Abstract
It is generally thought that muscle excitability is almost exclusively controlled by currents responsible for generation of action potentials. We propose that smaller ion channel currents that contribute to setting the resting potential and to subthreshold fluctuations in membrane potential can also modulate excitability in important ways. These channels open at voltages more negative than action potential threshold and are thus termed subthreshold currents. As subthreshold currents are orders of magnitude smaller than the currents responsible for the action potential, they are hard to identify and easily overlooked. Discovery of their importance in regulation of excitability opens new avenues for improved therapy for muscle channelopathies and diseases of the neuromuscular junction.
Keywords: myotonia, periodic paralysis, action potential, persistent inward current, sodium channel, chloride channel, muscle contraction, neuromuscular junction, endplate
The inherited muscle channelopathies are a family of disorders arising from mutations of ion channels that are critical for regulation of muscle excitability. The primary symptoms of muscle channelopathies are myotonia and attacks of weakness. In the channelopathies with myotonia, delayed relaxation of muscle is a prominent symptom. Myotonia is caused by either downregulation/loss-of-function of the muscle chloride channel (ClC-1)1–4 or gain-of-function mutations of the muscle Na channel5, 6. The primary approach to treating myotonic channelopathies is block of Na+ channels using drugs such as mexiletine7–9. However, many patients suffer side effects or incomplete symptom resolution; thus there is a need for improved therapy.
The periodic paralyses are a subgroup of muscle channelopathies caused by gain-of-function mutations of Na+ and Ca2+ channels or by loss-of-function mutations in K+ channels such as Kir 2.1 (KCNJ2)5, 6, 10–13. Attacks of weakness are triggered by changes in extracellular K+, either elevations (hyperkalemic periodic paralysis) or reductions (hypokalemic periodic paralysis)8, 14–17. Recently, a carbonic anhydrase inhibitor, dichlorphenamide, was approved by the FDA for treatment of both hypo- and hyperkalemic periodic paralysis17–19. The mechanism whereby inhibition of carbonic anhydrase improves weakness in periodic paralysis remains incompletely understood17, and the benefits appear to be greater in hypo- vs. hyperkalemic periodic paralysis17, 18.
While the mutations responsible for muscle channelopathies have largely been identified, a complete understanding of how these mutations cause dysregulation of muscle excitability remains elusive. As some mechanisms remain unknown, identification of these mutations has not led to significant advances in therapy. One difficulty is that an effective therapy for a muscle excitability disorder must preserve normal muscle function. However, because the mutated channels are still a necessary element of voluntary movement, they are difficult to target without affecting normal movement. To develop optimal therapies, it will be necessary to identify events unique to pathologic muscle excitation so that these mechanisms can be targeted. Do such events exist?
Normal muscle activation
To identify events unique to pathologic muscle excitation, it is necessary to understand all of the steps involved in normal muscle excitation. Normal activation of skeletal muscle is initiated at the neuromuscular junction. An action potential initiated in the motor neuron leads to release of acetylcholine from the nerve terminal at the neuromuscular junction, which activates acetylcholine receptors on muscle to cause depolarization20, 21. The depolarization of muscle due to opening of acetylcholine receptors is known as the endplate potential and each endplate potential triggers a single muscle action potential22, 23. Depolarization during muscle action potentials is driven by opening of voltage-activated Nav1.4 channels, and subsequent repolarization is driven by the voltage-activated K+ channels14, 15. Muscle action potentials propagate along the surface membrane (sarcolemma) and into the transverse tubules (t-tubules), which are invaginations of the surface membrane24 into the center of the muscle fiber. Action potential propagation into the t-tubules triggers release of Ca2+ from the intracellular sarcoplasmic reticulum compartment to trigger muscle contraction via a process known as excitation-contraction coupling25–27.
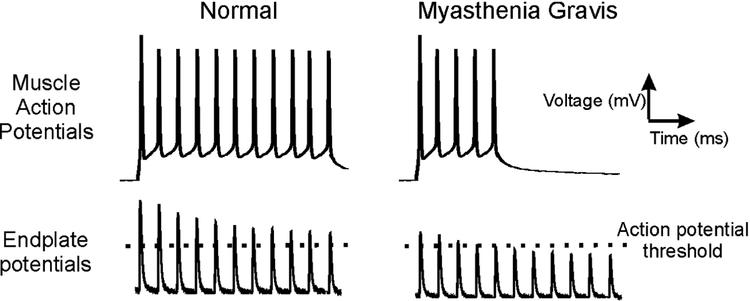
A key concept to understanding neuromuscular transmission is safety factor22, 23. One definition of safety factor is the difference between the peak of the endplate potential and the voltage at which a muscle fiber action potential is triggered (action potential threshold). Having an adequate safety factor is essential for maintaining neuromuscular transmission during the repetitive activation of muscle that is necessary for normal voluntary movement (Fig 1).
Figure 1:
Neuromuscular transmission in healthy and myasthenia gravis muscle. In normal muscle, the endplate potentials that trigger muscle action potentials go beyond the muscle fiber action potential threshold. This extra depolarization is known as the safety factor. During repetitive firing of the nerve, the endplate potential amplitude undergoes a normal decline in amplitude such that the safety factor decreases. However, as long as the endplate potential triggers sufficient depolarization to reach action potential threshold, there is no failure of neuromuscular transmission. On the right, myasthenia gravis is shown, in which the number of acetylcholine receptors is reduced. The safety factor is reduced such that, by the sixth endplate potential, it is insufficient to reach action potential threshold to trigger a muscle action potential. This failure of neuromuscular transmission is responsible for weakness. Note, all traces were generated using records from mouse muscle fibers. Since suprathreshold endplate potentials and action potentials cannot be recorded at the same time, the recordings in the top and bottom panels were obtained from different fibers. Action potentials in the traces were triggered by sustained injection of current rather than repeated brief depolarization as occurs during voluntary activation of muscle. The recordings in the right column were obtained from healthy wild type muscle and edited to mimic the changes seen in myasthenia gravis. For example, the endplate potential traces (which were generated from endplate current recordings) in the right column are the same as those in the left column but were scaled down 30% and the action potential stimulation was cut short to mimic failure of neuromuscular transmission.
