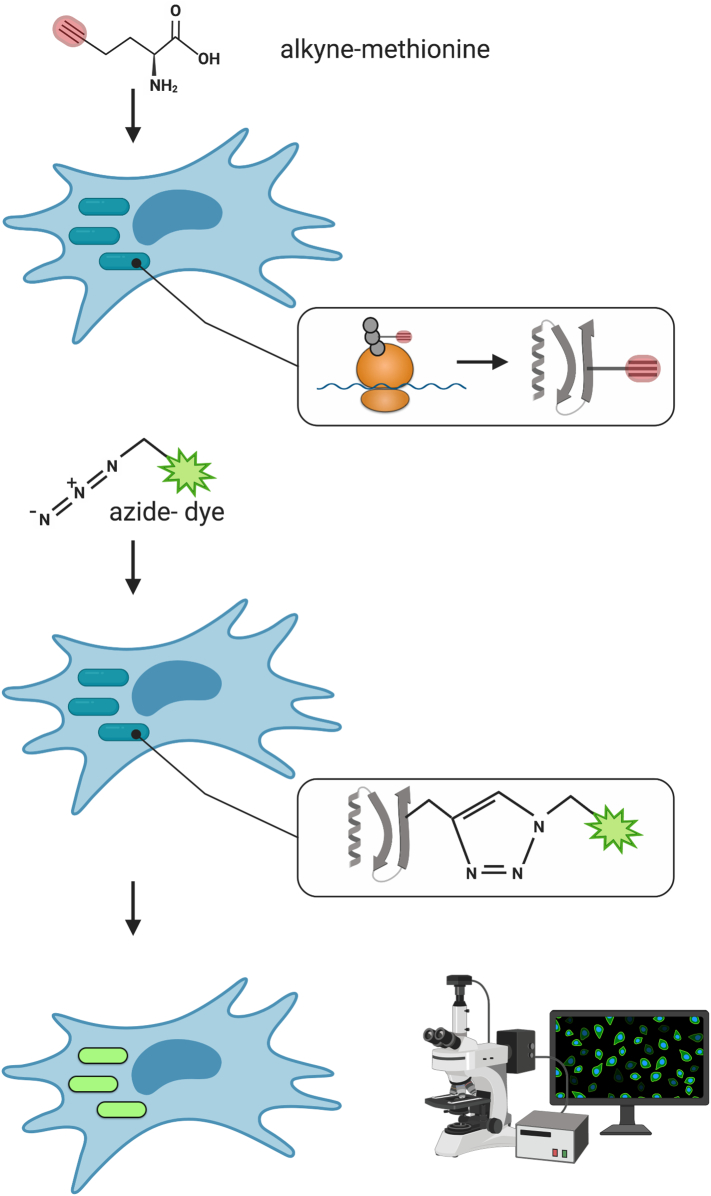
Here, we have used a clickable, non-toxic methionine analog probe (L-Homopropargylglycine, HPG) which readily incorporates into newly synthesized proteins to label a range of obligate intracellular bacteria from the order Rickettsiales (Beatty et al., 2005). The methionine derivative is conjugated to an alkyne (or azide) moiety and is added to growing bacterial cells. Cells are fixed, and then the incorporated methionines are conjugated to an azide (or alkyne) coupled fluorophore using a copper catalyzed click reaction (Fig. 1). This allows metabolically active bacteria to be visualized by fluorescence microscopy techniques.
Fig. 1.
Schematic overview of alkyne-methionine (HPG) labelling of intracellular bacteria. Intracellular bacteria are grown in the presence of an alkyne-methionine probe, which is incorporated into nascent polypeptide chains. After fixation, a fluorescent dye-azide conjugate is reacted with incorporated alkyne-methionine using a click chemistry reaction. Thus bacteria that were undergoing protein synthesis during the time of incubation with alkyne-methionine can be detected using fluorescence microscopy. Created with BioRender.
