Abstract
Background
Aging is a spontaneous and inevitable phenomenon of biology, which can lead to the gradual deterioration of tissues and organs. One of the age-related deterioration processes is immunosenescence, which leads to changes in the function of immune systems, including immune cells and associated cytokines. A proper modulation of immune responses can improve the age-related immunosenescence process and then reach healthy aging. Schisandra sphenanthera, a traditional Chinese medicine, has been used as both a medicine and a nutritional supplement for thousands of years. Anwulignan, a monomer compound of Schisandra sphenanthera lignans, has been reported to possess an immunomodulatory effect. Therefore, this study was designed to further explore whether Anwulignan could also modulate the immune functions in aging model mice and the underlying mechanism.
Methods
D-galactose (D-gal) is often used as an inducer of immunosenescence in animals. In this study, a mice model was created by subcutaneous D-gal (220 mg kg−1) for successive 42 days. Then, the blood and spleen tissue samples were taken for the analysis and observation of cytokine levels, immunoglobulin levels, leukocyte numbers, and the phagocytic activity of macrophages, as well as the histological changes, the proliferation ability of lymphocytes, and the biochemical parameters in the spleen tissue.
Results
Anwulignan significantly increased the serum levels of IL-2, IL-4, IFN-γ, lgG, lgM, and lgA, decreased the content of TNF-α and IL-6 in the aging mice, and increased the blood leukocyte number, the phagocytic activity, the lymphocyte proliferation, and the spleen index in vitro. Anwulignan also significantly increased the activities of SOD and GSH-Px, decreased the contents of MDA and 8-OHdG in the spleen tissue, up-regulated the expressions of Nrf2, HO-1, and Bcl2, down-regulated the expressions of Keap1, Caspase-3, and Bax in the spleen cells, and reduced the apoptosis of spleen lymphocytes.
Conclusion
Anwulignan can restore the immune function that is declined in D-gal-induced aging mice partly related to its antioxidant capacity by activating the Nrf2/ARE pathway and downstream enzymes, as well as its anti-apoptotic effect by regulating Caspase-3 and the ratio of Bcl2 to Bax in the spleen.
Keywords: Anwulignan, immunosenescence, antioxidation, anti-apoptosis
Introduction
With the increasing aging of the global population, the research and development of anti-aging medicines and health care products have been becoming a hot spot.1 In 1969, an American pathologist, Walford, first proposed the concept of immunosenescence,2,3 which refers to the gradual deterioration of the immune system in the process of aging, leading to immune response dysfunction, inefficiency, or ineffectiveness.4,5 This age-associated immune deficiency manifests as: i) diminished self-renewal capacity of hematopoietic stem cells (HSC) due to the accumulation of oxidative damage to DNA and cellular metabolic activity by aging;6 ii) a notable decline in the total number of phagocytes coupled with an intrinsic reduction of their bactericidal activity;7 iii) reduced cytotoxicity of natural killer (NK) cells and the antigen-presenting function of dendritic cells with aging;8 and iv) declined humoral immunity caused by a reduction in the population of B cells along with a smaller immunoglobulin diversity and affinity.9 In addition, immunosenescence also leads to subclinical accumulation of pro-inflammatory factor and inflamm-aging, which exhibits an ever-increasing chronic background of inflammation from sources such as senescent cells, cell debris, and changes in the gut microbiota. This inflamm-aging then drives immune system dysfunction.10 Immunosenescence affects both the capacity of an elderly individual to respond to infections and the development of immune memory by vaccination, standing at the origin of most diseases of the elderly including cancer, infection, and autoimmune diseases. A correct modulation of immune responses can be useful to reduce age-related immunosenescence process in order to reach healthy aging.4,5
Schisandra sphenanthera Rehd. et Wils (S. sphenanthera,Schisandraceae), a well-known Chinese traditional medicine, has been used in antioxidation and hepatoprotection for thousands of years.11 Anwulignan is a symbolic monomer lignan from S. sphenanthera. Our previous studies found that Anwulignan had significant protective effects on the injured liver and brain tissues in an aging mice model induced by D-gal,12,13 showing a significant anti-aging function. However, its effect on immune function remains elusive. Therefore, in this study, we sought to determine the regulatory effect of Anwulignan on the immune function also by using an aging mice model induced by D-gal and explore the underlying mechanism. We hope this study will provide a basis for the development of anti-aging medicine or health care products.
Materials and Methods
Experimental Animals, Materials, and Reagents
Clean-grade healthy male ICR mice, weighing 20±2 g and aged from 6 to 8 weeks, were purchased from Changchun Yisi Experimental Animal Co., Ltd with the Certificate of Quality No. SCXK (JI)- 2016-0003 (Changchun, China), and were kept in separate cages in a light/dark cycle of 12:12 h with free access to food and water. The animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of Beihua University. All of the experimental procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals (China).
Anwulignan (Chengdu Pufei De Biotech Co., Ltd, Chengdu, China); sodium carboxymethyl cellulose (AR) (Shandong Weifang Lite Composite Materials Co., Ltd, Weifang, China); Twain-20 (AR) (Tianjin Yungtay Reagent Co., Ltd, Tianjin, China); D-galactose (Sigma Co., Ltd, USA); PVDF (polyvinylidene fluoride) film, HCl–Tris, 30% acrylamide, TEMED (N,N,N’,N’-tetramethylethylenediamine), ammonium persulfate, Tris, and glycine (Beijing Dinguo Reagent Co., Ltd, Beijing, China); 8-OHdG, GSH-Px, SOD, and MDA kits (Nanjing Jiancheng Bioengineering Institute Co., Ltd, Nangjing, China); skim milk powder (BD Co., Ltd, San Francisco, CA, USA); rabbit anti-Keap1, rabbit anti-Nrf2 (EPR1390Y), and rabbit anti-HO-1 (EP1808Y) antibodies (Abcam, San Francisco, CA, USA); rabbit anti-Caspase-3 (0206130101), rabbit anti-Bcl2 (0206130101), and rabbit anti-Bax (0206130101) (ABclonal Biotechnology Co., Ltd, Wuhan, China); ECL (ElectroChemi-Luminescence) color liquid (Biyuntian Biological Products Co., Ltd, Beijing, China); broad spectrum protein marker (Beijing Soledao Technology Co., Ltd, Beijing, China); and DMEM medium and other culture reagents were obtained from Hyclone (Logan Co., Ltd, UT, USA). IL-2, IL-4, IL-6, TNF-α, and IFN-γ kits (Shanghai MLBIO Biotechnology Co., Ltd, Shanghai, China); lgG, lgM, and lgA kits (Shanghai Lengton Bioscience Co., Ltd, Shanghai, China); ANNEXIN-V-FITC/PI Apoptosis kit (Beijing Solarbio Science & Technology Co., Ltd, Beijing, China); Concanavalin A (ConA; Sigma Co., Ltd, USA); Indian ink (Shanghai Ruiyong Biotechnology Co., Ltd, Shanghai, China); Na2CO3 (Beijing Beihua Fine Chemicals Co., Ltd, Beijing, China); and Isopropanol (Tianjin Fuyu Fine Chemical Co., Ltd, Tianjin, China). All of the reagents were of analytical grade or chromatographically pure.
