Abstract
Coronary artery disease is the leading cause of death worldwide. After an acute myocardial infarction, early and successful myocardial intervention via recanalization of the coronary artery is the most effective strategy for reducing the size of ischemic myocardium. The coronary microvasculature cannot be visualized and imaged in vivo, but there are several invasive and noninvasive techniques that can be used to assess parameters which depend directly on coronary microvascular function. The endothelial function after ischemia reperfusion can be assessed also at the level of the coronary circulation via the coronary flow reserve (CFR). In this study, peak velocity of left anterior descending (LAD) coronary arteries was measured in rats in vivo via Transthoracic Doppler Echocardiography during resting and stress challenge (induced by Dobutamine). A normal heart can increase its coronary blood flow up to four times above the resting values during stress induction. Following ischemia reperfusion, we found a significantly diminished CFR, which can be used as a marker of coronary microvascular dysfunction. CFR has opened a window on the importance of microvascular dysfunction and has been shown to predict cardiovascular risk independent of whether the severe obstructive disease is present.
Keywords: Medicine, Issue 148, Coronary flow reserve, Ischemia/reperfusion, Left anterior descending artery, Coronary blood flow, Microcirculation, Coronary artery disease
Introduction
Myocardial ischemia reperfusion (IR) is a condition where blood supply is restricted to the heart followed by the restoration of perfusion and simultaneous reoxygenation1. Occlusion of coronary arteries can be caused by an embolus or cholesterol plaque rupture, which results in a severe imbalance of metabolic supply and demand, causing tissue hypoxia. Salvage of jeopardized myocardium, improve left ventricular function, and enhance survival in patients with acute myocardial infarction have been observed by the reperfusion therapy. However, after recanalization of the coronary artery, functional abnormalities of small coronary vessels may occur2, 3, 4, 5. A significant proportion of patients, perhaps as many as 40%, do not regain microvascular and myocardial perfusion despite the restoration of coronary flow. Visualization and evaluation of the coronary microvasculature can be difficult in vivo, but there are a number of invasive and noninvasive techniques that can be used to assess parameters directly depending on the coronary microvascular function6, 7. Also, the endothelial function can be assessed at the level of the coronary circulation via the CFR5.
Transthoracic Doppler echocardiography is a noninvasive tool which allows us to study coronary artery flow velocity and CFR5. CFR represents the ratio of maximal coronary blood flow to the resting coronary blood flow8. During the stress challenge, a normal heart increases coronary blood flow up to four times above the resting value. Cardiovascular risk increases when CFR is diminished9. Ishihara et al showed that the CFR was severely impaired immediately after the coronary angioplasty5. In the absence of coronary artery stenosis, CFR decreases during the coronary microvascular dysfunction and is present in about half of the patients with stable coronary artery disease10.
The overall goal of this method is noninvasive visualization of left anterior descending coronary artery (LAD) function in rats via echocardiography, which may be used to calculate CFR. This offers an important assessment tool for diagnosing microvascular dysfunction and evaluating potential therapeutic treatments.
Protocol
All procedures were performed in accordance with protocols approved by the University of Louisville Institutional Animal Care and Use Committee (IACUC-approved protocol 18223) and the NIH Guide for the Care and Use of Laboratory Animals11.
1. Animals
Use 4-month-old female Fisher 344 rats (BW~150-180 g) for the study.
2. Ultrasound Imaging before IR surgery
Anesthetize the rat with isoflurane - induction chamber at 5% with 1.5–2.0 L/min O2 flow followed by 1.5–2.0% with 1.5–2.0 L/min O2 flow. This anesthesia is maintained throughout the experiment, during the resting and stress stages.
-
Place the animal in a supine position and shave the thorax. Maintain the body temperature at 37-38 °C using the built-in warming platform. Monitor the heart rate using the software.
NOTE: Proper anesthesia is crucial for maintaining the heart rate at normal physiological rates (between 295-350 beats/min).
Apply ophthalmic vet ointment to prevent the dryness of eyes prior to the imaging.
