NONACCIDENTAL TRAUMA AND ABUSIVE HEAD TRAUMA
Nonaccidental trauma (NAT) is a leading cause of preventable death in the pediatric population. Abusive head trauma (AHT) is the most common cause of NAT related mortality. Clinicians must always consider abuse in their differential diagnosis when evaluating and treating children, particularly when the mechanism of injury is unclear or when reported history does not match the injuries or the child’s developmental age.
Definitions
Child abuse, child neglect, child maltreatment, and NAT are often used interchange-ably. Although subtle distinctions apply, the authors recommend use of the inclusive “child abuse and neglect” definition by the US Centers for Disease Control and Prevention: “Any act or series of acts of commission or omission by a parent or other caregiver that results in harm, potential for harm, or threat of harm to a child.”1,2 NAT is the commission subset of this definition. AHT is a subset of NAT. The American Academy of Pediatrics defines AHT as “the constellation of cerebral, spinal, and cranial injuries that result from inflicted head injury to infants and young children.”3
Epidemiology of Nonaccidental Trauma and Abusive Head Trauma
In 2016 the US Department of Health and Human Services (HHS) estimated a yearly death rate from child abuse of 2.36 per 100,000 children. Children younger than one year old are at highest risk of death, with a ten-fold greater rate of abuse-related mortality compared with older children.4 Because of the difficulty of identifying abuse cases and variation in data collection, these are likely underestimates.5 AHT is not directly tracked by HHS despite AHT being the deadliest form of child abuse and the most common cause of severe traumatic brain injury (TBI) in infants.6 One nongovernmental study estimated the yearly rate of AHT at 27.5 to 32.2 per 100,000 infants less than one year old.7 Children between two and seven years old can also suffer from AHT, albeit it at a lower rate than that of infants.8
Legal Reporting Recommendations and Requirements
All US states have statutes requiring clinicians to report suspected or known child abuse. The criteria, standards, and circumstances vary by state. Some states have criminal penalties for failing to report child abuse. Clinicians reporting suspected abuse are immune from criminal and civil liability pursuant to the federal Child Abuse Prevention and Treatment Act of 1974.9
Nonaccidental Trauma Injury Overview
NAT includes trauma to the head, abdomen, mouth, esophagus, sex organs, bones, and skin. Sexual abuse can be a contributing factor. Minor abuse may precede major abuse. Sentinel abuse signs may foreshadow a future catastrophe; therefore, recognizing a sentinel sign and taking action can save a child’s life and rescue other at-risk children in the household. One study showed that sentinel signs of injury were present in 30% of infants with AHT (Table 1).
Table 1.
Sentinel injuries observed in infants with abusive head trauma, with incidence on presentation
| Bruising (face, forehead, ear, extremity, trunk) | 80% |
| Intraoral injury (frenulum injury, tongue contusion) | 11% |
| Fracture (including both acute and healing) | 7% |
Data from Sheets LK, Leach ME, Koszewski IJ, et al. Sentinel injuries in infants evaluated for child physical abuse. Pediatrics 2013;131(4):701–7.
Nonaccidental Trauma Management, Examination, Documentation, and Referral
Management starts with constant awareness and vigilance for signs of potential abuse. Identifying NAT and AHT are not core competencies in anesthesia education, and education about abuse must be improved. Anesthesiologists often care for infants and children soon after traumatic injury during the critical time frame when signs of recent or chronic abuse are still visible. Clinicians should conduct thorough head-to-toe skin examinations during patient undressing for surgery (Box 1).
Box 1. Skin lesions associated with abuse.
Bruises in early and late stages of healing
Patterns: loops, belts or belt buckles, cords, cigarette burns, hand prints, finger prints Tongue and lip injuries: may be from forcing a utensil or object into the child’s mouth Lacerations in different stages of healing
Bruises in a child who cannot walk or who has limited mobility
Data from Ward MG, Ornstein A, Niec A, et al. The medical assessment of bruising in suspected child maltreatment cases: A clinical perspective. Paediatr Child Health 2013;18(8):433–42
Routine clinical care may alter physical evidence of abuse and can confound investigation. Such care interventions include line placement, intubation, Foley catheter placement, skin preparation, surgery, and bandaging. Anesthesiologists and operating room teams must document evidence of abuse before beginning clinical procedures (Table 2).
Table 2.
Documentation before initiating clinical care that could alter skin findings
| Take photographs of skin lesions |
|
| Describe skin lesions with location and size |
|
| Document genital and perianal injuries |
|
| Use plain language that people without medical knowledge can understand |
|
| Report concerns of abuse |
|
Abbreviation: HIPAA, Health Insurance Portability and Accountability Act.
Child abuse pediatricians work with interdisciplinary teams that include social workers and the police. They can guide forensic examination, advise on documentation and collection of evidence, and provide work-up continuity.