Animal Grouping and Administration
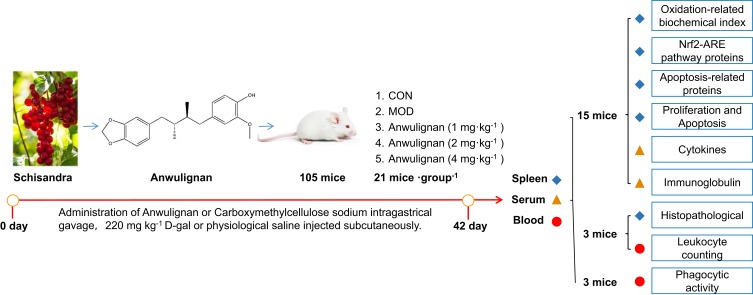
One hundred and five ICR mice were randomly and evenly divided into 5 groups, ie blank control group (distilled water by gavage and saline by subcutaneous injection), model group (distilled water by gavage and 220 mg kg−1 D-gal by subcutaneous injection), and three Anwulignan groups (1, 2, and 4 mg kg−1 by gavage and 220 mg kg−1 D-gal by subcutaneous injection), 21 mice in each group, and administered once daily for consecutive 42 days. After 30 min of the last dose, the blood was collected by removing the eyeball after anesthesia with ether, and spleen tissue was collected after the mice were sacrificed. Among the mice, 15 mice were used to detect the serum cytokine content and immunoglobulin level, to observe their spleen index, spleen tissue oxidation-related factors, the expression level of Nrf2/AER pathway and apoptosis-related proteins, as well as the proliferation ability and apoptosis of spleen lymphocytes. Three mice were used for the detection of phagocytic activity of macrophages in peripheral blood. The left 3 mice were used for observation of white blood cell count in peripheral blood and spleen histopathology. The experimental protocol is shown in Figure 1.
Figure 1.
The experimental protocol.
Serum IL-2, IL-4, IL-6, TNF-α, and IFN-γ Level Analysis
The peripheral blood samples of 15 mice from each group were centrifuged at 3500 rpm for 10 min to obtain the serum. Then, the serum contents of IL-2, IL-4, IL-6, TNF-α, and IFN-γ were analyzed by enzyme-linked immunosorbent assay (ELISA) as the instructions of the corresponding kits.
Serum lgG, lgM, and lgA Level Analysis
The serum samples were prepared as described in the previous section. The serum levels of lgG, lgM, and lgA were detected by ELISA as the instructions of the corresponding kits.
Blood Leukocyte Counting
The blood samples (0.02 mL for each) of 3 mice from each group were added to 0.38 mL leukocyte diluent and mixed. The number of leukocytes was counted with a counting plate under a microscope and calculated according to the following formula:
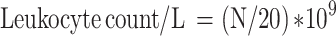 |
Phagocytic Activity of Macrophages Assessment
Thirty minutes after the last dose by gavage, 3 mice from each group were anesthetized with ether and injected with 50% diluted India ink (0.1 mL 10 g−1) through their tail veins. Then, 0.025 mL of blood of mice was collected from the inner canthus venous plexus 3 min and 11 min after the ink injection, respectively. The blood samples were immediately added to 2 mL of 0.1% Na2CO3 solution, which was used as the control. The absorbance values of the samples of two times (A3 and A11, respectively) were measured at 600 nm wavelength by a microplate reader (Infinite M200; Tecan, Maennedorf, Switzerland). The carbon clearance index (K) and phagocytosis index (α) were calculated according to the following equations:
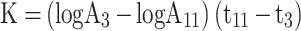 |
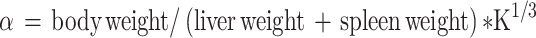 |
Histopathologic Examination
The spleen tissues from 3 mice of each group were isolated, and the excess tissues and fascia were stripped, rinsed with normal saline, dried with filter paper, and weighed. Then, the spleen tissues were fixed in 10% formalin, dehydrated, paraffin-embedded routinely and cut into slices 5 to 10 μm thick. Finally, the slices were stained with HE and the histopathological changes were examined under an optical microscope.
Spleen Index Measurement
The spleen index was calculated according to the following formula:
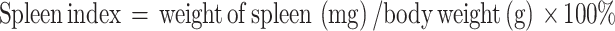 |
Proliferation Ability of Splenic Lymphocytes Assessment
The splenic lymphocytes were prepared and the proliferation ability of them was detected by MTT assay as reported.14,15 The optical density (OD) values were measured at a wavelength of 570 nm with a spectrophotometer (UV2550; Shimadzu, Kyoto, Japan). The difference in OD values between the samples that were incubated with and without ConA represented the proliferation ability of splenic lymphocytes.
Oxidative Biomarker Measurement
SOD and GSH-Px activities in the spleen homogenate were detected by the WST-1 method and colorimetry respectively. MDA and 8-OHdG levels in the spleen were also measured by the TBA method and ELISA respectively by referring to the instructions of the corresponding kits.
Western Blot Analysis
In order to analyze the proteins related to antioxidation and apoptosis, the splenic lymphocyte suspension was prepared and added with the lysis buffer, incubated on ice for 30 min, and then centrifuged at 12,000 rpm for 10 min to obtain the supernatant. The tissue protein concentration in the supernatant was determined by the BCA method. Antioxidation-related proteins Keap1, Nrf2, and HO-1, and apoptosis-related proteins Caspase-3, Bcl-2, and Bax in the supernatant were separated by 10% SDS-PAGE gel electrophoresis for 2 h, and then transferred onto PVDF membranes. The membranes were rinsed with Tris buffer (TBST) for 5 min and then blocked with the blocking TBST buffer (containing 5% skimmed milk powder) for 2 h at room temperature. Then, the blocking buffer was discarded, and the primary antibodies of Keap1 (1:1000), Nrf2 (1:1000), HO-1 (1:1000), Caspase-3 (1:1000), Bcl-2 (1:1000), and Bax (1:1000) were added onto the membranes. The membranes were incubated at 4 °C overnight and washed with TBST 5 times, for 5 min each time, and the corresponding second antibodies (1:5000) were added onto the membranes and the membranes were incubated for 2 h. The membranes were washed again with TBS-T 5 times, for 5 min each time. Finally, ECL chromogenic solution was added onto the membranes for the color development. The optical density of each band was analyzed by IPP software and the final results were expressed as the ratio of the target proteins to the internal reference β-actin.
Flow Cytometry
The spleen lymphocytes were adjusted to 5×106 mL−1 with DMEM (including 10% FBS), and the apoptosis of spleen lymphocytes was analyzed by an Epics-XL Flow Cytometer (Beckman Coulter, Brea, CA, USA) according to the instructions of the ANNEXIN-V-FITC/PI apoptosis kit.
Statistical Analysis
The experimental data were represented by mean±SD. One-way analysis of variance was used for the statistical analysis, and then Tukey’s multiple comparison test was carried out with SPSS software (SPSS 19.0; IBM Corporation, Armonk, NY, USA). P<0.05 was considered significant statistically.