Weakness can occur when the safety factor is reduced by either reducing the endplate potential or by raising action potential threshold. Endplate potential amplitude can be reduced by either decreasing release of acetylcholine, as occurs in Lambert-Eaton myasthenic syndrome, or by reducing the number of available acetylcholine receptors, as occurs in myasthenia gravis28–32 (Fig 1). If, however, endplate potentials are normal, but threshold becomes too elevated, endplate potentials will not reach threshold and neuromuscular transmission fails in a manner similar to that seen with diseases of the neuromuscular junction33, 34. To understand how threshold can be elevated, it is necessary to understand the determinants of threshold. At the action potential threshold, depolarizing current is equal and opposite to hyperpolarizing current35, 36. Elevation of threshold occurs if depolarizing currents are decreased or hyperpolarizing currents are increased, as either changes the voltage at which the currents are equal and opposite.
Pathologic depolarization in muscle in channelopathies.
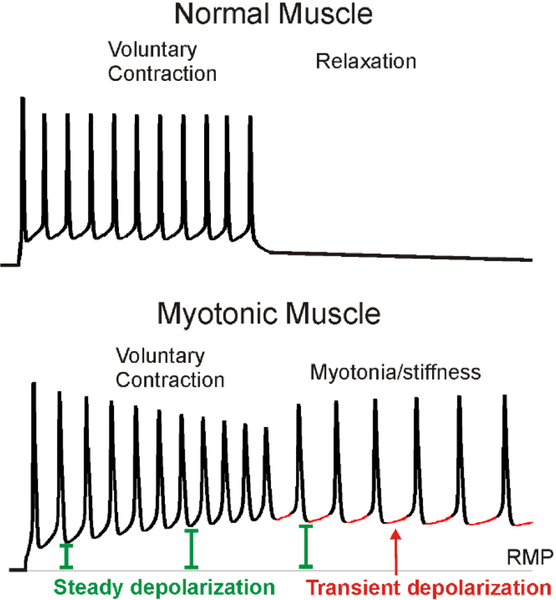
As mentioned above, identification of the ion channel mutations responsible for muscle channelopathies has not led to significant advances in therapy. The reason for this is an incomplete understanding of the mechanisms responsible for pathologic depolarization. As an example we will consider in detail how loss-of-function mutations in Cl− channels causes myotonia (involuntary firing of muscle action potentials). There are both a steady and a transient depolarization that combine to trigger involuntary firing of muscle action potentials in myotonia37 (Fig 2). One contributor to steady depolarization is build-up of K in the t-tubules, which shifts the Nernst potential for K+ to cause depolarization of the resting membrane potential38–41. Normally, ClC-1-mediated Cl− current, which accounts for 70%−80% of resting muscle membrane conductance, offsets the depolarizing influence of K+ accumulation and thus lessens the steady depolarization2, 27, 38, 42, 43. Whether K+ build-up is the sole cause of the steady depolarization remains unknown. The transient depolarization, however, cannot be due to K+ build-up: It occurs during a time when no action potentials are being fired and therefore there is no efflux of K+ from the fiber into the t-tubules.
Figure 2:
Two contributors to the depolarization that triggers myotonia. On top is an intracellular recording of action potentials from a normal mouse skeletal muscle fiber. In normal muscle, as soon as voluntary firing of muscle action potentials stops, muscle hyperpolarizes and relaxes. On the bottom are action potentials from a myotonic mouse muscle. Unlike normal muscle, there is continued firing of action potentials following cessation of voluntary firing. The cause of involuntary firing is a combination of a steadily increasing depolarization (green) such that the membrane potential does not return to the resting membrane potential (RMP, indicated by a thin gray line) between action potentials, and a transient depolarization (red) which occurs prior to each myotonic action potential.
We recently determined that a Na+ persistent inward current (NaPIC) is an important contributor to the repetitive firing occurring during myotonia37. NaPIC is present in normal skeletal muscle and lacks the fast inactivation which is the hallmark of the Na+ channels that are responsible for triggering action potentials44. In muscle, NaPIC is sensitive to low doses of tetrodotoxin, suggesting it is carried by Nav1.4 channels37. It is likely that muscle NaPIC derives from a small subset of Nav1.4 channels that are in a different conformation from fast-inactivating Nav1.4 channels. This understanding is based on recordings from frog skeletal muscle, in which single Na+ channels shifted modes between a normal, fast-inactivating mode and a mode lacking fast inactivation45. For clarity as we move forward, we will term the Na+ channels responsible for action potentials “fast-inactivating Na+ channels” as they stay open for no more than a few milliseconds before inactivating. This is in contrast to channels in the NaPIC mode, which can stay open for seconds without inactivating.
The role of channels in the NaPIC mode in regulating excitability has primarily been studied in neurons. It is well established that channels in the NaPIC mode help maintain repetitive firing of neurons in response to sustained depolarization46, 47. Neurons have many weak synapses that are activated asynchronously. Thus, neurons experience steady depolarization from asynchronous activation of their many inputs, which is converted to repetitive firing. The greater the depolarization of the neuron, the greater the firing rate. This is known as the F-I relationship – F represents firing frequency and I represents injected current. In lower motor neurons, this relationship is one of the primary means of muscle force regulation47.