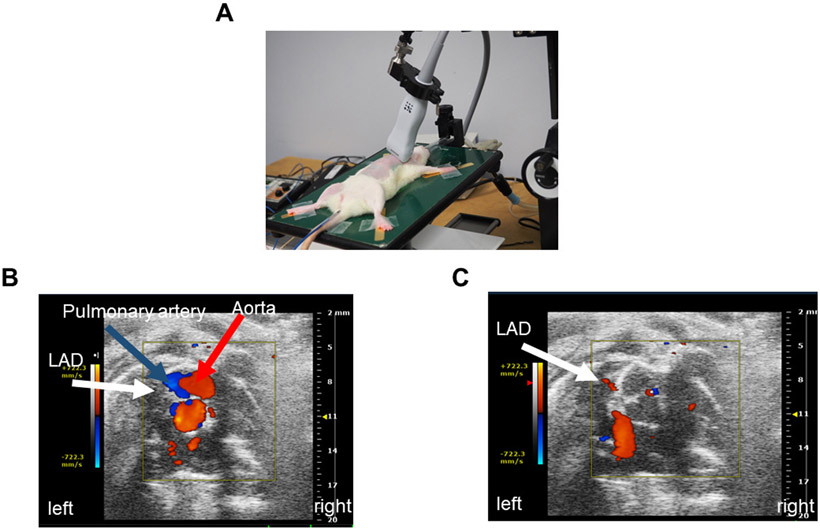
Perform echocardiography using 13–24 MHz linear probe (e.g., MS250) (Figure 1A).
Place the animal in the supine position on the heated platform. Ensure that the anesthesia is controlled via the nose cone. Then position the probe to obtain the parasternal short axis view (PSAX) using the rail system (Figure 1B).
Moving the probe in the rostral direction from the PSAX, locate the pulmonary artery (Figure 1B).
-
Move the probe slightly in the direction caudal from the pulmonary artery to view the LAD and store the image.
NOTE: The LAD is difficult to find without Color Doppler, so B-Mode images of the LAD are not always possible.
- When individual differences make it difficult to locate the LAD coronary artery, follow the technique mentioned below:
- Move the probe lateral to the pulmonary artery.
- Angle the platform so that the animal is inclined, inverted, or slightly towards the right side so that the left ventricle is more readily visible with the probe.
Once the image in B Mode is captured or cine-stored, click the Color Doppler on the touch screen (Figure 1C). Visualize the coronary artery (white arrow indicates LAD) in the short axis (Figure 1C). The red color, as seen in real time, is indicative of the direction of the flow (i.e., blood flow is towards the probe).
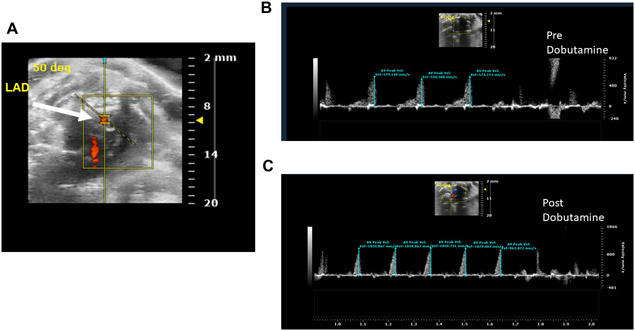
After visualizing LAD on a color-Doppler mode, change the mode to the pulse wave (PW)-mode. Look for the presence of a yellow-indicator line on the coronary artery (Figure 2A).
-
Place the yellow PW-line in the middle of the coronary artery. Ensure that the angle is parallel to the direction of the flow.
NOTE: Velocity of the flow is highly dependent on the angle of the PW line, so be sure to match the angle of the on-screen probe with the angle of the LAD. Use the touch screen to adjust the angle; PW angle should be less than 60°.
Use cine store to capture the velocity of the resting LAD coronary flow at the peak diastole as wave forms (Figure 2B).
-
After obtaining of resting LAD flow velocity, measure the maximum flow velocity of LAD during the stress to calculate CFR. To measure the maximum flow velocity during the stress, infuse Dobutamine at a dose of 20 μg/kg/min via tail vein8 (Figure 2C).
