Abstract
Background
Competing risk analysis determines the probability of survival and considers competing events. This retrospective study aimed to undertake a competing risk analysis of prognosis in patients with esophageal carcinoma between 2006–2015 using data from the Surveillance, Epidemiology, and End Results (SEER) database.
Material/Methods
Clinicopathological, demographic, and survival data were analyzed for patients with esophageal carcinoma registered in the SEER database between 2006–2015. The competing risk model calculated the cumulative incidence function (CIF) of events of interest and prognosis. The Cox proportional-hazards model and the cause-specific hazard function (CS) were used to generalize the hazard function for competing risks. The Fine-Gray model was used for multivariate analysis. More accurate prognostic factors were analyzed by comparing the hazard ratio (HR) values between groups.
Results
There were 14,695 patients identified with esophageal carcinoma, 9,621 died from esophageal carcinoma, and 1,251 patients died from other causes. The cumulative incidence of events of interest was significant for age at diagnosis, race, primary tumor site, grade, stage, and treatment with surgery, radiotherapy, and chemotherapy (P<0.001). Multivariate analysis showed that age at diagnosis, primary tumor site, grade, stage, and treatment with surgery, radiotherapy, and chemotherapy statuses were independent prognostic factors (P<0.05). The Fine-Gray and the CS model showed that grade, stage, and treatments with surgery, radiotherapy, and chemotherapy were significant independent prognostic factors (P<0.05).
Conclusions
A competing risk model used data from the SEER database to obtain a more accurate estimate of the CIF of esophageal carcinoma-specific mortality and prognostic factors.
MeSH Keywords: Esophageal Neoplasms, Proportional Hazards Models, SEER Program
Background
Worldwide, in 2018 there were 572,000 (3.2%) new cases of esophageal carcinoma, with 508,585 (5.3%) esophageal carcinoma-associated deaths [1]. There are two main histological subtypes of esophageal carcinoma, esophageal adenocarcinoma, and squamous cell carcinoma. Although esophageal squamous cell carcinoma is the main histological type, the epidemiology has recently changed. In Australia, the UK, the USA, and some Western European countries, the incidence of esophageal adenocarcinoma has exceeded esophageal squamous cell carcinoma [2]. In Western countries, the main risk factors for esophageal squamous cell carcinoma are smoking and drinking alcohol, while esophageal adenocarcinoma predominates in high-income countries, with the main risk factors being obesity and chronic gastroesophageal reflux disease (GERD) [3–5]. Due to advances in imaging technology, advances in surgical techniques, and the development of new chemotherapeutic agents, the survival rate of patients with esophageal carcinoma is expected to increase. However, due to the lack of accurate prognostic indicators to guide clinical practice, the survival rate remains unchanged.
There are several statistical methods used to analyze patient prognosis and survival in epidemiological studies, including Kaplan-Meier regression analysis of survival [6], the log-rank test for comparing survival curves, and the Cox proportional hazards model for the analysis of multiple factors [7]. Classical survival analysis usually evaluates one endpoint, such as the impact of a risk factor on patient survival. However, clinically, multiple endpoints often coexist [8], and these endpoints compete with each other to produce competing risk data [9]. In the case of multiple endpoint events, the use of single-endpoint analysis methods will result in bias in the estimated probabilities of the endpoint events due to the existence of competing risks. However, competing risk analysis determines not only the probability of survival but also considers competing events.
Therefore, this retrospective study aimed to undertake a competing risk analysis of prognosis in patients with esophageal carcinoma between 2006–2015 using data from the Surveillance, Epidemiology, and End Results (SEER) database. In this study, the competing risk model calculated the cumulative incidence function (CIF) of events and prognosis. The Cox proportional-hazards model and the cause-specific hazard function (CS) were used to generalize the hazard function for competing risks. The Fine-Gray model was used for multivariate analysis, and prognostic factors were analyzed by comparing the hazard ratio (HR) values between groups.
Material and Methods
Data collection and selection of patients with esophageal carcinoma
Data were collected from the Surveillance, Epidemiology, and End Results (SEER) database of the National Cancer Institute (NCI) [8]. The SEER program collects cancer incidence data from population-based cancer registries that cover approximately 34.6% of the US population [8]. The SEER registries collect data on patient demographics, primary tumor site, tumor morphology, the American Joint Committee on Cancer (AJCC) stage at diagnosis, and the first course of treatment, with patient follow-up [8].
The SEER registry was searched for all cases of esophageal carcinoma, using the tumor site ICD-9 codes of C15.0 and C15.2–C15.5 for diagnoses between 2006–2015 (N=31,641). There were 16,676 patients who were excluded due to lack of information on race, differentiation grade, AJCC stage, or surgery or radiotherapy status, who did not have primary tumors, and patients whose histology code was not 8140/3 or 8170/3 (Figure 1). All tumors were staged according to the AJCC staging system, version 6. Also, the primary tumor site and morphological codes C15.0 and C15.3 were used to identify tumors located in the upper esophagus, C15.4 was used to identify tumors located in the mid-esophagus, and C15.2 and C15.5 were used to identify tumors located in the lower esophagus [10]. To facilitate the analysis of the competing model according to the SEER cause-specific death classification and vital status recode recorded in the SEER database, all patient follow-up outcomes were divided into three categories. The three patient categories studied included esophageal cancer-specific mortality, competing events, and censored events. The final analysis included 14,965 eligible patients with esophageal carcinoma. Because the individual patient data were de-identified, no Ethics Committee approval or Institutional Review Board approval was required.
