Abstract
Rationale
Results from clinical trials in patients with severe eosinophilic asthma have demonstrated that mepolizumab is well tolerated and is associated with improved asthma control as evidenced by reductions in both exacerbations and maintenance oral corticosteroid use, and improvements in lung function, asthma control, and quality of life. However, real-world data are lacking on the impact of mepolizumab treatment.
Objective
To assess the effect of mepolizumab treatment on the rate of asthma exacerbations and asthma exacerbation-related costs in a real-world setting.
Methods
This retrospective cohort study (GSK ID: 209017; HO-18-19168) analyzed data from patients with severe asthma ≥12 years of age at mepolizumab treatment initiation (index date) with ≥12 months pre- (baseline) and post-index (follow-up) data from a commercial claims database (patients were identified from November 1, 2015 to March 31, 2017). Asthma exacerbations (primary objective) and asthma exacerbation-related costs (secondary objective) in the baseline and follow-up periods were compared. Other analyses included the number of mepolizumab administrations and the use of concomitant asthma medications.
Results
Data were analyzed from 346 patients. Mepolizumab significantly reduced the proportion of patients with any exacerbation and exacerbations requiring hospitalization, compared with baseline. Significant reductions in the rate of all exacerbations of 38.4% (from 2.68 to 1.65 events/patient/year; P<0.001) and of exacerbations requiring hospitalization of 72.7% (from 0.11 to 0.03 events/patient/year; P=0.004) were observed, compared with baseline. Mean total asthma exacerbation-related costs (excluding mepolizumab acquisition and administrative costs) per person were significantly lower during follow-up compared with baseline (P<0.05) and the use of asthma medications, including oral and inhaled corticosteroids, was also lower.
Conclusion
This study confirms the clinical benefit observed in previous mepolizumab clinical trials and demonstrates that mepolizumab is effective in a real-world setting.
Keywords: asthma exacerbation, mepolizumab, cost, real world, effectiveness
Plain-Language Summary
Why was the study done? Mepolizumab is a biologic therapy given as an add-on treatment to patients with severe asthma whose symptoms are not controlled with controller therapy. Mepolizumab has been shown to reduce the rate of asthma exacerbations and use of oral corticosteroids in clinical trials, but there is currently a lack of data on the impact of mepolizumab treatment initiation in a real-world setting.
What did the researchers do and find? This retrospective study used claims data from privately insured patients with severe asthma who were prescribed mepolizumab. The rate of asthma exacerbations, associated costs, and use of other asthma medications were compared 12 months before and 12 months after the start of mepolizumab treatment.
Patients experienced significantly fewer exacerbations during the 12 months after mepolizumab treatment initiation compared with the 12 months before. Asthma exacerbation-related costs per person were also significantly lower, and in general, patients used fewer asthma medications after treatment initiation compared with before.
What do these results mean? These results confirm earlier findings from clinical trials showing mepolizumab is associated with reductions in the number of asthma exacerbations. The study also demonstrates that the efficacy of mepolizumab is replicated in a real-world setting, outside of the controlled environment of a clinical trial.
Introduction
Asthma is a common, heterogeneous respiratory disease characterized by chronic airway inflammation that can generally be controlled with inhaled therapy in the majority of patients.1 Severe asthma is defined as asthma that requires maximal, optimized inhaled corticosteroid (ICS) therapy plus another controller to remain under control or that is uncontrolled despite this therapy,1,2 and is estimated to affect 5–10% of the asthma population.2 Severe eosinophilic asthma is a phenotype of severe asthma, and is characterized primarily by increased blood eosinophils and frequent exacerbations despite corticosteroid therapy.1–3
Despite the relatively low prevalence of severe asthma among patients with asthma, it places a high burden on health-care systems. In particular, it has been shown that health-care costs, and rates of health-care utilization are higher for patients with severe asthma compared with those with asthma.4–6 In addition, severe asthma is estimated to account for approximately 50% of all annual asthma-related health-care costs in the UK,7 and annual asthma-related health-care costs for severe uncontrolled asthma have been found to be approximately double those of non-severe uncontrolled asthma in the USA.4
There are several biologics approved to treat severe asthma in the USA.8 The first anti-interleukin 5 monoclonal antibody, mepolizumab, selectively inhibits eosinophilic inflammation by reducing the number of blood eosinophils, and is indicated as an add-on maintenance treatment in patients with severe eosinophilic asthma.9–11 Clinical trials in patients with severe eosinophilic asthma have shown that mepolizumab reduces the rate of any asthma exacerbations, as well as those that require hospitalization. It is also associated with reductions in maintenance oral corticosteroids (OCS) use, and improvements in lung function, asthma control, and quality of life.9,12–14 However, there are currently limited data available on the effectiveness of mepolizumab in a real-world setting, particularly in relation to disease severity and health-care use. The aim of this study was to examine the impact of mepolizumab treatment initiation on asthma exacerbations and health-care costs experienced by patients with severe asthma in the real world using data from a commercial insurance claims database in the USA.