2. Materials and methods
2.1. Growth of bacteria and cell lines
The following bacterial strains were used: Orientia tsutsugamushi strain Karp, Rickettsia canadensis (gift from Nancy Connell, Rutgers University), Anaplasma marginale strain Oklahoma 291, Wolbachia pipientis endosymbiont of Aedes albopictus, Ehrlichia chaffeensis strain AR (all gifts from Ulrike Munderloh, University of Minnesota) and Anaplasma phagocytophilum strain HGE1 (gift from Thomas Bakken, University of Minnesota).
Macrophage-like DH82 cells (ATCC CRL-10389) were grown in 25 cm2 flasks with Eagle's Minimum Essential Medium (EMEM) (Sigma, M0325, USA) with 10% heat inactivated FBS at 37 °C and 5% CO2. Human leukemia HL-60 cells (ATCC CCL-240) were grown in 25 cm2 flasks with Iscove's Modified Dulbecco's Medium (IMDM) (ATCC 30–2005) with 10% heat inactivated FBS at 37 °C and 5% CO2. L929 cells (ATCC CCL-1) were grown in RPMI 1640 Medium with HEPES (Thermo Fisher Scientific, 22–400-071, USA) supplemented with 10% heat inactivated FBS (Thermo Fisher Scientific, 16,140,071, USA) in 25 cm2 flasks at 35 °C and 5% CO2. Kidney epithelial Vero cells (ATCC CCL-81) were grown in RPMI 1640 Medium with HEPES, supplemented with 10% heat inactivated FBS in 25 cm2 flasks at 37 °C and 5% CO2.
The bacterial strains were grown in the following cell lines: Orientia in L929 cells (as shown previously (Giengkam et al., 2015)), R. canadensis and A. marginale in Vero cells, A. phagocytophilum in HL-60 cells, E. chaffeensis in DH82 cells and Wolbachia in Vero cells.
Chloramphenicol was used at 150 μg/ml and was added to infected host cells at the same time and for the same duration as the methionine probe. Cycloheximide was used at 40 μg/ml and added to infected host cells for 4 h prior to addition of the methionine probe.
2.2. Methionine labelling and click chemistry
Probes Click-IT™ L-Homopropargylglycine (HPG) (Thermo Fisher Scientific, C10186, USA) or L-Azidohomoalanine (Click Chemistry Tools, 1066–25, USA) were added to growing bacteria in host cells at 50 μM in methionine-free DMEM culture media (Thermo Fisher Scientific, 21,013,024, USA) or methionine-supplemented media (Thermo Fisher Scientific, 11,965,092, USA). After 30 min, the probe and culture media were discarded, and the cells were fixed with 4% PFA for 10 min at room temperature. Cells were washed with 3× PBS and permeabilized with 0.5% triton X100 for 10 min on ice. Click chemistry was performed using the Click-iT™ Cell Reaction Buffer Kit (Invitrogen, C10269, USA) with an azide or alkyne Alexa Fluor 488/594 (AF 594 Alkyne, 1297–1 or AF 488 Picolyl Azide 1276–5, Click Chemistry Tools, USA) for 1 h in the dark. Cells were washed with PBS 3× and covered with mounting media (20 mM Tris-HCl, pH 8.0, 0.5% N- propyl-gallate and 90% glycerol). All imaging was performed using either Zeiss LSM700 (Carl Zeiss, Jena, Germany) or Leica DMi8 laser scanning confocal microscopes (Leica Microsystems, Wetzlar, Germany).
2.3. Quantification of fluorescence intensity
Images for quantitative analysis were acquired under constant imaging parameters. Pixel intensity measurements were made using the software ICY (version 2.0.3.0) (de Chaumont et al., 2012) and statistical analyses were done using GraphPad Prism (version 8.3.0 (358)).