Results
Anwulignan Regulated the Serum Levels of Cytokines Differently
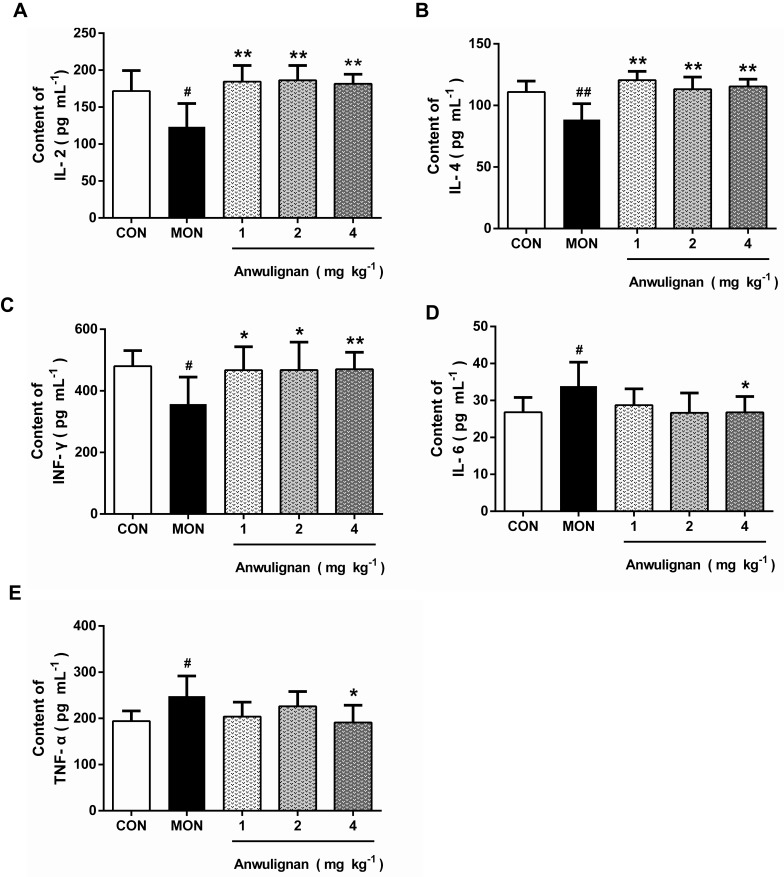
The results showed that Anwulignan up-regulated some cytokine levels but down-regulated others. On one hand, Anwulignan increased the levels of IL-2, IL-4, and IFN-γ significantly in all three dose groups (P<0.05, P<0.01) in comparison to the MOD group, in which these serum cytokines were significantly lower than the CON group (Figure 2A–C). On the other hand, Anwulignan at 4 mg kg−1 decreased the serum levels of IL-6 and TNF-α when compared with the MOD group (P<0.05), in which these two serum cytokines were significantly higher than the CON group (P<0.05) (Figure 2D and E). Given that up-regulation of pro-inflammatory cytokines (such as IL-6) and down-regulation of anti-inflammatory cytokines (IL-2 and IL-4) are hallmarks of inflamm-aging related to immunosenescence,16 our results indicated that: i) the aging animal model induced by D-gal faithfully represents the characteristics of naturally aging animals and is suitable for aging research; and ii) treatment with Anwulignan reversed the course of inflamm-age process in the D-gal-induced aging model mice.
Figure 2.
Effects of Anwulignan on the serum IL-2, IL-4, IL-6, TNF-α, and IFN-γ contents in mice (mean±SD, n=15).
Notes: (A) Effect of Anwulignan on the serum IL-2 content; (B) Effect of Anwulignan on the serum IL-4 content; (C) Effect of Anwulignan on the serum IFN-γ content; (D) Effect of Anwulignan on the serum IL-6 content; (E) Effect of Anwulignan on the serum TNF-α content. ##P<0.01, #P<0.05, compared with the CON group; **P<0.01, *P<0.05, compared with the MOD group.
Abbreviations: CON, control; MOD, model.
Anwulignan Increased the Serum Levels of Immunoglobulins
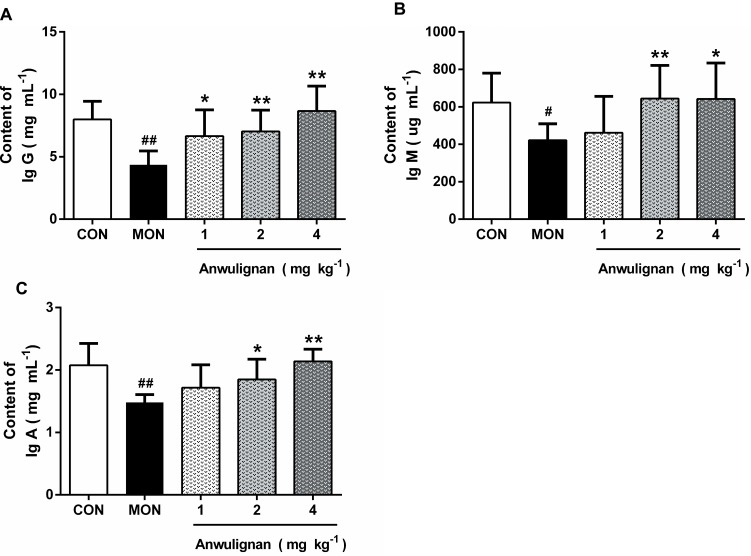
Immunoglobulin (Ig) is a common index of humoral immune status and plays an important role in immune response and adaptation.15 Our results showed that the serum levels of IgG, IgM, and lgA in the MOD group were significantly lower than those in the CON group (P<0.01, P<0.05), however, Anwulignan increased them significantly (P<0.01, P<0.05) (Figure 3A–C), suggesting that Anwulignan is able to restore the humoral immunity that is declined in aging model mice caused by a reduction in the population of B cells.
Figure 3.
Effects of Anwulignan on the serum IgG, IgM, and lgA levels in mice (mean±SD, n=15).
Notes: (A) Effect of Anwulignan on the serum IgG content; (B) Effect of Anwulignan on the serum IgM content; (C) Effect of Anwulignan on the serum lgA content. ##P<0.01, #P<0.05, compared with the CON group; **P<0.01, *P<0.05, compared with the MOD group.
Abbreviations: CON, control; MOD, model.
Anwulignan Increased the Number of Blood Leukocytes and Phagocytic Activity
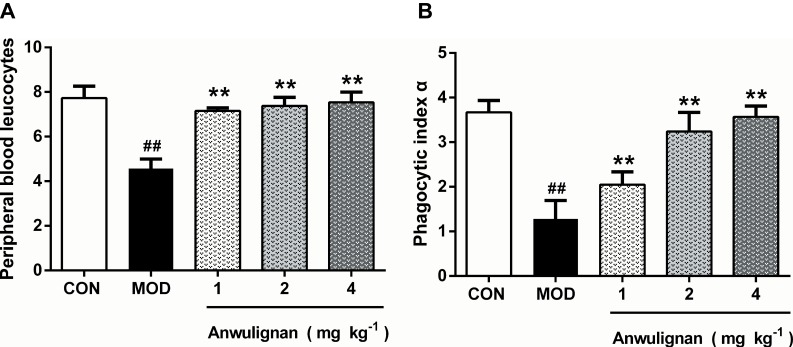
There is a notable decline in the total number of phagocytes in aged hosts, coupled with an intrinsic reduction of the phagocytic activity.17 Therefore, it is very necessary to examine the leukocyte numbers and their phagocytic activity. Our results showed that the blood leukocyte numbers in the MOD group were significantly lower than those of the CON group (P<0.01). However, Anwulignan increased the blood leukocyte numbers significantly in comparison to the MOD group (P<0.01) (Figure 4A).
Figure 4.
Effects of Anwulignan on the blood leukocyte count and the phagocytosis of macrophages (mean±SD, n=3).
Notes: (A) Effect of Anwulignan on the blood leukocyte count in mice; (B) Effect of Anwulignan on the phagocytosis of macrophages of mice. ##P<0.01, compared with the CON group; **P<0.01 compared with the MOD group.
Abbreviations: CON, control; MOD, model.