Materials and Methods
Study Design
This was a retrospective cohort study of patients with asthma receiving mepolizumab, using data from the IBM Watson Health MarketScan® Commercial Claims Database (GSK ID: 209017, HO-18-19168). From 1995 to 2017, this database contained health-care data for approximately 148 million privately insured individuals covered under a variety of fee-for-service, fully capitated, and partially capitated health plans, sourced from employers and health plans. Patients were identified from November 1, 2015 to March 31, 2017 (patient selection period); the index date was the first administration of mepolizumab. Data were examined for each patient during the 12 months prior to index (baseline period) and the 12 months following the index date (follow-up period).
This study builds on data from a previously conducted pilot study (GSK ID: 209019, HO-18-19166) in which patients were identified from November 1, 2015 to September 30, 2016. Owing to the shorter patient identification period, the pilot study included fewer patients than the main study (GSK ID: 209017, HO-18-19168; see Supplementary section 1 for further details).
Study Population
Eligible patients were ≥12 years of age at the index date, with a medical or pharmacy claim between November 1, 2015 and March 31, 2017 that included a health-care common procedure coding system (HCPCS) code or a national drug code (NDC) indicating administration of mepolizumab (HCPCS: C9473, J2182; NDC: 00173-0881-01). Patients were required to have continuous enrollment with medical and pharmacy benefits in the baseline and follow-up periods. Further eligibility criteria included a diagnosis of asthma during the baseline period, and a minimum of two doses of mepolizumab in the first 180 days of the follow-up period. Patients with missing data on gender or age were not included in the study. Patients with evidence of mepolizumab use during the baseline period, or evidence of omalizumab, reslizumab, benralizumab, or dupilumab use during the baseline or follow-up periods were excluded.
For the analysis of exacerbation rates, a subcohort was identified that closely resembled the patient population that participated in the clinical trials (where patients were monitored closely ensuring administration of the product at the recommended 4-weekly interval). This clinical trial-like subcohort included patients with ≥2 exacerbations/year during the baseline period and ≥10 injections/year of mepolizumab during the follow-up period.
Objectives and Assessments
The primary objective was to compare the proportions of patients who experienced any asthma exacerbations and asthma exacerbations requiring hospitalization, and the rate of asthma exacerbations (events/patient/year) in the baseline period with those in the follow-up period. Asthma exacerbations were identified by an outpatient or emergency department visit with a diagnosis of asthma in any position (International Classification of Diseases [ICD]-9: 493.xx, ICD-10: J45.xx) and ≥1 prescription of systemic corticosteroids (SCS; intramuscular, intravenous, or oral) within ±5 days of the encounter. Asthma exacerbations were also identified by exacerbations requiring hospitalization, which were identified by inpatient hospital admissions with a primary diagnosis of asthma (ICD-9: 493.xx, ICD-10: J45.xx).
Exacerbations occurring in the 14 days following the exacerbation start date for outpatient exacerbations and in the 14 days following discharge date for hospitalizations were considered a single exacerbation. The start date of the exacerbation was the service date of the medical visit (even if corticosteroid prescription/administration occurred prior to this visit date); the end date of the exacerbation was defined as 14 days following the exacerbation episode start date (or discharge date for an inpatient). Exacerbations that occurred on the index date were considered baseline exacerbations.
The secondary objective was to compare asthma exacerbation-related costs among patients in the baseline period versus those in the follow-up period. Costs were based on paid amounts of adjudicated claims, including insurer and health plan payments as well as patient cost-sharing in the form of co-payment, deductible, and coinsurance. Claims with asthma-exacerbation costs were identified as inpatient claims with a primary diagnosis of asthma, outpatient claims with an asthma diagnosis in any position, or medical or pharmacy claims for SCS, rescue medications, and asthma medications during the exacerbation event. Costs measured on the index date were considered part of the baseline period and excluded those costs associated with mepolizumab acquisition (identified by medical or pharmacy claims with NDC and HCPCS codes associated with mepolizumab) or administration (identified by medical claims with HCPCS codes associated with mepolizumab and by Current Procedural Terminology [CPT] codes for drug administration). CPT codes had to occur on the same date as the HCPCS code for mepolizumab, and had to occur in the 28 days following a drug claim for mepolizumab and prior to the next drug claim for mepolizumab. Only the first outpatient encounter for mepolizumab administration was classified as administration related. All costs were adjusted for inflation using the Consumer Price Index and standardized to 2017 US dollars.
Other analyses included the number of mepolizumab administrations and the use of asthma medications (short-acting β2-agonists [SABA] and short-acting muscarinic antagonist [SAMA], and long-acting muscarinic antagonist [LAMA], leukotriene receptor antagonist [LTRA], ICS/long-acting β2-agonist [LABA] dual therapy, triple therapy [ICS/LABA/LAMA], and others [LABA, mast-cell stabilizers, and methylxanthines]). The total number of asthma exacerbations by calendar month, the use of ICS stratified by dose category (low, medium, high; according to the Global Initiative for Asthma guidelines), and the use of chronic OCS were assessed post hoc. Chronic OCS was defined as a mean daily dose of ≥5 mg/day of prednisone equivalents measured during the 12-month baseline and follow-up periods. Use of asthma treatments was assessed in terms of the proportion of patients with prescriptions.