Figure 1.
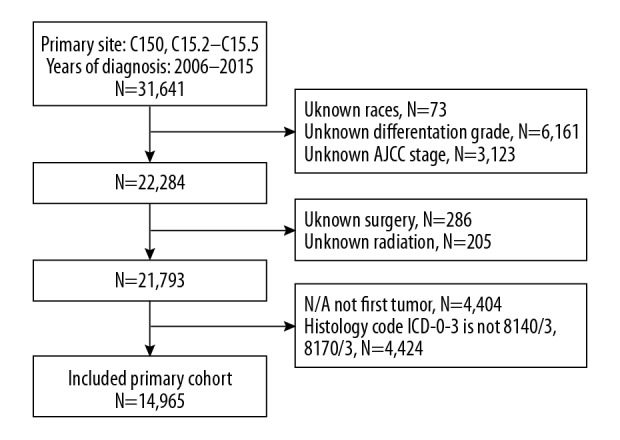
The inclusion and exclusion criteria used to identify patients with esophageal carcinoma between 2006–2015 using data from the Surveillance, Epidemiology, and End Results (SEER) database.
Statistical analysis
The baseline data were described using numerical counts and percentage values. The cumulative risk rate was estimated using the competing risk model in the single-factor analysis, and Gray’s test [11] was used for between-group comparisons. The multifactor analysis used the Fine-Gray model [12] and the cause-specific risk (CS) model [13] to explore the cumulative rate of esophageal carcinoma-specific mortality. Patient data were investigated using a classic survival analysis method to analyze single endpoint events. For single endpoint events, the Kaplan-Meier method was used to estimate the cumulative incidence of events of interest. In single-factor analysis, the log-rank test was used for intergroup comparison, and the Cox proportional risk model was used for multifactor analysis. Data were analyzed using SAS software version 9.4 (SAS Software, Cary, NC, USA) and SPSS version 24.0 software (SPSS Inc., Chicago, IL, USA). A P-value <0.05 was considered to be statistically significant.
Results
Patient characteristics
There were 14,965 patients with esophageal carcinoma identified between 2006–2015 using data from the Surveillance, Epidemiology, and End Results (SEER) database. There were 10,872 patient deaths, including 9,621 patient deaths from esophageal carcinoma, and 1,251 deaths from other events. There were 4,093 patients who were excluded from the study, giving a censoring rate of 27.35%. The patients with esophageal carcinoma were aged between 18–103 years and had a mean age of 65.40 years. In terms of gender, the 12,166 male patients included 7,859 who died from esophageal carcinoma and 1,030 who died from competing events. Among the 2,799 female patients, 1,762 experienced esophageal carcinoma-specific mortality, and 221 died from competing events. The survival period among all patients ranged from 0–119 months, with a median of 10 months (Table 1).
Table 1.
Baseline demographic and clinical characteristics of patients with esophageal carcinoma and all events (n=14,965) between 2006–2015 using data from the Surveillance, Epidemiology, and End Results (SEER) database.