Skin Injuries
Skin injuries, such as bruises, are the most common manifestation of child abuse and the most common sentinel injury.10,11 Bruises can also occur from normal pediatric behavior, and bleeding disorders can make bruising more severe. Bruise-like skin findings may also be congenital malformations, dyes and inks, or side effects of cultural practices such as cupping.11
Babies less than nine months old are younger than cruising age and rarely show bruising (1% compared with 40% to 90% of children older than nine months old). Most accidental bruises are small, oval, and associated with a bony prominence. Abusive bruises are associated with patterned imprints of inflicting objects, such as hands, fingers, belts, and shoe soles. Bruises in various stages of healing are also associated with abuse (Box 1).11
Abusive Trauma of the Abdomen, Oral Cavity, and Esophagus
More than half of child abuse cases involve trauma to the head, face, or neck. Intraoral injuries account for 12% of documented abuse injuries; however, this likely underestimates the true rate because of poor clinician awareness of how to assess intraoral injuries and the frequent absence of additional abuse signs. Concerning signs include dental neglect, dental trauma, mucosal injuries, tongue lacerations, and frenulum injury.5 Intraoral injuries may be associated with sexual abuse.
Abdominal trauma may include splenic injury, liver lacerations, or duodenal injury that present as bowel obstruction, hematoma, or stricture. Note that one-third of pediatric abdominal injuries from abuse do not have visible external signs of injury.12
Abusive Orthopedic Injuries
Orthopedic injuries can involve any bones, including the skull, spine, ribs, and long bones.13 A radiographic full-body skeletal survey is critical when evaluating suspected abuse, especially in children younger than two years old.13 Skeletal surveys may reveal subtle abuse injuries, including fracture fragments at the growth plate, growth plate irregularities, and subperiosteal new bone formation.14
Abusive Head Trauma
AHT is the deadliest form of nonaccidental trauma. It can happen to any child in any family or care setting. Some studies suggest increased AHT risk with young parents, delays in prenatal care, low birth weight, and socioeconomic stress.15,16 The AHT perpetrator is often but not always a parent. Box 2 lists confessions that describe acts of AHT.
Box 2. Confessions from perpetrators of abusive head trauma.
I didn’t want to choke him, but I wanted him to stop crying. I picked him up and I shook him; I threw him on the bed and he bounced on the sheet.
I shook her so she’d be quiet, it lasted maybe 5 minutes; I was exasperated; I shook her up and down, in front of me, without holding her against me; I was shaking her hard; I was crying just like she was, and I was worked up.
Data from Adamsbaum C, Grabar S, Mejean N, et al. Abusive head trauma: judicial admissions highlight violent and repetitive shaking. Pediatrics 2010;126(3):546–55
Diagnosis and Recognition of Abusive Head Trauma
AHT is missed in one-third of cases despite signs of head trauma, including vomiting, irritability, superficial face and scalp injuries, seizures, or respiratory abnormalities.17 Misdiagnoses attributed these findings to a medical process or accidental injury.17 Socioeconomic cognitive bias may play a role. Of missed AHT cases, families tend to be younger, white, and have two parents living with the child.17
Families or guardians accompanying the victim may present vague stories that are inconsistent with the observed injury. One AHT study that analyzed perpetrator confessions found that cases involved a single shaking episode in 45% of cases and repeated episodes in 55%, with a range of two to 30 episodes. In some cases, shaking was habitual for weeks or months and was often in response to the baby crying. Perpetrators often said the child would sleep after shaking, which led to habitual repeated shaking episodes. Some described verbal abuse and others described blunt impact to the head. Almost all cases involved a significant delay between the abuse and bringing the child to medical care.18
Clinical findings associated with AHT include subdural hematoma, hypoxic ischemic encephalopathy, and bilateral retinal hemorrhage (Figs. 1–3). The mechanisms and presentation AHT are complex (Table 3). Altered mental status may manifest as irritability, inconsolable crying, or drowsiness. Rapid brain degeneration with multifocal leukoencephalopathy and diffuse atrophy has been described after AHT.19 When cases of AHT or accidental TBI of the same Glasgow Coma Scale (GCS) score are compared, AHT groups generally have poorer outcomes. Children with moderate AHT and GCS score of 9 to 11 have a mortality risk similar to that of children with severe accidental TBI and GCS score less than 9.20
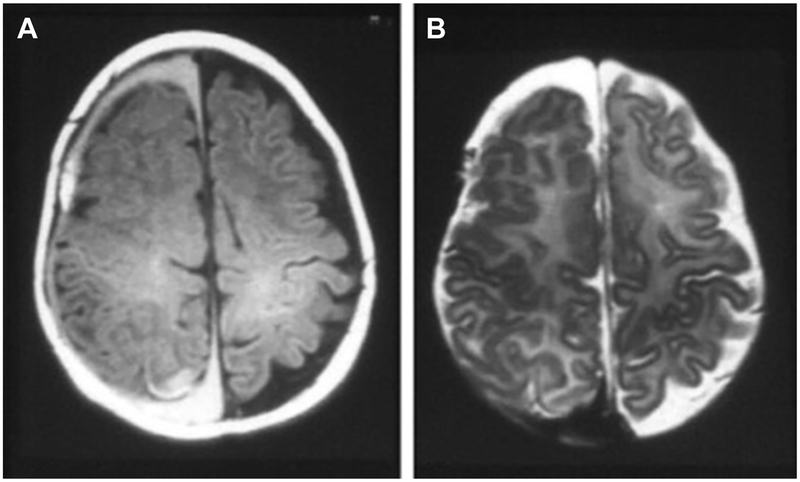
Fig. 1.
Abusive head trauma. Axial T1-weighted (A) and T2-weighted (B) MRI scans of an infant reveal bilateral subdural blood collections of different ages. Right collection shows blood in the late subacute phase (2–4 weeks old), and left shows blood in the chronic phase (>1 month). This finding is highly suggestive of repeated abuse. (From Michelson DJ, Ashwal S. Neuroimaging. In: Vincent JL, Abraham E, Moore FA, et al, editors. Textbook of critical care. 6th edition. Philadelphia: Saunders; 2011; with permission.)