Statistical Analysis
Bivariate analyses were performed to compare asthma exacerbations in the baseline and follow-up periods, as well as to compare asthma exacerbation-related costs in these same periods. Chi-squared tests were used to evaluate the statistical significance of differences for dichotomous or categorical variables; t-tests or analysis of variance (ANOVA) were used for comparison of continuous variables. A P-value of 0.05 was the maximum P-value for which differences between utilization and costs were considered statistically significant.
Results
Patient Population
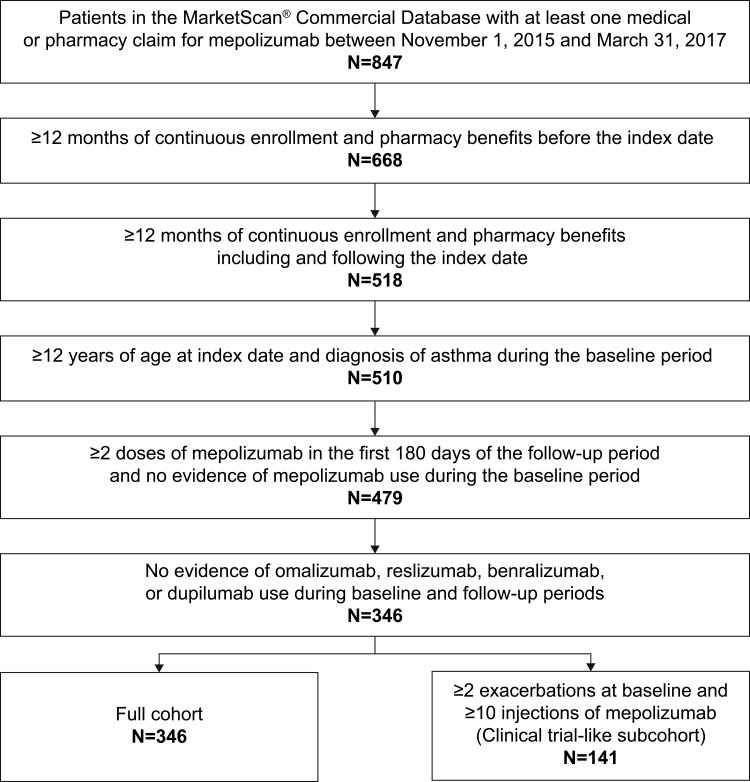
In total, 346 patients met the study criteria (Figure 1); the mean age was 49.3 years and the majority (61.6%) were female. Most patients (58.4%) had exclusive provider organization (EPO)/preferred provider organization (PPO) insurance (Table 1). The most common comorbidities (n>150 patients) included allergic rhinitis, sinusitis, and respiratory infections (Table 1).
Figure 1.
Patient inclusion.
Table 1.
Demographic Characteristics and Baseline Comorbidities
| N=346 | |
|---|---|
| Age, years, mean (SD) | 49.3 (12.0) |
|
Gender, n (%) Female |
213 (61.6) |
| Geographic region, n (%) | |
| South | 141 (40.8) |
| Northeast | 79 (22.8) |
| North Central | 74 (21.4) |
| West | 51 (14.7) |
| Unknown | 1 (0.3) |
| Insurance plan type, n (%) | |
| EPO/PPO | 202 (58.4) |
| CDHP/HDHP | 67 (19.4) |
| HMO | 39 (11.3) |
| POS/POS with capitation | 21 (6.1) |
| Comprehensive/indemnity | 11 (3.2) |
| Unknown | 6 (1.7) |
| Deyo-Charlson index, mean (SD) | 1.4 (0.9) |
| Comorbid conditions, n (%) | |
| Allergic rhinitis | 230 (66.5) |
| Sinusitis | 202 (58.4) |
| Acute | 116 (33.5) |
| Chronic | 148 (42.8) |
| Respiratory infections | 155 (44.8) |
| Gastroesophageal reflux disease | 122 (35.3) |
| Hypertension | 119 (34.4) |
| Chronic obstructive pulmonary disease | 101 (29.2) |
| Nasal polyps | 75 (21.7) |
| Diabetes | 44 (12.7) |
| Eosinophilic granulomatosis with polyangiitis | 13 (3.8) |
Note: Data shown are for the full cohort.
Abbreviations: CDHP, consumer-directed health plan; EPO, exclusive provider organization; HDHP, high-deductible health plan; HMO, health maintenance organization; POS, point-of-service; PPO, preferred provider organization; SD, standard deviation.
Asthma Exacerbations
During the baseline period, most patients (n=292/346; 84.4%) in the full cohort experienced an exacerbation. Mepolizumab treatment in the follow-up period resulted in a significant 19.9% reduction in the proportion of patients experiencing an exacerbation (n=223/346; 64.5%; P<0.001) (Figure 2). Likewise, the proportion of patients experiencing exacerbations requiring hospitalization was also significantly reduced following mepolizumab treatment (n=9/346; 2.6%) compared with those in the baseline period (n=26/346; 7.5%) (P=0.003). Following mepolizumab treatment, the rate of exacerbations significantly decreased by 38.4% (from 2.68 events/patient/year to 1.65 events/patient/year; P<0.001), and the rate of exacerbations requiring hospitalization was significantly reduced by 72.7% (from 0.11 to 0.03 events/patient/year; P=0.004), compared with baseline (Figure 2).
Figure 2.