Macrophages, as a kind of leukocyte, secrete a variety of complement components, cytokines, and inflammatory mediators and produce phagocytosis.17,18 We compared phagocytic activities of leukocytes with and without Anwulignan in D-gal-induced aging model mice. The results showed that the phagocytic activity of macrophages in the MOD group was significantly lower than that of the CON group (P<0.01). After Anwulignan treatment, the phagocytic activity of blood macrophages was significantly increased (P<0.01) (Figure 4B). Taken together, these results demonstrate that Anwulignan is able to restore the leukocyte number and phagocytosis that have declined in the course of age-related immunosenescence.
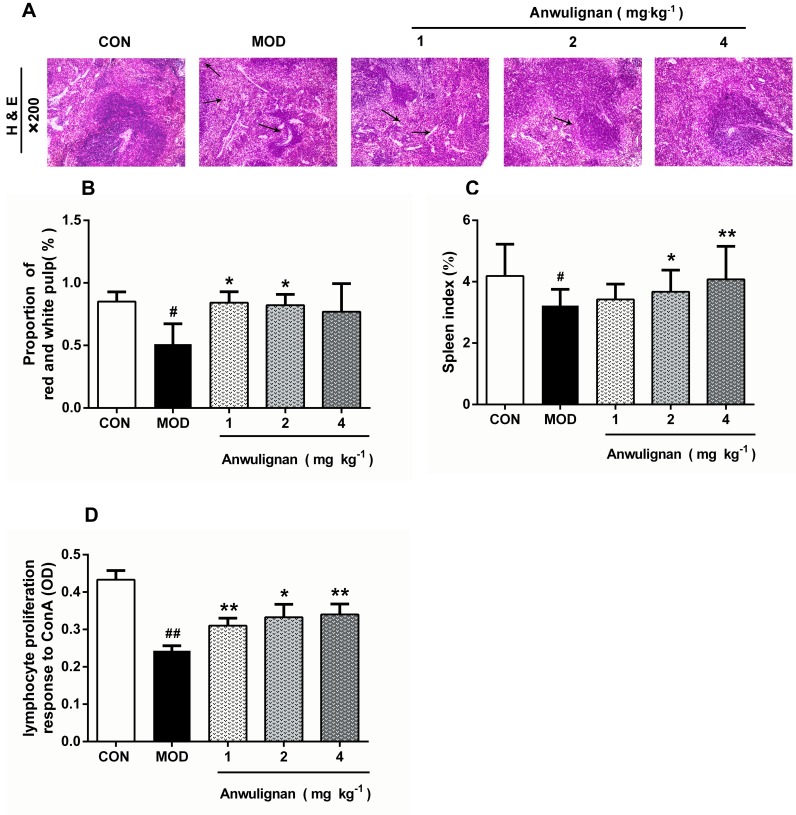
Anwulignan Ameliorated the Histopathological Changes in the Spleen
In the aging process, structural changes occur to the spleen, affect the functioning of the immune cells, and ultimately result in less effective or decreased immune responses. In order to evaluate the effect of Anwulignan on the histopathology of the spleen in aging mice, the morphological changes of the spleen were observed under an optical microscope by HE staining. The results showed that in the CON group, the splenocytes were tightly and well arranged with clear nuclei and boundary between the red and white medulla. In contrast, in MOD mice, the spleen cells were sparsely and disorderly arranged, with visible nuclear pyknosis, many vacuoles under the capsule of the white pulp and spleen, and some macrophages. However, Anwulignan improved the pathological changes as observed in the MOD group, and increased macrophage numbers with the dose increase (Figure 5A).
Figure 5.
Protective effect of Anwulignan on the histopathological changes in the spleen of mice.
Notes: (A) Effects of Anwulignan on histopathological changes in the spleen tissue (HE×200) (n=3); (B) Effect of Anwulignan on the proportion of white pulp in the spleen (n=3); (C) Effect of Anwulignan on the spleen index (n=15); (D) Effect of Anwulignan on the proliferation of spleen lymphocytes (n=3). ##P<0.01, #P<0.05, compared with the CON group; **P<0.01, *P<0.05, compared with the MOD group.
Abbreviations: CON, control; MOD, model. The arrows indicate vacuoles and macrophages.
The ratio of red and white pulps of the spleen tissue in the MOD group was significantly lower than that in the CON group (P<0.05). However, Anwulignan increased the ratio of red and white pulp significantly (P<0.05) (Figure 5B).
The spleen index reflects the immune status of the body.19 The results showed that the spleen index in the MOD group was significantly lower than that of the CON group (P<0.05). Anwulignan increased the spleen index dose dependently (P<0.05, P<0.01) (Figure 5C). It is suggested that Anwulignan may improve the body weight and spleen weight of mice and thus protect the spleen in the aging mice.
Aging can reduce the proliferation and transformation ability of spleen lymphocytes in mice.20,21 Our results showed that the spleen lymphocyte proliferation ability was significantly lower in the MOD group than that of the CON group (P<0.01). However, Anwulignan increased the proliferation of lymphocytes significantly (P<0.05, P<0.01) (Figure 5D).
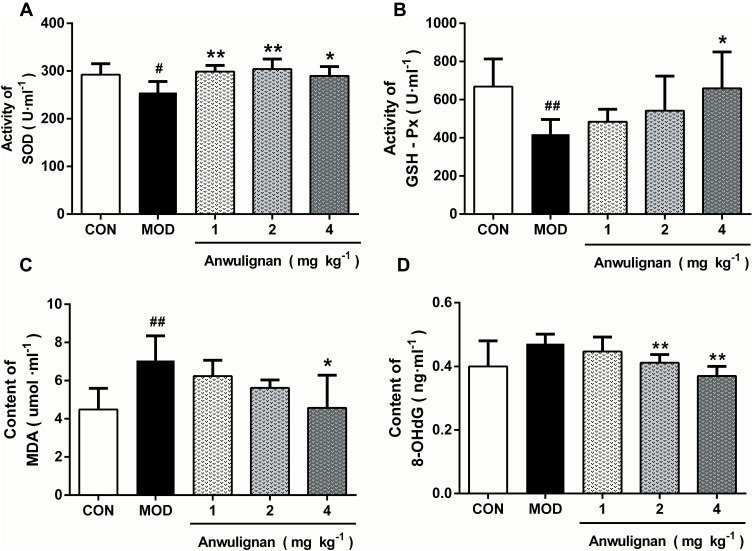
Anwulignan Increased SOD and GSH-Px Activities and Decreased MDA and 8-OHdG Contents in the Spleen
The reduction in immune functions in immunosenescence is owing to oxidative damage of proteins, lipids, and carbohydrates and apoptotic cell death triggered by the accumulation of oxidative debris. SOD, the most important antioxidant enzyme in the body, can effectively eliminate free radicals in the process of biological oxidation.22 GSH-Px, an important peroxidase in the body, can protect the body from oxidative damage.23 MDA is the product of lipid peroxidation and an important index to reflect the injury caused by free radicals, and its content increases with the aging of the body.24 The accumulation of free radicals can also damage intracellular DNA, and 8-OHdG is the most commonly used biomarker in the oxidative damage of DNA.25 The results showed that SOD and GSH-Px activities in spleen tissue of the MOD group were significantly decreased in comparison to the CON group (P<0.05, P<0.01) (Figure 6A and B). Anwulignan increased SOD activity (P<0.01, P<0 05). GSH-Px activity was not significantly changed in the 1 mg kg−1 and 2 mg kg−1 Anwulignan groups (P>0 05), but was significantly higher than those in the control group in the 4 mg kg−1Anwulignan group (P<0. 05) (Figure 6A and B). On the contrary, the MDA and 8-OHdG contents in the MOD group decreased (P<0.01, P<0.05) (Figure 6C and D). Anwulignan decreased the MDA and 8-OHdG contents significantly in the 4 mg kg–1 Anwulignan group (P<0.05, P<0.01) (Figure 6C and D). It is suggested that Anwulignan has an antioxidant effect, which may be related to its protective effect on immune injury induced by D-gal in mice.