Fig. 3.
(A) RetCam photographs of a 3-month-old boy diagnosed with AHT who subsequently died. The posterior pole and periphery showed no hemorrhages, but there were severe multilayered hemorrhages noted in the left eye, with preretinal hemorrhage layered in the inferior macula of the left eye. (B) RetCam photographs of a 4-month-old boy diagnosed with AHT showing bilateral severe retinal hemorrhages greater in the left eye. The hemorrhages are asymmetric, with the left side having more hemorrhages than the right, but both show multilayered retinal hemorrhages in the posterior pole and periphery. (From Longmuir SQ, McConnell L, Oral R, et al. Retinal hemorrhages in intubated pediatric intensive care patients. J AAPOS 2014;18(2):132; with permission.)
Table 3.
Abusive head trauma is multifactorial
| Shaking | Cervical Spine Injury | Bridging Vein Tears | Intracranial Hypertension |
| Blunt force | Thoracic spine injury | Subdural hematomas in the brain and spine | Hypoventilation, apnea |
| Impact | Hypoxic ischemic injury (especially if medical care is delayed) | Retinal hemorrhages | Coagulopathy |
| Acceleration-deceleration | Brain stem injury | Immature cervical ligaments and muscles in infants | Hypothermia |
| Diffuse axonal injury | Shearing forces | Seizures | Hypotension |
Compared to those with accidental TBI, children with AHT tend to be younger, are more likely to be brought in from home, and are more likely to have both apnea and seizures. Both groups are at equal risk of hypotension and hypoxemia. The ultimate cause of death in both groups is related to intracranial hypertension.21
Management of Abusive Head Trauma
Guidelines on the management of pediatric TBI largely stem from studies that either group accidental TBI and AHT together or exclude AHT because of its complex nature. Some clinicians suggest that AHT should be treated more aggressively than accidental TBI, specifically with more aggressive intracranial pressure (ICP) management. However, this strategy is not supported by current data.24 Therefore, current recommendations are to follow pediatric TBI guidelines without any cause-specific modifications (Box 3). Please also refer to Table 4 regarding calculation of pediatric GCS. The pediatric GCS scale is recommended for children two years old and younger, as their verbalization cannot be assessed using adult criteria.
Box 3. Summary of pediatric traumatic brain injury guidelines.
Maintain ICP <20 mm Hg.
Maintain cerebral perfusion pressure (CPP) ≥40 to 50 mm Hg, and adjust target CPP ranges for age.
Use methods to decrease the ICP while simultaneously supporting the CPP through hemodynamic support. It is not sufficient to increase blood pressure alone.
Avoid hypoxia.
Avoid hypercarbia.
Reserve hyperventilation for treatment of refractory intracranial hypertension with impending brain herniation. Hyperventilation causes cerebral vasoconstriction, which may cause cerebral ischemia.
Avoid hyperthermia.
Consider treatment of early post-traumatic seizures.
Elevate the head of bed.
Use hypertonic saline, barbiturates, or decompressive craniectomy to treat intracranial hypertension. Mannitol is second line to hypertonic saline.
Use ICP monitoring in patients with GCS score <9.
Data from Shein SL, Bell MJ, Kochanek PM, et al. Risk factors for mortality in children with abusive head trauma. J Pediatr 2012;161(4):716–22.e1; and Hardcastle N, Benzon HA, Vavilala MS. Update on the 2012 guidelines for the management of pediatric traumatic brain injury - information for the anesthesiologist. Paediatr Anaesth 2014;24(7):703–10
Table 4.
Modified Glasgow Coma Scale for young children and infants
| Pediatric | Infants | Score | |
|---|---|---|---|
| Eyes | |||
| No response | No response | 1 | |
| Verbal | |||
| No response | No response | 1 | |
| Motor | |||
| No response | No response | 1 |
Based on Institute of Neurological Sciences NHS Greater Glasgow and Clyde. Glasgow Coma Scale: Do it this way. Available at: http://www.glasgowcomascale.org/downloads/GCS-Assessment-Aid-English.pdf?v53. Accessed October 16, 2018.
Preoperative planning begins in the trauma bay with initiation of vascular access, volume resuscitation as required, and ordering of blood products. Airway management must focus on first-attempt success to avoid periods of prolonged apnea, which can lead to hypoxia and hypercarbia. An ideal approach includes rapid sequence induction, and the choice of induction medications and depolarizing or nondepolarizing muscle relaxants is at the discretion of the anesthesiologist. Etomidate, although cardiac stable, is associated with potential future adrenal suppression. Ketamine also maintains blood pressure but carries a theoretic risk of increase in ICP, although this is rarely seen in clinical practice. Propofol is familiar and reduces cerebral metabolic rate but can cause transient hypotension.
For patients with severe intracranial hypertension, intermittent ventilation with low ventilator pressure may be needed to avoid the aforementioned hypercarbia and hypoxia, which may cause catastrophic increases in ICP. Cervical spine in-line stabilization is essential, even if radiographic films are normal because pediatric spine injuries may be missed on radiographs. AHT may cause significant bleeding from mechanical tearing of bridging veins and potential late injury presentation.