Mean rate ± SD of asthma exacerbations and exacerbations requiring hospitalization during the baseline and follow-up periodsa. aBaseline period defined as the 12 months before first mepolizumab treatment; follow-up period defined as the 12 months following first mepolizumab treatment. bCohort with ≥2 exacerbations at baseline and ≥10 injections of mepolizumab. Outpatient visits with a HCPCS code indicating mepolizumab administration were excluded from the exacerbation definition, to prevent the inclusion of planned provider visits for mepolizumab administration. Additionally, the first outpatient visit with a Current Procedural Terminology code indicating subcutaneous/intramuscular drug administration (96372, 96401) either in the 28 days following an outpatient prescription (NDC) claim for mepolizumab or prior to the next outpatient prescription claim (the earlier of the two) was excluded from the exacerbation definition. N, numbers are the numbers of patients with data available.
Abbreviations: HCPCS, health-care common procedure coding system; NDC, national drug code; SD, standard deviation.
In the clinical trial-like subcohort, all patients (N=141) experienced ≥2 exacerbations during the baseline period. The proportion of patients experiencing an exacerbation was significantly reduced by 31.9% in the follow-up period (n=96/141; 68.1%) compared with the baseline period (n=141; 100.0%) (P<0.001); a significant reduction of 81.3% was also observed in the proportion of patients who experienced exacerbations requiring hospitalization (baseline: n=16/141; 11.3%; follow-up: n=3/141; 2.1%) (P=0.002) (Figure 2). The rates of all exacerbations and of exacerbations requiring hospitalization were significantly reduced by 54.4% (P<0.001) from 3.97 to 1.81 events/patient/year and by 86.7% (P=0.003) from 0.15 to 0.02 events/patient/year, respectively, in the follow-up period compared with the baseline period (Figure 2). When comparing exacerbations by calendar month, the number of exacerbations per month was consistent across both the baseline and follow-up periods, although there was a slight increase from December to January and in September during the baseline period (Supplementary Figure 1).
Asthma Exacerbation-Related Costs
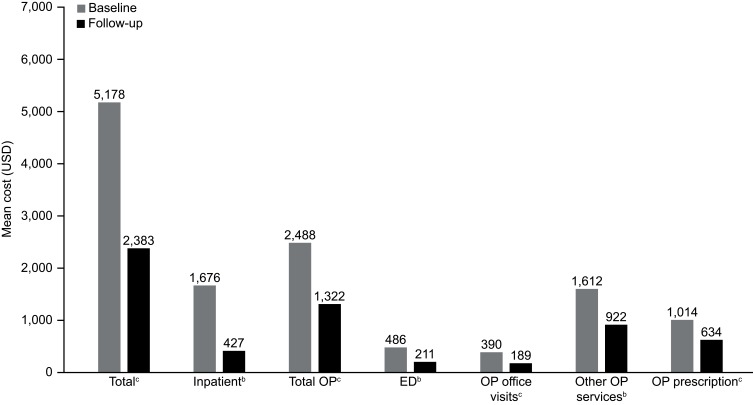
Mean total costs (as previously noted, excluding mepolizumab acquisition and administrative costs) per person were significantly lower in the follow-up period compared with the baseline period (P<0.001), and for all other components included in total costs (P<0.05 and P<0.001; Figure 3).
Figure 3.
Mean costs related to asthma exacerbations per patient during the 12-month baseline and 12-month follow-up periodsa. Data shown are for the full cohort; costs exclude mepolizumab acquisition and administration costs. aBaseline period defined as the 12 months before first mepolizumab treatment; follow-up period defined as the 12 months following first mepolizumab treatment. bP<0.05. cP<0.001.
Abbreviations: ED, emergency department; OP, outpatient; USD, US dollars.
Administrations of Mepolizumab
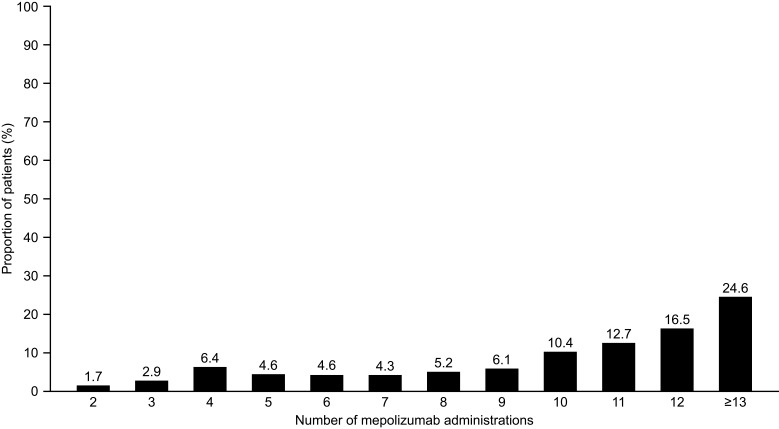
The number of mepolizumab administrations during the follow-up period (including index administration) was 10.3 per year. Approximately one-third (35.8%) of patients received fewer than 10 administrations of mepolizumab per year, and 41.0% of patients had ≥12 or more administrations of mepolizumab in the follow-up period (Figure 4).
Figure 4.