Figure 6.
Effects of Anwulignan on the activity of SOD and GSH-Px and the content of MDA and 8-OHdG in the spleen tissue of aging mice (mean±SD, n=15).
Notes: (A) Effect of Anwulignan on the SOD content in the spleen tissue; (B) Effect of Anwulignan on the GSH-Px content in the spleen tissue; (C) Effect of Anwulignan on the MDA content in the spleen tissue; (D) Effect of Anwulignan on the 8-OHdG content in the spleen tissue. ##P<0.01, #P<0.05, compared with the CON group; **P<0.01, *P<0.05, compared with the MOD group.
Abbreviations: CON, control; MOD, model.
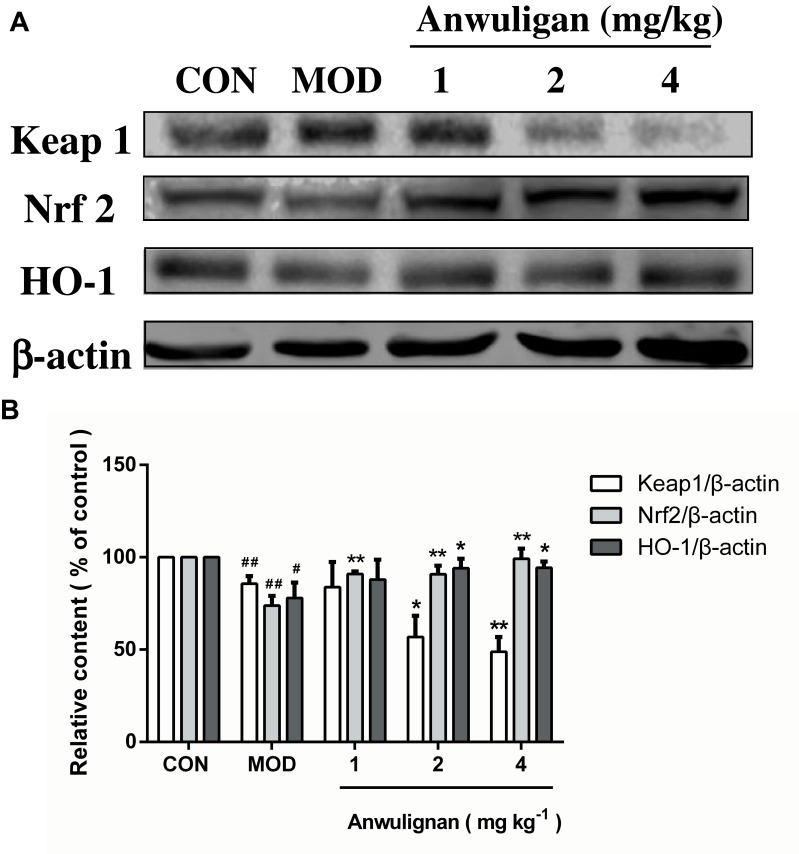
Anwulignan Regulated the NRF2-ARE Pathway in the Spleen
NRF2-ARE is the key antioxidant regulatory pathway of aging in macrophages and epithelial cells.26,27 We examined the effect of Anwulignan on this pathway in the spleen cells. The results showed that in the MOD group, D-gal decreased the expressions of Nrf2 and HO-1, but increased that of Keap1 significantly (P<0.05 or P<0.01) in comparison to the CON group. On the contrary, Anwulignan increased Nrf2 and HO-1 expressions, but decreased Keap1 expression significantly and dose dependently (P<0.05 or P<0.01) (Figure 7A and B). It is suggested that Anwulignan may exert its effects via the NRF2-ARE antioxidant pathway.
Figure 7.
Effects of Anwulignan on the expressions of Keap1, Nrf2, and HO-1 proteins in the spleen cells of mice (mean±SD, n=3).
Notes: (A) Keap1, Nrf2, HO-1, and β-actin protein electrophoresis images (western blotting); (B) Keap1/β-actin, Nrf2/β-actin, and HO-1/β-actin column charts (western blotting). ##P<0.01, #P<0.05, compared with the CON group; **P<0.01, *P<0.05, compared with the MOD group.
Abbreviations: CON, control; MOD, model.
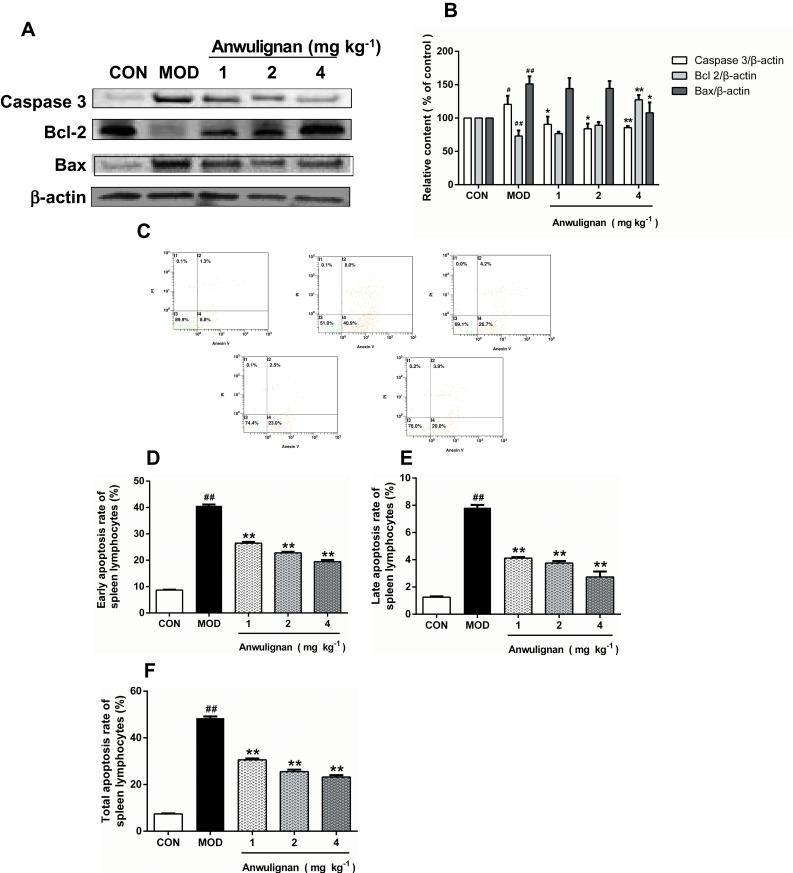
Anwulignan Inhibited the Apoptosis of Spleen Cells
Apoptosis is one of many typical changes of immunosenescence. Anti-apoptotic Bcl2 and pro-apoptotic Bax and Caspase-3 play an important role in the regulation of apoptosis.28 We traced the expression changes of these apoptosis-related genes in the spleen to assess their response to aging and Anwulignan treatment. The results showed that in the MOD group, the expressions of Caspase-3 and Bax in splenocytes increased (P<0.05, P<0.01), while the expression of Bcl2 decreased (P<0. 01) in comparison to the CON group. However, Anwulignan reduced the expressions of Caspase-3 and Bax proteins (P<0.05, P<0.01), but increased the expression of Bcl2 significantly (4 mg kg−1, P<0.01) (Figure 8A and B), suggesting that Anwulignan exerts its anti-aging partly via anti-apoptosis in spleen cells.