If the infant or child requires additional radiologic studies and is hemodynamically stable, it is helpful to obtain such studies promptly after surgery to prevent delay in diagnosing additional injuries. If the patient received an ophthalmologic examination, the anesthesiologist must know which eye was dilated preoperatively. Consider also that biomarker use to detect or predict TBI over the last decade is limited by small sample size, variable practices in sample collection, inconsistent biomarker-related data elements, and disparate outcome measures. Future studies of biomarkers for pediatric TBI are needed.25
Prevention of Abusive Head Trauma
Several states mandate AHT education in the postnatal period, and the results have been mixed. The Safe Babies New York program taught parents about normal crying behavior, how to comfort crying babies, how to reduce caregiver frustration or anger, and about the medical impact of shaking an infant. This program showed a 47% reduction in incidence in AHT over six years.26 However, similar programs in Pennsylvania and North Carolina showed no reduction in the overall incidence of AHT.27,28 With continued improvements in data collection and analysis, clinicians hope to better identify high-risk groups and create more effective AHT prevention strategies.29
Second Victim Considerations
Child abuse cases cause significant psychological impact on clinicians, thereby creating a second victim. Second victim symptoms include anxiety, depression, confusion, loss of confidence, and sleep derangement. Each of these increase the risk of future medical errors.30 Clinicians must recognize second victim harm and support fellow clinicians during and after treatment of child abuse.
THE PEDIATRIC TRAUMATIC BRAIN INJURY SPECTRUM: SPORTS-RELATED CONCUSSIONS
This overview thus far focuses on AHT with associated NAT as a subset of pediatric TBI. AHT involves younger children and is both acute and devastating. Sports-related mild TBI tends to affect older children, with a peak in adolescence, and represents the other end of the pediatric TBI spectrum. It is subacute with more subtle presentation and leads to potentially debilitating neurocognitive deficits. The rest of this overview focuses on sports-related TBI with associated anesthetic considerations.
Sports-related Traumatic Brain Injury in Children and Adolescents
Concussion and other forms of mild TBI constitute approximately 75% of TBI’s that present per year, and mild TBI accounts for 16% of children less than ten years of age presenting for medical attention.31,32 Although TBI in the pediatric population is most commonly caused by falls and motor vehicle accidents, approximately 1.9 million youth have sports concussion annually, and 60% to 80% of pediatric sports-related hospitalizations are a result of pediatric sports-related TBI.33,34 Most common causes of sports-related concussion among boys include football, hockey, and lacrosse, whereas soccer, lacrosse, and basketball account for the leading causes among girls.31,35 Between 1996 and 2010, there were more than 78,000 snow sports–related head injuries among children and adolescents.36 The incidence of pediatric sports-related concussion is highest among the adolescent group, particularly in the 15-year-old to 19-year-old age group, and recent studies have indicated that the incidence of diagnosed pediatric sports-related concussion is increasing.37,38
Concussion is defined as brain injury resulting from biomechanical forces that may or may not present with loss of consciousness but leads to brief changes in mental status and neurologic function impairment in the absence of visible abnormalities on standard structural neuroimaging studies.39 Concussion typically presents with a range of symptoms including headache, lethargy, memory impairment, cognitive deficits, dizziness, problems with balance, sleep disturbances, and psychiatric symptoms. Symptoms are generally rapid in onset and resolve within a week to ten days from injury. However, up to 30% of children with concussion have symptoms that extend beyond 28 days after injury, a phenomenon known as persistent postconcussive symptoms.40,41
Pathophysiology of Concussion
Although the pathophysiology of concussion is not definitively elucidated, it is known that changes in cerebrovascular homeostatis commonly occur following concussion injury and likely contribute to brain injury and brain vulnerability. Impairment of cerebral autoregulation after concussion is well established and renders the brain susceptible to injury from cerebral ischemia or hyperemia.42 Mild to severe TBI, specifically in the pediatric population, has been shown to not only impair cerebral autoregulation; however, this impairment increases with younger age and is associated with worse six-month prognosis.43–45 Recently, Vavilala and colleagues46 examined cerebral autoregulation in youth who were hospitalized after sports-related mild TBI. By measuring middle cerebral artery flow velocity and cerebral autoregulation index using transcranial Doppler ultrasonography and tilt testing, the study showed that impaired cerebral autoregulation is common in sports-related TBI and occurs even when all of the patients’ presenting and serial daily GCS scores were 15.46
Sports-related concussion in teenage athletes has been shown to result in dimin-ished changes in cerebral flow in response to hypocapnia and hypercapnia compared with healthy controls.47 Prolonged decreases in cerebral blood flow can continue for weeks to months after injury and correlate with persistent postconcussive symptoms.48,49 In addition, imaging studies have shown that cerebral metabolic rates often increase after concussion, and the increased oxygen demand may further render the brain vulnerable to injury, particularly in the setting of compromised supply caused by decreased cerebral blood flow.47,50 Increased neurotransmitter release and inflammatory cytokine expression can occur after concussion, which may lead to cytotoxic cerebral edema and diffuse axonal injury.51,52
Anesthesia and Surgery Following Concussion in the Pediatric Population
The effects of general anesthesia or surgery on the brain in the postconcussive period have not been well researched. However, anesthesiologists should be concerned with the potential risks that both general anesthesia and surgery may pose to postconcussive patients. For instance, volatile anesthetics may further undermine the cerebrovascular autoregulation impairment known to be present in even mild pediatric TBI. It is known that, in pediatric TBI in general, blood pressure and glucose control and maintenance of normal oxygenation and ventilation directly affect patient outcomes.53 Hence, the so-called secondary insults commonly produced by general anesthesia and surgery, which include hypotension and decreased cerebral perfusion, hypocarbia or hypercarbia, and hyperglycemia, could potentially exacerbate concussive brain injury and delay recovery of symptoms.54
A recent poll of attendees of the Fall 2016 Washington State Society of Anesthesiologists meeting revealed that although 93% of providers responded that screening for concussion is important, only 5% reported that they conducted routine screening.55 In a study conducted by Ferrari and colleagues,56 a questionnaire was administered during the preoperative assessment to patients between the ages of five and 21 years who presented for surgical repair of orthopedic traumatic injury or nasal fracture, and who had precipitating injury within four weeks of the proposed surgery. Depending on age, at-risk patients were given either the Sport Concussion Assessment Tool (SCAT-3) or the Child Sport Concussion Assessment Tool (Child SCAT-3) to elicit self-reported symptoms of concussion. The prevalence of recent concussion diagnosed during the preoperative period for children undergoing semielective orthopedic surgery was approximately 6%. Moreover, only one in seven patients who had SCAT-3 or Child SCAT-3 scores suggestive of concussion had a preoperative diagnosis of concussion before the preoperative assessment.