3. Results and discussion
3.1. HPG specifically labels bacterial protein synthesis
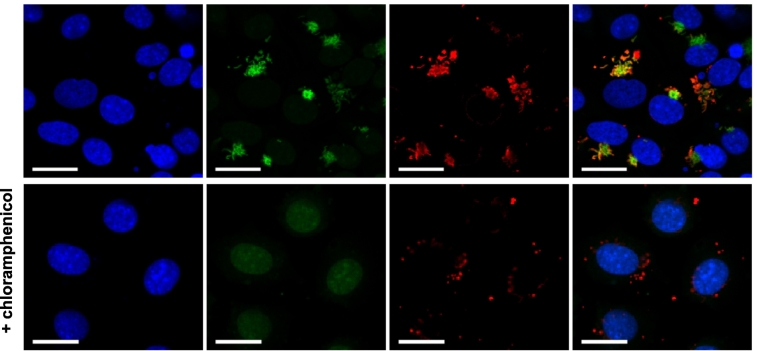
To confirm that the methionine probes were specifically labelling bacterial protein synthesis, chloramphenicol was added to infected host cells. Chloramphenicol targets the bacterial ribosome and inhibits protein synthesis. Bacterial labelling was inhibited by chloramphenicol treatment indicating specific incorporation of HPG into nascent proteins (Fig. 2).
Fig. 2.
The bacterial ribosome inhibitor chloramphenicol blocks nascent protein synthesis in intracellular bacteria, as measured by a loss of labelling with a clickable alkynemethionine (HPG) probe. Orientia tsutsugamushi were grown in L929 host cells in the presence of HPG for 30 mins, cells were fixed, and HPG was reacted with an azide-conjugated fluorophore to reveal cells actively undergoing protein synthesis (green). Cells grown in the presence of the bacterial ribosome inhibitor chloramphenicol (150 μg/ml) did not contain bacteria undergoing detectable protein synthesis. Bacteria are counterstained in red (TSA56 antibody) and host cell nuclei are shown in blue (DAPI). Scale bar = 20 μm. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.2. The signal from HPG-labelled bacteria can be detected over the signal from host cell protein synthesis
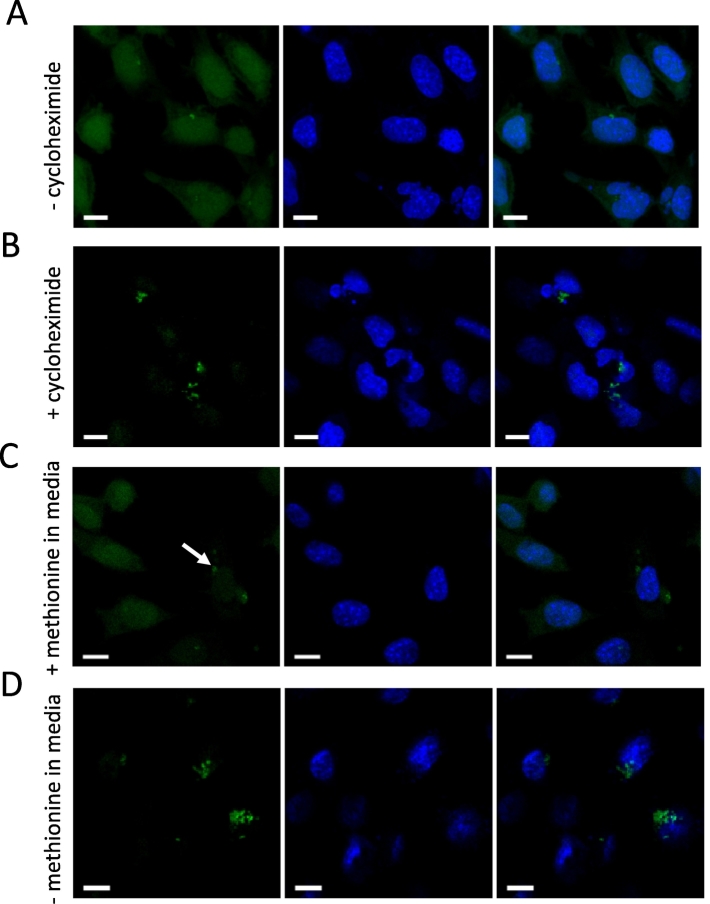
Both bacteria and host cells undergo protein synthesis and therefore both will incorporate HPG that is present in the growth media. We found that when incubating with the probe for 1 to 3 h the signal from intracellular bacteria could be readily detected against the background of host cells (Fig. 3A). However, host cell background could be further reduced using a specific inhibitor of eukaryotic protein synthesis, cycloheximide (Fig. 3B). A concentration of 40μg/ml reduced the host signal but did not affect host morphology. This may be beneficial in those cases where the bacterial signal is weak or when a very strong signal to noise ratio is required. It is worth noting, however, that inhibition of host protein synthesis will likely affect the host-pathogen interaction and thus this approach will not always be appropriate.