Figure 8.
Effects of Anwulignan on Caspase-3, Bcl-2, and Bax protein expressions in the spleen cells of mice (mean±SD, n=3).
Notes: (A) Caspase-3, Bcl-2, Bax, and β-actin protein electrophoresis images (western blotting); (B) Caspase-3/β-actin, Bcl-2/β-actin, and Bax/β-actin column charts (western blotting); (C) Effects of Anwulignan on the apoptosis of spleen lymphocytes; (D) Earlyapoptosis rate; (E) Late apoptosis rate; (F) Total apoptosis rate. ##P<0.01, #P<0.05, compared with the CON group; **P<0.01, *P<0.05, compared with the MOD group.
Abbreviations: CON, control; MOD, model.
To confirm the above inference, the spleen lymphocytes of each group were isolated, and their apoptosis status was further analyzed by flow cytometry. The results showed that the apoptosis rate in the MOD group was significantly higher than that in the CON group (P<0. 01), whereas Anwulignan decreased the apoptosis rate significantly (P<0. 01) (Figure 8C–F).
Discussion
The aging immunosenescence theory holds that the immune system is fundamentally involved in the aging process of normal vertebrates and is the regulator of this process.29,30 With the progression of aging, the immune organs and cells will become progressively atrophied and degenerated, resulting in the degenerative changes of immune function and then an increased incidence of immune-related diseases.30 D-gal is a reducing sugar, which causes the increase in free radicals and induces the oxidative damage,31 finally leading to the gradual aging and damage of the body similar to those in natural aging.32,33 It has been reported that in the aging host, the levels of cytokines also change, and they play an important regulatory role in the immune system.34,35 In the current study, we found that Anwulignan increased the serum levels of IL-2, IL-4, and IFN-γ in the aging mice, while it decreased the serum levels of IL-6 and TNF-α, suggesting that Anwulignan can restore the immune function that is declined in the process of aging by regulating the level of the cytokines in mice and reversing the inflamm-aging process.
At the molecular level, we detected the serum contents of IgG, IgM, and IgA, which can reflect the immune response ability and humoral immune level of the body, and also change in the aging body.15,35–38 D-gal used to produce an aging model in the others’ and our present studies decreased these immunoglobulins and declined the level of humoral immune response.39,40 Anwulignan reversed these effects of D-gal and increased these immunoglobulins, suggesting that Anwulignan has a regulatory function on the humoral immune response of aging mice.
At the cellular level, the number of leukocytes and the phagocytic activity of macrophages were examined, which are also important indexes for the evaluation of the immune function.41 Anwulignan could increase the number of leukocytes and enhance the phagocytic activity of macrophages in the blood, and then restore the macrophage immunity that once damaged in D-gal-induced aging.
At the organ level, the spleen, one of the important organs of the immune system, was chosen, because it contains a large number of immune cells, and can produce immunoglobulin, complement, and other immune substances for immunity.42,43 Based on the theory of aging immunosenescence, the injury and functional decline of immune organs are one of the causes of aging.43,44 During immune activation, proliferation of spleen lymphocytes increases the weight of the spleen. But with the progression of aging, the spleen index decreases, its structure is damaged, and the proliferation ability of spleen lymphocytes decreases, finally resulting in declining immune function.45 Anwulignan could significantly improve the atrophy of the spleen induced by D-gal, increase the spleen index, and enhance the proliferation of spleen lymphocytes in vitro, demonstrating a significant protective role of Anwulignan in the spleen of aging mice induced by D-gal.
At the antioxidant pathway level, the Nrf2-ARE pathway was detected because it is the most important endogenous antioxidant stress pathway,46 and Nrf2 is the key regulator of antioxidant activity.47,48 Elevated activity of Nrf2 can effectively resist the oxidative stress damage and then reduce the immune decline in the body.49 Keap1 is a negative regulator of Nrf2 and HO-1 is a strong antioxidant in the body, which is regulated by Nrf2.50 Antioxidation enzyme SOD and peroxidase GSH-Px are the downstream enzymes of the Nrf2-ARE pathway and can convert superoxide free radicals into hydrogen peroxide and quickly decompose into water. The combined action of SOD and GSH-Px can effectively prevent tissue cells from being damaged by peroxides. Then, as for the free radicals, the ultimate factors of oxidative stress, there is a free radical theory of aging, which indicates that the accumulation of free radical products destroys the structure and function of immune organs such as the spleen.45 Our present results showed that Anwulignan up-regulated the expression of Nrf2 and HO-1 protein and down-regulated the expression of Keap1 protein in the spleen of aging mice; and then, increased the activities of antioxidation enzyme SOD and peroxidase GSH-Px and accordingly decreased the contents of the oxidative damage marker MDA and the DNA oxidative damage biomarker 8-OHdG in the spleen of mice treated with D-gal. Our previous study also found that Anwulignan could significantly increase the activity of SOD and GSH-Px and decrease the contents of MDA and 8-OHdG, but in the serum, liver, and brain tissues of aging mice induced by D-gal, confirming the antioxidant effect of Anwulignan.12,13 By taking all of the above effects together, we find that Anwulignan possesses an antioxidant function and is therefore able to protect against aging deterioration by acting on all processes of the Nrf2-ARE pathway, its downstream enzymes, and their substrates.
Apoptosis is another important factor involved in aging by eliminating the aging cells. Appropriate apoptosis can maintain the homeostasis of the body, but abnormal apoptosis inevitably indicates the disorder of the internal environment of the body.51,52 The apoptosis of immune cells is closely related to the regulation of the immune system, and is an important index to evaluate the changes of immune function.53 As important regulators of apoptosis, the expressions of both anti-apoptotic proteins represented by Bcl2 and pro-apoptotic proteins represented by Bax and Caspase-3 determine the trend of apoptosis.54,55 Interestingly, apoptosis is also related to Nrf2. Several studies have shown that Nrf2 can regulate the expression of Bcl2 by binding to its antioxidant response element (ARE), and Nrf2 inhibitors can reduce the expression of Bcl2.56,57 Our results showed that Anwulignan could up-regulate the expression of Bcl2 and down-regulate the expression of Bax and Caspase-3 in the spleen tissue of aging mice. Furthermore, the results of flow cytometry also confirmed that Anwulignan could decrease the apoptosis of spleen lymphocytes in aging mice.
Conclusions
In conclusion, Anwulignan can restore the immune function that is declined in D-gal-induced aging mice partly related to its antioxidant capacity by activating the Nrf2/ARE pathway and downstream enzymes, as well as its anti-apoptotic effect by regulating Caspase-3 and the ratio of Bcl2 to Bax in the spleen. The present research contributes an experimental basis for Anwulignan to be a potential candidate for a novel antioxidative and anti-aging medicine or health care product.