A retrospective analysis identified patients who were diagnosed with concussion at the Mayo Clinic (Rochester, MN) between 2005 and 2015 who underwent a surgical procedure and anesthesia under the care of a physician anesthesiologist. Of the 1038 patients who were identified by these criteria, 93% (n = 965) had anesthesia one week after injury. Of note, 7% of patient with concussion had a delay in diagnosis of more than one week after injury, and several of these patients had undergone anesthesia before a concussion diagnosis. Of the patients who had surgery within one week after concussive injury, 5% underwent anesthesia for surgical procedures that were considered to be elective and unrelated to the concussive injury.57
The results of both of the studies signify that anesthesia and surgery may often confer a real and clinically significant risk of secondary insult soon after initial injury before the brain has had time to recover. Second, the studies show that a formal diagnosis of concussion is often missed, and that many patients with concussion fail to report symptoms of head injury to medical providers. Patients and families may fail to disclose these symptoms for multiple reasons, including symptoms being vague or mild and that recognition of signs of concussion are unknown, particularly when patients present in outpatient settings. In spite of official statements made by the National Football League, the American Academy of Pediatrics, and the American Academy of Neurology on recognition of concussion symptoms and return-to-play guidelines, studies show that around one-third of athletes do not recognize their symptoms as resulting from concussion.58 Distracting injury can obscure recognition of neurocognitive symptoms. Moreover, the neurocognitive side effects of opioids administered for pain control may also cloud the observation and monitoring of cognitive dysfunction caused by concussion.59
Persistent Postconcussion Symptoms
Although most concussion symptoms resolve within 28 days of injury, a prospective multicenter cohort study enrolling more than 3000 patients between the ages of five and 17 years who presented with acute concussion in the emergency department revealed that almost one-third had persistent postconcussive symptoms lasting for longer than one month.41 A retrospective case control study of 294 children presenting to a tertiary care concussion clinic identified risk factors that may result in delayed recovery from pediatric sports-related concussion. The study also found that approximately one-third of patients with concussion had persistent postconcussive symptoms beyond 28 days. Previous history of concussion, concussion injury resulting from nonhelmeted sports, female gender, presenting SCAT-2 score less than 80, SCAT-2 symptom severity score greater than 20, and previous diagnosis of attention-deficit/hyperactivity disorder were factors that predicted for concussion symptoms lasting greater than 28 days.60 Similarly, a retrospective cohort study that evaluated children undergoing MRI for persistent symptoms after pediatric sports-related concussion identified female gender and history of previous TBI to be associated with a delayed resolution of postconcussive symptoms.61 Although both of these studies are retrospective analyses, they provide evidence suggesting that persistent postconcussive symptoms are a relevant issue that anesthesiologists should be aware of and actively evaluate for during their assessments.
Summary of Pediatric Sports Related Concussions
Although there is no evidence to delineate optimal management of patients for semielective surgeries or provide definitive guidelines for timing of elective procedures, it is clear that anesthesiologists play a crucial role in providing optimal care for patients who have had concussion. As such, anesthesiologists may be pivotal in routine screening for risk and symptoms of concussion during preanesthesia evaluations, regardless of whether the patients carry prior diagnoses. Routine screening should include a detailed evaluation and clear, direct questioning of risk factors and concussive symptoms, which could include a modified version of the SCAT questionnaire (https://bjsm.bmj.com/content/bjsports/47/5/259.full.pdf). Screening of concussion symptoms even beyond one month after the concussion injury may be important in identifying the at-risk population. At a minimum, it may be prudent to defer elective surgeries and procedures requiring anesthesia until postconcussive symptoms have fully resolved. For nonelective procedures, the anesthetic management should be precise and follow the general principles of TBI care outlined earlier to minimize secondary insults. To effectively establish best-practice guidelines, further discussion, education on awareness, and research need to be prioritized in order to identify optimal means of screening for and man-aging postconcussive patients in the perioperative period (Box 4). Prospective studies will be critical to evaluate the effect of anesthesia and surgery on postconcussion patient outcomes.