| Variable | All patients | Censored | Concerned | Competition |
|---|---|---|---|---|
| N | 14,965 | 4,093 | 9,621 | 1,251 |
| Age (years) | ||||
| ≤65 | 7,689 (51.4) | 2,281 (55.7) | 4,905 (51.0) | 503 (40.2) |
| >65 | 7,276 (48.6) | 1,812 (44.3) | 4,716 (49.0) | 748 (59.8) |
| Gender | ||||
| Male | 12,166 (81.3) | 3,277 (80.1) | 7,859 (81.7) | 1,030 (82.3) |
| Female | 2,799 (18.7) | 816 (19.9) | 1,762 (18.3) | 221 (17.7) |
| Race | ||||
| White | 12,872 (86.0) | 3,646 (89.1) | 8,160 (84.8) | 1,066 (85.2) |
| African-American | 1,381 (9.2) | 241 (5.9) | 1,007 (10.5) | 133 (10.6) |
| Other | 712 (4.8) | 206 (5.0) | 454 (4.7) | 52 (4.2) |
| Site of cancer | ||||
| Upper | 1,013 (6.8) | 256 (6.3) | 674 (7.0) | 83 (6.6) |
| Middle | 2,501 (16.7) | 596 (14.6) | 1,674 (17.4) | 231 (18.5) |
| Lower | 11,451 (76.5) | 3,241 (79.2) | 7,273 (75.6) | 937 (74.9) |
| Histology | ||||
| Squamous | 4,261 (28.5) | 1,008 (24.6) | 2,861 (29.7) | 392 (31.3) |
| Adenocarcinoma | 10,704 (71.5) | 3,085 (75.4) | 6,760 (70.3) | 859 (68.7) |
| Grade | ||||
| 1 | 915 (6.1) | 420 (10.3) | 395 (4.1) | 100 (8.0) |
| 2 | 6,534 (43.7) | 1,987 (48.5) | 3,950 (41.1) | 597 (47.7) |
| 3 and 4 | 7,516 (50.2) | 1,686 (41.2) | 5,276 (54.8) | 554 (44.3) |
| Stage | ||||
| I | 2,392 (16.0) | 1,100 (26.9) | 975 (10.1) | 317 (25.3) |
| II | 3,232 (21.6) | 1,251 (30.6) | 1,634 (17.0) | 347 (27.7) |
| III | 3,561 (23.8) | 1,041 (25.4) | 2,221 (23.1) | 299 (23.9) |
| IV | 5,780 (38.6) | 701 (17.1) | 4,791 (49.8) | 288 (23.0) |
| Surgery | ||||
| Yes | 4,804 (32.1) | 2,441 (59.6) | 1,929 (20.0) | 434 (34.7) |
| No | 10,161 (67.9) | 1,652 (40.4) | 7,692 (80.0) | 817 (65.3) |
| Radiotherapy | ||||
| Yes | 9,103 (60.8) | 2,652 (64.8) | 5,695 (59.2) | 756 (60.4) |
| No | 5,862 (39.2) | 1,441 (35.2) | 3,926 (40.8) | 495 (39.6) |
| Chemotherapy | ||||
| Yes | 10,130 (67.7) | 2,921 (71.4) | 6,421 (66.7) | 788 (63.0) |
| No | 4,835 (32.3) | 1,172 (28.6) | 3,200 (33.3) | 463 (37.0) |
| Year of diagnosis | ||||
| 2006–2010 | 7,395 (49.4) | 1,092 (26.7) | 5,520 (57.4) | 783 (62.6) |
| 2011–2015 | 7,570 (50.6) | 3,001 (73.3) | 4,101 (42.6) | 468 (37.4) |
Univariate analysis
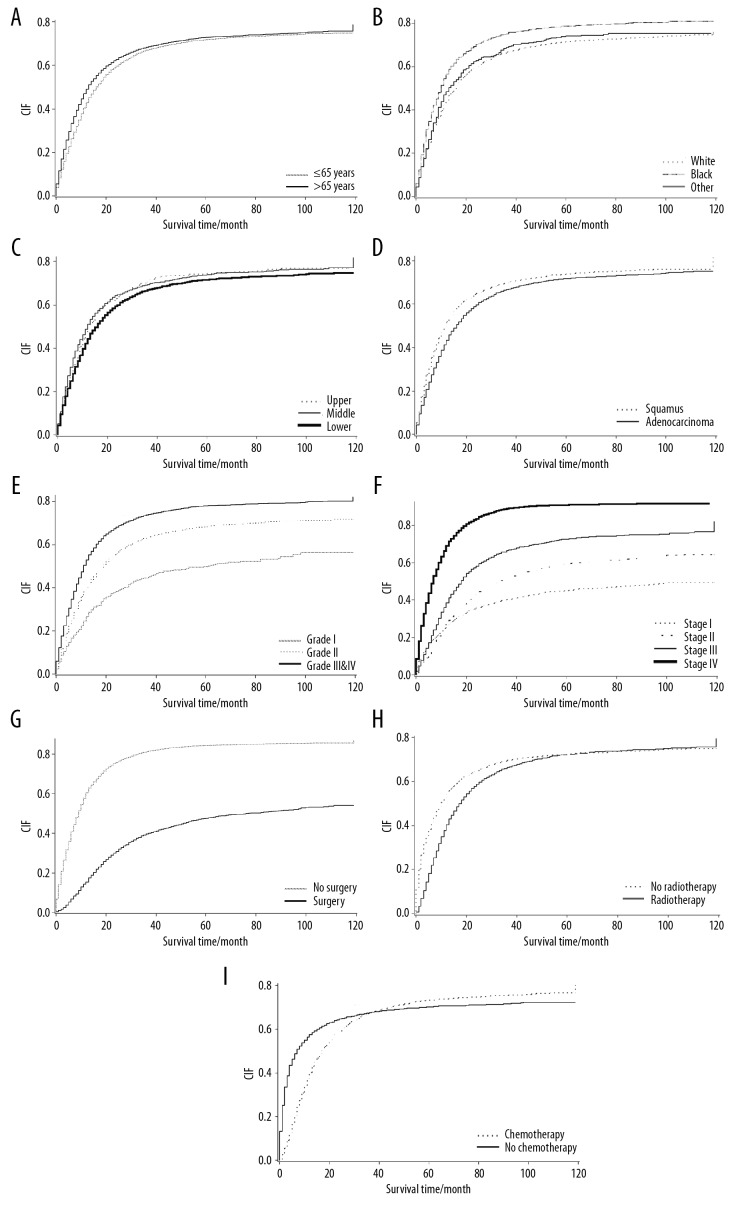
When the patients with esophageal carcinoma were divided into groups with different ages at diagnosis, the cumulative incidence of esophageal carcinoma-specific mortality increased with increasing follow-up time. When the risk of competition existed, the cumulative incidence rates of events of interest at one year, three years, and five years in the two groups of patients were 0.429, 0.669, and 0.718, and 0.490, 0.683, and 0.728, respectively (Table 2, Figure 2A). In patients with esophageal carcinoma at a different primary tumor site, the cumulative incidence rates of one-year mortality due to esophageal carcinoma-specific mortality in the upper, mid, and lower esophagus were 0.494, 0.511, and 0.444, respectively. The cumulative esophageal carcinoma-specific mortality rate during the follow-up period was the highest in the mid esophagus. The cumulative esophageal carcinoma-specific mortality rate beyond five years was highest in the upper esophagus and lowest in the lower esophagus, indicating the effects of the primary tumor site of esophageal carcinoma. The cumulative incidence rates of esophageal carcinoma-specific mortality were significantly different between the groups (Table 2, Figure 2C, 2D) (P<0.001). The competing risk (P=0.915) and rate of single endpoint events did not differ significantly with gender (P=0.612) (Table 2).