The F-I relationship in motor neurons is governed by currents that are active at membrane potentials more negative than action potential threshold (subthreshold currents). These currents consist of both non-voltage gated (leak) currents and voltage-gated currents which activate at potentials negative to action potential threshold48, 49. In neurons, subthreshold currents include depolarizing currents carried by channels in the NaPIC mode and Ca2+ channels, as well as hyperpolarizing currents carried by K+ channels46, 47. The ratio of depolarizing to hyperpolarizing current determines whether the neuron will reach threshold and fire. When the ratio of depolarizing to hyperpolarizing subthreshold current is high, there is rapid depolarization toward action potential threshold and a high F-I gain (a high firing rate for a given current injection). When the depolarizing to hyperpolarizing subthreshold current ratio is low, the F-I gain is low and, in the extreme case, neurons can fire a single action potential, but cannot fire repetitively49. Manipulation of NaPIC in neurons has a dramatic effect on the F-I relationship and ability of neurons to fire repetitively49.
One factor that may affect the percent of channels in the NaPIC mode present in neurons is the expression level of Nav1.6. Nav1.6, the primary voltage gated Na channel at nodes of Ranvier, is also present at the axon initial segment, and produces a relatively large NaPIC50, 51. The NaPIC carried by Nav1.6 at Nodes of Ranvier may play an important role in sustaining normal repetitive firing of peripheral nerve and could contribute to pathologic repetitive firing and/or degeneration of peripheral nerve51, 52.
If NaPIC has a similar function in triggering repetitive firing in muscle, it could play a central role in triggering myotonia. How does it do this? Part of the answer is that the “persistent” part of NaPIC’s name can be misleading. Channels in the NaPIC mode have a more negative voltage dependence of activation relative to fast-inactivating Na channels44. Thus, they open at voltages negative to action potential threshold and bring the fiber to threshold such that an action potential is triggered. The channels then close during repolarization following the action potential. Therefore, during myotonia, NaPIC is not a persistent current, but is instead a transient current that contributes to the transient depolarization that triggers each action potential. This is not to say that NaPIC never contributes to steady depolarization. Nav1.4 mutations that cause hyperkalemic periodic paralysis increase the percent of channels in the NaPIC mode and cause both myotonia and attacks of weakness17, 53, 54. Our interpretation is that, in affected patients, when the membrane potential is relatively negative between attacks, there is only a slight increase in the depolarizing to hyperpolarizing subthreshold current ratio. In this setting, this increase causes myotonia due to transient activation of the NaPIC current as described above. As K+ rises during an attack, the resting membrane potential depolarizes continuously such that channels in the NaPIC mode become continuously activated (truly persistent). This causes a large increase in the ratio of depolarizing to hyperpolarizing subthreshold current, which causes inactivation of fast-inactivating Na+ channels, electrical inexcitability, and weakness. Thus, depending on the membrane potential, NaPIC contributes to either transient or steady depolarization that causes either myotonia or weakness.
The ion channels responsible for subthreshold currents in muscle
Subthreshold currents are active at voltages more negative than action potential threshold. The balance of inward and outward subthreshold currents involves both a) ion channels that are open at rest and contribute to regulation of resting potential and b) ion channels not open at rest, which activate in the voltage range between resting potential and action potential threshold. This second type of ion channel can have profound effects on repetitive firing without having any effect on resting potential or properties of single action potentials37, 55. While subthreshold currents may be small, they can determine whether an action potential fires, and thus play a major role in regulation of muscle excitability.
We propose that disorders of muscle excitability are caused by an elevation of the depolarizing to hyperpolarizing subthreshold current ratio. This framework could explain why mutations in both Na+ channels and Cl− channels can trigger myotonia. Either increasing subthreshold depolarizing current (due to increases in NaPIC caused by mutation of Na channels53, 54) or decreasing subthreshold hyperpolarizing current (due to loss-of-function mutations of ClC-1 Cl− channels2) will increase the depolarizing-to-hyperpolarizing subthreshold current ratio.
The realization that subthreshold currents are important regulators of excitability makes it desirable to identify all channels that are responsible for subthreshold currents. There are few channels that have been well characterized in skeletal muscle that could carry depolarizing subthreshold currents. As Cav1.1 channels primarily open at membrane potentials more depolarized than action potential threshold, they likely do not contribute significantly to depolarizing subthreshold currents. This leaves Nav1.4 channels as the only well characterized candidate. While fast-inactivating Nav1.4 channels open at potentials more negative than threshold, they do not stay open long enough to fully account for the transient depolarization occurring over 20 to 50 ms that triggers myotonia. Nav1.4 channels in the NaPIC mode, activate significantly at membrane potentials more negative than action potential threshold37, 44 and remain open long enough to account for the slow depolarization to threshold.
Hyperpolarizing subthreshold currents are carried by Cl− and K+ channels. Understanding the role of ClC-1 chloride channels in regulation of muscle excitability is complicated by the fact that the Cl− reversal potential can be either more depolarized or more hyperpolarized than the resting membrane potential. Normally, the Cl− reversal potential is slightly more depolarized than the resting potential56, 57. This might make one think that ClC-1 channels function to increase excitability. However, during periods of depolarization, such as at the end of action potentials and after K build-up in t-tubules, ClC-1 chloride channels function to hyperpolarize muscle as the Cl- reversal potential is more negative than the depolarized membrane potential8, 58. In this situation, ClC-1 channels are the biggest contributor to hyperpolarizing subthreshold currents because they are responsible for 70–80% of resting membrane conductance42, 59. Unfortunately, opening ClC-1 channels is not generally a viable option for treatment of myotonia congenita as the disorder is due to loss of function mutations of ClC-1 channels.