Number of administrations of mepolizumab during the follow-up perioda. Data shown are for the full cohort. aBaseline period defined as the 12 months before first mepolizumab treatment; follow-up period defined as the 12 months following first mepolizumab treatment.
Use of Asthma Medications
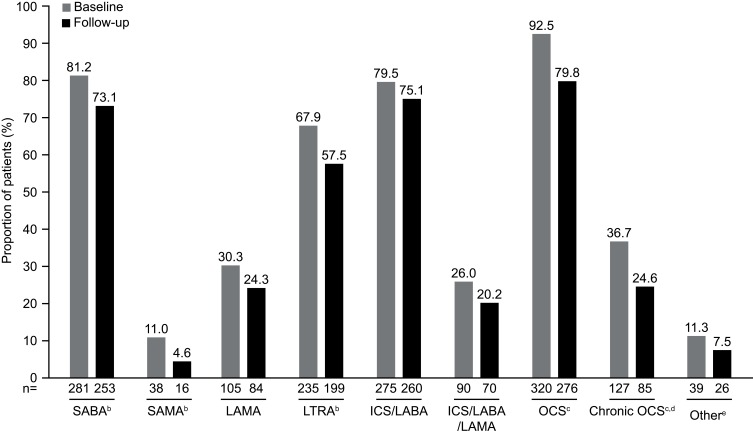
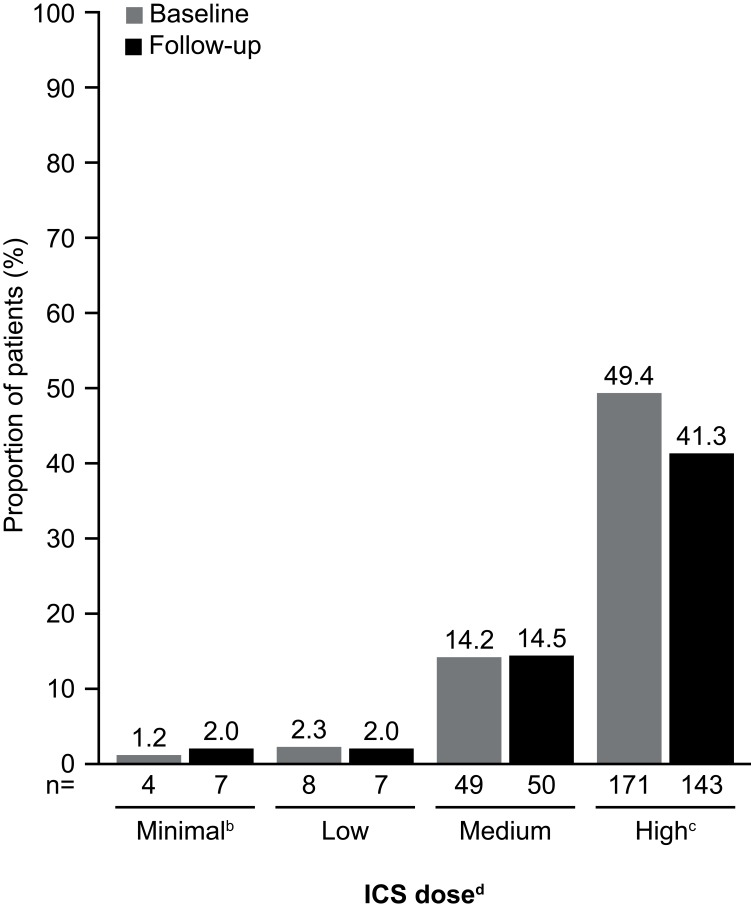
In general, there was a trend toward decreased proportions of patients using asthma medications during the follow-up period when compared with the baseline period (Figure 5). A significantly smaller proportion of patients used SABAs (P=0.011), SAMAs (P=0.002), and LTRAs (P=0.005) during follow-up as compared with during baseline. In addition, there were significant decreases in the proportion of patients requiring the use of OCS or chronic OCS during the follow-up period (P<0.001; Figure 5). The proportion of patients receiving high-dose ICS during the follow-up period was 16% lower than that during the baseline period (Figure 6).
Figure 5.
Proportion of patients using asthma medications during the baseline and follow-up periodsa. Data shown are for the full cohort. aBaseline period defined as the 12 months before first mepolizumab treatment; follow-up period defined as the 12 months following first mepolizumab treatment. bP<0.05. cP<0.001. dChronic OCS use defined as an average daily dose of ≥5 mg/day of prednisone equivalents measured during the baseline and follow-up periods. e‘Other’ includes LABA, mast-cell stabilizers, and methylxanthines. N numbers are the numbers of patients with data available.
Abbreviations: ICS, inhaled corticosteroids; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist; LTRA, leukotriene receptor antagonist; OCS, oral corticosteroid; SABA, short-acting β2-agonist; SAMA, short-acting muscarinic antagonist.
Figure 6.