Table 2.
Univariate analysis of prognostic factors in patients with esophageal carcinoma between 2006–2015 using data from the Surveillance, Epidemiology, and End Results (SEER) database, including the cumulative incidence function (CIF) of events at 12 months, 36 months, and 60 months.
| Prognostic factors | CIF 12 months | CIF 36 months | CIF 60 months | P-value |
|---|---|---|---|---|
| Age (years) | <0.001•/<0.001** | |||
| ≤65 | 0.429*/0.465** | 0.669*/0.726** | 0.718*/0.789** | |
| >65 | 0.490*/0.549** | 0.683*/0.776** | 0.728*/0.842** | |
| Gender | 0.915*/0.612** | |||
| Male | 0.457*/0.505** | 0.678*/0.753** | 0.724*/0.816** | |
| Female | 0.462*/0.508** | 0.668*/0.739** | 0.716*/0.807** | |
| Race | <0.001*/<0.001** | |||
| White | 0.445*/0.492** | 0.667*/0.740** | 0.715*/0.806** | |
| African-American | 0.566*/0.623** | 0.752*/0.843** | 0.787*/0.891** | |
| Other | 0.484*/0.526** | 0.690*/0.761** | 0.742*/0.823** | |
| Site of cancer | <0.001*/<0.001** | |||
| Upper esophagus | 0.494*/0.536** | 0.707*/0.774** | 0.745*/0.837** | |
| Mid-esophagus | 0.511*/0.564** | 0.695*/0.776** | 0.740*/0.841** | |
| Lower esophagus | 0.444*/0.490** | 0.669*/0.743** | 0.717*/0.807** | |
| Histological type | <0.001*/<0.001** | |||
| Squamous cell carcinoma | 0.519*/0.570** | 0.698*/0.779** | 0.738*/0.839** | |
| Adenocarcinoma | 0.434*/0.480** | 0.667*/0.739** | 0.717*/0.805** | |
| Grade | <0.001*/<0.001** | |||
| 1 | 0.266*/0.316** | 0.447*/0.534** | 0.499*/0.626** | |
| 2 | 0.403*/0.452** | 0.634*/0.716** | 0.685*/0.787** | |
| 3 and 4 | 0.530*/0.575** | 0.740*/0.806** | 0.7820*/0.861** | |
| AJCC stage | <0.001*/<0.001** | |||
| I | 0.266*/0.327** | 0.403*/0.509** | 0.451*/0.591** | |
| II | 0.271*/0.318** | 0.516*/0.609** | 0.593*/0.712** | |
| III | 0.385*/0.432** | 0.664*/0.741** | 0.726*/0.821** | |
| IV | 0.690*/0.732** | 0.889*/0.939** | 0.909*/0.963** | |
| Surgery | <0.001*/<0.001** | |||
| Yes | 0.154*/0.186** | 0.396*/0.465** | 0.475*/0.572** | |
| No | 0.605*/0.659** | 0.811*/0.889** | 0.842*/0.932** | |
| Radiotherapy | <0.001*/<0.001** | |||
| Yes | 0.404*/0.446** | 0.663*/0.739** | 0.723*/0.819** | |
| No | 0.543*/0.598** | 0.696*/0.770** | 0.725*/0.811** | |
| Chemotherapy | <0.001*/<0.001K | |||
| Yes | 0.403*/0.442** | 0.676*/0.746** | 0.734*/0.823** | |
| No | 0.575*/0.640** | 0.678*/0.762** | 0.702*/0.800** |
M – month; CIF – cumulative incidence function; AJCC – American Joint Committee on Cancer.