Opening K+ channels is an attractive approach to treatment of both myotonia and hypokalemic periodic paralysis as the channels are present, and in both situations the K+ reversal potential is negative relative to the membrane potential. A number of types of K+ channels are present in skeletal muscle. Kir channels are open at the resting membrane potential and are likely carry the largest hyperpolarizing subthreshold current other than ClC-160, 61. KCNQ type (Kv7) K+ channels might also contribute to hyperpolarizing subthreshold currents. We and others have found that retigabine, an activator of Kv7 channels, can lessen myotonia55, 62. Retigabine has no effect on the number of K+ channels open at rest, nor does it have any effect on excitability that can be identified by measuring properties of single action potentials55, 62. Instead it appears likely that, by causing a hyperpolarized shift in the voltage dependence of Kv7 channel activation63, 64, retigabine leads to activation of Kv7 channels only when the membrane potential is relatively depolarized during runs of myotonia. Unfortunately, retigabine is no longer FDA-approved. Despite its effectiveness in vitro, it had no beneficial effect on motor function of mice55. One potential explanation for the lack of motor improvement is the finding that retigabine has significant CNS side effects that contribute to motor dysfunction65. If openers of Kv7 channels that do not cross the blood brain barrier are identified, they might provide effective therapy for myotonia without the CNS side effects.
Another class of K+ channels expressed in skeletal muscle is KATP channels66, 67. These channels are thought to play a role in regulation of excitability during repetitive firing and are opened by drugs such as cromakalim or pinacidil68–70. It has been shown that treatment with cromakalim greatly improves the contractile force of muscle fibers from patients with hypokalemic periodic paralysis71. Unfortunately, treatment of patients by activating KATP channels may be limited by side effects such as hypoglycemia72. Finally, Ca-activated, large conductance (BK) K+ channels are expressed in skeletal muscle73, 74. An opener of BK channels has been developed and found to be safe for use in humans75 such that targeting these channels might be an option.
It seems unlikely that all subthreshold currents have been identified. A number of members of the transient receptor potential (TRP) ion channel family are also expressed in skeletal muscle76, 77. TRP channels depolarize neurons when activated and could also be important contributors to depolarizing subthreshold currents. TRP channels are opened by a number of different stimuli, including voltage, Ca2+, pH, muscle stretch, and temperature changes78, which may help explain phenomena such as stretch- or cold-induced myotonia. Another channel type that could contribute to involuntary firing are hyperpolarization-activated cyclic-nucleotide-gated (HCN) channels. HCN channels are responsible for the rhythmic depolarization in cardiac pacemaker cells and have been implicated in diseases of excitability such as epilepsy and pain79, 80. Low levels of RNA encoding for various HCN channels have been found in skeletal muscle81.
A novel approach to development of therapy for disorders of muscle excitability
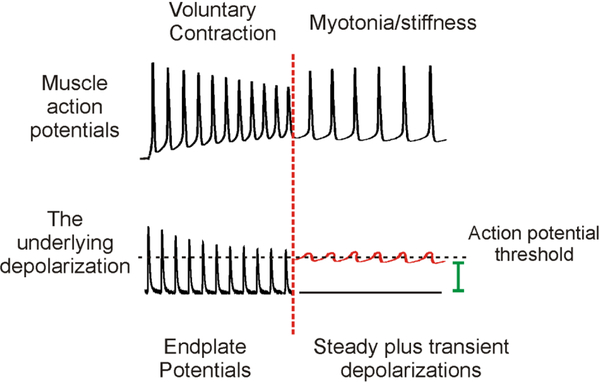
Identification of the role of subthreshold currents in repetitive firing has led us to hypothesize that the currents which trigger repetitive firing of voluntary action potentials are not the same currents which trigger involuntary myotonic action potentials. Voluntary repetitive firing of muscle fibers is triggered by repetitive depolarization due to opening of acetylcholine receptors at the neuromuscular junction, as described in Fig 1. At the point when voluntary contraction ends and myotonia begins, the source of depolarization triggering action potentials switches from opening of acetylcholine receptors to the combination of subthreshold currents responsible for steady and transient depolarization (Fig 3).
Figure 3:
Distinct currents trigger repetitive firing during voluntary contraction versus myotonia. The top trace shows the muscle fiber’s action potentials during voluntary firing vs. myotonia. A vertical, dotted red line indicates the time at which voluntary firing ceases and myotonia begins. The bottom trace shows the depolarization type responsible for the repetitive firing of muscle action potentials. During voluntary contraction, repeated firing of the motor neuron triggers repeated endplate potentials, each of which triggers a muscle action potential. When voluntary contraction ends, motor neuron firing stops, as do endplate potentials. At this time, there is a switch in the type of current responsible for firing the muscle action potential, from endplate potentials to a combination of steady (green) and transient (red) depolarizations. The subthreshold oscillations in membrane potential in the bottom right of the figure were generated from a real recording of myotonia by erasing the part of the trace more depolarized than action potential threshold and drawing the missing part of the oscillation by hand.