Acknowledgment
This study was financially supported by Grants from Jilin Provincial Health and Family Planning Commission (No. 2018J089, 2018J094), Jilin Provincial Administration of Traditional Chinese Medicine (2018108), Post-graduate Innovation Program of Beihua University (No. 2019071), Jilin Provincial Research Foundation for Education Department (JJKH20180356KJ), and Jilin City Bureau of Science and Technology (No. 20190601177).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Robert L. The three avenues of gerontology: from basic research to clinical gerontology and anti-aging medicine. Another French paradox. J Gerontol a Biol Sci Med Sci. 2004;59(6):B540–B542. doi: 10.1093/gerona/59.6.B540 [DOI] [PubMed] [Google Scholar]
- 2.Walford RL. The immunologic theory of aging. Immunol Rev. 1969;2:195. doi: 10.1111/imr.1969.2.issue-1 [DOI] [PubMed] [Google Scholar]
- 3.Walford RL. Immunologic theory of aging: current status. Fed Proc. 1974;33:2020. [PubMed] [Google Scholar]
- 4.Vallet H, Fali T, Sauce D. Aging of the immune system: from fundamental to clinical data. Rev Med Interne. 2019;40:105–111. doi: 10.1016/j.revmed.2018.07.005 [DOI] [PubMed] [Google Scholar]
- 5.Xin L, Jiang TT, Kinder JM, Ertelt JM, Way SS. Infection susceptibility and immune senescence with advancing age replicated in accelerated aging Lmna(Dhe) mice. Aging Cell. 2015;14:1122–1126. doi: 10.1111/acel.12385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ito K, Hirao A, Arai F, et al. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature. 2004;431:997–1002. doi: 10.1038/nature02989 [DOI] [PubMed] [Google Scholar]
- 7.Stout RD, Suttles J. Immunosenescence and macrophage functional plasticity: dysregulation of macrophage function by age-associated microenvironmental changes. Immunol Rev. 2005;205:60–71. doi: 10.1111/imr.2005.205.issue-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mocchegiani E, Malavolta M. NK and NKT cell functions in immunosenescence. Aging Cell. 2004;3:177–184. doi: 10.1111/ace.2004.3.issue-4 [DOI] [PubMed] [Google Scholar]
- 9.Gibson KL, Wu YC, Barnett Y, et al. B-cell diversity decreases in old age and is correlated with poor health status. Aging Cell. 2009;8:18–25. doi: 10.1111/j.1474-9726.2008.00443.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franceschi C, Bonafè M, Valensin S, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x [DOI] [PubMed] [Google Scholar]
- 11.Chen X, Tang R, Liu T, et al. Physicochemical properties, antioxidant activity and immunological effects in vitro of polysaccharides from Schisandra sphenanthera and Schisandra chinensis. Int J Biol Macromol. 2019;131:744–751. doi: 10.1016/j.ijbiomac.2019.03.129 [DOI] [PubMed] [Google Scholar]
- 12.Gao J, Yu Z, Jing S, et al. Protective effect of Anwulignan against D-galactose-induced hepatic injury through activating p38 MAPK-Nrf2-HO-1 pathway in mice. Clin Interv Aging. 2018;13:1859–1869. doi: 10.2147/CIA.S173838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu Z, Jing S, Gao J, et al. Anwulignan improves d-galactose-induced learning and memory impairment via regulating P38 MAPK-Nrf2-HO-1 pathway in mice. Nat Prod Commun. 2019;14:1934578X84631. doi: 10.1177/1934578X19846316 [DOI] [Google Scholar]
- 14.Zheng X, Li P, Pan X, et al. Effects of polysaccharide from anoectochilus formosanus on murine splenic lymphocytes proliferation, cell cycle and cytokine secretion. J Chin Inst Food Sci Technol. 2017;17:47–52. [Google Scholar]
- 15.Dai YF, Niu Y, Cheng J, et al. Immunological mechanism in development of allergic dermatitis in guinea pig induced by trichloroethylene in vitro. Chin J Ind Hyg Occup Dis. 2005;23:129–131. [PubMed] [Google Scholar]
- 16.Ventura MT, Casciaro M, Gangemi S, Buquicchio R. Immunosenescence in aging: between immune cells depletion and cytokines up-regulation. Clin Mol Allergy. 2017;15:21–28. doi: 10.1186/s12948-017-0077-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gren ST, Grip O. Role of monocytes and intestinal macrophages in Crohn’s disease and ulcerative colitis. Inflamm Bowel Dis. 2016;22:1992–1998. doi: 10.1097/MIB.0000000000000824 [DOI] [PubMed] [Google Scholar]
- 18.Lloberas J, Modolell M, Celada A, et al. l-arginine and macrophages: role in classical and alternative activation Nat Rev Immunol 2017:117–129. [Google Scholar]
- 19.Song H, Zhang S, Zhang L, et al. Effect of orally administered collagen peptides from bovine bone on skin aging in chronologically aged mice. Nutrients. 2017;9(11):1209–1223. doi: 10.3390/nu9111209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fan Y, Liu J, Wang D, et al. The preparation optimization and immune effect of epimedium polysaccharide-propolis flavone liposome. Carbohydr Polym. 2013;94:24–30. doi: 10.1016/j.carbpol.2012.12.071 [DOI] [PubMed] [Google Scholar]
- 21.Zhu YH, Meng XS, Bao YR, TG Kang. The inhibition of cancer cell proliferation and regulating the immune function by compatibility of ratio emblica total phenolic acids and flavonoids. Chin J Exp Tradit Medl Formulae. 2012;18:3–6. [Google Scholar]
- 22.Szibor M, Richter C, Ghafourifar P. Redox control of mitochondrial functions. Antioxid Redox Signal. 2001;3:515–523. doi: 10.1089/15230860152409149 [DOI] [PubMed] [Google Scholar]
- 23.Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001;30:1191–1212. doi: 10.1016/S0891-5849(01)00480-4 [DOI] [PubMed] [Google Scholar]
- 24.Moldovan L, Moldovan NI. Oxygen free radicals and redox biology of organelles. Histochem Cell Biol. 2004;122:395–412. doi: 10.1007/s00418-004-0676-y [DOI] [PubMed] [Google Scholar]
- 25.Zhou X, Zhao L, Luo J, et al. The toxic effects and mechanisms of Nano-Cu on the spleen of rats. Int J Mol Sci. 2019;20:1469–1488. doi: 10.3390/ijms20061469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li N, Alam J, Venkatesan MI, et al. Nrf2 is a key transcription factor that regulates antioxidant defense in macrophages and epithelial cells: protecting against the proinflammatory and oxidizing effects of diesel exhaust chemicals. J Immunol. 2004;173:3467–3481. doi: 10.4049/jimmunol.173.5.3467 [DOI] [PubMed] [Google Scholar]
- 27.Zhou L, Zhang H, Davies KJA, et al. Aging-related decline in the induction of Nrf2-regulated antioxidant genes in human bronchial epithelial cells. Redox Biol. 2018;14:35–40. doi: 10.1016/j.redox.2017.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu Q, Zheng Y-J, Hu Y-X, et al. Mechanism of Paris Forrestii (Takht.) H. Li-Suppressing the proliferation of acute myeloid leukemia cell lines. J Exp Hematol Chin Assoc Pathophysiol. 2019;27(1):7–13. [DOI] [PubMed] [Google Scholar]
- 29.Gorczynski RM, Terzioglu E. Aging and the immune system. Int Urol Nephrol. 2008;40:1117–1125. doi: 10.1007/s11255-008-9412-1 [DOI] [PubMed] [Google Scholar]