Use of ICS during the baseline and follow-up periodsa. aBaseline period defined as the 12 months before first mepolizumab treatment; follow-up period defined as the 12 months following first mepolizumab treatment. bPatients on a lower dose than the ‘low” category, or on ICS but missing dosage information on the claims. cP=0.033. dICS dosing categories (low, medium, and high) were determined according to GINA guidelines;1 ICS/LABA combination prescriptions were included. ICS dose was determined based on ICS utilization within the last 90 days before the end of the baseline and follow-up periods. Prescription fills that overlapped into these periods were included in the calculations. N numbers are the numbers of patients with data available. The proportion of patients not on ICS or with data missing was 32.9% (n=114) and 40.2% (n=139) at baseline and follow-up, respectively. Patients were not on ICS in the last 90 days of the baseline or follow-up periods.
Abbreviations: GINA, Global Initiative for Asthma; ICS, inhaled corticosteroid; LABA, long-acting β2-agonist.
Discussion
In this study we assessed the impact of mepolizumab treatment on exacerbations and health-care costs in patients with severe asthma. We found that mepolizumab treatment led to a significant reduction in the annual rate of exacerbations and all asthma exacerbation-related cost (excluding mepolizumab acquisition and administrative cost) compared with those during the baseline period. This study represents the first examination of the impact of mepolizumab using an insurance claims database in this context. These results demonstrate the effectiveness of mepolizumab in a real-world setting.
We found that both the proportion of patients experiencing an exacerbation and the rate of exacerbations were significantly reduced following mepolizumab treatment, compared with the baseline period. These results are consistent with those from the earlier, smaller pilot study we conducted, in which the rate of exacerbations was significantly reduced by 27.9% (Supplementary Figure 2). The rate of exacerbations requiring hospitalization in the pilot study was also reduced, although this reduction was not statistically significant (Supplementary Figure 2); the number of patients with exacerbations requiring hospitalization in the pilot study was too small to be sufficiently powered to evaluate changes in this outcome. Our data also indicated that the number of exacerbations per calendar month was fairly constant throughout the baseline and follow-up periods, and that mepolizumab reduced the number of exacerbations across all months and seasons, consistent with data on other biologics.15,16 Recently, several smaller studies based on real-world data have also shown that mepolizumab is associated with a reduction in exacerbations.17,18 Taken together with our findings, this highlights the beneficial effect of mepolizumab on exacerbation frequency irrespective of seasonality.
Our data also support the efficacy of mepolizumab demonstrated in clinical trials (of the licensed dose, 100 mg subcutaneously), where significant reductions of 53–58% in the rate of all exacerbations versus placebo and 69% in the rate of exacerbations requiring hospitalization versus placebo were observed.13,14 Importantly, physician prescribing of mepolizumab that is reflected in our real-world data may not mimic trial inclusion criteria, and patients rarely have near-complete adherence to therapy. It should also be noted that the patient profile for inclusion in the aforementioned clinical studies (specifically exacerbation history), differed from that in this real-world study. Thus, the rate of exacerbations shown for the full cohort in this study (38.4%) is, as expected, lower than those found in the clinical trials.
To better compare these real-world data on exacerbation reduction with data from the previous mepolizumab clinical trials, we identified a clinical trial-like subcohort of patients who had ≥2 exacerbations at baseline and ≥10 injections of mepolizumab during the follow-up period. All patients in this subcohort experienced exacerbations during the baseline period, versus 84.4% in the full cohort. Patients in the clinical trial-like subcohort experienced a greater reduction in exacerbations than those patients in the full cohort. The difference in exacerbation rate reduction between the clinical trial-like cohort and the full cohort may be explained by the fact that 15.6% (n=54/346) of patients in the full cohort appeared to have no exacerbations during the baseline period (and therefore a reduction would not be expected in these patients). In addition, 34% of patients in the full cohort had <10 administrations of mepolizumab in the 12-month follow-up period. It is likely that not all patients receive an injection every month, as per label,10 in real-world clinical practice owing to several reasons, one of which could be missed appointments. Recently, a new liquid formulation of mepolizumab has been approved that allows administration at home via an autoinjector or prefilled syringe by a patient or caregiver.10 It is possible that the use of these devices may positively impact on patient adherence to mepolizumab treatment. Nonetheless, our results highlight that mepolizumab is associated with a clinically meaningful reduction in annual exacerbation rate outside the controlled environment of a clinical trial.
The secondary objective of this study was to assess asthma exacerbation-related costs, and we found that both total and individual component costs were significantly reduced following mepolizumab treatment, compared with the baseline period. Reductions in exacerbation-related costs were also seen in the pilot study, but were only statistically significant for outpatient office visits and outpatient prescriptions (Supplementary Figure 3), potentially as a result of the low patient numbers. Our results therefore provide evidence that mepolizumab not only helps to reduce symptoms, but may also result in a reduction in health-care resource use and costs for treating asthma exacerbations. Of note, other asthma medications were reduced during the mepolizumab treatment period, suggesting that improved asthma control can have a positive impact on the need for additional controller medications.
Although not a formal objective of this study, we observed reductions in chronic OCS use with mepolizumab treatment, a finding that is consistent with results from the previous clinical trials of mepolizumab,12,19,20 as well as other recent analyses of real-world data on mepolizumab treatment.17,18 A recent longitudinal database study showed that increasing the number of OCS prescriptions is associated with increased odds of experiencing an adverse event. Thus, a reduction in the use of OCS may improve outcomes in patients with asthma.21 Another longitudinal database study showed that increasing exposure (measured in terms of low, medium, and high doses of SCS) in patients with severe asthma was also significantly associated with a higher risk of experiencing any SCS-related complication.22 Our results and those from clinical studies show that mepolizumab reduces the need for OCS treatment, and therefore may also improve patient health outcomes by reducing the dependence on OCS.