NOTE: Dobutamine infusion should not be more than 8 min. Use an infusion pump and set the diameter of the infusion pump to 14.43 for BD 10 mL syringe.- Use 25-G infusion butterfly set for the tail vein cannulation. To place the infusion needle, place a small strip of gauze around the base of the rat’s tail, then grab with hemostats and twist the tourniquet to apply pressure and cause the vein to enlarge.
-
Place the needle while directly connected to Dobutamine syringe.NOTE: Take extra care not to accidentally introduce drug when drawing up blood to ensure proper placement of the needle in the tail vein. Also, be sure to avoid introducing an air bubble into the vein, as an embolism may be fatal to the animal.
- Once the needle is placed, stabilize it with a glue and a piece of surgical tape, securing the infusion line to the tail.
- Remove the hemostats and tourniquet to recover the flow.
- Place Dobutamine syringe in the infusion pump and set it to inject 20 μg/kg/min.
-
During Dobutamine infusion, carefully monitor the LAD peak and heart rate. Periodically record LAD PW peaks in the Doppler mode, especially whenever it increases in response to Dobutamine.NOTE: The stress challenge induced by Dobutamine causes the heart to work harder; this often results in the movement of the heart and the LAD. Be prepared to move the animal, the probe, or both in order to keep the LAD in view.
- After LAD peaks and heart rate have plateaued during the challenge, stop the Dobutamine infusion, remove the tail vein infusion set and remove the animal from the platform. Allow the animal to recover in its home cage.
Select Peak Vel tool to obtain the peak diastolic velocities from the images shown in Figure 2B and C.
Calculate the CFR index as the ratio of the LAD stress (Dobutamine) peak diastolic flow velocity to the resting LAD peak diastolic flow velocity (Figure 2A).
Figure 1: Coronary artery location.
A. Probe position on the rat while obtaining LAD coronary artery velocity. B. Short axis anatomical representation of the pulmonary artery, aorta, and LAD coronary artery. C. Anatomical visualization of LAD coronary artery on echocardiography.
Figure 2: The Pulse Wave Velocity imaging of the LAD coronary artery.
A. Representation of the pulse wave velocity sensor placement on LAD coronary artery. B. LAD coronary artery pulse wave image during the rest condition. C. LAD coronary artery pulse wave image during the stress (Dobutamine) condition.
3. Ischemia Reperfusion Injury
Anesthetize rat with isoflurane - induction chamber at 5% with 1.5–2.0 L/min O2 flow followed by 1.5–2.0% with 1.5–2.0 L/min O2 flow.
Confirm the depth of anesthesia by the lack of the pedal withdrawal reflex. Use ophthalmic vet ointment on the eyes to prevent dryness. Maintain the proper body temperature of 37-38 °C using a heating pad or an animal temperature controller.
Administer pre-surgical Meloxicam, 5 mg/kg intramuscularly, 15 min before the surgery, followed by 2 mL subcutaneous bolus of 0.9% saline to prevent dehydration during the surgery. Sterile gloves and instruments were used, and aseptic techniques were employed. Using the fiberoptic light source, intubate rat using 18-gauge IV catheter and connect to the ventilator.
Ligate LAD using 8-0 monofilament suture through 15 mm opening at the 5th intercostal space. Tie a plain knot and leave for 30 min. Visually confirm ischemia in all rats via discoloration of the heart surface12. Release the ligature after 30 min and verify reperfusion by the reddening of the previously discolored area of heart muscle for 1-2 min12.
Close the rib cage using 4-0 absorbable suture with an interrupted suture pattern, then close the skin using 5-0 non-absorbable silk suture with continuous suture pattern.
Remove the animal from anesthesia. Allow the animal to recover in the home cage. 5mg/kg Meloxicam was injected intramuscular in every 24 hours before euthanizing the animal (72hours after surgery).
4. Ultrasound imaging after IR surgery
72 h after IR surgery, measure the coronary flow and CFR again. Compare the measurements to before IR measurements. Repeat steps 2.1 to 2.15 as described above.