The results of Gray test using the competing risk model;
The results of Kaplan-Meier analysis when comparing single events.
Figure 2.
Cumulative incidence function (CIF) curves for the characteristics of patients with esophageal carcinoma between 2006–2015 using data from the Surveillance, Epidemiology, and End Results (SEER) database. (A) Cumulative incidence functions for age. (B) Cumulative incidence functions for race. (C) Cumulative incidence functions for the site of cancer. (D) Cumulative incidence functions for histological type. (E) Cumulative incidence functions for the tumor grade. (F) Cumulative incidence functions for the American Joint Committee on Cancer (AJCC) stage. (G) Cumulative incidence functions for the primary site of surgery. (H) Cumulative incidence functions for radiotherapy. (I) Cumulative incidence functions for chemotherapy.
Multivariate analysis
The factors that were statistically significant in the univariate analysis (P<0.05) were introduced into the Cox regression model and the competing risk model for the multivariate analysis. Variables with a P>0.10 were culled. The Cox proportional hazards model results showed that the independent risk factors for the prognosis of patients with esophageal carcinoma were as follows: an age at diagnosis of >65 years (HR=1.137; 95% CI, 1.094–1.182; P<0.001), African-American ethnicity (HR=1.175; 95% CI, 1.098–1.257; P<0.001), a primary tumor site in the middle esophagus (HR=1.176, 95% CI, 1.080–1.280; P=0.0002), a primary tumor site in the lower esophagus (HR=1.145, 95% CI, 1.052–1.246; P=0.0018), histological grade 2 (HR=1.283; 95% CI, 1.169–1.409; P<0.001), histological grade 3 or 4 (HR=1.597; 95% CI, 1.455–1.753; P<0.001), American Joint Committee on Cancer (AJCC) stage II (HR=1.547; 95% CI, 1.436–1.666; P<0.001), AJCC stage III (HR=2.334; 95% CI, 2.169–2.511; P<0.001), AJCC stage IV (HR=3.151, 95% CI, 2.936–3.382; P<0.001), no surgery (HR=3.039; 95% CI, 2.881–3.206; P<0.001), no radiotherapy (HR=1.154; 95% CI, 1.104–1.207; P<0.001), and no chemotherapy (HR=2.227; 95% CI, 2.125–2.334; P<0.001).
In the Fine-Gray model, the independent risk factors for the prognosis of patients with esophageal carcinoma were African-American ethnicity (HR=1.097; 95% CI, 1.011–1.190; P=0.0255), histological grade 2 (HR=1.331; 95% CI, 1.201–1.474; P<0.001), histological grade 3 or 4 (HR=1.654; 95% CI, 1.493–1.833; P<0.001), AJCC stage II (HR=1.572; 95% CI, 1.442–1.713; P<0.001), AJCC stage III (HR=2.365; 95% CI, 2.168–2.579; P<0.001), AJCC stage IV (HR=3.335; 95% CI, 3.059–3.637; P<0.001), no surgery (HR=2.566; 95% CI, 2.428–2.712; P<0.001), no radiotherapy (HR=1.093; 95% CI, 1.040–1.149; P<0.0004), and no chemotherapy (HR=1.903; 95% CI, 1.797–2.015; P<0.001).
In the cause-specific hazard function (CS) model, the independent risk factors for the prognosis of patients with esophageal carcinoma were an age at diagnosis of >65 years (HR=1.088; 95% CI, 1.044–1.134; P<0.001), African-American ethnicity (HR=1.162; 95% CI, 1.081–1.249; P<0.001), primary tumor site in the mid-esophagus (HR=1.160; 95% CI, 1.060–1.270; P<0.0012), primary tumor site in the lower esophagus (HR=1.118; 95% CI, 1.022–1.223; P<0.0151), grade 2 (HR=1.361; 95% CI, 1.227–1.511; P<0.001), grade 3 or 4 (HR=1.756; 95% CI, 1.584–1.948; P<0.001), stage II (HR=1.704; 95% CI, 1.568–1.852; P<0.001), stage III (HR=2.756; 95% CI, 2.540–2.991; P<0.001), stage IV (HR=3.968; 95% CI, 3.667–4.294; P<0.001), no surgery (HR=3.168; 95% CI, 2.989–3.357; P<0.001), no radiotherapy (HR=1.166; 95% CI, 1.113–1.222; P<0.001), and no chemotherapy (HR=2.399; 95% CI, 2.284–2.520; P<0.001).
These findings indicated that the risk factors and stratification of prognostic factors differed significantly between the three models. The results of the analysis are presented in Table 3.
Table 3.