Fig. 3.
Methionine-free media and eukaryotic protein synthesis inhibitor cycloheximide can improve intracellular bacterial labelling. A, B. Orientia tsutsugamushi-infected L929 host cells were treated with and without the eukaryotic ribosome inhibitor cycloheximide (50μg/ml) for 4 h. Methionine HPG probe was then added to infected and treated cells for 30mins. Cells were fixed, and HPG was reacted with an azide-conjugated fluorophore to reveal cells actively undergoing protein synthesis (green). The higher signal-to-noise ratio in treated (B) compared with untreated (A) cells reflects the loss of signal from HPG incorporated into nascent host cell proteins. C, D. Orientia tsutsugamushi cells were grown in L929 host cells with HPG for 30mins in media supplemented with methionine (C) or methionine-free media (D). Cells were fixed, and HPG was reacted with an azide-conjugated fluorophore to reveal cells actively undergoing protein synthesis (green). The increased signal in bacteria grown in the absence of methionine in the growth media (D) reflects the loss of competition for incorporating alkyne-methionine rather than native methionine. Host cell nuclei are shown in blue (DAPI). White arrows show HPG-labelled bacteria. Scale bar = 10 μm. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.3. Use of methionine-free media can improve signal-to-noise
Mammalian cell culture media usually contains a supplement of methionine (e.g. RPMI contains 0.015 g/L and DMEM contains 0.03 g/L). This will compete with the clickable methionine analog for incorporation into growing polypeptides. We tested the effect of this by comparing HPG labelling in media with and without unlabelled methionine. In this experiment bacteria were first grown in the presence of methionine as usual, and then the media was replaced with methionine-free (or regular) media during the 30 min incubation with HPG. Whilst bacterial labelling could be clearly resolved in both cases there was an increase in signal when incubation was performed using methionine-free growth media (Fig. 3C,D).
3.4. Using HPG labelling as a reporter of differential metabolic activity
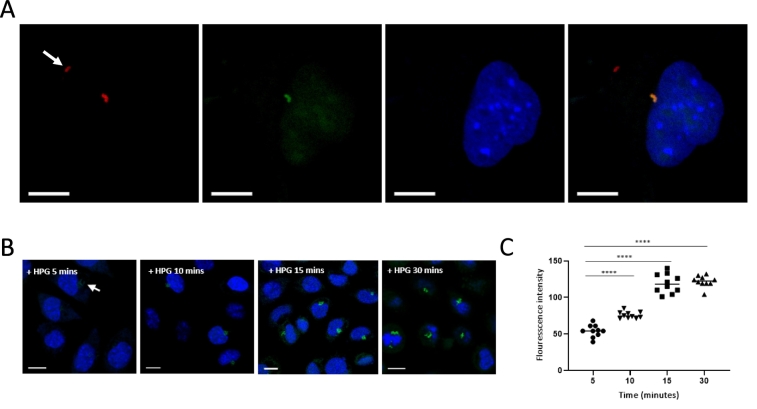
We tested whether HPG could be used to distinguish bacterial subpopulations of differing metabolic activity. Obligate intracellular bacteria are dependent on their host cells for metabolic intermediates and therefore extracellular bacteria are expected to be metabolically inactive. We found that HPG never labelled O. tsutsugamushi when located outside host cells (Fig. 4A) unlike intracellular bacteria. We next assessed whether fluorescence intensity could be quantified as a measure of the amount of HPG incorporated. In order to address this, we exposed bacteria to HPG for 5, 10, 15 and 30 min (Fig. 3B). The concentration of HPG and labelling reagents as well as all other conditions were kept constant. We found that the fluorescence intensity increased up to 15 min, and that this count be quantified by measuring the pixel intensity of images acquired under identical settings (Fig. 3C). This showed that the relative amount of HPG incorporated could be quantified using pixel intensity measurements, and this assay could be used to quantify natural variations in metabolic activity within a bacterial population.