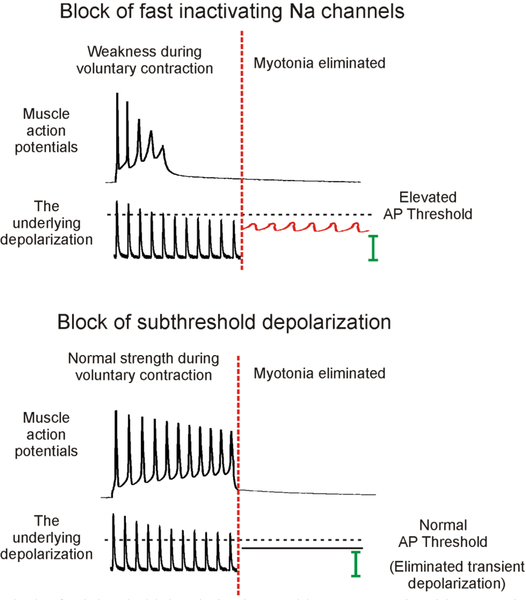
The idea that different currents are responsible for voluntary versus myotonic action potentials changes the way one approaches therapy. The present approach to therapy focuses on blocking the fast-inactivating Na+ channels responsible for generation of action potentials. This raises action potential threshold to make it harder to fire, which can lead to weakness (Fig 4). In fact, elevation of action potential threshold is the cause of neuromuscular transmission failure in patients with congenital myasthenia gravis due to loss-of-function mutations of Nav1.4 Na+ channels33, 34. However, focusing treatment on subthreshold currents may leave action potential threshold unaltered such that voluntary contraction is unaffected (Fig 4).
Figure 4:
Block of subthreshold depolarization could treat myotonia without causing the weakness that can accompany block of fast-inactivating Na channels. The top trace shows the effects of treating myotonia by raising action potential threshold via block of fast-inactivating Na channels with mexiletine. Note that the action potentials become steadily smaller, due to reduced muscle excitability, until they fail. This is in contrast to the muscle action potentials in myasthenia gravis depicted in Fig. 1, which are normal; then suddenly disappear when the endplate potentials fail to reach threshold. While block of fast-inactivating Na+ channels can effectively eliminate myotonia by raising threshold above the voltage reached by subthreshold depolarizations, it runs the risk of causing failure of neuromuscular transmission, such that there is weakness. In the lower trace, myotonia is eliminated by blocking transient subthreshold depolarization. Alternately, if the steady subthreshold depolarization could be blocked it would also be possible to eliminate myotonia. As subthreshold currents play little to no role in setting action potential threshold, activation of muscle during voluntary contraction is unaffected such that there is no weakness. AP = action potential.
While block of subthreshold depolarization is theoretically attractive as a treatment option, we do not yet have the right pharmacologic tools. Blocking NaPIC should have profound effects on the balance of subthreshold currents while having little to no effect on action potential threshold. Unfortunately, there is currently no drug that is specific for NaPIC. Available drugs that block NaPIC, such as riluzole and ranolazine, may be partially effective as therapy55 but as they also partially block the fast inactivating Na channels responsible for generating action potentials47, 53, 82 they are not optimal.
Another therapeutic approach would be to increase subthreshold hyperpolarizing currents by activating K+ currents. As mentioned above, this appears to be the mechanism of action of retigabine. Perhaps another activator of Kv7 channels can be identified that does not cross the blood brain barrier. Such a drug might effectively treat myotonia without CNS side effects or detrimental effects on normal muscle excitation.
Subthreshold currents may contribute to neuromuscular transmission
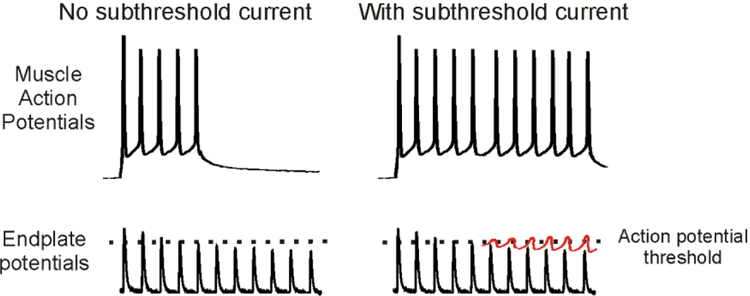
We do not currently know the normal function of subthreshold currents in skeletal muscle. Given that the subthreshold currents identified to date are voltage-activated and tend to induce depolarization, it is possible that they contribute to fidelity of neuromuscular transmission. As described above, the endplate potential normally decrements during repetitive stimulation. This is especially true at high rates of stimulation. Our studies of NaPIC37 suggest it is activated during high frequency firing, such that it may boost the depolarization caused by endplate potentials that would otherwise be subthreshold (Fig 5).
Figure 5:
A potential role of subthreshold currents in neuromuscular transmission. Shown on the left is failure of neuromuscular transmission occurring in a situation, such as myasthenia gravis, in which the endplate potential is not large enough to reach action potential threshold. In the absence of a subthreshold current, depression of the endplate potential causes failure of neuromuscular transmission. On the right is shown how gradual activation of a subthreshold current (shown in red) during repetitive firing of muscle might counteract depression of the endplate potential to maintain neuromuscular transmission.
It was recently discovered that there is failure of neuromuscular transmission in aged mice that worsens at high rates of stimulation (William Arnold, personal communication). Measures of presynaptic and postsynaptic function at the neuromuscular junction are normal, suggesting failure of transmission is due to a problem with muscle fiber excitability. Further studies of muscle fiber excitability will be needed to determine the mechanism underlying failure of transmission, but preliminary data favor the possibility that a reduction in subthreshold current contributes.
Summary
The realization that subthreshold currents play a central role in dysregulation of excitability in the muscle channelopathies leads to a new way of thinking about disorders of muscle excitability. Identification of new subthreshold currents paves the way for identification of other channel types to target. This will allow for development of novel therapies that may be more effective than current options.
Acknowledgements:
We would like to thank William Arnold for helpful comments and Sonja Kraner of Alpha Writing Resource for editorial assistance. This work was supported by NIH grants AR074985 (M.M.R.), NS099850 (A.A.V.) and MDA grant 602459 (M.M.R.).