Multivariate analysis of the three analytical models used to identify prognostic factors in patients with esophageal carcinoma between 2006–2015 using data from the Surveillance, Epidemiology, and End Results (SEER) database.
| Prognostic factors | Cox model | Fine-Gray model | Cause-specific hazard function (CS) model | ||||||
|---|---|---|---|---|---|---|---|---|---|
| P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | |
| Age (years) | |||||||||
| ≤65 | 1 (reference) | ||||||||
| >65 | <0.001 | 1.137 | 1.094–1.182 | 0.069 | 1.042 | 0.997–1.089 | <0.001 | 1.088 | 1.044–1.134 |
| Race | |||||||||
| white | 1 (reference) | ||||||||
| African-American | <0.001 | 1.175 | 1.098–1.257 | 0.026 | 1.097 | 1.011–1.190 | <0.001 | 1.162 | 1.081–1.249 |
| Other | 0.065 | 0.917 | 0.837–1.005 | 0.217 | 0.937 | 0.846–1.039 | 0.120 | 0.926 | 0.841–1.020 |
| Site of cancer | |||||||||
| Upper esophagus | 1 (reference) | ||||||||
| Mid-esophagus | <0.001 | 1.176 | 1.080–1.280 | 0.095 | 1.087 | 0.986–1.199 | 0.001 | 1.160 | 1.060–1.270 |
| Lower esophagus | 0.002 | 1.145 | 1.052–1.246 | 0.340 | 1.048 | 0.952–1.154 | 0.015 | 1.118 | 1.022–1.223 |
| Histology | |||||||||
| Squamous | 1 (reference) | ||||||||
| Adenocarcinoma | 0.708 | 0.989 | 0.936–1.046 | 0.930 | 1.003 | 0.939–1.071 | 0.832 | 0.994 | 0.936–1.055 |
| Grade | |||||||||
| 1 | 1 (reference) | ||||||||
| 2 | <0.001 | 1.283 | 1.169–1.409 | <0.001 | 1.331 | 1.201–1.474 | <0.001 | 1.361 | 1.227–1.511 |
| 3 and 4 | <0.001 | 1.597 | 1.455–1.753 | <0.001 | 1.654 | 1.493–1.833 | <0.001 | 1.756 | 1.584–1.948 |
| Stage | |||||||||
| I | 1 (reference) | ||||||||
| II | <0.001 | 1.547 | 1.436–1.666 | <0.001 | 1.572 | 1.442–1.713 | <0.001 | 1.704 | 1.568–1.852 |
| III | <0.001 | 2.334 | 2.169–2.511 | <0.001 | 2.365 | 2.168–2.579 | <0.001 | 2.756 | 2.540–2.991 |
| IV | <0.001 | 3.151 | 2.936–3.382 | <0.001 | 3.335 | 3.059–3.637 | <0.001 | 3.968 | 3.667–4.294 |
| Surgery | |||||||||
| Yes | 1 (reference) | ||||||||
| No | <0.001 | 3.039 | 2.881–3.206 | <0.001 | 2.566 | 2.428–2.712 | <0.001 | 3.168 | 2.989–3.357 |
| Radiotherapy | |||||||||
| Yes | 1 (reference) | ||||||||
| No | <0.001 | 1.154 | 1.104–1.207 | <0.001 | 1.093 | 1.040–1.149 | <0.001 | 1.166 | 1.113–1.222 |
| Chemotherapy | |||||||||
| Yes | 1 (reference) | ||||||||
| No | <0.001 | 2.227 | 2.125–2.334 | <0.001 | 1.903 | 1.797–2.015 | <0.001 | 2.399 | 2.284–2.520 |
HR – hazard radio; CI – confidence interval; CS – cause-specific hazard function.
Discussion
Worldwide, esophageal carcinoma is a significant cause of morbidity and mortality, and the incidence in Western countries has recently risen [1]. Although the management and treatment of patients with esophageal carcinoma have improved, the overall five-year survival rate, of approximately 10%, and the five-year survival rate after surgical resection of between 15–40% are still poor [14]. The high morbidity and mortality rates in patients with esophageal carcinoma highlight the importance of accurate analysis of prognostic factors to improve the survival of patients with esophageal carcinoma. Previous survival analysis methods have only addressed specific outcomes. However, there are often multiple outcome events, each of which competes with the other events. Neglecting the existence of such competition will result in inaccurate calculations of the cumulative mortality when using the classic survival analysis methods, such as the single-factor Kaplan-Meier method [15], or incorrect estimations of the hazard ratio (HR) values when using multivariate Cox regression analysis.
The competing risk model is an analysis method for dealing with competing risk events. Currently, there are two main competing risk models, the cause-specific hazard function (CS) model and the Fine-Gray model. The CS model is suitable for etiological studies [16], and the Fine-Gray model is suitable for estimating disease risk and prognostic factors [17]. In the present study, using the Cox proportional hazards model and the CS model, age at diagnosis was an independent risk factor for prognosis of esophageal carcinoma (Table 3). Also, the cumulative incidence of esophageal carcinoma-specific mortality was higher in older age groups (Table 2). In contrast, the Fine-Gray model showed that age was not an independent risk factor for the prognosis of esophageal carcinoma (P=0.069). Previous studies have shown that age did not affect the survival rate of patients with esophageal carcinoma [18–20]. Wolbers et al. [21] showed that the Fine-Gray model was more suitable for the analysis of clinical prediction models. Therefore, when analyzing survival data, it is necessary to fully consider the possibility of competing risks being present, and then use an appropriate statistical model for the analysis to avoid drawing erroneous conclusions.