This study has some limitations. The MarketScan® Commercial Claims Database relies on administrative claims data for clinical detail. As such, the data collected are subject to coding limitations and data entry errors, which could potentially lead to the underestimation of treatment effects. However, provider reimbursement requires the submission of accurate data on medical claims, and it is expected that such errors are likely to be rare and non-differential when they occur. In addition, the source population included individuals in the USA with private commercial insurance only, and therefore the data are not generalizable to those patients with asthma with other types of insurance (or no insurance) in the USA, or to patients with asthma in other countries. The study design also assumed there was no disease progression and therefore that exacerbation rates would remain stable irrespective of treatment. In addition, the criteria defining asthma exacerbations were linked to the asthma diagnosis code and it is possible that asthma exacerbations occurring during the study period may have been underestimated, for example, if the asthma diagnosis code was missing. It should also be noted that medications of interest administered in the inpatient setting could also not be identified; therefore, the total number of patients who received a medication of interest may have been underestimated in the subset of patients with an inpatient admission during the study period. Finally, time-varying covariates that may be important risk factors for exacerbations were not accounted for during the 12-month follow-up period, since comorbid conditions were measured during the 12-month baseline period only. Despite these limitations, this study provides important insights into the impact of mepolizumab in real-world clinical practice.
Conclusions
The results of this study indicate that mepolizumab is associated with reductions in asthma exacerbations, associated costs, and the use of asthma medications. These real-world data show that mepolizumab is effective in patients with severe asthma, confirming findings from previous clinical trials. These findings provide valuable information for health-care professionals and payers on the impact of mepolizumab treatment in the real-world setting.
Acknowledgments
This study and the related pilot study were funded by GSK (GSK IDs: 209017/HO-18-19168 and 209019/HO-18-19166, respectively). Editorial support (in the form of writing assistance, including development of the initial draft from the study report, assembling tables and figures, collating author comments, grammatical editing, and referencing) was provided by Roisin McCorkell, MSc, of Fishawack Indicia Ltd, UK, and was funded by GSK.
Funding Statement
This study and the related pilot study were funded by GlaxoSmithKline (GSK IDs: 209017/HO-18-19168 and 209019/HO-18-19166, respectively).
Abbreviations
ANOVA, analysis of variance; CPT, Current Procedural Terminology; EPO, exclusive provider organization; HCPCS, health-care common procedure coding system; ICD, International Classification of Diseases; ICS, inhaled corticosteroids; LABA, long-acting β2-agonists; LAMA, long-acting muscarinic antagonist; LTRA, leukotriene receptor antagonist; NDC, national drug code; OCS, oral corticosteroids; PPO, preferred provider organization; SABA, short-acting β2-agonists; SAMA, short-acting muscarinic antagonist; SCS, systemic corticosteroids.
Ethics Approval and Informed Consent
This study used fully deidentified data and as such was not classified as research involving human participants as defined by 45 CFR 46.102(f)(2). Therefore, Institutional Review Board approval was not required.
Consent for Publication
Consent for publication was not applicable for this work.
Data Sharing Statement
GSK makes available anonymized individual participant data and associated documents from interventional clinical studies which evaluate medicines, upon approval of proposals submitted to www.clinicalstudydatarequest.com. To access data for other types of GSK sponsored research, for study documents without patient-level data and for clinical studies not listed, please submit an enquiry via the website. The data that support the findings of this study are available from the MarketScan® Commercial Claims Database but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are, however, available from the authors upon reasonable request and with permission, including appropriate data use agreements and potential licenses, of the MarketScan® Commercial Claims database.
Author Contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
J-PL and HO are former employees of GSK. J-PL’s current affiliation is Global Medical Affairs, Amgen, Thousand Oaks, CA, USA. HO’s current affiliation is Clinical Development, Gossamer Bio, San Diego, CA, USA. MB, CFB, and BH are current employees of GSK and hold stocks/shares in GSK. EP and JM are employees of IBM Watson Health, which received funds from GSK for this study. The authors report no other conflicts of interest in this work.
References
- 1.Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention; 2019. Available from: https://ginasthma.org/wp-content/uploads/2019/06/GINA-2019-main-report-June-2019-wms.pdf. Accessed July2, 2019.