Footnotes
Potential conflicts of Interest: Nothing to report.
References:
- 1.Koch MC, Steinmeyer K, Lorenz C et al. The skeletal muscle chloride channel in dominant and recessive human myotonia. Science. 1992;257:797–800 [DOI] [PubMed] [Google Scholar]
- 2.Steinmeyer K, Klocke R, Ortland C et al. Inactivation of muscle chloride channel by transposon insertion in myotonic mice. Nature. 1991;354:304–308 [DOI] [PubMed] [Google Scholar]
- 3.Charlet BN, Savkur RS, Singh G et al. Loss of the muscle-specific chloride channel in type 1 myotonic dystrophy due to misregulated alternative splicing. Mol Cell. 2002;10:45–53 [DOI] [PubMed] [Google Scholar]
- 4.Lueck JD, Mankodi A, Swanson MS et al. Muscle chloride channel dysfunction in two mouse models of myotonic dystrophy. J Gen Physiol. 2007;129:79–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ptacek LJ, George AL Jr., Barchi RL et al. Mutations in an S4 segment of the adult skeletal muscle sodium channel cause paramyotonia congenita. Neuron. 1992;8:891–897 [DOI] [PubMed] [Google Scholar]
- 6.Rojas CV, Wang JZ, Schwartz LS et al. A Met-to-Val mutation in the skeletal muscle Na+ channel alpha-subunit in hyperkalaemic periodic paralysis. Nature. 1991;354:387–389 [DOI] [PubMed] [Google Scholar]
- 7.Lehmann-Horn F, Jurkat-Rott K, Rudel R. Diagnostics and therapy of muscle channelopathies--Guidelines of the Ulm Muscle Centre. Acta Myol. 2008;27:98–113 [PMC free article] [PubMed] [Google Scholar]
- 8.Cannon SC. Channelopathies of skeletal muscle excitability. Compr Physiol. 2015;5:761–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trivedi JR, Cannon SC, Griggs RC. Nondystrophic myotonia: challenges and future directions. Exp Neurol. 2014;253:28–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ptacek LJ, George AL Jr., Griggs RC et al. Identification of a mutation in the gene causing hyperkalemic periodic paralysis. Cell. 1991;67:1021–1027 [DOI] [PubMed] [Google Scholar]
- 11.Ptacek LJ, Tawil R, Griggs RC et al. Dihydropyridine receptor mutations cause hypokalemic periodic paralysis. Cell. 1994;77:863–868 [DOI] [PubMed] [Google Scholar]
- 12.Ryan DP, da Silva MR, Soong TW et al. Mutations in potassium channel Kir2.6 cause susceptibility to thyrotoxic hypokalemic periodic paralysis. Cell. 2010;140:88–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Plaster NM, Tawil R, Tristani-Firouzi M et al. Mutations in Kir2.1 cause the developmental and episodic electrical phenotypes of Andersen’s syndrome. Cell. 2001;105:511–519 [DOI] [PubMed] [Google Scholar]
- 14.Cannon SC. Sodium Channelopathies of Skeletal Muscle. Handb Exp Pharmacol. 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burge JA, Hanna MG. Novel insights into the pathomechanisms of skeletal muscle channelopathies. Curr Neurol Neurosci Rep. 2012;12:62–69 [DOI] [PubMed] [Google Scholar]
- 16.Fialho D, Griggs RC, Matthews E. Periodic paralysis. Handb Clin Neurol. 2018;148:505–520 [DOI] [PubMed] [Google Scholar]
- 17.Statland JM, Fontaine B, Hanna MG et al. Review of the Diagnosis and Treatment of Periodic Paralysis. Muscle Nerve. 2018;57:522–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sansone VA, Burge J, McDermott MP et al. Randomized, placebo-controlled trials of dichlorphenamide in periodic paralysis. Neurology. 2016;86:1408–1416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greig SL. Dichlorphenamide: A Review in Primary Periodic Paralyses. Drugs. 2016;76:501–507 [DOI] [PubMed] [Google Scholar]
- 20.Katz B, Miledi R. The Effect of Calcium on Acetylcholine Release from Motor Nerve Terminals. Proc R Soc Lond B Biol Sci. 1965;161:496–503 [DOI] [PubMed] [Google Scholar]
- 21.Katz B, Miledi R. The binding of acetylcholine to receptors and its removal from the synaptic cleft. J Physiol. 1973;231:549–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wood SJ, Slater CR. Safety factor at the neuromuscular junction. Prog Neurobiol. 2001;64:393–429 [DOI] [PubMed] [Google Scholar]
- 23.Rich MM. The control of neuromuscular transmission in health and disease. Neuroscientist. 2006;12:134–142 [DOI] [PubMed] [Google Scholar]
- 24.Peachey LD. Structure and Function of the T-System of Vertebrate Skeletal Muscle In: Tower DB, ed. The Nervous System. Vol. 1 New York: Raven Press, 1975:81–89 [Google Scholar]
- 25.Hernandez-Ochoa EO, Schneider MF. Voltage sensing mechanism in skeletal muscle excitation-contraction coupling: coming of age or midlife crisis? Skelet Muscle. 2018;8:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Franzini-Armstrong C The relationship between form and function throughout the history of excitation-contraction coupling. J Gen Physiol. 2018;150:189–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev. 2008;88:287–332 [DOI] [PubMed] [Google Scholar]
- 28.Heinemann S, Merlie J, Lindstrom J. Modulation of acetylcholine receptor in rat diaphragm by anti-receptor sera. Nature. 1978;274:65–68 [DOI] [PubMed] [Google Scholar]
- 29.Bevan S, Heinemann S, Lennon VA, Lindstrom J. Reduced muscle acetylcholine sensitivity in rats immunised with acetylcholine receptor. Nature. 1976;260:438–439 [DOI] [PubMed] [Google Scholar]