In terms of racial differences, our analysis of the three models showed that the cumulative esophageal carcinoma-specific mortality rate was higher in patients of African-American ethnicity when compared with Caucasians (Figure 2B), which may be associated with socioeconomic factors [22] and lower surgical rates [23]. The results are consistent with a previous study of racial differences in treatments for esophageal cancer [24], which showed that African-American patients with esophageal carcinoma had a worse prognosis.
In this study, in the multivariate analysis of the primary tumor site, the Cox proportional hazards model showed that locations in the mid esophagus (P<0.001) and the lower esophagus (P=0.002) were independent factors influencing the prognosis of patients with esophageal carcinoma. Similar results were obtained using the CS model (P=0.001 and P=0.015, respectively). In contrast, the Fine-Gray model indicated that locations in the mid esophagus (P=0.095) and the lower esophagus (P=0.340) were not independent factors affecting the prognosis of patients with esophageal carcinoma (Table 3). Univariate analysis of the primary tumor site showed no significant difference in the cumulative incidence of esophageal carcinoma-specific mortality at one-year, three-years, and five-years at each site (Table 2). The findings from a previously reported study showed that tumors located in the upper, middle, or lower esophagus were associated with a similar five-year survival rate in patients with esophageal carcinoma [25]. Previous studies have also shown that the tumor location does not affect survival [25,26]. These studies further confirmed that there were competing risks [25,26], indicating that multifactor analysis using the Fine-Gray model is necessary to obtain more reasonable and accurate conclusions.
However, all three models used in this study showed that histological grade (Figure 2E), American Joint Committee on Cancer (AJCC) stage (Figure 2F), and surgery (Figure 2G), radiotherapy (Figure 2H), and chemotherapy status (Figure 2I) were independent factors that influenced the prognosis of patients with esophageal carcinoma. The cumulative incidence for patients with early-stage tumors and esophageal carcinoma-specific mortality was lower for those who received chemotherapy. However, the reverse was true for the long-term cumulative incidence (Figure 2I), which may be related to the hematological toxicity of chemotherapy drugs. Hematologic toxicity in chemotherapy may be the reason for the increased incidence of long-term cumulative esophageal carcinoma-specific mortality rates in chemotherapy patients [27].
The main advantage of the competing risk model was that it directly established the dependency relationship between the incidence of esophageal carcinoma and the covariates, which enabled a better and more intuitive explanation of the covariate effect. The competing risk model realized the standardization of distribution functions of different types of competing risks and avoided overestimating the incidence of outcomes concerned when the impact of competing risks was significant.
This study had several limitations. This study had a retrospective design, and bias in the data selection may have been present. The Surveillance, Epidemiology and End Results (SEER) database lacks descriptions of the specific dose of radiotherapy, and the chemotherapy record does not specify the chemotherapy regimen. Also, the SEER database is a US database, and the findings might not be applicable to other countries and populations.
Conclusions
This study aimed to undertake a competing risk analysis of prognosis in patients with esophageal carcinoma between 2006–2015 using data from the Surveillance, Epidemiology, and End Results (SEER) database. Two survival analysis methods were used to compare the P-value, the cumulative incidence function (CIF), and the cause-specific hazard function (CS) to generalize the hazard function for competing risks. The findings showed that the tumor grade, the American Joint Committee on Cancer (AJCC) stage, and surgery, radiotherapy, and chemotherapy status were independent prognostic factors for patients with esophageal carcinoma. Although the retrospective analysis of data from the SEER database has some limitations, the findings provide useful clinical information. The study also showed that when competing clinical risks are present, prognostic factors should be analyzed using a competing risk model to calculate the CIF of prognostic factors. Also, the use of the Fine-Gray model may obtain more reliable and clinically applicable results.
Acknowledgments
The authors thank the staff of the Surveillance, Epidemiology, and End Results (SEER) program for providing open access to the SEER database.