Representative Results
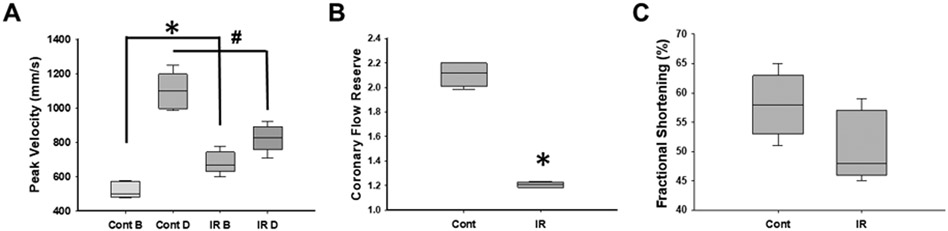
For this study, we used 12 female Fisher 344 rats. We performed a stress test with Dobutamine and measured LAD coronary artery velocity before and 72 hours after the IR surgery. Before the IR surgery, resting LAD coronary artery velocity was measured as 423 ± 59 mm/s, which was increased after Dobutamine infusion (1005 ± 77mm/s) (Figure 3A). After 72 h of ischemia reperfusion, resting LAD coronary artery velocity was significantly higher compared to the resting LAD coronary artery velocity before the IR surgery (743 ± 40mm/s vs 423 ± 59 mm/s) (Figure 3A). Stress response to Dobutamine test after the IR surgery was significantly reduced compared to the before IR surgery responses (937 ± 67ms/s vs 1005 ± 77mm/s) (Figure 3A).
Figure 3. Measurement of the coronary flow using Doppler echocardiography.
A. Pulse wave velocity measured in Control during the rest (Cont B) and during Dobutamine infusion (Cont D) and in animals after 72 h of IR surgery during the rest (IR B) and during Dobutamine challenge (IR D), p < 0.05 Cont B vs. ischemia reperfusion B (*). B. CFR was calculated from pulse wave in the experimental animals (n=9), p < 0.05 Cont vs. IR (*). C. Fractional shortening assessment from the experimental groups (n=9). Data are presented as mean ± SD, analyzed with one-way ANOVA.
CFR is calculated as the ratio of the peak flow velocity during stress (Dobutamine) to the resting flow velocity (measured prior to Dobutamine infusion)8. CFR was 2.1 ± 0.35 in young rats before the IR surgery (Figure 3B) but significantly reduced (1.1 ± 0.25) after 72 h of the IR surgery, even though the resting LAD coronary artery velocity was higher in these rats compared to data obtained before IR surgery (Figure 3C). In addition, there were no significant changes in the systolic function of the left ventricle in rats after 72 h of the IR surgery (Figure 3C).
Discussion
The major findings from the present study are that IR increases the resting LAD coronary artery velocity and impairs CFR, even in the absence of any residual angiographic stenosis.
Understanding the coronary physiology is an essential part of the clinical decision-making for cardiologists to treat coronary artery disease. CFR is one of the important functional parameters in understanding the pathophysiology of coronary microcirculation7,13. CFR is a noninvasive method to assess both coronary artery stenosis and coronary microvascular circulation and is an indicator of myocardial blood supply, explicitly the ability of the coronaries to increase blood flow under stress conditions7. A normal CFR (≥.2.0) often reflects a good prognosis, while CFR less than 1.90 provides incremental diagnostic information for the identification of high-risk coronary artery disease14,15,16.
Our results show that, even though the systolic function was preserved after ischemia reperfusion (Figure 3C), CFR was significantly lower (Figure 3B). Thus, recanalization of stenotic coronary arteries does not improve microvascular perfusion. Decreased CFR enables detection of impaired microvascular vasodilation after ischemia reperfusion.