- 30.Cull-Candy SG, Miledi R, Trautmann A, Uchitel OD. On the release of transmitter at normal, myasthenia gravis and myasthenic syndrome affected human end-plates. J Physiol. 1980;299:621–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Engisch KL, Rich MM, Cook N, Nowycky MC. Lambert-Eaton antibodies inhibit Ca2+ currents but paradoxically increase exocytosis during stimulus trains in bovine adrenal chromaffin cells. J Neurosci. 1999;19:3384–3395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang X, Rich MM. Homeostatic synaptic plasticity at the neuromuscular junction in myasthenia gravis. Ann N Y Acad Sci. 2018;1412:170–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsujino A, Maertens C, Ohno K et al. Myasthenic syndrome caused by mutation of the SCN4A sodium channel. Proc Natl Acad Sci U S A. 2003;100:7377–7382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arnold WD, Feldman DH, Ramirez S et al. Defective fast inactivation recovery of Nav 1.4 in congenital myasthenic syndrome. Ann Neurol. 2015;77:840–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fitzhugh R. Thresholds and plateaus in the Hodgkin-Huxley nerve equations. J Gen Physiol. 1960;43:867–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Noble D, Stein RB. The threshold conditions for intitiation of action potentials by excitable cells. Journal of Physiology. 1966;187:129–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hawash AA, Voss AA, Rich MM. Inhibiting persistent inward sodium currents prevents myotonia. Ann Neurol. 2017;82:385–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adrian RH, Bryant SH. On the repetitive discharge in myotonic muscle fibres. J Physiol. 1974;240:505–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adrian RH, Marshall MW. Action potentials reconstructed in normal and myotonic muscle fibres. J Physiol. 1976;258:125–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wallinga W, Meijer SL, Alberink MJ et al. Modelling action potentials and membrane currents of mammalian skeletal muscle fibres in coherence with potassium concentration changes in the T-tubular system. Eur Biophys J. 1999;28:317–329 [DOI] [PubMed] [Google Scholar]
- 41.Fraser JA, Huang CL, Pedersen TH. Relationships between resting conductances, excitability, and t-system ionic homeostasis in skeletal muscle. J Gen Physiol. 2011;138:95–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palade PT, Barchi RL. Characteristics of the chloride conductance in muscle fibers of the rat diaphragm. J Gen Physiol. 1977;69:325–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steinmeyer K, Ortland C, Jentsch TJ. Primary structure and functional expression of a developmentally regulated skeletal muscle chloride channel. Nature. 1991;354:301–304 [DOI] [PubMed] [Google Scholar]
- 44.Gage PW, Lamb GD, Wakefield BT. Transient and persistent sodium currents in normal and denervated mammalian skeletal muscle. J Physiol. 1989;418:427–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patlak JB, Ortiz M. Two modes of gating during late Na+ channel currents in frog sartorius muscle. J Gen Physiol. 1986;87:305–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bean BP. The action potential in mammalian central neurons. Nat Rev Neurosci. 2007;8:451–465 [DOI] [PubMed] [Google Scholar]
- 47.Heckman CJ, Enoka RM. Motor unit. Compr Physiol. 2012;2:2629–2682 [DOI] [PubMed] [Google Scholar]
- 48.Iglesias C, Meunier C, Manuel M et al. Mixed mode oscillations in mouse spinal motoneurons arise from a low excitability state. J Neurosci. 2011;31:5829–5840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nardelli P, Powers R, Cope TC, Rich MM. Increasing motor neuron excitability to treat weakness in sepsis. Ann Neurol. 2017;82:961–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Caldwell JH, Schaller KL, Lasher RS et al. Sodium channel Na(v)1.6 is localized at nodes of ranvier, dendrites, and synapses. Proc Natl Acad Sci U S A. 2000;97:5616–5620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chatelier A, Zhao J, Bois P, Chahine M. Biophysical characterisation of the persistent sodium current of the Nav1.6 neuronal sodium channel: a single-channel analysis. Pflugers Arch. 2010;460:77–86 [DOI] [PubMed] [Google Scholar]
- 52.Cross KP, Robertson RM. Ionic mechanisms maintaining action potential conduction velocity at high firing frequencies in an unmyelinated axon. Physiol Rep. 2016;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.El-Bizri N, Kahlig KM, Shyrock JC et al. Ranolazine block of human Na v 1.4 sodium channels and paramyotonia congenita mutants. Channels (Austin). 2011;5:161–172 [DOI] [PubMed] [Google Scholar]
- 54.Cannon SC, Brown RH Jr., Corey DP. A sodium channel defect in hyperkalemic periodic paralysis: potassium-induced failure of inactivation. Neuron. 1991;6:619–626 [DOI] [PubMed] [Google Scholar]
- 55.Dupont C, Denman KS, Hawash AA et al. Treatment of myotonia congenita with retigabine in mice. Exp Neurol. 2019;315:52–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aickin CC, Betz WJ, Harris GL. Intracellular chloride and the mechanism for its accumulation in rat lumbrical muscle. J Physiol. 1989;411:437–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Heiny JA, Cannon SC, DiFranco M. A four-electrode method to study dynamics of ion activity and transport in skeletal muscle fibers. J Gen Physiol. 2019;151:1146–1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baekgaard Nielsen O, de Paoli FV, Riisager A, Pedersen TH. Chloride Channels Take Center Stage in Acute Regulation of Excitability in Skeletal Muscle: Implications for Fatigue. Physiology (Bethesda). 2017;32:425–434 [DOI] [PubMed] [Google Scholar]