Fig. 4.
HPG can be used as a reporter of differential metabolic activity in bacterial subpopulations. A. Orientia tsutsugamushi were grown in L929 host cells with HPG for 30 mins in methionine-free media. After cell fixation HPG was reacted with an azide-conjugated fluorophore to reveal cells actively undergoing protein synthesis (green). Bacteria were counterstained in red (TSA56 antibody). This shows that metabolically inactive bacteria, located outside the host cell and indicated with a white arrow, cannot be labelled with HPG because they are not undergoing protein synthesis. B, C. The relative amount of HPG incorporated into bacteria can be estimated using fluorescence intensity measurements. Bacteria were grown in the presence of HPG for 5, 10, 15 and 30 min. The fluorescence intensity after 5 min incubation is lower than after 15 and 30 min as shown by confocal microscopy images (B) and fluorescence intensity measurements (C). Statistical significance was calculated using an unpaired t-test with GraphPad Prism software. ****p value ≤ .0001. The host cell nuclei are shown in blue (DAPI). Scale bar = 10 μm. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
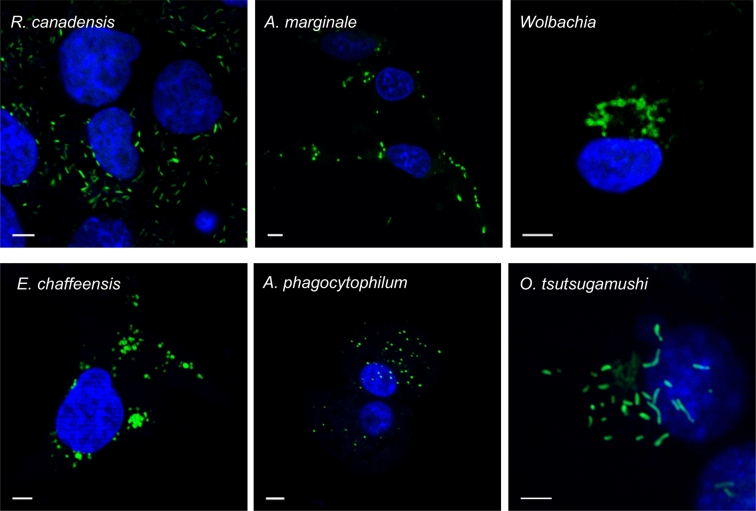
3.5. Using HPG labelling to image a panel of Rickettsiales obligate intracellular bacteria
We tested the ability of HPG to label a diverse group of 6 obligate intracellular Rickettsiales bacterial species (Anaplasma marginale, Ehrlichia chaffeensis, Anaplasma phagocytophilum, Wolbachia, Orientia tsutsugamushi, Rickettsia canadensis) (Fig. 5). Despite differences in host cell type, replication cycle and generation time all could be labelled successfully using the same protocol. Obligate intracellular bacteria are dependent on host cells for provision of nutrients, therefore any bacteria outside host cells were metabolically inactive and not labelled with the methionine probe.