Box 4. Knowledge Gap Areas in Perioperative Concussion.
Some potential questions regarding perioperative care of pediatric patients with concussion:
What do anesthesiologists know, think, and believe?
What is the current anesthesia practice?
Should anesthesiologists or surgeons screen?
Should anesthesiologists modify anesthesia care?
Should patients with concussion receive opioids at discharge?
What is the best anesthetic technique?
Should anesthesiologists delay surgery for symptomatic patients?
Does timing of concussion affect decision to proceed and/or anesthetic technique?
Should guidelines be developed for the care of concussed patients requiring elective surgery?
Should hemodynamic targets be different in concussion?
From Vavilala MS, Ferrari LR, Herring SA. Perioperative care of the concussed patient: making the case for defining best anesthesia care. Anesth Analg 2017;125(3):1054; with permission.
Fig. 2.
Watershed pattern of supratentorial hypoxic ischemic injury. Four-month-old boy with acute mental status change and seizures. (A) Computed tomography on admission to the emergency room shows bilateral chronic subdural collections with evidence of acute bleed on the left (arrow). Loss of gray-white matter discrimination because of cortical edema is present bilaterally. (B) MRI axial diffusion, B1000, shows bilateral watershed areas of restricted diffusion in watershed distribution. (C) MRI coronal diffusion, B1000, shows bilateral watershed areas of restricted diffusion. (From Zimmerman RA, Bilaniuk LT, Farina L. Non-accidental brain trauma in infants: diffusion imaging, contributions to understanding the injury process. J Neuroradiol 2007;34(2):111; with permission.)
KEY POINTS.
Nonaccidental trauma must be considered in all pediatric trauma cases.
Abusive head trauma is the most common cause of death from child abuse.
Perioperative and intensive care unit management of pediatric traumatic brain injury (TBI) aims to ensure adequate cerebral perfusion, prevention of hypoxia and hypercarbia, and aggressive control of intracranial pressure and core temperature.
Children with sports-related TBI who undergo surgery and anesthesia soon after injury may be vulnerable to worsening neurologic injury caused by impaired cerebral autoregulation, among other effects.
It is important to screen for potential TBI in sports-related injuries and factor in perioperative management.
Disclosures:
J.K. Lee received research support from Medtronic and was also a paid advisory board member for Medtronic. This arrangement has been reviewed and approved by the Johns Hopkins University in accordance with its conflict of interest policies. All other authors have no disclosures.
REFERENCES
- 1.Leeb RT, Paulozzi LJ, Melanson C, et al. Child maltreatment surveillance uniform definitions for public health and recommended data elements version 1.0. 2008. Available at: https://www.cdc.gov/violenceprevention/pdf/CM_Surveillance-a.pdf. Accessed July 5, 2018.
- 2.Leeb RT, Fluke JD. Child maltreatment surveillance: enumeration, monitoring, evaluation and insight. Health Promot Chronic Dis Prev Can 2015;35(8–9): 138–40. Available at: http://www.ncbi.nlm.nih.gov/pubmed/26605561. Accessed July 5, 2018. [PMC free article] [PubMed] [Google Scholar]
- 3.Christian CW, Block R, Committee on Child Abuse and Neglect, American Academy of Pediatrics. Abusive head trauma in infants and children. Pediatrics 2009; 123(5):1409–11. [DOI] [PubMed] [Google Scholar]
- 4.US Department of Health and Human Services, Administration for Children and Families, Administration on Children Youth and Families, Children’s Bureau. Child Maltreatment 2016. 2018. Available at: https://www.acf.hhs.gov/sites/default/files/cb/cm2016.pdf. Accessed June 26, 2018.
- 5.Yu DTY, Ngo TL. Child abuse—a review of inflicted intraoral, esophageal, and abdominal visceral injuries. Clin Pediatr Emerg Med 2016;17(4):284–95. [Google Scholar]
- 6.Beers SR, Berger RP, Adelson PD. Neurocognitive outcome and serum biomarkers in inflicted versus non-inflicted traumatic brain injury in young children. J Neurotrauma 2007;24(1):97–105. [DOI] [PubMed] [Google Scholar]
- 7.Ellingson KD, Leventhal JM, Weiss HB. Using hospital discharge data to track inflicted traumatic brain injury. Am J Prev Med 2008;34(4):S157–62. [DOI] [PubMed] [Google Scholar]
- 8.Salehi-Had H, Brandt JD, Rosas AJ, et al. Findings in older children with abusive head injury: does shaken-child syndrome exist? Pediatrics 2006;117(5): e1039–44. [DOI] [PubMed] [Google Scholar]
- 9.Fishe JN, Moffat FL. Child abuse and the law. Clin Pediatr Emerg Med 2016;17(4): 302–11. [Google Scholar]
- 10.Sheets LK, Leach ME, Koszewski IJ, et al. Sentinel injuries in infants evaluated for child physical abuse. Pediatrics 2013;131(4):701–7. [DOI] [PubMed] [Google Scholar]
- 11.Ward MG, Ornstein A, Niec A, et al. , Canadian Paediatric Society, Child and Youth Maltreatment Section. The medical assessment of bruising in suspected child maltreatment cases: a clinical perspective. Paediatr Child Health 2013;18(8) 433–42. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24426797. Accessed July 3, 2018. [PMC free article] [PubMed] [Google Scholar]