Footnotes
Source of support: Departmental sources
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. 2013;381:400–12. doi: 10.1016/S0140-6736(12)60643-6. [DOI] [PubMed] [Google Scholar]
- 3.Renehan AG, Tyson M, Egger M, et al. Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–78. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 4.Whiteman DC, Sadeghi S, Pandeya N, et al. Combined effects of obesity, acid reflux and smoking on the risk of adenocarcinomas of the oesophagus. Gut. 2008;57:173–80. doi: 10.1136/gut.2007.131375. [DOI] [PubMed] [Google Scholar]
- 5.Lagergren J, Lagergren P. Recent developments in esophageal adenocarcinoma. Cancer J Clin. 2013;63(4):232–48. doi: 10.3322/caac.21185. [DOI] [PubMed] [Google Scholar]
- 6.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. Journal of American Statistical Association. 1958;53:457–81. [Google Scholar]
- 7.Cox DR. Regression models and life-tables. J R Stat Soc Series B Stat Methodol. 1972;34:187–220. [Google Scholar]
- 8.Huque MF, Sankoh AJ. A reviewer’s perspective on multiple endpoint issues in clinical trials. J Biopharm Stat. 1997;7:545–64. doi: 10.1080/10543409708835206. [DOI] [PubMed] [Google Scholar]
- 9.Dignam JJ, Kocherginsky MN. Choice and interpretation of statistical tests used when competing risks are present. J Clin Oncol. 2008;26:4027–34. doi: 10.1200/JCO.2007.12.9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amin RN, Parikh SJ, Gangireddy VG, et al. Early esophageal cancer specific survival is unaffected by anatomical location of tumor: A population-based study. Can J Gastroenterol Hepatol. 2016;2016 doi: 10.1155/2016/6132640. 6132640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gray RJ. A Class of K-Sample Tests for comparing the cumulative incidence of a competing risk. Ann Statist. 1988;16:1141–54. [Google Scholar]
- 12.Fine JP, Gray RJ. A Proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 13.Fiocco M, Putter H, Van Houwelingen JC. Reduced rank proportional hazards model for competing risks. Biostatistics. 2005;6:465–78. doi: 10.1093/biostatistics/kxi022. [DOI] [PubMed] [Google Scholar]
- 14.Huang FL, Yu SJ. Esophageal cancer: Risk factors, genetic association, and treatment. Asian J Surg. 2018;41:210–15. doi: 10.1016/j.asjsur.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Varadhan R, Weiss CO, Segal JB, et al. Evaluating health outcomes in the presence of competing risks: A review of statistical methods and clinical applications. Medical Care. 2010;48:S96–105. doi: 10.1097/MLR.0b013e3181d99107. [DOI] [PubMed] [Google Scholar]
- 16.Lau B, Cole SR, Gange SJ. Competing risk regression models for epidemiologic data. Am J Epidemiol. 2009;170:244–56. doi: 10.1093/aje/kwp107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koller MT, Raatz H, Steyerberg EW, Wolbers M. Competing risks and the clinical community: irrelevance or ignorance? Stat Med. 2012;31:1089–97. doi: 10.1002/sim.4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Markar SR, Karthikesalingam A, Low DE. Outcomes assessment of the surgical management of esophageal cancer in younger and older patients. Ann Thorac Surg. 2012;94:1652–58. doi: 10.1016/j.athoracsur.2012.06.067. [DOI] [PubMed] [Google Scholar]
- 19.Mori M, Ohno S, Tsutsui S, et al. Esophageal carcinoma in young patients. Ann Thorac Surg. 1990;49:284–86. doi: 10.1016/0003-4975(90)90152-v. [DOI] [PubMed] [Google Scholar]
- 20.Kasagi Y, Morita M, Otsu H, et al. Clinicopathological characteristics of esophageal squamous cell carcinoma in patients younger than 50 years. Ann Surg Oncol. 2015;22:311–15. doi: 10.1245/s10434-014-3856-6. [DOI] [PubMed] [Google Scholar]
- 21.Wolbers M, Koller MT, Witteman JC, Steyerberg EW. Prognostic models with competing risks: Methods and application to coronary risk prediction. Epidemiology. 2009;20:555–61. doi: 10.1097/EDE.0b013e3181a39056. [DOI] [PubMed] [Google Scholar]
- 22.Morris AM, Rhoads KF, Stain SC, Birkmeyer JD. Understanding racial disparities in cancer treatment and outcomes. J Am Coll Surg. 2010;211:105–13. doi: 10.1016/j.jamcollsurg.2010.02.051. [DOI] [PubMed] [Google Scholar]
- 23.Bach PB, Cramer LD, Warren JL, Begg CB. Racial differences in the treatment of early-stage lung cancer. N Engl J Med. 1999;341:1198–205. doi: 10.1056/NEJM199910143411606. [DOI] [PubMed] [Google Scholar]
- 24.Greenstein AJ, Litle VR, Swanson SJ, et al. Racial disparities in esophageal cancer treatment and outcomes. Ann Surg Oncol. 2008;15:881–88. doi: 10.1245/s10434-007-9664-5. [DOI] [PubMed] [Google Scholar]
- 25.Otterstatter MC, Brierley JD, De P, et al. Esophageal cancer in Canada: Trends according to morphology and anatomical location. Can J Gastroenterol. 2012;26:723–27. doi: 10.1155/2012/649108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doki Y, Ishikawa O, Takachi K, et al. Association of the primary tumor location with the site of tumor recurrence after curative resection of thoracic esophageal carcinoma. World J Surg. 2005;29:700–7. doi: 10.1007/s00268-005-7596-4. [DOI] [PubMed] [Google Scholar]
- 27.Liou SY, Stephens JM, Carpiuc KT, et al. Economic burden of haematological adverse effects in cancer patients: A systematic review. Clin Drug Investig. 2007;27(6):381–96. doi: 10.2165/00044011-200727060-00002. [DOI] [PubMed] [Google Scholar]