- 30.Smallwood HS, Daniel LF, Squier TC. Aging enhances the production of reactive oxygen species and bactericidal activity in peritoneal macrophages by upregulating classical activation pathways. Biochemistry. 2011;50:9911–9922. doi: 10.1021/bi2011866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma J, Wang H, Liu B, et al. Combination of chick embryo and nutrient mixture prevent D-galactose-induced cognitive deficits, immune impairment and oxidative stress in aging rat model. Sci Rep. 2019;9:4092–4101. doi: 10.1038/s41598-019-40953-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kong SZ, Li JC, Li SD, et al. Anti-aging effect of chitosan oligosaccharide on d-galactose-induced subacute aging in mice. Mar Drugs. 2018;16(6):181–193. doi: 10.3390/md16060181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li WJ, Nie SP, Peng XP, et al. Ganoderma atrum polysaccharide improves age-related oxidative stress and immune impairment in mice. J Agric Food Chem. 2012;60(6):1413–1418. doi: 10.1021/jf204748a [DOI] [PubMed] [Google Scholar]
- 34.Kedzierska K, Crowe SM. Cytokines and HIV-1: interactions and clinical implications. Antivir Chem Chemother. 2001;12:133–150. doi: 10.1177/095632020101200301 [DOI] [PubMed] [Google Scholar]
- 35.Schulte W, Bernhagen J, Bucala R. Cytokines in sepsis: potent immunoregulators and potential therapeutic targets–an updated view. Mediators Inflamm. 2013;2013:165974. doi: 10.1155/2013/165974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Han M, Sun P, Li Y, Wu G, Nie J. Structural characterization of a polysaccharide from Sargassum henslowianum, and its immunomodulatory effect on gastric cancer rat. Int J Biol Macromol. 2018;108:1120–1127. doi: 10.1016/j.ijbiomac.2017.12.109 [DOI] [PubMed] [Google Scholar]
- 37.Liu Y, Zheng D, Wang D, et al. Immunomodulatory activities of polysaccharides from white button mushroom, agaricus bisporus (Agaricomycetes), fruiting bodies and cultured mycelia in healthy and immunosuppressed mice. Int J Med Mushrooms. 2019;21:13–27. doi: 10.1615/IntJMedMushrooms.v21.i1 [DOI] [PubMed] [Google Scholar]
- 38.Li MY, Ouyang WQ, Wu XL, Zheng Y, Gao R, Tang JX. Effect of kinetin on immunity and splenic lymphocyte proliferation in vitro in D-galactose-induced aging rats. Sheng Li Xue Bao. 2014;66:605–611. [PubMed] [Google Scholar]
- 39.Wu YN, Yan FF, Hu JY, et al. The effect of chronic ammonia exposure on acute-phase proteins, immunoglobulin, and cytokines in laying hens. Poult Sci. 2017;96:1524–1530. doi: 10.3382/ps/pew454 [DOI] [PubMed] [Google Scholar]
- 40.Zhao Y, Xiao Y, Xie Y. Clinical study on changes of serum levels of immunoglobulin and cytokines in anaphylaxis patients induced by Qingkailing injection. China J Chin Mater Med. 2011;36:1382–1385. [PubMed] [Google Scholar]
- 41.Yamamoto T. Roles of the ribosomal protein S19 dimer and the C5a receptor in pathophysiological functions of phagocytic leukocytes. Pathol Int. 2010;57:1–11. doi: 10.1111/j.1440-1827.2007.02049.x [DOI] [PubMed] [Google Scholar]
- 42.Straub RH. Complexity of the bi-directional neuroimmune junction in the spleen. Trends Pharmacol Sci. 2004;25:640–646. doi: 10.1016/j.tips.2004.10.007 [DOI] [PubMed] [Google Scholar]
- 43.Miquel J. An update of the oxidation-inflammation theory of aging: the involvement of the immune system in oxi-inflamm-aging. Curr Pharm Des. 2009;15(26):3003–3026. doi: 10.2174/138161209789058110 [DOI] [PubMed] [Google Scholar]
- 44.Lee S, Kim MS, Lee D, et al. The comparative immunotoxicity of mesoporous silica nanoparticles and colloidal silica nanoparticles in mice. Int J Nanomedicine. 2013;8:147–158. doi: 10.2147/IJN.S39534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun J, Zhang L, Zhang J, et al. Protective effects of ginsenoside Rg1 on splenocytes and thymocytes in an aging rat model induced by d -galactose. Int Immunopharmacol. 2018;58:94–102. doi: 10.1016/j.intimp.2018.03.017 [DOI] [PubMed] [Google Scholar]
- 46.Peng S, Hou Y, Yao J, Fang J.. Activation of Nrf2-driven antioxidant enzymes by cardamonin confers neuroprotection of PC12 cells against oxidative damage. Food Funct. 2017;8:997–1007. doi: 10.1039/C7FO00054E [DOI] [PubMed] [Google Scholar]
- 47.Sykiotis GP, Habeos IG, Samuelson AV, Bohmann D. The role of the antioxidant and longevity-promoting Nrf2 pathway in metabolic regulation. Curr Opin Clin Nutr Metab Care. 2011;14:41–48. doi: 10.1097/MCO.0b013e32834136f2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yuanxiang J, Wenyu M, Xiaojian L, et al. Acute exposure to 3-methylcholanthrene induces hepatic oxidative stress via activation of the Nrf2/ARE signaling pathway in mice. Environ Toxicol. 2015;29:1399–1408. [DOI] [PubMed] [Google Scholar]
- 49.Hamdam J. Role of the Redox Responsive Transcription Factor, NRF2, in Immune Cell Function. University of Liverpool; 2013:1–231. [Google Scholar]
- 50.Li L, Dong H, Song E, Xu X, Liu L, Song Y. Nrf2/ARE pathway activation, HO-1 and NQO1 induction by polychlorinated biphenyl quinone is associated with reactive oxygen species and PI3K/AKT signaling. Chem Biol Interact. 2014;209:56–67. doi: 10.1016/j.cbi.2013.12.005 [DOI] [PubMed] [Google Scholar]
- 51.Mkrtchian GM, Boiadzhian AS, Avetian DG, et al. The involvement of abnormal apoptosis in the disturbance of synaptic plasticity in posttraumatic stress disorder. Zh Nevrol Psikhiatr Im SS Korsakova. 2013;113(1):26–29. [PubMed] [Google Scholar]
- 52.Zheng J, Edelman SW, Tharmarajah G, et al. Differential patterns of apoptosis in response to aging in Drosophila. Proc Natl Acad Sci U S A. 2005;102:12083–12088. doi: 10.1073/pnas.0503374102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu J, Cong L, Wang C, et al. Immunomodulatory effect of Schisandra polysaccharides in cyclophosphamide-induced immunocompromised mice. Exp Ther Med. 2018;15:4755–4762. doi: 10.3892/etm.2018.6073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yoo ES, Choo GS, Kim SH, et al. Antitumor and apoptosis-inducing effects of piperine on human melanoma cells. Anticancer Res. 2019;39:1883–1892. [DOI] [PubMed] [Google Scholar]
- 55.Wang K, Chen Z, Huang J, et al. Naringenin prevents ischaemic stroke damage via anti-apoptotic and anti-oxidant effects. Clin Exp Pharmacol Physiol. 2017;44:862–871. doi: 10.1111/cep.2017.44.issue-8 [DOI] [PubMed] [Google Scholar]
- 56.Zhang J, Su L, Ye Q, et al. Discovery of a novel Nrf2 inhibitor that induces apoptosis of human acute myeloid leukemia cells. Oncotarget. 2017;8:7625–7636. doi: 10.18632/oncotarget.13825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Niture SK, Jaiswal AK. Nrf2 protein up-regul3ates antiapoptotic protein Bcl-2 and prevents cellular apoptosis. J Biol Chem. 2012;287:9873–9886. doi: 10.1074/jbc.M111.312694 [DOI] [PMC free article] [PubMed] [Google Scholar]