- 12.Naik-Mathuria B, Akinkuotu A, Wesson D. Role of the surgeon in non-accidental trauma. Pediatr Surg Int 2015;31(7):605–10. [DOI] [PubMed] [Google Scholar]
- 13.Narain A Skeletal manifestations of child maltreatment. Clin Pediatr Emerg Med 2016;17(4):274–83. [Google Scholar]
- 14.Perez-Rossello JM, McDonald AG, Rosenberg AE, et al. Absence of rickets in infants with fatal abusive head trauma and classic metaphyseal lesions. Radiology 2015;275(3):810–21. [DOI] [PubMed] [Google Scholar]
- 15.Niederkrotenthaler T, Xu L, Parks SE, et al. Descriptive factors of abusive head trauma in young children—United States, 2000–2009. Child Abuse Negl 2013; 37(7):446–55. [DOI] [PubMed] [Google Scholar]
- 16.Kesler H, Dias MS, Shaffer M, et al. Demographics of abusive head trauma in the Commonwealth of Pennsylvania. J Neurosurg Pediatr 2008;1(5):351–6. [DOI] [PubMed] [Google Scholar]
- 17.Jenny C, Hymel KP, Ritzen A, et al. Analysis of missed cases of abusive head trauma. JAMA 1999;281(7):621. [DOI] [PubMed] [Google Scholar]
- 18.Adamsbaum C, Grabar S, Mejean N, et al. Abusive head trauma: judicial admissions highlight violent and repetitive shaking. Pediatrics 2010;126(3):546–55. [DOI] [PubMed] [Google Scholar]
- 19.Duhaime A-C, Durham S. Traumatic brain injury in infants: the phenomenon of subdural hemorrhage with hemispheric hypodensity (“Big Black Brain”). Prog Brain Res 2007;161:293–302. [DOI] [PubMed] [Google Scholar]
- 20.Shein SL, Bell MJ, Kochanek PM, et al. Risk factors for mortality in children with abusive head trauma. J Pediatr 2012;161(4):716–22.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller Ferguson N, Sarnaik A, Miles D, et al. Abusive head trauma and mortality–an analysis from an international comparative effectiveness study of children with severe traumatic brain injury. Crit Care Med 2017;45(8):1398–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Longmuir SQ, McConnell L, Oral R, et al. Retinal hemorrhages in intubated pediatric intensive care patients. J AAPOS 2014;18(2):129–33. [DOI] [PubMed] [Google Scholar]
- 23.Lee JK, Brady KM, Deutsch N. The anesthesiologist’s role in treating abusive head trauma. Anesth Analg 2016;122(6):1971–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hardcastle N, Benzon HA, Vavilala MS. Update on the 2012 guidelines for the management of pediatric traumatic brain injury - information for the anesthesiologist. Paediatr Anaesth 2014;24(7):703–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Papa L, Ramia MM, Kelly JM, et al. Systematic review of clinical research on biomarkers for pediatric traumatic brain injury. J Neurotrauma 2013;30(5):324–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dias MS, Smith K, DeGuehery K, et al. Preventing abusive head trauma among infants and young children: a hospital-based, parent education program. Pediatrics 2005;115(4):e470–7. [DOI] [PubMed] [Google Scholar]
- 27.Dias MS, Rottmund CM, Cappos KM, et al. Association of a postnatal parent education program for abusive head trauma with subsequent pediatric abusive head trauma hospitalization rates. JAMA Pediatr 2017;171(3):223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zolotor AJ, Runyan DK, Shanahan M, et al. Effectiveness of a statewide abusive head trauma prevention program in North Carolina. JAMA Pediatr 2015;169(12): 1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kelly P, Thompson JMD, Koh J, et al. Perinatal risk and protective factors for pediatric abusive head trauma: a multicenter case-control study. J Pediatr 2017; 187:240–6.e4. [DOI] [PubMed] [Google Scholar]
- 30.Marmon LM, Heiss K. Improving surgeon wellness: the second victim syndrome and quality of care. Semin Pediatr Surg 2015;24:315–8. [DOI] [PubMed] [Google Scholar]
- 31.Barlow KM, Crawford S, Stevenson A, et al. Epidemiology of postconcussion syndrome in pediatric mild traumatic brain injury. Pediatrics 2010;126(2):e374–81. [DOI] [PubMed] [Google Scholar]
- 32.National Center for Injury Prevention and Control. Report to Congress on mild traumatic brain injury in the United States: steps to prevent a serious public health problem. Atlanta (GA): Centers for Disease Control and Prevention; 2003. [Google Scholar]
- 33.Bryan MA, Rowhani-Rahbar A, Comstock RD, et al. Sports- and recreation-related concussions in US youth. Pediatrics 2016;138(1) [pii:e20154635]. [DOI] [PubMed] [Google Scholar]
- 34.Yue JK, Winkler EA, Burke JF, et al. Pediatric sports-related traumatic brain injury in United States trauma centers. Neurosurg Focus 2016;40(4):E3. [DOI] [PubMed] [Google Scholar]
- 35.Noble JM, Hesdorffer DC. Sport-related concussions: a review of epidemiology, challenges in diagnosis, and potential risk factors. Neuropsychol Rev 2013; 23(4):273–84. [DOI] [PubMed] [Google Scholar]