- 59.Pedersen TH, Riisager A, de Paoli FV et al. Role of physiological ClC-1 Cl- ion channel regulation for the excitability and function of working skeletal muscle. J Gen Physiol. 2016;147:291–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Struyk AF, Cannon SC. Paradoxical depolarization of BA2+- treated muscle exposed to low extracellular K+: insights into resting potential abnormalities in hypokalemic paralysis. Muscle Nerve. 2008;37:326–337 [DOI] [PubMed] [Google Scholar]
- 61.Hibino H, Inanobe A, Furutani K et al. Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol Rev. 2010;90:291–366 [DOI] [PubMed] [Google Scholar]
- 62.Su TR, Zei WS, Su CC et al. The Effects of the KCNQ Openers Retigabine and Flupirtine on Myotonia in Mammalian Skeletal Muscle Induced by a Chloride Channel Blocker. Evid Based Complement Alternat Med. 2012;2012:803082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Main MJ, Cryan JE, Dupere JR et al. Modulation of KCNQ2/3 potassium channels by the novel anticonvulsant retigabine. Mol Pharmacol. 2000;58:253–262 [DOI] [PubMed] [Google Scholar]
- 64.Wickenden AD, Yu W, Zou A et al. Retigabine, a novel anti-convulsant, enhances activation of KCNQ2/Q3 potassium channels. Mol Pharmacol. 2000;58:591–600 [DOI] [PubMed] [Google Scholar]
- 65.Hayashi H, Iwata M, Tsuchimori N, Matsumoto T. Activation of peripheral KCNQ channels attenuates inflammatory pain. Mol Pain. 2014;10:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Banas K, Clow C, Jasmin BJ, Renaud JM. The KATP channel Kir6.2 subunit content is higher in glycolytic than oxidative skeletal muscle fibers. Am J Physiol Regul Integr Comp Physiol. 2011;301:R916–925 [DOI] [PubMed] [Google Scholar]
- 67.Koganti SR, Zhu Z, Subbotina E et al. Disruption of KATP channel expression in skeletal muscle by targeted oligonucleotide delivery promotes activity-linked thermogenesis. Mol Ther. 2015;23:707–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tricarico D, Barbieri M, Antonio L et al. Dualistic actions of cromakalim and new potent 2H-1,4-benzoxazine derivatives on the native skeletal muscle K ATP channel. Br J Pharmacol. 2003;139:255–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pedersen TH, de Paoli FV, Flatman JA, Nielsen OB. Regulation of ClC-1 and KATP channels in action potential-firing fast-twitch muscle fibers. J Gen Physiol. 2009;134:309–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reimann F, Gribble FM, Ashcroft FM. Differential response of K(ATP) channels containing SUR2A or SUR2B subunits to nucleotides and pinacidil. Mol Pharmacol. 2000;58:1318–1325 [DOI] [PubMed] [Google Scholar]
- 71.Grafe P, Quasthoff S, Strupp M, Lehmann-Horn F. Enhancement of K+ conductance improves in vitro the contraction force of skeletal muscle in hypokalemic periodic paralysis. Muscle Nerve. 1990;13:451–457 [DOI] [PubMed] [Google Scholar]
- 72.McTaggart JS, Clark RH, Ashcroft FM. The role of the KATP channel in glucose homeostasis in health and disease: more than meets the islet. J Physiol. 2010;588:3201–3209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tricarico D, Barbieri M, Mele A et al. Carbonic anhydrase inhibitors are specific openers of skeletal muscle BK channel of K+-deficient rats. FASEB J. 2004;18:760–761 [DOI] [PubMed] [Google Scholar]
- 74.Dinardo MM, Camerino G, Mele A et al. Splicing of the rSlo gene affects the molecular composition and drug response of Ca2+-activated K+ channels in skeletal muscle. PLoS One. 2012;7:e40235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jensen BS. BMS-204352: a potassium channel opener developed for the treatment of stroke. CNS Drug Rev. 2002;8:353–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brinkmeier H TRP channels in skeletal muscle: gene expression, function and implications for disease. Adv Exp Med Biol. 2011;704:749–758 [DOI] [PubMed] [Google Scholar]
- 77.Gailly P TRP channels in normal and dystrophic skeletal muscle. Curr Opin Pharmacol. 2012;12:326–334 [DOI] [PubMed] [Google Scholar]
- 78.Moran MM. TRP Channels as Potential Drug Targets. Annu Rev Pharmacol Toxicol. 2018;58:309–330 [DOI] [PubMed] [Google Scholar]
- 79.Sartiani L, Mannaioni G, Masi A et al. The Hyperpolarization-Activated Cyclic Nucleotide-Gated Channels: from Biophysics to Pharmacology of a Unique Family of Ion Channels. Pharmacol Rev. 2017;69:354–395 [DOI] [PubMed] [Google Scholar]
- 80.Cao Y, Pang J, Zhou P. HCN Channel as Therapeutic Targets for Heart Failure and Pain. Curr Top Med Chem. 2016;16:1855–1861 [DOI] [PubMed] [Google Scholar]
- 81.Terry EE, Zhang X, Hoffmann C et al. Transcriptional profiling reveals extraordinary diversity among skeletal muscle tissues. Elife. 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Desaphy JF, Carbonara R, Costanza T, Conte Camerino D. Preclinical evaluation of marketed sodium channel blockers in a rat model of myotonia discloses promising antimyotonic drugs. Exp Neurol. 2014;255:96–102 [DOI] [PMC free article] [PubMed] [Google Scholar]