This study demonstrates the serial CFR evaluations to explore the effect of various pharmacological therapies using noninvasive transthoracic Doppler echocardiography. This method of coronary functional assessment can be used in small animal research as a feasible and viable clinical diagnostic tool. This will lead to minimizing the requirement of animal use, euthanasia, or necropsy in the small animal models. Critical steps in this protocol are visualizing the coronary artery and obtaining the PW velocity images of good quality. Another critical step is to maintain LAD visualization during the stress state. During Dobutamine challenge, the heart rate increases and the LAD may move from the field of view; researchers should be prepared to move the field in order to follow the coronary artery. Limitations in the current study include the relatively small sample size, the lack of correlation between CFR and the coronary artery lumen diameter in vivo in rats, due to the difficulty in obtaining accurate visualization for the size measurement of the coronary artery. However, the methods described here are reliable, reproducible, and offer insightful information on the damage inflicted on the cardiac microvasculature following ischemia reperfusion.
Acknowledgments
We would like to thank the Helmsley foundation for providing ultrasound equipment to perform our experiments. This work was supported by NIA R01 053585 grant.
Footnotes
Disclosures
The authors have nothing to disclose
Video Link
The video component of this article can be found at https://www.jove.com/video/59406/
References
- 1.Eltzschig HK, & Eckle T Ischemia and reperfusion--from mechanism to translation. Nature medicine. 17 (11), 1391–1401 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.French CJ, Zaman AK, & Sobel BE Failure of erythropoietin to render jeopardized ischemic myocardium amenable to incremental salvage by early reperfusion. Coronary Artery Disease. 20 (4), 295–299 (2009). [DOI] [PubMed] [Google Scholar]
- 3.Marzilli M, & Mariani M Ischemia-reperfusion and microvascular dysfunction: implications for salvage of jeopardized myocardium and reduction of infarct size. Italian Heart Journal. 2 Suppl 3 40s–42s (2001). [PubMed] [Google Scholar]
- 4.Prasad A, & Gersh BJ Management of microvascular dysfunction and reperfusion injury. Heart (British Cardiac Society). 91 (12), 1530–1532 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ishihara M et al. Impaired coronary flow reserve immediately after coronary angioplasty in patients with acute myocardial infarction. British heart journal. 69 (4), 288–292 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Camici PG, d'Amati G, & Rimoldi O Coronary microvascular dysfunction: mechanisms and functional assessment. Nature Reviews Cardiology. 12 (1), 48–62 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Chung KS, & Nguyen PK Non-invasive measures of coronary microcirculation: Taking the long road to the clinic. Journal of Nuclear Cardiology. (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelm NQ et al. Adipose-derived cells improve left ventricular diastolic function and increase microvascular perfusion in advanced age. PLoS One. 13 (8), e0202934 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pan M et al. Late recovery of coronary flow reserve in patients successfully treated with a percutaneous procedure. Revista Espa–ola de Cardiologia. 56 (5), 459–464 (2003). [DOI] [PubMed] [Google Scholar]
- 10.Samim A, Nugent L, Mehta PK, Shufelt C, & Bairey Merz CN Treatment of angina and microvascular coronary dysfunction. Current treatment options in cardiovascular medicine. 12 (4), 355–364 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guide for the Care and Use of Laboratory Animals. The National Academies Collection: Reports funded by National Institutes of Health (8th edition). (2011). [Google Scholar]
- 12.Ciuffreda MC et al. Rat experimental model of myocardial ischemia/reperfusion injury: an ethical approach to set up the analgesic management of acute post-surgical pain. PloS one. 9 (4), e95913–e95913 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van de Hoef TP, Siebes M, Spaan JAE, & Piek JJ Fundamentals in clinical coronary physiology: why coronary flow is more important than coronary pressure. European Heart Journal. 36 (47), 3312–3319 (2015). [DOI] [PubMed] [Google Scholar]
- 14.Naya M et al. Preserved coronary flow reserve effectively excludes high-risk coronary artery disease on angiography. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 55 (2), 248–255 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cortigiani L et al. Diagnostic and prognostic value of Doppler echocardiographic coronary flow reserve in the left anterior descending artery. Heart (British Cardiac Society). 97 (21), 1758 (2011). [DOI] [PubMed] [Google Scholar]
- 16.Kawata T et al. Prognostic value of coronary flow reserve assessed by transthoracic Doppler echocardiography on long-term outcome in asymptomatic patients with type 2 diabetes without overt coronary artery disease. Cardiovascular diabetoiogy. 12 121–121 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]