Fig. 5.
HPG can label Intracellular Rickettsiales species.
The methionine probe HPG was added to infected cells for 30 min prior to fixation. HPG was reacted with an azide-conjugated fluorophore to reveal cells actively undergoing protein synthesis (green). Host cell nuclei are shown in blue (DAPI). Scale bar = 5 μm. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Conclusion
Taken together, these results show that a clickable methionine probe can be used to fluorescently label obligate intracellular bacteria. This probe can be used as a universal visual label for pathogens for which other tools are not available. In addition to delineating bacterial organisms it can be used to probe for differential metabolic activity within bacterial populations.
Acknowledgments
Acknowledgements
HF was supported by NIH F32GM122266 (USA); JD was supported by R01 GM114213, R21AI135427, and is a Burroughs-Welcome Investigator in the Pathogenesis of Infection Disease (USA). JS was supported by a Royal Society Dorothy Hodgkin Research Fellowship (UK) DH140154 and MRC-NSTDA research grant MR/N012380/1 (UK/Thailand)
Declaration of Competing Interest
The authors declare no competing interests.
References
- Atwal S. Live imaging of the genetically intractable obligate intracellular bacteria Orientia tsutsugamushi using a panel of fluorescent dyes. J. Microbiol. Methods. 2016;130:169–176. doi: 10.1016/j.mimet.2016.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battilani M. Genetic diversity and molecular epidemiology of Anaplasma. Infect. Genet. Evol. 2017;49:195–211. doi: 10.1016/j.meegid.2017.01.021. [DOI] [PubMed] [Google Scholar]
- Beatty K.E. Selective dye-labeling of newly synthesized proteins in bacterial cells. J. Am. Chem. Soc. 2005;127(41):14150–14151. doi: 10.1021/ja054643w. [DOI] [PubMed] [Google Scholar]
- de Chaumont F. Icy: an open bioimage informatics platform for extended reproducible research. Nat. Methods. 2012;9(7):690–696. doi: 10.1038/nmeth.2075. [DOI] [PubMed] [Google Scholar]
- Fang R., Blanton L.S., Walker D.H. Rickettsiae as emerging infectious agents. Clin. Lab. Med. 2017;37(2):383–400. doi: 10.1016/j.cll.2017.01.009. [DOI] [PubMed] [Google Scholar]
- Giengkam S. Improved quantification, propagation, purification and storage of the obligate intracellular human pathogen Orientia tsutsugamushi. PLoS Negl. Trop. Dis. 2015;9(8) doi: 10.1371/journal.pntd.0004009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocan K.M. Antigens and alternatives for control of Anaplasma marginale infection in cattle. Clin. Microbiol. Rev. 2003;16(4):698–712. doi: 10.1128/CMR.16.4.698-712.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luce-Fedrow A. A review of scrub typhus (Orientia tsutsugamushi and related organisms): then, now, and tomorrow. Trop. Med. Infect. Dis. 2018;3(1) doi: 10.3390/tropicalmed3010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure E.E. Engineering of obligate intracellular bacteria: progress, challenges and paradigms. Nat. Rev. Microbiol. 2017;15(9):544–558. doi: 10.1038/nrmicro.2017.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller W.J. Bugs in transition: the dynamic world of Wolbachia in insects. PLoS Genet. 2013;9(12) doi: 10.1371/journal.pgen.1004069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T.B., Walker D.H. Ehrlichioses: an important one health opportunity. Vet. Sci. 2016;3(3) doi: 10.3390/vetsci3030020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salje J. Orientia tsutsugamushi: a neglected but fascinating obligate intracellular bacterial pathogen. PLoS Pathog. 2017;13(12) doi: 10.1371/journal.ppat.1006657. [DOI] [PMC free article] [PubMed] [Google Scholar]