- 36.Graves JM, Whitehill JM, Stream JO, et al. Emergency department reported head injuries from skiing and snowboarding among children and adolescents, 1996–2010. Inj Prev 2013;19(6):399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gessel LM, Fields SK, Collins CL, et al. Concussions among United States high school and collegiate athletes. J Athl Train 2007;42(4):495. [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang AL, Sing DC, Rugg CM, et al. The rise of concussions in the adolescent population. Orthop J Sports Med 2016;4(8). 2325967116662458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCrory P, Meeuwisse WH, Aubry M, et al. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Br J Sports Med 2013;47(5):250–8. [DOI] [PubMed] [Google Scholar]
- 40.Eisenberg MA, Meehan WP, Mannix R. Duration and course of post-concussive symptoms. Pediatrics 2014;133(6):999–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zemek R, Barrowman N, Freedman SB, et al. Clinical risk score for persistent postconcussion symptoms among children with acute concussion in the ED. JAMA 2016;315(10):1014–25. [DOI] [PubMed] [Google Scholar]
- 42.Obrist WD, Gennarelli TA, Segawa H, et al. Relation of cerebral blood flow to neurological status and outcome in head-injured patients. J Neurosurg 1979; 51(3):292–300. [DOI] [PubMed] [Google Scholar]
- 43.Vavilala MS, Lee LA, Boddu K, et al. Cerebral autoregulation in pediatric traumatic brain injury. Pediatr Crit Care Med 2004;5(3):257–63. [DOI] [PubMed] [Google Scholar]
- 44.Freeman SS, Udomphorn Y, Armstead WM, et al. Young age as a risk factor for impaired cerebral autoregulation after moderate to severe pediatric traumatic brain injury. Anesthesiology 2008;108(4):588–95. [DOI] [PubMed] [Google Scholar]
- 45.Chaiwat O, Sharma D, Udomphorn Y, et al. Cerebral hemodynamic predictors of poor 6-month Glasgow Outcome Score in severe pediatric traumatic brain injury. J Neurotrauma 2009;26(5):657–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vavilala MS, Farr CK, Watanitanon A, et al. Early changes in cerebral autoregulation among youth hospitalized after sports-related traumatic brain injury. Brain Inj 2018;32(2):269–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Len T, Neary J. Cerebrovascular pathophysiology following mild traumatic brain injury. Clin Physiol Funct Imaging 2011;31(2):85–93. [DOI] [PubMed] [Google Scholar]
- 48.Bonne O, Gilboa A, Louzoun Y, et al. Cerebral blood flow in chronic symptomatic mild traumatic brain injury. Psychiatry Res Neuroimaging 2003;124(3):141–52. [DOI] [PubMed] [Google Scholar]
- 49.Maugans TA, Farley C, Altaye M, et al. Pediatric sports-related concussion produces cerebral blood flow alterations. Pediatrics 2012;129(1):28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lovell MR, Pardini JE, Welling J, et al. Functional brain abnormalities are related to clinical recovery and time to return-to-play in athletes. Neurosurgery 2007; 61(2):352–60. [DOI] [PubMed] [Google Scholar]
- 51.Patterson ZR, Holahan MR. Understanding the neuroinflammatory response following concussion to develop treatment strategies. Front Cell Neurosci 2012; 6:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Choe MC, Babikian T, DiFiori J, et al. A pediatric perspective on concussion pathophysiology. Curr Opin Pediatr 2012;24(6):689–95. [DOI] [PubMed] [Google Scholar]
- 53.Kannan N, Ramaiah R, Vavilala MS. Pediatric neurotrauma. Int J Crit Illn Inj Sci 2014;4(2):131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vavilala MS, Ferrari LR, Herring SA. Perioperative care of the concussed patient: making the case for defining best anesthesia care. Anesth Analg 2017;125(3): 1053–5. [DOI] [PubMed] [Google Scholar]
- 55.NIH NIONDAS. Pediatric Concussion Workshop. 2016, October 13-14 Available at: https://meetings.ninds.nih.gov/Home/General/15077. Accessed November 18, 2016.
- 56.Ferrari LR, O’Brien MJ, Taylor AM, et al. Concussion in pediatric surgical patients scheduled for time-sensitive surgical procedures. J Concussion 2017;1 2059700217704775. [Google Scholar]
- 57.Abcejo AS, Savica R, Lanier WL, et al. Exposure to surgery and anesthesia after concussion due to mild traumatic brain injury. Mayo Clin Proc 2017;92(7): 1042–52. [DOI] [PubMed] [Google Scholar]
- 58.McCrea M, Hammeke T, Olsen G, et al. Unreported concussion in high school football players: implications for prevention. Clin J Sport Med 2004;14(1):13–7. [DOI] [PubMed] [Google Scholar]
- 59.Pinchefsky E, Dubrovsky AS, Friedman D, et al. Part II—Management of pediatric post-traumatic headaches. Pediatr Neurol 2015;52(3):270–80. [DOI] [PubMed] [Google Scholar]
- 60.Miller JH, Gill C, Kuhn EN, et al. Predictors of delayed recovery following pediatric sports-related concussion: a case-control study. J Neurosurg Pediatr 2016;17(4): 491–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bonow RH, Friedman SD, Perez FA, et al. Prevalence of abnormal magnetic reso-nance imaging findings in children with persistent symptoms after pediatric sports-related concussion. J Neurotrauma 2017;34(19):2706–12. [DOI] [PMC free article] [PubMed] [Google Scholar]