- 2.Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343–373. doi: 10.1183/09031936.00202013 [DOI] [PubMed] [Google Scholar]
- 3.Wenzel S. Severe asthma: from characteristics to phenotypes to endotypes. Clin Exp Allergy. 2012;42(5):650–658. doi: 10.1111/j.1365-2222.2011.03929.x [DOI] [PubMed] [Google Scholar]
- 4.Zeiger RS, Schatz M, Dalal AA, et al. Utilization and costs of severe uncontrolled asthma in a managed-care setting. J Allergy Clin Immunol Pract. 2016;4(1):120–129.e123. doi: 10.1016/j.jaip.2015.08.003 [DOI] [PubMed] [Google Scholar]
- 5.Chastek B, Korrer S, Nagar SP, et al. Economic burden of illness among patients with severe asthma in a managed care setting. J Manag Care Spec Pharm. 2016;22(7):848–861. doi: 10.18553/jmcp.2016.22.7.848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kerkhof M, Tran TN, Soriano JB, et al. Healthcare resource use and costs of severe, uncontrolled eosinophilic asthma in the UK general population. Thorax. 2018;73(2):116–124. doi: 10.1136/thoraxjnl-2017-210531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nunes C, Pereira AM, Morais-Almeida M. Asthma costs and social impact. Asthma Res Pract. 2017;3:1. doi: 10.1186/s40733-016-0029-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krings JG, McGregor MC, Bacharier LB, Castro M. Biologics for severe asthma: treatment-specific effects are important in choosing a specific agent. J Allergy Clin Immunol Pract. 2019;7(5):1379–1392. doi: 10.1016/j.jaip.2019.03.008 [DOI] [PubMed] [Google Scholar]
- 9.Pavord ID, Korn S, Howarth P, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380(9842):651–659. doi: 10.1016/S0140-6736(12)60988-X [DOI] [PubMed] [Google Scholar]
- 10.GlaxoSmithKline. Mepolizumab (NUCALA) highlights of prescribing information; 2019. Available from: https://gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Nucala/pdf/NUCALA-PI-PIL.PDF. Accessed July8, 2019.
- 11.Menzella F, Lusuardi M, Galeone C, Taddei S, Zucchi L. Profile of anti-IL-5 mAb mepolizumab in the treatment of severe refractory asthma and hypereosinophilic diseases. J Asthma Allergy. 2015;8:105–114. doi: 10.2147/JAA.S40244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bel EH, Wenzel SE, Thompson PJ, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371(13):1189–1197. doi: 10.1056/NEJMoa1403291 [DOI] [PubMed] [Google Scholar]
- 13.Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371(13):1198–1207. doi: 10.1056/NEJMoa1403290 [DOI] [PubMed] [Google Scholar]
- 14.Chupp GL, Bradford ES, Albers FC, et al. Efficacy of mepolizumab add-on therapy on health-related quality of life and markers of asthma control in severe eosinophilic asthma (MUSCA): a randomised, double-blind, placebo-controlled, parallel-group, multicentre, phase 3b trial. Lancet Respir Med. 2017;5(5):390–400. doi: 10.1016/S2213-2600(17)30125-X [DOI] [PubMed] [Google Scholar]
- 15.Staton TL, Arron JR, Olsson J, Holweg CTJ, Matthews JG, Choy DF. Seasonal variability of severe asthma exacerbations and clinical benefit from lebrikizumab. J Allergy Clin Immunol. 2017;139(5):1682–1684.e1683. doi: 10.1016/j.jaci.2017.01.028 [DOI] [PubMed] [Google Scholar]
- 16.DuBuske L, Newbold P, Wu Y, Trudo F. Seasonal variability of exacerbations of severe, uncontrolled eosinophilic asthma and clinical benefits of benralizumab. Allergy Asthma Proc. 2018;39(5):345–349. doi: 10.2500/aap.2018.39.4162 [DOI] [PubMed] [Google Scholar]
- 17.Montero-Perez O, Contreras-Rey MB, Sanchez-Gomez E. Effectiveness and safety of mepolizumab in severe refractory eosinophilic asthma: results in clinical practice. Drugs Context. 2019;8:212584. doi: 10.7573/dic.212584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strauss RA, Jawhari N. Mepolizumab in the treatment of severe eosinophilic asthma: results from a physician in the field. Ann Allergy Asthma Immunol. 2018;121(1):121–123. doi: 10.1016/j.anai.2018.04.016 [DOI] [PubMed] [Google Scholar]
- 19.Khurana S, Brusselle GG, Bel EH, et al. Long-term safety and clinical benefit of mepolizumab in patients with the most severe eosinophilic asthma: the COSMEX study. Clin Ther. 2019;41:2041–2056.e5. epub. doi: 10.1016/j.clinthera.2019.07.007 [DOI] [PubMed] [Google Scholar]
- 20.Lugogo N, Domingo C, Chanez P, et al. Long-term efficacy and safety of mepolizumab in patients with severe eosinophilic asthma: a multi-center, open-label, phase IIIb study. Clin Ther. 2016;38(9):2058–2070. e2051. doi: 10.1016/j.clinthera.2016.07.010 [DOI] [PubMed] [Google Scholar]
- 21.Sullivan PW, Ghushchyan VH, Globe G, Schatz M. Oral corticosteroid exposure and adverse effects in asthmatic patients. J Allergy Clin Immunol. 2018;141(1):110–116.e117. doi: 10.1016/j.jaci.2017.04.009 [DOI] [PubMed] [Google Scholar]
- 22.Lefebvre P, Duh MS, Lafeuille MH, et al. Acute and chronic systemic corticosteroid-related complications in patients with severe asthma. J Allergy Clin Immunol. 2015;136(6):1488–1495. doi: 10.1016/j.jaci.2015.07.046 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention; 2019. Available from: https://ginasthma.org/wp-content/uploads/2019/06/GINA-2019-main-report-June-2019-wms.pdf. Accessed July2, 2019.